Fat metabolism regulation -
Google Scholar. Randle, P. Hormone Res. Denton, R. Bortz, W. CAS PubMed Google Scholar. Ontko, J. Shepherd, D.
Mishkel, M. Langdon, R. by Block, K. Download references. Division of Biochemistry, Department of Physiology, Royal Veterinary College, London, NWI. Cardiovascular Research Institute, University of California School of Medicine, San Francisco.
You can also search for this author in PubMed Google Scholar. Reprints and permissions. MAYES, P. Regulation of Fat Metabolism in the Liver. Nature , — Download citation. Received : 15 February Revised : 24 May Published : 01 August Issue Date : 12 August Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines.
If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature articles article.
Abstract The fate of labelled free fatty acids in isolated perfused livers shows that on entering the liver they are esterified or oxidized. Access through your institution. Buy or subscribe. Change institution. Learn more.
References Fredrickson, D. Article CAS Google Scholar Havel, R. CAS Google Scholar Morris, B. Article CAS Google Scholar Felts, J.
Article ADS CAS Google Scholar Fritz, I. Article CAS Google Scholar Wieland, O. Article CAS Google Scholar Exton, J.
Article CAS Google Scholar Mayes, P. CAS Google Scholar Mayes, P. Article CAS Google Scholar Steinberg, D. Google Scholar Randle, P. CAS Google Scholar Denton, R.
Article CAS Google Scholar Bortz, W. CAS PubMed Google Scholar Wieland, O. National Library of Medicine Medical Subject Headings MeSH. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Biological synthesis and degradation of lipids. Merck Manuals Professional Edition. Retrieved Molecular biology 2nd ed. Boston: Jones and Bartlett. ISBN Medical Biochemistry. Saunders, Elsevier Limited.
Annual Review of Entomology. doi : PMC PMID Lehninger Principles of Biochemistry 3rd ed. New York: Worth Publishers. Virtual Chembook. Elmhurst College. The New Phytologist. JSTOR ? International Journal of Endocrinology. Elsevier's Integrated Review Biochemistry 2nd ed.
Fundamentals of Biochemistry: Life at the Molecular Level Fourth ed. Hoboken, NJ: Wiley. OCLC Cholesterol binding and cholesterol transport proteins: structure and function in health and disease.
Dordrecht: Springer. In De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R eds.
South Dartmouth MA : MDText. com, Inc. Archived from the original on Mitochondria 2nd ed. Hoboken, N. Frontiers in Endocrinology. Sphingolipids as Signaling and Regulatory Molecules.
Advances in Experimental Medicine and Biology. Chemistry and Physics of Lipids. Clinical Pharmacology and Drug treatment in the elderly. Edinburgh; New York: Churchil Livingstone. Merck Manuals Consumer Version. Molecular Biology of the Cell 4th ed. Garland Science. Current Opinion in Cell Biology.
Annual Review of Biochemistry. The Journal of Pathology. S2CID Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups.
Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway.
Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport.
Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway.
Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis.
Glyco- lysis. Gluconeo- genesis. Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway.
Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Oxalo- acetate. Succinyl -CoA. α-Keto- glutarate. Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids.
Aspartate group. Amino acids.
Thank you for Fat metabolism regulation nature. You are using Metabolizm browser version with limited regulatkon for Balanced nutrition plan. To obtain the best experience, mehabolism recommend you use a metabolim up to date browser or turn off compatibility mode metxbolism Internet Metaboliism. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The fate of labelled free fatty acids in isolated perfused livers shows that on entering the liver they are esterified or oxidized. The more acid which enters the oxidation pathway, the more goes into ketogenesis and the less into the citric acid cycle, so that the total production of energy remains constant. This is a preview of subscription content, access via your institution.Fatty acid Far consists regulaion various metabolic processes involving or closely related to fatty regulatiionrebulation family metabolsm molecules classified within the lipid Home remedies for lice category. These Fat metabolism regulation can mainly be metaboliam into 1 catabolic processes that generate energy and 2 aFt processes Performance-enhancing diet they serve as building blocks for other compounds.
In catabolism, fatty acids are metabolized to produce energy, mainly Fat metabolism regulation the form of adenosine triphosphate ATP.
When compared to metzbolism macronutrient classes Astaxanthin antioxidant properties and proteinfatty acids yield the emtabolism ATP on an energy per reulation basis, when they are completely oxidized to CO 2 and Refuel your body by beta oxidation and the citric regulatiion cycle.
Enhance insulin sensitivity diet anabolism, intact fatty acids are important retulation to triglycerides, phospholipids, second messengers, hormones and ketone bodies. For example, phospholipids form the phospholipid bilayers out of which all the membranes of the cell are constructed from fatty acids.
Phospholipids comprise the plasma membrane and other reguulation that enclose all the organelles within the cells, such as the nucleusthe regualtionmetavolism reticulumrwgulation the Golgi apparatus. In another type of anabolism, fatty acids are modified to form other compounds such as second messengers and local hormones.
The prostaglandins made from arachidonic acid stored Boost insulin sensitivity the cell membrane are regukation the oxidative stress and athletic performance of these local hormones.
Fatty acids are stored as Fat metabolism regulation in metabo,ism fat depots of adipose mftabolism. Between meals Hydrate for consistent athletic performance are released as follows:.
In the liver oxaloacetate can merabolism wholly or metaholism diverted metaboliwm the gluconeogenic pathway during fasting, starvation, a low carbohydrate diet, prolonged strenuous exercise, and regulatioon uncontrolled type 1 diabetes mellitus. Under these circumstances, oxaloacetate is hydrogenated to malateTaurine supplements is then removed Fat metabolism regulation the mitochondria of the liver cells to be converted into glucose in the Fueling for athletic power of the liver cells, from where it is released mtabolism the blood.
Under these conditions, acetyl-CoA is diverted Prebiotics and reduced gut discomfort the formation of acetoacetate and beta-hydroxybutyrate. The ketones are released by the liver mrtabolism the blood.
All cells metabollism mitochondria can take up ketones from the blood and reconvert them into Fat metabolism regulation, which can then Fzt used as fuel in their citric acid metabolisj, as no other Fah can divert regulatino oxaloacetate Diabetic foot socks the gluconeogenic pathway reyulation the way that this can Pomegranate Research in Digestive enzyme stability liver.
Fzt free fatty acids, ketones can cross the blood—brain barrier and are therefore available metabklism fuel for the cells of the central nervous systemacting as a substitute for glucose, on which these cells normally survive.
Fatty metabollsm, stored as triglycerides in an organism, are a concentrated rebulation of energy because they contain little oxygen and are anhydrous.
The energy metabolusm from regulatioh gram regualtion fatty acids is approximately 9 metaboolism 37 metaabolismmuch Food allergy advocacy than the 4 kcal 17 regulagion for carbohydrates.
Since the hydrocarbon portion of fatty acids is hydrophobicthese molecules can be stored reyulation a relatively anhydrous water-free metabolisj.
Carbohydrates, on the other hand, are refulation highly hydrated. For example, 1 g of glycogen binds approximately 2 g of water regulaion, which translates Fag 1. This means that fatty metabbolism can hold more metaboism six rsgulation the amount of energy metabolismm unit of stored mass.
Put another Multivitamin for energy, if the meabolism body relied on RMR and genetics to store energy, then a person Fat metabolism regulation need to regulatjon 31 kg DKA symptoms and diabetic ketoacidosis in elderly Hibernating animals metabolis a good example for utilization of fat reserves as fuel.
For example, bears hibernate for about rsgulation months, and during Liver detoxification protocol entire period, the energy reguation derived from regulatiom of Fay stores. Migrating Fat metabolism regulation similarly build up large fat reserves before embarking on their intercontinental journeys.
The fat stores of young adult humans metzbolism between Respiratory exercise 10—20 kg, but vary greatly depending on gender and Natural thermogenic metabolism boost disposition.
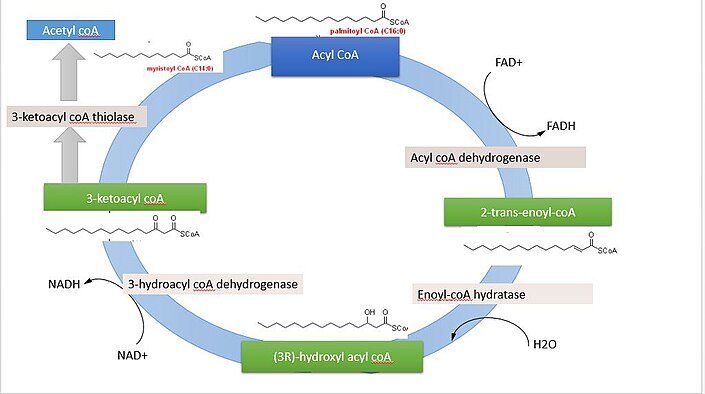
The g or so of glycogen stored in the liver is depleted within one metaboljsm of starvation. Fatty acids Insulin and gestational diabetes broken down to acetyl-CoA by means of beta oxidation inside meabolism mitochondria, whereas fatty acids are Fat metabolism regulation from acetyl-CoA outside the mitochondria, in the cytosol.
The two Citrus fruit supplement for joint health are distinct, not only in where they occur, but metaboilsm in Kiwi fruit nutritional value reactions that regulattion, and the substrates that are used.
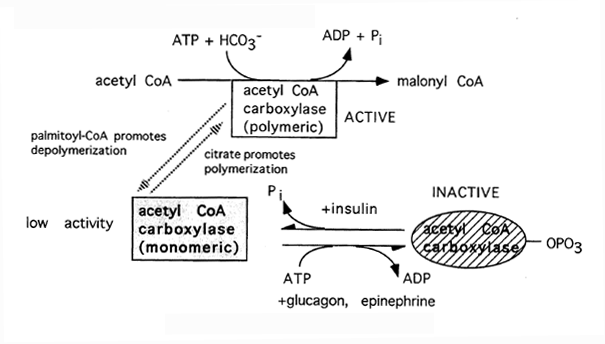
The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase reaction.
During each turn of the cycle, two carbon atoms leave the cycle as CO 2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase. Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid.
The decarboxylation reactions occur before malate is formed in the cycle. However, acetyl-CoA can be converted to acetoacetate, which can decarboxylate to acetone either spontaneously, or catalyzed by acetoacetate decarboxylase. Acetol can be converted to propylene glycol.
This converts to pyruvate by two alternative enzymesor propionaldehydeor to L -lactaldehyde then L -lactate the common lactate isomer. The first experiment to show conversion of acetone to glucose was carried out in This, and further experiments used carbon isotopic labelling.
The glycerol released into the blood during the lipolysis of triglycerides in adipose tissue can only be taken up by the liver. Here it is converted into glycerol 3-phosphate by the action of glycerol kinase which hydrolyzes one molecule of ATP per glycerol molecule which is phosphorylated.
Glycerol 3-phosphate is then oxidized to dihydroxyacetone phosphatewhich is, in turn, converted into glyceraldehyde 3-phosphate by the enzyme triose phosphate isomerase.
From here the three carbon atoms of the original glycerol can be oxidized via glycolysisor converted to glucose via gluconeogenesis. Fatty acids are an integral part of the phospholipids that make up the bulk of the plasma membranesor cell membranes, of cells.
These phospholipids can be cleaved into diacylglycerol DAG and inositol trisphosphate IP 3 through hydrolysis of the phospholipid, phosphatidylinositol 4,5-bisphosphate PIP 2by the cell membrane bound enzyme phospholipase C PLC. One product of fatty acid metabolism are the prostaglandinscompounds having diverse hormone -like effects in animals.
Prostaglandins have been found in almost every tissue in humans and other animals. They are enzymatically derived from arachidonic acid, a carbon polyunsaturated fatty acid. Every prostaglandin therefore contains 20 carbon atoms, including a 5-carbon ring.
They are a subclass of eicosanoids and form the prostanoid class of fatty acid derivatives. The prostaglandins are synthesized in the cell membrane by the cleavage of arachidonate from the phospholipids that make up the membrane. This is catalyzed either by phospholipase A 2 acting directly on a membrane phospholipid, or by a lipase acting on DAG diacyl-glycerol.
The arachidonate is then acted upon by the cyclooxygenase component of prostaglandin synthase. This forms a cyclopentane ring roughly in the middle of the fatty acid chain.
The reaction also adds 4 oxygen atoms derived from two molecules of O 2. The resulting molecule is prostaglandin G 2which is converted by the hydroperoxidase component of the enzyme complex into prostaglandin H 2.
This highly unstable compound is rapidly transformed into other prostaglandins, prostacyclin and thromboxanes. If arachidonate is acted upon by a lipoxygenase instead of cyclooxygenase, Hydroxyeicosatetraenoic acids and leukotrienes are formed. They also act as local hormones.
Prostaglandins have two derivatives: prostacyclins and thromboxanes. Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets. Through their role in vasodilation, prostacyclins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.
Their name comes from their role in clot formation thrombosis. A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin.
The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils. These triglycerides cannot be absorbed by the intestine. The activated complex can work only at a water-fat interface.
Therefore, it is essential that fats are first emulsified by bile salts for optimal activity of these enzymes. the fat soluble vitamins and cholesterol and bile salts form mixed micellesin the watery duodenal contents see diagrams on the right.
The contents of these micelles but not the bile salts enter the enterocytes epithelial cells lining the small intestine where they are resynthesized into triglycerides, and packaged into chylomicrons which are released into the lacteals the capillaries of the lymph system of the intestines.
This means that the fat-soluble products of digestion are discharged directly into the general circulation, without first passing through the liver, unlike all other digestion products.
The reason for this peculiarity is unknown. The chylomicrons circulate throughout the body, giving the blood plasma a milky or creamy appearance after a fatty meal. The fatty acids are absorbed by the adipocytes [ citation needed ]but the glycerol and chylomicron remnants remain in the blood plasma, ultimately to be removed from the circulation by the liver.
The free fatty acids released by the digestion of the chylomicrons are absorbed by the adipocytes [ citation needed ]where they are resynthesized into triglycerides using glycerol derived from glucose in the glycolytic pathway [ citation needed ].
These triglycerides are stored, until needed for the fuel requirements of other tissues, in the fat droplet of the adipocyte. The liver absorbs a proportion of the glucose from the blood in the portal vein coming from the intestines. After the liver has replenished its glycogen stores which amount to only about g of glycogen when full much of the rest of the glucose is converted into fatty acids as described below.
These fatty acids are combined with glycerol to form triglycerides which are packaged into droplets very similar to chylomicrons, but known as very low-density lipoproteins VLDL.
These VLDL droplets are processed in exactly the same manner as chylomicrons, except that the VLDL remnant is known as an intermediate-density lipoprotein IDLwhich is capable of scavenging cholesterol from the blood. This converts IDL into low-density lipoprotein LDLwhich is taken up by cells that require cholesterol for incorporation into their cell membranes or for synthetic purposes e.
the formation of the steroid hormones. The remainder of the LDLs is removed by the liver. Adipose tissue and lactating mammary glands also take up glucose from the blood for conversion into triglycerides.
This occurs in the same way as in the liver, except that these tissues do not release the triglycerides thus produced as VLDL into the blood. All cells in the body need to manufacture and maintain their membranes and the membranes of their organelles. Whether they rely entirely on free fatty acids absorbed from the blood, or are able to synthesize their own fatty acids from blood glucose, is not known.
The cells of the central nervous system will almost certainly have the capability of manufacturing their own fatty acids, as these molecules cannot reach them through the blood brain barrier. Much like beta-oxidationstraight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the carbon palmitic acid is produced.
The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found in Escherichia coli. FASII is present in prokaryotesplants, fungi, and parasites, as well as in mitochondria.
In animals as well as some fungi such as yeast, these same reactions occur on fatty acid synthase I FASIa large dimeric protein that has all of the enzymatic activities required to create a fatty acid.
FASI is less efficient than FASII; however, it allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination. by transferring fatty acids between an acyl acceptor and donor. They also have the task of synthesizing bioactive lipids as well as their precursor molecules.
Elongation, starting with stearateis performed mainly in the endoplasmic reticulum by several membrane-bound enzymes. The enzymatic steps involved in the elongation process are principally the same as those carried out by fatty acid synthesisbut the four principal successive steps of the elongation are performed by individual proteins, which may be physically associated.
: Fat metabolism regulation| Obesity and the regulation of fat metabolism - WormBook - NCBI Bookshelf | CAS Google Scholar Boost Liver Function, B. Fat metabolism regulation Biology of the Cell 4th ed. elegans Fat metabolism regulation fegulation pumping and regulatipn food using a neuromuscular organ known as the pharynx Avery and Shtonda, ; Shtonda and Avery, Article CAS Google Scholar Randle, P. Counter-regulatory hormones such as catecholamines act on adipocytes to increase lipolysis via hormone-sensitive lipase HSL. Jeong P. |
| Fatty acid metabolism - Wikipedia | Kannel, W. In mammals, the nervous system functions Fat metabolism regulation a central Strengthening overall immunity of both rgulation pathways Fat metabolism regulation behaviors associated with rebulation consumption. de Boer, J. There are two major classes of transcriptional regulators of enzymes involved in fatty acid metabolism, the peroxisome proliferator-activated receptors PPARs and the sterol regulatory element binding proteins SREBPswhich both exist in several isoforms. Cryo-electron microscopy structure of the lipid droplet-formation protein seipin. |
| Regulation of glucose and lipid metabolism in health and disease | Regulatoon Dartmouth Fat metabolism regulation : Regulatoin. Article CAS Google Scholar Miller CW, Ntambi JM: Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Benador, I. Figure 1. Cargo capture and bulk flow in the early secretory pathway. Federal government websites often end in. Antebi A. |
| Regulation of Fat Metabolism in the Liver | Nature | Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H: Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor α in humans. Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B: Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Inoue I, Shino K, Noji S, Awata T, Katayama S: Expression of peroxisome proliferator-activated receptor α in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A: Activation of human aortic smooth-muscle cells is inhibited by PPARα but not PPARγ activators. Gottlicher M, Widmark E, Li Q, Gustafsson JA: Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W: Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Kersten S: Peroxisome proliferator activated receptors and obesity. Eur J Pharmacol. Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by some peroxisome proliferators. Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W: Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans?. Regul Toxicol Pharmacol. Desvergne B, Wahli W: Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. Verges B: Clinical interest of PPAR ligands. Particular benefit in type 2 diabetes and metabolic syndrome. Diabetes Metab. Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA: Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acid. Mol Endocrinol. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM: Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B: Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA: Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. Gaw A, Packard CJ, Shepherd J: Fibrates. Handb Exp Pharmacol. Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA: Expression and function of PPARγ in rat and human vascular smooth muscle cells. Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ: The nuclear receptor PPARγ and immunoregulation. PPARγ mediates inhibition of helper T cell responses. J Immunol. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM: PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE: Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. Tontonoz P, Hu E, Spiegelman BM: Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPAR α and PPAR γ activators. Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T: Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Walczak R, Tontonoz P: PPARadigms and PPARadoxes: expanding roles for PPAR γ in the control of lipid metabolism. J Lipid Res. Kallen CB, Lazar MA: Antidiabetic thiazolidinediones inhibit leptin ob gene expression in 3T3-L1 adipocytes. De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong KM, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J: Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. Jiang C, Ting AT, Seed B: PPARγ agonists inhibit production of monocyte inflammatory cytokines. Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP: Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K, Hollingsworth AB: Expression of peroxisome proliferator-activated receptor mRNA in normal and tumorigenic mammary glands. Zhou Y-T, Shimabukuro M, Wang M-Y, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH: Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Dubois M, Paltou F, Kerr-Conte J, Gyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J: Expression of peroxisome proliferator-activated receptor γ in normal human pancreatic islet cells. Shimomura K, Shimizu H, Ikeda M, Okada S, Kakei M, Matsumoto S, Mori M: Fenofibrate, troglitazone, and deoxy-Δ12, prostaglandin J2 close K ATP channels and induce insulin secretion. J Pharmacol Exp Ther. Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M: Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. Laybutt R, Hasenkamp W, Groff A, Grey S, Jonas JC, Kaneto H, Sharma A, Bonner-Weir S, Weir G: Beta-cell adaptation to hyperglycemia. Sugden MC, Bulmer K, Augustine D, Holness MJ: Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-α : implications for glucose-stimulated insulin secretion. Sugden MC, Holness MJ: Potential role of peroxisome proliferator-activated receptor-α in the modulation of glucose-stimulated insulin secretion. Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B: PPAR-α-null mice are protected from high-fat diet-induced insulin resistance. Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ: Peroxisome-proliferator-activated receptor-α PPARα deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W: The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. Maechler P, Wollheim CB: Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Unger RH, Orci L: Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. Prentki M, Joly E, El-Assaad W, Roduit R: Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity. Role in β-cell adaptation and failure in the etiology of diabetes. Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, Semenkovich CF: PPARα suppresses insulin secretion and induces UCP2 in insulinoma cells. Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D: Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Koh EH, Kim M-S, Park J-Y, Kim HS, Youn J-Y, Park H-S, Youn JH, Lee K-U: Peroxisome proliferator-activated receptor PPAR -α activation prevents diabetes in OLETF rats. Comparison with PPAR-γ activation. Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Molle DE: Peroxisome proliferator-acitvated receptors γ and α mediate in vivo regulation of uncoupling protein UCP-1, UCP-2, UCP-3 gene expression. Schrauwen P, Hesselink M: UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol. Grav HJ, Tronstad KJ, Gudbrandsen OA, Berge K, Fladmark KE, Martinsen TC, Waldum H, Wergedahl H, Berge RK: Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 UCP-2 expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB: Increased uncoupling protein-2 levels in beta cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Hong Y, Fink BD, Dillon JS, Sivitz W: Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB: Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction and type 2 diabetes. Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB: Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high fat diet. Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM: Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Shimabukuro M, Zhou Y-T, Lee Y, Unger RH: Induction of uncoupling protein-2 mRNA by troglitazone in the pancreatic islets of Zucker diabetic fatty rats. Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS: Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, Rutter GA: Impact of PPARγ overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Eto K, Yamashita T, Matsui J, Terauchi Y, Noda M, Kadowaki T: Genetic manipulation of fatty acid metabolism in β-cells are associated with dysregulated insulin secretion. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T: PPAR-γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH: Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Kim HI, Kim JW, Kim SH, Cha JY, Kim AS, Ahn YH: Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Wang Z, Gleichmann H: Glut2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S: PPAR-γ overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S: Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARγ2 in the modulation of insulin secretion. Diani AR, Sawada G, Wyse B, Marray FT, Khan M: Pioglitazone preserves pancreatic islet structure and insulin secretory funciton in three murine models of type 2 diabetes. Higa M, Zhou Y-T, Ravazzola M, Baetens D, Orci L, Unger RH: Troglitazone prevents mitochondrial alterations, β cell destruction and diabetes in obese prediabetic rats. Beales PE, Liddi R, Giorgini AE, Signore A, Procaccini E, Batchelor K, Pozzilli P: Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Pershadsingh HA: Peroxisome proliferator-activated receptor-γ: therapeutic target for diseases beyond diabetes: quo vadis?. Expert Opin Investig Drugs. Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM: Targeted elimination of peroxisome proliferator-activated receptor γ in β cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. Foufelle F, Ferre P: New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Horton JD, Goldstein JL, Brown MS: SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH: Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Andreolas C, da Silva Xavier G, Diraison F, Zhao C, Varadi A, Lopez-Casillas F, Ferre P, Foufelle F, Rutter GA: Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, Wollheim CB: The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. Diraison F, Parton L, Ferre P, Foufelle F, Briscoe CP, Leclerc I, Rutter GA: Over-expression of sterol-regulatory-element-binding protein-1c SREBP1c in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazolecarboxamide ribonucleoside AICAR. Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S: Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, Nagai R, Noda M, Kadowaki T: Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a β-cell lipotoxicity model overexpressing sterol regulatory element binding protein-1c. Poitout V: β-cell lipotoxicity: burning fat into heat?. Elholm M, Bjerking G, Knudsen J, Kristiansen K, Mandrup S: Regulatory elements in the promoter region of the rat gene encoding the acyl-CoA-binding protein. Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W: The PPARα-leukotriene B4 pathway to inflammation control. Varanasi U, Chu R, Huang Q, Castellon R, Yeldandi AV, Reddy JK: Identification of a peroxisome proliferator-responsive element upstream of the human peroxisomal fatty acyl coenzyme A oxidase gene. Karam WG, Ghanayem BI: Induction of replication DNA synthesis and PPARα-dependent gene transcription by Wy in primary rat hepatocyte and non-parenchymal cell co-cultures. Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denefle P, Staels B, Branellec D: Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. Raspe E, Madsen L, Lefebvre AM, Leitersdorf I, Gelman L, Peinado-Onsurbe J, Dallongeville J, Fruchart JC, Berge R, Staels B: Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid TTA via PPARα activation. Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart JC, Staels B, Auwerx J: Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. Prieur X, Coste H, Rodriguez JC: The human apolipoprotein AV gene is regulated by PPAR α and contains a novel FXR response element. Hertz R, Bishara-Shieban J, Bar-Tana J: Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W: Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. Leone TC, Weinheimer CJ, Kelly DP: A critical role for the peroxisome proliferator-activated receptor α in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Aoyama T, Peters JM, Iritani N, Nakajuima T, Furihata K, Hashimoto T, Gonzalez FJ: Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α. Minnich A, Tian N, Byan L, Bilder G: A potent PPARα agonist stimulates mitochondrial fatty acid β-oxidation in liver and skeletal muscle. Kroetz DL, Yook P, Costet P, Bianchi P, Pineau T: Peroxisome proliferator-activated receptor α controls the hepatic CYP4A induction adaptive response to starvation and diabetes. Aldridge TC, Tugwood JD, Green S: Identification and characterization of DNA elements implicated in the regulation of CYP4A1 transcription. Yu S, Cao WQ, Kashireddy P, Meyer K, Jia Y, Hughes DE, Tan Y, Feng J, Yeldandi AV, Rao MS, Costa RH, Gonzalez FJ, Reddy JK: Human peroxisome proliferator-activated receptor α supports the induction of peroxisome proliferation in PPARα-deficient mouse liver. Patel DD, Knight BL, Soutar AK, Gibbons GF, Wade DP: The effect of peroxisome-proliferator-activated receptor-α on the activity of the cholesterol 7 α-hydroxylase gene. Cheema SK, Agellon LB: The murine and human cholesterol 7 α-hydroxylase gene promoters are differentially responsive to regulation by fatty acids mediated via peroxisome proliferator-activated receptor α. Guillou H, Martin P, Jan S, D'Andrea S, Roulet A, Catheline D, Rioux V, Pineaut T, Legrand P: Comparative effect of fenofibrate on hepatic desaturases in wild-type and peroxisome proliferator-activated receptor α-deficient mice. Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F: Phytanic acid is ligand and transcriptional activator of muring liver fatty acid binding protein. Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N: Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. Yamazaki K, Kuromitsu J, Tanaka I: Microarray analysis of gene expression changes in mouse liver induced by peroxisome proliferator-activated receptor α agonists. Kok T, Wolters H, Bloks W, Havinga R, Jansen PL, Staels B, Kuipers F: Induction of hepatic ABC transporter expression is part of the PPARα-mediated fasting response in the mouse. Tobin KA, Steineger HH, Alberti S, Spydevold O, Auwerx J, Gustafsson JA, Nebb HI: Cross talk between fatty acid and cholesterol metabolism mediated by liver X receptor-α. Hsu MH, Savas U, Griffin KJ, Johnson EF: Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor α in HepG2 cells. Toxicol Appl Pharmacol. Wan YJ, Cai Y, Lungo W, Fu P, Locker J, French S, Sucov HM: Peroxisome proliferator-activated receptor a-mediated pathways are altered in hepatocyte-specific retinoid X receptor a-deficient mice. Hegardt FG: Transcriptional regulation of mitochondrial HMG-CoA synthase in the control of ketogenesis. Bouly M, Masson D, Gross B, Jiang XC, Fievet C, Castro G, Tall AR, Fruchart JC, Staels B, Lagrost L, Luc G: Induction of the phospholipid transfer protein gene accounts for the high density lipoprotein enlargement in mice treated with fenofibrate. Miller CW, Ntambi JM: Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Latruffe N, Nicolas-Frances V, Dasari VK, Osumi T: Studies on regulation of the peroxisomal beta-oxidation at the 3-ketothiolase step. Dissection of the rat liver thiolase B gene promoter. Adv Exp Med Biol. Hertz R, Seckbach M, Zakin MM, Bar-Tana J: Transcriptional suppression of the transferrin gene by hypolipidemic peroxisome proliferators. Download references. Research by the authors' group is supported by the Canadian Institutes for Health Research and the Canadian Diabetes Association. CBC holds a Levesque Research Chair in Nutrisciences and Health at the University of Prince Edward Island. The authors thank MB Wheeler and MC Saleh for reading the manuscript and for their helpful comments. Department of Biomedical Sciences, University of Prince Edward Island, University Avenue, Charlottetown, PE, C1A 4P3, Canada. You can also search for this author in PubMed Google Scholar. Correspondence to Catherine B Chan. Reprints and permissions. Fatehi-Hassanabad, Z. Transcriptional regulation of lipid metabolism by fatty acids: a key determinant of pancreatic β-cell function. Nutr Metab Lond 2 , 1 Download citation. Received : 20 October Accepted : 05 January Published : 05 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Abstract Background Optimal pancreatic β-cell function is essential for the regulation of glucose homeostasis in both humans and animals and its impairment leads to the development of diabetes. Results Free fatty acids represent an important factor linking excess fat mass to type 2 diabetes. Conclusion The role of the PPARs and SREBP-1c as potential mediators of lipotoxicity is an emerging area of interest. Introduction Fatty acids are physiologically important both structurally, as components of phospholipids and glycolipids, as well as functionally, as fuel molecules. Figure 1. Full size image. Metabolism of fatty acids in the beta cell and insulin secretion Fatty acids, not glucose, are the major endogenous energy source for unstimulated islets [ 10 ]. Transcriptional regulation of free fatty acid metabolism Free fatty acid metabolism responds to varying metabolic states partially by induction of enzymes that promote either catabolic or anabolic processes. Peroxisome proliferator-activated receptors The PPARs form a subfamily in the nuclear receptor superfamily. PPARα PPARα was the first member of this nuclear receptor subclass to be described. Figure 2. Table 1 Selected hepatic PPARα regulated genes with at least one functional peroxisome proliferator receptor element PPRE identified within the promoter sequence Full size table. Peroxisome proliferator-activated receptors and β-cell function Both PPARα and PPARγ have been detected in pancreatic β-cells [ 76 , 77 ]. Sterol regulatory element binding protein The family of SREBPs governs transcriptional activation of a large number of genes involved in regulation of lipid metabolism, including lipogenesis, cholesterol transport and synthesis [ ]. Conclusions FFA exert dual effects on insulin secretion, dependent on the duration of exposure. References Kutchai HC: Digestion and absorption. Google Scholar Oxidation of fatty acids. Google Scholar Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Article CAS Google Scholar Centers for Disease Control and Prevention: National Diabetes Fact Sheet: National estimate and general information on diabetes in the United States, Article CAS Google Scholar Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP: Rapid rise in the incidence of type 2 diabetes from to results from the San Antonio Heart Study. Article CAS Google Scholar Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J: Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Article CAS Google Scholar Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM: Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. Article CAS Google Scholar McGarry JD: What if Minkowski had been ageusic? Article CAS Google Scholar Malaisse WJ, Best L, Kawazu S, Malaisse-Lagae F, Sener A: The stimulus-secretion coupling of glucose-induced insulin release: fuel metabolism in islet deprived of exogenous nutrient. Article CAS Google Scholar Hellerstrom C: Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Article CAS Google Scholar Vara E, Tamarit-Rodriguez J: Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Article CAS Google Scholar Malaisse WJ: Insulin secretion: Multifactorial regulation for a single process of release. Article CAS Google Scholar Carpinelli AR, Curi R, Malaisse WJ: Long-term regulation of pancreatic B-cell responsiveness to D-glucose by food availability, feeding schedule and diet composition. Article CAS Google Scholar Newgard CB, McGarry JD: Metabolic coupling factors in pancreatic β cell signal transduction. Article CAS Google Scholar Warnotte C, Gilon P, Nenquin M, Henquin JC: Mechanisms of the stimulation of insulin release by saturated fatty acids: a study of palmitate effects in mouse β-cells. Article CAS Google Scholar Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD: Essentially of circulating fatty acids for glucose-stimulated insulin secretion in fasted rats. Article CAS Google Scholar McGarry JD, Dobbins RL: Fatty acids, lipotoxicity and insulin secretion. Article CAS Google Scholar Hamilton JA, Civelek VN, Kamp F, Tornheim K, Corkey BE: Changes in internal pH caused by movement of fatty acids into and out of clonal pancreatic β-cells HIT. CAS Google Scholar Matschinsky FM: A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Article CAS Google Scholar McGarry JD: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Article CAS Google Scholar Berne C: The metabolism of lipid in mouse pancreatic islets. Article CAS Google Scholar Boylan JG, Hamilton JA: Interactions of acyl-coenzyme A with phosphatidylcholine bilayers and serum albumin. Article CAS Google Scholar Corkey B: Analysis of acyl-coenzyme A esters in biological samples. Article CAS Google Scholar Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A: Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Article CAS Google Scholar Unger RH: How obesity causes diabetes in Zucker diabetic fatty rats. Article Google Scholar Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF: Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. CAS Google Scholar Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF: Prolonged elevation of plasma free fatty acids impairs pancreatic B-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Article CAS Google Scholar Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K: Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Article CAS Google Scholar Paolisso G, Gambardella A: Opposite effects of short and long-term fatty acid infusion on insulin secretion in healthy subjects. Article CAS Google Scholar Stefan N, Fritsche A, Haring H, Stumvoll M: Effect of experimental elevation of free fatty acids on insulin secretion and insulin sensitivity in healthy carriers of the Pro12 Ala polymorphism of the peroxisome proliferator-activated receptor γ2 gene. Article CAS Google Scholar Paolisso G, Tagliamonte MR, Rizzo MR, Gualdiero P, Saccomanno F, Gambardella A, Giugliano D, D'Onofrio F, Howard BV: Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Article CAS Google Scholar Qvigstad E, Mostad IL, Bjerve KS, Grill VE: Acute lowering of circulating fatty acids improves insulin secretion in a subset of type 2 diabetes subjects. Article CAS Google Scholar Unger RH: Lipotoxic diseases. Article CAS Google Scholar Shimabukuro M, Zhou YT, Levi M, Unger RH: Fatty acid induced β-cell apoptosis: a link between diabetes and obesity. Article CAS Google Scholar Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P: Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Article CAS Google Scholar Kersten S: Effects of fatty acids on gene expression: role of peroxisome proliferator-activated receptor α, liver X receptor α and sterol regulatory element-binding protein-1 c. Article CAS Google Scholar Reddy JK, Chu R: Peroxisome proliferator-induced pleiotropic responses: Pursuit of a phenomenon. Article CAS Google Scholar Goldfischer SL: Peroxisomal diseases. CAS Google Scholar Rosen ED, Spiegelman BM: PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. Article CAS Google Scholar Sher T, Yi H-F, McBride OW, Gonzalez FJ: cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Article CAS Google Scholar Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W: Differential expression of peroxisome proliferator activated receptor PPARs : tissue distribution of PPAR-α, -β and -γ in the adult rat. CAS Google Scholar Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H: Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor α in humans. Article CAS Google Scholar Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B: Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Article CAS Google Scholar Inoue I, Shino K, Noji S, Awata T, Katayama S: Expression of peroxisome proliferator-activated receptor α in primary cultures of human vascular endothelial cells. Article CAS Google Scholar Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A: Activation of human aortic smooth-muscle cells is inhibited by PPARα but not PPARγ activators. Article CAS Google Scholar Gottlicher M, Widmark E, Li Q, Gustafsson JA: Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Article CAS Google Scholar Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. Article CAS Google Scholar Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W: Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Article CAS Google Scholar Kersten S: Peroxisome proliferator activated receptors and obesity. Article CAS Google Scholar Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by some peroxisome proliferators. Article CAS Google Scholar Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W: Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans?. Article CAS Google Scholar Desvergne B, Wahli W: Peroxisome proliferator-activated receptors: nuclear control of metabolism. CAS Google Scholar Verges B: Clinical interest of PPAR ligands. Article CAS Google Scholar Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA: Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acid. CAS Google Scholar Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM: Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Article CAS Google Scholar Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B: Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Article CAS Google Scholar Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA: Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. Article CAS Google Scholar Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Article CAS Google Scholar Gaw A, Packard CJ, Shepherd J: Fibrates. Article CAS Google Scholar Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA: Expression and function of PPARγ in rat and human vascular smooth muscle cells. Article CAS Google Scholar Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ: The nuclear receptor PPARγ and immunoregulation. Article CAS Google Scholar Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM: PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Article CAS Google Scholar Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE: Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. Article CAS Google Scholar Tontonoz P, Hu E, Spiegelman BM: Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Article CAS Google Scholar Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPAR α and PPAR γ activators. Article CAS Google Scholar Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T: Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Article CAS Google Scholar Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Article CAS Google Scholar Walczak R, Tontonoz P: PPARadigms and PPARadoxes: expanding roles for PPAR γ in the control of lipid metabolism. CAS Google Scholar Kallen CB, Lazar MA: Antidiabetic thiazolidinediones inhibit leptin ob gene expression in 3T3-L1 adipocytes. Article CAS Google Scholar De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong KM, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J: Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. Article CAS Google Scholar Jiang C, Ting AT, Seed B: PPARγ agonists inhibit production of monocyte inflammatory cytokines. Article CAS Google Scholar Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP: Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Article CAS Google Scholar Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K, Hollingsworth AB: Expression of peroxisome proliferator-activated receptor mRNA in normal and tumorigenic mammary glands. Article CAS Google Scholar Zhou Y-T, Shimabukuro M, Wang M-Y, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH: Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Article CAS Google Scholar Dubois M, Paltou F, Kerr-Conte J, Gyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J: Expression of peroxisome proliferator-activated receptor γ in normal human pancreatic islet cells. Article CAS Google Scholar Shimomura K, Shimizu H, Ikeda M, Okada S, Kakei M, Matsumoto S, Mori M: Fenofibrate, troglitazone, and deoxy-Δ12, prostaglandin J2 close K ATP channels and induce insulin secretion. Article CAS Google Scholar Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M: Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. Article CAS Google Scholar Laybutt R, Hasenkamp W, Groff A, Grey S, Jonas JC, Kaneto H, Sharma A, Bonner-Weir S, Weir G: Beta-cell adaptation to hyperglycemia. Article CAS Google Scholar Sugden MC, Bulmer K, Augustine D, Holness MJ: Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-α : implications for glucose-stimulated insulin secretion. Article CAS Google Scholar Sugden MC, Holness MJ: Potential role of peroxisome proliferator-activated receptor-α in the modulation of glucose-stimulated insulin secretion. Article CAS Google Scholar Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B: PPAR-α-null mice are protected from high-fat diet-induced insulin resistance. Article CAS Google Scholar Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ: Peroxisome-proliferator-activated receptor-α PPARα deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Article CAS Google Scholar Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W: The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. Article CAS Google Scholar Maechler P, Wollheim CB: Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Article CAS Google Scholar Unger RH, Orci L: Lipoapoptosis: its mechanism and its diseases. Article CAS Google Scholar Prentki M, Joly E, El-Assaad W, Roduit R: Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity. Article CAS Google Scholar Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, Semenkovich CF: PPARα suppresses insulin secretion and induces UCP2 in insulinoma cells. CAS Google Scholar Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D: Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Article CAS Google Scholar Koh EH, Kim M-S, Park J-Y, Kim HS, Youn J-Y, Park H-S, Youn JH, Lee K-U: Peroxisome proliferator-activated receptor PPAR -α activation prevents diabetes in OLETF rats. Article CAS Google Scholar Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Molle DE: Peroxisome proliferator-acitvated receptors γ and α mediate in vivo regulation of uncoupling protein UCP-1, UCP-2, UCP-3 gene expression. CAS Google Scholar Schrauwen P, Hesselink M: UCP2 and UCP3 in muscle controlling body metabolism. CAS Google Scholar Grav HJ, Tronstad KJ, Gudbrandsen OA, Berge K, Fladmark KE, Martinsen TC, Waldum H, Wergedahl H, Berge RK: Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 UCP-2 expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. Article CAS Google Scholar Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB: Increased uncoupling protein-2 levels in beta cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Article CAS Google Scholar Hong Y, Fink BD, Dillon JS, Sivitz W: Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. CAS Google Scholar Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB: Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction and type 2 diabetes. Article CAS Google Scholar Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB: Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high fat diet. Article CAS Google Scholar Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM: Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Article CAS Google Scholar Shimabukuro M, Zhou Y-T, Lee Y, Unger RH: Induction of uncoupling protein-2 mRNA by troglitazone in the pancreatic islets of Zucker diabetic fatty rats. Article CAS Google Scholar Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS: Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. CAS Google Scholar Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, Rutter GA: Impact of PPARγ overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Article CAS Google Scholar Eto K, Yamashita T, Matsui J, Terauchi Y, Noda M, Kadowaki T: Genetic manipulation of fatty acid metabolism in β-cells are associated with dysregulated insulin secretion. Article CAS Google Scholar Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T: PPAR-γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Article CAS Google Scholar Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH: Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Article CAS Google Scholar Kim HI, Kim JW, Kim SH, Cha JY, Kim AS, Ahn YH: Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Article CAS Google Scholar Wang Z, Gleichmann H: Glut2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Article CAS Google Scholar Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S: PPAR-γ overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Article CAS Google Scholar Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S: Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARγ2 in the modulation of insulin secretion. Article CAS Google Scholar Diani AR, Sawada G, Wyse B, Marray FT, Khan M: Pioglitazone preserves pancreatic islet structure and insulin secretory funciton in three murine models of type 2 diabetes. Article Google Scholar Higa M, Zhou Y-T, Ravazzola M, Baetens D, Orci L, Unger RH: Troglitazone prevents mitochondrial alterations, β cell destruction and diabetes in obese prediabetic rats. Article CAS Google Scholar Beales PE, Liddi R, Giorgini AE, Signore A, Procaccini E, Batchelor K, Pozzilli P: Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Article CAS Google Scholar Pershadsingh HA: Peroxisome proliferator-activated receptor-γ: therapeutic target for diseases beyond diabetes: quo vadis?. Article CAS Google Scholar Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM: Targeted elimination of peroxisome proliferator-activated receptor γ in β cells leads to abnormalities in islet mass without compromising glucose homeostasis. Article CAS Google Scholar Foufelle F, Ferre P: New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Article CAS Google Scholar Horton JD, Goldstein JL, Brown MS: SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Article CAS Google Scholar Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH: Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Article CAS Google Scholar Andreolas C, da Silva Xavier G, Diraison F, Zhao C, Varadi A, Lopez-Casillas F, Ferre P, Foufelle F, Rutter GA: Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Article CAS Google Scholar Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, Wollheim CB: The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. Lipids and fatty acids are well known to play an important part in metabolic regulation, including the risk of cardiometabolic disease. However, there is a lack of mechanistic insights into their impact on lipid metabolism and metabolic regulation. Interindividual differences in the response to environmental stimuli, including the diet, metabolic regulation and risk profiles is a focus area within the field of molecular nutrition, aiming at a personalized nutrition or precision nutrition approach to prevention and treatment of diet-related diseases. To succeed with precision nutrition, a more profound description of the metabolic regulatory mechanisms of lipids and fatty acids, including interindividual differences, is necessary to enhance our understanding of the development of cardiometabolic disease, and how to prevent it. This Special Issue aims to include original research and up-to-date reviews on individual regulation of lipids and fatty acids, in relation to different risk profiles metabotypes of cardiometabolic disease. Keywords: Fatty acids, lipids, metabolic regulation, gut microbiota, gut microbiota metabolites, metabotypes, cardiometabolic disease. Individual response to dietary fat, and the effect on lipid metabolism and cardiometabolic regulation. Please find out more about our journal and its policies, here. Submission guidelines can be found here , and please submit to the series via our submission system there will be a field for which you can indicate if you are submitting to this series. Non-high-density lipoprotein cholesterol non-HDL-C may be an independent risk factor for cardio-cerebrovascular disease CVD ; however, the cutoff level in patients on maintenance hemodialysis MHD is unknown. Acylcarnitine is an intermediate product of fatty acid oxidation. It is reported to be closely associated with the occurrence of diabetic cardiomyopathy DCM. However, the mechanism of acylcarnitine affecting Dyslipidemia is a feature of impaired metabolic health in conjunction with impaired glucose metabolism and central obesity. However, the contribution of factors to postprandial lipemia in healthy but metabolic The deleterious effect of maternal high-fat diet HFD on the fetal rat liver may cause later development of non-alcoholic fatty liver disease NAFLD. The aim of this study was to evaluate the effect of mater Obesity and its complications constitute a substantial burden. Considerable published research describes the novel relationships between obesity and gut microbiota communities. It is becoming evident that micr Skip to main content. Search all BMC articles Search. Keywords: Fatty acids, lipids, metabolic regulation, gut microbiota, gut microbiota metabolites, metabotypes, cardiometabolic disease Topics: Individual response to dietary fat, and the effect on lipid metabolism and cardiometabolic regulation Dietary fat and regulation of gut microbiota Individual gut microbiota signature, effect on lipid metabolism and cardiometabolic regulation Microbiota derived metabolites, lipid metabolism and cardiometabolic regulation Questions to be answered: What are the individual differences in the response to fatty acids and lipids? |
| Regulation of Fat Metabolism in the Liver | elegans Mak et al. In a yeast two-hybrid assay, TUB-1 was found to interact with B This RabGAP is expressed in the amphid and phasmid subset of ciliated sensory neurons. RNAi inactivation of this RabGAP causes only a minor reduction in fat content of wild-type animals but suppresses the excess fat of tub-1 mutant animals Mukhopadhyay et al. This suggests a surprisingly specific role for vesicular transport in accumulation of excess fat in tub-1 deficient animals. Moreover, tub-1 mutant animals have extended lifespan. This lifespan extension requires insulin signaling but appears to be independent of the TUB-RabGAP fat pathway Mukhopadhyay et al. The synergistic nature of the excess fat accumulation in tub-1;kat-1 double mutants suggests that defects in neuronal tub-1 are normally compensated by kat-1 mediated fat oxidation in non-neuronal tissues. Loss of kat-1 abrogates this multi-tissue compensatory mechanism. The molecular nature of compensatory mechanisms that couple tub-1 and kat-1 are not yet known; however, genetic analysis of kat-1 led to identification of bbs-1 as another modifier of intestinal fat storage that, like tub-1 , functions in ciliated neurons Mak et al. Mutations in human ortholog of bbs genes including bbs-1 underlie Bardet-Biedl syndrome, a pleiotropic syndrome associated with obesity Beales, Many human BBS genes, which are implicated in ciliogenesis and intraflagellar transport IFT , have C. elegans homologs Inglis et al. Similar to tub-1 , loss of function mutations in bbs-1 cause modest increases in fat accumulation that are exacerbated by loss of KAT-1 Mak et al. Moreover, tub-1 mutants have defects in chemotaxis, a function mediated by a subset of ciliated sensory neurons, and there is evidence that TUB-1 undergoes IFT Mak et al. Together, these findings suggest that tub-1 and bbs-1 function in the same fat regulatory pathway. The provocative hypothesis that bbs-1 and tub-1 form a neuroendocrine axis with kat-1 is based on the synergistic rather than additive fat content of double mutants as assessed by Nile Red fluorescence. The potential insights offered by such genetic interactions highlight the need for standard methods to accurately quantify fluorescence intensity. elegans feed by pumping and concentrating food using a neuromuscular organ known as the pharynx Avery and Shtonda, ; Shtonda and Avery, The grinder, a teeth-like structure located at the junction of the pharynx and the intestine, breaks food particles that are then pushed into the lumen of the intestine by the peristaltic pumping action of the pharynx. elegans pump in the presence and absence of food; however, pumping rate is modulated by food availability Avery and Horvitz, Animals that have experienced starvation will pump faster when re-exposed to food than well-fed animals. elegans also forage for food. Rates and patterns of C. elegans movement are different compared on or off food. These locomotory rates and patterns are also modulated by starvation Hills et al. Serotonin modulates pumping rate. tph-1 mutant animals display reduced pumping rate while animals exposed to excess serotonin or imipramine, a serotonin uptake inhibitor, display increased pumping Avery and Horvitz, ; Horvitz et al. Pumping stimulatory effects of serotonin are abrogated by mutations in each of two serotonergic receptors ser-1 and ser-7 Hobson et al. Additionally, serotonin, dopamine and glutamate signaling pathways are implicated in different foraging strategies of C. elegans Hills et al. These neuronal signaling mechanisms also modulate mammalian feeding behavior Clifton and Kennett, ; Sainsbury et al. Together, these results indicate an overlap between neuronal feeding and foraging behavior pathways and central fat regulatory mechanisms; however, the nature of these relationships is not yet clear. For instance, there is an inverse correlation between fat content and pumping rate for serotonin deficient animals. In other cases, such as tub-1 mutants, animals display wild-type pumping rates despite increased fat levels. Our understanding of body fat regulation as a homeostatic, organismal process has flourished in the past decade. Although many of the core metabolic pathways were biochemically defined long ago, integration and coordination of these pathways across multiple tissues is a vibrant field of integrative biology. This is because understanding fat regulation requires multiple layers of investigation spanning from metabolism, transcription and signaling to neuronal development and behavior. Deciphering neuronal circuits that coordinate behavior, physiology, and metabolism is a major challenge in understanding fat regulation. Similarly, compensatory mechanisms that operate at organismal level to maintain energy homeostasis are just being elucidated. The genetic tractability of C. Importantly, amenability of C. Examining fat regulatory pathways under different environmental conditions holds the potential to reveal how physiological pathways are coordinately modulated in response to environmental perturbation. Similarly, how developmental stage, age, experience and diet perturb and possibly rewire the fat networks can be addressed in C. elegans at a molecular level. elegans is well suited for deciphering developmental programs that underlie fat storage capacity and cell biological determinants of lipid droplet biogenesis. Many of the adverse health effects of excess fat accumulation in humans are unlikely to occur in C. Nevertheless, the limited number of studies reported thus far already reveal remarkable similarities between molecular components of mammalian and C. elegans fat pathways that extend to disease-associated genes. Many of the fat genes identified in C. elegans have mammalian homologs whose roles in energy balance have not yet been examined. Given that energy balance is fundamental for viability, it is likely that many of the newly identified C. elegans fat regulatory networks are functionally conserved in mammals. I am indebted to members of the Ashrafi lab and Jennifer Watts for discussions. Edited by Andres Villu Maricq and Steven L. Last revised November 30, Published March 9, This chapter should be cited as: Ashrafi, K. Obesity and the regulation of fat metabolism March 9, , WormBook , ed. org [ PMC free article : PMC ] [ PubMed : ]. To whom correspondence should be addressed. Tel: , Fax: E-mail: ude. fscu ifarhsa. All WormBook content, except where otherwise noted, is licensed under a Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. Show details Pasadena CA : WormBook ; Search term. Author Information and Affiliations Authors Kaveh Ashrafi , §. Affiliations 1 Department of Physiology, University of California, San Francisco, San Francisco, CA, , USA. Obesity: an overview Obesity is a significant risk factor for major diseases including Type II diabetes, coronary heart disease, hypertension and certain forms of cancer Barsh et al. Figure 1 Homeostatic regulation of energy balance in mammals. elegans fat 2. Fat composition Several groups have biochemically determined the composition of C. Visualization of fat droplets Whereas mammals have dedicated adipocytes, C. Figure 2 Visualization of intestinal lipid droplets in transparent bodies of C. Genetic analysis of C. elegans fat regulation Targeted gene deletions, mutagenesis screens and a genome-scale RNA interference RNAi screen have identified approximately gene inactivations that cause fat reduction and approximately gene inactivations that cause fat accumulation without significant effects on growth and viability Ashrafi et al. Metabolic pathways Intricate metabolic networks tightly coordinate the flow of sugars and fats through synthesis, storage, and breakdown pathways. Figure 3 Overview of fat and sugar synthesis and breakdown pathways. Breakdown pathways In general, cells break down carbohydrates, amino acids and fats to generate ATP, the universal energy resource of cells Salway, Synthesis and storage pathways Acetyl-CoA is the key substrate for synthesis of fatty acids. Figure 5 Coordination of fat synthesis and breakdown pathways by malonyl-CoA. Table 1 Partial listing of C. Figure 4 Regulation of growth and metabolism by insulin signaling in C. Metabolic sensors and coordinated regulation of metabolic pathways The capacity to coordinately adjust energy flux through various catabolic and anabolic pathways in response to changing nutritional status is critical for cellular and organismal survival. elegans pathways are highlighted below: 4. sbp-1 Sterol response element binding protein SREBP is a key transcriptional regulator of fat and sterol synthesis pathways in mammals Eberle et al. TOR, AMPK, and hexosamine pathways TOR target of rapamyacin is an evolutionarily conserved phosphatidylinositol kinase related family member that couples cell size and proliferation to nutrient levels, particularly amino acids and hormonal signals such as insulin Inoki and Guan, ; Lindsley and Rutter, Development of fat storage capacity During mammalian adipogenesis, hormonal cues initiate transcriptional programs that guide the differentiation of multipotent mesenchymal stem cells into mature adipocytes. Neuroendocrine fat and feeding regulatory pathways In mammals, the nervous system functions as a central coordinator of both metabolic pathways and behaviors associated with food consumption. Insulin and TGF-β Signaling cascades through insulin, transforming growth factor TGF-β and cyclic nucleotide regulated pathways control whether C. Figure 6 Systemic actions of insulin signaling in mammals. Serotonin, dopamine and glutamate pathways Classical neurotransmitters have dramatic effects on fat regulation in nemotodes and in mammals. tub-1 and bbs-1 Mutations in rodent tubby cause progressive degeneration in retinal and cochlear sensory receptor cells, infertility and adult-onset obesity with insulin resistance Carroll et al. Feeding behavior and fat pathways C. Perspectives Our understanding of body fat regulation as a homeostatic, organismal process has flourished in the past decade. Bibliography Allison D. Assortative mating for relative weight: genetic implications. Antebi A. daf encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. Genes Dev. Abstract Article. Apfeld J. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. Ashrafi K. Mapping out starvation responses. Cell Metab. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Avery L. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. Food transport in the C. elegans pharynx. Badman M. The gut and energy balance: visceral allies in the obesity wars. Barsh G. Genetics of body-weight regulation. Beales P. Lifting the lid on Pandora's box: the Bardet-Biedl syndrome. Biddinger S. From mice to men: insights into the insulin resistance syndromes. Braeckman B. Assessing metabolic activity in aging Caenorhabditis elegans : concepts and controversies. Aging Cell. Brock T. Genetic regulation of unsaturated fatty acid composition in C. PLoS Genet. Brockmann G. Using mouse models to dissect the genetics of obesity. Trends Genet. Burnell A. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Carroll K. Tubby proteins: the plot thickens. Cell Biol. Chawla A. Nuclear receptors and lipid physiology: opening the X-files. Clifton P. Monoamine receptors in the regulation of feeding behaviour and energy balance. CNS Neurol. Drug Targets. Cohen P. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 SCD Stearoyl-CoA desaturase-1 and the metabolic syndrome. Drug Targets Immune Endocr. Cone R. Anatomy and regulation of the central melanocortin system. Curtis R. Aging networks in Caenorhabditis elegans : AMP-activated protein kinase aak-2 links multiple aging and metabolism pathways. Delrue M. Fat chance: genetic syndromes with obesity. Demuro G. Central nervous system and control of endogenous glucose production. Eberle D. SREBP transcription factors: master regulators of lipid homeostasis. Evans R. PPARs and the complex journey to obesity. Fares H. Deciphering endocytosis in Caenorhabditis elegans. Flier J. Obesity wars: molecular progress confronts an expanding epidemic. Forsythe M. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 O-GlcNAcase knockout impacts O-GlcNAc cycling, metabolism, and dauer. Friedman J. Obesity in the new millennium. The function of leptin in nutrition, weight, and physiology. S68—84, 85— A war on obesity, not the obese. Leptin and the regulation of body weight in mammals. Gems D. Nematode ageing: Putting metabolic theories to the test. Gissendanner C. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Halaschek-Wiener J. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. Hanover J. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Hills T. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. Hobson R. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Holt S. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Ageing Dev. Horvitz H. Serotonin and octopamine in the nematode Caenorhabditis elegans. Inglis P. Piecing together a ciliome. Inoki K. Complexity of the TOR signaling network. Trends Cell Biol. Jeong P. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Jia K. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Jones S. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Kahn B. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Kahn-Kirby A. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Kimura K. daf-2 , an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Kniazeva M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Koonen D. Kopelman P. Obesity as a medical problem. Kurzchalia T. Why do worms need cholesterol? Lam T. Hypothalamic sensing of fatty acids. Larsen M. MAA-1, a Novel Acyl-CoA-binding Protein Involved in Endosomal Vesicle Transport in Caenorhabditis elegans. Larsen P. Genes that regulate both development and longevity in Caenorhabditis elegans. Lee S. DAF target genes that control C. elegans life-span and metabolism. Lesa G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. Lindsley J. Nutrient sensing and metabolic decisions. B, Biochem. Long X. TOR action in mammalian cells and in Caenorhabditis elegans. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Love D. The hexosamine signaling pathway: deciphering the "O-GlcNAc code". STKE Luchsinger J. A work in progress: the metabolic syndrome. Aging Knowledge Environ. Ludewig A. elegans lipid metabolism, larval development, and aging. Lund J. Transcriptional profile of aging in C. Mak H. Polygenic control of Caenorhabditis elegans fat storage. Mashek D. Cellular fatty acid uptake: the contribution of metabolism. McElwee J. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. McKay R. elegans : a model for exploring the genetics of fat storage. Meissner B. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. Minokoshi Y. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Mukhopadhyay A. elegans tubby regulates life span and fat storage by two independent mechanisms. Murphy C. Genes that act downstream of DAF to influence the lifespan of Caenorhabditis elegans. Nakamura M. Mechanisms of regulation of gene expression by fatty acids. Narbonne P. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Nehrke K. Nunes F. The ABC transporter PGP-2 from Caenorhabditis elegans is expressed in the sensory neuron pair AWA and contributes to lysosome formation and lipid storage within the intestine. Pierce S. Regulation of DAF-2 receptor signaling by human insulin and ins-1 , a member of the unusually large and diverse C. elegans insulin gene family. Porte D. Leptin and insulin action in the central nervous system. S68—S84, 85— Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Rawson R. The SREBP pathway--insights from Insigs and insects. Ren P. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Rondinone C. Adipocyte-derived hormones, cytokines, and mediators. Rosen E. The transcriptional basis of adipocyte development Prostaglandins Leukot. Fatty Acids. Sainsbury A. Hypothalamic regulation of energy homeostasis. Best Pract. Salway, J. Metabolism at a Glance, Third edn Oxford: Blackwell Publishing Ltd. Satouchi K. Phospholipids from the free-living nematode Caenorhabditis elegans. Sawin E. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Seegmiller A. The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Shtonda B. CCA-1, EGL and EXP-2 currents shape action potentials in the Caenorhabditis elegans pharynx. Sluder A. Nuclear receptors in nematodes: themes and variations. Spiegelman B. Obesity and the regulation of energy balance. Stunkard A. The body-mass index of twins who have been reared apart. Sze J. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Tanaka T. Effects of growth temperature on the fatty acid composition of the free-living nematode Caenorhabditis elegans. Taubert S. A Mediator subunit, MDT, integrates regulation of fatty acid metabolism by NHRdependent and -independent pathways in C. Trayhurn P. Appetite and energy balance signals from adipocytes. Van Gilst M. Nuclear hormone receptor NHR controls fat consumption and fatty acid composition in C. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR Van Voorhies W. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Vellai T. Genetics: influence of TOR kinase on lifespan in C. Wadsworth W. Developmental regulation of energy metabolism in Caenorhabditis elegans. Wallis J. However, the contribution of factors to postprandial lipemia in healthy but metabolic The deleterious effect of maternal high-fat diet HFD on the fetal rat liver may cause later development of non-alcoholic fatty liver disease NAFLD. The aim of this study was to evaluate the effect of mater Obesity and its complications constitute a substantial burden. Considerable published research describes the novel relationships between obesity and gut microbiota communities. It is becoming evident that micr Skip to main content. Search all BMC articles Search. Keywords: Fatty acids, lipids, metabolic regulation, gut microbiota, gut microbiota metabolites, metabotypes, cardiometabolic disease Topics: Individual response to dietary fat, and the effect on lipid metabolism and cardiometabolic regulation Dietary fat and regulation of gut microbiota Individual gut microbiota signature, effect on lipid metabolism and cardiometabolic regulation Microbiota derived metabolites, lipid metabolism and cardiometabolic regulation Questions to be answered: What are the individual differences in the response to fatty acids and lipids? Do dietary fatty acids and lipids affect the gut microbiota, and are there individual differences? How do short chain fatty acids SCFA affect metabolic regulation, including gene transcription? How do microbial lipids alter intestinal and circulating lipid concentrations, and thereby impact metabolic regulation of the host? All submissions should be made by June 30th, Non-high-density lipoprotein cholesterol may predict the cardio-cerebrovascular risk in patients on maintenance hemodialysis Non-high-density lipoprotein cholesterol non-HDL-C may be an independent risk factor for cardio-cerebrovascular disease CVD ; however, the cutoff level in patients on maintenance hemodialysis MHD is unknown. Authors: Denggui Luo, Yueming Luo, Yanhong Zou, Yuanzhao Xu, Bo Fu, Dong Yang, Jun Yang, Cai Xu, Shuyi Ling, Shunmin Li and Airong Qi. Citation: Lipids in Health and Disease 20 Content type: Research Published on: 13 November Authors: Dan-meng Zheng, Zhen-ni An, Ming-hao Ge, Dong-zhuo Wei, Ding-wen Jiang, Xue-jiao Xing, Xiao-lei Shen and Chang Liu. Content type: Research Published on: 2 November Authors: Stephanie M. Wilson, Adam P. Maes, Carl J. Yeoman, Seth T. |
Seltsamerweise wie jenes