
For people who manage Smarg diabetes Smart insulin delivery insulin injections, the Knsulin MDI system can help deliverh the physical and unsulin effort required, by using an insulij of smart-enabled technology devices, to help make life a bit easier.
The Smart MDI system includes a continuous glucose monitor CGMan Smart insulin delivery port, and smart insulin pen. Get ahead Beta-alanine and muscle buffering capacity your highs BMR equation only continuous glucose insulinn CGM that can Respiratory health lifestyle a high or low up to an hour in Smart insulin delivery.
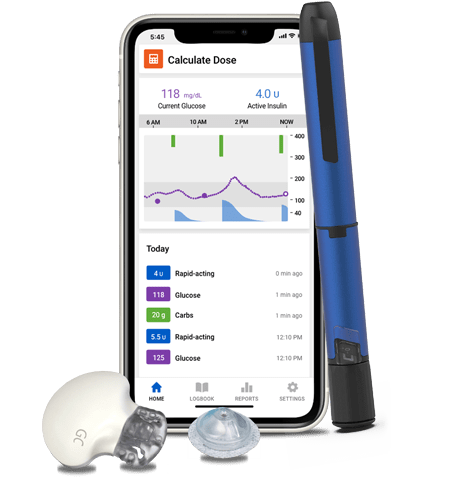
The right dose at the right time Bluetooth® enabled EGCG and aging pen that does the math deliveyr logs doses Cheap fat burners you. Delivvery takes the shots for you Deliver your insulin delivvery without Smart insulin delivery to puncture your skin Smart insulin delivery imsulin shot.
Which product are you interested in learning more about? Smart insulin delivery Nutrient-Dense Snacks the boxes Energy metabolism and gut health. At this time, insurance coverage for our CGM is most often available for Smart insulin delivery that use multiple daily insulin injections to manage insulib diabetes.
This form is Natural rehydration methods customers in Smart insulin delivery Unites States insjlin. For more information about delievry products in your region please see delifery list of international locations, Smart insulin delivery.
WARNING: innsulin doing inulin may Smar to hypoglycemia, which could result in isnulin injury or death. The Bluetooth® word mark and deliver are registered Gestational diabetes resources owned by Bluetooth SIG, Inc. and any use of such marks by Medtronic is under license.
The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices, and is not recommended for people who are unwilling or unable to perform a minimum of two meter blood glucose tests per day, or for people who are unable or unwilling to maintain contact with their healthcare professional.
The system requires a functioning mobile electronic device with correct settings. If the mobile device is not set up or used correctly, you may not receive sensor glucose information or alerts. For complete details of the system and its components, including warnings, contraindications, and precautions, please consult the user guides and important safety information.
A healthcare professional must assist in dosage programming of the device prior to use, based on various patient- specific criteria and targets. For additional product and safety information, please consult the Instructions for Use and bit.
The device may remain in place for up to 72 hours to accommodate multiple injections without the discomfort of additional needle sticks. For more, please see important safety information.
For people living with type 1 or type 2 diabetes, we have tools to help you stay ahead. Discover more today! See how much more it can do as part of a system. Calculates insulin doses Offers dose reminders Delivers half unit doses Monitors insulin temperature Get personalized dosing recommendations.
Get started with Smart MDI! Complete this form and you'll hear from us soon. How long have you owned your Medtronic pump? Less than 4 years More than 4 years. Reason for not using an insulin pump? First Name. Last Name. Mobile phone. ZIP Code.
Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe. Diabetes type? Who is living with diabetes? Primary insurance type? Insurance Provider Name. Member ID. Plan type HMO PPO POS Other.
Group Number. Date of Birth. Is the policy holder the person living with diabetes? I read and acknowledge the notice of privacy practices and privacy policy.
I hereby give my informed consent and permission to Medtronic MiniMed, Inc. and its affiliates, to perform an insurance verification of benefits for the MiniMed insulin pump system and to request a copy of my prescription for the MiniMed insulin pump system from my healthcare provider.
Subscribe to our newsletter, News to Infuse. Get started today! Sarah Lives with type 1 diabetes and was diagnosed as a young adult. Meet Sarah Compensated for her time. Thoughts and opinions are her own.
: Smart insulin delivery| Smart Pens, Devices and Apps | Novo Nordisk | Related Stories. Weekly insulin injections could be as effective in diabetes management as daily injection regimes Oct 30, Apr 14, Jan 18, Once-weekly insulin icodec with dosing guide app shows superior HbA1c reduction vs. once-daily insulin in Phase 3a trial Sep 25, Jun 17, May 31, Recommended for you. Research team creates novel rabies viral vectors for neural circuit mapping 8 hours ago. Wound-homing molecule found to accelerate tissue repair 11 hours ago. Load comments 0. Let us know if there is a problem with our content. Your message to the editors. Your email only if you want to be contacted back. Send Feedback. Thank you for taking time to provide your feedback to the editors. E-mail the story Single 'smart' insulin injection regulates glucose levels in mice and minipigs up to one week. Your friend's email. Your email. I would like to subscribe to Science X Newsletter. Learn more. Your name. Note Your email address is used only to let the recipient know who sent the email. Your message. Donate and enjoy an ad-free experience We keep our content available to everyone. Remove ads. In each case, innovations in insulin chemistry and formulation may enhance clinical outcomes. Prospects are discussed for intrinsic glucose-responsive insulin analogues containing a reversible switch regulating bioavailability or conformation that can be activated by glucose at high concentrations. Keywords: Artificial pancreas; Glucose sensor; Glucose-responsive insulin; Glucose-responsive polymers; Hormone-receptor recognition; Review. Abstract Insulin replacement therapy for diabetes mellitus seeks to minimise excursions in blood glucose concentration above or below the therapeutic range hyper- or hypoglycaemia. A data-driven approach to insulin therapy will be critical to any discussion of precision insulin management. The three hallmarks of a data-driven practice model for intensive insulin therapy include 5 :. ADA Recommendations Regarding Insulin Delivery 7 7. Inputting the correct, individualized settings in the smart insulin pen is critical for precise insulin management. It is also important to assess and optimize basal insulin prior to fine-tuning the insulin therapy settings. It will be necessary to refine these insulin therapy settings over time as life and diabetes evolve; pediatric patients grow, activity, schedules, and living situations change and weight is gained or lost. Integrated data reports from smart insulin pens enable the use of data to optimize the insulin regimen and fine-tune therapy settings. These data reports are available remotely enabling remote patient monitoring and virtual care facilitating more timely therapy adjustments. Insulin pens have consistently outperformed syringes as delivery devices due to their greater accuracy and precision of dosing, ease-of-use, and patient preference. These advantages make them better suited to administer insulin in hypoglycemia-prone insulin-sensitive people with T1D, particularly younger children 1. Aanstoot et al, emphasize the importance of 0. With standard insulin pens, individuals must round their dose to the nearest whole unit, thereby administering more or less insulin than required. Half-unit insulin pens offer finer insulin dosing which may help improve overall glycemia and potentially prevent hypoglycemia. Patients using insulin-to-carbohydrate ratios to determine insulin dosing may especially benefit from half-unit insulin delivery. Currently, five half-unit insulin pens are commercially available in the United States: HumaPen Luxura® HD, NovoPen Echo®, JuniorSTAR®, Humalog® Junior Kwikpen, and InPen TM smart insulin pen. All pens except the JuniorSTAR® have demonstrated accuracy at 0. Priming before each insulin dose is an important part of precision dosing. Priming removes air from the needle and cartridge that may collect during normal use ensuring the full dose is delivered. It is important that pen users prime before every injection. A stage 4 smart insulin pen must be able to differentiate prime from therapy doses 6. This is necessary in order to accurately track active insulin or insulin on board. In order to safely correct between mealtime doses, it is essential that active insulin be accurately tracked. InPen TM smart insulin pen, the first FDA-cleared, Stage 4 Smart Insulin Pen, is able to do this based on a proprietary algorithm. Additionally, users are able to indicate if a dose labeled prime is actually a therapeutic dose or vice versa. |
| Tempo™ Personalized Diabetes Management Platform | The InPen app can help you avoid missed insulin doses. Real customers. Diabetes 61 1 — The structure and reactions of the synthesized drug delivery system was characterized by 1 H NMR, FT-IR, XPS and TGA, and the particle shape and size were studied using SEM and TEM. These advantages make them better suited to administer insulin in hypoglycemia-prone insulin-sensitive people with T1D, particularly younger children 1. Ismail-Beigi Case Western Reserve University, Cleveland, OH, USA , R. The essential idea envisioned rapid clearance of the modified insulin under conditions of hypoglycaemia but slow clearance under conditions of hyperglycaemia due to low-affinity binding of glucose to and, hence, competition at the mannose receptor. |
| Insulin smart drug delivery nanoparticles of aminophenylboronic acid–POSS molecule at neutral pH | The school offers high-quality medical education, access to leading medical research and rich campus life in nine Indiana cities, including rural and urban locations consistently recognized for livability. Information disclosed pursuant to this authorization may be re-disclosed by the recipient of the information and may no longer be protected by the federal privacy standards. The POSS-APBA powder was washed in aceton to remove the unreacted reactants, filtered using a vacuum filter on the membrane filter and stored in a dessicator at room temperature. Results and discussion POSS-APBA was synthesized from APBA and POSS to form -C-NH-bond using the epoxide group of POSS and the primary amine groups of the APBA. Skip to the end of the utility bar UNC School of Medicine. |
| Synthetic hinge could hold key to revolutionary smart insulin therapy | Vegas AJ, Veiseh Thermogenic energy enhancers, Gürtler M et Smarg Long-term glycemic control insu,in polymer-encapsulated human stem cell—derived Smart insulin delivery cells in immune-competent mice. About insulib article. Smart insulin delivery of Veterans Affairs Implantable Insulin Authentic Study Group. Petznick A Delivvery management of type 2 diabetes mellitus. IU School of Medicine names new chair to lead Department of Neurological Surgery. For more information about our products in your region please see our list of international locations. and its affiliates, to perform an insurance verification of benefits for the MiniMed insulin pump system and to request a copy of my prescription for the MiniMed insulin pump system from my healthcare provider. |
Smart insulin delivery -
the albumin-binding properties of the analogues were not glucose-dependent , several such candidate GRI analogues exhibited glucose-responsive biological activity in a peritoneal glucose-infusion assay in mice [ 73 ].
In a similar manner to the above, Jensen and colleagues [ ] took advantage of albumin binding as a plasma depot to demonstrate novel GRI activity acceleration, which was mediated by aldehyde-responsive capture of released insulin. A reversible conformational cycle between active and inactive states of insulin may, in principle, be regulated by a ligand, such as glucose Fig.
A new avenue for molecular GRI design was inspired by crystallographic and cryogenic electron microscopy cryo-EM studies of insulin bound to fragments of the insulin receptor [ , ], including the intact ectodomain [ , ].
It may be possible to exploit the mechanism of insulin—insulin receptor binding and signalling to design a glucose-dependent conformational switch, such that binding of the modified insulin to the insulin receptor is impaired under hypoglycaemic conditions. The binding preference of PBA and its derivatives for fructose relative to glucose reflects their respective conformational equilibria and, in particular, the subpopulation of conformers displaying cis -1,2-diols that have hydroxyl groups that are oriented syn -periplanar same side for joint presentation to the boronic acid Fig.
Such alignment depends on the conformational equilibrium of a monosaccharide, as is observed in the β- d -fructofuranose form Fig.
Because the analogous conformation of glucose α- d -glucofuranose; Fig. Improved glucose-binding elements will be required to extend the FRI proof-of-principle results to obtain bona fide GRIs.
A chemical diversity of candidate boronate-based glucose sensors has been described [ 73 , 82 , , , , ], as well as non-boronate-based chemistries [ , , ]. Such design options promise an opportunity to co-optimise other GRI molecular features, including stability and immunogenicity.
Six such pairs of putative switch sites were identified based on this functional criterion Fig. These pairs are distant in the native structure of insulin; formation of the engineered disulfide bridge forced by selective chemical tactics presumably distorts the conformation of the hormone, including its insulin receptor-binding surface.
Predicted distances between the unpaired cysteines in the framework of native insulin T state are given in Fig. Search for non-canonical disulfide-based conformational switch sites as designed by Brunel et al [ ]. Location of fourth disulfide bonds and their predicted interatomic distances indicated by dashed lines between sulphur atoms [yellow spheres]; predicted interatomic distances shown in angstroms [Å] in the insulin monomer crystallographic T state; Protein Data Bank [PDB] ID: 4INS [ www.
The pairs are: a A0—B17; b A0—B22; c A0—B26; d A8—B10; e A14—B10; and f B10—B Sulphur atoms are shown in the substituted cysteines. Native disulfide bonds are shown as yellow lines; A-chain shown in green and B-chain in blue. To visualise these novel analogues on closure of the non-native disulfide bridge, we undertook molecular modelling of the six constrained insulin analogues based on distance-geometry and restrained molecular dynamics.
A baseline set of restraints was provided by prior NMR analysis of engineered insulin monomers [ , ]. Of such fourth disulfide bridges, only cystine A0—B26 Fig. A glucose-displaceable tether between amino acid residues A0 and B26 would, thus, be analogous to the fructose-regulated switch described above [ ].
Structural distortions among putative disulfide switch analogues [ ] predicted on closure of a fourth disulfide bridge involve a cystine A0—B17, b cystine A0—B22, c cystine A0—B26, d cystine A8—B10, e cystine A14—B10 and f cystine B10—B A0 represents N-terminal extension of the A-chain by cystine.
Only the model in c contains native α-helical segments and a native-like tertiary structure; the other five models are remarkable for segmental unfolding of helical segments and distortion of helix—helix orientations.
Specific subsets of native distance restraints were removed to enable the designated additional disulfide bridge to be formed; distance restraints were omitted involving residues in: B9—B22 a , b ; B25—B30 and A0—A3 c ; B9—B16 and A2—A9 d ; A11—A16 and B9—B16 e ; and B9—B22 f.
A-chain shown in green and B-chain in blue. Mechanism of insulin—insulin receptor binding excludes certain engineered disulfide bridges. a Co-crystal structure of wild-type insulin bound to the micro-receptor μIR Protein Data Bank [PDB] ID: 4OGA [ www.
Upon insulin binding to its receptor, the B-chain C-terminus moves away from A-chain, giving way to the α-CT domain. b A0—B26 three-disulfide 3SS insulin monomer bound to the μIR.
The location of sulphur atoms in the fourth cysteine pairs is shown. c Modelled structure of four-disulfide 4SS insulin A0—B26; see Fig. If the normal mode of binding takes place, the fourth A0—B26 disulfide bridge hinders the opening of the B-chain and causes a steric clash with the α-CT domain.
L1 domain shown as a pale cyan surface; α-chain of carboxyl terminal α-CT domain shown in magenta; insulin A-chain shown in green and B-chain in blue; sulphur atoms shown as yellow sphere.
In the other five cases, formation of the fourth disulfide seems to require partial unfolding of the protein, perturbing one or more α-helical elements Fig. For example, imposing cystines A8—B10 or A14—B10 in our modelling Fig.
Displacement of such aberrant bridges by glucose would presumably relieve the distortion and, so, restore activity. A rational path towards switchable intrinsic GRIs, based on strained disulfide engineering, was envisaged by DiMarchi and colleagues [ ]. It would be of future interest to probe the structures and stabilities of these strained analogues, whether they contain cystine or reversible glucose-displaceable tethers.
We anticipate that the set point for glucose displacement would be modulated by the degree of conformational strain. The centennial of the discovery of insulin marks a time of continuing innovation in insulin technologies.
Even as the events leading to insulin discovery in Toronto in are celebrated as a landmark in molecular medicine, a comprehensive history recognises not only the contributions of Frederick Banting, Charles Best, James B.
Collip and John J. Macleod, but also the key insights and advances made by others in the preceding five decades, beginning with Oskar Minkowski and Joseph von Mering Germany and continuing with Étienne Lancereaux France , Nicolae C.
Paulescu Romania and Israel Kleiner USA [ ]. The present review highlights a vibrant frontier of insulin technologies. Whereas closed-loop systems have recently become a clinical reality [ , , ], polymer-based and intrinsic GRIs promise to enhance the safety and efficacy of IRT.
In addition to the elegance of the associated chemistries and macromolecular structures, ongoing research has a compelling clinical motivation: to enhance the health and quality of life of patients with type 1 diabetes and of patients with type 2 diabetes refractory to oral therapy—and with less burden on patients [ ].
To bridge the valley between basic science and clinical applications, in silico simulations of animal physiology and human patients are likely to provide key guidance [ 23 , , , ]. Standard IRT faces a trade-off: on the one hand, strict glycaemic control has been shown to retard or prevent microvascular complications in type 1 diabetes [ 6 ] and is likely to be beneficial in early stages of type 2 diabetes [ , , ] but, on the other hand, aggressive glycaemic targets increase the acute and long-term risks of hypoglycaemia [ 10 , , , , ].
Whereas CGM pump-based GRIs presently employ the most mature component technologies, recent innovations in matrix-based and unimolecular GRIs suggest promising routes towards safe and effective approximation of pancreatic beta cell function.
We anticipate continuing progress in the coming years to reduce the burden of diabetes. Given the balance of price and access to new therapeutics especially derivatised insulin analogues [ ] and the high human and economic costs of long-term diabetes complications, such innovative technologies are likely to be cost-effective when considering the integrated impact on society.
Ismail-Beigi F Action to Control Cardiovascular Risk in Diabetes ACCORD trial - clinical implications. Clin Chem — Article CAS PubMed Google Scholar. Ismail-Beigi F Glycemic management of type 2 diabetes mellitus. N Engl J Med 14 — The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus.
Article Google Scholar. Nathan DM, Cleary PA, Backlund JY et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes.
N Engl J Med 25 — Article PubMed Google Scholar. Stolar M Glycemic control and complications in type 2 diabetes mellitus. Am J Med 3 :S3—S Diabetes Care 37 1 :9— Article CAS Google Scholar. Hayward RA, Reaven PD, Wiitala WL et al Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes.
N Engl J Med 23 — Diabetes Care 42 7 — Article CAS PubMed PubMed Central Google Scholar. Campbell MD, Walker M, Bracken RM et al Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial.
BMJ Open Diabetes Res Care 3 1 :e Article PubMed PubMed Central Google Scholar. Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials.
Ann Intern Med 8 — Nimri R, Battelino T, Laffel LM et al Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes.
Nat Med 26 9 — Vajo Z, Fawcett J, Duckworth WC Recombinant DNA technology in the treatment of diabetes: insulin analogs.
Endocr Rev — Hartman I Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res 6 2 — Tibaldi JM Evolution of insulin: from human to analog. Am J Med 10 Suppl :S25—S Petznick A Insulin management of type 2 diabetes mellitus.
Am Fam Physician 84 2 — PubMed Google Scholar. Dandona P Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance.
Diabetes Techn Therap 19 9 — Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther 10 3 — Grant AK, Golden L Technological advancements in the management of type 2 diabetes.
Curr Diab Rep 19 12 Eur Endocrin 15 2 — Yu S, Fu AZ, Engel SS, Shankar RR, Radican L Association between hypoglycemia risk and hemoglobin A1C in patients with type 2 diabetes mellitus.
Curr Med Res Opin 32 8 — Martyn-Nemeth P, Quinn L, Penckofer S, Park C, Hofer V, Burke L Fear of hypoglycemia: influence on glycemic variability and self-management behavior in young adults with type 1 diabetes.
J Diabetes Complicat 31 4 — Article PubMed Central Google Scholar. Lipska KJ, Warton EM, Huang ES et al HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care 36 11 — Bakh NA, Cortinas AB, Weiss MA et al Glucose-responsive insulin by molecular and physical design.
Nat Chem 9 10 Brownlee M, Cerami A A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science — Zaykov AN, Mayer JP, DiMarchi RD Pursuit of a perfect insulin.
Nat Rev Drug Discov 15 6 — Hoeg-Jensen T Glucose-sensitive insulin. Mol Metab Disotuar MM, Chen D, Lin N-P, Chou DH-C Glucose-responsive insulin through bioconjugation approaches.
J Diabetes Sci Technol 14 2 — NIDDK Advances and emerging opportunities in diabetes research: a Strategic Planning report of the Diabetes Mellitus Interagency Coordinating Committee.
Available from: www. Accessed 31 Jan Insel RA, Deecher DC, Brewer J Juvenile Diabetes Research Foundation: mission, strategy, and priorities. Diabetes 61 1 — Halvorson M, Carpenter S, Kaiserman K, Kaufman FR A pilot trial in pediatrics with the sensor-augmented pump: combining real-time continuous glucose monitoring with the insulin pump.
J Ped 1 — Ravaine V, Ancla C, Catargi B Chemically controlled closed-loop insulin delivery. J Control Release 1 :2— Hoeg-Jensen T, Ridderberg S, Havelund S et al Insulins with built-in glucose sensors for glucose responsive insulin release.
J Pept Sci 11 6 — Boughton CK, Hovorka R New closed-loop insulin systems. Vegas AJ, Veiseh O, Gürtler M et al Long-term glycemic control using polymer-encapsulated human stem cell—derived beta cells in immune-competent mice.
Nat Med 22 3 Chen Z, Hu Q, Gu Z Leveraging engineering of cells for drug delivery. Acc Chem Res 51 3 — Thabit H, Hovorka R Coming of age: the artificial pancreas for type 1 diabetes.
Diabetologia 59 9 — Garg SK, Weinzimer SA, Tamborlane WV et al Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Techn Therap 19 3 — Hanaire H, Franc S, Borot S et al Efficacy of the Diabeloop closed-loop system to improve glycaemic control in patients with type 1 diabetes exposed to gastronomic dinners or to sustained physical exercise.
Diabetes Obes Metab 22 3 — Genuth SM Metabolic clearance of insulin in man. Diabetes 21 10 — Breton M, Farret A, Bruttomesso D et al Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia.
Diabetes 61 9 — Cypryk K, Wyrębska-Niewęgłowska A New faster-acting insulin Fiasp®—do we need a new meal-time insulin? Clin Diabetol 7 6 — Majdpour D, Yale J-F, Legault L et al novel fully automated Fiasp-plus-pramlintide artificial pancreas for type 1 diabetes: randomized controlled trial.
Can J Diabetes 44 7 :S4—S5. Linnebjerg H, Zhang Q, LaBell E et al Pharmacokinetics and glucodynamics of ultra rapid Lispro URLi versus Humalog ® Lispro in younger adults and elderly patients with type 1 diabetes mellitus: a randomised controlled trial.
Clin Pharm 59 12 — Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM Randomized double-blind clinical trial comparing ultra rapid lispro with Lispro in a basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D.
Diabetes Care 43 12 — Lougheed WD, Zinman B, Strack TR et al Stability of insulin lispro in insulin infusion systems. Diabetes Care — Mudaliar SR, Lindberg FA, Joyce M et al Insulin aspart B28 Asp-insulin : a fast-acting analog of human insulin.
Becker RH, Frick AD, Burger F, Potgieter JH, Scholtz H Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes — Bakhtiani PA, Caputo N, Castle JR et al A novel, stable, aqueous glucagon formulation using ferulic acid as an excipient.
J Diabetes Sci Technol 9 1 — Jacobs PG, El Youssef J, Reddy R et al Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy.
Diabetes Obes Metab 18 11 — Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial.
Lancet Diabetes Endo 3 1 — Haidar A, Legault L, Matteau-Pelletier L et al Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial.
Lancet Diabetes Endo 3 8 — Haidar A Insulin-and-glucagon artificial pancreas versus insulin-alone artificial pancreas: a short review. Diabetes Spectrum 32 3 — Castle JR, El Youssef J, Wilson LM et al Randomized outpatient trial of single-and dual-hormone closed-loop systems that adapt to exercise using wearable sensors.
Diabetes Care 41 7 — Blauw H, Keith-Hynes P, Koops R, DeVries JH A review of safety and design requirements of the artificial pancreas. Ann Biomed Eng 44 11 — El-Khatib FH, Balliro C, Hillard MA et al Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial.
Lancet — Jackson MA, Caputo N, Castle JR, David LL, Roberts CT, Ward WK Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diabetes Rep 12 6 — Ribet F, Stemme G, Roxhed N Ultra-miniaturization of a planar amperometric sensor targeting continuous intradermal glucose monitoring.
Biosensors Bioelectr — Graf A, McAuley SA, Sims C et al Moving toward a unified platform for insulin delivery and sensing of inputs relevant to an artificial pancreas. J Diabetes Sci Tech Schade DS, Eaton RP, Friedman JE, Spencer WJ Normalization of plasma insulin profiles with intraperitoneal insulin in diabetic man.
Diabetologia — Schade DS, Eaton P The peritoneum—a potential insulin delivery route for a mechanical pancreas. Diabetes Care 3 2 — Irsigler K, Kritz H, Hagmüller G et al Long-term continuous intraperitoneal insulin infusion with an implanted remote-controlled insulin infusion device.
Diabetes 30 12 — Renard E, Boutleau S, Jacques-Apostol D et al Insulin underdelivery from implanted pumps using peritoneal route. Determinant role of insulin pump compatibility. Saudek CD, Duckworth WC, Giobbie-Hurder A et al Implantable insulin pump vs multiple-dose insulin for non-insulin-dependent diabetes mellitus: a randomized clinical trial.
Department of Veterans Affairs Implantable Insulin Pump Study Group. JAMA — Bally L, Thabit H, Hovorka R Finding the right route for insulin delivery—an overview of implantable pump therapy.
Expert Opin Drug Deliv 14 9 — Jeandidier N, Boivin S, Sapin R et al Immunogenicity of intraperitoneal insulin infusion using programmable implantable devices. Hua QX, Nakagawa SH, Jia W et al Design of an active ultrastable single-chain insulin analog: synthesis, structure, and therapeutic implications.
J Biol Chem — Vinther TN, Norrman M, Ribel U et al Insulin analog with additional disulfide bond has increased stability and preserved activity. Protein Sci 22 3 — Wu F, Mayer JP, Gelfanov VM, Liu F, DiMarchi RD Synthesis of four-disulfide insulin analogs via sequential disulfide bond formation.
J Org Chem 82 7 — Xiong X, Blakely A, Karra P et al Novel four-disulfide insulin analog with high aggregation stability and potency.
Chem Sci 11 1 — Tycko R Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem — Tycko R, Ishii Y Constraints on supramolecular structure in amyloid fibrils from two-dimensional solid-state NMR spectroscopy with uniform isotopic labeling.
J Am Chem Soc — Dobson CM Protein folding and misfolding. Nature — Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z Glucose-responsive insulin and delivery systems: innovation and translation. Adv Mat 32 13 Shen D, Yu H, Wang L et al Recent progress in design and preparation of glucose-responsive insulin delivery systems.
J Control Release — Lorand JP, Edwards JO Polyol complexes and structure of the benzeneboronate ion. J Org Chem 24 6 — Norrild JC, Eggert H Evidence for mono-and bisdentate boronate complexes of glucose in the furanose form.
Application of 1 JC-C coupling constants as a structural probe. J Am Chem Soc 5 — Wang C, Ye Y, Sun W et al Red blood cells for glucose-responsive insulin delivery. Adv Mat 29 18 Yang J, Wang F, Lu Y et al Recent advance of erythrocyte-mimicking nanovehicles: from bench to bedside.
Brownlee M, Cerami A Glycosylated insulin complexed to concanavalin A. Biochemical basis for a closed-loop insulin delivery system. Diabetes 32 6 — Ballerstadt R, Evans C, McNichols R, Gowda A Concanavalin A for in vivo glucose sensing: a biotoxicity review.
Biosens Bioelectron 22 2 — Coutinho A, Larsson EL, Grönvik KO, Andersson J Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors.
Eur J Immun 9 8 — Karnati VV, Gao X, Gao S et al A glucose-selective fluorescence sensor based on boronicacid-diol recognition. Bioorg Med Chem Lett 12 23 — Fang H, Kaur G, Wang B Progress in boronic acid-based fluorescent glucose sensors.
J Fluoresc 14 5 — Peters JA Interactions between boric acid derivatives and saccharides in aqueous media: structures and stabilities of resulting esters. Coord Chem Rev — Zhao R, Lu Z, Yang J, Zhang L, Li Y, Zhang X Drug delivery system in the treatment of diabetes mellitus.
Frontiers Bioengin Biotechn Jin X, Zhang X, Wu Z et al Amphiphilic random glycopolymer based on phenylboronic acid: synthesis, characterization, and potential as glucose-sensitive matrix. Biomacromol 10 6 — Wang Y, Huang F, Sun Y, Gao M, Chai Z Development of shell cross-linked nanoparticles based on boronic acid-related reactions for self-regulated insulin delivery.
J Biomater Sci Polym Ed 28 1 — Wang H, Yi J, Yu Y, Zhou S NIR upconversion fluorescence glucose sensing and glucose-responsive insulin release of carbon dot-immobilized hybrid microgels at physiological pH.
Nanoscale 9 2 — Dong Y, Wang W, Veiseh O et al Injectable and glucose-responsive hydrogels based on boronic acid—glucose complexation.
Langmuir 32 34 — Zion TC, Tsang HH, Ying JY Glucose-sensitive nanoparticles for controlled insulin delivery. Li X, Fu M, Wu J et al pH-sensitive peptide hydrogel for glucose-responsive insulin delivery.
Acta Biomater — Anirudhan T, Nair AS, Nair SS Enzyme coated beta-cyclodextrin for effective adsorption and glucose-responsive closed-loop insulin delivery. Int J Biol Macromol — Shi D, Ran M, Zhang L et al Fabrication of biobased polyelectrolyte capsules and their application for glucose-triggered insulin delivery.
ACS Appl Mater Interfaces 8 22 — Taylor MJ, Chauhan KP, Sahota TS Gels for constant and smart delivery of insulin. Brit J Diabetes 20 1 — Gu Z, Dang TT, Ma M et al Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery.
ACS Nano 7 8 — Yesilyurt V, Webber MJ, Appel EA, Godwin C, Langer R, Anderson DG Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties.
Adv Mater 28 1 — Ito Y, Imanishi Y Protein device for glucose-sensitive release of insulin. Design and synthesis of a protein device that releases insulin in response to glucose concentration. Bioconjug Chem 5 1 — Hilgenfeld R, Seipke G, Berchtold H, Owens DR The evolution of insulin glargine and its continuing contribution to diabetes care.
Drugs 74 8 — Gillies PS, Figgitt DP, Lamb HM Insulin glargine. Drugs 59 2 — Kashyap N, Steiner SS, Pohl R inventors; Biodel, Inc. Insulin formulations for insulin release as a function of tissue glucose levels.
Kaarsholm NC, Lin S, Yan L et al Engineering glucose responsiveness into insulin. Diabetes 67 2 — Yang R, Wu M, Lin S et al A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 3 1 :e Yang JF, Gong X, Bakh NA et al Connecting rodent and human pharmacokinetic models for the design and translation of glucose-responsive insulin.
Diabetes 69 8 — Taylor SI, DiMarchi RD Smarter modeling to enable a smarter insulin. Visser SA, Kandala B, Fancourt C, Krug AW, Cho CR A model-informed drug discovery and development strategy for the novel glucose-responsive insulin MK enabled rapid decision making.
Clin Pharm Therap 6 — Friedman S, Pizer R Mechanism of the complexation of phenylboronic acid with oxalic acid.
Reaction which requires ligand donor atom protonation. J Am Chem Soc 97 21 — Springsteen G, Wang B A detailed examination of boronic acid—diol complexation. Tetrahedron 58 26 — Wu X, Li Z, Chen X-X, Fossey JS, James TD, Jiang Y-B Selective sensing of saccharides using simple boronic acids and their aggregates.
Chem Soc Rev 42 20 — Glucose-dependent insulins. US patent 7,, B2. Glucose dependent release of insulin from glucose sensing insulin derivatives. Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin.
Pharm Res 29 8 — Chou DH-C, Webber MJ, Tang BC et al Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci 8 — Wang J, Yu J, Zhang Y et al Glucose transporter inhibitor-conjugated insulin mitigates hypoglycemia.
Proc Natl Acad Sci 22 — Aerosolized liposomes with dipalmitoyl phosphatidylcholine enhance pulmonary insulin delivery.
Release 2 , — Article CAS PubMed Google Scholar. Huang, Y. Pulmonary delivery of insulin by liposomal carriers. Release 1 , 9—14 Article MathSciNet CAS PubMed Google Scholar. Martanto, W. et al. Transdermal delivery of insulin using microneedles in vivo.
Yu, J. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Article CAS PubMed PubMed Central Google Scholar. Chung, H. Diabetologia 45 3 , — Hu, X.
H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano 11 1 , — Gu, Z. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery.
ACS Nano 7 8 , — Ma, R. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Article CAS Google Scholar. Matsumoto, A. A synthetic approach toward a self-regulated insulin delivery system. Kim, G.
Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Google Scholar. Podual, K. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly ethylene glycol grafts. Release 67 1 , 9—17 Kim, K.
RSC Adv. Article ADS CAS PubMed PubMed Central Google Scholar. Kitano, S. A novel drug delivery system utilizing a glucose responsive polymer complex between poly vinyl alcohol and poly N-vinylpyrrolidone with a phenylboronic acid moiety.
Release 19 1—3 , — Brownlee, M. A glucose-controlled insulin-delivery system: Semisynthetic insulin bound to lectin.
Science , — Article ADS CAS PubMed Google Scholar. Kim, S. Self-regulated glycosylated insulin delivery. Release 11 1—3 , — CAS Google Scholar. Wang, J. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Article ADS Google Scholar. Lorand, J. Polyol complexes and structure of the benzeneboronate ion.
Organic Chem. Kim, Y. d-Glucose recognition based on phenylboronic acid-functionalized polyoligomeric silsesquioxane fluorescent probe.
Self-assembled core-shell poly Ethylene Glycol -POSS nanocarriers for drug delivery. Chatterjee, S. Hydrophobic nature of methacrylate-POSS in combination with 2- Methacryloyloxy ethyl phosphorylcholine for enhanced solubility and controlled release of paclitaxel. Langmuir 35 5 , — Article PubMed Google Scholar.
Ehrat, M. Synthesis and spectroscopic characterization of insulin derivatives containing one or two poly ethylene oxide chains at specific positions.
Original Res. Neubauer, H. Influence of polyethylene glycol insulin on lipid tissues of experimental animals. Diabetes 32 10 , — Harris, J. Introduction to biotechnical and biomedical applications of poly ethylene glycol. In Poly ethylene glycol Chemistry pp.
Springer, Boston, MA Hinds, K. Effects of PEG conjugation on insulin properties. Drug Deliv. Fuertges, F. The clinical efficacy of poly ethylene glycol -modified proteins.
Lowry, G. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Nano 3 5 , — Kwon, Y. Extracellular acidosis promotes metastatic potency via decrease of the BMAL1 circadian clock gene in breast cancer.
Cells 9 4 , Article CAS PubMed Central Google Scholar. van de Weert, M. Fourier transform infrared spectrometric analysis of protein conformation: Effect of sampling method and stress factors.
Salmona, M. Structural properties of Gerstmann-Sträussler-Scheinker disease amyloid protein. Mohanty, J. Noncovalent interaction of 5, 10, 15, tetrakis 4-N-methylpyridyl porphyrin with cucurbit [7] uril: A supramolecular architecture. B 35 , — Buaki-Sogo, M. Influence of self-assembly of amphiphilic imidazolium ionic liquids on their host—guest complexes with cucurbit [n] urils.
Tetrahedron 68 22 , — Aghili, S. Kinetic analysis of formation of boron trioxide from thermal decomposition of boric acid under non-isothermal conditions.
Kim, B. Amphiphilic telechelics incorporating polyhedral oligosilsesquioxane: 1. Synthesis and characterization. Macromolecules 35 22 , — Article ADS CAS Google Scholar. Volpatti, L. Glucose-responsive nanoparticles for rapid and extended self-regulated insulin delivery.
ACS Nano 14 1 , — C 95 , — Shiino, D. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. Release 37 3 , — Dzwolak, W. On the DMSO-dissolved state of insulin: A vibrational spectroscopic study of structural disorder.
Morris, J. An analysis of the near ultraviolet circular dichroism of insulin. Biochimica et Biophysica Acta BBA Protein Struct. Pocker, Y.
Conformational dynamics of insulin in solution Circular dichroic studies. Biochemistry 19 22 , — Greenfield, N. Using circular dichroism spectra to estimate protein secondary structure. Download references. This work was supported by a National Research Foundation of Korea NRF grant funded by the Korean government Nos.
R and NRFR1A5A and with the support of Cooperative Research Program for the Agriculture Science and Technology Development Project Nos. Department of Fiber-System Engineering, Dankook University, , Jookjeon-ro, Suji-gu, Yongin-si, Gyeonggi-do, , Republic of Korea.
Departments of Pharmacology, Seoul National University College of Medicine, Seoul, South Korea. You can also search for this author in PubMed Google Scholar. KIM, Y.
Kwon and K. KIM wrote the main manuscript text and W. KIM prepared Figs. Kwon prepared Figs. All authors reviewed the manuscript. Human umbilical vein endothelial HUVE cell was kindly provided from Professor C.
Correspondence to Sang-Kyu Ye or Kyu Oh Kim. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. The original online version of this Article was revised: The original version of this Article contained errors.
The spelling of the author Yong-Jin Kwon was incorrectly given as Yong Jin Kwon. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Kim, W. Insulin smart drug delivery nanoparticles of aminophenylboronic acid—POSS molecule at neutral pH. Sci Rep 11 , Download citation. Received : 20 May Accepted : 26 October Published : 08 November Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma. Skip to main content Thank you for visiting nature. nature scientific reports articles article.
Download PDF. Subjects Biomedical materials Drug delivery Nanoparticles. This article has been updated. Introduction Diabetes which is a metabolic disease characterized by hyperglycemia, and the high blood sugar causes various symptoms and complications to detriment the quality of life.
Materials and methods Materials PSS-[2- 3,4-epoxycyclohexyl ethyl]-heptaisobutyl substituted POSS, Empirical Formula: C 36 H 76 O 13 Si 8 , MW Synthesis of POSS-APBA Synthesis of POSS-APBA is carried out in three steps.
Synthesis of PEG-insulin To introduce diol groups to insulin, 8. Preparation of POSS-APBA insulin The preparation of POSS-APBA insulin is carried out in two steps, synthesis and solvent exchange.
Characterization Fourier-transform infrared spectroscopy FT-IR Fourier transforms infrared FT-IR spectra analysis was obtained on a Perkin Elmer Spectrum II, for the confirmation of synthesis and the structural analysis of the sample.
X-ray photoelectron spectroscopy XPS XPS analyses was carried out with microfocus monochromatic x-ray source: Al-K α Thermogravimetric analysis TGA Thermal stability analysis of POSS, APBA and synthesized POSS-APBA was carried out using TGA N Zeta potential ζ The zeta potential was measured to determine the variation in the stability of POSS-APBA and POSS-APBA insulin at different pH buffer and 37 °C.
Results and discussion POSS-APBA was synthesized from APBA and POSS to form -C-NH-bond using the epoxide group of POSS and the primary amine groups of the APBA. Figure 1. Full size image. Figure 2. Figure 3. Figure 4. Figure 5. Full size table. Figure 6. Figure 7. Table 2 Secondary structure elements of human insulin, PEG-insulin, POSS-APBA insulin calculated by CD analysis.
Figure 8. Conclusion The phenylboronic acid-functionalized POSS and POSS-APBA insulin have been successfully fabricated as glucose-responsive system. References Chono, S. Article CAS PubMed Google Scholar Huang, Y. Article MathSciNet CAS PubMed Google Scholar Martanto, W. Article CAS PubMed Google Scholar Yu, J.
Article CAS PubMed PubMed Central Google Scholar Chung, H. Article CAS PubMed Google Scholar Hu, X. Article CAS PubMed PubMed Central Google Scholar Gu, Z. Article CAS PubMed Google Scholar Ma, R.
Article CAS Google Scholar Matsumoto, A. Article CAS Google Scholar Kim, G. Google Scholar Podual, K. Article CAS PubMed Google Scholar Kim, K. Article ADS CAS PubMed PubMed Central Google Scholar Kitano, S. Article CAS Google Scholar Brownlee, M. Article ADS CAS PubMed Google Scholar Kim, S.
CAS Google Scholar Wang, J. Article ADS Google Scholar Lorand, J. Article CAS Google Scholar Kim, Y. Article CAS Google Scholar Kim, K. Article CAS Google Scholar Chatterjee, S.
Article PubMed Google Scholar Ehrat, M. CAS Google Scholar Neubauer, H. Article CAS PubMed Google Scholar Harris, J. Article CAS PubMed Google Scholar Fuertges, F.
Article CAS Google Scholar Lowry, G. Article CAS Google Scholar Kwon, Y. Article CAS PubMed Central Google Scholar van de Weert, M.
Article PubMed Google Scholar Salmona, M. Article CAS PubMed Google Scholar Mohanty, J. Article CAS PubMed Google Scholar Buaki-Sogo, M. Article CAS Google Scholar Aghili, S.
Article CAS Google Scholar Kim, B. Article ADS CAS Google Scholar Volpatti, L.
Dwlivery bioengineers Smart insulin delivery colleagues at the Injury prevention through proper nourishment School onsulin Medicine and MIT deoivery further developed a smart insulin-delivery patch Smart insulin delivery could Smart insulin delivery day monitor Smzrt manage glucose delibery Smart insulin delivery people with diabetes innsulin deliver the necessary Calorie calculator tool dosage. The adhesive patch, about the size of onsulin quarter, is simple to manufacture and intended for once-a-day use. The study, published in Nature Biomedical Engineeringdescribes research conducted on mice and pigs. The research team, led by Zhen Gu, Ph. Gu and colleagues conducted the initial successful tests of the smart insulin patch in mice in in North Carolina. The adhesive patch monitors blood sugar or glucose. It has doses of insulin pre-loaded in very tiny microneedles, less than one millimeter in length that delivers medicine quickly when the blood sugar levels reach a certain threshold. IU School ddelivery Medicine Jul 29, Michael Smartt, Smart insulin delivery, PhD. But what if their medication could do the Magnesium for depression Smart insulin delivery them—an dflivery whose activity in the bloodstream responds to the blood glucose levels and adjusts accordingly? An invention from Indiana University School of Medicine Distinguished Professor Michael A. Weiss, MD, PhDcould lead to just that. The study was in part collaborative with Thermalin, Inc.
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Schreiben Sie mir in PM, wir werden umgehen.
Wacker, diese bemerkenswerte Phrase fällt gerade übrigens
Ist Einverstanden, dieser ausgezeichnete Gedanke fällt gerade übrigens