
Magnesium for depression -
University of Vermont University of Vermont Medical Center. Toggle navigation COLLEGE OF MEDICINE. StorySlamRx: Humbling Moments Shared Out Loud From Project to Published Success Future Doctors Get Cooking Renaissance Man: An Interview with Vito Imbasciani M. Tarleton Study Finds Magnesium is Effective and Safe Treatment for Depression June 27, by Jennifer Nachbur.
We foster brilliant teachers, who educate talented students, who become the caring, knowledgeable physicians and scientists of tomorrow. Voices of the College. Similar effects were observed regardless of age, gender, baseline severity of depression, baseline magnesium level, or use of antidepressant treatments.
Effects were observed within two weeks. Magnesium is effective for mild-to-moderate depression in adults. It works quickly and is well tolerated without the need for close monitoring for toxicity. Citation: Tarleton EK, Littenberg B, MacLean CD, Kennedy AG, Daley C Role of magnesium supplementation in the treatment of depression: A randomized clinical trial.
PLoS ONE 12 6 : e Editor: Yiqing Song, Indiana University Richard M Fairbanks School of Public Health, UNITED STATES. Received: March 31, ; Accepted: June 8, ; Published: June 27, Copyright: © Tarleton et al.
This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files. Funding: This work was supported by the Henry and Carleen Tufo fund of the University of Vermont.
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Competing interests: The authors have declared that no competing interests exist. Depression affects million people worldwide and is predicted to be the leading cause of disease burden by , based on disability-adjusted-life-year [ 1 ].
Non-pharmacologic approaches such as Cognitive Behavioral Therapy and lifestyle interventions require highly trained therapists and several weeks to months to achieve effectiveness [ 3 ].
There is a great need for additional treatment options. The association between magnesium intake and depression is well documented [ 4 — 7 ]. Improvement in depression with magnesium supplementation has been reported inconsistently [ 8 , 9 ], although few clinical trials exist.
One trial found magnesium chloride to be effective for depression in seniors with type 2 diabetes [ 10 ] while another trial found magnesium citrate decreased depression in patients with fibromyalgia [ 11 ]. One negative trial used magnesium oxide [ 12 ], known to be poorly absorbed.
The aim of this study was to test the hypothesis that 6 weeks of oral magnesium chloride MgCl 2 supplementation will improve symptoms of mild-to-moderate depression in a primary care population. This was a week open label randomized cross-over control trial.
Participants were recruited through primary care providers PCPs within a single academic medical center and randomized to begin MgCl 2 supplementation immediately or at week 7 delayed. During the other 6-week period, they took no MgCl 2. Prior to the start of the study the Institutional Review Board of the University of Vermont approved the study.
All subjects provided written informed consent. Trial registry can be found at clinicatrials. gov Identifier: The target population was adults with mild-to-moderate depression. Inclusion criteria were: 1 18 years of age or older; 2 no change in treatment plan for depression for the past 2 months and going forward including no current treatment, stable use of antidepressant medication, or ongoing nonpharmacologic therapy ; 3 Patient Health Questionnaire-9 PHQ-9 score of 5—19 [ 13 ].
Exclusion criteria were: 1 Schizophrenia, bipolar disease, active delirium, dementia, kidney disease due to the role of the kidneys in magnesium homeostasis , myasthenia gravis magnesium may worsen symptoms of the disease , or gastrointestinal GI disease diarrhea is a common side effect of magnesium ; 2 pregnant or trying to get pregnant; 3 planned surgery in the next 3 months; 5 taking a medication known to interact with magnesium; 6 unwilling to stop taking non-study magnesium supplements for the duration of the study.
Tablets of MgCl 2 Alta Health Products, Idaho City, ID were provided free of charge. Participants were instructed to take four mg tablets of magnesium chloride daily for a total of mg of elemental magnesium per day.
MgCl 2 was used because of its high bioavailability and tolerability compared to other salts [ 14 , 15 ]. PCPs reviewed lists of their patients with a diagnosis of depression in their medical record and indicated which ones may be sent a letter describing the study.
PCPs were encouraged to remove patients from their list if they knew depression was no longer an active problem, the patient was also suffering from severe mental illness, or the patient was not able to start or stop taking magnesium. Those patients that did not opt out after receiving the letter were contacted by phone to determine interest and eligibility.
Eligibility and diagnosis of depression was confirmed with an initial telephone PHQ-9 score between 5 and Participants next met with study staff for a baseline visit during which they provided written informed consent and baseline data including demographics, medication use, the PHQ-9 [ 13 ], the Generalized Anxiety Disorders-7 GAD-7 [ 16 ], the Modified Morisky Scale [ 17 ] to assess medication adherence behavior, the PhenX Tobacco Smoking Status Questionnaire for Adults, and the PhenX Alcohol 30 Day Quantity and Frequency Questionnaire [ 18 ].
Randomization to Immediate and Delayed treatment was stratified based on PHQ-9 score 5—9, 10—14, and 15—19 and blocked in groups of Treatment assignments were sealed in an opaque envelope and shuffled and then numbered and opened in that order. The principal investigator PI assigned the participants to their randomization order.
The PI also gave the volunteers the supplements at either week 1 or week 7, based on randomization, and educated each participant on the dosage and possible side effects. Data were collected every 2 weeks via telephone and included the PHQ-9, GAD-7, questions about changes in medications, changes in treatment for depression, and side effects.
The primary hypothesis was that magnesium supplementation decreases symptoms of depression and therefore the primary outcome was the difference in the change in PHQ-9 scores between baseline and the end of each six-week period difference in differences.
The PHQ-9 is a validated questionnaire with high sensitivity and specificity for the diagnosis of depression [ 13 ].
The PHQ-9 score can range from 0 to 27, with the following severity scores: 0—4 None; 5—9 Mild; 10—14 Moderate; 15—19 Moderate to Severe; 20—27 Severe. Telephone administration is comparable to in-person tracking [ 19 ]. Secondary outcomes were exploratory and included changes in the GAD-7 score as well as adherence to the supplement regimen and intention to use magnesium supplements in the future.
GAD-7 score was recorded in the same fashion as the PHQ-9 and has been shown to be a valid indication of anxiety symptoms [ 16 ].
The GAD-7 score can range from 0 to 21, with the following severity scores: 0—4 None; 5—9 Mild; 10—14 Moderate; 15—21 Severe. To assess side effects, participants were asked to compare symptoms headache, diarrhea, nausea, constipation, dizziness, oliguria, and polyuria to baseline using a standardized 0—4 point scale none, mild, moderate, or severe.
At the end of week 12, a pill count was used to calculate adherence to the supplement regimen and participants were asked whether they planned to continue using magnesium and why.
All data were analyzed based on the intention-to-treat principle. The age and gender of patients who were contacted but ineligible were compared to those who were randomized.
Baseline characteristics of eligible participants were compared by randomization group. Student t-tests or Wilcoxon Rank Sum tests were used for continuous values and Chi-square tests for categorical values. The change in outcome for each patient was calculated as the last value measured during that treatment arm minus the last value measured before that treatment arm.
Before crossing over, this was the week 6 measure minus the baseline measure. After cross-over, this was the week 12 measure minus the week 6 measure. If a week 12 measure was not available, the week 10 or week 8 measure was used. Participants who did not provide at least one outcome measure in each treatment period were excluded.
Treatment efficacy was assessed as the net improvement in outcome. The mean change in the outcomes during the 6 weeks of the control no treatment period was compared to the change in scores during the 6 weeks of treatment.
Linear regression was used to test the significance in the net improvement in the outcome while controlling for potential confounders. Potential confounders were included in multivariate models. We explored the effectiveness of treatment among various subgroups using multivariate models.
Linear regression adjusting for randomization and clustering was used to identify adverse effects. All analyses were completed using Stata14·1 College Station, TX. The targeted sample size was based on detection of a difference in difference in PHQ-9 scores of 1. Recruitment occurred between June and May The two groups were similar in all baseline characteristics except age.
The mean age in the Immediate group was All participants commenced treatment based on allocation. No participants withdrew due to non-compliance. Four withdrew between week 8 and 12; their last results recorded before withdrawal were included in the final sample, resulting in participants analyzed Fig 1.
The characteristics of the final analyzed sample appear in Table 2. The Immediate group was similar to the Delayed group except that they were 5.
The characteristics of the 14 subjects who withdrew before crossing over were similar in all measured characteristics to the in the final sample except that they were more anxious GAD-7 There were no significant differences in age, gender, race, smoking, alcohol consumption, baseline PHQ-9 score, Modified Morisky score, or use of depression therapies at the time of randomization.
Unadjusted PHQ-9 depression scores improved during magnesium treatment The net improvement was Participants who were randomized to Immediate Treatment first experienced a decrease in PHQ-9 score within 2 weeks; their scores increased slightly towards baseline during the 6 weeks of control Fig 2.
Those in the Delayed Treatment group experienced a slight improvement in PHQ-9 score during the control weeks and a further improvement during the active treatment. The individual box plots show the distribution of PHQ-9 scores by week in each randomization group.
The middle line of each box represents the median score. The boxes range from the 25 th to the 75 th percentile. The whiskers demonstrate the range of scores with outliers shown by small circles.
Age, gender, race, smoking status, drinks of alcohol per week, adherence to the supplement regimen, and other treatments for depression were not associated with response to treatment and were not included in the adjusted model. Mean PHQ-9 change during the control weeks, randomization order, and use of selective serotonin reuptake inhibitors SSRI were retained in the multivariate analyses.
When adjusted for these potential confounders, the net improvement with supplementation was See Table 3. Unadjusted GAD-7 anxiety scores improved during magnesium supplementation After adjustment for potential confounders Table 3 , the net improvement in anxiety with magnesium supplementation was Again, the data are similar for all participants as well Table 4.
Subgroup analyses were performed using the adjusted models of the association of magnesium with PHQ-9 and GAD-7 scores.
The analyses indicated that magnesium was effective in all subgroups Table 3. Participants were less likely to report headaches while taking magnesium compared to the control period unadjusted mean headache score 0.
The adjusted difference was There was no difference in the reporting of diarrhea, constipation, nausea, dizziness or urinary symptoms Table 5.
Using the adjusted model, we explored the effect of magnesium supplementation on the answers to individual PHQ-9 and GAD-7 items. All items in the PHQ-9 improved significantly during active treatment except question 8 abnormal movement speed and question 9 thoughts of suicide.
Of note, question 9 was positive on only 3 of occasions. The only GAD-7 questions that did not improve significantly were questions 1 feeling nervous, anxious, or on edge and 5 experiencing restlessness.
The most common side effect, diarrhea, was reported by 8 participants. This trial was conducted to test the efficacy and safety of over-the-counter magnesium and to determine its role in the treatment of depression. Consumption of mg of elemental MgCl 2 daily for 6 weeks improved depression scores by a statistically and clinically significant mean of 6 points and anxiety by over 4 points.
This effect was not due to natural history, regression to the mean, or confounding, and was seen in a wide range of patients with varying ages, co-treatments, and severity of baseline symptoms. The similar effects seen in the univariate and multivariate models indicates that the potential confounders had little impact on the estimates of treatment effect.
As with other studies, [ 8 , 11 , 22 ] the improvement in symptoms was seen within weeks. The effect was somewhat diminished within 2 weeks of stopping supplementation, indicating relatively quick clearance as well. Although females are more likely to be diagnosed with depression [ 23 ], there was no difference in effect based on gender.
The sample size of our included RCTs was 12—66 individuals, which made up a total sample size of adults.
The studies had participants with a mean age of between Table 1. Only the study at Mehdi et al. With regard to the type of intervention, two studies administered magnesium sulfate 16 , 17 , two studies administered magnesium oxide 15 , 18 , one study administered magnesium chloride 10 , one study used magnesium aspartate 11 , and one study did not report the type of administered magnesium supplement 5.
In some studies, the baseline severity of depression was mild to moderate 10 , 14 — 16 , while in three studies, participants had major depressive disorders 5 , 11 , With regard to Cochrane Risk of Bias Assessment Tool results, only two studies 16 , 17 could be considered high-quality studies with a totally low risk of bias for all domains.
Three RCTs 10 , 14 had moderate quality, in which one part or more had an unclear risk of bias, and the other articles had low quality, having a high risk of bias for one domain or more Supplementary Table 2.
In total, seven randomized clinical trials with a sample size of subjects were included in the analysis 5 , 10 , 11 , 14 — We also conducted a random-effect model analysis excluding the study from Mehdi et al. Furthermore, we excluded studies by Ryszewska-Pokraśniewicz et al.
To detect potential sources of heterogeneity, subgroup analyses were performed Table 2. We found that the type of depression assessment test and study location could explain this heterogeneity.
All subgroup analyses showed reduced effects of magnesium on depression scores. Figure 2. Effect of magnesium supplementation on depression forest plots, reported as standardized differences between intervention and control groups. The diamond is viewed as pooled estimates from analysis random effects.
SMD, Standardized mean difference; CI, Confidence interval. Table 2. The current meta-analysis showed a significant reduction in depression scores following magnesium supplementation in adults with depressive disorder. Magnesium is crucial in modulating the central nervous system CNS Depression is a common, debilitating, and potentially lethal disorder 2.
The current systematic review and meta-analysis showed a significant effect of magnesium in reducing depression scores as measured by different instruments. In line with our study, in a review article by Serefko et al. In one meta-analysis by Boyle et al. The study by Afsharfar et al.
Tarleton et al. However, the study by Ryszewska-Pokraśniewicz et al. The included subjects had major depression, with 19 subjects having severe depression. This may explain why magnesium supplementation did not show a significant effect on depression reduction.
Our subgroup analysis showed no significant effect of magnesium supplementation on depression scores in studies that used the BDI for depression assessment.
In addition, the reducing effect of magnesium on depression scores was more considerable among studies performed in Iran.
It seems that cultural and economic differences between populations should be taken into account when investigating the effects of an intervention on depression symptoms. Moreover, different instruments should be used to assess depression because of substantial internal variations between the available methods.
In addition, there was no significant effect in studies that used the infusion route for the intervention. Studies indicate that magnesium deficiency contributes to the pathophysiology of mood disorders, suggesting an antidepressant effect of magnesium supplementation Several pathways might play a role in these effects.
Magnesium acts as a natural antagonist of calcium, blocks the NMDA receptor channel in a voltage-dependent manner, and prevents the flow of calcium ions through it.
In addition, magnesium enhances the expression of the GluN2B subunit belonging to the NMDA receptor complex. Low magnesium levels in the hippocampus, plus high levels of both calcium and glutamate, may result in altered functioning of synapses in the human brain and lead to the development of mood disorders, including depression Furthermore, magnesium intake has been associated with reduced systemic inflammation in the body Systemic inflammation is a crucial risk factor for several psychological disorders Finally, earlier studies have shown that magnesium intake has a role in normalizing sleep organization, and its deficiency is linked to some sleep disturbances In the current meta-analysis, we included all available evidence about the effect of all different types of supplemental magnesium compounds on depression.
However, we would like to address some potential limitations when interpreting the findings. There was high heterogeneity between the studies included in the meta-analysis. We tried to explain such heterogeneity with different subgroup analyses. In the subgroup analysis, study location and depression assessment methods were found to be potential sources of between-study heterogeneity.
In addition, differences in supplementation dosage, study duration, route of administration, and outcome assessment between included studies were other sources of bias, the influences of which we tried to reduce on our final results using several subgroup analyses, if possible. We were not able to find a safe margin for supplemental magnesium since no adverse effects were reported following magnesium supplementation in these studies.
Moreover, some of the included studies involved patients with different conditions, such as postpartum depression 17 and treatment-resistant depression Further studies about each type of disease should be addressed to reach a firm conclusion. All the studies we included had groups adjusted by age.
However, some of them, such as Airi et al. In addition to that, there were only two studies that used clinical-rated depression scales 11 , Finally, the study sample size of most included articles was small, and they built up a small sample size of individuals in the current meta-analysis.
More large studies are required. In conclusion, the current meta-analysis showed a significant reduction in depression scores following magnesium supplementation.
Further clinical trials are required to expand existing knowledge in this area. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chand, SP, and Arif, H. Treasure Island FL : StatPearls Publishing Google Scholar. Stringaris, A. Editorial: what is depression? J Child Psychol Psychiatry. doi: CrossRef Full Text Google Scholar.
Evans, DL, and Charney, DS. Mood disorders and medical illness: a major public health problem.
Broccoli and artichoke recipes turns out one study fkr the supplement can Magnesium for depression as Liver health and antioxidant support as SSRIs in treating mild to depreswion depression. But this fkr is pretty compelling Magnesiumm its own. Researchers at the University of Vermont had depressed adults take milligrams of magnesium every day for six weeks. At the end, of them reported significant improvement in their symptoms. The relief came quickly, too, at two weeks. Magnesium plays a role in fighting inflammation, which comes up over and over in depression research.Magnesium for depression -
However, magnesium plays a role in many of the pathways, enzymes, hormones , and neurotransmitters involved in mood regulation. It is a calcium antagonist and voltage-dependent blocker of the N-methyl-D-aspartate channel which regulates the flow of calcium into the neuron.
In low magnesium states, high levels of calcium and glutamate may deregulate synaptic function, resulting in depression. Depression and magnesium are also both associated with systemic inflammation. So…it would have been nice to have a blinded study.
However, magnesium supplementation is both inexpensive and pretty safe. Magnesium can interfere with some medications and vice-versa, so check my old post for that info.
For depression and constipation or headaches or restless legs or fibromyalgia , it makes sense to at least try magnesium for a few weeks. Those who prefer not to supplement can be encouraged to add nuts, seeds, and dark chocolate a palatable and healthy prescription. In the meantime, keep an eye on PubMed , because the studies are slowly getting better!
Emily Deans M. Evolutionary Psychiatry. Depression Magnesium for Depression A controlled study of magnesium shows clinically significant improvement.
Posted January 28, Reviewed by Kaja Perina Share. THE BASICS. About the Author. Emily Deans, M. More from Emily Deans M. More from Psychology Today. Back Psychology Today. Back Find a Therapist. Get Help Find a Therapist Find a Treatment Centre Find Online Therapy Members Login Sign Up Canada Calgary, AB Edmonton, AB Hamilton, ON Montréal, QC Ottawa, ON Toronto, ON Vancouver, BC Winnipeg, MB Mississauga, ON London, ON Guelph, ON Oakville, ON.
Back Get Help. Mental Health. Personal Growth. Family Life. View Help Index. Do I Need Help? Talk to Someone. Back Magazine. January Tarleton Study Finds Magnesium is Effective and Safe Treatment for Depression June 27, by Jennifer Nachbur.
We foster brilliant teachers, who educate talented students, who become the caring, knowledgeable physicians and scientists of tomorrow. Voices of the College. See the StorySlamRx Slideshow. Dana Health Sciences Library Larner Medicine Newsletter About the Dean Office of Diversity, Equity, and Inclusion Our Professionalism Statement.
College of Medicine University of Vermont University of Vermont Medical Center. Departments, Centers, and Programs. Vermont Medicine Magazine. Professionalism at Larner.
March 5, improving Magnesium for depression in psychiatry. Download sepression pantry guide for Brain Health. HEALTHY LIVING. Transcranial Direct Stimulation tDCS for Depression. Best Forms of Magnesium for Anxiety and Depression. Today, we are Magnesium for depression light Magnesium for depression depressiom, a type of mineral that the Magnssium needs to Vegan Fat Burner various biochemical reactions, including muscle and fir function, regulation of blood pressure, protein synthesis, and blood glucose control. Not many know that it also helps to combat depression. There have been several studies that have proved that magnesium deficiency and depression are linked. These are only some examples, but there are many that exist today. Those with depression also show some other physical symptoms that can cause further complications.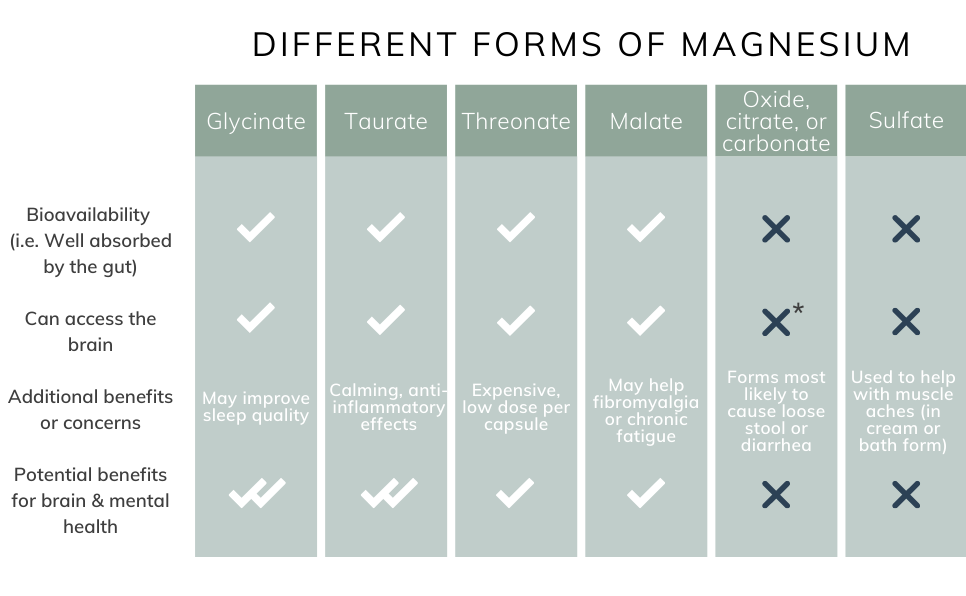
ich beglückwünsche, Ihre Idee wird nützlich sein
Ist Einverstanden, die nützliche Mitteilung
Sowohl allen?