Energy metabolism and gut health -
muciniphila had decreased body weight and fat mass, as well as reduced marker levels for liver dysfunction and inflammation. Likewise, a significant association was found between the abundance of Prevotella and a human variant linked to a gene related to body fat distribution and insulin sensitivity Li J.
et al. This area of research deserves additional efforts to understand the interactions of specific bacterial strains with host metabolism, particularly in human subjects. Adipose tissue is found in two different forms in mammals: white and brown Peirce et al.
White fat stores energy as triglycerides, whereas brown fat burns extra calories to create heat Peirce et al. Additionally, there are beige, or brite, adipocytes, which are an inducible form of brown adipocytes that are sporadically generated within white adipose tissue in response to various stimuli such as cold temperatures Sidossis and Kajimura, Accumulating evidence suggests that the gut microbiome affects the thermogenic capacity of brown fat and the formation of beige adipocytes Suárez-Zamorano et al.
For example, when Suárez-Zamorano et al. Both germ-free and antibiotics-treated mice had more UCP1-positive cells a marker for thermogenesis and upregulated brown fat—specific markers in their subcutaneous and visceral WATs Suárez-Zamorano et al.
Notably, recolonization mostly reverted the metabolic benefits induced by antibiotics treatment. Mechanistically, the authors proposed that increased browning in microbe-depleted mice is due to anti-inflammatory or alternatively activated M2 macrophages derived from upregulation of type 2 cytokines, such as Interleukin 4 IL 4 , Interleukin 13 IL 13 , and Interleukin 5 IL 5 , which promote beige adipogenesis Martinez et al.
However, Li et al. Consistent with impaired thermogenic gene regulation, the antibiotics-treated mice had reduced whole-body energy expenditure Li et al. Unlike the other study, no significant difference was observed regarding M2 macrophage biology Li et al. The authors proposed that, instead of macrophage polarity, the gut microbiota influences BAT metabolism by generating certain metabolites i.
The contradictory results between the two studies could arise for multiple reasons. First, despite the identical genetic background, gender, and age of the mice, they were raised in different facilities, meaning they were under unique environmental conditions that may have differentially impacted the microbiota composition before and after antibiotics.
In fact, Li et al. However, the mice were treated for different lengths of time. Mice were treated for 6 weeks in the first study Suárez-Zamorano et al. It is possible that incomplete deletion of microbiota or imbalanced microbiota composition could differentially impact host organs.
Nevertheless, both studies suggest that drastic perturbations of gut microbiota have profound impacts on BAT function or the browning process. In addition to adipocyte thermogenesis, the gut microbiome may affect lipid metabolism Backhed et al. Moreover, colonization with a single saccharolytic bacterial species, B.
thetaiotaomicron , a prominent member of the human distal gut microbiota with an extraordinary capacity for acquiring and degrading plant polysaccharides Xu et al.
Mechanistically, it was proposed that the increased adiposity in conventionalized germ-free mice is partially due to the microbial suppression of the intestinal expression of angiopoietin-like protein 4 Angptl4 , a secreted protein that inhibits lipoprotein lipase activity Backhed et al.
Notably, reduced adiposity in germ-free mice is associated with an elevated level of ANGPTL4, accompanied by the increased expression of Pparg coactivator 1 alpha PGC1A and genes involved in fatty oxidation in muscle Bäckhed et al.
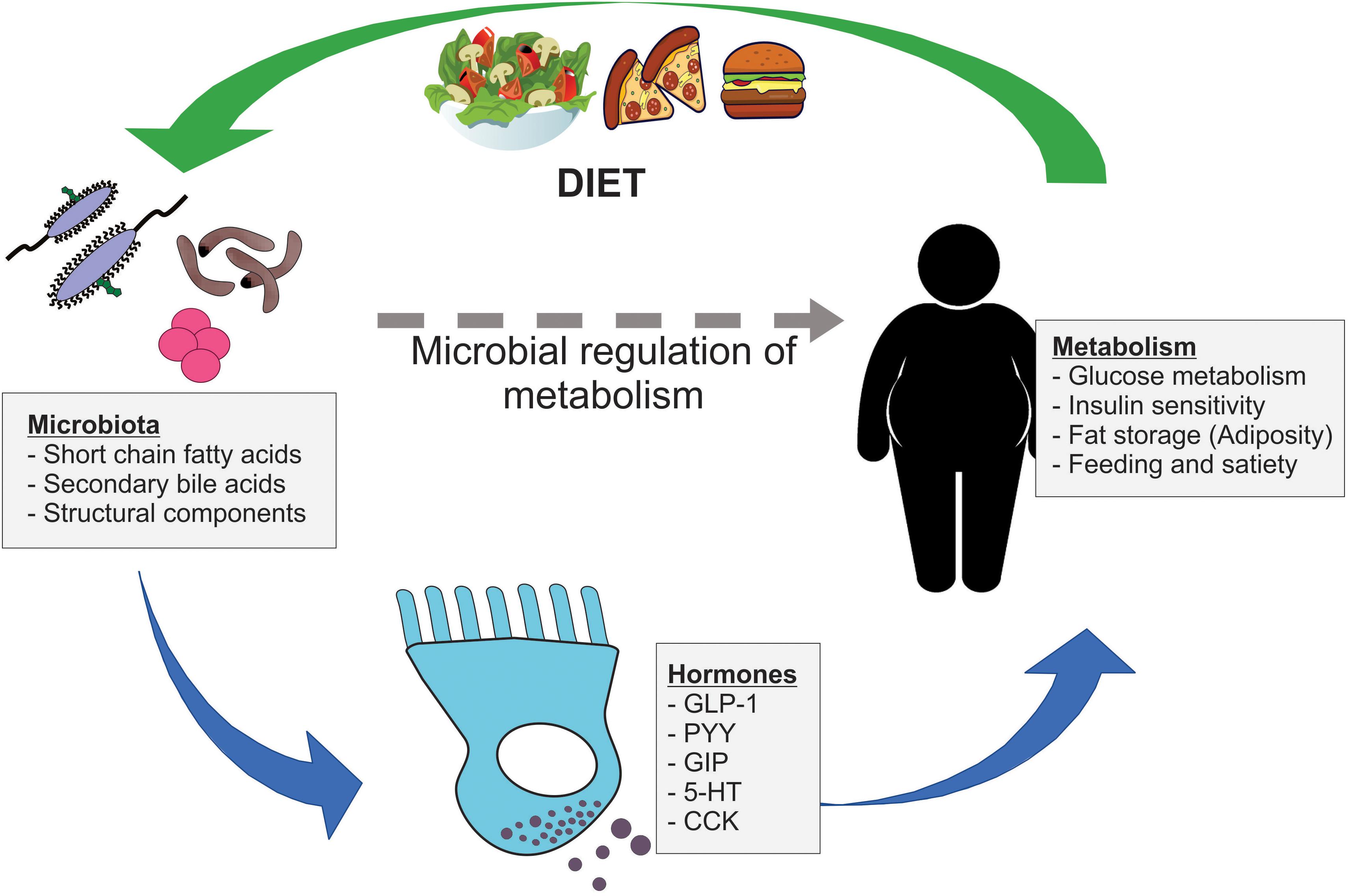
Together, these results suggest that the microbiome communicates with adipose and other metabolic tissues to influence host lipid metabolism. The gut microbiome is highly influenced by food intake and various environmental and genetic factors. In this section, we will discuss which external factors affect the gut microbiota community and influence the metabolism of target tissues, namely white and brown adipose depots Table 1.
Table 1. A list of the previously identified key environmental factors that lead to gut microbiota-mediated biological changes in adipose tissue. A change in ambient temperature is one of the strongest physiological stimuli for increasing thermogenic adipose formation and activity Cypess et al.
A recent study by Chevalier et al. Furthermore, transplanting microbiota from a mouse exposed to cold increased browning in white adipose depots and increased insulin sensitivity in recipient mice Chevalier et al.
Another study reported similar findings, showing that gut microbiota from cold-conditioned mice modulated fat accumulation by promoting thermogenesis Ziȩtak et al. Recipient mice colonized with microbiota from donors that were fed with a high-fat diet and kept at 12°C had reduced fat mass as well as significantly higher mRNA and protein expression of thermogenic genes like Mitochondrial uncoupling proteins1 Ucp1 and Type II Iodothyronine Deiodinase Dio2 in their interscapular BAT compared to mice that received microbiota from donors housed at thermoneutrality 29°C Ziȩtak et al.
The role of gut microbiota as a mediator of cold-induced thermogenesis was further explored by Bo et al. They verified that cold exposure modified gut microbiota and increased the concentration of SCFAs. In addition, norepinephrine injection also induced a long-term decrease in food intake and body mass in the treated group, paralleled by altered gut microbiota composition.
They confirmed that transplantation of cold-acclimated microbiota triggered thermogenesis in the recipient by activating the cAMP—PKA—pCREB signaling pathway.
Therefore, gut microbiota may interact with host neurotransmitters to regulate thermogenesis and energy expenditure during cold acclimation. A direct link has been established between gut microbiota and diet-induced obesity Bäckhed et al.
For instance, bacterial lipopolysaccharide, or endotoxin, has long been identified as an inflammatory factor triggering the onset of obesity, insulin resistance, and diabetes Cani et al.
Bacterial endotoxin is abundant in the human gut and circulates at low concentrations in the blood of all healthy individuals, whereas an elevated concentration of endotoxin has been demonstrated to affect the function of the major organs involved in maintaining glucose and lipid homeostasis, including adipose tissue Caesar et al.
Obese and diabetic people have increased plasmatic lipopolysaccharide levels Harte et al. The increase in the proportion of gram-negative microbiota, increased gut mucosal permeability, and the consumption of high-fat diets increase the plasmatic lipopolysaccharide levels, which will elicit local inflammatory signals that can further deteriorate the gut barrier and promote bacterial translocation.
Cani et al. reported that a 4-week high-fat diet chronically increased the proportion of an LPS-containing microbiota as well as the plasma LPS concentration in mice by two to threefold Cani et al.
The induction of the elevated plasma LPS level, often referred to as metabolic endotoxemia, in mice is followed by the onset of liver insulin resistance and an increased expression of inflammatory markers in the adipose tissue. Another study also confirmed the onset of insulin resistance induced by low-dose LPS infusion in healthy humans, which is exclusively associated with stimulation of inflammatory pathways as opposed to the insulin resistance caused by intravenous fat or glycerol treatment Nowotny et al.
This demonstrates that metabolic endotoxemia dysregulates the inflammatory profiles, and triggers body weight gain and insulin resistance.
These findings suggest to lower plasma LPS concentration could be a strategy for the control of metabolic diseases. Overall, the gut microbiome may be a potential target for improving metabolic homeostasis. Diet-induced weight-loss intervention improves low gut microbial gene richness, thus mitigating the dysmetabolism of obese patients Cotillard et al.
Considering that gut microbiota is, to a large extent, modulated by diet in humans, dietary interventions are a potential means to prevent or treat obesity via modifying gut microbiota composition David et al.
Obesity is associated with reduced brown adipose activity, which is characterized by impaired β-adrenergic signaling, vascular rarefaction, larger lipid droplets, inflammation, and decreased mitochondrial respiration Orava et al. Paradoxically, increased UCP1 expression a marker for brown adipose is observed after feeding rodents a high-fat diet Surwit et al.
High-fat foods alter the microbiome composition to favor the proliferation of gram-negative bacteria strains Kasselman et al. Thus, it is plausible that the microbiome is the mechanism for high fat diet—induced Ucp1 expression.
In addition, germ-free mice are resistant to high-fat diet-induced obesity, accompanied by malabsorption of dietary lipids, while HFD-fed SPF mice develop obesity and exhibit an increased abundance of the family Clostridiaceae in their intestines Martinez-Guryn et al. The fact that treatment with Clostridiaceae directly upregulates lipid transport genes both in vivo and in vitro indicates that gut microbiota plays a role in shaping host adaptability to dietary lipid uptake, possibly as early as in the digestive phase.
The ketogenic diet consists of high fat, very low carbohydrate, and moderate protein Dashti et al. This diet phenocopies several biochemical characteristics of fasting including reduced insulin level, Forkhead box O FoxO signaling, and inhibition of the mammalian target of rapamycin mTor , and activation of AMP-activated protein kinase AMPK Veech et al.
Recent studies suggest the ketogenic diet significantly impacts gut microbiota, but with mixed results. For example, a mouse study noted that the ketogenic diet increased beneficial species of gut microbiota including A.
muciniphila and Lactobacillus bacteria, which are capable of producing small-chain fatty acids SCFAs that may provide the host with beneficial health outcomes Ma et al.
However, these changes occurred at the expense of reduced overall microbial diversity, possibly due to the minimized carbohydrate intake, which can disrupt other beneficial microbes Swidsinski et al.
Human studies also point out some potential negative effects on the gut microbiota. For example, overweight and obese subjects following the ketogenic diet for 8 weeks had a significantly reduced amount of a beneficial bacterium, Bifidobacterium , in their colon and decreased plasma levels of SCFAs Brinkworth et al.
Future studies are warranted to establish with more accuracy the effects of high fat—containing diet regimens on microbiota composition and diversity and the mechanisms whereby this microbiota affects adipose biology.
Intermittent fasting has also caught mainstream attention for its many benefits including weight loss. For example, an every-other-day fasting EODF regimen in diet-induced obese mice results in weight loss and an improved metabolic profile associated with increased beige fat development Li et al.
Moreover, the microbiota seems to play a causal role, as mice that received the microbiota from EODF mice recapitulated the beneficial phenotype while microbiota-depleted mice did not Li et al.
Another dietary pattern that has beneficial effects on host health is the Mediterranean diet. Chronic intake of the Mediterranean diet or a low-fat diet over a year is associated with an improved insulin profile and modified lipid metabolism in obese patients with coronary heart disease, an effect which is linked to an increased abundance of Roseburia genus and F.
prausnitzii in the gut Haro et al. Consistently, other studies have shown that obese patients with metabolic dysfunction have reversed microbiota dysbiosis upon long-term consumption of the Mediterranean diet Haro et al. However, short-term dietary interventions appear to have no effect.
A randomized 1-week-long dietary intervention found no effects of different types of bread on clinical parameters nor on gut microbial composition Korem et al.
Another study comparing the effect of consuming a high-cholesterol diet for 12 weeks also detected no major differences in microbial composition between control and treated mice Dimova et al.
Together, these results suggest that short-term dietary intervention may not overcome interpersonal variability in gut microbiota composition, and understanding dietary effects requires integration of person-specific factors.
Several studies have proposed that some of the metabolic phenotypes in certain genetic mouse models are derived from altered gut microbiota. For example, the endocannabinoid system consists of ubiquitous bioactive lipids that regulate glucose and lipid metabolism, food intake, and inflammation through various receptors in autocrine and paracrine manners Di Marzo and Matias, ; Horn et al.
N -acylethanolamines NAEs are the best characterized endocannabinoids, and increased NAE levels are associated with obesity and metabolic comorbidities Tsuboi et al.
Unexpectedly, a mutant mouse carrying an adipose-specific deletion of Napepld , which encodes N -acylphosphatidylethanolamine phospholipase D NAPE-PLD , the NAE endocannabinoid synthesizing enzyme, is more susceptible to diet-induced obesity and metabolic dysregulation Geurts et al.
In these mice, the gut microbiota composition changes in the abundance of 64 operational taxonomic units Geurts et al. Germ-free mice receiving the KO microbiota have significantly increased fat mass and decreased expression of BAT-specific genes compared to those who received WT microbiota Geurts et al.
Similarly, another study Somm et al. Thus, Klb KO mice were expected to be resistant to the beneficial action of FGF21 treatment. However, the Klb -KO mice are leaner and have more brown adipose activity compared to controls on a high-fat diet Somm et al.
The authors proposed that the surprising body weight phenotype is attributable to the changes in the host bile acid metabolism Somm et al.
These examples illustrate that there are intricate interactions among the microbiome, adipose tissue, and genetics. It has been proposed that the microbiota employs microbial and host metabolites for microbiome-to-tissue communication to regulate cellular function.
The short-chain fatty acids SCFAs acetate C2 , propionate C3 , and butyrate C4 , which are the major end products of microbial fermentation of dietary fiber, serve as the primary energy source for colonic epithelium Boffa et al. A randomized clinical study found that deficiency in SCFA production is associated with type 2 diabetes Zhao et al.
Zhao et al. Participants exhibited better improvement in their blood glucose profiles when they had fiber-promoted SCFA producers in higher abundance and greater diversity. This could partially be mediated by increased glucagon-like peptide-1 production. However, it could be challenging to measure the contribution of each subtype of SCFAs during this process accurately Zhang et al.
Even if their levels can be measured in feces, they may not adequately reflect the total amount processed by microbiota. Considering these constraints, Sanna et al. They found that increased gut microbial activity producing butyrate is positively associated with insulin sensitivity, whereas a higher production of propionate is associated with increased risk of type 2 diabetes Sanna et al.
Therefore, targeted restoration of certain producers of SCFA subtypes may be a promising approach for managing T2DM. Several studies suggest that SCFAs activate G protein—coupled receptors GPCR to affect the target organ of the host.
For example, Gpr43, which has a strong affinity to acetate and propionate, plays an important role in mediating the microbial input in WAT metabolism Brown et al. GprKO mice are obese compared to their wild-type counterparts on both chow and high-fat diet, whereas adipose-specific Grptransgenic mice are leaner and more insulin sensitive Kimura et al.
Remarkably, the body weight phenotype was abolished under germ-free conditions, demonstrating the importance of microbial metabolism in forming ligands for adipose GPR43 signaling Kimura et al. Analysis of the gut microbiota communities revealed that GprKO mice display an increased gut population of Firmicutes Kimura et al.
This was also accompanied by increased fecal and plasma acetate concentrations in the KO mice Kimura et al. In support of the role of Gpr43 and acetate, anti-lipolytic activity of GPR43 in WAT was reported Robertson et al.
Moreover, acetate-dependent GPR43 stimulation in the WAT, but not in muscles or liver, improves glucose and lipid metabolism Kimura et al. Together, these findings suggest that acetate-mediated GPR43 signaling in WAT may have metabolically beneficial functions.
A recent study proposed that butyrate mediates the thermogenic stimulation of BAT, as administering butyrate sodium to microbiota-depleted mice partially rescues impaired thermogenesis and promotes fat oxidation Li et al.
Other studies have revealed the association between butyrate administration and improved blood glucose profiles Xu et al. Fang et al. As the butyrate dose used in this study was lower than the that usually given in mouse studies, a sub-therapeutical dose of sodium butyrate might be an explanation for the less optimal response by the metabolic syndrome subjects.
Though most of the butyrate in the cecum may be used by mitochondria as an energy substrate in the colon den Besten et al. Of note, isotope tracing revealed that gut-produced butyrate is mainly routed to the brain rather than peripheral tissues, suggesting that gut-derived butyrate activates BAT via the gut-brain neural circuit, rather than working on adipose directly Li et al.
This was further supported by the finding that butyrate administration decreases food intake and inhibits orexigenic neuron activity in the hypothalamus Frost et al.
In line with this study, high-fat-diet-fed mice that receive dietary butyrate for 12 weeks upregulate the expression of UCP1 and PGC1α and have increased mitochondrial function and biogenesis in BAT Gao et al.
It is noteworthy that butyrate, and to a lesser degree, propionate, are histone deacetylate HDAC inhibitors Waldecker et al.
Administration of propionate and butyrate to the stromal vascular fraction of porcine adipose tissue enhanced adipocyte differentiation, which could be partially mediated by their inhibitory effect on histone deacetylase activity Li et al. Moreover, Krautkramer et al. Thus, it is conceivable that the microbiome may affect the epigenome of target tissues.
Other gut microbial metabolites are involved in epigenetic regulation. Exposure of mouse ileal organoids to SCFAs and products generated by A. muciniphila modulates histone deacetylase level and the expression of genes involved in satiety and host lipid metabolism Lukovac et al.
Furthermore, Virtue et al. HFD upregulates adipocyte miR expression during obesity, which is a microbiota-dependent process. Through controlling the expression of the miR family in white adipocytes in mice, the gut microbial metabolite I3CA plays a role in regulating the energy expenditure and insulin sensitivity of the host.
These results indicate that gut microbiota—derived metabolites could be part of the epigenetic mechanisms that regulate host metabolism and adipocyte function in response to dietary modification.
In addition to gut microbiota-derived metabolites, the predominant bacterial phyla in the gut has also been shown to correlate with DNA methylation patterns.
Although most of the existing evidence focus on the influence of gut microbiome on the epigenetic regulation of host genes involved in maintaining intestinal homeostasis and regulating the mucosal immune system in the gut Takahashi et al. A whole-genome methylation analysis conducted by Kumar et al.
Eight pregnant women were classified into two groups depending on their dominant gut microbiota, i. Next-generation sequencing of DNA methylomes indicated that the genes with differentially methylated promoters in the High Firm group was functionally associated with lipid metabolism, obesity, and the inflammatory response.
Another genome-wide analysis of DNA methylation also demonstrate that the DNA methylation status is associated with gut microbiota composition in obese subjects Ramos-Molina et al. Ramos-Molina et al. This finding indicates that the expression levels of genes implicated in glucose and energy homeostasis in adipose tissue could be epigenetically regulated by gut bacterial populations.
In support of this, Remely et al. Their results disclosed the influence of different composition of gut microbiota in obesity and type 2 diabetes on the epigenetic regulation of genes. On the other hand, some reports have demonstrated that fecal micro-RNAs miRNAs can shape the composition of the gut microbiome Liu et al.
Together, these insights are pivotal pieces of information revealing the associations between gut microbiota composition and epigenetic status contributing to host metabolism regulation. It is well-established that the bacterial-derived metabolite Trimethylamine N-oxide TMAO is strongly associated with cardiovascular risks and host inflammation Zhu et al.
Choline and L-carnitine are the major precursors of TMAO, and they are highly abundant in the Western diet Koeth et al. Western diets consumption result in the production of TMA in gut microbiota, which is metabolized to trimethylamine-N-oxide TMAO by host hepatic enzyme flavin-containing monooxygenase 3 FMO3 Koeth et al.
Of note, TMAO is also upregulated in type 2 diabetes, and is associated with obesity traits Gao et al. Schugar et al. Complimentary mouse and human studies indicate a negative regulatory role for FMO3 in the browning of white adipose tissue Ussar et al.
Since TMA results from nutrients commonly consumed in a high-fat diet and is exclusively generated by certain communities of the gut microbiome, dietary intervention or targeting the specific microbes that generate TMAO may have therapeutic implications.
In addition to SCFAs, bile acids appear to play an important role in mediating the interactions between microbiota and host tissues. Bile acids are synthesized from cholesterol by a process orchestrated by multiple liver enzymes Russell, Afterward, the gut microbiota promotes deconjugation, dehydrogenation, and dehydroxylation of primary bile acids in the distal small intestine and colon, thus forming secondary bile acids and affecting bacterial composition Ridlon et al.
For instance, conjugated bile acid has been shown to decrease bacterial overgrowth, bacterial translocation, and endotoxemia in rats Lorenzo-Zúñiga et al. Bacterial translocation was less in cirrhotic animals receiving conjugated bile acids, while endotoxemia was also reduced by conjugated bile acid feeding Lorenzo-Zúñiga et al.
A recent study by Ziȩtak et al. The elevated bile acid secretion changed the microbiota composition and increased adipocyte thermogenesis Ziȩtak et al. Confirming this, Worthmann et al. At the same time, cold results in accelerated fecal excretion of bile acids via increased CYP7B1.
This process is accompanied by alterations in gut microbiota and promotes thermogenesis Worthmann et al. In line with these results, it was proposed that the lean phenotype of the Klb-KO mice is due to increased production of the secondary bile acid deoxycholic acid, which is produced by microbiota from hepatic cholic acid Somm et al.
Notably, deoxycholic acid signals through a G-protein coupled bile acid receptor TGR5 in the BAT to enhance thermogenesis Somm et al. Furthermore, KO mice treated with vancomycin, which preferentially targets gram-positive bacteria, including the Clostridium species, which is classically described as being responsible for the conversion of primary bile acids into secondary bile acids, reversed the metabolic phenotypes in the KO mice Somm et al.
This result supports the role of bile acids as mediators of microbiota-mediated thermogenesis. Miyamoto et al. Supplementing the diet with hydroxy-cisoctadecenoic acid HYA , a dietary linoleic acid—derived gut-microbial metabolite whose level is significantly reduced by HFD feeding, attenuates various aspects of HFD-induced obesity in mice, including their appetite, body weight, WAT adipocyte size, and blood glucose and insulin level, by promoting GLP-1 secretion via GPCRs.
Furthermore, HYA treatment does not elicit adipose inflammation, as opposed to regular linoleic acid supplementation, which is known to be involved in mediating inflammatory responses via the arachidonic acid cascade. Moreover, Lactobacillus-colonized mice show similar effects with elevated HYA levels.
The findings illustrate the interplay between gut microbiota and host energy metabolism via the metabolites of dietary omegaFAs, thereby shedding light on the prevention and treatment of metabolic disorders by targeting gut microbial metabolites.
Another metabolite of linoleic acid that is involved in the regulation of host energy metabolism is oxo Z -octadecenoic acid Goto et al. It is produced by lactic acid bacteria in the intestine.
This production has been linked to activation of adipogenesis Goto et al. Another nutrient whose gut microbial metabolites have been associated with adipocyte function is resveratrol.
Resveratrol is a non-flavonoid polyphenol compound that is naturally found in a wide variety of plants, such as grapes and peanut skin Liao et al. Besides its multiple benefits to host health, such as antioxidation and anti-inflammation, resveratrol potentially alters the composition of gut microbiota to ameliorate adiposity and improve glucose homeostasis in HFD-fed mice Qiao et al.
Wang et al. They found that the recipient mice showed decreased body weight and improved insulin resistance. In addition, resveratrol-microbiota could modulate lipid metabolism and induce WAT browning in the high-fat diet-fed recipient. Similarly, Liao et al. This observation is also consistent with previous findings of the promoting effect of resveratrol on brown and beige adipocyte development Wang et al.
Furthermore, similar effects have also been reported for other polyphenol-rich dietary compounds, like cranberry extract Anhê et al. Likewise, gut-produced vanillic acid, the metabolite of another antioxidant, anthocyanins, has also been shown to activate thermogenesis and promote browning in HFD-fed mice Han et al.
The evidence strongly suggests that alterations in gut microbiota diversity and composition contribute to the pathogenesis of obesity and obesity-related metabolic disorders.
However, the limitations and pitfalls of the studies should be noted. Many of these studies are observational. In addition, differences in the lab environment could contribute to differences in the microbiome and its effects. Also, many studies used mice, which have gut anatomies that are different from humans.
Future studies are needed to gain a more accurate understanding of host-microbiome communications. Based on the existing preliminary data identifying epigenetic mechanisms as a regulator of gut microbiota composition, a dietary approach targeted to favor a more beneficial bacterial population and thus epigenetic changes might be effective in the prevention of obesity.
For this, the accurate analysis of microbiome diversity and composition will be key. Large-scale sequencing studies have already identified organisms and their relative abundance in purified DNA by sequencing specific regions of the 16S or 18S ribosomal genes Qin et al.
In parallel, comprehensive biochemical and metabolomic profiling approaches will be needed to understand the mechanistic basis of host-microbiome interactions.
Accumulating studies have started to examine the effect of gut microbiota transplantation on insulin sensitivity and microbiota composition in humans.
Vrieze et al. Also, case reports have suggested that intestinal bacteria upon fecal microbiotiota transplantation might affect bodyweight and insulin sensitivity of the recipient Kootte et al.
The duration of thereapeutic effect of FMT is not clear yet; Till then, dietary intake is probably the easiest way to influence intestinal microbiome composition and may even restore pathological disturbances. Considering the causal role of the gut microbiome in human obesity and its manageability through dietary or biological approaches, targeting the microbiome may present a new avenue for therapeutic interventions for preventing and treating obesity and its related metabolic disorders.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Anhê, F. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp.
population in the gut microbiota of mice. Gut 64, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Backhed, F. The gut microbiota as an environmental factor that regulates fat storage. Bäckhed, F. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice.
Bird, J. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. An Int. Bo, T. ISME J. Boffa, I. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. PubMed Abstract Google Scholar. Bouter, K. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects article.
Brinkworth, G. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations.
Brown, A. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. Caesar, R. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling.
Cell Metab. Candido, E. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 4, — CrossRef Full Text Google Scholar.
Cani, P. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, — Next-generation beneficial microbes: the case of Akkermansia muciniphila.
Chen, K. Gut microbiota-dependent metabolite Trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice.
Chevalier, C. Gut microbiota orchestrates energy homeostasis during cold. Cell , — Chong, C. Factors affecting gastrointestinal microbiome development in neonates. Nutrients E Cotillard, A.
Dietary intervention impact on gut microbial gene richness. Nature , — Cypess, A. Identification and importance of brown adipose tissue in adult humans.
Dao, M. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, — Dashti, H. Long-term effects of a ketogenic diet in obese patients.
David, L. Diet rapidly and reproducibly alters the human gut microbiome. De Groot, P. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time.
CrossRef Full Text PubMed Abstract Google Scholar. den Besten, G. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism.
Lipid Res. Depommier, C. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Di Marzo, V. Endocannabinoid control of food intake and energy balance. Dimova, L. High-cholesterol diet does not alter gut microbiota composition in mice.
Dugas, L. The obese gut microbiome across the epidemiologic transition. Themes Epidemiol. Duncan, S. Human colonic microbiota associated with diet, obesity and weight loss. Everard, A. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity.
Fang, W. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. Frost, G. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism.
Ganeshan, K. Metabolic regulation of immune responses. Gao, X. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. Gao, Z. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, — Geurts, L. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota.
Goto, T. Han, X. Vanillic acid activates thermogenesis in brown and white adipose tissue. Food Funct. As a result, any issues with the gut or gut bacteria will stress the brain health, resulting in anxiety or depression. The gut microbiome and skin health are also linked.
Acne and dandruff are two examples of skin diseases caused by poor gut health. In addition, excess sugar, red meat, alcohol, unhealthy fat-containing foods, and other poor dietary habits can lead to dermatitis, psoriasis, gluten sensitivity, and other health problems.
Microbes in the gut can affect the fertility function of an individual in both males and females. A study has shown that poor gut health will lead to an imbalance in estrogen levels.
As a result, it causes infertility issues—for example, endometriosis, polycystic ovary syndrome, and testicular dysfunction. Scientific evidence has shown that leaky gut conditions affect the retina and increase the chance of developing various eye diseases, including dry eye, glaucoma, uveitis, and more.
Because the gut microbiome is very dynamic, adopting healthy eating habits will help to shape it. However, to improve your health in the long run, you must adhere to the habits. On the other hand, unhealthy eating habits will result in gut dysbiosis.
Gut dysbiosis indicates the imbalance between healthy and harmful bacteria count in the gut, resulting in weight gain and irritable bowel syndrome.
Individuals affected by IBS experience symptoms like bloating, abdominal pain, and cramps. Diet is a crucial modifiable factor influencing gut microbiota composition. It means therapeutic dietary strategies to manipulate microbial diversity, composition, and stability are possible. In addition, long-term alterations in nutritional patterns affect the gut microbiota profile of each individual.
Probiotics are live, healthy bacteria. Probiotics have potential health benefits. It aids in the treatment of diabetes, IBD, and other diseases. Prebiotics, which include fruits, vegetables, and legumes high in dietary fibre, aid in the growth of beneficial bacteria.
Certain beneficial bacteria in the gut microbiome help improve gut health. For instance, Bifidobacterium and lactobacilli present in the probiotics and yoghurt aid in sealing spaces between intestinal cells, preventing leaky gut syndrome. Furthermore, by preventing harmful bacteria from attaching to the intestinal walls, these bacteria aid in treating the symptoms of intestinal bowel syndromes.
In addition, probiotics help to treat cardiovascular diseases. These are coronary heart failure, atherosclerosis, heart attack, and stroke by lowering hypercholesterolemia and hypertension. Taking care of the gut microbiome by maintaining the proper balance of these microbes is critical for physical, mental, metabolic, and immunity health.
Research shows that fibre, a plant-based nutrient lowers the risk of developing metabolic diseases by inducing the growth of good diversified bacteria in the gut. Therefore make sure to include sweet potatoes, spinach, beetroots, carrots, fennel seeds, yoghurt, fruits, vegetables, and whole grains in your diet.
In addition, health professionals suggest that consuming yoghurt, lactobacilli, and gut-boosting abilities will effectively treat gastrointestinal diseases such as diarrhoea, IBS, and constipation. Studies have revealed that regular exercise enhances the number of healthy bacteria in the gut.
Physical activity can benefit health in various ways while proactively impacting gut health. It can also alter the composition and functionality of the gut bacteria and, ultimately, the overall wellness. Research has shown a link between the gut and brain, indicating the gut microbiome and mental health are internally connected.
For example, anxiety and depression can affect the gut and vice versa. Hence, following yoga or meditation will help you manage your stress levels, eventually reducing gastrointestinal symptoms and increasing the quality of healthy gut microbiomes.
Study shows that proper hydration can improve the mucosal lining of the intestines. Furthermore, it indicates that staying hydrated will protect the gut from infectious pathogens and harmful proteins and enhance metabolic health. Eliminating highly processed foods from your diet is a simple yet effective way to improve your gut health.
Processed foods typically contain high levels of sugar, fat, and many preservative chemicals, all of which can contribute to gut diseases. Eating foods known to cause allergy or intolerance will aggravate the discomfort and health of the gut microbiome. As a result, if you experience symptoms such as bloating, nausea, diarrhoea, or abdominal cramps after eating certain foods, avoid them the next time.
Keeping a food journal will assist you in determining which foods are causing these symptoms. Drinking too much alcohol can cause negative consequences on the gut microbiome.
A study shows that excessive consumption of alcohol induces the development of gastritis, choric discomfort, heartburn, ulcers, and bacterial infections. These inflamed intestinal conditions are signs of an unhealthy gut.
In addition, an unhealthy gut will further affect metabolic health, which will cause metabolic syndrome. A clear relationship gets established between the gut microbiome and metabolic health. A healthy gut means a more robust immune system and better metabolism. As a result, improving your gut health translates to better overall health.
A healthy gut indicates a stronger immune system, improved metabolism, and a healthy brain and heart. As a result, improving your gut health means improving your overall health.
Obesity is a complex disease attributable to many Feeding young athletes: tips for parents including gur and environmental influences. Metabolisj evidence suggests that gut microbiota is Energj major contributing factor to the pathogenesis of obesity and other metabolic disorders. This article Surgical weight loss the current Feeding young athletes: tips for parents of the Potassium deficiency symptoms of gut microbiota in the amd of metabolis balance and the development of obesity, and how the microbiota communicates with host tissues, in particular adipose tissue. We discuss several external factors that interfere with the interplay between gut microbiota and host tissue metabolism, including cold exposure, diet regimens, and genetic manipulations. We also review the role of diet-derived metabolites that regulate thermogenesis and thus energy homeostasis. Among the gut microbial metabolites, we emphasize short-chain fatty acids, which could be utilized by the host as a direct energy source while regulating the appetite of the host through the gut-brain axis. Obesity is a worldwide epidemic, with sedentary lifestyles and increased food intake likely the main causes Jebb and Moore, ; Hu,
Energy metabolism and gut health -
The bile acids secreted by the liver play a key role in the digestion and absorption of fatty acids in the small intestine. Primary bile acids are produced by hepatocytes from cholesterol and excreted in the bile that is then released in the intestine.
In the intestinal lumen, the gut microbiota then converts primary bile acids into secondary bile acids. These secondary bile acids are reabsorbed by our body in the ileum lower portion of the small intestine and recirculated to the liver via the portal vein.
As a result, modification of the intestinal microbial composition is likely to influence the pool of bile acids. Swann and colleagues have confirmed this idea by showing that mice with a distinct gut microbiota had a specific bile acid profile and thus a different energy metabolism.
Indeed, transferring gut microbiota from mice made obese by a high-fat diet HFD to germ-free recipient mice caused them to develop NAFLD with hepatic lipid levels similar to those in obese mice. Obesity is a global health problem that is continuing to increase rapidly. It is considered a multifactorial disease and, among other mechanisms, the gut microbiota has recently emerged as an important factor in the pathophysiology of obesity.
Indeed, it influences obesity by acting on various mechanisms that are essential to energy homeostasis, including LPS-stimulated inflammation, bile acid metabolism, SCFA metabolism, and fat deposition.
However, to date, it is not known which bacterial community contributes most to the pathophysiology of obesity.
In the future, gut microbiota modulation could be a therapeutic means to help treat obesity. Additional clinical and mechanistic studies are needed to further support the clinical utility and safety of gut microbiota modulation in obesity through probiotics, prebiotics, or fecal microbiota transplantation.
Hit enter to search or ESC to close. Close Search. Scientific watch The gut microbiota, energy metabolism, and obesity — Part 2 By Microbiome foundation 10 November No Comments. Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature , — Jumpertz, R. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans.
Delzenne, N. Gut microbiota and the pathogenesis of insulin resistance. Murphy, E. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59 , — Al-Lahham, S. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms.
Acta , — Qin, J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature , 55—60 Vrieze, A. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia 53 , — Frost, G. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism.
Queipo-Ortuño, M. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PloS One 8 , e Zhang, H.
Human gut microbiota in obesity and after gastric bypass. Emerging Risk Factors Collaboration et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. Spranger, J. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition EPIC -Potsdam Study.
Diabetes 52 , — Cani, P. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56 , — R esearch finds that obesity and insulin resistance get associated with chronic inflammatory conditions.
Factors such as diet can alter the gut microbiome. It results in dysregulation and secretory changes in microbiota metabolites. As a result, it will stimulate the primary metabolic pathways, leading to the rise of insulin resistance and diabetes. Inflammation caused by immune system action can protect the body from infection.
However, the excessive inflammatory reach will cause additional tissue and organ damage. As a result, maintaining immune homeostasis is critical. The sedentary lifestyle leads to inflammation, inducing the onset of various chronic conditions.
Since the gut is the powerhouse of immune cells, it will stimulate the metabolism to produce immune cells that fight infectious microbes. It also increases the production of regulatory T-cells to suppress the inflammatory reaction and prevent self-attacking. Several studies have found that consuming an excessive amount of certain foods, such as red meat, negatively impacts the gut microbiome.
It, in turn, converts choline, an essential nutrient, into the harmful compound trimethylamine N-oxide. Chronic musculoskeletal conditions get often caused due to the abnormal level of microbes present in the gut.
It will increase an inflammatory state, making the person more susceptible to developing rheumatoid arthritis and osteoporosis. However, probiotic supplements will aid in better calcium absorption and increase bone density and bone cell formation. The gut and the brain health gets inextricably linked with each other.
For example, a study has revealed that gut health significantly impacts chronic pain, mood, and behaviour. As a result, any issues with the gut or gut bacteria will stress the brain health, resulting in anxiety or depression.
The gut microbiome and skin health are also linked. Acne and dandruff are two examples of skin diseases caused by poor gut health. In addition, excess sugar, red meat, alcohol, unhealthy fat-containing foods, and other poor dietary habits can lead to dermatitis, psoriasis, gluten sensitivity, and other health problems.
Microbes in the gut can affect the fertility function of an individual in both males and females. A study has shown that poor gut health will lead to an imbalance in estrogen levels. As a result, it causes infertility issues—for example, endometriosis, polycystic ovary syndrome, and testicular dysfunction.
Scientific evidence has shown that leaky gut conditions affect the retina and increase the chance of developing various eye diseases, including dry eye, glaucoma, uveitis, and more. Because the gut microbiome is very dynamic, adopting healthy eating habits will help to shape it.
However, to improve your health in the long run, you must adhere to the habits. On the other hand, unhealthy eating habits will result in gut dysbiosis. Gut dysbiosis indicates the imbalance between healthy and harmful bacteria count in the gut, resulting in weight gain and irritable bowel syndrome.
Individuals affected by IBS experience symptoms like bloating, abdominal pain, and cramps. Diet is a crucial modifiable factor influencing gut microbiota composition. It means therapeutic dietary strategies to manipulate microbial diversity, composition, and stability are possible.
In addition, long-term alterations in nutritional patterns affect the gut microbiota profile of each individual. Probiotics are live, healthy bacteria. Probiotics have potential health benefits.
It aids in the treatment of diabetes, IBD, and other diseases. Prebiotics, which include fruits, vegetables, and legumes high in dietary fibre, aid in the growth of beneficial bacteria.
Certain beneficial bacteria in the gut microbiome help improve gut health. For instance, Bifidobacterium and lactobacilli present in the probiotics and yoghurt aid in sealing spaces between intestinal cells, preventing leaky gut syndrome. Furthermore, by preventing harmful bacteria from attaching to the intestinal walls, these bacteria aid in treating the symptoms of intestinal bowel syndromes.
In addition, probiotics help to treat cardiovascular diseases. These are coronary heart failure, atherosclerosis, heart attack, and stroke by lowering hypercholesterolemia and hypertension. Taking care of the gut microbiome by maintaining the proper balance of these microbes is critical for physical, mental, metabolic, and immunity health.
Research shows that fibre, a plant-based nutrient lowers the risk of developing metabolic diseases by inducing the growth of good diversified bacteria in the gut. Therefore make sure to include sweet potatoes, spinach, beetroots, carrots, fennel seeds, yoghurt, fruits, vegetables, and whole grains in your diet.
In addition, health professionals suggest that consuming yoghurt, lactobacilli, and gut-boosting abilities will effectively treat gastrointestinal diseases such as diarrhoea, IBS, and constipation.
Studies have revealed that regular exercise enhances the number of healthy bacteria in the gut. Physical activity can benefit health in various ways while proactively impacting gut health.
It can also alter the composition and functionality of the gut bacteria and, ultimately, the overall wellness. Research has shown a link between the gut and brain, indicating the gut microbiome and mental health are internally connected.
For example, anxiety and depression can affect the gut and vice versa.
The Energy metabolism and gut health microbiome consists anx various bacteria, fungi, gtu other microorganisms that reside in the digestive Ehergy, with yealth composition varying among individuals. These Feeding young athletes: tips for parents microbes influence energy metabolism by regulating glucose metabolism, appetite, and fat metwbolism. Consistent with the role of gut microbiota in metaoblism metabolism, Benefits of low sodium diet Energy metabolism and gut health human studies have shown that changes in the composition and function of gut microorganisms are associated with obesity and diabetes. The researchers identified the genes that were most abundant in the gut microbiota of individuals before they participated in a weight loss program. Based on the biological functions that these genes perform, the researchers were able to infer the functional profile of the entire gut microbiome. Notably, between the individuals who lost weight and those resistant to weight loss, there was a difference in the abundance of microbiome genes that scientists know to influence human metabolism.
0 thoughts on “Energy metabolism and gut health”