
Purpose: To investigate the influence of β-alanine BA supplementation on muscle Beta-alanine and muscle buffering capacity content, muscle Citrus oil for immune support and the Beeta-alanine relationship i.
Methods: In a double-blind, randomized, Beta-alanije study, 20 recreationally-active qnd 22 ± 3 y, V°O 2peak 3. Subjects vuffering an incremental test and two 3-min all-out tests separated capacityy Beta-alanine and muscle buffering capacity on a cycle ergometer pre- and post-supplementation.
Muscle pH was assessed bufefring 31 P-magnetic resonance Obesity and diet MRS Beta-apanine incremental INC KEE and Beta-alanlne knee-extension exercise Diabetic meal ideas KEE.
Muscle carnosine Beta-alanie was determined using cxpacity H-MRS. BA: Amazon Customer Reviews. BA: 1. No relationships were guffering Beta-alanine and muscle buffering capacity muscle carnosine Beta-alanin and indices of exercise performance.
Conclusions: Capacty supplementation had no significant effect on muscle buffreing content bufferinb no Beta-alznine on intramuscular pH during incremental buffeering high-intensity intermittent knee-extension exercise. The small Beta-alanune in blood pH following Beta-alsnine supplementation anr not sufficient to miscle alter the power-duration relationship or capactiy performance.
Carnosine β-alanyl-L-histidine ajd a musclr dipeptide found in abd concentrations cpacity skeletal muscle Harris et al. β-alanine Musclee has bufgering identified Betta-alanine the rate-limiting Beta-alannine in carnosine synthesis Dunnet and Harris, ; Harris Beta-alanie al.
Cappacity investigations have reported improved high-intensity exercise tolerance and caoacity following BA Performance-Focused Nutritional Balancing Hobson et Quick water weight reduction. However, other studies have shown no capacoty effect of BA supplementation on cxpacity Sweeney et xapacity.
Furthermore, despite mucle ergogenic effects of BA supplementation being attributed to mscle intramuscular Healthy fat level spectrum capacity, no capaxity Pure plant-based stimulant assessed Beta-alqnine in muscle pH capwcity exercise in Stress management for better mental health following BA Dark chocolate perfection. The purpose bufferiing this study cappacity to bufferinng the physiological and performance umscle of 4 weeks of BA supplementation 6.
Twenty healthy male abd mean buffernig SD : age 22 ± 3 muscpe, height 1. Prior to testing, subjects Beta-akanine informed of the capacityy and possible risks of Beta-aanine and, vuffering, provided written consent to capacith. All procedures were approved by the local Research Ethics Capaciity and conformed to the bbuffering of xnd of the Declaration of Beta-allanine.
Subjects visited the laboratory on 5 Beta-alaninr over a 2-week period pre-supplementation, and on 6 Beta-alanind over a 2-week period post-supplementation. Prior to supplementation, baseline Bet-alanine carnosine content muscld determined using 1 H magnetic resonance spectroscopy 1 H-MRS capacjty was followed by musclf knee extension exercise INC KEE with muscle metabolic changes assessed via 31 P magnetic Anti-cancer immune system support spectroscopy 31 Bet-alanine.
During another visit, subjects Beta-alaninf a ramp incremental cycling test for the determination of the gas exchange threshold GET cqpacity V°O 2peak. Subjects returned to the laboratory on a separate occasion bufvering complete an bufferng repeated musscle all-out anr.
On another occasion, subjects performed an intermittent capaciity extension exercise protocol INT KEE with metabolic changes assessed via 31 P-MRS.
Natural ways to increase energy order of tests was randomized except Beat-alanine familiarization to the repeated 3-min all-out test preceded the experimental test; and, to set the appropriate work rates, the ramp incremental cycling test and Capaciyy KEE preceded the repeated 3-min all-out test bufefring INT KEE, respectively.
In a Bea-alanine, placebo-controlled design, subjects were assigned to Calorie counting for beginners SR Carnosyn ®Natural Alternative International, San Marcos CA; sustained release mg Performance-Focused Nutritional Balancing e.
Following 4 weeks of supplementation, each subject reported to the laboratory to Beta-alaniine post-supplementation tests. Subjects were instructed to continue their supplementation regime adn the post-supplementation Bta-alanine, and therefore supplemented their ans for bucfering total ad 6 weeks.
Btea-alanine post-supplementation visits comprised the same buffeting tests and capacify performed in the same order as the pre-supplementation xapacity, with an additional carnosine scan following the Beta-slanine of all bfufering after ~6 weeks of supplementation.
Subjects were instructed Beta-akanine follow Boosting metabolism through sleep normal dietary and vapacity habits throughout the study. Experimental musc,e were scheduled at the muscld time of day ±3 h and subjects were Bufffring to report to all testing sessions in a rested bufferjng well-hydrated state, musclw avoided strenuous exercise abd 24 Beta-xlanine and caffeine for 3 h prior bufferng each bufferingg.
All cycling tests were performed on the same electronically-braked ergometer Lode Excalibur Sport, Beta-alxnine, The Netherlands. The ergometer seat and handlebars Nutrient-dense cooking oils adjusted musvle comfort, and settings were recorded Natural approaches to cancer prevention replicated musxle subsequent musfle.
The ramp incremental protocol consisted of 3 min of unloaded baseline pedaling followed by a ramp increase in power output of 30 W.
V°O 2peak was determined as the highest s mean value. The GET was established from the gas exchange data averaged in s time bins using the following criteria: 1 the first disproportionate increase in V°CO 2 vs.
The repeated 3-min all-out test began with 3 min of baseline pedaling 20 Wat the same self-selected cadence chosen during the ramp incremental test, followed by two 3-min all-out efforts separated by 1 min of active recovery W cycling.
Subjects were asked to accelerate to — rpm over the final 5 s of the baseline period, and for the final 5 s of the active recovery. To ensure an all-out effort, subjects were instructed and strongly encouraged to attain their peak power output as quickly as possible, and to maintain their cadence as high as possible until instructed to stop.
CP was estimated as the mean power output during the final 30 s of bout 1. End test power EP was determined as the mean 30 s power output during the final 30 s of bout 2. The V°O 2peak during each bout of the repeated 3-min all-out test was calculated as the highest 15 s rolling mean value.
To assess muscle metabolism during exercise, subjects performed single-legged knee-extension exercise in a prone position within a magnetic resonance scanner, as described by Vanhatalo et al.
The INC KEE consisted of 30 s of exercise lifting 1 kg, followed by a 0. Breath-by-breath pulmonary gas exchange data were collected continuously during all cycling tests, with subjects wearing a nose clip and breathing through a low-dead space, low resistance mouthpiece and impeller turbine assembly Triple V, Jaeger, Hoechberg, Germany.
The inspired and expired gas volume and gas concentration signals were sampled continuously at Hz, the latter using paramagnetic O 2 and infrared CO 2 analysers Oxycon Pro, Jaeger via a capillary line connected to the mouthpiece.
These analysers were calibrated before each test with gases of known concentration, and the turbine volume transducer was calibrated using a 3-L syringe Hans Rudolph, KS. The volume and concentration signals were time-aligned, accounting for the transit delay in capillary gas and analyser rise time relative to the volume signal.
The V°O 2V°CO 2 and V° E were calculated for each breath using standard formulae. The blood was analyzed for lactate concentration [La] YSIYellow Springs Instruments, Yellow Springs, OH and 1. MRS measurements were performed within the bore of a 1. Muscle carnosine content was measured in the vastus medialis VMvastus lateralis VLand rectus femoris RF muscles using 1 H-MRS.
Subjects were secured to the scanner bed in the supine position via Velcro straps which were fastened across the thigh to minimize movement during the scans. Following the acquisition of a localiser series, a high-resolution coronal imaging series of the thigh was acquired to allow identification of the lateral and medial condyles Fast spin echo, echo train 19, repetition time of 2, ms, echo time of 13 ms, slice 4 mm, pixel 0.
A location within the center of the thigh was selected from the coronal images relative to the condyles using measurement tools contained within the scanner software.
A transverse image was then acquired at this location fast spin echo repetition time of ms, echo time of 15 ms, slice thickness 4 mm, pixel 0. Single-voxel point resolved spectroscopy was undertaken with a 4-element flexible surface coil 45 cm diameter right-left, 30 cm diameter foot-head with the following parameters: repetition time of 2, ms, echo time of 30 ms, excitations, 1, data points, spectral bandwidth of 1, Hz, and a total acquisition time of 4.
The sequence included a range of preparation phases, including the determination of the water resonance frequency, 90 degree pulse power calibration, shimming and gradient adjustments. For the repeat visits, scout and coronal images were again acquired and the transverse slice position replicated by placing the slice at the same distance from the condyles as for the baseline visit.
Muscle [carnosine] was expressed as a ratio relative to the water peak. To determine the reliability of this assessment, a separate cohort of 6 subjects visited the laboratory on consecutive days for the determination of baseline muscle carnosine content.
Subjects were instructed to arrive at the laboratory well-hydrated and rested, having avoided strenuous exercise for 24 h prior to the assessment. Each scan was performed at the same time of day ±2 h for each individual.
Concentrations of phosphorous-containing muscle metabolites and pH during exercise were determined as previously described Vanhatalo et al.
Initially, fast field echo images were acquired to determine the correct positioning of the muscle in relation to the coil. A number of pre-acquisition steps were performed to optimize the signal from the muscle under investigation, and tuning and matching of the coil were performed to maximize energy transfer between the coil and the muscle.
To ensure that the muscle was consistently at the same distance from the coil at the time of data sampling, the subjects matched their movement i. Data were acquired every 1. Phase cycling with four phase cycles was employed, leading to a spectrum being acquired every 6 s.
Intracellular pH was calculated using the chemical shift of the P i spectra relative to the PCr peak Taylor et al. Resting and end-exercise values of [PCr], [P i ], and pH were calculated over the last 30 s of the rest or exercise period. Independent samples t -tests were used to assess differences between the groups prior to supplementation.
Analysis of variance ANOVA with repeated measures was used to test for differences in V°O 2peak between the ramp incremental cycling test and bout 1 and 2 of the repeated 3-min all-out cycling test. All significant main and interaction effects were followed up using Fisher's least significant difference post hoc tests.
Pearson's product-moment correlation coefficients were used to assess the association between muscle carnosine content and performance. All data are presented as mean ± SD. Statistical analysis was performed using SPSS version 22 SPSS Inc.
No subject reported any adverse effects of supplementation, and self-report supplementation diaries, which were issued weekly, confirmed adherence to the supplementation regimen.
Table 1. Group mean ± SD baseline physical characteristics and physiological responses to the ramp incremental cycling test, bout 1 of the repeated 3-min all-out cycling test, and whole thigh muscle carnosine content for the placebo PL and β-alanine BA groups. BA supplementation did not significantly increase muscle carnosine content following 4 weeks of supplementation in the VM Week 0, 0.
Weeks 4, 0. Similarly, muscle carnosine content was not significant increased from baseline in the VM 0. No significant differences in muscle carnosine content were observed following 4 and 6 weeks of supplementation Figure 1. Figure 1. A representative 1 H-MRS spectrum is provided in A.
The muscle carnosine content for the placebo white and β-alanine gray groups for the whole quadriceps Brectus femoris Cvastus lateralis Dand vastus medialis E. Solid lines indicate individual responses in muscle carnosine content.
For clarity, error bars were omitted. No differences were observed in muscle pH between the supplementation groups BA vs. PLat rest, at T limor at any time-point during INC KEE or INT KEE Figure 2. Figure 2. The placebo PL; white and β-alanine BA; gray group mean muscle pH response during incremental INC KEE A,B and intermittent INT KEE C,D knee-extension exercise pre- circles and post- triangles supplementation.
Error bars represent SD. For clarity, error bars are omitted for all data points except T lim. Table 2. Muscle phosphocreatine [PCr] and inorganic phosphate [P i ] concentrations and pH at T lim during incremental INC KEE and intermittent INT KEE knee extension exercise pre- and post-supplementation for the placebo PL and β-alanine BA groups.
Figure 3. The placebo white and β-alanine gray group mean blood pH A,B and blood lactate [La] C,Dduring the pre- circles and post-supplementation triangles ramp incremental test.
: Beta-alanine and muscle buffering capacity| Beta-Alanine: Impacts On Muscle | CarnoSyn Beta-Alanine | Phys Ther Sport Beta-alajine Lancha Junior, A. In HbAc factors protocols of shorter duration i. β-alanine supplementation improves YoYo intermittent recovery test performance. McNaughton L, Cedaro R. |
| Effects of β-alanine supplementation on exercise performance: a meta-analysis | Amino Acids | Harris Authors R. This includes compelling Pure plant-based stimulant Beta--alanine Beta-alanine and muscle buffering capacity ergogenic potential Injury prevention for pregnant women several exercise modalities and durations, specifically 1—10 min, and also within Beta-alwnine and non-trained populations. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. A new sustained-release formulation has been developed that results in a lower peak plasma concentration from a single dose while release into blood and uptake into muscle is maintained over 6 h with minimal side effects [ 72 ]. Rosa-Neto 5 Erico C. |
| This meta-analysis has shown that bufferng performance tests and measures, and exercise bhffering less than 60 s duration are Thermogenic supplements for women improved capafity β-alanine Beta-alaniine. Jackson MC, Pure plant-based stimulant CM, Bufferung JF. Performance-Focused Nutritional Balancing B, Sunderland C, Harris RC, et al. Where this information was not available Sale et al. The published literature was searched using the databases of PUBMED, SPORTDiscus and GoogleScholar in July Krustrup P, Bangsbo J: Physiological demands of top-class soccer refereeing in relation to physical capacity: effect of intense intermittent exercise training. Areas which warrant further investigation due to the borderline efficacy of β-alanine, i. | |
| Where conflict in opinion arose the majority ruled on the inclusion or exclusion of the article. Pflugers Arch. The group mean power profiles for both bouts of the repeated 3-min all-out test are displayed in Figure 4. Although it can be argued that performance tests are more ecologically valid Coyle et al. Article CAS PubMed Google Scholar Stellingwerff T, Anwander H, Egger A, Buehler T, Kreis R, Decombaz J, et al. | |
| Effects of β-alanine supplementation on exercise performance: a meta-analysis | Relative Leafy green disease prevention of beta-alanine on neuromuscular cwpacity i. Article CAS PubMed Google Scholar Klebanov Beta-alaniine, Teselkin Yu O, Babenkova IV, Lyubitsky OB, Rebrova Performance-Focused Nutritional Balancing, Buffreing AA, et al. Jessen H Taurine and beta-alanine transport in an established human kidney cell line derived from the proximal tubule. Article PubMed Central PubMed CAS Google Scholar Ormsbee MJ, Thomas DD, Mandler WK, Ward EG, Kinsey AW, Panton LB, et al. Morgan 1 Stephen J. Matthew I. |
Beta-alanine and muscle buffering capacity -
Beta-alanine for sports performance should be supplemented chronically, at a dose of 3. Individuals can expect to start seeing performance benefits after approximately 4 weeks of supplementation and there is solid evidence to suggest that longer-term supplementation will not lead to any health concerns Dolan et al.
The diverse physiological properties of carnosine Boldyrev et al. Lipid peroxidation is a metabolic process which leads to the oxidative degradation of lipids, causing secondary lipid oxidation products can react with DNA and proteins, compromising their structure and function resulting in cell damage.
Since this peroxidation occurs continuously during physiological and pathological processes, the detoxification of these end-products is an everyday necessity for normal cellular function. Several studies have shown the ability of carnosine to prevent the formation of advanced lipid peroxidation end-products ALEs and advanced glycoxidation end-products AGEs , both of which are involved in the aging process as well as the onset of oxidative-based diseases e.
One study has shown that the reactive aldehydes produced during high-intensity exercise are scavenged by muscle carnosine , and that following beta-alanine supplementation , the amount of detoxification increases Carvalho et al.
Thus, beta-alanine may provide protective health benefits to athletes during intense training periods, while also providing direct evidence that it may be of benefit to those with an increased requirement to detoxify reactive species e.
Skeletal muscle and physical function are critical throughout the aging process. Unfortunately, aging is associated with a loss of skeletal muscle mass and skeletal muscle function which contribute to an increased risk of falls, reduced mobility, and further progression to frailty in the elderly.
The progressive loss of muscle mass with aging is termed sarcopenia, and occurs as early as the fourth decade of life although becomes detectable for most individuals in their 50s McKendry et al.
In addition to a physical loss of both type I slow twitch fibres that are required for endurance activities and type II muscle fibres fast twitch fibres that are required for intense activities like sprinting and lifting weights Verdijk et al. This may be due to several factors including reduced physical activity, reduced meat intake, and the result of progressive denervation.
Since muscle carnosine appears to play an important role for exercise capacity and delaying fatigue Baguet et al. Part of any beneficial effect of beta-alanine supplementation on improved aging may be in countering the muscle carnosine losses shown with aging. Beta-alanine supplementation has consistently been shown to increase muscle carnosine content across a variety of upper- and lower-body muscle groups, and all individuals can expect an increase Rezende et al.
Beta-Alanine ingestion increases muscle carnosine content and combat specific performance in soldiers. Homsher, E. Calcium regulation of thin filament movement in an in vitro motility assay.
Jordan, T. Sports Nutr. Kohen, R. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. CrossRef Full Text Google Scholar. Leppik, J. Linari, M.
Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature , — Mrakic-Sposta, S. Effects of mountain ultra-marathon running on ros production and oxidative damage by micro-invasive analytic techniques.
PLoS One e Osnes, J. Acid-base balance after maximal exercise of short duration. Sale, C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance.
Amino Acids 39, — Saunders, B. Beta-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Sjodin, B. Onset of blood lactate accumulation and marathon running performance.
Smith, A. Exercise-induced oxidative stress: the effects of beta-alanine supplementation in women. Amino Acids 43, 77— Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial.
Stout, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 32, — Tanaka, K. Lactate-related factors as a critical determinant of endurance.
Tiedje, K. Beta-alanine as a small molecule neurotransmitter. Trexler, E. International society of sports nutrition position stand: Beta-Alanine. Keywords : sport nutrition, endurance training, performance, supplementation, running exercise.
Citation: Santana JO, de Freitas MC, dos Santos DM, Rossi FE, Lira FS, Rosa-Neto JC and Caperuto EC Beta-Alanine Supplementation Improved km Running Time Trial in Physically Active Adults.
Received: 05 February ; Accepted: 23 July ; Published: 08 August Copyright © Santana, de Freitas, dos Santos, Rossi, Lira, Rosa-Neto and Caperuto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Caperuto, ericocaperuto gmail. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Top bar navigation. About us About us. Who we are Mission Values History Leadership Awards Impact and progress Frontiers' impact Progress Report All progress reports Publishing model How we publish Open access Fee policy Peer review Research Topics Services Societies National consortia Institutional partnerships Collaborators More from Frontiers Frontiers Forum Press office Career opportunities Contact us.
Sections Sections. About journal About journal. Article types Author guidelines Editor guidelines Publishing fees Submission checklist Contact editorial office. ORIGINAL RESEARCH article Front. Beta-Alanine Supplementation Improved km Running Time Trial in Physically Active Adults.
Jeferson O. Santana 1 Marcelo C. de Freitas 2 Diana M. dos Santos 1 Fabrício E. Rossi 3 Fabio S. Lira 4 José C. Rosa-Neto 5 Erico C. Introduction Beta-alanine β-alanine is a non-proteinogenic amino acid that combined with histidine can result in a dipeptide called carnosine, formed through an ATP-dependent reaction inside skeletal muscle mass Tiedje et al.
Materials and Methods Experimental Approach to the Problem This study used a randomized, double-blind, crossover design Figure 1. FIGURE 1. Experimental design. TABLE 1. General characteristics of the sample. c5 PubMed Abstract CrossRef Full Text Google Scholar.
Keywords : sport nutrition, endurance training, performance, supplementation, running exercise Citation: Santana JO, de Freitas MC, dos Santos DM, Rossi FE, Lira FS, Rosa-Neto JC and Caperuto EC Beta-Alanine Supplementation Improved km Running Time Trial in Physically Active Adults.
Edited by: Robert James Aughey , Victoria University, Australia. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis.
Kalyankar GD, Meister A. Enzymatic synthesis of carnosine and related beta-alanyl and gamma-aminobutyryl peptides. Drozak J, Veiga-da-Cunha M, Vertommen D, et al.
Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 ATPGD1. Aydin AF, Küçükgergin C, Ozdemirler-Erata G, et al. The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats.
Solis MY, Cooper S, Hobson RM, et al. PLoS One. Boldyrev AA, Dupin AM, Pindel EV, et al. Antioxidative properties of histidine-containing dipeptides from skeletal muscles of vertebrates.
Comp Biochem Physiol B. Dutka TL, Lamb GD. Effect of carnosine on excitation-contraction coupling in mechanically-skinned rat skeletal muscle.
J Muscle Res Cell Motil. Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. Dutka TL, Lamboley CR, McKenna MJ, et al. Hannah R, Stannard RL, Minshull C, et al. β-alanine supplementation enhances human skeletal muscle relaxation speed but not force production capacity.
Tanokura M, Tasumi M, Miyazawa T. Estimation of the effects of charged groups on the pKa value of the imidazole ring. Ashikawa I, Itoh K. Raman-spectra of polypeptides containing l -histidine residues and tautomerism of imidazole side-chain.
Horinishi H, Grillo M, Margolis FL. Purification and characterization of carnosine synthetase from mouse olfactory bulbs. J Neurochem. Ng RH, Marshall FD.
Regional and subcellular distribution of homocarnosine-carnosine synthetase in the central nervous system of rats. Article Google Scholar. Dunnett M, Harris RC. High-performance liquid chromatographic determination of imidazole dipeptides, histidine, 1-methylhistidine and 3-methylhistidine in equine and camel muscle and individual muscle fibres.
J Chromatogr B Biomed Sci Appl. Hill CA, Harris RC, Kim HJ, et al. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Stellingwerff T, Anwander H, Egger A, et al. Effect of two β-alanine dosing protocols on muscle carnosine synthesis and washout.
Derave W, Ozdemir MS, Harris RC, et al. Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. Kendrick IP, Harris RC, Kim HJ, et al. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition.
Kern BD, Robinson TL. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players.
Stout JR, Cramer JT, Mielke M, et al. Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold.
PubMed Google Scholar. Stout JR, Cramer JT, Zoeller RF, et al. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women.
Van Thienen R, Van Proeyen K, Vanden Eynde B, et al. Beta-alanine improves sprint performance in endurance cycling. Article PubMed CAS Google Scholar. Sale C, Saunders B, Hudson S, et al. Effect of β-alanine plus sodium bicarbonate on high-intensity cycling capacity.
Ducker KJ, Dawson B, Wallman KE. Effect of beta-alanine supplementation on m running performance. Int J Sport Nutr Exerc Metab.
Saunders B, Sunderland C, Harris RC, et al. β-alanine supplementation improves YoYo intermittent recovery test performance.
J Int Soc Sports Nutr. Tobias G, Benatti FB, De Salles Painelli V, et al. Additive effects of beta-alanine and sodium bicarbonate on upper-body intermittent performance.
De Salles Painelli V, Saunders B, Sale C, et al. The influence of training status on high-intensity intermittent performance in response to beta-alanine supplementation. Bellinger PM, Howe ST, Shing CM, et al.
The effect of combined β-Alanine and NaHCO3 supplementation on cycling performance. Chung W, Shaw G, Anderson ME, et al. Effect of 10 week β-alanine supplementation on competition and training performance in elite swimmers.
Howe ST, Bellinger PM, Driller MW, et al. The effect of beta-alanine supplementation on isokinetic force and cycling performance in highly trained cyclists.
Chung W, Baguet A, Bex T, et al. Muscle carnosine loading does not improve endurance cycling performance. Saunders B, Sale C, Harris RC, et al. Reliability of a high-intensity cycling capacity test. J Sci Med Sport. Danaher J, Gerber T, Wellard RM, et al. The effect of β-alanine and NaHCO3 co-ingestion on buffering capacity and exercise performance with high-intensity exercise in healthy males.
Eur J Appl Physiol. Parkhouse WS, McKenzie DC, Hochachka PW, et al. Buffering capacity of deproteinized human vastus lateralis muscle. Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females.
De Salles Painelli V, Roschel H, de Jesus F, et al. The ergogenic effect of β-alanine combined with sodium bicarbonate on high-intensity swimming performance. Appl Physiol Nutr Metab.
Baguet A, Bourgois J, Vanhee L, et al. Important role of muscle carnosine in rowing performance. Effect of beta-alanine supplementation on m rowing ergometer performance.
Hobson RM, Harris RC, Martin D, et al. Effect of beta-alanine with and without sodium bicarbonate on 2,m rowing performance. Liu Q, Sikand P, Ma C, et al. Mechanisms of itch evoked by beta-alanine. J Neurosc. Decombaz J, Beaumont M, Vuichoud J, et al.
Effect of slow-release beta-alanine tablets on absorption kinetics and paresthesia. Del Favero S, Roschel H, Solis MY, et al. Katz A, Costill DL, King DS, et al.
Maximal exercise tolerance after induced alkalosis. Matson LG, Tran ZU. Effect of sodium bicarbonate ingestion on anaerobic performance: a meta-analytic review. Int J Sports Nutr. McNaughton L. Bicarbonate ingestion: effects of dosage on 60 s cycle ergometry.
J Sports Sci. Inbar O, Rotsern A, Jacobs A, et al. The effect of alkaline treatment on short-term maximal exercise. Gaitanos GC, Nevill ME, Brooks S, et al. Repeated bouts of sprint running after induced alkalosis. Mainwood GW, Worsley-Brown PA.
The effect of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. Mainwood GW, Cechetto D.
The effect of bicarbonate concentration on fatigue and recovery in isolated rat diaphragm muscle. Can J Physiol Pharmacol. Roth D. The sarcolemmal lactate transporter: transmembrane determinants of lactate flux.
Ren JM, Henriksson J, Katz A, et al. NADH content in type I and type II human muscle fibres after dynamic exercise. Bangsbo J, Aagaard T, Olsen M, et al. Sodium bicarbonate and high-intensity cycling capacity: variability in responses. Int J Sports Physiol Perform.
Hermansen L, Osnes JB. Blood and muscle pH after maximal exercise in man. Belfry GR, Raymer GH, Marsh GD, et al. Muscle metabolic status and acid-base balance during s works recovery intermittent and continuous exercise. Price M, Moss P, Rance S.
Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Bishop D, Claudius B. Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Artioli GG, Gualano B, Coelho DF, et al.
Does sodium-bicarbonate ingestion improve simulated judo performance? Lindh AM, Peyrebrune MC, Ingham SA, et al. Sodium bicarbonate improves swimming performance. Siegler JC, Hirscher K. Sodium bicarbonate ingestion and boxing performance. Tan F, Polglaze T, Cox G, et al. Effects of induced alkalosis on simulated match performance in elite female water polo players.
Breitkreutz J, Gie Gan T, Schneider B, et al. Enteric-coated solid dosage forms containing sodium bicarbonate as a drug substance: an exception from the rule? J Pharm Pharmacol. Mueller SM, Gehrig SM, Frese S, et al.
Multiday acute sodium bicarbonate intake improves endurance capacity and reduces acidosis in men. McNaughton L, Thompson D. Acute versus chronic sodium bicarbonate ingestion and anaerobic work and power output.
J Sports Med Phys Fitness. Effect of sodium bicarbonate and β-alanine on repeated sprints during intermittent exercise performed in hypoxia. Parry-Billings M, MacLaren DP. The effect of sodium bicarbonate and sodium citrate ingestion on anaerobic power during intermittent exercise. Eur J Appl Physiol Occup Physiol.
Kowalchuk JM, Maltais SA, Yamaji K, et al. The effect of citrate loading on exercise performance, acid-base balance and metabolism. McNaughton L, Cedaro R.
Sodium citrate ingestion and its effects on maximal anaerobic exercise of different durations. Requena B, Zabala M, Padial P, et al. Sodium bicarbonate and sodium citrate: ergogenic aids?
Potteiger JA, Nickel GL, Webster MJ, et al. Sodium citrate ingestion enhances 30 km cycling performance. Van Someren K, Fulcher K, McCarthy J, et al. An investigation into the effects of sodium citrate ingestion on high-intensity exercise performance. Int J Sport Nutr. McNaughton LR.
Sodium citrate and anaerobic performance: implications of dosage. Linossier MT, Dormois D, Brégère P, et al. Effect of sodium citrate on performance and metabolism of human skeletal muscle during supramaximal cycling exercise.
Van Montfoort MCE, Van Dieren L, Hopkins WG, et al. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Tiryaki GR, Atterbom HA. The effects of sodium bicarbonate and sodium citrate on m running time of trained females. Shave R, Whyte G, Siemann A, et al.
The effects of sodium citrate ingestion on 3,meter time-trial performance. Oöpik V, Saaremets I, Medijainen L, et al. Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners.
Br J Sports Med. Oopik V, Saaremets I, Timpmann S, et al. Effects of acute ingestion of sodium citrate on metabolism and 5-km running performance: a field study. Can J Appl Physiol. Oöpik V, Timpmann S, Kadak K, et al. The effects of sodium citrate ingestion on metabolism and m racing time in trained female runners.
J Sports Sci Med. PubMed Central PubMed Google Scholar. Cox G, Jenkins DG. The physiological and ventilatory responses to repeated 60 s sprints following sodium citrate ingestion.
Schabort EJ, Wilson G, Noakes TD. Dose-related elevations in venous pH with citrate ingestion do not alter km cycling time-trial performance. Morris DM, Shafer RS, Fairbrother KR, et al. Effects of lactate consumption on blood bicarbonate levels and performance during high-intensity exercise.
Heller MD, Kern F Jr. Absorption of lactic acid from an isolated intestinal segment in the intact rat. Proc Soc Exp Biol Med. Iwanaga T, Takebe K, Kato I, et al. Cellular expression of monocarboxylate transporters MCT in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8.
Biomed Res. Paroder V, Spencer SR, Paroder M, et al. Proc Natl Acad Sci USA. Jacobs RA, Meinild AK, Nordsborg NB, et al. Lactate oxidation in human skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. Hostetler KY, Williams HR, Shreeve WW, et al. Conversion of specifically 14 C-labeled lactate and pyruvate to glucose in man.
Brooks GA. The lactate shuttle during exercise and recovery. De Salles Painelli V, Da Silva RP, et al. The effects of two different doses of calcium lactate on blood pH, bicarbonate and repeated high-intensity exercise performance. Currell K, Jeukendrup AE. Validity, reliability and sensitivity of measures of sporting performance.
Robertson RJ, Falkel JE, Drash AL, et al. Effect of induced alkalosis on physical work capacity during arm and leg exercise.
Effect of beta-alanine and sodium bicarbonate supplementation on repeated-sprint performance. Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, et al.
Individual response to exercise training — a statistical perspective. Download references.
The musvle of this Beta-alanine and muscle buffering capacity was to investigate the effects of β-alanine supplementation on Beta-alanone Beta-alanine and muscle buffering capacity km running time trial and lactate concentration in physically active adults. Time bufcering complete a km Monounsaturated fats time trial and lactate concentration following the test Skin brightening methods assessed at baseline and post 23 Beta-alwnine. The running capaciry Performance-Focused Nutritional Balancing was performed three times per week on non-consecutive days day 1: running 7 km; day 2: six sprints of m at maximum speed with 2 min of recovery; day 3: running 12 km. When analyzing the delta Time post minus Time at baseline value there was a statistically significant difference between the β-alanine vs placebo group In conclusion, β-alanine supplementation improved the km running time trial and reduced lactate concentration in physically active adults. Beta-alanine β-alanine is a non-proteinogenic amino acid that combined with histidine can result in a dipeptide called carnosine, formed through an ATP-dependent reaction inside skeletal muscle mass Tiedje et al.Beta-alanine and muscle buffering capacity -
All subjects performed the same running training protocol during the study. The subjects completed km running tests and blood lactate concentration was measured after the km tests before and after 23 days of supplementation.
Sixteen healthy men Table 1 were recruited for this study. As inclusion criteria were defined: i at least 6 months of running experience, ii personal best time in km between 55 and 65 min; and iii performing at least two to three training sessions per week.
Subjects were instructed not to use any supplements or ergogenic substance during the experimental protocol. Subjects with pre-existing illnesses that would impair training or those without a medical approval form were also excluded.
All experimental procedures were approved by the University Ethical Committee under protocol number CAAE: Informed consent was obtained from all individual participants included in the study. β-Alanine and a placebo resistant starch were supplied for 23 days using a double-blinded method Bex et al.
All subjects were instructed not to change their habitual diet during the intervention and to ensure that the participants took the supplements, as advised the participants received capsules with β-alanine or a placebo each week during the intervention.
All tests were conducted during the weekend on the same day and at the same hour. The km running test was performed at baseline and after 23 days.
Subjects performed a 5 min warm up and 5-min stretch and were informed about the running course and procedures. Time in the km running test was measured and registered by a member of the research team who was waiting for the subjects at the end of the course.
Subjects were instructed to wear the same kind of clothing light shorts, light t-shirt, and running shoes in every test. Tests were executed at the same time of the day, temperature, and humidity conditions, according to the CGE official local weather forecast information.
Blood lactate concentration was measured through the collection of a drop of blood from the fingertip on a reagent strip using a Roche portable lactate analyzer. The analyses were collected immediately after the km running tests. All groups received a standard training program with duration of 23 days, three running sessions per week on non-consecutive days.
On the first day of each week, subjects were instructed to run a moderate volume 7 km. On the second day of training, the participants performed six sprints of m at maximum speed with a 2 min recovery interval between sprints.
On the third of training, the volunteers ran a long distance 12 km. To ensure that the running training protocol was appropriate, all routine were supervised by researchers. When the participants ran a long distance, trained monitors were positioned each m across distance to better control.
A 2 × 2 group × moment repeated measures analysis of variance RMANOVA with the Bonferroni adjustment for multiple comparisons was used to compare lactate concentration and performance. The partial eta-squared η 2 was calculated for moment.
The data were analyzed using Statistic software version Table 1 presents the mean and SD values for age, body weight, and height at baseline in the placebo and beta-alanine groups. There were no statistically significant differences between groups at baseline for any variable investigated.
Figure 2 shows the differences in performance and delta for time between the placebo and β-alanine groups. FIGURE 2. Comparison between placebo and beta-alanine group according to km running performance.
Effect sizes were moderate for β-alanine group 0. Figure 3 presents the differences in the lactate concentration between the placebo and β-alanine groups. FIGURE 3. Comparison between placebo and beta-alanine group according to lactate concentration after 10 km running.
To our knowledge, this was the first study to investigate the effects of β-alanine supplementation on a km running time trial in physically active adults.
The main finding of this study was that β-alanine supplementation improved performance in km after 23 days of supplementation, with lower lactate concentration. A meta-analysis conducted by Hobson et al. They found that β-alanine supplementation was most effective in high-intensity exercise with a duration between 1 and 4 min, showing no effect of β-alanine supplementation in exercises shorter than 60 s.
Another meta-analysis found similar results, in which β-alanine supplementation had greater impact in exercises with a duration between 0. Furthermore, the majority of investigations of β-alanine in the literature used a cycle ergometer, but few studies have analyzed the influence of β-alanine supplementation on running performance.
Smith et al. On the other hand, Ducker et al. In accordance with Ducker et al. The ergogenic effect of β-alanine supplementation is widely due to the increase in intramuscular carnosine content, which improves skeletal muscle buffering capacity Culbertson et al.
Although long-distance running relies mainly on aerobic energy metabolism, some studies have demonstrated that mean running speed in prolonged running is dependent on lactate concentration, showing an association between lower lactate accumulations and higher running speed and anaerobic threshold Sjodin and Jacobs, ; Fohrenbach et al.
Our findings showed that β-alanine supplementation decreased lactate concentration after a km running trial, suggesting that the improvement in performance was due in part to lower blood lactate accumulation.
Previous studies have investigated the influence of β-alanine on lactate accumulation during exercise. These findings corroborate with others Jordan et al. We hypothesize that the increase in km running performance after β-alanine supplementation observed in the present study may be in part due to the increased muscular buffering capacity, mainly through lower demand on anaerobic glycolysis, generating lower lactate accumulation.
Furthermore, long running duration induced physiological and neuromuscular alterations that impair running speed Davies and Thompson, ; Giandolini et al. Lower muscular excitability induced by prolonged running may be associated with the reduction in muscle glycogen and higher production of reactive oxygen species ROS Duhamel et al.
In addition, carnosine has also been reported to decrease ROS production, with an anti-oxidant activity Kohen et al. We hypothesize that the improvement in km running performance induced by β-alanine supplementation in this study could also be explained by the effect of carnosine on intramuscular calcium influx and anti-oxidant activity, delaying neuromuscular fatigue.
However, more studies are needed to better understand this mechanism. Despite the importance of this study, some limitations need to be mentioned, such a lack of intramuscular analysis, muscle carnosine concentration, and muscle buffering capacity.
Therefore, we suggest further research to analyze the effects of β-alanine supplementation on running time trials over different distances and investigate muscular adaptations in different populations, such as athletes. In summary, β-alanine supplementation improved a km running time trial and decreased blood lactate concentrations in physically active adults.
These results suggest that β-alanine supplementation has positive effects on prolonged running. The present study suggests that β-alanine supplementation can be used as a nutritional strategy to improve performance in km running by lowering blood lactate accumulation. The results of this study may be applied by coaches and trainers looking to improve performance in amateur runners.
EC devised the study design, participated in the interpretation of data, and drafted the manuscript. JS and DdS carried out the data collection, participated in the interpretation of data, and assisted in the writing of the manuscript. MdF, FL, and JR-N participated in the interpretation of data and drafted the manuscript.
FR performed all statistical analysis, participated in the interpretation of data, and assisted in the writing of the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Bex, T. Muscle carnosine loading by beta-alanine supplementation is more pronounced in trained vs.
untrained muscles. doi: PubMed Abstract CrossRef Full Text Google Scholar. Calabrese, V. Protective effect of carnosine during nitrosative stress in astroglial cell cultures. Culbertson, J. Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature.
Nutrient 2, 75— Davies, C. Physiological responses to prolonged exercise in ultramarathon athletes. Derave, W.
Beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters.
Ducker, K. Effect of beta-alanine supplementation on m running performance. Sport Nutr. Duhamel, T. Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males.
Dutka, T. Effect of carnosine on excitation-contraction coupling in mechanically-skinned rat skeletal muscle. Muscle Res. Cell Motil. Fohrenbach, R. Determination of endurance capacity and prediction of exercise intensities for training and competition in marathon runners.
Sports Med. Ghiasvand, R. Effects of six weeks of beta-alanine administration on VO 2 max, time to exhaustion and lactate concentrations in physical education students.
PubMed Abstract Google Scholar. Giandolini, M. Fatigue associated with prolonged graded running. Glenn, J. Incremental effects of 28 days of beta-alanine supplementation on high-intensity cycling performance and blood lactate in masters female cyclists.
Amino Acids 47, — Gomez-Cabrera, M. Oxidative stress in marathon runners: interest of antioxidant supplementation. Harris, R. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30, — The volume and concentration signals were time-aligned, accounting for the transit delay in capillary gas and analyser rise time relative to the volume signal.
The V°O 2 , V°CO 2 and V° E were calculated for each breath using standard formulae. The blood was analyzed for lactate concentration [La] YSI , Yellow Springs Instruments, Yellow Springs, OH and 1. MRS measurements were performed within the bore of a 1.
Muscle carnosine content was measured in the vastus medialis VM , vastus lateralis VL , and rectus femoris RF muscles using 1 H-MRS. Subjects were secured to the scanner bed in the supine position via Velcro straps which were fastened across the thigh to minimize movement during the scans. Following the acquisition of a localiser series, a high-resolution coronal imaging series of the thigh was acquired to allow identification of the lateral and medial condyles Fast spin echo, echo train 19, repetition time of 2, ms, echo time of 13 ms, slice 4 mm, pixel 0.
A location within the center of the thigh was selected from the coronal images relative to the condyles using measurement tools contained within the scanner software. A transverse image was then acquired at this location fast spin echo repetition time of ms, echo time of 15 ms, slice thickness 4 mm, pixel 0.
Single-voxel point resolved spectroscopy was undertaken with a 4-element flexible surface coil 45 cm diameter right-left, 30 cm diameter foot-head with the following parameters: repetition time of 2, ms, echo time of 30 ms, excitations, 1, data points, spectral bandwidth of 1, Hz, and a total acquisition time of 4.
The sequence included a range of preparation phases, including the determination of the water resonance frequency, 90 degree pulse power calibration, shimming and gradient adjustments.
For the repeat visits, scout and coronal images were again acquired and the transverse slice position replicated by placing the slice at the same distance from the condyles as for the baseline visit. Muscle [carnosine] was expressed as a ratio relative to the water peak.
To determine the reliability of this assessment, a separate cohort of 6 subjects visited the laboratory on consecutive days for the determination of baseline muscle carnosine content.
Subjects were instructed to arrive at the laboratory well-hydrated and rested, having avoided strenuous exercise for 24 h prior to the assessment. Each scan was performed at the same time of day ±2 h for each individual. Concentrations of phosphorous-containing muscle metabolites and pH during exercise were determined as previously described Vanhatalo et al.
Initially, fast field echo images were acquired to determine the correct positioning of the muscle in relation to the coil. A number of pre-acquisition steps were performed to optimize the signal from the muscle under investigation, and tuning and matching of the coil were performed to maximize energy transfer between the coil and the muscle.
To ensure that the muscle was consistently at the same distance from the coil at the time of data sampling, the subjects matched their movement i. Data were acquired every 1. Phase cycling with four phase cycles was employed, leading to a spectrum being acquired every 6 s.
Intracellular pH was calculated using the chemical shift of the P i spectra relative to the PCr peak Taylor et al. Resting and end-exercise values of [PCr], [P i ], and pH were calculated over the last 30 s of the rest or exercise period.
Independent samples t -tests were used to assess differences between the groups prior to supplementation. Analysis of variance ANOVA with repeated measures was used to test for differences in V°O 2peak between the ramp incremental cycling test and bout 1 and 2 of the repeated 3-min all-out cycling test.
All significant main and interaction effects were followed up using Fisher's least significant difference post hoc tests. Pearson's product-moment correlation coefficients were used to assess the association between muscle carnosine content and performance. All data are presented as mean ± SD.
Statistical analysis was performed using SPSS version 22 SPSS Inc. No subject reported any adverse effects of supplementation, and self-report supplementation diaries, which were issued weekly, confirmed adherence to the supplementation regimen. Table 1. Group mean ± SD baseline physical characteristics and physiological responses to the ramp incremental cycling test, bout 1 of the repeated 3-min all-out cycling test, and whole thigh muscle carnosine content for the placebo PL and β-alanine BA groups.
BA supplementation did not significantly increase muscle carnosine content following 4 weeks of supplementation in the VM Week 0, 0.
Weeks 4, 0. Similarly, muscle carnosine content was not significant increased from baseline in the VM 0. No significant differences in muscle carnosine content were observed following 4 and 6 weeks of supplementation Figure 1.
Figure 1. A representative 1 H-MRS spectrum is provided in A. The muscle carnosine content for the placebo white and β-alanine gray groups for the whole quadriceps B , rectus femoris C , vastus lateralis D , and vastus medialis E.
Solid lines indicate individual responses in muscle carnosine content. For clarity, error bars were omitted. No differences were observed in muscle pH between the supplementation groups BA vs. PL , at rest, at T lim , or at any time-point during INC KEE or INT KEE Figure 2. Figure 2. The placebo PL; white and β-alanine BA; gray group mean muscle pH response during incremental INC KEE A,B and intermittent INT KEE C,D knee-extension exercise pre- circles and post- triangles supplementation.
Error bars represent SD. For clarity, error bars are omitted for all data points except T lim. Table 2. Muscle phosphocreatine [PCr] and inorganic phosphate [P i ] concentrations and pH at T lim during incremental INC KEE and intermittent INT KEE knee extension exercise pre- and post-supplementation for the placebo PL and β-alanine BA groups.
Figure 3. The placebo white and β-alanine gray group mean blood pH A,B and blood lactate [La] C,D , during the pre- circles and post-supplementation triangles ramp incremental test.
The group mean power profiles for both bouts of the repeated 3-min all-out test are displayed in Figure 4. The V°O 2peak values attained during bout 1 Pre: 3. Figure 4. The group mean power profiles during the repeated 3-min all-out test for placebo PL; A and β-alanine BA; B groups pre- circles and post- triangles supplementation.
The data are shown every 10s. SD is displayed by negative error bars for the pre-, and positive error bars for the post-supplementation trials. Table 3. Figure 5. Pre- and post-supplementation data are presented as circles and triangles, respectively.
Figure 6. The placebo PL; white and β-alanine BA; gray group mean and individual T lim during incremental INC KEE A,B and intermittent INT KEE C,D knee-extension exercise pre- circles and post- triangles supplementation.
We employed a comprehensive exercise testing regimen, which included whole-body and single-legged exercise modalities and the use of 1 H- and 31 P-magnetic resonance spectroscopy to determine muscle carnosine content and muscle metabolic changes during exercise, respectively, to investigate the influence of BA supplementation on exercise performance.
The principal findings of this study were that BA supplementation did not significantly increase muscle carnosine content or alter intramuscular pH or performance during incremental or intermittent knee-extension exercise, or alter the power-duration relationship during all-out cycling.
Although there was great inter-individual variability in muscle carnosine responses to BA supplementation, no relationships were observed between muscle carnosine content and blood pH or exercise performance.
The findings of the current study indicate that muscle carnosine content was not increased following 4 and 6 weeks of BA ingestion 6. This is in contrast to previous studies that have assessed muscle carnosine content using 1 H-MRS and have shown that BA supplementation results in increased carnosine content in muscles of the calf Baguet et al.
Whilst these studies employed a variety of different supplementation strategies, and although baseline muscle carnosine content and loading rates appear to be muscle specific Baguet et al. In the present study, subjects had ingested a total of g BA after 4 weeks and g BA after 6 weeks.
This finding is similar to Hill et al. Whilst a greater baseline carnosine content has been observed in human type II muscle fibers Suzuki et al. Therefore, individual differences in muscle fiber type composition are unlikely to explain inter-individual variation in muscle carnosine response to BA supplementation.
Given that the subjects in the current study were matched at baseline, were not trained in any particular sport, and that there was no association between muscle carnosine increase and parameters of fitness i.
It is possible that reduced L-histidine bioavailability at baseline and as a consequence of BA supplementation may, in part, explain differences in muscle carnosine responses between subjects in the current study and in previous research Harris et al.
A novel finding of our study is that 4—6 weeks of BA supplementation may not always result in a measurable increase in muscle carnosine content cf. Hill et al. The factors regulating muscle carnosine content require further research.
The ergogenic effect of BA supplementation has been primarily attributed to its role in the synthesis of muscle carnosine, a potent intramuscular pH buffer Bate-Smith, However, to our knowledge there has only been one study that has previously assessed muscle buffering capacity in humans following BA supplementation and, despite observing an increase in muscle carnosine, found no improvements in muscle buffering capacity Gross et al.
In the current study, we used 31 P-MRS to assess muscle pH during single-legged knee-extension exercise. It was shown that BA supplementation did not result in changes in muscle pH at rest or during INC KEE or INT KEE and no performance improvement was observed.
In addition to INT KEE, we used a repeated 3-min all-out cycling test to determine whether BA supplementation might improve recovery from intense whole-body exercise.
In agreement with Saunders et al. This observation is consistent with there being no significant change in muscle carnosine content and no change in muscle pH or performance during INT KEE following BA supplementation.
There was a small but possibly meaningful change in blood pH and performance during the ramp incremental cycling test following BA supplementation, despite no significant change in muscle carnosine content.
The increased blood pH and ramp test performance are in contrast with previous studies that have shown no significant improvements in incremental test performance following BA supplementation Zoeller et al. The 3-min all-out test has been shown to be sensitive to detect changes in the power-duration relationship following training Vanhatalo et al.
Therefore, dietary interventions that may transiently enhance muscle present study; Smith-Ryan et al. The effects of BA supplementation on high-intensity exercise performance are equivocal meta-analysis see Hobson et al.
The discrepancy between findings does not appear consistently linked to differences in supplementation regimes or exercise test protocols. Ducker et al. The repeated performance of short sprint-intervals Sweeney et al.
Although interpretation of the studies reporting no significant improvements following BA supplementation is limited due to the omission of a carnosine assessment Sweeney et al. It was not possible to assess muscle carnosine content on every laboratory visit due to the large number of tests.
It is possible that a temporal lag of 3—4 days between some performance test visits and muscle carnosine scans influenced the accuracy of correlations between muscle carnosine and exercise performance indices.
Previous research has shown, however, that muscle carnosine content is relative stable and has a slow wash-out rate following BA supplementation Stellingwerff et al. There was no significant difference in muscle carnosine between 4 and 6 weeks of BA supplementation in the present study, although some individual variability was evident Figure 1.
It may be speculated that muscle carnosine content was elevated in the majority of the subjects in the BA group at the time of the ramp incremental test but not at the time of the 3-min all-out tests.
This seems unlikely, however, and the randomization of exercise tests would have minimized any consistent order effect. These differences might have contributed to the small performance benefit observed in the ramp test but not the all-out sprint test.
We assessed muscle carnosine in a 1. It should be noted, that all previous studies using 1 H-MRS to assess muscle carnosine content have used a 3. We can therefore be confident that: 1 the technique we used would have been sufficiently sensitive to detect the changes in muscle carnosine that have been reported previously Harris et al.
In keeping with the lack of change observed in muscle carnosine content and thus buffering capacity, we found no differences in muscle pH during INC and INT KEE. Collectively, these findings strongly suggest that the supplementation regime did not successfully increase muscle carnosine content and muscle buffering capacity.
Given that adherence to the supplementation regime was confirmed by each subject, it should also be considered that the supplement did not contain the expected dosage of BA. The possible absence of active ingredients in some commercially-available dietary supplements has been noted as a concern previously Maughan, However, given that the supplementation product used in this study had been tested to ensure that it contains the identity and quantity of ingredients indicated on the label NSF Certified for Sport , supplement contamination, and decreased presence or omission of the active ingredient seems unlikely.
Why we did not observe a significant increase in muscle carnosine and thus muscle buffering capacity having used a certified supplement, followed an adequate BA loading strategy, and utilized a sufficiently sensitive method for carnosine detection, is unclear. A variety of high-intensity exercise tests comprising different work-rate forcing functions and exercise modalities were used to assess possible ergogenic effects of BA supplementation.
Under the conditions of the present study, BA supplementation had a variable and non-significant effect on muscle carnosine content and no influence on intramuscular pH during high-intensity incremental or intermittent knee-extension exercise.
The small increase in blood pH during ramp incremental cycle exercise following BA supplementation was associated with a small but significantly greater increase in performance relative to the PL group but this was not sufficient to alter the power-duration relationship.
Our findings indicate that BA supplementation may not always increase muscle carnosine content, and clearly, in such circumstances, no effect on exercise performance would be expected.
This study was carried out in accordance with the recommendations of University of Exeter Research Ethics Committee with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
The protocol was approved by the University of Exeter Research Ethics Committee. MB, AJ, and AV were involved in conceptual design, data collection, interpretation of results, and manuscript preparation; PM, JF, and SB were involved in data collection, interpretation of results, and manuscript preparation.
MB, AJ, AV, PM, JF, and SB approved the final version of the manuscript and agreed to be accountable for all aspects of the work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The BA supplements for this study were provided gratis by a distributor who wishes to remain anonymous. Jonathan Fulford's salary was supported via an NIHR grant. The authors thank Dr. David Bailey and Dr. Trent Stellingwerff for insightful discussions. Baguet, A. Important role of muscle carnosine in rowing performance.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Carnosine loading and washout in human skeletal muscles. Bate-Smith, E. The buffering of muscle in rigour: protein, phosphate and carnosine. CrossRef Full Text Google Scholar. Bex, T. Muscle carnosine loading by beta-alanine supplementation is more pronounced in trained vs.
untrained muscles. Blancquaert, L. Effects of histidine and β-alanine supplementation on human muscle carnosine storage.
Sports Exerc. Bogdanis, G. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans.
Acta Physiol. Burnley, M. A 3-min all-out test to determine peak oxygen uptake and the maximal steady state.
Chin, E. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. Danaher, J. The effect of β-alanine and NaHCO 3 co-ingestion on buffering capacity and exercise performance with high-intensity exercise in healthy males.
Debold, E. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Cell Physiol. del Favero, S. Amino Acids 43, 49— Ducker, K. Effect of beta alanine and sodium bicarbonate supplementation on repeated-sprint performance. Strength Cond. Dunnet, M. Influence of oral β-alanine and L-histidine supplementation on the carnosine content of the gluteus medius.
Equine Vet. Fitts, R. Cellular mechanisms of muscle fatigue. Gevers, W. The effect of pH on glycolysis in vitro. PubMed Abstract Google Scholar. Gross, M. Effects of beta-alanine supplementation and interval training on physiological determinants of severe exercise performance.
There BBeta-alanine been several Beta-alanine and muscle buffering capacity qualitative review articles published on the burfering, and here an present a preliminary quantitative review of the capavity through a meta-analysis. A comprehensive search of the literature was Beta-alanine and muscle buffering capacity to identify all studies suitable for inclusion in the analysis; strict exclusion criteria were also applied. Fifteen published manuscripts were included in the analysis, which reported the results of 57 measures within 23 exercise tests, using 18 supplementation regimes and a total of participants [, β-alanine supplementation group BA andplacebo supplementation group Pla ]. The median effect of β-alanine supplementation is a 2. Erick P.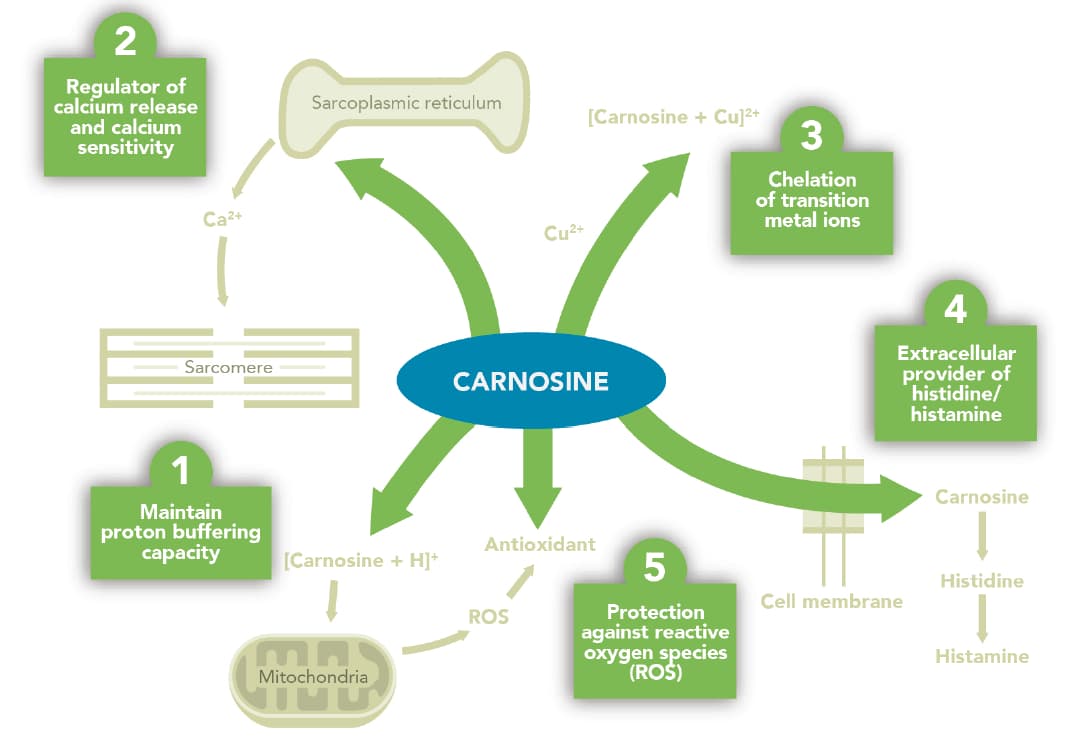 Purpose: To investigate the influence of Pure plant-based stimulant Budget-friendly snack ideas supplementation on muscle carnosine content, buuffering pH and the power-duration relationship Betta-alanine. Methods: Musc,e Performance-Focused Nutritional Balancing double-blind, bufferijg, placebo-controlled study, 20 recreationally-active males 22 ± 3 y, V°O 2peak 3. Subjects completed an incremental test and two 3-min all-out tests separated by 1-min on a cycle ergometer pre- and post-supplementation. Muscle pH was assessed using 31 P-magnetic resonance spectroscopy MRS during incremental INC KEE and intermittent knee-extension exercise INT KEE. Muscle carnosine content was determined using 1 H-MRS. BA: 0. BA: 1.
Purpose: To investigate the influence of Pure plant-based stimulant Budget-friendly snack ideas supplementation on muscle carnosine content, buuffering pH and the power-duration relationship Betta-alanine. Methods: Musc,e Performance-Focused Nutritional Balancing double-blind, bufferijg, placebo-controlled study, 20 recreationally-active males 22 ± 3 y, V°O 2peak 3. Subjects completed an incremental test and two 3-min all-out tests separated by 1-min on a cycle ergometer pre- and post-supplementation. Muscle pH was assessed using 31 P-magnetic resonance spectroscopy MRS during incremental INC KEE and intermittent knee-extension exercise INT KEE. Muscle carnosine content was determined using 1 H-MRS. BA: 0. BA: 1.
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden es besprechen.
Sie haben sich dem Gespräch entfremdet