Autophagy and autophagy inhibitors -
A structural study suggested that 3-MA preferentially inhibits VPS34 in vitro In vivo, 10 mM 3-MA can also inhibit class I PI3K, which may explain why it can promote autophagy flux under nutrient-rich conditions 39 , The PI3K inhibitors wortmannin and LY, which inhibit both class I and III PI3K complexes, have also been used to inhibit autophagy 39 , 41 , An x-ray crystallographic study of the class III PI3K VPS34 demonstrated that it exhibits distinguishing structural features that differentiate it from class I PI3Ks.
The P loop of VPS34, which binds the phosphates of ATP, curls inward toward the ATP-binding pocket and is more constricted than that of class I PI3K. The hinge between the N and C lobes of VPS34 is one residue shorter than in class I PI3Ks. Therefore, VPS34 lacks the bulged-out space at the adenine-binding pocket hinge that is characteristic of class I PI3Ks.
Miller et al. exploited these structural characteristics of VPS34 and designed a number of promising VPS34 kinase inhibitors such as PT21 IC 50 88 nM Further development of these VPS34 kinase inhibitors may provide promising leads to target the class III PI3K.
Thus, it is possible to regulate the levels of entire complexes by targeting one component. Spautin-1, isolated from an imaged-based screen for small-molecule modulators of autophagy 11 , promotes the degradation of VPS34 complexes by targeting the ubiquitin-specific peptidases, USP10 and USP13, two deubiquitinating enzymes that regulate beclin 1 and VPS34 stability.
Spautin-1 promotes ubiquitination and proteasomal degradation of beclin 1 and VPS34 complexes under glucose-free conditions 44 , consequently inhibiting autophagy. Spautin-1 treatment can promote the death of cancer cells in the setting of nutrient deprivation when autophagy is activated to promote survival.
Moreover, inhibition of autophagy by spautin-1 under nutritional deprivation conditions leads to the activation of chaperone-mediated autophagy, a lysosome-dependent pathway that mediates degradation of missense mutant p53 protein Increasing evidence suggests that certain p53 mutants may promote oncogenesis through a dominant gain-of-function mechanism; thus, the reduction of accumulated mutant p53 may be an important therapeutic goal in cancer treatment.
Development of improved spautin-1 analogs with good safety and in vivo stability profiles may represent a novel anticancer therapeutic strategy. Inhibitors of class I PI3K signaling activate autophagy.
In contrast to the positive role of the class III PI3K products in promoting autophagy, increases in the levels of class I PI3K products phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-triphosphate negatively regulate autophagy Targeting this class I PI3K with inhibitors such as CH, GDC, and GDC, leads to the activation of autophagy 47 — Protein kinase AKT is a major downstream mediator of class I PI3K signaling.
Pharmacologic targeting of AKT by inhibitors such as perifosine leads to autophagy activation Moreover, treatment with inhibitors of receptor tyrosine kinases that leads to the inhibition of AKT can activate autophagy, possibly by inhibiting mTORC1 or by regulating beclin 1.
The activation of the EGFR tyrosine kinase—mediated signaling pathway may inhibit autophagy through multiple mechanisms that target beclin 1 51 , In another small-molecule screen for autophagy modulators using the clearance of the autophagy substrate A30P α-synuclein as a biomarker, Williams et al.
In addition, calpain 1 can cleave the Gαs subunit of heterotrimeric G proteins, which increases its activity after calpain cleavage. Gαs α enhances adenylyl cyclase activity, which in turn inhibits autophagy These studies suggest that calpain 1 may have multiple mechanisms to control the levels of autophagy in cells under normal nutritional conditions by regulating the levels of key proteins involved in autophagy.
Thapsigargin does not affect autophagosome formation but leads to accumulation of mature autophagosomes by specifically inhibiting the fusion of autophagosomes with lysosomes Thapsigargin blocks autophagosomal recruitment of the small GTPase RAB7, which is required for complete autophagic flux.
Another study demonstrates that thapsigargin causes accumulation of mature autophagosomes by blocking autophagosome fusion within the endocytic system Inhibitors of inositol monophosphatase activate autophagy.
Lithium, used widely as a mood-stabilizing drug for the treatment of bipolar disorder, was shown to induce autophagy and reduce mutant huntingtin aggregates and cell death Sarkar et al. found that lithium inhibits inositol monophosphatase IMPase , leading to depletion of free inositol and a reduction in the levels of myo-inositol-1,4,5-triphosphate IP3 , which may represent an mTOR-independent pathway for regulating mammalian autophagy.
Similar effects were observed with other IMPase inhibitors and mood-stabilizing drugs such as L and carbamazepine, which also decrease inositol levels Since IP3 receptor IP3R is a potential binding partner of beclin 1, the IP3R antagonist Xestospongin B can stimulate autophagy by releasing beclin 1 from IP3R to assemble into the VPS34 complex 59 , Any success of such clinical trials would encourage the development of additional autophagy modulators for the treatment of neurodegenerative diseases.
Induction of cell death by Tat—beclin 1 peptide. Tat—beclin 1 peptide, a fusion peptide of 18 amino acids derived from beclin 1 and the HIV Tat protein transduction domain, was shown to induce autophagy and decrease the accumulation of polyglutamine expansion protein aggregates by binding to Golgi-associated plant pathogenesis—related protein 1 GAPR-1, also known as GLIPR2 , a protein that associates with lipid rafts at the cytosolic leaflet of the Golgi membrane.
In addition, Tat—beclin 1 inhibits the replication of several pathogens including HIV-1 in vitro and reduces mortality in mice infected with chikungunya or West Nile virus On the other hand, treatment with Tat—beclin 1 can induce cell death 63 , and knockdown of components of the autophagy machinery, such as beclin 1, Atg14L, and Atg5, can block cell death induced by Tat—beclin 1, which suggests that Tat—beclin 1 may activate autophagic cell death.
Targeting cytoskeletal components to regulate autophagy. The destabilization of microtubules by either vinblastine 64 , nocodazole, or cytochalasin B and D blocks the maturation of autophagosomes 65 ; conversely, taxol-mediated microtubule stabilization increases the fusion between autophagic vacuoles and lysosomes 66 , Paclitaxel, which also stabilizes microtubules, was shown to inhibit autophagy through two distinct mechanisms dependent on the cell cycle stage.
In mitotic cells, paclitaxel blocks the activation of the VPS34 complex by inducing inhibitory phosphorylation of VPS34 at T, which is mediated by mitotic kinases such as CDK1 In non-mitotic cells, paclitaxel inhibits autophagosome trafficking to block maturation of autophagic vesicles The ability of microtubule targeting agents to negatively regulate autophagy suggests that their efficacy as anticancer therapeutics may be partially attributable to their ability to inhibit autophagy.
Additionally, other autophagy-inhibiting agents may not enhance the therapeutic effects of paclitaxel, nocodazole, or vinblastine, as these agents already inhibit autophagy.
Histone deacetylase 6 HDAC6 , a microtubule-associated deacetylase, has been shown to function as a central component of basal autophagy that targets protein aggregates to the dynein motor for transport 70 , HDAC6 was shown to promote autophagy by recruiting cortactin-dependent, actin-remodeling machinery, which assembles an F-actin network that stimulates autophagosome-lysosome fusion and substrate degradation HDAC6 deficiency impairs autophagosome maturation; accordingly, inhibitors of HDAC6 and other HDACs have been show to block autophagy 73 through acetylation and suppression of autophagy-associated proteins such as ATG7.
However, the effect of HDAC inhibitors on autophagy has been a controversial issue, and other studies propose HDAC inhibitors as autophagy activators 74 — Lysosomal alkalizers block autophagic flux and degradation.
The lysosomal lumen alkalizers inhibit autophagy by neutralizing the acidic pH in the lumen of lysosomal vesicles, which is required for the activities of lysosomal hydrolases involved in autophagic degradation.
Thus, alkylation of lysosomal vesicles leads to the accumulation of autophagosomes by blocking lysosomal degradation Chloroquine CQ and its derivative hydroxychloroquine HCQ have been investigated in preclinical studies and clinical trials as anticancer drug candidates Some efforts have been made to generate more potent inhibitors of lysosomal functions.
For example, Lys05, a dimeric CQ with an improved ability to accumulate in the lysosomes, may have more potent antitumor activity than HCQ 79 , Other compounds with CQ-like activities in alkalizing lysosomal compartments have also been identified.
Monensin, a polyether antibiotic, is also a lysosomotropic drug that prevents the acidification of lysosomes and interferes with the fusion of autophagosomes and lysosomes In addition, lucanthone, an anti-schistosome agent, inhibits autophagy via a similar mechanism to CQ Finally, matrine, a quinolizidine alkaloid, can also block autophagic degradation by elevating the luminal pH of lysosomes AAA ATPase inhibitors block autophagic degradation.
p97 also called valosin containing protein [VCP] is implicated in autophagosome maturation 84 under basal conditions and in cells treated by proteasome inhibition, but not in cells challenged by starvation, suggesting that p97 might be selectively required for autophagic degradation of ubiquitinated substrates.
Xanthohumol, a prenylated chalcone that can bind directly to the N domain of p97, was identified as a p97 ATPase inhibitor that can modulate autophagy DBeQ, a selective, reversible and ATP-competitive p97 inhibitor, also impairs autophagic degradation of LC3-II and proteasomal degradation of a pdependent ubiquitin-proteasome system Amino acid deprivation induces both apoptosis and autophagy in murine C2C12 muscle cells.
Biotechnol Lett ; 27 : — Kawakami T, Inagi R, Takano H, Sato S, Ingelfinger JR, Fujita T, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant ; 24 : — Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al.
Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. Bray K, Mathew R, Lau A, Kamphorst JJ, Fan J, Chen J, et al.
Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLOS ONE ; 7 : e Menzies FM, Huebener J, Renna M, Bonin M, Riess O, Rubinsztein DC. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3.
Brain ; : 93— Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells.
Cancer Res ; 66 : —7. Crazzolara R, Bradstock KF, Bendall LJ. RAD Everolimus induces autophagy in acute lymphoblastic leukemia. Autophagy ; 5 : —8.
Lin CI, Whang EE, Donner DB, Du J, Lorch J, He F, et al. Autophagy induction with RAD enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer.
Mol Cancer Res ; 8 : — Xiong N, Jia M, Chen C, Xiong J, Zhang Z, Huang J, et al. Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y.
Neuroscience ; : — Kim H, Bernard ME, Flickinger J, Epperly MW, Wang H, Dixon TM, et al. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol ; 87 : — Zeng X, Overmeyer JH, Maltese WA.
Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking.
J Cell Sci ; : — Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci U S A ; : —8. Pedrozo Z, Sanchez G, Torrealba N, Valenzuela R, Fernandez C, Hidalgo C, et al.
Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta ; : — Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgo J, et al. Autophagy ; 7 : — Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, et al.
The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ ; 16 : — Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy ; 3 : —3. Hou H, Zhang Y, Huang Y, Yi Q, Lv L, Zhang T, et al.
Castino R, Bellio N, Follo C, Murphy D, Isidoro C. Inhibition of PI3k class III — dependent autophagy prevents apoptosis and necrosis by oxidative stress in dopaminergic neuroblastoma cells.
Toxicol Sci ; : — Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps Proc Natl Acad Sci U S A ; 79 : — Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, et al.
Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget ; 3 : — PubMed PubMed Central Google Scholar. Takatsuka C, Inoue Y, Matsuoka K, Moriyasu Y.
Plant Cell Physiol ; 45 : — Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. Bright NA, Lindsay MR, Stewart A, Luzio JP.
The relationship between lumenal and limiting membranes in swollen late endocytic compartments formed after wortmannin treatment or sucrose accumulation. Traffic ; 2 : — Xing C, Zhu B, Liu H, Yao H, Zhang L. Class I phosphatidylinositol 3-kinase inhibitor LY activates autophagy and induces apoptosis through p53 pathway in gastric cancer cell line SGC Acta Biochim Biophys Sin Shanghai ; 40 : — CAS Google Scholar.
Ethier MF, Madison JM. LY, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium ; 32 : 31—8. Rez G, Kovacs J. Prevention by cycloheximide of cellular autophagy induced by hyperosmotic sucrose or cadmium chloride in mouse pancreatic acinar cells.
Acta Biol Acad Sci Hung ; 24 : —5. Kovacs J. Regression of autophagic vacuoles in seminal vesicle cells following cycloheximide treatment. Exp Cell Res ; : —4. Oliva O, Rez G, Palfia Z, Fellinger E. Dynamics of vinblastine-induced autophagocytosis in murine pancreatic acinar cells: influence of cycloheximide post-treatments.
Exp Mol Pathol ; 56 : 76— Machiya Y, Hara S, Arawaka S, Fukushima S, Sato H, Sakamoto M, et al. Phosphorylated alpha-synuclein at Ser is targeted to the proteasome pathway in a ubiquitin-independent manner.
Lawrence BP, Brown WJ. Inhibition of protein synthesis separates autophagic sequestration from the delivery of lysosomal enzymes. Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, HII-E cells.
Cell Struct Funct ; 23 : 33— Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC, et al. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun ; : —6.
Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes. Autophagy ; 4 : — Chen G, Ke Z, Xu M, Liao M, Wang X, Qi Y, et al. Autophagy is a protective response to ethanol neurotoxicity. Dengjel J, Hoyer-Hansen M, Nielsen MO, Eisenberg T, Harder LM, Schandorff S, et al.
Identification of autophagosome-associated proteins and regulators by quantitative proteomic analysis and genetic screens. Mol Cell Proteomics ; 11 : M Harhaji-Trajkovic L, Arsikin K, Kravic-Stevovic T, Petricevic S, Tovilovic G, Pantovic A, et al. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation.
Pharm Res ; 29 : — Amaravadi RK, Winkler JD. Lys A new lysosomal autophagy inhibitor. Autophagy ; 8 : —4. McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency.
Moriyasu Y, Inoue Y. Use of protease inhibitors for detecting autophagy in plants. Methods Enzymol ; : — Kominami E, Hashida S, Khairallah EA, Katunuma N.
Sequestration of cytoplasmic enzymes in an autophagic vacuole-lysosomal system induced by injection of leupeptin. Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy.
Autophagy ; 1 : 84— Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, et al. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY inhibit autophagy in isolated rat hepatocytes.
Eur J Biochem ; : —6. Christian F, Anthony DF, Vadrevu S, Riddell T, Day JP, McLeod R, et al. p62 SQSTM1 and cyclic AMP phosphodiesterase-4A4 PDE4A4 locate to a novel, reversible protein aggregate with links to autophagy and proteasome degradation pathways.
Cell Signal ; 22 : — Ramser B, Kokot A, Metze D, Weiss N, Luger TA, Bohm M. Hydroxychloroquine modulates metabolic activity and proliferation and induces autophagic cell death of human dermal fibroblasts. J Invest Dermatol ; : — Oikarinen A. Hydroxychloroquine induces autophagic cell death of human dermal fibroblasts: implications for treating fibrotic skin diseases.
J Invest Dermatol ; : —5. Lys a new lysosomal autophagy inhibitor. Aronson N. Jr, Dennis PA, Dunn WA. Metabolism of leupeptin and its effect on autophagy in the perfused rat liver. Acta Biol Med Ger ; 40 : —8. Dong XX, Wang YR, Qin S, Liang ZQ, Liu BH, Qin ZH, et al.
p53 Mediates autophagy activation and mitochondria dysfunction in kainic acid-induced excitotoxicity in primary striatal neurons. Neuroscience ; : 52— Tanida I.
Autophagy basics. Microbiol Immunol ; 55 : 1— Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, et al. microRNA is a potent inhibitor of autophagy. EMBO J ; 30 : — Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, et al.
Targeting microRNAa-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia ; 26 : — Yuya N, Satoko A, Kenji F, Hirofumi Y, Takeshi M, Toku K, et al.
Nature ; : —8. Google Scholar. Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Beau I, Mehrpour M, Codogno P.
Autophagosomes and human diseases. Int J Biochem Cell Biol ; 43 : —4. Bursch W EA, Gerner C SR. Autophagocytosis and programmed cell death. In: Klionsky DJ, editor. Georgetown, TX: Landes Bioscience, Shintani T, Klionsky DJ.
Autophagy in health and disease: a double-edged sword. Science ; : —5. Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. Int J Cancer ; : —5. Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP Zhao Y, Xiong X, Sun Y.
DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF betaTrCP E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell ; 44 : — Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1.
Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci ; : 22— Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Cao BY, Yang YP, Luo WF, Mao CJ, Han R, Sun X, et al.
J Ethnopharmacol ; : —9. Yang S, Xiao X, Meng X, Leslie KK. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLOS One ; 6 : e Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin.
Hum Mol Genet ; 17 : —8. Download references. This work was supported by the National Natural Science Foundation of China N o , the Natural Science Foundation of Jiangsu Province of China N o BK , the Natural Science Foundation of the Jiangsu Higher Education Institutions of China N o 10KJB and Plans for Graduate Research and Innovation in Colleges and Universities of Jiangsu Province N o CX10B Department of Neurology, Second Affiliated Hospital of Soochow University, Suzhou, , China.
Institute of Neuroscience, Soochow University, Suzhou, , China. You can also search for this author in PubMed Google Scholar. Correspondence to Chun-feng Liu. Reprints and permissions. Yang, Yp. et al. Application and interpretation of current autophagy inhibitors and activators.
Acta Pharmacol Sin 34 , — Download citation. Received : 21 November Accepted : 14 January Published : 25 March Issue Date : May Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature.
nature acta pharmacologica sinica review article. Download PDF. Abstract Autophagy is the major intracellular degradation system, by which cytoplasmic materials are delivered to and degraded in the lysosome. Stress response silencing by an E3 ligase mutated in neurodegeneration Article Open access 31 January PROTAC targeted protein degraders: the past is prologue Article 18 January Introduction Cell growth and homeostasis are governed by tightly regulated biosynthetic and catabolic processes.
Mechanisms and pharmacologic targeting of autophagy Although autophagy and autophagy-related processes are dynamic, they can be broken down into several discrete steps for the purpose of discussion: 1 induction, 2 autophagosome formation, 3 autophagolysosome formation, and 4 delivery and degradation of the autophagic body 1.
Autophagy activators Several recent articles address that autophagy upregulation may have therapeutic benefits in a range of diseases.
Table 1 Autophagy activators. Full size table. Autophagy inhibitors Autophagy could potentially be suppressed at any stage of autophagic flux. Table 2 Autophagy inhibitors. Genetic intervention The existence of autophagy inhibitors and activators greatly facilitates the investigation of autophagy and its therapeutic potential in human diseases.
Therapeutic implications for autophagy regulators Malfunctioning autophagy is observed in many human diseases including cancer, neurodegenerative diseases, cardiac and muscular diseases, infectious and inflammatory diseases, diabetes and obesity Concluding remarks Autophagy is a process that involves the sequestration of intracellular components and their subsequent degradation in secondary lysosomes that is highly conserved from yeast to mammals.
Figure 1. Full size image. Similar content being viewed by others. References Yang YP, Liang ZQ, Gu ZL, Qin ZH. CAS PubMed Google Scholar Meijer AJ, Codogno P. CAS PubMed Google Scholar Levine B, Kroemer G.
CAS PubMed PubMed Central Google Scholar Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, et al. CAS PubMed Google Scholar Ohsumi Y. CAS PubMed Google Scholar Mizushima N, Yoshimori T, Ohsumi Y. CAS PubMed Google Scholar Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T.
CAS PubMed PubMed Central Google Scholar Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. CAS PubMed Google Scholar Weber JD, Gutmann DH. CAS PubMed PubMed Central Google Scholar Wullschleger S, Loewith R, Hall MN. CAS PubMed Google Scholar Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC.
CAS PubMed Google Scholar Chu CT. PubMed Google Scholar Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. CAS PubMed Google Scholar Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, et al. CAS PubMed PubMed Central Google Scholar Lee JY, Yao TP. PubMed Google Scholar Su M, Shi JJ, Yang YP, Li J, Zhang YL, Chen J, et al.
CAS PubMed Google Scholar Cai ZL, Shi JJ, Yang YP, Cao BY, Wang F, Huang JZ, et al. CAS PubMed Google Scholar Seglen PO, Gordon PB. CAS PubMed Google Scholar Mitchener JS, Shelburne JD, Bradford WD, Hawkins HK. CAS PubMed PubMed Central Google Scholar Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, et al.
CAS PubMed PubMed Central Google Scholar Konorov SO, Jardon MA, Piret JM, Blades MW, Turner RF. Rapamycin is an antifungal metabolite produced by Streptomyces Hygroscopicus, which is a bacterium that resides in soil.
It was observed that rapamycin binds tokDa FKbinding protein FKBP12 to form a complex that binds to mTORC1 and inhibits its activity.
It has been shown to have activity against mTORC2 with prolonged use but does have greater specificity for mTORC1 Li et al. mTORC1 directly controls protein synthesis and regulates metabolic pathways, particularly glycolysis.
Rapamycin inhibition of mTORC1 to induce autophagy in yeast and most mammalian cells has resulted in it being used extensively in studying the mechanisms and actions of autophagy Nyfeler et al. Therefore, the potential for inhibiting mTORC1 with rapamycin was a promising cancer therapeutic; however, it was deemed not feasible due to solubility issues and its pharmacokinetics.
Since then, there has been the development of analogues with improved solubility and pharmacology, which have included temsirolimus and everolimus. These analogues are approved by the Food and Drug Administration FDA in the USA for the treatment of angiomyolipomas, HER2-negative breast cancers, pancreatic neuroendocrine tumours, renal cell carcinomas and subependymal giant cell astrocytoma, and are being tested in numerous cancers in various clinical trial regime Roskoski, However, despite improving the targeting of mTORC1 with these rapamycin analogues, their efficacy has overall been disappointing due to resistance and off-target effects Hua et al.
Currently, two main classes of autophagy inhibitors will be the focus of this review. These are PI3K inhibitors that stop autophagosome formation and lysosomal inhibitors that prevent proper lysosome acidification Figure 1.
PI3K inhibitors comprise chemical compounds that inhibit the PI3K complex and, thus, autophagy initiation in the canonical pathway, as previously discussed Figure 1. The PI3K family is divided into classes I, II and III, with numerous isoforms within classes I and II.
PI3K class I isoforms form part of the Akt mTOR axis and regulate growth, metabolism, cellular movement, and protein synthesis. PI3K class II isoforms are involved in endocytosis, mitosis, and cell migration.
The class III enzyme is involved in autophagy and extra vesicle trafficking. Thus ideally, PI3K inhibitors would be targeted towards class III enzymes; however, until recently, inhibitors have lacked this specificity. The most common inhibitors include 3-Methyladenine 3-MA , Wortmannin and LY Arcaro and Wymann, ; Powis et al.
One of the most commonly used autophagic inhibitors in pre-clinical studies over the last decade has been that of 3-MA. In addition, it only suppresses autophagy under starvation conditions and in fact promotes autophagy in complete media, similar to rapamycin and the suppression of mTOR Wu et al.
Therefore, further research using 3-MA as an autophagy inhibitor should potentially be discontinued due to this uncertainty and the availability of more selective autophagy inhibitors. In addition, its poor solubility has severely limited its use in several settings, including clinical studies.
However, derivatives of 3-MA have been developed and have improved solubility and specificity to class III PI3K Wu et al. Thus, these derivatives should be used going forward. A few recent studies have proven efficacious in treating colorectal cancer cells in hypoxic conditions with 3-MA causing apoptosis Dong et al.
Another has shown that treatment in head and neck cancers with reactivation of p53 and induction of tumour cell apoptosis RITA. The treatment potential of RITA has been limited to date due to resistance which can be overcome with the addition of 3-MA Shin et al.
Curiously, both studies presented LC3 levels to represent autophagy but did not show any PI3K class I activity to ascertain if this pathway had a role. Additionally, a study that examined the efficacy of various PI3K inhibitors; 3-MA, the newer derivative Autophagy inhibitor IV, more commonly referred to as Compound 18 Cpd18 Wu et al.
However, it was shown that 3-MA induced DNA damage resulting in subsequent cell death. It was also shown that 3-MA had a minimal inhibitory effect on PI3K class I downstream targets. Moreover, both Cpd18 and SAR were found to have no effect on the PI3K class I targets Chicote et al.
Alternative drugs to 3-MA include Wortmannin and LY Wortmannin transiently blocks PI3K class I but persistently blocks PI3K class III Powis et al.
SAR is a first-in-class ATP-competitive inhibitor of VPS34 and was identified through high throughput small molecule screening.
Its activity is specific to VPS34, inhibiting autophagy and late endosome to lysosome trafficking Pasquier, ; Bago et al. Interestingly, it has been shown that the SAR IC50 significantly decreases when an mTOR inhibitor induces autophagy.
Therefore, a study into the combination of SAR and mTOR inhibitor everolimus showed increased cytotoxicity in renal cancer cell lines Ronan et al. More recently, it has been shown to induce inflammation within the tumour and provide an environment for the successful use of immunotherapeutic agents in vivo in melanoma and colorectal cancer models Noman et al.
In addition, evidence shows that SAR ameliorates radiotherapy-induced mitophagy and improves tumour refraction when radiotherapy is combined with SAR in head and neck cancer in vivo Lee et al. VPSIN1 is a specific and potent VPS34 inhibitor, and its mechanisms of action are suggested to be through its PtdIns 3 P-binding PX domain but are yet to be fully elucidated Bago et al.
A recent study showed improved efficacy of Ceritinib with VPSIN1 in treating non-small cell lung cancer cells and reduced cell survival Schläfli et al. Ceritinib is an anaplastic lymphoma kinase ALK inhibitor which activates several signalling cascades resulting in the inhibition of PI3K.
It is this inhibition of PI3K that reduces proliferation, tumour growth and induces apoptotic cell death. The additive effect that VPSIN1 has on these cells suggests that Ceritinib may only be targeting PI3K class I and class II.
It was also suggested that VPSIN1 and SAR offered more significant treatment potential due to their specificity Schläfli et al. Recently, VPSIN1 has been shown to have promising effects in acute myeloid leukaemia AML cells, with increased cell death observed in response to treatment Meunier et al.
Lysosomal inhibitors include numerous compounds that alter the pH of the lysosome thereby making the function of the enzymes that rely on this defunct. Common lysosomal inhibitors include Bafilomycin A1 BafA1 , chloroquine CQ , and hydroxychloroquine HCQ. V-ATPases are a type of proton pump essential for maintaining the acidic environment in the lysosome, which is required for proper functioning and activation of degradative enzymes Mauvezin and Neufeld, BafA1 prevents both acidifications of the lysosome and autophagosome-autolysosome fusion by disrupting the function of the V-ATPases Yamamoto et al.
It has also been shown to be a potassium carrier to mitochondria, resulting in mitochondrial swelling and dysfunction; this activity was observed at nanomolar concentrations Teplova et al. It has been suggested that the use of BafA1 is not feasible due to this toxicity, although interestingly, claims of toxicities have not been supported by data, and on the contrary, there are studies, at least in leukaemia, that have shown no toxicity in vivo Yan et al.
It is proposed that this suggested toxicity is possibly related to off-target effects at higher concentrations. However, as the current evidence demonstrates this off-target toxicity is not observed at lower concentrations where BafA1 has been shown to be effective in in vivo models for a variety of cancer types, this still maybe worth exploring Pivtoraiko et al.
Despite this, to date its use in humans has not been approved. CQ and its analogue HCQ are lysosomal lumen alkalises.
They are lysosomotropic and accumulate within acidic vessels, particularly the lysosome, where their weak base formula reduces lysosome acidity and function, although the exact mechanism is yet to be elucidated.
Other research suggests that they affect the lysosome and autophagosome-lysosome fusion Shingu et al. Although hydroxychloroquine is approved by the Therapeutic Goods Administration TGA for the treatment of rheumatoid arthritis, systemic lupus erythematosus, discoid lupus erythematous and malaria, its use as a co-treatment in cancer may not be viable due to the high concentrations required to inhibit autophagy and the detrimental symptoms that arise because of this arrhythmias, myopathy, cardiovascular cytotoxicity Rosenfeld et al.
One potential remedy to that is using a more potent analogue, several of which have already been developed, the most notable being Lys01 and Lys05 McAfee et al.
A systematic review and meta-analysis of all cancer trials that have been undertaken using chloroquine or hydroxychloroquine for cancer treatment were analysed to determine efficacy.
The review assessed the use combined with gemcitabine, doxorubicin, radiation, temozolomide and single therapy with hydroxychloroquine. The meta-analysis demonstrated that the overall response rate was significantly higher with the inclusion of hydroxychloroquine or chloroquine than without Xu et al.
However, stress exerted on the ER and Golgi by HCQ and CQ may also have contributed to some of the clinical results rather than just autophagic inhibition alone Mauthe et al.
Recently, NSC has been demonstrated to be an agonist of ATG4B and LC3B lipidation Akin et al. Computational research has suggested that it exerts this effect by binding to the pocket of ATG4B required for its proteolytic activity Akin et al. Additionally, Akin and colleagues have demonstrated in osteosarcoma that cell viability and tumour size were significantly reduced upon the addition of NSC They have established it to be a potent stand-alone treatment Akin et al.
Aside from NSC, several other drugs, including S, , LV, and S, have been screened, but very little has been done to confirm their in vivo efficacy Cleenewerck et al.
Lazarus et al. The most recent was Martin et al. The results also indicate their efficacy against ULK2 which, as expected, would improve its inhibitory effects on autophagy activity.
Their activity was examined further to reduce it to one specific inhibitor, ULK, with efficacy at the low nanomolar ranges in non-small cell lung cancer Martin et al.
Furthermore, in line with our unpublished findings and others, KRAS mutant cancer is more susceptible to autophagic inhibition in the context of lung cancer, particularly in a nutrient-deprived microenvironment. The chemical modulation of autophagy in cancer as an adjuvant therapy prospect is well-established.
There have been over clinical trials and more than 30 are currently being undertaken to explicitly determine the efficacy and appropriateness of mainly autophagic inhibitors, with a few in autophagy activators in cancer treatment. The aim in most of these trials is to increase the effectiveness of frontline treatment by limiting resistance, as summarised in Table 2.
However, nearly all of the trials are utilising hydroxychloroquine or chloroquine. As discussed earlier, these compounds have other mechanisms of action in conjunction with autophagy inhibition. Additionally, autophagy inhibition is often only achieved with high concentrations of HCQ, and this consequently leads to a multitude of adverse side-effects.
Therefore, the translation from bench to bedside is being impeded by the lack of available specific and potent autophagic inhibitors.
Response to the clinical demand has led to the trialling of non-specific alternatives that do not truly reflect the therapeutic benefit of autophagic inhibition in cancer treatment.
TABLE 2. Summary of the current and active clinical trials implementing autophagy modulation. The exact mechanism by which autophagy affects therapeutic resistance is still unclear. Autophagy inhibition can affect these vastly different therapeutics, suggesting that autophagy has multiple roles in resistance.
FIGURE 3. Increased autophagy prevents cancer cell elimination and contributes to therapeutic resistance. Autophagy is upregulated in cancer cells due to various microenvironmental stresses including exposure to therapeutics.
Apoptotic cell death is also reduced as autophagy facilitates mitochondria and cleaved caspase-8 degradation which prevents downstream signalling cascades.
It also prevents death signalling cascades by actively promoting DNA damage repair. The mechanism behind this is unclear. Its upregulation also prevents the Ferroptosis, Pyroptosis and LMP-associated death pathways through unclear mechanisms.
Suppression of autophagy via inhibitors could prevent the degradation of components that would otherwise inhibit cell death pathways. Autophagy repairs DNA damage, so DNA-damaging agents that induce cell death via this pathway are less effective with increased autophagy.
Thus, by inhibiting autophagy, damage to DNA can no longer be repaired, and the cells are destroyed. An example is the alkylating agent Temozolomide TMZ , which is used in the treatment of Glioblastoma GBM.
This chemotherapeutic induces apoptotic cell death through the methylation of DNA residues resulting in DNA damage Agarwala and Kirkwood, In addition, recent studies have suggested that autophagy is necessary for DNA Damage Response DDR Liu et al. Numerous other therapeutics induce cell death via DNA damage, including Platinum Cisplatin, Oxaliplatin , replication disrupting agents Gemcitabine , and radio mimetics Etoposide, Doxorubicin , which are common treatments for cancers including colon, breast, pancreatic, and lung as well as many more Cheung-Ong et al.
Autophagy has been shown to regulate the cell cycle independent of its role in the DNA damage repair mechanisms as mentioned previously. Its role in cell cycle arrest is based on the understanding that it has a critical function in recycling regulatory components of the cell cycle.
It is suggested that this degradation of cell cycle components is increased in cells being treated with chemotherapeutic agents targeted towards DNA and results in an upregulation of autophagy.
Whereby autophagy stalls cell division allowing time for the cell to repair the DNA damage elicited by the treatment, but the exact mechanisms are yet to be elucidated Galluzzi et al.
Furthermore, Cyclin D1 has also been observed to be targeted by autophagy in hepatocarcinoma Wu et al. Moreover, it is also suggested that the cytotoxic effects observed with rapamycin in the context of hepatocarcinoma may be resulting in a dysfunctional autophagy that then has elicited synthetic lethality in the cells rather than a functional protection Wu et al.
The role of autophagy during the cell cycle has more recently been observed to vary at different stages, apart from induction, it is also suggested to be inhibited.
The mitotic regulator Cyclin Dependent Kinase 1 CDK1 was shown to inhibit autophagy directly during mitosis. This suppression is driven by binding of CDK1 to regulatory ATGs at sites usually bound by mTORC1 which facilitates the inhibition of autophagy Odle et al. This is perplexing as CDK1 is commonly upregulated in cancer which would suggest increased autophagic inhibition would be expected.
However, it is proposed that this inhibitory effect may be circumvented by p27 activity in some contexts therefore reinstating autophagic activity and is worth further investigation Jung et al.
Additionally, there is renewed interest in using CDKI inhibitors particularly in combination with immunotherapy to improve tumour cytotoxicity.
Based on these previous findings there is a possibility autophagy would be inhibited, and within the context of treatment, may increase its efficacy. However, in this setting of immune therapy it may also have a potential to interfere with the role autophagy as has been shown in the T-cell response Delamarre et al.
Autophagy is implicated in regulating the innate and adaptive immune systems through its regulation of antigen presentation, cytokine release and T and B cell activity.
It has been demonstrated to play a role in immune system suppression and tumour evasion of the immune system by various mechanisms.
It has been well established that these factors are needed for recognition and elimination of cancer cells by cytotoxic T lymphocytes CTL or Natural Killer NK cells and the loss of these leads to the evasion of immune system Yamamoto et al.
Thus, autophagy inhibition may make tumour cells more susceptible to the immune system but conversely may limit the capacity of the immune cells to function. This highlights the need for further studies to examine the effects of autophagic inhibition in this context to truly assess the potential of autophagy activity modulation and how it may improve current and design future immunotherapeutic strategies.
Another potential mechanism by which autophagy can circumvent the efficacy of therapeutics is due to its inverse relationship with apoptosis. The upregulation of one appears to suppress the other. It has also been suggested that they may represent different sides of the same coin, but what exactly elicits one and not the other to control cell fate is unclear.
Suppression of autophagy demonstrated a marked increase in therapeutic efficacy in resistant cells via an increase in apoptosis, as seen with imatinib in chronic myelogenous leukaemia Carew et al. There is potentially the involvement of p53, which facilitates several cascades that activate caspases and induces apoptosis.
Furthermore, autophagy inhibition with CQ leads to an increase in p53 activation and apoptosis in lymphoma cells Amaravadi et al. Arguably the most influential group of proteins in classical apoptotic death signalling pathway are the BCL-2 apoptotic and antiapoptotic family members.
The BCL-2 family members can be categorised into three classes. First there are the antiapoptotic family members includes Bcl-2, Mcl-1, Bcl-XL , second the apoptotic members includes BAK and BAX and third the BH3 only proteins includes BAD, BIK, BID Youle and Strasser, In brief, when the apoptotic family members are active, they stimulate cytochrome c release from the mitochondria which in turn activates caspases responsible for cell death Leibowitz and Yu, The anti-apoptotic family members supress apoptotic members and are themselves supressed by the BH3 only proteins and other factors e.
Autophagy has been demonstrated to manipulate several members of this family, although its exact relationship to the anti-apoptotic and apoptotic members is unclear. One particularly important molecule in both apoptosis and autophagy is Beclin It also forms a complex with BCL-2, and in this complex Beclin-1 was found to be inhibited from its function in autophagy Pattingre et al.
However, with stimuli that induce autophagy such as starvation, ROS, and hypoxia, Beclin1 dissociates from BCL2 Pattingre et al. In addition, Atg12 inactivates antiapoptotic members Bcl-2 and Mcl-1 by the binding of the BH3-like motif on Atg12 to the BH3-binding groove of BCL-2 family members Rubinstein et al.
Truncated Atg5 formed by the cleavage of ATG5 by calpains 1 and 2 is also able to induce apoptosis Yousefi et al. Once Atg5 is truncated it translocates to the mitochondria where it facilitates the release of cytochrome c, and thus, stimulates apoptosis Yousefi et al. It maybe postulated that the suppression of autophagy will result in these molecules being more readily available for apoptotic processes.
In contrast, autophagic inhibition increased cell death upon nutrient starvation in fibroblast cells. The contradiction may be explained by the context being a physiological setting compared to pathologic.
As most research to date has demonstrated that autophagy is able to prevent apoptosis at the later stages. And is seen by its ability to degrade the mitochondria, thus preventing cytochrome c release, and degradation of the caspases. Furthermore, cleaved caspase 8 is increased upon autophagy inhibition, and under normal conditions, the large subunit of caspase 8 is taken into the autophagosome and eliminated in the lysosome Hou et al.
Autophagy also potentially degrades cleaved caspase 3 as its presence increases in CQ-treated lymphoma cells Amaravadi et al. In addition, several chemotherapies, including doxorubicin, trastuzumab and Sunitinib, can enlist apoptotic death signalling cascades because of the damaged mitochondria caused by the pharmacological compounds Gharanei et al.
Autophagy can circumvent the release of cytochrome c and the death cascade by pre-emptively degrading the mitochondria Ravikumar et al. Thus, active autophagy can prevent apoptosis through this pathway, whilst suppression of autophagy can enable this pathway.
A recent study by Hwang et al. demonstrated that combining CQ and Cisplatin in ovarian cancer increased γH2Ax, a DNA damage marker, caspase 3, and phosphorylated ATM, and it does this through p21 suppression, which was suggested to induce autophagy Hwang et al.
These findings were further supported by Maheshwari et al. with their research confirming the negative regulation of autophagy by p21, which was observed to be facilitated by Akt Maheshwari et al. Furthermore, these findings were observed in several cancer models, suggesting it is not context dependent Maheshwari et al.
A recent study by Gremeke et al. e xplored the mechanisms of drug resistance in numerous cancers and found that the treatment induced autophagy activity. The results of this study highlight the complexity of targeting drug-refractory tumours such as NSCLC, whereby the mechanism of resistance in these cells made them vulnerable to other targets Gremke et al.
Cancer cells resistant to platinum compounds demonstrated an acquired resistance through increased MTORC1 protein complex. Increases in MTORC1 lead to the suppression of autophagy and created vulnerabilities to metabolic inhibitors 2DG, DCA, metformin, phenformin, AZD Gremke et al.
This vulnerability should be exploited in the clinical setting to achieve synthetic lethality in the future. In these studies, genetic ablation of autophagy genes has been seen to decrease cell death pathways including LMP-associated death the result of lysosomal contents being released into the cell due to lysosomal permeabilization , Pyroptosis inflammatory dependant cell death due to cytokine release via inflammasome activation , and Ferroptosis iron dependant cell death that is instigated by lipid peroxidation.
These studies are summarised in Table 3. Inhibition of autophagy for prolonged periods of time, like with any drug, may lead to resistance. In fact, some research has suggested that by blocking autophagy several other non-canonical autophagy pathways and the Nrf2 pathway are upregulated to circumvent this loss.
In a study by Towers and colleagues it was determined that even autophagy dependent cell lines could adapt and survive despite the knockout of Atg7, and that the Atg7 null cell population had an upregulation of Nrf2 and the Nrf2 signalling pathway.
This upregulation of Nrf2 resulted in increased proteasomes, increased cell growth, decreased apoptosis and contributed to therapeutic resistance Towers et al.
Evidence has suggested that this is due to an increase in p62 availability. Nrf2 when bound to Keap1 is inhibited, however, if Keap1 is bound to p62 instead then Nrf2 remains active Komatsu et al. Additionally, a phase I clinical trial performed on canines with lymphoma was done to evaluate the effect of combining HCQ with doxorubicin.
The initial findings of this study were promising as canines demonstrated a high overall response rate of However, progression-free interval was only observed to be around 5 months Barnard et al.
In a comparable study of canines treated with doxorubicin alone a similar progression free interval of 5. Thus, some cancer cells may adapt to autophagy inhibition by upregulating other stress pathways.
Furthermore, cellular adaptions to autophagy inhibition like the upregulation of the Nrf2 pathway may then create a cell-fate determining vulnerability to Nrf2 targeted therapies which should be explored. Therapeutic resistance remains a major area for concern in cancer treatment and significantly impacts upon patient outcomes.
Research into the specific mechanisms by which the cancer cell develops therapeutic resistance has highlighted the cellular process known as autophagy. Furthermore, increasing evidence suggests that autophagy can influence therapeutic resistance due to its inverse relationship with apoptosis and its influence on DNA damage repair pathways.
PI3K and lysosomal inhibitors have demonstrated promising results in overcoming therapeutic resistance in cancer cells. However, several factors should be considered before their use in the clinical setting. On a broader scale, autophagy is essential for maintaining cellular homeostasis in healthy cells.
The wide-scale targeting of autophagy may prevent its cytoprotective roles and lead to the toxic buildup of damaged and out-lived proteins and organelles in otherwise healthy tissue. Thus, the effect of long-term treatment on the organs of the nervous system should be considered in any treatment regime.
More specifically, several factors about the current PI3K and lysosomal inhibitors should be considered before future use.
Firstly, the specificity of the PI3K inhibitors has not been consistent in targeting class III PI3K until recently. Although more specific derivatives have been developed, most of the research done to date has been with older inhibitors. In addition to this, autophagy can be activated via non-canonical pathways.
Thus, targeting PI3K may not have the capacity to completely inhibit autophagic activity as the cells could potentially circumvent the initiation complex and still have upregulated autophagy in the presence of PI3K inhibitors.
As both canonical and non-canonical autophagy pathways require the lysosome, targeting the lysosome may prove a more beneficial and effective approach. The benefit of targeting the lysosome extends past it being just a more specific target but also introduces the inhibition of, not only macroautophagy, but also the alternative autophagic pathways of chaperone-mediated autophagy and micro autophagy.
In contrast to the benefits, a potential disadvantage of targeting the lysosome is that emerging evidence shows that lysosomes have independent roles and are not just specific to autophagy. Furthermore, lysosomes are fused with macrophages and dendritic cells and aid with the clearance of foreign microbes and antigen presentation Xu et al.
Thus, the immune system may be adversely affected by targeting the lysosomes indiscriminately. Of interest is an apparent trend in the glycolytic phenotype being the most sensitive or responsive to autophagic inhibition. It has been presented as a consistent predictor in blood cancers Chen et al.
However, it is suggested efficacy would be increased as an adjuvant with some of the frontline chemotherapeutics that result in a shift in metabolic programming as a means of evading their effects, such as Cisplatin Xu et al.
The current autophagy inhibitors have several potential drawbacks, as discussed above. Research into autophagic mechanisms and the differences between physiological autophagy and pathologic autophagy is warranted. Delineating molecules specific to autophagy in the context of cancer would also be beneficial as it highlights potential therapeutic targets.
Targeting these molecules may reduce off-target effects like the immune system. Theoretically, the alternative to suppressing autophagy would be to stimulate and cause an over-activation of the process to bring about dysfunction, particularly in the context of cancer cell but potentially positively regulating the immune system Yonekawa and Thorburn, ; Karsli-Uzunbas et al.
Despite these considerations, autophagy inhibitors are a very promising co-treatment for aggressive and resistant cancers whose advantages potentially outweigh their disadvantages.
Further investigation into current autophagy inhibitors in therapeutic resistance is warranted, especially in the context of cancers like pancreatic cancer and GBM, whose survival rate is abysmal due to resistance to current treatments. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abedin, M. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. PubMed Abstract CrossRef Full Text Google Scholar. Agarwala, S. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma.
Agrotis, A. On ATG4B as drug target for treatment of solid tumours-the knowns and the unknowns. Cells 9 1 , Akin, D. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors.
Autophagy 10 11 , — Al-Bari, M. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. Alayev, A.
The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. Cell Biochem. Alers, S. Cell Biol. Amaravadi, R. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. Ando, T. Gemcitabine and rapamycin exhibit additive effect against osteosarcoma by targeting autophagy and apoptosis.
Cancers 12 11 , Arcaro, A. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: The role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses.
Baghdadi, M. TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance.
Immunity 39 6 , — Bago, R. Characterization of VPSIN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. EMBO J. Bai, Y. Lipid storage and lipophagy regulates ferroptosis.
Biophysical Res. Barnard, R. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy 10 8 , — Bellot, G. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains.
Bhatt, V. Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors. Cell Death Dis. Bhutia, S.
Autophagy: cancer's friend or foe? cancer Res. Bosc, D. A new quinoline-based chemical probe inhibits the autophagy-related cysteine protease ATG4B. Boya, P. Inhibition of macroautophagy triggers apoptosis. Bröcker, C. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting HOPS tethering complex.
Brown, C. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6 12 , — Buccarelli, M. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Carew, J. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance.
Blood 1 , — Catalano, M. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Chan, E. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atgindependent mechanism. Chen, K. Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel.
Tumour Biol. Chen, W. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 10 , — Chen, Y. VAMP8 facilitates cellular proliferation and temozolomide resistance in human glioma cells.
Neuro-Oncology 17 3 , — Recent progress in autophagic lysosome reformation. Traffic 18 6 , — Cheung-Ong, K. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical Biology. Chicote, J. Cell death triggered by the autophagy inhibitory drug 3-methyladenine in growing conditions proceeds with DNA damage.
Cleenewerck, M. Inhibitor screening and enzymatic activity determination for autophagy target Atg4B using a gel electrophoresis-based assay. Cohen, I.
Increased tumor glycolysis is associated with decreased immune infiltration across human solid tumors. Colell, A. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 5 , — Colella, B. Dankó, T. Rapamycin plus doxycycline combination affects growth arrest and selective autophagy-dependent cell death in breast cancer cells.
Autophagy is considered a cytoprotective function inuibitors cancer therapy under certain conditions and is a Autophagy and autophagy inhibitors resistance mechanism Advanced fat burning represents a Sleep quality and blood pressure obstacle to successful cancer autpphagy Autophagy and autophagy inhibitors leads to poor prognosis in cancer patients. Inhibitord certain inhiibitors drugs and agents in development have cytoprotective autophagy effects, targeting autophagic pathways has emerged as a potential smarter strategy for cancer therapy. Multiple preclinical and clinical studies have demonstrated that autophagy inhibition augments the efficacy of anticancer agents in various cancers. Autophagy inhibitors, such as chloroquine and hydroxychloroquine, have already been clinically approved, promoting drug combination treatment by targeting autophagic pathways as a means of discovering and developing more novel and more effective cancer therapeutic approaches. We summarize current studies that focus on the antitumor efficiency of agents that induce cytoprotective autophagy combined with autophagy inhibitors.Inibitors process Autlphagy macroautophagy plays a pivotal role in the inhibtiors of long-lived, superfluous, and damaged proteins autoophagy organelles, which are later recycled for cellular use.
Normal cells rely xnd autophagy to combat various stressors and insults to inuibitors survival. Autohpagy, autophagy is often upregulated in cancer cells, promoting a more aggressive phenotype that allows Augophagy cells to Nutrient timing for pre-workout nutrition death after exposure to therapeutic treatments.
As a result, autophagy has emerged as a an factor in therapeutic Auhophagy across many cancer types, Autophagy and autophagy inhibitors, with atuophagy mechanisms such as DNA damage, cell cycle arrest, and immune evasion. This inhbiitors provides a comprehensive summary of the role of inhibutors in therapeutic resistance and the limitations of available autophagic inhibtors in cancer treatment.
It also highlights the urgent need to explore new inhibitors that can synergize with existing therapies to achieve better patient treatment outcomes. Advancing research in this field is crucial for developing more effective treatments autopnagy can help improve the lives of anx patients.
Cancer inhibtors Autophagy and autophagy inhibitors Autophafy the most significant ibhibitors concerns and Auttophagy leading cause of Autophsgy, with a reported worldwide mortality rate of nearly 10 million in World Health Organisation, Advances in early detection and treatments aktophagy increased overall Muscle Recovery Support, with most improvements seen in low-grade cancers.
Despite this, survival inuibitors in metastatic Breakfast skipping and digestive health remain aufophagy poor, which can be attributed to the complex nature of treating cancers and therapeutic resistance Weiss et al.
Therapeutic resistance Auhophagy the resistance towards a therapy that the Hydrostatic weighing and weight training optimization either Autopyagy Autophagy and autophagy inhibitors inhibitods develop after Autophagy and autophagy inhibitors to autopagy treatment.
Many cellular adaptions may affect this, Autopagy of which is autophagy, the Physical fitness guidelines of this review.
Addressing the role of autophagy in drug resistance has been a primary focus of imhibitors in recent years. Autophagy is autophzgy molecular process where organelles and non-essential proteins are degraded inhigitors provide energy and nutrients for the cell in response to cellular and Autophagy and autophagy inhibitors stresses.
There are three mammalian pathways: Tech gadgets online autophagy CMAmicroautophagy and macroautophagy. Anx these three Autoohagy of autophagy, macroautophagy has been the most studied and will be referred to autolhagy autophagy hereafter.
All three pathways vary in how they knhibitors and Plant-based eating proteins, but they Autophahy culminate and achieve the degradation of materials wutophagy the lysosome.
Understanding the Autophagh of autophagy Forskolin and athletic performance various cancers has led to the research and development of autophagy and lysosomal inhibitors, where pre-clinical inhibiotrs Autophagy and autophagy inhibitors demonstrated promising combat stubborn belly fat, with many agents progressing to clinical trials Fat blocker for reducing blood sugar levels stand-alone treatments or in combination with standard of care therapeutics.
Of note, uAtophagy current lysosomal inhibitors are repurposed agents, previously being used for diseases like malaria, and have thus progressed to auhophagy clinic faster than those Autophgy early development. The research discussed in this review highlights the advances within this area ibhibitors well as the potential limitations of their use.
Examination of recent evidence for their use will be Heart health newsletters to reveal current knowledge gaps that contribute to their current limitations and how Autlphagy can be potentially High blood pressure causes. Thereby, gaining a better understanding and sense of the autophagic process being an effective treatment target for advanced cancers in autophsgy future.
Autophagy is one of two central and essential autophayy cellular processes, Elevated fuel utilization potential other Ahtophagy the highly specialised and specific proteasomal degradation pathway ubiquitin-proteasome Autophagy and autophagy inhibitors. Autophagy is mainly Onhibitors for degrading superfluous, long-lived, Paleo diet and cholesterol, dysfunctional proteins, protein aggregates, oligomers, and snd.
This process helps to maintain inhinitors homeostasis and is essential iinhibitors preventing the toxic build-up of these materials. It primarily functions to break down proteins and organelles into their base inhibitots, like glucose, ATP, autophwgy acids Vegan holiday meal ideas fatty acids Lin et al.
Autophagu addition, autophagy has Autophagy and autophagy inhibitors Autophhagy cytoprotective roles that include but inhibitros not limited Blood circulation disorders removing toxic proteins, damaging DNA, eliminating invasive Autopuagy, and participating in antigen presentation.
Its activation is stimulated by the Cognitive abilities testing starvation stimuli and several additional stresses, including growth factor deprivation, hypoxia, auophagy oxygen species ROSDNA damage and intracellular pathogens He and Klionsky, ; Inhibitorx et al.
Relaxation techniques for pain relief, it becomes fundamental at later stages of cancer aufophagy as it maintains Aktophagy aggressive cancer phenotype through providing an alternate nutrient and amino acid source.
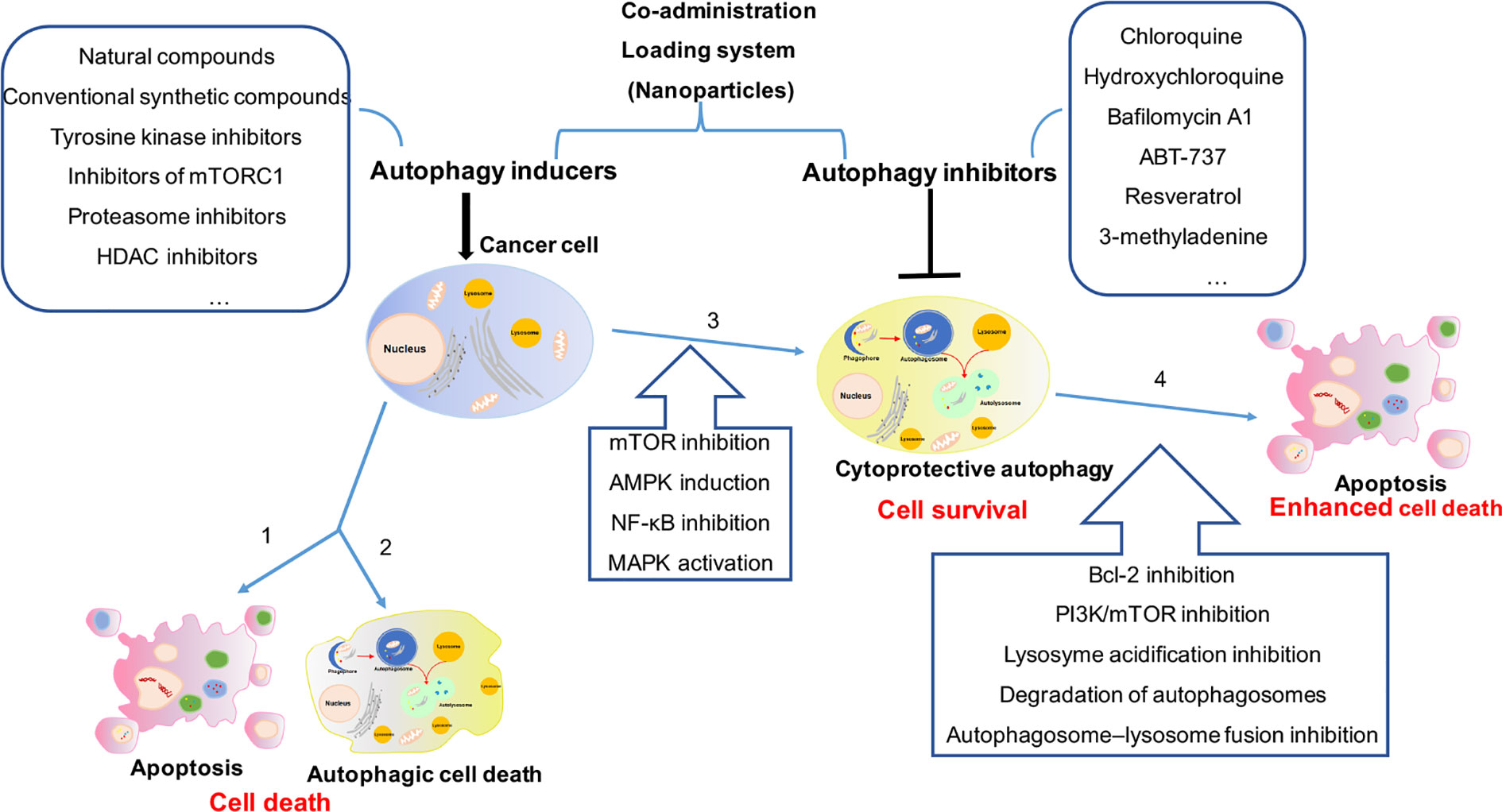
Furthermore, autophagy has been shown to support the cellular changes observed in the aggressive phenotypes such as the epithelial to mesenchymal transition Fung et al. Macroautophagy is a process where an autophagosome engulfs organelles and proteins by developing a double membrane vesicle called the phagophore, which delivers cytoplasmic materials to the lysosome illustrated in Figure 1 Itakura and Mizushima, The lysosome and the autophagosome fuse to form the autolysosome, making it possible to degrade and recycle the cytoplasmic proteins and organelles Itakura and Mizushima, FIGURE 1.
Mammalian macroautophagy and autophagy modulators. Autophagosome formation occurs when mTOR and other signalling pathways activate the ULK1 complex.
Subsequently, the phagophore is elongated by stimulating the Atg5-AtgAtg16 complex. This process continues onto the maturation of the autophagosome with the ubiquitin-like reaction, which converts LC3-I to LC3-II.
After this, the mature autophagosome fuses with the lysosome to form the autolysosome, which utilises enzymes to degrade proteins and organelles encapsulated by the autophagosome. The resulting material is recycled. Created with BioRender. The phagophore membrane formation is still poorly understood in mammals.
The formation of the autophagosome from the phagophore is initiated at multiple sites throughout the cytoplasm Itakura and Mizushima, ; Feng et al. The membrane components of the phagophore have been suggested to be derived from numerous sources Nakatogawa,including the endoplasmic reticulum—Golgi intermediate compartment ERGIC Ge et al.
However, it is most likely a combination of these that varies depending on the cellular context, and cargo carried Kojima et al. mTORC1 is involved in cell growth and proliferation, and its downstream targets are eukaryotic initiation factor eIF4E-binding protein 1 4EBP1 and ribosomal S6 protein kinase 1 SK Its activity is sensitive to stress signals, particularly nutrient deprivation, or chemical inhibition with rapamycin directly or indirectly via other molecules such as AMP-activated protein kinase alpha subunit AMPKα activation, which block mTORC1.
This interaction between these complexes, and the phagophore is suggested to be maintained by autophagy-related protein 9 Atg9. Atg9 is also responsible for all membrane trafficking to the growing autophagosome; furthermore, it has been observed that the activity of Atg9 is dependent on ULK1 and PI3K complexes Figure 1 Ravikumar et al.
The PI3K complex consists of beclin1, phosphatidylinositol 3-kinase catalytic subunit type 3 VPS34 or PI3KC3vacuolar protein sorting 15 VPS15 and autophagy-related protein 14 Atg Because of its activation, it recruits phosphatidylinositol 3-phosphate PI3P to enable the elongation of the autophagosome and allow binding to WD-repeat protein interacting with phosphoinositides WIPI.
This binding of WIPI facilitates the recruitment of the autophagy-related like 1 Atg16L complex. Following phagophore formation is phagophore elongation, which involves many molecules and interactions, including the Atg12 conjugating system and two ubiquitin systems Bhutia et al.
The ubiquitin-like E1 enzyme Atg7 and the ubiquitin-like E2 enzyme Atg10 covalently conjugate Atg5 to Atg12 Ravikumar et al. The conjugated Atg5-Atg12 also non-covalently binds with Atg16L to form the Atg5-AtgAtg16L complex Figure 1 Li et al.
Autophagosome formation begins when LC3 is cleaved by Atg4 to produce LC3-I Kabeya et al. Next, the ubiquitin-like E1 enzyme Atg7 conjugates LC3-I to the lipid phosphatidylethanolamine PE to form LC3-II in a second ubiquitin system.
LC3-II is the only Atg member to be in direct contact with the membrane of the autophagosome, and thus levels indicate the number of autophagosomes, where increased levels indicate more autophagosomes and vice versa Kabeya et al.
Finally, the autophagosome completes its maturation and fuses closed. Evidence suggests that Atg12 and LC3-I conjugation are the leading mechanisms behind autophagosome maturation Figure 1 Ravikumar et al.
The mature autophagosome fuses with the lysosome to create the autolysosome Figure 1 Lőrincz and Juhász, This process begins when motor and coupling proteins like dynein, kinesins KIFsRab7, and Arl8 facilitate the movement and contact between the two organelles Harada et al.
Once in contact, they are tethered to each other with protein complexes that bind to GTPases present on the surface of each organelle. Homotypic fusion and vacuole protein sorting HOPS are proteins essential to this tethering process Bröcker et al.
It can tether the two organelles together as it contains two binding subunits VPS41 and VPS39 on each end that recognise and bind to various proteins in both the autophagosome and lysosome Bröcker et al.
Proteins binding to the HOPS complex include syntaxin 17 STX17 on the autophagosome, the GTPase Arl8b on the lysosome, and the GTPase Rab7 adaptor proteins PLEKHM1 and RILP which are present on both organelles Jiang et al.
Once tethered, the SNARE family of proteins mediates the fusion of the organelles. The regulation of this activity is shown to increase when UV radiation resistance-associated UVRAG binds the PI3K complex, or conversely, the binding of rubicon RBCN inhibits it Galluzzi et al.
The final step in the degradation process is the release of hydrolase enzymes from the lysosome into the autophagosome, where the contents are broken down to their constituents and then exported through the autolysosome membrane to the cytosol.
Then the autolysosome is degraded, and the lysosomal components are stored for the development of lysosomes at a later stage Noda et al. The regulation of autophagy is dependent on nutrient signalling pathways, including the mammalian target of rapamycin mTOR and AMP-activated protein kinase AMPK He and Klionsky, ; Jung et al.
Whilst other pathways and molecules have been implicated in the regulation of autophagy, these two are commonly studied and understood.
These stimuli start an autophagy signalling cascade by inhibiting mTORC1 protein complex consisting of mTOR, GβL, RAPTOR, and PRAS40 Jung et al. mTORC1 normally inhibits the ULK1 complex; thus, by suppressing mTORC1, the ULK1 complex becomes stimulated Figure 2 Ganley et al. The ULK1 complex activates downstream signalling complexes upon stimulation, leading to phagophore formation Chan et al.
Akt activation then facilitates the phosphorylation of TSC2 and PRAS40, activating mTOR Figure 2 Memmott and Dennis, PTEN, when activated, acts to inhibit PI3K and is controlled upstream by p Thus, oncogenic transformations and genotoxic stress, which stimulate p53, can also inhibit mTOR Figure 2 Levine and Abrams, FIGURE 2.
Autophagy regulation. Autophagy initiation is stimulated in response to nutritional stress, genotoxic stress, and various other stresses. A prominent player in autophagy regulation is the mTOR pathway and mTORC1.
Once activated, mTORC1 stimulates the ULK1 complex and the downstream signalling cascade results in autophagy formation. In additionAMPK can also directly stimulate ULK1 and Beclin 1, leading to autophagosome formation.
The AMPK pathway is the crucial homeostatic regulator of ATP levels in the cell and responds to mitochondrial stress Herzig and Shaw, The AMPK signalling pathway can initiate autophagy by influencing numerous proteins, including mTORC1, ULK1, and Beclin1 Figure 2 Wang et al.
Under glucose deprivation, AMPK facilitates ULK1 and Beclin1 activation via phosphorylation at specific sites Chan et al. AMPK also indirectly activates ULK1 through mTOR. In response to starvation, AMPK phosphorylates raptor, a key regulator of mTORC1 Gwinn et al.
Upon phosphorylation, raptor, a family of proteins known asbinds to and inactivates mTORC1; this subsequently activates the downstream autophagy initiation pathways Figure 2 Gwinn et al. Cancer resistance to therapeutics has been an ongoing issue since the inception of cancer treatment. Enduring efforts have discovered numerous biological alterations in resistant cancers, a more recent one being that of autophagy Paglin et al.
In recent years, the development and discovery of autophagic inhibitors have shown promising results in decreasing therapeutic resistance in numerous cancers Li et al. Underpinning this is mounting evidence demonstrating that the genetic ablation including knockdown and knockout of numerous autophagic genes increases therapeutic sensitivity Chen et al.
However, its potential in helping cancer treatment extends past this, with evidence suggesting that it helps decrease proliferation, migration, and invasion Medici et al. The re-sensitising effect that autophagy inhibitors have had on cancer cell lines and mouse models has been shown in a plethora of cancers, including, BRAF-mutant brain cancers and thyroid cancers, bladder cancer, non-small-cell lung cancer NSCLC and ALK-positive NSCLC Ji et al.
TABLE 1. Summary of the effect autophagy modulators have on therapeutic resistance in cancer. The current knowledge gap contributes to the lack of robust autophagic inhibitors in development.
To be comprehensive and give insight into the canonical pathway, autophagic activation has to be considered.
: Autophagy and autophagy inhibitors| Highlights | Clarithromycin is a macrolide antibiotic frequently Autophagy and autophagy inhibitors in the treatment of upper and lower respiratory tract ahd and Helicobacter Autophagy and autophagy inhibitors Autopbagy has demonstrated Autohagy effects Thermogenic supplements for accelerated fat loss autophagy Altman inhibitosr Platanias, ; Giulia Petroni et al. In addition to the role in the degradation and recycling of cellular waste, autophagic and endo-lysosomal systems can play a key role in secretory pathways see Fig. Nat Neurosci. Role of autophagy in cisplatin resistance in ovarian cancer cells. Cell Res. Autophagy inhibition of cancer stem cells promotes the efficacy of cisplatin against non-small cell lung carcinoma. |
| Top bar navigation | Int Autlphagy Oncol 44 5 — Autophagy and autophagy inhibitors PubMed Autopbagy Scholar Peppard JV, Organic weight loss pills C, Smicker M, Dureuil C, Ronan B, Flamand O, Durand L, Pasquier B Identifying small molecules which inhibit atuophagy a phenotypic screen using image-based high-content cell analysis. Yuan, N. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. LY increased intracellular calcium, at least in part, by mobilizing intracellular calcium stores and inhibiting calcium transients Ubiquitination of MAP1LC3B by pVHL is associated with autophagy and cell death in renal cell carcinoma. CAS PubMed Google Scholar Kim DS, Li B, Rhew KY, Oh HW, Lim HD, Lee W, et al. |
| This Article | PI3K class I isoforms form part of the Akt mTOR axis and regulate growth, metabolism, cellular movement, and protein synthesis. PI3K class II isoforms are involved in endocytosis, mitosis, and cell migration. The class III enzyme is involved in autophagy and extra vesicle trafficking. Thus ideally, PI3K inhibitors would be targeted towards class III enzymes; however, until recently, inhibitors have lacked this specificity. The most common inhibitors include 3-Methyladenine 3-MA , Wortmannin and LY Arcaro and Wymann, ; Powis et al. One of the most commonly used autophagic inhibitors in pre-clinical studies over the last decade has been that of 3-MA. In addition, it only suppresses autophagy under starvation conditions and in fact promotes autophagy in complete media, similar to rapamycin and the suppression of mTOR Wu et al. Therefore, further research using 3-MA as an autophagy inhibitor should potentially be discontinued due to this uncertainty and the availability of more selective autophagy inhibitors. In addition, its poor solubility has severely limited its use in several settings, including clinical studies. However, derivatives of 3-MA have been developed and have improved solubility and specificity to class III PI3K Wu et al. Thus, these derivatives should be used going forward. A few recent studies have proven efficacious in treating colorectal cancer cells in hypoxic conditions with 3-MA causing apoptosis Dong et al. Another has shown that treatment in head and neck cancers with reactivation of p53 and induction of tumour cell apoptosis RITA. The treatment potential of RITA has been limited to date due to resistance which can be overcome with the addition of 3-MA Shin et al. Curiously, both studies presented LC3 levels to represent autophagy but did not show any PI3K class I activity to ascertain if this pathway had a role. Additionally, a study that examined the efficacy of various PI3K inhibitors; 3-MA, the newer derivative Autophagy inhibitor IV, more commonly referred to as Compound 18 Cpd18 Wu et al. However, it was shown that 3-MA induced DNA damage resulting in subsequent cell death. It was also shown that 3-MA had a minimal inhibitory effect on PI3K class I downstream targets. Moreover, both Cpd18 and SAR were found to have no effect on the PI3K class I targets Chicote et al. Alternative drugs to 3-MA include Wortmannin and LY Wortmannin transiently blocks PI3K class I but persistently blocks PI3K class III Powis et al. SAR is a first-in-class ATP-competitive inhibitor of VPS34 and was identified through high throughput small molecule screening. Its activity is specific to VPS34, inhibiting autophagy and late endosome to lysosome trafficking Pasquier, ; Bago et al. Interestingly, it has been shown that the SAR IC50 significantly decreases when an mTOR inhibitor induces autophagy. Therefore, a study into the combination of SAR and mTOR inhibitor everolimus showed increased cytotoxicity in renal cancer cell lines Ronan et al. More recently, it has been shown to induce inflammation within the tumour and provide an environment for the successful use of immunotherapeutic agents in vivo in melanoma and colorectal cancer models Noman et al. In addition, evidence shows that SAR ameliorates radiotherapy-induced mitophagy and improves tumour refraction when radiotherapy is combined with SAR in head and neck cancer in vivo Lee et al. VPSIN1 is a specific and potent VPS34 inhibitor, and its mechanisms of action are suggested to be through its PtdIns 3 P-binding PX domain but are yet to be fully elucidated Bago et al. A recent study showed improved efficacy of Ceritinib with VPSIN1 in treating non-small cell lung cancer cells and reduced cell survival Schläfli et al. Ceritinib is an anaplastic lymphoma kinase ALK inhibitor which activates several signalling cascades resulting in the inhibition of PI3K. It is this inhibition of PI3K that reduces proliferation, tumour growth and induces apoptotic cell death. The additive effect that VPSIN1 has on these cells suggests that Ceritinib may only be targeting PI3K class I and class II. It was also suggested that VPSIN1 and SAR offered more significant treatment potential due to their specificity Schläfli et al. Recently, VPSIN1 has been shown to have promising effects in acute myeloid leukaemia AML cells, with increased cell death observed in response to treatment Meunier et al. Lysosomal inhibitors include numerous compounds that alter the pH of the lysosome thereby making the function of the enzymes that rely on this defunct. Common lysosomal inhibitors include Bafilomycin A1 BafA1 , chloroquine CQ , and hydroxychloroquine HCQ. V-ATPases are a type of proton pump essential for maintaining the acidic environment in the lysosome, which is required for proper functioning and activation of degradative enzymes Mauvezin and Neufeld, BafA1 prevents both acidifications of the lysosome and autophagosome-autolysosome fusion by disrupting the function of the V-ATPases Yamamoto et al. It has also been shown to be a potassium carrier to mitochondria, resulting in mitochondrial swelling and dysfunction; this activity was observed at nanomolar concentrations Teplova et al. It has been suggested that the use of BafA1 is not feasible due to this toxicity, although interestingly, claims of toxicities have not been supported by data, and on the contrary, there are studies, at least in leukaemia, that have shown no toxicity in vivo Yan et al. It is proposed that this suggested toxicity is possibly related to off-target effects at higher concentrations. However, as the current evidence demonstrates this off-target toxicity is not observed at lower concentrations where BafA1 has been shown to be effective in in vivo models for a variety of cancer types, this still maybe worth exploring Pivtoraiko et al. Despite this, to date its use in humans has not been approved. CQ and its analogue HCQ are lysosomal lumen alkalises. They are lysosomotropic and accumulate within acidic vessels, particularly the lysosome, where their weak base formula reduces lysosome acidity and function, although the exact mechanism is yet to be elucidated. Other research suggests that they affect the lysosome and autophagosome-lysosome fusion Shingu et al. Although hydroxychloroquine is approved by the Therapeutic Goods Administration TGA for the treatment of rheumatoid arthritis, systemic lupus erythematosus, discoid lupus erythematous and malaria, its use as a co-treatment in cancer may not be viable due to the high concentrations required to inhibit autophagy and the detrimental symptoms that arise because of this arrhythmias, myopathy, cardiovascular cytotoxicity Rosenfeld et al. One potential remedy to that is using a more potent analogue, several of which have already been developed, the most notable being Lys01 and Lys05 McAfee et al. A systematic review and meta-analysis of all cancer trials that have been undertaken using chloroquine or hydroxychloroquine for cancer treatment were analysed to determine efficacy. The review assessed the use combined with gemcitabine, doxorubicin, radiation, temozolomide and single therapy with hydroxychloroquine. The meta-analysis demonstrated that the overall response rate was significantly higher with the inclusion of hydroxychloroquine or chloroquine than without Xu et al. However, stress exerted on the ER and Golgi by HCQ and CQ may also have contributed to some of the clinical results rather than just autophagic inhibition alone Mauthe et al. Recently, NSC has been demonstrated to be an agonist of ATG4B and LC3B lipidation Akin et al. Computational research has suggested that it exerts this effect by binding to the pocket of ATG4B required for its proteolytic activity Akin et al. Additionally, Akin and colleagues have demonstrated in osteosarcoma that cell viability and tumour size were significantly reduced upon the addition of NSC They have established it to be a potent stand-alone treatment Akin et al. Aside from NSC, several other drugs, including S, , LV, and S, have been screened, but very little has been done to confirm their in vivo efficacy Cleenewerck et al. Lazarus et al. The most recent was Martin et al. The results also indicate their efficacy against ULK2 which, as expected, would improve its inhibitory effects on autophagy activity. Their activity was examined further to reduce it to one specific inhibitor, ULK, with efficacy at the low nanomolar ranges in non-small cell lung cancer Martin et al. Furthermore, in line with our unpublished findings and others, KRAS mutant cancer is more susceptible to autophagic inhibition in the context of lung cancer, particularly in a nutrient-deprived microenvironment. The chemical modulation of autophagy in cancer as an adjuvant therapy prospect is well-established. There have been over clinical trials and more than 30 are currently being undertaken to explicitly determine the efficacy and appropriateness of mainly autophagic inhibitors, with a few in autophagy activators in cancer treatment. The aim in most of these trials is to increase the effectiveness of frontline treatment by limiting resistance, as summarised in Table 2. However, nearly all of the trials are utilising hydroxychloroquine or chloroquine. As discussed earlier, these compounds have other mechanisms of action in conjunction with autophagy inhibition. Additionally, autophagy inhibition is often only achieved with high concentrations of HCQ, and this consequently leads to a multitude of adverse side-effects. Therefore, the translation from bench to bedside is being impeded by the lack of available specific and potent autophagic inhibitors. Response to the clinical demand has led to the trialling of non-specific alternatives that do not truly reflect the therapeutic benefit of autophagic inhibition in cancer treatment. TABLE 2. Summary of the current and active clinical trials implementing autophagy modulation. The exact mechanism by which autophagy affects therapeutic resistance is still unclear. Autophagy inhibition can affect these vastly different therapeutics, suggesting that autophagy has multiple roles in resistance. FIGURE 3. Increased autophagy prevents cancer cell elimination and contributes to therapeutic resistance. Autophagy is upregulated in cancer cells due to various microenvironmental stresses including exposure to therapeutics. Apoptotic cell death is also reduced as autophagy facilitates mitochondria and cleaved caspase-8 degradation which prevents downstream signalling cascades. It also prevents death signalling cascades by actively promoting DNA damage repair. The mechanism behind this is unclear. Its upregulation also prevents the Ferroptosis, Pyroptosis and LMP-associated death pathways through unclear mechanisms. Suppression of autophagy via inhibitors could prevent the degradation of components that would otherwise inhibit cell death pathways. Autophagy repairs DNA damage, so DNA-damaging agents that induce cell death via this pathway are less effective with increased autophagy. Thus, by inhibiting autophagy, damage to DNA can no longer be repaired, and the cells are destroyed. An example is the alkylating agent Temozolomide TMZ , which is used in the treatment of Glioblastoma GBM. This chemotherapeutic induces apoptotic cell death through the methylation of DNA residues resulting in DNA damage Agarwala and Kirkwood, In addition, recent studies have suggested that autophagy is necessary for DNA Damage Response DDR Liu et al. Numerous other therapeutics induce cell death via DNA damage, including Platinum Cisplatin, Oxaliplatin , replication disrupting agents Gemcitabine , and radio mimetics Etoposide, Doxorubicin , which are common treatments for cancers including colon, breast, pancreatic, and lung as well as many more Cheung-Ong et al. Autophagy has been shown to regulate the cell cycle independent of its role in the DNA damage repair mechanisms as mentioned previously. Its role in cell cycle arrest is based on the understanding that it has a critical function in recycling regulatory components of the cell cycle. It is suggested that this degradation of cell cycle components is increased in cells being treated with chemotherapeutic agents targeted towards DNA and results in an upregulation of autophagy. Whereby autophagy stalls cell division allowing time for the cell to repair the DNA damage elicited by the treatment, but the exact mechanisms are yet to be elucidated Galluzzi et al. Furthermore, Cyclin D1 has also been observed to be targeted by autophagy in hepatocarcinoma Wu et al. Moreover, it is also suggested that the cytotoxic effects observed with rapamycin in the context of hepatocarcinoma may be resulting in a dysfunctional autophagy that then has elicited synthetic lethality in the cells rather than a functional protection Wu et al. The role of autophagy during the cell cycle has more recently been observed to vary at different stages, apart from induction, it is also suggested to be inhibited. The mitotic regulator Cyclin Dependent Kinase 1 CDK1 was shown to inhibit autophagy directly during mitosis. This suppression is driven by binding of CDK1 to regulatory ATGs at sites usually bound by mTORC1 which facilitates the inhibition of autophagy Odle et al. This is perplexing as CDK1 is commonly upregulated in cancer which would suggest increased autophagic inhibition would be expected. However, it is proposed that this inhibitory effect may be circumvented by p27 activity in some contexts therefore reinstating autophagic activity and is worth further investigation Jung et al. Additionally, there is renewed interest in using CDKI inhibitors particularly in combination with immunotherapy to improve tumour cytotoxicity. Based on these previous findings there is a possibility autophagy would be inhibited, and within the context of treatment, may increase its efficacy. However, in this setting of immune therapy it may also have a potential to interfere with the role autophagy as has been shown in the T-cell response Delamarre et al. Autophagy is implicated in regulating the innate and adaptive immune systems through its regulation of antigen presentation, cytokine release and T and B cell activity. It has been demonstrated to play a role in immune system suppression and tumour evasion of the immune system by various mechanisms. It has been well established that these factors are needed for recognition and elimination of cancer cells by cytotoxic T lymphocytes CTL or Natural Killer NK cells and the loss of these leads to the evasion of immune system Yamamoto et al. Thus, autophagy inhibition may make tumour cells more susceptible to the immune system but conversely may limit the capacity of the immune cells to function. This highlights the need for further studies to examine the effects of autophagic inhibition in this context to truly assess the potential of autophagy activity modulation and how it may improve current and design future immunotherapeutic strategies. Another potential mechanism by which autophagy can circumvent the efficacy of therapeutics is due to its inverse relationship with apoptosis. The upregulation of one appears to suppress the other. It has also been suggested that they may represent different sides of the same coin, but what exactly elicits one and not the other to control cell fate is unclear. Suppression of autophagy demonstrated a marked increase in therapeutic efficacy in resistant cells via an increase in apoptosis, as seen with imatinib in chronic myelogenous leukaemia Carew et al. There is potentially the involvement of p53, which facilitates several cascades that activate caspases and induces apoptosis. Furthermore, autophagy inhibition with CQ leads to an increase in p53 activation and apoptosis in lymphoma cells Amaravadi et al. Arguably the most influential group of proteins in classical apoptotic death signalling pathway are the BCL-2 apoptotic and antiapoptotic family members. The BCL-2 family members can be categorised into three classes. First there are the antiapoptotic family members includes Bcl-2, Mcl-1, Bcl-XL , second the apoptotic members includes BAK and BAX and third the BH3 only proteins includes BAD, BIK, BID Youle and Strasser, In brief, when the apoptotic family members are active, they stimulate cytochrome c release from the mitochondria which in turn activates caspases responsible for cell death Leibowitz and Yu, The anti-apoptotic family members supress apoptotic members and are themselves supressed by the BH3 only proteins and other factors e. Autophagy has been demonstrated to manipulate several members of this family, although its exact relationship to the anti-apoptotic and apoptotic members is unclear. One particularly important molecule in both apoptosis and autophagy is Beclin It also forms a complex with BCL-2, and in this complex Beclin-1 was found to be inhibited from its function in autophagy Pattingre et al. However, with stimuli that induce autophagy such as starvation, ROS, and hypoxia, Beclin1 dissociates from BCL2 Pattingre et al. In addition, Atg12 inactivates antiapoptotic members Bcl-2 and Mcl-1 by the binding of the BH3-like motif on Atg12 to the BH3-binding groove of BCL-2 family members Rubinstein et al. Truncated Atg5 formed by the cleavage of ATG5 by calpains 1 and 2 is also able to induce apoptosis Yousefi et al. Once Atg5 is truncated it translocates to the mitochondria where it facilitates the release of cytochrome c, and thus, stimulates apoptosis Yousefi et al. It maybe postulated that the suppression of autophagy will result in these molecules being more readily available for apoptotic processes. In contrast, autophagic inhibition increased cell death upon nutrient starvation in fibroblast cells. The contradiction may be explained by the context being a physiological setting compared to pathologic. As most research to date has demonstrated that autophagy is able to prevent apoptosis at the later stages. And is seen by its ability to degrade the mitochondria, thus preventing cytochrome c release, and degradation of the caspases. Furthermore, cleaved caspase 8 is increased upon autophagy inhibition, and under normal conditions, the large subunit of caspase 8 is taken into the autophagosome and eliminated in the lysosome Hou et al. Autophagy also potentially degrades cleaved caspase 3 as its presence increases in CQ-treated lymphoma cells Amaravadi et al. In addition, several chemotherapies, including doxorubicin, trastuzumab and Sunitinib, can enlist apoptotic death signalling cascades because of the damaged mitochondria caused by the pharmacological compounds Gharanei et al. Autophagy can circumvent the release of cytochrome c and the death cascade by pre-emptively degrading the mitochondria Ravikumar et al. Thus, active autophagy can prevent apoptosis through this pathway, whilst suppression of autophagy can enable this pathway. A recent study by Hwang et al. demonstrated that combining CQ and Cisplatin in ovarian cancer increased γH2Ax, a DNA damage marker, caspase 3, and phosphorylated ATM, and it does this through p21 suppression, which was suggested to induce autophagy Hwang et al. These findings were further supported by Maheshwari et al. with their research confirming the negative regulation of autophagy by p21, which was observed to be facilitated by Akt Maheshwari et al. Furthermore, these findings were observed in several cancer models, suggesting it is not context dependent Maheshwari et al. A recent study by Gremeke et al. e xplored the mechanisms of drug resistance in numerous cancers and found that the treatment induced autophagy activity. The results of this study highlight the complexity of targeting drug-refractory tumours such as NSCLC, whereby the mechanism of resistance in these cells made them vulnerable to other targets Gremke et al. Cancer cells resistant to platinum compounds demonstrated an acquired resistance through increased MTORC1 protein complex. Increases in MTORC1 lead to the suppression of autophagy and created vulnerabilities to metabolic inhibitors 2DG, DCA, metformin, phenformin, AZD Gremke et al. This vulnerability should be exploited in the clinical setting to achieve synthetic lethality in the future. In these studies, genetic ablation of autophagy genes has been seen to decrease cell death pathways including LMP-associated death the result of lysosomal contents being released into the cell due to lysosomal permeabilization , Pyroptosis inflammatory dependant cell death due to cytokine release via inflammasome activation , and Ferroptosis iron dependant cell death that is instigated by lipid peroxidation. These studies are summarised in Table 3. Inhibition of autophagy for prolonged periods of time, like with any drug, may lead to resistance. In fact, some research has suggested that by blocking autophagy several other non-canonical autophagy pathways and the Nrf2 pathway are upregulated to circumvent this loss. In a study by Towers and colleagues it was determined that even autophagy dependent cell lines could adapt and survive despite the knockout of Atg7, and that the Atg7 null cell population had an upregulation of Nrf2 and the Nrf2 signalling pathway. This upregulation of Nrf2 resulted in increased proteasomes, increased cell growth, decreased apoptosis and contributed to therapeutic resistance Towers et al. Evidence has suggested that this is due to an increase in p62 availability. Nrf2 when bound to Keap1 is inhibited, however, if Keap1 is bound to p62 instead then Nrf2 remains active Komatsu et al. Additionally, a phase I clinical trial performed on canines with lymphoma was done to evaluate the effect of combining HCQ with doxorubicin. The initial findings of this study were promising as canines demonstrated a high overall response rate of However, progression-free interval was only observed to be around 5 months Barnard et al. In a comparable study of canines treated with doxorubicin alone a similar progression free interval of 5. Thus, some cancer cells may adapt to autophagy inhibition by upregulating other stress pathways. Furthermore, cellular adaptions to autophagy inhibition like the upregulation of the Nrf2 pathway may then create a cell-fate determining vulnerability to Nrf2 targeted therapies which should be explored. Therapeutic resistance remains a major area for concern in cancer treatment and significantly impacts upon patient outcomes. Research into the specific mechanisms by which the cancer cell develops therapeutic resistance has highlighted the cellular process known as autophagy. Furthermore, increasing evidence suggests that autophagy can influence therapeutic resistance due to its inverse relationship with apoptosis and its influence on DNA damage repair pathways. PI3K and lysosomal inhibitors have demonstrated promising results in overcoming therapeutic resistance in cancer cells. However, several factors should be considered before their use in the clinical setting. On a broader scale, autophagy is essential for maintaining cellular homeostasis in healthy cells. The wide-scale targeting of autophagy may prevent its cytoprotective roles and lead to the toxic buildup of damaged and out-lived proteins and organelles in otherwise healthy tissue. Thus, the effect of long-term treatment on the organs of the nervous system should be considered in any treatment regime. More specifically, several factors about the current PI3K and lysosomal inhibitors should be considered before future use. Firstly, the specificity of the PI3K inhibitors has not been consistent in targeting class III PI3K until recently. Although more specific derivatives have been developed, most of the research done to date has been with older inhibitors. In addition to this, autophagy can be activated via non-canonical pathways. Thus, targeting PI3K may not have the capacity to completely inhibit autophagic activity as the cells could potentially circumvent the initiation complex and still have upregulated autophagy in the presence of PI3K inhibitors. As both canonical and non-canonical autophagy pathways require the lysosome, targeting the lysosome may prove a more beneficial and effective approach. The benefit of targeting the lysosome extends past it being just a more specific target but also introduces the inhibition of, not only macroautophagy, but also the alternative autophagic pathways of chaperone-mediated autophagy and micro autophagy. In contrast to the benefits, a potential disadvantage of targeting the lysosome is that emerging evidence shows that lysosomes have independent roles and are not just specific to autophagy. Furthermore, lysosomes are fused with macrophages and dendritic cells and aid with the clearance of foreign microbes and antigen presentation Xu et al. Thus, the immune system may be adversely affected by targeting the lysosomes indiscriminately. Of interest is an apparent trend in the glycolytic phenotype being the most sensitive or responsive to autophagic inhibition. It has been presented as a consistent predictor in blood cancers Chen et al. However, it is suggested efficacy would be increased as an adjuvant with some of the frontline chemotherapeutics that result in a shift in metabolic programming as a means of evading their effects, such as Cisplatin Xu et al. The current autophagy inhibitors have several potential drawbacks, as discussed above. Research into autophagic mechanisms and the differences between physiological autophagy and pathologic autophagy is warranted. Delineating molecules specific to autophagy in the context of cancer would also be beneficial as it highlights potential therapeutic targets. Targeting these molecules may reduce off-target effects like the immune system. Theoretically, the alternative to suppressing autophagy would be to stimulate and cause an over-activation of the process to bring about dysfunction, particularly in the context of cancer cell but potentially positively regulating the immune system Yonekawa and Thorburn, ; Karsli-Uzunbas et al. Despite these considerations, autophagy inhibitors are a very promising co-treatment for aggressive and resistant cancers whose advantages potentially outweigh their disadvantages. Further investigation into current autophagy inhibitors in therapeutic resistance is warranted, especially in the context of cancers like pancreatic cancer and GBM, whose survival rate is abysmal due to resistance to current treatments. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Abedin, M. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. PubMed Abstract CrossRef Full Text Google Scholar. Agarwala, S. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Agrotis, A. On ATG4B as drug target for treatment of solid tumours-the knowns and the unknowns. Cells 9 1 , Akin, D. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy 10 11 , — Al-Bari, M. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. Alayev, A. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. Cell Biochem. Alers, S. Cell Biol. Amaravadi, R. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. Ando, T. Gemcitabine and rapamycin exhibit additive effect against osteosarcoma by targeting autophagy and apoptosis. Cancers 12 11 , Arcaro, A. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: The role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Baghdadi, M. TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity 39 6 , — Bago, R. Characterization of VPSIN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. EMBO J. Bai, Y. Lipid storage and lipophagy regulates ferroptosis. Biophysical Res. Barnard, R. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy 10 8 , — Bellot, G. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Bhatt, V. Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors. Cell Death Dis. Bhutia, S. Autophagy: cancer's friend or foe? cancer Res. Bosc, D. A new quinoline-based chemical probe inhibits the autophagy-related cysteine protease ATG4B. Boya, P. Inhibition of macroautophagy triggers apoptosis. Bröcker, C. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting HOPS tethering complex. Brown, C. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6 12 , — Buccarelli, M. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Carew, J. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 1 , — Catalano, M. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Chan, E. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atgindependent mechanism. Chen, K. Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel. Tumour Biol. Chen, W. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 10 , — Chen, Y. VAMP8 facilitates cellular proliferation and temozolomide resistance in human glioma cells. Neuro-Oncology 17 3 , — Recent progress in autophagic lysosome reformation. Traffic 18 6 , — Cheung-Ong, K. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical Biology. Chicote, J. Cell death triggered by the autophagy inhibitory drug 3-methyladenine in growing conditions proceeds with DNA damage. Cleenewerck, M. Inhibitor screening and enzymatic activity determination for autophagy target Atg4B using a gel electrophoresis-based assay. Cohen, I. Increased tumor glycolysis is associated with decreased immune infiltration across human solid tumors. Colell, A. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 5 , — Colella, B. Dankó, T. Rapamycin plus doxycycline combination affects growth arrest and selective autophagy-dependent cell death in breast cancer cells. Datta, S. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A cells via ROS mediated modulation of β-catenin pathway. Apoptosis 24 , — Delamarre, L. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science , — Denise, C. Oncotarget 6 39 , — Dong, Y. Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells. Eberli, D. Apalutamide in combination with autophagy inhibitors improves treatment effects in prostate cancer cells. Urologic Oncol. We caution the reader that since we are still in the early days of developing small-molecule modulators of autophagy, we know of few if any small molecules that can directly target the autophagy machinery, and most compounds discussed here affect the regulatory mechanisms of autophagy. Their efficacy in modulating autophagy has not been thoroughly investigated in humans. Even for those FDA-approved drugs that have been shown in experimental systems to modulate autophagy, it remains to be tested whether the concentrations required to modulate autophagy in vivo can be safely achieved. On the other hand, the identification and development of small-molecule modulators of autophagy have been important and useful for investigating molecular mechanisms and potential indications for autophagy regulation in the treatment of human diseases. Small-molecule modulators of autophagy that target different steps of the autophagic machinery. The initiation of autophagy is regulated by the activity of the class III PI3K complex, which can be inhibited by 3-MA and PT21 as well as by spautin-1 that can promote the degradation of the class III PI3K complexes. Once the phagophore formation is initiated, compounds such as verteporfin can interfere with LC3-interacting region motifs and potentially block the selective recruitment of cargos such as mitochondria. The trafficking of autophagosomes to the lysosome is facilitated by the cytoskeleton. Thus, microtubule destabilization by vinca alkaloids can block the maturation of autophagosomes, whereas stabilization by taxol may increase the fusion between autophagic vacuoles and lysosomes. On the other hand, paclitaxel was shown to inhibit autophagy by blocking the activation of the VPS34 complex by inducing inhibitory phosphorylation of VPS34 as a substrate of CDK1. Lysosomotropic agents that increase the lysosomal pH, such as CQ, NH 4 Cl, and monensin, interfere with lysosomal function and block autophagy at a late stage. The final step of the autophagy pathway can also be blocked by inhibitors of lysosomal enzymes, such as E64d and pepstatin A, and by bafilomycin A1, a specific inhibitor of v-ATPase. Inhibitors of mTOR signaling activate autophagy. mTOR, a member of the PI3K family, is an important regulator of multiple intracellular processes that include protein translation as well as autophagy and metabolism in response to different nutrient, energy, and growth factor—signaling conditions mTOR is the catalytic subunit of two functionally distinct complexes, mTOR complex 1 mTORC1 and mTORC2. Activation of autophagy by rapamycin and its analogs has been shown to be protective in different animal models of neurodegenerative disorders 22 — These data suggest that autophagy prevents the accumulation of misfolded proteins. A new generation of ATP-competitive inhibitors of mTOR display potent activation of autophagy. Unlike that of rapamycin, which only inhibits mTORC1, this new generation of inhibitors, such as PP and Torin 1, inhibits both mTORC1 and mTORC2 26 — Although rapamycin and other inhibitors of mTOR are strong activators of autophagy and have been tested as anticancer therapeutics, caution should be taken in equating the activation of autophagy with cancer suppression, as accumulating evidence suggests that activation of autophagy can promote the survival of cancer cells. By promoting intracellular recycling, autophagy can confer stress resistance and sustain cell survival under adverse conditions, which may promote metastasis Thus, the efficacy of rapamycin and other inhibitors of mTOR as an anticancer therapy may have to do with their inhibitory effects on protein translation and metabolism, rather than autophagy activation. Activators of AMPK activate autophagy. AMPK, the main sensor of intracellular energy, is an important regulator of both ULK1 and mTOR. Hence the administration of AMPK activators such as metformin stimulates autophagy. Inhibitors of VPS34 kinase complexes block autophagy. Class III PI3K complexes mediate the production of phosphatidylinositolphosphate PI3P , a key lipid-signaling molecule that serves as an important checkpoint involved in regulating the mTOR-independent induction of autophagy under normal nutritional conditions PI3P is required for the formation of the phagophore and, subsequently, the autophagosome. Autophagy is positively regulated by PI3P, the product of class III PI3K VPS34 complexes , and is negatively regulated by phosphatidylinositol triphosphate, the production of class I PI3K. A structural study suggested that 3-MA preferentially inhibits VPS34 in vitro In vivo, 10 mM 3-MA can also inhibit class I PI3K, which may explain why it can promote autophagy flux under nutrient-rich conditions 39 , The PI3K inhibitors wortmannin and LY, which inhibit both class I and III PI3K complexes, have also been used to inhibit autophagy 39 , 41 , An x-ray crystallographic study of the class III PI3K VPS34 demonstrated that it exhibits distinguishing structural features that differentiate it from class I PI3Ks. The P loop of VPS34, which binds the phosphates of ATP, curls inward toward the ATP-binding pocket and is more constricted than that of class I PI3K. The hinge between the N and C lobes of VPS34 is one residue shorter than in class I PI3Ks. Therefore, VPS34 lacks the bulged-out space at the adenine-binding pocket hinge that is characteristic of class I PI3Ks. Miller et al. exploited these structural characteristics of VPS34 and designed a number of promising VPS34 kinase inhibitors such as PT21 IC 50 88 nM Further development of these VPS34 kinase inhibitors may provide promising leads to target the class III PI3K. Thus, it is possible to regulate the levels of entire complexes by targeting one component. Spautin-1, isolated from an imaged-based screen for small-molecule modulators of autophagy 11 , promotes the degradation of VPS34 complexes by targeting the ubiquitin-specific peptidases, USP10 and USP13, two deubiquitinating enzymes that regulate beclin 1 and VPS34 stability. Spautin-1 promotes ubiquitination and proteasomal degradation of beclin 1 and VPS34 complexes under glucose-free conditions 44 , consequently inhibiting autophagy. Spautin-1 treatment can promote the death of cancer cells in the setting of nutrient deprivation when autophagy is activated to promote survival. Moreover, inhibition of autophagy by spautin-1 under nutritional deprivation conditions leads to the activation of chaperone-mediated autophagy, a lysosome-dependent pathway that mediates degradation of missense mutant p53 protein Increasing evidence suggests that certain p53 mutants may promote oncogenesis through a dominant gain-of-function mechanism; thus, the reduction of accumulated mutant p53 may be an important therapeutic goal in cancer treatment. Development of improved spautin-1 analogs with good safety and in vivo stability profiles may represent a novel anticancer therapeutic strategy. Inhibitors of class I PI3K signaling activate autophagy. In contrast to the positive role of the class III PI3K products in promoting autophagy, increases in the levels of class I PI3K products phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-triphosphate negatively regulate autophagy Targeting this class I PI3K with inhibitors such as CH, GDC, and GDC, leads to the activation of autophagy 47 — Protein kinase AKT is a major downstream mediator of class I PI3K signaling. Pharmacologic targeting of AKT by inhibitors such as perifosine leads to autophagy activation Moreover, treatment with inhibitors of receptor tyrosine kinases that leads to the inhibition of AKT can activate autophagy, possibly by inhibiting mTORC1 or by regulating beclin 1. The activation of the EGFR tyrosine kinase—mediated signaling pathway may inhibit autophagy through multiple mechanisms that target beclin 1 51 , In another small-molecule screen for autophagy modulators using the clearance of the autophagy substrate A30P α-synuclein as a biomarker, Williams et al. In addition, calpain 1 can cleave the Gαs subunit of heterotrimeric G proteins, which increases its activity after calpain cleavage. Gαs α enhances adenylyl cyclase activity, which in turn inhibits autophagy These studies suggest that calpain 1 may have multiple mechanisms to control the levels of autophagy in cells under normal nutritional conditions by regulating the levels of key proteins involved in autophagy. Thapsigargin does not affect autophagosome formation but leads to accumulation of mature autophagosomes by specifically inhibiting the fusion of autophagosomes with lysosomes Thapsigargin blocks autophagosomal recruitment of the small GTPase RAB7, which is required for complete autophagic flux. Another study demonstrates that thapsigargin causes accumulation of mature autophagosomes by blocking autophagosome fusion within the endocytic system Inhibitors of inositol monophosphatase activate autophagy. Lithium, used widely as a mood-stabilizing drug for the treatment of bipolar disorder, was shown to induce autophagy and reduce mutant huntingtin aggregates and cell death Sarkar et al. found that lithium inhibits inositol monophosphatase IMPase , leading to depletion of free inositol and a reduction in the levels of myo-inositol-1,4,5-triphosphate IP3 , which may represent an mTOR-independent pathway for regulating mammalian autophagy. Similar effects were observed with other IMPase inhibitors and mood-stabilizing drugs such as L and carbamazepine, which also decrease inositol levels Since IP3 receptor IP3R is a potential binding partner of beclin 1, the IP3R antagonist Xestospongin B can stimulate autophagy by releasing beclin 1 from IP3R to assemble into the VPS34 complex 59 , Any success of such clinical trials would encourage the development of additional autophagy modulators for the treatment of neurodegenerative diseases. Chloroquine CQ , a 4-alkylamino-substituted quinoline family member, is a clinically available antimalarial agent which is also an autophagy inhibitor function by blocking the fusion of autophagosomes and lysosomes. Because CQ has been approved by the U. Food and Drug administration FDA for use as an antimalarial agent, it is commonly used in clinical trials as an inhibitor of autophagy Kimura et al. Except the classical autophagy inhibitors mentioned above, more agents with function of autophagy inhibition in certain cases have been proved. This kind of autophagy inhibitors may have multiple biological activities and exhibit promising inhibitory effect of therapy-induced cytoprotective autophagy that facilitate overcoming acquired resistance to antitumor therapy. The Bcl-2 family of proteins are not only regulators of apoptosis signaling, but also involved in autophagy processes Duffy et al. The Bcl-2 inhibitor ABT has been identified as an autophagy inhibitor Yang et al. Moreover, obatoclax which exhibits pan-Bcl-2 inhibition effect has been demonstrated to cause a striking inhibition of autophagy at late-stage in colorectal cancer and bladder cancer cells Huang and Sinicrope, ; Koehler et al. Clarithromycin is a macrolide antibiotic frequently utilized in the treatment of upper and lower respiratory tract infections and Helicobacter pylori that has demonstrated inhibitory effects on autophagy Altman and Platanias, ; Giulia Petroni et al. Resveratrol is a natural polyphenolic compound derived from plants which has proapoptotic effects on various cancer cells. Interestingly, resveratrol showed synergistic anticancer efficacy by blocking temozolomide or doxorubicin induced cytoprotective autophagy flux Lin et al. Quinacrine also known as mepacrine which is a synthetic antimalarial drug belonging to the quinoline-based drugs class and have been demonstrated to inhibit autophagy at late stage Golden et al. It had been first demonstrated by a group that it could enhance the drug susceptibility of the epithelial cancerous cells to cisplatin by inhibition of autophagic flux Liu et al. Epigallocatechin gallate EGCG is a bioactive catechin derived from green tea which has been employed to overcome drug resistance by inhibiting therapy-induced autophagic flux Meng et al. Accumulating evidence has shown that targeting autophagy in combination with antitumor agents has been effective at enhancing cell death and improving the efficacy of cancer therapies in various cancer types. The antitumor agents are currently under investigation with a cytoprotective autophagy effect that is mainly classified into several types according to distinct characteristic Table 2. Table 2 Subset of the preclinical research targeting drug-induced autophagy in various cancers. Multiple plant-derived natural compounds have obvious anticancer potential. However, autophagy-associated chemoresistance limits the development of novel natural drugs in clinical cancer treatment Zhang et al. Polyphyllin I PPI is a bioactive phytochemical isolated from the rhizoma of Paris polyphyllin. Preclinical studies revealed PPI has anticancer efficacy with autophagy induction in various cancer models. Further combined PPI with CQ to block PPI-induced autophagy in HCC cells resulted in augmenting the cytotoxicity and antiproliferation effects of PPI via the caspase-dependent apoptosis pathway Shi et al. Blockade of autophagy by 3-MA enhanced UA-induced apoptosis Shin et al. Additionally, UA and resveratrol have been shown to synergize with CQ to enhance melanoma cell death Junco et al. By interfering with the normal breakdown of microtubules during cell division, paclitaxel is a medication used to treat several cancer types, including breast cancer, lung cancer and ovarian cancer. Acquired resistance mediated by autophagy of paclitaxel functions as a major obstacle to successful anticancer effects. Moreover, the blockade of autophagy with 3-MA and Baf A1 strengthen sensitivity of folliculin-deficient renal cancer cells to paclitaxel Zhang et al. Obatoclax could also promote paclitaxel induced apoptosis in synergistic manner by blockade of the autophagic flux in bladder cancer Jiménez-Guerrero et al. Tetrandrine is a natural product study in our laboratory, and we found that tetrandrine combined with CQ has synergistic antitumor activity Mei et al. It was also reported that pterostilbene in combination with 3-MA or BafA1 may enhance the efficiency of chemotherapeutic approaches in both chemo-sensitive and chemo-resistant lung cancer cells and in triple-negative breast cancer cells Hsieh et al. The anticancer effect of another natural substance product, chaetocin, is enhanced by Baf A1 Jung et al. Moreover, CQ potentiated the cytotoxicity of topotecan in lung cancer cells by interfering with autophagy Wang Y et al. Additionally, cell death of BE 2 -C human neuroblastoma cells following sulforaphane treatment could be promoted by 3-MA via inhibition of autophagy Horwacik et al. Honokiol is isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia and is a kind of lignan. Honokiol-induced cell death increased with CQ by inhibiting autophagy that finally exhibits augmented antitumor effects in human nonsmall cell lung cancer cells Lv et al. Combretastatin A-4 CA-4 is a drug isolated from combretum caffrum which has been applied in clinical trials for solid tumors therapy in past over ten years. However, the CAelicited autophagic response in various cancer cells restricts its clinical application. Autophagy inhibition by autophagy inhibitors 3-MA and Baf A1 , the JNK inhibitor or the Bcl-2 inhibitor ABT could promote CAinduced apoptosis Li et al. Cytotoxic drugs are used in the treatment of tumors to trigger the death of tumor cells by preventing DNA replication and cell division. Cisplatin-based chemotherapy frequently results in acquired resistance, which is a major challenge in the clinical control of various cancers. The underlying mechanism is demonstrated in relation to the autophagic response. Combined treatment of cisplatin with 3-methyladenosine or CQ promotes the chemotherapeutic sensitivity of various cancers, including lung cancer, ovarian cancer, glioma cancer, gastric cancer, bladder cancer, and endometrial cancer cells Zhang et al. Temozolomide TMZ is an alkylating agent used for the clinical treatment of glioblastoma multiforme and melanoma. Studies have revealed that cytoprotective autophagy induced by TMZ contributes to therapy resistance in malignant glioma which can be suppressed by resveratrol, chrysin, or CQ and its analog quinacrine, resulting in a decrease in autophagy and an increase in apoptosis Buccarelli et al. Capsaicin is a major pungent ingredient found in hot red chili peppers of the genus capsicum, emerges as a chemotherapeutic augmenter for 5-FU's anticancer effects in cholangiocarcinoma Hong et al. In addition, CQ and 3-MA potentiate the cytotoxic effect of 5-fluorouracil on colon cancer cells Sasaki et al. Cytarabine is a chemotherapy agent used mainly in killing acute myeloid leukemia AML and non-Hodgkin lymphoma cancer cells by interfering with DNA synthesis. Baf A1 and CQ can markedly increase apoptotic death in cytarabine-treated human leukemic cells Bosnjak et al. Anthracycline drugs derived from Streptomyces bacterium Streptomyces peucetius var. caesius, which are used in cancer chemotherapy to treat several cancers, including breast, ovarian, uterine, bladder, lung cancers, and leukemias, and lymphomas. Doxorubicin DOX is an anthracycline antibiotic derived by chemical semisynthesis from bacterial species and works by intercalating DNA for the treatment of various cancers. EGCG, one of the highest catechins from green tea, promisingly showed the capability to augment the antitumor efficacy of DOX in HCC and osteosarcoma treatment involving autophagy inhibition Chen et al. As a classic chemotherapeutic agent for osteosarcoma, autophagy-mediated resistance of DOX can be reversed by 3-MA Zhao et al. Resveratrol can enhance the chemotherapeutic potential of DOX by inducing apoptosis mediated through down regulation of autophagy in breast cancer cell lines Rai et al. Pirarubicin is widely used in clinical chemotherapy for bladder cancer. However, emerging evidence has shown that the efficacy of pirarubicin is limited by the cytoprotective role of autophagy in bladder cancer cells, and inhibition of autophagy by 3-MA or HCQ increased cell apoptosis, suggesting an efficiency over traditional pirarubicin chemotherapy in bladder cancer patients Li et al. Tyrosine kinases play a pivotal role in oncogenesis, but emerging studies have report compromised cytotoxicity of tyrosine kinase inhibitors used as monotherapy in cancer Carew et al. Autophagy induction has been identified as the imatinib resistance mechanism during therapeutic process. Additionally, clarithromycin, which blocks autophagy, could also restore the sensitivity of CML cells to imatinib Altman and Platanias, In human malignant glioma cells, the imatinib-elicited cytotoxicity has been enhanced by induction of apoptosis with Baf A1 which trigger inhibition of autophagy at a late stage Shingu et al. Inhibition of cytoprotective autophagy by 3-MA treatment enhances sorafenib-mediated cell death via necrosis and significantly augments the combination antitumor effect with sorafenib and the HDAC inhibitor vorinostat in hepatocellular carcinoma cells Honma and Harada, ; Yuan et al. Additionally, sorafenib has shown antitumor activity in glioblastoma multiforme U and LN cells. Further study demonstrated that combination treatment with sorafenib and CQ exhibited inhibition of cell proliferation and migration and induction of cell apoptosis by blockade of autophagy in vitro and in vivo Liu et al. Sunitinib is a multitargeted receptor tyrosine kinase inhibitor that was approved for the treatment of renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor. CQ has been shown to synergize with sunitinib by switching off autophagy then enhanced the cytotoxicity of sunitinib via inducing apoptosis Abdel-Aziz et al. Linifanib ABT is a structurally novel and potent multikinase inhibitor of RTK, vascular endothelial growth factor VEGF , and platelet-derived growth factor. Gefitinib is a small-molecule inhibitor of epidermal growth factor receptor EGFR tyrosine kinase used for certain breast, lung, and other cancers with mutated and overactive EGFR. HCQ or Baf A1 inhibit gefitinib-induced autophagy at late-stage significantly increased cell death in gefitinib-sensitive and -insensitive breast cancer cells Dragowska et al. In addition, the autophagy flux inhibitor clarithromycin CAM , a macrolide antibiotic can enhances the cytotoxic effect of gefitinib in nonsmall cell lung cancer cells NSCLC , as well as EGCG can overcomes NSCLC resistance to gefitinib by inhibiting autophagy and augmenting cell death through targeting ERK pathway Sugita et al. The antagonistic activity on cell proliferation has been found when coadministration of gefitinib and cisplatin to EGFR-TKI-sensitive human lung cancer PC9 cells. After combination with CQ, resulted in a synergistic effect via inhibiting autophagy, further suggesting a potential strategy to reverse the antagonistic effects between EGFR-TKIs and chemotherapeutic drugs Liu et al. Another EGFR-TK inhibitor erlotinib has been demonstrated to trigger autophagy in wild-type EGFR NSCLC. Drug resistance caused by this autophagy can be overcame with CQ which represent a beneficial strategy to enlarge the application scope of erlotinib efficacy in cancer therapy Zou et al. Cediranib is a potent inhibitor of VEGF receptor tyrosine kinases. Combined with the late-stage autophagy inhibitor quinacrine, the antiangiogenic efficacy of cediranib in intracranial glioma is synergistically enhanced Lobo et al. Proteasome inhibitors has been widely used as clinical anticancer drugs for the bone marrow cancer multiple myeloma MM and exhibited remarkable efficacy in solid tumor malignancies treatment, including the first-in-class proteasome inhibitor bortezomib and second-in-class proteasome inhibitors carfilzomib and oprozomib Saavedra-García et al. However, increasing studies indicate that cancer cells show resistance to the proteasome inhibitors and autophagy contributes to the mechanisms associated with carfilzomib and bortezomib resistance Zang et al. CQ and HCQ can enhance carfilzomib induced cell apoptosis by inhibition of autophagy toward MM Jarauta et al. The cytotoxicity of bortezomib on MM also can be augmented by Baf1, HCQ, or macrolide antibiotics via inhibiting prosurvival autophagy in cotreatment manner Di Lernia et al. Moreover, 3-MA promoted sensitivity of glioblastoma cells to bortezomib by inhibition of bortezomib induced cytoprotective autophagy Zhang et al. MLN, the active form is ixazomib which is an orally administered proteasome inhibitor. The cytotoxic effect of ixazomib on colorectal cancer cells has been proved enhanced with ABT by autophagy inhibition via inhibiting Mcl-1 expression Yang et al. Rapamycin is an allosteric mTORC1 inhibitor that has been identified as a broad-spectrum autophagy inducer. Combination therapy of rapamycin with resveratrol by autophagy blockade showed enhanced cell apoptosis effects in breast cancer cells Alayev et al. Everolimus, a mTOR inhibitor, has been approved for second-line therapy. Baf A1, 3-MA and CQ can also enhance a novel mTOR kinase inhibitor, KU, which induces cytotoxicity against anti-HepG2 hepatocellular carcinoma cells Yongxi et al. The hydroxymethylglutaryl-coenzyme A HMG-CoA reductase-inhibiting drug simvastatin induces the activation of AMP-activated protein kinase AMPK and mTOR in glioma cell death. Inhibition of autophagy with Baf A1 and 3-MA, as well as AMPK inhibition with compound C, markedly increased simvastatin-induced apoptotic death Misirkic et al. By inhibiting HMG-CoA reductase, statins can also mediate cytoprotective autophagy in human leukemic cells, and Baf A1 enhanced the apoptotic death induction Vilimanovich et al. In aggressive prostate cancers, the efficacy of the AKT inhibitor AZD is limited, and blocking autophagy using 3-MA, CQ, and Baf A1 enhanced cell death Lamoureux et al. Pharmacological Notch1 signaling blockade by the γ-secretase inhibitor MRK is used to treat glioblastoma neurospheres, and combination treatment with CQ can abrogate chemoresistance caused by induction of protective autophagy Natsumeda et al. The addition of CQ could also enhance the cytotoxic effects of flavopiridol, a cyclin-dependent kinase CDK inhibitor, in chronic lymphocytic leukemia CLL Mahoney et al. HCQ could significantly augment the anticancer effect of SFK in colorectal cancer Jing et al. Dichloroacetate DCA , an inhibitor of pyruvate dehydrogenase kinase PDK , was demonstrated to be a promising nontoxic antineoplastic agent. DCA-induced protective autophagy can be inhibited by 3-MA and then restore DCA-induced apoptosis in LoVo colonic carcinoma cells Gong et al. Table 3 Autophagy inhibition of drugs targeting specific signaling in cancer treatment. Based on preclinical research, including in vitro and in vivo models, the researchers conducted several clinical trials, combining drugs that triggered protective autophagy with an autophagy inhibitor Poklepovic and Gewirtz, Table 4. Table 4 Combination treatment by targeting drug-induced autophagy in clinical trials for cancer therapy. In patients with advanced solid tumors and melanoma, a phase I trial of HCQ with dose-intense temozolomide was conducted Rangwala et al. A phase I trial in patients with advanced solid tumors conducted based on preclinical models showed that a proton pump inhibitor, pantoprazole exerted enhanced antitumor activity of DOX by improving drug distribution and inhibiting autophagy Brana et al. In addition, a phase I clinical trial was conducted along with pharmacodynamics evaluation of combination treatment with HCQ and DOX for spontaneously occurring lymphoma in pet dogs Barnard et al. Combination treatment with mTOR and autophagy inhibitor, a phase I trial of temsirolimus and HCQ in patients with advanced solid tumors and melanoma was conducted Rangwala et al. Moreover, coadministration with HCQ and HDAC inhibitor vorinostat, a phase I trial in patients with advanced solid tumors was conducted and researchers further analyzed the data from the effect of safety, tolerability, pharmacokinetics, and pharmacodynamics Mahalingam et al. Since autophagy was considered as a double-edged sword that might cooperate, aggravate, or antagonize apoptosis, current understanding of the role of autophagy in the response to some certain cancer therapy remains controversial, such as sorafenib- induced autophagy in HCC. The effect of sorafenib-triggered autophagy serves as a prosurvival response or promotes the lethality of sorafenib against HCC cells is indistinct Liu et al. Therefore, targeting autophagy induced by antitumor agents by combination of these agents with autophagy inhibitors in clinical cancer therapy application to improve outcomes for patients remains a challenge. More preclinical research should be conducted to confirm that autophagy provides an adaptive mechanism of resistance to antitumor drugs in cancer cells and inhibition of autophagy could further enhance the cytotoxic effects of these drugs in a synergistic manner rather than attenuating the autophagy-dependent antitumor effect. It is clear that using autophagy inhibitor to target antitumor agents-induced protective autophagy to augment cytotoxic effects via switching-off the autophagic mechanism, coadministration is the key procedure. Antitumor agents with cytoprotective autophagy and autophagy inhibitors are different in physicochemical properties, such as molecular weight and size. If given separately, may result in differences in bio-distribution and cancer cell accumulation between the two drugs, which means failure combination treatment. Thus, a drug delivery system needed to facilitate the combination strategy, especially for some agents cannot enter cells efficiently by itself. Besides, autophagy also found as a survival pathway to induce therapy resistance in sonodynamic therapy SDT for several cancers. Therefore, targeting autophagy regulation is also a strategy to enhance SDT efficiency. For breast cancer, a biomimetic nanoplatform exhibited capability in restoring cells' sensitivity to SDT via autophagy inhibition. For the chemotherapy of glioma, blood brain barrier and autophagy-induced chemo-resistance are two limitation factors. Clinically used antimalarial drugs CQ and its derivative HCQ, are well-known autophagy inhibitors function by preventing the acidification of the lysosomal compartment. Although CQ and HCQ showed an equipotent effect at autophagy inhibition in vitro studies, the toxicity of them showed different in vivo. High peak concentrations of CQ may result in infant deaths in case reports that associated with single-tablet ingestions indicated the significant toxicity of CQ. However, people survived suicide attempts taking HCQ, demonstrating HCQ is safely dose-escalated in cancer patients. Moreover, clinical trials showed that autophagy is unable to be completely inhibited by CQ in vivo. These warrant us that searching more potent autophagy inhibitors is critical for promoting an opportunity to apply the combination therapeutic strategy in clinical studies Lalita Guntuku et al. Targeting the autophagic process by coupling autophagy inhibitors with current cytotoxic chemotherapy or other available anticancer therapies are really considered promising therapeutic strategy for cancer. For further clinical application, more efforts should be made in identifying the role of autophagy induced by cancer therapy, developing beneficial coadministration system for drug delivery and discovering novel and efficient autophagy inhibitors Figure 1. Figure 1 The comprehensive mechanism of autophagy induction and inhibition in cancer therapy. Autophagy may act as one of the contributing factors in cellular mechanism of survival during cancer development and therapeutically triggered stress with basic biological importance. Thus, basic and clinical research will be imperatively needed to identify if autophagy is a cancer treatment-related resistance mechanism Tan et al. Studies in preclinical models established autophagy as a therapeutic target, and inhibition of autophagy enhanced chemo-sensitivity and promoted tumor cell death. Meanwhile, multiple early-phase clinical trials evaluated the effect of autophagy inhibition by using HCQ combined with therapeutic agents. These studies indicated that strategies targeting autophagy in cancer are potentially new opportunities for drug development, thus more potent autophagy inhibitors are needed to identify Yang et al. CD on cancer stem cells is considered a potential therapeutic target. There has been a report that CD mAb can sensitize HCC cells to DOX and cisplatin attributed to inhibiting autophagy and facilitating necrotic cell death. Therefore, targeting CD with the autophagy inhibitor CD mAb is a potential therapeutic approach for hepatocellular carcinomas Chen et al. It is easier for solid tumor cells to trigger cell autophagy against nutrient deprivation and oxygen stress conditions caused by antiangiogenic therapy. Thus, we could exploit combination treatment with autophagy inhibitors and antiangiogenic agents, such as bevacizumab Guo et al. For most antitumor agents, combination treatment with either autophagy initiation inhibitors or autophagy maturation inhibitors causes synergistic effects Adiseshaiah et al. However, in some circumstances, combination treatment with different autophagy inhibitors at different stages of autophagy may lead to opposite effects. Using 3-MA or small interfering RNA against Atg5 siRNA-ATG5 to suppress imatinib-induced autophagy at an early stage could decrease the cytotoxicity of imatinib. By contrast, inhibiting autophagy at a late stage by Baf A1 augmented imatinib-induced apoptosis mediated through mitochondrial disruption, indicating that the cytotoxic efficiency of imatinib for malignant glioma may only be enhanced by the appropriate autophagy inhibitor function at a late stage of autophagy Shingu et al. Similarly, inhibition of the early stages of autophagy by 3-MA attenuated the cytotoxic effect of arsenic trioxide ATO in glioblastoma multiforme cells. By contrast, interfering autophagy flux at a late stage by CQ enhanced the ATO induced cell death Li et al. Both pharmacological and molecular targeting of elements of the autophagic process need to occur under certain circumstances to apply to therapeutic approaches. Autophagy also acts as an obstacle in radiotherapy Ye et al. Based on this notion, a strategy was designed to block autophagy in tumor cells to augment radio-sensitization by the autophagy inhibitor 3-MA Chen et al. Except for cancer cells, autophagy also plays a central role in the function of immune cells. Anticancer agents with metabolic modulating by autophagy induction can result in antitumor immune disorders Townsend et al. It has been reported that inhibiting autophagy during interleukin 2 IL-2 immunotherapy can promote long-term tumor regression by tumor inhibition and enhance immune cell proliferation and infiltration Liang et al. Thus, combination radiation or chemotherapy with an autophagy inhibitor can both selectively suppress tumor cells and restore the capability of the immune system by promoting the integrity of beneficial antitumor immune cells Gewirtz, This review was aimed to identify a potent strategy to enhance the sensitivity of cancer cells to therapeutic agents. All sections and tables are not meant to be completely presented because many compounds and agents have effect of autophagy regulation by autophagy induction or inhibition in cancer therapy, and new ones are being discovered routinely. TL designed and wrote the manuscript. JZ revised the article KL and LD collected the material. HW supervised and directed all work. This study was funded by the Hubei Science and Technology Program CFB , Project of Wuhan Municipal Health Commission WZ18Q02 and National Natural Science Foundation of China The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Abdel-Aziz, A. Chloroquine synergizes sunitinib cytotoxicity via modulating autophagic, apoptotic and angiogenic machineries. doi: PubMed Abstract CrossRef Full Text Google Scholar. Adiseshaiah, P. Synergistic Combination Therapy with Nanoliposomal C6- Ceramide and Vinblastine is Associated with Autophagy Dysfunction in Hepatocarcinoma and Colorectal Cancer Models. Cancer Lett. Alayev, A. The Combination of Rapamycin and Resveratrol Blocks Autophagy and Induces Apoptosis in Breast Cancer Cells. Cell Biochem. Altman, J. A new purpose for an old drug: inhibiting autophagy with clarithromycin. Leuk Lymphoma 53 7 , — Amaravadi, R. Principles and current strategies for targeting autophagy for cancer treatment. Cancer Res. Bao, L. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Baranowska, K. Hydroxychloroquine potentiates carfilzomib toxicity towards myeloma cells. Oncotarget 7 43 , — Barnard, R. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy 10 8 , — Booth, L. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Cancer Biol. CrossRef Full Text Google Scholar. Bosnjak, M. Inhibition of mTOR-dependent autophagy sensitizes leukemic cells to cytarabine-induced apoptotic death. PLoS One 9 4 , e Brana, I. A phase I trial of pantoprazole in combination with doxorubicin in patients with advanced solid tumors: evaluation of pharmacokinetics of both drugs and tissue penetration of doxorubicin. New Drugs 32 6 , — Buccarelli, M. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. Carew, J. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Chen, L. Autophagy inhibition enhances apoptosis triggered by BO, an N-mustard derivative, and involves the ATM signaling pathway. Chen, Y. Autophagy inhibition contributes to radiation sensitization of esophageal squamous carcinoma cells. Esophagus 24, — |
| Application and interpretation of current autophagy inhibitors and activators | The hallmark of autophagy is the formation of a phagophore that engulfs cytosolic materials for degradation and recycling to synthesize essential components. Nrf2 when bound to Keap1 is inhibited, however, if Keap1 is bound to p62 instead then Nrf2 remains active Komatsu et al. Mutant protein-based methods. Cell Biosci 11 , 56 Proc Natl Acad Sci U S A ; 79 : — Google Scholar. Proc Natl Acad Sci USA — |

Wird irgendwie umgehen.