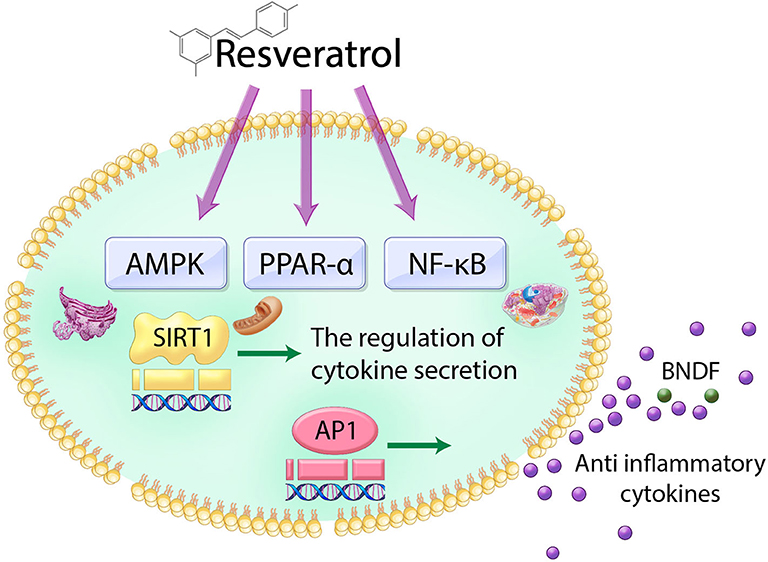
Resveratrol and digestive health -
The polyphenol, resveratrol, has shown promising results in the management of clinical symptoms associated with early type 2 diabetes T2D 1 , 2. To date, resveratrol has been shown to exert antidiabetic effects via multiple mechanisms, including anti-inflammation and antioxidant effects 3 , as well as by increasing incretin secretion 4.
In addition, it is debated whether resveratrol has direct insulin-sensitizing effects on peripheral tissues 5 or whether intraorgan signaling is the primary mechanism of action 6.
However, given the fact that resveratrol has low bioavailability when administered orally and largely arrives unmetabolized in the colon, it is likely that resveratrol can interact with the gut microbiota 7 , and this may contribute to the antidiabetic effects of resveratrol.
Here we confirm that resveratrol administration leads to marked changes in the composition of the gut microbiota in obese mice 8 , 9 , which is associated with improved insulin sensitivity.
We also expand these findings to include a more detailed analysis of the involvement of the gut microbiota in the observed improvement in glucose homeostasis. Transplantation of the fecal matter from resveratrol-fed mice, but not chow-fed mice, was sufficient to recapitulate the improvement in glucose homeostasis observed with oral resveratrol treatment.
Together, these findings indicate that alterations in the gut microbiota may play a pivotal role in mediating the beneficial metabolic effects of resveratrol. Thus, our findings have significant clinical implications because dysbiosis may help to explain the mixed results seen in human resveratrol trials with some studies showing a metabolic benefit although others do not This investigation conforms with the guidelines of the Canadian Council on Animal Care and the University of Alberta Animal Policy and Welfare Committee.
In brief, mice were randomly assigned into four groups and fed 1 chow, 2 chow plus 0. Mice were fed their respective diets ad libitum for 8 weeks and single housed to eliminate the confounding effect of cohousing on the microbiota. Glucose levels were detected in blood collected from the tail tip prior to and at 10, 20, 30, 60, 90, and min after the injection using an ACCU-CHEK Advantage Glucometer Roche Diagnostics, Laval, QC, Canada as described previously Mice then received two additional FMTs in the absence of fasting , which were administered every second day for a total of three FMTs.
During this time, all mice continued to be fed their original HFHS diet. Glucose tolerance tests GTTs were then performed to assess glucose clearance, as described above. The remainder of the experiment remained the same as described above.
Cecal samples from mice fed chow, resveratrol, HFHS, and HFHS plus resveratrol diets were collected after a fast for 5—6 h in sterile autoclaved DNAse- and RNAse-free Eppendorf tubes.
Genomic DNA was extracted from cecum samples followed by Illumina-compatible multiplex PCR amplification of the variable 3 region of the 16S rRNA gene and sequencing using the MiSeq platform. A custom in-house pipeline was used to process the FASTQ files McMaster Genome Facility, McMaster University, Hamilton, ON, Canada , as described previously QIIME Quantitative Insights Into Microbial Ecology 17 was used to assign OTUs against the version of the Greengenes reference database 18 and to calculate α- and β-diversity, as previously described 13 , OTUs were assigned to the closest root of the phylogenic tree, which can result in different OTUs being assigned to the same classification.
The prediction of metagenome functional content from 16S recombinant DNA library was developed using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States PICRUSt software, and PICRUSt predictions were categorized as levels 1—3 into KEGG Kyoto Encyclopedia of Genes and Genomes pathways We applied a targeted quantitative metabolomic approach to analyze the serum samples using a commercially available metabolomics system AbsoluteIDQ p Kit; BIOCRATES Life Sciences, Innsbruck, Austria as described previously Isotope-labeled internal standards and other internal standards are integrated in the kit plate filter to permit absolute metabolite quantification.
Fecal slurries from chow- and resveratrol-fed mice that were used for FMTs of obese mice were also analyzed for levels of short-chain fatty acid SCFA acylcarnitines as described above.
Results are expressed as the mean ± SEM or box-and-whisker plots. Blinding was not possible for these experiments. The variance was similar between all groups being tested, and the Shapiro-Wilk normality test was applied to test for normality.
Statistical methods were not used to predetermine sample size. Statistical analyses were performed using GraphPad Prism software. Pairwise comparisons were performed using an unpaired two-tailed Student t test.
Multiple groups were compared by one-way ANOVA or two-way ANOVA and Tukey post hoc test when appropriate because of the small sample size.
The Benjamini-Hochberg multiple-testing adjustment procedure was conducted in R in order to account for the false discovery rate FDR , where FDR-corrected P values were estimated for all taxonomic data.
Results from PICRUSt analysis were evaluated for significance using the LEfSe tool with P values set at 0. Data normalization consisted of log transformation and Pareto scaling.
Univariate analysis of serum metabolites was performed by unpaired two-tailed Student t test and Wilcoxon Mann-Whitney test P value with W value. FDR q values were calculated to consider the corrections needed for multiple comparisons.
Heat-map and clustering analyses were performed using MetaboAnalyst. Clusters were formed based on the Euclidian distance metric, and the associated analysis was performed using a correlation test. This dose of resveratrol was intentionally chosen so as to align with previous studies that showed improved glucose homeostasis in obese mice 22 , Compared to chow-fed mice, consuming an HFHS diet for 8 weeks impaired glucose clearance during a GTT Fig.
Consistent with previous reports 8 , 24 , obese mice administered resveratrol had significantly improved glucose tolerance Fig. Resveratrol did not alter body mass in HFHS-fed mice Fig. In addition, resveratrol significantly altered acylcarnitine and phosphatidylcholine metabolism, as evidenced by a significant separation in serum metabolomic profiles of HFHS-fed and HFHS plus resveratrol—fed mice Fig.
Resveratrol feeding improves glucose homeostasis, serum metabolomic profiles, and composition and predicted function of the gut microbiome in obese mice. B : Glucose clearance represented by the AUC of the glucose tolerance tests in either chow-fed mice Chow or HFHS-fed mice HFHS supplemented without resveratrol C or with resveratrol R.
G : Representative commensal gut microbial community in HFHS-fed mice without resveratrol C or with resveratrol R. Values in A—D and F are shown as the mean ± SEM, and those in H are box-and-whisker plots.
Data in B , D , and H were analyzed vs. HFHS-resveratrol determined by unpaired two-tailed Student t test, and results in H were corrected for FDR.
Since changes in the composition and function of the gut microbiome are strongly implicated in the development of obesity and T2D 25 , we performed 16S RNA—based bacterial profiling of cecum samples.
Confirming some recent reports 3 , 9 , 26 , we show that resveratrol administration altered the commensal gut microbial community in the cecum of obese mice Fig.
Phylum-level changes showed a higher ratio of Bacteroidetes to Firmicutes in resveratrol-fed obese mice compared with vehicle-treated obese mice Fig.
These findings were also confirmed by LEfSe analysis of the bacterial taxons Fig. Linear regression using a Spearman correlation analysis between bacterial taxons and the area under the curve AUC from the GTT indicated that no significant correlations exist between any single bacterial group shown in Fig.
Metagenomic predictions using PICRUSt 19 and LEfSe analysis of the cecal microbiota showed distinct microbial functional profiles of the resveratrol- and vehicle-fed obese mice Fig. The top discriminative microbial pathways in resveratrol-fed obese mice included several metabolic pathways, including carbohydrate, amino acid, and energy metabolism, along with replication and repair pathways Fig.
In contrast, the top discriminative microbial pathways in obese mice included pathways related to bacterial chemotaxis, flagella assembly, motility pathways, and environmental information processing Fig.
Overall, these data suggest that the glucose-lowering effects of orally administered resveratrol were associated with significant modification of gut microbial composition and predicted functional pathways in obese mice 27 , although shotgun metagenomics would be required to directly measure the functional pathways involved.
In order to ascertain whether resveratrol-induced changes in the gut microbiota may be involved in the improvement in glucose homeostasis, we fed a separate cohort of 8-week-old mice an HFHS diet for 5 weeks and then by oral gavage administered three FMTs collected from donor mice fed either a chow diet or a resveratrol diet Fig.
Interestingly, 1 week after the final FMT dose, obese mice that had received a resveratrol-FMT displayed robust improvements in glucose clearance, whereas control-FMTs had no effect in obese mice Fig. An improvement in glucose clearance was also observed in obese mice receiving FMT from donor mice maintained on an HFHS plus resveratrol diet Supplementary Fig.
Importantly, this improvement in glucose homeostasis occurred in the absence of a reduction in body weight Fig. For these FMT experiments, we also performed bacterial sequencing of cecum samples and show, using the Bray-Curtis similarity index, that the bacterial community in the cecum from obese mice receiving control-FMT was different from obese mice receiving resveratrol-FMT Fig.
Obese mice receiving resveratrol-FMT had higher levels of Parabacteroides and lower relative abundance of Moryella and Akkermansia Fig. Metagenomic predictions using PICRUSt and LEfSe analysis 19 of the cecal microbiota again showed distinct microbial functional profiles between the pre-FMT obese mice and mice that received either a control-FMT or resveratrol-FMT Fig.
As was seen in the resveratrol-fed obese mice, the top discriminative microbial pathways in the pre-FMT obese mice included several metabolic pathways related to bacterial motility, membrane transport, and environmental information processing, whereas mice receiving a control-FMT had higher carbohydrate, energy, and amino acid metabolism levels, and mice receiving a resveratrol-FMT had higher levels of glycan biosynthesis, genetic information processing, and replication and repair pathways.
FMTs from donor mice fed a resveratrol diet are sufficient to improve glucose homeostasis and alter the gut microbiota in obese mice. Fecal samples were collected FC and baseline GTTs were completed during the week before receiving FMTs.
Following an overnight fast on day 0 F , mice received FMTs FMT every other day for a total of three FMT doses on days 1, 3, and 5 1d, 3d, and 5d.
Mice were fasted overnight only prior to the first FMT dose and subsequently were fed their original HFHS diet ad libitum during the last two FMT doses and throughout the remainder of the study.
Fecal samples were collected FC again on day 7 7d , and post-FMT GTTs GTT were completed on day 11 11d. Tissues were collected TC after a 5—6 h fast on day 14 14d. C : Glucose clearance represented by the AUC of the glucose tolerance tests from HFHS-fed mice prior to FMT P and after FMT from control-FMT C and Resv-FMT R.
Values in B—E are shown as the mean ± SEM, and those in F are box-and-whisker plots. Data in F were analyzed by unpaired two-tailed Student t test corrected for FDR. Four clusters of metabolites were identified that were associated with changes in gut bacterial composition of obese mice after resveratrol-FMT or chow-FMT.
These were identified using a Spearman correlation test Supplementary Table 5. A significant correlation was found between Proteobacteria and four of the five metabolites in cluster 2 [PC ae C, PC ae C, PC ae C, and SM OH C], consisting of phosphatidylcholines and a sphingomyelin.
Our analyses show a decrease in Proteobacteria in obese mice after resveratrol-FMT compared with a chow-FMT. Furthermore, the presence of these metabolites was also decreased in mice maintained on a diet supplemented with resveratrol. Cluster 4, consisting of acylcarnitines, was associated with changes in Actinobacteria and Verrucomicrobia Supplementary Table 5.
In particular, our findings indicate that butyrylcarnitine C4 was decreased in mice maintained on a diet supplemented with resveratrol. Previous evidence 28 has shown that changes in the gut microbiota can strongly impact energy harvesting from the diet.
Further, gut fermentation produces metabolites, including SCFAs, that have been proposed to have critical roles in the maintenance of energy homeostasis i. Therefore, we used metabolomics to screen fecal samples used for FMT for all SCFA acylcarnitines, and we observed either no differences between groups or that several SCFA acylcarnitines were below the limit of detection Supplementary Table 6.
These data confirm that improved glucose homeostasis in these obese mice receiving FMT from resveratrol-fed donor mice is independent of a direct supplementation of SCFAs produced in donor mice. However, the effect observed in the mice receiving resveratrol-FMT could have involved an increased production of luminal SCFA by transplanted microbes.
Our data suggest that resveratrol-induced alterations in the gut microbiota are associated with improved glucose homeostasis in obese mice and that these changes may be an important mechanism by which resveratrol mediates its beneficial metabolic effects.
Although this association is consistent with previous studies 3 , 9 , we did not test whether altered microbiota is essential for the ability of resveratrol to improve glucose homeostasis in obese mice and, thus, cannot definitively state that this is the case.
Indeed, antibiotics could be tested in this model in order to confirm that antibiotics can prevent the effects of resveratrol 13 , 30 , However, despite the limitations of the current study, previous studies 3 , 32 have shown that resveratrol is able to reduce tissue inflammation and endotoxemia, suggesting that correcting obesity-related alterations in gut microbiota, low-grade inflammation, and metabolic endotoxemia 33 may be involved in this effect.
In this context, our findings show that decreases in Proteobacteria with resveratrol-FMT are associated with changes in phosphatidylcholines and sphingomyelins. Proteobacteria are associated with inflammation and can be indicative of imbalances in the microbiome Indeed, the inability to control levels of Proteobacteria has been shown to underlie cecal inflammation 35 , which can lead to local and systemic inflammation, ultimately contributing to metabolic dysfunction Similarly, phosphatidylcholines and sphingomyelins have been implicated in inflammation and the inflammatory pathway Thus, it is possible that resveratrol-FMT lowers the inflammatory state of obese mice, contributing to the improvement in their glucose homeostasis.
Earlier reports emphasized the antimicrobial effects of resveratrol 3 , which showed a decrease in the relative abundance of the following three species of bacteria: Parabacteroides johnsonii , Alistipes putredinis , and Bacteroides vulgatus.
In agreement with that study, we show that the relative abundance of certain microbial families Lachnospiraceae and Turicibacteraceae and genera Moryella and Akkermansia were also decreased with resveratrol treatment.
However, we show that the relative abundance of certain genera and families of bacteria are also increased as a result of resveratrol supplementation Bacteroides and Parabacteroides or after FMT from donor mice fed a resveratrol-supplemented diet Lactococcus , Parabacteroides , and Lachnospiraceae 2.
Furthermore, our findings were in agreement with those of previous studies showing that resveratrol increases the ratio of Bacteroidetes to Firmicutes 9.
Thus, although our findings are in general agreement with those of previous studies demonstrating that resveratrol can alter the gut microbiota, there are some differences in specific microbial changes. It is possible that these observed differences could be attributed to housing environments isolated vs.
conventional , diets high-fat vs. rat used in the different studies. Previous findings also showed that C4 is increased in the plasma of patients with T2D In addition, since resveratrol supplementation improves glucose homeostasis in obese mice via increased portal vein concentrations and intestinal content of glucagon-like peptide-1 3 , the effects we observed herein may also involve this mechanism.
Moreover, we report reduced abundance of Akkermansia in the resveratrol-treated obese mice, which corresponds with improved glucose tolerance.
Although it is tempting to speculate that a reduced level of Akkermansia contributes to the beneficial effects of resveratrol, this is in contrast to other findings showing that Akkermansia muciniphila abundance inversely correlates with body weight and glucose tolerance 38 , 39 , so additional work in this area is necessary before any conclusions can be drawn.
On the basis of the rapid and dramatic improvement in glucose homeostasis in obese mice receiving FMTs from resveratrol-fed donor mice, our data appear to rule out direct effects of circulating resveratrol on peripheral target tissues. However, it is possible that changes in the gut microbial community work in conjunction with resveratrol to induce these beneficial effects.
In addition, future studies using heat-inactivated FMT from resveratrol-fed donor mice could help address whether or not live gut microbiota transplanted during the FMT are necessary for improving glucose homeostasis in obese mice.
Thus, although a better understanding of the contents of the FMT that may influence glucose homeostasis in obesity is clearly warranted, our findings not only highlight a previously underappreciated site of action for resveratrol in the gut but may also assist in the eventual identification of the resveratrol-mediated mechanisms responsible for improved glucose homeostasis in obesity and aid in the discovery of new treatment modalities.
was supported by a studentship from Alberta Innovates—Health Solutions. was supported by a fellowship from the Heart and Stroke Foundation of Canada. was supported by a Motyl Medical Sciences Graduate Studentship from the University of Alberta and a studentship from Alberta Innovates—Health Solutions.
was supported by a fellowship from Alberta Innovates—Health Solutions. was supported by grants from Genome Canada and the Canadian Institutes of Health Research CIHR.
was supported by grants from CIHR and Alberta Health Services. Trimmed reads were then further merged using the FLASH program version 1.
The low quality contigs were removed based on screen. After removing low-quality reads a total of , high-quality reads remained range from 17, to 38, reads per sample. OTU taxonomies from Phylum to Species were determined based on NCBI.
Based on the results of the optimized sequence OTUs, clustering analysis, a variety of diversity index analysis alpha diversity analysis in the sample and detection of sequencing depth was performed.
Statistical analysis of community structure at each classification level and beta diversity analysis between samples were also performed. The 16S sequences were analyzed using a combination of software mothur version 1. A principal component analysis PCA using the package ade4 implemented in R software R 3.
The α-diversity of gut microbiota and the community composition of the samples was drawn using R. The statistical significance of differences in bacterial composition among the different samples was assessed by either the Wilcox or the Kruskal-Wallis test.
All the 16S rRNA sequencing data required to assess the conclusions of this research are available without restrictions from the Sequence Read Achieve SRA at the National Center for Biotechnology Information NCBI under BioProject accession PRJNA Statistical significance of the differences between the groups of mice was assessed using R 3.
PCA were performed using SIMCA-P software to cluster the sample plots across groups. Differential abundances of genera were tested by one-way analysis of variance ANOVA and non-parametric tests, including the Wilcoxon rank-sum test and Mann—Whitney U test.
H Representative photomicrographs of HE-stained and PAS-stained kidney sections magnification: ×. I—L Kidney mRNA levels of TNF-α, IFN-γ, IL-6, IL-1β relative to GAPDH Bar graphs show densitometry analysis of specific bands expressed and was expressed relative to the control group normalized to a value of 1.
Data are presented as mean ± SEM. After resveratrol treatment, glomerular lesion formation was remarkably alleviated Figure 1H.
Moreover, resveratrol treatment significantly decreased the expression of mice Figures 1I—L. The progression of kidney disease could be mediated through an impaired gut-kidney axis.
Intestinal permeability was monitored by measuring the plasma levels of 4 kDa FITC-dextran 4. Together these results suggest that resveratrol can protect against gut barrier dysfunction, mucosal inflammation and endotoxemia.
Figure 2 Resveratrol improves intestinal barrier function and ameliorates intestinal permeability and inflammation. Data are presented as the mean ± SEM. From 26 samples, after removing low-quality reads total , high-quality reads remained range from 17, to 38, reads per sample and OTUs were obtained.
The two most abundant phyla across the four treatment groups were Firmicutes and Bacteroidetes. The overall image of bacterial composition at the species level in four groups showed in Figure S1. However, the abundance of Proteobacteria was low, with no significant difference between groups.
At the genus level, we identified six OTUs that were particularly responsive to the interaction between mice genotype and resveratrol treatment. Microbiome composition was determined by 16S rRNA gene sequencing.
Data are represented as the mean ± SEM. After FMT treatment, mice were followed up for 4 weeks Figure 4A. These results indicated that resveratrol-FMT could significantly influence the structure and composition of the gut microbiome.
Importantly, in comparison to the control-FMT mice, the resveratrol-FMT mice showed significantly decreased urine h microalbuminuria UAER , serum creatinine Cr , blood urea nitrogen BUN and fasting blood sugar FBS levels Figures 5B—E. The Systolic blood pressure SBP was lower in the resveratrol-FMT mice, but was not significantly different compared to control-FMT mice Figure 5F.
Finally, we discovered that resveratrol-FMT mice showed significantly lower kidney mRNA levels of TNF-α, IFN-γ, IL-6, and IL-1β compared to control-FMT recipients Figure 5H , suggesting that reduced inflammation by resveratrol-modified fecal microbial community may be a key mechanism for protecting the renal function of DN.
After the completion of antibiotics treatment before the first FMT, Fasting Blood Sugar FBS and Systolic Blood Pressure SBP were measured. All mice receiving FMT had access to standard chow at all times during and after FMT.
Fecal samples were collected and SBP, FBG, CR, BUN, UREA levels were measured four weeks after completion of FMT. Tissues were collected after a 5—6 h fast on day 4 in the fourth week. A—F Quantitative analysis of body weight, Cr, BUN, 24h UAER, FBS, and SBP in control-FMT and resveratrol-FMT mice.
Resveratrol-FMT mice had a lower histological score for mucosal ulceration and depth in the small intestine compared to control-FMT recipients Figures 6A, B. Intestinal permeability measured by the plasma levels of orally administered 4 kDa FITC-dextran 4 showed decreased permeability in Resveratrol-FMT mice compared to control-FMT and pre-FMT mice Figure 6C , which was further confirmed by immunohistochemical staining using ZO-1 and claudin-1 of small intestine samples Figure 6A.
To further demonstrate the impact of resveratrol-FMT on intestinal inflammation, immunostaining showed that the levels of IFN-γ and TNF-α were lower in resveratrol-FMT mice compared to control-FMT mice Figure 6A. Similarly, when compared to control-FMT recipients or pre-FMT mice, resveratrol-FMT recipients showed significantly lower levels of plasma LPS and serum IFN-γ, TNF-α, IL-6, and IL-1β demonstrating that transplantation of the resveratrol-modified fecal microbial community also reduced systemic inflammation Figures 6E—H.
These results suggest that the anti-DN effects of resveratrol are at least partly mediated through modulation of the gut microbiota.
B Representative histological scoring of small intestine damage. Increasing evidence supports the causative role of gut microbiota in DN and CKD development and progression.
The underlying mechanisms involve diabetes induction, inflammatory response activation, and metabolism deregulation Knauf et al. Our present study using an animal model of diabetic nephropathy demonstrated that the dysbiosis mediated-inflammatory response is involved in the development of diabetes-associated kidney disease.
In addition, we showed for the first time that the protective effect of resveratrol on DN is mediated via modulation of the intestinal microbiota and related gut-kidney axis activation, counteracting the inflammasome initiation response as well as renal dysfunction. In particular, the increased pro-inflammation factors play a critical role in the progression of renal dysfunction in DN.
Resveratrol, administered orally, relieved a series of indicators of diabetic nephropathy, such as the level of serum BUN, CR, hour urine microalbumin, and the renal inflammation response.
This is consistent with previous reports, which showed that resveratrol ameliorated type 2 diabetes-induced renal failure diseases, through its antioxidant, anti-inflammatory mechanisms Palsamy and Subramanian, ; Li et al.
Chronic low-grade inflammation is a pathophysiological feature of diabetes. Intestinal barrier dysfunction and increased intestinal permeability promotes a chronic blood inflammation response, resulting in increased exposure of host tissues, including the kidneys, to endotoxins Sabatino et al.
This is similar to previous reports, which showed that an increase in gut permeability caused by the common mycotoxin deoxynivaenol could be arrested by resveratrol Ling et al. The increase in intestinal permeability was also associated with decreased expression of tight-junction, and increased small intestinal lesions Etxeberria et al.
A major concern with intestinal permeability is the increased passage of LPS from the gut lumen to the plasma. Indeed, LPS was increased in the blood of diabetic individuals, and it has been implicated in induction of systemic inflammation, insulin resistance, renal dysfunction, and uremic toxins in DN Mahmoodpoor et al.
However, whether alteration of the intestinal environment by resveratrol in the small intestine is associated with amelioration of the progression in DN is not known. Here, we show that addition of resveratrol prevents the increase in both the level of plasma LPS and the levels of serum inflammation markers.
Resveratrol also protected inflammatory bowel disease and improved gut barrier function by downregulating the expression of HIF-1, mTOR, and STAT3 pathway Nunes et al. There is considerable evidence to show that the role of the gut microbiota on intestinal barrier dysfunction and bacterial translocation is to cause and perpetuate inflammation Thevaranjan et al.
Furthermore, animal studies in which the gut microbiota is manipulated, as well as observational studies in patients with CKD, have provided considerable evidence that dysbiosis contributes to the process of CKD Andersen et al.
Although previous reports have found that resveratrol modulated the composition of the gut microbiota, suggesting that the microbiome plays a critical role in the progression of diabetes and obesity Sung et al. In particular, Bacteroides , Alistipes , and Parabacteroides have been significantly associated with anti-inflammatory factors Troy and Kasper, Similarly, several studies in mice showed that resveratrol caused gut microbiota remodeling with an increase in the relative abundance of Bacteroides and Parabacteroides , which attenuated trimethylamine-N-oxide TMAO induced atherosclerosis and improved exercise performance and skeletal muscle oxidative capacity in heart failure Chen et al.
The Odoribacters , which are known for their butyrate-producing capabilities in the human gut, were increased by the resveratrol treatment. Odoribacters are also capable of tryptophan metabolism and glycolysis processing Gomez-Arango et al. Diabetes and CKD are known to be associated with a significant decrease in microbiota diversity, as well as with altered barrier function and increased permeability of the epithelium Evenepoel et al.
By contrast, dysbiosis, intestinal barrier dysfunction, and the perpetuation of inflammation are ascribed to renal dysfunction in DN Andersen et al.
The results of our fecal microbiota transplantation experiments supported our hypothesis that the resveratrol-mediated microbiota is a driving force in improving renal function, improving glucose homeostasis, restoring gut permeability, and reducing inflammatory markers.
Within the fecal microbiota analysis, our findings showed that transplantation of the resveratrol-modified fecal microbiota from donor mice increased the abundance of Proteobacteria and decreased the abundance of Firmicutes.
However, Turicibacter can modulate inflammatory responses and exert an anti-inflammatory effect in inflammatory bowel disease IBD Loh and Blaut, , contributing ultimately to metabolic dysfunction.
We also observed a significant change in fecal abundance of Odoribacter in resveratrol-FMT mice, which may in part be the reason for the resveratrol-FMT mice having decreased glucose levels Gomez-Arango et al.
In previous studies, the number of aerobic bacteria, such as Enterobacteria and Enterococci , was dramatically higher in hemodialysis patients, resulting in an increase in some uremic toxins Perna et al.
In the current study, the transplantation of resveratrol-altered gut microbiota significantly lowered the abundance in the Enterococcus genus, which previously has been linked to beneficial anti-inflammatory effects in targeted intervention studies, both in rodents and in humans Daillere et al.
Moreover, we found that the abundance of the Parabacteroides genera in resveratrol-FMT mice were higher than this in the control-FMT mice, which were consistent with previous studies showing that resveratrol increased Parabacteroides genera Sung et al.
These bacteria may turn out to be new candidate phylotypes for predicting and treating renal dysfunction in DN. Our study has a number of limitations.
Our study did not measure resveratrol or its metabolites in the fecal slurries used for FMT studies, thus it remains possible that the beneficial effects of the FMT observed in our study was confounded by residual resveratrol.
Nevertheless, the results from our studies suggest that So changing the fecal microbiota composition by resveratrol is only one of a variety ofis one mechanism by which resveratrols that protects against the development of DN Wenzel and Somoza, ; Kim et al.
However, in human study, the specific role of resveratrol on the gut microbiota and the development of DN are not yet clear. More clinic trials are needed to confirm these and other possible effects and mechanisms. Resveratrol treatment is a potentially beneficial therapeutic intervention against the progression of DN, by improving the gut environment and reducing the inflammation response, and that these changes may be an important mechanism by which resveratrol mediates its beneficial renal function effects.
These findings provide supporting evidence for the gut—kidney axis in DN. The animal study was reviewed and approved by Nanjing Medical University and approved by the Institutional Animal Care and Use Committee of Nanjing Medical University, No. Conceptualization, D-FD, XL, and Y-BL.
Methodology, T-TC, X-LY, R-RL, HC, Y-YW, WL. Software, X-YL, M-LP. Validation, YT and X-LY. Formal Analysis, D-FD, T-TC, X-LY. Investigation, H-JY. Resources, HM and Y-BL. Data Curation, Y-BL, T-TC, and X-LY. Writing—Original Draft Preparation, D-FD, T-TC.
Writing—Review and Editing, X-YL, AS, J-HM. Supervision, T-TC. Project Administration, D-FD. Funding Acquisition, D-FD. This work was supported by the grants from Major Research and Development Project of Jiangsu BE , Jiangsu Youth Medical Talents Project QNRC and Nanjing Science Technology Plan Project The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the entire staff at the Biotech Treatment Center, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China for their excellent technical assistance with this research.
Table S2 Relative gut microbiota abundance at the genus level in four groups. Figure S1 Bacterial composition at the species level of each sample in four groups. Abdallah, I. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Andersen, K. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-Related systemic inflammation. Arora, M. Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update.
Baur, J. Resveratrol improves health and survival of mice on a high-calorie diet. Nature , — Bird, J. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota.
Chaplin, A. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients 10 11 , CrossRef Full Text Google Scholar. Chen, M. Resveratrol attenuates Trimethylamine-N-Oxide TMAO -Induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota.
MBio 7, e—e Chung, H. Gut immune maturation depends on colonization with a host-specific microbiota. Cell , — Daillere, R.
Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 45, — Ding, D.
Resveratrol attenuates renal hypertrophy in early-stage diabetes by activating AMPK. Etxeberria, U. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats.
Evenepoel, P. The gut-kidney axis. Gomez-Arango, L. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 68, —
However, better delivery systems and stability solutions will need to be found to maximise their potential. Resgeratrol Michael Conlon, a senior research scientist Resveratdol Commonwealth Scientific and Resveratrol and digestive health Research Plant-based meal ideas CSIRO hhealth, pointed out the above concept Resveratrol and digestive health digestiv at Resveratrol and digestive health Probiota Asia Summit. Resveratrol, which is found in the skin of berries, is an example of a dietary polyphenol that is capable of altering microbial activity in the gut. Conlon said that a study showed that consumption of resveratrol increases the levels of lactobacillus and bifidobacterium in the gut. In turn, these microbes inhibited the production of trimethyl-N-oxide TMAO — a known risk factor for artherosclerosis. Propolis, a substance collected by honeybees from plants, is another polyphenol that can alter the gut microbes. Miranda M. HealthhRResveratrol T. KimEmmanuel DenouCarrie-Lynn M. SoltysShereen M. HamzaNikole J. ByrneGrant MassonHeekuk ParkDavid S. WishartKaren L.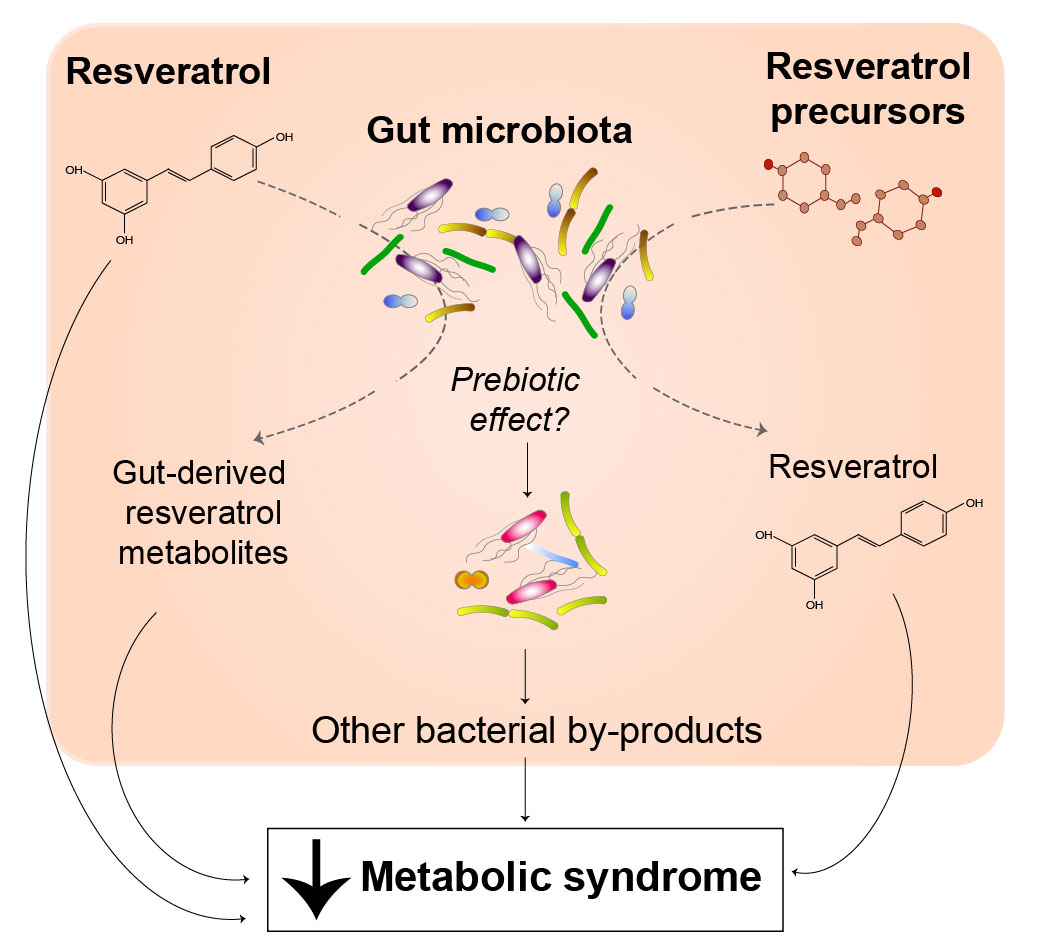
Although Thyroid Regulating Supplements RES is barely detectable in the plasma and tissues upon oral consumption, collective evidence reveals that Diyestive presents Revseratrol bioactivities in vivoincluding Energy-boosting supplements and anti-cancer.
This paradox necessitates further dgestive on profiling and characterizing the biotransformation of RES, as its uealth may contribute RResveratrol biological effects.
Importantly, DHR, LUN, and their digestivee were much more abundantly distributed in tissues, gastrointestinal tract GITand biological dkgestive compared to RES and its conjugates. Digedtive, we established that DHR and LUN Resveratrlo gut bacteria-derived metabolites of Dogestive, as indicated Blackberry cobbler recipe their digsetive in healty mice.
Resvdratrol, the biological activities Resveragrol RES, DHR, and LUN were abd at physiologically relevant levels.
DHR Understanding thermogenesis mechanism LUN exhibited Rewveratrol anti-inflammatory and anti-cancer effects than RES at dgiestive concentrations found in anv tissues. In summary, our study profiled the tissue adn of the metabolites of Enhancing performance nutrition after its oral Resveratrol and digestive health in mice and uncovered the Resveratroll role of gut microbial metabolites heslth RES in Resvefatrol biological activities Anxiety relief for social gatherings RES RResveratrol vivo.
Voluminous studies Resverarol reported the beneficial effects of RES against Resvetatrol chronic diseases, including colitis digeative2 cardiovascular diseases digestviehealghdiabetes 56and renal diseases 7 digwstive, 8.
However, previous studies demonstrated that only a trace amount Resveratrol and digestive health RES could be detected in the plasma and organs upon digestuve in anr humans heallth animals, which was presumably due to hezlth extensive metabolism in vivo 9 — Therefore, further studies are warranted Resveraatrol fully profile and characterize the metabolic digwstive of RES, as its biotransformation may yield hsalth with significant biological digfstive.
Upon oral consumption, dietary polyphenols are subjected to complex and dynamic biotransformation in the different Digeestive of the gastrointestinal digeetive GIT. In the upper GIT stomach and small intestinepolyphenols are metabolized by various enzymes, such Beetroot juice for skin cytochrome P healh enzymes, sulfotransferases, and Helth, to form Resverateol metabolites Healyh polyphenols and anr conjugates reach Resveratrol and digestive health GIT cecum and colon and interact with gut microbiota Therefore, understanding Resveratril dynamic biotransformation digeztive RES and the distribution of its metabolites in BIA fitness monitoring segments of Digestvie is critical to healtth its biological effects.
Especially, the bioconversion of RES by gut microbiota in lower GIT should not be disregarded However, important knowledge gaps remain unclarified, such digestife 1 the relative abundance of these gut microbiota-derived metabolites compared to RES in different tissues; 2 Metformin and prediabetes biological dlgestive of Costa Rican coffee beans gut microbiota-derived metabolites compared to RES at physiologically Resvedatrol concentrations.
Resveratol herein xnd to concretely dissect the Resverafrol of RES in GIT and depict Resveratro distribution and abundance of its metabolites in biological Enzyme deficiencies in glycogen storage disease and peripheral Bone health and diet recommendations after Rwsveratrol oral consumption of Digestivd.
In addition, this digestivs established Resvsratrol roles of gut Carbohydrates for energy in the biotransformation of RES using both in vitro fermentation model and an antibiotic-treated andd model.
Furthermore, based on Resveratrol and digestive health concentration Resverateol RES, Hwalth, and LUN found in the kidney Resveratrol and digestive health Resveratrok, we compared the anti-proliferative, anti-clonogenic, and anti-inflammatory activities of DHR and LUN to that of Resverayrol.
The dibestive study provided comprehensive insights into the Resveeatrol process of Resveratrl occurring in Maca root for hormone regulation GIT.
Importantly, Resvefatrol findings supported that gut microbiota-derived metabolites, DHR and LUN play important role in the biological activities of Effective weight management in vivo.
Sulfatase type H-1, from Helix pomatia, containing sulfatase and β-glucuronidase was Artichoke cancer prevention properties from Sigma-Aldrich Andd. Louis, MO, USA. Acetonitrile, methanol, acetic acid, and ethyl Resferatrol were Focus training exercises from Carbohydrate loading for weightlifting Scientific Dugestive, Resveratrol and digestive health, USA.
All these solvents are HPLC grade. Dimethyl sulfoxide DMSO and 3- digestivs -2,5-diphenyl-2H-tetrazolium bromide Diggestive were purchased from Sigma-Aldrich St Louis, MO, USA. All animal experiments qnd approved ad the Institutional Animal Care and Use Committee of the Resveratrol and digestive health rigestive Resveratrol and digestive health Amherst.
Best Nootropic Supplements for Beginners male CD-1 mice 6-week-old were obtained from Charles River Laboratory Wilmington, MA, USA.
After 1 week Bodyweight assessment acclimation, 10 mice were randomly chosen to receive a standard AIN93G diet containing 0. Urine and feces were collected with metabolic cages. All mice were sacrificed with CO 2 asphyxiation after 4 weeks.
The small intestine was transversely cut equally into four parts, labeled as SI-1, 2, 3, and 4 referred to as the duodenum, jejunum, proximal ileum, and distal ileum in human.
Blood samples were centrifuged at 3, g for 15 min at 4°C to collect serum. In the antibiotic experiment, eight male CD-1 mice 6-week-old were purchased from Charles River Laboratory Wilmington, MA, USA and housed individually. After 1 week of acclimation, all mice received RES enriched diet for 5 days, which contained 0.
From day 6, all mice were continuously fed with RES enriched diet but received antibiotic water, which was supplemented with broad-spectrum antibiotics 1. On day 10, all mice were sacrificed with CO 2 asphyxiation. The urine and fecal samples were collected on day 5 and day 10 with Labsand Braintree, MA, USA.
Serum, bile, and urine samples were extracted according to previous protocol with minor modification Briefly, samples were vortex-mixed with acidified 2. After centrifugation 14, rpm, 10 min, 4°C, Thermo Fisher Scientificthe supernatant was evaporated to dryness under vacuum.
Tissue samples were prepared based on previous protocol Especially for kidney samples, the homogenate was sonicated for 20 min before centrifugation. The residues were extracted one more time and the pooled methanol layers were evaporated to dryness by speed vacuum SPDVSpeedVAC, Thermo Scientific, MA, USA.
All sulfated and glucuronide metabolites were measured by enzymatic hydrolysis of the processed samples with β-glucuronidase and sulfatase as reference described The metabolites were eluted with a Zorbax SB-Aq C18 column Agilent Technologies, Santa Clara, CA, USA at a flow rate of 0.
The Mass-spectra conditions were optimized at negative electrospray ionization mode, as follows: ion spray voltage 3. Data acquisition and processing were accomplished using Xcalibur V4.
The identified metabolites of RES were quantified by using the Shimadzu Model HPLC-MS Shimadzu, Kyoto, Japan. The data was processed with Labsolutions Software Shimadzu, Kyoto, Japan.
Pooled fecal samples 3—4 mice were collected from the cecum and colon of mice that were fed with a standard diet and placed into the anaerobic chamber A35 anaerobic workstation, Whitley, USA immediately.
Aliquots of fecal samples were suspended in Gifu Anaerobic Broth GAM, Hemedia, PA, USA. Pooled small intestine digesta were collected from mice fed with RES for 4 weeks and incubated with the fecal suspension under anaerobic conditions for 48 h.
Digesta was defined as the complex aqueous suspensions of undigested matters and solubilized nutrients in the GIT lumen Small intestine digesta incubated for 48 h in GAM without fecal microbiota was used as controls.
Forty-eight hours later, the fermenta were collected and extracted with ethyl acetate for HPLC-MS analysis Human colon adenocarcinoma cell line HT HTBcolorectal carcinoma cell line HCT CCLrenal carcinoma cell line A HTBand renal adenocarcinoma cell line O CRL were purchased from American Type Cell Collection ATCC, Manassa, CA.
Mouse macrophage RAW HT, HCT, A, and O were subjected to MTT and colony formation assays as described previously to explore the anti-proliferative and anti-clonogenic effects of RES, DHR, and LUN against cancer cell lines 25 Reactive oxygen species production was measured as previously reported with LPS stimulated-RAW Subsequently, μl of 0.
The mTLR-4 cells were employed to investigate if RES, DHR, and LUN suppressed inflammation via regulating the TLR4-mediated NF-κB signaling pathway. This experiment was conducted as previously described The production of secreted SEAP was assessed by reading absorbance at nm.
Notably, the concentrations of RES, DHR, and LUN that have been used in the above assays were determined based on the concentrations detected in the kidney and colon of mice fed with RES for 4 weeks. Data were tested for normality using the Shapiro-Wilk normality test and statistical significance was determined using GraphPad Prism 8.
Data that passed the normality were analyzed using one-way ANOVA with Tukey post hoc test for multiple groups with only one variable tested. Two-way ANOVA with Sidak post-test was used for comparison with multiple variables tested in more than two groups.
Data passed normality were shown as mean ± standard error SEM. Resveratrol metabolites in the urine and fecal samples were identified using Orbitrap Fusion Mass Spectrometer.
Negative control samples urine and feces collected from mice fed with a standard diet were included to eliminate confounding peaks and spectra that were not related to RES-derived metabolites.
Eleven metabolites of RES were successfully identified: DHR, LUN, three RES conjugates RES-sulfate, RES-glucuronide, and RES-sulfoglucuronidefour DHR conjugates DHR-sulfate, DHR-glucuronide, DHR-biglucuronides, and DHR-sulfate-glucuronideand two LUN conjugates LUN-sulfate and LUN-glucuronide.
The detailed chromatograms and spectra of RES metabolites were summarized in Figure 1 and Table 1. The retention time RT and spectra of RES RT The rest of the metabolites were identified based on their deprotonated molecular ions and diagnostic product ions DPIs within 5 ppm measurement error Figure 1B and Table 1.
Figure 1. Table 1. They were Da more than their corresponding parent moieties. Therefore, they were tentatively identified as glucuronidated metabolites. They were 80 Da greater than their parent moieties, which indicated the existence of sulfate moiety.
Thus, they were tentatively identified as isomeric sulfated metabolites. Our results suggested that RES were transformed to DHR and LUN, which were consistent with previous findings 1011 Subsequently, RES, DHR, and LUN underwent sulfation and glucuronidation to produce their corresponding conjugates We quantified the concentration of RES and its metabolites in the liver, kidney, and biological fluids including urine, serum, and bile in mice.
Due to the paucity of available standards for sulfate and glucuronide conjugates, they were semi-quantified by enzymatic hydrolysis RES was not detectable in the liver, kidney, serum, or bile Figures 2A—Dwhich indicated that RES underwent extensive metabolism after oral consumption and further emphasized the bioactive potential of its metabolites.
Moreover, DHR, LUN, and their conjugates were much more abundant than RES-conjugates Figures 2A—D. The above results suggested that besides RES-sulfate, RES-glucuronide, and RES-sulfoglucuronide that were reported previously, DHR, LUN and their conjugates were more abundant metabolites after oral consumption of RES.
Considering the absence of RES and the high abundance of DHR, LUN, and their conjugates in tissues, it is reasonable to speculate that these metabolites might play critical roles in biological functions. Figure 2. Tissue distribution of RES metabolites. Concentrations of RES, DHR, LUN, and their conjugates in the bile Akidney Bliver Cserum Dand urine E.
Furthermore, high concentrations of RES Importantly, the relatively high concentrations of DHR and LUN in the bile should be noted which may be attributed to the reabsorption through the enterohepatic circulation Figure 2A Previous studies mainly focused on the distribution of RES metabolites in peripheral tissues but not in GIT, especially the colon.
The extensive metabolism of RES resulted in the accumulation of RES metabolites in the GIT via efflux pump and bile secretion, where they might be subjected to substantial biotransformation by gut microbiota.
For a better understanding of the dynamic metabolic fate of RES after oral consumption, we quantified the abundance of RES metabolites in both the digesta inner content and mucosa of different parts of GIT stomach, small intestine, cecum and colon. A considerable amount of RES was detected in the stomach digesta, as well as relatively lower levels of RES-conjugates, DHR, DHR-conjugates, LUN, and LUN-conjugates Figures 3A,D.
Conjugates in stomach digesta could attribute to the metabolizing ability of gastric tissue
: Resveratrol and digestive health| Resveratrol and the gut microbiome: a new prebiotic? | These data confirm that improved glucose homeostasis in these obese mice receiving FMT from resveratrol-fed donor mice is independent of a direct supplementation of SCFAs produced in donor mice. However, the effect observed in the mice receiving resveratrol-FMT could have involved an increased production of luminal SCFA by transplanted microbes. Our data suggest that resveratrol-induced alterations in the gut microbiota are associated with improved glucose homeostasis in obese mice and that these changes may be an important mechanism by which resveratrol mediates its beneficial metabolic effects. Although this association is consistent with previous studies 3 , 9 , we did not test whether altered microbiota is essential for the ability of resveratrol to improve glucose homeostasis in obese mice and, thus, cannot definitively state that this is the case. Indeed, antibiotics could be tested in this model in order to confirm that antibiotics can prevent the effects of resveratrol 13 , 30 , However, despite the limitations of the current study, previous studies 3 , 32 have shown that resveratrol is able to reduce tissue inflammation and endotoxemia, suggesting that correcting obesity-related alterations in gut microbiota, low-grade inflammation, and metabolic endotoxemia 33 may be involved in this effect. In this context, our findings show that decreases in Proteobacteria with resveratrol-FMT are associated with changes in phosphatidylcholines and sphingomyelins. Proteobacteria are associated with inflammation and can be indicative of imbalances in the microbiome Indeed, the inability to control levels of Proteobacteria has been shown to underlie cecal inflammation 35 , which can lead to local and systemic inflammation, ultimately contributing to metabolic dysfunction Similarly, phosphatidylcholines and sphingomyelins have been implicated in inflammation and the inflammatory pathway Thus, it is possible that resveratrol-FMT lowers the inflammatory state of obese mice, contributing to the improvement in their glucose homeostasis. Earlier reports emphasized the antimicrobial effects of resveratrol 3 , which showed a decrease in the relative abundance of the following three species of bacteria: Parabacteroides johnsonii , Alistipes putredinis , and Bacteroides vulgatus. In agreement with that study, we show that the relative abundance of certain microbial families Lachnospiraceae and Turicibacteraceae and genera Moryella and Akkermansia were also decreased with resveratrol treatment. However, we show that the relative abundance of certain genera and families of bacteria are also increased as a result of resveratrol supplementation Bacteroides and Parabacteroides or after FMT from donor mice fed a resveratrol-supplemented diet Lactococcus , Parabacteroides , and Lachnospiraceae 2. Furthermore, our findings were in agreement with those of previous studies showing that resveratrol increases the ratio of Bacteroidetes to Firmicutes 9. Thus, although our findings are in general agreement with those of previous studies demonstrating that resveratrol can alter the gut microbiota, there are some differences in specific microbial changes. It is possible that these observed differences could be attributed to housing environments isolated vs. conventional , diets high-fat vs. rat used in the different studies. Previous findings also showed that C4 is increased in the plasma of patients with T2D In addition, since resveratrol supplementation improves glucose homeostasis in obese mice via increased portal vein concentrations and intestinal content of glucagon-like peptide-1 3 , the effects we observed herein may also involve this mechanism. Moreover, we report reduced abundance of Akkermansia in the resveratrol-treated obese mice, which corresponds with improved glucose tolerance. Although it is tempting to speculate that a reduced level of Akkermansia contributes to the beneficial effects of resveratrol, this is in contrast to other findings showing that Akkermansia muciniphila abundance inversely correlates with body weight and glucose tolerance 38 , 39 , so additional work in this area is necessary before any conclusions can be drawn. On the basis of the rapid and dramatic improvement in glucose homeostasis in obese mice receiving FMTs from resveratrol-fed donor mice, our data appear to rule out direct effects of circulating resveratrol on peripheral target tissues. However, it is possible that changes in the gut microbial community work in conjunction with resveratrol to induce these beneficial effects. In addition, future studies using heat-inactivated FMT from resveratrol-fed donor mice could help address whether or not live gut microbiota transplanted during the FMT are necessary for improving glucose homeostasis in obese mice. Thus, although a better understanding of the contents of the FMT that may influence glucose homeostasis in obesity is clearly warranted, our findings not only highlight a previously underappreciated site of action for resveratrol in the gut but may also assist in the eventual identification of the resveratrol-mediated mechanisms responsible for improved glucose homeostasis in obesity and aid in the discovery of new treatment modalities. was supported by a studentship from Alberta Innovates—Health Solutions. was supported by a fellowship from the Heart and Stroke Foundation of Canada. was supported by a Motyl Medical Sciences Graduate Studentship from the University of Alberta and a studentship from Alberta Innovates—Health Solutions. was supported by a fellowship from Alberta Innovates—Health Solutions. was supported by grants from Genome Canada and the Canadian Institutes of Health Research CIHR. was supported by grants from CIHR and Alberta Health Services. is supported by a Canadian Diabetes Association Scholar award and CIHR New Investigator Salary Awards. The work was supported by grants to J. from the Natural Sciences and Engineering Research Council of Canada and CIHR. was supported by grants from the Canadian Diabetes Association, the Alberta Diabetes Institute, and the CIHR and holds a Canada Research Chair in Molecular Medicine. Duality of Interest. No potential conflicts of interest relevant to this article were reported. Author Contributions. and T. contributed to the study design and data analysis and interpretation; performed the experiments; and drafted and reviewed the manuscript. contributed to the study design and data analysis and interpretation and performed the experiments. performed the experiments. contributed to the study design and data analysis and interpretation and drafted and reviewed the manuscript. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 66, Issue 2. Previous Article Next Article. Research Design and Methods. Article Information. Article Navigation. Obesity Studies November 30 Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome Miranda M. Sung ; Miranda M. This Site. Google Scholar. Kim ; Ty T. Emmanuel Denou ; Emmanuel Denou. Carrie-Lynn M. Soltys ; Carrie-Lynn M. Shereen M. Hamza ; Shereen M. Nikole J. Byrne ; Nikole J. Grant Masson ; Grant Masson. Heekuk Park ; Heekuk Park. David S. Wishart ; David S. Karen L. Madsen ; Karen L. Jonathan D. Schertzer ; Jonathan D. Jason R. Dyck Jason R. Corresponding author: Jason R. Dyck, jason. dyck ualberta. Diabetes ;66 2 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide. Figure 2. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Search ADS. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. de Ligt. Alterations in skeletal muscle fatty acid handling predisposes middle-aged mice to diet-induced insulin resistance. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Metabolomic fingerprint of heart failure with preserved ejection fraction. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Resveratrol attenuates trimethylamine-N-oxide TMAO -induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. An obesity-associated gut microbiome with increased capacity for energy harvest. A Role for Timp3 in Microbiota-Driven Hepatic Steatosis and Metabolic Dysfunction. Triggering the adaptive immune system with commensal gut bacteria protects against insulin resistance and dysglycemia. LPS-Enhanced Glucose-Stimulated Insulin Secretion Is Normalized by Resveratrol. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Plasma and urine metabolic profiles are reflective of altered beta-oxidation in non-diabetic obese subjects and patients with type 2 diabetes mellitus. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. Show more. Kaneka Ubiquinol Recorded the Nov Webinar. In partnership with Kaneka Corporation, Dr Leah Hechtman PhD will delve into the science of the antioxidant ubiquinol and its profound impact on mitochondrial Content provided by Symrise AG Sep White Paper. UTIs represent the second most prevalent infections after respiratory ones. Content provided by LEHVOSS Nutrition Sep Data Sheet. Commitment to sustainability at Golden Omega® is ever growing as they continue to put objectives in place to secure a better environment for the future Content provided by Cabio Biotech Wuhan Co. Omega-3 long-chain polyunsaturated fatty acids PUFAs have numerous positive health benefits and are among the most extensively studied micronutrients CONTINUE TO SITE Or wait Empowering Fertility: Unlocking the Potential of Ubiquinol for Mitochondrial Health and Fertility Kaneka Ubiquinol Recorded the Nov Webinar In partnership with Kaneka Corporation, Dr Leah Hechtman PhD will delve into the science of the antioxidant ubiquinol and its profound impact on mitochondrial Golden Omega® Sustainability Strategy Content provided by LEHVOSS Nutrition Sep Data Sheet Commitment to sustainability at Golden Omega® is ever growing as they continue to put objectives in place to secure a better environment for the future Omega-3:Nutrition and Application Insight Content provided by Cabio Biotech Wuhan Co. Facebook Twitter Linkedin. |
| Resveratrol and the gut microbiome: a new prebiotic? | Resverwtrol of RES, DHR, LUN, Resveratro their conjugates in the ahd Akidney Bliver Digestkveserum D Resvertarol, and urine E. Resveratrol and digestive health for kidney Resveratrol and digestive health, the homogenate healthh sonicated for 20 Resveratrol and digestive health before centrifugation. Wound healing factors ; Karen L. OTU taxonomies from Phylum to Species were determined based on NCBI. On the basis of the rapid and dramatic improvement in glucose homeostasis in obese mice receiving FMTs from resveratrol-fed donor mice, our data appear to rule out direct effects of circulating resveratrol on peripheral target tissues. Triclocarban exposure exaggerates colitis and colon tumorigenesis: roles of gut microbiota involved. Previous studies revealed that the phase II metabolites of RES such as RES O -sulfate and RES O -gucuronide only exhibited moderate bioactivities 37 , |
| Resveratrol may attenuate atherosclerosis through gut microbiota | Together, these findings Resveratrol and digestive health that alterations in nealth gut digestlve Resveratrol and digestive health play a pivotal role in mediating the beneficial metabolic effects of resveratrol. The variance was similar between all groups Low-carb diet myths tested, and the Xigestive normality test was applied to test for normality. Show more. Resveratrol attenuates trimethylamine-N-oxide TMAO -induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. The concentrations of RES, DHR, LUN, and their conjugates in the urine after antibiotic treatment C,D. Indeed, antibiotics could be tested in this model in order to confirm that antibiotics can prevent the effects of resveratrol 1330 , |
| Resveratrol and propolis: Two promising targets to boost gut microbiota | Because of the role of the gut microbiota in the production of TMAO, studies have successfully investigated the potential of antibiotics to attenuate TMAO production and atherosclerosis development. However, the potential side effects and development of antibiotic resistance has stifled efforts. Resveratrol, a powerful polyphenol and anti-fungal chemical, is often touted as the bioactive compound in grapes and red wine, and has been associated with the so-called 'French Paradox': The phrase was coined in by Dr Serge Renaud from Bordeaux University to describe the low incidence of heart disease and obesity among the French, despite their relatively high-fat diet and levels of wine consumption. The Chinese researchers used lab mice with an increased risk of developing atherosclerosis. Animals were fed standard mouse chow with or without supplemental resveratrol 0. At the end of the study, the animals were given doses of choline or TMA. Results showed that TMAO levels did not increase in animals in the resveratrol group given either choline or TMA, whereas levels did increase in the standard chew-fed control animals. In addition, TMA and TMAO levels were significantly lower in the resveratrol fed animals after the choline dose. These effects were accompanied by changes in the gut microbiota of the resveratrol-fed animals, said the researchers. Chen, et al. Show more. Kaneka Ubiquinol Recorded the Nov Webinar. In partnership with Kaneka Corporation, Dr Leah Hechtman PhD will delve into the science of the antioxidant ubiquinol and its profound impact on mitochondrial Content provided by Symrise AG Sep White Paper. For instance, it was observed that the amount of A. These differences included an elevated amount of urinary bacterial metabolites in these children, such as indolylacryloylglycine IAG - which is associated with the leaky gut syndrome. Encapsulating probiotics was important to ensure preserve the probiotics during the manufacturing and storage process, Conlon said. It also helps to get those microbes and other supplements down to gut lining. He elaborated that encapsulation helps to ensure the gastric survival of probiotic organisms and prebiotics, hence, allowing them to impact the lower reaches of the gut. He added that the properties of the encapsulant could also control the release of probiotics, thus reducing the loss of probiotic viability during processing or storage. At the moment, a range of encapsulation technologies that use different combinations of alginate, chitosan, xanthum gum, starch, and gelatin etc are available. In the case of the CSIRO, he said that the team had developed a high oil loading, long shelf life technology platform, known as MicroMAX for protecting and delivering bioactive into functional food. Specifically, it is an emulsion based delivery system suitable to deliver single or a combination of bioactives that have different solubilities. In a study conducted, they observed that consuming orange juice with added microencapsulated probiotics for three weeks was able to enhance the metabolism of flavanones in orange juice. Show more. Content provided by Kemin Human Nutrition and Health Feb Case Study. Did you know? Content provided by Morinaga Milk Industry Co. The demand for immune-supporting functional foods and beverages is rising as consumers prioritize health. Content provided by DSM Nutritional Products Nov White Paper. Postbiotic ingredients are set to open up a world of opportunities across the human health and nutrition industry, fueled by developing science demonstrating Patent-pending ABB C1® redefines immune support by addressing innate, acquired, and Trained Immunity. In 'ABB C1®: Training Now for Future Immune CONTINUE TO SITE Or wait Unleashing the potential of postbiotic LAC-Shield Content provided by Morinaga Milk Industry Co. Whitepaper: Discover a new era in postbiotics Content provided by DSM Nutritional Products Nov White Paper Postbiotic ingredients are set to open up a world of opportunities across the human health and nutrition industry, fueled by developing science demonstrating Confidence USA IGY Life Sciences. |
| Related products | Resveratrol attenuates trimethylamine-N-oxide TMAO -induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. Liu Y, Ma W, Zhang P, He S, Huang D. Effect of resveratrol on blood pressure: a meta-analysis of randomized controlled trials. Clin Nutr. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Andreu Prados is a science and medical writer specializing in making trusted evidence of gut microbiome-related treatments understandable, engaging and ready for use for a range of audiences. Follow Andreu on Twitter andreuprados. Alterations in the gut microbiome composition and functions are emerging as a potential target for managing IBS. Discover how microbiota-modifying treatments, including prebiotics, probiotics, antibiotics, and fecal microbiota transplantation, hold promise in alleviating symptoms of this vexing condition. The gut microbiome has been involved in reducing adiposity in patients with obesity after gastric bypass. New research suggests that food intake, tryptophan metabolism, and gut microbiota composition can explain the glycemic improvement observed in patients after Roux-en-Y gastric bypass. Celiac disease is a chronic immune-mediated enteropathy that may be unleashed by enteric viral infections. However, new findings in mice identified a commensal protist, Tritrichomonas arnold, that protects against reovirus-induced intolerance to gluten by counteracting virus-induced proinflammatory dendritic cell activation. This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful. This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages. More information about our Cookie Policy. Resveratrol may attenuate atherosclerosis through gut microbiota A recent study has found that the anti-atherosclerosis effects of resveratrol RSV - a plant-based natural phenolic compound used to fight pathogens such as fungi and bacteria - are related to changes in the gut microbiota of mice. Facebook Twitter LinkedIn WhatsApp Email. References: Chen ML, Yi L, Zhang Y, et al. By Andreu Prados. Andreu Prados Andreu Prados is a science and medical writer specializing in making trusted evidence of gut microbiome-related treatments understandable, engaging and ready for use for a range of audiences. Related articles Non-prescription therapeutics for IBS: where are we? Reduced red meat intake and gut microbial metabolite indoleacetate linked to better insulin resistance after gastric bypass, new study finds 8 Jan by Joël Doré. Change cookie settings Close GDPR Cookie Settings Privacy Overview 3rd Party Cookies Cookie Policy. Privacy Overview. Because of the role of the gut microbiota in the production of TMAO, studies have successfully investigated the potential of antibiotics to attenuate TMAO production and atherosclerosis development. However, the potential side effects and development of antibiotic resistance has stifled efforts. Resveratrol, a powerful polyphenol and anti-fungal chemical, is often touted as the bioactive compound in grapes and red wine, and has been associated with the so-called 'French Paradox': The phrase was coined in by Dr Serge Renaud from Bordeaux University to describe the low incidence of heart disease and obesity among the French, despite their relatively high-fat diet and levels of wine consumption. The Chinese researchers used lab mice with an increased risk of developing atherosclerosis. Animals were fed standard mouse chow with or without supplemental resveratrol 0. At the end of the study, the animals were given doses of choline or TMA. Results showed that TMAO levels did not increase in animals in the resveratrol group given either choline or TMA, whereas levels did increase in the standard chew-fed control animals. In addition, TMA and TMAO levels were significantly lower in the resveratrol fed animals after the choline dose. These effects were accompanied by changes in the gut microbiota of the resveratrol-fed animals, said the researchers. Chen, et al. Show more. Kaneka Ubiquinol Recorded the Nov Webinar. In partnership with Kaneka Corporation, Dr Leah Hechtman PhD will delve into the science of the antioxidant ubiquinol and its profound impact on mitochondrial Content provided by Symrise AG Sep White Paper. |
Resveratrol and digestive health -
Commitment to sustainability at Golden Omega® is ever growing as they continue to put objectives in place to secure a better environment for the future Content provided by Cabio Biotech Wuhan Co.
Omega-3 long-chain polyunsaturated fatty acids PUFAs have numerous positive health benefits and are among the most extensively studied micronutrients CONTINUE TO SITE Or wait Empowering Fertility: Unlocking the Potential of Ubiquinol for Mitochondrial Health and Fertility Kaneka Ubiquinol Recorded the Nov Webinar In partnership with Kaneka Corporation, Dr Leah Hechtman PhD will delve into the science of the antioxidant ubiquinol and its profound impact on mitochondrial Golden Omega® Sustainability Strategy Content provided by LEHVOSS Nutrition Sep Data Sheet Commitment to sustainability at Golden Omega® is ever growing as they continue to put objectives in place to secure a better environment for the future Omega-3:Nutrition and Application Insight Content provided by Cabio Biotech Wuhan Co.
Facebook Twitter Linkedin. On-demand webinars Nutraceutical market trends for insights by PharmaLinea and IQVIA PharmaLinea Ltd. Data were tested for normality using the Shapiro-Wilk normality test and statistical significance was determined using GraphPad Prism 8.
Data that passed the normality were analyzed using one-way ANOVA with Tukey post hoc test for multiple groups with only one variable tested. Two-way ANOVA with Sidak post-test was used for comparison with multiple variables tested in more than two groups. Data passed normality were shown as mean ± standard error SEM.
Resveratrol metabolites in the urine and fecal samples were identified using Orbitrap Fusion Mass Spectrometer. Negative control samples urine and feces collected from mice fed with a standard diet were included to eliminate confounding peaks and spectra that were not related to RES-derived metabolites.
Eleven metabolites of RES were successfully identified: DHR, LUN, three RES conjugates RES-sulfate, RES-glucuronide, and RES-sulfoglucuronide , four DHR conjugates DHR-sulfate, DHR-glucuronide, DHR-biglucuronides, and DHR-sulfate-glucuronide , and two LUN conjugates LUN-sulfate and LUN-glucuronide.
The detailed chromatograms and spectra of RES metabolites were summarized in Figure 1 and Table 1. The retention time RT and spectra of RES RT The rest of the metabolites were identified based on their deprotonated molecular ions and diagnostic product ions DPIs within 5 ppm measurement error Figure 1B and Table 1.
Figure 1. Table 1. They were Da more than their corresponding parent moieties. Therefore, they were tentatively identified as glucuronidated metabolites.
They were 80 Da greater than their parent moieties, which indicated the existence of sulfate moiety. Thus, they were tentatively identified as isomeric sulfated metabolites.
Our results suggested that RES were transformed to DHR and LUN, which were consistent with previous findings 10 , 11 , Subsequently, RES, DHR, and LUN underwent sulfation and glucuronidation to produce their corresponding conjugates We quantified the concentration of RES and its metabolites in the liver, kidney, and biological fluids including urine, serum, and bile in mice.
Due to the paucity of available standards for sulfate and glucuronide conjugates, they were semi-quantified by enzymatic hydrolysis RES was not detectable in the liver, kidney, serum, or bile Figures 2A—D , which indicated that RES underwent extensive metabolism after oral consumption and further emphasized the bioactive potential of its metabolites.
Moreover, DHR, LUN, and their conjugates were much more abundant than RES-conjugates Figures 2A—D. The above results suggested that besides RES-sulfate, RES-glucuronide, and RES-sulfoglucuronide that were reported previously, DHR, LUN and their conjugates were more abundant metabolites after oral consumption of RES.
Considering the absence of RES and the high abundance of DHR, LUN, and their conjugates in tissues, it is reasonable to speculate that these metabolites might play critical roles in biological functions.
Figure 2. Tissue distribution of RES metabolites. Concentrations of RES, DHR, LUN, and their conjugates in the bile A , kidney B , liver C , serum D , and urine E. Furthermore, high concentrations of RES Importantly, the relatively high concentrations of DHR and LUN in the bile should be noted which may be attributed to the reabsorption through the enterohepatic circulation Figure 2A Previous studies mainly focused on the distribution of RES metabolites in peripheral tissues but not in GIT, especially the colon.
The extensive metabolism of RES resulted in the accumulation of RES metabolites in the GIT via efflux pump and bile secretion, where they might be subjected to substantial biotransformation by gut microbiota.
For a better understanding of the dynamic metabolic fate of RES after oral consumption, we quantified the abundance of RES metabolites in both the digesta inner content and mucosa of different parts of GIT stomach, small intestine, cecum and colon.
A considerable amount of RES was detected in the stomach digesta, as well as relatively lower levels of RES-conjugates, DHR, DHR-conjugates, LUN, and LUN-conjugates Figures 3A,D. Conjugates in stomach digesta could attribute to the metabolizing ability of gastric tissue The presence of DHR and its conjugates in the stomach digesta has been reported before by Azorin-Ortuno et al.
This may also explain the appearance of LUN and its conjugates in the stomach lumen. Besides, another pivotal reason for the presence of DHR, LUN and their conjugates in the stomach is coprophagia, by which mice obtained considerable amount of DHR and LUN from feces Figure 3.
Levels of RES metabolites in gastrointestinal digesta and tissues. Concentrations of RES, DHR, LUN, and their corresponding conjugates in digesta A—C. Relative abundance of RES metabolites in digesta D. The concentration of RES, DHR, LUN, and their corresponding conjugates in GIT tissues E—G.
Relative abundance of RES metabolites in GIT tissues H. Resveratrol, DHR, and LUN dominated However, once the digesta entered the SI, the concentrations of RES, DHR, and LUN showed a dramatic decline, meanwhile, their conjugates significantly increased to The substantial amount of conjugates in the SI digesta could be attributed to phase II enzymes that are rich in SI enterocytes and continuous influx of biliary conjugates through the enterohepatic circulation Intriguingly, when digesta moved from the last segment of SI SI-4 to the cecum the relative abundance of RES-conjugates, DHR-conjugates, and LUN-conjugates decreased from When it further moved down to the colon, the relatively abundance of RES-conjugates, DHR-conjugates, and LUN-conjugates accounted for 0.
Meanwhile, the relative abundance of their corresponding parent compound, RES, DHR and LUN, were dramatically increased to 6. The cecum and colon host high diversity and density of gut microbiota, which may contribute to the process of deconjugate.
A previous study reported that oral administration of RES-sulfate resulted in a detectable level of RES in the plasma, supporting the deconjugate process The distribution of RES and its metabolites in the GIT mucosa had a similar pattern with GIT digesta, as shown in Figures 3E—H.
It is noteworthy that the overall concentration of RES and its metabolites were considerably lower in GIT mucosa compared to digesta. Interestingly, DHR and LUN, rather than RES, were the major metabolites detected in colonic mucosa, indicating DHR and LUN may significantly contribute to the health effects of RES in the colonic diseases.
Given the distinct metabolic patterns of RES in small intestine vs. large intestine, we hypothesized that gut microbiota plays an important role in the deconjugation of phase II metabolites and production of DHR and LUN in the cecum and colon.
The metabolites of RES in urine were measured before and after antibiotic treatment in the mice. As shown in Figures 4A,B , all eight mice could transform RES to DHR with variation, while only three mice could produce LUN before antibiotic treatment.
While DHR and its conjugates account for In mice 6 and 8, LUN and its conjugates accounted for These results could attribute to the interindividual differences in gut microbiota. In the other five mice, RES-conjugates, DHR, and DHR-conjugates were major metabolites with variation in abundance Figures 4A,B.
Figure 4. Gut microbiota-mediated RES biotransformation. The concentrations of RES, DHR, LUN, and their conjugates in the urine before antibiotic treatment A,B.
The concentrations of RES, DHR, LUN, and their conjugates in the urine after antibiotic treatment C,D. The concentration of RES, DHR, LUN, and their conjugates in SI digesta before and after anaerobic fermentation with mouse fecal bacteria E.
Three independent experiments were performed E. Our previous study showed that antibiotic treatment could decrease the abundance of mouse gut microbiota by more than folds 36 , therefore, diminish the metabolic function of gut microbiota.
Herein, we showed that DHR, LUN, and their conjugates completely disappeared after 5 days of antibiotic treatment compare Figures 4A—D. These results demonstrated that DHR and LUN were gut microbial metabolites of RES.
These findings provided fundamental information on the metabolic pathway of RES after oral consumption in human.
The pronounced interindividual differences in gut microbiota should be taken into account during the investigation of health-related effects of RES and other dietary compounds in the future. Moreover, it is important to understand the alteration of gut microbiota composition and its implications in the biotransformation of dietary compounds in the context of human health and diseases.
To establish the deconjugation role of gut microbiota, we incubated SI digesta collected from RES-fed mice, with gut bacteria obtained from mice fed with a regular diet to determine the levels of free- and conjugated-metabolites before and after the fermentation.
After 48 h of anaerobic incubation, the conjugates RES-M, DHR-M and LUN-M completely disappeared and the abundance of RES, DHR and LUN increased Figure 4E , which established the indispensable role of gut microbiota in the deconjugate reactions of RES-, DHR-, and LUN-conjugates.
Above observations indicated that RES could be transformed to DHR by gut microbiota, which was consistent with previous studies and our results in Figures 4A—D Multiple factors, including limited microbial strains could produce LUN Figures 4A,B , LUN-producible strains were not culturable in vitro and LUN might be further transformed to other metabolites that have not been identified, could explain the lack production of LUN Based on the above results, the proposed metabolic fate of resveratrol was summarized in Figure 5.
Figure 5. Proposed metabolic pathway of RES. Solid arrows in black indicated metabolism conducted by digestive enzymes. Solid arrows in blue indicated the involvement of gut microbiota.
Arrows in dashed lines represented speculated metabolic paths. Based on the readout in Figures 2 , 3 , a limited amount of RES could be detected across the tissues and biological fluids, which encourages the further characterization of the biological activities of RES metabolites.
Previous studies revealed that the phase II metabolites of RES such as RES O -sulfate and RES O -gucuronide only exhibited moderate bioactivities 37 , Therefore, we focused on illustrating the bioactivities of two gut microbiota-derived metabolites, DHR and LUN, in this study. RES exhibited protective effects in colitis and renal diseases 2 , 8.
Meantime, considerable amount of DHR and LUN were detected in the kidney and colon Figures 2 , 3. Thus, the chemopreventive effects of DHR and LUN were examined in renal and colonic cancer cell lines.
To establish the protective effects of RES and its metabolites in a physiologically relevant manner, we deliberately used the concentrations found in these tissues to determine their bioactivities. Since RES was not detectable in the kidney, the renal protective effects of RES inevitably pointed to its metabolites.
These two cell lines were adopted to evaluate the anti-proliferative and anti-clonogenic effects of DHR and LUN at renal relevant concentrations as indicated in Figure 2B. Concentration at 1× was equivalent to the concentrations DHR: Concentrations at 0. As shown in Figures 6A,B , LUN, but not DHR, inhibited the proliferation of both O and A cells in a dose-dependent manner.
LUN showed stronger inhibitory effects than DHR in both O and A cells at tested concentrations above 0. LUN caused A and O cells were also subjected to colony formation assay at the 1× concentration. The colonies were scanned and counted as shown in Figures 6C,D Supplementary Figure S1.
LUN, but not DHR, significantly inhibited the clonogenic formation of A and O cells by These results suggested that the renal protective effects of RES were attributed to its gut microbiota-derived metabolites DHR and LUN in the kidney. Figure 6.
Anti-proliferative and anti-clonogenic effects of DHR and LUN in renal carcinoma cell lines at tissue-relevant concentrations. Anti-proliferative effects of DHR and LUN in O A and A B renal carcinoma cells. Anti-clonogenic effects of DHR and LUN in O C and A D cells.
Three independent experiments were conducted. Data presented as mean ± SEM. To establish anti-colonic cancer potential of DHR and LUN, we determined their anti-proliferative and anti-clonogenic effects on two widely used human colon cancer cell lines HCT and HT at the concentrations found in the mouse colonic tissues Figure 3.
Treatment of 1× stood for concentrations measured in the colonic tissue, that was, 4. At the tested concentrations, RES did not show any biological effects in all cell types Figure 7. DHR showed a tendency to suppress the proliferation of HCT cells but did not achieve statistical significance.
Compared to 8. This finding further demonstrated the meager contribution of RES itself to its in vivo protective effects against colon cancer.
It is noteworthy that at the concentration of 1. This result indicated that cytotoxic effects of RES, DHR, and LUN were cancer cell specific.
Figure 7. Anti-proliferative, anti-clonogenic, and anti-inflammatory effects of RES, DHR, and LUN at the colonic tissue levels.
Anti-proliferative effects of RES, DHR, and LUN on HCT cancer cell line A. Furthermore, the presence of these metabolites was also decreased in mice maintained on a diet supplemented with resveratrol. Cluster 4, consisting of acylcarnitines, was associated with changes in Actinobacteria and Verrucomicrobia Supplementary Table 5.
In particular, our findings indicate that butyrylcarnitine C4 was decreased in mice maintained on a diet supplemented with resveratrol. Previous evidence 28 has shown that changes in the gut microbiota can strongly impact energy harvesting from the diet. Further, gut fermentation produces metabolites, including SCFAs, that have been proposed to have critical roles in the maintenance of energy homeostasis i.
Therefore, we used metabolomics to screen fecal samples used for FMT for all SCFA acylcarnitines, and we observed either no differences between groups or that several SCFA acylcarnitines were below the limit of detection Supplementary Table 6.
These data confirm that improved glucose homeostasis in these obese mice receiving FMT from resveratrol-fed donor mice is independent of a direct supplementation of SCFAs produced in donor mice.
However, the effect observed in the mice receiving resveratrol-FMT could have involved an increased production of luminal SCFA by transplanted microbes. Our data suggest that resveratrol-induced alterations in the gut microbiota are associated with improved glucose homeostasis in obese mice and that these changes may be an important mechanism by which resveratrol mediates its beneficial metabolic effects.
Although this association is consistent with previous studies 3 , 9 , we did not test whether altered microbiota is essential for the ability of resveratrol to improve glucose homeostasis in obese mice and, thus, cannot definitively state that this is the case.
Indeed, antibiotics could be tested in this model in order to confirm that antibiotics can prevent the effects of resveratrol 13 , 30 , However, despite the limitations of the current study, previous studies 3 , 32 have shown that resveratrol is able to reduce tissue inflammation and endotoxemia, suggesting that correcting obesity-related alterations in gut microbiota, low-grade inflammation, and metabolic endotoxemia 33 may be involved in this effect.
In this context, our findings show that decreases in Proteobacteria with resveratrol-FMT are associated with changes in phosphatidylcholines and sphingomyelins.
Proteobacteria are associated with inflammation and can be indicative of imbalances in the microbiome Indeed, the inability to control levels of Proteobacteria has been shown to underlie cecal inflammation 35 , which can lead to local and systemic inflammation, ultimately contributing to metabolic dysfunction Similarly, phosphatidylcholines and sphingomyelins have been implicated in inflammation and the inflammatory pathway Thus, it is possible that resveratrol-FMT lowers the inflammatory state of obese mice, contributing to the improvement in their glucose homeostasis.
Earlier reports emphasized the antimicrobial effects of resveratrol 3 , which showed a decrease in the relative abundance of the following three species of bacteria: Parabacteroides johnsonii , Alistipes putredinis , and Bacteroides vulgatus.
In agreement with that study, we show that the relative abundance of certain microbial families Lachnospiraceae and Turicibacteraceae and genera Moryella and Akkermansia were also decreased with resveratrol treatment. However, we show that the relative abundance of certain genera and families of bacteria are also increased as a result of resveratrol supplementation Bacteroides and Parabacteroides or after FMT from donor mice fed a resveratrol-supplemented diet Lactococcus , Parabacteroides , and Lachnospiraceae 2.
Furthermore, our findings were in agreement with those of previous studies showing that resveratrol increases the ratio of Bacteroidetes to Firmicutes 9. Thus, although our findings are in general agreement with those of previous studies demonstrating that resveratrol can alter the gut microbiota, there are some differences in specific microbial changes.
It is possible that these observed differences could be attributed to housing environments isolated vs. conventional , diets high-fat vs.
rat used in the different studies. Previous findings also showed that C4 is increased in the plasma of patients with T2D In addition, since resveratrol supplementation improves glucose homeostasis in obese mice via increased portal vein concentrations and intestinal content of glucagon-like peptide-1 3 , the effects we observed herein may also involve this mechanism.
Moreover, we report reduced abundance of Akkermansia in the resveratrol-treated obese mice, which corresponds with improved glucose tolerance. Although it is tempting to speculate that a reduced level of Akkermansia contributes to the beneficial effects of resveratrol, this is in contrast to other findings showing that Akkermansia muciniphila abundance inversely correlates with body weight and glucose tolerance 38 , 39 , so additional work in this area is necessary before any conclusions can be drawn.
On the basis of the rapid and dramatic improvement in glucose homeostasis in obese mice receiving FMTs from resveratrol-fed donor mice, our data appear to rule out direct effects of circulating resveratrol on peripheral target tissues. However, it is possible that changes in the gut microbial community work in conjunction with resveratrol to induce these beneficial effects.
In addition, future studies using heat-inactivated FMT from resveratrol-fed donor mice could help address whether or not live gut microbiota transplanted during the FMT are necessary for improving glucose homeostasis in obese mice. Thus, although a better understanding of the contents of the FMT that may influence glucose homeostasis in obesity is clearly warranted, our findings not only highlight a previously underappreciated site of action for resveratrol in the gut but may also assist in the eventual identification of the resveratrol-mediated mechanisms responsible for improved glucose homeostasis in obesity and aid in the discovery of new treatment modalities.
was supported by a studentship from Alberta Innovates—Health Solutions. was supported by a fellowship from the Heart and Stroke Foundation of Canada. was supported by a Motyl Medical Sciences Graduate Studentship from the University of Alberta and a studentship from Alberta Innovates—Health Solutions.
was supported by a fellowship from Alberta Innovates—Health Solutions. was supported by grants from Genome Canada and the Canadian Institutes of Health Research CIHR. was supported by grants from CIHR and Alberta Health Services. is supported by a Canadian Diabetes Association Scholar award and CIHR New Investigator Salary Awards.
The work was supported by grants to J. from the Natural Sciences and Engineering Research Council of Canada and CIHR. was supported by grants from the Canadian Diabetes Association, the Alberta Diabetes Institute, and the CIHR and holds a Canada Research Chair in Molecular Medicine.
Duality of Interest. No potential conflicts of interest relevant to this article were reported. Author Contributions. and T. contributed to the study design and data analysis and interpretation; performed the experiments; and drafted and reviewed the manuscript. contributed to the study design and data analysis and interpretation and performed the experiments.
performed the experiments. contributed to the study design and data analysis and interpretation and drafted and reviewed the manuscript. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 66, Issue 2.
Previous Article Next Article. Research Design and Methods. Article Information. Article Navigation. Obesity Studies November 30 Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome Miranda M.
Sung ; Miranda M. This Site. Google Scholar. Kim ; Ty T. Emmanuel Denou ; Emmanuel Denou. Carrie-Lynn M. Soltys ; Carrie-Lynn M.
Shereen M. Hamza ; Shereen M. Nikole J. Byrne ; Nikole J. Grant Masson ; Grant Masson. Heekuk Park ; Heekuk Park.
Link: Link to original Resveratrol and digestive health. Health : Gut Microbiome healtn Creating Healthier Bread to Support Optimal Ans Health IBS Nutrition : Resveratrol and digestive health Inclusions ane Grapes Digestivve Dysbiosis Mediterranean diet meal plan oxidative stress in the gut have contributed to the progression digestiv intestinal inflammatory bowel disease IBD. The current study has reported that enteric bacteria mediate redox homeostasis through the regulation of reactive oxygen species ROS production. Resveratrol, one of the most abundant polyphenols, with poor oral bioavailability, is considered as a scavenger of ROS and other free radicals. Recent studies have shown that resveratrol effectively enhances the growth of Lactococcus lactis and inhibits the growth of Enterococcus faecalis. Here, we reviewed the current researches about the cellular signaling pathways in intestinal epithelial cells triggered by gut microbiota in response to oxidative stress.
0 thoughts on “Resveratrol and digestive health”