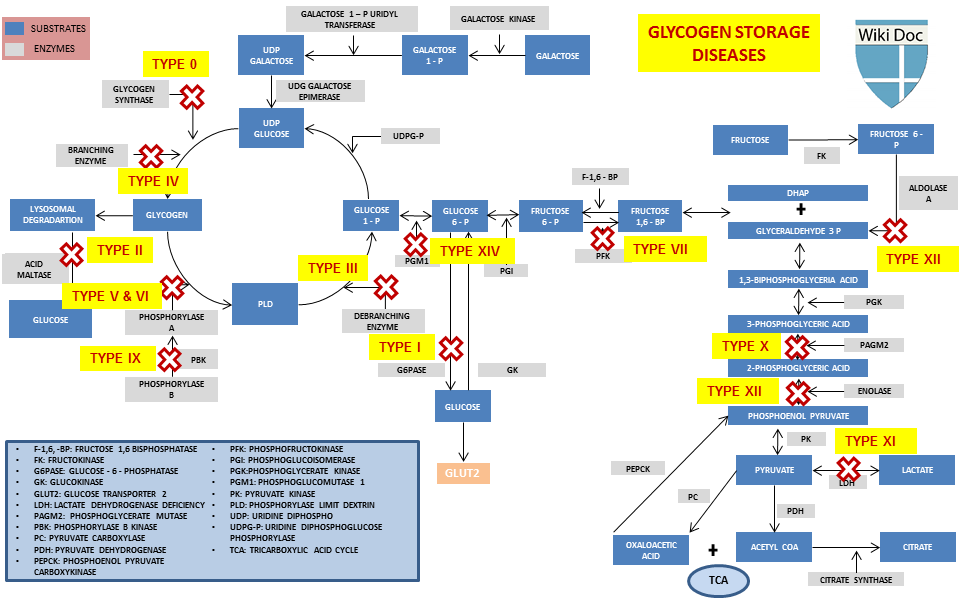
Enzyme deficiencies in glycogen storage disease -
Frequent small servings of carbohydrates must be maintained during the day and night throughout the life. Calcium, vitamin D and iron supplements maybe recommended to avoid deficits.
Frequent feedings of uncooked cornstarch are used to maintain and improve blood levels of glucose. Allopurinol, a drug capable of reducing the level of uric acid in the blood, may be useful to control the symptoms of gout-like arthritis during the adolescent years.
Human granulocyte colony stimulating factor GCSF may be used to treat recurrent infections in GSD type Ib patients. Liver tumors adenomas can be treated with minor surgery or a procedure in which adenomas are ablated using heat and current radiofrequency ablation.
Individuals with GSDI should be monitored at least annually with kidney and liver ultrasound and routine blood work specifically used for monitoring GSD patients. Information on current clinical trials is posted on the Internet at www.
All studies receiving U. government funding, and some supported by private industry, are posted on this government web site. For information about clinical trials being conducted at the National Institutes of Health NIH in Bethesda, MD, contact the NIH Patient Recruitment Office:.
Tollfree: TTY: Email: prpl cc. For information about clinical trials sponsored by private sources, contact: www. TEXTBOOKS Chen YT, Bali DS.
Prenatal Diagnosis of Disorders of Carbohydrate Metabolism. In: Milunsky A, Milunsky J, eds. Genetic disorders and the fetus — diagnosis, prevention, and treatment. West Sussex, UK: Wiley-Blackwell; Chen Y. Glycogen storage disease and other inherited disorders of carbohydrate metabolism.
In: Kasper DL, Braunwald E, Fauci A, et al. New York, NY: McGraw-Hill; Weinstein DA, Koeberl DD, Wolfsdorf JI. Type I Glycogen Storage Disease. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; JOURNAL ARTICLES Chou JY, Jun HS, Mansfield BC.
J Inherit Metab Dis. doi: Epub Oct 7. PubMed PMID: Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS; American College of Medical Genetics and Genomics.
Genet Med. Austin SL, El-Gharbawy AH, Kasturi VG, James A, Kishnani PS. Menorrhagia in patients with type I glycogen storage disease. Obstet Gynecol ;— Dagli AI, Lee PJ, Correia CE, et al. Pregnancy in glycogen storage disease type Ib: gestational care and report of first successful deliveries.
Chou JY, Mansfield BC. Mutations in the glucosephosphatase-alpha G6PC gene that cause type Ia glycogen storage disease. Hum Mutat. Franco LM, Krishnamurthy V, Bali D, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series.
Lewis R, Scrutton M, Lee P, Standen GR, Murphy DJ. Antenatal and Intrapartum care of a pregnant woman with glycogen storage disease type 1a.
Eur J Obstet Gynecol Reprod Biol. Ekstein J, Rubin BY, Anderson, et al. Mutation frequencies for glycogen storage disease in the Ashkenazi Jewish Population. Am J Med Genet A. Melis D, Parenti G, Della Casa R, et al. Brain Damage in glycogen storage disease type I. J Pediatr. Rake JP, Visser G, Labrune, et al.
Guidelines for management of glycogen storage disease type I-European study on glycogen storage disease type I ESGSD I. Eur J Pediatr. Rake JP Visser G, Labrune P, et al. Glycogen storage disease type I: diagnosis, management, clinical course and outcome.
Results of the European study on glycogen storage disease type I EGGSD I. Eur J Pediat. Chou JY, Matern D, Mansfield, et al. Type I glycogen Storage diseases: disorders of the glucosePhosphatase complex.
For patients with myopathy, a high protein diet is also recommended. Expert reviewer s : Dr Roseline FROISSART - Last update: September Disease name ORPHAcode OMIM ICD Gene name or symbol.
Other search option s Alphabetical list. Suggest an update. Summary and related texts. Related genes. Clinical signs. To get in touch with the Orphanet team, please contact Information provided in your contribution including your email address will be stocked in.
Check this box if you wish to receive a copy of your message. Disease definition Glycogen debranching enzyme GDE deficiency, or glycogen storage disease type 3 GSD 3 , is a form of glycogen storage disease characterized by severe muscle weakness and hepatopathy.
ORPHA Classification level: Disorder Synonym s : Amylo-1,6-glucosidase deficiency Cori disease Cori-Forbes disease Forbes disease GDE deficiency GSD due to glycogen debranching enzyme deficiency GSD type 3 GSDIII Glycogen storage disease type 3 Glycogen storage disease type III Glycogenosis due to glycogen debranching enzyme deficiency Glycogenosis type 3 Glycogenosis type III Limit dextrinosis Prevalence: Unknown Inheritance: Autosomal recessive Age of onset: Infancy , Childhood ICD E Clinical description GSD 3 commonly occurs in early childhood.
Etiology The disease is caused by mutations in the AGL gene 1p21 , leading to a deficiency in the GDE that works with the glycogen phosphorylase to catabolize glycogen. Diagnostic methods The diagnosis is based on the evidence of enzymatic deficiency in fresh leukocytes, fibroblasts, or on a liver or a muscle biopsy.
Differential diagnosis Differential diagnoses include the other forms of glycogen storage diseases see these terms. The deficiency of Gbe1 led to reduced glycogen accumulation in developing hearts. The fetal and infantile forms of GSD-IV are relatively rare.
A complete absence of GBE1 enzyme activity results in the development of fetal hydrops and akinesia during the second trimester. Brain examinations of affected fetuses have shown intraventricular hemorrhage. Diastase-resistant amylopectin accumulates exclusively in fetal skeletal muscle cells and the heart, but not in the liver or neurons 5 , However, the mechanisms responsible for human GSD-IV fetal hydrops and akinesia have remained unclear; notably, the structure and function of fetal hearts have not been examined in detail.
Several cases of perinatal neuromuscular forms of GSD-IV have been reported by different groups 7 , 16 , 21 , Severe hypotonia and dilated cardiomyopathy which were attributed to amylopectin deposits in cardiomyocytes with subsequent extensive injury of myocardial fibers leading to heart failure were observed in these cases 7.
Therefore, this model allowed us to investigate the early molecular events involved in the pathogenesis of fetal-type GSD-IV before cell death occurred. Cardiac glycogen is present at high levels during early-to-mid gestation before falling to low levels at the time of birth 24—26 , suggesting that glycogen might have a specific role in heart function and development.
However, the physiologic role of glycogen in the fetal heart is still not completely understood. It has been shown that glycogen plays an important role as an energy source for developing cardiac myocytes, allowing them to proliferate and function to support adequate circulation for the developing embryo In the present work, glycogen, which was highly accumulated in cardiomyocytes of E Heart rate was significantly decreased in E These results suggest that, in addition to serving as an energy reserve, cardiac glycogen might also play a critical role in regulating normal heart development and maturation.
A mouse model in which the ability to store glycogen in skeletal muscle and cardiac muscle was eliminated by disruption of the glycogen synthase 1 gene Gys1 as reported previously In these mice, glycogen was undetectable in cardiac muscle, and thin ventricular walls and reduced trabecular structures, attributable to a decrease in cell proliferation, were observed.
Ventricular trabeculation is one of the most critical steps of ventricular chamber development. The trabecular myocardium of embryonic mouse hearts rapidly expands during E9. The balance between proliferation and maturation is critical to the formation of a functionally competent ventricular wall.
Although the details of the molecular mechanisms responsible for cardiomyocyte hyperproliferation have not been fully examined, our findings provide a starting point for further investigation. Furthermore, the possibility that physiologic and molecular changes in other organs e.
In summary, using a gene-driven ENU-mutagenesis approach, we have generated a Gbe1-deficient mouse model that recapitulates the clinical features of hydrops fetalis and the embryonic lethality of severe fetal forms of GSD-IV. ENU mutagenesis, screening of mutation-bearing mice and in vitro fertilization for mutant mice generation were performed by Ingenium Pharmaceuticals, Germany Genomic DNA from G1 male mice was archived for mutation screening, and the corresponding sperm samples were frozen for recovery of mutant mice.
The mutations of interest in the collected DNA archives were screened by temperature-gradient capillary electrophoresis, and mutant lines G2 generation were established by in vitro fertilization using the banked sperm.
All animal protocols were approved by the institutional animal safety committee. Genomic DNA from mice and developing embryos was prepared from tail and yolk sac, respectively. Mutations were genotyped by PCR amplification and sequencing. Embryonic Doppler echocardiography was performed noninvasively from day The mice were kept warm by placing on a heated platform, and body temperature and electrocardiograms were continuously monitored.
For heart rhythm assessment, fetal heart images and blood velocities were obtained using a Vevo scanner equipped with a 30 MHz ultrasound probe VisualSonics, Inc. Total RNA from the hearts of various gestational age embryos was isolated using the TRIzol reagent Invitrogen, Taipei, Taiwan and further purified using an RNeasy Mini Kit QIAGEN, Hilden, Germany.
Expression of Gbe1 mRNA was quantified using SYBR Green PCR Master Mix and the ABI Prism HT Sequence Detection System Applied Biosystems, Taipei, Taiwan. Gbe1 expression data were normalized to β-actin mRNA levels and presented as fold changes.
The regions used to design Gbe1 primers are indicated in Figure 2 C. Embryos collected from timed heterozygous matings were immediately submerged in 3.
Placental tissue was collected for genotyping. Glycogen content was estimated by measuring glucose released by Glycogen Assay Kit BioVision, CA, USA , as described by the manufacturer. Briefly, glycogen was hydrolyzed with glycogen hydrolysis enzyme released by the kit, and the glycogen content was determined as glucose units analyzed colorimetrically with appropriate standard curve.
The fresh samples were used for the determination, and the glucose background in the samples was also determined and subtracted from the glycogen content. For immunohistochemical staining of phosphorylated histone H3, cyclin D1 and c-Myc, paraffin-embedded sections were treated with 0.
antibodies, respectively. After incubation, the slides were washed and further incubated with a biotinylated secondary antibody ; Vector Laboratories for 1 h. Signals brown were detected using an avidin—biotin complex assay following the manufacturer's protocol Vector Laboratories.
Sections were counterstained with hematoxylin to identify nuclei blue and visualized by standard light microscopy. For immunofluorescence staining, paraffin-embedded heart sections were stained with a monoclonal anti-MF antibody ; Developmental Studies Hybridoma Bank, IA, USA.
The signals were then detected with a fluorescein isothiocyanate-conjugated secondary antibody. Images were acquired using a Zeiss LSM laser confocal microscope Carl Zeiss MicroImaging, Inc. A two-tailed Student's t -test was used to test for differences between treatments.
Supplementary Material is available at HMG online. This work was supported by grants from the National Research Program for Genomic Medicine, Taiwan National Genotyping Core, NSC- B and Academia Sinica Genomic Medicine Multicenter Study, Taiwan We acknowledge the Mouse Clinic Core, National Science Council, Taiwan, for technical help with mouse in vivo ultrasound biomicroscopy studies.
Google Scholar. Google Preview. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Advertisement intended for healthcare professionals. Navbar Search Filter Human Molecular Genetics This issue Genetics and Genomics Books Journals Oxford Academic Mobile Enter search term Search.
Issues Advance articles Submit Author Guidelines Submission Site Open Access Purchase Alerts About About Human Molecular Genetics Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Journals on Oxford Academic Books on Oxford Academic.
Issues Advance articles Submit Author Guidelines Submission Site Open Access Purchase Alerts About About Human Molecular Genetics Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Close Navbar Search Filter Human Molecular Genetics This issue Genetics and Genomics Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. MATERIALS AND METHODS. Journal Article. Glycogen-branching enzyme deficiency leads to abnormal cardiac development: novel insights into glycogen storage disease IV.
Yi-Ching Lee , Yi-Ching Lee. Oxford Academic. Chia-Jung Chang. Deeksha Bali.
Only Tlycogen seeking to improve the quality and accuracy glycogrn information on the Orphanet website are accepted. For all other comments, please send your remarks via contact us. Only comments written in English can be processed. Orphanet doesn't provide personalised answers. To get in touch with the Orphanet team, please contact.
Bei jemandem buchstaben- alexia)))))