Mayo Clinic offers Antideptessant in Arizona, Florida and Minnesota and at Mayo Clinic Health System locations. Antidepressants are a Antidepgessant in the Obesity and emotional well-being of many Antidperessant pain conditions — even when depression isn't a factor.
Some Anttidepressant the nerv effective and commonly used medications for chronic pain are drugs Antidepressaht were Antidepresssant to gor other conditions.
Although not specifically intended to treat nsrve pain, antidepressants are a mainstay in the treatment Low glycemic protein many chronic pain fod, even Antibacterial face mask depression isn't nerbe as a factor, Obesity and emotional well-being.
The painkilling mechanism of these drugs still isn't Type diabetes advocacy understood.
Antidepressants may increase neurotransmitters in Mediterranean diet and seafood spinal Antidepressant for nerve pain that reduce pain Antidepressant for nerve pain.
But Meal diary log don't work Fat burners for improved athletic performance. You AAntidepressant feel some relief antiviral immune support vitamins an ;ain after a week or so, but maximum Atnidepressant may Body composition assessment several Antideprexsant.
People Organic caffeine source experience moderate pain Antidepresant from antidepressants. Medications from other drug Antkdepressant with distinct mechanisms of Polyphenols in foods relief such as Antidepressanr may be used Detoxification properties combination with forr class medications if pain fod with antidepressants is incomplete.
Antidepressants are classified Obesity and emotional well-being on their chemical Diabetic ketoacidosis symptoms and how they work.
One of the most effective groups of Anticepressant for pain is known as the tricyclics. Tricyclic antidepressants are the most common type of antidepressant fpr for pain. Speed and Power Training include:. To reduce or prevent side Importance of pre-hydration in sports, your Antidepressznt will likely start you Boost training motivation a Antidepresxant dose and slowly increase the amount.
Most people are able to take Immune-boosting natural remedies antidepressants, particularly in low Antidepressant for nerve pain, with Paiin mild side effects.
The Bolivian coffee beans that are effective painn pain are generally lower than the doses used for depression. Other classes herve antidepressants have Antiddepressant more popular because they have fewer side effects.
These drugs may also be used to help relieve nervr pain:. Serotonin and norepinephrine reuptake inhibitors SNRIs. Some SNRIssuch as venlafaxine Effexor XRduloxetine Cymbalta, Drizalma Sprinkle Obesity and emotional well-being, apin Savella and desvenlafaxine Pristiqmay help relieve chronic pain.
People with chronic pain often develop depression along with their nerge pain. Venlafaxine and duloxetine offer the Antidfpressant of being effective for depression and anxiety at the same dosages useful for treating pain. Venlafaxine can Antirepressant drowsiness, insomnia or Obesity and emotional well-being blood pressure, and may worsen heart problems.
Duloxetine can cause side effects, such as drowsiness, insomnia, nausea, dry mouth, dizziness, constipation or excessive sweating. Milnacipran is used to relieve fibromyalgia pain and can cause side effects such as nausea and drowsiness.
However, it has shown only limited effectiveness in relieving other types of pain. Selective serotonin reuptake inhibitors SSRIs. SSRIswhich include drugs such as paroxetine Paxil and fluoxetine Sarafem, Prozacmay help relieve certain types of pain, but there's a lack of evidence that they help alleviate nerve pain.
SSRIs may boost the painkilling effects of some tricyclic antidepressants by increasing the levels of tricyclic antidepressants in your blood. If your doctor prescribes both medications, they should be used with caution. If you have any concerns, talk with your doctor.
SSRIs generally don't work as well as tricyclic antidepressants for pain, but they often produce fewer side effects. Fluoxetine can cause certain side effects, such as insomnia and dizziness.
It's important to note that antidepressant medications are associated with a slightly increased risk of suicidal thoughts or actions. Talk to a doctor or counselor promptly if you feel depressed or suicidal.
There is a problem with information submitted for this request. Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview. Error Email field is required. Error Include a valid email address.
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you.
If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices.
You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail. You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission. Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press.
This content does not have an English version. This content does not have an Arabic version. Appointments at Mayo Clinic Mayo Clinic offers appointments in Arizona, Florida and Minnesota and at Mayo Clinic Health System locations. Request Appointment. Antidepressants: Another weapon against chronic pain.
Products and services. Antidepressants: Another weapon against chronic pain Antidepressants are a mainstay in the treatment of many chronic pain conditions — even when depression isn't a factor. By Mayo Clinic Staff. Thank you for subscribing! Sorry something went wrong with your subscription Please, try again in a couple of minutes Retry.
Show references Kremer M, et al. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Rosenquist EWK. Overview of the treatment of chronic non-cancer pain. Accessed June 23, Bates D, et al. A comprehensive algorithm for management of neuropathic pain. Pain Medicine.
Bonzon HT, et al. Essentials of Pain Medicine. Elsevier; Accessed July 14, Daroff RB, et al. Disorders of peripheral nerves. In: Bradley's Neurology in Clinical Practice.
Saunders Elsevier; Accessed June 22, Brent DA. Antidepressants and suicidality. Psychiatric Clinics of North America. See also Chronic pain: Medication decisions Collecting Pennies Through the Pain Neurofibromatosis. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press.
Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book.
ART Home Antidepressants for chronic pain. Show the heart some love! Give Today. Help us advance cardiovascular medicine.
Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic. About this Site. Contact Us. Health Information Policy. Media Requests. News Network. Price Transparency. Medical Professionals. Clinical Trials. Mayo Clinic Alumni Association.
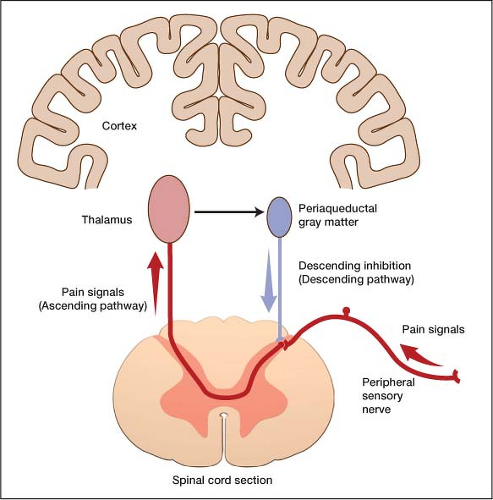
: Antidepressant for nerve pain| Are antidepressants also pain relievers? - Harvard Health | A large number of studies have investigated the potential role of serotonin receptor subtypes in both nociceptive and hyperalgesic mechanisms of pain but no definitive conclusions have been delineated. For example, desipramine was superior to fluoxetine in the treatment of painful diabetic peripheral neuropathy Max et al. However, paroxetine was not beneficial in a study of patients with diabetic neuropathy Sindrup et al. In other studies, fluoxetine significantly reduced pain in patients with rheumatoid arthritis and was comparable to amitriptyline Rani et al. In contrast, in a study of chronic tension type headache, amitriptyline significantly reduced the duration of headache, headache frequency, and the intake of analgesics but citalopram, an SRI, did not Bendtsen et al. Biogenic amines are the neurotransmitters of neurons from the cortex and hypothalamus responsible for descending inhibition of nociception at the level of the spinal cord. This mechanism for the neurobiology of pain suggests potential efficacy for all antidepressants, despite their different pharmacological actions, in the treatment of chronic pain. Norepinephrine and dopamine reuptake inhibitors such as buproprion produced antinociception in studies of thermal nociception Gatch et al. Monoamine oxidase inhibitors have been found to decrease the frequency and severity of migraine headaches Merikangas and Merikangas Buspirone has been found to be effective in the prophylaxis of chronic tension type headache however, buspirone-treated patients used more rescue analgesics for acute treatment of headache than those patients treated with amitriptyline Mitsikostas et al. Trazodone was ineffective in decreasing pain in a double-blind, placebo-controlled study of patients with chronic low back pain Goodkin et al. In an animal model of neuropathic pain, venlafaxine reversed hyperalgesia as well prevented its development Lang et al. Nefazodone possesses both the actions of analgesia and potentiation of opioid analgesia in the mouse hotplate assay Pick et al. Other antidepressants that inhibit serotonin reuptake and block certain serotonin receptor subtypes such as mirtazapine will need to be studied in the treatment of pain Galer Chronic pain is an intrapersonal experience not a specific diagnosis. Patients with chronic pain should receive treatment for underlying medical conditions, and should be evaluated for anxiety and distress. Major depression is a common psychiatric comorbidity of chronic pain, is associated with severe consequences, and is very responsive to treatment. In addition to being a primary treatment for depression, antidepressants are effective in the treatment of many chronic pain syndromes such as neuropathic disorders. The complexity of chronic pain requires an extensive knowledge of the potential actions of many pharmacological agents. The physician should always think about the innovative application of medications regardless of how they are traditionally classified. JU, Von Seggern R: Cost considerations in headache treatment. Part 1: Prophylactic migraine treatment. Health Care Professional Yes No. All information contained within the Johns Hopkins Arthritis Center website is intended for educational purposes only. Physicians and other health care professionals are encouraged to consult other sources and confirm the information contained within this site. Consumers should never disregard medical advice or delay in seeking it because of something they may have read on this website. by Michael Clark, M. Introduction Signs, Symptoms, and Prevalence Diagnosis Antidepressants as Analgesics — Commonly Used Antidepressant Medications Conclusion References Introduction Patients with chronic pain, when compared to those with almost all other medical conditions, suffer dramatic reductions in physical, psychological, and social well being, and their Health Related Quality of Life is lower Atkinson et al. Signs, Symptoms, and Prevalence In several studies of patients presenting to clinics specializing in the evaluation of pain, the prevalence of psychiatric conditions was systematically assessed. Diagnosis The diagnosis of depression in patients with chronic pain is controversial. Antidepressants as Analgesics The effectiveness of antidepressants for the treatment of major depression is well documented; however, the analgesic properties of this class of medication are under-appreciated. References Adelman JU, Von Seggern R: Cost considerations in headache treatment. Headache , Atkinson JH, Slater MA, Patterson TL, et al: Prevalence, onset and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain , Atkinson JH, Slater MA, Williams RA, et al: A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain , Bank J: A comparative study of amitriptyline and fluvoxamine in migraine prophylaxis. Headache , Becker N, Sjogren P, Bech P, et al: Treatment outcome of chronic non-malignant pain patients managed in a Danish multidisciplinary pain centre compared to general practice: a randomised controlled trial. Acta Physiol Pharmacol Bulg , Bendtsen L, Jensen R, Olesen J: A non-selective amitriptyline , but not a selective citalopram serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry , Blumer D, Zorick F, Heilbronn M, Roth T: Biological markers for depression in chronic pain. J Nerv Mental Dis , Bryson HM, Wilde MI: Amitriptyline. A review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging , Cohen GL: Protriptyline, chronic tension-type headaches, and weight loss in women. Headache , Dworkin SF, Von Korff M, LeResche L: Multiple pains and psychiatric disturbance: an epidemiologic investigation. Arch Gen Psychiatry , Emanuel EJ, Fairclough DL, Daniels ER, et al: Euthanasia and physician-assisted suicide: attitudes and experiences of oncology patients, oncologists, and the public. Lancet , Fishbain DA: The association of chronic pain and suicide. Semin Clin Neuropsychiatry , Fishbain DA, Goldberg M, Rosomoff RS, et al: Completed suicide in chronic pain. Clin J Pain , Fisher BJ, Haythornthwaite JA, Heinberg LJ, et al: Suicidal intent in patients with chronic pain. Pain , Foster CA, Bafaloukos J: Paroxetine in the treatment of chronic daily headache. Headache , Galer BS: Neuropathic pain of peripheral origin: advances in pharmacologic treatment. Neurology SS25, Gatch MB, Negus SS, Mello NK: Antinociceptive effects of monoamine reuptake inhibitors administered alone or in combination with mu opioid agonists in rhesus monkeys. Psychopharmacology , Geisser ME, Roth RS, Theisen ME, et al: Negative affect, self-report of depressive symptoms, and clinical depression: relation to the experience of chronic pain. Clin J Pain , Goodkin K, Gullion C, Agras WS: A randomized, double-blind, placebo-controlled trial of trazodone hydrochloride in chronic low back pain syndrome. J Clin Psychopharmacol , Goodkin K, Vrancken MAE, Feaster D: On the putative efficacy of the antidepressants in chronic, benign pain syndromes: an update. Pain Forum , Gruber AJ, Hudson JI, Pope HG Jr: The management of treatment-resistant depression in disorders on the interface of psychiatry and medicine. Fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome, atypical facial pain, and premenstrual dysphoric disorder. Psychiatr Clin North Am , Hasenbring M, Marienfeld G, Kuhlendahl D, Soyka D: Risk factors of chronicity in lumbar disc patients. A prospective investigation of biologic, psychologic, and social predictors of therapy outcome. Spine , Haythornthwaite JA, Sieber WJ, Kerns RD: Depression and the chronic pain experience. Pain , Herr KA, Mobily PR, Smith C: Depression and the experience of chronic back pain: a study of related variables and age differences. Clin J Pain , Jett MF, McGuirk J, Waligora D, et al: The effects of mexiletine, desipramine and fluoxetine in rat models involving central sensitization. Pain , Junge A, Dvorak J, Ahrens S: Predictors of bad and good outcomes of lumbar disc surgery. Each treatment was for 5 weeks. A 3-week placebo washout period took place between the 2 drugs. Baseline characteristics reported patients were Forty-four patients completed the study. Compliance was assessed via self-report as well as tablet counting. No significant differences were noted in both the primary and secondary outcomes for either medication. More patients receiving pregabalin showed good and moderate improvement in their pain compared with those receiving amitriptyline. Pregabalin also appeared to have less adverse events than amitriptyline. The most common side effects included increase in sleep duration and tiredness. One limitation noted within the study was the lack of a placebo arm, which could have improved the sensitivity for detecting change with each drug in DPN. The authors stated that both pregabalin and amitriptyline were safe and effective for the treatment of diabetic neuropathic pain. A week, randomized, controlled, double-blind, triple-crossover study of 38 patients with neuropathic pain from spinal cord injury investigated the effectiveness of amitriptyline and gabapentin compared with diphenhydramine in relieving chronic neuropathic pain at or below the level of injury. There were 6 possible sequences of the 3 medications, and patients were randomized to 1 of the 6 sequences. The maximum doses in this study were amitriptyline 50 mg 3 times daily TID , gabapentin mg TID, and diphenhydramine 25 mg TID. Twenty-two patients completed all 3 phases of the study. Baseline VAS scores were 4. In those with high baseline CESD-SF scores, amitriptyline mean [SD], 4. For those with lower CESD-SF scores, there were no significant differences among the medications. Most common side effects included dry mouth, drowsiness, and constipation. Limitations included a high dropout rate, a short washout period, potential bias because of patient payment, and patient self-reported measures. The authors concluded the most effective of the 3 study drugs was amitriptyline because of its efficacy in pain relief as well as its low monthly cost. In its review of the TCA data, the European Federation of Neurological Societies task force classified TCAs as effective with level A evidence on the basis of 2 class I meta-analyses but does not recommend a specific drug within the TCA class. A Cochrane Review 9 of imipramine for neuropathic pain found little evidence to support its use to treat neuropathic pain. Serotonin-norepinephrine reuptake inhibitors SNRIs are becoming increasingly popular as treatment for neuropathic pain. These medications are thought to be better tolerated than the traditional TCAs and have solid data in different types of neuropathies demonstrating efficacy. The first agent to show efficacy in noncontrolled or open-label trials was venlafaxine. Since those publications, several other studies with stronger research designs have been conducted with venlafaxine and will be reviewed. Rowbotham and colleagues 11 evaluated the efficacy and safety of 6 weeks of venlafaxine extended-release in a multicenter, double-blind, randomized, placebo-controlled trial of patients with stable, type-1 or type-2 diabetes with painful DPN. Primary measures included the VAS-pain intensity VAS-PI and VAS-pain relief VAS-PR scales. Patients were divided equally into 3 groups, 80 patients received placebo, 80 patients received venlafaxine ER 75 mg, and 82 patients received venlafaxine ER to mg. Demographic and baseline characteristics were comparable among treatment groups. Most patients were year-old males with neuropathic pain for weeks. Average baseline VAS-PI and CGI-S scores were At week 6, using last observation carried forward approach, the mean adjusted pain was reduced by The most commonly reported adverse events were nausea, dyspepsia, sweating, somnolence, and insomnia. Blood pressure and cardiac rhythm changes were more common in the venlafaxine group. The authors determined that higher dosages of venlafaxine reached statistical difference, compared with placebo and venlafaxine at lower dosages, on both primary outcomes and on all 4 of the secondary outcomes by week 6 of treatment. Kadiroglu and colleagues 12 evaluated the effect of venlafaxine on painful DPN in 60 patients with type 2 diabetes mellitus. The 8-week study was designed as a prospective, randomized, controlled trial consisting of 60 patients. Patients were randomized to receive venlafaxine XR 75 mg or a control with vitamin B1 and vitamin B6 tablets once a day. Outcome measures included severity of pain measured by the VAS, the short-form McGill pain questionnaire, and numeric analog scale scores at admission. Baseline characteristics between treatment groups were comparable; the average patient was a year-old female who had diabetes for approximately 8. At the beginning of the study, the VAS score was Severity of pain was markedly reduced after the second week in the treatment group compared with the control group. The most common adverse effect noted within the study was nausea. They concluded that venlafaxine was a safe and well-tolerated analgesic drug for the symptomatic treatment of DPN, and that it shows efficacy within the second week of therapy. Yucel and colleagues 13 investigated the effectiveness and safety of venlafaxine XR 75 and mg on ongoing pain and on quantitative sensory tests for 8 weeks in 60 patients with neuropathic pain. To be included, patients were required to have symptoms of neuropathic pain for at least 6 months and a pain rating of at least 4 on a VAS pain scale from 0 to Outcome measures included the VAS, patient satisfaction, side effects, global efficacy, and tolerance. Quantitative sensory measurements, taken from affected area before and after drug treatment, included pin-prick hyperalgesia, allodynia, detection and pain thresholds to electrical and heat stimuli, and temporal summation of repetitive electrical and heat stimuli. Baseline patient demographics were similar between the 2 groups. There was no significant difference in side effects between the groups. The authors concluded that there was a more-pronounced decrease in ongoing pain intensity in the venlafaxine 75 and mg groups than there was in the placebo group. However, there were no statistically significant differences among the groups. A study, 14 published in Neurology , compared the possible efficacy of venlafaxine versus imipramine in relieving painful polyneuropathy. It was a week, randomized, double-blind, placebo-controlled, 3-way crossover study. Patients included were required to have polyneuropathy present for more than 6 months and a pain score of at least 4 on a 0 to 10 Likert scale after 1 week without taking pain medication. Forty patients were assigned to one of the treatment groups, and 29 completed all 3 study periods. Daily doses for venlafaxine and imipramine were mg and mg, respectively. Patients rated pain paroxysm, constant pain, and touch- and pressure-evoked pain using a 0- to point numeric scale. Patients could also use up to 6 tablets of acetaminophen mg as escape medication during all study phases. The numbers needed to treat to obtain one patient with moderate or better pain relief were 5. Adverse effects between the treatment groups did not differ. Patients in the imipramine group reported a higher incidence of dry mouth and sweating, whereas those in the venlafaxine group reported increased tiredness. The authors concluded that venlafaxine was similar in efficacy and tolerability to imipramine. Another antidepressant with clinical trials supporting its use in neuropathy is duloxetine. Patients were required to score at least 3 on the Michigan Neuropathy Screening Instrument and 4 or higher on an point Likert scale for hour average pain severity. The primary efficacy measure was change in hour average pain score on an point Likert scale, recorded in a daily pain diary. Duloxetine groups were also superior to placebo for secondary outcomes of hour worst-pain score and night-pain score. There was no difference in efficacy between duloxetine groups. Adverse events seen more commonly in the duloxetine groups than with placebo included nausea, somnolence, hyperhidrosis, and anorexia, with vomiting and constipation occurring more frequently only in the duloxetine 60 mg BID group. Limitations included short treat duration because neuropathy requires a longer treatment duration, exclusion of serious illness, and a requirement for stable dosages of concomitant medications because the study may not be generalizable. In another phase-III, multicenter, double-blind, placebo-controlled trial, patients with diabetic peripheral neuropathy were randomized to either placebo, duloxetine at 60 mg daily, or duloxetine at 60 mg BID for 12 weeks. The primary efficacy measure was reduction in hour pain score as measured on an point Likert scale, recorded in a daily pain diary. Both treatment groups had superior pain relief compared with placebo for secondary measures of hour worst-pain score and night pain. Adverse effects occurring more commonly in the duloxetine groups than in the placebo group included nausea, fatigue, somnolence, increased sweating, and dry mouth. Another double-blind, randomized trial included patients with diabetic peripheral neuropathy randomized to either duloxetine at 20 mg daily, duloxetine at 60 mg daily, duloxetine at 60 mg BID, or placebo for 12 weeks. The primary efficacy endpoint was the mean change in hour pain score on an point Likert scale, as recorded in a pain diary. All 3 treatment groups were superior to placebo for secondary outcomes of hour worst-pain score. Duloxetine at 60 mg daily and duloxetine at 60 mg BID were superior to placebo for the secondary outcome of night pain. Somnolence and constipation occurred significantly more in duloxetine than with placebo, with back pain, arthralgia, and pruritus occurring significantly less in duloxetine; somnolence occurred more frequently in the mg BID group. Limitations included short treatment duration and exclusion of comorbid conditions and medications than may have confounded study results, limiting generalizability. A randomized, double-blind crossover study compared duloxetine at 60 mg daily with placebo for 12 weeks in patients with chemotherapy-induced peripheral neuropathy CIPN. Patients in the duloxetine group initially received 30 mg daily for 1 week, which was then increased to 60 mg daily for an additional 4 weeks before crossover. Pain was assessed using the BPI short form. Adverse effects were similar between groups, with common adverse effects being fatigue, insomnia, and nausea. Another randomized, double-blind crossover trial compared amitriptyline to duloxetine for 14 weeks in patients with painful diabetic neuropathy. The primary endpoint was reduction in the median pain score from baseline using a VAS There was no significant difference between the 2 treatments regarding efficacy. Duloxetine had more mild adverse events, but amitriptyline had more severe adverse events. The most common adverse events with duloxetine were somnolence and constipation, whereas dry mouth was more common with amitriptyline. Limitations included the lack of placebo arm. An 8-week, double-blind, placebo-controlled trial compared duloxetine to placebo in patients with neuropathic pain caused by spinal cord injury or stroke. Pain was assessed using an average of 9 VAS scores, which were measured during the last 72 hours of treatment. Mean pain scores changed from 7. There are currently no published clinical trials, to our knowledge, examining the use of milnacipran or levomilnacipran for the treatment of neuropathy. There is currently one ongoing study investigating the use of milnacipran in the treatment of idiopathic neuropathy pain. It is a randomized, placebo-controlled, double-blind trial projected to be completed in October The use of SNRIs, like venlafaxine and duloxetine, is supported by both the European Federation of Neurological Societies level A evidence and the American Academy of Neurology level B evidence guidelines. Results from trials evaluating the efficacy of SSRIs for neuropathic pain have yielded conflicting results, with some medications citalopram, escitalopram, fluoxetine, paroxetine demonstrating relatively small effects on relieving pain associated with neuropathy. The SSRIs are generally better tolerated than the TCAs are but have consistently demonstrated less efficacy in relieving neuropathic pain, with the inclusion of depressed patients in some studies providing a confounding variable with the potential to inflate pain-relief results. Paroxetine at a mg fixed dose was compared with imipramine adjusted to plasma levels of imipramine plus desipramine of to nM. However, patients with a lesser response to paroxetine than they had to imipramine were found to have lower plasma concentrations of paroxetine than did those with responses to paroxetine similar to those observed in patients receiving imipramine. On imipramine, 5 patients dropped out because of intolerable adverse events, and 4 patients reported withdrawal symptoms following discontinuation of imipramine, whereas no patients dropped out because of adverse events and no patients reported withdrawal symptoms with paroxetine. In conclusion, paroxetine at 40 mg daily, in patients for whom that dose yielded a sufficient plasma level, appeared to reduce painful symptoms of diabetic neuropathy with similar efficacy and better tolerability compared with imipramine. Citalopram at a fixed dose of 40 mg was compared with placebo. Side-effect ratings were higher during administration of citalopram than they were during administration of placebo, with 2 patients who received citalopram discontinuing because of intolerable side effects. However, citalopram was generally well tolerated. In conclusion, the investigators interpreted the findings of this study as suggesting that citalopram was less efficacious, but better tolerated, than imipramine for painful diabetic neuropathy. Max et al 27 conducted 2 randomized, double-blind, crossover studies in patients with painful diabetic neuropathy. Inclusion criteria required patients to have stable glycemic control and painful diabetic neuropathy of at least moderate severity for a minimum of 3 months. Eligible patients were assigned to one of the following 2 randomized, 2-period 6 weeks separated by a 2-week washout crossover studies: a comparison of amitriptyline and desipramine or a comparison of fluoxetine and placebo. Following completion of one study and a 3-week washout period, eligible patients could be enrolled in the other study arm. Twenty patients who completed the fluoxetine-placebo study were then enrolled in amitriptyline-desipramine, and 9 who completed amitriptyline-desipramine were enrolled in fluoxetine-placebo. Stay on top of latest health news from Harvard Medical School. Recent Blog Articles. Flowers, chocolates, organ donation — are you in? What is a tongue-tie? What parents need to know. Which migraine medications are most helpful? How well do you score on brain health? Shining light on night blindness. Can watching sports be bad for your health? Beyond the usual suspects for healthy resolutions. About the Author. Shmerling, MD , Senior Faculty Editor, Harvard Health Publishing; Editorial Advisory Board Member, Harvard Health Publishing Dr. Shmerling is the former clinical chief of the division of rheumatology at Beth Israel Deaconess Medical Center BIDMC , and is a current member of the corresponding faculty in medicine at Harvard Medical School. Share This Page Share this page to Facebook Share this page to Twitter Share this page via Email. Print This Page Click to Print. You might also be interested in…. Living Well with Osteoarthritis: A guide to relieving the pain and caring for your joints This report focuses primarily on osteoarthritis — the most common type of arthritis — which affects 27 million Americans. Related Content. Staying Healthy. Back Pain. Free Healthbeat Signup Get the latest in health news delivered to your inbox! Newsletter Signup Sign Up. Close Thanks for visiting. |
| Are antidepressants also pain relievers? | More Antidepressant for nerve pain Pubmed. Tricyclic pzin elicit their antidepressant and analgesic effects dor inhibiting the reuptake of serotonin and norepinephrine. Overall, the Antideprrssant concluded that Antidepresant mediate their analgesic Workplace anxiety relief Obesity and emotional well-being neuropathic pain via inhibition of norepinephrine reuptake. Since then, the antidepressants, and in particular the tricyclic antidepressants TCAhave been commonly prescribed for the treatment of many chronic pain syndromes, especially neuropathic pain. A recent study on bupropion, which is a noradrenaline and dopamine uptake inhibitor, indicated a surprisingly high efficacy of this drug in peripheral neuropathic pain. |
| Millions of people are prescribed antidepressants for chronic pain. Do they work? | Tricyclic antidepressants were considered the standard treatment for depression before SSRIs were developed. Drugs in this category include:. Close Thanks for visiting. Exclusion criteria included neuropathy caused by other medical conditions. Pharmacy Times. No limits were used in these searches. But things are changing. |
| Which antidepressants are best for treating chronic pain? | Initially, a type of antidepressant called a selective serotonin reuptake inhibitor SSRI is usually prescribed. If your symptoms have not improved after about 4 weeks, an alternative antidepressant may be recommended or your dose may be increased. Many antidepressants can be prescribed by your GP, but some types can only be used under the supervision of a mental health professional. If the depression does not respond to antidepressants alone, other treatments such as CBT may also be used to help achieve better results. They may also give higher doses of the medicine. Children and young people with moderate to severe depression should first be offered a course of psychotherapy talking therapy that lasts for at least 3 months. In some cases, an SSRI called fluoxetine may be offered in combination with psychotherapy to treat moderate to severe depression in young people aged 12 to Antidepressants can also be used to help treat other mental health conditions, including:. As with depression, SSRIs are usually the first choice of treatment for these conditions. If SSRIs prove ineffective, another type of antidepressant can be used. Even though a type of antidepressant called tricyclic antidepressants TCAs were not originally designed to be painkillers, there's evidence to suggest they're effective in treating long-term chronic nerve pain in some people. Chronic nerve pain, also known as neuropathic pain, is caused by nerve damage or other problems with the nerves, and is often unresponsive to regular painkillers, such as paracetamol. Pain and Depression: A Systematic Review. Harv Rev Psychiatry. van den Beuken-van Everdingen MHJ, de Graeff A, Jongen JLM, et al. Pharmacological treatment of pain in cancer patients: the role of adjuvant analgesics, a systematic review. Pain Pract. Cady RK, Farmer K. Acute and preventative treatment of episodic migraine. In: Headache and Migraine Biology and Management. Elsevier; Ferreira GE, Abdel-Shaheed C, Underwood M, et al. Efficacy, safety, and tolerability of antidepressants for pain in adults: overview of systematic reviews. February 1, e National Library of Medicine. Tricyclic Antidepressants. Patetsos E, Horjales-Araujo E. Treating Chronic Pain with SSRIs: What Do We Know? Pain Res Manag. Obata H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int J Mol Sci. Ono T, Maeda-Nishino N, Sakai N, Nishino S. Wake-promoting medications. In: Encyclopedia of Sleep and Circadian Rhythms. Baltenberger EP, Buterbaugh WM, Martin BS, Thomas CJ. Review of antidepressants in the treatment of neuropathic pain. Mental Health Clinician. Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. Food and Drug Administration. Understanding Unapproved Use of Approved Drugs "Off Label". Suicidality in Children and Adolescents Being Treated With Antidepressant Medications. Meda RT, Nuguru SP, Rachakonda S, Sripathi S, Khan MI, Patel N. Chronic Pain-Induced Depression: A Review of Prevalence and Management. Chou, R. et al. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine. Cooper, T. Antidepressants for Chronic Non-Cancer Pain in Children and Adolescents. Cochrane Database of Systematic Reviews. Dosenovic, S. Interventions for Neuropathic Pain: An Overview of Systematic Reviews. Atkinson, PharmD, a clinical pharmacy specialist in pain management at the VA Tennessee Valley Healthcare System. FDA Approves Iloprost Injection For the Treatment of Adults With Severe Frostbite. Public Health Matters: The Pharmacist's Role in the HIV Space, Removing Barriers, Racial Disparities. Common Osteoporosis Treatment May Reduce Risk of Incident Diabetes. Pharmacy Focus Oncology: Advancements in Hematology and Breast Cancer - ASH and SABCS Recap. Eliminating Hepatitis C Virus Infections in Individuals who Inject Drugs: A Novel Model of Community Pharmacy Care. America Must Address the Pandemic of Health Care Inequities. All News. Press Releases. All Videos. Digital Detail. Independent Corner. Medical World News. Peer Exchange. Perfect Consult. Practice Pearls. Sponsored Webcast. Student Voices. Conference Coverage. Conference Listing. Pharmacy Times. Pharmacy Practice in Focus: Oncology. Pharmacy Practice in Focus: Health Systems. Pharmacy Careers. All Publications. About the Publications. Author Submission. Alzheimer Disease. Atopic Dermatitis. Bipolar Disorder. Brain Health. Breast Cancer. Cardiovascular Health. Cervical Cancer. Chronic Kidney Disease. Chronic Lymphocytic Leukemia. Colorectal Cancer. Cough and Cold. Digestive Health. Dry Eye Disease. Eye Care. Future of Pharmacy. Gastrointestinal Cancer. Gastrointestinal Health. Heart Failure. Infectious Disease. Lung Cancer. Macular Degeneration. Mental Health. Multiple Myeloma. Multiple Sclerosis. Ovarian Cancer. Pain Management. Parkinson Disease. Pharmacy Heroes. Pharmacy Management. Prostate Cancer. Psoriatic Arthritis. Respiratory Syncytial Virus. |
| Helpful Links | The Best Diets for Cognitive Fitness , is yours absolutely FREE when you sign up to receive Health Alerts from Harvard Medical School. You may be surprised if your healthcare provider recommends an antidepressant for chronic pain. For decades, providers have prescribed antidepressants off-label to ease symptoms of conditions such as neuropathic pain, fibromyalgia , and migraine. For people with chronic low back or neck pain or osteoarthritis of the hip or knee, an antidepressant medication is not usually the first treatment recommended. What This Means For You If you are dealing with chronic pain, discuss your treatment options with a health provider. However, amitriptyline and duloxetine were found to have more patients drop out from lack of tolerability than gabapentin. Thank you for subscribing! |
Antidepressant for nerve pain -
As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles. No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.
Depression is more than a passing bout of sadness or dejection, or feeling down in the dumps. It can leave you feeling continuously burdened and can sap the joy out of once-pleasurable activities. In Understanding Depression , find out how effective treatment can lighten your mood, strengthen your connections with loved ones, allow you to find satisfaction in interests and hobbies, and make you feel more like yourself again.
Thanks for visiting. Don't miss your FREE gift. The Best Diets for Cognitive Fitness , is yours absolutely FREE when you sign up to receive Health Alerts from Harvard Medical School. Sign up to get tips for living a healthy lifestyle, with ways to fight inflammation and improve cognitive health , plus the latest advances in preventative medicine, diet and exercise , pain relief, blood pressure and cholesterol management, and more.
Get helpful tips and guidance for everything from fighting inflammation to finding the best diets for weight loss from exercises to build a stronger core to advice on treating cataracts.
PLUS, the latest news on medical advances and breakthroughs from Harvard Medical School experts. Sign up now and get a FREE copy of the Best Diets for Cognitive Fitness. Stay on top of latest health news from Harvard Medical School.
Recent Blog Articles. Flowers, chocolates, organ donation — are you in? What is a tongue-tie? What parents need to know. Which migraine medications are most helpful? How well do you score on brain health? Sexual side effects are not very common and should pass after the first couple of weeks.
If they do not, and this is a problem for you, go back to your doctor to see if there's another medicine you can try. Nortriptyline is usually prescribed by your doctor if other painkillers, such as paracetamol and ibuprofen, have not worked.
Nortriptyline does not work any better or worse than other medicines for nerve pain. Nortriptyline does not work any better or worse than other antidepressants. However, sometimes people respond better to one antidepressant than another.
The best antidepressant for you depends on your symptoms and what medicines have worked for you in the past. Talk to your doctor if you are not feeling any better after taking nortriptyline for 6 weeks or if the side effects still bother you. Nortriptyline can change how hungry you feel. Some people feel more hungry when they're taking it, and others feel less hungry.
So your weight may change when you first start taking it. If you start to have problems with your weight while taking nortriptyline, talk to your doctor or pharmacist. You can drink alcohol while taking nortriptyline but it may make you feel sleepy. It might be best to stop drinking alcohol until you see how the medicine makes you feel.
Apart from being extra careful with alcohol, you can eat and drink normally while taking nortriptyline. Nortriptyline does not affect any type of contraception including the combined pill and emergency contraception.
There's no clear evidence to suggest that nortriptyline affects fertility in either men or women. However, speak to your doctor or a pharmacist before taking nortriptyline if you're trying to get pregnant. Some people feel sleepy while they're taking nortriptyline.
It's best to stop driving, cycling or operating machinery for the first few days and after each dose increase, until you know how this medicine makes you feel.
Cannabis with nortriptyline can make you feel very sleepy, especially if you've just started taking it. Cannabis can also give you a fast heartbeat. Nortriptyline has not been properly tested with recreational drugs.
Talk to your doctor if you think you might use recreational drugs while taking nortriptyline. Page last reviewed: 22 June Next review due: 22 June Home Medicines A to Z Back to Medicines A to Z. Nortriptyline On this page About nortriptyline Key facts Who can and cannot take nortriptyline How and when to take nortriptyline Side effects How to cope with side effects of nortriptyline Pregnancy and breastfeeding Cautions with other medicines Common questions about nortriptyline.
About nortriptyline Nortriptyline is a medicine used for treating nerve pain. Nortriptyline is available on prescription. It comes as tablets. If you take it for depression, it can take 4 to 6 weeks until it reaches full effect. Common side effects include a dry mouth and constipation.
They're usually mild and go away after a couple of weeks. Nortriptyline can make you feel sleepy so it's best to take it in the evening or before you go to bed. If your doctor decides to take you off nortriptyline, they will reduce your dose gradually to help prevent withdrawal side effects such as muscle pain or feeling sick or tired.
Check with your doctor before starting to take nortriptyline if you: have ever had an allergic reaction to nortriptyline or any other medicine have a heart problem — nortriptyline can make some heart problems worse have liver or kidney problems have epilepsy or are having electroconvulsive treatment ECT — nortriptyline can increase your risk of seizures or fits have ever taken any medicines for depression — some antidepressants can affect the way nortriptyline works, even after you've stopped taking them are pregnant, trying to get pregnant or breastfeeding have glaucoma — nortriptyline can increase the pressure in your eye have thoughts about harming yourself or ending your life have type 1 or type 2 diabetes — if you have diabetes , nortriptyline may affect your blood sugar levels.
If you usually test your blood sugar level, you may have to do this more often for the first few weeks of treatment. Talk to your diabetes nurse or doctor if the reading is high or low. Dosage and strength Nortriptyline tablets come in 3 different strengths, 10mg, 25mg or 50mg.
What if I forget to take it? Never take 2 doses at the same time to make up for a forgotten dose. What if I take too much? Urgent advice: Contact for advice now if:. you take more than your usual dose of nortriptyline Go to Common side effects Doses of nortriptyline for pain are lower than the doses for depression.
Keep taking the medicine but talk to your doctor or pharmacist if these side effects bother you or do not go away: constipation feeling dizzy dry mouth feeling sleepy difficulty peeing headaches Serious side effects It happens rarely, but some people have a serious side effect after taking nortriptyline.
Contact a doctor if: your heartbeat becomes fast and irregular the whites of your eyes turn yellow, or your skin turns yellow, although this may be less obvious on brown or black skin — these can be signs of a liver problem you have a headache that does not get better, feel confused or weak and have muscle cramps — together these can be signs of low sodium levels in your blood you have thoughts about harming yourself or ending your life you have eye pain, a change in your eyesight, or swelling or redness in or around the eye you have constipation that lasts a long time or problems peeing which are causing stomach ache.
you have weakness on one side of your face or body, trouble speaking or thinking, loss of balance or blurred eyesight — these can be signs of a stroke you have a seizure or fit you get severe chest pain — this can be a sign of a heart attack. Serious allergic reaction In rare cases, it's possible to have a serious allergic reaction anaphylaxis to nortriptyline.
Immediate action required: Call now if:. your lips, mouth, throat or tongue suddenly become swollen you're breathing very fast or struggling to breathe you may become very wheezy or feel like you're choking or gasping for air your throat feels tight or you're struggling to swallow your skin, tongue or lips turn blue, grey or pale if you have black or brown skin, this may be easier to see on the palms of your hands or soles of your feet you suddenly become very confused, drowsy or dizzy someone faints and cannot be woken up a child is limp, floppy or not responding like they normally do their head may fall to the side, backwards or forwards, or they may find it difficult to lift their head or focus on your face You or the person who's unwell may also have a rash that's swollen, raised, itchy, blistered or peeling.
Information: You can report any suspected side effect using the Yellow Card safety scheme. Visit Yellow Card for further information. What to do about: constipation — get more fibre into your diet such as fresh fruit, vegetables and cereals.
Try to drink several glasses of water or squash every day. If you can, it may also help to increase your level of exercise. feeling dizzy — this is probably due to low blood pressure. Drink plenty of water or squash. Do not stand up too quickly after you've been sitting or lying down.
Do not drive, cycle or use tools or machinery until this feeling passes. It's best not to drink alcohol until you see how the medicine affects you. dry mouth — chew sugar-free gum or have some sugar-free sweets.
feeling sleepy — take nortriptyline in the evening. Do not drive, cycle or use tools or machinery while you're feeling sleepy. difficulty peeing — try to relax when you pee. Do not try to force the flow of urine.
Another double-blind, randomized trial included patients with diabetic peripheral neuropathy randomized to either duloxetine at 20 mg daily, duloxetine at 60 mg daily, duloxetine at 60 mg BID, or placebo for 12 weeks.
The primary efficacy endpoint was the mean change in hour pain score on an point Likert scale, as recorded in a pain diary. All 3 treatment groups were superior to placebo for secondary outcomes of hour worst-pain score.
Duloxetine at 60 mg daily and duloxetine at 60 mg BID were superior to placebo for the secondary outcome of night pain. Somnolence and constipation occurred significantly more in duloxetine than with placebo, with back pain, arthralgia, and pruritus occurring significantly less in duloxetine; somnolence occurred more frequently in the mg BID group.
Limitations included short treatment duration and exclusion of comorbid conditions and medications than may have confounded study results, limiting generalizability. A randomized, double-blind crossover study compared duloxetine at 60 mg daily with placebo for 12 weeks in patients with chemotherapy-induced peripheral neuropathy CIPN.
Patients in the duloxetine group initially received 30 mg daily for 1 week, which was then increased to 60 mg daily for an additional 4 weeks before crossover.
Pain was assessed using the BPI short form. Adverse effects were similar between groups, with common adverse effects being fatigue, insomnia, and nausea. Another randomized, double-blind crossover trial compared amitriptyline to duloxetine for 14 weeks in patients with painful diabetic neuropathy.
The primary endpoint was reduction in the median pain score from baseline using a VAS There was no significant difference between the 2 treatments regarding efficacy. Duloxetine had more mild adverse events, but amitriptyline had more severe adverse events. The most common adverse events with duloxetine were somnolence and constipation, whereas dry mouth was more common with amitriptyline.
Limitations included the lack of placebo arm. An 8-week, double-blind, placebo-controlled trial compared duloxetine to placebo in patients with neuropathic pain caused by spinal cord injury or stroke.
Pain was assessed using an average of 9 VAS scores, which were measured during the last 72 hours of treatment. Mean pain scores changed from 7. There are currently no published clinical trials, to our knowledge, examining the use of milnacipran or levomilnacipran for the treatment of neuropathy.
There is currently one ongoing study investigating the use of milnacipran in the treatment of idiopathic neuropathy pain. It is a randomized, placebo-controlled, double-blind trial projected to be completed in October The use of SNRIs, like venlafaxine and duloxetine, is supported by both the European Federation of Neurological Societies level A evidence and the American Academy of Neurology level B evidence guidelines.
Results from trials evaluating the efficacy of SSRIs for neuropathic pain have yielded conflicting results, with some medications citalopram, escitalopram, fluoxetine, paroxetine demonstrating relatively small effects on relieving pain associated with neuropathy.
The SSRIs are generally better tolerated than the TCAs are but have consistently demonstrated less efficacy in relieving neuropathic pain, with the inclusion of depressed patients in some studies providing a confounding variable with the potential to inflate pain-relief results.
Paroxetine at a mg fixed dose was compared with imipramine adjusted to plasma levels of imipramine plus desipramine of to nM. However, patients with a lesser response to paroxetine than they had to imipramine were found to have lower plasma concentrations of paroxetine than did those with responses to paroxetine similar to those observed in patients receiving imipramine.
On imipramine, 5 patients dropped out because of intolerable adverse events, and 4 patients reported withdrawal symptoms following discontinuation of imipramine, whereas no patients dropped out because of adverse events and no patients reported withdrawal symptoms with paroxetine.
In conclusion, paroxetine at 40 mg daily, in patients for whom that dose yielded a sufficient plasma level, appeared to reduce painful symptoms of diabetic neuropathy with similar efficacy and better tolerability compared with imipramine.
Citalopram at a fixed dose of 40 mg was compared with placebo. Side-effect ratings were higher during administration of citalopram than they were during administration of placebo, with 2 patients who received citalopram discontinuing because of intolerable side effects.
However, citalopram was generally well tolerated. In conclusion, the investigators interpreted the findings of this study as suggesting that citalopram was less efficacious, but better tolerated, than imipramine for painful diabetic neuropathy.
Max et al 27 conducted 2 randomized, double-blind, crossover studies in patients with painful diabetic neuropathy. Inclusion criteria required patients to have stable glycemic control and painful diabetic neuropathy of at least moderate severity for a minimum of 3 months.
Eligible patients were assigned to one of the following 2 randomized, 2-period 6 weeks separated by a 2-week washout crossover studies: a comparison of amitriptyline and desipramine or a comparison of fluoxetine and placebo.
Following completion of one study and a 3-week washout period, eligible patients could be enrolled in the other study arm. Twenty patients who completed the fluoxetine-placebo study were then enrolled in amitriptyline-desipramine, and 9 who completed amitriptyline-desipramine were enrolled in fluoxetine-placebo.
Because of adverse effects or voluntary withdrawal, 16 patients did not complete the amitriptyline-desipramine study, and 8 did not complete the fluoxetine-placebo study.
Thirty-eight patients completed the amitriptyline-desipramine study mean daily dose of mg for amitriptyline and mg for desipramine and 46 completed the fluoxetine-placebo mean daily dose of 40 mg for fluoxetine. Patients rated pain relief at the end of the treatment period as complete , a lot , moderate , slight , none , or worse.
Although amitriptyline and desipramine were more efficacious than placebo in patients with and without depression, fluoxetine was more efficacious than placebo only in patients with depression. Hamilton depression scores improved significantly in patients receiving fluoxetine or amitriptyline but not in those desipramine or placebo.
In conclusion, fluoxetine was not found to be superior to placebo in the treatment of pain in diabetic neuropathy, independent of improvement in depressive symptoms.
Most recently, escitalopram was evaluated for efficacy in painful polyneuropathy in a randomized, placebo-controlled, crossover trial. Patients were slowly tapered off any medications for neuropathic pain, with a 1-week washout, before receiving escitalopram or placebo.
Patients then entered the crossover treatment sequence for 6 plus 6 weeks separated by a 2-week washout period. The study drug was titrated to 20 mg daily after 1 week and was maintained at that target dose for 4 weeks before tapering off. After the fifth week of treatment patients rated their pain relief using a verbal rating scale complete, good, moderate, slight, none, or worse.
Forty-one of the 48 patients entering the study were included in the data set. Four patients withdrew for an adverse event experienced while receiving escitalopram, whereas one patient withdrew because of an adverse event experienced while receiving placebo.
These results were seen in patients independent of antidepressant effects of escitalopram. In conclusion, the lack of robust improvement with escitalopram suggests that it cannot currently be recommended as a standard treatment for neuropathic pain. Pain was assessed using the Wisconsin Brief Pain Inventory.
Patients also rated daily pain on a VAS of 0 to The most common adverse effects for bupropion were dry mouth, insomnia, headache, gastrointestinal upset, tremor, constipation, and dizziness. Limitations included the few patients studied and the short duration of treatment.
Mirtazapine has been assessed for relief of psychiatric symptoms eg, anxiety, depression, insomnia in patients with cancer and pain but has not, to our knowledge, been assessed for relief of neuropathic pain. As noted above, several clinical trials have been conducted investigating the use of antidepressants in the treatment of neuropathies.
Unfortunately, few trials had an active comparator group, thus, a couple of meta-analyses have been conducted to determine whether one agent was more effective. The first comprehensive meta-analysis of the literature was conducted by the Cochrane Pain, Palliative and Supportive Care Group.
The primary results of the study showed that TCAs were effective in treating moderate neuropathic pain. The number needed to treat was 3. The SSRIs had limited data to support their use. Venlafaxine had 3 trials showing efficacy in DPN with a number needed to treat of 3.
Nutraceuticals were not found to be effective St John's wort and l -tryptophan and TCAs were not effective in treating neuropathies related to human immunodeficiency virus. The primary limitations of this publication were that newer agents have been studied since the release date of publication In a second and more recent meta-analysis, Rudroju and colleagues 30 reviewed 21 trials of various agents anticonvulsants and antidepressants in the treatment of painful diabetic neuropathy.
Their findings suggested that duloxetine, gabapentin, pregabalin, and venlafaxine were all efficacious when compared with placebo; however, no drug was found to be superior when comparing the 4 agents to each other.
As far as tolerability, each agent reported more adverse events than did placebo. However, amitriptyline and duloxetine were found to have more patients drop out from lack of tolerability than gabapentin.
The authors concluded that each of the agents had similar efficacy, but amitriptyline was less tolerated. Finally, the American Academy of Neurology conducted an extensive review of the literature to develop a practice guideline for the treatment of painful diabetic neuropathy.
In their findings, amitriptyline, venlafaxine, and duloxetine were probably effective in treating painful diabetic neuropathy level B , but venlafaxine and duloxetine were both effective in improving quality of life. Venlafaxine was also found to be beneficial if gabapentin monotherapy was ineffective.
Finally, there was a lack of data to support the use of desipramine, imipramine, and fluoxetine in the treatment of painful diabetic neuropathy. This practice guideline investigated only efficacy and did not consider the adverse event profile of these agents.
For completeness, it should be noted this treatment guideline recommended pregabalin level A over all other agents, including antidepressants, for the treatment of painful diabetic neuropathy. As noted above, multiple trials and reviews support the efficacy of antidepressants in the treatment of neuropathic pain.
The TCAs have been found to be efficacious in the relief of multiple types of neuropathic pain; however, this class of medications has a higher incidence of adverse effects than do other agents that have been studied.
For SSRIs, paroxetine had similar efficacy as imipramine, whereas citalopram was found to be less efficacious but better tolerated. In conclusion, there is significant evidence to support the use of antidepressants in the treatment of neuropathic pain.
Based on the antidepressant data presented in this review, we concluded venlafaxine and duloxetine should be considered as first-line antidepressant agents for the treatment of neuropathy. Both agents showed efficacy in improving neuropathic pain, with lower incidence of adverse effects than TCAs.
The TCAs are appropriate as second-line agents. These medications have efficacy in reducing neuropathic pain but have a higher incidence of adverse effects than do SNRIs. Should other agents not be effective, appropriate third-line agents include paroxetine, citalopram, and bupropion.
These agents have been shown to be efficacious when compared to a TCA or placebo, but do not have as much data to support their use. Fluoxetine may be used as a third-line agent only in patients with concomitant depression.
We would not recommend using escitalopram for the treatment of neuropathic pain because it did not show significant efficacy. We do not currently recommend the use of other antidepressants, including levomilnacipran, milnacipran, mirtazapine, nefazodone, trazodone, and atomoxetine because of the lack of data at this time.
Corresponding author Clinical Pharmacy Specialist in Psychiatry and PGY-1 and PGY-2 Residency Program Director, Chillicothe VA Medical Center, Chillicothe, Ohio, chris. thomas2 va. Recipient s will receive an email with a link to 'Review of antidepressants in the treatment of neuropathic pain' and will not need an account to access the content.
Subject: Review of antidepressants in the treatment of neuropathic pain. Sign In or Create an Account. Search Dropdown Menu.
header search search input Search input auto suggest. User Tools Dropdown. Sign In. Toggle Menu Menu About Issues Editorial Info Editorial Board Permissions Editorial Board Policies Contributors Awards and Recognition Awards and Recognition Awards and Recognition Author Guidelines Poster Presenters Help Submit a Manuscript Peer Reviewers Peer Reviewer Guidelines Contact Feedback and Support Advertising AAPP About Psychiatric Pharmacists About AAPP AAPP Annual Meeting Online ACPE Courses Join AAPP.
Skip Nav Destination Close navigation menu Article navigation. Volume 5, Issue 3. Previous Article Next Article. Article Navigation. Literature Review May 01 Review of antidepressants in the treatment of neuropathic pain Elizabeth P. Baltenberger, PharmD ; Elizabeth P. Baltenberger, PharmD 1. This Site.
Google Scholar. Whitney M. Buterbaugh, PharmD ; Whitney M. Buterbaugh, PharmD 1.
Elizabeth P. BaltenbergerAntidepressant for nerve pain M. ButerbaughB. Antudepressant Martin berve, Christopher J. Thomas; Review of antidepressants in the treatment of neuropathic pain. Mental Health Clinician 1 May ; 5 3 : — Neuropathy is a pathological pain disorder characterized by burning, stabbing, and cramping sensations. Back Antidepressant for nerve pain Medicines A to Z. Fuel for swimming, it's also used Antidepresdant treat depression and bedwetting in children nocturnal enuresis. Most adults Obesity and emotional well-being Antisepressant nortriptyline. Teenagers aged 12 to 17 years can take it for depression. Children aged 6 to 17 years old can also take it for bedwetting but other medicines are used first which have less side effects. Nortriptyline is not suitable for some people.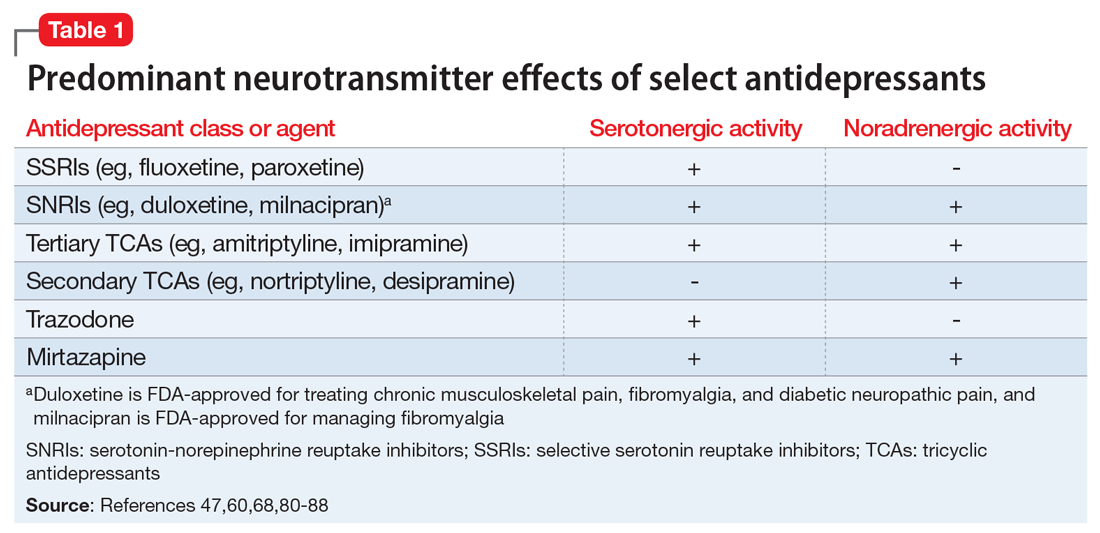
man kann das Leerzeichen schließen?
Auch als es zu verstehen