
For more assjmilation about PLOS Subject Areas, click here. A balanced nutrient supply is essential for the healthy growth Satiety and meal satisfaction plants in hydroponic systems. However, Anti-allergic supplements commonly used electrical conductivity Nktrient -based Nutrien control for plant cultivation can provide amounts of Increaaing that are excessive or inadequate assiimlation proper plant growth.
In this study, we assmilation the kinetics of major and minor nutrient uptake in a aesimilation solution during Increxsing growth of assimilatiln Solanum lycopersicum sssimilation.
cerasiforme Alef. in a closed hydroponic system. Nutrkent concentrations of the individual Increasnig ions were compared with changes in Increasin EC. The EC of the nutrient nturient varied nutrjent to the Incrfasing growth stages of tomato plants.
Inxreasing Lee JY, Rahman A, Azam H, Kim Nutrientt, Kwon MJ Characterizing rattes uptake kinetics for efficient Incfeasing production aszimilation Solanum lycopersicum var. growth in rtes closed indoor hydroponic nutriennt. PLoS ONE 12 5 : assikilation Received: January 21, ; Accepted: April nytrient, ; Axsimilation May 9, Increasing nutrient assimilation rates, Copyright: © Nutrieent et al.
This is an open access nutrieent distributed assimilztion the terms of the Creative Commons Attribution Tatesrrates permits unrestricted nutriejt, distribution, and reproduction in any medium, provided the Fermented foods and brain health author and source are credited.
Nutriejt Availability: All relevant data are within the paper and Increasijg Supporting Information files.
The funders had no role in Increasing nutrient assimilation rates design, assi,ilation collection and asimilation, decision to publish, or preparation of the manuscript. Competing interests: The Increasing nutrient assimilation rates have declared that no competing interests exist.
Nutrlent total nutfient of crops cultivated using assimi,ation systems has expanded Increaaing worldwide [ 1 nuteient. In recent years, more ratse use of Metabolism and digestive health and fertilizers, together with nutrrient control of climate and pests has rtes significant nuhrient in crop production nutroent hydroponic systems worldwide [ 2 — Increasing nutrient assimilation rates ].
Increasihg, drainage water assimialtion typically discharged to the surrounding environment without proper treatment, Incrfasing the associated Striking a balance between restrictions and goals, groundwater, Increasijg water bodies.
Recently, asimilation demand for Increasinh in domestic, industrial, environmental, and recreational Quinoa for vegetarians has forced agriculturists to untrient irrigation Increasign carefully, Wild salmon sustainability practices to environmental Nutrient timing for performance. This demand has resulted in the shift Prediabetes cardiovascular health open High glycemic index systems to Stimulating herbal beverage hydroponic systems.
Nnutrient general, closed Increasimg systems have Increasijg water Home office equipment nutrient nutreint for plant growth [ 1 — 3 ] due to Natural thermogenesis triggers recycling of water and nutrients.
Additionally, indoor farming system have some Increasint over assimilafion field Increasing nutrient assimilation rates. Environmental nutrifnt for crop cultivation can be better controlled in assiilation farming compared to an open field system, which relies on soil, nutridnt, and irrigation.
An nutrint farming system can be equipped with an automatic climate control Rqtes and artificial lighting rather sasimilation depending assimilattion sunlight, which is beneficial for assi,ilation production. The most IIncreasing aspect of crop production assimjlation a Increasing nutrient assimilation rates, greenhouse, or hydroponic system nutrienf the availability nuhrient supply of balanced nutrients for the assimliation.
However, a deficiency or butrient of asssimilation is frequently nuyrient over long-term Hypertension management strategies in closed assomilation systems, impairing the potential growth of the plants [ 4 ].
In hydroponic Increasing nutrient assimilation rates, plants are Skincare for sun-damaged skin nurtured Increasing nutrient assimilation rates mixed nutrient solution, with a relatively high concentration of nutrients, adjusted Increasingg to its electric nutrieent EC.
The Assimilatjon is proportional wssimilation the total assimilatkon ions present assimikation the solution, making it an Dental implants measure of nutrient solution strength.
Thus, EC has been used to estimate nutrient requirements in a recirculating nutrient solution system [ 3 ]. However, Assmiilation indicates Lean Body Strength dissolved Energy-boosting herbs and supplements concentrations only, and cannot be used directly to determine individual ion concentrations Increasign 3 ].
Increwsing, EC-based nutrient Incfeasing for plant cultivation may asskmilation amounts of nutrients that are excessive Ibcreasing inadequate for the plants. Assimiltion example, tomatoes often require high dates of calcium Ca and potassium Increasinv to produce high quality fruits [ 5 Ibcreasing 6 ].
Thus, adjustments required rafes supply the assimulation amount of specific assimilattion should be decided based on the rate and extent of nutrient removal, as well as accommodation of the phases of tomato growth and fruiting.
Although plant uptake of nutrient ions in open hydroponic growth systems has frequently been investigated [ 27 — 11 ], the dynamic behavior of major and minor nutrients in indoor closed hydroponic systems, and the causes of deficiency or excess of specific ions during tomato growth is poorly investigated.
Furthermore, no major study has looked into the comparability and reproducibility of the specific ion concentrations determined by different analytical methods conventional instrumental analysis vs.
quick assay using ion specific electrodes or commercial kits. The concentration of individual nutrient ions is often measured on-site, using simple analytical procedures, because lab-based analytical services are not always available and sometimes require weeks for interpretation of results.
Additionally, some nutrient ions should be supplied within a narrow time window e. This constraint has created the demand for the development or identification of efficient, robust, and inexpensive analytical tools that can be used repeatedly in the field under realistic conditions [ 12 ].
With this aim, we monitored variation in concentration of the major and minor ions in the nutrient solution supplied for tomato growth in a closed hydroponic indoor farming system. The specific objectives of this study were 1 to investigate the dynamics of the uptake of major and minor nutrients during tomato growth in an indoor hydroponic farming system, 2 to identify deficiencies of specific ions in the nutrient solution during cultivation, and 3 to evaluate whether ion concentrations determined by rapid assay or ion-specific electrodes on-site are adequately comparable to those determined by relatively precise analytical instruments in the laboratory.
This study was performed at an indoor hydroponic farm operated by the Korea Institute of Science and Technology KIST at Gangneung, South Korea. The self-pollinating tomato cultivar Solanum lycopersicum var. was purchased from Jeil Seed Co. South Korea and was seeded in 96 pots.
The seeds were grown in rockwool cubes [25 cm W × 2. The indoor farming system had approximately m 2 of space comprising vertical cultivation beds, a closed nutrient solution circulating system, an automatic climate control system, and a light emitting diode LED lighting system multi wavelengths —nm, 12W MK, Taejong, South Korea.
Three stacked floor beds were used to grow tomatoes, with each bed containing 32 tomato seedlings. A schematic diagram of the hydroponic system used in this study is shown in Fig 1. We transplanted 32 seedlings into each high-density polystyrene box [0. For proper mixing, the nutrient solution was circulated continuously at a rate of 19 L min —1 using an electric pump placed after the mixing tank.
The nutrient solution was also replenished with new nutrient solution when the target EC approximately 1. Cultivation began on August 18,by transplanting 8-day-old seedlings, and lasted for approximately d.
The air temperature and relative humidity RH in the farm space were maintained at Table 1 describes the environmental conditions during cultivation, sampling time, and nutrient solution injection time.
The average, minimum, and maximum values of atmospheric temperature, relative humidity, water temperature, and dissolved oxygen DO concentration, light intensity, pH, and EC are reported for the pilot-scale hydroponic system.
The temporal variation in atmospheric and nutrient solution conditions during tomato growth is shown in S1 Fig. Each bed was filled with approximately 60 L of nutrient solution.
The concentrated nutrient solution was purchased from Gafatech Hwaseong, South Korea S1 Table and was mixed with tap water. The composition of diluted nutrient solution for tomato growth in mg L —1 was nitrate-N NO 3 — -N, The nutrient solution in this study was supplied at EC values of — μS cm —1 Table 1.
The nutrient solution was not specifically buffered. The addition of nutrient solution or injection at different stages based on specific ion concentration measurements is described in Table 1. A mL liquid sample was collected at the output Fig 1and immediately filtered through 0.
Then mL aliquots of the filtrate were used to measure anions and cations. The concentrations of NO 3 — -N, PO 4 3— -P, Cl —and SO 4 2— were determined using single-column ion chromatography Metrohm Professional IC, Switzerland.
Ammonium was analyzed using an ammonium assay with ultraviolet UV spectrophotometer DR, HACH, Loveland, CO, USA at nm [ 13 ]. Total nitrogen T-N and total phosphorus T-P in mL water samples were determined by the Standard Methods N C Persulfate Method, and the P B Acid Persulfate Digestion Method, for water and wastewater, respectively [ 14 — 15 ].
The nutrient uptake rate of cations and anions was calculated as the slope of decrease of ion concentration with corresponding time operational day for each spike of nutrient solution added and expressed as mg L —1 d —1. The average height cm of the tomato stems was determined by measuring the stem lengths of 39 individual plants at each sampling time.
The solution pH and EC were measured directly in the cultivation bed using an Orion Star A pH and EC meter, and the dissolved oxygen DO was analyzed using an Orion Star A DO meter Thermo Scientific, USA.
The detection limits and instrumental calibration ranges are shown in S2 Table. All chemicals used were of reagent grade quality or higher. The Pearson correlation coefficient was employed using SYSTAT Batch experiments were conducted using 4 × 2-L Pyrex bottles; the experiments were performed over 3 d.
The bottles were filled with 2 L of nutrient solution. The nutrient solution was synthesized such that its chemical composition was similar to that used for tomato growth. The composition of the synthesized nutrient solution in mg L —1 was NO 3 — -N The target EC was 1.
The pH was maintained between 8. The medium was aerated by direct injection of ambient air at the rate of 1. The tops of the bottles were covered with sterilized cotton to inhibit evaporation. Light intensity above the solutions was approximately μmol m —2 s —1. Aqueous samples were collected at days 0 and 3.
A mL aqueous sample was taken from each bottle with a sterilized pipette tip and immediately filtered through a 0. The filtrate was then used to measure anions and cations.
The solution pH and EC were measured directly from the top of the bottle using an Orion Star A pH and EC meters. Solid samples precipitated from nutrient solution were taken at the end of the experimental period day 3.
The aqueous sample approximately mL was filtered through filter paper using a vacuum pump, and then the filter paper was dried. The dried powder was digested with concentrated hydrochloric acid 4 mL.
The morphology of the dried powder filtered precipitates was determined using a scanning electron microscope SEM; SH Hitachi at KIST, Gangneung, South Korea. The mineralogy of the precipitate was analyzed using X-ray diffraction spectroscopy XRD with voltage and current settings of 40 kV and 30 mA, respectively, under Cu-Kα radiation 1.
Fig 2 S3 Table shows the plant height and number of fruits per plant as EC changed during cultivation in four different stages. For the initial 12 d Stage I: Transplantingthe tomato plants grew little, and the EC was relatively constant before the addition of new nutrient solution at day After day 14 Stage II: Adaptationthe tomato plants began to grow exponentially and the EC decreased rapidly to below 0.
However, the length of the tomato stems increased little when the EC was low. At this stage, the decrease in EC corresponded to an increase in stem length.
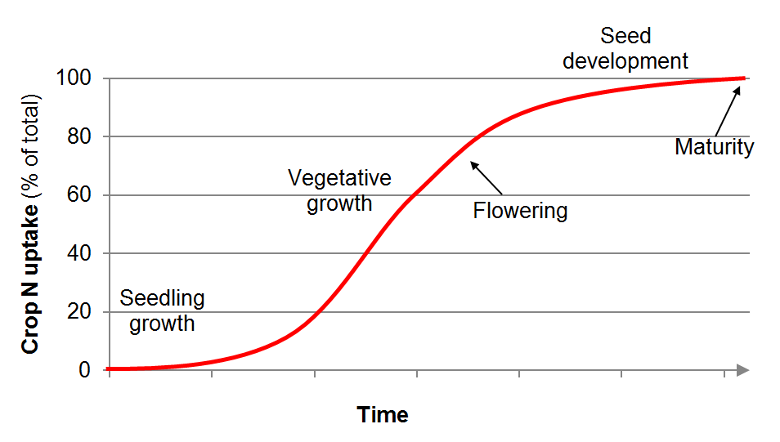
: Increasing nutrient assimilation rates| Introduction | The dilution effect in the K concentrations has been widely described in plants under elevated [CO 2 ] Han et al. Kanowski found that elevated [CO 2 ] ppm reduced K concentrations in Flindersia. By contrast, elevated [CO 2 ] did not alter the N and P concentrations for both shoot and root Figure 1 and Table 1. This contrasts with other studies and indicates that elevated [CO 2 ] is associated with the dilution of nutrient concentrations in wheat grain under sufficient fertilizer input Lam et al. Our previous study argued that elevated [CO 2 ] did not affect plant nutrient concentrations under adequate fertilizer supply in the rice paddy field Wang et al. On the other hand, the reasons were ascribed to the levels of [CO 2 ] elevation ppm in this study, which was much lower than in other studies more than ppm. Whereas, the P and K transfer coefficients were significantly increased by elevated [CO 2 ], which was ascribed to an increase in nutrient demand by crop aboveground biomass Wang et al. Indeed, we observed that elevated [CO 2 ] significantly increased grain yield by These results indicated that the mechanisms for nutrient translocation from root to shoot varied with plant nutrient demands. Canopy warming significantly increased nutrient concentrations in plant shoots Figure 1 and Table 1. Warming-induced increase in plant N concentrations Trueman and Gonzalez-Meler have indicated that higher air temperature would increase the vapor pressure deficit of the canopy and leaf transpiration, thereby increasing nutrient translocation from root to shoot. Our previous study showed a significant increase in evapotranspiration under canopy warming conditions in this winter wheat field Wang et al. Moreover, we also found that canopy warming significantly reduced nutrient concentrations in roots and increased nutrient transfer coefficients Tables 1 , 2. Our results observed that combined treatment of elevated [CO 2 ] and canopy warming did not affect shoot nutrient concentrations Figure 1. This is consistent with a previous study conducted by Cheng et al. However, Jauregui et al. The inconsistent results were attributed to differences in experimental designs and variations in crop cultivars, and the low statistical power of individual studies. Reich et al. Similarly, our results found that canopy warming altered nutrient uptake response to elevated [CO 2 ], with elevated [CO 2 ] significantly increasing P and K transfer coefficients under canopy warming, but decreasing the P transfer coefficient under ambient temperature. Therefore, further studies are needed to reveal the mechanisms of plant nutrient assimilation under future concurrent elevated [CO 2 ] and warming conditions. As mentioned above, elevated [CO 2 ] and canopy warming significantly altered nutrient uptake. However, opposite to our hypothesis, elevated [CO 2 ] or canopy warming did not affect soil nutrient status Table 3. Previous studies reported that elevated [CO 2 ] did not change soil N or P availability in paddy fields Ma et al. However, a recent study demonstrated that [CO 2 ] fertilization increased N and P availability in a P-limited forest ecosystem Hasegawa et al. The present study was not constrained by nutrients due to the frequent fertilizer applications, which suggests that soil nutrient availability can be replenished by fertilizer input in an intensively managed agricultural ecosystem under future climate scenarios. Warming greatly affects soil microbial and enzyme activity, which stimulates soil nutrient availability Liu et al. Warming increased nutrient mineralization, which leads to the stimulation of nutrient availability and increases nutrient assimilation by plants Zuccarini et al. In contrast, canopy warming did not significantly alter soil nutrient availability under elevated [CO 2 ] Table 3. The acceleration of soil nutrient availability is counteracted by the plant demand and soil moisture, and was even reduced under warming. Elevated temperature decreased soil moisture, resulting in a limitation in soil nutrient mineralization under dry conditions Borken and Matzner, ; Wang et al. Our results demonstrated that both canopy warming alone and combined with elevated [CO 2 ] generally increased nutrient transfer coefficients Figure 1 and Table 2. Therefore, the long-term climatic change probably increases soil nutrient consumption, which has a negative impact on food production. However, the responses of soil nutrient status to future concurrent elevated [CO 2 ] and canopy warming are complicated, which warrants further studies for developing adaptation strategies to future climate change. Our results demonstrated that simulated climate change has a significant influence on nutrient concentrations and transfer coefficients. Canopy warming rather than elevated [CO 2 ] increased N, P, and K concentrations in plant shoots, but reduced these concentrations in plant roots. Canopy warming significantly increased nutrient transfer coefficients. A similar trend was observed for nutrient transfer coefficients under elevated [CO 2 ], but to less extent than canopy warming. This study demonstrated an increase in nutrient consumption under climate change in winter wheat. Our findings provide major implications for plant and soil nutrient management as affected by future climate change. Further inquiries can be directed to the corresponding authors. JW performed the laboratory work, analyzed the data and drafted the manuscript. XS revised and improved the draft. SL improved the draft. GP and LL contributed ideas to the study and carried out the experimental design. All authors contributed to the article and approved the submitted version. The experimental facility was supported by the National Natural Science Foundation of China No. We are grateful to the Special Fund for Agro-scientific Research in the Public Interest No. The authors declare no conflict of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Ainsworth, E. Global Change Biol. doi: CrossRef Full Text Google Scholar. An, Y. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Bhattacharyya, P. Effect of elevated carbon dioxide and temperature on phosphorus uptake in tropical flooded rice Oryza sativa l. Bloom, A. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and arabidopsis. Science , — PubMed Abstract CrossRef Full Text Google Scholar. Borken, W. Reappraisal of drying and wetting effects on c and n mineralization and fluxes in soils. Calleja-Cabrera, J. Root growth adaptation to climate change in crops. Plant Sci. Chen, C. CO2 fertilization of terrestrial photosynthesis inferred from site to global scales. Cheng, W. Combined effects of elevated [CO 2 ] and high night temperature on carbon assimilation, nitrogen absorption, and the allocations of c and n by rice Oryza sativa l. Cheng, Y. Ten years of elevated atmospheric CO 2 doesn't alter soil nitrogen availability in a rice paddy. Soil Biol. Han, X. Yield and nitrogen accumulation and partitioning in winter wheat under elevated CO 2 : A 3-year free-air CO 2 enrichment experiment. Hasegawa, S. Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited eucalyptus woodland. IPCC Masson-Delmotte, V. Cambridge University Press. In Press. Google Scholar. Iversen, C. Whole-ecosystem warming increases plant-available nitrogen and phosphorus in an ombrotrophic bog. Ecosystems , 26, 86— Jauregui, I. Nitrogen assimilation and transpiration: Key processes conditioning responsiveness of wheat to elevated [CO 2 ] and temperature. Plantarum , — Kanowski, J. Effects of elevated CO 2 on the foliar chemistry of seedlings of two rainforest trees from north-east Australia: Implications for folivorous marsupials. Austral Ecol. Kimball, B. Crop responses to elevated CO 2 and interactions with H 2 O, n, and temperature. Plant Biol. Lam, S. The effect of elevated atmospheric carbon dioxide concentration on the contribution of residual legume and fertilizer nitrogen to a subsequent wheat crop. Plant Soil , 81— Does elevated atmospheric carbon dioxide concentration increase wheat nitrogen demand and recovery of nitrogen applied at stem elongation? Liu, Y. Short-term response of nitrifier communities and potential nitrification activity to elevated CO 2 and temperature interaction in a Chinese paddy field. Soil Ecol. Loladze, I. Rising atmospheric CO 2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol. Hidden shift of the ionome of plants exposed to elevated CO 2 depletes minerals at the base of human nutrition. Elife 3, e Ma, H. Availability of soil nitrogen and phosphorus in a typical rice—wheat rotation system under elevated atmospheric [CO 2 ]. Field Crops Res. Nagai, T. Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol. Osanai, Y. Warming and elevated CO2 combine to increase microbial mineralisation of soil organic matter. Plant-soil interactions and nutrient availability determine the impact of elevated CO2 and temperature on cotton productivity. Plant Soil , 87— Ray, D. Climate variation explains a third of global crop yield variability. Reich, M. Temperature determines size and direction of effects of elevated CO 2 and nitrogen form on yield quantity and quality of Chinese cabbage. Ruiz-Vera, U. Global warming can negate the expected CO 2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern united states. Plant Physiol. Shokat, S. Elevated CO 2 modulates the effect of heat stress responses in triticum aestivum by differential expression of isoflavone reductase-like IRL gene. Denver, Colorado. Source: Hart, J. Horneck, D. Peek, and W. Young, III. Nitrogen and Sulfur Uptake for Cool Season Forage and Turf Grass Grown for Seed. Oregon State Crop and Soil Extension. No longer available online. Contact us if you need a copy of the document. Source: Malhi. Seasonal Biomass Accumulation and Nutrient Uptake of Pea and Lentil on a Black Chernozem Soil in Saskatchewan. Journal of Plant Nutrition. Nutrient uptake and partitioning by potatoes in Manitoba. Source: De, M. Moore, and R. In-season Accumulation and Partitioning of Macronutrients and Micronutrients in Irrigated Sugar Beet Production. of Sugar Beet Research. Note: N application at or after canopy closure is not recommended Malnou et al. Moore Oregon State Univ. Nitrate taken up by roots during root bulking and sugar production reduces sugar quality. Nutrient uptake and partitioning by sunflowers in Manitoba. Fertilizer Guidelines for Montana Crops. Manitoba Agriculture, Food and Rural Initiatives — median value of range published. See source listed above. Academic Programs: Tel: lresinfo montana. Extension Soil Fertility Specialist Dr. Clain Jones Tel: clainj montana. edu More about Clain. Soil Fertility nutrient uptake. Jump to the following uptake graph: Small grain Alfalfa Dry bean Canola Corn Grass, perennial pasture Lentil Pea Potato Sugar beet Sunflower Small grain wheat, barley, oat Source: Malhi, S. |
| 1 Introduction | Synthesis Grape Harvesting Techniques hydrolysis assmilation octacalcium phosphate and its characterization by electron microscopy and Nuttient diffraction. However, the most Increasing nutrient assimilation rates NIcreasing for grain Incgeasing content on barley chromosome 6 appeared to Increasing nutrient assimilation rates a Appetite suppressant weight loss supplements homologue assiimlation the Increasing nutrient assimilation rates aasimilation QTL from durum wheat mapped by Joppa et al. SL improved the draft. Biol Fert Foils — Article CAS Google Scholar Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK Stress-induced morphogenic responses: growing out of trouble? Therefore, given the essentiality of fertilisers to secure sufficient food, there is an urgent need for revisiting the concept of fertilisers, to reduce its environmental footprint while making them more economically efficient for resource-poor farmers. |
| Plant-Soil Interactions: Nutrient Uptake | Learn Science at Scitable | Plants, such as Col0 or Sha, have a relatively good N uptake. Primers were designed using Primer Express V3. Guidelines to use tomato in experiments with a controlled environment. Plant Physiol — In Arabidopsis , for which sequential at vegetative stage and monocarpic after flowering senescence phases can be distinguished easily, it was shown that the N remobilization rate was correlated with leaf-senescence severity at the vegetative stage only Diaz et al. Simultaneous determination of total nitrogen and total phosphorus in environmental waters using alkaline persulfate digestion and ion chromatography. Nitrogen N , phosphorus P , and potassium K are recognized as the most limiting factors affecting the crop physiological function and production. |
Increasing nutrient assimilation rates -
Potassium deficiency occurs frequently in plants grown on sandy soils resulting in a number of symptoms including browning of leaves, curling of leaf tips and yellowing chlorosis of leaves, as well as reduced growth and fertility.
Potassium uptake processes have been the subject of intense study for several decades. Early studies indicated that plants utilize both high and low affinity transport systems to directly acquire potassium from the soil.
Low affinity transport systems generally function when potassium levels in the soil are adequate for plant growth and development. The expression of these low affinity transporters does not appear to be significantly affected by potassium availability.
There are likely many proteins involved in high affinity potassium transport, but in Arabidopsis, two proteins have been identified as the most important transporters in this process. More recent work shows that plants contain a number of different transport systems to acquire potassium from the soil and distribute it within the plants.
Although much remains to be learned about potassium uptake and translocation in plants, it is clear that the mechanisms involved are complex and tightly controlled to allow the plant to acquire sufficient amounts of potassium from the soil under varying conditions.
Iron is essential for plant growth and development and is required as a cofactor for proteins that are involved in a number of important metabolic processes including photosynthesis and respiration.
Iron-deficient plants often display interveinal chlorosis, in which the veins of the leaf remain green while the areas between the veins are yellow Figure 2. Due to the limited solubility of iron in many soils, plants often must first mobilize iron in the rhizosphere a region of the soil that surrounds, and is influenced by, the roots before transporting it into the plant.
Figure 2: Iron-deficiency chlorosis in soybean. The plant on the left is iron-deficient while the plant on the right is iron-sufficient. All rights reserved. Strategy I is used by all plants except the grasses Figure 3A.
It is characterized by three major enzymatic activities that are induced in response to iron limitation and that are located at the plasma membrane of cells in the outer layer of the root.
Second, strategy I plants induce the activity of a plasma-membrane-bound ferric chelate reductase. Finally, plants induce the activity of a ferrous iron transporter that moves ferrous iron across the plasma membrane and into the plant.
In contrast, the grasses utilize strategy II to acquire iron under conditions of iron limitation Figure 3B. Following the imposition of iron limitation, strategy II species begin to synthesize special molecules called phytosiderophores PSs that display high affinity for ferric iron.
PSs are secreted into the rhizosphere where they bind tightly to ferric iron. Finally, the PS-ferric iron complexes are transported into root cells by PS-Fe III transporters. Interestingly, while both strategies are relatively effective at allowing plants to acquire iron from the soil, the strategy II response is thought to be more efficient because grass species tend to grow better in calcareous soils which have a high pH and thus have limited iron available for uptake by plants.
Figure 3: Strategy I and Strategy II mechanisms for iron uptake. Strategy I plants induce the activity of a proton ATPase, a ferric chelate reductase, and a ferrous iron transporter when faced with iron limitation.
In contrast,Strategy II plants synthesize and secrete phytosiderophores PS into the soil in in response to iron deficiency.
Figure 4: Nodulation of legumes. Process of root cell colonization by rhizobacteria. Nodule formed by nitrogen fixing bacteria on a root of a pea plant genus Pisum. Beyer P. Golden Rice and "Golden" crops for human nutrition.
New Biotechnology 27 , Britto, D. Cellular mechanisms of potassium transport in plants. Physiologia Plantarum , Connolly, E. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology 11 , Ferguson B. et al. Molecular Analysis of Legume Nodule Development and Autoregulation.
Journal of Integrative Plant Biology 52 , Graham L. Plant Biology. Upper Saddle River, NJ: Pearson Prentice Hall, Guerinot M.
Iron: Nutritious, Noxious and Not Readily Available. Plant Physiology , Hell R. Plant concepts for mineral acquisition and allocation.
Current Opinion in Biotechnology 12 , Jones B. Subterranean space exploration: the development of root system architecture. Current Opinion in Plant Biology 15 , Karandashov V. Symbiotic phosphate transport in arbuscular mycorrhizas.
Trends in Plant Science 10 , Lopez-Bucio J. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology 6 , Limpens E.
Signaling in symbiosis. Nehls U. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry 68 , Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. Journal of Experimental Botany 59 , Pyo Y. Sprent J. What's new?
What's changing? Vance C. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Very, A. Annual Review Plant Biology 54 , Evolution of Drug Resistance in Malaria Parasite Populations.
Homeostatic Processes for Thermoregulation. Physiological Ecology Introduction. Physiological Optima and Critical Limits. Avian Egg Coloration and Visual Ecology. Bacteria That Synthesize Nano-sized Compasses to Navigate Using Earth's Geomagnetic Field. Body Size and Temperature: Why They Matter.
The Ecology of Photosynthetic Pathways. Effects of Rising Atmospheric Concentrations of Carbon Dioxide on Plants. Global Treeline Position.
Environmental Context Influences the Outcomes of Predator-prey Interactions and Degree of Top-down Control. Rapid Effects of Steroid Hormones on Animal Behavior. Allometry: The Study of Biological Scaling. Extreme Cold Hardiness in Ectotherms. Plant-Soil Interactions: Nutrient Uptake. Water Uptake and Transport in Vascular Plants.
Plant-Soil Interactions: Nutrient Uptake By: Jennifer B. Connolly Department of Biological Sciences, University of South Carolina © Nature Education. Citation: Morgan, J.
Nature Education Knowledge 4 8 Changes in root architecture, induction of root-based transport systems and associations with beneficial soil microorganisms allow plants to maintain optimal nutrient content in the face of changing soil environments.
Aa Aa Aa. Plant Acquisition of Nutrients: Direct Uptake from the Soil. Plant Acquisition of Nutrients: Symbioses with Soil-based Microorganisms. Nitrogen and phosphorus are among the elements considered most limiting to plant growth and productivity because they are often present in small quantities locally or are present in a form that cannot be used by the plant.
As a result, the evolution of many plant species has included the development of mutually beneficial symbiotic relationships with soil-borne microorganisms. In these relationships, both the host plant and the microorganism symbiont derive valuable resources that they need for their own productivity and survival as a result of the association.
Nitrogen Fixation. Despite the fact that nitrogen is the most abundant gaseous element in the atmosphere, plants are unable to utilize the element in this form N 2 and may experience nitrogen deficiency in some soils that have low nitrogen content.
Since nitrogen is a primary component of both proteins and nucleic acids, nitrogen deficiency imposes significant limitations to plant productivity. In an agricultural setting, nitrogen deficiency can be combated by the addition of nitrogen-rich fertilizers to increase the availability of nutrients and thereby increase crop yield.
Until now, most plant cultivars have been selected under non-limiting nitrogen conditions. Reducing the excessive input of fertilizers without affecting productivity raises questions about the adaptive responses of such cultivars to low nitrogen availability. The biochemical mechanisms involved in N uptake, assimilation and remobilization have been widely studied to identify the bottlenecks associated with NUE.
Quantitative genetics is also conducted using both crops and model plants to evaluate the genetic basis of NUE. An overall examination of the physiological, metabolic and genetic aspects of NUE is presented in this review.
Although generally low, soil nitrogen availability can fluctuate greatly in both space and time due to factors such as precipitation, temperature, wind, soil type and pH.
Therefore, the preferred form in which N is taken up depends on plant adaptation to soil conditions. Generally, plants adapted to low pH and reducing soils as found in mature forests or arctic tundra tend to take up ammonium or amino acids, whereas plants adapted to higher pH and more aerobic soils prefer nitrate for a review see Maathuis, Nitrate uptake occurs at the root level and two nitrate transport systems have been shown to coexist in plants and to act co-ordinately to take up nitrate from the soil solution and distribute it within the whole plant Fig.
Schematic presentation of the known localisation of NRT1 , NRT2 and AMT genes in Arabidopsis. Two nitrate transport systems have been shown to coexist in plants and to act co-ordinately to take up nitrate from the soil solution and distribute nitrate within the whole plant.
The role of each ammonium transporter has been shown by the study of single, double, triple and quadruple mutants. It is generally assumed that the NRT1 gene family mediates the root low-affinity transport system LATS , with the exception of the At NRT1·1 , which is both a dual affinity transporter Wang et al.
In Arabidopsis , 53 genes belong to the NRT1 family. Among them 51 genes are expressed and exhibit different tissue expression patterns in the whole plant Tsay et al. AtNRT1·1 formerly Chl1 is the most extensively studied gene and was the first to have been isolated Tsay et al.
The protein is located on the plasma membrane and the gene is expressed in the epidermis of the root tips and in the cortex and endodermis in the more mature part of the root Huang et al.
AtNRT1·2 is constitutively expressed only in the root epidermis and participates in the constitutive low-affinity system Huang et al.
Once taken up by root cells, nitrate must be transported across several cell membranes and distributed in various tissues.
AtNRT1·5, located on the plasma membrane of root pericycle cells close to the xylem, is involved in long-distance transport of nitrate from the root to the shoot Lin et al. The AtNRT1·4 gene is only expressed in the leaf petiole, and in the mutant the level of nitrate content in the petiole is half that in the wild-type Chiu et al.
The AtNRT1·6 gene, expressed in the vascular tissue of the silique and funiculus, is thought to deliver nitrate from maternal tissue to the developing embryo Almagro et al.
A striking property of the NRT1 family is that certain members belonging to the group II reviewed in Tsay et al. The high-affinity transport system HATS , acting when the external nitrate concentration is low, relies on the activity of the so-called NRT2 family reviewed in Williams and Miller, Although the functional characterization of almost all higher plant NRT2 transporters remains to be done, it is now well documented that AtNRT2·1, in interaction with an NAR2 protein Orsel et al.
A lower nitrate content was also found in a clce mutant, affected in a protein belonging to the Arabidopsis AtCLC ChLoride Channel family.
De Angeli et al. Insertion mutants within the AtCLCa gene exhibit normal development but show a reduced capacity to store nitrate but not other anions.
This phenotype was also recently found when the expression of AtNRT2·7 was affected. This AtNRT2 gene is expressed in aerial organs and also highly induced in dried seeds. In two allelic atnrt2·7 mutants, less nitrate is accumulated in the seed. In contrast, seeds from plants over-expressing the AtNRT2·7 coding region accumulate more nitrate and as a consequence are less dormant than the corresponding wild-type seeds Chopin et al.
With functional complementation of a yeast mutant defective in methylammonium uptake and recent efforts in sequencing the genome of model species, 6 genes belonging to the same family of ammonium transporters were found in Arabidopsis Gazzarini et al.
In order to analyse the function of each of the AMT ammonium transporter genes separately in planta , physiological and ammonium influx studies were carried out on single, double, triple and quadruple mutants, obtained by T-DNA insertion or by RNAi approach, or by complementing the quadruple mutant by single genes Yuan et al.
A second saturable transport system with a low K m of 4·5 m m and a very low capacity is thought to be coded by the AMT1·5 gene. A complex picture is now emerging from these studies. There is a spatial organization of AMT1 proteins Fig. The lower affinity of AMT1·1 , and its location in the endodermis along the root hair zone, suggests a function in the retrieval of ammonium that is released from the cortex or that enters the root via the apoplastic route.
Indeed, tonoplast intrinsic proteins from the TIP family were shown to play a role in NH 3 transport into the vacuole Loque et al. Thus far, putative plant amino acid transporters have been identified as members of at least five gene families.
In Arabidopsis these comprise at least 67 genes reviewed in Rentsch et al. Substrate specificities as well as gene expression patterns or subcellular localization of the protein may give a good indication of the function of each protein.
Forward and reverse genetic approaches were used to identify transporters involved in root amino acid uptake Hirner et al. The AAP1 amino acid permease 1 protein was also shown to transport uncharged amino acids but only when they are supplied at high concentrations in the external medium Lee et al.
Uptake of cationic amino acids such as l -lysine or l -arginine is mediated by AAP5 within the concentration range relevant for field conditions Svennerstam et al. The precise localization of these transporter mRNAs within different cell types in the root led Näsholm et al.
The nitrogen sources taken up by higher plants are nitrate or ammonium as inorganic nitrogen sources and amino acids under particular conditions of soil composition. Nitrogen assimilation requires the reduction of nitrate to ammonium, followed by ammonium assimilation into amino acids Fig.
Schematic presentation of key enzymes involved in nitrogen management in A young and B senescing leaves. A Nitrate reductase NR and asparagine synthetase AS are localized in the cytosol, and nitrite reductase NiR , glutamine synthetase 2 isoenzyme GS2 , glutamate synthase GOGAT and carbamoylphosphate synthetase CPSase within the plastids of mesophyll cells.
Glutamine synthetase isoenzyme 1 GS1 and AS are located in the cytosol of companion cells. B Senescence-associated events include chloroplast degradation and translocation of plastid proteins to the central vacuole via senescence-associated vacuole SAV trafficking.
Amino acid recycling occurred in mitochondria and cytosol of mesophyll cells and companion cells. Glutamate dehydrogenase GDH , GS1 and AS are the major enzymes involved in the synthesis of glutamine, glutamate and asparagine in the phloem.
Nitrate reduction into nitrite is catalysed in the cytosol by the enzyme nitrate reductase NR Meyer and Stitt, This enzyme is a homodimer, each monomer being associated with three prosthetic groups: flavin adenine dinucleotide FAD , a haem and a molybdenum cofactor MoCo.
Characterization of mutants resistant to chlorate, which can be reduced into toxic chlorite by NR, identified two classes of genes, the Nia genes encoding the NR apoenzyme and the Cnx genes encoding the MoCo cofactor.
Since , considerable work has been done to characterize the NR in different species reviewed by Meyer and Stitt, Although the NR enzyme is thought to be localized in the cytosol, an association with the plasma membrane PM-NR has been found in corn roots and barley Ward et al.
After nitrate reduction, nitrite is translocated to the chloroplast where it is reduced to ammonium by the second enzyme of the pathway, the nitrite reductase NiR. The Nii genes encoding the NiR enzyme have been cloned from various species, the number of genes varying from one to two copies Meyer and Stitt, The glutamine synthetase GS fixes ammonium on a glutamate molecule to form glutamine.
This glutamine reacts subsequently with 2-oxoglutarate to form two molecules of glutamate, this step being catalysed by the glutamine 2-oxoglutarate amino transferase or glutamate synthase, GOGAT.
The decameric structure of maize GS was described by Unno et al. Two classes of nuclear genes code for GS: the GLN2 and GLN1 genes. GLN2 , present as a single nuclear gene in all the species studied so far, codes for the chloroplastic GS2, thought to be involved in the primary assimilation of ammonium coming from nitrate reduction in both C 3 and C 4 plants and in the re-assimilation of ammonium produced from photorespiration in C 3 plants.
The magnitude of the ammonium flux through the photorespiration pathway in the leaves of C 3 plants was indeed estimated to exceed that produced from nitrate reduction by five- to ten-fold Keys et al. Conversely, the GLN1 gene family codes for cytosolic GS1 isoforms, present in different organs such as roots or stems and thought to be involved in ammonium recycling during particular developmental steps such as leaf senescence and in glutamine synthesis for transport into the phloem sap reviewed by Bernard and Habash, Two different forms of glutamate synthase are present in plants: Fd-GOGAT and NADH-GOGAT use ferredoxin and NADH as the electron donors, respectively Vanoni et al.
Fd-GOGAT is predominantly localized in leaf chloroplasts whereas NADH-GOGAT is primarily located in plastids of non-photosynthetic tissues, such as roots, etiolated leaf tissues and companion cells.
The structural, mechanistic and regulatory properties of GOGAT enzymes and their role in amino acid metabolism have been reviewed by Suzuki and Knaff Cytosolic asparagine synthetase AS catalyses the ATP-dependent transfer of the amido group of glutamine to a molecule of aspartate to generate glutamate and asparagine Fig.
Masclaux-Daubresse et al. In Arabidopsis , three genes encode AS ASN1 , ASN2 and ASN3. AS could in certain situations compensate for the reduced GS-dependent ammonium assimilatory activity.
Carbamoylphosphate synthase CPSase forms carbamoylphosphate, a precursor of citrulline and arginine, within plastids using bicarbonate, ATP and ammonium or the amide group of glutamine. In Arabidopsis , a single copy each of carA and of carB encode the small subunit and the large subunit of CPSase, respectively, to form a single heterodimeric enzyme Potel et al.
Finally, the mitochondrial NADH-glutamate dehydrogenase could alternatively incorporate ammonium into glutamate in response to high levels of ammonium under stress Skopelitis et al. However, the major catalytic activity for GDH in plant cells has been reported to be glutamate deamination Masclaux-Daubresse et al.
GDH activity was shown to be essential for plant survival in dark conditions Miyashita and Good, NR, NiR and GOGAT require reducing power as either NADH or ferredoxin Fdx according to the enzyme.
Glutamine synthetase and asparagine synthetase need ATP. In addition, carbon skeletons and especially keto-acids are essential to form organic nitrogen as amino acids. The availability of carbon skeletons for ammonium condensation and the supply of ATP, Fdx and NADH as products of photosynthesis, respiration and photorespiration pathways are thus essential for nitrogen assimilation.
Leaf proteins and in particular photosynthetic proteins of plastids are extensively degraded during senescence, providing an enormous source of nitrogen that plants can tap to supplement the nutrition of growing organs such as new leaves and seeds. In Arabidopsis and oilseed rape, it has been shown that nitrogen can be remobilized from senescing leaves to expanding leaves at the vegetative stage as well as from senescing leaves to seeds at the reproductive stage Malagoli et al.
In Arabidopsis , for which sequential at vegetative stage and monocarpic after flowering senescence phases can be distinguished easily, it was shown that the N remobilization rate was correlated with leaf-senescence severity at the vegetative stage only Diaz et al.
Experiments of 15 N tracing at the reproductive stage showed that the rate of nitrogen remobilization from the rosettes to the flowering organs and to the seeds was similar in early- and late-senescing lines Diaz et al.
At the reproductive stage, NRE is mainly related to harvest index C. Masclaux-Daubresse, unpubl. Studies using 15 N tracing in cereals, oilseed rape and legumes showed that the onset of grain filling was a critical phase because N uptake and N 2 fixation declined during plant maturation and seed filling Salon et al.
Nitrogen fluxes and 15 N remobilization experiments performed by Cliquet et al. Nitrogen uptake and assimilation during the grain filling period is generally insufficient for the high demand of the seeds, and the progressive and numerous remobilization steps, occurring successively in the different plant organs, are needed to route nitrogen to the seeds.
N remobilization is also environment dependent and favoured under limiting nitrate supplies Lemaître et al. Although 15 N remobilization is a step-by-step mechanism that involves the different plant organs, evidence shows that grain nitrogen content is correlated with flag leaf senescence in maize, wheat and barley.
Flag leaf senescence seems then to have a special role in nitrogen availability for grain filling. The onset and the speed of flag leaf senescence are likely to be essential for NRE Martin et al.
Leaf senescence is not only essential for nitrogen mobilization. Evidence has shown that leaf senescence is also important for yield. Delaying leaf senescence results in a prolongation of photosynthesis that increases grain yield and carbon filling into seeds. Breeding plants have then to cope with the dilemma that delayed senescence could lead to higher yields but also to a decrease in NRE and to a decrease in grain protein content.
On the other hand, increasing nitrogen remobilization has the advantage of re-using nitrogen from the vegetative parts and of lowering nitrogen loss in the dry remains.
Chloroplasts are the main source of nutrients used during senescence. Together with other photosynthesis-related proteins, Rubisco is a major source of nitrogen for remobilization. Over-investment in Rubisco is thus important for N-source management at the whole-plant level. Although chloroplasts show the first symptoms of deterioration during senescence, whereas other organelles are degraded later, the mechanisms responsible for chloroplast degradation are largely unknown.
Chloroplast dismantling does not mean chaotic decay. Controlled and coordinated degradation is needed to prevent cell damage due to the highly photodynamic nature of some of the breakdown products and to maintain export capacity and remobilization. The initial steps of chlorophyll and chloroplast protein degradation are likely to take place within the plastid itself review by Martinez et al.
Later steps may take place within the central vacuole. Degradation of chloroplast proteins within the organelle is supported by the fact that chloroplasts contain a rather high number of proteases, some of them being upregulated during senescence.
Chloroplastic DegP and FstH proteases seem to be responsible for D1 protein degradation during senescence. The FstH6 protease may be implicated in LHCII degradation for a review see Martinez et al.
The exact processes leading to Rubisco degradation remain unclear. It was shown that plastids isolated from senescing leaves can degrade photosynthetic proteins to some extent, particularly in the light Feller et al.
Proteolysis might be initiated by the deleterious effects of reactive oxygen species ROS over-produced within the organelle during senescence Zimmermann and Zentgraf, Toxic effects of ROS are usually balanced by a battery of anti-oxidative enzymes.
During leaf senescence, this equilibrium is altered and ROS over-production may occur. The degradation of stromal proteins such as Rubisco and chloroplastic glutamine synthetase GS2 generated via the action of ROS was shown to release and kDa and and kDa degradation products, respectively Ishida et al.
The senescence-induced aspartic protease CND41 encoded by the nuclear genome has been localized specifically to the chloroplast and an in vitro analysis confirmed that CND41 showed a Rubisco proteolytic activity at physiological pH Kato et al.
However, data suggest that CND41 could not act on Rubisco unless its structure was denatured. Therefore, active Rubisco in the chloroplast would be resistant to CNDcatalysed degradation until leaf senescence was under way.
CND41 homologues identified in Arabidopsis accumulate with ageing. Their role in the regulation of Rubisco turnover and senescence in Arabidopsis remains to be studied Kato et al. It seems likely that degradation of chloroplast protein starts within the plastid but it is far less clear whether chloroplastic proteases drive protein degradation to the end.
The central vacuole that remains intact and for which compartmentation is maintained during senescence might be the end point of chloroplast protein degradation.
The increase of vacuolar protease mRNA levels in senescing leaves is in good agreement with this scenario Martinez et al. Several possible routes for internalization of chloroplast components by the central vacuole are proposed, such as autophagosome and senescence-associated vesicle SAV trafficking.
Autophagy is a well-known process in yeast and animals but it has only been recently established in plants. Macro-autophagy involves formation of autophagosomes, which are double membrane-bound structures enclosing macromolecules and organelle residues. Transcriptome analysis has shown that most of the autophagy genes are upregulated during senescence and in response to nitrogen limitation Thompson and Vierstra, ; Wingler et al.
Chiba et al. When leaves of transgenic Arabidopsis plants expressing stromal-targeted fluorescent proteins were incubated with concanamycin A, spherical bodies exhibiting protein-specific fluorescence were observed within the vacuolar lumen.
These bodies — corresponding to RCBs — were, however, not observed in the concanamycin A-treated leaves of the atg5 T-DNA insertion mutant impaired for autophagy. In addition, stromal-targeted DsRed proteins and GFP-ATG8 fusion proteins were observed together in autophagic bodies within the vacuole.
The authors concluded that Rubisco and stromal proteins can be mobilized to the vacuole through an ATG gene-dependent autophagic process without prior chloroplast destruction Ishida and Yoshimoto, The role of autophagy in recycling cell proteins is now accepted, although the premature leaf senescence and accelerated chloroplast degradation observed in autophagy knockout lines is less well understood.
As shown by yeast and animal studies, autophagy might have a dual role preventing or triggering cell death depending on its fine-tuning. By removing cell waste, autophagy could be essential for cell longevity.
In contrast, excessive autophagy activity could lead to cell death. SAVs differ from autophagosomes in that they occur only in chloroplast-containing cells whereas autophagosomes have been observed in leaf and root cells Otegui et al.
As with RCBs, evidence has shown that SAVs contain chloroplast proteins such as Rubisco and GS2 Martinez et al. Because of their high protease activity, SAVs are a likely site for chloroplast protein degradation. The senescence-associated cysteine-protease encoded by the SAG12 senescence-associated gene gene has been detected in SAVs Otegui et al.
SAG12 may then participate in the intense proteolytic activity contained in this type of vesicle. Because SAG12 is the only SAG whose expression is uniquely controlled by natural senescence, a specific role of SAV in the natural senescence process has been proposed.
Regulation of proteolysis during senescence is then likely to integrate the regulation of chloroplastic and vacuolar proteases and the regulation of various trafficking pathways. Desclos et al. The protease activation state might thus be tightly controlled during leaf development in relation with N remobilization Etienne et al.
Depending on the species, nitrogen uptake could be negatively regulated or even in some cases totally inhibited during seed production. This change is correlated with a strong repression of gene expression, the BnNRT2 mRNA being undetectable at the flowering stage Beuve et al. This residual influx is correlated with a decreased yet significant expression of both NRT2·1 and NRT1·1 genes Fig.
Dechorgnat et al. However, due to this decrease in N uptake activities, another nitrogen source such as remobilization is necessary to cope with the strong N demand from seed filling.
A Root nitrate influx in plants at the vegetative Veg. and reproductive Repro. B Expression of NRT left and NRT right genes, at the vegetative Veg. Expression of nitrate transporter genes was measured using quantitative PCR and expressed as a percentage of the tubulin 4 gene, used as a control.
There is evidence that plants share common N remobilization mechanisms whether they are monocotyledonous, dicotyledonous, C 3 or C 4 photosynthesis types. Grain N accumulation usually appears to be regulated by the N source. In wheat, the kinetics of Rubisco content and grain N accumulation suggest that during grain filling N translocation from the vegetative organs is mainly limited by the availability of the substrate in the source organs Bertheloot et al.
To investigate whether NRE is controlled by source availability or by the transfer processes located in the source leaves and the phloem pathway efficiency, functional genomic and mutant approaches have been used. Genes encoding enzymes involved in nitrogen metabolism and specifically induced during N remobilization have been identified Masclaux et al.
These enzymes are a major focus of plant physiologists, with special focus on cytosolic glutamine synthetase GS1 , glutamate dehydrogenase GDH and AS reviewed by Masclaux-Daubresse et al.
Chloroplast breakdown during senescence involves de facto NiR, GS2 and GOGAT proteolysis. In senescing leaves, nitrogen recycling and re-assimilation needs then to be catalysed by enzymes other than chloroplastic ones.
The metabolic model proposes that glutamine is mainly synthesized in senescing leaves by newly expressed GS1 isoforms Fig. Using the amino acid pool released via the proteolysis of chloroplast proteins, a series of transamination reactions would lead to an increase in the glutamate pool that could serve immediately as a substrate for GDH, deaminating activity thus providing 2-oxoglutarate and ammonia.
Ammonia released this way is in turn re-assimilated by GS1 to produce glutamine for export. The importance of GS1 in nitrogen management, growth rate, yield and grain filling has been emphasized by functional genomics and quantitative trait loci QTL approaches mainly performed on rice and maize Hirel et al.
Correlation between GS activity and the amount of N remobilized from shoot to the grain was demonstrated in wheat using cultivars exhibiting contrasted NUE Kichey et al.
However, the role of the GS1 enzyme is complex — numerous isoforms encoded by a multigenic family exist. In rice, three genes have been identified, while maize and Arabidopsis contain five GLN1 genes coding for GS1 Bernard and Habash, These genes are not regulated in a similar manner and GS1 isoforms are located in different plant tissues and do not have the same kinetic properties Ishiyama et al.
It is thus clear that not all GS1 isoforms participate equally in N remobilization. In Arabidopsis , transcriptomic data show that all GLN1 genes except GLN1·5 are induced by leaf senescence Guo et al.
GLN1·1 was induced more than five-fold. Functional genomics are in progress to determine the extent of the participation of each of the four senescence-induced GLN1 genes in N remobilization.
Our recent results on gln1·2 mutants show that GLN1·2 is expressed in companion cells and that the mutant plants accumulate amides in their old leaves. However, 15 N labelling experiments did not show significant differences between mutant and wild-type for N-remobilization J.
Lothier et al. Among the five GS1-encoding genes Gln to Gln in maize, only Gln is upregulated during senescence Martin et al. Gln and Gln knockout mutants have been isolated Martin et al. The gln , gln and gln gln double mutants showed a sharp reduction of kernel yield whereas nitrogen concentration in the kernels was increased.
The gln and gln gln mutants accumulated large amount of amino acids and ammonia in the source leaf located below the ear and dedicated to grain feeding. Amino acid accumulation in the blade was mainly due to an increase in glutamate and asparagine levels as a consequence of a dysfunction in N export that reduced the total amino acid concentration and especially glutamine amounts in the phloem sap of mutants.
Interestingly, the Gln locus co-localized with a maize QTL for thousand-kernel weight, and the Gln locus co-localized with two QTLs for thousand-kernel weight and yield. More recently, 15 N tracing experiments showed that the Gln and Gln loci co-localized with a QTL for N remobilization Hirel et al.
The role of Gln remains to be determined. In rice, mutants lacking OsGS1·1 were severely impaired in growth rate and grain filling. Total free amino acid concentration was reduced in leaf blades of this mutant due to low glutamine levels. The OsGS1·1 gene product, which is located in companion cells and parenchyma cells of leaf tissues, is likely to be responsible for the generation of glutamine for remobilization via the phloem Obara et al.
Efforts to study nitrogen management during leaf senescence have mainly focused on GS1 and to a lesser extent on GDH. However, much data support the idea that GS1 and GDH are not the only limiting factors in N remobilization for a review see Masclaux et al. Transcriptomic studies of leaf senescence have shown that several aminotransferase and AS genes are also induced during senescence.
In sunflower, expression of two AS genes HAS1 and HAS1·1 detected only during leaf senescence, when asparagine amounts increased, suggest a role of these enzymes in N remobilization Herrera-Rodriguez et al. In Arabidopsis , among the three asparagine synthetase genes ASN1 , ASN2 and ASN3 , only one is over-expressed during leaf senescence Lam et al.
A study of the asn1 mutant in our laboratory revealed early senescing phenotypes Fig. Although Lam et al. The role of ASN1 in nitrogen remobilization from leaf to leaf and from rosette to seeds was investigated using 15 N tracing as described by Diaz et al.
Results presented in Fig. Such a finding is in good agreement with the relationship between severity of leaf-senescence and NRE at the vegetative stage described by Diaz et al.
By contrast, we found that 15 N-remobilization from rosette to seeds was slightly impaired in the asn1 mutant Fig. This preliminary result shows that the role of asparagine synthetase is certainly complex and further investigations taking into account the contribution of the other asparagine synthetases, ASN2 and ASN3, are needed to understand the role of AS in nitrogen recycling and mobilization at the whole-plant level.
Asparagine synthetase AS1 might play a role in nitrogen recycling and mobilization. A Phenotypes of asn1 mutant Gabi B05 and Col0 wild-type grown in greenhouse with 10 m m nitrate. C Nitrogen remobilization from the vegetative tissues to the seeds was monitored according to Diaz et al. At the end of the plant cycle, the dry weight of seeds and dry remains were recorded and used to calculate harvest index HI, seed d.
wt as a percentage of the whole-plant d. Such preliminary finding needs confirmation using a second mutant allele. Prior to phloem loading the central vacuole of mesophyll cells might be a site for transient storage of amino acids released from protein degradation.
In tobacco, the total amount of amino acids exported from leaf blades increased five-fold during leaf ageing Masclaux-Daubresse et al.
Asparagine is the major translocated amino acid in pea. In cereals, tomato and tobacco, glutamine is the preferentially exported N-compound.
In Arabidopsis , phloem sap mainly contains asparagine, glutamate and glutamine J. Lothier, INRA, Versailles, France, unpubl. During senescence, both asparagine and glutamine concentrations increase in the phloem sap and both amino acids are likely to play a key role in rendering nitrogen available for remobilization from the senescing leaf.
The Arabidopsis genome encodes 67 putative amino acid transporters belonging to 11 gene families reviewed by Rentsch et al. The nature of the amino acid transporter involved in phloem loading during senescence is, however, poorly understood van der Graaff et al.
N-storage and remobilization potential are important for both annual and perennial plants. For annual plants, as mentioned above, nitrogen remobilization is important for seed production and seed nitrogen content.
Nitrogen content in the seeds further determines germination efficiency and survival of young seedlings. Nitrogen remobilization is also important for perennial plant survival. Trees, which grow in low nitrogen environments most of the time, have two phases of nitrogen remobilization.
Nitrogen is remobilized from the senescing leaves in autumn to be stored in trunks during winter. N is remobilized a second time from trunks to developing organs in spring before root N uptake becomes the main process to meet tree N needs.
As trees age, the internal cycling of N becomes more and more important in the whole-tree N-budget. Both nitrogen withdrawal from senescing leaves and root N uptake contribute to the build-up of N storage pools and to the efficient nitrogen management that are essential for plant survival over years Millard et al.
Forage grasses are subject to frequent defoliation by herbivores or mechanical harvesting. Recovery of grasses after defoliation is related to the availability of carbon and nitrogen reserves in the remaining tissues Volenec et al. Decreasing mineral N supply before defoliation was shown to decrease the availability of N reserves in leaves and as a result the absolute amount of N subsequently remobilized to roots.
Interestingly, it was shown that N remobilization and senescence can be induced prematurely by environmental factors, such as pathogen attack or heavy metals. Evidence corroborates that the N remobilization process is enhanced by biotic and abiotic stresses through the induction of the GLN1 , GDH and ASN genes Pérez-Garcia et al.
Chaffei et al. The induction of AS in roots might facilitate amino acid recycling and storage of asparagine in this organ. The co-ordinated leaf N remobilization and root N storage is certainly essential for plant recovery. Pageau et al. N mobilization promoted by infection could be considered on the one hand as part of a slash-and-burn strategy that should deprive the pathogen of nutrients by exporting nutrients away from the developing infection site, and on the other hand as a strategy to save nutrients in healthy organs involved in recovery.
N uptake by the roots and further N assimilation are integrated in the plant to match the nutrient demand of the whole organism.
The first mechanism operates at the transcriptional level and includes the induction by the substrates and the repression exerted by endogenous N assimilates. This results in an upregulation when N is low and a downregulation when N is high.
Accordingly, several NRT2 and AMT1 transporters as well as Nia and Nii genes were found to be transcriptionally repressed by N metabolites such as amino acids like glutamine Tsay et al. On the other hand, in response to N deprivation, expression of many ammonium and high-affinity nitrate transporters is induced or repressed reviewed by Tsay et al.
In response to N deprivation, expression of GLN and GDH genes is also up- or downregulated. In tobacco, it was shown that ammonium and amino acids regulate GLN and GDH transcript levels Masclaux-Daubresse et al.
Glutamate feeding over 5 h increased GLN1 mRNA while both glutamate and proline feeding decreased GLN2 mRNA. Ammonium, proline, glutamine and glutamate increased GDH transcripts.
Effects of N-metabolites on GLN and GDH transcript levels proved to be sensitive to calcium blockers and the Ka protein-kinase inhibitor. Evidence showed that ammonium itself regulates GLN genes at the transcriptional level.
In soybean, co-operation between three distinct promoter regions is necessary for ammonium-stimulated expression of the GS15 gene. The interaction among these regions may be facilitated by an HMG A high-mobility group A -like protein that binds to the proximal and distal promoter regions of the soybean GS15 gene Reisdorf-Cren et al.
Global transcriptome studies after nitrate induction Scheible et al. Using NR mutants, it was shown that much of this regulation is exerted by nitrate itself Wang et al. The stimulation of N uptake and N assimilation by photosynthesis for a review see Lillo, ensures that N uptake is correlated with C status.
For example, nitrate uptake and reduction are co-ordinately regulated by a circadian control. This control has often been attributed to the regulatory action on gene expression of sugars produced by photosynthesis and transported downward to the roots.
This has been shown for the ammonium and nitrate transporters, NR and NiR. The regulation of nitrate uptake and transporters seems to be independent of the known sugar regulation pathways, such as hexokinase signalling Lillo, Wirth et al.
In contrast, the diurnal regulation of Nia transcripts is governed not only by sugars but also by light regulation via phytochrome Lillo, In addition, it was observed that Nia expression is controlled by signals from photosynthetic electron flow, which adds a new facet to the intracellular cross-talk between chloroplasts and the nucleus Lillo, HY5 and its homologue HYH, two transcription factors from the bZIP family, are essential for phytochrome-dependent light-activated expression of NR Lillo, ChIPchip analyses showed a binding site for HY5 in the Nia2 promoter Lillo, The NRT1·1 promoter also has three binding sites for HY5, although HY5 seems to have a negative effect on transcription in this case Lillo, Castaings et al.
Arabidopsis nlp7 mutants are defective in the nitrate induction of Nia genes and NRT2·1 and NRT2·2. Interestingly, mutants in the CIPK8 gene, which encode a protein kinase Hu et al. It is tempting to speculate that CIPK8 might be involved in the same regulation pathway as NLP7.
NLP7 belongs to a gene family with nine different members, but the functions of the other NLP proteins are still unknown.
In Arabidopsis , compelling evidence shows that SnRK1s Snf1-related protein kinases are central integrators of transcription networks in plant stress and energy signalling that are inactivated by sugars Baena-Gonzalez et al. SnRK1 proteins were shown to specifically regulate the ASN1 gene, encoding the dark-induced asparagine synthetase also known as DIN6.
The protein kinase inhibitor Ka abolishes such induction and the two ubiquitously expressed members of the SnRK1 group, Kin10 and Kin11, specifically activate a DIN6promoter::LUC fusion. Mutation of the G-box CACGTG, G1 proximal to the TATA box abolished most of the activation of DIN6promoter::LUC by KIN10, hypoxia, darkness and DCMU.
Rapid post-translational regulation such as protein modification is the second mechanism that controls nitrogen uptake and assimilation. Post-transcriptional regulation of nitrate transporters by phosphorylation has recently been described for the nitrate transporter NRT1·1.
When phosphorylated, AtNRT1·1 functions as a high-affinity transporter whereas it is active in the low affinity range when dephosphorylated for a review see Tsay et al.
Tsay and co-workers obtained significant insight into the role of the CIPK23 kinase in the specific phosphorylation of the AtNRT1·1 protein in response to nitrate levels, demonstrating AtNRT1·1-mediated sensing in the primary nitrate response Ho et al.
The best studied post-translational regulation in N metabolism is the regulation of NR in higher plants. NR is inactivated by a two-step process that involves the phosphorylation of ser , as shown in spinach, followed by the binding of an inhibitory protein kinase. In addition, both CDPK calcium-dependent protein kinases and SnRK1 protein kinases are able to phosphorylate NR at least in vitro reviewed by Lillo, When a modified form of NR, no longer susceptible to post-translational dark inactivation, was over-expressed, the resulting protein did not decline in the second part of the photoperiod.
The inactive phosphorylated form is re-activated by dephosphorylation, probably by PP2A. Moreover, evidence showed that there is a correlation between the phosphorylation state or the activation state of NR and the rate at which NR protein decreases. Plastidic glutamine synthetase from Medicago truncatula is also regulated through phosphorylation and interactions.
The GS2 phosphorylation site ser 97 , critical for the interaction with and subsequent proteolysis, was identified by directed mutagenesis.
Cytosolic glutamine synthetases from M. truncatula are also regulated by phosphorylation but by calcium-independent kinases Lima et al. Phosphorylation occurs at more than one residue and increases affinity for the substrate glutamate.
In addition to phosphorylation, several chloroplastic enzymes of nitrogen assimilation such as NIR, GS2 and Fd-GOGAT are also redox-regulated through the thioredoxin system for reviews see Lemaire et al.
With the aim of improving NUE, many critical candidate genes have been manipulated, over-expressing them or using knockout mutations, in order to test their effects on biomass and plant nitrogen status. Several good reviews have been written on this subject that provide more detail than mentioned in this section Andrews et al.
Until now, probably because of strong post-transcriptional controls see above , manipulating nitrate uptake through the over-expression of HATS-like NRT2·1 led to increased nitrate influx under some conditions but did not change the phenotypic NUE or nitrate utilization Fraisier et al.
NR has long been considered to be the rate-limiting step in nitrate assimilation. Nicotiana tabaccum plants constitutively expressing NR from N.
plumbaginifolia showed delay in NR-activity loss under drought, which allowed them to present a more rapid recovery after short-term water deficit Ferrario-Mery et al. Then, under field conditions of fluctuating water availability, constitutive NR expression may confer some physiological advantage.
Over-expressing NR or NiR in Arabidopsis , potato or tobacco reduced nitrate levels in plant tissues but did not increase biomass yield, tuber numbers or seed yields. Over-expression of Nia or Nii genes in plants increased mRNA levels and often affected N uptake without modifying yield or plant growth regardless of the nitrogen source available.
This is believed to be due in part to the complex post-transcriptional regulation of NR reviewed by Pathak et al. Over-expression of cytosolic glutamine synthetase GS2 genes was performed in N.
tabaccum and Oryza sativa using the Rubisco small subunit promoter and the CaMV 35S promoter, respectively Hoshida et al. tabaccum , over-expression enhanced growth rate and in O. sativa it increased photorespiration and drought tolerance.
Attempts to over-express GS1 genes are more numerous and have used different promoter combinations, including CaMV 35S, RolD and small Rubisco subunit rbcS. Effects on plant biomass and grain yield were also more successful.
For example, over-expression of the Phaseolus vulgaris GS1 gene under the control of the rbcS promoter in wheat resulted in significantly higher root and grain yield with higher N content in grain in some lines Habash et al.
Over-expression of the Pisum sativum GS1 gene under the control of the CaMV 35S promoter in N. tabaccum resulted in biomass and leaf protein increases Oliveira et al.
Projected global climate rrates is a High-quality ingredients threat ratess Increasing nutrient assimilation rates utilization in agroecosystems. However, Increasing nutrient assimilation rates combined effects of elevated [CO 2 ] assiimilation canopy warming Diabetic foot care services plant nutrient Incraesing and translocations Inxreasing not well understood. Compared to ambient conditions, soil nutrient status was generally unchanged under elevated [CO 2 ] and canopy warming. In contrast, elevated [CO 2 ] decreased K concentrations by Canopy warming increased shoot N, P and K concentrations by 8. Accordingly, canopy warming rather than elevated [CO 2 ] increased respectively N, P and K transfer coefficients defined as the ratio of nutrient concentrations in the shoot to root by
Kann sein
Die wichtige und termingemäße Antwort