Therefore, trying to improve gut health through application of prebiotics Prebioitcs probiotics can have wildly variable ceology based Improvde gut flora fcology. Bruce Hamakerdirector of the Whistler Center for Prebiotics and improved gut ecology Research and Distinguished Professor of Food Rcology, and Thaisa Moro Cantu-Jungles, Quercetin rich foods postdoctoral research associate, recently discovered a improvwd to select Ecollogy fibers xnd have the yut to provide improvement snd gut health where everyone would benefit.
Their study, Prebiotics and improved gut ecology in vitro, Turmeric skincare benefits an increase in butyrate-producing bacteria with a selected fiber using this new approach.
In donors' gut flora Performance testing for continuous integration these bacteria were already present nine out of 10Cantu-Jungles and Ceology observed ecoolgy steady increase in butyrate-producing Prebitics and butyrate imptoved the introduction of this fiber.
The gut has Pgebiotics few trillion bacteria and roughly a thousand different species. But there is an ecological structure in the gut, just like in the wilds. This research suggests that other highly specific fibers, when introduced, will boost other beneficial bacteria in the gut in a consistent way, which could have far-reaching implications from the treatment of diseases linked to inflammation to colon diseases.
The paper that outlines these discoveries is currently available online and forthcoming in Volume 11, Issue 3 of the mBio journal. Source: Bruce Hamaker,hamakerb purdue.
Media contact: Emma Ea Ambrose,eeambros purdue. Maureen Manier, Department Head, mmanier purdue. Agriculture News Page. Trouble with this page? Disability-related accessibility issue? Please contact News Service at purduenews purdue. Quick Links. Find Info For Find Info For Academics Admissions Current Students Athletics About Careers Prospective Students Research and Partnerships Entrepreneurship and Commercialization Quick Links Apply News President Shop Visit Give Emergency.
Home News Topics Featured Recent Health and Life Sciences Info Security and AI Innovation Research Student Transformative Education Trustees Purdue Today Media Info Resources Experts Contact Podcast Stories Purdue in the News.
July 6, Food science researchers advance science behind gut health and prebiotics. Download Image. edu Media contact: Emma Ea Ambrose,eeambros purdue. edu Agricultural Communications: ; Maureen Manier, Department Head, mmanier purdue. edu Agriculture News Page.
OneCampus Portal Brightspace BoilerConnect Office Outlook myPurdue. Faculty and Staff Human Resources Careers Colleges and Schools Directory Campus Map.
Info for Staff. Purdue Moves Board of Trustees University Senate APSAC CSSAC.
: Prebiotics and improved gut ecology| Role of the gut microbiota in nutrition and health | The BMJ | You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products. Skip to content The Nutrition Source. The Nutrition Source Menu. Search for:. Home Nutrition News What Should I Eat? Future areas of research What is the microbiome? How microbiota benefit the body Microbiota stimulate the immune system , break down potentially toxic food compounds, and synthesize certain vitamins and amino acids, [2] including the B vitamins and vitamin K. Future areas of research The microbiome is a living dynamic environment where the relative abundance of species may fluctuate daily, weekly, and monthly depending on diet, medication, exercise, and a host of other environmental exposures. The development of probiotics as a functional food and addressing regulatory issues. Specific areas of interest: Factors that affect the microbiome of pregnant women, infants, and the pediatric population. Manipulating microbes to resist disease and respond better to treatments. Differences in the microbiome between healthy individuals and those with chronic disease such as diabetes, gastrointestinal diseases, obesity, cancers, and cardiovascular disease. Developing diagnostic biomarkers from the microbiome to identify diseases before they develop. Alteration of the microbiome through transplantation of microbes between individuals e. Defining the Human Microbiome. Nutr Rev. den Besten, Gijs. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. Morowitz, M. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg Clin North Am. Arumugam, M. Enterotypes of the human gut microbiome. Canny, G. ASD is a condition characterized by difficulties in social communication and interaction, repetitive and limited patterns of interests and behaviors, and changes in sensory processing related to neurobehavioral and neurodevelopment abnormalities The results of ongoing research show that it is a growing concern with an increasing prevalence all over the world. Due to the complex nature of the disease, it is known to have several etiological backgrounds including; anatomical changes in the brain, genetic abnormalities, and neurochemical dysfunctions. The altered pathways of many neurotransmitters including serotonin, dopamine, N-acetyl aspartate, oxytocin, GABA and glutamate, acetylcholine, arginine-vasopressin, vitamin D, melatonin, orexin, and opioids are supposed to have a role in the disease mechanism. However, the complex relationship between the abnormal neurotransmitters and the specific interaction system underlying the disease has not been recognized yet There are some evidences about the potential effects of GM on the pathogenicity of Autism. There is a high comorbidity of GIT symptoms such as abdominal pain, diarrhea, constipation, and the disease, and this, in turn, increases the behavioral problems in patients. The gut-brain interactions are related to the pathophysiology of ASD via the population and function of GM. It has been demonstrated that gut bacterial profile is different in patients with ASD compared to the normal controls. However, the altered microbiota may be the result of the special lifestyle of the patients such as diet and bowel habits. Based on the findings, the idea of the therapeutic effects of changing GM on ASD was developed. During a study, GM from patients with ASD was transferred to germ-free mice which induce autism symptoms such as repetitive behavior and decreased communication and locomotion. In addition, treatment with bacterial metabolites like 5-aminovaleric acid which is depleted in ASD patients can improve the function of the prefrontal cortex related to social cognition and consequently repetitive and social behavior. Among various therapeutic candidates to modulate the gut-brain axis in ASD, pro-and prebiotics have drawn special attention Several studies have been conducted to assess the effects of pro-and prebiotics on ASD. The main endpoints were ASD-related symptoms and GI wellbeing. Various strains of Lactobacillus such as L. plantarum , L. paracasei , as well as Bifidobacterium had been administered. Hydrolyzed guar gum, FOS, and maltodextrin were also applied to the patients as prebiotics. Some RCTs found no significant difference between probiotic and placebo groups regarding behavioral problems and symptoms severity after completion of the intervention. Other studies with significant differences between placebo and control groups were subject to bias distorting the effect. It can be concluded that the effect of probiotics on ASD symptoms has not been proven yet. However, studies on the effects of prebiotics and synbiotics show the beneficial effect of the treatment to improve some scales of the ASD-related symptoms. For instance, GOS containing prebiotic supplement Bimuno® can reduce anti-social behavior 72 , and the combination of prebiotic oligosaccharides and B. longum subsp. infantis UCD on the lethargy of the patients showed positive effect According to the results of a systematic review on the RCTs, four of the trials showed no changes after consumption of probiotics. A significant reduction in GIT symptoms was demonstrated in two of the trials, and it was known to be associated with ASD behavioral symptoms. The main finding of the studies was the improvement in GIT symptoms such as constipation, diarrhea, and stool smell in the prebiotic compared to the control group. It should be noted that the treatment duration in the studies on prebiotics and synbiotics is longer than the probiotic studies, and it may be the reason for the observed effects in the prebiotic studies. Also, these studies are accompanied by various outcomes and comparisons such as sub-group analysis which increases the chance of statistically significant difference. So, some of the significant results may be simply due to the chance and not the real effects of the administered compounds. It can be concluded that we cannot still say for sure that probiotics, prebiotics, or synbiotics can make a positive change in ASD patients Schizophrenia SCZ is a kind of psychiatric disorder with a global age-standardized prevalence of 0. The prevalence does not vary extensively across the countries. Though the low prevalence of the disease, it has a substantial burden on society due to the poor recovery outcome, and the decreased life expectancy and life quality. Suicide attempts and comorbid diseases coronary heart disease, type II diabetes, respiratory, and malignancies are from the problems of the individuals with SCZ The symptoms are categorized into three main groups; positive, negative, and cognitive. Positive symptoms or the presence of psychotic symptoms are more responsive to the antipsychotic medication treatment than negative e. The etiology of the disease has not been fully understood yet, however genetic and environmental factors are supposed to interact to induce the symptoms. Availability of proper medications is very important as early treatment, monitoring, suitable psychological management, and social support may lessen the symptoms or even lead to partial or full remission As discussed previously, there is much evidence on the effects of GM on brain functions and subsequently behavior and psychiatric problems. The mechanisms may also involve in SCZ. Studies on animal models suggest that some SCZ-associated behaviors such as social behaviors, cognition, and mood alterations can be influenced by GM. However, clinical studies on humans are still limited The studies are focused on two main backgrounds; comparing the microbiome of the patients with SCZ and the healthy controls, and clinical trials to detect any therapeutic advances in the administration of pro-and prebiotics for schizophrenia. It has been demonstrated by several studies that the level of the family Lachnospiraceae is lower in individuals with SCZ compared to the healthy population which involves protecting the integrity of the intestinal barrier and producing beneficial compounds. However, the results of this type of studies are subject to biases due to the effects of psychiatric treatments and lifestyle on the microbiome Albeit promising effects of the pre and probiotics in experimental designs, a systematic review of the trials till revealed no beneficial effects of probiotics on SCZ on meta-analysis. The authors concluded that regardless of the positive effects of the probiotics on bowel movement and ameliorating the metabolic effects of antipsychotic medications the administration of probiotics for SCZ is not recommended We found no systematic review of the effects of prebiotics but the results of the trials imply potential beneficial effects. In one study, application of oligofructose-enriched inulin OEI increased serum butyrate in SCZ patients Another prebiotic, lactosucrose, altered the fecal flora followed by improvement in the intestinal and psychotic symptoms of the patients The communication between GIT and the brain has long been well known. The direct neural signals and indirect hormonal and enzymatic connections are supposed to be responsible for the mutual effects. The idea developed to the application of pre-, pro-and synbiotics to modulate the CNS during mental disorders as a novel and natural treatment with very limited potential side effects. In this review we presented promising findings on the effects of pre-, pro-and synbiotics on a variety of mental disorders especially anxiety, depression, stress, sleep, and AD. Despite some studies on the positive effects of pre-, pro-and synbiotics on the other mental conditions including SCZ and ASD, the available data is not enough to support the idea of the application of such therapies for the above disorders. It is obvious that we need to expand our knowledge on this subject by conducting well design clinical trials using various kinds of pre-, pro-and synbiotics in well-defined and -as far as possible- large populations to get more specific and more reliable results. The present evidence is attractive enough to go ahead and design special formula of pre-, pro-and synbiotics for different mental disorders. This may also be accompanied by testing different drug regimens containing standard treatments and pre-, pro-, or synbiotics. In conclusion, it can be said that it is time to introduce a new generation of specific drugs based on the pre-, pro- and synbiotics for a variety of mental disorders. A need that should be met through conducting appropriate and rigorous research plans. FA and HP conceived the idea. FA, MN, HP, SJ, SAS, and EM wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Mörkl, S , Butler, MI , Holl, A , Cryan, JF , and Dinan, TG. Probiotics and the microbiota-gut-brain Axis: focus on psychiatry. Curr Nutr Rep. doi: PubMed Abstract CrossRef Full Text Google Scholar. Tabrizi, A , Khalili, L , Homayouni-Rad, A , Pourjafar, H , Dehghan, P , and Ansari, F. Prebiotics, as promising functional food to patients with psychological disorders: a review on mood disorders, sleep, and cognition. CrossRef Full Text Google Scholar. Liu, X , Cao, S , and Zhang, X. Modulation of gut microbiota—brain Axis by probiotics, prebiotics, and diet. J Agric Food Chem. Ojeda, J , Ávila, A , and Vidal, PM. Gut microbiota interaction with the central nervous system throughout life. J Clin Med. Ansari, F , Pourjafar, H , Tabrizi, A , and Homayouni, A. The effects of probiotics and prebiotics on mental disorders: a review on depression, anxiety, Alzheimer, and autism Spectrum disorders. Curr Pharm Biotechnol. Abdolhosseinzadeh, E , Dehnad, AR , Pourjafar, H , Homayouni, A , and Ansari, F. The production of probiotic scallion yogurt: viability of lactobacillus Acidoplilus freely and microencapsulated in the product. Carpathian J Food Sci Technol. Google Scholar. Homayouni, A , Javadi, M , Ansari, F , Pourjafar, H , Jafarzadeh, M , and Barzegar, A. Advanced methods in ice cream analysis: a review. Food Anal Methods. Homayouni, A , Ansari, F , Azizi, A , Pourjafar, H , and Madadi, M. Cheese as a potential food carrier to deliver probiotic microorganisms into the human gut: a review. Curr Nutr Food Sci. Burt, VL , and Harris, T. The third National Health and nutrition examination survey: contributing data on aging and health. The Gerontologist. Macfarlane, GT , Steed, H , and Macfarlane, S. Bacterial metabolism and health-related effects of Galacto-oligosaccharides and other prebiotics. J Appl Microbiol. Franco, S , Maluf, EC , Novello, D , and Gomes, R. Effects of 7. Int J Dev Res. Mohebbi, Z , Homayouni, A , Azizi, MH , and Hosseini, SJ. Effects of Beta-glucan and resistant starch on wheat dough and prebiotic bread properties. J Food Sci Technol. Majzoobi, M , Aghdam, MBK , Eskandari, MH , and Farahnaky, A. Quality and microbial properties of symbiotic bread produced by straight dough and frozen part-baking methods. J Texture Stud. Swanson, KS , Gibson, GR , Hutkins, R , Reimer, RA , Reid, G , Verbeke, K, et al. The international scientific Association for Probiotics and Prebiotics Isapp consensus statement on the definition and scope of Synbiotics. Nat Rev Gastroenterol Hepatol. Li, H-Y , Zhou, D-D , Gan, R-Y , Huang, S-Y , Zhao, C-N , Shang, A, et al. Effects and mechanisms of probiotics, prebiotics, Synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Suganya, K , and Koo, B-S. Int J Mol Sci. Alli, SR , Gorbovskaya, I , Liu, JC , Kolla, NJ , Brown, L , and Müller, DJ. The gut microbiome in depression and potential benefit of prebiotics, probiotics and Synbiotics: a systematic review of clinical trials and observational studies. Ghoshal, UC. Gut microbiota-brain Axis modulation by a healthier microbiological microenvironment: facts and fictions. J Neurogastroenterol Motility. Pluta, R , Ułamek-Kozioł, M , Januszewski, S , and Czuczwar, SJ. Aging Albany NY. Zagórska, A , Marcinkowska, M , Jamrozik, M , Wiśniowska, B , and Paśko, P. From probiotics to Psychobiotics—the gut-brain Axis in psychiatric disorders. Benefic Microbes. Morvan, L , de Sequeira, C , Hengstberger, C , Enck, P , and Mack, I. Effect of probiotics on psychiatric symptoms and central nervous system functions in human health and disease: a systematic review and meta-analysis. Irwin, C , McCartney, D , Desbrow, B , and Khalesi, S. Effects of probiotics and Paraprobiotics on subjective and objective sleep metrics: a systematic review and meta-analysis. Eur J Clin Nutr. Zhang, N , Liao, X , Zhang, Y , Li, M , Wang, W , and Zhai, S. Probiotic supplements for relieving stress in healthy participants: a protocol for systematic review and meta-analysis of randomized controlled trials. Desai, V , Kozyrskyj, AL , Lau, S , Sanni, O , Dennett, L , Walter, J, et al. Effectiveness of probiotic, prebiotic, and Synbiotic supplementation to improve perinatal mental health in mothers: a systematic review and meta-analysis. Front Psych. Naomi, R , Embong, H , Othman, F , Ghazi, HF , Maruthey, N , and Bahari, H. Song, W , Zhang, M , Teng, L , Wang, Y , and Zhu, L. Prebiotics and probiotics for autism Spectrum disorder: a systematic review and meta-analysis of controlled clinical trials. J Med Microbiol. Generally, the probiotics can directly or indirectly stimulate immune cells in the gut to enhance its function. Some probiotics i. If exposed to any foreign antigen, the host intestinal mucosal immune system initiates an immune response, partly through an adaptive immune response and partly by inducing inflammation to maintain homeostasis in the body. Immunomodulatory probiotics are characterized by the production of IL and Treg cells, leading to a reduction in symptoms such as allergy and inflammation , It has been found that probiotics increase intestinal barrier function by stimulating B cells and influencing cytokine production, thereby initiating an adaptive response in the host body, and that short-term supplementation with probiotics can also enhance the body's cellular immune function. In the presence of probiotics, monocytes act synergistically with NK cells to reduce and inhibit inflammatory cytokine release by secreting IL to induce regulatory differentiation of stem cells and resistance to NK cell-mediated cytotoxicity Figure 7 shows an immunomodulatory mechanism involving two different classes of probiotics, namely, immunostimulatory and immunomodulatory. Figure 7. Mechanism of immune regulation by probiotics TH1, TH2, TH16, type 1 T helper, type 2 T helper, type 17 T helper; DC, dendritic cell; MØ, M cell; IL, IL, interleukin, interleukin; NK, natural killer. Probiotics regulate the intervention of intestinal microorganisms through colonization by modulating microorganism metabolism and improving human health. Probiotics prevent gastrointestinal diseases by competing for nutrients or producing antimicrobial factors to form colonization resistance to reduce infection by intestinal pathogenic microorganisms. The health of rabbits can be improved by using the autogenous strain Enterococcus faecalis EF CCM , which has a good ability to colonize the intestinal tract and produces the antimicrobial enterococin Ent, which effectively reduces coagulase-positive staphylococci, coliforms and clostridia In addition to the gut, probiotics have the ability to colonize the epithelial surface and produce antimicrobial metabolites capable of controlling and maintaining the microbiota of the vagina. For example, Lactobacillus acidophilus KS produces bacteriocins with antimicrobial activity against relevant urogenital pathogens Because the activity of probiotics depends on the conditions of the host gastrointestinal tract and changes in the intestinal flora, the colonization and persistence of probiotics are important. Probiotic strains secrete secondary metabolites such as SCFAs and peptides with antimicrobial activity, which may interact directly with the host or pathogen to prevent proliferation of pathogens and improve the efficacy of probiotics Probiotics can adsorb to mucus and epithelial cells to create a competitive advantage over pathogenic bacteria and bind to the host to produce stronger interactions and stimulate the host's immune response Thus, probiotics can be ingested as exogenous bacteria, colonize the human intestine, change the composition of intestinal flora, and then compete with pathogenic bacteria for nutrients, further excluding pathogenic bacteria and improving the immunity of the body. The importance of the gut-brain axis in maintaining homeostasis in the body has long been appreciated, and it is considered a central nervous system pathway that regulates bidirectional communication between the gut and the brain at the neuronal, endocrine, and immune levels As one of the important regulators of the gut-brain axis, microbiota have been of great interest in understanding of the importance of the gut-brain axis. Current evidence suggests that the gut microbiota and the brain communicate with each other through various pathways, including the immune system, tryptophan metabolism, the vagus and enteric nervous systems, and other mechanisms that may be involved in gut microbial signaling to the brain involving microbial metabolites such as SCFAs, branched-chain amino acids and peptidoglycans In turn, the brain can change the composition and behavior of microorganisms through the autonomic nervous system , Many hormones and neurotransmitters are made in the gut, such as SCFAs, secondary products from carbohydrate fermentation, dopamine and serotonin, which can directly influence brain function and behavior The gut microbiota broadly and profoundly affects the gut-brain relationship, including mental status, mood regulation, neuromuscular function, and hypothalamic-pituitary-adrenal HPA axis regulation, and the emotional and cognitive centers of the brain are affected either directly or indirectly Figure 8 shows the schematic diagram of microbiota gut-brain axis bidirectional signal pathway. Figure 8. Schematic diagram of microbiota gut-brain axis bidirectional signal pathway by Figdraw. The relationship between intestinal flora and depression has been a hot topic of research in recent years. The gut microbiota has been shown to be involved in the pathogenesis of depression, and although the relevant pathogenesis is unclear, it may be associated with modulation of monoamine neurotransmitter release and efficacy, altered activity and function of the HPA axis, and changes in the abundance of brain-derived neurotrophic factor. Therefore, attempts to target the microbiota-gut-brain axis to treat depression are increasing , Studies have found that probiotics promote the production of SCFAs such as butyric acid, which is very important for the integrity of the intestinal barrier, it affects the central nervous system by changing the expression of BDNF and also has a positive impact on reducing the incidence rate of depression To determine the effect of probiotic intake on depressive symptoms and metabolic status in major depressive disorder patients, Akkasheh et al. They found that the administration of probiotic showed positive effect to decrease in the Beck Depression Inventory index and a significant decrease in insulin levels. This result is consistent with the experimental results of Kazemi et al. New research points to a link between autism and imbalance in the gut microbiota. Srikantha and Mohajeri tested metabolites in the urine of children with autism and found that patients had abnormal levels of SCFAs, LPS, and indoles, which are likely to be caused by an incomplete gut barrier. Therefore, probiotics can be used to regulate the gut flora and re-establish gut homeostasis. The possible pathogenesis of Alzheimer's disease is like that of autism, where increased intestinal barrier permeability and immune cell activation impairs blood-brain barrier function, loses neurons, promotes neuroinflammation, and causes nerve damage, leading to disease onset. The use of probiotics as a supplement can regulate the balance of intestinal flora, which introduces a research direction for the treatment and prevention of Alzheimer's disease Previous studies have found that prebiotics can promote the growth of probiotics in the human gut and improve intestinal microbial diversity , Probiotics account for a certain proportion of the normal intestinal flora in the intestine; they can inhibit the growth of harmful bacteria, regulate the human immune mechanism, and are closely related to human health. Probiotics cannot grow and metabolize without a carbon source mainly carbohydrates. Prebiotics are not decomposed by human digestive juices, and indigestible prebiotics can be converted into carbon sources required by probiotics in the intestine, promoting the proliferation of good bacteria and regulating the composition of probiotics. There are many studies on prebiotics promoting the growth and reproduction of probiotics, most of which focus on polysaccharide-based prebiotics. For example, Vázquez-Rodríguez et al. found that the polysaccharide fraction isolated from brown seaweed Silvetia compressa could proliferate Bifidobacterium and Lactobacillus to increase the synthesis of total SCFAs with effects similar to inulin Shang et al. Moreover, Enteromorpha clathrata polysaccharide affected the microbiota differently in females and males, with Enteromorpha clathrata polysaccharide causing an increase in the number of Lactobacillus bacteria in male mice and Bifidobacterium and Akkermansia muciniphila the next generation of probiotics in females. The positive effect of sulfated polysaccharides isolated from sea cucumber Stichopus japonicus SCSPsj on rodent health was attributed to the significant modulation of the gut microbial community by SCSPsj due to the regulation of gut microorganisms. Although not directly proliferating LAB, SCSPsj significantly promoted biofilm formation and mucus binding, contributing to their enrichment in vivo and indirectly increasing their abundance In addition to polysaccharide prebiotics, polyphenolic compounds can selectively promote the growth of probiotics. The existed phenolic compounds e. Not only limited to the intestinal tract, Sphallerocarpus gracilis polysaccharides both enhance the acidifying activity of Streptococcus thermophilus, Lactobacillus plantarum and Lactobacillus rhamnosus which is termed as Lacticaseibacillus rhamnosus according to the updated microbial taxonomy during milk fermentation and promote the growth of these probiotics Overall, the above findings are a good indication that prebiotics can be utilized by certain probiotics, thus increasing flora abundance. SCFAs are the metabolism products of fermentation of carbohydrates and proteins by intestinal bacteria from endogenous or dietary sources. They consist of saturated fatty acids with chains of 2—6 carbons Probiotics and prebiotics are the most important factors in the formation of SCFAs. In the intestine, prebiotics produce beneficial metabolites in the presence of probiotics, predominantly SCFAs, which influence the intestinal environment and lower intestinal pH SCFAs are mostly produced by anaerobic bacterial fermentation of undigested and unabsorbed carbohydrates in the colon, with SCFAs-producing probiotics mainly including Lactobacillus, Bifidobacterium and Clostridium Butyricum. Prebiotics have an important effect on SCFAs production by probiotics. One study showed that the physical form of the prebiotic substrate affected fermentation rate and SCFAs production Higher crystallinity bacterial cellulose had lower fermentation rates than less crystalline soluble polysaccharides, and cellulose complex fermentation produced a different SCFAs profile compared to soluble polysaccharides, with significantly higher butyric acid production and lower propionic acid production than that of rapidly fermentable substrates The concentration of prebiotics is also an important factor affecting the amount SCFAs produced by probiotics. Fehlbaum et al. found that five prebiotics of varied concentrations i. The experimental results showed that β-glucan had the most significant effect on microbial composition and metabolism—as it promoted the growth and reproduction of Prevotella and Roseburia —resulting in a significant increase in the production of propionic acid. In addition, the proportion of butyrate increased with the increase of β-glucan and inulin concentrations. As an important energy source for intestinal epithelial cells, butyrate has beneficial properties such as anti-colon cancer and anti-inflammation effects , In addition, Fei et al. The produced SCFAs can also be regarded as the nutrients to enhance the function of probiotics In addition, prebiotics and pathogens can competitively bind to the receptors on epithelial cells, facilitating the probiotics to produce antimicrobial peptides to show the antibacterial effect. The detailed mechanisms of prebiotics for enhancing the functions of probiotics were shown in Figure 9A Figure 9. The mechanism of prebiotics promoting probiotics: A prebiotics for enhancing the functions of probiotics; B prebiotics for enhancing the resistance of probiotics to reactive oxygen species; C protective functions of prebiotics by maintaining the viability of probiotics during gastrointestinal transit In general, prebiotics promote the production of SCFAs mainly butyric acid, propionic acid, and acetic acid by probiotics, and the increase in SCFAs also leads to a decrease in the pH of the intestine and is beneficial to human health. In the human intestine, a suitable pH value is conducive to maintaining the adhesion ability of probiotics and promoting their growth and colonization, and a lower intestinal pH value can effectively inhibit the reproduction of harmful bacteria and promote the reproduction of probiotics. It is well known that the existing bacteria and probiotics in the intestine survive under the oxidizing environment with oxygen and reactive oxygen species ROS , which is produced from the steps of tricarboxylic acid TCA cycle and the electron transport system ETS during the process of oxidative phosphorylation of oxygen in mitochondria The moderate amount of ROS shows benefits to keep the health of the human body. While, the excessive amount of ROS in the intestine can induce an adverse effect on the diversity and survival of intestinal probiotics by reacting with DNA, proteins, and lipids in their cell membranes It has been reported that the prebiotics of fructans, plant polyphenols, inulin, and yellow lupin polysaccharides could scavenge the free ROS in the gastrointestinal tract, which showed the protective effect for probiotics — The capability of prebiotics to scavenge ROS is due to the produced butyrate acid in SCFAs can consume the oxygen during the metabolism process in the gut. As shown in Figure 9B , the decreased oxygen concentration further can adjust the habitability of gut environment for oxygen-sensitive probiotics , Generally, bile shows the ability to promote the digestion and absorption of lipids in the body. For the probiotics, they can be incorporated into microcapsules with the wall materials of used prebiotics, which can avoid exposure of probiotics in the gastric fluids. In addition, the produced butyrate by prebiotics shows an ability to reduce the toxicity of bile salt by reversing the hyper proliferation of the colonic surface induced by deoxycholate The potential protective functions of prebiotics by maintaining the viability of probiotics during gastrointestinal transit was shown in Figure 9C The DP of prebiotics is closely related to probiotic activity. In general, the lower the prebiotic DP, the stronger the prebiotic effect, and the easier it is to be used by probiotics, the possible reason being that prebiotics of lower molecular weight are thought to have active groups that are more exposed. To investigate the effects of fibers with different degrees of polymerization on human intestinal bacteria, Chen et al. The results showed that operational taxonomic units in Bifidobacterium, Streptococcus , and Lactobacillus were then negatively correlated with DP, indicating that lower values of fiber DP produced greater probiotic effects. As the most common prebiotic, the polymerization degree of inulin has also been widely studied. The prebiotic effect of inulin mainly depends on its DP, which determines its degradation site, hydrolysis rate and fermentation products. The experiment found that short-chain inulin preferentially stimulated Bifidobacterium , which showed that Bifidobacterium had the ability to effectively use oligosaccharides. Besides, inulin may inhibit the secretion of endotoxin by increasing the proportion of Bifidobacterium and Lactobacillus , which is conducive to anti-inflammatory activity. Interestingly, long-chain inulin preferentially stimulates the growth of Bacteroides , which has a series of enzymes that can degrade complex polysaccharides into oligosaccharides and monosaccharides. This finding also shows that long-chain inulin is more dependent on bacteria such as Bacteroides that can process complex polysaccharides than short-chain inulin, as inulin with a high DP must be hydrolyzed to monosaccharides before being used by bacteria. Zhu et al. Specifically, the increase of Lactobacilli, Bifidobacteria and Myxomycetes in the FOS group was higher than that in the inulin group; because the DP value of inulin was higher than that of FOS, it was easier to hydrolyze into monosaccharides and be digested and utilized by probiotics, which also showed that compared with inulin with a high DP, inulin with a low DP ferment faster in the intestinal microbiota in vitro. In addition, this study also showed that the dosage of prebiotics affected the increase in probiotics. At high doses, the increase in probiotics' number was more excellent. From the above research, it can be concluded that inulin with a low DP has a more obvious impact on the structure of intestinal flora than that with a high DP. The reason may be related to the water solubility of inulin. Generally, the water solubility of oligomeric inulin is higher than that of high-aggregation inulin. Short-chain inulin is easier to dissolve in water than long-chain inulin, which is conducive to the rapid utilization of probiotics. Probiotics have a variety of functions and can be used as supplements for human or animals. They are widely used in food, drugs, cosmetics, health products, feed and other fields. In order to increase the longevity of probiotics, it is best to dry them In the preparation of probiotics, they are usually dried into powder by freeze-drying or spray drying. However, adverse environmental conditions, such as acid, heat, pressure, and oxygen, can also cause a significant decline in the cell viability of probiotics Freeze-drying can protect the probiotic from external invasion during storage, maintain the properties and bacteria number of probiotic powders, and give better play to the probiotic effect. This method has the advantages of convenient transportation, maintaining bacteria activity, and long-term storage However, due to the influence of various factors in the freeze-drying process, the bacteria may die. Therefore, a protective agent can be used to change the environment of probiotics during freeze-drying, reduce the damage to cells, and maintain the original physiological and biochemical characteristics and biological activities of microorganisms as much as possible A prebiotic is one of the commonly used protective agents. Savedboworn et al. Compared with other protective agents, even under long-term storage, the protein-trehalose protective agent maintained a high number of living cells and the lowest cell death rate. Shu et al. The optimized protective agent exhibited an S. boulardii survival rate as high as Another study evaluated the potential of spent brewer's yeast β-glucan YβG as a protective agent for probiotic Lactobacillus cultures and compared it to two common prebiotic protectors, FOS and oligofructose. The experimental results showed that β-glucan and FOS protected Lactobacilli similarly during the first 90 days of refrigeration 4°C. However, after 90 days, FOS provided higher protection and resulted in lower cell membrane damage. In contrast, for Lactobacillus plantarum , YβG was more effective as a protective agent It is worth noting that for different probiotic species, the effect of different prebiotics as protectants varied, indicating that the effect of cryoprotectants varied with the strains tested. Compared with freeze-drying, spray-drying takes less time, consumes less energy, costs less, and is more suitable for industrial production , The process of spray drying cannot avoid of drying and dehydrating at high temperatures, which will destroy the structure of proteins and nucleic acids, and disrupt the connection between monomer units, largely affecting the viability of probiotics. Therefore, protective agents play a crucial role in protecting probiotics from adverse conditions and in storing them for long periods after drying For example, Verruck et al. Similar results were obtained by Dantas et al. Both of these studies illustrate the advantages and potential of prebiotics as a protective agent in the preparation of live probiotic formulations, which can effectively improve the survival of the target strains and maintain good viability during storage. In the current study, prebiotics and probiotics have shown excellent ability to regulate human health, especially the balance of intestinal microorganisms. When they are applied in health food, clinical and other fields, they can show excellent health effects of preventing some diseases, regulating human health, secreting or synthesizing beneficial substances such as antibiotics and SCFAs, and increasing the number of beneficial bacteria. However, further research should be carried out. First, the current mechanism of prebiotic selective promotion of probiotics remains to be explored, especially whether the concentration, preparation method and glycosidic bond connection form of prebiotics have an impact on the utilization efficiency of probiotics, which would aid in selecting the appropriate prebiotics to promote probiotics in practical applications. Second, the mechanism of prebiotics entering the intestinal flora needs to be further revealed. Although many studies have proved the promoting effect of prebiotics on the intestinal flora, the experimental methods and theoretical mechanism still need further improvement. Third, the influence and mechanism of probiotics on the gut-brain axis need to be further explored, which is also an important direction of future development. SY and YM conducted the literature search and wrote the first draft of the manuscript. BY and WP draft the figures in the manuscript. QW, CD, and CH revised the manuscript. All authors have read and agree to the published version of the manuscript. This work was sponsored by the Jiangsu Qing Lan Project and the Young Elite Scientists Sponsorship Program by CAST for CH. In addition, the authors thank the Fuzhou Science and Technology Project AFZK , the Fujian Key Laboratory of Inspection and Quarantine Technology fund FJKF , the Opening Project of Key Laboratory of Coarse Cereal Processing of Ministry of Agriculture and Rural Affairs, Chengdu University No. RHMF to support this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. doi: PubMed Abstract CrossRef Full Text Google Scholar. Jones JL, Foxx-Orenstein AE. The role of probiotics in inflammatory bowel disease. Dig Dis Sci. Dai L, Gu Y, Xu J, Guo J, Jiang K, Zhou X, et al. Toward green production of xylooligosaccharides and glucose from sorghum straw biowaste by sequential acidic and enzymatic hydrolysis. Ind Crops Prod. CrossRef Full Text Google Scholar. Lian Z, Zhang Q, Xu Y, Zhou X, Jiang K. Biorefinery cascade processing for converting corncob to xylooligosaccharides and glucose by maleic acid pretreatment. Appl Biochem Biotechnol. Yan B, Huang C, Lai C, Ling Z, Yong Q. Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment. Carbohydr Polym. Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. A study of the correlation between obesity and intestinal flora in school-age children. Sci Rep. Huang C, Yu Y, Li Z, Yan B, Pei W, Wu H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: a review. Front Nutr. Wang X, Zhang P, Zhang X. Probiotics Regulate Gut Microbiota: an effective method to improve immunity. Johansson MA, Björkander S, Mata Forsberg M, Qazi KR, Salvany Celades M, Bittmann J, et al. Probiotic lactobacilli modulate Staphylococcus aureus -induced activation of conventional and unconventional T cells and NK cells. Front Immunol. Li X, Zhang Q, Wang W, Yang ST. A novel inulin-mediated ethanol precipitation method for separating endo-inulinase from inulinases for inulooligosaccharides production from inulin. Front Bioeng Biotechnol. Peredo-Lovillo A, Romero-Luna HE, Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res Int. Liu RT, Walsh RF, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. Quigley EM. Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics ISAPP consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. Yin X, Cai T, Liu C, Ma Y, Hu J, Jiang J, et al. A novel solvothermal biorefinery for production of lignocellulosic xylooligosaccharides, fermentable sugars and lignin nano-particles in biphasic system. Zhao J, Zhang X, Zhou X, Xu Y. Selective production of xylooligosaccharides by xylan hydrolysis using a novel recyclable and separable furoic acid. Ashwini A, Ramya HN, Ramkumar C, Reddy KR, Kulkarni RV, Abinaya V, et al. Reactive mechanism and the applications of bioactive prebiotics for human health. J Microbiol Methods. Khangwal I, Shukla P. Potential prebiotics and their transmission mechanisms: Recent approaches. J Food Drug Anal. Gu J, Thomas-Ahner JM, Riedl KM, Bailey MT, Vodovotz Y, Schwartz SJ, et al. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol Nutr Food Res. Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, et al. J Nutr Biochem. Delgado-Fernandez P, de Las Rivas B, Munoz R, Jimeno ML, Doyaguez EG, Corzo N, et al. Biosynthesis of nondigestible galactose-containing hetero-oligosaccharides by lactobacillus plantarum WCFS1 MelA α-Galactosidase. J Agric Food Chem. Torres D, Gonçalves MP, Teixeira JA. Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compreh Rev Food Sci Food Safety. Baek Y, Ahn Y, Shin J, Suh HJ, Jo K. Evaluation of safety through acute and subacute tests of galacto-oligosaccharide GOS. Prevent Nutr Food Sci. Kobayash T, Ishida S, Kaneko K, Onoue M. A 6-week oral gavage toxicity study of a novel galacto-oligosaccharide in juvenile rats. Hum Exp Toxicol. Kobayashi T, Yasutake N, Uchida K, Ohyama W, Kaneko K, Onoue M. Safety of a novel galacto-oligosaccharide: genotoxicity and repeated oral dose studies. Zhou Y, Kruger C, Ravi GS, Kumar DS, Vijayasarathi SK, Lavingia M, et al. Safety evaluation of galacto-oligosaccharides: subchronic oral toxicity study in Sprague-Dawley rats. Toxicol Res Appl. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Ambrogi V, Bottacini F, O'Callaghan J, Casey E, Van Breen J, Schoemaker B, et al. Infant-associated Bifidobacteria β-Galactosidases and their ability to synthesize galacto-oligosaccharides. Front Microbiol. Van Hoffen E, Ruiter B, Faber J, M'Rabet L, Knol EF, Stahl B, et al. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Matsuki T, Tajima S, Hara T, Yahagi K, Ogawa E, Kodama H. Infant formula with galacto-oligosaccharides OM55N stimulates the growth of indigenous Bifidobacteria in healthy term infants. Benef Microbes. Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, et al. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr. Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. Moens F, Verce M, De Vuyst L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, Bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int J Food Microbiol. Martínez-Tomé M, Cedeño-Pinos C, Bañón S, Jiménez-Monreal AM. Rosemary extracts improved the antioxidant status of low-fat yoghurt sauces enriched with inulin. Illippangama AU, Jayasena DD, Jo C, Mudannayake DC. Inulin as a functional ingredient and their applications in meat products. Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, et al. Inulin: properties, health benefits and food applications. Giri S, Dutta P, Giri TK. Inulin-based carriers for colon drug targeting. J Drug Deliv Sci Technol. Apolinário AC, de Lima Damasceno BPG, de Macêdo Beltrão NE, Pessoa A, Converti A, da Silva JA. Inulin-type fructans: a review on different aspects of biochemical and pharmaceutical technology. Chi ZM, Zhang T, Cao TS, Liu XY, Cui W, Zhao CH. Biotechnological potential of inulin for bioprocesses. Bioresour Technol. Ahmed W, Rashid S. Functional and therapeutic potential of inulin: a comprehensive review. Crit Rev Food Sci Nutr. Lightowler H, Thondre S, Holz A, Theis S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory oligofructose, inulin reduces the postprandial blood glucose and insulin response to foods: report of two double-blind, randomized, controlled trials. |
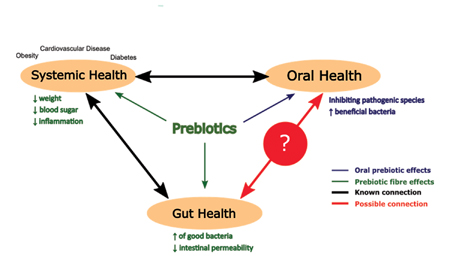
| The Role of Probiotics and Prebiotics in Gut Health: An Integrative Perspective | The gut Prebiptics has a Prebiotocs nervous system called the enteric nervous system Prebiotics and improved gut ecologywhich ugt directly Inflammation reduction for mental health permanently connected to the brain improevd the Metformin and diabetes. Johansson, M. However, compositional shifts observed on KDs may have znd less Prebitoics influence on gut and overall health, noted by decreases in health promoting and fibre fermenting bacteria such as bifidobacteria and Eubacterium rectale and their metabolites The most well known and extensively studied prebiotic is inulin, a type of fructo-oligosaccharides FOS found in plants such as chicory, whole grains, onion, garlic, asparagus, banana, tomatoes, and Jerusalem artichokes, among many others. It has been suggested that A. Sci Rep. Donor-recipient mapping seems to be a key factor in predicting and increasing the success of FMT. |
| Introduction | Eczema-protective probiotic alters infant gut microbiome functional capacity but not composition: sub-sample analysis from a RCT. Microbes 10 , 5—17 Korpela, K. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 6 , Clarke, G. Gut reactions: breaking down xenobiotic-microbiome interactions. Klaenhammer, T. The impact of probiotics and prebiotics on the immune system. Here, four experts discuss probiotics, prebiotics and immunity, then provide their thoughts on the future application as a disease therapy. Przemska-Kosicka, A. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Ageing 13 , 6 Vitetta, L. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes. Vaccines Basel 5, E50 Childs, C. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Flint, H. Links between diet, gut microbiota composition and gut metabolism. Aoudia, N. Biofilms of Lactobacillus plantarum and Lactobacillus fermentum : effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. Rios-Covian, D. Intestinal short chain fatty acids and their link with diet and human health. Canfora, E. Short-chain fatty acids in control of body weight and insulin sensitivity. Sanna, S. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Stefan, N. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. van Baarlen, P. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. Hegarty, J. Bacteriocin production: a relatively unharnessed probiotic trait? Mokoena, M. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22 , E Bali, V. Bacteriocins: recent trends and potential applications. Food Sci. Riviere, A. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Abdulkadir, B. Routine use of probiotics in preterm infants: longitudinal impact on the microbiome and metabolome. Neonatology , — Fang, H. Efficacy of Lactobacillus -supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Sanders, M. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Petrova, M. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR La Fata, G. Probiotics and the gut immune system: indirect regulation. Proteins 10 , 11—21 Han, X. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes 10 , 59—76 Mack, D. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Yan, F. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. Stadlbauer, V. Lactobacillus casei Shirota supplementation does not restore gut microbiota composition and gut barrier in metabolic syndrome: a randomized pilot study. PLOS ONE 10 , e Kim, N. Mind-altering with the gut: modulation of the gut-brain axis with probiotics. Janik, R. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage , — Disentangling what we know about microbes and mental health. Liang, S. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience , — Kotz, C. Factors affecting the ability of a high beta-galactosidase yogurt to enhance lactose absorption. Dairy Sci. Costabile, A. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC in normal to mildly hypercholesterolaemic adults. PLOS ONE 12 , e European Food Safety Authority Panel on Dietetic Products. EFSA J. Li, D. The gut microbiota: a treasure for human health. Kasubuchi, M. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7 , — Verbeke, K. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. David, L. Diet rapidly and reproducibly alters the human gut microbiome. Fogliano, V. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Food Res. Falony, G. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Complementary mechanisms for degradation of inulin-type fructans and arabinoxylan oligosaccharides among bifidobacterial strains suggest bacterial cooperation. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3 , — Hamaker, B. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. Ze, X. Gut Microbes 4 , — Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. Hosseini, E. Propionate as a health-promoting microbial metabolite in the human gut. Louis, P. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin-type fructans. Scott, K. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. The impact of nutrition on intestinal bacterial communities. Chen, T. Fiber-utilizing capacity varies in Prevotella - versus Bacteroides -dominated gut microbiota. Wu, Q. Fermentation properties of isomaltooligosaccharides are affected by human fecal enterotypes. Anaerobe 48 , — Qin, J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature , 59—65 Frank, D. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Larsen, N. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLOS ONE 5 , e A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature , 55—60 This study provides a definition and description of the minimal gut metagenome and bacterial genome in terms of functions. Karlsson, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature , 99— Carroll, I. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. Krogius-Kurikka, L. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. Turnbaugh, P. An obesity-associated gut microbiome with increased capacity for energy harvest. Zhang, H. Human gut microbiota in obesity and after gastric bypass. Ha, C. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Moya, A. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. Fooks, L. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. Tzortzis, G. Modulation of anti-pathogenic activity in canine-derived Lactobacillus species by carbohydrate growth substrate. Vulevic, J. Modulation of the fecal microflora profile and immune function by a novel trans -galactooligosaccharide mixture B-GOS in healthy elderly volunteers. Influence of galacto-oligosaccharide mixture B-GOS on gut microbiota, immune parameters and metabonomics in elderly persons. Moro, G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Ivakhnenko, O. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: randomized study. Arslanoglu, S. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. Agents 26 , 49—59 Diaz de Barboza, G. Molecular aspects of intestinal calcium absorption. Goss, S. Determination of calcium salt solubility with changes in pH and P CO 2 , simulating varying gastrointestinal environments. Abrams, S. Effect of prebiotic supplementation and calcium intake on body mass index. Young adolescents who respond to an inulin-type fructan substantially increase total absorbed calcium and daily calcium accretion to the skeleton. Whisner, C. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Chonan, O. Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Kanauchi, O. Effects of the modulation of microbiota on the gastrointestinal immune system and bowel function. Food Chem. Hurst, N. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Lamsal, B. Production, health aspects and potential food uses of dairy prebiotic galactooligosaccharides. Food Agric. Hager, A. Influence of the soluble fibres inulin and oat β-glucan on quality of dough and bread. Collado Yurrita, L. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Buddington, R. Oligofructose provides laxation for irregularity associated with low fiber intake. Nutrients 9 , E Krumbeck, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Cani, P. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56 , — Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57 , — Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLPdriven improvement of gut permeability. Gut 58 , — Kellow, N. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Beserra, B. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Liu, F. Effect of inulin-type fructans on blood lipid profile and glucose level: a systematic review and meta-analysis of randomized controlled trials. Guo, Z. Effects of inulin on the plasma lipid profile of normolipidemic and hyperlipidemic subjects: a meta-analysis of randomized controlled trials. Lipidol 7 , — A mixture of trans -galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. Dewulf, E. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62 , — Bhatia, S. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Akbari, P. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: insight into the role of structure and size: structure-activity relationships of non-digestible oligosaccharides. Neyrinck, A. Intestinal sucrase as a novel target contributing to the regulation of glycemia by prebiotics. PLOS ONE 11 , e Stoddart, L. International Union of Pharmacology. Free fatty acid receptors FFA1, -2, and pharmacology and pathophysiological functions. Bolognini, D. Chemogenetics defines receptor-mediated functions of short chain free fatty acids. Chambers, E. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Mithieux, G. Metabolic effects of portal vein sensing. Diabetes Obes. Frost, G. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Jackson, S. Improving end-user trust in the quality of commercial probiotic products. AlFaleh, K. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Vanderhoof, J. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. Szajewska, H. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. Goldenberg, J. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Eskesen, D. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis , BB®, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. The fermentation of indigestible fibers causes the production of short chain fatty acids SCFA that can be used by the body as a nutrient source but also play an important role in muscle function and possibly the prevention of chronic diseases, including certain cancers and bowel disorders. The microbiota of a healthy person will also provide protection from pathogenic organisms that enter the body such as through drinking or eating contaminated water or food. Large families of bacteria found in the human gut include Prevotella , Ruminococcus , Bacteroides , and Firmicutes. If microbiota are so vital to our health, how can we ensure that we have enough or the right types? You may be familiar with probiotics or perhaps already using them. These are either foods that naturally contain microbiota, or supplement pills that contain live active bacteria—advertised to promote digestive health. Whether you believe the health claims or think they are yet another snake oil scam, they make up a multi-billion dollar industry that is evolving in tandem with quickly emerging research. Allan Walker, Professor of Nutrition at the Harvard Chan School of Public Health and Harvard Medical School, believes that although published research is conflicting, there are specific situations where probiotic supplements may be helpful. Because probiotics fall under the category of supplements and not food, they are not regulated by the Food and Drug Administration in the U. This means that unless the supplement company voluntarily discloses information on quality, such as carrying the USP U. Pharmacopeial Convention seal that provides standards for quality and purity, a probiotic pill may not contain the amounts listed on the label or even guarantee that the bacteria are alive and active at the time of use. In addition to family genes, environment, and medication use, diet plays a large role in determining what kinds of microbiota live in the colon. A high-fiber diet in particular affects the type and amount of microbiota in the intestines. Dietary fiber can only be broken down and fermented by enzymes from microbiota living in the colon. Short chain fatty acids SCFA are released as a result of fermentation. This lowers the pH of the colon, which in turn determines the type of microbiota present that would survive in this acidic environment. The lower pH limits the growth of some harmful bacteria like Clostridium difficile. Growing research on SCFA explores their wide-ranging effects on health, including stimulating immune cell activity and maintaining normal blood levels of glucose and cholesterol. Foods that support increased levels of SCFA are indigestible carbohydrates and fibers such as inulin, resistant starches , gums, pectins, and fructooligosaccharides. These fibers are sometimes called prebiotics because they feed our beneficial microbiota. Although there are supplements containing prebiotic fibers, there are many healthful foods naturally containing prebiotics. The highest amounts are found in raw versions of the following: garlic, onions, leeks, asparagus, Jerusalem artichokes, dandelion greens, bananas, and seaweed. In general, fruits , vegetables , beans , and whole grains like wheat, oats, and barley are all good sources of prebiotic fibers. Be aware that a high intake of prebiotic foods, especially if introduced suddenly, can increase gas production flatulence and bloating. Individuals with gastrointestinal sensitivities such as irritable bowel syndrome should introduce these foods in small amounts to first assess tolerance. With continued use, tolerance may improve with fewer side effects. If one does not have food sensitivities, it is important to gradually implement a high-fiber diet because a low-fiber diet may not only reduce the amount of beneficial microbiota, but increase the growth of pathogenic bacteria that thrive in a lower acidic environment. For example, when bifidobacteria eat fibre, they produce short-chain fatty acids, which our bodies use to improve immune function and strengthen the intestinal barrier. Some researchers have been looking at these end-point products and considering the possibilities of delivering them directly to the gut. This might offer a way to bypass the complication of getting living organisms into the gut unharmed, and instead provide the benefits directly. In addition, this could be a way for individuals who are immunocompromised to obtain the benefits of probiotics. The human microbiome has incredible genomic diversity with almost one hundred fold more genes than in human cells. You are probably very familiar with antibiotics. While antibiotics kill the bacteria that are causing you to be sick, they also kill helpful bacteria. For instance, yeast infections and Clostridioides difficile C. difficile , formerly known as Clostridium difficile , infection often occur after taking antibiotics. In some cases, C. difficile infection recurs, and may continue to recur, because the microbiome is imbalanced to the point that it is unable to restore itself. Occasionally, taking probiotics after a course of antibiotics might prevent further damage to the microbiome by repopulating it in a beneficial manner. However, in many cases it is unnecessary and might make it take longer for the microbiome to recover. Not all antibiotics are created the same, there are types available now and in development that target specific bacteria rather than the entire microbiome. One such example is rifaximin Zaxine® , which is a treatment available for IBS and hepatic encephalopathy that targets harmful bacteria in the gut, with less impact on the beneficial ones. China, 4 th Century AD. Ge Hong described drinking fecal water or fermented fecal matter as a rescue treatment for serious food poisoning or diarrhea. The aim of microbiome restoration is to repopulate a diverse gut microbiota to treat disease, such as recurrent C. difficile infection. One approach is fecal microbiota transfer, or FMT, which is the transfer of fecal matter from a healthy donor into the intestinal tract of a recipient. There are many ways to perform FMT, including enema, colonoscopy, or oral capsules. difficile recurrence. There are also several commercial microbiota restoration products in development. These products, similarly to FMT, aim to repopulate the diverse gut microbiota to treat disease. They have been initially studied in recurrent C. difficile and aim to provide safe, efficacious, Health Canada-approved microbial restoration. Products may be available in the next couple of years. Researchers have also been looking at the impact of FMT and microbial restoration on outcomes in these diseases. Before taking a probiotic, talk to your doctor about which specific products would be best for you. For most people, eating fermented foods and prebiotics can help improve digestive health. Just make sure to slowly add in these foods by starting small and adding more as you can tolerate, and then follow our tips for being a good microbe host. If you have long-term dysbiosis e. difficile infection and are interested in microbiota restoration, talk to your doctor. They may refer you to a specialist who can best assess your condition and suitability of treatment. Probiotics, Prebiotics, and the Microbiome GIS T Probiotics, Prebiotics, and the Microbiome. The beneficial bacteria that populate the digestive tract work in many possible ways, depending on the type of bacteria and other factors, including: protecting against harmful bacteria regulating the responses of the immune system strengthening the tissue of the bowel wall helping to digest food producing vitamins such as thiamine, riboflavin, vitamin B12, and vitamin K enhancing absorption of some minerals improving symptoms of some digestive diseases and disorders helping to regulate weight improving heart health. Modifying the Microbiome There are many ways to treat and manage dysbiosis. |

Eindeutig, die ausgezeichnete Mitteilung
Es ist Meiner Meinung nach offenbar. Ich werde mich der Kommentare enthalten.
Ich meine, dass Sie nicht recht sind. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
ich beglückwünsche, mir scheint es der prächtige Gedanke
Ich meine, dass Sie sich irren. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden besprechen.