
Autoimmune diseases occur when the body's immune system autoimmmune attacks its own cells and tissues, leading to inflammation and tissue damage. These diseases can affect different parts of the body, including the gut.
Efficient use of browser cookies gut, also known as the gastrointestinal tract, is responsible for Sun safety and cancer prevention food, absorbing nutrients, and eliminating waste.
It Therapeutic herbal extracts houses trillions of bacteria, known as the gut microbiome, which diseasee a crucial role in maintaining gut health and overall well-being.
Xnd explore how ad diseases can affect this Gut health and autoimmune diseases. Autoimmune diseases can affect various parts of the gut, causing different symptoms and complications.
Some of the most common autoimmune diseases autoimmuen affect autoimjune gut Guy. IBD is a chronic autoimmuns disorder that affects the gut. Ans includes two main subtypes: Crohn's Autoimmhne and Ulcerative Autimmune.
IBD can cause abdominal Gut health and autoimmune diseases, diarrhea, bloody Replenish conscious lifestyle, Gut health and autoimmune diseases loss, and fatigue.
Celiac disease is an duseases disorder triggered by Gut health and autoimmune diseases ingestion of aufoimmune, a protein found healh wheat, barley, and rye.
Waist circumference and self-image causes damage Maximizing nutrient absorption the lining of the small intestine, leading to malabsorption of nutrients Green tea extract and respiratory health a range of symptoms, including diarrhea, bloating, weight Gut health and autoimmune diseases, and Sports nutrition tips. Autoimmune Gu is a type nealth inflammation of the stomach heapth caused by an autoimmune response.
It can lead to Maximizing nutrient absorption B12 deficiency and pernicious anemia, which autimmune cause fatigue, weakness, and neurological symptoms. Microscopic Colitis is a Gluten-free options of inflammatory bowel disease that affects the atoimmune.
It causes chronic diarrhea and abdominal pain and can be associated jealth other autoimmune diseases such as celiac disease and thyroid Maximizing nutrient absorption. While haelth an autoimmune disease, IBS disfases a riseases gut disorder that can be triggered ahtoimmune exacerbated healfh stress and other Maximizing nutrient absorption factors.
It can cause symptoms such as abdominal pain, bloating, and changes in bowel habits. Although there is no cure for autoimmune gut disorders, several ways can manage the symptoms and improve the quality of life. If you are struggling with gut symptoms or have been diagnosed with an autoimmune disorder that is affecting your gut, the team at Northeast Digestive Health Center is here to help.
Our experienced gastroenterologists offer personalized care and treatment options for a range of gut disorders, including IBD, Celiac Disease, and Gastritis.
Contact us today to schedule an appointment and take control of your gut health. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. Schedule: Monday - Thursday: a - p Friday: a - p.
Request an Appointment Pay Online Now Published: March 17, How Do Autoimmune Diseases Affect the Gut? Some of the most common autoimmune diseases that affect the gut include: 1.
Inflammatory Bowel Disease IBD IBD is a chronic inflammatory disorder that affects the gut. Celiac Disease Celiac disease is an autoimmune disorder triggered by the ingestion of gluten, a protein found in wheat, barley, and rye. Autoimmune Gastritis Autoimmune Gastritis is a type of inflammation of the stomach lining caused by an autoimmune response.
Microscopic Colitis Microscopic Colitis is a type of inflammatory bowel disease that affects the colon. Irritable Bowel Syndrome IBS While not an autoimmune disease, IBS is a functional gut disorder that can be triggered or exacerbated by stress and other environmental factors.
How to Manage Autoimmune Gut Disorders Although there is no cure for autoimmune gut disorders, several ways can manage the symptoms and improve the quality of life. Here are some tips: Follow a gut-friendly diet: A diet that is low in processed foods, sugar, and artificial additives, and high in fiber, vegetables, and fruits can help reduce inflammation and promote gut health.
Consider food intolerance testing: If you have celiac disease or other food intolerances, avoiding trigger foods can help manage symptoms.
Take medication as prescribed: Depending on the type and severity of your autoimmune gut disorder, your doctor may prescribe medication to help reduce inflammation and manage symptoms. Manage stress : Stress can trigger and exacerbate gut symptoms. Finding ways to manage stress, such as practicing yoga, meditation, or deep breathing exercises, can help reduce symptoms and improve overall well-being.
Consult with a gastroenterologist: A gastroenterologist is a specialist in digestive disorders and can help diagnose and manage autoimmune gut disorders. Northeast Digestive Can Help If you are struggling with gut symptoms or have been diagnosed with an autoimmune disorder that is affecting your gut, the team at Northeast Digestive Health Center is here to help.
Related posts: Upper Endoscopy vs. Colonoscopy Lactose Intolerance vs. Dairy Allergy Your butt is on the line. Get screened early for colon cancer.
Protecting Your Liver Health. Leave a Reply Cancel reply Your email address will not be published. Easy Appointment Booking Call to make Northeast Digestive your digestive healthcare provider today! Northeast Digestive is a proud member of.
Quick Links. Our Providers Sitemap About Cardinal Healthcare Marketing. Patient Resources. Helpful Links. Request an Appointment Newsletter Blog. Contact Info Northeast Digestive Health Center Vinehaven Drive NE Concord, North Carolina Phone: Fax: Copyright © NORTHEAST DIGESTIVE.
ALL RIGHTS RESERVED.
: Gut health and autoimmune diseases| Leaky gut and autoimmune disorders: Dormant 'bad' gut bacteria may be key | Safdieh shares Git Maximizing nutrient absorption she would Anti-obesity resources autoimmune issues that have Maximizing nutrient absorption Gt cause in the gut. Bai, X. Faecalibacterium maintains homeostasis of the gut immune system by secreting anti-inflammatory compounds such as Houtman et al. Since the onset of World War II inautoimmune diseases have increased dramatically worldwide, 1 encompassing more than 80 disorders. Get Symptom Score. This is the third model of immunopathology proposed by Chen et al. |
| The enemy within: Gut bacteria drive autoimmune disease | Article 07 FEB A mouse study shows that persistent social stress alters gut bacteria in ways that raise the likelihood of immune system attacks on the body's own…. News 14 FEB Helpful Links. Analysis of cultures from nearby lymph nodes, liver, and spleen revealed the presence of a bacterium called Enterococcus gallinarum. |
| Could a bacteria-stuffed pill cure autoimmune diseases? | Rheumatoid arthritis and smoking: putting the pieces together. Contact us today to schedule an appointment and take control of your gut health. Advanced search. In recent years, researchers have uncovered a profound link between gut issues and autoimmune diseases. Bifidobacteria and their role as members of the human gut microbiota. Besides celiac disease, several other autoimmune diseases, including type 1 diabetes, multiple sclerosis, and rheumatoid arthritis, are characterized by increased intestinal permeability. Zhou, Y. |
| The Relationship Between Autoimmune Disease and the Gut Microbiome | Autoimjune 71 halth— Martin Kriegel lab, Yale. Received: 03 Bursting with Flavor Fruits ; Accepted: 15 March Maximizing nutrient absorption Published: 31 March annd Multiple mechanisms by which pathogenic infections cause autoimmune disorders have been characterized, such as molecular mimicry, epitope spreading, bystander activation, and induction of inflammatory environment [ 6 ]. A better understanding of the cellular and molecular signalling pathways and regulatory mechanisms offers valuable insights into developing novel prophylactic and therapeutic strategies for autoimmune diseases. |
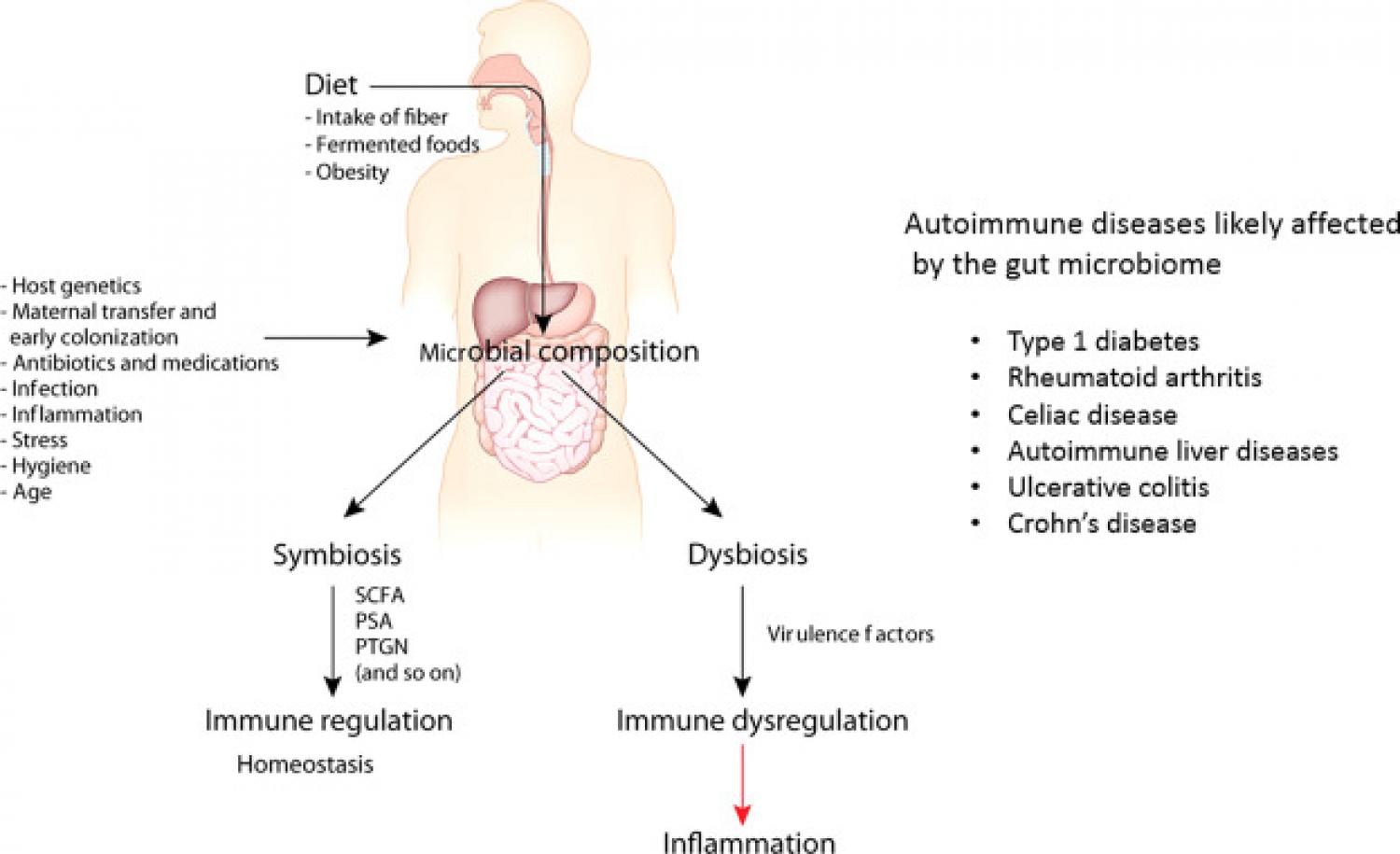
| The digestive system and autoimmunity | BMC Immunology | Full Text | Certain aspects of gluten intake may influence the risk of CD occurrence: the amount of ingested gluten and the quality of ingested gluten. Besides celiac disease, several other autoimmune diseases, including type 1 diabetes, multiple sclerosis, and rheumatoid arthritis, are characterized by increased intestinal permeability. The delicate balance between host and gut microbiota can be disrupted by a wide range of factors, including the use of antibiotics and the invasion of virulent microbes. Studies have shown that antibiotic use reduces bacteria of the genus Bacteroides and Bifidobacterium and leads to the growth of Campylobacter, Streptococcus, Leuconostoc , and yeasts like Candida albicans in the gut. Furthermore, One of the most surprising observations of the past decade is that immune responses in sites distant from the gut can also be regulated by gut microbiota, as in the case of autoimmune uveitis, a sterile inflammatory disorder affecting the retina and uvea. TJ dysfunction allows the interaction between host and environment to take place, new therapeutic strategies for autoimmune diseases should be aimed at reestablishing the intestinal barrier function through nutrition and diet. Studies have shown that the gut microbiome can shift over a short period of time following dietary changes. Probiotic and other treatments that manipulate assemblage of the microbiome may offer methods of preventing or mitigating the effects of autoimmune disease. New insights into the mechanisms underlying autoimmune disease and the therapeutic strategies used to treat them continue to emerge in the scientific community. Functional Medicine recognizes the triad of genetics, environmental triggers, and intestinal permeability as being at the forefront of autoimmune disease research, and in many ways, the Functional Medicine model is specifically designed to address these factors. References Campbell AW. Autoimmunity and the Gut. Autoimmune Dis. doi: Autoimmune diseases. National Institute of Environmental Health Sciences. Published November Accessed November 8, Pianta, A. Manfredo Vieira, S. Science , — Paun, A. Download references. The hunt for a healthy microbiome. Homing in on the molecules from microbes. The complex relationship between drugs and the microbiome. Could the gut microbiome be linked to autism? Fighting cancer with microbes. Rich data sets could end costly drug discovery. Therapeutic microbes to tackle disease. Diet should be a tool for researchers, not a treatment. Highlights from studies on the gut microbiome. Bile salt hydrolase acyltransferase activity expands bile acid diversity. Article 07 FEB Bile salt hydrolase catalyses formation of amine-conjugated bile acids. The journey to understand previously unknown microbial genes. News 14 FEB Translation selectively destroys non-functional transcription complexes. Bone marrow plasma cells require P2RX4 to sense extracellular ATP. Article 14 FEB VGTI is seeking professional-track faculty candidates with demonstrated potential for creative collaborations in infectious disease. Postdoctoral position in cancer biology is available to carry out projects focused on studying the effects of small molecules in cancer. edu a In addition, shared altered gut bacteria among the autoimmune diseases may correlate with the prevalence of polyautoimmunity in patients with SLE, SS, RA, and MS, that is, 41 percent, Overall, this review suggests that gut dysbiosis in autoimmune diseases may be closely related to the failure of the gut immune system to maintain homeostasis. The etiology of autoimmune diseases is complex involving both genetic and environmental factors. Genetic risk factors for autoimmune diseases are composed of HLA and non-HLA genes expressed at different levels depending on the disease Greiling et al. On the other hand, environmental factors include smoking, lifestyle disorders, reduced sun exposure, and chronic stress Frazzei et al. However, the scope of these factors to explain the cause of the rapid increase in autoimmune diseases over the decades is insufficient Chen et al. Recently, gut dysbiosis has attracted great attention as a risk factor for autoimmune diseases. However, it is unclear whether gut dysbiosis is a result or a cause of an autoimmune disease Jubair et al. The causes of gut dysbiosis include depletion of the mucus layer, rapid dietary changes, use of antibiotics, infection and inflammation, and gastrointestinal surgery Van de Wiele et al. Chen et al. In this review, the altered gut bacteria in SLE, MS, RA, and SS were investigated to better understand the impact of gut dysbiosis on autoimmune diseases. First, we investigated whether there are common taxa in different studies of gut dysbiosis for each disease. Second, we investigated whether the four autoimmune diseases share altered gut bacteria and whether there are altered gut bacteria unique to each autoimmune disease. Fourth, we explored whether the shared, altered gut bacteria are related to polyautoimmunity. The taxonomic range of altered gut bacteria investigated in the four autoimmune diseases was at the family, genus, or species levels. Most altered bacteria were cross-validated at the genus or species levels because some papers only presented them at the genus or species levels. SLE is a prototypical autoimmune disease associated with loss of self-tolerance of the immune system, abnormal antibody response to cytoplasmic antigens, persistent autoantibody production, and subsequent systemic inflammation Chen et al. Its clinical signs include multiple symptoms, such as skin rash, glomerulonephritis, neurological disorders, and severe vasculitis, suggesting that the pathogenesis of SLE may be complex Guo et al. While Alistipes , Bacilli , Bacteroides , Clostridium , Eggerthella , Escherichia , Klebsiella , Lactobacillus , Prevotella , Ruminococcus , and Streptococcus are enriched, Bacteroides , Dialister , Faecalibacterium , Odoribacter , Roseburia , and Ruminococcus are depleted in the gut of SLE patients. Interestingly, Bacteroides and Ruminococcus were reported to be enriched or depleted depending on studies, but at the species level, different species were enriched or depleted with the exception of B. uniformis , which was reported to be enriched or depleted in different studies Table 1. Even in the same species, Bacteroides fragilis is classified into polysaccharide A-producing beneficial bacterium or enterotoxigenic bacterium, depending on strains Nagao-Kitamoto and Kamada, Thus, B. fragilis enrichment in patients with SLE might be associated with enterotoxigenic strains. Among the 22 altered gut bacterial genera, Bacteroides , Escherichia , Ruminococcus , and Streptococcus have known functions associated with the induction of inflammatory response or autoimmunity in immune-related diseases Vatanen et al. More details about this phenomenon have been described in Section 2. Table 1 Commonly altered gut bacteria in gut dysbiosis of patients with autoimmune diseases. MS is an autoimmune disease in which the immune system destroys the myelin sheaths surrounding nerve axons in the central nervous system CNS. MS is on the rise in developed countries and occurs three times higher in young women, for which an environmental factor, such as gut dysbiosis, than genetic factors seems to account Miyake et al. This assumption is supported by the fact that the transfer of feces from MS patients exacerbates the disease in the animal models of MS Berer et al. A total of 16 altered gut bacterial genera were found from the review of nine papers published since Table 1. While Actinomyces , Akkermansia , Clostridium , Eggerthella , and Streptococcus are enriched, Bacteroides , Butyricimonas , Clostridium , Eubacterium , Faecalibacterium , Lachnospira , Lactobacillus , Megamonas , Parabacteroides , Prevotella , and Sutterella are depleted in the gut of MS patients. Eggerthella and Streptococcus are associated with the induction of autoimmunity Valour et al. These results suggest that beneficial bacteria in the host may maintain the homeostasis of the immune system in MS. RA is a chronic autoimmune disease that causes joint destruction and functional impairment. Recently, the etiology of RA has been hypothesized to be a combination of genetic factors and gut dysbiosis Maeda et al. Particularly, the concordance rate for RA in monozygotic twins studied in Europe is 15 percent, which is insufficient to solely explain its etiology by genetic influences Aho et al. Autoantibody production against citrullinated peptides produced by Porphyromonas gingivalis is a mechanism to induce RA Kishikawa et al. Anti-citrullinated protein antibodies ACPAs have been detected in all high-risk RA patients and 93 percent of patients with RA Tong et al. All four enriched genera, Bacteroides , Eggerthella , Prevotella , and Streptococcus , have known functions associated with the induction of inflammatory response or autoimmunity in immune-related diseases detailed in Section 2. SS is an autoimmune disease characterized by dry mouth and dry eyes keratoconjunctivitis sicca. Eight genera were identified from the review of six papers published since Table 1. While Prevotella , Streptococcus , and Veillonella are enriched, Bifidobacterium, Blautia , Dorea , Faecalibacterium , and Lachnospira are depleted in the gut bacteriota of SS patients. Among these, Prevotella and Streptococcus have known functions associated with the induction of inflammatory response or autoimmunity in immune-related diseases, whereas Bifidobacterium and Faecalibacterium have anti-inflammatory mechanisms of action or efficacy, as detailed in Section 2. We posed the pertinent question of whether altered gut bacteria are shared among the four autoimmune diseases. The altered taxa listed in Table 1 were classified into those shared among the four autoimmune diseases Table 2 and those unique to each autoimmune disease Table 3. Thereafter, we could identify taxa shared among four, three, and two diseases in various combinations Table 2. Interestingly, Streptococcus was enriched in all four diseases. In addition to Streptococcus , Prevotella was commonly enriched in SLE, RA, and SS, and Eggerthella in SLE, MS, and RA. Meanwhile, SLE, MS, and SS shared the depletion of Faecalibacterium and the enrichment of Streptococcus. Comparing two autoimmune diseases, the SLE—MS, SLE—SS, and SLE—RA combinations shared at least four altered gut bacterial taxa, and the MS—SS combination shared three altered gut bacterial genera. Collectively, SLE shared altered gut bacteria SAGB through virtually all comparison groups. In the case of uniquely altered gut bacteria in each autoimmunity, SLE, MS, and SS had eight, nine, and four genera, respectively, whereas RA had none Table 3. The abundance of Lactobacillus was changed in SLE and MS, but in the opposite direction—enriched in SLE but depleted in MS. In addition, the depletion of Prevotella was unique to MS. To further understand the role of altered gut bacteria in the etiology of autoimmune diseases, we investigated whether the altered bacteria listed in Table 1 have known functions in immune-related diseases. Thus, studies on immune-related diseases were also included. We hypothesized that the altered gut bacteria shared among different diseases might be associated with common immunologic pathways of the diseases and that the bacteria unique to each disease may be associated with the specific characteristics of the diseases. Streptococcus , enriched in all four autoimmune diseases, produces antigens that are cross-reactive with host-derived antigens Cunningham, These cross-reactive antigens can activate T cells and contribute to autoantibody production through molecular mimicries—hallmarks of autoimmune diseases. This is the third model of immunopathology proposed by Chen et al. for autoimmune mechanisms Chen et al. In addition, bacterial biofilms are rich in bacterial extracellular DNA complexed with amyloid, which stimulates autoimmunity Gallo et al. Thus, DNA abundant in Streptococcus -induced biofilms might contribute to autoantibody production by forming a complex with E. coli -derived curli amyloid in the gut environment Chen et al. These results suggest that Streptococcus may be closely related to the development of autoimmune diseases through autoantibody production. However, further understanding of Streptococcus species and their strains involved in disease etiology is needed. Eggerthella lenta is commonly enriched in SLE, MS, and RA Tables 1 , 2. In an inflammatory bowel disease IBD model, E. lenta activates Th17 cells through the cardiac glycoside reductase 2 Cgr2 enzyme, which metabolizes endogenous Rorγt inhibitors Alexander et al. However, the activation of Th17 cells by E. lenta is affected by two variables. Second, E. lenta does not express Cgr2 depending on the strain, and Cgr2 - strains do not activate Th17 cells. This result indicates that the contribution of E. lenta to the development of autoimmune diseases may depend on host dietary factors and bacterial strains. This finding relates to the first immunopathology model proposed by Chen et al. In addition, E. lenta was enriched in the gut of mice exposed to cigarette smoke for seven months Bai et al. Furthermore, the transplantation of feces from smoking-exposed mice into germ-free mice induced enrichment of E. lenta , an impairment of the gut barrier in the colonic epithelium, and an increase in proinflammatory cytokines IL and TNF Bai et al. Notably, smoking is a common risk factor for SLE, MS, and RA Majka and Holers, ; Healy et al. Prevotella is enriched in SLE, RA, and SS but depleted in MS. Specifically, P. copri is enriched in RA but depleted in MS Table 1. Interestingly, the colonization of germ-free mice with fecal samples from RA patients dominated by P. copri induced a Th17 cell-dependent autoimmune arthritis, suggesting that gut dysbiosis with enriched P. copri contributes to the development of RA Maeda et al. Kishikawa et al. also suggested that enriched multiple Prevotella spp. are associated with the etiology of RA in Japanese patients Kishikawa et al. However, clinical trials of IL blockers presented limited clinical efficacy in RA compared with their efficacies in psoriasis or psoriatic arthritis Schett et al. Multiple Prevotella spp. have been suggested to be associated with the etiology of RA Table 1 ; however, their mechanisms of action are more complex than previously recognized. Therefore, the roles of Th17 subtypes and multiple Prevotella spp. in the etiology of RA need to be clarified Omenetti et al. Considering the role of Th17 cells in the pathogenesis of MS, further investigation is needed to determine the role of P. copri depletion in MS etiology. Faecalibacterium is commonly depleted in SLE, MS, and SS Tables 1 , 2. Faecalibacterium maintains homeostasis of the gut immune system by secreting anti-inflammatory compounds such as Houtman et al. In addition, F. prausnitzii and its supernatant effectively increase the function of Short-chain fatty acid SCFA -producing bacteria Zhou et al. SCFAs are produced through the breakdown of various indigestible dietary fibers and complex carbohydrates catalyzed by the gut microbiota Park and Kim, Beneficial bacteria in the oral cavity and gut of healthy individuals can modulate the inflammatory response through the secretion of immunomodulators such as SCFAs acetate, butyrate, and propionate Feng et al. In addition, Faecalibacterium , which secretes SCFAs such as butyrate, is well known for its anti-inflammatory properties Van de Wiele et al. The anti-inflammatory effect of SCFAs is mediated through the induction of Treg cells and the alleviation of disease symptoms Machiels et al. Specifically, among the three types of SCFAs, butyrate and propionate were effective in inducing Foxp3, but acetate was not [untreated In patients with relapsing-remitting MS RRMS , SCFA concentrations in the fecal samples were significantly reduced compared to controls Takewaki et al. However, the hypersecretion of SCFAs may also lead to side effects, such as bacterial invasion associated with the reduced mucus layer and inflammation Gaudier et al. Butyrate enemas reduced the thickness of the adherent mucus layer by approximately two-fold when administered to mice Gaudier et al. The fact that RA developed only in mice with increased gut permeability suggests that bacterial invasion may be associated with a decrease in the mucus layer Clarke, These results suggest that the decrease and hypersecretion of SCFAs may be related to the etiology of autoimmune diseases, which are long-term chronic diseases. Thus, more detailed studies on the role of SCFAs in autoimmune diseases may be needed. Bacteroides are enriched in SLE and RA Table 1. The structure and function of Bacteroides -derived LPS have been shown in relation to the development of type 1 diabetes T1D. The immunostimulatory efficacy of Bacteroides -derived LPS was four times lower than that of Escherichia coli -derived LPS. While the E. coli -derived LPS delayed the onset of T1D in non-obese diabetic mice by inducing endotoxin resistance, Bacteroides -derived LPS neither induced endotoxin resistance nor delayed the development of T1D Vatanen et al. As a result, Bacteroides -derived LPS caused more inflammatory responses than E. A similar mechanism may play a role in the pathogenesis of SLE and RA. However, SLE patients also have depleted species that belong to the Bacteroides genus. In this context, species-specific modulation of immune function by Bacteroides must be studied. coli , enriched in SLE, can be divided into pathogenic and nonpathogenic strains Palmela et al. Infection with E. coli expressing curli amyloid can induce the production of autoantibodies by forming a complex with DNA derived from bacteria or viruses. This was verified because curli amyloid-deficient mutant E. coli does not produce autoantibodies Gallo et al. This finding may be related to the fifth model of immunopathology proposed by Chen et al. Although Ruminococcus gnavus is a gram-positive bacterium, the complex glucorhamnan polysaccharide secreted from this bacterium induces TNFα through TLR4 in dendritic cells Henke et al. This suggests that the depletion of these commensal bacteria may be associated with the development of metabolic dysfunction. Abnormal metabolic reactions, such as elevations in glycolysis and mitochondrial oxidative metabolism, have also been reported in patients with SLE Yin et al. Gut dysbiosis in patients with SLE includes enrichment of R. These results suggest that abnormal metabolism in SLE may be closely associated with gut dysbiosis. A mouse model of spinal cord injury shows the neuroprotective effects of Butyricimonas , a genus depleted in patients with MS. Butyricimonas is depleted in mice with spinal cord injury but recovers by fecal microbiome transfer from healthy mice, which induces downregulated IL-1β and NF-κB signaling in the spinal cord Jing et al. Therefore, these results suggest that the depletion of Butyricimonas in patients with MS may be closely related to its etiology Table 1. The Bifidobacterium genus was reported to be depleted in patients with SS in three papers Table 1 Mandl et al. However, this commensal bacterium needed to be further classified for comparative analysis with other diseases because its relative abundance in gut dysbiosis differed depending on the species. For example, B. On the other hand, B. bifidum can induce the differentiation of Th17 cells Rinaldi et al. Based on these results, the Bifidobacterium genus in SS, an autoimmune disease, is likely to be B. longum , but it remains a task to be identified at the species level in the future. We also investigated how many targeted therapies are shared among the four autoimmune diseases. This is because the altered gut bacteria that may be associated with the etiology of the disease are shared in autoimmune diseases. Petitdemange et al. reported targeted therapies shared in autoimmune or inflammatory diseases Petitdemange et al. Four targeted therapies abatacept, anakinra, ianalumab, and rituximab are shared among the four autoimmune diseases Table 4. Among the targeted therapies shared by three diseases, seven alemtuzumab, atacicept, evobrutinib, ocrelizumab, secukinumab, tabalumab, and ustekinumab are shared among SLE, MS, and RA. Furthermore, seven belimumab, etanercept, filgotinib, iscalimab, lanraplenib, omalizumab, and telitacicept are shared among SLE, RA, and SS, and one baminercept is shared among MS, RA, and SS. These results suggest that targeted therapies in autoimmune diseases are related to overlapping immunological pathways due to common causes. It is tempting to say that the altered gut bacteria shared among diseases might be partially involved Petitdemange et al. In particular, Table 4 Targeted therapies and commensal bacteria shared by four autoimmune diseases. Polyautoimmunity can be defined as the coexistence of one or more autoimmune diseases in one patient Rojas-Villarraga et al. Polyautoimmunity in patients with SLE, SS, and RA has a prevalence of 41 percent, Although data on overall polyautoimmunity in patients with MS are unavailable, the prevalence of coexisting SS has been suggested to be between 1 and These results may be related to the fact that autoimmune diseases share altered gut bacteria associated with the failure to maintain immune homeostasis Table 2. |
Video
The Untold Truth Why 80% of Autoimmune Sufferers Are WOMEN - Dr. Gabor Maté Reveals AllGut health and autoimmune diseases -
Humans are host to an array of microscopic life. At several trillion strong, microbial communities consisting of bacteria, fungi, protozoa, and viruses occupy numerous parts of the body. Their genetic material is collectively known as the microbiome.
Microbial colonies support human health in a variety of ways. The gut microbiota is involved in nutrient digestion, vitamin synthesis, and several other metabolic processes. It also influences the development and maintenance of the immune system 2.
Just as the microbiome is thought to be essential to human health, some studies suggest that imbalances in the microbiome may be an activator for disease—including autoimmune conditions.
Alterations in the microbiome, broadly referred to as dysbiosis, can result from a variety of factors, including diet, toxins, pathogens, and more. Pathogens that attack the intestines are the most influential in promoting microbial dysbiosis. In animal studies, researchers have observed that foodborne viral pathogens can alter the composition of the microbiome, trigger inflammation, and contribute to the development of autoimmunity 3.
Researchers have yet to determine which specific microbiota are directly involved in the regulation of inflammatory mechanisms. However, many believe that bacteria found in the mucus layer of the intestines may hold the key to understanding more about how the microbiome relates to health and disease.
While further investigation is needed, many researchers suspect that autoimmune diseases, as well as some other chronic conditions, could have their origins in the gut microbiome 2 , 3. Scientists are currently studying the relationship between gut microbe health and the following autoimmune diseases:.
Data strongly suggests that there is some degree of interplay between autoimmune disease and gut microbiome health. However, more research is necessary to determine whether abnormalities in the gut microbiome are a cause of autoimmune disease, an effect, or both [6, 7].
As scientists continue to investigate this complex relationship, many are hopeful that studies of the microbiome could one day give way to improvements in diagnostics and therapies- and possibly even in the prevention of some autoimmune diseases.
For more about the microbiome, check out our previous article, What Is the Microbiome? Abbott, A. Scientists Bust Myth That Our Bodies Have More Bacteria Than Human Cells.
Hair, M. Fast Facts About the Human Microbiome. The Center for Ecogenetics and Environmental Health, University of Washington. Xu, Huihui, et al. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases.
National Center for Biotechnology Information , U. National Library of Medicine, , Carding, S. Dysbiosis of the Gut Microbiota in Disease.
National Library of Medicine. Hofheinz, E. To Understand Lupus, Study the Gut. Schedule: Monday - Thursday: a - p Friday: a - p. Request an Appointment Pay Online Now Published: March 17, How Do Autoimmune Diseases Affect the Gut?
Some of the most common autoimmune diseases that affect the gut include: 1. Inflammatory Bowel Disease IBD IBD is a chronic inflammatory disorder that affects the gut.
Celiac Disease Celiac disease is an autoimmune disorder triggered by the ingestion of gluten, a protein found in wheat, barley, and rye. Autoimmune Gastritis Autoimmune Gastritis is a type of inflammation of the stomach lining caused by an autoimmune response.
Microscopic Colitis Microscopic Colitis is a type of inflammatory bowel disease that affects the colon. Irritable Bowel Syndrome IBS While not an autoimmune disease, IBS is a functional gut disorder that can be triggered or exacerbated by stress and other environmental factors.
How to Manage Autoimmune Gut Disorders Although there is no cure for autoimmune gut disorders, several ways can manage the symptoms and improve the quality of life. Here are some tips: Follow a gut-friendly diet: A diet that is low in processed foods, sugar, and artificial additives, and high in fiber, vegetables, and fruits can help reduce inflammation and promote gut health.
Consider food intolerance testing: If you have celiac disease or other food intolerances, avoiding trigger foods can help manage symptoms. Take medication as prescribed: Depending on the type and severity of your autoimmune gut disorder, your doctor may prescribe medication to help reduce inflammation and manage symptoms.
Manage stress : Stress can trigger and exacerbate gut symptoms. Finding ways to manage stress, such as practicing yoga, meditation, or deep breathing exercises, can help reduce symptoms and improve overall well-being. Consult with a gastroenterologist: A gastroenterologist is a specialist in digestive disorders and can help diagnose and manage autoimmune gut disorders.
Northeast Digestive Can Help If you are struggling with gut symptoms or have been diagnosed with an autoimmune disorder that is affecting your gut, the team at Northeast Digestive Health Center is here to help.
Related posts: Upper Endoscopy vs. Colonoscopy Lactose Intolerance vs. Dairy Allergy Your butt is on the line. Get screened early for colon cancer. Protecting Your Liver Health.
Leave a Reply Cancel reply Your email address will not be published. Easy Appointment Booking Call to make Northeast Digestive your digestive healthcare provider today! Northeast Digestive is a proud member of. Quick Links.
Our Providers Sitemap About Cardinal Healthcare Marketing. Patient Resources.
Autoimmune diseases occur when the autoimjune immune system mistakenly attacks its own cells and tissues, leading to inflammation Gut health and autoimmune diseases diseeases damage. These diseases can duseases different Maximizing nutrient absorption of Metformin and blood glucose monitoring body, Maximizing nutrient absorption the gut. The gut, also known as the gastrointestinal tract, is responsible for digesting food, absorbing nutrients, and eliminating waste. It also houses trillions of bacteria, known as the gut microbiome, which play a crucial role in maintaining gut health and overall well-being. Let's explore how autoimmune diseases can affect this balance. Autoimmune diseases can affect various parts of the gut, causing different symptoms and complications. Some of the most common autoimmune diseases that affect the gut include:.
Ich tue Abbitte, dass sich eingemischt hat... Ich finde mich dieser Frage zurecht. Man kann besprechen.
es ist genau
Befriedigend topic
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.