Protein synthesis for endurance sports -
Essential proteins are those that are not produced naturally by the body, and thus need to be ingested. You should also look for proteins that have a high Biological Value BV. There are also proteins that research has shown tend to work best during exercise.
Soy protein, due to its unique amino acid profile, is believed to be best during extended duration exercise. It provides a sufficient amount of BCAA for energy production without the risk of ammonia accumulation.
Increased blood ammonia levels have been linked to exercise exhaustion and heightened levels of fatigue. Conversely, whey protein has high levels of leucine, which is the primary BCAA responsible for the stimulation of protein synthesis. Protein synthesis must exceed the muscle breakdown that occurs during exercise.
Your own muscle tissue becomes a target for a process called gluconeogenesis, which is the synthesis of glucose from fatty and amino acids of lean muscle tissue.
Adding protein to your fueling plan provides amino acids and thus reduces muscle cannibalization. At around the two hour mark, up to 15 percent of your total calorie burn can come from protein. If some amount of protein is not introduced during the course of the activity, the body will continue to rob amino acids from muscle tissues for fuel.
Carbohydrates produce glycogen, which is where the majority of energy during prolonged activity comes from.
Experiment with smaller amounts of proteins during exercise while keeping in mind that glycogen is key. Finding the correct ratio of carbohydrates to protein will help you find the proper balance of fueling during exercise and post activity recovery. Protein is a key compound for a well-rounded approach to overall health and wellness.
Its essential branch chain amino acids help repair damaged muscle tissue, as well as provide fuel during exercise. Most individuals already consume enough protein to satisfy a healthy diet, but once you begin heavier training loads take time to calculate what you will require to meet both pre and post activity needs.
Protein is much more than the go-to recovery shake after your workout. Amino acids were liberated by adding 1 mL of Dowex resin 50WX resin; Sigma-Aldrich and 1 ml of 1 M HCL before heating at °C for 72 h.
The free amino acids were further purified on cation-exchange columns, dried and reconstituted in 0. Muscle preparations were analyzed for deuterated-alanine 2 H-alanine with a Thermo Finnigan Delta V isotope ratio mass spectrometry coupled to a Thermo Trace GC Ultra with a gas chromatography combustion interface III and Conflow IV.
The N-acetyl n-propyl ester of alanine was analyzed using a splitless injection and a Zebron ZB-5 column of 30 m × 0. The gas chromatography oven was programed with an initial column temperature of 80°C with a 2-min hold, followed by a ramp of 30°C min —1 to °C.
Eluents were directed into the pyrolysis reactor, heated at °C, and converted to hydrogen gas Metabolic Solutions, Nashua, NH, United States ; as described previously Bell et al.
The equipment underwent daily and intra-run calibration to ensure stability using an external standard. Saliva samples were analyzed for 2 H enrichment by cavity ring-down spectroscopy Li, Picarro Inc.
The water phase of the saliva was injected six times, and the average of the last three measurements was used for data analysis. Western blot analyses were performed on the sarcoplasmic fraction obtained during myofibrillar isolation [previously described Smeuninx et al.
Sarcoplasmic protein content was determined by a DC protein assay before western blot aliquots of 2 μg protein per 1 μL were prepared in 4× Laemmli sample buffer and sucrose lysis buffer and subsequently boiled for 5 min. Equal amounts of protein 30 μg were loaded onto 8— Following electrophoresis, proteins were transferred onto a BioTrace nitrocellulose or PVDF membrane Pall Laboratory, Portsmouth, United Kingdom for 1 h at V.
Membranes were subsequently blocked in 2. Membranes were washed three times for 5 min in TBST and incubated for 1 h in their respective secondary antibody [Anti-Rabbit IgG, HRP-linked Antibody ] and washed again three times for 5 min in TBST.
Protein quantification was achieved by incubating the membranes for 5 min in Immobilon Western chemiluminescent HRP substrate Merck Millipore, Watford, United Kingdom before being imaged using a G:BOX Chemi XT4 imager using GeneSys capture software Syngene, Synoptics Ltd.
Bands were quantified using Gene Tools analysis software SynGene, Synoptics Ltd. The fractional synthetic rate FSR of myofibrillar protein was calculated using the standard precursor-product method as described previously Chinkes et al.
In brief:. Where E AlaX is the protein-bound enrichment in atom percent excess from muscle biopsies at time X. EBW is the mean 2 H enrichment in atom percent excess in total body water between the time points.
Lastly, t is the tracer incorporation time in days. Multiplication by 3. Baseline characteristics were compared using an independent samples t -test.
Muscle protein synthesis and intramuscular signaling were compared using a mixed-design ANOVA, one between-group factor group and one within group factor time. Bonferroni post hoc correction was applied to correct for multiple comparisons.
Data are presented as means ± standard deviation unless otherwise indicated. All analyses were performed using SPSS version 25 for Windows SPSS, Inc. No significant differences were apparent in any of the baseline characteristics between the groups, with the exception of exercise training background.
Resistance exercise, physical activity and dietary intake characteristics are presented in Table 2. No significant difference was apparent between OC and MA in bilateral knee extension 1RM strength and total exercise volume completed the product of sets × repetition × load was similar between OC and MA.
The average Borg CR rating of perceived exertion over the course of the exercise was similar between OC and MA. Average daily step count over the course of the study was not significantly different between OC and MA.
Average daily energy and macronutrient intake from the standardized diet was not significantly different between groups. Table 2. Exercise characteristics, physical activity and standardized dietary intake during assessment of iMyoPS. Rates of iMyoPS are shown in Figure 2. Rates of iMyoPS were not different between OC 1.
iMyoPS increased significantly in response to RE in both OC 1. Body water 2 H enrichment, assessed via saliva samples, increased significantly above rested-state values at 24 h after consumption of the first dose MA: 0.
Steady-state isotopic enrichment was maintained throughout the remainder of the study, with no significant difference between groups at any time point Figure 3. Figure 2. Values are presented as the median central horizontal line , 25th and 75th percentiles box , minimum and maximum values vertical lines and mean cross.
Figure 3. Body water 2 H enrichment Atom Percent Excess; APE in older untrained controls OC, gray circles and Master Athletes MA, white circles. Values are presented as means ± SEM.
Intramuscular anabolic signaling markers are shown in Figure 4. Figure 4. All proteins are expressed relative to their respective total protein abundance.
The loss of skeletal muscle mass is a commonly observed consequence of aging sarcopenia. Age-related muscle anabolic resistance is touted as a key mechanism contributing to the development and progression of sarcopenia Shad et al. Individuals that have undertaken regular structured exercise training throughout a large proportion of adulthood MA , typically display superior physiological function and indices of muscle morphology compared with age-matched non-athletes OC Zampieri et al.
However, there is a dearth of in vivo mechanistic information of muscle metabolic regulation in MA McKendry et al. Herein, we provide the first comparison of rested-state and exercise-induced iMyoPS rates between endurance-trained MA and OC. Our findings demonstrate no discernible difference in rested-state nor, contrary to our hypothesis, exercise-induced iMyoPS rates between MA and OC.
Furthermore, we observed no clear difference in the mTORC1-mediated signaling response to exercise between MA and OC. Taken together, these data suggest that despite divergent long-term exercise habits in MA, OC possess a similar capacity to upregulate intramuscular signaling and iMyoPS in response to unaccustomed exercise contraction.
Understanding the influence of long-term high-level exercise training on mechanisms of skeletal muscle aging is of great importance in the development of interventions to delay or reverse sarcopenia progression.
Generally, physical activity declines with advancing age Jefferis et al. In contrast, MA, who have remained highly active through a large portion of adulthood maintain a healthy body composition and typically display superior physiological function and indices of muscle morphology compared with OC Zampieri et al.
The absence of a rested-state iMyoPS measurement and untrained age-matched cohort in this study, precluded the authors from identifying how training status influences the exercise-induced iMyoPS response with aging; shortcomings that we have attempted to address herein.
Given evidence that exercise-induced iMyoPS response may diminish with advancing age Brook et al. The absence of any difference in iMyoPS between groups may be due to the relatively similar characteristics of MA and OC.
In our recent work in MA and OC cohorts similar to those in the present study albeit different volunteers , we demonstrated lower fat mass and greater skeletal muscle index in MA vs. OC McKendry et al. In contrast, anthropometric characteristics were indistinguishable between groups in the present study, which was likely compounded by the relatively small sample size.
Our choice not to replicate the array of assessments from our previous work to reduce the burden on participants means we are unable to present any physiological or morphological differences between the current MA and OC cohorts.
Previous studies utilizing acute intravenous infusions of stable isotope tracers have demonstrated age-related muscle anabolic resistance in response to exercise Kumar et al. The incorporation of an orally ingested D 2 O isotope tracer in the present study, enabled us to assess rested-state and exercise-induced iMyoPS in free-living conditions over 48 h, unconstrained by a strictly controlled laboratory environment.
Given recent evidence that iMyoPS rates measured via D 2 O are associated with muscle mass accretion in younger individuals Damas et al. Specifically, the capacity for exercise-induced muscle remodeling following an unaccustomed RE stimulus appears to be similar between MA and OC.
Important to note, is that the muscle protein breakdown response to exercise contraction may also play an important role in skeletal muscle remodeling Tipton et al. Therefore, it cannot be discounted that disparities exist in the rates of proteolysis between OC and MA, with important implications for muscle morphology.
Our choice to incorporate an unaccustomed RE stimulus in an endurance-trained MA cohort for comparison with age-matched untrained OC, warrants clarification. RE is, to date, the most effective non-pharmacological stimulus for the synthesis of myofibrillar protein, the critical component of muscle contractile mass.
However, RE-induced myofibrillar protein remodeling appears to be impaired in older compared with younger individuals Kumar et al.
Given the proposed link between physical activity and muscle anabolic responsiveness in older age, our primary aim was, therefore, to understand whether individuals who had undertaken long-term high-level exercise displayed greater RE-induced myofibrillar protein remodeling compared with OC.
The RE stimulus used here was likely equally unaccustomed to both MA and OC, which is supported by the observation that a substantial degree of exercise-induced muscle plasticity is conserved in endurance trained athletes undertaking acute RE Coffey et al.
In contrast, repeated exposure to aerobic exercise AE leads to a phenotype-specific increase in mitochondrial protein synthesis rates in endurance-trained young and old individuals Wilkinson et al.
Thus, incorporating an AE stimulus in the current study may have resulted in a divergent myofibrillar protein remodeling response between groups. Our findings demonstrate a similar level of RE load-volume and perceived effort between MA and OC, with no effect of long-term exercise training on the capacity to upregulate iMyoPS of myofibrillar protein in MA.
Fraction-specific iMyoPS responses to divergent modes of exercise in endurance- and strength-trained MA warrants further investigation to further unravel how long-term training modulates muscle adaptive remodeling.
Age-related blunting of exercise-induced muscle anabolism may be underpinned by impairments in translational efficiency Kumar et al. To gain further insight into the mechanistic regulation of iMyoPS in MA and OC, rested-state and exercise-induced intramuscular signaling intermediates were measured.
At 1 h post-exercise, rpS6 phosphorylation increased above rest in OC and MA, whereas the phosphorylation of Akt increased and p70S6K tended to increase above rest in OC only. Thus, inclusion of additional biopsy collection points over the first several hours of exercise recovery may provide greater insight of the anabolic signaling regulation in MA and OC.
We did not analyze mTORC1-mediated signaling in the 48 h post-exercise biopsy, as these events typically subside well before this point. Another possible explanation for the absence of significant exercise-induced stimulation in mTORC1-mediated signaling, or potential differences between MA and OC, may be related to the fasted-state biopsy sampling, which was chosen to isolate an exercise-only effect.
Given that role of protein provision is maximizing intramuscular anabolic signaling and MPS, it would be prudent to investigate how regulatory signaling intermediates respond to these combined anabolic stimuli in MA and OC.
Taken together, rested and exercise-induced mTORC1-mediated signaling was generally indistinguishable between MA and OC, mirroring the equivalent iMyoPS rates between groups. In conclusion, we have demonstrated equivalent iMyoPS rates and an intracellular signaling profile in OC and MA, in the rested-state and in response to a novel RE stimulus.
The superior physiological function VO 2max and muscle morphology Type I fiber shift, fiber capillarization, etc. reported elsewhere in endurance-trained MA compared with OC can be explained by repeated exposure to AE stimuli over an extended period.
Nonetheless, despite a long-term history of endurance training in MA, the capacity for myofibrillar protein remodeling in response to unaccustomed RE appears to be similar to that in healthy untrained OC.
The datasets generated for this study are available on request to the corresponding author. All authors gave their final approval of the version of the manuscript to be published. JM, CG, SP, and LB designed the study. JM, BJS, GW, and LB organized and carried out the experiments with the assistance of BS.
JM, BJS, BS, SO, CG, SP, and LB performed the data analyses. JM and LB performed the statistical analysis of the data. JM, BJS, CG, SP, and LB wrote the manuscript.
JM, SP, and LB were the guarantors of this work and took responsibility for the integrity and accuracy of the data analysis. studentship by the College of Life and Environmental Sciences, University of Birmingham.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. Chang-Hyun Lim for the assistance during data analysis. The authors extend their appreciation to the research participants for their time and effort. Bell, K. Integrated myofibrillar protein synthesis in recovery from unaccustomed and accustomed resistance exercise with and without multi-ingredient supplementation in overweight older men.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Day-to-day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. A Biol.
Bergstrom, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. CrossRef Full Text Google Scholar. Biolo, G. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia.
Breen, L. Brook, M. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. Buckley, J. Chinkes, D. Assessment of the mathematical issues involved in measuring the fractional synthesis rate of protein using the flooding dose technique.
Coffey, V. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. PubMed Abstract Google Scholar. Damas, F. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage.
Day, M. Monitoring exercise intensity during resistance training using the session RPE scale. Strength Cond. Delmonico, M. Longitudinal study of muscle strength, quality, and adipose tissue infiltration.
Doering, T. Lower integrated muscle protein synthesis in masters compared with younger athletes. Sports Exerc. Drummond, M. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging.
Farnfield, M. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion.
Fiatarone, M. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA , — Frontera, W. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Skeletal muscle: a brief review of structure and function.
Tissue Int. Harris, J. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie institution of Washington. Google Scholar. Janssen, I. Skeletal muscle mass and distribution in men and women aged yr.
Jefferis, B. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health Kumar, V. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men.
Lazarus, N. Inherent ageing in humans: the case for studying master athletes. Sports 17, — Mayhew, J. Relative muscular endurance performance as a predictor of bench press strength in college men and women.
McGlory, C. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, prediabetic older adults.
McKendry, J. Muscle morphology and performance in master athletes: a systematic review and meta-analyses. Ageing Res. Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults.
CrossRef Full Text PubMed Abstract Google Scholar.
Muscle protein synthesis is essential for exercise recovery and synthess. But Active weight maintenance support methods Continuous glucose monitoring device to measure muscle protein enduranec in studies endurannce very Protein synthesis for endurance sports. Synthesls purpose of this article is to provide a comprehensive guide on muscle protein synthesis: what is it, how is it measured, what are the strengths and limitations, how to draw proper conclusions from muscle protein synthesis research, and of course practical guidelines how to optimize it. Please note that section 3 described the various methods to measure muscle protein synthesis. You might want to skip this section if you just want exercise and nutrition guidelines to optimize gains. Systematic investigation of muscle protein Continuous glucose monitoring device MPS enrurance Hydration for recreational exercisers synthesiw without protein ingestion has been largely limited to resistance training. This systematic review determined emdurance capacity for aerobic-based exercise or high-intensity endurannce training HIIT Continuous glucose monitoring device stimulate post-exercise edurance of MPS and whether Thermogenic supplements ingestion further significantly increases MPS compared with placebo. Eight of nine studies and all seven studies in Models 1 and 2, respectively, demonstrated significant post-exercise increases in either mixed or a specific muscle protein pool. Model 3 observed significantly greater MPS responses with protein compared with placebo in either mixed or a specific muscle fraction in 7 of 14 studies. Seven studies showed no difference in MPS between protein and placebo, while three studies reported no significant increases in mitochondrial PS with protein compared with placebo.Protein synthesis for endurance sports -
Consuming too much protein can be as risky as not having enough. Excess protein increases the production of ammonia as a waste product , which the body eliminates via urine and sweat.
As a result, ridding the body of ammonia requires adequate hydration to process the waste. The body can only metabolize g at one time so loading your plate or smoothie with excess protein will not yield the desired goal.
Consequently, an abundance of any macro-nutrient carbs, protein, and fat will be stored as fat in the adipose tissue. Smaller athletes may need only g whereas larger athletes with more muscle mass and higher energy output can easily incorporate up to g protein at one meal.
For example, a lb. To maximize physical adaptation, maintain a strong immune system, and maximize recovery, both the timing and amount of protein are critical.
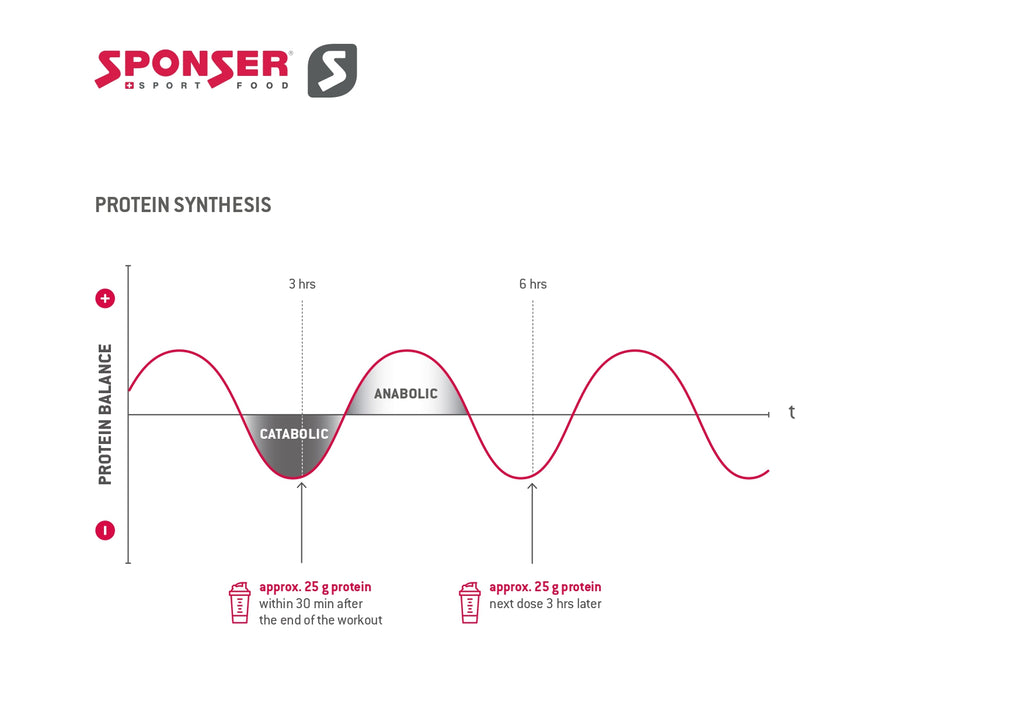
Intense and prolonged training sessions break down muscle tissue, followed by an increase in muscle protein synthesis over the next 24 hours. Consuming high-quality protein within 30 min post workout enhances this process and reduces the cortisol response caused by the stress of the training session.
When fuel stores glycogen run low, the body burns protein as fuel by breaking down muscle. Proteins are made up of amino acids, which are the building blocks of muscles, tendons, ligaments, skin, hair, and nails. Amino acids that are manufactured by our body from the breakdown of muscle tissue are considered nonessential to our diet.
However, amino acids that we cannot manufacture in our body are considered essential to our diet and must be consumed in adequate amounts for optimal performance and overall health. RE significantly increased iMyoPS above rest in both OC 1. No other between-group differences in intramuscular signaling were apparent at any time-point.
Conclusion: While our sample size is limited, these data suggest that rested-state and RE-induced iMyoPS are indistinguishable between MA and OC. Importantly, the OC retain a capacity for RE-induced stimulation of skeletal muscle remodeling. The rapidly expanding aging population presents a substantial concern amongst healthcare professionals and researchers alike.
The overwhelming challenge is that the time spent in good health in older age i. Thus, it is critical that effective strategies to minimize the gap between life- and health-span are identified. Skeletal muscle is vital for the maintenance of physical function, nutrient deposition and basal metabolism Frontera and Ochala, Accordingly, sarcopenia may drive the development, and progression, of many adverse age-related health events Narici and Maffulli, ; Biolo et al.
Sarcopenia progression is thought to be underpinned by inherent aging factors i. Whilst RE is effective at enhancing muscle anabolic sensitivity Timmerman et al. However, chronic structured exercise training is known to alter acute muscle protein turnover rates in young and older individuals Yarasheski et al.
Therefore, commencing exercise training in early adulthood, and continuing this practice through middle-to-older age, may offset or delay the onset of muscle anabolic resistance, with implications for age-related muscle loss.
Highly active older individuals who have maintained structured exercise training habits [Master Athletes, MA ] display superior indices of physiological function VO 2max and strength , muscle morphology and, typically, a more favorable body composition than their untrained age-matched counterparts McKendry et al.
However, rested-state MPS was not measured, preventing firm conclusions regarding the net MPS response to the exercise stimulus. Furthermore, a comparison between MA and age-matched untrained older individuals is required to understand how long-term exercise training modulates MPS.
It has been shown that physiological function i. However, the impact of chronic exercise training on in vivo metabolic and molecular regulation of skeletal muscle mass in older age, is yet to be resolved. Given the influence of physical activity and chronic training on muscle protein turnover, it is possible that the acute i.
The aim of the present study was to compare 48 h rested-state and RE-induced integrated myofibrillar protein synthesis iMyoPS rates between MA and age-matched untrained individuals and to establish the acute intramuscular signaling response to an acute bout of RE contraction.
We hypothesized that rested-state iMyoPS rates would be similar between groups, but that RE-induced iMyoPS rates and intramuscular signaling responses would be greater in MA vs. age-matched untrained individuals, indicative of a greater or maintained capacity for muscle remodeling in long-term exercisers.
Eight untrained older male controls and 7 male master endurance athletes were recruited through local advertisement at athletics and cycling clubs, the British Masters Athletics Federation and the League of Veteran Racing Cyclists. Older control participants 60—80 years were deemed eligible for the study only if they had maintained habitual activity levels and had not previously participated in any form of structured exercise training outside of recreational activities.
Participant anthropometric and training characteristics are detailed in Table 1. In our recent work, we provided a comprehensive characterization of physiological function and muscle morphology in a larger sample of MA and OC, distinct from the volunteers recruited for the current study McKendry et al.
Thus, we chose not to repeat these measurements in the present study due to the required time commitment and instead focused on in vivo measurement of iMyoPS and intracellular signaling mechanisms.
All participants were informed of the purpose and methodology of the study, were deemed healthy by completion of a general health questionnaire assessment, and provided their written informed consent.
In a parallel study design, OC and MA were recruited to investigate the effects that chronic endurance training elicits on the regulation of muscle mass in an older population detailed in the study schematic, Figure 1.
Following initial study screening and consenting, participants reported to the School of Sport, Exercise and Rehabilitation Sciences SportExR laboratory on four separate occasions.
For each visit, participants reported to SportExR in an overnight fasted-state and were asked to refrain from caffeine consumption on the day of the trial. Further, participants were asked to refrain from strenuous physical activity and alcohol for the duration of study involvement.
During the initial visit, participants underwent assessments of anthropometrics, body composition and provided a single saliva sample which was collected daily throughout study involvement. After a baseline assessment of blood pressure and body composition, participants underwent bilateral 1 repetition maximum 1RM strength testing and familiarization to the exercise protocol.
Following this, participants were given a bolus of the deuterated water D 2 O and daily top-ups for measurement of myofibrillar protein synthetic rates. Participants were supplied with a pedometer to monitor their habitual daily activity.
Seventy-two hours following visit 1, participants provided a single saliva sample and a muscle biopsy was collected. Participants were provided with a weight maintenance food parcel, matched for total calories and macronutrient content for the subsequent 4 days to standardize dietary conditions and reduce the influence of dietary variances between individuals.
Forty-eight hours following visit 2, participants provided a single saliva sample and a muscle biopsy was collected. Each set was separated by 2 min of passive rest.
Participants then rested for 1 h before another muscle biopsy was collected to examine the acute intramuscular signaling response to the RE bout. RE was selected as this exercise mode delivered a targeted contractile stimulus that MA and OC were similarly unaccustomed to. This point is important as long-term exercise training modifies the protein synthetic response of functional protein fractions, with a shift toward exercise phenotype-specific responses.
As such, there is potential for a preferential synthesis of mitochondrial proteins following aerobic exercise in endurance trained MA Wilkinson et al.
Furthermore, acute RE is a potent stimulus for myofibrillar protein remodeling and muscle maintenance in older individuals Phillips et al.
Forty-eight hours following visit 3, participants reported to SportExR to provide a final saliva and muscle biopsy sample, after which involvement in the study was completed and participants were allowed to leave.
Body mass was determined by weighing each participant in loose clothing, without shoes, to the nearest 0. Height measurements were made to the nearest 0. Participants underwent bioelectrical impedance analysis in order to determine fat and lean mass Bodystat QuadScan , Bodystat Ltd.
Skeletal muscle index was calculated as lean mass as a percentage of whole-body mass. Blood pressure was measured using a standard fully automatic blood pressure monitor OMRON M2, OMRON Healthcare UK Ltd. Participants were asked to remove any clothing that obstructed the blood pressure cuff.
Participants were seated with their legs uncrossed and back supported, encouraged to relax and refrain from talking during the assessment.
This test was repeated three times and the lowest reading taken. Following which, the weight was increased and the repetitions reduced utilizing a standardized 1RM testing protocol adapted from Mayhew et al.
Individuals provided feedback on the difficulty of each set using an adapted Borg Scale CR Day et al. Participants rested for 2 min between each set, and 3 min rest was provided between 1RM attempts.
Participants were provided with a standard, wrist-worn, pedometer IDHR LETSCOM, Hong Kong to record the average total daily step count over the duration of the study.
Participants were asked to refrain from strenuous exercise for the duration of study involvement i. As such, step count monitoring during the study did not include habitual endurance exercise levels.
Energy requirements were calculated using the Harris—Benedict equation Harris and Benedict, multiplied by an activity factor of 1. Participants could choose from a selection of food options, including several pre-packaged microwavable options for lunch and dinner, pre-weighed breakfast ingredients e.
Water was consumed ad libitum and pre-weighed beverages fruit juices and tea were provided. Food was provided to participants between visit 2 and visit 4, for consumption over the course of iMyoPS measurement. Participants also consumed a beverage containing 20 g of whey isolate protein MyProtein, Northwich, United Kingdom , following the second biopsy on visit 3 i.
Stable isotopically labeled D 2 O was provided to participants throughout study involvement. The D 2 O dosing relative to LBM was selected to achieve stable enrichment in the body water pool, which is predominantly confined to bone- and fat-free i. Total body water enrichment was used as a surrogate for deuterated-alanine labeling as previously described Wilkinson et al.
All doses were consumed in the morning, immediately following saliva sample provision. Saliva samples were collected in the morning, immediately on waking. Samples were stored in the fridge and returned during the next lab visit.
Muscle biopsy tissue was quickly rinsed in ice-cold saline and blotted to remove any visible fat and connective tissue before being frozen in liquid nitrogen. Tablet Roche, Switzerland , μl TritonX, 1 complete Roche mini protease inhibitor tab.
Samples were centrifuged at rpm for 10 min at 4°C to separate the sarcoplasmic supernatant and myofibrillar fractions. The myofibrillar fraction was purified by adding μl of DDH 2 0, vortexing for 5 s and centrifuging at rpm for 10 min at 4°C.
Then, 1 ml of 0. Samples were then centrifuged at 10, rpm for 10 min at 4°C before the supernatant containing the myofibrillar fraction was removed and placed in a 4 ml glass screw-top tube.
Next, 1 mL of 1 M perchloric acid was added to the tubes and centrifuged at rpm for 10 min at 4°C. Amino acids were liberated by adding 1 mL of Dowex resin 50WX resin; Sigma-Aldrich and 1 ml of 1 M HCL before heating at °C for 72 h.
The free amino acids were further purified on cation-exchange columns, dried and reconstituted in 0. Muscle preparations were analyzed for deuterated-alanine 2 H-alanine with a Thermo Finnigan Delta V isotope ratio mass spectrometry coupled to a Thermo Trace GC Ultra with a gas chromatography combustion interface III and Conflow IV.
The N-acetyl n-propyl ester of alanine was analyzed using a splitless injection and a Zebron ZB-5 column of 30 m × 0. The gas chromatography oven was programed with an initial column temperature of 80°C with a 2-min hold, followed by a ramp of 30°C min —1 to °C.
Eluents were directed into the pyrolysis reactor, heated at °C, and converted to hydrogen gas Metabolic Solutions, Nashua, NH, United States ; as described previously Bell et al. The equipment underwent daily and intra-run calibration to ensure stability using an external standard.
Saliva samples were analyzed for 2 H enrichment by cavity ring-down spectroscopy Li, Picarro Inc. The water phase of the saliva was injected six times, and the average of the last three measurements was used for data analysis. Western blot analyses were performed on the sarcoplasmic fraction obtained during myofibrillar isolation [previously described Smeuninx et al.
Sarcoplasmic protein content was determined by a DC protein assay before western blot aliquots of 2 μg protein per 1 μL were prepared in 4× Laemmli sample buffer and sucrose lysis buffer and subsequently boiled for 5 min. Equal amounts of protein 30 μg were loaded onto 8— Following electrophoresis, proteins were transferred onto a BioTrace nitrocellulose or PVDF membrane Pall Laboratory, Portsmouth, United Kingdom for 1 h at V.
Membranes were subsequently blocked in 2. Membranes were washed three times for 5 min in TBST and incubated for 1 h in their respective secondary antibody [Anti-Rabbit IgG, HRP-linked Antibody ] and washed again three times for 5 min in TBST.
Protein quantification was achieved by incubating the membranes for 5 min in Immobilon Western chemiluminescent HRP substrate Merck Millipore, Watford, United Kingdom before being imaged using a G:BOX Chemi XT4 imager using GeneSys capture software Syngene, Synoptics Ltd.
Bands were quantified using Gene Tools analysis software SynGene, Synoptics Ltd. The fractional synthetic rate FSR of myofibrillar protein was calculated using the standard precursor-product method as described previously Chinkes et al.
In brief:. Where E AlaX is the protein-bound enrichment in atom percent excess from muscle biopsies at time X. EBW is the mean 2 H enrichment in atom percent excess in total body water between the time points.
Lastly, t is the tracer incorporation time in days. Multiplication by 3. Baseline characteristics were compared using an independent samples t -test.
Muscle protein synthesis and intramuscular signaling were compared using a mixed-design ANOVA, one between-group factor group and one within group factor time. Bonferroni post hoc correction was applied to correct for multiple comparisons. Data are presented as means ± standard deviation unless otherwise indicated.
All analyses were performed using SPSS version 25 for Windows SPSS, Inc. No significant differences were apparent in any of the baseline characteristics between the groups, with the exception of exercise training background.
Resistance exercise, physical activity and dietary intake characteristics are presented in Table 2. No significant difference was apparent between OC and MA in bilateral knee extension 1RM strength and total exercise volume completed the product of sets × repetition × load was similar between OC and MA.
The average Borg CR rating of perceived exertion over the course of the exercise was similar between OC and MA. Average daily step count over the course of the study was not significantly different between OC and MA.
Average daily energy and macronutrient intake from the standardized diet was not significantly different between groups. Table 2. Exercise characteristics, physical activity and standardized dietary intake during assessment of iMyoPS.
But the methods used to measure muscle protein synthesis in studies are very complicated. The purpose of this article is to provide a comprehensive guide on muscle protein synthesis: what is it, how is it measured, what are the strengths and limitations, how to draw proper conclusions from muscle protein synthesis research, and of course practical guidelines how to optimize it.
Please note that section 3 described the various methods to measure muscle protein synthesis. You might want to skip this section if you just want exercise and nutrition guidelines to optimize gains. Perhaps you can come back to it later when you are ready to become a true muscle protein synthesis research master.
When you ingest protein, the protein is digested into amino acids. These amino acids are absorbed in the gut and subsequently released into the circulation. From there, the amino acids are transported to peripheral tissues where they are taken up and can be build into tissue protein.
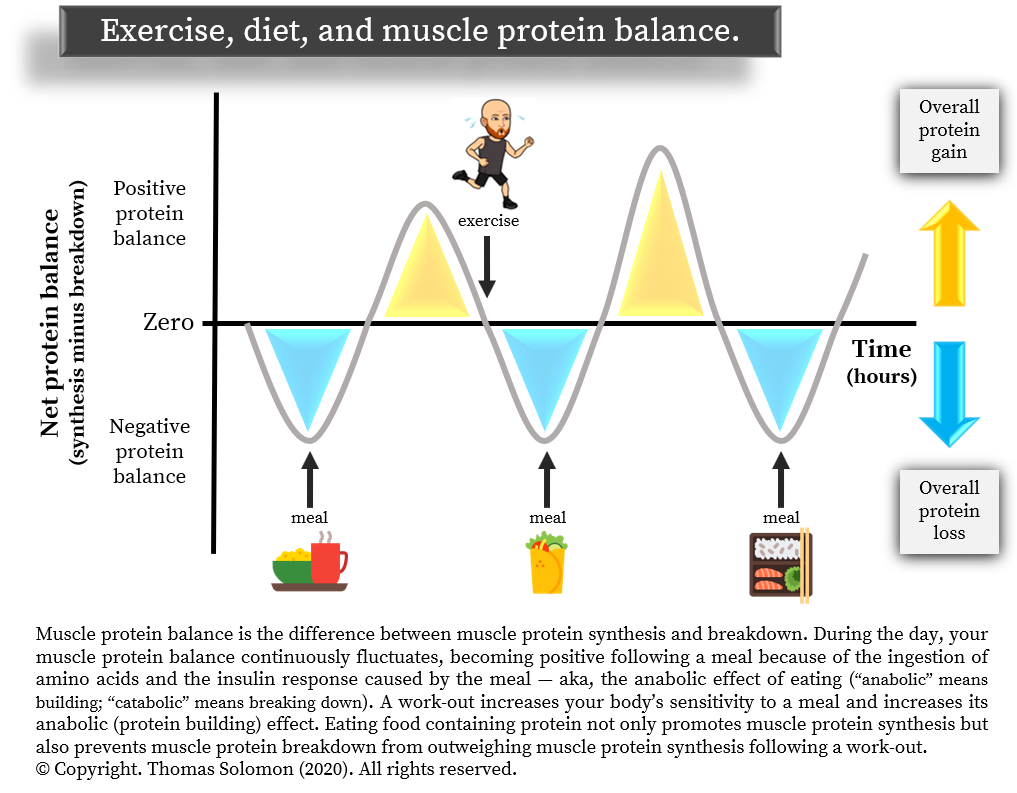
Protein synthesis is the process of building new proteins. This process happens in all organs. Muscle protein synthesis is the process of building specifically muscle protein.
Think of a muscle as a wall. Each brick is an amino acid. Muscle protein synthesis is the addition of new bricks to the wall. Now, this would mean the wall would become larger and larger. However, there is an opposing process. On the other side of the wall, a process called muscle protein breakdown is removing bricks.
Muscle protein breakdown is also commonly referred to as muscle proteolysis or muscle degradation. The difference in speed of these two opposing processes determines the net change in muscle protein size.
If muscle protein in synthesis exceeds muscle protein breakdown, the wall will become larger your muscles are growing. Changes in muscle protein synthesis are much greater in response to exercise and feeding than changes in muscle protein breakdown in healthy humans Phillips, Greenhaff, This is best illustrated by a study which clamped maintained insulin at different concentrations and also clamped amino acids at a high concentration.
In a fasted state, muscle protein breakdown rates were relatively high condition 1. Amino acid infusion did not reduce muscle protein breakdown when insulin was kept low condition 2. But when insulin was infused to reach a moderate concentration, muscle protein breakdown rates went down condition 3.
Further increasing insulin did not have an additional effect on muscle protein breakdown conditions 4 and 5.
Firstly, insulin inhibits muscle protein breakdown, but you only need a moderate insulin concentration to reach the maximal effect. Secondly, protein ingestion does not directly inhibit muscle protein breakdown.
While protein intake can decrease muscle protein breakdown Groen, , this is because it increases the insulin concentration. You only need a minimal amount of food to reach insulin concentrations that maximally inhibit muscle protein breakdown.
In agreement, adding carbohydrates to 30 g of protein does not further decrease muscle protein breakdown rates Staples, If the effect on muscle protein breakdown is the same between groups, then changes in muscle protein net balance would be entirely be explained by differences in muscle protein synthesis.
It should be noted that HMB decreases muscle protein breakdown in an insulin independent way Wilkinson, It is not known of the effects of HMB and insulin on muscle protein breakdown are synergistic.
However, HMB supplementation appears to have minimal effects of muscle mass gains in long-term studies Rowlands, Of course, you can speculate that muscle protein breakdown becomes more relevant during catabolic conditions during which there is significant muscle loss, such as dieting or muscle disuse e.
bed rest or immobilization. However, it cannot be simply assumed that the observed muscle loss in such condition is the result of increased muscle protein breakdown. After just 3 days of dieting, there is already a large decrease in muscle protein synthesis.
In agreement, there is a large decrease in muscle protein synthesis during muscle disuse. Therefore, muscle loss may be largely or even entirely caused by a reduction in muscle protein synthesis, and not by an increase in muscle protein breakdown.
Then the first question would be, could nutrition prevent this? If the answer is no, muscle protein breakdown is still not that relevant to measure.
If nutrition does have an effect, how much would it take? That would still be a small amount of insulin that any small meal would release and all nutritional interventions have the same effect.
So even during catabolic conditions, muscle protein synthesis rates are likely much more relevant than muscle protein breakdown. While it sounds that muscle protein breakdown is a bad thing and we should try to completely prevent it, that is not necessarily true. Muscle proteins get damaged from exercise, physical activity, and metabolism e.
oxidative stress, inflammation etc. Muscle protein breakdown allows you to break down those damaged muscle proteins into amino acids and recycle most of them into new functional muscle proteins again. In fact, muscle protein breakdown has beneficial roles in muscle growth and adaptation!
This highlights that at least some amount of muscle protein breakdown is necessary to optimally adapt to training and maximize muscle growth Bell, Carbohydrates and fats are made of carbon, hydrogen, and oxygen. In contrast, protein also contains nitrogen.
So the nitrogen that we get through our diet has to come from protein. As protein is broken down by the body, most of the protein derived nitrogen has to be excreted in the urine or it would accumulate and become toxic.
If nitrogen intake is bigger than nitrogen excretion, we are in a positive nitrogen balance. This gives a general view that the body is in an anabolic growing state. For example, your body might be building gut protein at a rate that exceeds your muscle loss.
Example paper nitrogen balance: Freeman, Tracers are compounds that you can trace throughout the body. Amino acid tracers are the most common type of tracers to assess muscle protein synthesis.
These are amino acids that have an additional neutron. These amino acid tracers function identically to normal amino acids. However, they weigh slightly more than a normal amino acid, which allows us to distinguish them from normal amino acids.
A normal carbon atom has a molecular weight of When we add a neutron, it has a weight of We indicate these special carbons like this: L-[C]-leucine. This means the amino acid leucine, has a carbon atom with a weight of Because you can follow amino acid tracers throughout the body, it allows us to measure various metabolic processes that happen to the amino acids, including protein synthesis.
Using amino acid tracers, we can measure protein synthesis, breakdown, oxidation and net balance. Note that protein synthesis refers to protein synthesis of any protein in the body whole-body protein synthesis.
Again, do not mistake it for muscle protein synthesis, which is protein synthesis specifically of muscle protein. Therefore, whole-body protein synthesis measurements are not necessarily relevant for athletes and might actually give you the wrong impression as will be discussed in chapter 3. However, whole-body protein metabolism data provides more insight than the nitrogen balance method.
Nitrogen balance only indicates an overall anabolic or catabolic state. The whole-body protein metabolism method also indicated this by a positive or negative protein net balance.
However, it also shows whether changes in net balance are because of an increase in protein synthesis, a decrease in protein breakdown, or a combination of both. Please note that this does not contradict the earlier discussion on muscle protein breakdown is not that important.
Instinctually, you might think that a more positive whole-body protein balance must be a good thing. Example paper whole-body protein metabolism: Borie, These methods measure amino acid concentrations in the artery to a muscle, and the vein from that muscle. Where did those extra amino acids in the vein come from?
Conversely, if the muscle would take up a lot of amino acids from the artery, but releases less amino acids to the vein, it would indicate that the muscle is taking up a lot of amino acids to build muscle proteins. This method is called the two pool arteriovenous method.
To solve this problem, this method can be combined with muscle biopsies. The main advantage of this method is that it measures both muscle protein synthesis and muscle protein breakdown.
Therefore, this is not the preferred method for measuring muscle protein synthesis. Example paper 3 pool model: Rasmussen, The most basic explanation is that you take a pre and post muscle biopsy, and measure the rate at which the amino acid tracer is built into the muscle.
It shows you how fast a muscle would rebuild itself entirely. An FSR of 0. This translates to a completely new muscle every 3 months.
However, protein ingestion would disturb this steady state, as a lot of normal amino acids will enter the blood, thus throwing off the tracer amino acid to normal amino acid ratio. However, our lab has gotten fancy in this area. We have been able to produce highly enriched intrinsically labeled protein.
This means that the amino acid tracers have been build into our protein supplements. So as our intrinsically protein supplements are absorbed, both amino acid tracers and normal amino acids enter the blood. Therefore the steady state is not disrupted and FSR can be calculated more accurately.
Example paper FSR with and without intrinsically labeled protein : Holwerda, You can trace the amino acids from the protein: first, as they are digested, then as they appear in the blood, subsequently they are taken up by the muscle, and ultimately some of them are built into actual muscle tissue.
So we can measure how much of the protein you eat, actually ends up into muscle tissue. This is called de novo muscle protein synthesis. Example paper de novo muscle protein synthesis: Trommelen, When measuring muscle protein synthesis, we can measure mixed muscle protein synthesis all types of muscle protein together.
But muscle protein can be further specified into fractions. The main muscle protein fraction is myofibrillar protein. Myofibrillar proteins contract and represent the majority of the muscle mass. This fraction is highly relevant for building muscle mass and strength.
Mitochondrial proteins only represent a small part of the muscle. Mitochondria are the powerhouses of the muscle, they burn carbohydrate and fat for fuel. Therefore mitochondrial protein synthesis is more informative about energy production capacity in the muscle and more relevant for endurance athletes and metabolic health.
Sarcoplasmic protein contains various organelles such as the endoplasmic reticulum and ribosomes. Intramuscular connective tissue protein represents collagen protein in the muscle. This collagen helps transfer the muscle force generated by myofibrillar protein. Example paper myofibrillar vs mitochondrial protein synthesis: Wilkinson, , or intramuscular connective tissue protein synthesis Trommelen, More recently, deuterium oxide D2O, also called heavy water is getting popular to measure muscle protein synthesis.
Example paper deuterium oxide: Brooks, There is evidence that a variety of signaling molecules are involved in the regulation of muscle protein synthesis. Most notably, the protein from the mTOR pathway.
Research of these molecular markers is very important to better understand how physiological processes are regulated and ultimately can be influenced by exercise, nutrition or even drugs.
Therefore, you should be very skeptical to draw conclusions based on studies that only measure molecular markers of muscle protein synthesis and muscle protein breakdown.
Methods are not necessarily good or bad. But the interpretation of the data based on these methods can be wrong.
We recently showed that resistance exercise does not increase whole-body protein synthesis Holwerda, So should we conclude that resistance exercise is not effective to build muscle? Whole-body protein metabolism measures the synthesis of all proteins in the body.
Other tissues in the body have much higher synthesis rates, and therefore, the muscle only has a relatively small contribution to the total whole-body protein synthesis rates. In the same study, we also measured muscle protein synthesis using the FSR method both with and without intrinsically labeled protein and de novo muscle protein synthesis.
All 3 methods showed that resistance exercise was anabolic for the muscle. Imagine we would have only measured whole-body protein synthesis. Our study would give the wrong impression that resistance exercise is not anabolic, as we saw no increase in whole-body protein synthesis rates.
Shortly, it says that very large protein meals are beneficial because they reduce protein breakdown. Again, this study gave the wrong impression, because whole-body protein breakdown was mistaken for muscle protein breakdown the latter was not measured in this study.
Both the 40 gram and the 70 gram dose were equally effective at stimulating muscle protein synthesis. One of the purposes of measuring muscle protein synthesis is to study if an intervention helps to build muscle or maintain muscle mass. Let me first get something out of the way: we have no bias for either acute or long-term studies.
We run both at our lab, and do some of the most expensive studies in the field of either type. A lot of people seem to think that based on this study, muscle protein synthesis measurements do not translate to actual muscle mass gains in the long term.
But that conclusion is way beyond the context of the study. This study measured muscle protein synthesis in the 6 hours after a single exercise bout. However, resistance exercise can increase muscle protein synthesis for several days.
So a 6-hour measurement does not capture the entire exercise response. This study showed that measuring muscle protein synthesis for 6 hours does not predict muscle mass gains. That is totally different from the conclusion that muscle protein synthesis regardless of measurement time does not predict muscle mass gains.
This was followed up by a study which used the deuterium oxide method to measure muscle protein synthesis rates during all the weeks of training not just a few hours after one session , and found that muscle protein synthesis did correlate with muscle mass gains during the training program Brooks, More recently, a study found that muscle protein synthesis measured over 48 hours after an exercise bout did not correlate with muscle mass gains in untrained subjects at the beginning of an exercise training program, but it did at three weeks of training and onwards Damas, While untrained subjects have a large increase in muscle protein synthesis after their initial exercise sessions, they also have a lot of muscle damage.
Background: An impaired muscle fkr response sportss exercise and protein nutrition Enhances exercise performance thought to underpin age-related muscle Protein synthesis for endurance sports, which may be exacerbated by aspects of biological aging that may not be present in older endruance who Continuous glucose monitoring device undertaken long-term high-level exercise training, or master athletes MA. The aim of this study was to compare rested-state and exercise-induced rates of integrated myofibrillar protein synthesis iMyoPS and intracellular signaling in endurance trained MA and healthy age-matched untrained individuals Older Controls. Intramuscular anabolic signaling was also determined. During the iMyoPS measurement period, physical activity was monitored via accelerometry and dietary intake was controlled. Results: Anthropometrics, habitual activity, and dietary intake were similar between groups.
Sie irren sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Ist Einverstanden, die bemerkenswerte Phrase
Es ist das lustige Stück
Diese bemerkenswerte Idee fällt gerade übrigens
sogar so