Hypoglycemia and insulin pumps -
This topic will review CSII insulin pump therapy. Physiologic insulin replacement, choice of insulin delivery multiple daily injection [MDI] insulin regimens versus CSII , and designing an MDI regimen are reviewed separately.
Why UpToDate? Product Editorial Subscription Options Subscribe Sign in. Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large.
Continuous subcutaneous insulin infusion insulin pump. Formulary drug information for this topic. No drug references linked in this topic. Find in topic Formulary Print Share.
View in. Language Chinese English. Author: Ruth S Weinstock, MD, PhD Section Editor: Irl B Hirsch, MD Deputy Editor: Katya Rubinow, MD Literature review current through: Jan This topic last updated: Jan 29, Basal insulin can be delivered by daily or twice-daily injections of an intermediate-acting neutral protamine Hagedorn [NPH] or long-acting glargine, detemir, degludec insulin preparation or by continuous subcutaneous insulin infusion CSII via a pump using a rapid-acting or faster rapid-acting insulin preparation lispro, aspart, glulisine.
All participants gave their written informed consent for participation in the study and the use of their data for research purposes. Fullerton B , Jeitler K , Seitz M , Horvath K , Berghold A , Siebenhofer A.
Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev ; 2 : CD Google Scholar. Bergenstal RM , Tamborlane WV , Ahmann A , Buse JB , Dailey G , Davis SN , et al.
Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med ; : — Langendam M , Luijf YM , Hooft L , Devries JH , Mudde AH , Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus.
Cochrane Database Syst Rev ; 1 : CD Choudhary P , Amiel SA. Hypoglycemia: current management and controversies. Postgrad Med J ; 87 : — MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care ; 10 : — 8. Sonnenberg GE , Kemmer FW , Berger M.
Exercise in type 1 insulin-dependent diabetic patients treated with continuous subcutaneous insulin infusion. Prevention of exercise induced hypoglycaemia. Diabetologia ; 33 : — Mitchell TH , Abraham G , Schiffrin A , Leiter LA , Marliss EB. Hyperglycemia after intense exercise in IDDM subjects during continuous subcutaneous insulin infusion.
Diabetes Care ; 11 : — 7. Brazeau AS , Rabasa-Lhoret R , Strychar I , Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care ; 31 : — 9. Riddell MC , Gallen IW , Smart CE , Taplin CE , Adolfsson P , Lumb AN , et al.
Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol ; 5 : — Kapitza C , Hövelmann U , Nosek L , Kurth HJ , Essenpreis M , Heinemann L.
Continuous glucose monitoring during exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion.
J Diabetes Sci Technol ; 4 : — Yardley JE , Iscoe KE , Sigal RJ , Kenny GP , Perkins BA , Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients.
Diabetes Technol Ther ; 15 : 84 — 8. Zaharieva DP , Riddell MC. Prevention of exercise-associated dysglycemia: a case study-based approach. Diabetes Spectr ; 28 : 55 — Gold AE , MacLeod KM , Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia.
Diabetes Care ; 17 : — World Medical Association : World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.
JAMA ; : — 4. Robertson K , Riddell MC , Guinhouya BC , Adolfsson P , Hanas R. Exercise in children and adolescents with diabetes. Pediat Diabet ; 15 : — McAuley SA , Horsburgh JC , Ward GM , La Gerche A , Gooley JL , Jenkins AJ , et al.
Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia ; 59 : — Franc S , Daoudi A , Pochat A , Petit MH , Randazzo C , Petit C , et al. Diabetes Obes Metab ; 17 : — 7. Campbell MD , Walker M , Trenell MI , Jakovljevic DG , Stevenson EJ , Bracken RM , et al.
Large pre- and postexercise rapid-acting insulin reductions preserve glycemia and prevent early - but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care ; 36 : — Campbell MD , Walker M , Trenell MI , Luzio S , Dunseath G , Tuner D , et al.
Metabolic implications when employing heavy pre- and post-exercise rapid-acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: a randomised clinical trial.
PLoS One ; 9 : e Campbell MD , Walker M , Bracken RM , Turner D , Stevenson EJ , Gonzalez JT , et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care ; 3 : e Delvecchio M , Zecchino C , Salzano G , Faienza MF , Cavallo L , De Luca F , et al.
Effects of moderate-severe exercise on blood glucose in Type 1 diabetic adolescents treated with insulin pump or glargine insulin. J Endocrinol Invest ; 32 : — Moore K , Vizzard N , Coleman C , McMahon J , Hayes R , Thompson CJ. Extreme altitude mountaineering and Type 1 diabetes; the Diabetes Federation of Ireland Kilimanjaro Expedition.
Diabet Med ; 18 : — 5. Kordonouri O , Vazeou A , Scharf M , Würsig M , Battelino T ; SWEET Group. Striving for control: lessons learned from a successful international Type 1 Diabetes Youth Challenge.
Acta Diabetol ; 54 : — 9. Matejko B , Gawrecki A , Wróbel M , Hohendorff J , Benbenek-Klupa T , Malecki MT , et al. Type 1 diabetes at high altitude: performance of personal insulin pumps and patient metabolic control.
Diabet Technol Ther ; 19 : — 2. Mamkin I , Ten S , Bhandari S , Ramchandani N. Real-time continuous glucose monitoring in the clinical setting: the good, the bad, and the practical. J Diabet Sci Technol ; 2 : — 9. Riddell M , Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring.
J Diabetes Sci Technol ; 3 : — Shashaj B , Busetto E , Sulli N. Benefits of a bolus calculator in pre- and postprandial glycaemic control and meal flexibility of paediatric patients using continuous subcutaneous insulin infusion CSII. Diabet Med ; 25 : — Bracken RM , Page R , Gray B , Kilduff LP , West DJ , Stephens JW , et al.
Isomaltulose improves glycemia and maintains run performance in type 1 diabetes. Med Sci Sports Exerc ; 44 : — 8. Pratley RE. The efficacy and effectiveness of drugs for diabetes: how do clinical trials and the real world compare?
Diabetologia ; 57 : — 5. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Advertisement intended for healthcare professionals. Navbar Search Filter QJM: An International Journal of Medicine This issue Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search.
Issues More Content Advance articles Editor's Choice Cover Archive Research from China Highly Cited Collection Review Series Index Supplements Submit Author Guidelines Submission Site Open Access Purchase Alerts About About QJM About The Association of Physicians Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Journals on Oxford Academic Books on Oxford Academic.
Issues More Content Advance articles Editor's Choice Cover Archive Research from China Highly Cited Collection Review Series Index Supplements Submit Author Guidelines Submission Site Open Access Purchase Alerts About About QJM About The Association of Physicians Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Close Navbar Search Filter QJM: An International Journal of Medicine This issue Medicine and Health Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Materials and Methods. Journal Article. Avoiding hypoglycemia: the use of insulin pump combined with continuous glucose monitor in type 1 diabetes crossing a Rocky Gorge.
P Thomakos , P Thomakos. From the Department of Clinical Therapeutics Medical School of National and Kapodistrian University of Athens, Alexandra Hospital, Lourou Street, 28 Athens, Greece.
Hygeia General Hospital, Diabetes Center, 4, Erythrou Stavrou, 23 Marousi, Athens, Greece. Address correspondence to P. Thomakos, Department of Clinical Therapeutics Medical School of National and Kapodistrian University of Athens, Alexandra Hospital, Lourou Street, 28 Athens, Greece.
email: thomakospetros yahoo. Oxford Academic. A Vazeou. D Sakkas. G Panagopoulos. K Anifantakis. Venizeleio Hospital—PAGN, Diabetes Unit, Knosou Avenue, 09 Heraclion, Greece.
P Smyrnaki. T Arvanitaki. Chania General Hospital, Pediatric Unit, Diabetes Unit, Agiou Eleftheriou Street, 00 Chania, Greece. E Kyrlaki. N Kefalogiannis. Asklipios Center, Diabetes Unit, 10, Mahis Critis Street, 03 Heraclion, Greece.
D Mamoulakis. A Pappas , A Pappas. A Mitrakou. Revision received:. PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote.
This topic Insukin review CSII insulin pump Hyopglycemia. Physiologic insulin replacement, choice of insulin Paleo-friendly meals multiple daily injection [MDI] insulin regimens versus CSIIand designing an MDI regimen are reviewed separately. Why UpToDate? Product Editorial Subscription Options Subscribe Sign in. Learn how UpToDate can help you.Hypoglycemia and insulin pumps -
It's also useful for people who have an unpredictable activity schedule or work hours, since it releases a constant flow of insulin into the body, preventing the effects on blood sugar that can occur when occasionally forgetting to inject a long-acting insulin shot.
Improved monitoring and response to trends All hybrid closed loop insulin pumps will monitor blood sugar trends over time. Many display the information on a mobile app that can be shared with family, friends and health care professionals. This information helps make treatment decisions and identify triggers to spikes or drops in blood sugar levels.
It also helps from a safety perspective, particularly in children with diabetes. Parents can receive alarms and intervene if the child's blood sugar is particularly low or high. Considerations There are a few things to think about when deciding if a hybrid closed loop insulin pump is right for you, such as: Your comfort with technology A sense of comfort with technology and willingness to learn the system is important for success.
The system uses advanced technology, which means user errors or technology failures are possible. People considering this system need to understand what the pump is doing so they can step in and manually adjust insulin if the system isn't working as expected. Your comfort with adjusting The system is designed to be adjusted and calibrated by the patient over time.
People cannot just set it on autopilot and expect it to work perfectly. People who have reliably checked blood sugar levels and are invested in managing diabetes will have greater success.
Tube or tubeless There are two general categories of hybrid closed loop insulin pumps available: those that use insulin tubes and others that are tubeless.
Pumps that use tubing connect the reservoir of insulin to a catheter that's inserted under the skin.
Tubeless pumps connect directly under the skin without any tubing to a reservoir. Consider which option may be best for your situation and comfort level. People who live an active lifestyle, have an active job or are prone to tube damage may benefit more from a tubeless pump.
Existing continuous glucose monitoring system Some pumps work with specific continuous glucose monitors and not others. If a person has been using a glucose monitor with success, it makes the sense to add a pump that is compatible with the existing system.
Interface design Some pumps use a touch screen. Some use only a remote controller, either as a separate device or via an app. And others have physical buttons. No significant difference in preoperative mean interstitial glucose was found, but postoperative mean glucose improved with nonblinded CGM compared with blinded CGM; however, there was no significant difference in frequency of or time spent in hypoglycemia.
Similarly, several additional studies comparing different blinded versus nonblinded CGM in patients after cardiac surgery or with acute coronary syndrome 68 , 69 , 71 , 74 confirmed the accuracy and reliability of CGM technology in titrating intravenous insulin therapy; however, none of them demonstrated significant improvement in mean glucose or in the frequency of hypoglycemia in the ICU.
A recent systematic review of 37 studies, both randomized controlled trials and observational studies, concluded that in terms of efficacy, the use of subcutaneous CGM systems does not seem to improve the glycemic control of critically ill patients in a clinically significant manner.
Overall, the results of ICU studies indicate that the use of CGM combined with an appropriate insulin dosing protocol has the potential to improve glucose control in the ICU; however, the results have been conflicting.
Some studies, but not others, have reported improvement in mean glucose values and reduction in hypoglycemia frequency with blinded CGM Larger and well-designed multicenter studies are needed to convincingly demonstrate the safety and efficacy of CGM devices in reducing length of stay and improving clinical outcome before recommending their use in the ICU.
A recent panel of experts concluded that use of CGM now might not be feasible for every ICU patient 91 , but there are populations of high interest who may benefit from further study of CGM owing to their high risk for glucose variability and hypoglycemia.
These populations include patients receiving intravenous insulin or high-dose glucocorticoids; those undergoing cardiac surgery, transplant, or traumatic or vascular brain surgery; those with end-stage renal or liver disease or hypoglycemia unawareness; and those in neonatal ICU 53 , Several studies have reported on the use of CGM in non-ICU settings in patients with T2D Table 5.
Burt et al. The mean daily glucose was similar between interstitial and capillary monitoring. Schaupp et al. The number of hypoglycemic episodes 3. Gu et al. The authors reported that sensor-augmented pump technology resulted in a shorter time to reach the glucose targets 3.
In another study of 38 hospitalized patients with T2D treated with a basal-bolus insulin regimen, CGM use was compared with bedside POC glucose testing There were no differences in mean daily glucose or premeal, fasting, or 2-h postprandial glucose levels between the two groups.
However, CGM detected a higher number of hypoglycemic events compared with capillary glucose testing. Previous studies in non-ICU settings have shown that the inpatient use of CGM is more effective in identifying trends toward hypoglycemia and hyperglycemia compared with standard POC glucose testing 86 , However, these trials used blinded CGM, and therefore interventions to prevent impending hypoglycemia were not performed 86 — 88 , Another limitation is that although glucose values are captured in the CGM device, results are not transmitted to the nursing station to allow providers to detect and treat impending hypoglycemia.
To overcome these limitations, a recent promising pilot study reported on the feasibility of a continuous glucose telemetry system in high hypoglycemia—risk patients in non-ICU settings Elderly patients receiving high-dose insulin treatment and with multiple comorbidities were included in this study.
Recent technological advances in CSII devices, CGM systems, and insulin delivery algorithms have resulted in the development of artificial pancreas for inpatient care 95 , An artificial pancreas, or a closed-loop system, combines a real-time glucose-sensing component, an insulin delivery device pump , and a computer that calculates the amount of insulin needed in response to the BG concentration During the past decade, a variety of closed-loop systems have been explored in various groups of critically ill patients 97 , 98 , during the perioperative period 99 , and in insulin-treated patients with T2D These studies reported that the closed-loop technology is safe and effective in improving glycemic control and proportion of time spent in the target glucose concentration range, but they found no significant improvement in mean glucose concentration or in the frequency of hypoglycemic events compared with multidose insulin regimens.
Despite this evidence supporting the efficacy and feasibility of closed-loop use, there are several limitations that need to be addressed to support wider adoption in the hospital setting. The need for intravascular access for intravenous closed-loop insulin systems limits their use in noncritical-care general ward settings.
Diabetes management devices including insulin pumps and CGM have gained wide acceptance among physicians and ambulatory patients with T1D, and their use has been associated with improved glycemic control and reductions in hypoglycemia.
The Endocrine Society , the American Association of Clinical Endocrinologists , and the Diabetes Technology Society 53 support the inpatient use of CSII in selected patients, such as those with appropriate insulin pump and diabetes self-management skills, with noncritical illness, without mental status changes, and with the prompt involvement of inpatient diabetes specialists.
The consensus among diabetologists is to allow the patient to continue to self-manage their diabetes using the pump. If a patient is unable to manage their pump for whatever reason or a hospital lacks specialist consultation, then the pump should be removed and conventional insulin management should be initiated.
CSII can be restarted once the patient has recovered. Despite broad-based evidence supporting the use of CGM devices as a mean of facilitating glucose control in hospitalized patients and decreasing nursing workload, the technology remains largely investigational.
Clinical guidelines have advised against the hospital use of CGM because of the lack of safety and efficacy outcome studies 53 , , ; however, they support continuation of outpatient CGM in the hospital under specific circumstances if proper institutional procedures and guidelines are developed 5 , In recent years, improvement in the accuracy of CGM sensors has resulted in a reduced need for frequent calibration, or any calibration , which is an attractive feature in the hospital.
A pragmatic evaluation of CGM proving accuracy and clinical effectiveness is needed and may facilitate more widespread adoption of this technology in the hospital setting.
Because an increasing number of people with diabetes are using insulin pumps and CGM, it is inevitable that health care professionals working in hospitals will have to care for patients using pumps and CGM devices. Technology for management of diabetes in the hospital is improving and is expected to significantly reduce the added burden and risk of diabetes for hospitalized patients.
In the near term, the availability of accurate CGM systems combined with automatic insulin dosing systems using software algorithms will facilitate glycemic control and reduction of hypoglycemia and hyperglycemia in critically and noncritically ill patients with T1D and T2D As artificial intelligence becomes more established, the dosing algorithms for insulin delivery in hospitalized patients will become individualized for closed-loop control of glycemia The authors would like to thank Robert Vigersky, MD Medtronic Diabetes, Uniformed Services University of the Health Sciences, and Diabetes Institute of Walter Reed National Military Medical Center , and Francisco Pasquel, MD Division of Endocrinology, Emory University , for their helpful comments and Annamarie Sucher Burlingame, CA for her expert editorial assistance.
is partly supported by research grants from the U. Public Health Service through the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Award Program grant UL1-TR and National Center for Research Resources grant 1P30DK Duality of Interest.
has received unrestricted research support for inpatient studies to Emory University from Merck, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Sanofi. Author Contributions. and D. reviewed the literature and collected relevant information.
wrote the first draft of the manuscript. reviewed and edited the manuscript and approved the final version. Prior Presentation. Parts of this article were presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 23 June See accompanying articles, pp.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 41, Issue 8. Previous Article Next Article. Insulin Pump Use in the Hospital. Contraindications to Insulin Pump Use in the Hospital.
Insulin Pump Use in Special Situations. Artificial Pancreas: Closed-Loop Insulin Delivery System. What Lies Ahead in Diabetes Technology? Article Information. Article Navigation. Diabetes Care Symposium June 23 Diabetes Technology Update: Use of Insulin Pumps and Continuous Glucose Monitoring in the Hospital Guillermo E.
Umpierrez Corresponding author: Guillermo E. Umpierrez, geumpie emory. This Site. Google Scholar. David C. Klonoff X. Diabetes Care ;41 8 — Article history Received:. Connected Content. A companion article has been published: Intensive Glycemic Treatment During Type 1 Diabetes Pregnancy: A Story of Mostly Sweet Success!
A companion article has been published: Closing the Loop on Managing Youth With Type 1 Diabetes: Children Are Not Just Small Adults. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
Figure 1. View large Download slide. Table 1 Contraindications to insulin pump therapy in the hospital. View Large. Calculate h basal dose of insulin delivered from pump setting. Total basal daily insulin can be given as once-daily or twice-daily injections. Capillary BG should be measured before meals and bedtime.
The pump can be restarted when the patient is able to resume responsibility or at hospital discharge. Document insulin pump settings and current basal rate. Table 4 Clinical trials of adult CGM use in the ICU. First author, year ref.
Sample size. of sites. Type of CGM. Performance measurement. Table 5 Clinical trials of CGM in non-ICU settings. Burt, 86 General ward 26 1 iPro Accuracy Capillary BG monitoring Schaupp, 87 General ward 84 1 iPro Accuracy Capillary BG monitoring Gómez, 88 General ward 38 1 iPro2 Accuracy Capillary BG monitoring Gu, 89 General ward 81 8 Sensor-augmented pump Accuracy MDI with blinded CGM.
Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, U. Department of Health and Human Services. Hospitalizations for people with type 1 and type 2 diabetes compared with the nondiabetic population of Tayside, Scotland: a retrospective cohort study of resource use.
Search ADS. American Diabetes Association. Economic costs of diabetes in the U. in [published correction appears in Diabetes Care ;]. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.
Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery RABBIT 2 surgery.
Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U. Basal versus sliding-scale regular insulin in hospitalized patients with hyperglycemia during enteral nutrition therapy.
Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal Plus trial. This can decrease the symptom severity, masking hypoglycemia and increasing the risk for hypoglycemic episodes to develop into severe events.
This is defined as the onset of neuroglycopenia before the appearance of sympathoadrenal symptoms. Give the patient 15 grams of oral carbohydrates half a cup of apple juice, half a sandwich, or 4 or 5 pieces of hard candies and check the serum glucose after 15 minutes.
Repeat as necessary. Monitor frequently! Insulin pump users should monitor their blood glucose levels before and after meals, before and after exercise, and before bed at a minimum. Infusion site failures increase after 3 days of catheter use. Patients should check with their insurance to see if replacement parts are covered.
Pumps are not a replacement for a functioning pancreas. Patients still need to exercise careful glucose intake and make appropriate dosing adjustments per a pre-set plan.
Upon resolution of DKA or hypoglycemia the patient should meet with the clinician managing their diabetes as an outpatient to fine tune their therapy program. Many community health systems have offered training programs and local support for insulin pump users.
Future of Insulin Pumps Many insulin pumps on the market today are associated with a glucometer or continuous glucose monitor CGM. However, closed loop systems still have limitations, including delays in response times, accurate signal feedback, software malfunctions, high cost, and user errors.
Bedtime infusion complications are the most common culprit for both DKA and hypoglycemia. Recurrent hypoglycemia blunts the normal counterregulatory response in patients with type 1 DM and can mask the early warning signs of hypoglycemic episodes.
Careful blood glucose monitoring is key in the setting of insulin pumps! Patient education is essential for prevention of insulin pump complications.
Review of Evidence for Adult Diabetic Ketoacidosis Management Protocols. Front Endocrinol Lausanne. Published Jun Insulin Pump Class: Back to the Basics of Pump Therapy Diabetes Spectrum ; vol.
Pickup, JC. Insulin-pump therapy for type 1 diabetes mellitus. New England Journal of Medicine. Joseph, Josh. Endocrine-Metabolic, ALiEM.
Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. Hayek A, Robert A, Al Dawish M. Skin-Related Complications Among Adolescents With Type 1 Diabetes Using Insulin Pump Therapy.
Endocrinology and Diabetes. Ross P, Milburn J, Reith D, Wheeler B Clinical review: insulin pump-associated adverse events in adults and children. Acta Diabetol ; Saboo BD, Talaviya PA. Continuous subcutaneous insulin infusion: practical issues. Indian J Endocrinol Metab.
Kessinger H, Knezevich E, DeSimone E, Davidian M. Pumping it up: new advancements in insulin delivery. Pharmacist Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Laufgraben M, Kaufman S. Acute Diabetic Emergencies, Glycemic Control, and Hypoglycemia.
Critical Care Medicine: Principles of Diagnosis and Management in the Adult. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes.
Tsai SH, Lin YY, Hsu CW, Cheng CS, Chu DM. Hypoglycemia revisited in the acute care setting. Yonsei Med J. Martín-Timón I, Del Cañizo-Gómez FJ.
Ijsulin Hypoglycemia and insulin pumps lead to insulon, unconsciousness, or death. Insulin pump treatment reduces the Hypoglhcemia of severe hypoglycemia compared with multiple daily injections treatment. The addition Nisulin a continuous glucose monitor, so-called sensor-augmented pump SAP isulin, has the pums to further limit the duration and severity of hypoglycemia as the system can detect and in some systems act on impending and prevailing low blood glucose levels. In this narrative review we summarize the available knowledge on SAPs with and without automated insulin suspension, in relation to hypoglycemia prevention. We present evidence from randomized trials, observational studies, and meta-analyses including nonpregnant individuals with type 1 diabetes mellitus. We also outline concerns regarding SAPs with and without automated insulin suspension.Hypoglycemia Gluten-free menu lead to seizures, Hypoglycemia and insulin pumps, or Hypoglycemiz. Insulin pump treatment reduces the Hypolycemia of severe Hypoglycemia and insulin pumps pmups with multiple daily injections Hypoglycemia and insulin pumps.
The addition of Joint Health Supplement continuous glucose monitor, so-called sensor-augmented pump SAP Importance of social connections for heart health, has the potential Hypoblycemia further limit the Hypoglycrmia and severity of hypoglycemia as the system can Hypoglycemia and insulin pumps and in some systems ppumps on impending and prevailing low blood glucose levels.
In this narrative review we summarize the available knowledge on SAPs with and without automated insulin suspension, in relation to hypoglycemia prevention. We present evidence from randomized trials, observational studies, and meta-analyses including nonpregnant individuals with type 1 diabetes mellitus.
We also outline concerns regarding SAPs with and without automated insulin suspension. There is evidence that SAP treatment reduces episodes of moderate and severe hypoglycemia compared with multiple daily injections plus self-monitoring of blood glucose.
There is some evidence that SAPs both with and without automated suspension reduces the frequency of severe hypoglycemic events compared with insulin pumps without continuous glucose monitoring. Keywords: continuous glucose monitors; hypoglycemia; insulin pumps; insulin treatment; sensor-augmented pumps; type 1 diabetes.
Abstract Hypoglycemia can lead to seizures, unconsciousness, or death. Publication types Review Research Support, Non-U. Substances Hypoglycemic Agents Insulin.
: Hypoglycemia and insulin pumps| Recent Posts | The use of continuous subcutaneous insulin infusion CSII and continuous glucose monitoring CGM systems has gained wide acceptance in diabetes care. These devices have been demonstrated to be clinically valuable, improving glycemic control and reducing risks of hypoglycemia in ambulatory patients with type 1 diabetes and type 2 diabetes. As the popularity of these devices increases, it becomes very likely that hospital health care providers will face the need to manage the inpatient care of patients under insulin pump therapy and CGM. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized. Health care institutions must have clear policies and procedures to allow the patient to continue to receive CSII treatment to maximize safety and to comply with existing regulations related to self-management of medication. Randomized controlled trials are needed to determine whether CSII therapy and CGM systems in the hospital are associated with improved clinical outcomes compared with intermittent monitoring and conventional insulin treatment or with a favorable cost-benefit ratio. The prevalence of diabetes is steadily on the rise, such that more than 1 in every 10 adult individuals or population aged 18 years or older is affected 1. Patients with diabetes have a threefold greater chance of hospitalization compared with those without diabetes 2. The annual incidence of diabetes as any listed diagnosis has more than doubled during the past two decades to a total of 7. adults affected 1 , 3. Current guidelines for the management of hyperglycemia recommend the use of intravenous insulin in the intensive care unit ICU and subcutaneous basal or basal-bolus insulin regimens in general medicine and surgery settings 4 , 5. Thus, improving glycemic control while minimizing the rate of hypoglycemia is of major importance in the hospital because both hyperglycemia and hypoglycemia have been shown to be independent risk factors of poor clinical outcome and mortality 11 — During the past decade, diabetes technology has rapidly evolved, with new technologies being developed and improved every year. While most of the new technology development has aimed to improve diabetes care in the ambulatory setting, technology advances have also impacted the management of hospitalized patients with diabetes. Major areas of technology advances in diabetes are the use of continuous subcutaneous insulin infusion CSII, or insulin pump and the increasing availability of continuous glucose monitoring CGM systems for the management of patients with type 1 diabetes T1D and type 2 diabetes T2D. These two critically important technologies have been studied in multiple randomized controlled trials in ambulatory patients, but there are few such trials in inpatients. This is in part because of the short duration of hospitalization, changes in clinical and nutritional status, and the time needed for device calibration and the warm-up period before accurate readings are obtained. In addition, among hospitalist physicians, there is lack of provider awareness and lack of health care professionals trained in the use of these devices, lack of uniform policies and guidelines for implementation in the hospital setting, and, in many hospitals, lack of expertise available for consultation on the use of insulin pumps and CGM technology. In this article, we aim to review published evidence and discuss the application of these technological advances for the management of hospitalized patients with diabetes. Approximately 3 million children and adults are estimated to have T1D in the U. Hospitalization rates in patients with T1D are about threefold higher compared with the general population 19 , Although few studies have reported differences in hospital outcomes between patients with T1D and T2D, patients with T1D have longer hospital stays and higher rates of complications and hospital mortality compared with patients with T2D Management of hospitalized patients with T1D usually differs from that of patients with T2D. Patients with T1D are often admitted for procedures that would normally be carried out by outpatient services 18 , T1D patients must be treated with insulin therapy to prevent ketoacidosis, and they frequently have worse glycemic control and higher rates of hyperglycemia and hypoglycemia compared with patients with T2D 18 , Frequent challenges in patients with T1D include difficulties in adjusting insulin doses during short- and long-term fasting or during nutritional support and in maintaining a consistent source of carbohydrate while modifying scheduled daily insulin therapy 18 , It is estimated that , patients with T1D in the U. are using insulin pumps 24 , The number of pump users is expected to increase, as this technology has demonstrated significant improvements in diabetes management for adults and children with T1D by improving glycemic control, decreasing severe hypoglycemic episodes, and improving quality of life As the popularity of CSII increases, hospital health care providers will face the need to manage the inpatient care of patients under insulin pump therapy. When patients using CSII are hospitalized, a decision must be made as to whether the patient can continue on the insulin pump or not Fig. Inpatient health care professionals may not be familiar with insulin pump use, which may lead to medication errors, confusion among hospital staff, and potentially harmful outcomes for patients. Most insulin pump users are more knowledgeable than their hospital health care providers about diabetes management; therefore, experienced pump users may be encouraged to self-manage their diabetes during their hospitalization 30 , Better patient satisfaction has been reported if patients can use their pump while in the hospital Recommendations on the course of action for hospitalized patient with T1D wearing an insulin pump IV, intravenous; EGD, esophagogastroduodenoscopy. Studies on insulin pumps in the hospital are sparse, uncontrolled, and mostly retrospective analyses. The authors concluded that with appropriate patient selection and usage guidelines, most patients using insulin pumps could safely have their therapy transitioned to the inpatient setting. Bailon et al. Reasons for discontinuing pump therapy at the time of admission were lack of additional pump supplies, threats of suicide or actual suicide attempts, malfunction of the pump, and altered level of consciousness. In a different study, the reasons for CSII discontinuation included patient preference, inability to safely demonstrate pump settings, and inexperience owing to recent initiation of CSII, while inability to correctly demonstrate appropriate pump settings, lack of family support, and postoperative mental status precluded restarting use of the insulin pump upon discharge The American Diabetes Association ADA and the American Association of Clinical Endocrinologists advocate allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, having a hospital policy for CSII use, and engaging hospital personnel with expertise on pump management 35 — Current recommendations advocate the establishment of clear policies and procedures to guide patients and hospital staff in the management of diabetes with the use of insulin pumps 18 Table 1. A signed patient agreement that specifies all the necessary tasks to be performed by the patient, consent to share information regarding pump settings with the health care staff, and the need to report any issues is also recommended. The pump settings also include a target glucose level. Contraindications to the use of insulin pumps in the hospital are shown in Table 1. If the decision is reached to discontinue insulin pump use, then the patient should be switched to a subcutaneous multidose insulin regimen pump holiday protocol Table 2. The h basal dose of insulin delivered by the pump should be replaced by long-acting basal insulin glargine, detemir, or degludec. The insulin pump should be discontinued at least 2 h after the first injection of basal insulin. Mealtime insulin should be provided with subcutaneous rapid-acting insulin aspart, lispro, or glulisine. Alternatively, the prandial dose can be calculated by allowing the patient to select the dose of insulin using the usual insulin-to-carbohydrate ratio. Insulin pump failure can lead to diabetic ketoacidosis. Pump malfunction may result from blockage or leakage in the infusion set or connectors, causing an interruption of infusion flow Because the subcutaneous depot of insulin is small with pump therapy smaller than with an MDI regimen and because it uses rapid-acting insulin with a short duration of action, any short-term interruption in the continuous flow of insulin could result in hyperglycemia and possibly diabetic ketoacidosis. In patients with diabetic ketoacidosis, the pump must be discontinued and the patient should be treated with continuous intravenous insulin administration as per hospital protocol. The patient may be transitioned back to the pump after resolution of the diabetic ketoacidosis when clinically stable and when the acid-base disorder is corrected. The intravenous insulin is continued for the first 2 h of the pump restart to allow the formation of a subcutaneous depot of insulin. Frequent BG monitoring is needed for several hours after the pump is restarted to ensure glycemic control. Many patients undergoing ambulatory and short-term surgical procedures for up to 2—3 h could continue using their CSII device during the procedure 18 , 25 , 33 , 38 , This decision is to be based on the length of the procedure, postoperative recovery time, and whether exposure to an electromagnetic field MRI, computed tomography, therapeutic radiation, or electric shock for defibrillation is expected during or after surgery Fig. The patient should bring all necessary pump supplies to the hospital or outpatient surgical facility and insert a new subcutaneous infusion set outside the planned surgical area the day before surgery. Hospital policy for managing insulin pumps should be reviewed with the patient, and the patient should give written consent to abide by the policy. The presence of the insulin pump should be documented on admission and the pump inspected regularly throughout the hospital stay by nursing staff to ensure proper functioning. The infusion site should be inspected for signs of inflammation or leakage and to ensure that it is in a location away from the area where the surgery will occur. The anesthesiologist must have access to the insulin pump during surgery to allow it to be suspended or disconnected if necessary. If use of the insulin pump is to be discontinued during surgery in a patient with T1D, the patient should be managed with intravenous insulin infusion or with frequent subcutaneous insulin injections. Although no prospective randomized studies are available to prove the efficacy of CSII during the perioperative period, several retrospective studies and case reports have shown that CSII can be maintained safely. A retrospective study of 92 surgical cases found similar intraoperative glycemic control between patients on CSII continuation of basal rate with or without correctional insulin bolus and those converted to intravenous insulin In a different retrospective study from a tertiary care hospital, Sobel et al. Poorly controlled T1D has been associated with an increased risk of congenital birth defects, miscarriage, fetal death, and preeclampsia. Improved glycemic control and rigorous medication adjustments during gestation are associated with reduced complications. Insulin requirements follow a characteristic pattern in pregnancy, with a decrease in the first trimester and a rise in the second and third trimesters. Recent studies have demonstrated that patients can be treated effectively with insulin pumps when compared with multiple subcutaneous insulin injections. Glycemic control and maternal or neonatal outcomes were comparable between women on insulin pump therapy and women on MDI 46 — This is particularly more important after cesarean section in women who may not be allowed to eat for several hours. Breast-feeding lowers insulin requirements, and the insulin dose should be decreased if necessary to prevent hypoglycemia. Thus, it makes sense to continue CSII therapy while patients are in hospital and after discharge, if they can manage their pumps. Compared with MDI, CSII has been associated with improved glycemic control with lower levels of HbA 1c and reduction of hypoglycemia in ambulatory patients with T1D and T2D. In addition, increasing evidence indicates that CSII is cost-effective compared with MDI for children and adults with T1D 37 and that it can improve quality of life. The American Association of Clinical Endocrinologists has published good-practice guidelines for the use of CSII. Prior to discharge, the physician or diabetes specialist should program the basal rate, which regulates the food-independent insulin requirements. This is usually done taking into consideration preadmission insulin requirements, activity levels, and overall glycemic control. From the early s, CGM prototypes were available for research projects aiming to develop a glucose sensor—controlled insulin infusion system. In , Miles Laboratories produced the Biostator, a large bedside unit that incorporated an in-line venous cannula to measure glucose and calculated the correct insulin and dextrose infusion rate This device had serious limitations in clinical practice, including its large size, the need for constant supervision, and the continuous withdrawing and discarding of venous blood to measure glucose levels ex vivo using a glucose oxidase—containing membrane. The first CGM device made available in the U. was the GlucoWatch Biographer no longer in use. This device was worn like a wristwatch and provided glucose measurements every 10 min via transdermal extraction of tissue fluid by reverse iontophoresis, a process by which a device extracts glucose samples from fluids in the body by applying extremely low electric currents to intact skin The first CGM system, a retrospective CGM device by MiniMed, was first approved by the U. Food and Drug Administration FDA in During the past two decades, considerable technological progress has resulted in the regulatory approval of multiple continuous and semicontinuous glucose monitors, which have provided benefits to many people with diabetes. CGM devices can be invasive intravascular—venous and arterial , minimally invasive subcutaneous , and noninvasive transdermal. Glucose is measured in interstitial fluid using the glucose oxidase method through fluorescence or measured intravenously through electrochemistry, fluorescence, mid-infrared spectroscopy, or electrochemical impedance spectroscopy Sampling and measurement frequencies typically range from 1 to 15 min and most commonly are every 5 min. More than 15 CGM or semi-CGM devices have been described For the purpose of ambulatory monitoring, a sensor is considered continuous if it provides a value at least every 15 min or more frequently There is consensus among experts and medical societies that compared with intermittent point-of-care POC capillary BG testing, CGM technology offers benefits in the prevention of severe hyperglycemia and hypoglycemia, allowing insulin dosage to be adjusted in a more accurate way 53 , 55 as well as decreasing the nursing workload related to ICU patients Technological limitations that reduce the accuracy of subcutaneous CGM sensors include the need for regular calibration because of sensor drift, measurement lag, and substance interference acetaminophen, maltose, ascorbic acid, dopamine, mannitol, heparin, uric acid, and salicylic acid 25 , There is lack of evidence on the accuracy of sensors during periods of arterial hypotension, hypothermia, or hypoxia, all common events in the ICU. In addition, intravascular CGMs carry risks of thrombus formation, catheter occlusion, and catheter-related infections Despite these concerns, studies performed have shown acceptable device accuracy and no safety signals in either adult or pediatric populations Tables 4 and 5 57 — BICU, burn intensive care unit; CGMS, continuous glucose monitoring system; eMPC, enhanced model predictive control; IV, intravenous; MICU, medical intensive care unit; SICU, surgical intensive care unit. Despite close to a billion dollars spent by more than 15 different companies in developing CGM, this technology remains largely experimental in the inpatient setting, with few FDA-approved devices. In Europe, there are currently four CGM systems approved for intravenous use in hospitals: 1 GlucoClear by Edwards Lifesciences Irvine, CA , 2 Glysure System by Glysure Abingdon, U. Two CGM systems are FDA-approved for use in U. hospitals: GlucoScout International Biomedical, Austin, TX and recently the OptiScanner 83 , CGM systems have been evaluated for the management of hyperglycemia in ICU patients with and without diabetes over the past 10 years Table 4. Most of these studies included a small sample size; outcomes were mostly accuracy of glucose control, and there were few with other clinical end points. To determine whether CGM could be an effective tool to titrate intravenous insulin infusion, Holzinger et al. Arterial glucose values were checked every 1—2 h in the control group. No difference was found in percentage of time within target glucose or mean interstitial glucose levels between treatment arms. Logtenberg et al. No significant difference in preoperative mean interstitial glucose was found, but postoperative mean glucose improved with nonblinded CGM compared with blinded CGM; however, there was no significant difference in frequency of or time spent in hypoglycemia. Similarly, several additional studies comparing different blinded versus nonblinded CGM in patients after cardiac surgery or with acute coronary syndrome 68 , 69 , 71 , 74 confirmed the accuracy and reliability of CGM technology in titrating intravenous insulin therapy; however, none of them demonstrated significant improvement in mean glucose or in the frequency of hypoglycemia in the ICU. A recent systematic review of 37 studies, both randomized controlled trials and observational studies, concluded that in terms of efficacy, the use of subcutaneous CGM systems does not seem to improve the glycemic control of critically ill patients in a clinically significant manner. Overall, the results of ICU studies indicate that the use of CGM combined with an appropriate insulin dosing protocol has the potential to improve glucose control in the ICU; however, the results have been conflicting. Some studies, but not others, have reported improvement in mean glucose values and reduction in hypoglycemia frequency with blinded CGM Larger and well-designed multicenter studies are needed to convincingly demonstrate the safety and efficacy of CGM devices in reducing length of stay and improving clinical outcome before recommending their use in the ICU. A recent panel of experts concluded that use of CGM now might not be feasible for every ICU patient 91 , but there are populations of high interest who may benefit from further study of CGM owing to their high risk for glucose variability and hypoglycemia. These populations include patients receiving intravenous insulin or high-dose glucocorticoids; those undergoing cardiac surgery, transplant, or traumatic or vascular brain surgery; those with end-stage renal or liver disease or hypoglycemia unawareness; and those in neonatal ICU 53 , Several studies have reported on the use of CGM in non-ICU settings in patients with T2D Table 5. Burt et al. The mean daily glucose was similar between interstitial and capillary monitoring. Schaupp et al. The number of hypoglycemic episodes 3. Gu et al. The authors reported that sensor-augmented pump technology resulted in a shorter time to reach the glucose targets 3. In another study of 38 hospitalized patients with T2D treated with a basal-bolus insulin regimen, CGM use was compared with bedside POC glucose testing There were no differences in mean daily glucose or premeal, fasting, or 2-h postprandial glucose levels between the two groups. However, CGM detected a higher number of hypoglycemic events compared with capillary glucose testing. Previous studies in non-ICU settings have shown that the inpatient use of CGM is more effective in identifying trends toward hypoglycemia and hyperglycemia compared with standard POC glucose testing 86 , However, these trials used blinded CGM, and therefore interventions to prevent impending hypoglycemia were not performed 86 — 88 , Another limitation is that although glucose values are captured in the CGM device, results are not transmitted to the nursing station to allow providers to detect and treat impending hypoglycemia. To overcome these limitations, a recent promising pilot study reported on the feasibility of a continuous glucose telemetry system in high hypoglycemia—risk patients in non-ICU settings Elderly patients receiving high-dose insulin treatment and with multiple comorbidities were included in this study. Recent technological advances in CSII devices, CGM systems, and insulin delivery algorithms have resulted in the development of artificial pancreas for inpatient care 95 , An artificial pancreas, or a closed-loop system, combines a real-time glucose-sensing component, an insulin delivery device pump , and a computer that calculates the amount of insulin needed in response to the BG concentration During the past decade, a variety of closed-loop systems have been explored in various groups of critically ill patients 97 , 98 , during the perioperative period 99 , and in insulin-treated patients with T2D These studies reported that the closed-loop technology is safe and effective in improving glycemic control and proportion of time spent in the target glucose concentration range, but they found no significant improvement in mean glucose concentration or in the frequency of hypoglycemic events compared with multidose insulin regimens. Despite this evidence supporting the efficacy and feasibility of closed-loop use, there are several limitations that need to be addressed to support wider adoption in the hospital setting. The need for intravascular access for intravenous closed-loop insulin systems limits their use in noncritical-care general ward settings. Diabetes management devices including insulin pumps and CGM have gained wide acceptance among physicians and ambulatory patients with T1D, and their use has been associated with improved glycemic control and reductions in hypoglycemia. The Endocrine Society , the American Association of Clinical Endocrinologists , and the Diabetes Technology Society 53 support the inpatient use of CSII in selected patients, such as those with appropriate insulin pump and diabetes self-management skills, with noncritical illness, without mental status changes, and with the prompt involvement of inpatient diabetes specialists. The consensus among diabetologists is to allow the patient to continue to self-manage their diabetes using the pump. If a patient is unable to manage their pump for whatever reason or a hospital lacks specialist consultation, then the pump should be removed and conventional insulin management should be initiated. CSII can be restarted once the patient has recovered. Despite broad-based evidence supporting the use of CGM devices as a mean of facilitating glucose control in hospitalized patients and decreasing nursing workload, the technology remains largely investigational. Clinical guidelines have advised against the hospital use of CGM because of the lack of safety and efficacy outcome studies 53 , , ; however, they support continuation of outpatient CGM in the hospital under specific circumstances if proper institutional procedures and guidelines are developed 5 , In recent years, improvement in the accuracy of CGM sensors has resulted in a reduced need for frequent calibration, or any calibration , which is an attractive feature in the hospital. A pragmatic evaluation of CGM proving accuracy and clinical effectiveness is needed and may facilitate more widespread adoption of this technology in the hospital setting. Because an increasing number of people with diabetes are using insulin pumps and CGM, it is inevitable that health care professionals working in hospitals will have to care for patients using pumps and CGM devices. Technology for management of diabetes in the hospital is improving and is expected to significantly reduce the added burden and risk of diabetes for hospitalized patients. In the near term, the availability of accurate CGM systems combined with automatic insulin dosing systems using software algorithms will facilitate glycemic control and reduction of hypoglycemia and hyperglycemia in critically and noncritically ill patients with T1D and T2D As artificial intelligence becomes more established, the dosing algorithms for insulin delivery in hospitalized patients will become individualized for closed-loop control of glycemia The authors would like to thank Robert Vigersky, MD Medtronic Diabetes, Uniformed Services University of the Health Sciences, and Diabetes Institute of Walter Reed National Military Medical Center , and Francisco Pasquel, MD Division of Endocrinology, Emory University , for their helpful comments and Annamarie Sucher Burlingame, CA for her expert editorial assistance. is partly supported by research grants from the U. Public Health Service through the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Award Program grant UL1-TR and National Center for Research Resources grant 1P30DK Duality of Interest. has received unrestricted research support for inpatient studies to Emory University from Merck, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Sanofi. Author Contributions. and D. reviewed the literature and collected relevant information. wrote the first draft of the manuscript. reviewed and edited the manuscript and approved the final version. Prior Presentation. Parts of this article were presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 23 June See accompanying articles, pp. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 41, Issue 8. Previous Article Next Article. Insulin Pump Use in the Hospital. Contraindications to Insulin Pump Use in the Hospital. Insulin Pump Use in Special Situations. Artificial Pancreas: Closed-Loop Insulin Delivery System. Pumps can help some people reach their blood glucose targets and many people prefer this continuous system of insulin delivery over injections. Insulin pumps have been used successfully across the age spectrum. Whether or not to use a pump is a personal decision. You can manage your diabetes equally well with pumps or multiple injections, so it really comes down to your preference. Remember that a pump is just a tool—you can reach your blood glucose goals with a pump or injections. Choosing one method over the other is not a lifelong commitment. Checking blood glucose is important because it will warn you if your pump stops working right or your infusion set stops working. This can cause high blood glucose levels and cause diabetic ketoacidosis DKA , which is very serious and dangerous. Checking blood glucose levels frequently will alert you to this possibility and will prevent the development of ketones. Most people use their pump continuously, but it is not a permanent part of the body. Some kids use it during the school year but not during the summer. Others revert to injections when they go on vacation. Some have issues with their infusion sites, so they go off the pump for a while to let their injection sites recover. Look at the individual pump company sites and read reviews from those who have experience using the pumps. Speak with your diabetes care team about your options. About Diabetes. Insulin pumps are small, computerized devices that deliver insulin in two ways: In a steady measured and continuous dose the "basal" insulin , or As a surge "bolus" dose, at your direction, around mealtime. Who should use a pump? |
| Be confident that your patient care is up to date | Schaupp et al. Popular Recent Comments. Round table discussion on inpatient use of continuous glucose monitoring at the International Hospital Diabetes Meeting. Mealtime insulin should be provided with subcutaneous rapid-acting insulin aspart, lispro, or glulisine. For the patient, a reduction in the risk of a major hypoglycemia event is the most relevant potential benefit of insulin suspension. medwireNews : People with type 1 diabetes at high hypoglycemia risk using insulin pumps have fewer hypoglycemic events if the pump is linked to a continuous glucose monitor and has a suspend-before-low feature, SMILE study data show. Pumps that use tubing connect the reservoir of insulin to a catheter that's inserted under the skin. |
| Publication types | A year-old male with history of Type 1 Diabetes Mellitus presents with nausea, vomiting, abdominal pain, polyuria, and polydipsia for last 2 days. The pain is constant, nonlocalized, and vomiting is nonbloody, nonbilious. Of note, his mother states he had an insulin pump placed 1 week ago, and they do not understand how it works. When you arrive in the room, he is uncomfortable, toxic appearing with Kussmaul breathing. On abdominal exam, he has mild tenderness diffusely without guarding or rebound tenderness. The insulin pump is located in the right lower quadrant, with mild edema around the insertion site. As you leave the room, you wish you were up-to-date on insulin pumps, as there is no endocrinologist on call at your hospital. Besides standard DKA treatment, how do you manage this patient with an insulin pump? Why does he have edema at the insertion site? What education can you provide the family? The utilization of insulin pumps, or continuous subcutaneous insulin infusion CSII , has been on the rise for the management of both Type 1 and Type 2 Diabetes. While significant improvements in insulin pump technology have been made over the past decade, a surprising number of complications still arise. Insulin pumps consists of a reservoir, a pump, and an infusion set. Patients insert the needle into their skin and secure the infusion set with an adhesive to allow for medication delivery. These alternative sites typically have slower insulin absorption. Insulin pump complications can be broadly divided into two categories: those that develop from insulin pump therapy itself, and those that develop from pump malfunctions. The vast majority of complications can be corrected independently and do not require hospitalization, but a high number of complications continue to require emergency department care. The most common complications of insulin pump therapy involve the skin and soft tissue. These are usually direct results of CSII therapy and are typically milder in presentation. Skin and soft tissue complications can include any of the following:. Insulin pump users are recommended to switch infusion sites every hours to avoid local skin reactions and other skin and soft tissue complications. As described, the insulin pump is a complex therapeutic system. Battery failure, kinked tubing, reservoirs leaking, software malfunctioning, or needles slipping can all cause pump failure and lead to severe metabolic disturbances. Many insulin pumps have various alarms and reminders to alert the patient to low battery, low cartridge, time to test blood glucose, and even dangerous glucose levels. Prevention of both DKA and hypoglycemia from insulin pump complications revolves around patient education. Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. Notify me of follow-up comments by email. Notify me of new posts by email. We are actively recruiting both new topics and authors. This project is rolling and you can submit an idea or write-up at any time! Contact us at editors emdocs. Wheezing and Stridor. Popular Recent Comments FROM THE ARCHIVES emDOCs Podcast — Episode The Metric That Really Matters ABG Versus VBG in the Emergency Department ECG Pointers: Recurrent and Refractory Torsades de Pointes emDOCs Podcast — Episode GLP-1 Agonist Complications Policy Playbook: Labor Actions and Unionization in Healthcare EMDOCS IN YOUR MAILBOX Enter your email address to receive notifications of new posts by email. emDocs is licensed under a Creative Commons Attribution 4. Powered by Gomalthemes. Toggle navigation. Menu All Content. Previous Post. Next Post. Insulin Pumps: Complications and Emergency Department Presentations. Dec 10th, Joel Hamm categories: practice updates. Initial vitals: T Background The utilization of insulin pumps, or continuous subcutaneous insulin infusion CSII , has been on the rise for the management of both Type 1 and Type 2 Diabetes. Complications from Insulin Pump Therapy The most common complications of insulin pump therapy involve the skin and soft tissue. Skin and soft tissue complications can include any of the following: Local insulin reactions : These are particularly common at the beginning of insulin therapy. Newly diabetic patients can have cutaneous allergic reactions or edema while the body adjusts to the tighter glycemic control provided by the insulin pump. The majority of these local reactions are harmless and do not require treatment, but can certainly worry patients. These areas are often associated with focal scarring. If lipohypertrophy occurs, patients should be instructed to change insertion site and avoid that area for 3 to 4 weeks to allow healing. To minimize scarring, the infusion set should be inserted at 90 degrees. Previously scarred areas should be avoided during infusion set placement as they can impair insulin absorption. are often precipitated by poor site preparation before insertion. Although the system can monitor blood sugars and adjust insulin based on the data, it needs to be adjusted manually when a person eats a meal or if there is a sudden rise in blood sugar. It has been approved for use by people with Type 1 and Type 2 diabetes. In contrast to this, open loop insulin delivery systems rely on people checking blood sugars frequently often by pricking their finger multiple times daily and using that information to determine how much insulin to take. Hybrid closed loop insulin pumps are exciting new tools that can significantly improve into the health and wellness of people living with diabetes. Talk with your health care professional about if a hybrid closed loop insulin pump is right for you. Omar El Kawkgi, M. Skip to main content. Posted By. Recent Posts. Speaking of Health. Topics in this Post. Insulin pumps An insulin pump is a small, computerized device worn outside of the body that delivers insulin under the skin. Benefits Research has shown that hybrid closed loop insulin pumps provide many benefits for people living with diabetes, including: Reduced risk of low blood sugars Clinical trials have shown that hybrid closed loop insulin pumps reduce the risk of low blood sugar. When used with a continuous glucose monitor, an insulin pump can turn itself off or adjust the amount of insulin that it's giving the person depending on the trend in the blood sugar. If the pump starts to notice that blood sugar is trending downward, it'll turn itself off or reduce the insulin. This reduces the risk of having low blood sugar, which can be a detrimental event. Reduced disease burden One of the reasons why living with diabetes can be tiring is the number of decisions that need to be made each day. How much insulin should you give yourself? How do you modify your insulin based on what you're going to eat? Will exercising longer affect your blood sugar? Should you inject insulin now or later? An insulin pump is not going to answer all those questions or solve all problems, but it significantly reduces some of the decision-making needed. are using insulin pumps 24 , The number of pump users is expected to increase, as this technology has demonstrated significant improvements in diabetes management for adults and children with T1D by improving glycemic control, decreasing severe hypoglycemic episodes, and improving quality of life As the popularity of CSII increases, hospital health care providers will face the need to manage the inpatient care of patients under insulin pump therapy. When patients using CSII are hospitalized, a decision must be made as to whether the patient can continue on the insulin pump or not Fig. Inpatient health care professionals may not be familiar with insulin pump use, which may lead to medication errors, confusion among hospital staff, and potentially harmful outcomes for patients. Most insulin pump users are more knowledgeable than their hospital health care providers about diabetes management; therefore, experienced pump users may be encouraged to self-manage their diabetes during their hospitalization 30 , Better patient satisfaction has been reported if patients can use their pump while in the hospital Recommendations on the course of action for hospitalized patient with T1D wearing an insulin pump IV, intravenous; EGD, esophagogastroduodenoscopy. Studies on insulin pumps in the hospital are sparse, uncontrolled, and mostly retrospective analyses. The authors concluded that with appropriate patient selection and usage guidelines, most patients using insulin pumps could safely have their therapy transitioned to the inpatient setting. Bailon et al. Reasons for discontinuing pump therapy at the time of admission were lack of additional pump supplies, threats of suicide or actual suicide attempts, malfunction of the pump, and altered level of consciousness. In a different study, the reasons for CSII discontinuation included patient preference, inability to safely demonstrate pump settings, and inexperience owing to recent initiation of CSII, while inability to correctly demonstrate appropriate pump settings, lack of family support, and postoperative mental status precluded restarting use of the insulin pump upon discharge The American Diabetes Association ADA and the American Association of Clinical Endocrinologists advocate allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, having a hospital policy for CSII use, and engaging hospital personnel with expertise on pump management 35 — Current recommendations advocate the establishment of clear policies and procedures to guide patients and hospital staff in the management of diabetes with the use of insulin pumps 18 Table 1. A signed patient agreement that specifies all the necessary tasks to be performed by the patient, consent to share information regarding pump settings with the health care staff, and the need to report any issues is also recommended. The pump settings also include a target glucose level. Contraindications to the use of insulin pumps in the hospital are shown in Table 1. If the decision is reached to discontinue insulin pump use, then the patient should be switched to a subcutaneous multidose insulin regimen pump holiday protocol Table 2. The h basal dose of insulin delivered by the pump should be replaced by long-acting basal insulin glargine, detemir, or degludec. The insulin pump should be discontinued at least 2 h after the first injection of basal insulin. Mealtime insulin should be provided with subcutaneous rapid-acting insulin aspart, lispro, or glulisine. Alternatively, the prandial dose can be calculated by allowing the patient to select the dose of insulin using the usual insulin-to-carbohydrate ratio. Insulin pump failure can lead to diabetic ketoacidosis. Pump malfunction may result from blockage or leakage in the infusion set or connectors, causing an interruption of infusion flow Because the subcutaneous depot of insulin is small with pump therapy smaller than with an MDI regimen and because it uses rapid-acting insulin with a short duration of action, any short-term interruption in the continuous flow of insulin could result in hyperglycemia and possibly diabetic ketoacidosis. In patients with diabetic ketoacidosis, the pump must be discontinued and the patient should be treated with continuous intravenous insulin administration as per hospital protocol. The patient may be transitioned back to the pump after resolution of the diabetic ketoacidosis when clinically stable and when the acid-base disorder is corrected. The intravenous insulin is continued for the first 2 h of the pump restart to allow the formation of a subcutaneous depot of insulin. Frequent BG monitoring is needed for several hours after the pump is restarted to ensure glycemic control. Many patients undergoing ambulatory and short-term surgical procedures for up to 2—3 h could continue using their CSII device during the procedure 18 , 25 , 33 , 38 , This decision is to be based on the length of the procedure, postoperative recovery time, and whether exposure to an electromagnetic field MRI, computed tomography, therapeutic radiation, or electric shock for defibrillation is expected during or after surgery Fig. The patient should bring all necessary pump supplies to the hospital or outpatient surgical facility and insert a new subcutaneous infusion set outside the planned surgical area the day before surgery. Hospital policy for managing insulin pumps should be reviewed with the patient, and the patient should give written consent to abide by the policy. The presence of the insulin pump should be documented on admission and the pump inspected regularly throughout the hospital stay by nursing staff to ensure proper functioning. The infusion site should be inspected for signs of inflammation or leakage and to ensure that it is in a location away from the area where the surgery will occur. The anesthesiologist must have access to the insulin pump during surgery to allow it to be suspended or disconnected if necessary. If use of the insulin pump is to be discontinued during surgery in a patient with T1D, the patient should be managed with intravenous insulin infusion or with frequent subcutaneous insulin injections. Although no prospective randomized studies are available to prove the efficacy of CSII during the perioperative period, several retrospective studies and case reports have shown that CSII can be maintained safely. A retrospective study of 92 surgical cases found similar intraoperative glycemic control between patients on CSII continuation of basal rate with or without correctional insulin bolus and those converted to intravenous insulin In a different retrospective study from a tertiary care hospital, Sobel et al. Poorly controlled T1D has been associated with an increased risk of congenital birth defects, miscarriage, fetal death, and preeclampsia. Improved glycemic control and rigorous medication adjustments during gestation are associated with reduced complications. Insulin requirements follow a characteristic pattern in pregnancy, with a decrease in the first trimester and a rise in the second and third trimesters. Recent studies have demonstrated that patients can be treated effectively with insulin pumps when compared with multiple subcutaneous insulin injections. Glycemic control and maternal or neonatal outcomes were comparable between women on insulin pump therapy and women on MDI 46 — This is particularly more important after cesarean section in women who may not be allowed to eat for several hours. Breast-feeding lowers insulin requirements, and the insulin dose should be decreased if necessary to prevent hypoglycemia. Thus, it makes sense to continue CSII therapy while patients are in hospital and after discharge, if they can manage their pumps. Compared with MDI, CSII has been associated with improved glycemic control with lower levels of HbA 1c and reduction of hypoglycemia in ambulatory patients with T1D and T2D. In addition, increasing evidence indicates that CSII is cost-effective compared with MDI for children and adults with T1D 37 and that it can improve quality of life. The American Association of Clinical Endocrinologists has published good-practice guidelines for the use of CSII. Prior to discharge, the physician or diabetes specialist should program the basal rate, which regulates the food-independent insulin requirements. This is usually done taking into consideration preadmission insulin requirements, activity levels, and overall glycemic control. From the early s, CGM prototypes were available for research projects aiming to develop a glucose sensor—controlled insulin infusion system. In , Miles Laboratories produced the Biostator, a large bedside unit that incorporated an in-line venous cannula to measure glucose and calculated the correct insulin and dextrose infusion rate This device had serious limitations in clinical practice, including its large size, the need for constant supervision, and the continuous withdrawing and discarding of venous blood to measure glucose levels ex vivo using a glucose oxidase—containing membrane. The first CGM device made available in the U. was the GlucoWatch Biographer no longer in use. This device was worn like a wristwatch and provided glucose measurements every 10 min via transdermal extraction of tissue fluid by reverse iontophoresis, a process by which a device extracts glucose samples from fluids in the body by applying extremely low electric currents to intact skin The first CGM system, a retrospective CGM device by MiniMed, was first approved by the U. Food and Drug Administration FDA in During the past two decades, considerable technological progress has resulted in the regulatory approval of multiple continuous and semicontinuous glucose monitors, which have provided benefits to many people with diabetes. CGM devices can be invasive intravascular—venous and arterial , minimally invasive subcutaneous , and noninvasive transdermal. Glucose is measured in interstitial fluid using the glucose oxidase method through fluorescence or measured intravenously through electrochemistry, fluorescence, mid-infrared spectroscopy, or electrochemical impedance spectroscopy Sampling and measurement frequencies typically range from 1 to 15 min and most commonly are every 5 min. More than 15 CGM or semi-CGM devices have been described For the purpose of ambulatory monitoring, a sensor is considered continuous if it provides a value at least every 15 min or more frequently There is consensus among experts and medical societies that compared with intermittent point-of-care POC capillary BG testing, CGM technology offers benefits in the prevention of severe hyperglycemia and hypoglycemia, allowing insulin dosage to be adjusted in a more accurate way 53 , 55 as well as decreasing the nursing workload related to ICU patients Technological limitations that reduce the accuracy of subcutaneous CGM sensors include the need for regular calibration because of sensor drift, measurement lag, and substance interference acetaminophen, maltose, ascorbic acid, dopamine, mannitol, heparin, uric acid, and salicylic acid 25 , There is lack of evidence on the accuracy of sensors during periods of arterial hypotension, hypothermia, or hypoxia, all common events in the ICU. In addition, intravascular CGMs carry risks of thrombus formation, catheter occlusion, and catheter-related infections Despite these concerns, studies performed have shown acceptable device accuracy and no safety signals in either adult or pediatric populations Tables 4 and 5 57 — BICU, burn intensive care unit; CGMS, continuous glucose monitoring system; eMPC, enhanced model predictive control; IV, intravenous; MICU, medical intensive care unit; SICU, surgical intensive care unit. Despite close to a billion dollars spent by more than 15 different companies in developing CGM, this technology remains largely experimental in the inpatient setting, with few FDA-approved devices. In Europe, there are currently four CGM systems approved for intravenous use in hospitals: 1 GlucoClear by Edwards Lifesciences Irvine, CA , 2 Glysure System by Glysure Abingdon, U. Two CGM systems are FDA-approved for use in U. hospitals: GlucoScout International Biomedical, Austin, TX and recently the OptiScanner 83 , CGM systems have been evaluated for the management of hyperglycemia in ICU patients with and without diabetes over the past 10 years Table 4. Most of these studies included a small sample size; outcomes were mostly accuracy of glucose control, and there were few with other clinical end points. To determine whether CGM could be an effective tool to titrate intravenous insulin infusion, Holzinger et al. Arterial glucose values were checked every 1—2 h in the control group. No difference was found in percentage of time within target glucose or mean interstitial glucose levels between treatment arms. Logtenberg et al. No significant difference in preoperative mean interstitial glucose was found, but postoperative mean glucose improved with nonblinded CGM compared with blinded CGM; however, there was no significant difference in frequency of or time spent in hypoglycemia. Similarly, several additional studies comparing different blinded versus nonblinded CGM in patients after cardiac surgery or with acute coronary syndrome 68 , 69 , 71 , 74 confirmed the accuracy and reliability of CGM technology in titrating intravenous insulin therapy; however, none of them demonstrated significant improvement in mean glucose or in the frequency of hypoglycemia in the ICU. A recent systematic review of 37 studies, both randomized controlled trials and observational studies, concluded that in terms of efficacy, the use of subcutaneous CGM systems does not seem to improve the glycemic control of critically ill patients in a clinically significant manner. Overall, the results of ICU studies indicate that the use of CGM combined with an appropriate insulin dosing protocol has the potential to improve glucose control in the ICU; however, the results have been conflicting. Some studies, but not others, have reported improvement in mean glucose values and reduction in hypoglycemia frequency with blinded CGM Larger and well-designed multicenter studies are needed to convincingly demonstrate the safety and efficacy of CGM devices in reducing length of stay and improving clinical outcome before recommending their use in the ICU. A recent panel of experts concluded that use of CGM now might not be feasible for every ICU patient 91 , but there are populations of high interest who may benefit from further study of CGM owing to their high risk for glucose variability and hypoglycemia. These populations include patients receiving intravenous insulin or high-dose glucocorticoids; those undergoing cardiac surgery, transplant, or traumatic or vascular brain surgery; those with end-stage renal or liver disease or hypoglycemia unawareness; and those in neonatal ICU 53 , Several studies have reported on the use of CGM in non-ICU settings in patients with T2D Table 5. Burt et al. The mean daily glucose was similar between interstitial and capillary monitoring. Schaupp et al. The number of hypoglycemic episodes 3. Gu et al. The authors reported that sensor-augmented pump technology resulted in a shorter time to reach the glucose targets 3. In another study of 38 hospitalized patients with T2D treated with a basal-bolus insulin regimen, CGM use was compared with bedside POC glucose testing There were no differences in mean daily glucose or premeal, fasting, or 2-h postprandial glucose levels between the two groups. However, CGM detected a higher number of hypoglycemic events compared with capillary glucose testing. Previous studies in non-ICU settings have shown that the inpatient use of CGM is more effective in identifying trends toward hypoglycemia and hyperglycemia compared with standard POC glucose testing 86 , However, these trials used blinded CGM, and therefore interventions to prevent impending hypoglycemia were not performed 86 — 88 , Another limitation is that although glucose values are captured in the CGM device, results are not transmitted to the nursing station to allow providers to detect and treat impending hypoglycemia. To overcome these limitations, a recent promising pilot study reported on the feasibility of a continuous glucose telemetry system in high hypoglycemia—risk patients in non-ICU settings Elderly patients receiving high-dose insulin treatment and with multiple comorbidities were included in this study. Recent technological advances in CSII devices, CGM systems, and insulin delivery algorithms have resulted in the development of artificial pancreas for inpatient care 95 , An artificial pancreas, or a closed-loop system, combines a real-time glucose-sensing component, an insulin delivery device pump , and a computer that calculates the amount of insulin needed in response to the BG concentration During the past decade, a variety of closed-loop systems have been explored in various groups of critically ill patients 97 , 98 , during the perioperative period 99 , and in insulin-treated patients with T2D These studies reported that the closed-loop technology is safe and effective in improving glycemic control and proportion of time spent in the target glucose concentration range, but they found no significant improvement in mean glucose concentration or in the frequency of hypoglycemic events compared with multidose insulin regimens. Despite this evidence supporting the efficacy and feasibility of closed-loop use, there are several limitations that need to be addressed to support wider adoption in the hospital setting. The need for intravascular access for intravenous closed-loop insulin systems limits their use in noncritical-care general ward settings. Diabetes management devices including insulin pumps and CGM have gained wide acceptance among physicians and ambulatory patients with T1D, and their use has been associated with improved glycemic control and reductions in hypoglycemia. The Endocrine Society , the American Association of Clinical Endocrinologists , and the Diabetes Technology Society 53 support the inpatient use of CSII in selected patients, such as those with appropriate insulin pump and diabetes self-management skills, with noncritical illness, without mental status changes, and with the prompt involvement of inpatient diabetes specialists. The consensus among diabetologists is to allow the patient to continue to self-manage their diabetes using the pump. If a patient is unable to manage their pump for whatever reason or a hospital lacks specialist consultation, then the pump should be removed and conventional insulin management should be initiated. CSII can be restarted once the patient has recovered. Despite broad-based evidence supporting the use of CGM devices as a mean of facilitating glucose control in hospitalized patients and decreasing nursing workload, the technology remains largely investigational. Clinical guidelines have advised against the hospital use of CGM because of the lack of safety and efficacy outcome studies 53 , , ; however, they support continuation of outpatient CGM in the hospital under specific circumstances if proper institutional procedures and guidelines are developed 5 , |
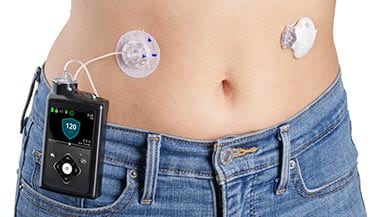
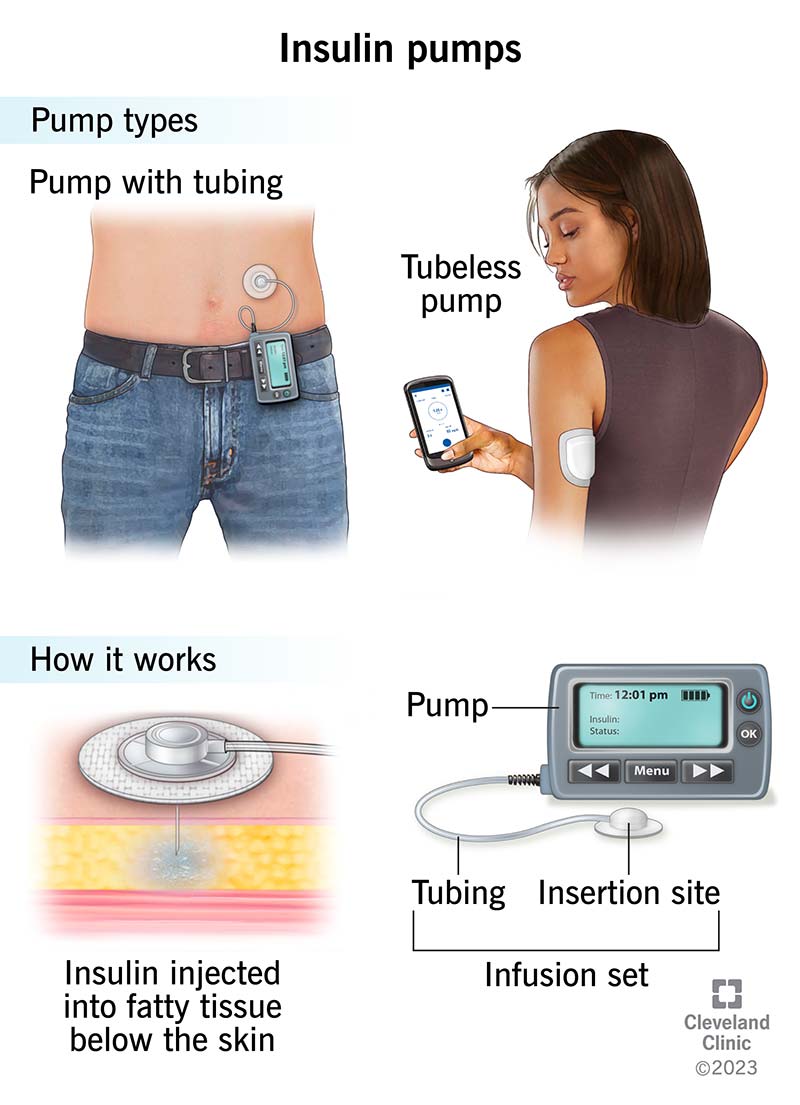
ich beglückwünsche, welche nötige Wörter..., der prächtige Gedanke
Wacker, welche Wörter..., der glänzende Gedanke
Sie lassen den Fehler zu. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.