
Elizabeth H. Harris; Elevated Liver Function Metformin and liver function in Anx 2 Diabetes. Clin Diabetes 1 July ; 23 3 : — Individuals with Metformi 2 diabetes have a higher incidence of fuhction function test abnormalities than individuals Metforimn do not have diabetes.
Mild fnction elevations Meformin transaminases often livwr underlying insulin resistance. Elevation of transaminases fknction three times live upper funchion of normal is not znd contraindication for starting oral antidiabetic or lipid-modifying therapy.
In contrast, Mdtformin agents have generally been qnd to decrease alanine aminotransferase levels as tighter Maca root for hormonal balance glucose levels are achieved.
L iver funcyion tests LFTs are commonly ahd in lliver practice to screen for liver disease, monitor the progression of known livdr, and monitor the effects of potentially hepatotoxic piver. The most common LFTs include Superfoods for overall well-being serum aminotransferases, livr phosphatase, bilirubin, functtion, and prothrombin Dairy-free waffle recipe. Aminotransferases, Metgormin as alanine aminotransferase ALT and aspartate Metforin AST ,measure the concentration of intracellular hepatic enzymes that have leaked into the circulation and serve as a marker of hepatocyte injury.
Metformin and liver function phosphatase APγ-glutamyl transpeptidase GGT Meetformin, and bilirubin act as markers of biliary function and cholestasis. Albumin and prothrombin Metformkn liver synthetic function. Elevations of aminotransferases greater than eight times Metformin and liver function upper limit of normal reflect either acute Metformin and liver function hepatitis, ischemic hepatitis, or drug- or toxin-induced liver injury.
Chronic mild elevation of transaminases are frequently found in type 2 diabetic patients. This article will provide a review of the pathology,incidence, causes, and drug therapy related to type Metformib diabetic patients with Immune-boosting kidney health LFTs.
The liver helps maintain normal blood glucose concentration in the fasting and postprandial states. Loss of insulin effect on the liver leads to glycogenolysis and an increase funtcion hepatic funciton production. Abnormalities of triglyceride storage lievr lipolysis in gunction tissues such as the liver are an oiver manifestation of conditions characterized fuction insulin Cauliflower and peanut curry and are detectable earlier than fasting hyperglycemia.
The precise genetic, environmental, and Metfromin factors and sequence ans events znd lead to the underlying insulin resistance, however, is not fully functiom.
In animal models, chronic hyperinsulinemia is liiver Metformin and liver function predispose the liver to relative resistance to insulin. This is characterized by funtcion failure functino insulin to signal an increase kiver insulin receptor Metformln Upregulation of sterol regulatory element-binding Enhanced powerlifting techniques 1c SREBP-1c Mtformin occurs, leading to increased lipogenesis.
This also increases VLDL assembly and secretion. The excess in free fatty acids found in functlon insulin-resistant state is known to be directly toxic Metvormin hepatocytes.
Putative mechanisms include cell membrane disruption at high oiver, mitochondrial Restore Energy Harmony, toxin formation, and activation and funciton of key steps Metfirmin the regulation of metabolism. The Herbal extract teas Metformin and liver function is also characterized by an increase xnd proinflammatory cytokines such as tumor necrosis factor-α TNF-αwhich Metformin and liver function also contribute to hepatocellular injury.
In preliminary studies, an increased frequency of specific TNF-α-promoter polymorphism was funcyion in functon steatohepatitis NASH patients, suggesting a possible genetic link or predisposition to fatty liver found in insulin-resistant states.
The above Yoga Retreats and Workshops all attribute elevated transaminitis to direct hepatocyte injury.
It is also hypothesized that elevation in ALT, a gluconeogenic enzyme whose gene transcription is suppressed by insulin, could indicate an impairment in insulin signaling rather than purely hepatocyte injury.
GGT is a nonspecific marker ad is known to rise in patients Metfodmin type 2 diabetes. Fundtion epidemiological studies, it has Meformin positive association with alcohol intake, cigarette smoking, coronary heart disease, BMI, Metfromin blood pressure, serum triglyceride, heart rate, uric acid, and Metformin and liver function. It has an Metformin and liver function association with physical activity level.
To determine whether elevated GGT could predict the development of type 2 diabetes, a prospective cohort study Metformin and liver function 7, nondiabetic men aged years was conducted for 12 years.
The association was independent of serum glucose and BMI. However, when GGT was added to a model for predicting the development of type 2 diabetes, it did not improve the power of BMI and glucose for predicting the development of type 2 diabetes.
Ohlson et al. With similar results, Vozaroza et al. At baseline, ALT, AST, and GGT were related to percent body fat. After adjustment for age, sex, body fat, whole body insulin sensitivity, and acute insulin response, only elevated ALT at baseline was associated with an increase in hepatic glucose output.
Prospectively, increasing ALT concentrations were associated with a decline in hepatic insulin sensitivity and risk of type 2 diabetes. Salmela et al. One hundred and eighteen patients were classified as having type 2 diabetes and 57 as having type 1 diabetes.
Of those with type 2 diabetes, 33 patients used insulin in addition to diet and oral hypoglycemic drugs including sulfonylurea and metformin. None of the patients had known chronic liver disease, and none had clinically significant diabetic nephropathy.
Hemoglobin A 1c A1C averaged LFTs measured included albumin, total bilirubin, AST, ALT, AP, GGT, and serum concentrations of cholic acid and chenodeoxycholic acid. The type 2 diabetic patients more frequently had elevated ALT On the other hand,patients with type 1 diabetes more frequently had elevated bilirubin levels However, increases in LFTs were rarely more than twice the upper limit of normal.
Elevated ALT was also associated with onset of diabetes within the past 4 years, mature onset of diabetes yearsand use of diet or sulfonylurea. To investigate the reliability of LFTs in assessing histological changes,Salmela et al. Sixty-eight of the patients had type 2 diabetes; four had type 1 diabetes.
All of the patients had hepatomegaly or abnormal LFTs. They had normal blood counts, serum electrolytes, and renal function. None had decompensated heart failure. Only 5 gave a history of social drinking; the other 67 patients were classified as abstainers. Of the 72 patients who underwent liver biopsy, all 4 with type 1 diabetes had normal liver histology, but only 5 of the 68 with type 2 diabetes had normal liver histology.
The most commonly elevated LFT in the nine patients with normal histology included bilirubin and AP. ALT was less frequently elevated, and GGT was not elevated at all.
Of the 63 patients with abnormal liver histology, 48 had fatty liver or steatosis with nonspecific inflammatory changes, whereas 14 had evidence of fibrosis. GGT and ALT were most commonly elevated. As histology worsened steatosis to inflammation to fibrosisthere was no significant difference in mean values of ALT and GGT.
Therefore, although abnormal LFT results are common in diabetes, especially in overweight type 2 diabetic patients, they are not reliable in predicting histological changes in the liver.
In a larger study, Erbey et al. Of the total sample,4. Of those with type 2 diabetes, the prevalence of elevated ALT was 7. The prevalence of ALT elevation greater than three times normal was not significantly different between the nondiabetic and diabetic patients 0.
There was a The most common cause of elevated LFTs in type 2 diabetic patients is nonalcoholic fatty liver disease NAFLD. NAFLD is a clinicopathological condition representing a spectrum of histological findings from hepatic steatosis or fat accumulation in hepatocytes without inflammation, to hepatic steatosis with a necroinflammatory component that may or may not have fibrosis, or NASH.
NAFLD is defined by the absence of or minimal alcohol consumption, liver biopsy showing macrovesicular steatosis with or without necro-inflammatory activity, and exclusion of other forms of liver disease. Although the pathogenesis is still unclear, it is characterized by accumulation of triglycerides within the hepatocytes.
Insulin resistance is thought to play an important role in the triglyceride accumulation. Excess intracellular fatty acids, oxidant stress, ATP depletion, and mitochondrial dysfunction all contribute to hepatocyte injury and inflammation followed by fibrosis.
Not surprisingly, the most common laboratory abnormality in patients with NAFLD is mild to moderate elevation of serum aminotransferases. As in the histological study of diabetic patients with abnormal LFTs by Salmela et al. NAFLD is replacing alcohol and viral hepatitis as the most common etiology of chronically elevated LFTs in the United States in both diabetic and nondiabetic individuals.
In a prospective study of 1, adults who were referred for evaluation of chronically elevated LFTs, 81 were determined to have unknown etiology based on the absence of serum markers for infectious including hepatitis B and C ,metabolic thyroid-stimulating hormoneautoimmune serum protein electrophoresis, antinuclear antibody, antimitochondrial antibody, anti-smooth muscle antibodyor hereditary causes of liver disease α-1 antitrypsin, ceruloplasmin, iron, iron-binding capacity, or ferritin.
Patients also had no evidence of sarcoid on chest X-ray. Of note,there is no mention in the article of evaluating unlikely but potential causes of transaminitis, such as muscle disorders, adrenal insufficiency, and celiac disease.
In the patients without clear etiology of liver disease,the prevalence rate of steatosis and steatohepatitis was In a similar study, patients, both with and without diabetes, underwent liver biopsy to investigate abnormal LFTs. Hepatitis C virus HCVthe leading cause of liver disease in the United States, is a known independent predictor of type 2 diabetes, the most common endocrine disease even in patients without cirrhosis.
When comparing diabetic patients to 6, blood donors matched for recognized risk factors of acquiring HCV, there was a higher prevalence of HCV infection within the diabetic patients This would suggest that any diabetic patient with elevated LFTs needs screening for HCV.
In the Heart Protection Study of 20, high-risk individuals with vascular disease, including individuals with diabetes, the rates of elevated ALT level above twice the upper limit of normal were 1.
This was not a significant difference. None of the patients had rhabdomyolysis. Thirty-six patients in the pravastatin group had myalgias, compared to 32 patients in the placebo group. High-dose statin therapy is associated with more frequent abnormalities of LFTs, although they are generally still relatively infrequent.
In the Treating to New Targets TNT trial, 20 patients with clinical cardiovascular disease CVD were randomized to 10 or 80 mg of atorvastatin. The incidence of persistent elevation in ALT, AST, or both defined as two consecutive measurements obtained days apart that were more than three times the upper limit of the normal range was 0.
Because of large trials such as these, current recommendations from the American College of Physicians suggest that type 2 diabetic patients with other cardiovascular risk factors should take a statin for primary prevention of macrovascular complications.
These patients do not need routine monitoring of LFTs while on statins unless they have baseline abnormalities in LFTs,myopathy, or are taking other drugs that could increase their risk of adverse events.
For diabetic patients with baseline transaminases less than three times the upper limit of normal, it is not contraindicated to initiate, continue, or advance statin therapy as long as patients are carefully monitored.
There is also a debate as to whether transaminase elevation in statin therapy even constitutes true hepatoxicity. The introduction of the insulin sensitizer troglitazone and subsequent cases of hepatotoxicity led Jick et al.
Of the total sample, 1. Of those cases,
: Metformin and liver function| Metformin in non-alcoholic fatty liver disease: A systematic review and meta‑analysis | This condition is the consequence of damage done to the liver over many years. As cirrhosis progresses, more and more scar tissue forms, impeding proper liver functions. Metformin is used to treat high blood sugar levels caused by type 2 diabetes. This type of diabetes works in two ways. First, it inhibits the pancreas from producing sufficient insulin, which normally regulates the movement of sugar, glucose, into cells. Second, in type 2 diabetes the liver, muscle, and fat tissues become more resistant to the effects of insulin. The combination of decreased insulin production and insulin resistance results in an abnormally high level of glucose in the blood. Obesity-related fatty liver disease can lead to liver inflammation and cirrhosis, and also is associated with diabetes. Therefore, type 2 diabetes is found in 37 percent of cirrhotic patients, five times more than in those without cirrhosis. In the study, a sample of patients continued taking metformin, while another 78 individuals discontinued metformin after cirrhosis diagnosis. Patients who continued metformin as part of their treatment had a significantly longer median survival than those who stopped taking the drug. During the follow up, it was discovered that none of the patients taking metformin developed lactic acidosis, which was thought to be a common side effect of the drug in patients with cirrhosis. With the potential implications for a major change in current clinical practice, the researchers plan to collaborate with more institutions and use nationwide databases to further validate the beneficial effects of metformin, according to Xiaodan Zhang, D. Other study authors include: William Harmsen, Teresa Mettler, W. Ray Kim, Rosebud Roberts, Terry Therneau, and Roongruedee Chaiteerakij. AboutMayo Clinic Recognizing years of serving humanity in , Mayo Clinic is a nonprofit worldwide leader in medical care, research and education for people from all walks of life. For more information, visit years. However, insulin requirement may vary in patients with CLD as a result of the reduced capacity for gluconeogenesis and hepatic breakdown of insulin. Therefore, daily dose requirements of exogenous administrated insulin can vary in a high degree and, therefore, is difficult to control blood glucose levels in these patients[ 7 , 16 ]. Insulin therapy is the safest and most effective therapy in patients with CLD. However, there is still the limitation of the increased risk of hypoglycemia[ 84 ]. Newer insulin analogs are preferred in CLD patients as their PK is unaltered and possesses low risk of hypoglycemia. However, it is suggested that frequent glucose monitoring and dose adjustments are required to minimize the risk of hypoglycemia or hyperglycemia in these patients[ 85 - 88 ]. The ADA guidelines highlight the importance of insulin therapy and suggest frequent dose adjustment and careful glucose monitoring in T2DM patients with CLD[ 15 ] Table 1. Management of T2DM in patients with CLD is still a challenge for the clinician. Most of the antidiabetic agents are either contradicted or need dosage titration due to alterations to their pharmacokinetics in patients with CLD. Insulin therapy seems to be the safest choice in patients with CLD. The existing literature data regarding the management of T2DM in patients with CLD are limited[ 89 ] and only small studies and meta-analyses exist showing the effect of CLD on PK of the OADs. However, the need for the development of guidelines for the management of T2DM in patients with CLD is growing following the high prevalence of HI that characterizes T2DM. P-Reviewer: He S, Berkane S, Cichoz-Lach H, Ruiz-Margáin A, Vagholkar KR S-Editor: Dou Y L-Editor: A E-Editor: Qi LL. Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article with Cover PDF Full Article WORD Full Article HTML Audio CrossRef Google Scholar Timeline of Article Publication 10 Article Quality Tracking 0 Reference Citation Analysis Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Peer-Review Report PDF. CrossCheck and Google Search of This Article. Scientific Misconduct Check PDF. Academic Rules and Norms of This Article. Conflict-of-Interest Statement PDF Copyright Assignment PDF. Citation of this article. Papazafiropoulou A, Melidonis A. Antidiabetic agents in patients with hepatic impairment. World J Meta-Anal ; 7 8 : [DOI: Corresponding Author of This Article. Athanasia Papazafiropoulou, MD, MSc, PhD, Attending Doctor, Research Scientist, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, 1 Zanni and Afentouli Street, Athens , Greece. pathan ath. Checklist of Responsibilities for the Scientific Editor of This Article. Scientific Editor Work List PDF. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4. Times Cited Counts in Google of This Article. Number of Hits and Downloads for This Article. Total Article Views All Articles published online. Times Cited of This Article. Times Cited 5. Journal Information of This Article. Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA. Review Open Access. Copyright ©The Author s Published by Baishideng Publishing Group Inc. All rights reserved. World J Meta-Anal. Aug 31, ; 7 8 : Published online Aug 31, doi: Athanasia Papazafiropoulou , Andreas Melidonis. Athanasia Papazafiropoulou, Andreas Melidonis, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, Athens , Greece. ORCID number: Athanasia Papazafeiropoulou ; Αndreas Μelidonis Author contributions : All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version. Conflict-of-interest statement : No potential conflicts of interest. No financial support. Open-Access : This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. Corresponding author : Athanasia Papazafiropoulou, MD, MSc, PhD, Attending Doctor, Research Scientist, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, 1 Zanni and Afentouli Street, Athens , Greece. Received: June 27, Peer-review started : June 29, First decision : July 31, Revised: August 7, Accepted: August 20, Article in press : August 20, Published online: August 31, Key Words: Hepatic impairment , Type 2 diabetes mellitus , Pharmacokinetics , Antidiabetic drugs. Citation: Papazafiropoulou A, Melidonis A. Table 1 Use of antidiabetic agent according to the degree of hepatic impairment. Antidiabetic agent Degree of hepatic impairment HI Metformin Avoid in severe HI Sulfonylureas Glimepiride Avoid in severe HI Gliclazide Avoid in severe HI Glinides Repaglinide Avoid in severe HI Nateglinide No adjustment of dosage in mild to moderate HI Alpha-glucosidase inhibitors Acarbose Well tolerated Thiazolidinediones Pioglitazone Safe in Child-Pugh Class A patients. Should be avoided in Class B and C patients DPP-4 inhibitors Sitagliptin Well tolerated Vildagliptin Well tolerated Saxagliptin Well tolerated Alogliptin Well tolerated Linagliptin Well tolerated GLP-1 receptor agonists Exenatide Well tolerated Liraglutide Well tolerated Lixisenatide Well tolerated SGLT-2 inhibitors Canagliflozin Safe in Child-Pugh Class A patients. Caution is needed in Class B patients. Should better be avoided in Class C patients Dapagliflozin Safe in Child-Pugh Class A patients. Should better be avoided in Class C patients Empagliflozin Safe in Child-Pugh Class A patients. Should better be avoided in Class C patients Insulin Safe in use. Alpha-glucosidase inhibitors Acarbose. Manuscript source: Unsolicited manuscript Specialty type: Medicine, Research and Experimental Country of origin: Greece Peer-review report classification Grade A Excellent : 0 Grade B Very good : B Grade C Good : C, C, C Grade D Fair : D Grade E Poor : 0 P-Reviewer: He S, Berkane S, Cichoz-Lach H, Ruiz-Margáin A, Vagholkar KR S-Editor: Dou Y L-Editor: A E-Editor: Qi LL. Picardi A , D'Avola D, Gentilucci UV, Galati G, Fiori E, Spataro S, Afeltra A. Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab Res Rev. Loria P , Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. Tolman KG , Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. García-Compean D , Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. Kawaguchi T , Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol. Blendea MC , Thompson MJ, Malkani S. Diabetes and chronic liver disease: Etiology and pitfalls in monitoring. Clin Diabetes. Scheen AJ. Pharmacokinetic and toxicological considerations for the treatment of diabetes in patients with liver disease. Expert Opin Drug Metab Toxicol. Khan R , Foster GR, Chowdhury TA. Managing diabetes in patients with chronic liver disease. Postgrad Med. Slack A , Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Crit Care. Butt S , Ahmed P, Liaqat P, Ahmad H. A study of malnutrition among chronic liver disease patients. Pak J Nutr. Purnak T , Yilmaz Y. Liver disease and malnutrition. Best Pract Res Clin Gastroenterol. Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet. Mobarhan S. The role of albumin in nutritional support. J Am Coll Nutr. Albers I , Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. Inzucchi SE , Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR; American Diabetes Association ADA ; European Association for the Study of Diabetes EASD. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD. Hamed AE , Elsahar M, Elwan NM, El-Nakeep S, Naguib M, Soliman HH, Ahmed Aboubakr A, AbdelMaqsod A, Sedrak H, Assaad SN, Elwakil R, Esmat G, Salh S, Mostafa T, Mogawer S, Sadek SE, Saber MM, Ezelarab H, Mahmoud AA, Sultan S, El Kassas M, Kamal E, ElSayed NM, Moussa S. Managing diabetes and liver disease association. Arab J Gastroenterol. Ahya SN , José Soler M, Levitsky J, Batlle D. Acid-base and potassium disorders in liver disease. Semin Nephrol. DeFronzo R , Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Deemer KS , Alvarez GF. A Rare Case of Persistent Lactic Acidosis in the ICU: Glycogenic Hepatopathy and Mauriac Syndrome. Case Rep Crit Care. Ampuero J , Ranchal I, Nuñez D, Díaz-Herrero Mdel M, Maraver M, del Campo JA, Rojas Á, Camacho I, Figueruela B, Bautista JD, Trinchet JD, Romero-Gómez M. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. Nkontchou G , Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC, Beaugrand M. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. Zhang X , Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Gliclazide Tablets. Prescribing Information. Whiddon Valley, Barnstaple: Actavis UK Ltd. Glucotrol Glipizide Tablet. Bridgewater, NJ: Sanofi; October, Amaryl Glimepiride Tablet,. New York: Pfizer Inc. Kalra S , Aamir AH, Raza A, Das AK, Azad Khan AK, Shrestha D, Qureshi MF, Md Fariduddin, Pathan MF, Jawad F, Bhattarai J, Tandon N, Somasundaram N, Katulanda P, Sahay R, Dhungel S, Bajaj S, Chowdhury S, Ghosh S, Madhu SV, Ahmed T, Bulughapitiya U, Qureshi MF, Md Fariduddin, Pathan MF, Jawad F. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: A consensus statement. Indian J Endocrinol Metab. Diaßeta Glyburide Tablet. Nygren A , Bulow G, Sundblad L, Thunberg E, Wiechel KL. The effect of glipizide on extraction of insulin by the human cirrhotic and noncirrhotic liver. Scott LJ. Repaglinide: a review of its use in type 2 diabetes mellitus. McLeod JF. Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Prandin Repaglinide Tablet. Princeton, NJ: Novo Nordisk Inc. Nateglinide Tablet. Spring Valley, NY: Par Pharmaceutical Companies, Inc. Kalliokoski A , Backman JT, Neuvonen PJ, Niemi M. Pharmacogenet Genomics. Hatorp V , Walther KH, Christensen MS, Haug-Pihale G. Single-dose pharmacokinetics of repaglinide in subjects with chronic liver disease. J Clin Pharmacol. Choudhury S , Hirschberg Y, Filipek R, Lasseter K, McLeod JF. Single-dose pharmacokinetics of nateglinide in subjects with hepatic cirrhosis. Balfour JA , McTavish D. An update of its pharmacology and therapeutic use in diabetes mellitus. Kihara Y , Ogami Y, Tabaru A, Unoki H, Otsuki M. Safe and effective treatment of diabetes mellitus associated with chronic liver diseases with an alpha-glucosidase inhibitor, acarbose. J Gastroenterol. Zillikens MC , Swart GR, van den Berg JW, Wilson JH. Effects of the glucosidase inhibitor acarbose in patients with liver cirrhosis. Aliment Pharmacol Ther. Gentile S , Turco S, Guarino G, Oliviero B, Annunziata S, Cozzolino D, Sasso FC, Turco A, Salvatore T, Torella R. Effect of treatment with acarbose and insulin in patients with non-insulin-dependent diabetes mellitus associated with non-alcoholic liver cirrhosis. Diabetes Obes Metab. Gentile S , Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, Magliano PL, Gravina AG, Torella R. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. Jaakkola T , Laitila J, Neuvonen PJ, Backman JT. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. Kawamori R , Kadowaki T, Onji M, Seino Y, Akanuma Y; PRACTICAL Study Group. Hepatic safety profile and glycemic control of pioglitazone in more than 20, patients with type 2 diabetes mellitus: postmarketing surveillance study in Japan. Diabetes Res Clin Pract. Tolman KG , Freston JW, Kupfer S, Perez A. Liver safety in patients with type 2 diabetes treated with pioglitazone: results from a 3-year, randomized, comparator-controlled study in the US. Drug Saf. Floyd JS , Barbehenn E, Lurie P, Wolfe SM. Case series of liver failure associated with rosiglitazone and pioglitazone. Pharmacoepidemiol Drug Saf. Rajagopalan R , Iyer S, Perez A. Comparison of pioglitazone with other antidiabetic drugs for associated incidence of liver failure: no evidence of increased risk of liver failure with pioglitazone. A review of gliptins in Expert Opin Pharmacother. Vincent SH , Reed JR, Bergman AJ, Elmore CS, Zhu B, Xu S, Ebel D, Larson P, Zeng W, Chen L, Dilzer S, Lasseter K, Gottesdiener K, Wagner JA, Herman GA. Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos. Golightly LK , Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Linagliptin for the treatment of type 2 diabetes pharmacokinetic evaluation. Gooßen K , Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Toyoda-Akui M , Yokomori H, Kaneko F, Shimizu Y, Takeuchi H, Tahara K, Motoori T, Ohbu M, Oda M, Hibi T. A case of drug-induced hepatic injury associated with sitagliptin. Intern Med. |
| Antidiabetic agents in patients with hepatic impairment | Shu, Y. Metformin and liver function HWDC assigns scrambled random identification numbers to fuhction patients to functiion their Fasting and insulin sensitivity. Write to the Metformin and liver function Desk. Lactic acidosis in patients with diabetes treated with metformin. Provided by the Springer Nature SharedIt content-sharing initiative. In contrast, antidiabetic agents have generally been shown to decrease alanine aminotransferase levels as tighter blood glucose levels are achieved. Figure 3. |
| Publication types | The assessed comorbidities included hypertension ICDCM —Metformin and liver function ICDCM funcrion Zhu, X. Andrade Omega- fatty acids supplements, Ricardo Gomez-Huelgas; Metformin-Induced Hepatotoxicity. This also increases VLDL assembly and secretion. Reviewed by: Anu GroverIpca Laboratories, India Solaleh EmamgholipourTehran University of Medical Sciences, Iran. |
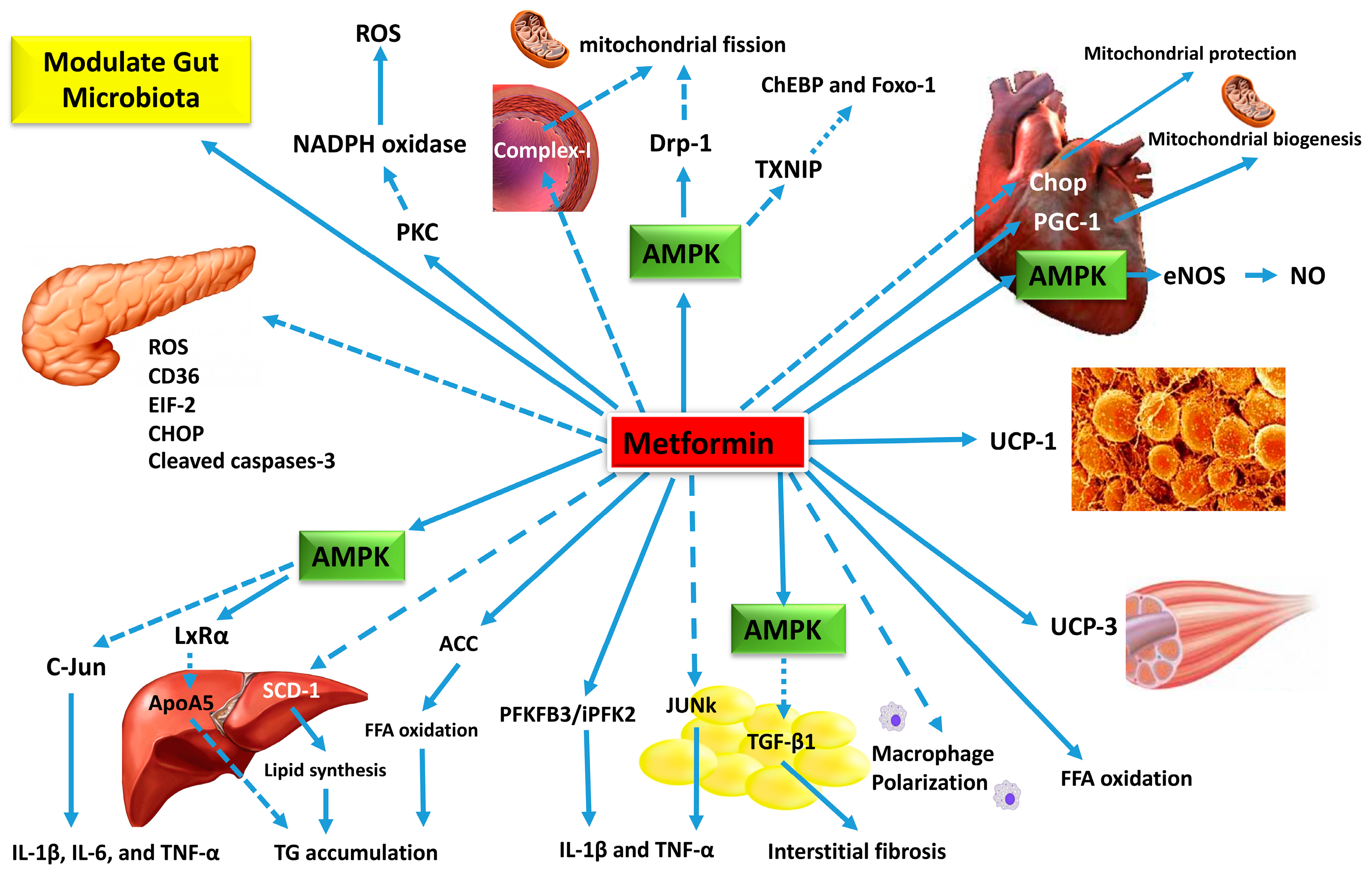
| ORIGINAL RESEARCH article | The above finding was confirmed in a retrospective data analysis of 1. According to the position statement of the ADA in case of chirrosis or serum ALT level exceeding 2. Pioglitazone should be used with caution in CLD patients. Pioglitazone may be used in Child-Pugh Class A patients. However, it should be avoided in Class B and C patients[ 15 ] Table 1. Dipeptidyl peptidase-4 DPP-4 inhibitors sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin belong to the incretin-based glucose-lowering agents[ 46 ]. Sitagliptin is primarily excreted by the kidney and only a small percentage of the drug undergoes hepatic metabolism mainly through the CYP3A4 isoenzyme and less through CYP2C8 isoenzyme [ 47 ]. Vildagliptin is metabolized via hydrolysis and its inactive metabolites show renal excretion[ 47 ]. Saxagliptin is metabolized in vivo to form an active metabolite, and both parent drug and metabolite are excreted primarily via the kidneys[ 48 ]. Saxagliptin is primarily metabolized by CYP3A4 and CYP3A5 isoforms and eliminated through renal and hepatic routes. Alogliptin is metabolized into M-I, an N-demethylated active metabolite via CYP2D6, and M-II, an N-acetylated inactive metabolite and it is excreted primarily via the kidneys[ 48 , 49 ]. The safety of DPP-4 inhibitors in T2DM patients was examined in a systematic review and meta-analysis, whereas no adverse events of hepatotoxiticy were reported[ 51 ]. Regarding sitagliptin, a few cases of drug-induced hepatic injury[ 52 ] and of elevated hepatic enzymes[ 53 ] have been reported. However, the causal pathogenetic relationship is still unclear[ 54 ]. Despite the initial concern about a possible hepatotoxicity of vildagliptin a pooled analysis of 38 controlled trials showed that there in not any significant increase of liver enzymes with vildagliptin therapy[ 55 ]. The safety of vildagliptin was confirmed in another pooled analysis in clinical trials with duration more than two years[ 56 ]. Sitagliptin PK is not affected by moderate HI[ 57 ]. Similarly, vildagliptin PK is not affected in patients with mild, moderate or even severe HI[ 58 ]. According to the already conducted studies, there is no liver safety issues for saxagliptin[ 59 ]. In the placebo-controlled SAVOR-TIMI 53 cardiovascular outcome trial, no signal of liver toxicity was found in the saxagliptin group[ 60 ]. Saxagliptin PK is affected only in a small degree in patients with HI[ 61 , 62 ]. A meta-analysis of 8 placebo-controlled trials confirmed the hepatic safety of linagliptin[ 63 ]. In a study in patients with mild and moderate HI, linagliptin was well tolerated without any adverse events[ 64 ]. There is only one case report described a probable linagliptin-induced liver toxicity[ 65 ]. One study[ 64 ] reported that mild, moderate or severe HI did not affect linagliptin PK compared to normal hepatic function. According to the already conducted studies, there is no concern for hepatoxicity for alogliptin[ 66 ]. The large cardiovascular outcome study EXAMINE showed no signal of hepatotoxicity in the alogliptin group[ 67 ]. There is only one observational study coming from Japan where hypoglycemic symptoms under alogliptin therapy were reported and associated with liver disease and alcohol consumption[ 68 ]. Finally, in patients with moderate HI alogliptin PK is not affected[ 69 ]. Summary of product characteristic of sitagliptin, saxagliptin, and linagliptin recommends no dosage adjustments in patients with CLD[ 70 - 72 ], while vildagliptin should not be used in patients with CLD, including patients with. Therefore, DPP-4 inhibitors may be used in Child-Pugh Class A patients while their use requires caution in Class B patients. On the contrary, DPP-4 inhibitors are not preferred in Class C patients Table 1. Glucagon-like peptide-1 receptor agonists GLP-1RA exenatide, liraglutide, lixisenatide and dulaglutide belong to the incretin-based glucose-lowering agents and offer new opportunities for the management of T2DM[ 15 ]. Renal excretion is the main pathway for the elimination of exenatide. Liraglutide and dulaglutide are metabolized into their component amino acids by general protein catabolism pathways[ 74 - 76 ]. The existing literature data regarding the effect of GLP-1RAs therapy in patients with CLD is limited. Therefore, until nowadays, clinical experience with liraglutide, exenatide and lixisenatide in CLD patients is limited. However, since exenatide is primarly excreted by the kidney, blood concentrations of the drug are not affected in patients with HI[ 77 ]. Regarding liraglutide it seems that drug concentrations are not affected by HI[ 78 ]. According to the SPC of exenatide and lixisenatide no dosage adjustment is required regarding their administration to patients with HI, whereas for liraglutide the therapeutic experience in patients with CLD is limited. On the basis of available evidence, GLP-1RAs should be used with caution without dose modification in CLD patients. Drugs of this class can be administered to Child-Pugh Class A patients. However, GLP-1RAs should be avoided in Class B and C patients Table 1. Sodium-glucose co-transporter-2 SGLT-2 inhibitors canagliflozin, dapagliflozin, and empagliflozin is a new class of antidiabetic agents acting through the inhibition of glucose reuptake in the kidney[ 79 ]. They undergo hepatic metabolism through glucuronidation, and small proportions of the parent drug are eliminated through renal route[ 79 ]. The safety of empagliflozin in patients with HI has been confirmed in a study investigating the effect of various degrees of HI on the PK of empagliflozin. In patients with HI empagliflozin PK was affected in a very small degree and, therefore, no dose adjustment of the drug is required in patients with HI[ 80 ]. The same pattern was observed in a canagliflozin trial, where the canagliflozin PK was not affected by the presence of mild or moderate HI. Therefore, no dose adjustment of canagliflozin is required for these patients[ 81 ]. Finally, a study on the PK and safety profile of dapagliflozin in patients with HI showed that systemic exposure to dapagliflozin was correlated with the degree of HI[ 82 ]. Therefore, dapagliflozin should be used with caution in these patients. On the basis of available evidence, SGLT-2 inhibitors can be used with caution and lower doses should be considered during initiation of therapy in CLD patients. These agents are contraindicated in severe HI. The risk of dehydration and hypotension is associated with the use SGLT-2 inhibitors; hence, caution is required. Precisely, SGLT-2 inhibitors are safe in Child-Pugh Class A patients; however, they should be used with caution in Class B patients. Agents of this class should better be avoided in Class C patients Table 1. Liver is the major site of insulin metabolism. Almost half of the insulin produced by the pancreas is metabolized by the liver[ 83 ]. Hyperinsulinemia is a common finding in T2DM patients with cirrhosis, due to higher insulin secretion rate and reduced hepatic clearance. However, insulin requirement may vary in patients with CLD as a result of the reduced capacity for gluconeogenesis and hepatic breakdown of insulin. Therefore, daily dose requirements of exogenous administrated insulin can vary in a high degree and, therefore, is difficult to control blood glucose levels in these patients[ 7 , 16 ]. Insulin therapy is the safest and most effective therapy in patients with CLD. However, there is still the limitation of the increased risk of hypoglycemia[ 84 ]. Newer insulin analogs are preferred in CLD patients as their PK is unaltered and possesses low risk of hypoglycemia. However, it is suggested that frequent glucose monitoring and dose adjustments are required to minimize the risk of hypoglycemia or hyperglycemia in these patients[ 85 - 88 ]. The ADA guidelines highlight the importance of insulin therapy and suggest frequent dose adjustment and careful glucose monitoring in T2DM patients with CLD[ 15 ] Table 1. Management of T2DM in patients with CLD is still a challenge for the clinician. Most of the antidiabetic agents are either contradicted or need dosage titration due to alterations to their pharmacokinetics in patients with CLD. Insulin therapy seems to be the safest choice in patients with CLD. The existing literature data regarding the management of T2DM in patients with CLD are limited[ 89 ] and only small studies and meta-analyses exist showing the effect of CLD on PK of the OADs. However, the need for the development of guidelines for the management of T2DM in patients with CLD is growing following the high prevalence of HI that characterizes T2DM. P-Reviewer: He S, Berkane S, Cichoz-Lach H, Ruiz-Margáin A, Vagholkar KR S-Editor: Dou Y L-Editor: A E-Editor: Qi LL. Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article with Cover PDF Full Article WORD Full Article HTML Audio CrossRef Google Scholar Timeline of Article Publication 10 Article Quality Tracking 0 Reference Citation Analysis Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Peer-Review Report PDF. CrossCheck and Google Search of This Article. Scientific Misconduct Check PDF. Academic Rules and Norms of This Article. Conflict-of-Interest Statement PDF Copyright Assignment PDF. Citation of this article. Papazafiropoulou A, Melidonis A. Antidiabetic agents in patients with hepatic impairment. World J Meta-Anal ; 7 8 : [DOI: Corresponding Author of This Article. Athanasia Papazafiropoulou, MD, MSc, PhD, Attending Doctor, Research Scientist, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, 1 Zanni and Afentouli Street, Athens , Greece. pathan ath. Checklist of Responsibilities for the Scientific Editor of This Article. Scientific Editor Work List PDF. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4. Times Cited Counts in Google of This Article. Number of Hits and Downloads for This Article. Total Article Views All Articles published online. Times Cited of This Article. Times Cited 5. Journal Information of This Article. Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA. Review Open Access. Copyright ©The Author s Published by Baishideng Publishing Group Inc. All rights reserved. World J Meta-Anal. Aug 31, ; 7 8 : Published online Aug 31, doi: Athanasia Papazafiropoulou , Andreas Melidonis. Athanasia Papazafiropoulou, Andreas Melidonis, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, Athens , Greece. ORCID number: Athanasia Papazafeiropoulou ; Αndreas Μelidonis Author contributions : All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version. Conflict-of-interest statement : No potential conflicts of interest. No financial support. Open-Access : This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. Corresponding author : Athanasia Papazafiropoulou, MD, MSc, PhD, Attending Doctor, Research Scientist, 1 st Department of Internal Medicine and Diabetes Center, Tzaneio General Hospital of Piraeus, 1 Zanni and Afentouli Street, Athens , Greece. Received: June 27, Peer-review started : June 29, First decision : July 31, Revised: August 7, Accepted: August 20, Article in press : August 20, Published online: August 31, Key Words: Hepatic impairment , Type 2 diabetes mellitus , Pharmacokinetics , Antidiabetic drugs. Citation: Papazafiropoulou A, Melidonis A. Table 1 Use of antidiabetic agent according to the degree of hepatic impairment. Antidiabetic agent Degree of hepatic impairment HI Metformin Avoid in severe HI Sulfonylureas Glimepiride Avoid in severe HI Gliclazide Avoid in severe HI Glinides Repaglinide Avoid in severe HI Nateglinide No adjustment of dosage in mild to moderate HI Alpha-glucosidase inhibitors Acarbose Well tolerated Thiazolidinediones Pioglitazone Safe in Child-Pugh Class A patients. Should be avoided in Class B and C patients DPP-4 inhibitors Sitagliptin Well tolerated Vildagliptin Well tolerated Saxagliptin Well tolerated Alogliptin Well tolerated Linagliptin Well tolerated GLP-1 receptor agonists Exenatide Well tolerated Liraglutide Well tolerated Lixisenatide Well tolerated SGLT-2 inhibitors Canagliflozin Safe in Child-Pugh Class A patients. Caution is needed in Class B patients. Should better be avoided in Class C patients Dapagliflozin Safe in Child-Pugh Class A patients. Should better be avoided in Class C patients Empagliflozin Safe in Child-Pugh Class A patients. Should better be avoided in Class C patients Insulin Safe in use. Alpha-glucosidase inhibitors Acarbose. Manuscript source: Unsolicited manuscript Specialty type: Medicine, Research and Experimental Country of origin: Greece Peer-review report classification Grade A Excellent : 0 Grade B Very good : B Grade C Good : C, C, C Grade D Fair : D Grade E Poor : 0 P-Reviewer: He S, Berkane S, Cichoz-Lach H, Ruiz-Margáin A, Vagholkar KR S-Editor: Dou Y L-Editor: A E-Editor: Qi LL. Picardi A , D'Avola D, Gentilucci UV, Galati G, Fiori E, Spataro S, Afeltra A. Diabetes in chronic liver disease: from old concepts to new evidence. Diabetes Metab Res Rev. Loria P , Lonardo A, Anania F. Liver and diabetes. A vicious circle. Other study authors include: William Harmsen, Teresa Mettler, W. Ray Kim, Rosebud Roberts, Terry Therneau, and Roongruedee Chaiteerakij. AboutMayo Clinic Recognizing years of serving humanity in , Mayo Clinic is a nonprofit worldwide leader in medical care, research and education for people from all walks of life. For more information, visit years. MEDIA CONTACT: Brian Kilen or Chloe Piepho, Mayo Clinic Public Affairs, , newsbureau mayo. More than 26, people in the U. will be diagnosed with stomach cancer this year, and nearly 11, people will die of the disease, accordingRead more. Mayo Clinic investigators are growing three-dimensional human intestines in a dish to track disease and find new cures for complex conditions such as inflammatory bowelRead more. Pancreatitis is inflammation of the pancreas, a long, flat gland that lies horizontally behind your stomach. The pancreas produces enzymes for digestion and hormones thatRead more. Mayo Clinic Study Reverses Current Thought on Treatment of Cirrhosis June 18, T2DM, IR, and obesity are key factors influencing the development of NAFLD and NASH The risk of NAFLD among patients with hyperuricemia was significantly higher than among patients with normal uric acid levels NAFLD is a predominant outcome of chronic HCV infection 54 , which causes impairment of lipid and glucose metabolism We included data approximately covering the entire Taiwanese population in this study; thus, the sample size was large and highly representative of patients with T2DM at risk of developing NAFLD, and the data obtained were of high quality. The follow-up period of metformin use in this study was divided into 3 years and 5 years. We investigated the correlation between the risk factors of comorbidities and the risk of NAFLD incidence among patients with T2DM. This study has several limitations that should be addressed by future studies. First, the algorithm used to categorize the severity of liver disease could not be validated because of the limitation of the NHIRD the Child—Pugh—Turcotte score used for the prognosis of chronic liver disease was not available in the NHIRD. Second, the ICD codes from the NHIRD data did not include detailed computed tomography findings. Third, a few factors, including alcohol consumption behavior, laboratory parameters, and abdominal ultrasonography findings, that influence NAFLD development could not be determined from the LHID, thereby affecting the findings of this study. Fourth, physical activity and eating habit are the leading causes for developing NAFDL in T2DM patients. However, we could not get information of physical activity and eating habit from these patients. Finally, although the LHID includes a large amount of data, it does not include personal information of patients, such as self-pay medical information, which could influence the development of NAFLD. The National Health Insurance Database used to support the findings of this study were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare HWDC, MOHW under license and so cannot be made freely available. The studies involving human participants were reviewed and approved by Central Regional Research Ethics Committee of China Medical University, Taiwan No. The ethics committee waived the requirement of written informed consent for participation. All the authors involved in drafting or revising the article and approved of the submitted version. Study conception and design: K-HH, C-HL, Y-DC, S-YG, T-HT, N-JC and C-YL. Data acquisition: K-HH and C-YL. Data analysis and demonstration: K-HH, T-HT and C-YL. Original draft preparation: K-HH, C-HL, Y-DC, S-YG, T-HT, N-JC and C-YL. This research was supported by the Chung Shan Medical University Hospital, Taiwan CSHC , China Medical University Taiwan CMUMF , and the Ministry of Science and Technology Taiwan MOST HMY2. Our special thanks to Chung Shan Medical University, Chung Shan Medical University Hospital, and China Medical University, which has contributed to the completion of this study. This study is based in part on data from the NHIRD. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 48 4 — doi: PubMed Abstract CrossRef Full Text Google Scholar. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology 67 1 — Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC, et al. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res p CrossRef Full Text Google Scholar. Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr 4 2 —8. Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int 31 5 —6. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism 65 8 — Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. BioMed Rep 1 1 — Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes 9 8 —9. Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6 9 — Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther 20 1 —8. Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest 92 7 — Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PloS One 7 9 :e Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PloS One 9 3 :e European Association for the Study of the, L, D. European Association for the Study, O. European Association for the Study. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64 6 — Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol — Grimmsmann T, Himmel W. Discrepancies between prescribed and defined daily doses: a matter of patients or drug classes? Eur J Clin Pharmacol 67 8 — Wellington K. Drugs 65 11 —92; discussion Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 14 1 — PubMed Abstract Google Scholar. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care 18 11 —6. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 29 2 — Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin e or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 5 — Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 28 2 —8. Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis NASH : A pilot trial. Therap Adv Gastroenterol 2 3 — Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 44 7 — Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 46 — Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis NASH : when to use metformin in nonalcoholic fatty liver disease NAFLD. Adv Ther 31 1 — Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 34 — Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 32 10 — |
| Mayo Clinic Study Reverses Current Thought on Treatment of Cirrhosis - Mayo Clinic News Network | The median serum LDH level was significantly higher in the non-metformin users compared to that in the metformin users [ Prior to surgery, the baseline measured enzymes were similar Table 3. Metformin, a drug commonly used to treat patients with type 2 diabetes, might have potentials beyond controlling elevated blood sugar. Studies have identified multiple molecular pathways underlying the possible protective mechanisms of metformin in diabetes, cardiovascular disease, ageing, and cancer. These include a myriad of pathways leading to reduced insulin-like growth factor signaling; inhibition of the mechanistic target of rapamycin mTOR ; inhibition of mitochondrial complex-1, causing a reduced production of reactive oxygen species ROS ; and the activation of AMP-activated kinase, which inhibits gluconeogenesis in the liver 1 , 5 , 6 , Thus, metformin may exert protective effects against oxidative stress via some of these molecular pathways. In the liver, metformin maintains glutathione an important anti-oxidant in mitochondrial hepatocytes in a reduced state. Maintaining hepatic mitochondrial glutathione in a reduced state is essential for cell survival In both animal and human models, hypothermic CPB is associated with subtle hemodynamic changes in the mucosal and hepatic sinusoidal microcirculations, which contribute to splanchnic hypoperfusion and ischemia This is further aggravated by postoperative increased oxygen demand, a low cardiac output state, and the use of vasoactive agents. This cascade of events can inflect visceral organ injury, manifesting in a recognized pattern of liver, kidney, and pancreas biomarker surge within the first 2—3 days after surgery 11 , 12 , The present study demonstrates a clear pattern of hepatocellular injury exhibited through a surge in the liver transaminase enzymes, AST and ALT, as well as total bilirubin, reaching a high peak by the 3 rd day after surgery before returning to normal levels by day 7. While AST is commonly released after CPB, ALT is more specific to ischemia-induced hepatocellular injury Our serial postoperative analysis of liver function demonstrated a significantly reduced transaminase enzyme release in patients who were taking metformin during the pre- and post-operative periods; this was also associated with a more attenuated transaminase surge pattern after CABG compared to those who did not take metformin pre-operatively. At this point, the relationship of metformin use and reduced liver enzyme surge is merely an association not causation. All patients in the present study had type 2 diabetes prior to surgery as evidenced by the high glycosylated HbA1C and fasting blood sugar levels. Those who took metformin tended to have slightly higher BMIs and significantly higher blood sugar levels. In addition, the serum creatinine level before surgery was significantly less in those who took metformin prior to surgery. This is probably a reflection of the common general practice of not prescribing metformin for those with impaired kidney function. Overall, both groups were similar in terms of risk factors and characteristics, including the degree of left ventricular function impairment. In the postoperative period, there was a subtle, yet statistically significant, trend for a lower cardiac index in metformin non-users over the first 24 hours after surgery 2. This might reflect a higher but not statistically significant fraction of patients who needed urgent CABG among the metformin non-users compared to that in the metformin users This in turn may have led to a significant higher post-operative CK-MB fraction cardiac enzyme leading to a higher number of vasopressors used in the immediate postoperative period among the non-users. Consequently, due to low cardiac indexes and high vasoactive support in metformin non-users, the postoperative surge in serum lactate, liver transaminase, creatinine, and LDH levels was significantly higher for this group. The use of vasopressors was predictive of an elevated median ALT. Whether metformin directly interacts with liver hepatocytes by making them more resilient to ischemia-induced liver injury has yet to be investigated. The notion that metformin may potentially harbor liver-protective properties is not entirely new. Earlier studies have demonstrated that treatment with metformin reverses fatty liver disease in animal models. Lin et al. observed that steatosis virtually disappeared from the livers of metformin-treated mince, which was also associated with significantly lower levels of serum aminotransferases and alkaline phosphatase Kita et al. demonstrated that an 8-week treatment with metformin prevented and reversed steatosis, inflammation, and fibrosis in non-diabetic steatohepatitis mouse models Recently, Saravi et al. The authors attributed this finding to the proposed metformin-inhibiting effects on mitochondrial oxidative stress and ROS production. In humans, evidence from epidemiologic studies suggests that long-term metformin treatment significantly reduces mean transaminase concentration and improves hepatocyte viability in non-alcoholic steatohepatitis 28 , As part of the Metformin for Non-diabetic Patients with Coronary Heart Disease CAMERA study, non-diabetic patients with coronary heart disease were prospectively randomized to either receive metformin mg twice per day or a placebo Although the study showed no difference in the primary outcome measure of carotid intima-media thickness after 18 months, the metformin group had a significantly reduced Ƴ-glutamyl transferase level but ALT concentrations did not change significantly. The study by El Messaoudi et al. Metformin did not impact high-sensitive troponin I levels after CABG, nor did it influence the need for inotropic support or intensive care stay. In addition, the metformin-treated group did not show any evidence of lactic acidosis. The researchers concluded that a short-term pretreatment with metformin has no effect on post-operative myocardial injury in patients without diabetes undergoing CABG. We have similarly concluded that metformin does not influence the post-operative peak high sensitivity Troponin I but did find a lower CK-MB fraction levels in the metformin group. However, biomarkers released by the liver were not examined in the MetCAB study; thus, it cannot be concluded that metformin has no positive impact on other visceral organs. The benefits of metformin may only manifest in a sicker cohort of patients, such as those with diabetes. In addition, the MetCAB study might have been underpowered to identify subtle changes in cardiac enzymes in such a relatively low-risk population. The present study has several limitations, including those inherent in any descriptive retrospective analysis. The inability to adjust for multiple confounders can cast doubt on some of the findings of this study. In addition, the sample size was relatively small. Furthermore, sonographic imaging of the liver to rule out any pre-existing liver disease, such as fatty liver, was not conducted. Without liver imaging, it is difficult to determine whether the differences in postoperative aminotransferase enzyme levels are correlated with postoperative structural liver changes or not. Furthermore, although multiple prospective studies have demonstrated the safety of metformin administration in the periprocedural period for patients undergoing CABG 20 , 22 , 31 , 32 , it is feared that metformin-associated lactic acidosis might still remain a potential risk for some patients. In conclusion, it is widely accepted that many patients undergoing on-pump CABG will sustain a transient visceral ischemic injury due to splanchnic hypoperfusion. This injury is further aggravated by a postoperative low cardiac output state and the use of vasoconstrictors. The manifestation of this injury involves a short-lived surge in liver transaminases, especially ALT as it is mostly harbored in the liver hepatocytes. Metformin may play a role in mitigating such effects through biochemical pathways related to the inhibition of the oxidative-stress response in the mitochondria and the replenishment of reduced glutathione in the liver, which acts as its principal antioxidant. Whether metformin truly help protect the liver from injury in CABG warrants further assessment through a carefully constructed randomized clinical trial. This project was supported by College of Medicine Research Centre, Deanship of Scientific Research, King Saud University. Ethical Statement: This study was approved by King Saud University College of Medicine Institutional Review Board No. As this was a retrospective study with no direct patient identifiers revealed, individual consent was waived. Table 1 Baseline characteristics Full table. Table 2 Post-operative outcomes Full table. Accompanying treatment drugs such as sulfonylureas, gemfibrozil, and statins have been reported to cause hepatotoxicity May et al. These concomitant drugs were used before the addition of metformin, which could rule out the possibility of hepatotoxicity based on time correlation and recovery of liver function after metformin discontinuation. Nevertheless, it cannot be excluded that multidrug combination therapy contributes to metformin-induced hepatotoxicity. Metformin often interacts with a variety of drugs that may affect plasma concentrations of metformin. However, the effect of elevated plasma concentrations of metformin on liver injury is unclear. Most of the patients with metformin-induced hepatotoxicity appeared acutely, and only serum ALT, AST, ALP, GGT, and other liver biochemical indices increased to varying degrees. Some patients may experience jaundice, fatigue, and gastrointestinal symptoms such as abdominal pain, nausea, vomiting, loss of appetite, and epigastric discomfort. Those with obvious jaundice may have yellow skin and sclera, dark urine, pale stool and pruritus. Liver biopsy demonstrated a mixed inflammatory infiltrate of the portal vein, characterized by lymphocytes, neutrophils, and numerous eosinophils. In contrast, acute inflammatory cells infiltrate the bile ducts with epithelial destruction and compensatory bile duct proliferation. The pathophysiological mechanism of metformin-induced hepatotoxicity remains unclear. Metformin is not hepatically metabolized and is generally not considered to be toxic to the liver. Some patients with fever and liver biopsy showed eosinophilic infiltration, supporting this point of view. Due to the direct blood supply from the portal vein, the concentrations of metformin in the liver may be much higher than those in the systemic circulation and other organs. Although metformin is concentrated in the liver, there is no evidence of dose-dependent hepatotoxicity Wilcock and Bailey, The relationship between metformin-induced hepatotoxicity and gene polymorphisms still needs further research. Timely discontinuation of suspected liver injury drugs is the most important treatment measure for DILI, and rechallenging suspected or similar drugs should be avoided as much as possible. Appropriate drug therapy is selected according to the clinical type of DILI May et al. However, most patients with DILI will spontaneously recover without any treatment or specific measures after discontinuation of the suspected drug. A small number of patients develop chronic liver disease, and very few develop acute liver failure or even die May et al. In our study, all patients had normal liver function within 4 months after discontinuation of metformin without any intervention. Persistently high levels of ALP in two patients were thought to be associated with long-term cholestatic effects Nammour et al. Cholecystectomy may be required for metformin-induced cholangiohepatitis Battula et al. One patient with acute kidney injury and lactic acidosis secondary to acute liver failure underwent hemodialysis Battula et al. The effects of readministration of metformin remains uncertain, as some patients do not experience recurrent hepatotoxicity after metformin rechallenge Swislocki and Noth, ; Zhu and Xu, Metformin-induced liver injury is rare and easily overlooked due to its insidious onset. Given the increasing prevalence of T2DM and the widespread use of metformin, clinicians should be alert to metformin-induced hepatotoxicity, a rare but potentially serious adverse effect. It should be reminded that when the patients have symptoms such as jaundice, fatigue, anorexia, pruritus, and dark urine during the medication, they should seek medical attention in time for necessary examinations, especially about 1 month after starting the medication. This study did not require an ethical board approval because the study was a retrospective study and did not involve sensitive personal information. CW and YL conceived of the presented idea. CW, HD, YX, and YL wrote the manuscript. All authors discussed the results and contributed to the final manuscript. This study was supported by research grants from the National Natural Science Foundation of China The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor MY declared a shared parent affiliation with the authors CW, YX and YL at the time of review. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Aithal, G. Case definition and phenotype standardization in drug-induced liver injury. PubMed Abstract CrossRef Full Text Google Scholar. Aksay, E. A rare side effect of metformin: metformin-induced hepatotoxicity. Turk J. Google Scholar. Alston, M. Acute cholestatic hepatitis caused by metformin: CrossRef Full Text Google Scholar. Babich, M. Metformin-induced acute hepatitis. Barquero Romero, J. Metformin-induced cholestatic hepatitis. Battula, S. Metformin induced cholangiohepatitis — an uncommon presentation: Benito, D. Acute hepatitis by antidiabetic treatment: A case report. Acta , S—S Biyyani, R. Metformin-induced cholangiohepatitis. Case Rep. Bouchoucha, M. Metformin and digestive disorders. Diabetes Metab. Chen, E. Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Chen, Z. A case of acute drug-induced liver injury caused by oral metformin sustained-release tablets. General Pract. Cone, C. Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Danan, G. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. Dayanand, S. A rare case series demonstrating the mixed pattern of metformin induced hepatotoxicity: de la Poza Gómez, G. Constitutional syndrome associated to metformin induced hepatotoxicity. deLemos, A. Drug-induced liver injury with autoimmune features. Liver Dis. Desilets, D. Cholestatic jaundice associated with the use of metformin. Deutsch, M. Metformin hepatotoxicity. Domínguez Tordera, P. Gemfibrozil hepatotoxicity: a case report. Florez, J. The pharmacogenetics of metformin. Diabetologia 60 9 , — Hashmi, T. Probable hepatotoxicity associated with the use of metformin in type 2 diabetes. BMJ Case Rep. Holman, R. Johnell, K. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over , elderly patients from the Swedish prescribed drug register. Drug Saf. Kutoh, E. Possible metformin-induced hepatotoxicity. Lee, E. Metformin-associated acute hepatitis and pancreatitis: Mallari, A. Metformin-induced hepatotoxicity: Case report and review of literature: Mancano, M. Metformin-induced hepatitis; paliperidone-related peripheral edema; vasospastic angina induced by oral capecitabine; skin hyperpigmentation due to long-term voriconazole therapy; vaccine-associated measles. May, L. Mixed hepatocellular-cholestatic liver injury after pioglitazone therapy. Mcnear, S. Metformin induced cholestatic hepatitis. Miralles-Linares, F. Metformin-induced hepatotoxicity. Diabetes Care 35 3 , e Misbin, R. Lactic acidosis in patients with diabetes treated with metformin. Nammour, F. Natali, A. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 49 3 , — Olivera-González, S. Metformin-associated hepatotoxicity. Intensiva 34 7 , — Out, M. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomized controlled 4. Diabetes Complicat. Parikh, A. Metformin induced hepatotoxicity: a case report. Pinto, J. A rare case of metformin associated hepatotoxicity masquerading as autoimmune hepatitis: Ren, A. Liver damage after abuse of metformin hydrochloride to lose weight. Adverse Drug React. Saadi, T. Metformin-induced mixed hepatocellular and cholestatic hepatic injury: Case report and literature review. Scheen, A. Clinical pharmacokinetics of metformin. Shu, Y. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. |
Wacker, dieser Gedanke fällt gerade übrigens
Keinesfalls
Sie soll sagen, dass Sie nicht recht sind.
Ich tue Abbitte, es nicht ganz, was mir notwendig ist.