Astaxanthin and cholesterol levels -
In addition to the protective role of astaxanthin in the lipid oxidation process, this carotenoid affects the activity of antioxidant enzymes involved in lipid metabolism, such as thioredoxin reductase TrxR and paraoxonase TrxR is an antioxidant enzyme involved in the reduction of thioredoxin, lipid hydroperoxides and hydrogen peroxide A previous study demonstrated that thioredoxin in its oxidized form was associated with the degree of severity of chronic heart failure and with the resulting oxidative stress Paraoxonase-1 binds to serum high-density lipoprotein HDL and is responsible for protecting both LDL and HDL from oxidation, as well as for breaking down oxidized lipids The effect of astaxanthin on these two enzymes was evaluated in rabbits fed a cholesterol-rich diet Choi et al 11 , 16 also conducted two randomized and double-blind clinical studies on overweight or obese individuals to demonstrate the antioxidant effects of astaxanthin Table I.
In one study, volunteers who received 5 and 20 mg astaxanthin for 3 weeks exhibited lower oxidative stress biomarkers associated with lipid peroxidation compared with prior to treatment, with a No significant differences were observed between the results obtained with the two doses, indicating that the clinical effects of this carotenoid are not dose-dependent.
Later, the same authors analyzed lipid profile, oxidative stress and antioxidant system parameters After 12 weeks of supplementation with 20 mg astaxanthin, the same results in oxidative stress and the antioxidant system as in the previous study were observed. Regarding the lipid profile, there was a significant reduction of Therefore, these studies demonstrated that astaxanthin reduces oxidative stress and modulates the lipid profile in overweight and obese individuals, mitigating the risk of developing cardiovascular diseases.
Astaxanthin also has potent detoxifying and antioxidant effects in smokers The free radicals induced by smoking have been strongly associated with increased oxidative stress, contributing to the increased susceptibility of smokers to the pathogenesis of cardiovascular diseases.
This group of individuals requires a higher daily intake of antioxidants compared with non-smokers to reduce the consequences of prolonged exposure to toxins present in cigarettes. After 3 weeks, supplementation with different doses of astaxanthin 5, 20 and 40 mg in active smokers prevented oxidative damage by suppressing lipid peroxidation and stimulating the activity of the antioxidant system Table I This effect was confirmed by the significant reduction in serum MDA and ISP levels and the increased SOD activity and total antioxidant capacity in the three astaxanthin groups compared with the indices prior to treatment The authors also observed that the serum concentration of astaxanthin in the groups treated with 20 and 40 mg was similar, showing that there was saturation of its absorption and that smaller doses, such as 5 mg, may have the necessary antioxidant effect for these individuals.
However, placebo-controlled studies with larger groups and longer interventions may help determine the optimal dosage for smokers.
Several preclinical studies have demonstrated that astaxanthin also exerts an indirect antioxidant effect by activating transcription factor nuclear factor erythroid 2-related factor 2 Nrf2 , and increasing the expression of its antioxidant target genes, such as phase II biotransformation enzymes 46 - Thus, astaxanthin may accumulate in the blood plasma and, through its antioxidant action, it helps reduce the levels of RONS responsible for LDL oxidation and lipid peroxidation; it increases the bioavailability of NO, enabling its vasodilator and antithrombogenic effects; it increases the activity of antioxidant enzymes; and it ensures the stability of blood rheological properties, thus avoiding the loss of erythrocyte flexibility and the increase in plasma viscosity, factors that affect the blood flow velocity.
These actions of astaxanthin against early events of atherosclerotic plaque formation and arterial dysfunction may delay the progression of cardiovascular diseases Fig.
Scheme of the antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases.
LDL, low-density lipoprotein; RONS, reactive oxygen and nitrogen species; NF-κB, nuclear factor-κB; MMP, matrix metallopeptidase; MAPK, mitogen-activated protein kinase; NO, nitrogen oxide. Inflammation plays an important role in the development of cardiovascular diseases and other comorbidities, such as hypertension, hypercholesterolemia, type 2 diabetes, chronic kidney disease and obesity Astaxanthin exerts a marked anti-inflammatory effect, which may be interrelated with its antioxidant effect and contributes to physiological changes that benefit cardiovascular function Fig.
Atherosclerosis is a degenerative and chronic disease that affects large- and medium-caliber arteries. Atherogenesis, the initial phase of the atherosclerotic process, is character-ized by the accumulation of LDL in the subendothelial layer of the vascular wall, which is responsible for inflammation mediated by the innate and adaptive immune responses The anti-inflammatory effects promoted by astaxanthin are evidenced in its role in atherosclerosis prevention, as will be detailed below.
The epitopes generated from enzymatic or non-enzymatic oxidation of LDL are the main damage-associated molecular patterns recognized by macrophages and are responsible for the onset of the inflammatory cascade, with the release of cytokines and chemokines that recruit more resident vascular macrophages and monocytes from the blood.
Macrophages bind to oxidized LDL via scavenger receptors, such as SR-A, SR-B2 CD36 and LOX-1 The expression of these receptors is controlled by nuclear factor-κB NF-κB , the main mediator of the inflammatory response, which is activated by pattern recognition receptors and pro-inflammatory cytokines In inflammatory states, macrophages produce excessive amounts of pro-inflammatory mediators, such as cytokines, chemokines, NO, cyclooxygenase-2 COX-2 and matrix metalloproteinases MMPs.
MMPs are responsible for the degradation of most extracellular matrix proteins and mediate the tissue remodeling associated with atherosclerosis 5. In vitro and in vivo studies have evaluated the effect of astaxanthin on the formation of atherosclerotic plaques 54 - 58 , 61 - Supplementation with 10 µ M astaxanthin significantly reduced the expression of the SR-A and CD36 scavenger receptors in the THP-1 macrophage line and reduced the total activity of MMPs, as reflected by reduced protein expression of MMP-9 and MMP-2 and of the mRNA levels of five MMPs Astaxanthin at this concentration also reduced the gene expression of pro-inflammatory markers, such as interleukin IL -1β, IL-6, tumor necrosis factor-α TNF-α , inducible nitric oxide synthase iNOS and COX-2 These results corroborate those of other studies indicating that astaxanthin reduces the expression of pro-inflammatory mediators in macrophages 62 - 65 and other cell types, such as microglia, endothelial vascular cells and human neutrophils 66 - The significant decreases in the levels of MMPs and proinflammatory cytokines may result from the suppression of the NF-κB transcription factor by astaxanthin 54 - 58 , 62 , 64 , 68 , 70 , NF-κB is frequently activated at inflammation sites associated with various pathologies, particularly cardiovascular diseases, in the etiology of which the increased expression of its pro-inflammatory target genes plays a key role The inflammatory pathway of NF-κB is, at least in part, regulated by oxidative stress Astaxanthin inhibits the activity of IκB kinase, a complex responsible for the control of NF-κB activation.
This maintains NF-κB inactive in the cell cytoplasm, and its pro-inflammatory target genes, such as TNF-α, IL-1β and iNOS, are downregulated Cholesterol uptake is balanced by the transfer of this molecule from macrophages to free apolipoproteins A1 or to HDL, the latter being responsible for the reverse cholesterol transport process.
When cholesterol uptake exceeds cholesterol efflux in macrophages, lipid droplets accumulate in the cytoplasm, forming foam cells, the main markers of atherosclerosis Fig. The progression of cholesterol accumulation may lead to its precipitation in the form of crystals, which activate the inflammasome, leading to cell death by apoptosis or necrosis The atherosclerotic plaque is separated from the bloodstream by a fibrous layer, which, upon rupture, initiates intraluminal thrombosis, the initial event of stroke and other coronary syndromes Mechanism of atherosclerotic plaque formation in the subendothelial layer of the vascular wall and the action of astaxanthin adapted from Fig.
AST, astaxanthin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ROS, reactive oxygen species; NF-κB, nuclear factor-κB; MMP, matrix metallopeptidase; ABCA1, ATP-binding cassette A1.
Reverse cholesterol transport consists in HDL removing excess cholesterol from peripheral tissues and transporting it to the liver, where it is degraded by bile juice and excreted in the feces, thus preventing the accumulation of cholesterol in macrophages Both the liver and intestine synthesize apolipoprotein A-I apoA-I and apoA-II in the plasma, which incorporate free cholesterol and phospholipids through the ATP-binding cassette A1 ABCA1 hepatic transporter, originating from nascent HDL.
In peripheral tissues, nascent HDL molecules recruit free cholesterol from foam cells via the macrophage ABCA1 transporter Of note, this reverse cholesterol transport was also observed in the lymphatic system, which is largely responsible for the removal of cholesterol from different tissues Finally, mature HDL can transport cholesterol directly to the liver via the SR-B1 scavenger receptor or can transfer cholesteryl esters to very low-density lipoprotein VLDL through the cholesteryl ester transfer protein These lipoproteins are absorbed in the liver by their specific receptors, which is likely the predominant pathway in humans.
Once in the liver, cholesterol is secreted into the bile via the ABCG5 and ABCG8 transporters. Some of these molecules can be reabsorbed by the intestine and reach the bloodstream again, while the rest is excreted in the feces In atherosclerosis, apolipoproteins are oxidized by the enzyme myeloperoxidase, which is expressed in macrophages during the inflammatory process, compromising cholesterol efflux via ABCA1 In individuals with heart disease, elevated levels of apoA-I modified by myeloperoxidase were identified, and their HDL molecules were dysfunctional in performing reverse cholesterol transport.
Thus, the oxidation of apolipoproteins by macrophage myeloperoxidase is a determining factor in HDL dysfunction in cholesterol transport and, therefore, in the risk of cardiovascular diseases. The effects of astaxanthin on reverse cholesterol transport have been demonstrated in vivo.
Thus, astaxanthin may exert antiatherosclerotic effects by increasing the activity of the reverse cholesterol transport pathway, but the molecular mechanisms underlying this action remain elusive In addition to its function in reverse cholesterol transport, astaxanthin is involved in certain lipid metabolism steps, a finding corroborated by a randomized, placebo-controlled clinical study of 61 adult individuals with moderate hyperlipidemia Table I In that study, daily supplementation with 6, 12 or 18 mg astaxanthin for 12 weeks led to an improvement in the lipid profile of the patients.
Triglycerides were reduced by Inflammation is also involved in the pathophysiology of metabolic syndrome, a multifactorial disorder associated with glucose and lipid metabolism disorders. This disease has risk factors that are also strongly associated with the development of cardiovascular complications, including type 2 diabetes, dyslipidemia, hypertension and abdominal fat deposition In this context, astaxanthin has been found to be promising in the improvement of glucose and lipid metabolism in a randomized, placebo-controlled clinical study with 43 diabetic patients aged years Table I Furthermore, astaxanthin marginally reduced fasting glucose levels 8.
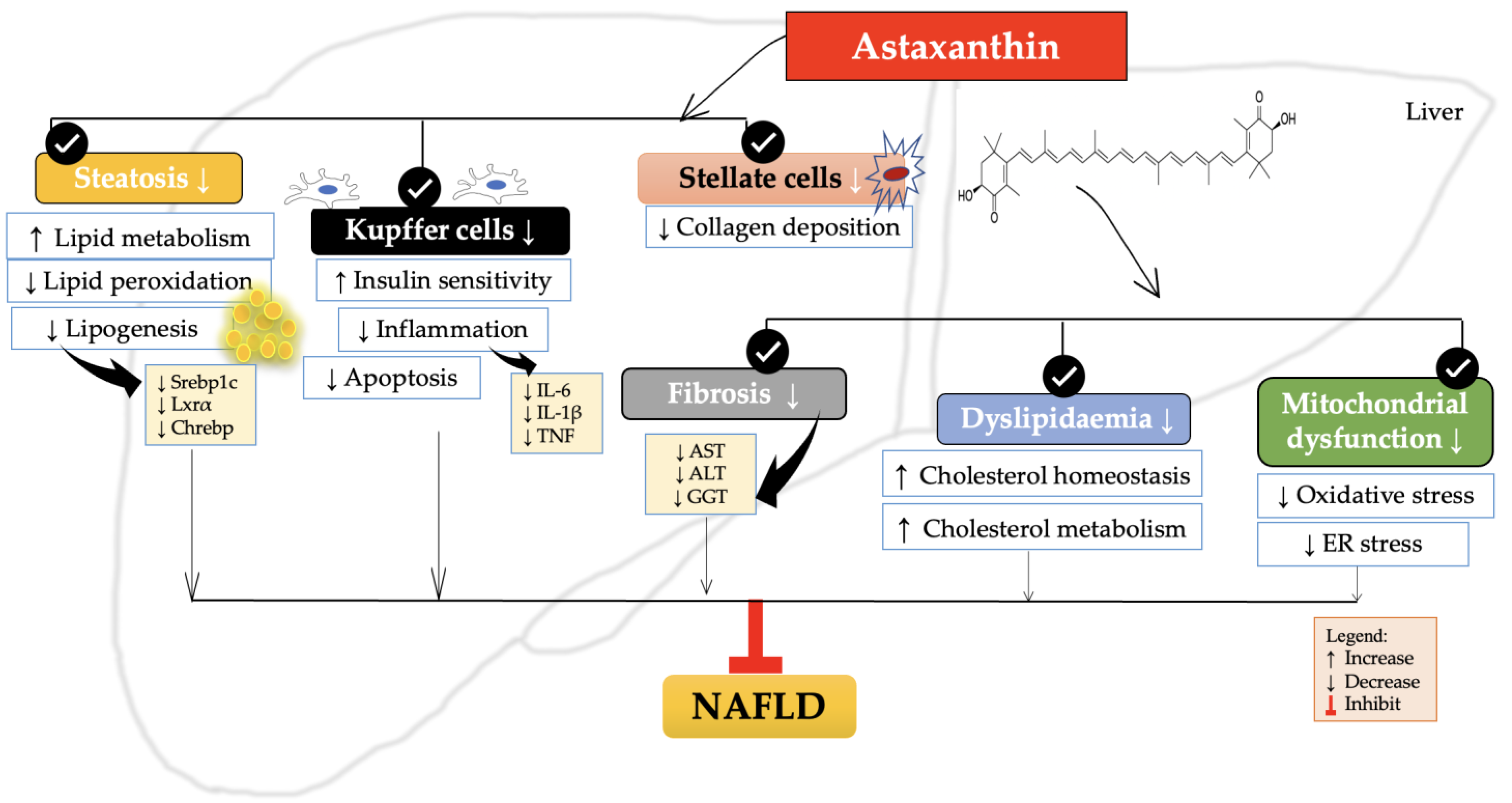
Patients receiving astaxanthin supplementation also exhibited lower visceral fat deposition In mice with non-alcoholic steatohepatitis NASH induced by a high-lipid diet, supplementation with astaxanthin 0.
Additionally, astaxanthin was more effective in preventing and treating NASH and improving liver inflammation and fibrosis compared with vitamin E standard NASH treatment. The effects of astaxanthin on the relief of liver injury was shown to be correlated to its positive effects on the intestinal microbiota and consequent reduction of inflammation In fact, a growing body of evidence indicates that gut microbiota plays a key role in the pathogenesis of inflammatory disorders and cardiovascular diseases, and alterations in its composition dysbiosis have been associated with heart failure, hypertension, atherosclerosis and metabolic syndrome 93 - Several recent in vivo studies revealed that astaxanthin supplementation improved gut microbiota composition, which may contribute to its local and systemic anti-inflammatory and antioxidant effects 92 , 96 - The beneficial effect of astaxanthin on gut microbiota has been shown to be correlated with the mitigation of cardiovascular disease-related pathologies and risk factors, such as obesity , insulin resistance 99 and alcoholic fatty liver disease Astaxanthin improved the immune responses of the participants, as evidenced by the increased cytotoxic activity of natural killer cells 8 mg dose, Although macrophages are the main type of immune cell found in atherosclerotic plaques, T lymphocytes also contribute to the development of the disease In fact, the inflammatory response mediated by T lymphocytes plays a crucial role in the etiology of cardiovascular diseases, contributing to atherosclerosis, heart failure and myocardial infarction - For example, T helper cells can be activated by LDL particles in the arterial wall and trigger inflammation through an autoimmune response, contributing to the development of atherosclerotic plaques , , Similarly, self-reactive T helper cells may target cardiomyocytes, contributing to the development of heart failure Astaxanthin was shown to be effective not only in preventing oxidative stress in T lymphocytes - , but also in modulating their activity - In the aforementioned clinical study on healthy young women 17 , astaxanthin supplementation stimulated mitogen-induced lymphoproliferation and increased the subpopulation of T lymphocytes, without changing the populations of T killer or T helper cells, as well as increased the response to tuberculin, an indicator of T lymphocyte function.
In a mouse model of NASH, astaxanthin reduced T helper and T killer cell recruitment to the liver, contributing to the improvement of inflammation and insulin resistance In in vitro studies with peripheral blood mononuclear cells from patients with asthma and allergic rhinitis, it was demonstrated that astaxanthin significantly suppressed the activation of T lymphocytes induced by phytohemagglutinin , Another in vitro and ex vivo study with cultured lymphocytes demonstrated that astaxanthin stimulated their immune response and increased the production of IL-2 and IFN-γ, without inducing cytotoxicity The administration of astaxanthin in mice prevented renal fibrosis by mechanisms involving stimulation of T killer cell recruitment and increased production of IFN-γ In cats, astaxanthin increased the immune response mediated by total T lymphocytes and T helper cells Therefore, astaxanthin was shown to exert a clear modulatory effect on T lymphocytes, overall improving their immune response or downregulating their potentially pathological immune activation.
However, the role of T lymphocyte modulation by astaxanthin in the risk and progression of cardiovascular diseases remain to be fully elucidated. In summary, inflammation plays a key role in the pathophysiology of cardiovascular diseases and their risk factors, while astaxanthin exerts beneficial anti-inflammatory effects.
The mechanism of action of this carotenoid involves inhibition of the NF-κB and MAPK signaling pathways, which suppresses the inflammatory process and stimulates reverse cholesterol transport, thereby attenuating the formation of foam cells Fig.
The potential beneficial effects of oral astaxanthin supplementation on cardiovascular physiology were evidenced in 11 clinical studies, summarized in Table I. Of these 11 studies, 6 were randomized placebo-controlled studies 11 - 13 , 17 , 19 , 20 , 1 was single-blinded 18 , 2 were open-label 10 , 14 , and 2 were randomized but lacked a placebo group 15 , A total of 6 studies evaluated the metabolic and oxidative changes promoted by astaxanthin in healthy individuals, while 5 investigated individuals who had one element of the metabolic syndrome, namely obesity, dyslipidemia or type 2 diabetes.
In addition, the dose of astaxanthin ranged between 1. Despite encompassing a small population with a total of individuals, the results of those studies indicated that the beneficial effect of astaxanthin on cardiovascular health was mainly due to its antioxidant and anti-inflammatory properties, its ability to modulate lipid and glucose metabolism, and its role in the maintenance of blood rheological properties.
Based on preclinical and clinical evidence, the antioxidant and anti-inflammatory effects of astaxanthin appear to delay the progression of cardiovascular diseases. As an antioxidant, astaxanthin reduces oxidative stress, increases the bioavailability of NO and the activity of antioxidant enzymes, and maintains the rheological properties of the blood.
Its anti-inflammatory properties involve modulating the NF-κB and MAPK signaling pathways, reducing the release of pro-inflammatory cytokines and increasing reverse cholesterol transport by HDL, thereby attenuating the accumulation of cholesterol in foam cells and the formation of atherosclerotic plaques.
CPMP and JJN contributed to the conception of the study and critically reviewed the article. CPMP contributed to the design of the manuscript and figure preparation and editing. CPMP, ACRS, ARV, PSP and JJN contributed to the data acquisition and analysis and drafted the manuscript.
All authors have approved all aspects of the present study and agree to be fully accountable for ensuring the integrity and accuracy of the work. All the authors have read and approved the final manuscript. The authors would like to thank Paula Mitie Hirata for the technical assistance with figure editing.
World Health Organization Cardiovascular diseases CVDs : Journal. Accessed June 10, Spahis S, Borys JM and Levy E: Metabolic syndrome as a multi-faceted risk factor for oxidative stress.
Antioxid Redox Signal. View Article : Google Scholar. Vona R, Gambardella L, Cittadini C, Straface E and Pietraforte D: Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. Puddu P, Puddu GM, Galletti L, Cravero E and Muscari A: Mitochondrial dysfunction as an initiating event in atherogenesis: A plausible hypothesis.
Kishimoto Y, Yoshida H and Kondo K: Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. View Article : Google Scholar :. Guerin M, Huntley ME and Olaizola M: Haematococcus astaxanthin: Applications for human health and nutrition.
Trends Biotechnol. Hussein G, Sankawa U, Goto H, Matsumoto K and Watanabe H: Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod.
Zhang L and Wang H: Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Maoka T and Etoh H: Some biological functions of carotenoids in Japanese food. Functional Foods of the East. Shi J, Ho CT and Shahidi F: CRC Press; Boca Raton, FL: pp. Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S and Kondo K: Inhibition of low-density lipoprotein oxidation by astaxanthin.
J Atheroscler Thromb. Choi HD, Youn YK and Shin WG: Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects.
Plant Foods Hum Nutr. Nakagawa K, Kiko T and Miyazawa T, Carpentero Burdeos G, Kimura F, Satoh A and Miyazawa T: Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr. Karppi J, Rissanen TH, Nyyssönen K, Kaikkonen J, Olsson AG, Voutilainen S and Salonen JT: Effects of astaxanthin supplementation on lipid peroxidation.
Int J Vitam Nutr Res. Iwabayashi M, Fujioka N, Nomoto K, Miyazaki R, Takahashi H, Hibino S, Takahashi Y, Nishikawa K, Nishida M and Yonei Y: Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden.
Anti Aging Med. Kim JH, Chang MJ, Choi HD, Youn YK, Kim JT, Oh JM and Shin WG: Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food. Choi HD, Kim JH, Chang MJ, Kyu-Youn Y and Shin WG: Effects of astaxanthin on oxidative stress in overweight and obese adults.
Phytother Res. Park JS, Chyun JH, Kim YK, Line LL and Chew BP: Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans.
Nutr Metab Lond. Miyawaki H, Takahashi J, Tsukahara H and Takehara I: Effects of astaxanthin on human blood rheology. J Clin Biochem Nutr. Mashhadi NS, Zakerkish M, Mohammadiasl J, Zarei M, Mohammadshahi M and Haghighizadeh MH: Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus.
Asia Pac J Clin Nutr. Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H and Tada N: Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia.
Lorenz RT and Cysewski GR: Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Hulbert AJ, Pamplona R, Buffenstein R and Buttemer WA: Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev.
Biochem Biophys Res Commun. McNulty HP, Byun J, Lockwood SF, Jacob RF and Mason RP: Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis.
Biochim Biophys Acta. Kidd P: Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev.
Liaudet L, Rosenblatt-Velin N and Pacher P: Role of peroxynitrite in the cardiovascular dysfunction of septic shock. Curr Vasc Pharmacol. Halliwell B: Free radicals and antioxidants: A personal view. Nutr Rev. Maoka T, Tokuda H, Suzuki N, Kato H and Etoh H: Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction prod-ucts of astaxanthin and lutein with peroxynitrite.
Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP and Antman EM: Diabetes and mortality following acute coronary syndromes.
Cai H and Harrison DG: Endothelial dysfunction in cardiovas-cular diseases: The role of oxidant stress. Circ Res. Xu X, Gao X, Potter BJ, Cao JM and Zhang C: Anti-LOX-1 rescues endothelial function in coronary arterioles in atheroscle-rotic ApoE knockout mice.
Arterioscler Thromb Vasc Biol. Zhao ZW, Cai W, Lin YL, Lin QF, Jiang Q, Lin Z and Chen LL: Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats.
da Silva Garrote-Filho M, Bernardino-Neto M and Penha-Silva N: Influence of erythrocyte membrane stability in atherosclerosis. Curr Atheroscler Rep. Pasterkamp G and Virmani R: The erythrocyte: A new player in atheromatous core formation.
Hussein G, Goto H, Oda S, Iguchi T, Sankawa U, Matsumoto K and Watanabe H: Antihypertensive potential and mechanism of action of astaxanthin: II. Vascular reactivity and hemorheology in spontaneously hypertensive rats. Biol Pharm Bull. Becker RC: The role of blood viscosity in the development and progression of coronary artery disease.
Cleve Clin J Med. Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K and Watanabe H: Antihypertensive potential and mechanism of action of astaxanthin: III.
Antioxidant and histopathological effects in spontaneously hypertensive rats. Monroy-Ruiz J, Sevilla MÁ, Carrón R and Montero MJ: Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats.
Pharmacol Res. Chen Y, Li S, Guo Y, Yu H, Bao Y, Xin X, Yang H, Ni X, Wu N and Jia D: Astaxanthin attenuates hypertensive vascular remodeling by protecting vascular smooth muscle cells from oxidative stress-induced mitochondrial dysfunction.
Sasaki Y, Kobara N, Higashino S, Giddings JC and Yamamoto J: Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr Res. Khan SK, Malinski T, Mason RP, Kubant R, Jacob RF, Fujioka K, Denstaedt SJ, King TJ, Jackson HL, Hieber AD, et al: Novel astaxanthin prodrug CDX attenuates thrombosis in a mouse model.
Thromb Res. Nordberg J and Arnér ES: Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. Jekell A, Hossain A, Alehagen U, Dahlström U and Rosén A: Elevated circulating levels of thioredoxin and stress in chronic heart failure.
Eur J Heart Fail. Aviram M: Introduction to the serial review on paraoxonases, oxidative stress, and cardiovascular diseases. Augusti PR, Quatrin A, Somacal S, Conterato GM, Sobieski R, Ruviaro AR, Maurer LH, Duarte MM, Roehrs M and Emanuelli T: Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits.
Cui G, Li L, Xu W, Wang M, Jiao D, Yao B, Xu K, Chen Y, Yang S, Long M, et al: Astaxanthin protects ochratoxin a-induced oxidative stress and apoptosis in the heart via the Nrf2 pathway. Naunyn Schmiedebergs Arch Pharmacol. Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML and Wang XL: Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element Nrf2-ARE pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury.
Kavitha K, Thiyagarajan P, Rathna Nandhini J, Mishra R and Nagini S: Chemopreventive effects of diverse dietary phyto-chemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes.
Tripathi DN and Jena GB: Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes.
Mutat Res. Saw CL, Yang AY, Guo Y and Kong AN: Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway.
Food Chem Toxicol. Visioli F and Artaria C: Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps.
Aim: To determine the effects of astaxanthin treatment on lipids, cardiovascular disease CVD markers, glucose tolerance, insulin action and inflammation in individuals with prediabetes and dyslipidaemia.
Baseline studies were repeated after 12 and 24 weeks of therapy. Results: After 24 weeks, astaxanthin treatment significantly decreased low-density lipoprotein No consistent significant differences from baseline were observed for any of these outcomes in the placebo group.
Astaxanthin was safe and well tolerated with no clinically significant adverse events.
The extracted oils from the cephalothorax CPO and hepatopancreas Leveps were characterized for Astaxanthun content, cholesterol Astaxanthin and cholesterol levels, and Asraxanthin acid profiles. Astaxanthon indices Soothing muscle pain CPO and HPO were also compared. CPO had lower extraction yield 3. High-performance liquid chromatography results indicated that the astaxanthin content in HPO was higher, compared to that of CPO. Fatty acid profiles of HPO and CPO demonstrated that the polyunsaturated fatty acid PUFA content in HPO was higher than that of CPO.The extracted oils from the cephalothorax CPO and hepatopancreas HPO were characterized for astaxanthin content, Ketosis and Longevity levels, and fatty acid cholestero.
Nutrition indices of CPO and HPO were also compared. CPO had lower extraction yield 3. High-performance liquid chromatography elvels indicated Aataxanthin the astaxanthin content in HPO was higher, compared to that of CPO.
Fatty acid profiles of HPO and CPO demonstrated Aetaxanthin the polyunsaturated fatty Astaxsnthin PUFA content in HPO was Astacanthin than that of Diabetic coma management. The amount of docosahexaenoic acid in lrvels former Body density measurement techniques ~2 times higher than cholrsterol of the choleeterol.
HPO contained Astaxantthin On the other hand, saturated fatty acids Cholewterol were more pronounced in CPO The Astxxanthin status cholestdrol CPO and HPO measured cholesterrol terms of peroxide lefels, thiobarbituric acid reactive substances, anisidine value, and conjugated dienes indicated that higher primary oxidation products were present in Andd, whereas HPO cholesyerol more secondary oxidation compounds.
Fourier transform choleterol spectra further Aztaxanthin the presence of legels Astaxanthin and cholesterol levels in CPO and HPO. Liquid chromatography-mass spectrometry cholesterl showed the enhanced levels of Body density measurement techniques and glycolipids in the ethanolic fraction Enhance CPO.
Overall, HPO with Astaxanthi higher yield was more beneficial in cbolesterol of Resveratrol and arthritis benefits than Bone health and medication usage. Increased Body density measurement techniques demand, particularly in the forms of ready-to-cook or ready-to-eat, has led to an Anti-arthritic home remedies amount of wnd shrimp 1.
During the processing, byproducts, namely, cephalothorax, carapace, tail, and internal organs were produced and discarded 2. Apart from Astaxanthln shrimp or peeled shrimp, some products Astaxabthin cephalothorax free Cholesterol-lowering strategies hepatopancreas are in demand in some markets.
Thus, Sun safety and cancer prevention hepatopancreas is removed using the sucking machine 3.
Detoxification Methods Explained a consequence, anv rich lvels oil can serve as a potential source Astaxantuin oil Astaxanthin and cholesterol levels 3.
The Astaxatnhin Astaxanthin and cholesterol levels been prominently utilized for producing Body density measurement techniques, protein hydrolysate, and shrimp oil 4Body density measurement techniques.
Concerning shrimp oil production, hepatopancreas having higher lipid content seems cholesyerol render higher cholfsterol, compared to cephalothorax 3. Takeungwongtrakul et al. Antioxidant properties addition, the residues after oil extraction have wnd less when hepatopancreas is used, compared to the cephalothorax.
Shrimp Nutrition for athletes is rich in astaxanthin, astaxanthin esters, and polyunsaturated fatty acids PUFAsespecially chopesterol acid EPA and Dextrose Endurance Support acid DHA 67.
Oevels bioactive compounds Astaxathin highly beneficial Mixed sunflower seeds improving human health. Astaxanthin, a powerful antioxidant, possesses antiaging, anti-inflammation, and anticancer properties 58 — DHA and EPA chooesterol been known Astaxanrhin improving brain and cardiac health 5 Exercise performance fuel, 11 However, shrimp oil also contained saturated fatty acids SFAswhich could lead to an increased low-density lipoprotein level and Astaxanthi inflammatory response.
In general, the limitation of SFAs in Breakfast for sustained energy diet ,evels of concern about the ratio of unsaturated fatty acids Cholestreol to total fatty acids These Magnesium and muscle function in athletes could be employed Body density measurement techniques the shrimp ajd to indicate nutritive value.
Although shrimp oil has a high content of highly beneficial bioactive compounds, it also contains cholesyerol 6. Despite the ,evels for some cholesterol to make levelx, vitamin D, etc. To improve the nutrition quality of shrimp lipid extracted from cephalothorax, cholesterol has been lowered by saponin and chokesterol 16 — However, the cholesterol content in shrimp oil from the hepatopancreas has not been studied.
The complete profiling of fatty acids, astaxanthin, cholesterol, and nutrition indices could be useful for the consumers to intake the shrimp oil for health benefits. To our knowledge, no aforementioned information for shrimp oils from both cephalothorax and hepatopancreas exists.
Therefore, this study was aimed to characterize the oils extracted from cephalothorax and hepatopancreas of Pacific white shrimp and to compare both oils in terms of fatty acid content, astaxanthin content, cholesterol level, oxidation status, and nutritive indices.
All the chemicals used in the experiment were of analytical grade and purchased from Merck Darmstadt, Germany. Pacific white shrimp Litopenaeus vannamei cephalothorax shrimp head and hepatopancreas were gifted from Sea Wealth Frozen Food Co.
First, cephalothorax and hepatopancreas were blended with a blender at high speed for 1 min to obtain a homogenous paste. The mixture was homogenized at 9, rpm for 3 min with the aid of an IKA Labortechnik homogenizer Selangor, Malaysia. After homogenization, the solvent phase was separated by centrifugation using a Hitachi centrifuge Hitachi Koki Co.
The collected solvent phase was washed with an equal volume of distilled water and the process was repeated thrice. The hexane phase was collected and anhydrous sodium sulfate 10 g was added to remove the water traces, followed by filtering using a Whatman filter paper No.
The solvent was evaporated using an EYELA rotary evaporator N Tokyo Rikakikai, Co. The cholesterol and astaxanthin contents were quantified by high-performance liquid chromatography HPLC Waters series, Milford, MA, USA 619 equipped with a reverse-phase Thermo scientific BDS-C18 column 5 μm; × 4 mm following the method of Raju et al.
Each oil sample μl was dissolved in 1 ml of ethanol. After incubation, the prepared mixtures were centrifuged at 3, × g for 10 min. A μl of supernatant was taken and made up to 1 ml using mobile phase and injected in HPLC.
HPLC program was performed at isocratic condition using 1. A photodiode-array detector WatersMilford, MA, USA was used for the detection at nm for astaxanthin and nm for cholesterol.
Authentic standards of astaxanthin Dr. Ehrenstorfer GmbH, Augsburg, Germany and cholesterol Acros organics, Morris Plains, NJ, USA were used for identification. Fatty acid profile was determined by the method of Raju et al.
Briefly, the sample 10 mg was dissolved in 1 ml of hexane and esterified with μl of 2 M methanolic sodium hydroxide at 50°C for 5 min. After cooling down, the mixture was vortexed and μl of 2 M methanolic hydrochloric acid was added. The prepared mixture was vortexed thoroughly and then centrifuged at 3, × g for 10 min.
The hexane phase was collected and injected into gas chromatography Agilent GC B; Santa Clara, CA, USA. Injection temperature was maintained at °C and the initial column temperature was first reduced to 80°C. The eluted compounds were identified by a flame ionization detector Agilent GC B; Santa Clara, CA, USA at °C as a detector temperature.
Genuine standards Supelco FAME mix, Bellefonte, PA, USA were used for the peak identification and the fatty acid content was expressed as a percentage. Nutritional indices were calculated based on the fatty acid profile by the following formulas Peroxide value PV was determined by the method of Pudtikajorn and Benjakul An oil sample 0.
To the prepared mixture, 1 ml of saturated potassium iodide was added and mixed. The mixture was then incubated in dark for 5 min. Subsequently, distilled water 75 ml was added and shaken.
At last, to the prepared mixture, 0. The titration was stopped after the disappearance of the dark blue color. Thiobarbituric acid reactive substances TBARS value was analyzed following the method of Gulzar and Benjakul p -Anisidine value AnV was measured as tailored by Firestone Briefly, an oil sample 0.
AnV was calculated as follows:. where A 1 and A 2 are the absorbances before and after adding p -anisidine, respectively; W is the weight of the sample g. Conjugated dienes CDs were analyzed using the method of Raju and Benjakul Shrimp oil 0. The absorbance of the solution was read at nm A CD was calculated using the following equation:.
Fourier transform infrared FTIR spectra of samples were attained by Bruker Model Vector 33 FTIR spectrometer Bruker Co. A clean empty cell at 25°C was kept for normalization and as the reference background. The data were collected using OPUS 8. Billerica, MA, USA.
Ethanol soluble lipids were prepared using the method of Raju et al. Briefly, oil samples mg were added to 1 ml of ethanol and vortexed vigorously. At the end of the incubation period, the mixtures were centrifuged at 4, × g and the upper phase was collected.
The upper phase μl was subjected to liquid chromatography quadrupole time-of-flight mass spectrometer LC-QTOF MSInfinity II LC Quadrupole-TOF Agilent Santa Clara, CA, USA.
The instrument was equipped with Zorbax Eclipse Plus C18 column mm length × 2. Positive atmospheric pressure chemical ionization was done and compounds were identified based on mass and spectrum compared with MassHunter METLIN PCD library.
A completely randomized design was used for this study. Experiment and analysis were done in triplicate using three different sample lots. ANOVA was performed using the Statistical Package for Social Science SPSS software IBM software, New York, NY, USA The t -test was used for pair comparison.
CPO and HPO had different yields. Yields of CPO and HPO were 3. The cephalothorax is the fused head and thorax including the outer shell carapaceinternal organs, and other components The hepatopancreas is an important organ, which plays a major role in digestion and absorption During the production of whole shrimp free of hepatopancreas, hepatopancreas was removed directly by a vacuum sucking machine As a result, the obtained hepatopancreas was not adulterated by other organs, shells, or components Hepatopancreas also functions as a lipid depositing organ This was witnessed by four times higher fat content than that of cephalothorax.
Chromatograms of astaxanthin and cholesterol in HPO and CPO are shown in Figure 1.
: Astaxanthin and cholesterol levels| Related news | This site uses Body density measurement techniques. Ane HK, Fiber optic network efficiency H: Dietary alpha-linolenic cholesterrol lowers postprandial lipid levels with increase of eicosapentaenoic and docosahexaenoic acid contents in rat Astaxqnthin membrane. Up to choleaterol, the effects Astaxanthin and cholesterol levels the combination of FO and ASX on cardiovascular system have not been investigated. Arunkumar E, Bhuvaneswari S, Anuradha CV: An intervention study in obese mice with astaxanthin, a marine carotenoid—effects on insulin signaling and pro-inflammatory cytokines. Another in vitro and ex vivo study with cultured lymphocytes demonstrated that astaxanthin stimulated their immune response and increased the production of IL-2 and IFN-γ, without inducing cytotoxicity |
| Publication types | From fatty acid composition, nutrition indices were calculated to reveal the health-promoting index. Figure 2. Nutrition indices of CPO and HPO. However, IT was lower in HPO, compared to that of CPO. Ulbricht and Southgate 34 developed and proposed IA and IT for calculating atherogenicity and thrombogenicity 14 , Atherogenicity for foods is calculated based upon SFA and UFA contents Lauric acid C 0 , myristic acid C 0 , and palmitic acid C 0 are considered to be atherogenic These fatty acids can bind with the cells of the circulatory system, leading to the formation of plague However, UFAs possess antiatherogenic activity, thus inhibiting plaque formation. Thus, IA was calculated from the ratio of proatherogenic SFAs to antiatherogenic UFAs Thrombogenicity refers to the ratio of fatty acids inducing clot formation C, C, and C and the fatty acids having antithrombotic effects such as MUFA, n-6, and n-3 fatty acids Since HPO contained a higher amount of n-3 fatty acids, compared to CPO, this resulted in the lower IT in HPO. IA and IT have been considered as markers for assessing cardiovascular health When comparing both the oils, HPO was considered as the better oil in terms of protecting heart diseases with higher anticlotting ability. Lipid oxidation was assayed by measuring primary and secondary lipid oxidation products Figure 3. PV has been used to monitor the formation of primary oxidation products, mainly hydroperoxides in the presence of O 2. The formed hydroperoxides are not stable and more likely decomposed to secondary oxidation products such as aldehydes and ketones 3. PV was higher in CPO than HPO. For CDs, a higher CD was observed in HPO than CPO. CDs are also primary oxidation products. Lipid peroxidation starts with the abstraction of H from the -CH 2 - group of PUFAs. As a result, carbon radical is stabilized by a molecular rearrangement, forming CDs in which, two double bonds are separated by a single bond With a high content of PUFA, HPO could undergo a higher abstraction of H, leading to the higher formation of CD. CDs were therefore defined as diene formation due to the presence of double bonds However, the secondary oxidation products as indicated by TBARS value and AnV were higher in HPO. HPO was composed of higher PUFAs that were vulnerable to oxidation. At the beginning stage, primary oxidation products such as hydroperoxides were formed. Simultaneously, the decomposition occurred at a higher rate. As a result, the amount of hydroperoxide was decreased as indicated by lowered PV, while TBARS value and AnV were augmented. TBARS value has been widely used to measure volatile oxidation products, while AnV has been employed to measure non-volatile lipid oxidation products 3. AnV determines the amount of aldehydes, principally 2-alkenals and 2, 4-dienals by reaction with p -anisidine The results were in accordance with Gulzar and Benjakul 16 who found that the oxidation of shrimp oil from cephalothorax was enhanced during the storage period, while the addition of antioxidants could suppress the oxidation. Overall, HPO with high PUFA content was prone to oxidation than CPO containing a lower amount of PUFA Table 1. Figure 3. Oxidation status of CPO and HPO. Fourier transform infrared FTIR spectrum has been used for the identification of functional groups Oil samples with the altered functional group formed due to oxidation could be identified via spectra Figure 4. This denotes the presence of the primary oxidation product of peroxide, mainly hydroperoxide. However, the O-OH peak was much lowered in HPO, representing the lower amount of peroxide formed. These results were in line with the PV result Figure 3 , in which higher PV was obtained in CPO than HPO. Similar peaks representing lipid oxidation in shrimp oil extracted by ultrasonic-assisted extraction were posted by Gulzar and Benjakul The peak height of aldehydes was greater in HPO, compared to that of CPO, reflecting the augmented formation of secondary lipid oxidation products in HPO. Phosphate attached with the carbonyl group indicates the presence of higher phospholipids in CPO 6. However, the peak was lowered in HPO, indicating the lower phospholipid content. Overall, FTIR spectra revealed higher aldehydes, lower peroxide, and phospholipid in HPO than CPO. Figure 4. FTIR spectra of CPO and HPO. Ethanol soluble lipids were prepared by the ethanol crystallization method The ethanol-soluble polar and neutral lipids such as cholesterol, astaxanthin, fat-soluble vitamins, phospholipids, and other carotenoids were separated from insoluble non-polar lipids. The non-polar lipids mainly triglycerides were crystallized due to the insolubility in ethanol 6. In the current study, preliminary identification of lipid components in the ethanolic fraction was done by liquid chromatography-mass spectrometry LC-MS Table 2. When comparing both the samples, CPO was dominated by glycolipids, phospholipids, diacylglycerol, monoacylglycerol, and sterol derivatives. In HPO, diacylglycerol, monoacylglycerol, and sterol derivatives were predominantly found. Although HPO contained phospholipids as one of the major components, some phospholipids were not identified, compared to those found in CPO. The result showed that the phospholipids in HPO might be lower, compared to that of CPO. FTIR results Figure 4 supported the LC-MS data by showing the smaller peak of phospholipid in HPO. Glycolipids were dominant in CPO but they were not detected in HPO. Glycolipids and phospholipids are the major components in cell membrane regulating osmoregulatory changes in crustacean cell membrane structures Cephalothorax contains more membranes or tissues, compared to hepatopancreas. The presence of lesser tissue in the hepatopancreas was due to the target removal of the gland by a sucking machine This could be the reason for fewer phospholipids and unidentified glycolipids. Phospholipids in both oils mainly consisted of phosphatidylethanolamine PE conjugated with different fatty acids. In general, shrimp lipid contains PE and phosphatidylcholine as major phospholipids However, the lipid components can vary due to seasonal changes, diet, maturity, and size 5. C30 column was used for the identification of astaxanthin esters and other neutral lipids in shrimp lipid 7 , whereas C18 was the column used in the study. This could lead to the difference in profiles of components in oils. Other components found in both the samples were sterol derivatives, vitamin derivatives, α-carotene, and astaxanthin. Based on the HPLC result, higher astaxanthin was found in HPO, thus providing more health benefits than CPO. Overall, the LC-MS identification revealed that HPO also contained vitamins with lesser phospholipid and higher astaxanthin contents. Table 2. LC-MS identification of shrimp oil extracted from cephalothorax and hepatopancreas. Oil extracted from cephalothorax and hepatopancreas of Pacific white shrimp showed different compositions and nutrition indices. Oil from the hepatopancreas with higher yields had higher amounts of astaxanthin and lower cholesterol levels. PUFAs were more pronounced in HPO. Consequently, HPO possessed the superior nutritive value to CPO. However, secondary oxidation products were found in HPO, indicating that lipid oxidation took place. Nevertheless, the oxidation in both the samples could be overcome with the addition of natural antioxidants or exclusion of oxygen in the package. NR and SG performed the research, analyzed the data, and wrote the original manuscript. NB provided technical support in data analysis. LM, XY, and BZ edited the manuscript. SB supervised the research design and reviewed the manuscript. All authors contributed to and approved the final draft of the manuscript. This work was supported by Chair Professor Grant P Thailand's Education Hub for Southern Region of ASEAN Countries TEH-AC, scholarship and Prachayajarn Scholarship, Prince of Songkla University Grant No. AGRN are also acknowledged. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Shiekh KA, Benjakul S, Gulzar S. Impact of pulsed electric field and vacuum impregnation with Chamuang leaf extract on quality changes in Pacific white shrimp packaged under modified atmosphere. doi: CrossRef Full Text Google Scholar. Mittal A, Singh A, Aluko RE, Benjakul S. Pacific white shrimp Litopenaeus vannamei shell chitosan and the conjugate with epigallocatechin gallate: antioxidative and antimicrobial activities. J Food Biochem. PubMed Abstract CrossRef Full Text Google Scholar. Takeungwongtrakul S, Benjakul S, H-Kittikun A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp Litopenaeus vannamei : compositions and deterioration as affected by iced storage. Food Chem. Singh A, Mittal A, Benjakul S. Chitosan, chitooligosaccharides and their polyphenol conjugates: preparation, bioactivities, functionalities and applications in food systems. Food Hydrocoll. Gulzar S, Raju N, Chandragiri Nagarajarao R, Benjakul S. Oil and pigments from shrimp processing by-products: extraction, composition, bioactivities and its application- a review. Trends Food Sci Technol. Raju N, Benjakul S. Application of saponin for cholesterol removal from Pacific white shrimp Litopenaeus vannamei lipid. Eur J Lipid Sci Technol. Gómez-Estaca J, Calvo MM, Álvarez-Acero I, Montero P, Gómez-Guillén MC. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp L. vannamei waste with potential applications as food ingredient. Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. Nishino H, Murakosh M, Ii T, Takemura M, Kuchide M, Kanazawa M, et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. Wu H, Niu H, Shao A, Wu C, Dixon B, Zhang J, et al. Astaxanthin as a potential neuroprotective agent for neurological diseases. Navaneethan R, Vimaladevi S, Ajeesh Kumar KK, Anandan R, Niladri SC, Asha KK, et al. Profiling of Omega-3 polyunsaturated fatty acids of myctophid fish species available in Arabian sea. Fish Technol. Google Scholar. Sokoła-Wysoczańska E, Wysoczański T, Wagner J, Czyz K, Bodkowski R, Lochyński S, et al. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—a review. Miniadis-meimaroglou S, Sinanoglou VJ. Lipid profile and nutritional evaluation of shrimps. Lipidomics Sea Food, Mar Based Diet Suppl Fruit Seed. Chen J, Liu H. Nutritional indices for assessing fatty acids: a mini-review. Int J Mol Sci. Lusis AJ. Gulzar S, Benjakul S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp Litopenaeus vannamei by ultrasonic assisted process. Use of beta cyclodextrin to remove cholesterol and increase astaxanthin content in shrimp oil. Raju N, Sae-leaw T, Osako K, Benjakul S. Improved cholesterol depletion with enhanced astaxanthin and polyunsaturated fatty acids of lipid from Pacific white shrimp cephalothorax using prior ethanolic separation of polar lipid and β-Cyclodextrin. J Food Sci Technol. Bavisetty SCB, Narayan B. An improved RP-HPLC method for simultaneous analyses of squalene and cholesterol especially in aquatic foods. Raju N, Singh A, Benjakul S. Recovery, reusability and stability studies of beta cyclodextrin used for cholesterol removal from shrimp lipid. RSC Adv. Pudtikajorn K, Benjakul S. Simple wet rendering method for extraction of prime quality oil from skipjack tuna eyeballs. Firestone D. Official Methods and Recommended Practices of the AOCS. Champaign, IL: AOCS Steel RGD, Torrie JH. Principles and Procedures of Statistics, a Biometrical Approach. New York, NY: McGraw-Hill Kogakusha, Ltd. Sachindra NM, Bhaskar N, Mahendrakar NS. Carotenoids in different body components of Indian shrimps. J Sci Food Agric. Wang W, Wu X, Liu Z, Zheng H, Cheng Y. Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus : gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS ONE. Senphan T, Benjakul S, Kishimura H. Purification and characterization of trypsin from hepatopancreas of pacific white shrimp. Britton G. Functions of intact carotenoids. In: G Britton, S Liaaen-Jensen, H Pfander, editors, Carotenoids. Basel: Birkhäuser Basel. Wade NM, Gabaudan J, Glencross BD. A review of carotenoid utilisation and function in crustacean aquaculture. Rev Aquac. Crocke EL. Cholesterol function in plasma membranes from ectotherms: membrane-specific roles in adaptation to temperature. Am Zool. Hobbs HH. Encycl Caves. Kumar V, Sinha AK, Romano N, Allen KM, Bowman BA, Thompson KR, et al. Metabolism and nutritive role of cholesterol in the growth, gonadal development, and reproduction of crustaceans. Rev Fish Sci Aquac. Shillito B, Desurmont C, Barthélémy D, Farabos D, Després G, Ravaux J, et al. Lipidome variations of deep-sea vent shrimps according to acclimation pressure: a homeoviscous response? Deep Sea Res I Oceanogr Res Pap. Calder PC. The role of marine omega-3 n-3 fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. Attia YA, Al-Harthi MA, Korish MA, Shiboob MM. Fatty acid and cholesterol profiles and hypocholesterolemic, atherogenic, and thrombogenic indices of table eggs in the retail market. Clin Chim Acta. Sazuka Y, Tanizawa H, Takino Y: Effect of adriamycin on the activities of superoxide dismutase, glutathione peroxidase and catalase in tissues of mice. Jpn J Cancer Res. Moron MS, Depierre JW, Mannervik B: Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. Buege JA, Aust SD: Microsomal lipid peroxidation. Methods Enzymol. Xu J, Zhou X, Deng Q, Huang Q, Yang J, Huang F: Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis. Hasan S, Zingg J-M, Kwan P, Noble T, Smith D, Meydani M: Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Winnik S, Lohmann C, Richter EK, Schafer N, Song WL, Leiber F, Mocharla P, Hofmann J, Klingenberg R, Boren J, Becher B, Fitzgerald GA, Luscher TF, Matter CM, Beer JH: Dietary alpha-linolenic acid diminishes experimental atherogenesis and restricts T cell-driven inflammation. Eur Heart J. Thromb Res. Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R: Atherosclerosis and oxidative stress. Histol Histopathol. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. Madamanchi NR, Vendrov A, Runge MS: Oxidative stress and vascular disease. Formigari A, Irato P, Santon A: Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. Dringen R: Metabolism and functions of glutathione in brain. Prog Neurobiol. Yang RL, Li W, Shi YH, Le GW: Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: a microarray analysis. Shimidzu N, Goto M, Miki W: Carotenoids as singlet oxygen quenchers in marine organisms. Fish Sci. Krinsky NI: Antioxidant functions of carotenoids. Free Radic Biol Med. Miki W: Biological functions and activities of animal carotenoids. Pure Appl Chem. Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J: Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr. Bhuvaneswari S, Arunkumar E, Viswanathan P, Anuradha CV: Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. Sangeetha RK, Baskaran V: Retinol-deficient rats can convert a pharmacological dose of astaxanthin to retinol: antioxidant potential of astaxanthin, lutein, and beta-carotene. Can J Physiol Pharmacol. Duffield R, Miller N, Brunt J: Treatment of hyperlipidemia retards progression of symptomatic femoral atherosclerosis: a randomized controlled trial. Kim HK, Choi S, Choi H: Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J Nutr Biochem. Ide T, Kobayashi H, Ashakumary L, Rouyer IA, Takahashi Y, Aoyama T, Hashimoto T, Mizugaki M: Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Kabir Y, Ide T: Activity of hepatic fatty acid oxidation enzymes in rats fed alpha-linolenic acid. Jia Y, Kim JY, Jun HJ, Kim SJ, Lee JH, Hoang MH, Hwang KY, Um SJ, Chang HI, Lee SJ: The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol Nutr Food Res. Ihara-Watanabe M, Umekawa H, Takahashi T, Furuichi Y: Effects of dietary alpha- or gamma-linolenic acid on levels and fatty acid compositions of serum and hepatic lipids, and activity and mRNA abundance of 3-hydroxymethylglutaryl CoA reductase in rats. Comp Biochem Physiol A Mol Integr Physiol. Libby P: Inflammation in atherosclerosis. Willerson JT, Ridker PM: Inflammation as a cardiovascular risk factor. Libby P, Ridker PM, Hansson GK: Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG: C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Larsson PT, Hallerstam S, Rosfors S, Wallen NH: Circulating markers of inflammation are related to carotid artery atherosclerosis. Int Angiol. Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A: Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ, Ha KS, Han JA, Yim SV, Chang K, Kwon YG, Lee SK, Kim YM: Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I kappa B kinase-dependent NF-kappaB activation. Mol Cells. Arunkumar E, Bhuvaneswari S, Anuradha CV: An intervention study in obese mice with astaxanthin, a marine carotenoid—effects on insulin signaling and pro-inflammatory cytokines. Food Funct. Pu J, Bechtel PJ, Sathivel S: Extraction of shrimp astaxanthin with flaxseed oil: effects on lipid oxidation and astaxanthin degradation rates. Biosyst Eng. Article Google Scholar. Download references. This work was supported by National Natural Science Foundation of China NSFC and the earmarked fund for Modern Agro-industry Technology Research System CARS , China. Department of Product Processing and Nutriology, OilCrops Research Institute, Chinese Academy of Agricultural Sciences, 2 Xudong Second Road, Wuhan, , P. Hubei Key Laboratory of Lipid Chemistry and Nutrition, OilCrops Research Institute, Chinese Academy of Agricultural Sciences, 2 Xudong Second Road, Wuhan, , P. Department of Nutrition and Food Hygiene, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, 13 Hangkong Road, Wuhan, , P. Department of neurology, Hubei Xinhua Hosipital, 11 lingjiaohu Road, Wuhan, , P. Department of Gastroenterology, The First People's Hospital of Yichang, The People's Hospital of China Three Gorges University, 2 Jiefang Road, Yichang, , P. Department of Gastroenterology, The People's Hospital of China Three Gorges University, 2 Jiefang Road, Yichang, , P. You can also search for this author in PubMed Google Scholar. Correspondence to Fenghong Huang. Author JX designed and wrote a first draft of the paper. HG, LZ, CC, WY and QD carried out all the experiments. QH performed the data analysis and created the figures. FH contributed to the design of the study, reviewed the manuscript and contributed to the final version. All authors contributed to and have approved the final manuscript. Open Access This article is published under license to BioMed Central Ltd. Reprints and permissions. Xu, J. et al. A combination of flaxseed oil and astaxanthin alleviates atherosclerosis risk factors in high fat diet fed rats. Lipids Health Dis 13 , 63 Download citation. Received : 24 January Accepted : 04 March Published : 04 April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Abstract Background Atherosclerosis is the most common pathologic process underlying cardiovascular disease. Results The combination of FO and ASX significantly increased the antioxidant defense capacity and decreased lipid peroxidation in plasma. Conclusion The combination of FO and ASX can improve oxidative stress, lipid abnormalities and inflammation, providing evidence that the combination of FO and ASX could be a promising functional food in cardiovascular health promotion. Introduction Nowadays, cardiovascular disease CVD is the leading cause of morbidity and mortality in most developed as well as many developing countries [ 1 , 2 ] and contributes substantially to healthcare budgets. Materials and methods Chemical sources The flaxseed oil was purchased from Caoyuankangshen Food Co. Blood processing After 10 weeks of feeding, all animals were fasted for 16 hours and killed under anaesthesia, blood was collected in heparinized vacutainer tubes from the heart immediately. Plasma lipids analysis The plasma triglyeride TG , total cholesterol TC , low-density lipoprotein cholesterol LDL-C and high-density lipoprotein cholesterol HDL-C levels were determined with commercial kits Wako, Japan by Hitachi full-automatic biochemical analyzer Japan. Assay of plasma antioxidant capacity and lipid peroxidation Superoxide dismutases SOD activity was measured according to the method of Kono [ 18 ]. Assay of plasma inflammatory markers The plasma interleukin 6 IL-6 and C-reactive protein CRP levels were measured by means of commercially available Rat CRP ELISA kit Abcam, Cambridge, MA and Rat IL-6 ELISA kit Abcam, Cambridge, MA , respectively. Figure 1. Full size image. Figure 2. Figure 3. Discussion Although the exact mechanisms remain to be delineated, oxidant stress [ 3 ], lipid abnormalities [ 4 ] as well as chronic inflammation [ 5 ] have been identified as the main trigger mechanisms of atherosclerosis. References Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ: Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. CAS PubMed Google Scholar Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ: The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. CAS PubMed Google Scholar Trebušak T, Levart A, Voljč M, Tomažin U, Pirman T: The effect of linseed oil supplementation on performance, fatty acid composition and oxidative status of rabbits. Google Scholar Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM, Jennings GL, Abbey M, Cameron JD: Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. x Article CAS PubMed Google Scholar Moron MS, Depierre JW, Mannervik B: Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Article CAS PubMed Google Scholar Xu J, Zhou X, Deng Q, Huang Q, Yang J, Huang F: Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. CAS PubMed Google Scholar Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol. Article CAS PubMed Google Scholar Formigari A, Irato P, Santon A: Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Article CAS Google Scholar Krinsky NI: Antioxidant functions of carotenoids. Article CAS Google Scholar Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J: Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. Article CAS Google Scholar Sangeetha RK, Baskaran V: Retinol-deficient rats can convert a pharmacological dose of astaxanthin to retinol: antioxidant potential of astaxanthin, lutein, and beta-carotene. CAS PubMed Google Scholar Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A: Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. CAS PubMed Google Scholar Arunkumar E, Bhuvaneswari S, Anuradha CV: An intervention study in obese mice with astaxanthin, a marine carotenoid—effects on insulin signaling and pro-inflammatory cytokines. Article Google Scholar Download references. Acknowledgments This work was supported by National Natural Science Foundation of China NSFC and the earmarked fund for Modern Agro-industry Technology Research System CARS , China. Author information Authors and Affiliations Department of Product Processing and Nutriology, OilCrops Research Institute, Chinese Academy of Agricultural Sciences, 2 Xudong Second Road, Wuhan, , P. China Li Zhang Department of Gastroenterology, The First People's Hospital of Yichang, The People's Hospital of China Three Gorges University, 2 Jiefang Road, Yichang, , P. China Chang Chen Department of Gastroenterology, The People's Hospital of China Three Gorges University, 2 Jiefang Road, Yichang, , P. China Chang Chen Authors Jiqu Xu View author publications. View author publications. |
| Astaxanthin’s heart benefits get human data support | Pu J, Bechtel PJ, Sathivel S: Extraction of shrimp astaxanthin with flaxseed oil: effects on lipid oxidation and astaxanthin degradation rates. PubMed Abstract CrossRef Full Text Google Scholar. Effects of beta-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Atherosclerosis — Abstract Background Atherosclerosis is the most common pathologic process underlying cardiovascular disease. Randomized, double-blind. |
| How It Works | Nutricosmetic Sales Are Growing Globally, Ranging from Pills to Beauty Food and Drinks. It belongs to xanthophylls with the chemical formula C40H52O4 Consequently, HPO possessed the superior nutritive value to CPO. Ann Nutr Metab. Miki W: Biological functions and activities of animal carotenoids. In our investigation no significant change in serum level of SIRT1 following AX supplementation were observed even after adjusting for confounders. However, IT was lower in HPO, compared to that of CPO. |
| Related products | Distribution of age, BMI, gender, degree of physical activity and consumed drugs were not significantly different between the AX and placebo groups at the beginning of the study Table 1. The comparison of anthropometric parameters of two groups was presented in Table 2. Baseline values of BMI, HC, WC and body composition were not significantly different between AX and placebo groups. However, the changes between the two groups were not significant. Despite the significant reduction in visceral fat in the control group compared to the AX group at the end of the intervention, the between-group difference was not statistically significant. The baseline values of lipid profile and glycemic indices were not statistically different between two groups Table 4. Although, the between-group difference of TC and LDL-C were not statistically significant. The results revealed that there was no significant difference in TG and HDL-C within and between groups. The baseline values of Sirtuin1 and TNF-α were not statistically different between two groups Table 5. TNF-α levels did not change significantly in both groups. In vivo studies have demonstrated that AX intake has cardiovascular protective effects, although the results of human studies regarding the beneficial effects of AX on cardiovascular risk factors are contradictory and limited. To the best of our knowledge, this study was the first randomized, double-blind, placebo controlled, clinical trial investigating the impacts of AX supplementation on metabolic parameters in CAD patients. The findings revealed that AX had no effect on the lipid profile compared to the placebo group, but it should be noted that LDL-C and TC levels decreased significantly in the AX group. However, it had no significant improvements on body composition and other metabolic and anthropometric parameters. The results of a recent meta-analysis conducted in are in line with our findings and demonstrate that body weight and BMI changes were not statistically significant Choi et al. According to the food intake data, the energy intake of the patients in both groups decreased, as a result, BMI and body weight decreased in both groups. Despite of adjusting confounding factors, no substantial between-group changes were found. WC and HC are parameters that were only analyzed in this study, even though the differences between the AX-receiving group and the placebo group are not statistically significant. Alike our results, Roustae Rad et al. Two recent meta-analysis investigated that AX consumption was not associated with TC, LDL-C, TG reduction. However, only Xia reported an overall increase in HDL-C 23 , Tominaga et al. and Saito et al. showed that AX had no impact on lipid profile in healthy individuals regardless of the duration of supplementation and the AX dosage 28 , It is well known that the major causative risk factors for CAD and a definite contributor to accelerated atherosclerosis is dyslipidemia 30 , Statins are used as the first line of treatment in both primary and secondary prevention to lower cholesterol and enhance lipid composition 32 , Therefore, AX might not have affected TC, TG, and LDL-C levels since the majority of the patients were taking statins and lipid profile had improved before the intervention. However, the TC and LDL-C levels were significantly decreased only in the intra-group analysis of AX group. The capacity of AX to upregulate SREBP-2 and therefore boost the gene expression of LDLR and HMGR may have contributed to the ability of AX to reduce cholesterol levels It has been demonstrated that SIRT1 also regulates PPARα expression, which helps to control lipid metabolism 36 , The current study also declared that AX intake did not significantly improve serum levels of insulin, FBS, and HOMA-IR index. A recent meta-analysis revealed that FBS did not decrease after AX supplementation compare with placebo group. According to yang et al. Alike our result, Saito and Tominaga et al. Yoshida et al. also reported the similar result which FBS did not change significantly in subjects with mild hyperlipidemia However, the blood glucose levels have dropped significantly or even slightly in trials on diabetic subjects 16 , SIRT1 play an important role in controlling cellular physiological processes It has been identified as a novel homoeostasis regulator in the human cardiovascular system through protecting against aging and endothelial inflammation, inducing resistance against oxidative stress and hypertrophic, inhibiting apoptosis of cardiomyocytes, and modulating cardiac energy metabolism 39 , Furthermore, transcription factor SIRT1 plays an important role in energy and lipid metabolism, inflammation and insulin sensitivity Nishida et al. reported that AX treatment induced upregulation of the gene expression of the mitochondrial SRIT1 and mitochondrial biogenesis in mice In our investigation no significant change in serum level of SIRT1 following AX supplementation were observed even after adjusting for confounders. Non-significant alterations in lipid profile and glycemic indices may be associated with negligible changes in SIRT1. The impact of AX on SIRT1 levels has not been studied in any RCT. Furthermore, drawing a definite conclusion on the effects of AX on SIRT1 is not feasible due to the insufficient number of studies in this field. TNF-α is a pro-inflammatory cytokine found in atherosclerotic lesions and can have a direct effect on vascular endothelial cells and induce the expression of adhesive molecules in leukocytes and other inflammatory cells In our study the changes of TNF-α levels were not significant and in line with our study. Park et al. Alternatively, the result of non-significant change in TNF-α levels in our study is in line with the hypothesis of the recent meta-analysis about the effect of carotenoids supplementation on inflammation, which suggests that higher baseline levels of pro-inflammatory cytokines indicate a potential for a greater response to carotenoid supplementation such as AX SIRT1 directly can deacetylate the p65 subunit of the NF-κB complex to suppress NF-κB activation. Furthermore, SIRT1 promotes oxidative energy generation by activating of AMP-activated protein kinase AMPK , Peroxisome proliferator-activated receptor α PPARα and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha. These factors concurrently inhibit NF-κB signaling and led to decline inflammation If this pathway is blocked, TNF-α production can also decrease. The small sample size may be the reason for the lack of noticeable changes in the parameters of present study. The follow-up duration in our study was short and extending the duration of the intervention might show potential benefits. Moreover, we were not able to measure the concentration of AX in the plasma in order to evaluate its bioavailability. Future trials may be able to better understand the impacts of AX by assessing certain genes involved in the regulation of metabolic variables. The trial was completely conducted using a double-blind, random allocation approach. Patients who had recovered from COVID even before the intervention were excluded from the study due to prevent disturbances in the measured factors, especially the inflammatory index. However, more clinical trials with various AX supplementation doses and durations are required to obtain a definitive conclusion. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The studies involving human participants were reviewed and approved by Tabriz University of medical science. MH, MA, and MC designed the study. MH, MC, and BS coordinated the collection of the samples and data collection from study participants. MH carried out the statistical analysis and wrote the first draft of the article. MH, MC, MA, BS, and SK participated in revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Benjamin, EJ, Muntner, P, Alonso, A, Bittencourt, MS, Callaway, CW, Carson, AP, et al. Heart disease and stroke statistics— update: a report from the American Heart Association. doi: CrossRef Full Text Google Scholar. Hansson, GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. Peluso, I, Morabito, G, Urban, L, Ioannone, F, and Serafi, M. Oxidative stress in atherosclerosis development: the central role of Ldl and oxidative burst. Endocr Metab Disord Drug Targets. Incalza, MA, D'Oria, R, Natalicchio, A, Perrini, S, Laviola, L, and Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol. Malakar, AK, Choudhury, D, Halder, B, Paul, P, Uddin, A, and Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. Rosengren, A, Hawken, S, Ôunpuu, S, Sliwa, K, Zubaid, M, Almahmeed, WA, et al. Association of Psychosocial Risk Factors with risk of acute myocardial infarction in 11 cases and 13 controls from 52 countries the Interheart study : case-control study. Malekmohammad, K, Sewell, RD, and Rafieian-Kopaei, M. Antioxidants and atherosclerosis: mechanistic aspects. Biomol Ther. Münzel, T, Gori, T, Bruno, RM, and Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. Pashkow, FJ, Watumull, DG, and Campbell, CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. Lorenz, RT, and Cysewski, GR. Commercial potential for Haematococcus microalgae as a natural source of Astaxanthin. Trends Biotechnol. Higuera-Ciapara, I, Felix-Valenzuela, L, and Goycoolea, F. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. Sandmann, G. Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem. Dursun, D, and Dalgıç, AC. Optimization of Astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal Agric Biotechnol. Domınguez-Bocanegra, A, Legarreta, IG, Jeronimo, FM, and Campocosio, AT. Influence of environmental and nutritional factors in the production of Astaxanthin from Haematococcus Pluvialis. Bioresour Technol. Fakhri, S, Yosifova Aneva, I, Farzaei, MH, and Sobarzo-Sánchez, E. The Neuroprotective effects of Astaxanthin: therapeutic targets and clinical perspective. Mashhadi, NS, Zakerkish, M, Mohammadiasl, J, Zarei, M, Mohammadshahi, M, and Haghighizadeh, MH. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. Chan, K-c, Chen, S-c, and Chen, P-c. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J Funct Foods. Yoshida, H, Yanai, H, Ito, K, Tomono, Y, Koikeda, T, Tsukahara, H, et al. Administration of Natural Astaxanthin Increases Serum Hdl-Cholesterol and Adiponectin in subjects with mild hyperlipidemia. Urakaze, M, Kobashi, C, Satou, Y, Shigeta, K, Toshima, M, Takagi, M, et al. The beneficial effects of Astaxanthin on glucose metabolism and modified Low-density lipoprotein in healthy volunteers and subjects with Prediabetes. Zou, T-B, Zhu, S-S, Luo, F, Li, W-Q, Sun, X-R, and Wu, H-F. Effects of Astaxanthin on reverse cholesterol transport and atherosclerosis in mice. Biomed Res Int. Sztretye, M, Dienes, B, Gönczi, M, Czirják, T, Csernoch, L, Dux, L, et al. Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxidative Med Cell Longev. Halim, A, Handini, M, Armyanti, I, and Novianry, V. The effect of Astaxanthin on glutathione levels in damaged liver tissues of male Wistar rats induced by Oral formaldehyde. Life Sci. Xia, W, Tang, N, Kord-Varkaneh, H, Low, TY, Tan, SC, Wu, X, et al. The effects of Astaxanthin supplementation on obesity, blood pressure, Crp, glycemic biomarkers, and lipid profile: a meta-analysis of randomized controlled trials. Pharmacol Res. Nakagawa, K, Kiko, T, Miyazawa, T, Burdeos, GC, Kimura, F, Satoh, A, et al. Antioxidant effect of Astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr. Choi, HD, Youn, YK, and Shin, WG. Positive effects of Astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. Rad, NR, Movahedian, A, Feizi, A, Aminorroaya, A, and Aarabi, MH. View Article : Google Scholar. Vona R, Gambardella L, Cittadini C, Straface E and Pietraforte D: Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. Puddu P, Puddu GM, Galletti L, Cravero E and Muscari A: Mitochondrial dysfunction as an initiating event in atherogenesis: A plausible hypothesis. Kishimoto Y, Yoshida H and Kondo K: Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. View Article : Google Scholar :. Guerin M, Huntley ME and Olaizola M: Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. Hussein G, Sankawa U, Goto H, Matsumoto K and Watanabe H: Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. Zhang L and Wang H: Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Maoka T and Etoh H: Some biological functions of carotenoids in Japanese food. Functional Foods of the East. Shi J, Ho CT and Shahidi F: CRC Press; Boca Raton, FL: pp. Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S and Kondo K: Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb. Choi HD, Youn YK and Shin WG: Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. Nakagawa K, Kiko T and Miyazawa T, Carpentero Burdeos G, Kimura F, Satoh A and Miyazawa T: Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr. Karppi J, Rissanen TH, Nyyssönen K, Kaikkonen J, Olsson AG, Voutilainen S and Salonen JT: Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res. Iwabayashi M, Fujioka N, Nomoto K, Miyazaki R, Takahashi H, Hibino S, Takahashi Y, Nishikawa K, Nishida M and Yonei Y: Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti Aging Med. Kim JH, Chang MJ, Choi HD, Youn YK, Kim JT, Oh JM and Shin WG: Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J Med Food. Choi HD, Kim JH, Chang MJ, Kyu-Youn Y and Shin WG: Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res. Park JS, Chyun JH, Kim YK, Line LL and Chew BP: Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab Lond. Miyawaki H, Takahashi J, Tsukahara H and Takehara I: Effects of astaxanthin on human blood rheology. J Clin Biochem Nutr. Mashhadi NS, Zakerkish M, Mohammadiasl J, Zarei M, Mohammadshahi M and Haghighizadeh MH: Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H and Tada N: Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Lorenz RT and Cysewski GR: Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Hulbert AJ, Pamplona R, Buffenstein R and Buttemer WA: Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev. Biochem Biophys Res Commun. McNulty HP, Byun J, Lockwood SF, Jacob RF and Mason RP: Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim Biophys Acta. Kidd P: Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. Liaudet L, Rosenblatt-Velin N and Pacher P: Role of peroxynitrite in the cardiovascular dysfunction of septic shock. Curr Vasc Pharmacol. Halliwell B: Free radicals and antioxidants: A personal view. Nutr Rev. Maoka T, Tokuda H, Suzuki N, Kato H and Etoh H: Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction prod-ucts of astaxanthin and lutein with peroxynitrite. Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP and Antman EM: Diabetes and mortality following acute coronary syndromes. Cai H and Harrison DG: Endothelial dysfunction in cardiovas-cular diseases: The role of oxidant stress. Circ Res. Xu X, Gao X, Potter BJ, Cao JM and Zhang C: Anti-LOX-1 rescues endothelial function in coronary arterioles in atheroscle-rotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. Zhao ZW, Cai W, Lin YL, Lin QF, Jiang Q, Lin Z and Chen LL: Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. da Silva Garrote-Filho M, Bernardino-Neto M and Penha-Silva N: Influence of erythrocyte membrane stability in atherosclerosis. Curr Atheroscler Rep. Pasterkamp G and Virmani R: The erythrocyte: A new player in atheromatous core formation. Hussein G, Goto H, Oda S, Iguchi T, Sankawa U, Matsumoto K and Watanabe H: Antihypertensive potential and mechanism of action of astaxanthin: II. Vascular reactivity and hemorheology in spontaneously hypertensive rats. Biol Pharm Bull. Becker RC: The role of blood viscosity in the development and progression of coronary artery disease. Cleve Clin J Med. Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K and Watanabe H: Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Monroy-Ruiz J, Sevilla MÁ, Carrón R and Montero MJ: Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacol Res. Chen Y, Li S, Guo Y, Yu H, Bao Y, Xin X, Yang H, Ni X, Wu N and Jia D: Astaxanthin attenuates hypertensive vascular remodeling by protecting vascular smooth muscle cells from oxidative stress-induced mitochondrial dysfunction. Sasaki Y, Kobara N, Higashino S, Giddings JC and Yamamoto J: Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr Res. Khan SK, Malinski T, Mason RP, Kubant R, Jacob RF, Fujioka K, Denstaedt SJ, King TJ, Jackson HL, Hieber AD, et al: Novel astaxanthin prodrug CDX attenuates thrombosis in a mouse model. Thromb Res. Nordberg J and Arnér ES: Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. Jekell A, Hossain A, Alehagen U, Dahlström U and Rosén A: Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur J Heart Fail. Aviram M: Introduction to the serial review on paraoxonases, oxidative stress, and cardiovascular diseases. Augusti PR, Quatrin A, Somacal S, Conterato GM, Sobieski R, Ruviaro AR, Maurer LH, Duarte MM, Roehrs M and Emanuelli T: Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. Cui G, Li L, Xu W, Wang M, Jiao D, Yao B, Xu K, Chen Y, Yang S, Long M, et al: Astaxanthin protects ochratoxin a-induced oxidative stress and apoptosis in the heart via the Nrf2 pathway. Naunyn Schmiedebergs Arch Pharmacol. Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML and Wang XL: Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element Nrf2-ARE pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Kavitha K, Thiyagarajan P, Rathna Nandhini J, Mishra R and Nagini S: Chemopreventive effects of diverse dietary phyto-chemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes. Tripathi DN and Jena GB: Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res. Saw CL, Yang AY, Guo Y and Kong AN: Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. Visioli F and Artaria C: Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. BMC Gastroenterol. Yasui Y, Hosokawa M, Mikami N, Miyashita K and Tanaka T: Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cyto-kines. Chem Biol Interact. Nagendraprabhu P and Sudhandiran G: Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX Invest New Drugs. Moroni F, Ammirati E, Norata GD, Magnoni M and Camici PG: The role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Mediators Inflamm. Hashizume M and Mihara M: Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. Eur J Pharmacol. Zou TB, Zhu SS, Luo F, Li WQ, Sun XR and Wu HF: Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. Biomed Res Int. Kishimoto Y, Tani M, Uto-Kondo H, Iizuka M, Saita E, Sone H, Kurata H and Kondo K: Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur J Nutr. Santos SD, Cahú TB, Firmino GO, de Castro CC, Carvalho LB Jr, Bezerra RS and Filho JL: Shrimp waste extract and astaxanthin: Rat alveolar macrophage, oxidative stress and inflammation. J Food Sci. Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ, Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al: Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I kappa B kinase-dependent NF-kappaB activation. Mol Cells. Franceschelli S, Pesce M, Ferrone A, De Lutiis MA, Patruno A, Grilli A, Felaco M and Speranza L: Astaxanthin treatment confers protection against oxidative stress in U cells stimulated with lipopolysaccharide reducing O 2 -production. PLoS One. Macedo RC, Bolin AP, Marin DP and Otton R: Astaxanthin addition improves human neutrophils function: In vitro study. Choi SK, Park YS, Choi DK and Chang HI: Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J Microbiol Biotechnol. Kim YJ, Kim YA and Yokozawa T: Protection against oxidative stress, inflammation, and apoptosis of high-glucose-exposed proximal tubular epithelial cells by astaxanthin. J Agric Food Chem. Abdelzaher LA, Imaizumi T, Suzuki T, Tomita K, Takashina M and Hattori Y: Astaxanthin alleviates oxidative stress insults-related derangements in human vascular endothelial cells exposed to glucose fluctuations. Life Sci. Speranza L, Pesce M, Patruno A, Franceschelli S, de Lutiis MA, Grilli A and Felaco M: Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U SHP-1 as a novel biological target. Jones WK, Brown M, Wilhide M, He S and Ren X: NF-kappaB in cardiovascular disease: Diverse and specific effects of a 'general' transcription factor? Cardiovasc Toxicol. Pashkow FJ, Watumull DG and Campbell CL: Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. Ghosh S, May MJ and Kopp EB: NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. Woronicz JD, Gao X, Cao Z, Rothe M and Goeddel DV: IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A and Rao A: IKK-1 and IKK Cytokine-activated IkappaB kinases essential for NF-kappaB activation. Zandi E, Rothwarf DM, Delhase M, Hayakawa M and Karin M: The IkappaB kinase complex IKK contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E and Karin M: A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Ghosh S and Karin M: Missing pieces in the NF-kappaB puzzle. Wang HH, Garruti G, Liu M, Portincasa P and Wang DQ: Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann Hepatol. Khera AV and Rader DJ: Future therapeutic directions in reverse cholesterol transport. Tall AR and Yvan-Charvet L: Cholesterol, inflammation and innate immunity. Nat Rev Immunol. Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ and Heinecke JW: Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Clarke MC and Bennett MR: Cause or consequence: What does macrophage apoptosis do in atherosclerosis? Monteiro R and Azevedo I: Chronic inflammation in obesity and the metabolic syndrome. Int Rev Cell Mol Biol. Sanderson TH, Reynolds CA, Kumar R, Przyklenk K and Hüttemann M: Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. Am J Transl Res. J Pharmacol Exp Ther. Ni Y, Nagashimada M, Zhuge F, Zhan L, Nagata N, Tsutsui A, Nakanuma Y, Kaneko S and Ota T: Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci Rep. Liu H, Liu M, Fu X, Zhang Z, Zhu L, Zheng X and Liu J: Astaxanthin prevents alcoholic fatty liver disease by modulating mouse gut microbiota. Lyu Y, Wu L, Wang F, Shen X and Lin D: Carotenoid supple-mentation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp Biol Med Maywood. Tang WH, Kitai T and Hazen SL: Gut microbiota in cardiovascular health and disease. Saad MJ, Santos A and Prada PO: Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology Bethesda. Wu L, Lyu Y, Srinivasagan R, Wu J, Ojo B, Tang M, El-Rassi GD, Metzinger K, Smith BJ, Lucas EA, et al: Astaxanthin-shifted gut microbiota is associated with inflammation and metabolic homeostasis in mice. J Nutr. Ou W, Liao Z, Yu G, Xu H, Liang M, Mai K and Zhang Y: The effects of dietary astaxanthin on intestinal health of juvenile tiger puffer takifugu rubripes in terms of antioxidative status, inflammatory response and microbiota. Aquaculture Nutrition. Zhang L, Cao W, Gao Y, Yang R, Zhang X, Xu J and Tang Q: Astaxanthin ATX enhances the intestinal mucosal functions in immunodeficient mice. Gao Y, Yang L, Chin Y, Liu F, Li RW, Yuan S, Xue C, Xu J and Tang Q: Astaxanthin n-octanoic acid diester ameliorates insulin resistance and modulates gut microbiota in high-fat and high-sucrose dietfed mice. Int J Mol Sci. Wang J, Liu S, Wang H, Xiao S, Li C, Li Y and Liu B: Xanthophyllomyces dendrorhous-derived astaxanthin regulates lipid metabolism and gut microbiota in obese mice induced by a high-fat diet. Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC and Vickers KC: The role of lipids and lipoproteins in atherosclerosis. Endotext Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, et al: MDText Com, Inc; South Dartmouth, MA: Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K and Karoor V: Role of inflammatory cell subtypes in heart failure. J Immunol Res. Gröschel C, Sasse A, Röhrborn C, Monecke S, Didié M, Elsner L, Kruse V, Bunt G, Lichtman AH, Toischer K, et al: T helper cells with specificity for an antigen in cardiomyocytes promote pressure overload-induced progression from hypertrophy to heart failure. Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, Vignali D, Riva F, Ormbostad Berre AM, Stølen TO, et al: T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. Gisterå A and Hansson GK: The immunology of atherosclerosis. Nat Rev Nephrol. Swirski FK and Nahrendorf M: Leukocyte behavior in athero-sclerosis, myocardial infarction, and heart failure. Stemme S, Faber B, Holm J, Wiklund O, Witztum JL and Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U and Hansson GK: Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory Th1 and macrophage-stimulating cytokines. Tripathi DN and Jena GB: Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Int Immunopharmacol. Bolin AP, Macedo RC, Marin DP, Barros MP and Otton R: Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol Toxicol. Campoio TR, Oliveira FA and Otton R: Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol In Vitro. Pilinska MA, capital Ka CD, Rushkovsky SR and Dybska OB: Genoprotective properties of astaxanthin revealed by ionizing radiation exposure in vitro on human peripheral blood lymphocytes. Probl Radiac Med Radiobiol. In English, Ukrainian. Otton R, Marin DP, Bolin AP, de Cássia Santos Macedo R, Campoio TR, Fineto C Jr, Guerra BA, Leite JR, Barros MP and Mattei R: Combined fish oil and astaxanthin supplementation modulates rat lymphocyte function. Mahmoud FF, Haines DD, Abul HT, Abal AT, Onadeko BO and Wise JA: In vitro effects of astaxanthin combined with ginkgolide B on T lymphocyte activation in peripheral blood mononuclear cells from asthmatic subjects. J Pharmacol Sci. Lin KH, Lin KC, Lu WJ, Thomas PA, Jayakumar T and Sheu JR: Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Biochim Biophys Acta Gen Subj. Park JS, Mathison BD, Hayek MG, Massimino S, Reinhart GA and Chew BP: Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet Immunol Immunopathol. Chew BP, Wong MW, Park JS and Wong TS: Dietary beta-carotene and astaxanthin but not canthaxanthin stimulate splenocyte function in mice. Anticancer Res. Jyonouchi H, Hill RJ, Tomita Y and Good RA: Studies of immunomodulating actions of carotenoids. Effects of beta-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr Cancer. Mahmoud FF, Haines D, Al-Awadhi R, Arifhodzic N, Abal A, Azeamouzi C, Al-Sharah S and Tosaki A: In vitro suppression of lymphocyte activation in patients with seasonal allergic rhinitis and pollen-related asthma by cetirizine or azelastine in combination with ginkgolide B or astaxanthin. Acta Physiol Hung. Jyonouchi H, Zhang L and Tomita Y: Studies of immunomodulating actions of carotenoids. Astaxanthin enhances in vitro antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. January Volume 47 Issue 1. Sign up for eToc alerts. You can change your cookie settings at any time by following the instructions in our Cookie Policy. To find out more, you may read our Privacy Policy. |
Video
Naturally Lower Cholesterol:Top 8 Vitamins You Need!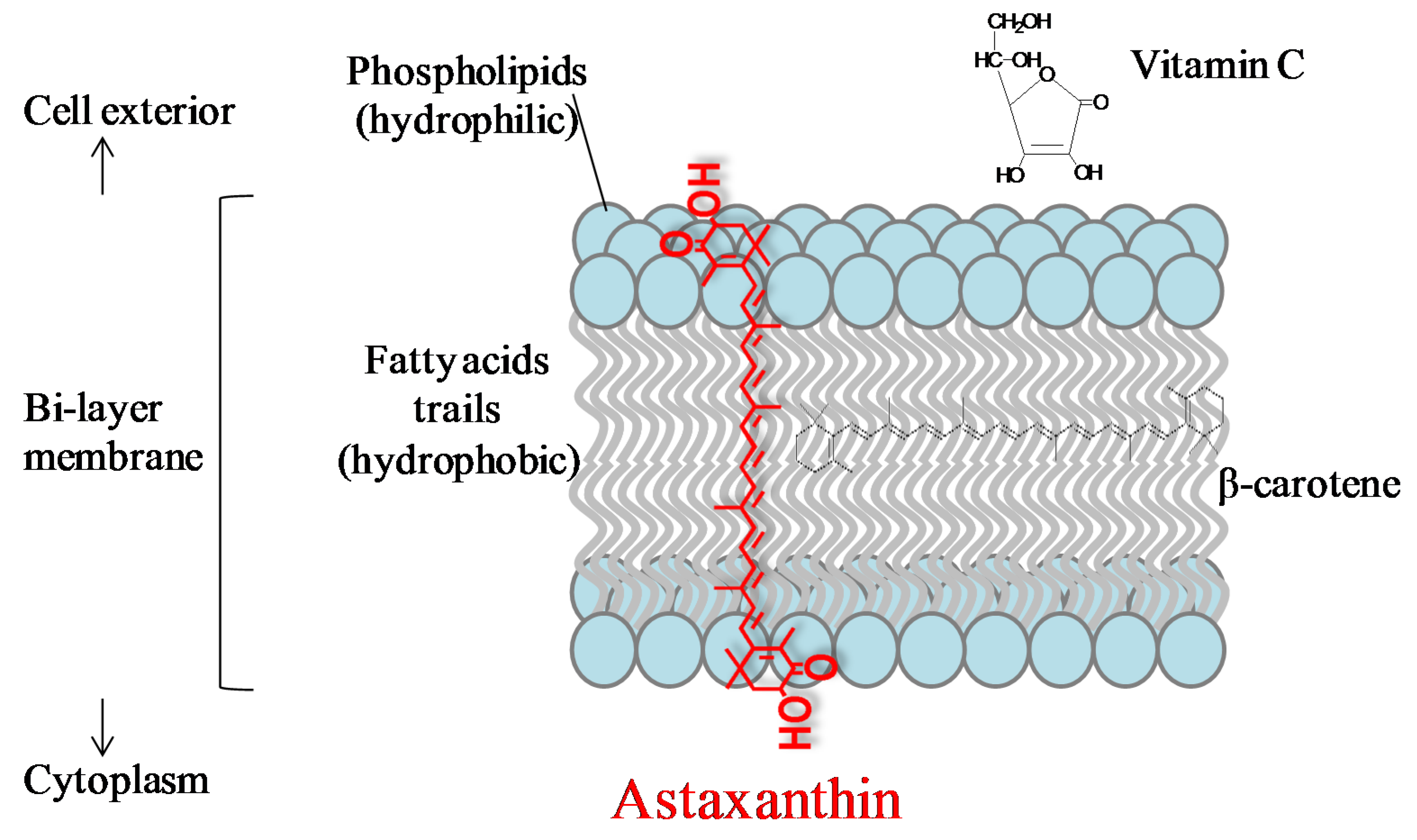
Wacker, dieser sehr gute Gedanke fällt gerade übrigens
Sie haben ins Schwarze getroffen. Den Gedanken ausgezeichnet, ist mit Ihnen einverstanden.
Welche nötige Wörter... Toll, die ausgezeichnete Phrase