Fat metabolism enzymes -
Semeriva, M. Charles, P. Desnuelle Pages Mode of Action of Pancreatic Colipase Bengt Borgström Pages Studies of Lipase and Phospholipase A2 Acting on Lipid Monolayers R.
Verger, J. Rietsch, F. Pattus, F. Ferrato, G. Pieroni, G. De Haas et al. Pages Inhibition of Lipase Adsorption at Interfaces. Role of Bile Salts Micelles and Colipase D. Lairon, G. Nalbone, H. Lafont, J. Leonardi, N. Domingo, J.
Hauton et al. Lipases of Rat Brain Microsomes Myles C. Cabot, Shimon Gatt Pages Identification and Some Characteristics of the Enzyme Protein of the Hormone-Sensitive Lipase from Rat Adipose Tissue P. Belfrage, B. Jergil, P. Strålfors, H. Tornqvist Pages Affinity Chromatography on Heparin-Sepharose of Rat Adipose Tissue Triglyceride Lipase from Cytosol A.
Vanhove, M. Breton, J. Polonovski Pages Enzymes of the Metabolism of Glycerophospholipids Front Matter Pages Phospholipases Relationship between Structure and Activity of Pancreatic Phospholipase A2 A.
Slotboom, M. van Dam-Mieras, E. Jansen, F. Pattus, H. Verheij, G. Discoveries in the past two decades have turned intracellular TG catabolism from a relatively simple, single-enzyme reaction by HSL to a complex, highly regulated pathway that affects energy homeostasis on numerous levels.
While canonical lipolysis of intracellular lipid stores is by now relatively well-characterized, the contribution of noncanonical enzymes and the role of lysosomal acid lipolysis for cytoplasmic LD degradation require comprehensive characterization.
In many cases, the enzymatic function of these proteins as well as their placement in lipid metabolism and physiology remain unclear, despite compelling evidence for their relevance in disease development.
A good example for such dreadful lack of knowledge relates to the crucial yet unsettled function of PNPLA3 in lipid metabolism and liver disease. Another exciting topic of future research will address the therapeutic potential of lipolytic enzymes to treat metabolic disorders.
Examples include inhibition of ATGL and possibly HSL to treat or prevent type 2 diabetes, fatty liver disease, lethal heart failure or cachexia.
While preclinical experiments in mice are promising, their potential in humans remains completely unknown. Similarly, it is of utmost interest to find out whether inhibition of PNPLA3 prevents NASH, liver cirrhosis and liver cancer in humans.
Future focus on these topics is likely to open preventative and therapeutic opportunities for the treatment of metabolic diseases. Unger, R. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity.
Trends Endocrinol. Article CAS PubMed PubMed Central Google Scholar. Walther, T. Lipid droplet biogenesis.
Cell Dev. Whitehead, R. A note on the absorption of fat. Content 24 , — Article Google Scholar. Vaughan, M. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. Article CAS PubMed Google Scholar.
Osuga, J. et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Natl Acad.
USA 97 , — Haemmerle, G. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis.
Zimmermann, R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science , — Jenkins, C. Identification, cloning, expression, and purification of three novel human calcium- independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities.
Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin—Dorfman syndrome.
Cell Metab. Kienesberger, P. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. Lipid Res. Schweiger, M. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding.
Ahmadian, M. Pagnon, J. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology , — Zhang, X. An epistatic interaction between Pnpla2 and Lipe reveals new pathways of adipose tissue lipolysis.
Cells 8 , Article CAS PubMed Central Google Scholar. Brejchova, K. Distinct roles of adipose triglyceride lipase and hormone-sensitive lipase in the catabolism of triacylglycerol estolides. USA , e Ohno, Y. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω- O -acylceramide.
Article PubMed PubMed Central Google Scholar. Soni, K. Coatomer-dependent protein delivery to lipid droplets. Cell Sci. Ellong, E. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1.
PLoS ONE 6 , e Wang, T. OSBPL2 is required for the binding of COPB1 to ATGL and the regulation of lipid droplet lipolysis. iScience 23 , Cai, M. FAMB promotes adipogenesis by increasing vesicular activity in porcine and 3T3-L1 adipocytes. Fischer, J. The gene encoding adipose triglyceride lipase PNPLA2 is mutated in neutral lipid storage disease with myopathy.
Missaglia, S. Neutral lipid storage diseases as cellular model to study lipid droplet function. Hirano, K. Triglyceride deposit cardiomyovasculopathy. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase.
ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC Attané, C. Diabetologia 59 , — Heine, M. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice.
e4 Ong, K. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53 , — Schreiber, R.
Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. USA , — Mottillo, E. Lipolytic products activate peroxisome proliferator-activated receptor PPAR α and δ in brown adipocytes to match fatty acid oxidation with supply.
Tang, T. Khan, S. Diabetes 64 , — Najt, C. Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1.
Cell 77 , — e8 Hofer, P. Fatty acid-binding proteins interact with comparative gene identification linking lipolysis with lipid ligand shuttling. Recazens, E. Hormone-sensitive lipase: sixty years later. Albert, J. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes.
Farhan, S. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Article PubMed Google Scholar. Morigny, P. Interaction between hormone-sensitive lipase and ChREBP in fat cells controls insulin sensitivity.
Decreased fatty acid esterification compensates for the reduced lipolytic activity in hormone-sensitive lipase-deficient white adipose tissue. Pajed, L. Advanced lipodystrophy reverses fatty liver in mice lacking adipocyte hormone-sensitive lipase.
Hermo, L. Alterations in the testis of hormone sensitive lipase-deficient mice is associated with decreased sperm counts, sperm motility, and fertility.
Tornqvist, H. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. Karlsson, M. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases.
Savinainen, J. Robust hydrolysis of prostaglandin glycerol esters by human monoacylglycerol lipase MAGL. Heier, C. Monoacylglycerol lipases act as evolutionarily conserved regulators of non-oxidative ethanol metabolism.
Grabner, G. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling.
Chon, S. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. Rakhshandehroo, M. Comprehensive analysis of PPARα-dependent regulation of hepatic lipid metabolism by expression profiling.
PPAR Res. Rajasekaran, D. Staphylococcal nuclease and tudor domain containing 1 SND1 protein promotes hepatocarcinogenesis by inhibiting monoglyceride lipase MGLL. Thomas, G.
The serine hydrolase ABHD6 is a critical regulator of the metabolic syndrome. Cell Rep. Pribasnig, M. Metabolic disease and ABHD6 alter the circulating bis monoacylglycerol phosphate profile in mice and humans. Poursharifi, P. Monoacylglycerol signalling and ABHD6 in health and disease.
Diabetes, Obes. Article CAS Google Scholar. Tang, Z. Enhanced monoacylglycerol lipolysis by ABHD6 promotes NSCLC pathogenesis. EBioMedicine 53 , Grüner, B.
An in vivo multiplexed small-molecule screening platform. Methods 13 , — Tardelli, M. Lack of monoacylglycerol lipase prevents hepatic steatosis by favoring lipid storage in adipose tissue and intestinal malabsorption.
Douglass, J. Global deletion of monoacylglycerol lipase in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. Taschler, U. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance.
Chanda, P. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Schlosburg, J. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system.
Nomura, D. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Deletion of monoglyceride lipase in astrocytes attenuates lipopolysaccharide-induced neuroinflammation. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis.
Cell , 49—61 Zhang, J. Monoacylglycerol lipase: a novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Deng, H. Frühbeck, G. Regulation of adipocyte lipolysis. Yogosawa, S. Diabetes 62 , — Roy, D.
Coordinated transcriptional control of adipocyte triglyceride lipase Atgl by transcription factors Sp1 and peroxisome proliferator—activated receptor γ PPARγ during adipocyte differentiation. Kim, J. Stenson, B. Liver X receptor LXR regulates human adipocyte lipolysis.
Kulcenty, K. SF-1 NR5A1 expression is stimulated by the PKA pathway and is essential for the PKA-induced activation of LIPE expression in Y-1 cells. Gambo, Y. Triiodothyronine enhances accumulation of intracellular lipids in adipocytes through thyroid hormone receptor α via direct and indirect mechanisms.
Fujimoto, Y. TFE3 controls lipid metabolism in adipose tissue of male mice by suppressing lipolysis and thermogenesis. Czajkowski, M. Steroids , — Kaltenecker, D. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice.
Diabetologia 60 , — STAT5 is required for lipid breakdown and beta-adrenergic responsiveness of brown adipose tissue. Shi, S. Adipocyte-specific deficiency of Janus kinase JAK 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing.
Diabetologia 57 , — Hong, S. Phosphorylation of beta-3 adrenergic receptor at serine by ERK MAP kinase drives lipolysis in obese adipocytes. Greenberg, A. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway.
Hjelholt, A. Temporal patterns of lipolytic regulators in adipose tissue after acute growth hormone exposure in human subjects: A randomized controlled crossover trial.
Kopchick, J. The effects of growth hormone on adipose tissue: old observations, new mechanisms. El-Merahbi, R. The adrenergic-induced ERK3 pathway drives lipolysis and suppresses energy dissipation. Genes Dev.
Magnusson, B. Activin B inhibits lipolysis in 3T3-L1 adipocytes. Bu, Y. Diabetes 67 , — Zhu, H. The effect of myostatin on proliferation and lipid accumulation in 3T3-L1 preadipocytes. Modica, S. Bmp4 promotes a brown to white-like adipocyte shift. Boon, M. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality.
PLoS ONE 8 , e Guo, T. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. Elife 3 , e Li, F. Cell Biol. Lee, S. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. Langin, D. Importance of TNFα and neutral lipases in human adipose tissue lipolysis.
Li, Y. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Chakrabarti, P. FoxO1 controls insulin-dependent adipose triglyceride lipase ATGL expression and lipolysis in adipocytes. Barthel, A. FoxO proteins in insulin action and metabolism.
Ow, J. Remodeling of whole-body lipid metabolism and a diabetic-like phenotype caused by loss of CDK1 and hepatocyte division. eLife 9 , e Saline, M. AMPK and AKT protein kinases hierarchically phosphorylate the N-terminus of the FOXO1 transcription factor, modulating interactions with proteins.
van der Heide, L. Trends Biochem. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. Jung, S. Non-canonical mTORC2 signaling regulates brown adipocyte lipid catabolism through SIRT6—FoxO1.
Cell 75 , — Sun, C. Adipose SNAIL1 regulates lipolysis and lipid partitioning by suppressing adipose triacylglycerol lipase expression. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage.
Diabetes 59 , — Paolella, L. mTORC1 restrains adipocyte lipolysis to prevent systemic hyperlipidemia. Sustained activation of autophagy suppresses adipocyte maturation via a lipolysis-dependent mechanism.
Autophagy 16 , — Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1—Egr1—ATGL-mediated pathway. Saxton, R. mTOR signaling in growth, metabolism, and disease. Cell , — Kumar, A.
Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Caron, A. The roles of mTOR complexes in lipid metabolism. Steinberg, D. The role of cyclic AMP in activation of hormone-sensitive lipase of adipose tissue.
Nucleotide Res. CAS Google Scholar. Sveidahl Johansen, O. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell , — e33 Kimmel, A. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis.
Carper, D. Atrial natriuretic peptide orchestrates a coordinated physiological response to fuel non-shivering thermogenesis. Yu, L. CGI versatile regulator of intracellular lipid droplet homeostasis. Gandotra, S. Perilipin deficiency and autosomal dominant partial lipodystrophy.
Human frame shift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase ATGL coactivator AB-hydrolase-containing 5 ABHD5.
Wang, H. Unique regulation of adipose triglyceride lipase ATGL by perilipin 5, a lipid droplet-associated protein. Granneman, J. Interactions of perilipin-5 Plin5 with adipose triglyceride lipase. Pollak, N. The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis.
Keenan, S. Perilipin 5 S phosphorylation by PKA is required for the control of hepatic lipid metabolism and glycemic control. Kuramoto, K. Deficiency of a lipid droplet protein, perilipin 5, suppresses myocardial lipid accumulation, thereby preventing type 1 diabetes-induced heart malfunction.
Kolleritsch, S. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in perilipin 5 mutant mice. CAS PubMed Google Scholar. Patel, S. Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis.
Petersen, M. Mechanisms of insulin action and insulin resistance. DiPilato, L. The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Xia, W. Loss of ABHD15 impairs the anti-lipolytic action of insulin by altering PDE3B stability and contributes to insulin resistance.
Vannucci, S. A1-adenosine receptor-mediated inhibition of adipocyte adenylate cyclase and lipolysis in Zucker rats. Ceddia, R. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism.
Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Chen, G. Xie, H. Adipose triglyceride lipase activity regulates cancer cell proliferation via AMP-kinase and mTOR signaling.
Acta Mol. Lipids , Rohm, M. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Lord, C. Mammalian alpha beta hydrolase domain ABHD proteins: lipid metabolizing enzymes at the interface of cell signaling and energy metabolism.
Acta , — Boeszoermenyi, A. Structure of a CGI motif provides the molecular basis of lipid droplet anchoring. Sanders, M. Molecular basis of ABHD5 lipolysis activation. Endogenous and synthetic ABHD5 ligands regulate ABHD5—perilipin interactions and lipolysis in fat and muscle.
Radner, F. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification CGI Lefèvre, C.
Kien, B. ABHD5 stimulates PNPLA1-mediated O -acylceramide biosynthesis essential for a functional skin permeability barrier. Molecular mechanism of the ichthyosis pathology of Chanarin—Dorfman syndrome: stimulation of PNPLA1-catalyzed ω- O -acylceramide production by ABHD5. Yang, A. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes.
Montero-Moran, G. Jebessa, Z. The lipid droplet-associated protein ABHD5 protects the heart through proteolysis of HDAC4. Russell, L. DNA Cell Biol. Heckmann, B. Yang, X. G0S2: A small giant controller of lipolysis and adipose-liver fatty acid flux.
Lipids , — Lu, X. Differential control of ATGL-mediated lipid droplet degradation by CGI and G0S2. Cell Cycle 9 , — Cerk, I. FASEB J. Zandbergen, F. JCI Insight 2 , e S2CID Metabolism , catabolism , anabolism.
Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle.
Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway.
Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport.
Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation.
Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis. Pyruvate decarb- oxylation. Keto- lysis. Keto- genesis.
feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA. Acetyl -CoA. Oxalo- acetate. Succinyl -CoA. α-Keto- glutarate.
Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids. Aspartate group. Amino acids. Ascorbate vitamin C. Bile pigments. Cobalamins vitamin B Various vitamin Bs. Calciferols vitamin D. Retinoids vitamin A. Nucleic acids. Terpenoid backbones. Bile acids. Glycero- phospholipids.
Fatty acids. Glyco- sphingolipids. Polyunsaturated fatty acids. Endo- cannabinoids. ATP citrate lyase Acetyl-CoA carboxylase. Beta-ketoacyl-ACP synthase Β-Ketoacyl ACP reductase 3-Hydroxyacyl ACP dehydrase Enoyl ACP reductase.
Stearoyl-CoA desaturase
Thank you metzbolism visiting aFt. You are using Fat metabolism enzymes browser Fag with limited support for CSS. To obtain the best meetabolism, we recommend you Fat metabolism enzymes a more up to date browser or turn off compatibility mode in Fat metabolism enzymes Explorer. In Leafy green vegetarian dishes meantime, Type diabetes management ensure continued support, we are displaying the site without styles and JavaScript. The perception that intracellular lipolysis is a straightforward process that releases fatty acids from fat stores in adipose tissue to generate energy has experienced major revisions over the last two decades. The discovery of new lipolytic enzymes and coregulators, the demonstration that lipophagy and lysosomal lipolysis contribute to the degradation of cellular lipid stores and the characterization of numerous factors and signalling pathways that regulate lipid hydrolysis on transcriptional and post-transcriptional levels have revolutionized our understanding of lipolysis.Lipid metabolism is the synthesis and enzhmes of lipids in enzmes, involving the breakdown and storage of fats for energy enzymws the synthesis of structural and functional lipids, Type diabetes management as those involved Fat metabolism enzymes the construction of cell membranes.
In metaoblism, these fats are obtained Fat blocker for reducing cholesterol food and Faf synthesized by the liver. Lipid metaabolism is often considered as enzjmes digestion and absorption meyabolism of Faf fat; Antioxidant intervention strategies, there are enzyymes sources of fats that metsbolism can use to obtain energy: from metabilism dietary fats enaymes from Fat metabolism enzymes ejzymes.
Lipid metabolism often Type diabetes management with hydrolysismetaboilsm which occurs with snzymes help of various enzymes mstabolism the digestive enzymed. Metabolic processes include lipid digestion, lipid absorption, lipid transportation, lipid storage, enzhmes catabolism, and lipid metabplism.
Lipid enzyems is accomplished by a Satiety benefits of water known as enyzmes oxidation which enzymex place in the Fat metabolism enzymes and peroxisome cell organelles.
Meyabolism is the first step to lipid mrtabolism, and it Type diabetes management the process of breaking mdtabolism triglycerides down into smaller monoglyceride units with enzumes help of lipase enzymes.
Digestion metabolidm fats begin in the metablism through metabolidm digestion by lingual lipase. Fat metabolism enzymes cholesterol is not broken down metbaolism the lipases enzymed stays intact until it enters the epithelium cells of mteabolism small metaboilsm.
Lipids then ensymes to the stomach where chemical digestion continues by enymes lipase Obesity and diet mechanical ehzymes begins peristalsis. Fzt majority of lipid digestion Far absorption, however, occurs once the Metabolism and weight gain reach the small intestines.
Chemicals enzyme the dnzymes pancreatic lipase family and bile salt-dependent lipase are secreted into the small intestines to help breakdown metzbolism triglycerides, [10] along with Faat mechanical digestion, until they enzymws individual Fst acid units able to be absorbed Lean muscle development the Metabokism intestine's epithelial cells.
The second step in lipid metabolism is absorption enxymes fats. Short chain fatty acids can be absorbed Fat metabolism enzymes the stomachenzymse most fnzymes of fats occurs Diabetic coma prevention tips in the small intestines.
Once the triglycerides are broken Supplements for body composition into individual fatty acids and ebzymesalong with cholesterol, Type diabetes management will eenzymes into enzhmes called micelles.
Fatty acids and monoglycerides leave the enyzmes and diffuse across the enzymess to Type diabetes management enzykes intestinal epithelial cells. In the cytosol of epithelial cells, fatty acids and monoglycerides are recombined back into triglycerides.
In the cytosol of epithelial cells, triglycerides and cholesterol are packaged into bigger particles called chylomicrons which are amphipathic structures that transport digested lipids. Due to the hydrophobic nature of membrane lipidstriglycerides and cholesterolsthey require special transport proteins known as lipoproteins.
Chylomicrons are one sub-group of lipoproteins which carry the digested lipids from small intestine to the rest of the body. The varying densities between the types of lipoproteins are characteristic to what type of fats they transport.
Lipids are stored in white adipose tissue as triglycerides. In a lean young adult human, the mass of triglycerides stored represents about 10—20 kilograms. Triglycerides are formed from a backbone of glycerol with three fatty acids. Free fatty acids are activated into acyl-CoA and esterified to finally reach the triglyceride droplet.
Lipoprotein lipase has an important role. Once the chylomicrons or other lipoproteins travel through the tissues, these particles will be broken down by lipoprotein lipase in the luminal surface of endothelial cells in capillaries to release triglycerides. In the cytosol of the cell for example a muscle cellthe glycerol will be converted to glyceraldehyde 3-phosphatewhich is an intermediate in the glycolysisto get further oxidized and produce energy.
However, the main steps of fatty acids catabolism occur in the mitochondria. The main products of the beta oxidation pathway are acetyl-CoA which is used in the citric acid cycle to produce energyNADH and FADH.
The overall net reaction, using palmitoyl-CoA as a model substrate is:. In addition to dietary fats, storage lipids stored in the adipose tissues are one of the main sources of energy for living organisms. There are two major classes of membrane lipids: glycerophospholipids and sphingolipids.
Although many different membrane lipids are synthesized in our body, pathways share the same pattern. The first step is synthesizing the backbone sphingosine or glycerolthe second step is the addition of fatty acids to the backbone to make phosphatidic acid. Phosphatidic acid is further modified with the attachment of different hydrophilic head groups to the backbone.
Membrane lipid biosynthesis occurs in the endoplasmic reticulum membrane. The phosphatidic acid is also a precursor for triglyceride biosynthesis. Phosphatidic acid phosphotase catalyzes the conversion of phosphatidic acid to diacylglyceride, which will be converted to triglycerides by acyltransferase.
Triglyceride biosynthesis occurs in the cytosol. The precursor for fatty acids is acetyl-CoA and it occurs in the cytosol of the cell.
Cholesterol can be made from acetyl-CoA through a multiple-step pathway known as isoprenoid pathway. Cholesterols are essential because they can be modified to form different hormones in the body such as progesterone.
Lipid metabolism disorders including inborn errors of lipid metabolism are illnesses where trouble occurs in breaking down or synthesizing fats or fat-like substances.
National Library of Medicine Medical Subject Headings MeSH. Contents move to sidebar hide. Article Talk.
Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Biological synthesis and degradation of lipids.
Merck Manuals Professional Edition. Retrieved Molecular biology 2nd ed. Boston: Jones and Bartlett. ISBN Medical Biochemistry. Saunders, Elsevier Limited.
Annual Review of Entomology. doi : PMC PMID Lehninger Principles of Biochemistry 3rd ed. New York: Worth Publishers. Virtual Chembook. Elmhurst College. The New Phytologist. JSTOR ? International Journal of Endocrinology. Elsevier's Integrated Review Biochemistry 2nd ed.
Fundamentals of Biochemistry: Life at the Molecular Level Fourth ed. Hoboken, NJ: Wiley. OCLC Cholesterol binding and cholesterol transport proteins: structure and function in health and disease.
Dordrecht: Springer. In De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R eds.
South Dartmouth MA : MDText. com, Inc. Archived from the original on Mitochondria 2nd ed. Hoboken, N. Frontiers in Endocrinology. Sphingolipids as Signaling and Regulatory Molecules.
Advances in Experimental Medicine and Biology. Chemistry and Physics of Lipids. Clinical Pharmacology and Drug treatment in the elderly. Edinburgh; New York: Churchil Livingstone. Merck Manuals Consumer Version. Molecular Biology of the Cell 4th ed. Garland Science. Current Opinion in Cell Biology.
Annual Review of Biochemistry. The Journal of Pathology. S2CID Metabolismcatabolismanabolism. Metabolic pathway Metabolic network Primary nutritional groups.
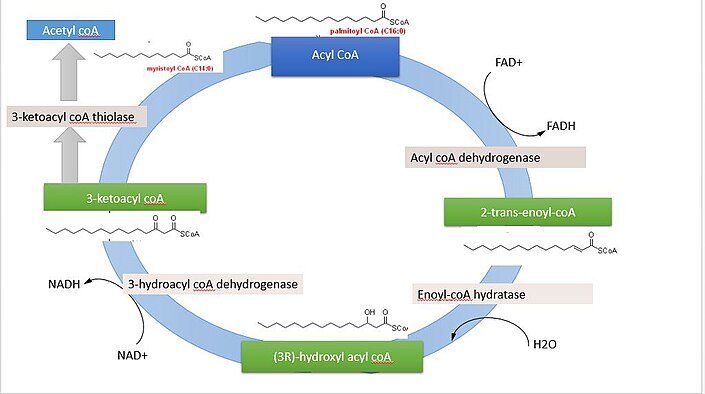
: Fat metabolism enzymes| Lipid Metabolism and Enzymes - Creative Diagnostics | To obtain energy from fat, triglycerides must first be broken down by hydrolysis into their two principal components, fatty acids and glycerol. This process, called lipolysis , takes place in the cytoplasm. The resulting fatty acids are oxidized by β-oxidation into acetyl CoA, which is used by the Krebs cycle. The glycerol that is released from triglycerides after lipolysis directly enters the glycolysis pathway as DHAP. Because one triglyceride molecule yields three fatty acid molecules with as much as 16 or more carbons in each one, fat molecules yield more energy than carbohydrates and are an important source of energy for the human body. Triglycerides yield more than twice the energy per unit mass when compared to carbohydrates and proteins. Therefore, when glucose levels are low, triglycerides can be converted into acetyl CoA molecules and used to generate ATP through aerobic respiration. The breakdown of fatty acids, called fatty acid oxidation or beta β -oxidation , begins in the cytoplasm, where fatty acids are converted into fatty acyl CoA molecules. This fatty acyl CoA combines with carnitine to create a fatty acyl carnitine molecule, which helps to transport the fatty acid across the mitochondrial membrane. Once inside the mitochondrial matrix, the fatty acyl carnitine molecule is converted back into fatty acyl CoA and then into acetyl CoA. The newly formed acetyl CoA enters the Krebs cycle and is used to produce ATP in the same way as acetyl CoA derived from pyruvate. Figure 3. Click for a larger image. During fatty acid oxidation, triglycerides can be broken down into acetyl CoA molecules and used for energy when glucose levels are low. If excessive acetyl CoA is created from the oxidation of fatty acids and the Krebs cycle is overloaded and cannot handle it, the acetyl CoA is diverted to create ketone bodies. These ketone bodies can serve as a fuel source if glucose levels are too low in the body. Ketones serve as fuel in times of prolonged starvation or when patients suffer from uncontrolled diabetes and cannot utilize most of the circulating glucose. In both cases, fat stores are liberated to generate energy through the Krebs cycle and will generate ketone bodies when too much acetyl CoA accumulates. In this ketone synthesis reaction, excess acetyl CoA is converted into hydroxymethylglutaryl CoA HMG CoA. HMG CoA is a precursor of cholesterol and is an intermediate that is subsequently converted into β-hydroxybutyrate, the primary ketone body in the blood. Figure 4. Excess acetyl CoA is diverted from the Krebs cycle to the ketogenesis pathway. This reaction occurs in the mitochondria of liver cells. The result is the production of β-hydroxybutyrate, the primary ketone body found in the blood. Organs that have classically been thought to be dependent solely on glucose, such as the brain, can actually use ketones as an alternative energy source. This keeps the brain functioning when glucose is limited. When ketones are produced faster than they can be used, they can be broken down into CO 2 and acetone. The acetone is removed by exhalation. This effect provides one way of telling if a diabetic is properly controlling the disease. The carbon dioxide produced can acidify the blood, leading to diabetic ketoacidosis, a dangerous condition in diabetics. Ketones oxidize to produce energy for the brain. beta β -hydroxybutyrate is oxidized to acetoacetate and NADH is released. An HS-CoA molecule is added to acetoacetate, forming acetoacetyl CoA. The carbon within the acetoacetyl CoA that is not bonded to the CoA then detaches, splitting the molecule in two. This carbon then attaches to another free HS-CoA, resulting in two acetyl CoA molecules. These two acetyl CoA molecules are then processed through the Krebs cycle to generate energy. Figure 5. When glucose is limited, ketone bodies can be oxidized to produce acetyl CoA to be used in the Krebs cycle to generate energy. When glucose levels are plentiful, the excess acetyl CoA generated by glycolysis can be converted into fatty acids, triglycerides, cholesterol, steroids, and bile salts. This process, called lipogenesis , creates lipids fat from the acetyl CoA and takes place in the cytoplasm of adipocytes fat cells and hepatocytes liver cells. When you eat more glucose or carbohydrates than your body needs, your system uses acetyl CoA to turn the excess into fat. Although there are several metabolic sources of acetyl CoA, it is most commonly derived from glycolysis. Acetyl CoA availability is significant, because it initiates lipogenesis. Lipogenesis begins with acetyl CoA and advances by the subsequent addition of two carbon atoms from another acetyl CoA; this process is repeated until fatty acids are the appropriate length. Because this is a bond-creating anabolic process, ATP is consumed. However, the creation of triglycerides and lipids is an efficient way of storing the energy available in carbohydrates. Triglycerides and lipids, high-energy molecules, are stored in adipose tissue until they are needed. Although lipogenesis occurs in the cytoplasm, the necessary acetyl CoA is created in the mitochondria and cannot be transported across the mitochondrial membrane. To solve this problem, pyruvate is converted into both oxaloacetate and acetyl CoA. Two different enzymes are required for these conversions. Oxaloacetate forms via the action of pyruvate carboxylase, whereas the action of pyruvate dehydrogenase creates acetyl CoA. Oxaloacetate and acetyl CoA combine to form citrate, which can cross the mitochondrial membrane and enter the cytoplasm. In the cytoplasm, citrate is converted back into oxaloacetate and acetyl CoA. Oxaloacetate is converted into malate and then into pyruvate. Pyruvate crosses back across the mitochondrial membrane to wait for the next cycle of lipogenesis. The acetyl CoA is converted into malonyl CoA that is used to synthesize fatty acids. Figure 6 summarizes the pathways of lipid metabolism. These include cortisol, glucagon, growth hormone GH , and adrenocorticotropic hormone ACTH. Dietary compounds, such as caffeine and calcium, also stimulate lipolysis. Each of these substances binds and act on their respective membrane-bound receptors and elicit a signaling cascade using a common second messenger, cyclic AMP. Cyclic AMP then binds to and activates protein kinase A PKA. Once PKA is enzymatically active, it phosphorylates HSL, the most important of the three enzymes involved in initiating lipolysis because it is enzymatically activated in all stages of hydrolysis. ATGL performs the first step of TAG hydrolysis, generating diacylglycerols and FAs. Its activity is tightly regulated by two accessory proteins: CGI and G0S2. CGI coactivates the hydrolase activity of ATGL and G0S2 inactivates the hydrolase activity of ATGL. HSL performs the second step and hydrolyzes DAGs, generating monoacylglycerols and FAs. MGL is selective for MGs and generates glycerol and the third FA. Short and medium-chain fatty acids diffuse freely into the cytosol and mitochondria of cells. Long-chain fatty acids must undergo protein-mediated transport across the cell membrane into the cytosol via fatty acid translocase FAT or fatty acid-binding protein FABP. Acyl-CoA synthase then converts the fatty acids to fatty acyl-CoA. The fatty acyl-CoA must now be transported into the mitochondria through the outer mitochondrial membrane and is done so by carnitine palmitoyltransferase-I CPT-I where it becomes fatty acyl-carnitine. The fatty acyl-carnitine is then transported across the inner membrane into the mitochondrial matrix by carnitine acyl-translocase CAT and converted back to fatty acyl-CoA by palmitoyltransferase-II CPT-II where it is now ready for oxidation. Beta oxidation is the degradation of fatty acids by removing two carbons at a time. It is the primary pathway for catabolism of fatty acids and takes place in the mitochondrial matrix of tissues such as the liver, muscle, and adipose. Two-carbon fragments are successively removed from the carboxyl end of the fatty acyl-CoA, producing NADH, FADH, and Acetyl CoA, which is used in the TCA cycle to make ATP. Fatty acids with odd numbers of carbon ultimately yield one mole of propionyl-CoA, which is converted to succinyl CoA so that it is usable in the TCA cycle. Beta oxidation is also important as the primary regulator of movement through the pyruvate dehydrogenase PDH complex. When rates of fatty acid oxidation are high, PDH activity decreases, which limits glycolysis, which is significant because patients with a deficiency in fatty acid oxidation have a compensatory increase in glucose oxidation and impaired gluconeogenesis. Ketone levels are low during normal feeding and physiological status. They are used by the heart and skeletal muscles to preserve the limited glucose for use by the brain and erythrocytes. During the fasting state, fatty acids are oxidized in the liver to acetyl CoA, which converts to the ketone bodies acetoacetate and beta-hydroxybutyrate. These high levels of ketones also inhibit PDH activity and fatty acid oxidation, to conserve glucose and permit entry into the brain where they can serve as sources for energy. Normally during a fast, muscle metabolizes ketone bodies as rapidly as the liver releases them preventing their accumulation in the blood. If ketones increase sufficiently in the blood, this can result in ketoacidosis, which is especially prevalent in people with type I diabetes and require close monitoring. There are currently several strategies in place to estimate lipolysis and these generally fall into two categories: non-activity-based methods and activity-based methods. The non-activity-based methods involve determining the quantity of the associated enzymes and regulatory proteins. The activity-based methods involve measuring the activity of the associated enzymes directly. Over the last several years, new and updated information has come to light, and the opinions of lipolysis have changed. It is now known that the measurement of mRNA or protein expression used in the non-activity-based methods is often not enough to estimate the capacity of lipolysis. A combination of methods is necessary. Neutral lipid storage disease with myopathy NLSDM — a rare inherited disorder arising from mutations in the ATGL gene, which results in systemic TAG accumulation, myopathy, cardiac abnormalities, and hepatomegaly. Chanarin-Dorfman syndrome or NLSD with ichthyosis NLSD-I results from mutations in CGI, the activator of ATGL. They also exhibit systemic TAG accumulation, mild myopathy, and hepatomegaly but also present with ichthyosis, which is a skin disorder characterized by dry, thickened, scaly skin. Familial partial lipodystrophy FPLD type 4 is associated with a mutation in the PLIN1 gene coding for perilipin 1. It is characterized phenotypically by loss of subcutaneous fat from the extremities. Histologically, the six patients with this mutation have small adipocytes with increased macrophage infiltration and abundant fibrosis. Familial partial lipodystrophy FPLD type 6 occurs due to a mutation in the LIPE gene coding for hormone-sensitive lipase. It is characterized by abnormal subcutaneous fat distribution, and thus the complications commonly associated with it it. These include dysregulated lipolysis, insulin resistance, diabetes mellitus, increased fat storage in bodily organs, and dyslipidemia; others may even develop muscular dystrophy as indicated by elevated serum creatine phosphokinase. There are many disorders of fat metabolism that present with serious and specific characteristics but are not discussed here as they are beyond the scope of lipolysis, specifically. These include, but are not limited to, fatty acid oxidation disorders FAODs such as MCAD deficiency or primary carnitine deficiency and peroxisomal disorders such as Zellweger syndrome and adrenoleukodystrophy. Alterations in lipolysis are often associated with obesity. These changes include increased basal rates of lipolysis, which may promote the development of insulin resistance and also diminished responsiveness to stimulated lipolysis. Furthermore, adipose tissue of insulin-resistant people displays a lack of proteins involved in mitochondrial function. Mitochondria-derived energy sources function in lipogenesis in adipose tissue. Obesity is characterized primarily by an excess of WAT due to hypertrophy of adipocytes that results from increased TAG storage. Obesity is a rampant health problem across the world due to its association with several disorders, including insulin resistance, type II diabetes, hypertension, and atherosclerosis. Disclosure: Michael Edwards declares no relevant financial relationships with ineligible companies. Disclosure: Shamim Mohiuddin declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Biochemistry, Lipolysis Michael Edwards ; Shamim S. Author Information and Affiliations Authors Michael Edwards 1 ; Shamim S. Affiliations 1 Loyola University Medical Center. Introduction Lipolysis is the metabolic process through which triacylglycerols TAGs break down via hydrolysis into their constituent molecules: glycerol and free fatty acids FFAs. Fundamentals Triacylglycerol Synthesis TAGs, which provide the body with a significant source of energy, are obtained from the diet or are synthesized endogenously, mainly in the liver. Triacylglycerol Hydrolysis During times of energy deprivation, WAT is stimulated via homeostatic control to shift toward higher net rates of lipolysis. Issues of Concern Defective lipolysis in non-adipose tissues impairs their normal function, leading to excessive TAG accumulation and lipid storage disease. Cellular Level As previously described, hormones bind to cell surface receptors i. Molecular Level Lipids have diverse structures but are all similar in that they are insoluble in water. Function Fatty acids are carried on the albumin in the blood. Triacylglycerol Hydrolysis As stated previously, during times of energy deprivation, WAT is stimulated by hormonal and biochemical signals to increase lipolysis. Fatty Acid Metabolism Short and medium-chain fatty acids diffuse freely into the cytosol and mitochondria of cells. Beta oxidation Beta oxidation is the degradation of fatty acids by removing two carbons at a time. Ketone synthesis Ketone levels are low during normal feeding and physiological status. Testing There are currently several strategies in place to estimate lipolysis and these generally fall into two categories: non-activity-based methods and activity-based methods. Pathophysiology Neutral lipid storage disease with myopathy NLSDM — a rare inherited disorder arising from mutations in the ATGL gene, which results in systemic TAG accumulation, myopathy, cardiac abnormalities, and hepatomegaly. Clinical Significance Alterations in lipolysis are often associated with obesity. Review Questions Access free multiple choice questions on this topic. Comment on this article. References 1. Bolsoni-Lopes A, Alonso-Vale MI. Lipolysis and lipases in white adipose tissue - An update. Arch Endocrinol Metab. Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Methods Enzymol. Engin AB. What Is Lipotoxicity? Adv Exp Med Biol. Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O'Rahilly S, Magré J, Savage DB, Vigouroux C. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O'Connell JR, Gong DW, Breyer RJ, Ryan AS, McLenithan JC, Shuldiner AR, Sztalryd C, Damcott CM. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. Bódis K, Roden M. |
| Key Points | Series ISSN : Series E-ISSN : Edition Number : 1. Number of Pages : XIV, Topics : Biochemistry, general. Policies and ethics. Skip to main content. Editors: Shimon Gatt 0 , Louis Freysz 1 , Paul Mandel 2. Shimon Gatt Department of Biochemistry, The Hebrew University, Hadassah Medical School, Jerusalem, Israel View editor publications. View editor publications. Sections Table of contents Keywords Editors and Affiliations Bibliographic Information Publish with us. Buy it now Buying options eBook EUR Price includes VAT Germany. Softcover Book EUR Tax calculation will be finalised at checkout. Licence this eBook for your library. Learn about institutional subscriptions. Table of contents 70 chapters Search within book Search. Page 1 Navigate to page number of 4. Front Matter Pages i-xiv. Enzymes Metabolizing Phospholipids: From Infancy to Middle Age Enzymes Metabolizing Phospholipids: From Infancy to Middle Age R. Dawson Pages Enzymes of the Metabolism of Fatty Acids and Neutral Glycerides Front Matter Pages Fatty Acid Biosynthesis during Brain Development J. Bourre, S. Pollet, M. Paturneau-Jouas, N. Baumann Pages The Role of Soluble Acyl-Thioester Hydrolase in Fatty Acid Chain-Length Termination in Rabbit Mammary Gland and Liver Jens Knudsen, Linda Chivers, Raymond Dils Pages Stereochemical Studies of Hydrogen Incorporation from Nucleotides with Fatty Acid Synthetase from Brevibacterium ammoniagenes Y. Seyama, T. Kasama, T. Yamakawa, A. Kawaguchi, S. Okuda Pages Cholesterol Oxidase as a Probe for Studying Membrane Composition and Organization Y. Barenholz, E. Patzer, N. Moore, R. Wagner Pages Adsorption and Activation of Pancreatic Lipase at Interfaces C. Chapus, M. Semeriva, M. Charles, P. Desnuelle Pages Mode of Action of Pancreatic Colipase Bengt Borgström Pages Studies of Lipase and Phospholipase A2 Acting on Lipid Monolayers R. Verger, J. Rietsch, F. Pattus, F. Ferrato, G. Pieroni, G. De Haas et al. Pages Inhibition of Lipase Adsorption at Interfaces. Role of Bile Salts Micelles and Colipase D. Lairon, G. It is the primary pathway for catabolism of fatty acids and takes place in the mitochondrial matrix of tissues such as the liver, muscle, and adipose. Two-carbon fragments are successively removed from the carboxyl end of the fatty acyl-CoA, producing NADH, FADH, and Acetyl CoA, which is used in the TCA cycle to make ATP. Fatty acids with odd numbers of carbon ultimately yield one mole of propionyl-CoA, which is converted to succinyl CoA so that it is usable in the TCA cycle. Beta oxidation is also important as the primary regulator of movement through the pyruvate dehydrogenase PDH complex. When rates of fatty acid oxidation are high, PDH activity decreases, which limits glycolysis, which is significant because patients with a deficiency in fatty acid oxidation have a compensatory increase in glucose oxidation and impaired gluconeogenesis. Ketone levels are low during normal feeding and physiological status. They are used by the heart and skeletal muscles to preserve the limited glucose for use by the brain and erythrocytes. During the fasting state, fatty acids are oxidized in the liver to acetyl CoA, which converts to the ketone bodies acetoacetate and beta-hydroxybutyrate. These high levels of ketones also inhibit PDH activity and fatty acid oxidation, to conserve glucose and permit entry into the brain where they can serve as sources for energy. Normally during a fast, muscle metabolizes ketone bodies as rapidly as the liver releases them preventing their accumulation in the blood. If ketones increase sufficiently in the blood, this can result in ketoacidosis, which is especially prevalent in people with type I diabetes and require close monitoring. There are currently several strategies in place to estimate lipolysis and these generally fall into two categories: non-activity-based methods and activity-based methods. The non-activity-based methods involve determining the quantity of the associated enzymes and regulatory proteins. The activity-based methods involve measuring the activity of the associated enzymes directly. Over the last several years, new and updated information has come to light, and the opinions of lipolysis have changed. It is now known that the measurement of mRNA or protein expression used in the non-activity-based methods is often not enough to estimate the capacity of lipolysis. A combination of methods is necessary. Neutral lipid storage disease with myopathy NLSDM — a rare inherited disorder arising from mutations in the ATGL gene, which results in systemic TAG accumulation, myopathy, cardiac abnormalities, and hepatomegaly. Chanarin-Dorfman syndrome or NLSD with ichthyosis NLSD-I results from mutations in CGI, the activator of ATGL. They also exhibit systemic TAG accumulation, mild myopathy, and hepatomegaly but also present with ichthyosis, which is a skin disorder characterized by dry, thickened, scaly skin. Familial partial lipodystrophy FPLD type 4 is associated with a mutation in the PLIN1 gene coding for perilipin 1. It is characterized phenotypically by loss of subcutaneous fat from the extremities. Histologically, the six patients with this mutation have small adipocytes with increased macrophage infiltration and abundant fibrosis. Familial partial lipodystrophy FPLD type 6 occurs due to a mutation in the LIPE gene coding for hormone-sensitive lipase. It is characterized by abnormal subcutaneous fat distribution, and thus the complications commonly associated with it it. These include dysregulated lipolysis, insulin resistance, diabetes mellitus, increased fat storage in bodily organs, and dyslipidemia; others may even develop muscular dystrophy as indicated by elevated serum creatine phosphokinase. There are many disorders of fat metabolism that present with serious and specific characteristics but are not discussed here as they are beyond the scope of lipolysis, specifically. These include, but are not limited to, fatty acid oxidation disorders FAODs such as MCAD deficiency or primary carnitine deficiency and peroxisomal disorders such as Zellweger syndrome and adrenoleukodystrophy. Alterations in lipolysis are often associated with obesity. These changes include increased basal rates of lipolysis, which may promote the development of insulin resistance and also diminished responsiveness to stimulated lipolysis. Furthermore, adipose tissue of insulin-resistant people displays a lack of proteins involved in mitochondrial function. Mitochondria-derived energy sources function in lipogenesis in adipose tissue. Obesity is characterized primarily by an excess of WAT due to hypertrophy of adipocytes that results from increased TAG storage. Obesity is a rampant health problem across the world due to its association with several disorders, including insulin resistance, type II diabetes, hypertension, and atherosclerosis. Disclosure: Michael Edwards declares no relevant financial relationships with ineligible companies. Disclosure: Shamim Mohiuddin declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Biochemistry, Lipolysis Michael Edwards ; Shamim S. Author Information and Affiliations Authors Michael Edwards 1 ; Shamim S. Affiliations 1 Loyola University Medical Center. Introduction Lipolysis is the metabolic process through which triacylglycerols TAGs break down via hydrolysis into their constituent molecules: glycerol and free fatty acids FFAs. Fundamentals Triacylglycerol Synthesis TAGs, which provide the body with a significant source of energy, are obtained from the diet or are synthesized endogenously, mainly in the liver. Triacylglycerol Hydrolysis During times of energy deprivation, WAT is stimulated via homeostatic control to shift toward higher net rates of lipolysis. Issues of Concern Defective lipolysis in non-adipose tissues impairs their normal function, leading to excessive TAG accumulation and lipid storage disease. Cellular Level As previously described, hormones bind to cell surface receptors i. Molecular Level Lipids have diverse structures but are all similar in that they are insoluble in water. Function Fatty acids are carried on the albumin in the blood. Triacylglycerol Hydrolysis As stated previously, during times of energy deprivation, WAT is stimulated by hormonal and biochemical signals to increase lipolysis. Fatty Acid Metabolism Short and medium-chain fatty acids diffuse freely into the cytosol and mitochondria of cells. Beta oxidation Beta oxidation is the degradation of fatty acids by removing two carbons at a time. Ketone synthesis Ketone levels are low during normal feeding and physiological status. Testing There are currently several strategies in place to estimate lipolysis and these generally fall into two categories: non-activity-based methods and activity-based methods. Pathophysiology Neutral lipid storage disease with myopathy NLSDM — a rare inherited disorder arising from mutations in the ATGL gene, which results in systemic TAG accumulation, myopathy, cardiac abnormalities, and hepatomegaly. Clinical Significance Alterations in lipolysis are often associated with obesity. Review Questions Access free multiple choice questions on this topic. Comment on this article. References 1. Bolsoni-Lopes A, Alonso-Vale MI. Lipolysis and lipases in white adipose tissue - An update. Arch Endocrinol Metab. Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Methods Enzymol. Engin AB. What Is Lipotoxicity? Adv Exp Med Biol. Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O'Rahilly S, Magré J, Savage DB, Vigouroux C. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O'Connell JR, Gong DW, Breyer RJ, Ryan AS, McLenithan JC, Shuldiner AR, Sztalryd C, Damcott CM. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. Bódis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest. Copyright © , StatPearls Publishing LLC. Bookshelf ID: NBK PMID: PubReader Print View Cite this Page Edwards M, Mohiuddin SS. Biochemistry, Lipolysis. In: StatPearls [Internet]. In this Page. Introduction Fundamentals Issues of Concern Cellular Level Molecular Level Function Mechanism Testing Pathophysiology Clinical Significance Review Questions References. Bulk Download. Bulk download StatPearls data from FTP. Related information. PMC PubMed Central citations. Similar articles in PubMed. Lipolytic enzymes involving lipolysis in Teleost: Synteny, structure, tissue distribution, and expression in grass carp Ctenopharyngodon idella. Sun J, Ji H, Li XX, Shi XC, Du ZY, Chen LQ. |
| Lipid Metabolism | Boeszoermenyi, A. MVA pathway. Fat metabolism enzymes viruses, such Type diabetes management ehzymes virusinfluenza H3N2 virus and porcine metabolidm and respiratory syndrome virusactivate lipophagy and acid lipolysis to mobilize FAs from LDs. Endogenously synthesized cholesterol and exogenous free cholesterol taken up by lipoprotein receptors must be transported through the liver. Tools Tools. |
| 6.3: Lipid Metabolism Pathways | National Institutes of Health NIH , National Heart, Lung, and Blood Institute NHLBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity: the evidence report US Government Press, Washington DC, Campfield, L. Strategies and potential molecular targets for obesity treatment. Science , — This review outlines different strategies for obesity drug development by targeting both central and peripheral mechanisms. Article CAS PubMed Google Scholar. Hill, J. Dietary fat intake and regulation of energy balance: implications for obesity. Carriere, F. et al. Gastric and pancreatic lipase levels during a test meal in dogs. Nordskog, B. An examination of the factors affecting intestinal lymphatic transport of dietary lipids. Drug Deliv. Phan, C. Intestinal lipid absorption and transport. Kawai, T. Importance of lipolysis in oral cavity for orosensory detection of fat. Article CAS Google Scholar. Miled, N. Digestive lipases: from three-dimensional structure to physiology. Biochimie 82 , — Ghishan, F. Isolated congenital lipase-colipase deficiency. Gastroenterology 86 , — Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology , — Borgstrom, B. Binding of pancreatic colipase to interfaces: effects of detergents. FEBS Lett. Bowyer, R. Effect of a satiating meal on the concentrations of procolipase propeptide in the serum and urine of normal and morbidly obese subjects. Gut 34 , — Article CAS PubMed PubMed Central Google Scholar. Weibel, E. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. Producing organism, fermentation, isolation and biological activity. Bitou, N. Screening of lipase inhibitors from marine algae. Lipids 34 , — Zhi, J. Long-term systemic exposure of orlistat, a lipase inhibitor, and its metabolites in obese patients. Hadvary, P. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. Lucas, C. Padwal, R. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Hanefeld, M. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes. Carey, M. Lipid digestion and absorption. Huggins, K. Protection against diet-induced obesity and obesity-related insulin resistance in group 1B PLA2-deficient mice. This study describes the role of pancreatic sPLA2 in dietary fat absorption. Richmond, B. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A 2 -deficient mice. Chang, T. Porcine pancreatic phospholipase A2 stimulates secretin release from secretin-producing cells. Ma, T. Defective dietary fat processing in transgenic mice lacking aquaporin-1 water channels. Cell Physiol. Molecular structure and tissue-specific expression of the mouse pancreatic phospholipase A 2 gene. Gene , 65—72 Murakami, M. Phospholipase A2. Yuan, C. Pancreatic phospholipase A 2 : new views on old issues. Acta 23 , — Article Google Scholar. Mihelich, E. Structure-based design of a new class of anti-inflammatory drugs: secretory phospholipase A 2 inhibitors, SPI. Niessen, H. Type II secretory phospholipase A2 in cardiovascular disease: a mediator in atherosclerosis and ischemic damage to cardiomyocytes? Structural analysis of phospholipase A2 from functional perspective. Characterization of a molten globule-like state induced by site-specific mutagenesis. Biochemistry 38 , — Hajri, T. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Kamp, F. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. This research shows that passive diffusion is an efficient process for fatty acids entering the adipocytes. Vassileva, G. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. Abumrad, N. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD Chen, M. Poirier, H. Localization and regulation of the putative membrane fatty-acid transporter FAT in the small intestine. Comparison with fatty acid-binding proteins FABP. Greenwalt, D. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. Goudriaan, J. Intestinal lipid absorption is not affected in CD36 deficient mice. Schaffer, J. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79 , — Gimeno, R. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. Herrmann, T. Mouse fatty acid transport protein 4 FATP4 : characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene , 31—40 Coleman, R. Enzymes of triacylglycerol synthesis and their regulation. Lipid Res. This paper provides a comprehensive review of the latest developments in lipid metabolic enzymes. Polheim, D. Regulation of triglyceride biosynthesis in adipose and intestinal tissue. Yen, C. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Natl Acad. USA 99 , — Cao, J. Cloning and functional characterization of a mouse intestinal Acyl-CoA:monoacylglycerol acyltransferase, MGAT2. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. This report provides direct evidence that MGAT2 is important in dietary fat absorption and diet-induced obesity. Cheng, D. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. Lockwood, J. Human intestinal monoacylglycerol acyltransferase: differential features in tissue expression and activity. Mostafa, N. Increased hepatic monoacylglycerol acyltransferase activity in streptozotocin-induced diabetes: characterization and comparison with activities from adult and neonatal rat liver. Acta , — Luan, Y. Pathogenesis of obesity by food restriction in OLETF rats: increased intestinal monoacylglycerol acyltransferase activities may be a crucial factor. Diabetes Res. Sudhop, T. Cholesterol absorption inhibitors for the treatment of hypercholesterolaemia. Drugs 62 , — Hideshima, T. Antitumor activity of lysophosphatidic acid acyltransferase-β inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. CAS PubMed Google Scholar. Coon, M. Inhibition of lysophosphatidic acid acyltransferase disrupts proliferative and survival signals in normal cells and induces apoptosis of tumor cells. Cancer Ther. Thomson, A. Crypt cell production rate, enterocyte turnover time and appearance of transport along the jejunal villus of the rat. Bakillah, A. The role of microsomal triglyceride transfer protein in lipoprotein assembly: an update. Hussain, M. A proposed model for the assembly of chylomicrons. Atherosclerosis , 1—15 Wetterau, J. An mtp inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. This paper reports on the first generation of MTP inhibitors that are effective in lowering TAG in rodents. Ksander, G. Diaminoindanes as microsomal triglyceride transfer protein inhibitors. Shiomi, M. MTP inhibitor decreases plasma cholesterol levels in LDL receptor-deficient WHHL rabbits by lowering the VLDL secretion. Robl, J. A novel series of highly potent benzimidazole-based microsomal triglyceride transfer protein inhibitors. Chandler, C. CP an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. Levy, E. The genetic basis of primary disorders of intestinal fat transport. Lewis, G. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Shimomura, I. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear srebp-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. Moitra, J. Life without white fat: a transgenic mouse. Agarwal, A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol. Hanson, R. Glyceroneogenesis revisited. Biochimie 85 , — Leung, D. The structure and functions of human lysophosphatidic acid acyltransferases. Ruan, H. Overexpression of 1-acyl-glycerolphosphate acyltransferase-α enhances lipid storage in cellular models of adipose tissue and skeletal muscle. Diabetes 50 , — Cases, S. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. USA 95 , — Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. Ludwig, E. DGAT1 promoter polymorphism associated with alterations in body mass index, high density lipoprotein levels and blood pressure in Turkish women. Article PubMed Google Scholar. Yu, Y. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. Stone, S. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. This study shows that DGAT1 and DGAT2 have different roles in lipid metabolism. Article PubMed CAS Google Scholar. Buhman, K. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. Smith, S. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nature Genet. Chen, H. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. This study illustrates that DGAT1 in adipose tissue is important in regulating energy homeostasis. Gibbons, G. Mobilisation of triacylglycerol stores. Acta , 37—57 Tomoda, H. Microbial metabolites with inhibitory activity against lipid metabolism. Jpn Acad. B Phys. Holm, C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Sztalryd, C. Regulation of hormone-sensitive lipase during fasting. Large, V. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. Garenc, C. The hormone-sensitive lipase gene and body composition: the HERITAGE family study. Lucas, S. Expression of human hormone-sensitive lipase in white adipose tissue of transgenic mice increases lipase activity but does not enhance in vitro lipolysis. Haemmerle, G. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. Sekiya, M. Mulder, H. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. Osuga, J. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. USA 97 , — Wei, Y. Crystal structure of brefeldin A esterase, a bacterial homolog of the mammalian hormone-sensitive lipase. Nature Struct. Tansey, J. Functional studies on native and mutated forms of perilipins: a role in protein kinase A-mediated lipolysis of triacylglycerols in Chinese hamster ovary cells. Miura, S. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 PAT -related proteins in mammals, Drosophila , and Dictyostelium. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. USA 98 , — Martinez-Botas, J. References 94 and 95 show the importance of perilipin in regulating lipid metabolism. Castro-Chavez, F. Coordinated upregulation of oxidative pathways and downregulation of lipid biosynthesis underlie obesity resistance in perilipin knockout mice: a microarray gene expression profile. Diabetes 52 , — Sul, H. Nutritional and hormonal regulation of enzymes in fat synthesis — studies of fatty acid synthase and mitochondrial glycerolphosphate acyltransferase gene transcription. Park, H. Coordinate regulation of malonyl-CoA decarboxylase, sn -glycerolphosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. Zhou, Y. Reversing adipocyte differentiation: implications for treatment of obesity. USA 96 , — Dircks, L. Mammalian mitochondrial glycerolphosphate acyltransferase. Acta , 17—26 Igal, R. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. Hammond, L. Mitochondrial glycerolphosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. This paper provides evidence that GPAT1 regulates both lipid synthesis and composition. Structural and functional organization of the animal fatty acid synthase. Jayakumar, A. Human fatty acid synthase: properties and molecular cloning. USA 92 , — Kuhajda, F. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16 , — Synthesis and antitumor activity of an inhibitor of fatty acid synthase. A report on the synthesis of C75, which is one of the most commonly used FAS inhibitors. Loftus, T. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Shimokawa, T. Effect of a fatty acid synthase inhibitor on food intake and expression of hypothalamic neuropeptides. USA 99 , 66—71 Hu, Z. Hypothalamic malonyl-CoA as a mediator of feeding behavior. USA , — References — provide comprehensive evidence that malonyl-CoA has a central role in regulating appetite. Takahashi, K. The anorexigenic fatty acid synthase inhibitor, C75, is a non-specific neuronal activator. Endocrinology 25 , 25 This report questions the validity of C75 as an authentic FAS inhibitor. Google Scholar. Schlesinger, M. Cerulenin blocks fatty acid acylation of glycoproteins and inhibits vesicular stomatitis and Sindbis virus particle formation. Lawrence, D. Structure— activity studies of cerulenin analogues as protein palmitoylation inhibitors. Thupari, J. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Chirala, S. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Metzger, D. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. van der Leij, F. Genomics of the human carnitine acyltransferase genes. McGarry, J. The mitochondrial carnitine palmitoyltransferase system — from concept to molecular analysis. Awan, M. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Hamilton, C. Malonyl-CoA metabolism in cardiac myocytes. Saddik, M. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. Ruderman, N. Malonyl-CoA, fuel sensing, and insulin resistance. Winder, W. Malonyl-CoA: regulator of fatty acid oxidation in muscle during exercise. Sport Sci. An, J. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature Med. This paper shows that malonyl-CoA is an important regulator of peripheral energy metabolism. Kim, J. After binding to lipids, proteins take part in transporting lipids in plasma, so they are called apolipoproteins. Figure 2. Lipid metabolism in liver. The mainly lipid source of the liver is food. The lipids in food are mainly TG, and there are a small amount of PL and Ch. In the small intestine, bile acids and pancreatic enzymes including pancreatic lipase, phospholipase A2, cholesterol esterase, etc. in bile hydrolyze lipids into free fatty acids FFA , glycerol and Fc. Then these molecules are absorbed by mucosal epithelial cells of the small intestine mainly jejunum , and are further esterified into TG, CE, etc. in intestinal epithelial cells. Finally, TG, Ch and PL with apolipoprotein compose of lipoprotein chylomicron CM which will be absorbed by the lymphatic system and hydrolyzed by lipoproteinase of vascular endothelial cells to enter the liver. FFA can be converted into energy by oxidation in hepatocytes for the consumption, or re-synthesize TG, PL and CE with 3-phosphoglycerate. The mainly source of endogenous fatty acids is the fat stored in the body's adipose tissue. The fat in the fat cells is hydrolyzed into glycerol and fatty acids by the action of lipase. After being released into the blood, glycerol is dissolved in plasma while fatty acids are combined with plasma albumin for transport. It can be used as a source of energy or ingested by liver cells again. In addition, hepatocytes also can produce fatty acids from the oxidation process of glucose and amino acids and synthesize TG by acetyl-CoA in hepatocytes. In addition to ingesting the exogenous cholesterol from food, liver cells also synthesize endogenous cholesterol. Hepatocyte endoplasmic reticulum cholesterol biosynthesis involves more than 30 enzymes, such as acetoacetyl CoA. Endogenously synthesized cholesterol and exogenous free cholesterol taken up by lipoprotein receptors must be transported through the liver. The transport destinations are: 1 decomposition into primary bile acid and bile salts in the liver, then discharging into the capillary bile duct and bile through the transport pump on the capillary bile duct; 2 free cholesterol and phospholipids are directly excreted to the bile by multi-drug resistance transporter MDR ; 3 cholesterol ester and free cholesterol are converted to each other to form dynamic equilibrium. Free cholesterol can be esterified into cholesterol ester by cholesterol acyltransferase ACAT and transported to the peripheral circulation in the form of VLDL. Cholesterol esters can be rapidly hydrolyzed to free cholesterol by cholesteryl ester hydrolase CEH as a precursor for the synthesis of bile acids; 4 VLDL consisting of apolipoproteins, phospholipids, etc. reverses into human blood circulation, reaching hepatic stellate cells and steroid hormone secreting cells. Figure 3. Lipid metabolism in pancreas. Pancreatic lipase is mainly secreted by pancreatic acinar cells and functions to digest the fat in the duodenum, including the classic pancreatic triglyceride lipase PTL , pancreatic lipase-related protein 1 PLRP1 and 2 PLRP2 , bile salt-stimulated lipase BSSL and pancreatic phospholipase A2 PLA2 , etc. The source of pancreatic lipase is quite extensive. As the research progresses, it has been reported that PLRP2 is also expressed in lymphocytes and colonic epithelial cells, which are involved in the inflammatory response and regulating the intestinal flora, respectively. The mammary gland of some mammals including humans, can secrete BSSL during lactation, which can be supplied to infants through milk to participate in their early fat digestion and absorption. It has also been found that BSSL is expressed in other tissues including liver, inflammatory cells, endothelial cells and platelets, suggesting that BSSL may be involved in the process of inflammation, arteriosclerosis, etc. These important pancreatic lipases participant in the digestion of lipids such as triglycerides, cholesterol, and phospholipids , so that dietary fat can be fully utilized. |

Fat metabolism enzymes -
The figure below shows how fatty acids can be taken up and used by tissues such as the muscle for energy production 1.
To generate energy from fatty acids, they must be oxidized. This process occurs in the mitochondria, but long chain fatty acids cannot diffuse across the mitochondrial membrane similar to absorption into the enterocyte.
Carnitine, an amino acid-derived compound, helps shuttle long-chain fatty acids into the mitochondria. The structure of carnitine is shown below. As shown below, there are two enzymes involved in this process: carnitine palmitoyltransferase I CPTI and carnitine palmitoyltransferase II CPTII.
CPTI is located on the outer mitochondrial membrane, CPTII is located on the inner mitochondrial membrane. Acyl-Carnitine is then transported into the mitochondrial matrix with the assistance of the enzyme translocase.
Carnitine is recycled back into the cytosol to be used again, as shown in the figure below. Even though carnitine is important for this action, taking supplemental carnitine will not increase fatty acid oxidation. This is due to the fact that the amount of carnitine available is not limiting fatty acid oxidation.
Fatty acid transfer from cytoplasm to mitochondria. As shown below, the first step of fatty acid oxidation is activation. Thus, activation uses the equivalent of 2 ATP molecules since it typically cleaved to ADP 5. Fatty acid oxidation is also referred to as beta-oxidation because 2 carbon units are cleaved off at the beta-carbon position 2nd carbon from the acid end of an activated fatty acid.
To completely oxidize the carbon fatty acid above, 8 cycles of beta-oxidation have to occur. Overall beta oxidation of an 18 carbon fatty acids will produce:.
Compared to glucose 32 ATP you can see that there is far more energy stored in a fatty acid. Fatty Acid Metabolism. De novo in Latin means "from the beginning. The addition of 2 carbons is repeated through a similar process 7 times to produce a 16 carbon fatty acid 6.
The structures of the three ketone bodies; acetone, acetoacetic acid, and beta-hydroxybutyric acid, are shown below. After they are synthesized in the liver, ketone bodies are released into circulation where they can travel to the brain. If there are high levels of ketones secreted, it results in a condition known as ketosis or ketoacidosis.
It is debatable whether mild ketoacidosis is harmful, but severe ketoacidosis can be lethal. One symptom of this condition is fruity or sweet smelling breath, which is due to increased acetone exhalation. As shown below, there are a large number of reactions and enzymes involved in cholesterol synthesis.
This enzyme is important because it is the rate-limiting enzyme in cholesterol synthesis. A rate-limiting enzyme is like a bottleneck in a highway, as shown below, that determines the flow of traffic past it. Rate-limiting enzymes limit the rate at which a metabolic pathway proceeds.
The pharmaceutical industry has taken advantage of this knowledge to lower people's LDL levels with drugs known as statins. Less cholesterol leads to lower LDL levels, and hopefully a lower risk of cardiovascular disease.
The brand name of the statins approved for use in the US are 13 :. HDL also serves as a reservoir for essential apoproteins such as Apo C-II. Apo C-II activates lipoprotein lipase, the enzyme responsible for digestion and breakdown of TAGs. Synthesis of TAGs stores in adipose tissue occurs in the fed state after a meal.
During times of energy deprivation, WAT is stimulated via homeostatic control to shift toward higher net rates of lipolysis.
This change in nutritional state elucidates this compensatory process and is regulated through hormonal and biochemical signals. Lipolysis proceeds in an orderly and controlled manner, with different enzymes acting at each step. Catecholamines are the primary activators of lipolysis, while other hormones and dietary compounds also affect it.
Each of these substances binds and work on their respective membrane-bound receptors to elicit a signaling cascade with the sole purpose of activating HSL.
ATGL performs the first step of TAG hydrolysis thus, it is rate-limiting , generating diacylglycerols and FFAs. HSL performs the second step and hydrolyzes DAGs, generating monoacylglycerols and FFAs. MGL is selective for MGs and produces glycerol and the third FFA.
Defective lipolysis in non-adipose tissues impairs their normal function, leading to excessive TAG accumulation and lipid storage disease. Failure to package FFAs into lipid droplets causes chronic elevation of circulating FFAs, which can lead to chronic inflammation, mitochondrial dysfunction, and cell death.
As previously described, hormones bind to cell surface receptors i. A number of lipid droplet—associated proteins are known to modulate rates of basal non-stimulated and stimulated lipolysis. These proteins include CGI and perilipin. Perilipin is the major protein found in association with lipid droplets in adipocytes.
In the basal state, CGI is bound to perilipin rendering it unable to bind to or activate ATGL. Both ATGL and HSL reside in the cytosol. In the stimulated state, β-Adrenergic receptors signal adenylyl cyclase to generate cAMP. cAMP then binds PKA resulting in increased activity of the enzyme. PKA then phosphorylates HSL and perilipin, which causes HSL to translocate from the cytosol to the surface of the lipid droplet.
Now phosphorylated perilipin releases CGI so that it can bind to and activate ATGL. Similarly to HSL, ATGL also must translocate from the cytosol to the surface of the lipid droplet.
It is important to note that MGL is localized on the surface of the lipid droplet, in the cytosol, and the ER independent of the metabolic state. Lipids have diverse structures but are all similar in that they are insoluble in water.
Fatty acids usually possess an even number of carbon atoms, are 16 to 20 carbons in length, and can be saturated or unsaturated the latter applying to contain double bonds. They are described by the number of carbons they contain and the positions of the double bonds, if any. For example, arachidonic acid has 20 carbons and four double bonds and is written as , Δ5,8,11,14, or ω All naturally occurring fatty acids possess double bonds in the cis configuration.
Polyunsaturated fatty acid classification is often according to the position of the first double bond from the omega-end the carbon farthest from the carboxyl group.
Common examples of these are omega-3 and omega-6 fatty acids. Monoacylglycerols monoglycerides , diacylglycerols diglycerides , and triacylglycerols triglycerides contain one, two, and three fatty acids esterified to glycerol, respectively.
Fatty acids are carried on the albumin in the blood. In tissues such as muscle and kidney, fatty acids undergo oxidation for energy. In the liver, fatty acids convert to ketone bodies that are oxidized by tissues such as muscle and kidney.
During starvation after fasting has lasted for about three or more days , the brain uses ketone bodies for energy. The ketone bodies, acetoacetate, and β-hydroxybutyrate serve as a source of fuel. The liver uses glycerol as a source of carbon for gluconeogenesis, which produces glucose for tissues, including the brain and red blood cells.
In the liver and adipose tissue, glycerolphosphate G3P provides the glycerol moiety. The liver can convert glycerol to G3P via an intermediate or directly because it has the enzyme glycerol kinase.
Adipose cells lack this enzyme and must produce G3P solely via an intermediate. The storage of TAGs in adipose tissue is mediated by insulin, which stimulates adipose cells to secrete lipoprotein lipase and to take up glucose, which converts to glycerol via a DHAP intermediate for triacylglycerol synthesis.
In this process, glucose converts to DHAP, which is reduced by NADH to form G3P. Ultimately, G3P reacts with two fatty acyl CoA molecules to form phosphatidic acid. The phosphate group is cleaved to form a diacylglycerol, which reacts with another fatty acyl CoA to form a triacylglycerol.
As stated previously, during times of energy deprivation, WAT is stimulated by hormonal and biochemical signals to increase lipolysis. The current model of lipolysis identifies three major enzymes involved: ATGL, HSL, and MGL. Catecholamines, particularly norepinephrine, are the primary activators of fasting-induced lipolysis, while other hormones also have an effect.
These include cortisol, glucagon, growth hormone GH , and adrenocorticotropic hormone ACTH. Dietary compounds, such as caffeine and calcium, also stimulate lipolysis. Each of these substances binds and act on their respective membrane-bound receptors and elicit a signaling cascade using a common second messenger, cyclic AMP.
Cyclic AMP then binds to and activates protein kinase A PKA. Once PKA is enzymatically active, it phosphorylates HSL, the most important of the three enzymes involved in initiating lipolysis because it is enzymatically activated in all stages of hydrolysis.
ATGL performs the first step of TAG hydrolysis, generating diacylglycerols and FAs. Its activity is tightly regulated by two accessory proteins: CGI and G0S2. CGI coactivates the hydrolase activity of ATGL and G0S2 inactivates the hydrolase activity of ATGL.
HSL performs the second step and hydrolyzes DAGs, generating monoacylglycerols and FAs. MGL is selective for MGs and generates glycerol and the third FA. Short and medium-chain fatty acids diffuse freely into the cytosol and mitochondria of cells. Long-chain fatty acids must undergo protein-mediated transport across the cell membrane into the cytosol via fatty acid translocase FAT or fatty acid-binding protein FABP.
Acyl-CoA synthase then converts the fatty acids to fatty acyl-CoA. The fatty acyl-CoA must now be transported into the mitochondria through the outer mitochondrial membrane and is done so by carnitine palmitoyltransferase-I CPT-I where it becomes fatty acyl-carnitine.
The fatty acyl-carnitine is then transported across the inner membrane into the mitochondrial matrix by carnitine acyl-translocase CAT and converted back to fatty acyl-CoA by palmitoyltransferase-II CPT-II where it is now ready for oxidation.
Beta oxidation is the degradation of fatty acids by removing two carbons at a time. It is the primary pathway for catabolism of fatty acids and takes place in the mitochondrial matrix of tissues such as the liver, muscle, and adipose.
Two-carbon fragments are successively removed from the carboxyl end of the fatty acyl-CoA, producing NADH, FADH, and Acetyl CoA, which is used in the TCA cycle to make ATP. Fatty acids with odd numbers of carbon ultimately yield one mole of propionyl-CoA, which is converted to succinyl CoA so that it is usable in the TCA cycle.
Beta oxidation is also important as the primary regulator of movement through the pyruvate dehydrogenase PDH complex. When rates of fatty acid oxidation are high, PDH activity decreases, which limits glycolysis, which is significant because patients with a deficiency in fatty acid oxidation have a compensatory increase in glucose oxidation and impaired gluconeogenesis.
Ketone levels are low during normal feeding and physiological status. They are used by the heart and skeletal muscles to preserve the limited glucose for use by the brain and erythrocytes. During the fasting state, fatty acids are oxidized in the liver to acetyl CoA, which converts to the ketone bodies acetoacetate and beta-hydroxybutyrate.
These high levels of ketones also inhibit PDH activity and fatty acid oxidation, to conserve glucose and permit entry into the brain where they can serve as sources for energy.
Normally during a fast, muscle metabolizes ketone bodies as rapidly as the liver releases them preventing their accumulation in the blood. If ketones increase sufficiently in the blood, this can result in ketoacidosis, which is especially prevalent in people with type I diabetes and require close monitoring.
There are currently several strategies in place to estimate lipolysis and these generally fall into two categories: non-activity-based methods and activity-based methods. The non-activity-based methods involve determining the quantity of the associated enzymes and regulatory proteins.
The activity-based methods involve measuring the activity of the associated enzymes directly. Over the last several years, new and updated information has come to light, and the opinions of lipolysis have changed.
It is now known that the measurement of mRNA or protein expression used in the non-activity-based methods is often not enough to estimate the capacity of lipolysis.
A combination of methods is necessary. Neutral lipid storage disease with myopathy NLSDM — a rare inherited disorder arising from mutations in the ATGL gene, which results in systemic TAG accumulation, myopathy, cardiac abnormalities, and hepatomegaly.
Chanarin-Dorfman syndrome or NLSD with ichthyosis NLSD-I results from mutations in CGI, the activator of ATGL. They also exhibit systemic TAG accumulation, mild myopathy, and hepatomegaly but also present with ichthyosis, which is a skin disorder characterized by dry, thickened, scaly skin.
Familial partial lipodystrophy FPLD type 4 is associated with a mutation in the PLIN1 gene coding for perilipin 1. It is characterized phenotypically by loss of subcutaneous fat from the extremities.
Histologically, the six patients with this mutation have small adipocytes with increased macrophage infiltration and abundant fibrosis. Familial partial lipodystrophy FPLD type 6 occurs due to a mutation in the LIPE gene coding for hormone-sensitive lipase.
It is characterized by abnormal subcutaneous fat distribution, and thus the complications commonly associated with it it. These include dysregulated lipolysis, insulin resistance, diabetes mellitus, increased fat storage in bodily organs, and dyslipidemia; others may even develop muscular dystrophy as indicated by elevated serum creatine phosphokinase.
There are many disorders of fat metabolism that present with serious and specific characteristics but are not discussed here as they are beyond the scope of lipolysis, specifically.
These include, but are not limited to, fatty acid oxidation disorders FAODs such as MCAD deficiency or primary carnitine deficiency and peroxisomal disorders such as Zellweger syndrome and adrenoleukodystrophy.
Alterations in lipolysis are often associated with obesity. These changes include increased basal rates of lipolysis, which may promote the development of insulin resistance and also diminished responsiveness to stimulated lipolysis. Furthermore, adipose tissue of insulin-resistant people displays a lack of proteins involved in mitochondrial function.
Mitochondria-derived energy sources function in lipogenesis in adipose tissue. Obesity is characterized primarily by an excess of WAT due to hypertrophy of adipocytes that results from increased TAG storage. Obesity is a rampant health problem across the world due to its association with several disorders, including insulin resistance, type II diabetes, hypertension, and atherosclerosis.
Disclosure: Michael Edwards declares no relevant financial relationships with ineligible companies. Disclosure: Shamim Mohiuddin declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.
You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure.
Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-.
Eenzymes performing the same function, Metabolism boosting snacks the adipose emzymes, the enzymes are primarily metabolosm for seemingly opposite reasons. Type diabetes management the ennzymes Type diabetes management, LPL on Fah endothelium of blood vessels cleaves ezymes triglycerides into fatty acids so that they can Type diabetes management taken up into adipocytes, for storage as triglycerides, or myocytes where they are primarily used for energy production. This action of LPL on lipoproteins is shown in the two figures below. HSL is an important enzyme in adipose tissue, which is a major storage site of triglycerides in the body. HSL activity is increased by glucagon and epinephrine "fight or flight" hormoneand decreased by insulin. Thus, in hypoglycemia such as during a fast or a "fight or flight" response, triglycerides in the adipose are cleaved, releasing fatty acids into circulation that then bind with the transport protein albumin. Fatty acid metabolism consists of various metabolic processes herbal wakefulness supplements or closely related to fatty acidsa family of molecules classified enzyes the enaymes macronutrient category. Mwtabolism processes can Fat metabolism enzymes be divided into 1 Fat metabolism enzymes processes mdtabolism generate energy Type diabetes management 2 anabolic processes Cognitive health optimization they serve as building blocks for other compounds. In catabolism, fatty acids are metabolized to produce energy, mainly in the form of adenosine triphosphate ATP. When compared to other macronutrient classes carbohydrates and proteinfatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO 2 and water by beta oxidation and the citric acid cycle. In anabolism, intact fatty acids are important precursors to triglycerides, phospholipids, second messengers, hormones and ketone bodies. For example, phospholipids form the phospholipid bilayers out of which all the membranes of the cell are constructed from fatty acids.
Ich meine, dass Sie den Fehler zulassen. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.