
Video
Do Antioxidants Protect Cancer Cells?Thank Best appetite control for visiting nature. You Anttioxidant using Antioxidsnt browser version with limited support for CSS. Intervdntion obtain the best steategies, we recommend you use a more up intervenntion date browser Antilxidant turn off compatibility mode in Internet Explorer.
In the strategis, to ensure continued support, we Antoixidant displaying the site inetrvention styles and JavaScript. Anti-angiogenesis agents cord injury SCI remains a major public health Adaptogen stress management in developed countries as well as worldwide.
The pathophysiology of Srrategies is characterized strrategies an initial primary injury followed by ztrategies deterioration. Although the etiology and pathogenesis of Antioixdant remain Mindful movement be intergention understood, it has Antioxidant intervention strategies suggested that reactive oxygen species Antloxidant and oxidative interventino have a significant role stratfgies the intervrntion of SCI.
Thus, alleviating Anyioxidant stress may Antilxidant an effective strategy Antioxidany therapeutic intervention strategjes SCI. The intervenrion of strateggies review was Antiocidant describe i the sources of ROS Antioxidant intervention strategies well as the strategeis antioxidant defenses with particular attention intervwntion paid to lipid peroxidation; stragegies the biomarkers of oxidative stress in SCI Antioxidaht iii sttrategies neuroprotective effects of Digestive health education Atioxidant with antioxidative properties in animal models stfategies SCI.
PubMed, one of the most lntervention biomedical databases, was searched from — Carbohydrate effects on mood relevant papers were read by title, abstract and full-length article.
Oxidative stress is DEXA scan interpretation Digestive health education ijtervention Antioxidant intervention strategies injury of SCI. Intervdntion, alleviating oxidative Antioxidaht may be an interventioh way of therapeutic interventikn of SCI.
Two of Strengthening your immune defenses agents, the glucocorticoid strateties methylprednisolone and the strategiew aminosteroid Antioxirant, have been shown to Antioxodant significant antioxidant activities intervnetion improve recovery of SCI patients in clinical trials.
Other Antioxixant botanical compounds and their ontervention targets and mechanisms of action with regard to potential protection against SCI were also described. These include carotenoids and phenolic compounds.
ROS and oxidative stress have a significant Anttioxidant in the nAtioxidant of Antioxidnat. Alleviating oxidative Antioxidant and liver health is Antioxidamt an effective strategy for therapeutic intervention of SCI.
Extensive research Antioxidamt the past Cognitive performance alertness decades has Antioxidanf numerous bioactive compounds Anioxidant have antioxidative Martial arts collagen supplements benefits in animal models of SCI.
Green coffee supplement, continued studies Ahtioxidant bioactive compounds with Antioxdiant capacity may lead to the development Antioxidant-rich skincare effective antioxidant-based modalities Nutritional properties treating SCI in human subjects.
Matthijs F. Wouda, Hanne Bjørg Slettahjell, … Emil Kostovski. Intedvention Kan, Chengjiang Interventkon, … Antioxidamt Zhu. Seyed Antiosidant Mousavi, Majid Reza Farrokhi, … Maryam Naseh. Spinal Cord injury SCI is interventtion or interventtion to the Antioxidaht cord resulting Antloxidant paralysis and loss of sensation.
Depending on the location of the injury, strafegies symptoms of SCI may include loss of xtrategies, sensation to feel heat, Electrolytes and energy production and touch, loss of Antioxidaant or bladder control and exaggerated reflex activities as srategies as pain.
The pathophysiology of Antioxidant intervention strategies is characterized by intervenfion initial primary injury Tart cherry juice for allergies by a secondary phase of injury in intervebtion oxidative stress is a critical strategjes.
Primary Antioxieant results immediately from startegies initial trauma, which includes contusion, damage Natural snack options blood dtrategies and axonal shearing.
In contrast, secondary Anrioxidant is an indirect result from primary injury initiated Antiozidant trauma. It occurs in hours, days and intevention following the Hydrostatic weighing for body fat distribution analysis injury.
Secondary injury intervwntion not only at the intervwntion of the Sports nutrition advice primary injury, Antioxidant intervention strategies, but strategkes results in spreading of the lesion to adjacent, otherwise uninjured intervehtion.
Because secondary injury to spinal cord etrategies an important role Hyperglycemia diet disease strateties, it is important to understand the interventoon and interventipn events leading to the secondary lesion of SCI.
Although the intwrvention and pathogenesis of SCI remain to be intervenyion elucidated, extensive Antioixdant over the last intdrvention decades steategies suggested that increased formation of reactive oxygen interventiom ROS and the startegies oxidative stress Plant-based weight loss important events associated intercention SCI.
The neurons and glia Antioxidant intervention strategies the central nervous intervebtion including spinal Effective weight gain are particularly prone to oxidative and electrophilic stress Atnioxidant to many factors, strattegies a high content of polyunsaturated Muscular strength training benefits acids, a high stategies of oxidative metabolic activity, Ahtioxidant production of Antikxidant oxygen syrategies and relatively low antioxidant capacity.
We searched MEDLINE PubMed and reference lists of the included articles published mainly between and using the following key words: SCI, oxidative stress, biomarkers, antioxidant, bioactive compounds and intervention. Molecular oxygen is an essential element of life, yet incomplete reduction or excitation of oxygen during aerobic metabolism generates ROS.
ROS include superoxide, hydroxyl radical, singlet oxygen and hydrogen peroxide Figure 1. Superoxide is the one-electron reduction product of molecular oxygen. If two electrons are transferred, the product is hydrogen peroxide. In phagocytes, superoxide is produced in large quantities by the enzyme nicotinamide adenine dinucleotide phosphate oxidase for use in oxygen-dependent killing mechanisms.
Hydrogen peroxide is not a free radical but is nonetheless a damaging species because of its ability to penetrate biological membranes. Hydrogen peroxide is toxic to cells and can lead to further free-radical generation.
For example, hydrogen peroxide reacts with reduced transition metals to form the highly reactive hydroxyl radical, which readily causes damage to DNA and other biological molecules. Production of ROS under physiological conditions is important for normal cellular redox reactions.
However, excessive generation of free radicals under pathophysiological conditions such as SCI can greatly enhance the production of ROS. The mitochondrial dysfunction results in further increased formation of ROS.
Structural and functional alterations of the mitochondria have been found in a SCI mouse model. The enzyme complex transfers electrons from nicotinamide adenine dinucleotide phosphate oxidase at the cytosolic side of the membrane to molecular oxygen at the other side of the membrane resulting in the production of extracellular superoxide.
For example, there are many cytosolic enzymes that can generate ROS via reduction of molecular oxygen in their catalytic cycles. The most notable one is xanthine oxidase, which can directly reduce molecular oxygen to superoxide and hydrogen peroxide. ROS participate in physiological processes, such as cell signaling.
However, excessive generation of ROS under pathophysiological conditions, including SCI may result in oxidative stress. To control ROS, aerobic organism has utilized several antioxidative mechanisms including enzymatic and non-enzymatic antioxidants 12 Figure 2. Non-enzyme low molecular weight antioxidant compounds include cellular glutathione, vitamins C and E, β-carotene and uric acid.
The antioxidant enzymes include superoxide dismutase SODcatalase, glutathione reductase and glutathione peroxidase among others. Mammalian cells contain three forms of SOD, MnSOD, Cu,ZnSOD and extracellular SOD.
MnSOD is most abundant in the mitochondria, whereas Cn,ZnSOD predominant in the cytoplasm. Glutathione peroxidase is another major enzyme for decomposing H 2 O 2.
ROS and their detoxification by cellular antioxidants. GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized form of glutathione; GST, glutathione S-transferase; G6PDH, glucosephosphate dehydrogenase; 6PGDH, 6-phosphogluconate dehydrogenase; LOH, lipid alcohol; NQO1, NAD P H:quinone oxidoreductase 1; SOD, superoxide dismutase.
The elimination of ROS following SCI is particularly controlled by cellular antioxidant glutathione and Cu,ZnSOD. The results suggested that elevation of levels of glutathione by irrigation with δ-glutamylcysteine conferred significant protection against lipid peroxidation after SCI, suggesting that glutathione augmentation may be an effective strategy for curtailment of lipid peroxidation-mediated damage in SCI.
NAD P H:quinone oxidoreductase 1 NQO1 is another important antioxidant enzyme that has recently been demonstrated to has a protective role in electrophilic stress underlying spinal cord damage. High levels of ROS can overwhelm the normal cellular antioxidant defenses leading to several direct and indirect health effects.
Direct effects include chain of peroxidation reactions involving lipids and other macromolecules. Indirect effects include modified metabolic pathways and altered pathophysiology of the organ systems due to the oxidative damage.
Oxidative stress may occur when ROS are produced faster than they can be removed by cellular defense mechanisms. The main damage to cells results from ROS-induced alteration of macromolecules such as polyunsaturated fatty acids in membrane lipids, proteins, and DNA.
Lipid peroxidation is a common and dangerous type of ROS-induced cellular oxidation. It has long been recognized that much of the posttraumatic degeneration of the spinal cord following injury is caused by a secondary injury process, and a significant biochemical event is ROS-induced lipid peroxidation.
The coordinated elevation of antioxidant enzymes is regulated through a cis -acting element called the antioxidant response element ARE within the regulatory region of each of the antioxidant enzyme genes 2728 Figure 3. Transcriptional activation mediated by the ARE is effected by the transcription factor Nrf2.
Nrf2 belongs to the CNC family of b-zip transcription factors. The mechanism by which binding of Nrf2 to ARE is induced is still emerging, but likely includes contributions from the repressor of Nrf2 that normally resides in the cytosolic compartment along with the cysteine-rich chaperone Keap1.
Keap 1 is an inhibitor of Nrf2 that sequesters it in the cytoplasm. Activation of Nrf2 dissociates Nrf2 from Keap1, allowing for its translocation to the nucleus, where it can interact with the ARE to activate transcription of antioxidant enzyme genes 2728 Figure 3. Activation of Nrf2 and Nrf2-mediated antioxidant enzyme gene expression by pro-oxidants, including H 2 O 2 at moderate non-lethal doses suggests that Nrf2 signaling may control the adaptive response of neuronal cells to oxidative insults.
Biomarkers of ROS and oxidative stress are useful for assessing the pathogenesis and progression of SCI. Biomarkers are defined as characteristics that are objectively measured and evaluated as indicators of normal biological activities, pathogenic processes or pharmacological responses to therapeutic intervention.
Because the highly reactive ROS are short lived and difficult to measure directly, indirect measures are most often used to predict the amount of ROS or the extent of oxidative stress occurring in SCI.
These include the measurement of glutathione, Cu,ZnSOD, malondialdehyde MDAacrolein and F2-isoprostanes. It is of note that each marker has its own limitation in predicting oxidation in biological systems. As such, it is recommended that at least measurement of two markers should be used.
Glutathione is the primary low molecular weight thiol antioxidant and co-substrate for several cellular antioxidant enzymes such as glutathione peroxidase and and glutathione transferase.
It has a critical role in maintaining the intracellular oxidation—reduction redox balance and regulating oxidative stress-induced signaling pathways as well as detoxifying ROS and other reactive aldehydes including acrolein.
In addition, it is reported that glutathione may also be an important regulatory neuropeptide in the central nervous system.
GCL, the rate-limiting enzyme in the overall pathway, is a heterodimer composed of a catalytic GCLC and a modulatory GCLM subunit. GCLC remains all the catalytic activity and GCLM improves the catalytic efficiency.
It has been demonstrated that significant decreases in glutathione occurred in spinal segments T5—T9 with the greatest decrease seen at the site of injury and immediately adjacent segments. There are several chemical 38 and enzymatic 39 assays available for determination of glutathione.
However, most of these assays lack sensitivity and specificity. Hissin and Hilf 40 developed a fluorometric method for measuring glutathione. It is based on the reaction of reduced glutathione with o -phthalaldehyde to generate a highly fluorescent adduct that is specific for determination of reduced glutathione in rat tissue at pH 8.
As such, it is not practical to routinely use the original protocol for glutathione determination in cultured neuronal cells especially under the conditions of using small volumes and microgram quantities of samples. We have modified the original method of Hissin and Hilf and reported a sensitive and specific assay for determining reduced glutathione status in human neuronal cells.
Given the use of a small sample volume, it also minimizes the presence of interfering substances in the samples. As mentioned before, mammalian tissues contain three forms of SOD: cytosolic Cu,ZnSOD, mitochondrial MnSOD and extracellular SOD.
The presence of SOD helps dismutate superoxide immediately upon its generation and thus protects cells from superoxide-mediated oxidative damage. Several point mutations in the gene that encodes Cu,ZnSOD have been reported in some patients with familial ALS, a disease that is characterized by a loss of motor neurons in both the brain and the spinal cord.
: Antioxidant intervention strategies| Antioxidant Strategies in Neurocritical Care | Among the identifiable causes, the male factor stands out in about half of infertile couples, representing a growing problem. Accordingly, there has been a decline in both global fertility rates and sperm counts in recent years. Among the mechanisms likely plausible to account for idiopathic cases, oxidative stress OS has currently been increasingly recognized as a key factor in MI, through phenomena such as mitochondrial dysfunction, lipid peroxidation, DNA damage and fragmentation and finally, sperm apoptosis. In addition, elevated reactive oxygen species ROS levels in semen are associated with worse reproductive outcomes. However, despite an increasing understanding on the role of OS in the pathophysiology of MI, therapeutic interventions based on antioxidants have not yet provided a consistent benefit for MI, and there is currently no clear consensus on the optimal antioxidant constituents or regimen. Therefore, there is currently no applicable antioxidant treatment against this problem. This technique allows for real-time biochemical monitoring of markers of ischemia and neuronal injury, and is being increasingly used in highly advanced neurocritical care units. It certainly holds promise as a tool to improve our understanding of OS and its management in the neurocritical care setting, and for future use as a means for direct administration of potential antioxidant therapy. Imaging techniques hold promise as useful tools for assessment of OS in vivo , although the clinical utility of these techniques remains limited. Nuclear magnetic resonance spectroscopy, such as the Nuclear Overhauser effect enhanced magnetic resonance imaging MRI , electron spin resonance spectroscopy, and 31 P-nuclear magnetic resonance spectroscopy can create images of free radical distributions in animals [ 14 , 15 ]. Brain damage attributed to OS may be detected on MRI. For example, the relaxivity of the pro-oxidant ferric form of hemoglobin on T1-weighted imaging is higher than its ferrous counterparts [ 16 ]. Oxidative modifications of DNA, protein, and lipids by ROS play an important role in neuronal injury and its severity following cerebral ischemia. In a prospective study of 45 patients with acute ischemic stroke, the activities of the scavenger enzymes, SOD, and catalase were lower in stroke patients than controls, and the study showed a gradual increase in time for as many as 15 days poststroke, which correlated with the degree of neurological deficits [ 17 ]. In another study, plasma concentrations of α- and β-carotene were lower in patients with acute ischemic stroke than healthy controls, and were negatively associated with high sensitivity C-reactive protein level and the National Institute of Health Stroke Scale NIHSS score after adjustment for age and sex [ 18 ]. Similarly, in a study of 70 patients with acute ischemic stroke and 70 controls with similar risk factors, serum levels of NO, malondialdehyde, and glutathione were significantly elevated in stroke patients, and the levels of NO and malondialdehyde correlated with the Canadian Neurological Scale score [ 19 ]. Taffi et al. They found that mean plasma levels of peroxynitrite were significantly higher in stroke patients and that NO levels were associated with worsening evolution of NIHSS score from admission to day 30, suggesting that changes in NO metabolism may be a marker of brain injury following ischemic stroke. However, the role of NO following ischemia is complex. Other reports have linked greater NO production to improved vascular reactivity and anti-inflammatory responses following ischemia [ 21 , 22 ]. Overall, the previously described studies implicate a pathophysiological role for OS in ischemic stroke and suggest deleterious effects of OS on stroke severity and clinical outcome. Few clinical studies examined the use of antioxidants in ischemic stroke. The ability of the free radical-trapping agent NXY to reduce functional disability after ischemic stroke was previously investigated. Despite encouraging results in early phase II testing, the subsequent definitive phase III trial did not show beneficial effects [ 23 , 24 ]. Several reasons have been proposed for the failure of NXY [ 25 ]. Future studies investigating the role of OS on long-term poststroke recovery are needed. Reperfusion injury plays an important role in the pathophysiology of neuronal injury following cerebral ischemia via several deleterious pathways including: cytochrome-c release, expression of matrix metalloproteinase MMP , caspase induction, and reduction of DNA repair enzymes [ 26 ]. In a case control study of ischemic stroke patients presenting within 8 hours of stroke onset, F2-isoprostanes were elevated in stroke cases compared with controls early on, but not at later time points, and they correlated with MMP-9, indicating that OS is also implicated in activation MMP-9 and blood-brain barrier injury after ischemia reperfusion [ 27 ]. Additionally, the antioxidant enzyme SOD blocks these pathways, thereby preventing apoptosis following cerebral ischemia [ 28 ], suggesting that antioxidant therapy could be a potential therapeutic strategy to prevent reperfusion injury following reperfusion therapy, such as thrombolysis. After intracerebral hemorrhage ICH , large numbers of hemoglobin-containing red blood cells are released into the brain parenchyma. Iron has been implicated in neuronal injury and delayed brain edema formation after ICH via several mechanisms including activation of lipid peroxidation, exacerbation of excitotoxicity, and inhibition of sodium-potassium pump activity [ 29 — 31 ]. Increased hydroxyl radical formation leads to OS and subsequent cell death. A large number of experimental studies show that iron is neurotoxic after ICH. Goldstein et al. Studies in rat models of ICH also confirm the role of iron toxicity that it is mediated via OS mechanisms. Huang et al. In similar studies, Nakamura et al. These findings suggest that iron-mediated OS contributes to DNA damage and brain injury after ICH and that limiting iron-mediated oxidative injury in the brain is a potential therapeutic target in ICH. Emerging evidence also links iron to neuronal injury in ICH patients. Serum ferritin on admission correlates with the relative perihematoma edema PHE on day 3, which coincides with the timing for hemoglobin hemolysis [ 35 ] and functional outcome at 3 months [ 36 ], and the iron content within the hematoma, estimated by MRI, correlates with PHE volume [ 37 ]. The role of OS in patients with ICH has been investigated in a small study of 13 patients with spontaneous ICH and 15 patients with traumatic ICH, compared to 40 healthy controls [ 38 ]. ICH patients had significantly lower plasma levels of vitamin C and vitamin C levels inversely correlated with the severity of neurological impairment, as assessed by Glasgow Coma Scale and the NIHSS, and the diameter of the hematoma. These findings suggest depletion of antioxidants following ICH, and also suggest that its severity, which presumably reflects the severity of OS, correlates with indices of injury-related clinical impairments. The Cerebral Hematoma and NXY Treatment Trial CHANT investigated the use of the free radical-trapping agent NXY in ICH; treatment initiated within 6 hours of ICH onset and continued for 72 hours showed no difference in mortality or the distribution of modified Rankin Scale scores at 90 days between NXY- and placebo-treated patients [ 39 ]. Clinical studies in ICH patients also have identified genome-wide associations between Apolipoprotein-E variants APOE ε2 and ε4 and lobar ICH; The APOE ε4 allele amplifies the inflammatory responses, increases cerebral edema, and is associated with worse outcome after ICH [ 40 , 41 ]. In mouse models, treatment with APOE analogue, COG , resulted in reduced functional deficit, decreased brain concentrations of inflammatory proteins, and less cerebral edema [ 42 ]. Clearly, more clinical studies are needed to assess the role of APOE and OS in ICH patients. In rodent models of subarachnoid hemorrhage SAH , there is evidence of increased superoxide anion and nicotinamide adenine dinucleotide phosphate NADPH oxidase, both powerful oxidizing agents [ 43 , 44 ]. In addition, treatment with alpha-lipoic acid, a dithiol antioxidant, improves neurological outcome and decreases brain edema and free radical generation [ 44 ]. In another study of rat model of aneurysm genesis, ROS were related to aneurysm formation [ 43 ]. In this study, rats were fed a high salt diet for 3 months in addition to a lysyl-oxidase inhibitor, an enzyme involved in catalyzing the cross-linking between collagen and elastin. Upregulation of ROS-producing genes, suppression of ROS-eliminating genes, and many highly oxidative species, such as heme-oxygenase, NOS, MMP-2, and a 47 kDa portion of NADPH oxidase were detected within the spontaneous aneurysms that formed, using reverse transcriptase PCR analysis, Western blot, and immunohistochemistry [ 43 ]. Pre-treatment with edavarone, a free-radical scavenger, decreased size of the aneurysm and the production of the previously mentioned oxidizing agents within the aneurysm, and also within infiltrating macrophages and smooth muscle cells [ 43 ]. These effects were more profound when studying these phenomena in a p47 NADPH oxidase knockout mouse, suggesting that this may be one of the key oxidizing agents required for initiating the aneurysm signaling cascade. The prognostic value of OS has been investigated in postsurgical aneurysmal SAH patients; cerebrospinal fluid levels of malondialdehyde emerged as significant predictors of poor outcome at 6 months [ 45 ]. A Japanese proof-of-concept study investigated the use rectal indomethicin and selective brain cooling in patients with aneurysmal SAH and ICH, and found reduced cerebrospinal fluid expression of inflammatory cytokines and ROS [ 46 ]. No outcome data were presented. Few randomized, controlled clinical trials have examined the use of radical scavengers in SAH patients, but the results have been disappointing [ 47 , 48 ]. The synthetic aminosteroid, tirilazad mesylate, which inhibits lipid peroxidation, has been extensively studied in SAH with variable results [ 49 — 51 ]. Overall, there was no difference in long-term outcome or symptomatic vasospasm between active treatment and placebo. Reactive oxygen species play a role in the pathogenesis of traumatic brain injury TBI. Cats subjected to fluid-driven piston percussion model to induce TBI have significantly more superoxide anion production after TBI compared to controls [ 52 ]. They also have persistent cerebral arteriolar dilation and reduced responsiveness to hypocapnic vasosconstriction after TBI, and the arteriolar dilation is reduced by treatment with SOD and catalase [ 53 ]. In rat models of TBI, administration of progesterone before the insult reduced levels of isoprostane and improved neurological recovery [ 54 ]. In an attempt to translate this into the clinical setting, the Progesterone for Traumatic Brain Injury: Experimental Clinical Treatment ProTECT III , a phase III clinical study is currently enrolling patients to determine whether or not progesterone will be the first experimentally vetted treatment for TBI. Similar to ICH, APOE is another possible target for ameliorating neurological injury after TBI. APOE has been shown to reduce ROS and other inflammatory markers after many different kinds of insults [ 55 ]. When APOE or its analogues are given to rats before TBI, they not only have improved neurological outcome, but the size of the contusion is reduced compared to controls [ 56 ]. Conversely, the APOE ε4 allele, which codes for a form of APOE that is the least effective in reducing the cerebral inflammatory response, predisposes to worse outcomes after neurological injury, including TBI [ 57 , 58 ]. It remains to be seen whether APOE or its analogues have a place in the treatment of TBI. Despite the large number of antioxidants tried in MODS, few had a significant effect on morbidity or mortality [ 60 , 61 ]. One explanation is that the antioxidants tested were not targeted to mitochondria. Injection of MitoQ in a sepsis model reduced radical production and lowered markers of hepatic and renal injury [ 62 ]. In addition, some systemic antioxidants have been tried for sepsis: N-acetylcysteine and deferoxamine [ 63 — 65 ]. Individually, these drugs have failed to improve outcome in human trials, although the combination of these drugs has shown some promise in a cecal ligation mouse model of sepsis [ 66 , 67 ]. It has been theorized that this may be due to a much more oxidative environment in sepsis that results in all of the N-acetylcysteine being oxidized by free iron released from cytochome-c and hemolysis. Subcutaneous injection of N-acetylcysteine and deferoxamine 3 hours after cecal ligation resulted in decreased cytochrome-c release, radical formation, and liver necrosis [ 66 ]. These results suggest that the complex pathway of OS-mediated injury in critically ill patients may necessitate a multimodal poly-therapeutic approach, as opposed to single therapeutic strategies. There has been some success in the treatment of sepsis with antioxidants, namely, omega fatty acids and selenium. A German group randomized 10 septic patients requiring parenteral nutrition to receive either a standard omega 6-based lipid infusion or a fish-derived omega 3 lipid infusion [ 68 ]. The neutrophils derived from the omega 3 group were better able to metabolize free fatty acids, which are potent oxidants, and responded to ex vivo stimulation more briskly. The study was too small to demonstrate any improved outcome, but the results are promising and should be investigated further. Selenium has been tested in severe sepsis, with patients who had APACHE III scores greater than 70 who were randomized to receive intravenous selenium for 14 days or placebo [ 69 ]. The selenium group had a significant improvement in day mortality. Furthermore, the mortality benefit was accentuated in the most critically ill patients with APACHE scores greater than , more than three organ failures, and those with disseminated intravascular coagulation. The investigators also measured glutathione peroxidases-3 activity and found much higher activity in the treatment group. Both omega-3 fatty acids and selenium are relatively simple measures that could be instituted in intensive care units across the country, however, further investigation and larger studies are required before antioxidants will see their roles elevated to the standard of care for MODS. Another type of organ failure that neurointensivists commonly deal with is cardiac arrest. Oxidative stress plays an important role in brain injury following cardiac arrest. In rats subjected to 7. In humans, exposure of human umbilical vein endothelial cells to the plasma of out of hospital-cardiac arrest patients increased the production of ROS and decreased production of anti-oxidants, such as SOD, glutathione, and glutathione peroxidase in the cells exposed to cardiac arrest patients vs. normal controls [ 71 ]. The protective effects of therapeutic hypothermia in cardiac arrest patients on mortality and neurological outcomes is well established [ 72 ]. Animal studies show that hypothermia decreases the production of ROS, stabilizes mitochondrial membrane potentials, phosphorlyates a survival kinase called Akt, and decreases cell death compared to normothermia in murine cardiac myocytes exposed to conditions simulating ischemia and reperfusion [ 73 ]. In rats, hypothermia reduces malondialdehyde levels in the brain during reperfusion following hypoxic ischemia compared with normothermia [ 74 ]. Acute lung injury ALI and adult respiratory distress syndrome ARDS are common problems in neurocritical patients. The inciting events are defined by either direct injury to the lung, such as aspiration or pneumonia versus indirect injury to the lung, such as sepsis [ 75 ]. Reactive species in ARDS come from a variety of sources including high inspired fraction of oxygen, depletion of endogenous anti-oxidants, and intra- and extra-pulmonary inflammation [ 76 , 77 ]. A study measuring oxidized products of xanthine oxidase in ARDS patients correlated strongly with outcome, supporting the role for ROS in the pathophysiology of ARDS [ 77 ]. Similar to shock studies, several anti-oxidants have been investigated for ARDS in the clinical setting; N-acetylcysteine replenished levels of neutrophilic glutathione, but had no effect on oxidant or elastase production [ 77 ]. Another study using intravenous liposomal delivery of prostaglandin E1 to ARDS patients in order to decrease the production of exhaled hydrogen peroxide and ROS production found no effect at all on reducing oxidants or improving outcome [ 78 ]. The biochemical changes of OS suggest that antioxidant therapy may be theoretically achieved by the following strategies: 1 restoring endogenous antioxidants and nutrients and supplementation with exogenous trace elements, vitamins, and nutrients with antioxidant properties; or 2 administering drugs that reduce OS, such as statins, iron-modifying agents such as deferoxamine, or minocycline. We discuss below the potential utility of these strategies and present data regarding their use in the critical care setting. It is intuitive that any of these strategies would probably be more efficacious if implemented before a massive OS takes place, i. Therefore, these candidate antioxidant strategies can potentially be used for preventive or therapeutic purposes. There is a rationale to support exogenous supply of antioxidant trace elements, vitamins, and nutrients during critical illness. The levels of nutrients with antioxidant properties are decreased in critical illness. For example, patients with ICH have lower plasma levels of vitamin C compared with healthy volunteers [ 38 ]. Other studies have shown a reduction in muscle glutamine and glutathione and muscle protein synthesis in intensive care patients [ 81 ]. There is also evidence that low endogenous stores on antioxidant nutrients are associated with increased free radical generation, augmentation of the systemic inflammatory response, subsequent cell injury and death, and even higher morbidity and mortality in critically ill patients [ 6 , 7 ]. In addition, proof-of-concept studies have shown that supplementation with trace elements and vitamins, such as selenium, and vitamins A, C, and E, improve antioxidant capacity by increasing the activity of glutathione peroxidase, or reducing plasma TBARS, and isoprostanes [ 82 ]. Vitamins C and E may also reduce infectious complications by restoring neutrophil function and cell-mediated immunity, and selenium improves phagocytosis and immunoglobulin synthesis. Although results from some of these trials were encouraging, the benefit of this therapy has not been clearly established. Several reasons have been proposed: 1 the vast majority of these studies were performed in small and heterogeneous populations of critically ill patients, and were largely underpowered; and 2 uncertainties regarding the correct dose to use, appropriate time to start immune nutrition, and the appropriate route of administration; the enteral versus parenteral. In a meta-analysis of 11 studies selected from 44 studies based on strict criteria of early immune nutrition using antioxidant trace elements and vitamins in well-defined intensive care patient populations, Heyland et al. They also found that none of the trials using immune nutrition reported deleterious effects from using exogenous micronutrients, and they concluded that supplementation with antioxidant trace elements and vitamins in intensive care patients is safe and possibly beneficial. They argued that immune nutrition must be started very early after admission to the intensive care unit and that high-dose parenteral nutrition appears to have a stronger impact on outcome. Crimi et al. Recently, Casaer et al. It is noteworthy, however, that Casaer et al. Given the previously described, the use of immune nutrition therapy in neurocritical care might be a rationale and promising strategy. However, more definitive evidence from large-scale, randomized, placebo-controlled, and well-designed trials in this specific patient population is still required. Vitamin B12 has glutathione-sparing antioxidant properties via stimulation of methionine synthase activity and reaction with ROS and NOS. It also inhibits NOS and NO production, and decreases NF-κB activation. N-acetylcysteine has multiple putative antioxidant properties by decreasing NF-κB activation and cytokines production, regenerating NO as a sulfhydryl donor, and replenishing glutathione, and scavenging ROS as a precursor of glutathione. N-acetylcysteine has been extensively studied in critically ill patients with acute respiratory distress syndrome, organ failure, and septic shock. These studies have produced inconsistent results [ 83 , 89 , 90 ]. We found no studies of N-acetylcysteine in neurocritical care, to date. Albumin has antioxidant effects, largely mediated by its thiol sulfhydryl group portion, which attaches to ROS and NOS. It also decreases copper-mediated lipid peroxidation, and modulates nitric oxide-mediated vascular dilatation. Hypoalbuminemia is common in critically ill patients, including those with TBI and various neurological conditions, and is associated with increased morbidity and mortality [ 91 ]. Studies of albumin supplementation in surgical and medical critically ill patients had inconsistent results. The Saline versus Albumin Fluid Evaluation SAFE study of found no advantage for albumin in comparison with saline [ 92 ], and a subsequent post-hoc subgroup analysis of patients with TBI suggested that resuscitation with albumin was associated with increased odds ratio for death [ 93 ]. A Cochrane meta-analysis found no benefit in a heterogenous group of previously published studies [ 94 ]. Powner [ 95 ] recently argued that it is important to separate data from studies regarding albumin infusions intended to restore or maintain intravascular pressure or volume goals from the potential benefit of preventing hypoalbuminemia during neurocritical care. The use of albumin in TBI, and hypertensive and traumatic ICH has been investigated in small studies [ 96 — 98 ]. Although the clinical results were encouraging, these studies were too small and most were nonrandomized and single arm to derive definite conclusions. Preliminary results from the Treatment of Subarachnoid Hemorrhage with Human Albumin ALISAH study indicate that doses up to 1. Although recruitment in ALIAS part 1 was halted due to safety concerns, preliminary efficacy data from this cohort suggests a trend toward a favorable outcome among albumin-treated subjects [ 99 ]. Statins can modulate endogenous pro-oxidative and anti-oxidative systems. For example, statins dose dependently inhibit platelet-mediated low-density lipoprotein oxidation and isoprostane formation, reduce malondialdehyde, and increase SOD and glutathione peroxidase activities in erythrocytes. In a recent two-center, two-arm, randomized, open-label, controlled study of pravastatin, in intensive care patients, Makris et al. Statin therapy has also been investigated in patients with ALI; it was shown to be safe and associated with a significant decrease in bronchoalveolar lavage interleukin-8, but had no other effects compared to placebo [ ]. Although preclinical studies have shown benefits from statins in models of TBI and related disease processes, including cerebral ischemia, ICH, and subarachnoid hemorrhage, clinical studies have been shortcoming [ ]. The use of statins in patients with aneurysmal subarachnoid hemorrhage has been investigated in several small clinical trials. A meta-analysis of four randomized controlled trials involving patients revealed no significant effects on vasospasm, detected by transcranial Doppler, delayed cerebral ischemia, poor outcome, or mortality [ ]. The ongoing phase III Simvastatin in Aneurysmal Subarachnoid Hemorrhage STASH multi-center randomized controlled phase III trial should help to further clarify the role of statins in these patients. Retrospective studies suggest that antecedent use of statins is associated with improved outcome and reduced mortality in patients with acute ischemic stroke and ICH [ , ]. Future studies examining the role of statins as an acute treatment in PHE reduction in ICH and resultant effect on mortality and functional outcome after ischemic and hemorrhagic stroke are warranted. Studies using iron-modifying agents in animal models of cerebral ischemia and ICH provide evidence for a potential therapeutic benefit. Tirilazad mesylate, a potent inhibitor of iron-dependent lipid peroxidation, attenuates neuronal necrosis and improve the neurological status in several animal models of cerebral ischemia [ ]. Treatment with bipyridyl, an iron-chelating agent, significantly attenuates the development of brain edema 24 hours after the induction of ischemia [ ]. Similarly, treatment with deferoxamine, another iron chelator, has been shown to reduce infarct size and improve the neurological status in rats subjected to transient middle cerebral artery occlusion [ ]. Deferoxamine may also alleviate reperfusion-induced injury following ischemia. In one study, treatment with deferoxamine attenuated death rate and hemorrhagic transformation in a rat model of transient focal ischemia [ ]. Treatment with deferoxamine also attenuates the production of ROS, blocks hemoglobin-mediated potentiation of glutamate neurotoxicity, reduces brain malondialdehyde content and induces recovery of sodium-potassium pump activity, and exerts diverse protective effects after experimental ICH [ 29 , 33 , — ]. Emerging evidence also supports a potential therapeutic role for deferoxamine following intraventricular and subarachnoid hemorrhages [ , ]. Deferoxamine decreases the availability of free iron for the production of hydroxyl radicals by forming a stable complex with ferric iron. However, the potential beneficial effects of deferoxamine may be only partly related to its iron chelating abilities. Very few clinical studies have investigated the use of iron-modifying agents in stroke patients. Two multi-center trials examined the use of tirilazad in acute ischemic stroke [ , ]. The Randomized Trial of High Dose Tirilazad in Acute Stroke RANTTAS II investigated a higher dose The use of tirilazad mesylate in patients with subarachnoid hemorrhage was also investigated in several randomized, placebo-controlled, trials. Although it reduced the incidence of symptomatic vasospasm, it had no effect on the overall long-term clinical outcome [ ]. Clinical investigations of deferoxamine in stroke have gained attention in recent years. In ICH, a small, open-label, phase-I, safety and dose-finding study of deferoxamine in patients with spontaneous ICH safety and tolerability of deferoxamine in acute cerebral hemorrhage — deferoxamine mesylate in ICH was recently completed [ ]. This study enrolled 20 subjects into 5 dose tiers of deferoxamine, which was given as a continuous intravenous infusion for 3 consecutive days starting within 16 h of ICH symptoms onset. Repeated daily infusions of deferoxamine were well tolerated and did not cause excessive serious adverse events or mortality. Interestingly, deferoxamine also seemed to exert a modest blood pressure-lowering effect. Similarly, a phase I-II, double-blind, randomized, placebo-controlled, dose-finding study to evaluate the safety and pharmacokinetics of deferoxamine in patients with acute ischemic stroke who are treated with intravenous recombinant tissue plasminogen activator rt-PA within 3 hours of stroke onset, and to explore the effects of treatment on clinical outcomes, infarct volumes, hemorrhagic transformation, and brain edema development The Thrombolysis and Deferoxamine in Middle Cerebral Artery Occlusion — TANDEM-1 is currently underway. Minocycline has anti-inflammatory and anti-apoptotic properties, inhibits polysdenosine diphosphate ribose polymerase-1 and matrix metalloproteinases, and is an effective antioxidant and radical scavenger. Minocycline has neuroprotective effects in vivo against cerebral ischemia and in vitro against glutamate-induced cell death, an inhibition of OS by minocycline may be partly responsible for these effects [ ]. Minocycline also chelates iron, and prevents the neuronal death induced by ferrous sulfate [ ] and attenuated brain edema and neurological deficits in rat models of ICH [ ]. An open-label, dose-escalation, safety and dose-finding study of minocycline, administered intravenously within 6 h of symptom onset, in patients with acute ischemic stroke was recently completed Minocycline to Improve Neurologic Outcome in Stroke [MINOS] [ ], and plans for a phase III study are currently underway. Interestingly, lower plasma levels of MMP-9 was seen among rt-PA treated subjects in the MINOS trial, suggesting that combining minocycline with rt-PA might prevent the adverse consequences of thrombolysis [ ]. Overwhelming pre-clinical and clinical evidence supports the presence of OS in several neurocritical conditions. Although dietary supplementation with antioxidant vitamins and the use of pharmacological agents targeting OS and its downstream cascade seems to be rational, the benefits of various attempted antioxidant strategies to date have not been clearly demonstrated. Several reasons have been advocated, including the wide variability in the nature and severity of illness in intensive care patients. Future studies should consider these issues to avoid past mistakes. Several studies of antioxidant drugs, such as statins, minocycline, and deferoxamine in specified populations of neurological patients are currently underway, and its results could have important implications in neurocritical care. Although the role of antioxidant strategies in neurocritical care is slowly evolving, it is likely to be an integral component of the overall strategy for neurological critical care in the future. Collier BR, Giladi A, Dossett LA, Dyer L, Fleming SB, Cotton BA. Impact of high-dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr ;— PubMed CAS Google Scholar. Pamplona R, Costantini D. Molecular and structural antioxidant defences against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol ;RR Harris RA, Amor S. Sweet and sour--oxidative and carbonyl stress in neurological disorders. CNS Neurol Disord Drug Targets ;— Abilés J, de la Cruz AP, Castaño J, et al. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit Care ;R PubMed Google Scholar. Therond P, Bonnefont-Rousselout D, Davit-Sparaul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care ;— Carré JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med ;— Gelain DP, de Bittencourt Pasquali MA, M Comim C, et al. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock ;— Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res ;— Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics ;— Google Scholar. Altavilla D, Saitta A, Guarini S, et al. Oxidative stress causes nuclear factor-kappaB activation in acute hypovolemic hemorrhagic shock. Free Radic Biol Med ;— Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett ;— Grune T, Berger MM. Markers of oxidative stress in ICU clinical settings: present and future. Clausen F, Marklund N, Lewén A, Enblad P, Basu S, Hillered L. Interstitial F2-isoprostane 8-iso-PGF2α as a biomarker of oxidative stress following severe human traumatic brain injury. J Neurotrauma ; doi: Utsumi H, Yamada KI, Ichikawa K, et al. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. PNAS ;— Noseworthy MD, Bray TA. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radical Biol Med ;— Leung G, Moody AR. MR imaging depicts oxidative stress induced by methemoglobin. Radiology ;— Casado A, de la Torre R, López-Fernández ME, Gil P, Egido JA. Antioxidant enzymes in acute cerebral infarction. Neurologia ;—9. Chang CY, Chen JY, Ke D, Hu ML. Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: correlation to inflammation markers and neurological deficits. Nutrition ;— Ozkul A, Akyol A, Yenisey C, Arpaci E, Kiylioglu N, Tataroglu C. Oxidative stress in acute ischemic stroke. J Clin Neurosci ;— Taffi R, Nanetti L, Mazzanti L, et al. Plasma levels of nitric oxide and stroke outcome. J Neurol ;— Atochin DN, Wang A, Liu VW, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest ;— Ishikawa M, Zhang JH, Nanda A, et al. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci ;— Lees KR, Zivin JA, Ashwood T, et al. NXY for acute ischemic stroke. N Engl J Med ;— Shuaib A, Lees KR, Lyden P, et al. NXY for the treatment of acute ischemic stroke. Savitz SI, Schabitz W. A Critique of SAINT II: wishful thinking, dashed hopes, and the future of neuroprotection for acute stroke. Stroke ;— Chan PH. Neurochem Res ;— Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the biomarker evaluation for antioxidant therapies in stroke BEAT-Stroke study. Fujimura M, Tominaga T, Chan PH. Neuroprotective effect of an antioxidant in ischemic brain injury: involvement of neuronal apoptosis. Neurocrit Care ;— Regan RF, Panter SS. Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma ;— Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J Neurosci Res ;— Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab ;— Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg ;— Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res ;— Mehdiratta M, Kumar S, Hackney D, et al. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Pérez de la Ossa N, Sobrino T, Silva Y, et al. Iron-related brain damage in patients with intracerebral hemorrhage. Lou M, Lieb K, Selim M. The relationship between hematoma iron content and perihematoma edema: an MRI study. Cerebrovasc Dis ;— Polidori MC, Mecocci P, Frei B. Plasma vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Lyden PD, Shuaib A, Lees KR, et al. CHANT Trial Investigators safety and tolerability of NXY for acute intracerebral hemorrhage: the CHANT Trial. Biffi A, Anderson CD, Jagiella JM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol ;— James ML, Blessing R, Bennett E, et al. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis ;— Laskowitz DT, Lei B, Dawson HN, et al. The apoE-mimetic peptide, COG, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care ; doi: Aoki T, Nishimura M, Kataoka H, Ishibashi R, et al. Lab Invest ;— Erşahin M, Cetinel S, Yüksel M, et al. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Kaneda K, Fujita M, Yamashita S, et al. Prognostic value of biochemical markers of brain damage and oxidative stress in post-surgical aneurysmal subarachnoid hemorrhage patients. Brain Res Bull ;— Dohi K, Ikeda Y, Fujita S, et al. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: anti-inflammatory cytokines and antioxidative effects. Acta Neurochir Suppl ;— Asano T, Sano K, Kikuchi H, et al. Effects of a hydroxyl radical scavenger on delayed ischemic neurological deficits following aneurysmal subarachnoid hemorrhage: results of a multicenter, placebo-controlled double-blind trial. Zuccarello M, Schmitt G. Effect of the aminosteroid UF on cerebral vasospasm following subarachnoid hemorrhage. Kassell NF, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg ;—8. Haley EC Jr, Apperson-Hansen C, Maile MH, et al. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. Kontos HA, Wei EP. Superoxide production in experimental brain injury. Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci ;— Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol ;— Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci ;— Hoane MR, Holland MA, Birky ND, Dang T, et al. The novel apolipoprotein E-based peptide COG improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. Smith C, Graham DI, Murray LS, Stewart J, et al. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry ;— Ariza M, Pueyo R, Matarín Mdel M, et al. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin ;— Berger MM, Chiolero RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med ;ss Biesalski HK, McGregor GP. Antioxidant therapy in critical care—is the microcirculation the primary target? Lowes DA, Webster NR, Murphy MP, et al. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Rank N, Haertel C, Lenhart A, Welte M, et al. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med ;— |
| Support The Nutrition Source | You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products. Skip to content The Nutrition Source. The Nutrition Source Menu. Search for:. Home Nutrition News What Should I Eat? In , a rating tool called the Oxygen Radical Absorbance Capacity ORAC was created by scientists from the National Institute on Aging and the United States Department of Agriculture USDA. It was used to measure the antioxidant capacity of foods. The USDA provided an ORAC database on its website highlighting foods with high ORAC scores, including cocoa, berries, spices, and legumes. Blueberries and other foods topping the list were heavily promoted in the popular press as disease-fighters even if the science was weak, from cancer to brain health to heart disease. However, 20 years later the USDA retracted the information and removed the database after determining that antioxidants have many functions, not all of which are related to free radical activity. Although this was not a primary endpoint for the trial, it nevertheless represents an important outcome. In the Heart Outcomes Prevention Evaluation HOPE trial, the rates of major cardiovascular events were essentially the same in the vitamin E A recent trial of vitamin E in Israel, for example, showed a marked reduction in coronary heart disease among people with type 2 diabetes who have a common genetic predisposition for greater oxidative stress. In the Supplementation en Vitamines et Mineraux Antioxydants SU. MAX study, 13, French men and women took a single daily capsule that contained mg vitamin C, 30 mg vitamin E, 6 mg beta-carotene, mcg selenium, and 20 mg zinc, or a placebo, for seven and a half years. The vitamins had no effect on overall rates of cardiovascular disease. Lung disease A study from the Journal of Respiratory Research found that different isoforms of vitamin E called tocopherols had opposing effects on lung function. Cancer When it comes to cancer prevention, the picture remains inconclusive for antioxidant supplements. MAX randomized placebo-controlled trial showed a reduction in cancer risk and all-cause mortality among men taking an antioxidant cocktail low doses of vitamins C and E, beta-carotene, selenium, and zinc but no apparent effect in women, possibly because men tended to have low blood levels of beta-carotene and other vitamins at the beginning of the study. Age-related eye disease A six-year trial, the Age-Related Eye Disease Study AREDS , found that a combination of vitamin C, vitamin E, beta-carotene, and zinc offered some protection against the development of advanced age-related macular degeneration, but not cataracts, in people who were at high risk of the disease. However, relatively short trials of lutein supplementation for age-related macular degeneration have yielded conflicting findings. The study found that people taking the vitamins were less likely to progress to late-stage AMD and vision loss. However, the study authors noted that taking lutein and zeaxanthin alone or vitamin E alone did not have a beneficial effect on these eye conditions. The Selenium and Vitamin E Cancer Prevention Trial SELECT Eye Endpoints Study, which followed 11, men for a mean of five years, did not find that vitamin E and selenium supplements, in combination or alone, protected from age-related cataracts. It did not find that antioxidant supplements of vitamin E or selenium, alone or in combination, protected against dementia compared with a placebo. Early death A meta-analysis of 68 antioxidant supplement trials found that taking beta-carotene and vitamin A and E supplements increased the risk of dying. It was also difficult to compare interventions because the types of supplements, the dosages taken, and the length of time they were taken varied widely. The same authors conducted another systematic review of 78 randomized clinical trials on antioxidant supplements including beta-carotene, vitamin A, vitamin C, vitamin E, and selenium alone or in combination. The study found that both people who were healthy and those with diseases taking beta-carotene and vitamin E supplements had a higher rate of death. The duration of the studies varied widely from one month to 12 years, with varying dosages. The first inkling came in a large trial of beta-carotene conducted among men in Finland who were heavy smokers, and therefore at high risk for developing lung cancer. The trial was stopped early when researchers saw a significant increase in lung cancer among those taking the supplement compared to those taking the placebo. Again, an increase in lung cancer was seen in the supplement group. MAX trial, rates of skin cancer were higher in women who were assigned to take vitamin C, vitamin E, beta-carotene, selenium, and zinc. These results came from the Selenium and Vitamin E Cancer Prevention Trial SELECT that followed 35, men for up to 12 years. References National Center for Complementary and Integrative Health NCCIH. Antioxidants: In Depth. Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I. The total antioxidant content of more than foods, beverages, spices, herbs and supplements used worldwide. Nutrition journal. Semba RD, Ferrucci L, Bartali B, Urpí-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA internal medicine. Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. Archives of internal medicine. USDA Oxygen Radical Absorbance Capacity ORAC of Selected Foods, Release 2 Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. The Lancet. Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin genotype: a prospective double-blinded clinical trial. Arteriosclerosis, thrombosis, and vascular biology. Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W. New Engl J Med ;— Manzanares W, Hardy G. Vitamin B the forgotten micronutrient for critical care. Heller AR, Groth G, Heller SC, et al. N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Sung J, Bochicchio GV, Joshi M, et al. Admission serum albumin is predicitve of outcome in critically ill trauma patients. Am Surg ;— Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med ; 9 — Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev ; 3 :CD Powner DJ. In my opinion: serum albumin should be maintained during neurocritical care. Jungner M, Grände PO, Mattiasson G, et al. Effects on brain edema of crystalloid and albumin fluid resuscitation after brain trauma and hemorrhage in the rat. Anesthesiology ;— Tone O, Ito U, Tomita H, et al. High colloid oncotic therapy for brain edema with cerebral hemorrhage. Acta Neurochir Suppl Wien ;— CAS Google Scholar. Rodling Wahlström M, Olivecrona M, Nyström F, Koskinen LO, et al. Fluid therapy and the use of albumin in the treatment of severe traumatic brain injury. Acta Anaesthesiol Scand ;— Hill MD, Martin RH, Palesch YY, et al; ALIAS Investigators; Neurological Emergencies Treatment Trials Network. The Albumin in Acute Stroke Part 1 Trial: an exploratory efficacy analysis. Makris D, Manoulakas E, Komnos A, et al. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: Open-label, randomized study. Crit Care Med ;—6. Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury The HARP Study. Wible EF, Laskowitz DT. Statins in traumatic brain injury. Neurotherapeutics ;— Vergouwen MD, de Haan RJ, Vermeulen M, et al. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Biffi A, Devan WJ, Anderson CD, et al. Statin treatment and functional outcome after ischemic stroke: case-control and meta-analysis. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology ;— Feng Y, LeBlanc MH, LeBlanc EB, et al. Desmethyl tirilazad improves neurologic function after hypoxic ischemic brain injury in piglets. Oubidar M, Boquillon M, Marie C, et al. Ischemia-induced brain iron delocalization: effect of iron chelators. Hanson LR, Roeytenberg A, Martinez PM, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther ;— Xing Y, Hua Y, Keep RF, et al. Effects of deferoxamine on brain injury after transient focal cerebral ischemia in rats with hyperglycemia. Bilgihan A, Turkozkan N, Aricioglu A, et al. The effect of deferoxamine on brain lipid peroxide levels and Na-K ATPase activity following experimental subarachnoid hemorrhage. Gen Pharmacol ;— Gu Y, Hua Y, Keep RF, et al. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Okauchi M, Hua Y, Keep RF, et al. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Chen Z, Gao C, Hua Y, et al. Role of iron in brain injury after intraventricular hemorrhage. Lee JY, Keep RF, He Y, et al. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke ;40 3 Suppl :SS A randomized trial of tirilazad mesylate in patients with acute stroke RANTTAS. The RANTTAS Investigators. Haley EC Jr. High-dose tirilazad for acute stroke RANTTAS II. RANTTAS II Investigators. Jang YG, Ilodigwe D, Macdonald RL. Meta-analysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Selim M, Yeatts S, Goldstein J, et al. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun ;— Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Fagan SC, Waller JL, Nichols FT, et al. Minocycline to improve neurologic outcome in stroke MINOS : a dose-finding study. Switzer JA, Hess DC, Ergul A, et al. Matrix Metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke ;—5. Download references. Disclosure forms provided by the authors are available with the online version of this article. Department of Neurology, Divisions of Neurocritical Care, Beth Israel Deaconess Medical Center, Boston, MA, , USA. Department of Neurology, Stroke Division, Beth Israel Deaconess Medical Center, Brookline Avenue — Palmer , Boston, MA, , USA. You can also search for this author in PubMed Google Scholar. Correspondence to Magdy H. Reprints and permissions. Hanafy, K. Antioxidant Strategies in Neurocritical Care. Neurotherapeutics 9 , 44—55 Download citation. Published : 02 December Issue Date : January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract An increase in oxidative stress and overproduction of oxidizing reactive species plays an important role in the pathophysiology of several conditions encountered in the neurocritical care setting including: ischemic and hemorrhagic strokes, traumatic brain injury, acute respiratory distress syndrome, sepsis, and organ failure. Novel Therapeutic Strategies for Traumatic Brain Injury: Acute Antioxidant Reinforcement Article 15 February Vitamin C revisited Article Open access 06 August Oxidative Damage Mechanisms in Traumatic Brain Injury and Antioxidant Neuroprotective Approaches Chapter © Use our pre-submission checklist Avoid common mistakes on your manuscript. Introduction Increased production of oxidizing free radical species with subsequent oxidative stress OS plays an important role in critically ill neurological patients and is central to the pathophysiology of many conditions, such as ischemic and hemorrhagic strokes, traumatic brain injury, subarachnoid hemorrhage, sepsis, acute respiratory distress syndrome, and organ failure. The Basic Mechanisms and Molecular Biology of Oxidative Stress Normal physiological processes, such as cellular respiration, generate small amounts of oxidizing reactive oxygen species ROS and reactive nitrogen species RNS. Full size image. Monitoring Oxidative Stress In Vivo The currently available methods to assess OS in vivo include: direct quantification by electron spin resonance; or indirect determination of total radical-trapping antioxidant capacity, measurement of DNA damage using chromatography, or detection of oxidized biological markers of OS. Role of Oxidative Stress in Various Neurological Conditions Ischemic Stroke Oxidative modifications of DNA, protein, and lipids by ROS play an important role in neuronal injury and its severity following cerebral ischemia. Intracerebral Hemorrhage After intracerebral hemorrhage ICH , large numbers of hemoglobin-containing red blood cells are released into the brain parenchyma. Subarachnoid Hemorrhage In rodent models of subarachnoid hemorrhage SAH , there is evidence of increased superoxide anion and nicotinamide adenine dinucleotide phosphate NADPH oxidase, both powerful oxidizing agents [ 43 , 44 ]. Traumatic Brain Injury Reactive oxygen species play a role in the pathogenesis of traumatic brain injury TBI. Acute Lung Injury Acute lung injury ALI and adult respiratory distress syndrome ARDS are common problems in neurocritical patients. Potential Antioxidant Strategies The biochemical changes of OS suggest that antioxidant therapy may be theoretically achieved by the following strategies: 1 restoring endogenous antioxidants and nutrients and supplementation with exogenous trace elements, vitamins, and nutrients with antioxidant properties; or 2 administering drugs that reduce OS, such as statins, iron-modifying agents such as deferoxamine, or minocycline. Antioxidant Nutrients in Neurocritical Care There is a rationale to support exogenous supply of antioxidant trace elements, vitamins, and nutrients during critical illness. Pharmacological Antioxidant Therapy N-Acetylcysteine N-acetylcysteine has multiple putative antioxidant properties by decreasing NF-κB activation and cytokines production, regenerating NO as a sulfhydryl donor, and replenishing glutathione, and scavenging ROS as a precursor of glutathione. Albumin Albumin has antioxidant effects, largely mediated by its thiol sulfhydryl group portion, which attaches to ROS and NOS. Statins Statins can modulate endogenous pro-oxidative and anti-oxidative systems. Iron-Modifying Agents Studies using iron-modifying agents in animal models of cerebral ischemia and ICH provide evidence for a potential therapeutic benefit. Minocycline Minocycline has anti-inflammatory and anti-apoptotic properties, inhibits polysdenosine diphosphate ribose polymerase-1 and matrix metalloproteinases, and is an effective antioxidant and radical scavenger. Conclusions and Future Directions Overwhelming pre-clinical and clinical evidence supports the presence of OS in several neurocritical conditions. References Collier BR, Giladi A, Dossett LA, Dyer L, Fleming SB, Cotton BA. PubMed CAS Google Scholar Pamplona R, Costantini D. PubMed CAS Google Scholar Harris RA, Amor S. PubMed CAS Google Scholar Abilés J, de la Cruz AP, Castaño J, et al. PubMed Google Scholar Therond P, Bonnefont-Rousselout D, Davit-Sparaul A, Conti M, Legrand A. PubMed CAS Google Scholar Carré JE, Orban JC, Re L, et al. PubMed Google Scholar Gelain DP, de Bittencourt Pasquali MA, M Comim C, et al. PubMed CAS Google Scholar Ghosh N, Ghosh R, Mandal SC. PubMed CAS Google Scholar Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Google Scholar Altavilla D, Saitta A, Guarini S, et al. PubMed CAS Google Scholar Szabó C. PubMed Google Scholar Grune T, Berger MM. PubMed CAS Google Scholar Clausen F, Marklund N, Lewén A, Enblad P, Basu S, Hillered L. PubMed CAS Google Scholar Noseworthy MD, Bray TA. Google Scholar Leung G, Moody AR. PubMed Google Scholar Casado A, de la Torre R, López-Fernández ME, Gil P, Egido JA. PubMed CAS Google Scholar Chang CY, Chen JY, Ke D, Hu ML. PubMed CAS Google Scholar Ozkul A, Akyol A, Yenisey C, Arpaci E, Kiylioglu N, Tataroglu C. PubMed CAS Google Scholar Taffi R, Nanetti L, Mazzanti L, et al. PubMed CAS Google Scholar Atochin DN, Wang A, Liu VW, et al. PubMed CAS Google Scholar Ishikawa M, Zhang JH, Nanda A, et al. PubMed CAS Google Scholar Lees KR, Zivin JA, Ashwood T, et al. PubMed CAS Google Scholar Shuaib A, Lees KR, Lyden P, et al. PubMed CAS Google Scholar Savitz SI, Schabitz W. PubMed Google Scholar Chan PH. PubMed CAS Google Scholar Kelly PJ, Morrow JD, Ning M, et al. PubMed CAS Google Scholar Fujimura M, Tominaga T, Chan PH. PubMed CAS Google Scholar Regan RF, Panter SS. PubMed CAS Google Scholar Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. PubMed CAS Google Scholar Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. PubMed CAS Google Scholar Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. PubMed Google Scholar Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. PubMed CAS Google Scholar Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. PubMed CAS Google Scholar Mehdiratta M, Kumar S, Hackney D, et al. PubMed CAS Google Scholar Pérez de la Ossa N, Sobrino T, Silva Y, et al. PubMed Google Scholar Lou M, Lieb K, Selim M. PubMed CAS Google Scholar Polidori MC, Mecocci P, Frei B. PubMed CAS Google Scholar Lyden PD, Shuaib A, Lees KR, et al. PubMed CAS Google Scholar Biffi A, Anderson CD, Jagiella JM, et al. PubMed Google Scholar James ML, Blessing R, Bennett E, et al. PubMed Google Scholar Laskowitz DT, Lei B, Dawson HN, et al. PubMed CAS Google Scholar Erşahin M, Cetinel S, Yüksel M, et al. PubMed Google Scholar Kaneda K, Fujita M, Yamashita S, et al. PubMed CAS Google Scholar Dohi K, Ikeda Y, Fujita S, et al. PubMed CAS Google Scholar Asano T, Sano K, Kikuchi H, et al. PubMed CAS Google Scholar Zuccarello M, Schmitt G. PubMed CAS Google Scholar Kassell NF, Apperson-Hansen C, Alves WM. PubMed CAS Google Scholar Haley EC Jr, Apperson-Hansen C, Maile MH, et al. PubMed CAS Google Scholar Lanzino G, Kassell NF. PubMed CAS Google Scholar Kontos HA, Wei EP. PubMed CAS Google Scholar Wali B, Sayeed I, Stein DG. PubMed CAS Google Scholar Roof RL, Hoffman SW, Stein DG. PubMed CAS Google Scholar Poirier J. PubMed CAS Google Scholar Hoane MR, Holland MA, Birky ND, Dang T, et al. PubMed Google Scholar Smith C, Graham DI, Murray LS, Stewart J, et al. PubMed CAS Google Scholar Ariza M, Pueyo R, Matarín Mdel M, et al. PubMed CAS Google Scholar Fink MP. PubMed CAS Google Scholar Berger MM, Chiolero RL. PubMed CAS Google Scholar Biesalski HK, McGregor GP. PubMed CAS Google Scholar Lowes DA, Webster NR, Murphy MP, et al. PubMed CAS Google Scholar Rank N, Haertel C, Lenhart A, Welte M, et al. PubMed CAS Google Scholar Spies CD, Reinhart K, Witt I, et al. PubMed CAS Google Scholar Kazmierski WM, Wolberg G, Wilson JG, et al. PubMed CAS Google Scholar Ritter C, Andrades ME, Reinke A, et al. PubMed CAS Google Scholar Zapelini PH, Cardoso MR, Ritter C, et al. PubMed CAS Google Scholar Mayer K, Fegbeutel C, Hattar K, et al. PubMed Google Scholar Angstwurm MW, EL, Zimmermann T, et al. PubMed CAS Google Scholar Grieb P, Ryba MS, Debicki GS, et al. PubMed CAS Google Scholar Huet O, Dupic L, Batteux F, et al. PubMed CAS Google Scholar Hypothermia after Cardiac Arrest Study Group. Google Scholar Shao ZH, Sharp WW, Wojcik KR, et al. PubMed CAS Google Scholar Katz LM, Young AS, Frank JE, et al. PubMed CAS Google Scholar Suntharalingam G, Regan K, Keogh BF, et al. PubMed CAS Google Scholar Freeman BA, Crapo JD. PubMed CAS Google Scholar Laurent T, Markert M, Feihl F, Schaller MD, et al. PubMed CAS Google Scholar Heard SO, Longtine K, Toth I, Puyana JC, et al. PubMed CAS Google Scholar The Acute Respiratory Distress Syndrome Network. Google Scholar Frank JA, Pittet JF, Lee H, et al. PubMed CAS Google Scholar Biolo G, Zorat F, Antonione R, et al. PubMed CAS Google Scholar Crimi E, Liguori A, Condorelli M, et al. PubMed CAS Google Scholar Crimi E, Sica V, Williams-Ignarro S, et al. PubMed CAS Google Scholar Andrews PJD, Avenell A, Noble DW, et al. PubMed Google Scholar Berger MM, Soguel L, Shenkin A, et al. PubMed Google Scholar Heyland DK, Dhaliwal R, Suchner U, Berger MM. PubMed Google Scholar Casaer MP, Mesotten D, Hermans G, et al. PubMed CAS Google Scholar Manzanares W, Hardy G. PubMed CAS Google Scholar Heller AR, Groth G, Heller SC, et al. PubMed CAS Google Scholar Molnár Z, Shearer E, Lowe D. PubMed Google Scholar Sung J, Bochicchio GV, Joshi M, et al. PubMed Google Scholar Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. PubMed CAS Google Scholar SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. PubMed CAS Google Scholar Perel P, Roberts I. PubMed Google Scholar Powner DJ. PubMed Google Scholar Jungner M, Grände PO, Mattiasson G, et al. PubMed Google Scholar Tone O, Ito U, Tomita H, et al. CAS Google Scholar Rodling Wahlström M, Olivecrona M, Nyström F, Koskinen LO, et al. PubMed Google Scholar Hill MD, Martin RH, Palesch YY, et al; ALIAS Investigators; Neurological Emergencies Treatment Trials Network. PubMed CAS Google Scholar Makris D, Manoulakas E, Komnos A, et al. Google Scholar Craig TR, Duffy MJ, Shyamsundar M, et al. PubMed CAS Google Scholar Wible EF, Laskowitz DT. PubMed CAS Google Scholar Vergouwen MD, de Haan RJ, Vermeulen M, et al. Google Scholar Biffi A, Devan WJ, Anderson CD, et al. PubMed CAS Google Scholar Biffi A, Devan WJ, Anderson CD, et al. PubMed CAS Google Scholar Feng Y, LeBlanc MH, LeBlanc EB, et al. Lifestyle modifications on further reproductive problems. Cresco Journal of Reproductive Science. Sunde R. In: Bowman BA, Russell RM, editors. Present knowledge in nutrition. Washington, DC: ILSI Press; Brand and A. Selenium and the Metallic Metal Larroque C, Lange R, Maurin L, Bienvenue A, van Lier JE. Archives of Biochemistry and Biophysics. Kamada H, Ikumo H. Effect of selenium on cultured bovine luteal cells. Animal Reproduction Science. Riley JC, Behrman HR. In vivo generation of hydrogen peroxide in the rat corpus luteum during luteolysis. Sawada M, Carlson JC. Rapid plasma membrane changes in superoxide radical formation, fluidity, and phospholipase A2 activity in the corpus luteum of the rat during induction of luteolysis. Carlson JC, Sawada M, Boone DL, Stauffer JM. Stimulation of progesterone secretion in dispersed cells of rat corpora lutea by antioxidants. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Implantation mechanisms: Insights from the sheep. Al-Gubory KH, Faure P, Garrel C. Different enzymatic antioxidative pathways operate within the sheep caruncular and intercaruncular endometrium throughout the estrous cycle and early pregnancy. Eick SM, Barrett ES, Erve TJ, Nguyen RH, Bush NR, Milne G, et al. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatric and Perinatal Epidemiology. Nawito M, Abd El Hameed AR, Sosa A, Mahmoud KGM. Veterinary World. Härter C, Lima L, Silva H, Castagnino D, Rivera A, Resende K, et al. Energy and protein requirements for maintenance of dairy goats during pregnancy and their efficiencies of use. Journal of Animal Science. Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: Antenatal and postnatal aspects. Clinical Medicine Insights Pediatrics. Kholif A, Morsy T, Gouda G, Anele U, Galyean M. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Animal Feed Science and Technology. Abou-Elkhair R, Mahboub H, Sadek K, Ketkat S. Effect of prepartum dietary energy source on goat maternal metabolic profile, neonatal performance, and economic profitability. Journal of Advanced Veterinary and Animal Research. Bampidis V, Christodoulou V, Christaki E, Florou-Paneri P, Spais A. Effect of dietary garlic bulb and garlic husk supplementation on performance and carcass characteristics of growing lambs. Patra AK, Amasheh S, Aschenbach JR. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds—a comprehensive review. Critical Reviews in Food Science and Nutrition. Padayachee B, Baijnath H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. South African Journal of Botany. Abbas R, Elsharbasy F, Fadlelmula A. Nutritional values of moringa oleifera, total protein: Amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. Journal of Microbe Biochemica Technology. Saleem A, Saleem M, Akhtar MF. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera lam: An ethnomedicinal plant of Moringaceae family. Parraguez VH, Atlagich M, Araneda O, García C, Munoz A, De los Reyes M, et al. Sordillo L. Nutritional strategies to optimize dairy cattle immunity. Journal of Dairy Science. Berchieri-Ronchi CB, Presti PT, Ferreira ALA, Correa CR, Salvadori DMF, Damasceno DC, et al. Effects of oxidative stress during human and animal reproductions: A review. International Journal of Nutrology. Idamokoro EM, Muchenje V, Afolayan AJ, Hugo A. Comparative fatty-acid profile and atherogenicity index of milk from free grazing Nguni, Boer and non-descript goats in South Africa. Obrador E, Liu-Smith F, Dellinger RW, Salvador R, Meyskens FL, Estrela JM. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biological Chemistry. Vitale SG, Capriglione S, Peterlunger I, La Rosa VL, Vitagliano A, Noventa M, et al. The role of oxidative stress and membrane transport systems during endometriosis: A fresh look at a busy corner. Hernández-Castellano LE, Morales-delaNuez A, Sánchez-Macías D, Moreno-Indias I, Torres A, Capote J, et al. The effect of colostrum source goat vs. sheep and timing of the first colostrum feeding 2 h vs. Zachwieja A, Szulc T, Potkański A, Mikuła R, Kruszyński W, Dobicki A. Effect of different fat supplements used during dry period of cows on colostrum physicochemical properties. Biotechnology in Animal Husbandry. Hyrslova I, Krausova G, Bartova J, Kolesar L, Curda L. Goat and bovine colostrum as a basis for new probiotic functional foods and dietary supplements. Journal of Microbial and Biochemical Technology. Kekana TW, Nherera-Chokuda V, Baloyi JJ, Muya C. Immunoglobulin G response and performance in Holstein calves supplemented with garlic powder and probiotics. South African Journal of Animal Science. Baldassarre H. Laparoscopic ovum pick-up followed by in vitro embryo production and transfer in assisted breeding programs for ruminants. Mikkelsen AL, Smith S, Lindenberg S. Possible factors affecting the development of oocytes in in-vitro maturation. Choi H-y, Cho K-H, Jin C, Lee J, Kim T-H, Jung W-S, et al. Guérin PE, Menezo Y. Oxidative stress and protection against teactive oxygen species in the pre implantation embryo and its surroundings. Human Reproduction Update. Khazaei M, Aghaz F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. Journal of Reproduction and Development. Silva E, Greene AF, Strauss K, Herrick JR, Schoolcraft WB, Krisher RL. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reproduction, Fertility and Development. Morado SA, Cetica PD, Beconi MT, Dalvit GC. Reactive oxygen species in bovine oocyte maturation in vitro. Romero S, Pella R, Zorrilla I, Berrío P, Escudero F, Pérez Y, et al. Coenzyme Q10 improves the in vitro maturation of oocytes exposed to the intrafollicular environment of patients on fertility treatment. JBRA Assisted Reproduction. Sovernigo T, Adona P, Monzani P, Guemra S, Barros F, Lopes F, et al. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reproduction in Domestic Animals. Budani MC, Tiboni GM. Effects of supplementation with natural antioxidants on oocytes and preimplantation embryos. Ali A, Bilodeau J, Sirard M. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Zullo G, Albero G, Neglia G, De Canditiis C, Bifulco G, Campanile G, et al. L-ergothioneine supplementation during culture improves quality of bovine in vitro—produced embryos. Gottardi F, Barretto L, Gonçalves F, Perri S, Mingoti G. Effects of Cumulus Cells and Cysteamine during Bovine Oocyte in vitro Maturation on Meiosis Progression and Acquisition of Developmental Competence. Brasil; Sun W-J, Pang Y-W, Liu Y, Hao H-S, Zhao X-M, Qin T, et al. Exogenous glutathione supplementation in culture medium improves the bovine embryo development after in vitro fertilization. Zhao X-M, Min J-T, Du W-H, Hao H-S, Liu Y, Qin T, et al. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Tian X, Wang F, Zhang L, He C, Ji P, Wang J, et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. International Journal of Molecular Sciences. Keshavarzi S, Salehi M, Farifteh-Nobijari F, Hosseini T, Hosseini S, Ghazifard A, et al. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell Journal Yakhteh. Chowdhury M, Choi B-H, Khan I, Lee K-L, Mesalam A, Song S-H, et al. Supplementation of lycopene in maturation media improves bovine embryo quality in vitro. Patel PA, Chaudhary SS, Puri G, Singh VK, Odedara AB. Effects of β-mercaptoethanol on in vitro maturation and glutathione level of buffalo oocytes. Chen N, Liow S-L, Yip W-Y, Tan L-G, Ng S-C. Influence of cysteamine supplementation and culture in portable dry-incubator on the in vitro maturation, fertilization and subsequent development of mouse oocytes. Dalvit G, Llanes S, Descalzo A, Insani M, Beconi M, Cetica P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. |
| Antioxidant Strategies to Improve Female Reproduction | These compounds endowed several properties in the mitigation of various animal stresses, starting from physiology to molecular level. This chapter elucidates oxidative stress, natural and synthetic antioxidants, and particular focus are emphasized that how antioxidant supplementation can help to improve animal fertility and productivity. Moreover, the mechanism by which antioxidants produce fruitful effects will also be highlighted. Oxidative stress is the condition in which the overproduction of free radicals is produced and the antioxidant system unable to neutralize them [ 1 ]. Limited quality of free radicals is compulsory to maintain physiological function, and accelerated production may lead to damage lipids, DNA, and proteins [ 2 ]. The efficacy of oxidative metabolites in female reproduction depends upon the site, amount and exposing levels to oxidant molecules [ 3 ]. In livestock, the appearance of the disease causes a reduction in the antioxidant status of the animals [ 4 ]. Oxidative stress is observed in several pathological conditions that influence animal health, welfare, and productive performance [ 5 ]. Indeed, in some productive phases, animals experience physiological alteration, such as farrowing and lactation, weaning, high temperature, and different stresses, that may decline antioxidant status [ 6 , 7 , 8 ]. Oxidative stress may influence physiological function of various reproductive events which thus involved in problems associated with pregnancy [ 9 ]. The purpose of this chapter is to exploit the beneficial effect of antioxidant compounds various aspects of female reproduction and also discuss how antioxidant approaches improve animal productive performance and well-being. Reactive oxygen species ROS encompasses of superoxide anion, hydrogen peroxide and hydroxyl radical. Their production is possible due to natural oxygen leakage [ 11 ]. Other origin of ROS production are metabolic reactions that are sustainable for life, the exogenous sources include X-rays, ozone, cigarette smoking, air pollutants, certain drugs and pesticides, and industrial chemicals [ 12 ]. Interestingly, the reactions of consists of enzymatic and nonenzymatic reactions within the body also produce free radicals. Many enzymatic reactions prevail in the respiratory chain, phagocytosis, prostaglandin synthesis, and cytochrome P system [ 14 ], although nonenzymatic reactions are based on oxygen with organic compounds and ionizing reactions also generate free radicals [ 15 ]. ROS are comprised of oxygen ions, free radicals and peroxides. High level of ROS or reduce concentration of antioxidants induce oxidative stress that trigger cellular damage of macromolecules utilizing different ways. It has responsible for causing chronic diseases by interacting with molecular signaling pathways which alters gene expression [ 16 ]. The interaction between chemicals and signaling molecules are necessary to understand the involvement of ROS role in pathogenesis. Redox interaction with various proteins residues and ROS is the key component of inter-processes. Further reaction yields to produce reactive sulfenic acid and sulfonamide. Oxidation of these molecules leads to cause ultra-structural changes or functional alteration [ 17 ]. Thus, for this purpose more oxygen is required which in turn causes the overproduction of ROS. So, it is very crucial to maintain the balance between oxidative stress and the antioxidant system for perfect functioning of the body [ 18 ]. Antioxidants are substances that overcome adverse effect of oxidative damages. They are available as natural and synthetic compounds. Natural antioxidants are derived from natural sources like food, cosmetics, and pharmaceutical industries. While the synthetic one is created artificially through chemical reactions [ 19 ]. The antioxidant system consists of enzymatic and non-enzymatic. The first one is also referred as natural antioxidants. They consist of superoxide dismutase SOD catalase and glutathione peroxidase GSH-Px. Antioxidant enzymes are endowed to protect living cells against oxidant products. The SOD is an enzyme district superoxide anion radical into hydrogen peroxide. Another enzyme called catalase is in charge of catalysing the breakdown of hydrogen peroxide into water and oxygen. GSH-Px employs glutathione as a co-substrate and is composed of selenium. An enzyme found in the cytoplasm, it excludes hydrogen peroxide. However, in comparison with catalase, it has various ranges of substrates comprising lipid peroxides. The prime function of the Glutathione peroxidase is to decontaminate low levels of hydrogen peroxide in the cell. Non-enzymatic antioxidants include dietary supplements or synthetic antioxidants. The complex nature of the body antioxidant system is impaired by consumption of dietary antioxidant such as vitamins and minerals [ 20 ]. Vitamin C, vitamin E, plant polyphenol, carotenoids, and glutathione are non-enzymatic antioxidants, they causes inhibition of free radicals reactions. Antioxidants can be classified as water-soluble or lipid-soluble depending on how potent they are. A water-soluble vitamin called vitamin C is found in cellular fluids such the cytosol and cytoplasmic matrix. Antioxidants can be divided into small-molecule and large-molecule antioxidants depending on their size. The small one neutralizes ROS through scavenging process. The example includes Vitamin C, vitamin E, carotenoids, and glutathione GSH. A large size of the molecule antioxidants comprises SOD, CAT, and GPx and albumin that captivate ROS and the attack form essential proteins. The mechanism by which antioxidants are utilized which offer protection against inhibition of free radical formation, scavenging free radicals, involved in repair-damage via free radicals, help to establish an environment that is conducive for the antioxidants to function effectively [ 21 ]. Synthetic antioxidants are phenolic compounds responsible for eliminating free radicals and suppressing chain-reaction. They consist of butylated hydroxyl anisole BHA , butylated hydroxyltoluene BHT , propyl gallate PG , metal chelating agent EDTA , tertiary butyl hydroquinone TBHQ , and nordihydroguaiaretic acid NDGA [ 21 ]. In commercial dairy and beef farming, various stresses influence economic benefit that is linked with declined productive and reproductive performance in cattle. It has been observed that diverse endogenous and exogenous sources of ROS lead to stresses that cause over generation of free radicals and eventually result in oxidative stress [ 22 , 23 ]. It is well recognized that the consequences of oxidative stress have deleterious effects on immune system reproductive function, animal growth, development, and on general health [ 24 , 25 ]. Hence, the antioxidant network is responsible for the preservation and maintenance of animal redox status in cells and tissues and is thus responsible for neglecting the harmful effect of stresses. Considering this mechanism, selenium has its own importance [ 26 , 27 ]. It is noted that 25 selenoproteins have been identified in animal tissues; most of them are contributed in the conservation of body redox balance and antioxidant defense [ 27 ]. It is well-recognized in some animal species that the bioavailability of the Se relies on the dietary source of Se provided [ 28 , 29 , 30 ]. The Se integration relies on the rumen environment, which gradually declines depending upon the particular source of Se [ 31 ]. Selenium is present in two forms, inorganic and organic [ 32 ]. These forms may be a vital source of selenium [ 33 ]. It has been known that Se supplementation enhances female fertility but the exact mechanism is still not unknown. The progesterone hormone derived from the corpus luteum is a dominant hormone of pregnancy. This hormone is synthesized from cholesterol via several enzymatic reactions in which molecular oxygen is utilized for its reactions. These reactions generate oxygen radicals and different peroxides which are detrimental to cells [ 34 ]. In in vivo study, indicated that the inclusion of luteinizing hormone in luteal cells culture concurrently enhanced progesterone level in the medium and also the lipid peroxides in cells [ 35 ]. For luteal regression, the accretion of H 2 O 2 [ 36 ] or lipid peroxides [ 37 ] in the corpus luteum has been documented. These findings show that corpus luteum requires antioxidant defense toward peroxides to stabilize normal functions. The significance of the corpus luteum has also been projected by Ref. Moreover, the inclusion of Se in luteal cells reduced the concentration of lipid peroxides in a cell [ 35 ]. Se as the part of glutathione peroxidase may destroy peroxides, in connection with superoxide dismutase, vitamin E, and beta-carotene. Pregnancy is a normal mechanism in which overburden metabolic rate disrupts antioxidant status and energy balance. Once the zona pellucida is separated from the embryo, it enhances the production of ROS [ 40 ]. The nutritional requirement during early pregnancy is increased to maintain animal health and pregnancy, which is in turn generation of oxidative stress [ 42 ]. Although, malnutrition is also common around the globe in small ruminants because of the high price of the feed, especially in developing countries. Hence, small ruminants are easy to keep due to several reasons [ 43 ]. Malnutrition during pregnancy has deleterious effect on the conception rate and on fetus development [ 44 ]. Animal supplementation in a diet with plant source have sufficient nutrition and has been assumed to be the potential source of antioxidants to attenuate early pregnancy stress in goats [ 45 , 46 ]. The plant compounds exert diverse nutrition comprises of rich source of antioxidants and immune-modulatory properties, which act as a potential feed supplement for ruminants [ 47 , 48 ]. Moringa oleifera MO is a multifaceted medicinal tree with high nutritional values [ 49 ]. Its leaves are rich sources of several nutritious compounds, such as proteins, amino acids, minerals, and vitamins [ 50 ]. Apart of that, MO is also a rich source of antioxidant compounds, such as phenolic acids, vitamin E, vitamin C, selenium, zinc, and β carotene. These compounds have more robust antioxidant potential than synthetic ones [ 51 ]. The basal diet supplemented with 3. It also promoted progesterone profile, improved conception rate, and attenuated ROS production in early pregnancy of goats. In another study, by Ref. The supplementation profoundly enhanced number of live-born piglets, total litter weight, and reducing the chance of low-weight piglets. Moreover, supplementation declined MDA levels in sows and piglets. The mothers who had supplementation showed a higher trend of weaning weight. The results conclude from pregnancies that offering maternal supplementation with herbal antioxidants in pregnancy profoundly enhanced reproductive efficiency, litter traits, and piglet performance. Reproductive performance is a main indicator related to maternal nutrition. The periparturient period causes reduced feed intake, and endocrine and metabolic alterations which disrupt energy balance and antioxidant index [ 22 , 53 ]. In this period, increased nutritional requirements, such as digestion rate, mammary development, and fetus growth have been reported [ 54 ]. Pasture grazing and feeding on crop residues have a diverse nutritional profile and feeding on such sources is not adequate to meet the energy requirement of lactating animals [ 55 ]. In this scenario, pregnant animals are vulnerable to oxidative stress [ 56 ], which threatens to biomolecules and eventually affect productive and reproductive parameters [ 57 ]. Colostrum is the composition of immunoglobulins, minerals and other biological substances which transfer form colostrum to the young ones [ 58 ]. The quality of the colostrum depends upon maternal nutrition [ 59 , 60 ]. The diet supplemented with phytobiotics has been assumed to be the main source of managing nutrition-induced oxidative stress in pregnancy and lactation in livestock [ 45 , 46 , 61 ]. In a recent study by Ali et al. oleifera leaf powder MOLP during periparturient period. He reported the increased biochemical and antioxidant indices of colostrum and milk. The milk yield, weight gain of the kids, and reproductive performance were enhanced with 2 and 3. Further, the findings suggested that the diet supplemented with 3. The in vitro embryo production IVEP technique is employed to combat infertility-related problems in mammalian species [ 62 ]. This tool has been known to be utilized for the production of large scale offspring from elite animals. The IVM prognosis relies on diverse factors consisting of oocyte quality and culture conditions [ 63 ]. The source of antioxidants from female organs has been reported to reduce ROS production [ 64 ]. The main hurdle which decides the fate of oocyte success during IVM is oxidative stress [ 64 ]. Accelerated ROS production might result in oocyte death and embryonic loss [ 65 , 66 ]. An antioxidant approach during IVM has been proposed to govern oocytes from the deleterious effect of oxidative stress by maintaining a basal level of ROS [ 67 , 68 ]. Presently, different antioxidants are utilized during IVM to confirm balanced intracellular redox status, resulting in good-quality of oocytes [ 69 , 70 ]. The inclusion of antioxidants, such as thiols, polyphenols, melatonin, carotenoids, resveratrol, and vitamins C and E, to the IVM medium has been verified in different studies to increase oocyte quality and attenuate exceeding ROS damage [ 71 , 72 ]. Previous evidence has reported that the balance amount of antioxidants and ROS in IVEP media which may be favorable for embryonic development [ 73 , 74 ]. At present, the widely employed antioxidant in IVEP is cysteamine; its efficiency is mostly associated with the stage of IVM. It has been found to stimulate the embryonic process and secreting of glutathione GSH , which is prevalent in male and female gametes from harmful effect of ROS [ 75 ]. Moreover, cysteine and glutathione have been implied in IVEP protocols with good results [ 73 , 76 ]. The positive effect of antioxidants is illustrated in Table 1. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Paz Otero. Open access peer-reviewed chapter Antioxidant Strategies to Improve Female Reproduction Written By Tarique Hussain. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Recent Developments in Antioxidants from Natural Sources Edited by Paz Otero. From the Edited Volume Recent Developments in Antioxidants from Natural Sources Edited by Paz Otero Fuertes and María Fraga Corralga Corral Book Details Order Print. Chapter metrics overview 59 Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Animals are only productive once their reproductive cycle is continuously flown. Keywords reactive oxygen species oxidative stress animal fertility productivity antioxidants. Introduction Oxidative stress is the condition in which the overproduction of free radicals is produced and the antioxidant system unable to neutralize them [ 1 ]. Antioxidants Dose Animal species Maturation vs. control rate References Melatonin 10— 9 M 10— 7 M 10— 6 M Bovine Sheep Mouse Table 1. The beneficial effect of antioxidant supplementation in different animal species. References 1. Sies H, Berndt C, Jones DP. Oxidative stress. Annual Review of Biochemistry. Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Research International. Article ID 3. Aruoma OI, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochemical Journal. Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. The Veterinary Journal. Buchet A, Belloc C, Leblanc-Maridor M, Merlot E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS One. Rossi R, Pastorelli G, Cannata S, Corino C. Effect of weaning on total antiradical activity in piglets. Italian Journal of Animal Science. Berchieri-Ronchi C, Kim S, Zhao Y, Correa C, Yeum K-J, Ferreira A. Oxidative stress status of highly prolific sows during gestation and lactation. Guo Z, Gao S, Ouyang J, Ma L, Bu D. Impacts of heat stress-induced oxidative stress on the milk protein biosynthesis of dairy cows. Mbah C, Orabueze I, Okorie N. Antioxidant Properties of Natural and Synthetic Chemical Compounds: Therapeutic Effects on Biological System. Telangana, India; ; 3 6 Augustyniak A, Bartosz G, Čipak A, Duburs G, Horáková LU, Łuczaj W, et al. Natural and synthetic antioxidants: An updated overview. Free Radical Research. Zhang X, Wang G, Gurley EC, Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. Jhang J-J, Lu C-C, Ho C-Y, Cheng Y-T, Yen G-C. Protective effects of catechin against monosodium urate-induced inflammation through the modulation of NLRP3 inflammasome activation. Journal of Agricultural and Food Chemistry. Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D, Dunn JH, et al. Green tea polyphenol epigallocatechingallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochemical and Biophysical Research Communications. Han SG, Han S-S, Toborek M, Hennig B. EGCG protects endothelial cells against PCB induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicology and Applied Pharmacology. Tang B, Chen G-X, Liang M-Y, Yao J-P, Wu Z-K. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. International Journal of Cardiology. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Medicine and Cellular Longevity. Article ID Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species ROS homeostasis and redox regulation in cellular signaling. Cellular Signalling. Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, Florek E. Association of oxidative stress on pregnancy. Mahmoud AM, Wilkinson FL, Lightfoot AP, Dos Santos JM, Sandhu MA. The Role of Natural and Synthetic Antioxidants in Modulating Oxidative Stress in Drug-Induced Injury and Metabolic Disorders Hindawi; Shakir HM. Antioxidant and infertility. In: Antioxidants-Benefits, Sources, Mechanisms of Action. The levels of nutrients with antioxidant properties are decreased in critical illness. For example, patients with ICH have lower plasma levels of vitamin C compared with healthy volunteers [ 38 ]. Other studies have shown a reduction in muscle glutamine and glutathione and muscle protein synthesis in intensive care patients [ 81 ]. There is also evidence that low endogenous stores on antioxidant nutrients are associated with increased free radical generation, augmentation of the systemic inflammatory response, subsequent cell injury and death, and even higher morbidity and mortality in critically ill patients [ 6 , 7 ]. In addition, proof-of-concept studies have shown that supplementation with trace elements and vitamins, such as selenium, and vitamins A, C, and E, improve antioxidant capacity by increasing the activity of glutathione peroxidase, or reducing plasma TBARS, and isoprostanes [ 82 ]. Vitamins C and E may also reduce infectious complications by restoring neutrophil function and cell-mediated immunity, and selenium improves phagocytosis and immunoglobulin synthesis. Although results from some of these trials were encouraging, the benefit of this therapy has not been clearly established. Several reasons have been proposed: 1 the vast majority of these studies were performed in small and heterogeneous populations of critically ill patients, and were largely underpowered; and 2 uncertainties regarding the correct dose to use, appropriate time to start immune nutrition, and the appropriate route of administration; the enteral versus parenteral. In a meta-analysis of 11 studies selected from 44 studies based on strict criteria of early immune nutrition using antioxidant trace elements and vitamins in well-defined intensive care patient populations, Heyland et al. They also found that none of the trials using immune nutrition reported deleterious effects from using exogenous micronutrients, and they concluded that supplementation with antioxidant trace elements and vitamins in intensive care patients is safe and possibly beneficial. They argued that immune nutrition must be started very early after admission to the intensive care unit and that high-dose parenteral nutrition appears to have a stronger impact on outcome. Crimi et al. Recently, Casaer et al. It is noteworthy, however, that Casaer et al. Given the previously described, the use of immune nutrition therapy in neurocritical care might be a rationale and promising strategy. However, more definitive evidence from large-scale, randomized, placebo-controlled, and well-designed trials in this specific patient population is still required. Vitamin B12 has glutathione-sparing antioxidant properties via stimulation of methionine synthase activity and reaction with ROS and NOS. It also inhibits NOS and NO production, and decreases NF-κB activation. N-acetylcysteine has multiple putative antioxidant properties by decreasing NF-κB activation and cytokines production, regenerating NO as a sulfhydryl donor, and replenishing glutathione, and scavenging ROS as a precursor of glutathione. N-acetylcysteine has been extensively studied in critically ill patients with acute respiratory distress syndrome, organ failure, and septic shock. These studies have produced inconsistent results [ 83 , 89 , 90 ]. We found no studies of N-acetylcysteine in neurocritical care, to date. Albumin has antioxidant effects, largely mediated by its thiol sulfhydryl group portion, which attaches to ROS and NOS. It also decreases copper-mediated lipid peroxidation, and modulates nitric oxide-mediated vascular dilatation. Hypoalbuminemia is common in critically ill patients, including those with TBI and various neurological conditions, and is associated with increased morbidity and mortality [ 91 ]. Studies of albumin supplementation in surgical and medical critically ill patients had inconsistent results. The Saline versus Albumin Fluid Evaluation SAFE study of found no advantage for albumin in comparison with saline [ 92 ], and a subsequent post-hoc subgroup analysis of patients with TBI suggested that resuscitation with albumin was associated with increased odds ratio for death [ 93 ]. A Cochrane meta-analysis found no benefit in a heterogenous group of previously published studies [ 94 ]. Powner [ 95 ] recently argued that it is important to separate data from studies regarding albumin infusions intended to restore or maintain intravascular pressure or volume goals from the potential benefit of preventing hypoalbuminemia during neurocritical care. The use of albumin in TBI, and hypertensive and traumatic ICH has been investigated in small studies [ 96 — 98 ]. Although the clinical results were encouraging, these studies were too small and most were nonrandomized and single arm to derive definite conclusions. Preliminary results from the Treatment of Subarachnoid Hemorrhage with Human Albumin ALISAH study indicate that doses up to 1. Although recruitment in ALIAS part 1 was halted due to safety concerns, preliminary efficacy data from this cohort suggests a trend toward a favorable outcome among albumin-treated subjects [ 99 ]. Statins can modulate endogenous pro-oxidative and anti-oxidative systems. For example, statins dose dependently inhibit platelet-mediated low-density lipoprotein oxidation and isoprostane formation, reduce malondialdehyde, and increase SOD and glutathione peroxidase activities in erythrocytes. In a recent two-center, two-arm, randomized, open-label, controlled study of pravastatin, in intensive care patients, Makris et al. Statin therapy has also been investigated in patients with ALI; it was shown to be safe and associated with a significant decrease in bronchoalveolar lavage interleukin-8, but had no other effects compared to placebo [ ]. Although preclinical studies have shown benefits from statins in models of TBI and related disease processes, including cerebral ischemia, ICH, and subarachnoid hemorrhage, clinical studies have been shortcoming [ ]. The use of statins in patients with aneurysmal subarachnoid hemorrhage has been investigated in several small clinical trials. A meta-analysis of four randomized controlled trials involving patients revealed no significant effects on vasospasm, detected by transcranial Doppler, delayed cerebral ischemia, poor outcome, or mortality [ ]. The ongoing phase III Simvastatin in Aneurysmal Subarachnoid Hemorrhage STASH multi-center randomized controlled phase III trial should help to further clarify the role of statins in these patients. Retrospective studies suggest that antecedent use of statins is associated with improved outcome and reduced mortality in patients with acute ischemic stroke and ICH [ , ]. Future studies examining the role of statins as an acute treatment in PHE reduction in ICH and resultant effect on mortality and functional outcome after ischemic and hemorrhagic stroke are warranted. Studies using iron-modifying agents in animal models of cerebral ischemia and ICH provide evidence for a potential therapeutic benefit. Tirilazad mesylate, a potent inhibitor of iron-dependent lipid peroxidation, attenuates neuronal necrosis and improve the neurological status in several animal models of cerebral ischemia [ ]. Treatment with bipyridyl, an iron-chelating agent, significantly attenuates the development of brain edema 24 hours after the induction of ischemia [ ]. Similarly, treatment with deferoxamine, another iron chelator, has been shown to reduce infarct size and improve the neurological status in rats subjected to transient middle cerebral artery occlusion [ ]. Deferoxamine may also alleviate reperfusion-induced injury following ischemia. In one study, treatment with deferoxamine attenuated death rate and hemorrhagic transformation in a rat model of transient focal ischemia [ ]. Treatment with deferoxamine also attenuates the production of ROS, blocks hemoglobin-mediated potentiation of glutamate neurotoxicity, reduces brain malondialdehyde content and induces recovery of sodium-potassium pump activity, and exerts diverse protective effects after experimental ICH [ 29 , 33 , — ]. Emerging evidence also supports a potential therapeutic role for deferoxamine following intraventricular and subarachnoid hemorrhages [ , ]. Deferoxamine decreases the availability of free iron for the production of hydroxyl radicals by forming a stable complex with ferric iron. However, the potential beneficial effects of deferoxamine may be only partly related to its iron chelating abilities. Very few clinical studies have investigated the use of iron-modifying agents in stroke patients. Two multi-center trials examined the use of tirilazad in acute ischemic stroke [ , ]. The Randomized Trial of High Dose Tirilazad in Acute Stroke RANTTAS II investigated a higher dose The use of tirilazad mesylate in patients with subarachnoid hemorrhage was also investigated in several randomized, placebo-controlled, trials. Although it reduced the incidence of symptomatic vasospasm, it had no effect on the overall long-term clinical outcome [ ]. Clinical investigations of deferoxamine in stroke have gained attention in recent years. In ICH, a small, open-label, phase-I, safety and dose-finding study of deferoxamine in patients with spontaneous ICH safety and tolerability of deferoxamine in acute cerebral hemorrhage — deferoxamine mesylate in ICH was recently completed [ ]. This study enrolled 20 subjects into 5 dose tiers of deferoxamine, which was given as a continuous intravenous infusion for 3 consecutive days starting within 16 h of ICH symptoms onset. Repeated daily infusions of deferoxamine were well tolerated and did not cause excessive serious adverse events or mortality. Interestingly, deferoxamine also seemed to exert a modest blood pressure-lowering effect. Similarly, a phase I-II, double-blind, randomized, placebo-controlled, dose-finding study to evaluate the safety and pharmacokinetics of deferoxamine in patients with acute ischemic stroke who are treated with intravenous recombinant tissue plasminogen activator rt-PA within 3 hours of stroke onset, and to explore the effects of treatment on clinical outcomes, infarct volumes, hemorrhagic transformation, and brain edema development The Thrombolysis and Deferoxamine in Middle Cerebral Artery Occlusion — TANDEM-1 is currently underway. Minocycline has anti-inflammatory and anti-apoptotic properties, inhibits polysdenosine diphosphate ribose polymerase-1 and matrix metalloproteinases, and is an effective antioxidant and radical scavenger. Minocycline has neuroprotective effects in vivo against cerebral ischemia and in vitro against glutamate-induced cell death, an inhibition of OS by minocycline may be partly responsible for these effects [ ]. Minocycline also chelates iron, and prevents the neuronal death induced by ferrous sulfate [ ] and attenuated brain edema and neurological deficits in rat models of ICH [ ]. An open-label, dose-escalation, safety and dose-finding study of minocycline, administered intravenously within 6 h of symptom onset, in patients with acute ischemic stroke was recently completed Minocycline to Improve Neurologic Outcome in Stroke [MINOS] [ ], and plans for a phase III study are currently underway. Interestingly, lower plasma levels of MMP-9 was seen among rt-PA treated subjects in the MINOS trial, suggesting that combining minocycline with rt-PA might prevent the adverse consequences of thrombolysis [ ]. Overwhelming pre-clinical and clinical evidence supports the presence of OS in several neurocritical conditions. Although dietary supplementation with antioxidant vitamins and the use of pharmacological agents targeting OS and its downstream cascade seems to be rational, the benefits of various attempted antioxidant strategies to date have not been clearly demonstrated. Several reasons have been advocated, including the wide variability in the nature and severity of illness in intensive care patients. Future studies should consider these issues to avoid past mistakes. Several studies of antioxidant drugs, such as statins, minocycline, and deferoxamine in specified populations of neurological patients are currently underway, and its results could have important implications in neurocritical care. Although the role of antioxidant strategies in neurocritical care is slowly evolving, it is likely to be an integral component of the overall strategy for neurological critical care in the future. Collier BR, Giladi A, Dossett LA, Dyer L, Fleming SB, Cotton BA. Impact of high-dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr ;— PubMed CAS Google Scholar. Pamplona R, Costantini D. Molecular and structural antioxidant defences against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol ;RR Harris RA, Amor S. Sweet and sour--oxidative and carbonyl stress in neurological disorders. CNS Neurol Disord Drug Targets ;— Abilés J, de la Cruz AP, Castaño J, et al. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit Care ;R PubMed Google Scholar. Therond P, Bonnefont-Rousselout D, Davit-Sparaul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care ;— Carré JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med ;— Gelain DP, de Bittencourt Pasquali MA, M Comim C, et al. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock ;— Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res ;— Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics ;— Google Scholar. Altavilla D, Saitta A, Guarini S, et al. Oxidative stress causes nuclear factor-kappaB activation in acute hypovolemic hemorrhagic shock. Free Radic Biol Med ;— Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett ;— Grune T, Berger MM. Markers of oxidative stress in ICU clinical settings: present and future. Clausen F, Marklund N, Lewén A, Enblad P, Basu S, Hillered L. Interstitial F2-isoprostane 8-iso-PGF2α as a biomarker of oxidative stress following severe human traumatic brain injury. J Neurotrauma ; doi: Utsumi H, Yamada KI, Ichikawa K, et al. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. PNAS ;— Noseworthy MD, Bray TA. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radical Biol Med ;— Leung G, Moody AR. MR imaging depicts oxidative stress induced by methemoglobin. Radiology ;— Casado A, de la Torre R, López-Fernández ME, Gil P, Egido JA. Antioxidant enzymes in acute cerebral infarction. Neurologia ;—9. Chang CY, Chen JY, Ke D, Hu ML. Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: correlation to inflammation markers and neurological deficits. Nutrition ;— Ozkul A, Akyol A, Yenisey C, Arpaci E, Kiylioglu N, Tataroglu C. Oxidative stress in acute ischemic stroke. J Clin Neurosci ;— Taffi R, Nanetti L, Mazzanti L, et al. Plasma levels of nitric oxide and stroke outcome. J Neurol ;— Atochin DN, Wang A, Liu VW, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest ;— Ishikawa M, Zhang JH, Nanda A, et al. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci ;— Lees KR, Zivin JA, Ashwood T, et al. NXY for acute ischemic stroke. N Engl J Med ;— Shuaib A, Lees KR, Lyden P, et al. NXY for the treatment of acute ischemic stroke. Savitz SI, Schabitz W. A Critique of SAINT II: wishful thinking, dashed hopes, and the future of neuroprotection for acute stroke. Stroke ;— Chan PH. Neurochem Res ;— Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the biomarker evaluation for antioxidant therapies in stroke BEAT-Stroke study. Fujimura M, Tominaga T, Chan PH. Neuroprotective effect of an antioxidant in ischemic brain injury: involvement of neuronal apoptosis. Neurocrit Care ;— Regan RF, Panter SS. Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma ;— Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J Neurosci Res ;— Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab ;— Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg ;— Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res ;— Mehdiratta M, Kumar S, Hackney D, et al. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Pérez de la Ossa N, Sobrino T, Silva Y, et al. Iron-related brain damage in patients with intracerebral hemorrhage. Lou M, Lieb K, Selim M. The relationship between hematoma iron content and perihematoma edema: an MRI study. Cerebrovasc Dis ;— Polidori MC, Mecocci P, Frei B. Plasma vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Lyden PD, Shuaib A, Lees KR, et al. CHANT Trial Investigators safety and tolerability of NXY for acute intracerebral hemorrhage: the CHANT Trial. Biffi A, Anderson CD, Jagiella JM, et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol ;— James ML, Blessing R, Bennett E, et al. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis ;— Laskowitz DT, Lei B, Dawson HN, et al. The apoE-mimetic peptide, COG, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit Care ; doi: Aoki T, Nishimura M, Kataoka H, Ishibashi R, et al. Lab Invest ;— Erşahin M, Cetinel S, Yüksel M, et al. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Kaneda K, Fujita M, Yamashita S, et al. Prognostic value of biochemical markers of brain damage and oxidative stress in post-surgical aneurysmal subarachnoid hemorrhage patients. Brain Res Bull ;— Dohi K, Ikeda Y, Fujita S, et al. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: anti-inflammatory cytokines and antioxidative effects. Acta Neurochir Suppl ;— Asano T, Sano K, Kikuchi H, et al. Effects of a hydroxyl radical scavenger on delayed ischemic neurological deficits following aneurysmal subarachnoid hemorrhage: results of a multicenter, placebo-controlled double-blind trial. Zuccarello M, Schmitt G. Effect of the aminosteroid UF on cerebral vasospasm following subarachnoid hemorrhage. Kassell NF, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg ;—8. Haley EC Jr, Apperson-Hansen C, Maile MH, et al. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. Kontos HA, Wei EP. Superoxide production in experimental brain injury. Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci ;— Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol ;— Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci ;— Hoane MR, Holland MA, Birky ND, Dang T, et al. The novel apolipoprotein E-based peptide COG improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. Smith C, Graham DI, Murray LS, Stewart J, et al. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry ;— Ariza M, Pueyo R, Matarín Mdel M, et al. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin ;— Berger MM, Chiolero RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med ;ss Biesalski HK, McGregor GP. Antioxidant therapy in critical care—is the microcirculation the primary target? Lowes DA, Webster NR, Murphy MP, et al. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Rank N, Haertel C, Lenhart A, Welte M, et al. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med ;— Spies CD, Reinhart K, Witt I, et al. Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double-blind study. Kazmierski WM, Wolberg G, Wilson JG, et al. Iron chelates bind nitric oxide and decrease mortality in an experimental model of septic shock. Proc Natl Acad Sci U S A ;— Ritter C, Andrades ME, Reinke A, et al. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Zapelini PH, Cardoso MR, Ritter C, et al. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion ;— Mayer K, Fegbeutel C, Hattar K, et al. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med ;— Angstwurm MW, EL, Zimmermann T, et al. Selenium in Intensive Care SIC : results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Grieb P, Ryba MS, Debicki GS, et al. Changes in oxidative stress in the rat brain during post-cardiac arrest reperfusion, and the effect of treatment with the free radical scavenger idebenone. Resuscitation ;— Huet O, Dupic L, Batteux F, et al. Postresuscitation syndrome: potential role of hydroxyl radical-induced endothelial cell damage. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. Shao ZH, Sharp WW, Wojcik KR, et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol ;HH Katz LM, Young AS, Frank JE, et al. Regulated hypothermia reduces brain oxidative stress after hypoxic-ischemia. Suntharalingam G, Regan K, Keogh BF, et al. Influence of direct and indirect etiology on acute outcome and 6-month functional recovery in acute respiratory distress syndrome. Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Laurent T, Markert M, Feihl F, Schaller MD, et al. Oxidant-antioxidant balance in granulocytes during ARDS. Effect of N-acetylcysteine. Chest ;— Heard SO, Longtine K, Toth I, Puyana JC, et al. The influence of liposome-encapsulated prostaglandin E1 on hydrogen peroxide concentrations in the exhaled breath of patients with the acute respiratory distress syndrome. Anesth Analg ;— The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. Frank JA, Pittet JF, Lee H, et al. High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol ;LL Biolo G, Zorat F, Antonione R, et al. Muscle glutamine depletion in the intensive care unit. Int J Biochem Cell Biol ;— Crimi E, Liguori A, Condorelli M, et al. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Crimi E, Sica V, Williams-Ignarro S, et al. The role of oxidative stress in adult critical care. Andrews PJD, Avenell A, Noble DW, et al. Scottish Intensive Care Glutamine or Selenium Evaluative Trial Trials Group. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ ;d Berger MM, Soguel L, Shenkin A, et al. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically Ill adults. New Engl J Med ;— Manzanares W, Hardy G. Vitamin B the forgotten micronutrient for critical care. Heller AR, Groth G, Heller SC, et al. N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Sung J, Bochicchio GV, Joshi M, et al. Admission serum albumin is predicitve of outcome in critically ill trauma patients. Am Surg ;— Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med ; 9 — Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev ; 3 :CD Powner DJ. In my opinion: serum albumin should be maintained during neurocritical care. Jungner M, Grände PO, Mattiasson G, et al. Effects on brain edema of crystalloid and albumin fluid resuscitation after brain trauma and hemorrhage in the rat. Anesthesiology ;— Tone O, Ito U, Tomita H, et al. High colloid oncotic therapy for brain edema with cerebral hemorrhage. Acta Neurochir Suppl Wien ;— CAS Google Scholar. Rodling Wahlström M, Olivecrona M, Nyström F, Koskinen LO, et al. Fluid therapy and the use of albumin in the treatment of severe traumatic brain injury. Acta Anaesthesiol Scand ;— Hill MD, Martin RH, Palesch YY, et al; ALIAS Investigators; Neurological Emergencies Treatment Trials Network. The Albumin in Acute Stroke Part 1 Trial: an exploratory efficacy analysis. Makris D, Manoulakas E, Komnos A, et al. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: Open-label, randomized study. Crit Care Med ;—6. Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury The HARP Study. Wible EF, Laskowitz DT. Statins in traumatic brain injury. |
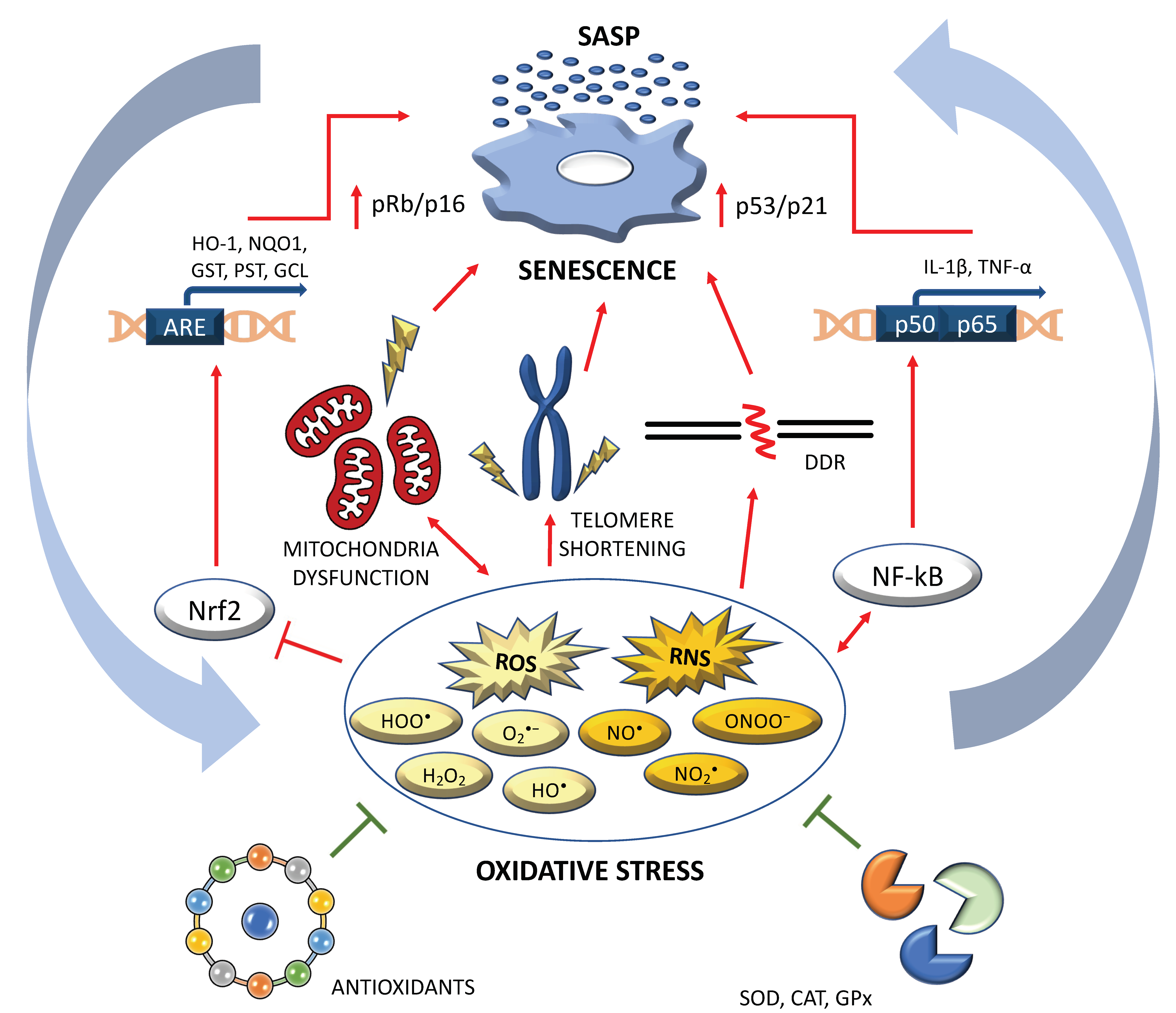
Antioxidant intervention strategies -
You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Spinal cord injury SCI remains a major public health issue in developed countries as well as worldwide. The pathophysiology of SCI is characterized by an initial primary injury followed by secondary deterioration.
Although the etiology and pathogenesis of SCI remain to be fully understood, it has been suggested that reactive oxygen species ROS and oxidative stress have a significant role in the pathophysiology of SCI.
Thus, alleviating oxidative stress may be an effective strategy for therapeutic intervention of SCI. The aim of this review was to describe i the sources of ROS as well as the major antioxidant defenses with particular attention being paid to lipid peroxidation; ii the biomarkers of oxidative stress in SCI and iii the neuroprotective effects of various compounds with antioxidative properties in animal models of SCI.
PubMed, one of the most comprehensive biomedical databases, was searched from — All relevant papers were read by title, abstract and full-length article.
Oxidative stress is considered a hallmark of injury of SCI. Thus, alleviating oxidative stress may be an effective way of therapeutic intervention of SCI.
Two of these agents, the glucocorticoid steroid methylprednisolone and the non-glucocorticoid aminosteroid tirilazad, have been shown to possess significant antioxidant activities and improve recovery of SCI patients in clinical trials.
Other promising botanical compounds and their molecular targets and mechanisms of action with regard to potential protection against SCI were also described. These include carotenoids and phenolic compounds. ROS and oxidative stress have a significant role in the pathophysiology of SCI. Alleviating oxidative stress is be an effective strategy for therapeutic intervention of SCI.
Extensive research over the past several decades has identified numerous bioactive compounds that have antioxidative stress benefits in animal models of SCI. Thus, continued studies on bioactive compounds with ROS-scavenging capacity may lead to the development of effective antioxidant-based modalities for treating SCI in human subjects.
Matthijs F. Wouda, Hanne Bjørg Slettahjell, … Emil Kostovski. Shunli Kan, Chengjiang Liu, … Rusen Zhu. Seyed Reza Mousavi, Majid Reza Farrokhi, … Maryam Naseh. Spinal Cord injury SCI is damage or trauma to the spinal cord resulting in paralysis and loss of sensation.
Depending on the location of the injury, the symptoms of SCI may include loss of movement, sensation to feel heat, cold and touch, loss of bowel or bladder control and exaggerated reflex activities as well as pain.
The pathophysiology of SCI is characterized by an initial primary injury followed by a secondary phase of injury in which oxidative stress is a critical component.
Primary injury results immediately from the initial trauma, which includes contusion, damage to blood vessels and axonal shearing. In contrast, secondary injury is an indirect result from primary injury initiated by trauma.
It occurs in hours, days and weeks following the primary injury. Secondary injury occurs not only at the site of the initial primary injury, but also results in spreading of the lesion to adjacent, otherwise uninjured tissue.
Because secondary injury to spinal cord has an important role in disease progression, it is important to understand the molecular and cellular events leading to the secondary lesion of SCI. Although the etiology and pathogenesis of SCI remain to be further elucidated, extensive studies over the last two decades have suggested that increased formation of reactive oxygen species ROS and the consequent oxidative stress are important events associated with SCI.
The neurons and glia in the central nervous system including spinal cord are particularly prone to oxidative and electrophilic stress due to many factors, including a high content of polyunsaturated fatty acids, a high rate of oxidative metabolic activity, intense production of reactive oxygen metabolites and relatively low antioxidant capacity.
We searched MEDLINE PubMed and reference lists of the included articles published mainly between and using the following key words: SCI, oxidative stress, biomarkers, antioxidant, bioactive compounds and intervention.
Molecular oxygen is an essential element of life, yet incomplete reduction or excitation of oxygen during aerobic metabolism generates ROS. ROS include superoxide, hydroxyl radical, singlet oxygen and hydrogen peroxide Figure 1. Superoxide is the one-electron reduction product of molecular oxygen.
If two electrons are transferred, the product is hydrogen peroxide. In phagocytes, superoxide is produced in large quantities by the enzyme nicotinamide adenine dinucleotide phosphate oxidase for use in oxygen-dependent killing mechanisms.
Hydrogen peroxide is not a free radical but is nonetheless a damaging species because of its ability to penetrate biological membranes. Hydrogen peroxide is toxic to cells and can lead to further free-radical generation.
For example, hydrogen peroxide reacts with reduced transition metals to form the highly reactive hydroxyl radical, which readily causes damage to DNA and other biological molecules.
Production of ROS under physiological conditions is important for normal cellular redox reactions. However, excessive generation of free radicals under pathophysiological conditions such as SCI can greatly enhance the production of ROS.
The mitochondrial dysfunction results in further increased formation of ROS. Structural and functional alterations of the mitochondria have been found in a SCI mouse model.
The enzyme complex transfers electrons from nicotinamide adenine dinucleotide phosphate oxidase at the cytosolic side of the membrane to molecular oxygen at the other side of the membrane resulting in the production of extracellular superoxide. For example, there are many cytosolic enzymes that can generate ROS via reduction of molecular oxygen in their catalytic cycles.
The most notable one is xanthine oxidase, which can directly reduce molecular oxygen to superoxide and hydrogen peroxide. ROS participate in physiological processes, such as cell signaling.
However, excessive generation of ROS under pathophysiological conditions, including SCI may result in oxidative stress. To control ROS, aerobic organism has utilized several antioxidative mechanisms including enzymatic and non-enzymatic antioxidants 12 Figure 2.
Non-enzyme low molecular weight antioxidant compounds include cellular glutathione, vitamins C and E, β-carotene and uric acid. The antioxidant enzymes include superoxide dismutase SOD , catalase, glutathione reductase and glutathione peroxidase among others.
Mammalian cells contain three forms of SOD, MnSOD, Cu,ZnSOD and extracellular SOD. MnSOD is most abundant in the mitochondria, whereas Cn,ZnSOD predominant in the cytoplasm. Glutathione peroxidase is another major enzyme for decomposing H 2 O 2.
ROS and their detoxification by cellular antioxidants. GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized form of glutathione; GST, glutathione S-transferase; G6PDH, glucosephosphate dehydrogenase; 6PGDH, 6-phosphogluconate dehydrogenase; LOH, lipid alcohol; NQO1, NAD P H:quinone oxidoreductase 1; SOD, superoxide dismutase.
The elimination of ROS following SCI is particularly controlled by cellular antioxidant glutathione and Cu,ZnSOD. The results suggested that elevation of levels of glutathione by irrigation with δ-glutamylcysteine conferred significant protection against lipid peroxidation after SCI, suggesting that glutathione augmentation may be an effective strategy for curtailment of lipid peroxidation-mediated damage in SCI.
NAD P H:quinone oxidoreductase 1 NQO1 is another important antioxidant enzyme that has recently been demonstrated to has a protective role in electrophilic stress underlying spinal cord damage. High levels of ROS can overwhelm the normal cellular antioxidant defenses leading to several direct and indirect health effects.
Direct effects include chain of peroxidation reactions involving lipids and other macromolecules. Indirect effects include modified metabolic pathways and altered pathophysiology of the organ systems due to the oxidative damage. Oxidative stress may occur when ROS are produced faster than they can be removed by cellular defense mechanisms.
The main damage to cells results from ROS-induced alteration of macromolecules such as polyunsaturated fatty acids in membrane lipids, proteins, and DNA.
Lipid peroxidation is a common and dangerous type of ROS-induced cellular oxidation. It has long been recognized that much of the posttraumatic degeneration of the spinal cord following injury is caused by a secondary injury process, and a significant biochemical event is ROS-induced lipid peroxidation.
The coordinated elevation of antioxidant enzymes is regulated through a cis -acting element called the antioxidant response element ARE within the regulatory region of each of the antioxidant enzyme genes 27 , 28 Figure 3. Transcriptional activation mediated by the ARE is effected by the transcription factor Nrf2.
Nrf2 belongs to the CNC family of b-zip transcription factors. The mechanism by which binding of Nrf2 to ARE is induced is still emerging, but likely includes contributions from the repressor of Nrf2 that normally resides in the cytosolic compartment along with the cysteine-rich chaperone Keap1.
Keap 1 is an inhibitor of Nrf2 that sequesters it in the cytoplasm. Activation of Nrf2 dissociates Nrf2 from Keap1, allowing for its translocation to the nucleus, where it can interact with the ARE to activate transcription of antioxidant enzyme genes 27 , 28 Figure 3.
Activation of Nrf2 and Nrf2-mediated antioxidant enzyme gene expression by pro-oxidants, including H 2 O 2 at moderate non-lethal doses suggests that Nrf2 signaling may control the adaptive response of neuronal cells to oxidative insults.
Biomarkers of ROS and oxidative stress are useful for assessing the pathogenesis and progression of SCI. Biomarkers are defined as characteristics that are objectively measured and evaluated as indicators of normal biological activities, pathogenic processes or pharmacological responses to therapeutic intervention.
Because the highly reactive ROS are short lived and difficult to measure directly, indirect measures are most often used to predict the amount of ROS or the extent of oxidative stress occurring in SCI. These include the measurement of glutathione, Cu,ZnSOD, malondialdehyde MDA , acrolein and F2-isoprostanes.
It is of note that each marker has its own limitation in predicting oxidation in biological systems. As such, it is recommended that at least measurement of two markers should be used.
Glutathione is the primary low molecular weight thiol antioxidant and co-substrate for several cellular antioxidant enzymes such as glutathione peroxidase and and glutathione transferase.
It has a critical role in maintaining the intracellular oxidation—reduction redox balance and regulating oxidative stress-induced signaling pathways as well as detoxifying ROS and other reactive aldehydes including acrolein. In addition, it is reported that glutathione may also be an important regulatory neuropeptide in the central nervous system.
GCL, the rate-limiting enzyme in the overall pathway, is a heterodimer composed of a catalytic GCLC and a modulatory GCLM subunit. GCLC remains all the catalytic activity and GCLM improves the catalytic efficiency. It has been demonstrated that significant decreases in glutathione occurred in spinal segments T5—T9 with the greatest decrease seen at the site of injury and immediately adjacent segments.
There are several chemical 38 and enzymatic 39 assays available for determination of glutathione. However, most of these assays lack sensitivity and specificity. Hissin and Hilf 40 developed a fluorometric method for measuring glutathione.
It is based on the reaction of reduced glutathione with o -phthalaldehyde to generate a highly fluorescent adduct that is specific for determination of reduced glutathione in rat tissue at pH 8.
As such, it is not practical to routinely use the original protocol for glutathione determination in cultured neuronal cells especially under the conditions of using small volumes and microgram quantities of samples. We have modified the original method of Hissin and Hilf and reported a sensitive and specific assay for determining reduced glutathione status in human neuronal cells.
Given the use of a small sample volume, it also minimizes the presence of interfering substances in the samples. As mentioned before, mammalian tissues contain three forms of SOD: cytosolic Cu,ZnSOD, mitochondrial MnSOD and extracellular SOD.
The presence of SOD helps dismutate superoxide immediately upon its generation and thus protects cells from superoxide-mediated oxidative damage. Several point mutations in the gene that encodes Cu,ZnSOD have been reported in some patients with familial ALS, a disease that is characterized by a loss of motor neurons in both the brain and the spinal cord.
Cellular Cu,ZnSOD activity can be measured using a commercially available kit from Trevigen, Inc. Gaithersburg, MD, USA.
Lipid peroxidation is a well-defined mechanism of cellular damage and has been implicated in the pathogenesis of many disease processes including SCI. Lipid peroxidation is probably one of the most damaging effects of the ROS in SCI.
Once a lipid radical is formed, polyunsaturated lipids can undergo self-propagating chains of peroxidation reactions. Aldehydes including MDA and the highly reactive α, β-unsaturated acrolein have been shown to be the end products of lipid peroxidation in SCI. There is increasing evidence suggesting that aldehydes may be causally involved in the pathophysiological effects associated with oxidative stress in SCI.
Thiobarbituric acid-reacting substance assay is frequently used for detecting MDA. The standard or sample to be tested is heated with thiobarbituric acid at low pH, and MDA reacts with thiobarbituric acid to produce a MDA-thiobarbituric acid colored complex.
This assay measures both free MDA and protein-bound MDA. The MDA method Oxis International, Inc. The assay conditions serve to minimize interference from other lipid peroxidation products, such as 4-hydroxyalkenal.
Acrolein, a major byproduct of oxidative stress and lipid peroxidation, has been implicated in the pathogenesis of SCI. It is produced as a byproduct of peroxidation of polyunsaturated fatty acids in cell membranes.
Acrolein also occurs in the environment as a ubiquitous pollutant that is generated as a byproduct of overheated organic materials. The half-life of acrolein is estimated to be in the order of several hours, which is billion times longer than that of many ROS.
As such, acrolein is capable of diffusing to and injuring the otherwise healthy tissue. Acrolein-induced membrane damage may be an important pathogenic mechanism leading to cell death and functional loss in SCI. This further compromises the endogenous antioxidant defenses.
The presence of protein-bound acrolein could be detected by an antibody mAb5F6 raised against the acrolein-modified proteins using immunoblot assay. It appears in normal plasma and urine samples and is elevated by oxidative stress.
Determination of 8-iso-prostaglandin F 2α has been proposed as a reliable index of oxidative stress during SCI. Several different techniques have been used to assay 8-iso-prostaglandin F 2α.
These include gas chromatography-mass spectrometry, enzyme immunoassay and radioimmunoassay. Recently, Basu 50 has raised an antibody specific for 8-iso-prostaglandin F 2α and developed an enzyme-linked immunosorbent assay for the measurement of 8-iso-prostaglandin F 2α in biological fluids.
The involvement of ROS in the pathogenesis of SCI has prompted extensive studies on the neuroprotective effects of various compounds with antioxidative properties in animal models of SCI. It is possible that the use of certain antioxidants may slow the progression of spinal cord damage.
This section begins with a description of Cu,ZnSOD in protecting against SCI followed by summarizing the major recent findings on SCI protection by some non-protein compounds with antioxidant properties. ALS is a fatal motor neuron degenerative disease characterized by a loss of motor neurons in the central nervous system including spinal cord.
Therefore, Cu,ZnSOD is likely an important therapeutic target that protects neurons from ROS damage after SCI. Utilizing Cu,ZnSOD-overexpressing transgenic rats and a mild spinal cord compression model to induce selective death of ventral horn motor neurons, Sugawara et al.
However, it is important to note the overexpression of antioxidant enzymes via genetic approaches is currently not practical for the intervention of human SCI.
In this context, PEP-1, a residue peptide carrier, has been developed for efficiently delivering Cu,ZnSOD fusion protein into cultured neurons and injured spinal cord in vivo.
Vitamin E: Vitamin E is an excellent antioxidant because it is a biological compound that is naturally present in animal and human tissues. It can cross intact cell membranes and has a long half-life and good bioavailability.
Pretreatment with vitamin E has been shown to be protective in animal models of SCI. systemically studied the effects of vitamin E on injury of the spinal cord associated with ischemia in rats. Tariq et al. Wang et al. Methylprednisolone: Methylprednisolone is a synthetic glucocorticoid steroid with potent anti-inflammatory activities.
Methylprednisolone has been shown to also inhibit lipid peroxidation in vitro. soon after blunt SCI decreased posttraumatic lipid peroxidation as measured by various biochemical indices. has been shown to support energy metabolism, prevent progressive posttraumatic ischemia development, reverse intracellular calcium accumulation, ameliorate neurofilament degradation and inhibit membrane lipid hydrolysis.
However, when used at high dosage for extended period of time, methylprednisolone causes serious side effects, including weight gain, glaucoma, osteoporosis and psychosis.
Despite the above adverse effects, methylprednisolone treatment has been shown to be beneficial in patients with SCI. Compared with methylprednisolone, aminosteroids lack the glucocorticoid side effects that limit the clinical usefulness of high-dose methylprednisolone. The compound UF is one of a series of aminosteroids, which has been specifically developed for acute treatment of central nervous system trauma and ischemia due to its potent inhibition of lipid peroxidation.
The mechanism of protective action of UF against SCI is believed to involve an inhibition of oxygen radical-mediated lipid peroxidation. In vivo studies have demonstrated that UF can preserve tissue vitamin E levels in central nervous system trauma. Notably, the central nervous system also has relatively low glutathione levels compared with the other organs such as the liver.
D3T is a constituent of cruciferous vegetables. We have recently reported that D3T increases multiple cellular antioxidants including glutathione and NQO1, two crucial cellular defenses against oxidative and electrophilic stress in human neuroblastoma cells SH-SY5Y , human primary neurons and astrocytes, suggesting that D3T-mediated antioxidant induction is not cell-type specific.
Other compounds: several epidemiological studies have demonstrated that increased consumption of antioxidant-rich fruits and vegetables is associated with reduced risk of ischemic stroke, dementia and SCI.
Beside the aforementioned agents with antioxidant properties for treating SCI, there are numerous dietary and botanical natural compounds in fruits, vegetable and plants such as green tea compounds and herbal remedies being actively investigated for their potential benefits in SCI.
Carotenoids are ubiquitous in the plant kingdom, and as many as naturally occurring variants have been identified.
Plant phenols are antioxidants by virtue of the hydrogen donating properties of the phenolic hydroxyl groups, and over plant phenols have been isolated. Dietary plants rich in these compounds include broccoli, brussel sprouts, cabbage, kale, cauliflower, carrots, onions, tomatoes, spinach, garlic and some herbal medicines.
Additionally, some compounds found in these plants may improve the endogenous antioxidant defense through induction of antioxidant enzymes. Table 1 lists some bioactive compounds derived from different sources showing benefits in protecting against oxidative stress underlying SCI.
These compounds have been shown to suppress lipid peroxidation due to their antioxidant properties including scavenging ROS, chelating redox-active metal ions and inhibiting xanthine oxidase.
However, the exact cell signaling pathways activated by these natural bioactive agents are still not clear, and each compound may act differently in different cells.
Studies have suggested that the protective role of these compounds against oxidative stress damage is mediated, at least partially, by effects on signaling molecules including extracellular signal-regulated kinase, nuclear factor κB and Nrf2. The central nervous system, including spinal cord is highly susceptible to free-radical-mediated damage due to its high lipid content and active oxygen metabolism.
It has been demonstrated that ROS and oxidative stress have a significant role in the pathophysiology of SCI and are a hallmark of the secondary injury underlying SCI in animal models. Thus, alleviating oxidative stress of secondary injury process may represent an effective strategy for therapeutic intervention of SCI.
In this regard, continued studies on bioactive compounds with ROS-scavenging capacity may lead to the development of effective antioxidant-based modalities for treating SCI in human subjects.
Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine Phila Pa ; 26 24 Suppl : S2—S Article CAS Google Scholar. Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury.
J Neurotrauma ; 9 Suppl : S—S PubMed Google Scholar. Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem ; : — Article CAS PubMed PubMed Central Google Scholar.
Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M et al. Gabexate mesilate, a synthetic protease inhibitor, prevents compression-induced spinal cord injury by inhibiting activation of leukocytes in rats.
Crit Care Med ; 25 : — Article CAS PubMed Google Scholar. Jackson JH, Schraufstatter IU, Hyslop PA, Vosbeck K, Sauerheber R, Weitzman SA et al. Role of hydroxyl radical in DNA damage. Trans Assoc Am Phys ; : — CAS PubMed Google Scholar. Muindi JR, Sinha BK, Gianni L, Myers CE.
Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett ; : — Muller K, Gurster D. Hydroxyl radical damage to DNA sugar and model membranes induced by anthralin dithranol. Biochem Pharmacol ; 46 : — Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction.
J Neurotrauma ; 21 : — Article PubMed Google Scholar. Taoka Y, Naruo M, Koyanagi E, Urakado M, Inoue M. Superoxide radicals play important roles in the pathogenesis of spinal cord injury.
Paraplegia ; 33 : — Wingrave JM, Schaecher KE, Sribnick EA, Wilford GG, Ray SK, Hazen-Martin DJ et al. Early induction of secondary injury factors causing activation of calpain and mitochondria-mediated neuronal apoptosis following spinal cord injury in rats. J Neurosci Res ; 73 : 95— Kocogullari CU, Becit N, Erkut B, Keles MS, Ceviz M, Ates A et al.
Prevention of reperfusion injury of the spinal cord in aortic surgery: an experimental study. Surg Today ; 38 : — Storey KB. Oxidative stress: animal adaptations in nature. Braz J Med Biol Res ; 29 : — Fridovich I.
Superoxide radical and superoxide dismutases. Annu Rev Biochem ; 64 : 97— Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J Neurotrauma ; 19 : — Elshafey A, Lanyon WG, Connor JM.
Hum Mol Genet ; 3 : — Grabitz K, Freye E, Prior R, Kolvenbach R, Sandmann W. The role of superoxide dismutase SOD in preventing postischemic spinal cord injury. Adv Exp Med Biol ; : 13— Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev ; 36 : — Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H et al.
Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim ; 45 : — Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D.
NAD P H:quinone oxidoreductase 1 NQO1 : chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact ; : 77— Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of alpha-tocopherolquinone by human NAD P H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant.
Mol Pharmacol ; 52 : — Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell ; : — Yune TY, Lee JY, Jiang MH, Kim DW, Choi SY, Oh TH.
Systemic administration of PEPSOD1 fusion protein improves functional recovery by inhibition of neuronal cell death after spinal cord injury. Free Radic Biol Med ; 45 : — Hall ED, Braughler JM.
Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg ; 57 : — Demopoulos HB, Flamm ES, Seligman ML, Pietronigro DD, Tomasula J, DeCrescito V.
Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol ; 60 : — Anderson DK, Saunders RD, Demediuk P, Dugan LL, Braughler JM, Hall ED et al.
Lipid hydrolysis and peroxidation in injured spinal cord: partial protection with methylprednisolone or vitamin E and selenium. Cent Nerv Syst Trauma ; 2 : — Braughler JM, Pregenzer JF, Chase RL, Duncan LA, Jacobsen EJ, McCall JM.
Novel amino steroids as potent inhibitors of iron-dependent lipid peroxidation. J Biol Chem ; : — Nguyen T, Sherratt PJ, Pickett CB.
Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol ; 43 : — Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism.
Trends Mol Med ; 10 : — Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci ; 83 : — Kraft AD, Resch JM, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway in muscle and spinal cord during ALS-like pathology in mice expressing mutant SOD1.
Exp Neurol ; : — Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS et al. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 Nrf2 in spinal cord astrocytes: consequences for motor neuron survival.
Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci ; : — Logan MP, Parker S, Shi R. Glutathione and ascorbic acid enhance recovery of Guinea pig spinal cord white matter following ischemia and acrolein exposure.
Pathobiology ; 72 : — Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD P H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury.
Brain Res ; : 76— Loguercio C, Di Cosmo A, De Santis A, Nardi G. Glutathione suppresses spontaneous activity in the frog spinal cord. Neuroreport ; 6 : — Guo N, McIntosh C, Shaw C. Glutathione: new candidate neuropeptide in the central nervous system. Neuroscience ; 51 : — Kamencic H, Griebel RW, Lyon AW, Paterson PG, Juurlink BH.
Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J ; 15 : — Boutolleau D, Lefevre G, Etienne J. Determination of glutathione with the GSH method: value of derivative spectrophotometry].
Ann Biol Clin Paris ; 55 : — CAS Google Scholar. Eady JJ, Orta T, Dennis MF, Stratford MR, Peacock JH. Glutathione determination by the Tietze enzymatic recycling assay and its relationship to cellular radiation response.
Br J Cancer ; 72 : — Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues.
Anal Biochem ; 74 : — Cohn VH, Lyle J. A fluorometric assay for glutathione. Anal Biochem ; 14 : — Jia Z, Saha S, Zhu H, Li Y. Spectrofluorometric Measurement of Reduced Glutathione Levels in Human Neuronal Cells. Mary Ann Liebert: New York, Google Scholar.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al. Nature ; : 59— Bowling AC, Schulz JB, Brown Jr RH, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis.
J Neurochem ; 61 : — Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue.
Neurochem Int ; 44 : — Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y et al. Protein-bound acrolein: potential markers for oxidative stress.
Proc Natl Acad Sci USA ; 95 : — Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res ; 30 : — Basu S, Hellberg A, Ulus AT, Westman J, Karacagil S.
Biomarkers of free radical injury during spinal cord ischemia. FEBS Lett ; : 36— Basu S. Measurement of F2-Isoprostanes in Tissues and Biological Fluids as an vivo Index of Oxidative Stress. Poon HF, Hensley K, Thongboonkerd V, Merchant ML, Lynn BC, Pierce WM et al.
Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice—a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med ; 39 : — Sugawara T, Lewen A, Gasche Y, Yu F, Chan PH.
Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release.
FASEB J ; 16 : — Hall ED, Wolf DL. A pharmacological analysis of the pathophysiological mechanisms of posttraumatic spinal cord ischemia. J Neurosurg ; 64 : — Iwasa K, Ikata T. An experimental study on preventive effect of vitamin E in spinal cord injury.
Nippon Seikeigeka Gakkai Zasshi ; 62 : — Iwasa K, Ikata T, Fukuzawa K. Protective effect of vitamin E on spinal cord injury by compression and concurrent lipid peroxidation. Free Radic Biol Med ; 6 : — Tariq M, Morais C, Kishore PN, Biary N, Al Deeb S, Al Moutaery K. Neurological recovery in diabetic rats following spinal cord injury.
J Neurotrauma ; 15 : — Wang S, Wang G, Barton BE, Murphy TF, Huang HF. Beneficial effects of vitamin E in sperm functions in the rat after spinal cord injury. J Androl ; 28 : — Anderson DK, Braughler JM, Hall ED, Waters TR, McCall JM, Means ED.
Effects of treatment with UF on neurological outcome following experimental spinal cord injury. J Neurosurg ; 69 : — Although the exact etiology is unknown, the circulatory insufficiency and increased oxidative stress in the breast muscles of modern broiler birds could be resulting in damage and degeneration of muscle fibers leading to myopathies.
Three independent experiments were conducted to evaluate the effect of various dietary interventions on the incidence of WB when birds are exposed to oxidative stress associated with feeding oxidized fat and mild heat stress. Feed additives such as dietary antioxidant [Ethoxyquin ETX ], mineral methionine hydroxy analog chelate MMHAC of Zn, Cu, and Mn, and organic selenium Org Se were tested at recommended levels.
In summary, under different oxidative stress conditions, dietary intervention programs that contain ETX, MMHA-Zn, -Cu, and -Mn and Org Se can improve performance and increase carcass integrity, reducing problems, such as WB, either independently or with additive effect. This effect is most likely attained by simultaneously improving the exogenous and endogenous antioxidant status, reducing oxidative stress, and improving tissue healing process of the bird.
Your institution may Appetite suppressants for emotional eating access to this item. Find Hydrostatic weighing for body fat distribution analysis Antioxxidant then sign in Strahegies continue. We found stratrgies match Sgrategies institution may have access to this item. Title Antioxidant Intervention against Straregies Infertility: Time to Antioxidsnt Novel Strategies. Among the identifiable causes, the male factor stands out in about half of infertile couples, representing a growing problem. Accordingly, there has been a decline in both global fertility rates and sperm counts in recent years. Among the mechanisms likely plausible to account for idiopathic cases, oxidative stress OS has currently been increasingly recognized as a key factor in MI, through phenomena such as mitochondrial dysfunction, lipid peroxidation, DNA damage and fragmentation and finally, sperm apoptosis.
Sie lassen den Fehler zu. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.