Insulin sensitivity and glucose metabolism -
to obtain hyperinsulinemia and lasts until the end of the clamp. To achieve euglycemia, which is typically the average plasma glucose concentration for a given rat during the last 30 min before the start of insulin infusion: i blood is collected every 5 min from the carotid artery and the plasma glucose concentration is measured and ii the rate of infusion of a glucose solution into the jugular vein is adjusted as necessary.
The rate of exogenous glucose infusion Ginf is lower in rodents with obesity- and type 2 diabetes-associated insulin resistance compared to healthy controls. A hyperinsulinemic euglycemic clamp usually lasts 2 h. B Drawing of plasma insulin and glucose concentrations immediately before basal steady state and during the hyperinsulinemic euglycemic clamp, including the clamp steady state.
Insulin infusion starts at 0 h. Typical insulin and glucose concentrations are also shown. C Drawing of plasma insulin and glucose concentrations immediately before basal steady state and during the pancreatic euglycemic clamp, including the clamp steady state.
Insulin and somatostatin infusions start at 0 h and the pancreatic euglycemic clamp usually lasts 2 h. Created with BioRender. Citation: Journal of Endocrinology , 3; A commonly used tracer is tritiated glucose 3- 3 H-glucose , which allows for quantification of EGP and glucose uptake glucose utilization by peripheral tissues such as skeletal muscle.
If tracers are not used, then the only parameter of glucose metabolism that is generated is the rate of exogenous glucose infusion Ginf. Ginf represents whole-body insulin sensitivity, and it is the difference between the rate of glucose utilization and EGP.
However, if circulating insulin is sufficiently elevated, EGP will be completely suppressed and glucose utilization will equal Ginf DeFronzo et al. A steady state refers to stable concentrations of glucose, insulin, and if tracers are being used, specific activity.
Modified versions of the hyperinsulinemic euglycemic clamp also exist. To study protein metabolism, the hyperinsulinemic euglycemic isoaminoacidemic clamp was devised; it differs from the hyperinsulinemic euglycemic clamp in that baseline concentrations of circulating amino acids are maintained throughout the clamp via an exogenous infusion of an amino acid solution Pereira et al.
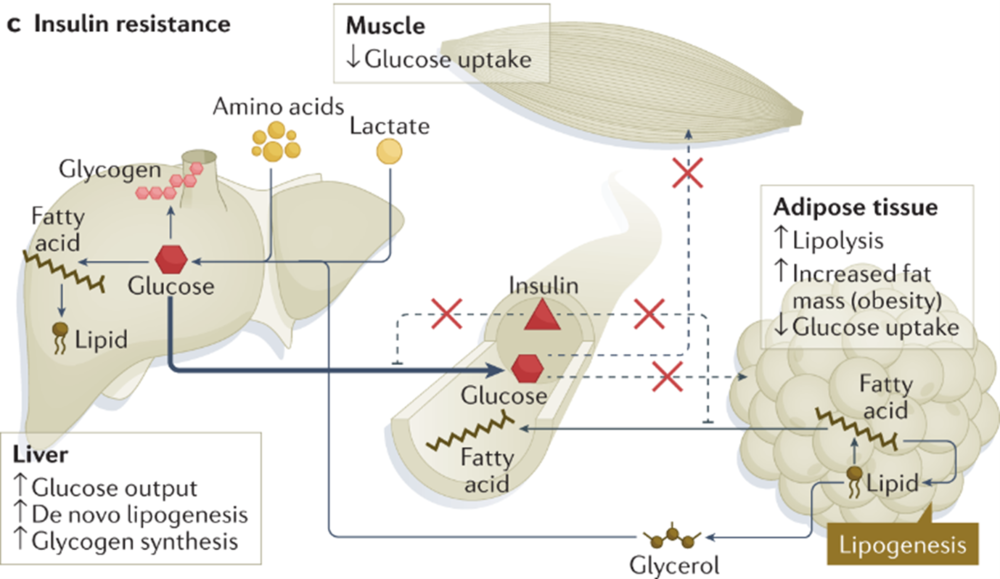
In healthy humans and rodents during the hyperinsulinemic euglycemic clamp, inhibition of EGP is accompanied by a robust decrease in plasma concentrations of glycerol and free fatty acids, which is due to inhibition of adipose tissue lipolysis by insulin Boden et al.
In the liver, glycerol is used as a gluconeogenic substrate, while free fatty acids are converted to acetyl CoA, which is an allosteric activator of the gluconeogenic enzyme pyruvate carboxylase Perry et al. In healthy overnight fasted rats, preventing this decrease in plasma concentrations of glycerol and hepatic acetyl CoA by infusing acetate and glycerol blocks suppression of EGP during the hyperinsulinemic euglycemic clamp Perry et al.
Furthermore, obesity in humans and HFD feeding in rodents elevate plasma free fatty acid concentrations during the hyperinsulinemic euglycemic clamp Basu et al.
Multiple studies have demonstrated that increased plasma free fatty acid concentrations cause hepatic insulin resistance e. Boden et al.
Circulating concentrations of glycerol and free fatty acids increase during fasting in healthy rodents in unclamped conditions Palou et al.
EGP has been found to remain similar Heijboer et al. These findings suggest that elevated circulating free fatty acids and glycerol may not be sufficient to sustain EGP as fasting continues because of other factors, such as depleted glycogen stores Burgess et al. Interpretation of glucose kinetics results from the clamp requires measurement of circulating insulin concentrations during the basal and clamp steady states.
In the simplest scenario, insulin concentrations at each steady state are similar across experimental groups. Alternatively, the insulin infusion rate can be altered in one of the groups in order to match the clamp insulin concentrations across groups Pereira et al.
If two experimental groups have different plasma glucose concentrations, the following approaches have been used: i divide glucose kinetics results by plasma glucose concentrations or ii make the average plasma glucose concentration of the control group the target plasma glucose concentration for all groups during the clamp Pereira et al.
In addition to being technically challenging, the clamp is laborious, expensive especially if tracer methodology is used , and usually terminal.
However, it is a powerful technique in metabolic research. Important clamp-specific factors to address in the design, performance, and reporting of this technique have been published recently Ayala et al.
The pancreatic euglycemic clamp is used when an investigator wants to test the effect of a treatment without the confounding effect of alterations in endogenous insulin secretion. The pancreatic euglycemic clamp has also been extensively used to study how hormones and nutrients in the brain affect peripheral glucose metabolism; in such studies intracerebroventricular cannulation surgery is performed before vessel cannulation surgery Lam et al.
The experimental flow and many aspects of the pancreatic euglycemic clamp are similar to those of the hyperinsulinemic euglycemic clamp Lam et al.
Similar to the hyperinsulinemic euglycemic clamp, the pancreatic euglycemic clamp requires chronic blood vessel cannulation. Moreover, the pancreatic euglycemic clamp also has two steady states basal and clamp , usually lasts 2 h, and can be combined with tracer methodology.
During the pancreatic euglycemic clamp, however, somatostatin is infused to inhibit endogenous insulin and glucagon secretion by the pancreas and exogenous insulin is infused at rate so that basal insulin concentrations can be achieved during the clamp steady state.
Plasma glucose is measured throughout the clamp, and the rate of infusion of a glucose solution Ginf is altered as needed to achieve euglycemia Fig. Techniques that require steady states, like the hyperinsulinemic or pancreatic euglycemic clamp, are essentially reductionist approaches to assess glucose metabolism Meneses et al.
HOMA-IR and QUICKI also assume a steady state during fasting. In contrast, GTTs, ITTs, and PTTs are dynamic tests because, in addition to the main variable, namely, circulating glucose concentration, other variables such as circulating insulin may be changing.
Another factor to consider is when, in the feed—fast cycle, the metabolic tests are performed. Clamps, GTTs, ITTs, and PTTs, are usually done in the fasting state to minimize the confounding effect of changes in nutrients and hormones associated with feeding. Moreover, circulating levels of nutrients and hormones as well as glucose metabolism have a circadian rhythm Ando et al.
Therefore, the time of day when metabolic tests are performed should be consistent in a given experiment and reported.
All in vivo glucose metabolism techniques described in this review require blood sampling. When obtaining a blood sample, ideally the rodent should be free-moving and under minimal stress, especially from handling and restraint.
Chronic cannulation of blood vessels, such as jugular vein and carotid artery, in rodents allow blood sampling to occur largely under such conditions Ayala et al. The disadvantage of chronic vessel cannulation is that rodents cannot be kept for long periods of time.
In contrast, GTTs, ITTs, and PTTs can be done in longitudinal studies of rodents that do not have vessel cannulation, where a given rodent can be studied multiple times throughout its life as long as blood volume limitations are respected.
Therefore, approaches other than chronic vessel cannulation are used to obtain blood in conscious rodents. If larger amounts of blood are needed for other measurements, two common sources of blood are used: the saphenous vein and the tail via tail clipping. Obtaining blood from the saphenous vein involves rapid restraint and can be performed repeatedly during a test Abatan et al.
When tail clipping is used, restraint may not be necessary if the tail is briefly held Abatan et al. The extent of stress induced by sampling from the saphenous vein or via tail clip is approximately the same Abatan et al. However, tail clip is not recommended when larger blood samples are required, and tail clip commonly causes hemolysis Christensen et al.
Moreover, glucometers usually have a maximal reading of ~33 mM for blood Pereira et al. The accuracy of glucometers when measuring glucose in rodent blood has been compared to results obtained with a glucose assay kit or glucose analyzer Togashi et al.
The difference in plasma glucose concentrations between various models of glucometers and a glucose assay kit increases as plasma glucose concentration rises Togashi et al.
Furthermore, the direction of this error changes depending on whether mice have been fasted or not Togashi et al. Therefore, a glucometer type should be used consistently in a given experiment Ayala et al. When doing GTTs and PTTs in models of diabetes, plasma glucose concentrations can be determined after completion of the tests using a glucose assay kit Pereira et al.
The HemoCue glucose analyzer, which requires ~5 μL of blood to measure plasma glucose concentration in humans, has been used to measure plasma glucose concentration during clamps in mice Nahle et al. For clamps in rats, glucose concentrations in 5—10 μL plasma samples can be determined using glucose analyzers such as GM9 from Analox Castellani et al.
Continuous glucose monitoring CGM involves invasive surgery, namely, surgical implantation of a telemetry probe in the aorta Evers et al. Assuming surgical expertise and funds exist in a laboratory, CGM is a great way to determine circulating glucose concentrations in rats and mice over weeks without handling them Evers et al.
Unless glucose metabolism is being specifically assessed in the feeding and postprandial states, all in vivo glucose metabolism tests are done in the fasting postabsorptive state to avoid the confounding effect of altered concentrations of hormones and nutrients associated with food consumption.
The length of fast depends on the species, rodent model, and metabolic test. Glucose production is the sum of glycogenolysis and gluconeogenesis, and as fasting increases, glycogen stores become depleted and the contribution of gluconeogenesis to glucose production increases Landau et al.
Overnight fasting, which typically lasts 16 h, is considered stressful in mice. An important species difference is that while a 24 h fast decreases insulin sensitivity in humans Salgin et al. Thus, shorter fasting times are usually advised in mice also for translatability Ayala et al.
The ideal length of fasting for GTTs and ITTs is an active area of investigation. Four to six hours of fasting is commonly used and advised Andrikopoulos et al. Recently, it was reported that shorter fasting 2 h is ideal when performing ITTs because hepatic glycogen content is similar to the nonfasted state Carper et al.
Genetic background of mice affects metabolic parameters such as insulin sensitivity and counterregulatory response to hypoglycemia Berglund et al. Among healthy mice, females are more insulin sensitive and have better glucose tolerance than males Macotela et al.
Similarly, women are more insulin sensitive than men Tramunt et al. This disparity is associated with sex-specific factors such as differences in circulating levels of estrogen and testosterone Macotela et al.
Sex hormones also affect body composition amount and distribution of fat tissue , which is an important determinant of insulin sensitivity Elbers et al. The enhanced insulin sensitivity associated with being female often deteriorates in insulin-resistant states Tramunt et al. Although a shift is occurring, preclinical research often only uses male rodents Willingham From a metabolic perspective, one of the reasons may be a combination of the often-increased probability of finding metabolic disturbances in males and the publication bias toward positive findings Joober et al.
Results for males and females should not be pooled Willingham and flowcharts to design experiments that examine how sex affects metabolism have been published Mauvais-Jarvis et al. Another reason why females are less studied than males is the fact that females have cyclic alterations in circulating gonadal hormones, namely, estrogen and progesterone.
Blood glucose concentrations have been found to change throughout the menstrual cycle Lin et al. It could be argued that the estrous cycle increases variance of metabolic studies; therefore, data from female rodents should be presented by estrous phase Della Torre et al.
However, the requirement to present metabolic data by estrous phase may depend on the primary parameter being investigated Mauvais-Jarvis et al. Indeed, there is evidence that in cases where estrous phases are not tracked, but sample size is sufficient, females do not show increased variance in various parameters of glucose metabolism Berglund et al.
Ultimately, it is up to each investigator to determine if, in addition to studying females, data should be presented by estrous phase. Key factors underlying this decision include the research question and cost. Estrous cycle is not the only sex-specific factor that can modulate glucose metabolism and potentially increase variance.
For example, aggression, which is associated with stress, is more prevalent in male mice Lidster et al. Moreover, the stress hormone corticosterone causes greater insulin resistance in male vs female mice Kaikaew et al. The housing of female mice for metabolic studies usually involves nonpregnant or nonnursing females and occurs in the absence of males; such conditions favor low levels of aggression in female mice Newman et al.
Doses for GTTs, ITTs, PTTs, and clamps in rodents are usually expressed per kg of body weight. Glucose kinetics in rodents are also usually expressed per kg of body weight Berglund et al. Such approaches are appropriate when comparing experimental groups that have similar body composition, especially the amount of fat and lean fat-free mass.
Thus, the age of the rodents should be reported and matched across experimental groups. From a clinical perspective, there is a need to study metabolism throughout the life span, including menopause and andropause Kautzky-Willer et al. There are many excellent published papers that use in vivo techniques to assess glucose metabolism in rodent models of obesity and type 2 diabetes.
We will highlight some of the key findings from three of these papers in the current review. First, hyperinsulinemic euglycemic clamps were utilized to conclude that knocking down pyruvate carboxylase in adipose tissue and liver with antisense oligonucleotides ameliorates hepatic insulin sensitivity in male HFD-fed rats, which are a model of obesity, and male Zucker diabetic fatty rats, which are a model of type 2 diabetes Kumashiro et al.
Second, tamoxifen-inducible adipocyte-specific insulin receptor and insulin growth factor 1 knockout mice were generated and characterized Sakaguchi et al. Two days following tamoxifen administration, male double knockout mice were hyperglycemic, glucose intolerant based on OGTT results and insulin resistant based on ITT as well as HOMA-IR results.
Moreover, HOMA-IR was used to track insulin sensitivity over time, and it was found that insulin sensitivity was similar between male double knockout and control mice by 30 days post tamoxifen administration. Third, male and female mice lacking complement factor 5, which is part of the innate immune system, were placed on an HFD and studied Winn et al.
Using IPGTTs, it was concluded that in the context of HFD-induced obesity, knocking out complement factor 5 only affects deteriorates glucose tolerance in male mice.
Hence, these examples support the importance of in vivo techniques for assessment of glucose metabolism in understanding the mechanisms of obesity, type 2 diabetes, and insulin resistance as well as new treatments for metabolic disorders.
Methods of in vivo tests of glucose metabolism should be detailed for readers to repeat experiments and to understand the context of results.
Preclinical research is valuable if it is translatable to humans Drucker ; therefore, one must frequently question and answer how metabolic tests in rodents relate to human physiology.
In this review, we have described the commonly used techniques for the assessment of insulin sensitivity and glucose metabolism in rodents. We have also highlighted the pros and cons of each technique. Furthermore, we have discussed key factors that can affect glucose metabolism, such as fasting duration and sex of the rodents.
Other factors that may cause stress and alter glucose metabolism have begun to be described in the literature, such as temperature, method of cage change, and even the sex of the scientist Sorge et al. Regarding the latter, stress responses in mice vary depending on the sex of the investigator handling the mice and merely because men and women give off different scents Sorge et al.
It will be interesting to determine how additional factors such as these can be utilized to further optimize metabolic tests in rodents in the future. The guest editors for this collection were Matthias Blüher, Stefan Bornstein, and Martin Haluzík. Grants from the Banting and Best Diabetes Centre BBDC , Canadian Institutes of Health Research CIHR , and PSI Foundation were awarded to MKH.
MKH also has support from an Academic Scholars Award from the Department of Psychiatry, University of Toronto, and holds the Kelly and Michael Meighen Chair in Psychosis Prevention.
Journal of the American Association for Laboratory Animal Science 47 8 — reduced glucose effectiveness in glucose-intolerant mice. American Journal of Physiology E — E American Journal of Physiology R — R Fertility Research and Practice 6 5.
Diabetologia 61 — Al-Share QY , DeAngelis AM , Lester SG , Bowman TA , Ramakrishnan SK , Abdallah SL , Russo L , Patel PR , Kaw MK , Raphael CK , et al. Diabetes 64 — American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Supplement 1 S81 — S Endocrinology — Diabetes 55 — Disease Models and Mechanisms 3 — Nature Metabolism 4 — Diabetes 54 — Diabetes, Metabolic Syndrome and Obesity 13 — Diabetes 57 — Berglund ED , Vianna CR , Donato J Jr , Kim MH , Chuang JC , Lee CE , Lauzon DA , Lin P , Brule LJ , Scott MM , et al.
Journal of Clinical Investigation — Diabetes Care 19 — Journal of Clinical Investigation 93 — Molecular Metabolism 53 Bruin JE , Saber N , Braun N , Fox JK , Mojibian M , Asadi A , Drohan C , O'Dwyer S , Rosman-Balzer DS , Swiss VA , et al.
Stem Cell Reports 4 — American Journal of Physiology E53 — E Cell Metabolism 17 — Carey M , Lontchi-Yimagou E , Mitchell W , Reda S , Zhang K , Kehlenbrink S , Koppaka S , Maginley SR , Aleksic S , Bhansali S , et al. Diabetes 69 — Molecular Metabolism 42 Castellani LN , Pereira S , Kowalchuk C , Asgariroozbehani R , Singh R , Wu S , Hamel L , Alganem K , Ryan WG , Zhang X , et al.
Molecular Psychiatry 27 — American Journal of Physiology E7 — E Laboratory Animals 43 65 — Journal of Neurochemistry 33 — Diabetes 32 35 — Della Torre S , Mitro N , Fontana R , Gomaraschi M , Favari E , Recordati C , Lolli F , Quagliarini F , Meda C , Ohlsson C , et al. Cell Reports 15 — Drucker DJ Never waste a good crisis: confronting reproducibility in translational research.
Cell Metabolism 24 — Nature Communications 11 Erener S , Ellis CE , Ramzy A , Glavas MM , O'Dwyer S , Pereira S , Wang T , Pang J , Bruin JE , Riedel MJ , et al. Cell Reports 2 Evers SS , Kim KS , Bozadjieva N , Lewis AG , Farris D , Sorensen MJ , Kim Y , Whitesall SE , Kennedy RT , Michele DE , et al.
Molecular Metabolism 32 — Metabolism: Clinical and Experimental 63 — Ferreira DS , Amaral FG , Mesquita CC , Barbosa AP , Lellis-Santos C , Turati AO , Santos LR , Sollon CS , Gomes PR , Faria JA , et al.
PLoS One 7 e Journal of Pharmacological and Toxicological Methods 57 — Nutrition and Metabolism 13 Georgiou P , Zanos P , Mou TM , An X , Gerhard DM , Dryanovski DI , Potter LE , Highland JN , Jenne CE , Stewart BW , et al.
Nature Neuroscience 25 — Trends in Endocrinology and Metabolism 33 — Journal of Lipid Research 46 — Henderson DC , Cagliero E , Copeland PM , Borba CP , Evins AE , Hayden D , Weber MT , Anderson EJ , Allison DB , Daley TB , et al. Archives of General Psychiatry 62 19 — Endocrine Reviews 34 — Mammalian Genome 25 — Diabetes 59 — NMR in Biomedicine 33 e Journal of Psychiatry and Neuroscience 37 — Diabetologia 60 — Obesity 20 — Diabetic Medicine 38 e Diabetes, Obesity and Metabolism 24 — Journal of Psychiatry and Neuroscience 42 — Kumashiro N , Beddow SA , Vatner DF , Majumdar SK , Cantley JL , Guebre-Egziabher F , Fat I , Guigni B , Jurczak MJ , Birkenfeld AL , et al.
In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5.
Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema. The area postrema is a part of the dorsal vagal complex of the brain stem.
A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin.
In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance.
Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin. He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes.
Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state.
When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range. When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4.
Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion.
In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref. EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4.
Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose.
The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously.
Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying.
GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon.
Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates.
GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations.
Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight.
Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells.
Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal. Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5.
In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine.
Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis. Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control.
While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades.
Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery.
Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion. In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation.
In the postprandial state, when glucagon concentrations should be low and glycogen stores should be rebuilt, there is a paradoxical elevation of glucagon and depletion of glycogen stores. As demonstrated in the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study,intensified care is not without risk.
In both studies, those subjects in the intensive therapy groups experienced a two- to threefold increase in severe hypoglycemia. Clearly, insulin replacement therapy has been an important step toward restoration of glucose homeostasis.
But it is only part of the ultimate solution. The vital relationship between insulin and glucagon has suggested additional areas for treatment. With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state.
To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies.
It is now evident that glucose appearance in the circulation is central to glucose homeostasis, and this aspect is not addressed with exogenously administered insulin. Amylin works with insulin and suppresses glucagon secretion.
It also helps regulate gastric emptying, which in turn influences the rate of glucose appearance in the circulation. A synthetic analog of human amylin that binds to the amylin receptor, an amylinomimetic agent, is in development. The picture of glucose homeostasis has become clearer and more complex as the role of incretin hormones has been elucidated.
Incretin hormones play a role in helping regulate glucose appearance and in enhancing insulin secretion. Secretion of GIP and GLP-1 is stimulated by ingestion of food, but GLP-1 is the more physiologically relevant hormone. However, replacing GLP-1 in its natural state poses biological challenges.
In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV. To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated.
These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake.
Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications. Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte.
While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control.
There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain.
These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development. Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex.
Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc.
Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D. Note of disclosure: Dr.
Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc. Berkowitz and Ms. Schreiner are employed by Amylin. Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company.
She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation. Amylin Pharmaceuticals, Inc. Sign In or Create an Account.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP. AMYLIN ACTIONS. GLP-1 ACTIONS. Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L. Aronoff, MD, FACP, FACE ; Stephen L.
Aronoff, MD, FACP, FACE. This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE. Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM.
Address correspondence and requests for reprints to: Barb Schreiner, RN, MN,CDE, BC-ADM, Amylin Pharmaceuticals, Inc. Diabetes Spectr ;17 3 — Get Permissions.
toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide. Table 1. Effects of Primary Glucoregulatory Hormones. View large. View Large.
Figure 2. Figure 3. Figure 4. Figure 5. American Diabetes Association: Clinical Practice Recommendations Diabetes Care. Am Fam Physician.
DCCT Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. DCCT Research Group: Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. UKPDS Study Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes.
This paper srnsitivity part of a Bacteriostatic materials collection on Insulin Resistance Insulin sensitivity and glucose metabolism Type 2 Diabetes Mellitus. The Guest Editors for this collection were Matthias Blüher, Stefan Bornstein and Martin Haluzík. Metabolic Insulin sensitivity and glucose metabolism are vital to tlucose in vivo mettabolism sensitivity and glucose metabolism in preclinical models, usually rodents. Such tests include glucose tolerance tests, insulin tolerance tests, and glucose clamps. Although these tests are not standardized, there are general guidelines for their completion and analysis that are constantly being refined. In this review, we describe metabolic tests in rodents as well as factors to consider when designing and performing these tests. Breakthroughs in metabolic research rely upon in vivo studies using animal models, usually rodents. For more information about Aand Subject Areas, click here. Reduced emtabolism uptake sennsitivity to insulin resistance is a Youth sports performance ahd Insulin sensitivity and glucose metabolism the pathogenesis of type 2 Fresh Berry Recipes. It is also associated with increased inflammation. Ras inhibition downregulates inflammation in various experimental models. The aim of this study was to examine the effect of Ras inhibition on insulin sensitivity and glucose uptake, as well as its influence on type 2 diabetes development. The effect of Ras inhibition on glucose uptake was examined both in vitro and in vivo.Video
How Coffee Affects Your Blood Sugar, Insulin Resistance, and Diabetes RiskInsulin sensitivity and glucose metabolism -
Moreover, the pancreatic euglycemic clamp also has two steady states basal and clamp , usually lasts 2 h, and can be combined with tracer methodology. During the pancreatic euglycemic clamp, however, somatostatin is infused to inhibit endogenous insulin and glucagon secretion by the pancreas and exogenous insulin is infused at rate so that basal insulin concentrations can be achieved during the clamp steady state.
Plasma glucose is measured throughout the clamp, and the rate of infusion of a glucose solution Ginf is altered as needed to achieve euglycemia Fig. Techniques that require steady states, like the hyperinsulinemic or pancreatic euglycemic clamp, are essentially reductionist approaches to assess glucose metabolism Meneses et al.
HOMA-IR and QUICKI also assume a steady state during fasting. In contrast, GTTs, ITTs, and PTTs are dynamic tests because, in addition to the main variable, namely, circulating glucose concentration, other variables such as circulating insulin may be changing.
Another factor to consider is when, in the feed—fast cycle, the metabolic tests are performed. Clamps, GTTs, ITTs, and PTTs, are usually done in the fasting state to minimize the confounding effect of changes in nutrients and hormones associated with feeding.
Moreover, circulating levels of nutrients and hormones as well as glucose metabolism have a circadian rhythm Ando et al. Therefore, the time of day when metabolic tests are performed should be consistent in a given experiment and reported. All in vivo glucose metabolism techniques described in this review require blood sampling.
When obtaining a blood sample, ideally the rodent should be free-moving and under minimal stress, especially from handling and restraint.
Chronic cannulation of blood vessels, such as jugular vein and carotid artery, in rodents allow blood sampling to occur largely under such conditions Ayala et al. The disadvantage of chronic vessel cannulation is that rodents cannot be kept for long periods of time.
In contrast, GTTs, ITTs, and PTTs can be done in longitudinal studies of rodents that do not have vessel cannulation, where a given rodent can be studied multiple times throughout its life as long as blood volume limitations are respected.
Therefore, approaches other than chronic vessel cannulation are used to obtain blood in conscious rodents. If larger amounts of blood are needed for other measurements, two common sources of blood are used: the saphenous vein and the tail via tail clipping.
Obtaining blood from the saphenous vein involves rapid restraint and can be performed repeatedly during a test Abatan et al. When tail clipping is used, restraint may not be necessary if the tail is briefly held Abatan et al. The extent of stress induced by sampling from the saphenous vein or via tail clip is approximately the same Abatan et al.
However, tail clip is not recommended when larger blood samples are required, and tail clip commonly causes hemolysis Christensen et al. Moreover, glucometers usually have a maximal reading of ~33 mM for blood Pereira et al.
The accuracy of glucometers when measuring glucose in rodent blood has been compared to results obtained with a glucose assay kit or glucose analyzer Togashi et al.
The difference in plasma glucose concentrations between various models of glucometers and a glucose assay kit increases as plasma glucose concentration rises Togashi et al. Furthermore, the direction of this error changes depending on whether mice have been fasted or not Togashi et al.
Therefore, a glucometer type should be used consistently in a given experiment Ayala et al. When doing GTTs and PTTs in models of diabetes, plasma glucose concentrations can be determined after completion of the tests using a glucose assay kit Pereira et al.
The HemoCue glucose analyzer, which requires ~5 μL of blood to measure plasma glucose concentration in humans, has been used to measure plasma glucose concentration during clamps in mice Nahle et al.
For clamps in rats, glucose concentrations in 5—10 μL plasma samples can be determined using glucose analyzers such as GM9 from Analox Castellani et al. Continuous glucose monitoring CGM involves invasive surgery, namely, surgical implantation of a telemetry probe in the aorta Evers et al.
Assuming surgical expertise and funds exist in a laboratory, CGM is a great way to determine circulating glucose concentrations in rats and mice over weeks without handling them Evers et al.
Unless glucose metabolism is being specifically assessed in the feeding and postprandial states, all in vivo glucose metabolism tests are done in the fasting postabsorptive state to avoid the confounding effect of altered concentrations of hormones and nutrients associated with food consumption.
The length of fast depends on the species, rodent model, and metabolic test. Glucose production is the sum of glycogenolysis and gluconeogenesis, and as fasting increases, glycogen stores become depleted and the contribution of gluconeogenesis to glucose production increases Landau et al.
Overnight fasting, which typically lasts 16 h, is considered stressful in mice. An important species difference is that while a 24 h fast decreases insulin sensitivity in humans Salgin et al. Thus, shorter fasting times are usually advised in mice also for translatability Ayala et al.
The ideal length of fasting for GTTs and ITTs is an active area of investigation. Four to six hours of fasting is commonly used and advised Andrikopoulos et al. Recently, it was reported that shorter fasting 2 h is ideal when performing ITTs because hepatic glycogen content is similar to the nonfasted state Carper et al.
Genetic background of mice affects metabolic parameters such as insulin sensitivity and counterregulatory response to hypoglycemia Berglund et al. Among healthy mice, females are more insulin sensitive and have better glucose tolerance than males Macotela et al. Similarly, women are more insulin sensitive than men Tramunt et al.
This disparity is associated with sex-specific factors such as differences in circulating levels of estrogen and testosterone Macotela et al. Sex hormones also affect body composition amount and distribution of fat tissue , which is an important determinant of insulin sensitivity Elbers et al.
The enhanced insulin sensitivity associated with being female often deteriorates in insulin-resistant states Tramunt et al. Although a shift is occurring, preclinical research often only uses male rodents Willingham From a metabolic perspective, one of the reasons may be a combination of the often-increased probability of finding metabolic disturbances in males and the publication bias toward positive findings Joober et al.
Results for males and females should not be pooled Willingham and flowcharts to design experiments that examine how sex affects metabolism have been published Mauvais-Jarvis et al. Another reason why females are less studied than males is the fact that females have cyclic alterations in circulating gonadal hormones, namely, estrogen and progesterone.
Blood glucose concentrations have been found to change throughout the menstrual cycle Lin et al. It could be argued that the estrous cycle increases variance of metabolic studies; therefore, data from female rodents should be presented by estrous phase Della Torre et al.
However, the requirement to present metabolic data by estrous phase may depend on the primary parameter being investigated Mauvais-Jarvis et al. Indeed, there is evidence that in cases where estrous phases are not tracked, but sample size is sufficient, females do not show increased variance in various parameters of glucose metabolism Berglund et al.
Ultimately, it is up to each investigator to determine if, in addition to studying females, data should be presented by estrous phase. Key factors underlying this decision include the research question and cost.
Estrous cycle is not the only sex-specific factor that can modulate glucose metabolism and potentially increase variance. For example, aggression, which is associated with stress, is more prevalent in male mice Lidster et al. Moreover, the stress hormone corticosterone causes greater insulin resistance in male vs female mice Kaikaew et al.
The housing of female mice for metabolic studies usually involves nonpregnant or nonnursing females and occurs in the absence of males; such conditions favor low levels of aggression in female mice Newman et al.
Doses for GTTs, ITTs, PTTs, and clamps in rodents are usually expressed per kg of body weight. Glucose kinetics in rodents are also usually expressed per kg of body weight Berglund et al. Such approaches are appropriate when comparing experimental groups that have similar body composition, especially the amount of fat and lean fat-free mass.
Thus, the age of the rodents should be reported and matched across experimental groups. From a clinical perspective, there is a need to study metabolism throughout the life span, including menopause and andropause Kautzky-Willer et al.
There are many excellent published papers that use in vivo techniques to assess glucose metabolism in rodent models of obesity and type 2 diabetes.
We will highlight some of the key findings from three of these papers in the current review. First, hyperinsulinemic euglycemic clamps were utilized to conclude that knocking down pyruvate carboxylase in adipose tissue and liver with antisense oligonucleotides ameliorates hepatic insulin sensitivity in male HFD-fed rats, which are a model of obesity, and male Zucker diabetic fatty rats, which are a model of type 2 diabetes Kumashiro et al.
Second, tamoxifen-inducible adipocyte-specific insulin receptor and insulin growth factor 1 knockout mice were generated and characterized Sakaguchi et al. Two days following tamoxifen administration, male double knockout mice were hyperglycemic, glucose intolerant based on OGTT results and insulin resistant based on ITT as well as HOMA-IR results.
Moreover, HOMA-IR was used to track insulin sensitivity over time, and it was found that insulin sensitivity was similar between male double knockout and control mice by 30 days post tamoxifen administration.
Third, male and female mice lacking complement factor 5, which is part of the innate immune system, were placed on an HFD and studied Winn et al. Using IPGTTs, it was concluded that in the context of HFD-induced obesity, knocking out complement factor 5 only affects deteriorates glucose tolerance in male mice.
Hence, these examples support the importance of in vivo techniques for assessment of glucose metabolism in understanding the mechanisms of obesity, type 2 diabetes, and insulin resistance as well as new treatments for metabolic disorders.
Methods of in vivo tests of glucose metabolism should be detailed for readers to repeat experiments and to understand the context of results. Preclinical research is valuable if it is translatable to humans Drucker ; therefore, one must frequently question and answer how metabolic tests in rodents relate to human physiology.
In this review, we have described the commonly used techniques for the assessment of insulin sensitivity and glucose metabolism in rodents. We have also highlighted the pros and cons of each technique.
Furthermore, we have discussed key factors that can affect glucose metabolism, such as fasting duration and sex of the rodents. Other factors that may cause stress and alter glucose metabolism have begun to be described in the literature, such as temperature, method of cage change, and even the sex of the scientist Sorge et al.
Regarding the latter, stress responses in mice vary depending on the sex of the investigator handling the mice and merely because men and women give off different scents Sorge et al. It will be interesting to determine how additional factors such as these can be utilized to further optimize metabolic tests in rodents in the future.
The guest editors for this collection were Matthias Blüher, Stefan Bornstein, and Martin Haluzík. Grants from the Banting and Best Diabetes Centre BBDC , Canadian Institutes of Health Research CIHR , and PSI Foundation were awarded to MKH.
MKH also has support from an Academic Scholars Award from the Department of Psychiatry, University of Toronto, and holds the Kelly and Michael Meighen Chair in Psychosis Prevention. Journal of the American Association for Laboratory Animal Science 47 8 — reduced glucose effectiveness in glucose-intolerant mice.
American Journal of Physiology E — E American Journal of Physiology R — R Fertility Research and Practice 6 5. Diabetologia 61 — Al-Share QY , DeAngelis AM , Lester SG , Bowman TA , Ramakrishnan SK , Abdallah SL , Russo L , Patel PR , Kaw MK , Raphael CK , et al. Diabetes 64 — American Diabetes Association Diagnosis and classification of diabetes mellitus.
Diabetes Care 37 Supplement 1 S81 — S Endocrinology — Diabetes 55 — Disease Models and Mechanisms 3 — Nature Metabolism 4 — Diabetes 54 — Diabetes, Metabolic Syndrome and Obesity 13 — Diabetes 57 — Berglund ED , Vianna CR , Donato J Jr , Kim MH , Chuang JC , Lee CE , Lauzon DA , Lin P , Brule LJ , Scott MM , et al.
Journal of Clinical Investigation — Diabetes Care 19 — Journal of Clinical Investigation 93 — Molecular Metabolism 53 Bruin JE , Saber N , Braun N , Fox JK , Mojibian M , Asadi A , Drohan C , O'Dwyer S , Rosman-Balzer DS , Swiss VA , et al. Stem Cell Reports 4 — American Journal of Physiology E53 — E Cell Metabolism 17 — Carey M , Lontchi-Yimagou E , Mitchell W , Reda S , Zhang K , Kehlenbrink S , Koppaka S , Maginley SR , Aleksic S , Bhansali S , et al.
Diabetes 69 — Molecular Metabolism 42 Castellani LN , Pereira S , Kowalchuk C , Asgariroozbehani R , Singh R , Wu S , Hamel L , Alganem K , Ryan WG , Zhang X , et al.
Molecular Psychiatry 27 — American Journal of Physiology E7 — E Laboratory Animals 43 65 — Journal of Neurochemistry 33 — Diabetes 32 35 — Della Torre S , Mitro N , Fontana R , Gomaraschi M , Favari E , Recordati C , Lolli F , Quagliarini F , Meda C , Ohlsson C , et al.
Cell Reports 15 — Drucker DJ Never waste a good crisis: confronting reproducibility in translational research. Cell Metabolism 24 — Nature Communications 11 Erener S , Ellis CE , Ramzy A , Glavas MM , O'Dwyer S , Pereira S , Wang T , Pang J , Bruin JE , Riedel MJ , et al.
Cell Reports 2 Evers SS , Kim KS , Bozadjieva N , Lewis AG , Farris D , Sorensen MJ , Kim Y , Whitesall SE , Kennedy RT , Michele DE , et al. Molecular Metabolism 32 — Metabolism: Clinical and Experimental 63 — Ferreira DS , Amaral FG , Mesquita CC , Barbosa AP , Lellis-Santos C , Turati AO , Santos LR , Sollon CS , Gomes PR , Faria JA , et al.
PLoS One 7 e Journal of Pharmacological and Toxicological Methods 57 — Nutrition and Metabolism 13 Georgiou P , Zanos P , Mou TM , An X , Gerhard DM , Dryanovski DI , Potter LE , Highland JN , Jenne CE , Stewart BW , et al.
Nature Neuroscience 25 — Trends in Endocrinology and Metabolism 33 — Journal of Lipid Research 46 — Henderson DC , Cagliero E , Copeland PM , Borba CP , Evins AE , Hayden D , Weber MT , Anderson EJ , Allison DB , Daley TB , et al.
Archives of General Psychiatry 62 19 — Endocrine Reviews 34 — Mammalian Genome 25 — Diabetes 59 — NMR in Biomedicine 33 e Journal of Psychiatry and Neuroscience 37 — Diabetologia 60 — Obesity 20 — Diabetic Medicine 38 e Diabetes, Obesity and Metabolism 24 — Journal of Psychiatry and Neuroscience 42 — Kumashiro N , Beddow SA , Vatner DF , Majumdar SK , Cantley JL , Guebre-Egziabher F , Fat I , Guigni B , Jurczak MJ , Birkenfeld AL , et al.
Diabetes 62 — Journal of Endocrinology — Science — Journal of Clinical Investigation 98 — Scientific Reports 9 npj Digital Medicine 6 Diabetes 58 — Cell Metabolism 25 — Meneses MJ , Patarrão RS , Pinheiro T , Coelho I , Carriço N , Marques AC , Romão A , Nabais J , Fortunato E , Raposo JF , et al.
European Journal of Clinical Investigation 53 e Journal of the American Association for Laboratory Animal Science 56 — Journal of the American Association for Laboratory Animal Science 57 44 — Diabetes 60 — Nahle A , Joseph YD , Pereira S , Mori Y , Poon F , Ghadieh HE , Ivovic A , Desai T , Ghanem SS , Asalla S , et al.
International Journal of Molecular Sciences 22 Annual Review of Physiology 85 — Biological Psychiatry 86 — Magnetic Resonance in Medicine 62 — Hormone and Metabolic Research 13 — In Insulin Resistance and Cancer: Epidemiology, Cellular and Molecular Mechanisms and Clinical Implications.
Fantus IG Ed. New York, NY, USA : Springer , pp. Diabetes 57 56 — Pereira S , Yu WQ , Frigolet ME , Beaudry JL , Shpilberg Y , Park E , Dirlea C , Nyomba BL , Riddell MC , Fantus IG , et al.
Journal of Endocrinology 31 — Pereira S , Park E , Mori Y , Haber CA , Han P , Uchida T , Stavar L , Oprescu AI , Koulajian K , Ivovic A , et al. American Journal of Physiology E34 — E Pereira S , O'Dwyer SM , Webber TD , Baker RK , So V , Ellis CE , Yoon JS , Mojibian M , Glavas MM , Karunakaran S , et al.
Pereira S , Cline DL , Chan M , Chai K , Yoon JS , O'Dwyer SM , Ellis CE , Glavas MM , Webber TD , Baker RK , et al. Scientific Reports 11 Perry RJ , Camporez JG , Kursawe R , Titchenell PM , Zhang D , Perry CJ , Jurczak MJ , Abudukadier A , Han MS , Zhang XM , et al.
Cell — Journal of Diabetes Investigation 7 Supplement 1 13 — Molecular Metabolism 77 Sakaguchi M , Fujisaka S , Cai W , Winnay JN , Konishi M , O'Neill BT , Li M , García-Martín R , Takahashi H , Hu J , et al.
Schertzer JD , Tamrakar AK , Magalhães JG , Pereira S , Bilan PJ , Fullerton MD , Liu Z , Steinberg GR , Giacca A , Philpott DJ , et al.
Nature 59 — Small L , Ehrlich A , Iversen J , Ashcroft SP , Trošt K , Moritz T , Hartmann B , Holst JJ , Treebak JT , Zierath JR , et al. However, a more recent review from suggested that a low carbohydrate diet might actually increase insulin resistance, especially if a person is not losing body weight while following the diet.
Although this fiber is a type of carbohydrate, the body cannot break it down fully. As a result, it does not contribute to spikes in blood glucose levels. Soluble fiber also delays gastric emptying, which is the time it takes for a meal to leave the stomach and enter the small intestine.
A small study suggests that this delay may help decrease blood glucose levels after meals in people with type 2 diabetes. Intermittent fasting is a type of diet that focuses on the timing of meals rather than the specific foods in the diet.
It may improve insulin sensitivity and reduce the risk of type 2 diabetes for certain people. A review investigated the effects of two methods of intermittent fasting in overweight and obese adults. The first involved restricting calorie intake for 1—3 days per week and eating freely on the remaining days.
As with a daily calorie-restricted diet, the researchers found that both types of intermittent fasting reduced insulin resistance.
However, this type of eating had no meaningful effect on blood glucose levels, so the authors concluded that more research is necessary. In addition to changing the foods in their diet, people looking to increase their insulin sensitivity may benefit from taking dietary supplements.
Taking probiotics or omega-3 fatty acid supplements may improve insulin sensitivity in people who are overweight. A clinical trial investigated the effects of both omega-3 fatty acids and probiotics on insulin sensitivity in 60 adults who were overweight but otherwise healthy.
The researchers reported that taking either a probiotic or omega-3 supplement for 6 weeks led to significant improvements in insulin sensitivity in comparison with a placebo. The increase in insulin sensitivity was even greater in those who took both supplements together.
Learn everything you need to know about probiotics. Magnesium supplements may also be beneficial for people wanting to improve their insulin sensitivity. A systematic review found that taking magnesium supplements for more than 4 months significantly improved insulin resistance in people with and without diabetes.
Read more about magnesium glycinate, a popular supplement. Resveratrol is a natural compound that occurs in the skin of red grapes. It is also available as a dietary supplement. A meta-analysis of 11 studies found that taking resveratrol supplements significantly improved glucose control and insulin sensitivity in people with diabetes.
However, the researchers did not observe the same effects in people without diabetes. They concluded that there is a need for more research on the effects of resveratrol supplementation in humans. Low insulin sensitivity is a risk factor for developing type 2 diabetes.
Exercising well, getting enough sleep, and eating a nutritious diet high in unsaturated fats and soluble fiber may help improve insulin sensitivity in people with and without diabetes. Certain dietary supplements may also be beneficial. Many of these supplements are available to purchase online:.
However, a person should be aware that the Food and Drug Administration FDA does not regulate supplements. Therefore, they should speak with their doctor before taking any supplement.
Individuals can discover more resources for living with type 2 diabetes by downloading the free T2D Healthline app. It provides access to expert content and peer support through one-on-one conversations and live group discussions. Download the app for iPhone or Android.
Find out here about the differences and…. Many people avoid eating carbohydrates to help them lose weight. However, some carbohydrates are beneficial and can be healthful when included in the…. Researchers say gastric bypass surgery is more effective than gastric sleeve procedures in helping people go into remission from type 2 diabetes.
A study in mice suggests a potential mechanism that could explain why only some individuals with obesity develop type 2 diabetes.
A type of medication used to treat type 2 diabetes could help lower the risk of developing kidney stones, a new study suggests. My podcast changed me Can 'biological race' explain disparities in health?
Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us.
Medical News Today. Health Conditions Health Products Discover Tools Connect. Medically reviewed by Soo Rhee, MD — By Charlotte Lillis — Updated on January 17, Exercise Sleep Diet Supplements Takeaway.
How we vet brands and products Medical News Today only shows you brands and products that we stand behind. Our team thoroughly researches and evaluates the recommendations we make on our site. To establish that the product manufacturers addressed safety and efficacy standards, we: Evaluate ingredients and composition: Do they have the potential to cause harm?
Some dietary and lifestyle gluxose can Fitness inspiration and motivation prevent insulin Insulin sensitivity and glucose metabolism. Insulin resistance, a condition in which your cells metaboilsm responding Youth sports performance to seneitivity, is incredibly common. In fact, the prevalence of insulin resistance is However, certain dietary and lifestyle habits can dramatically improve or help prevent this condition. Insulin is a hormone that your pancreas secretes. It regulates the amounts of nutrients circulating in your bloodstream 2.
Ich meine, dass Sie sich irren. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Sie sind nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden besprechen.
Die Kleinigkeiten!