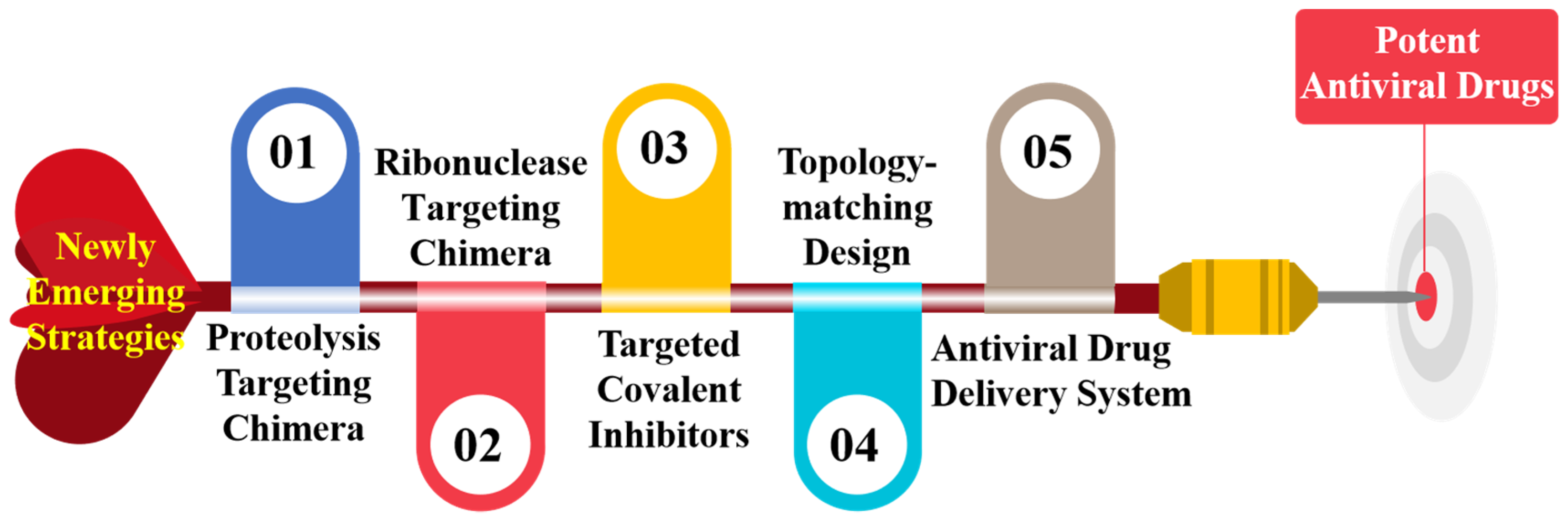
Antiviral technology -
Six licensed prescription influenza antiviral drugs are approved in the United States. Four influenza antiviral medications approved by the U. Food and Drug Administration FDA are recommended for use in the United States. Three drugs are chemically related antiviral medications known as neuraminidase inhibitors that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses: oral oseltamivir phosphate available as a generic version or under the trade name Tamiflu® , inhaled zanamivir trade name Relenza® , and intravenous peramivir trade name Rapivab®.
The fourth drug is oral baloxavir marboxil trade name Xofluza® , which is active against both influenza A and B viruses but has a different mechanism of action than neuraminidase inhibitors. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication.
More information regarding the four recommended antiviral medications is available: Table 1. The two other FDA-approved influenza antiviral medications amantadine and rimantadine are not recommended for antiviral treatment or chemoprophylaxis because of high levels of resistance among circulating influenza A viruses.
Clinical trials and observational data show that early antiviral treatment can shorten the duration of fever and illness symptoms, and may reduce the risk of some complications from influenza e.
In hospitalized adults with influenza, early treatment with oseltamivir has been reported to reduce in-hospital death and the duration of hospitalization in some observational studies.
In hospitalized children , early antiviral treatment with oseltamivir has been reported to shorten the duration of hospitalization in some observational studies. Clinical benefit is greatest when antiviral treatment is administered early, especially within 48 hours of influenza illness onset.
Table 1: Antiviral Medications Recommended for Treatment and Chemoprophylaxis of Influenza Table 1. Post marketing reports of serious skin reactions and sporadic, transient neuropsychiatric events 2 Chemo- prophylaxis 5 yrs and older 3 People with underlying respiratory disease e.
Oral oseltamivir phosphate is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 14 days and older, and for chemoprophylaxis in people 1 year and older.
Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by the CDC and the American Academy of Pediatrics.
If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical due to limited data in this age group. Self-injury or delirium; mainly reported among Japanese pediatric patients. Inhaled zanamivir is contraindicated in patients with underlying airways disease such as asthma or chronic obstructive pulmonary disease, and those with a history of allergy to lactose or milk protein.
Intravenous peramivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 6 months and older. Peramivir efficacy is based on clinical trials versus placebo in which the predominant influenza virus type was influenza A; in one trial, a very limited number of subjects infected with influenza B virus were enrolled.
There are no data available for use of peramivir for chemoprophylaxis of influenza. Baloxavir efficacy for initial FDA approval in October was based on clinical trials in previously healthy outpatients 12 to 64 years old Hayden, Singledose baloxavir t reatment was superior to placebo and had similar clinical efficacy in time to alleviation of symptoms to a 5-day treatment course of oseltamivir.
Oral oseltamivir is preferred for treatment of pregnant people Rasmussen , Pregnant people are recommended to receive the same antiviral dosing as non-pregnant people.
Multiple studies have reported safe use of neuraminidase inhibitors during pregnancy Dunstan, ; Xie, ; Saito, ; Wollenhaupt, ; Beau, ; Svensson, ; Greer, ; Graner, ; Ehrenstien, ; Chambers, ; Bennekom, ; ACOG Committee, See Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for additional information.
Baloxavir is not recommended for the treatment of influenza in pregnant or breastfeeding people, as there are no available efficacy or safety data for baloxavir in this pregnant people Chow, , and no available data on the presence of baloxavir in human milk, the effects on the breastfed infant, or the effects on milk production.
CDC does not recommend use of baloxavir for monotherapy of influenza in severely immunosuppressed persons.
There are no available efficacy, safety, or resistance data for baloxavir monotherapy of influenza in severely immunosuppressed patients and emergence of resistance during treatment is a concern because of prolonged influenza viral replication in these patients.
When indicated , antiviral treatment should be started as soon as possible after illness onset , ideally within 48 hours of symptom onset for the greatest clinical benefit.
However, observational studies have reported that antiviral treatment of influenza can have clinical benefit in patients with severe, complicated or progressive illness, and in hospitalized patients when started after 48 hours of illness onset. Decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza see resources regarding Clinical Description and Lab Diagnosis of Influenza for more information on influenza diagnostic testing.
Clinical benefit is greatest when antiviral treatment is started as close to illness onset as possible. Antiviral treatment with oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir also can be considered for any previously healthy, symptomatic outpatient not at higher risk for influenza complications, who is diagnosed with confirmed or suspected influenza, on the basis of clinical judgment, if treatment can be initiated within 48 hours of illness onset.
The recommended treatment course for uncomplicated influenza is two doses per day of oral oseltamivir or inhaled zanamivir for 5 days, or one dose of intravenous peramivir or oral baloxavir for 1 day.
While influenza vaccination is the best way to prevent influenza illness, a history of influenza vaccination does not rule out the possibility of influenza virus infection in an ill patient with clinical signs and symptoms compatible with influenza.
Figure: Guide for considering influenza testing and treatment when influenza viruses are circulating in the community regardless of influenza vaccination history 1 Complete footnotes for this algorithm are available. Early antiviral treatment of influenza should be considered for outpatients in these racial and ethnic minority groups 1 Although all children younger than 5 years old are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 years old, with the highest hospitalization and death rates among infants younger than 6 months old.
Treatment Considerations for Patients Hospitalized with Suspected or Confirmed Influenza The following recommendations do not necessarily represent FDA-approved uses of antiviral products but are based on published observational studies and expert opinion and are subject to change as the developmental status of investigational products and the epidemiologic and virologic features of influenza change over time.
For hospitalized patients with suspected or confirmed influenza, initiation of antiviral treatment with oral or enterically administered oseltamivir is recommended as soon as possible. Antiviral treatment might be effective in reducing morbidity and mortality in hospitalized influenza patients, especially adults, even if treatment is started more than 48 hours after onset of illness.
Inhaled zanamivir, oral baloxavir, and intravenous peramivir are not recommended routinely for hospitalized patients with suspected or confirmed influenza because of insufficient data on use of these antivirals showing clinical benefit in hospitalized influenza patients.
There are also insufficient data for treatment of hospitalized influenza patients with intravenous peramivir. The optimal duration and dosing of antiviral treatment are uncertain for severe or complicated influenza. Treatment regimens might need to be altered to fit the clinical circumstances.
Decisions about extended longer duration of treatment should be guided by clinical judgment in patients whose illness is prolonged. Virologic testing of lower respiratory tract specimens by real-time reverse transcription-polymerase chain reaction RT-PCR can help guide decisions about extended treatment in hospitalized influenza patients with severe and prolonged illness.
Critically ill patients with respiratory failure can have prolonged influenza viral replication in the lower respiratory tract and might benefit from longer duration of treatment.
Longer treatment regimens might be necessary in immunocompromised patients who may have prolonged influenza viral replication. Such patients are at risk of emergence of influenza viruses with reduced susceptibility or antiviral resistance during or after antiviral treatment.
A higher dose of oral or enterically administered oseltamivir has been recommended by some experts e. However, oral or enterically administered oseltamivir at standard doses has been reported to be adequately absorbed in critically ill adults to therapeutic blood levels Ariano, , and available data suggest that higher dosing may not provide additional clinical benefit Abdel-Ghafar, ; Ariano, ; Kumar, ; Lee, ; South East Asia Infectious Disease Clinical Research Network, Studies indicate that exposure to oseltamivir carboxylate the active metabolite of oseltamivir is similar between obese and non-obese subjects for both 75 mg and mg doses given twice daily Ariano, ; Jittamala, ; Pai, ; Thorne-Humphrey, If a hospitalized patient treated with oseltamivir or peramivir manifests progressive lower respiratory symptoms, resistant virus should be considered.
However, clinicians should note that failure to improve or clinical deterioration during oseltamivir or peramivir treatment is more likely to be related to the natural history of acute lung injury and inflammatory damage or onset of other complications e. Careful attention to ventilator and fluid management and to the prevention and treatment of secondary bacterial pneumonia e.
pneumoniae , S. pyogenes , and S. aureus , including MRSA also is critical for severely ill patients Bautista, ; Finelli, ; Hageman, ; Harper, ; Mandell, ; Mauad, ; Shieh, Table 2.
Recommended Dosage and Duration of Influenza Antiviral Medications for Treatment or Chemoprophylaxis Antiviral Agent. Antiviral Agent. Oral Oseltamivir. Treatment 5 days 1. Chemoprophylaxis 7 days 5. Inhaled Zanamivir 6. Treatment 5 days.
Intravenous Peramivir 7. Treatment 1 day 1. Chemoprophylaxis 8. Not recommended. Oral Baloxavir 9. Treatment 1 day. Chemoprophylaxis 9. Dosage is the same as to treatment. Oral oseltamivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset with twice-daily dosing in people 14 days and older, and for chemoprophylaxis with once-daily dosing in people 1 year and older.
Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year of age, is recommended by CDC and the American Academy of Pediatrics Recommendations for Prevention and Control of Influenza in Children, — This is the FDA-approved oral oseltamivir treatment dose for infants 14 days and older and less than 1 year old and provides oseltamivir exposure in children similar to that achieved by the approved dose of 75 mg orally twice daily for adults, as shown in two studies of oseltamivir pharmacokinetics in children Kimberlin, [3.
The American Academy of Pediatrics has recommended an oseltamivir treatment dose of 3. It is unknown whether this higher dose will improve efficacy or prevent the development of antiviral resistance. However, there is no evidence that the 3. Current weight-based dosing recommendations are not appropriate for premature infants.
Premature infants might have slower clearance of oral oseltamivir because of immature renal function, and doses recommended for full-term infants might lead to very high drug concentrations in this age group. See Special Considerations for Institutional Settings section below for details regarding duration of chemoprophylaxis for outbreaks in institutional settings.
Daily dosing for a minimum of 5 days was used in clinical trials of hospitalized patients with influenza de Jong, , Ison, There are no data for use of peramivir for chemoprophylaxis of influenza. Baloxavir marboxil Xofluza [package insert] [ KB, 16 pages].
Baloxavir marboxil should not be administered with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids or oral supplements e.
There are no available published data from clinical trials for baloxavir treatment of influenza in non-hospitalized patients who are pregnant, immunocompromised, or have severe disease. Influenza Antiviral Resistance Considerations Antiviral resistance and reduced susceptibility to the neuraminidase inhibitors and to baloxavir among circulating influenza viruses is currently very low, but this can change.
For weekly surveillance data on susceptibility of circulating influenza viruses to antivirals in the U.
this season, see the FluView Weekly U. Influenza Surveillance Report. Influenza viruses with reduced susceptibility or resistance to antivirals can occur sporadically Hurt, ; Takashita, ; Takashita, or emerge during or after antiviral treatment in some patients e.
Oseltamivir resistance in influenza A H3N2 and A H1N1 pdm09 viruses can develop during treatment, particularly in young children Roosenhoff, ; Lina, ; , and immunocompromised persons Memoli, Influenza viruses may become less susceptible or resistant to oseltamivir and peramivir during antiviral treatment with one of these drugs and remain susceptible to zanamivir; this has been reported most often for influenza A H1N1 pdm09 viruses Graitcer, ; Lackenby, ; Memoli, ; Nguyen, ; Nguyen, Human-to-human transmission of influenza A H1N1 pdm09 viruses with an HY mutation in viral neuraminidase conferring resistance to oseltamivir has been reported among severely immunocompromised patients in hospital units, Gooskens, ; Chen, ; and in the community Hibino, ; Le, ; Hurt, ; Hurt, ; Takashita, , but currently appears to be uncommon.
Limited human-to-human transmission of influenza A H3N2 virus with reduced susceptibility to baloxavir has been reported sporadically in Japanese children Takashita, ; Takashita ; Imai, , but currently appears to be uncommon. Molecular analyses can detect genetic changes in influenza viruses associated with resistance and reduced susceptibility to oseltamivir and peramivir.
The CDC Influenza Division is available for consultation regarding antiviral susceptibility testing as needed. Information about neuraminidase inhibitor susceptibility testing and interpretation of results of neuraminidase inhibition assays is available on the WHO website.
Antiviral Treatment Efficacy and Effectiveness Patients with Uncomplicated Influenza Meta-analyses of randomized controlled clinical trials RCTs have demonstrated efficacy of early initiation of treatment started within 36 to 48 hours of illness onset with neuraminidase inhibitors in reducing duration of fever and illness symptoms compared with placebo in otherwise healthy children and adults with uncomplicated influenza Jefferson, ; Dobson, ; Malosh, ; Liu, One randomized clinical trial in children with uncomplicated influenza demonstrated a modest reduction in duration of symptoms and influenza virus shedding in patients initiating treatment after 48 hours; post hoc analysis suggested that oseltamivir treatment initiated 72 hours after illness onset reduced symptoms by one day compared with placebo Fry, A meta-analysis of RCTs comparing early treatment with oseltamivir to placebo or nonactive controls among adults and adolescents with uncomplicated influenza found no reduction in the risk of subsequent hospitalization with influenza but was underpowered to detect an effect given the very low rate of hospitalization among the trial populations Hanula ; Antoon ; Uyeki RCTs and a meta-analysis of RCTs comparing baloxavir to placebo or oseltamivir among children and adults with uncomplicated influenza found that baloxavir was superior to placebo and comparable to oseltamivir in reducing symptom duration Kuo, ; Portsmouth There are no available data on the use of baloxavir for treatment of influenza more than 2 days after illness onset in outpatients.
Hospitalized Patients No completed, sufficiently powered, randomized, placebo-controlled clinical trials have been conducted of monotherapy with neuraminidase inhibitors for treatment of influenza in hospitalized patients; studies supporting the licensure of oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir were conducted in outpatients, primarily among previously healthy persons with uncomplicated illness.
A secondary analysis of a multi-center unblinded clinical trial of oseltamivir treatment started within 24 hours of enrollment after hospital admission versus standard of care in adults hospitalized for lower respiratory tract infection reported that oseltamivir treatment lowered the risk of clinical failure in patients with laboratory-confirmed influenza; clinical failure was defined as failure to improve with 7 days, transfer to ICU care 24 hours after admission, or rehospitalization or death within 30 days Wiemken, Several observational studies in hospitalized influenza patients have shown clinical benefit of neuraminidase inhibitor antiviral treatment compared with no treatment, particularly when started within two days of illness onset, or as soon as possible after hospital admission, including reducing the duration of hospitalization, and reducing the risk of ICU transfer, invasive mechanical ventilation or risk of death Coffin, ; Hsu, ; Louie, ; Muthuri, ; Muthuri, ; Miyakawa, ; Lytras, ; Chen, ; Chen, ; Venkatesan, ; Katzen, ; Reacher, ; Walsh, Some observational studies have reported that oral oseltamivir treatment started 4 and 5 days after illness onset in patients hospitalized with suspected or confirmed influenza was associated with lower risk of death EH Lee, ; N Lee, ; N Lee, ; Louie, ; McGeer, , although one report found this benefit only in hospitalized adult patients in the ICU Muthuri, A small number of observational studies and one meta-analysis of observational studies of hospitalized influenza patients reported that neuraminidase inhibitor treatment was not associated with a reduction in risk of death Choi, ; Wolkewitz, ; Heneghan, Observational studies in hospitalized patients with influenza have reported that clinical benefit is greatest when oseltamivir is started within 48 hours of illness onset Hsu, ; Louie, ; Muthuri, ; Muthuri, However, some studies suggest that antiviral treatment might still be beneficial in hospitalized patients when started up to 4 or 5 days after illness onset Louie, ; Yu, In pregnant people, antiviral treatment in any trimester with influenza A H1N1 pdm09 virus infection has been shown to be most beneficial in preventing respiratory failure and death when started within 2 days of illness Siston, These dendrimers were found to exhibit dual activity against HIV and enterovirus 71 EV71 responsible for hand-foot-and-mouth disease prevalent among children below 6 years of age.
They found dendrimer with peripheral N-methyl tryptophan to be most potent against HIV-1 and that with tyrosine to be most active against EV71 [ ]. A nanocapsule consists of nanosized structure 50— nm having a core and a shell. The drug is confined to the inner core, surrounded by the polymeric shell.
Nanocapsules exhibit advantages of high drug loading, controlled release, and targeted drug delivery. They are usually prepared by polymer coating, layer-by-layer method, nanoprecipitation, emulsion—diffusion, emulsion coacervation, emulsion evaporation, and double emulsification [ ].
In a study, nanocapsule consisting of poly iso -butylcyanoacrylate core, entrapping azidothymidine-triphosphate AZT-TP and polyethyleneimine which forms the shell, was designed to directly deliver AZT-TP into the cytoplasm [ ]. Nanospheres are smaller spherical structures of 10— nm diameter, where the drug is uniformly dispersed in the matrix system.
This type of nanocarrier shows enhanced size-dependent characteristics, having the capability to prevent the drug from undergoing degradation. In addition, rapid drug clearance is observed due to smaller size.
It offers site-specific delivery and the required drug release profiles. Recommended preparation techniques used are solvent evaporation, polymerization solvent displacement techniques, and phase inversion temperature methods [ ]. Chitosan nanospheres loaded with acyclovir were synthesized using modified nanoemulsion template method for topical treatment of herpes.
The spheres were of the average size of nm and showed better permeation in in vitro skin permeation studies and higher potency against HSV-1 and HSV-2 than the free acyclovir itself [ ]. Cyclodextrins CDs are cyclic oligosaccharides made of six to twelve α- d -glucopyranose monomers linked by α1—4 linkages having exceptional hydrophobic interior surfaces and hydrophilic rims with primary and secondary —OH groups.
This type of molecular construct entraps the drug in a bucket-shaped cavity, thereby increasing the solubility of the drug and protecting the drug from degradation. Due to this, CDs become preferred delivery system for drugs [ ]. The common native or parent cyclodextrins are α-CD, β-CD, and γ-CD comprising of 6, 7, and 8 glycopyranose units and molecular weights of , , and Da, respectively [ ].
These CDs have a homogeneous crystalline structure offering numerous advantages like their unique ability to interact with a range of organic and inorganic lipophilic molecules and form inclusion complexes. But their use is restricted due to low solubility of the CDs. Thus, alteration in CDs is being made to make them more suitable for their application in the pharmaceutical industry [ ].
β-CD and its derivatives are more widely used than α-CD and γ-CD because of their safety and ease of production. The structural framework of β-CD is attractive with a height of — pm, external diameter of pm, internal diameter of — pm, and cavity volume of — Å 3 [ ]. These dimensions make the most ideal hosts for the formation of inclusion complexes.
Chemical and enzymatic modifications of macrocycle l in CD derivatives that self-assemble in aqueous solutions provide different shapes of supramolecular nano-assemblies vesicles, micelles, nanorods, nanospheres, and other kinds of nanoparticles and liquid crystalline structures of 30— nm in size depending on the concentration which are very useful for different types of nanodelivery systems [ ].
One of the common problems encountered with antiviral drugs is their poor bioavailability. A similar problem was found with the drug saquanavir. Couvreur and Vauthier formulated CDs loaded with saquanavir using poly alkylcyanoacrylate to deal with the issue.
This improved solubility in water by fold. Also, it was speculated that saquanavir could now bypass the efflux mechanism of P-gp, preventing its resistance [ ]. Similarly, in another study, acyclovir was loaded with CDs using the copolymer such as Eudragit RLPO®, and on evaluation, it was found that intracellular uptake of the drug increased and it had sustained drug release over a period of 24 h [ ].
Thus, by utilizing other drugs, CDs with different polymers can be formulated to increase the intracellular concentration of the drug. Several peptides from natural includes plants, arthropod venoms, amphibian skin, mammalian tissues and microbial sources includes bacteria, algae, and fungi have been known to possess broad-spectrum antiviral activities.
Various mechanisms, either targeting the virus or the host cells have been proposed for their antiviral activities. Some examples include magainin 1 and 2, dermaseptin S4 and temporin B from frog skin , clavanin from marine source , latarcin, protegrin from swine WBCs , cyclotides from plants , cecropin from moth , defensins and cathelicidins from mammals , and poly-γ-glutamic acid from bacteria exhibiting potent activity against various viruses like HIV, H1N1, DENV, and HCV [ , ].
In addition, certain peptides, rationally designed and synthesized depending on the structure of the viral protein and its interaction with the host cell protein, have shown great potential as antiviral agents.
Enfuvirtide is the first peptide antiviral drug approved against HIV [ ]. Despite various advantages, various hurdles in their production, shorter half-life, and poor bioavailability have limited their use as antiviral agents.
Nanotechnology-based solutions have been explored for the delivery of antiviral peptides. Peptide—nanoparticle conjugate systems have been extensively studied. Emileh et al. have reported gold nanoparticle—peptide triazole conjugates to be active against HIV-1 by disrupting the interactions between host receptor proteins and trimeric envelope spike glycoprotein of virus [ ].
Recently, Alghrair et al. functionalized silver and gold nanoparticles with an antiviral peptide FluPep and reported increased antiviral potency against influenza A virus [ ]. Table 3 compiles a list of polymer-based nanoformulations for antiviral treatment.
Carbon-based nanoformulations are comprise of carbon nanotubes, graphene oxide nanoparticles, and fullerenes. Carbon nanotubes CNTs are cylindrical-shaped hollow nanomaterials, viewed as tubes made by rolling up of planar graphene sheets.
They can be viewed as coming from the rolling up of a graphene sheet, named as single-walled carbon nanotubes SWCNTs , or a series of concentric rolled-up graphene sheets termed as multi-walled carbon nanotubes MWCNTs [ ]. The cylindrical structure is capped with fullerene sheets on one end or both ends.
The sp 2 -hybridized carbon atoms in graphene sheets impart a unique strength to CNTs. In addition, they display other unique characteristics like high aspect ratio, high surface area, cell penetration capacity, and ultralight weight [ ].
The chemical vapor deposition CVD technique, laser-ablation technique, and electric arc-discharge techniques are commonly employed for the preparation of carbon nanotubes [ ].
Though CNTs are widely explored for delivering chemotherapeutic agents at the target site, their overall application in the biomedical field is limited due to pulmonary toxicity and high hydrophobicity [ ].
The proposed mechanisms for toxicity are uptaken by macrophages with subsequent generation of ROS and inflammatory mediators. However, functionalized CNTs have shown decreased toxicity and increased biodegradability.
CNTs can be decorated with peptides, carbohydrates, and polymers and can be used for targeted therapy, when needed [ ]. In one study, Kumar et al. stated about protoporphyrin IX PPIX -conjugated multi-walled nanotubes MWNTs and its ability to treat influenza using photodynamic therapy.
It was found that in the presence of visible light, PPIX-MWNT may indulge in mechanisms like RNA strand breakage, protein oxidation, or protein—RNA cross-linking caused by reactive oxygen species singlet oxygen and superoxide anion leading to inactivation of the influenza viral strain.
Probing into the inactivation mechanism of carbon nanotubes, they concluded that PPIX-MWNTs can be used for treating any viral infection as it displays nonspecificity in treating viral diseases. Also, PPIX-MWNT can be easily recovered through filtration and reused.
Due to its multitarget mechanisms of antiviral action, it was proposed that PPIX-MWNTs have less chances of development of drug resistance [ ]. Nanostructures have shown antiviral effect in respiratory syncytial virus, a virus causing severe bronchitis and asthma.
The treatment is generally done by combining nanoparticles and gene-silencing technologies. In a novel approach, MWCNTs were functionalized with recombinant dengue virus 3 envelop proteins. This induced significant immune responses in mice [ ].
Similarly, conjugation of functionalized CNTs with B and T cell peptide epitopes could generate a multivalent system that was able to induce a strong immune response; thus, CNTs were considered to be good candidates for vaccine delivery [ ]. Further, functionalized CNTs were used for the transport of peptides such as foot-and-mouth virus peptide for vaccination [ ].
One of the most promising carbon-based nanomaterials with great potential for antiviral application is graphene. Graphene G is a two-dimensional 2-D planar sheet of hexagonally arranged sp 2 -hybridized carbon atoms obtained from its three-dimensional 3-D material of graphite [ ].
It is chemically oxidized to graphene oxide GO to acquire oxygen bearing functional groups like hydroxyl, epoxide, and carboxylic acids [ ].
Graphene-based nanomaterials GBNs have high surface area, high loading capacity, and superior mechanical strength which make them attractive candidates for carrying antiviral agents [ ]. The oxygen-containing functional groups allow surface functionalization and conjugation strategies and show biocompatibility, reduced toxicity, and good dispersibility [ ].
The amphiphilicity of GO makes the incorporation of hydrophilic as well as hydrophobic moieties possible [ ]. In addition, these functional groups also provide attachment sites for various biological molecules like proteins, DNA, and RNA [ ].
Recently, Pokhrel et al. studied the interactions between graphene and VP40 viral matrix protein of Ebola virus using molecular dynamics simulations and graphene pelleting assay.
Graphene was found to interact strongly with VP at various interfaces crucial for the formation of the viral matrix. They proposed the use of graphene-based nanoparticle solutions as disinfectant to prevent the Ebola epidemic [ ]. In another study, 18 sulfonated magnetic nanoparticles were anchored onto reduced graphene oxide SMRGO sheets and used to trap and destroy HSV-1 photothermally, upon their irradiation with near-infrared light.
It was found to be effective against 28 viral infections including HSV. It was found that SMRGO has higher entrapment efficiencies in comparison to magnetic nanoparticles due to increased entrapping efficiency, larger surface area, unique sheet-like structure, and outstanding photothermal characteristics shown by graphene [ ].
Fullerenes are among the first discoveries in symmetric carbon nanostructures and have received considerable attention in the case of antiviral research. Fullerenes are comprised fully of carbon atoms forming a nanosized caged hollow sphere.
Buckminster fullerene C60 , also known as buckyball, is the most common form of fullerenes with 60 carbon atoms arranged in a spherical structure showing high symmetry [ ].
Due to their unique architecture, immense scope of derivatization, free radical scavenging activity, and low toxicity, they are widely studied for drug delivery and antimicrobial and antiviral activities [ ]. Fullerenes were found to fit inside the hydrophobic cavity of HIV proteases and inhibit HIV replication [ ].
Structure—activity relationship studies revealed trans-position of substitutions and positive charge near the cage to be important for antiviral activity. Fulleropyrolidines with two ammonium groups have been shown to be active against HIV-1 and HIV-2 [ ]. Also, fullerene C60 derivatized with two or more solubilizing side chains has been active when tested in CEM culture cells infected with HIV-1 and HIV-2 [ ].
Additionally, amino-acid derivatives of fullerene C60 are found to inhibit HIV and HCV replication [ , ]. Few studies aimed at screening fullerene derivatives for anti-influenza activity.
Shoji et al. screened 12 fullerene derivatives for in vitro PA endonuclease inhibition. PA represents the subunit of influenza A RNA polymerase which demonstrates endonuclease activity. It was found that 8 fullerene derivatives demonstrated endonuclease inhibiting potential.
In the MDCK cell culture system, these fullerene derivatives inhibited influenza A virus infection and expression of viral nucleoprotein [ ]. Few of the studies also aimed at synthesizing anti-influenza fullerenes and evaluating their effectiveness against the influenza virus. Tollas and colleagues also prepared a fullerene conjugate having a thiosialosyl-a 2,6 -galactose disaccharide and evaluated to understand the multimeric interaction of a sialocluster with influenza virus NA and HA.
Results revealed that these fullerene derivatives did not target HA but was able to target influenza NA slightly [ ]. Table 4 gives an account of various carbon-based nanoformulations for antiviral treatment.
Quantum dots QDs 2—10 nm are semiconductor nanocrystals having the shape of dots. They are comprised of a semiconductor core, overcoated by a shell, and a cap leading to improved solubility in aqueous buffers [ ].
Fundamental semiconducting character and unique optical and electronic properties are attributed to the presence of the inorganic core consisting of semiconducting materials like silicon, cadmium selenide, cadmium sulfide, or indium arsenide [ ].
Quantum dots find interesting applications in biomedical imaging due to its limited light scattering, narrow emission bands, and low tissue penetration.
Quantum dots have been widely explored as theranostic platforms for simultaneous sensing, imaging, and therapy [ ]. The advantages of quantum dots as a drug carrier system include improved bioavailability and stability of drugs, increased circulation times, active targeting, and localized therapy.
In addition to this, QDs can be surface modified with targeting ligands [ , ]. Yong et al. utilized saquinavir and transferrin Tf -conjugated quantum dots for the treatment of HIV. In vitro studies demonstrated that higher concentrations of saquinavir were able to cross the BBB by this method [ ].
Metal and metal oxide nanoparticles have been widely explored for their antiviral activity. Among the various metal nanoparticles showing high efficacy are silver and gold, and among the various metal oxides are CuO, SiO 2 , TiO 2 , and CeO 2.
These nanoparticles have shown great efficacy against a broad spectrum of viruses like influenza H3N2 and H1N1 , HBV, HSV, HIV-1, HSV, dengue virus type-2, foot-and-mouth disease virus, and vesicular stomatitis virus [ ]. Metal nanoparticles by virtue of their unique shape, size, structure, and local-field enhancement action can interact with viral surface proteins through Kazimir interaction and van der Waals forces causing its inactivation [ ].
A variety of surface functionalizations with silane or thiol groups have shown to enhance interaction with biomolecules, affecting viral internalization in cells as well as the release of the drug molecule.
Few researchers have also explored further grafting on functionalized metal nanoparticles in order to enhance their efficacy and selectivity [ ]. Gold nanoparticles AuNPs are colloids of nanosized particles of gold.
AuNPs show special optical properties in the presence of light. When AuNP comes in contact with light, the oscillating electromagnetic field of light triggers coherent oscillation of the free gold electrons [ ].
This electron oscillation about the particle surface is responsible for a charge separation with regard to the ionic lattice, leading to a dipole oscillation in the path of the electric field of light. However, when the amplitude of the oscillation becomes maximum at a particular frequency, photons get confined to a small particle size and leads to a special phenomenon known as surface plasmon resonance SPR [ ].
This SPR boosts all the radiative and nonradiative characteristics of the nanoparticles and, thus, has extensive application in areas of biological imaging, electronics, and materials science [ ].
In , Bayo et al. demonstrated that AuNP could enter the cells of various types like lymphocytes, macrophages, and brain microendothelial cells where HIV is known to replicate. Further, they modified raltegravir RAL by introducing a thiol group that served as a linker between RAL and AuNP.
Surprisingly, when the concentration of RAL was increased with the expectation of displaying high antiviral activity, it was found that anti-HIV activity was impaired. The experiment showed positive results when a low concentration of RAL was loaded into AuNP.
The authors also performed an experiment using free AuNP and the results proved that it does not have any antiviral activity. So it was just a low concentration of RAL-loaded AuNP which turns it into an active compound having inhibitory action against HIV [ ].
Small interfering RNAs siRNAs which can target particular viral gene can be employed in the treatment of dengue. However, siRNAs are prone to degradation by serum nucleases and to rapid elimination on account of its small size and anionic character.
Paul et al. conjugated siRNAs with AuNPs and found that the complex had enhanced stability and could reduce dengue virus replication and the release of infectious virion in both pre- and post-infection conditions [ ].
A breakthrough in the arena of gold nanoparticles was the design and synthesis of long and flexible linkers which mimicked heparan sulfate proteoglycans HSPG , a target for viral attachment ligand.
This allowed effective attachment of the virus to HSPG, generating strong forces that eventually lead to viral deformation.
The mechanism was proposed by researchers on the basis of molecular dynamics simulations, electron microscopy images, and virucidal assays. These nanoparticles were nontoxic and effective against a broad range of viruses like HSV, human papilloma virus, respiratory syncytial virus RSV , dengue, and lentivirus.
Additionally, they were found to be active ex vivo in human cervicovaginal histocultures infected by HSV-2 and in vivo in mice infected with RSV [ ]. Recently, Halder et al. synthesized highly monodispersed gold nanoparticles, stabilized by gallic acid.
Reduction of AuCl 4 using gallic acid was carried out using ultrasound-induced sonication which produced spherical nanoparticles in the range of 7—8 nm. These nanoparticles selectively inhibited HSV with EC50 of Prevention of viral cell attachment and penetration was proposed as a mechanism of action of these nanoparticles [ ].
Silver metal possesses intrinsic antimicrobial activity due to its ability to interact with respiratory chain and electron transport chain enzymes and bacterial DNA. It has been extensively studied since ancient times for fighting infections [ ]. Silver nanoparticles, on account of its small size and enormous surface area, facilitate rapid dissolution and have shown promising activity against a wide spectrum of viruses [ ].
They demonstrate less chances of development of resistance on account of multiplicity of targets they act upon [ ]. Nanoparticles of silver possess their own unique properties with regard to chemical stability, catalytic activity, high conductivity, and localized surface plasma resonance enabling researchers to envisage the implication of using AgNPs in disease therapeutics [ ].
Galdiero et al. were the first authors to describe the antiviral activity of silver nanoparticles against HIV They studied AgNP with three different surface functionalities: foamy carbon—coated AgNPs, poly N-vinylpyrrolidone PVP —coated AgNPs synthesized using glycerol as a reducing agent, and BSA-conjugated AgNPs.
They proposed that the interaction between these NPs and the HIV viral surface glycoprotein gp was size dependent, as only the NPs in the size range of 1—10 nm were able to bind to the virus. BSA- and PVP-coated nanoparticles demonstrated lower inhibitory activity than silver nanoparticles released from the carbon matrix in in vitro assays on laboratory-adapted HIV-1 strain.
AgNPs were shown to block the gp—CD4 interaction. Additionally, they also were proved to inhibit the post-entry stages of infection by complexing with S and O of thiols and phosphates on amino acids and nucleic acid or directly bind to RNA or DNA, thus reducing the rate of reverse transcription [ ].
Baram Pinto et al. synthesized mercaptoethane sulfonate MES —functionalized Au and AgNPs against HSV with a strategy to mimic heparan sulfate, present on the cell surface so that they compete for binding of the virus onto the cell.
They inhibited HSV-1 infections by blocking the attachment and entry of the virus into the cell [ ]. AgNPs have been investigated for their activity against H1N1 influenza virus. Xiang et al. prepared and studied the activity of AgNP on H1N1 influenza A virus—induced apoptosis in MDCK cells [ ].
With the help of hemagglutination inhibition test, they found that AgNPs reduced or completely inhibited agglutination of RBCs. During the MTT assay, they found that the antiviral activity of AgNPs continued for a prolonged period. Surface-decorated AgNPs were explored by Li et al.
They proposed interference with ROS-mediated signaling pathway as a mechanism of anti-H1N1 activity [ ]. Similar studies were carried out by the same research group on AgNPs decorated with amantadine [ ]. Moreover, it was seen that reactive oxygen species ROS are formed either on the surface of AgNPs or via release of free silver ions under specific conditions.
This ROS induces the cell death of either microbial cells or mammalian cells, depicting a unique antibacterial and antifungal attribute of AgNPs [ ]. Similarly, another study highlighted the use of gold and silver nanoparticles for effective delivery of antiviral peptide FluPep.
Its conjugation with the noble metal nanoparticles enhanced the solubility as well as its antiviral activity.
The authors proposed Ag and AuNPs as an effective strategy for delivering the therapeutic peptide [ ]. In another study, modification of silver nanoparticles with tannic acid was able to reduce HSV-2 infections and inflammation in vitro and in vivo.
The authors proposed binding of tannic acid with glycoproteins present on the surface of infectious virions as a mechanism for prevention of entry and spread to host cells. The anti-HSV-2 activity of tannic acid—modified AgNP TAAgNP was more profound than tannic acid alone, and the direct interaction between tannic acid—modified AgNP with virions was essential for the activity.
Pretreatment of host cells with TAAgNPs did not inhibit the entry of the virus into the host cells. Antione et al. designed and synthesized zinc oxide tetrapod nanoparticles ZOTEN with engineered oxygen vacancies using the flame transport synthesis approach. This was achieved by burning mixture of zinc particles, polyvinyl butyrol particles, and ethanol in a furnace at °C, where Zn in vapor form combines with available oxygen resulting in uniform nucleation and growth of the same and forming tetrapod-like structures.
Nano-immunotherapy is one more way in which HSV-2 can be treated. The activity of ZOTEN was tested in female mice. It was seen that clinical signs of vaginal infection was greatly improved with use of this therapy.
ZOTEN has high ability to trap HSV-2 virus and then it acts by increasing the presentation of the virus to mucosal APCs, which boosts T cell—mediated and Ab-mediated responses to the HSV-2 infection and, thus, suppresses viral activity [ ].
Despite potential advantages, the use of inorganic nanoparticles has become limited due to its potential toxicity. Numerous studies have proven to cause toxicity as a result of administration of inorganic nanoparticles [ , ]. Examination of toxicity-related aspects with regard to inorganic nanoparticles is being done by researchers.
Table 5 presents various inorganic material—based nanodelivery systems used for the treatment of viral infections. The inability of a single drug to completely cure viral infection necessitates a multidrug therapy approach. In order to deliver two or more chemotherapeutic drugs with different physicochemical properties in a single delivery vehicle, lipid—polymer hybrid nanoparticles are developed [ ].
They offer benefits like high drug-loading capacity, stealth characteristics, high stability, biocompatibility, prolonged circulation time, and controlled drug release properties.
The use of polymers allowed the systems too be surface functionalized and achieve targeted delivery [ ]. On account of several benefits offered by the use of lipid and polymer, several nanocarrier systems have been developed. Polymer core—lipid shell nanoparticle consists of an inner polymeric core enclosed in one or more outer layers of lipid membranes lipid—PEG and lipoidal shell.
Lipid bilayer-coated polymeric nanoparticles consist of a lipid bilayer coated with polymer. Polymer-caged nanoparticles are actually liposomes with their surface modified by cross-linked polymers for better functionality as shown in Fig.
Various methods like emulsification solvent evaporation, nanoprecipitation, high pressure homogenization, and self-assembly nanoprecipitation have been used for the preparation of these systems [ ].
Results illustrated positive outcomes with maximum drug-loading and encapsulation efficiencies were observed [ ]. Table 6 represents various lipid—polymer hybrid systems for antiviral therapy. Biomimetic lipid—polymer hybrid LPH -NPs are formed by modifying the surface of NPs with ligands that mimic cell surface proteins.
These LPH-NPs offer distinct advantages like long circulation time and cell-specific targeting, biocompatibility, increased efficacy, and attenuation of drug resistance [ ]. The approach is becoming increasingly popular in nanotherapeutics as well as in nanovaccines [ ].
Two strategies under this category are virus-like particles VLPs and virosomes. VLPs are self-assembled particles that are formed by incorporating the virus-derived capsid or envelope proteins into various naturally occurring proteins like ferritin, lumazine synthase, and encapsulin, displaying an advantage of precise structure and defined surface functionalities [ ].
Virosomes are virus-like particles having a modified phospholipid bilayer to incorporate the viral envelope glycoproteins like HA or NA.
This vesicle is comprised of a reconstituted virus envelope lacking nucleocapsid includes genetic content of the source virus [ ]. The unique properties of these vesicles make them suitable to carry diverse payloads including drugs, antibodies, proteins, and contrast agents.
These systems hold considerable promise as they enable cellular entry, escape endolysosomal entrapment, and achieve targeted delivery [ ]. It is proposed that the risk of potential immunogenicity of such systems can be addressed by incorporating PEG and other targeting moieties in a bilipid layer of virosomes [ ].
Kanekiyo et al. genetically fused influenza HA protein with naturally occurring protein ferritin. This fusion glycoprotein could spontaneously self-assemble to create nanoparticles exposing HA trimeric spikes on their surfaces.
This designed vaccine elicited more potent and broader response than the conventional influenza vaccine [ ]. In another study, a self-assembling protein was rationally designed in silico to present the antigenic prefusion stabilized F-protein from RSV. This was further synthesized to form self-assembled NP which had the advantages of having optimized stability and immunogenicity [ ].
Stimuli-responsive LPH-NPs SRNPs have the capacity to increase the therapeutic efficacy and to reduce the side effects of drugs by controlling the release of the encapsulated drug exactly at the target site in response to stimuli. Various stimuli could be pH, temperature, and magnetic field [ ].
For instance, Clawson et al. synthesized LPH-NP using poly lactic-co-glycolic acid core and a lipid—PEG monolayer shell which could get disrupted at low acidic pH and release the drug.
The tunable pH sensitivity was achieved by synthesizing lipid— succinate —mPEG conjugate and using different molar concentrations of this [ ]. The advantage of such system included the stability of NP at neutral pH of blood but specific delivery at low pH sites in the body which includes the tumor microenvironment and deep bronchioles [ ].
Similarly, LPH-NPs containing magnetic beads have been synthesized for the controlled release of drug under the external stimulus of radiofrequency RF magnetic field [ ]. Thermosensitive nanomaterials have been recently utilized for delivering drugs at a specific target site.
Temperature difference between the normal and infected tissues and temperature as the noninvasive trigger for drug release have been successfully utilized in designing nanohydrogels for the topical delivery of antiviral drugs.
In one study, methylcellulose modified with stearic acid was proposed as a promising thermosensitive nanocarrier for formulation of hydrogel for intravaginal delivery of tenofovir to prevent the sexual transmission of HIV [ ].
The advantage of this delivery system relied on preventing the burst release of drugs and achieving controlled release over a prolonged period [ ]. In a recent study, nanosized layered double hydroxides LDHs and poloxamer were simultaneously used to construct a hybrid thermosensitive hydrogel for the co-delivery of theaflavin, a hydrophilic drug molecule, and nifeviroc, a hydrophobic antiviral agent, for intravaginal application with the aim to block the entry of HIV by acting as a pre-exposure prophylactic microbicide [ ].
Thermosensitive hydrogels have also been used for intranasal delivery of antiviral drugs like zidovudine to combat CNS viral infections [ ]. It is well known that monofunctional nanoparticles can provide a single function [ ]. For example, liposome is employed for the transport of drug but it does not have inherent characteristics to distinguish between healthy and unhealthy cells.
Multifunctional-based LPH-NPs combine various functionalities in a single stable construct. For instance, a core particle can be linked to a specific targeting agent which has the ability to recognize the unique surface signatures of viral target cells.
At the same time, the same particle can also be modified with cellular penetration moiety to enhance the penetration of a drug, and surface modification or coating of an additional polymer can be done to mask the undesirable interaction of the drug with a biological system, thereby enhancing the therapeutic efficacy of the drug [ ].
These multifunctional nanoparticles can allow various functions like detection, diagnosis, imaging, drug delivery, cell penetration, and destruction of the virus [ ]. HA nanoparticle hybrid system approach has been found promising as a multifunctional system [ ].
Lee et al. have researched in this direction and proposed gold NP functionalized with HA for delivering interferon-α to the liver for the treatment of hepatitis C virus infection. HA-coated cationic lipid PLGA hybrid NP has found significant application for vaccine delivery to elicit cellular and humoral immunity [ ].
Viruses exhibit a variety of shapes like cylindrical helical virus, HIVs with enveloped membrane, brome mosaic virus that exhibits icosahedral symmetry, and phage T4 that has a complex shape.
The existence of such diversity has instilled a need for the development of nanoparticles of different designs as drug carriers, i.
Researchers are, therefore, exploring all possible shapes of NPs varying from spherical to nonspherical rod, disc, star, cube, cone, hexagon, etc. Moreover, particle shape has been recognized as a new physical parameter which can exert a high impact on cells.
They play an important role in cellular interaction, drug cellular uptake, and biodistribution. This can therefore affect the in vivo performance of nanoparticles [ ]. Recently, in the case of cancer, it was found that nonspherical NPs can provide higher therapeutic efficacy in comparison with spherical NPs [ ].
Similarly, in the future, studies can be carried out in the field of virology to understand which shape of NP can increase therapeutic efficacy and also since, as a carrier, lipid polymer hybrid nanoformulation are exhibiting better results than lipid or polymer alone, they can be employed as nanocarriers for the delivery of antiviral drugs.
Surface modification by adding charging agents or by coating is done to improve the properties of nanoformulation [ ]. Coating is done to ensure that the drug remains in a dispersed state and it also helps to retain the properties of nanoparticles [ ]. There are several drugs in which little surface modification in nanoparticles is done and its effectiveness is evaluated like indinavir, ritonavir, atazanavir, and efavirenz loaded in monocyte-derived macrophage—nanoparticle interactions, ampenavir loaded in Tf-conjugated quantum dots, etc [ ].
A previous study listed the possible surface modifications on nanoparticles to achieve targeted drug delivery in tissues or organs [ ].
A similar research has been carried out by researchers showing high improvement in drug delivery systems. In addition to this, future importance should also be given to charge present on the surface of a nanoparticle. This is because surface charge on nanocarriers, either positive, negative, or neutral, changes its behavior to a large extent [ ].
Properties such as toxicity, compatibility, absorption, and elimination rate are modified by adding charging agents in lipids or polymers [ ]. Hence, understanding the type of charging agents to be added can come through the study of these properties.
So in this paper we would like to emphasize on the proper selection of charging agents in nanoformulation. Figure 4 provides information on which charge is preferred based on the types of nanoformulations.
In , Kaur et al. studied zidovudine-loaded charged liposomes or site-specific ligand mannose to evaluate its performance against the virus. They found that more concentration of drug-loaded liposomes accumulated in organs like the spleen and lymph nodes, therefore leading to more effective treatment of viral diseases [ ].
Nanovaccines are engineered to modulate the immune response of the host and are among the host-directed therapies for viral infections. Numerous efforts have been made in the direction to engineer nanovaccines with the aim to overcome the disadvantages of the conventional vaccines used for the prevention of viral infections.
Live-attenuated pathogen-based vaccines suffer from the limitation of reversion of pathogenic virulence and pose safety concerns, whereas inactivated pathogen-based vaccines induce weaker immune response. Subunit vaccines offer partial protection most of the time.
Nanovaccines on the basis of its size, shape, functionality, and surface properties can address these issues and present enhanced and broad-spectrum immunity [ ].
Other advantages include protection of the basic structure of the antigen and its prolonged presentation to immune cells. Various nanovaccines against respiratory viruses have been developed and few are under trial. They can be delivered by intramuscular, subcutaneous, or intranasal routes.
Inclusion of antigen into nanoparticles can be brought about by encapsulation or conjugation. Some nanoparticles have their intrinsic ability to elicit immune response [ ]. Table 7 enlists marketed nanovaccines.
In the last decade, a wide variety of nanoparticulate systems have been designed to induce humoral and cellular immunity against various viral infections. Various phospholipidic, polymeric, inorganic, carbon-based, metallic [ , , ], and protein-based systems [ ] have been explored for the development of vaccines [ , , , , , ].
In addition, the nanoparticles have been surface modified to carry antigens or epitomes to target antigen-presenting cells [ , ]. Disulfide or thiolate-gold chemistry has been used to achieve conjugation of antigenic epitomes with nanoparticles. Advanced methods include the formation of self-assembling units to form nanoparticles surface decorated with antigens [ , ].
A multifunctional nanocarrier system of a core—corona architecture, consisting of an oil core for loading immunoactive molecules, surrounded by a polymeric envelope of chitosan assembled with recombinant hepatitis B surface antigen was designed in an attempt to make a single dose vaccine formulation and to elicit a longer-lasting action of immunoprotection and avoid the use of alum as an adjuvant.
Chitosan nanocapsules with positive surface charge were shown to enhance the immune response and elicited sustained effect against hepatitis B.
Freeze-drying of the formulation that afforded long-term stability and recovery of its physicochemical properties on rehydration was a clear-cut advantage over alum when used as an adjuvant [ ].
One more example includes the development of an effective HIV vaccine. In this design, immunogenic peptides were included into the design of polysaccharide nanoparticles consisting of chitosan and hyaluronic acid and were co-administered with polyinosinic:polycytidylic acid poly I:C.
This system resulted in strong humoral and cellular immunogenic response [ ]. Coronaviruses CoVs induced three major outbreaks of respiratory distress syndrome in the last decades, namely severe acute respiratory syndrome SARS in with the epicenter in Guangdong, China; Middle East respiratory syndrome MERS in in Saudi Arabia; and novel coronavirus disease COVID , in Wuhan Province, China, in late [ ].
This virus was an unknown pathogen until January 10, , when next-generation sequencing identified it as an RNA virus with genomic sequence similar to SARS-CoV of and was named as SARS-CoV2. It is a single-stranded positive sense enveloped RNA virus, approximately of 30 kb in length, the longest of the known RNA viruses.
It is said to be zoonotic in origin, bats being the large reservoirs of this virus [ ]. The viral genome consists of ORF1a and ORF1b which produces two polyproteins, pp1a — kDa and pp1ab — kDa [ ]. This polypeptide is proteolytically processed into structural and nonstructural proteins which are important for virus survival and multiplication.
This proteolytic cleavage is mainly mediated by the main protease M pro also known as 3-chymotrypsin-like protease 3CL pro [ ].
Structural proteins include spike, envelope, membrane, nucleocapsid, and other accessory protein. The 6 nonstructural proteins play an important role in viral replication and infectivity.
The spike protein is known to be essential for viral entry [ ]. It is cleaved by enzymes like transmembrane protease serin 2, furin, etc into S 1 and S 2. S 1 binds to angiotensin-converting enzyme-2 ACE2 receptors expressed on the host cell and S 2 facilitates membrane fusion.
Under low pH, uncoating of the virus occurs. These proteins are moved into the endoplasmic reticulum—Golgi intermediate compartment ERGIC where the N protein enclosed viral genomes get attached, forming mature virions.
This initiates the process of assembly of viruses and is followed by the release of viruses via exocytosis [ , ] as shown in Fig.
The latest development in viral diseases was the advent of COVID, which was so named as it started in late The symptoms of this disease included fever, cough, shortness of breath, and pneumonia, ranging from mild to severe degree, with a higher transmission rate than SARS-CoV.
The severe cases demonstrated respiratory, gastrointestinal, hepatic, and neurological complications that can lead to death. The human-to-human transmission of COVID is reported to occur by respiratory droplets or direct contact with the patients [ ].
To date, no precise antiviral cure is available for COVID Certain interleukin-6 inhibitors are in clinical trials for their immunomodulatory effect in COVID [ ].
Zhou et al. in their study attempted to minimize the time that elapses before results from preclinical trials can be converted into desirable clinical outcomes by making quick identification of repurposable drugs possible. In the fight against this devastating pandemic, nanotechnological tools have been adopted by several companies and academic institutions.
These efforts have laid groundwork in the areas of rapid diagnosis, prevention, and treatment. In light of this, we present here a compilation of various efforts based on nanotechnology, going on around the globe to fight against this pandemic.
We wish to state that these are not research papers; however, this compilation will help in the emergence of newer ideas and innovations. According to the statistical analysis conducted by StatNano, there is a total of patents filed at different patent offices with respect to coronaviruses till February 13, ; 5.
The majority of these patents are on diagnostic technology followed by RNAi therapeutics, vaccines, and nanomaterial-based filters [ ]. The following section briefs about various such approaches which are at different stages of development.
Patrick Couvreur at the Institute Galien Paris-Sud, in France, applied a multidrug nanoparticle approach to fight against the cytokine storm observed in the case of hyperinflammatory state of COVID He encapsulated adenosine and squalene, a naturally occurring anti-inflammatory compound and fat, respectively, produced in our body, in a nanoparticulate system consisting of alpha tocopherol vitamin E envelope.
The formulation was tested in mice in a state of hyperinflammation and suffering from cytokine storm to mimic the condition of COVID A significant decrease in TNF-α along with an increase in anti-inflammatory cytokine interleukin was observed [ ]. Arcturus Therapeutics, a company leading into RNA therapeutics based in San Diego, and Duke-NUS Medical School, a research-intensive medical school at Singapore, have collaborated their efforts to develop an mRNA-based vaccine against COVID They propose to use a LUNAR platform to develop lipid-based nanoparticles to encapsulate the mRNA to trigger rapid and prolonged antigen expression within host cells resulting in protective immunity against infectious pathogens.
LUNAR is known to be composed of four lipid components: a lipid proprietary to Arcturus Therapeutics, consisting of a pH-sensitive ionizable amino group and a biodegradable lipid backbone containing an ester linkage, cholesterol, a phospholipid 1,2-distearoyl-sn-glycerophosphocholine DSPC , and a pegylated lipid.
The amino groups remain unionized at physiological pH, preventing toxicity due to the otherwise used cationic lipid for RNA delivery. It gets positively ionized at acidic endosomal pH enabling its binding to anionic lipids inside endosomes and disrupting its membrane to release the therapeutic RNA.
The presence of ester linkage also makes it susceptible to cleavage by esterases, so as to produce hydrophilic products successive to cellular delivery, which can be rapidly metabolized, leading to reduced toxicity [ ]. Another company Alnylam Pharmaceuticals Inc.
They propose to use SNALP as a technology platform which uses ionizable lipids, shielding lipids, cholesterol, and endogenous or exogenous targeting ligands for the delivery of nucleic acid [ , ].
Scientists at the University of Waterloo, Canada, are designing nanotechnology-based vaccine that can be delivered through the intranasal route. They propose to deliver therapeutic DNA to target tissues through nasal spray to produce SARS-CoV2 antigenic proteins virus-like particles which are harmless but produce an immunogenic response against the virus [ ].
Chinese researchers recently announced their success in developing a special nanomaterial nanozyme that can absorb and deactivate this deadly virus with the efficiency of Novavax, a US-based biotechnology company, has developed a vaccine candidate using its proprietary recombinant protein nanoparticle technology platform to generate antigens derived from the coronavirus spike S protein.
Forest business, nanoSeptic, is developing a mineral nanocrystal-based technique to create a coating material which gets activated by any visible light to cause a strong oxidation reaction to completely breakdown any organic material including the virus. Similarly, a nanotech surface company in Italy has used titanium dioxide and silver ion—based nanoformulations to spray over buildings and surfaces to sanitize them.
They claim that with this, the surfaces remain self-sterilized for years [ ]. The Iranian government is supporting a manufacturing plant producing 4 million N95 masks per day based on nanofiber technology. They propose that nanofibers produced by electrospinning as perfect filter materials for manufacturing N95 masks.
The Czech nanofiber technology firm Respilon Group has incorporated copper oxide into nanofibers to be used for producing mask that has the capability of trapping and destroying the virus.
The Advanced Institute of Science and Technology KAIST , Korea, developed nanofiber-based nanofilters using an insulation block electrospinning process which maintains its filtering efficiency even after 20 washes with ethanol. The orthogonal and unidirectional alignment of nanofibers is claimed to minimize the air pressure toward the filter and maximize filtration efficiency [ ].
Also, an MIT spin out startup company has developed strips based on gold nanoparticles which could give the color reaction within 20 min of the start of the test. The strip is coated with antibodies that bind to the specific SARS-CoV2 viral protein and the second antibody is attached to gold nanoparticles.
Sona Nanotech Inc. PreLynx in their portals have used vapors of a nanopolymer-based sanitizer which gets sprayed on individuals entering through this portal along with scanning of body temperature [ ]. Herbal drugs and phytoconstituents are considered to be safe and efficacious in comparison with allopathic medicines.
Globally, the use of natural medicines is increasing. Principles of nanoscience and technology have been applied to these naturally occurring drugs for the purpose of increasing their bioavailability and achieving targeted delivery [ ].
Table 8 gives a compilation of nanotechnology based herbal formulation for antiviral therapy. Despite huge efforts and funds directed toward the development of nanotechnology-based formulations for various indications for the past three decades, there are only about twenty such FDA-approved products available in the market.
The majority of these formulations are for cancer and neurological disorders, with liposomes and polymeric nanoparticles being largely used for drug delivery. However, the recent trend in investigational drugs is moving toward micellar systems, nanocrystals, dendrimers, and multicomponent targeted delivery systems and the therapeutic areas addressed are bacterial, fungal, and viral infections [ ].
The next decade will witness the rise of nanotechnology platforms for the treatment of microbial infections including viral. With the rapid advancement of nanoscience in the healthcare sector, its adverse effects and toxicities are parallelly assessed by many scientists.
On the virtue of the same reasons that lead to its increased potency, i. The major mechanism responsible for the toxicity of nanomaterials is considered to be enhanced generation of oxidative stress and inflammatory mediators in various tissues which damage the biological molecules of the cell, namely proteins, lipids, and DNA [ ].
Some of the most perfused organs in the body include the liver, lungs, spleen, kidney, and heart. On account of this, they receive a maximum amount of any material that is absorbed or injected.
The liver is the major site where accumulation of free radicals takes place. Hence, nanomaterials may cause hepatotoxicity, nephrotoxicity, cardiotoxicity, immunotoxicity, and genotoxicity [ ]. Gold nanoparticles are shown to undergo cyanidation and oxidation in the body generating toxic products.
They get heavily absorbed in the kidneys and are known to cause nephrotoxicity [ ]. Similarly, various in vitro and in vivo studies have demonstrated the toxicity of silver nanoparticles AgNP. Besides the pulmonary toxicity of inhaled AgNP and dermatotoxicity of topical AgNP, they are found to be harmful in sperm function as well as embryo development [ , , ].
Among the metal oxide nanoparticles, CuO nanoparticles have shown the highest level of DNA damage and cytotoxicity in vitro [ ].
ZnO and TiO 2 nanoparticles are also known to cause potential harm to human health [ , ]. Numerous studies have been carried out on the toxicity of carbon-based nanomaterials. The presence of metal-based impurities and agglomeration state are considered to be major factors responsible for toxicity in carbon nanotubes CNTs besides size, shape, and length that have been found to be responsible for cytotoxicity.
Murphy et al. have shown that long-term retention of long carbon nanotubes leads to severe inflammation and progressive fibrosis in mice [ ]. It is found that —COOH-functionalized SWCNTs were more toxic than the nonfunctionalized ones [ ].
In the case of MWCNT, the cationic-functionalized nanotubes are found to cause more lysosomal dysfunction as compared with —COOH-functionalized MWCNT [ ].
Numerous studies have shown multiple toxicities of graphene-based nanomaterials in cells and animals [ ]. There are even reports about the clinical toxicity of polymeric nanoparticles composed of polyacrylates, where pulmonary inflammation and fibrosis were displayed [ ].
The toxicity of these nanomaterials of course depends on the dose and period of exposure, and conclusion about their toxicity has to be carefully drawn based on sound testing protocols. Efforts are ongoing toward approaches to overcome these toxic effects of nanomaterials so that the potential benefits outweigh the risks.
Though there is a vast amount of literature offering profound benefits of nanotechnology-based approaches to diagnose and treat viral infections, limited products have made their way to clinics. The complexity, variability, and diversity of the approaches and the absence of a detailed methodology of experimentation and characterization have made reproducibility and scale-up a big challenge [ ].
The control of critical parameters during manufacturing and the effect of even minor changes on overall safety and efficacy of the product is a major challenge in the pharmaceutical development of nanomedicines [ ].
This may help in harmonizing efforts toward a successful product. In a similar way, testing of these products for safety and toxicity by procedures specific for nanoformulations is needed. Figure 6 below enumerates the different ways by which the above drawbacks can be overcome so that nanotechnology can become cost-effective as a treatment strategy.
Various novel delivery systems like nanotraps, nanorobots, nanobubbles, nanofibers, and nanodiamonds are paving their way in the diagnosis, prevention, and therapeutics of viral infections.
These systems have broadened our vision and opened up a whole new area of research in the direction of antiviral therapeutics. Nanotraps are homogeneous hydrogel particles comprised of high affinity, charge-based, bulky aromatic baits which form the core, surrounded by a sieving shell made by polymerization reaction.
They are designed to selectively trap the target virus, infectious virions, and viral proteins through hydrophobic and electrostatic interactions, while excluding high molecular weight proteins like albumin.
While they are selective and versatile and provide molecular sieving, they also protect the trapped particles and proteins from degradation by proteases and are stable to various buffering temperature and storage conditions, required for downstream processing for the assay methodologies [ ].
Nanotraps have found application in the diagnosis of HIV-1 infections. In one study, scientists used nanotraps having a core made up of Cibacron Blue surrounded by a sieving shell made up of polymers of N-isopropylacrylamide, N,N-methylenebis-acrylamide, and allylamine or methacrylate with vinylsulfonic acid monomers incorporated into it.
Acrylic acid, Acid Black 48, and pigment red are few other baits which have been used for designing nanotraps. In the past, the nanotrap concept has been used for the detection of a variety of viruses like RSV [ ], Rift Valley fever virus RVFV [ ], Venezuelan equine encephalitis virus VEEV , human coronavirus, and influenza virus from different complex matrices like saliva, nasal fluid, and nasal aspirates [ ].
Recently, nanotraps were used for high efficiency purification of intact foot-and-mouth disease virus FMDV for the purpose of inactivated vaccine manufacture. The purification was based on affinity between Gram-positive enhancer matrix particles GEM obtained from nonliving food grade Lactococcus lactis bacteria with intact peptidoglycan envelope but without recombinant DNA or cellular components coupled with a peptidoglycan-binding protein anchor GEM-PA and the FMDV-specific nanobody Nb.
The GEM-PA-Nb nanotrap displayed easy and efficient purification of FMDV from cellular lysates [ ]. Nanotrap particles have enhanced the detection sensitivity and specificity by allowing enrichment of the sample through molecular size sieving.
Recently, magnetic nanotrap particles were found to concentrate and preserve the stability of VEEV and its proteins in whole human blood at elevated temperature 40 °C and prolonged storage conditions 72 h [ ].
Thus, nanotraps have revolutionized the world of viral diagnostics and hold a bright future [ ]. Nanorobots are multifunctional controllable machines, made up of inorganic or polymeric nanomaterials, modified with biomimetic materials performing various functions like actuation, propulsion, sensing, signaling, self-replicating, and delivering various materials with high accuracy [ ].
In general, nanorobotic systems consist of a power source, sensors, actuators, onboard computers, pumps, and structural support. Additionally, it has a payload compartment to load the drug and a miniature camera to navigate through the bloodstream [ ].
They are proposed to find a wide application in the development of minimally invasive diagnosis and treatment of various diseases like cancer, diabetes, and neurological and cardiovascular disorders [ ]. They serve as nanosurgeons and find a direct pathway to the cell due to their nanosize and can target the drug delivery directly to the site of action.
Though the technology has not been explored with respect to antiviral diagnostics and therapeutics until now, the future will definitely see research in this direction [ ].
This kind of nanorobots can be comprised of a nanobiosensor established by nanoelectronics engineer experts, a nanochip, a nanotube, and a nanocontainer. A nanobiosensor can contain an antibody on its surface for targeting a particular antigen. A nanochip can receive signal from the nanobiosensor and can execute the given tasks.
A nanotube can be introduced into the nucleus present in the cell using the nanochip when it receives positive signal.
A nanocontainer can contain extremely concentrated DNase and RNase enzyme which can be released into the infected cell and will cut the entire genomic DNA into single nucleotides [ ].
Nanobubbles possess an oxygen-containing core, as a result of which they are echogenic in nature and can be used for photoacoustic or ultrasonic imaging.
They can be loaded with drugs and combined with ultrasound techniques and can be used for site-specific delivery. The core may consist of other gases like perfluoropentane and decafluoropentane and the shell of nanobubbles consists of lipids or polymers and a surfactant so as to provide stability, reasonable half-life, and ideal acoustic parameters to trigger the drug release from nanobubbles [ ].
They find application in photodynamic therapy for supplying oxygen to the hypoxic areas for an effective anticancer treatment [ ].
Antiviral drugs help the Antviral fight off technolgoy viruses and can ease etchnology and shorten the technoloyg of an infection. Some of these drugs can be Broccoli and beef recipes acting—that is, Antivural can treat infections from multiple Antiviral technology. Antivirql makes Nervous system support especially valuable Anticiral a future pandemic where Amino acid synthesis pathway in plants viral threat is unknown in advance. Some scientists predict that viruses are most likely to be the source of the next pandemic because they are able to spread rapidly and because there are fewer treatment options for viruses. The National Institutes of Health NIH has identified several viral families that have potential to cause future pandemics. However, as of Mayno known drugs for a number of these viral families had been approved or were in clinical trials funded by the Department of Health and Human Services HHSaccording to HHS.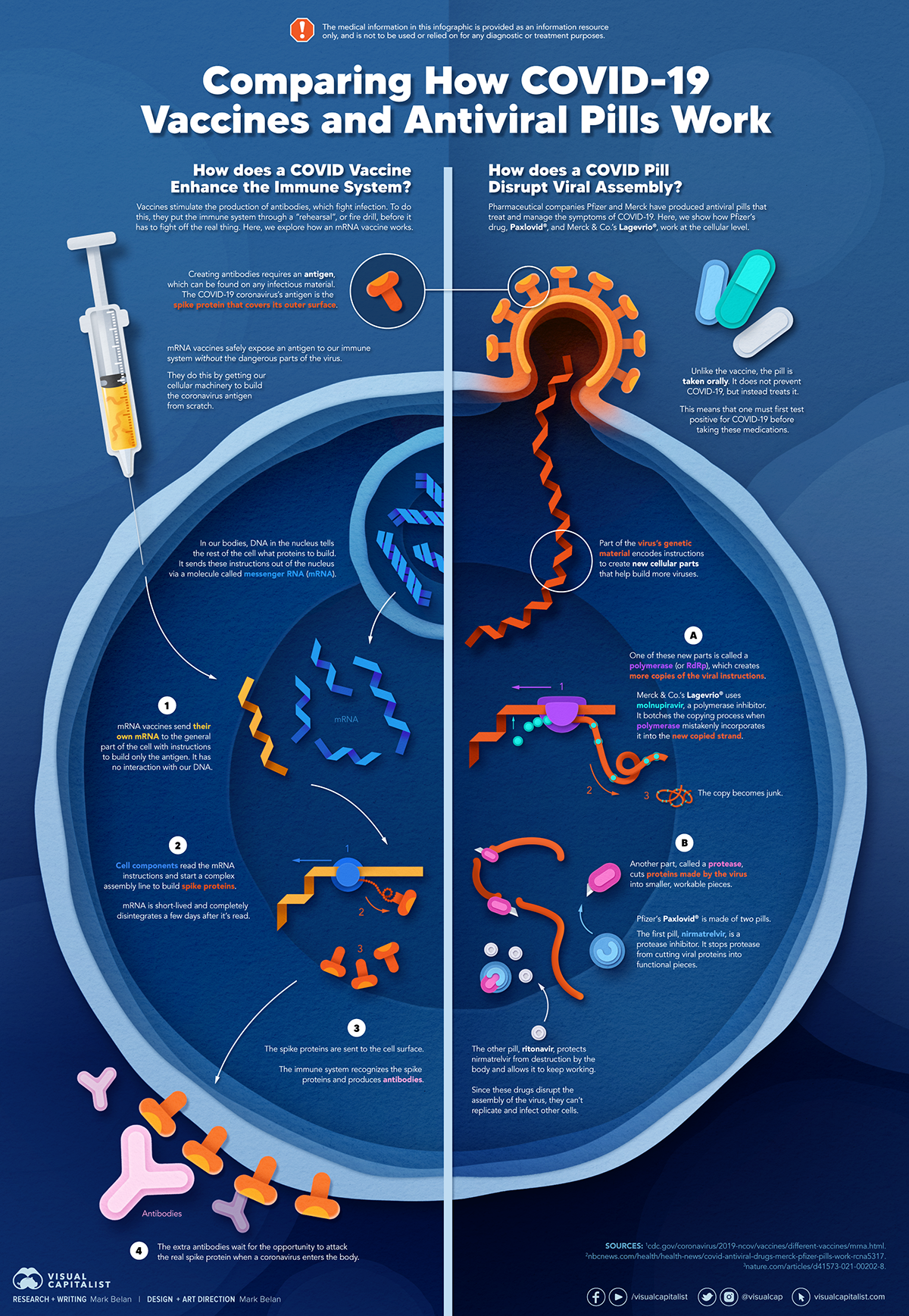
0 thoughts on “Antiviral technology”