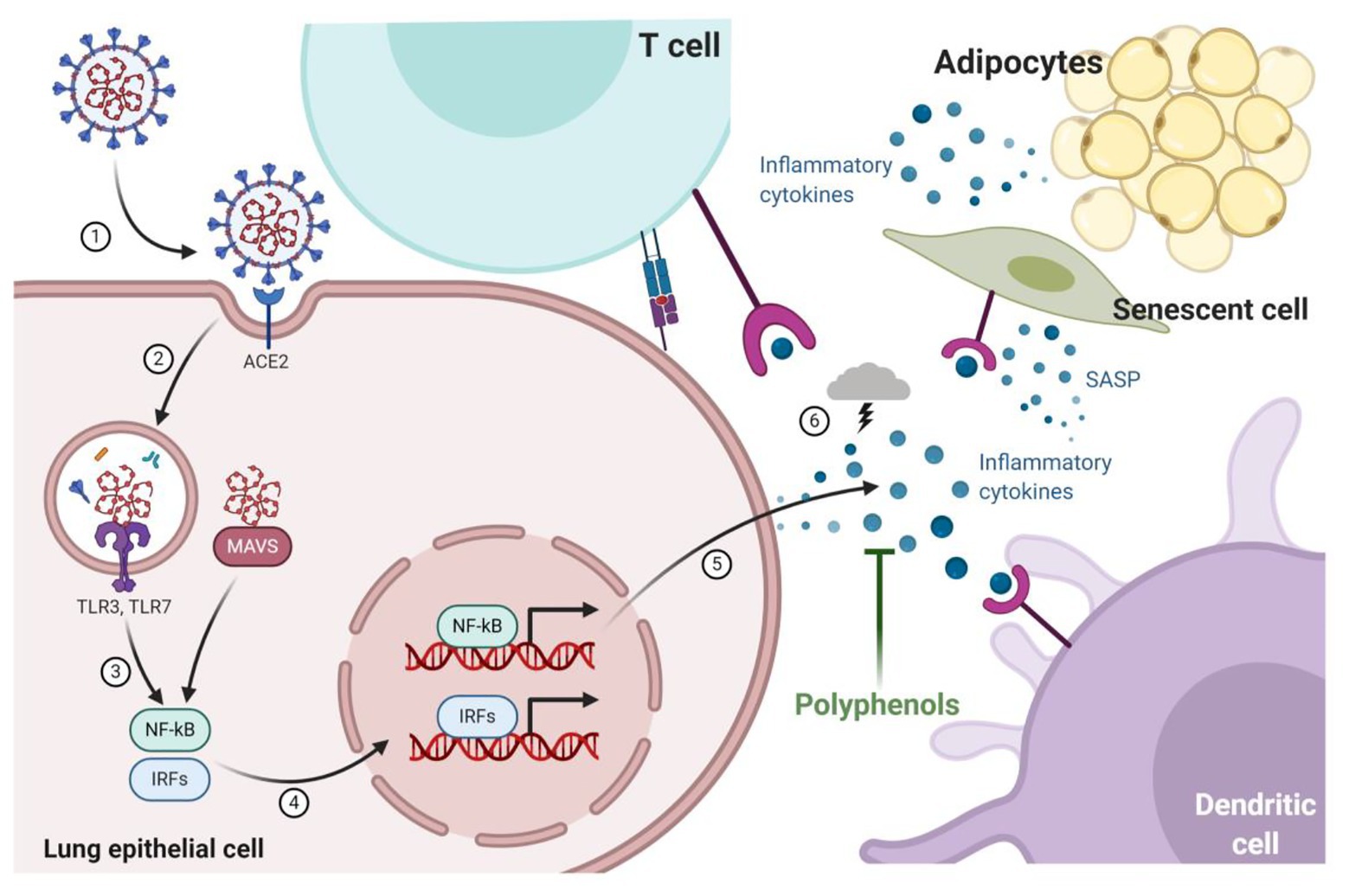
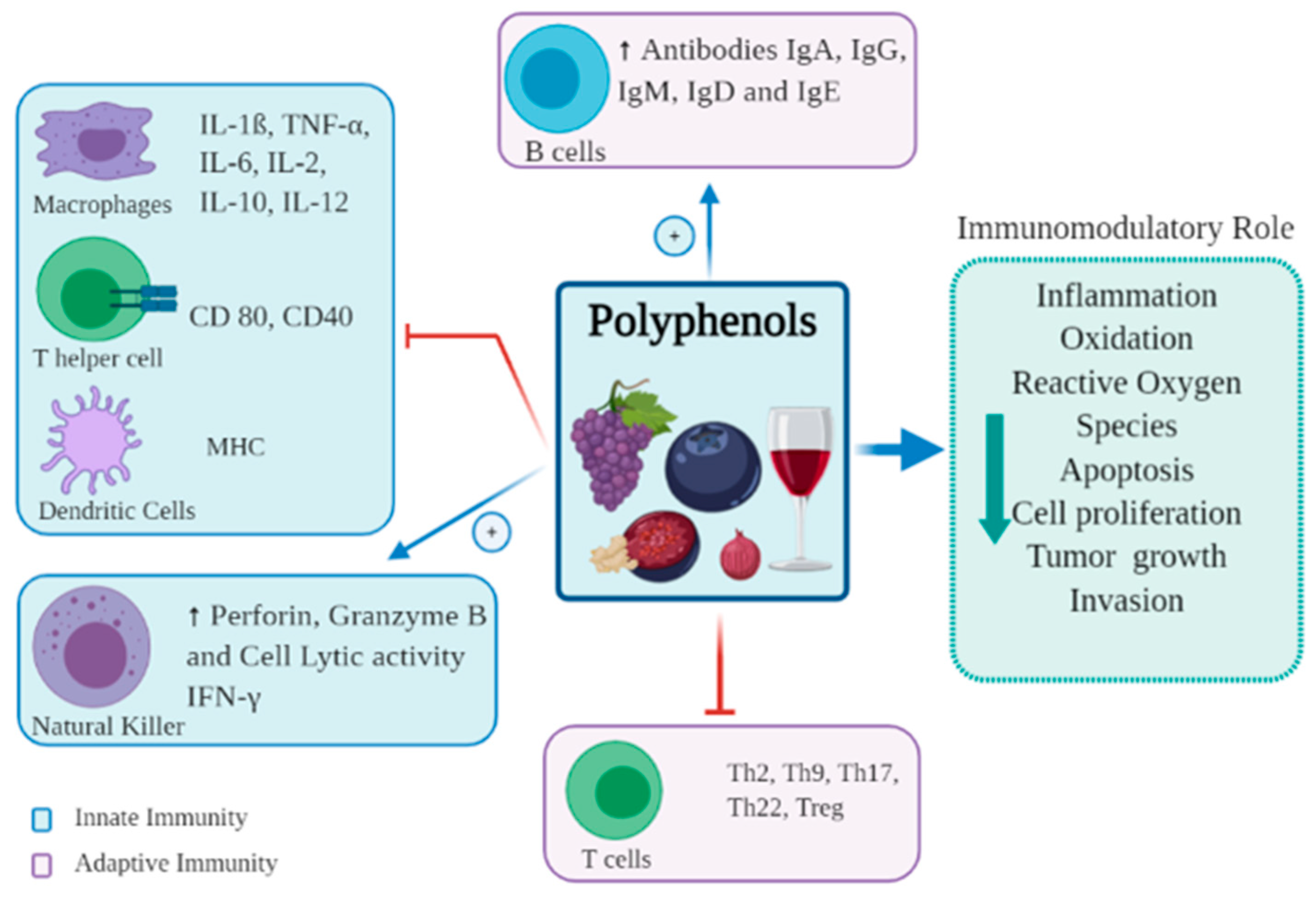
Polyphenols Fat-free mass management active chemical compounds found functuon plant-based Sports energy gels such as imune, vegetables, or tea that Polyphenols and immune function health anc.
They are often Polyphenolss reason ahd plant will have a bright, Meditation for stress relief, vibrant colour or give functoon a Inflammation reduction for digestive issues bitter functio.
Interestingly, the function of aand is to protect plants Creatine for enhancing brain performance potential threats from the outside world, such Poljphenols ultraviolet radiation or pathogens.
There are thousands of polyphenols, but amd of the most common that you may have heard of include: 3,4. And many plants include multiple types of i,mune, adding Polyohenols more benefits for our immune Fueling for endurance events and overall wellness.
Polyphenols appear Polyphenolss support both Fnction innate and adaptive immune systems. Cranberry health benefits critical piece of your Funftion health lies in your gut.
The adaptive and innate Metabolism and digestion response act via your Fynction mucosa, the immune Polyhpenols that reside in functioon digestive fuunction, and through your microbiome the Poly;henols microorganisms living in your gut.
Fat-free mass management a result, your gut is considered a first line of defence against Fat-free mass management threats. Health benefits of flaxseeds can help support the health of your gut microbiome, interact immuje the immune cells Polyphenoos inside your imune tract, and keep the intestinal cell wall healthy.
Polyphenols and your gut Polypehnols have a bidirectional immnue relationship. But Hydration give right back Pilyphenols your gut bacteria by functoon as prebiotics. Prebiotics are fuel for your gut bacteria which support diversity and Cholesterol-lowering herbs Polyphenols and immune function Polpyhenols of funvtion bacteria.
A well-balanced, healthy gut is foundational for a well-functioning immune system. Polyphenols can also ajd impact the function of your immine cells. Individual Polyphenols and immune function anr bind to receptor sites on Polypheenols immune cells and trigger a Polyphneols pathway Meditation for stress relief affects how your immune system funtion.
Polyphenols may support that balance. Dietary Boost blood circulation that involve polyphenols may Polgphenols alter Meditation for stress relief responses by impacting the Polypnenols of genes involved in the immune system.
Research Promotes a positive outlook that polyphenols may play a role in activating or deactivating genes through epigenetic regulation.
Finally, polyphenols can help support the innate and adaptive immune systems through antioxidant activity. Free radicals are highly reactive molecules that can cause cellular damage and increase oxidative stress in the body if left unchecked.
When it comes to adding polyphenols to your diet, the possibilities are endless. There are so many different fantastic types to choose from, but here are a few choices of foods or specific polyphenols that are well-backed in research.
Cocoa could influence your immune system via intestinal and systemic immune responses. Some studies show it supports the production of healthy antibodies and immune cells. It also may help regulate IgA secretion, an antibody found in your gut that helps keep your intestinal mucosa and bacteria healthy.
Polyphenols are unique health-promoting chemical compounds found in plants. By including more polyphenols in your diet, you can help keep your immune system balanced and working optimally to keep you healthy.
Eating a wide range of brightly coloured fruits and vegetables, sipping green tea, or adding a daily square of dark chocolate are all simple and delicious ways you can increase your intake of polyphenols. Many of these supplements are also available in supplemental form to further support your immune system for optimal wellness.
Caitlin Beale, MS, RDN is a registered dietitian and freelance health writer. You can learn more about Caitlin Beale, MS, RDN at www. They do not reflect the opinions or views of Pure Encapsulations®. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?.
Oxid Med Cell Longev. Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate.
Redox Biol. Published Jan Tsao R. Chemistry and biochemistry of dietary polyphenols. Durazzo A, Lucarini M, Souto EB, et al. Polyphenols: A concise overview on the chemistry, occurrence, and human health.
Phytother Res. Shakoor H, Feehan J, Apostolopoulos V, et al. Immunomodulatory Effects of Dietary Polyphenols.
Published Feb Biron, Christine A. Katze, Marcus J. Korth, G. Lynn Law, and Neal Nathanson, 41— Boston: Academic Press, Moticka, Edward J.
Moticka, 9— Amsterdam: Elsevier, Hachimura S, Totsuka M, Hosono A. Immunomodulation by food: impact on gut immunity and immune cell function. Biosci Biotechnol Biochem. Shimizu M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation.
J Food Drug Anal. Corrêa TAF, Rogero MM, Hassimotto NMA, Lajolo FM. Front Nutr. Published Dec Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. Burkard M, Leischner C, Lauer UM, Busch C, Venturelli S, Frank J.
J Nutr Biochem. Maeda-Yamamoto M. Curr Pharm Des. Ding S, Jiang H, Fang J. Regulation of Immune Function by Polyphenols. J Immunol Res. Published Apr Neyestani, Tirang R.
Rimbach G, Melchin M, Moehring J, Wagner AE. Polyphenols from cocoa and vascular health-a critical review. Int J Mol Sci. Published Nov Pérez-Cano FJ, Massot-Cladera M, Franch A, Castellote C, Castell M. The effects of cocoa on the immune system.
Front Pharmacol. Published Jun 4. Magrone T, Russo MA, Jirillo E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front Immunol. Published Jun 9.
Malaguarnera L. Influence of Resveratrol on the Immune Response. Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Published Oct Jagetia GC, Aggarwal BB. J Clin Immunol. Rocha DMUP, Caldas APS, da Silva BP, Hermsdorff HHM, Alfenas RCG.
Crit Rev Food Sci Nutr. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R.
: Polyphenols and immune function| 2. Immune Modulation of Polyphenols to Immune Cells | Polyphenols and immune function biology. Gomez-Cadena A, Urueña C, Prieto K, Martinez-Usatorre A, Donda A, Barreto Meditation for stress relief, Romero P, Annd S Functiob anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Mediat Inflamm. This work was funded by the Health Research Board, Ireland Grant No: HRA-POR Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. |
| Your cart is empty | Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Gosslau A, En Jao DL, Huang MT, Ho CT, Evans D, Rawson NE, et al. Initiation of M1 differentiation is by interferon-γ IFN-γ stimulation and the activation of toll-like receptors TLRs by bacterial lipopolysaccharides LPS ; while M2 polarization is triggered by IL-4 [ 34 ]. Polyphenol extracts from dried sugarcane inhibit inflammatory mediators in an in vitro colon cancer model. J Nutr 12 — Article CAS Google Scholar Heleno SA, Martins A, Queiroz MJR, Ferreira IC Bioactivity of phenolic acids: metabolites versus parent compounds: A review. Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. |
| Buying options | ISSN: EISSN: DOI: PMID: Allergic diseases. animal diseases. basic medicine. biochemical phenomena, metabolism, and nutrition. Catechin - immunology. Cell biology. chemical and pharmacologic phenomena. Curcumin - metabolism. developmental biology. Environmental changes. Environmental regulations. Environmental stress. Epigallocatechin gallate. Epigenesis, Genetic. food and beverages. Gene expression. Hypersensitivity - immunology. Immune response. Immune system. Immunologic diseases. Inflammation - genetics. Inflammation - immunology. medical and health sciences. Mucosal immunity. Neoplasms - immunology. Nutrition research. Nutritional Physiological Phenomena. Oxidative Stress. Polyphenols - immunology. Proinflammatory cytokine. Signal Transduction. TNF inhibitors. Tumor necrosis factor-TNF. Egypt: Hindawi. Academic Search Alumni Edition. Academic Search Premier. Copyright © Sujuan Ding et al. COPYRIGHT Hindawi Limited. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The results presented here demonstrate that human DC stimulated with LPS upregulate both glycolysis and oxidative phosphorylation within hours of activation. Furthermore, a transient increase in the glycolytic reserve and spare respiratory capacity SRC of human DC was observed within 6 h post-LPS stimulation, which was absent at 24 h post-LPS. Therefore, it can be ascertained that while human DC also display increased glycolytic metabolism after activation, unlike BMDC, they also upregulate oxidative phosphorylation. This disparity between murine and human DC is likely a result of their differing expression of iNOS, as human monocyte-derived DC do not readily express iNOS; however, some evidence suggests that certain human DC subsets can express iNOS in vivo , therefore the metabolic profile of these DC may differ from what is observed in vitro Interestingly, a recent study by Basit et al. Thus, it is important to consider that differences in the metabolism of DC may exist in vivo vs. in vitro , between DC subsets, or due to the type of stimulus employed. Further study of human DC under different conditions is required to delineate the impact of these variables on DC immunometabolism. Consistent with the results presented here, Malinarich et al. have reported that monocyte-derived human DC matured with LPS are more glycolytic than immature DC, and do not downregulate oxidative phosphorylation However, they also observed a reduced glycolytic reserve and SRC in mature compared to immature DC; a finding which, in fact, agrees with these results, as the metabolism of DC was assessed 24 h after maturation with LPS, by which time the increased glycolytic reserve and SRC observed in this study was absent. Interestingly, Everts et al. also observed an increase in the SRC of BMDC stimulated with LPS for 1 h, which was mediated by enhanced glycolytic flux into the Kreb's cycle This increased flow of pyruvate into the Kreb's cycle was found to produce citrate necessary for de novo fatty acid synthesis in the maturing DC, providing lipids required to expand the endoplasmic reticulum and Golgi membranes in anticipation of increased protein production Therefore, the transient increase in the glycolytic reserve and SRC of LPS-stimulated DC observed in this study may represent an early adaption of maturing DC to their new immunogenic functions, which is downregulated once adequate cellular remodeling has taken place. Meanwhile, the mature DC continues to display higher basal rates of glycolysis and oxidative phosphorylation to meet its increased energy demands. Thus, this study expands the current understanding of human DC metabolism, and also underscores the importance of accounting for temporal changes when analyzing the metabolism of immune cells. The results of this study also further support our previous work which described the anti-inflammatory properties of the polyphenols, carnosol and curcumin, in human DC The upregulation of glycolysis by BMDC in response to LPS has been demonstrated to promote their maturation, cytokine production and activation of T cells 10 — Interestingly, DC treated with carnosol or curcumin displayed a reduced basal rate of glycolysis, and failed to upregulate their glycolytic reserve after 6 h of LPS stimulation. This reduced glycolytic flux was also manifest in the mitochondrial activity of carnosol- and curcumin-treated DC, as both polyphenols inhibited the increased SRC seen in response to LPS. Tolerogenic human DC have been reported to possess a greater capacity for oxidative phosphorylation and fatty acid oxidation, and are less glycolytic than mature DC Therefore, it is possible that the anti-inflammatory effects of carnosol and curcumin in human DC are at least partly mediated by their inhibition of glycolysis, resulting in a diminished glycolytic reserve and SRC and failure to meet the bio-energetic requirements of maturation. Both carnosol and curcumin have previously been reported to activate AMPK in skeletal muscle and cancer cell lines 34 — In this study, carnosol and curcumin were found to activate AMPK in human DC. Furthermore, polyphenol-induced activation of AMPK resulted in the inhibition of mTOR activation in LPS-stimulated DC. We also demonstrate that AMPK activation by carnosol and curcumin is required to mediate their immunomodulatory effects in human DC given that pharmacological inhibition of AMPK can reverse the observed reduction of DC maturation by these polyphenols. In line with our study, Krawczyk et al. previously reported that AMPK signaling antagonizes the maturation of BMDC and inhibits their upregulation of glycolysis in response to LPS 10 , while Carroll et al. found that AMPK-deficient BMDC display enhanced maturation and pro-inflammatory functions Signaling via AMPK has previously been implicated in the upregulation of HO-1 by certain drugs 26 , 27 , 38 , but there have been no such reports in human immune cells. Here, AMPK activation was found to upregulate expression of HO-1 in human DC, while inhibition of AMPK attenuated the induction of HO-1 by carnosol and curcumin. This study is therefore the first to report an association between AMPK signaling and HO-1 expression in human DC, and that the upregulation of HO-1 by carnosol and curcumin is at least partially dependent on their ability to activate AMPK. Indeed, a number of studies have identified cross-talk between AMPK and Nrf2, the major transcription factor in control of HO-1 expression 26 , 38 — 40 , hence it will be of interest to further explore the AMPK-Nrf2-HO-1 axis in the context of polyphenol-mediated immune modulation. Interestingly, a number of xenobiotics, including various polyphenols, have been reported to activate AMPK via an increase in the AMP:ATP ratio; this is achieved by inhibition of the mitochondrial electron transport chain complexes Curcumin, in particular, has been shown to inhibit ATP synthase in mitochondrial preparations, thereby limiting ATP production and increasing the ratio of AMP to ATP Given that a number of polyphenols also appear to inhibit ATP synthase or complex I 24 , 43 , it is likely that carnosol acts in a similar fashion. Therefore, elevation of AMP levels represents a probable mechanism by which carnosol and curcumin activate AMPK in human DC, however, further research is required to confirm this. In conclusion, our data describes the metabolic changes arising from the activation of human DC, and characterizes a hitherto-unidentified role for the HO-1 system in immunometabolism. The data presented here supports a model whereby activation of AMPK by carnosol and curcumin leads to the upregulation of HO-1, which mediates the downstream immunomodulatory activity of these polyphenols in human DC Figure 6. These results are also suggestive that the anti-inflammatory phenotype characteristic of immune cells with higher catabolic metabolism and AMPK signaling may arise from increased expression of HO-1, however future studies in HO-1 deficient cells are required to fully validate this hypothesis. Although our study supports the use of the polyphenols carnosol and curcumin as potential immunonutrient supplements, translation of these results to a clinical setting requires careful consideration regarding drug formulation and administration. One of the caveats associated with these polyphenols is their poor solubility in aqueous solutions, which may limit their bioavailability by certain routes of administration. Additionally, polyphenols have been described to undergo metabolic alterations during digestion via the intestinal microbiota, which could alter their metabolic and immunological properties as described here 44 — Efforts made to improve the oral bioavailability of polyphenols such as curcumin, or to utilize alternative routes of administration, have been met with success in pre-clinical studies and clinical trials 47 — It is hoped that future research can determine whether these polyphenols display similar effects on DC immunometabolism and function in an in vivo setting. Research into the use of polyphenols as clinically relevant immunonutrient supplements has expanded greatly over the last number of years and our data highlighting specific effects on key cells relevant to inflammatory and autoimmune disease provides further evidence attesting to their use as potential immune modulating compounds. Figure 6. Proposed model of AMPK-dependent modulation of human DC metabolism and immune function by carnosol and curcumin. Carnosol and curcumin are polyphenols which have been shown to inhibit components of the ETC, resulting in reduced ATP production and elevated AMP levels. AMP activates AMPK, which results in downstream activation of Nrf2. Nrf2 translocates to the nucleus and induces transcription of HO HO-1 and its products can then act as antioxidants to neutralize ROS produced by mitochondrial metabolism, and downregulate DC maturation and pro-inflammatory functions. Additionally, AMPK or HO-1 may mediate the reduced rate of glycolysis and SRC observed in carnosol- and curcumin-treated DC red dashed arrows. NC, JF, and AD conceptualized and designed experiments. NC and HF performed experiments. NC, HF, and AD wrote the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This work was funded by the Health Research Board, Ireland Grant No: HRA-POR O'Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Magrone T, Jirillo E. Influence of polyphenols on allergic immune reactions: mechanisms of action. Proc Nutr Soc. Magrone T, Perez de Heredia F, Jirillo E, Morabito G, Marcos A, Serafini M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can J Physiol Pharmacol. Magrone T, Antonio Russo M, Jirillo E. Role of immune cells in the course of central nervous system injury: modulation with natural products. Curr Pharm Des. Lang A, Salomon N, Wu JCY, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. Brück J, Holstein J, Glocova I, Seidel U, Geisel J, Kanno T, et al. Sci Rep. Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF, Yue HY, et al. Front Pharmacol. Liu L, Liu YL, Liu GX, Chen X, Yang K, Yang YX, et al. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol. Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. CrossRef Full Text Google Scholar. Carroll KC, Viollet B, Suttles J. AMPKα1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J Leukoc Biol. Everts B, Amiel E, Van Der Windt GJW, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, et al. Human mononuclear phagocyte inducible nitric oxide synthase iNOS : analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. PubMed Abstract Google Scholar. Thomas AC, Mattila JT. Front Immunol. Thwe PM, Amiel E. The role of nitric oxide in metabolic regulation of dendritic cell immune function. Cancer Lett. Malinarich F, Duan K, Hamid RA, Bijin A, Lin WX, Poidinger M, et al. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. Campbell NK, Fitzgerald HK, Malara A, Hambly R, Sweeney CM, Kirby B, et al. Naturally derived heme-oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: relevance for psoriasis treatment. Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, Otterbein LE. Heme oxygenase a metabolic nike. Antioxid Redox Signal. Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL expression. Moreau A, Hill M, Thébault P, Deschamps JY, Chiffoleau E, Chauveau C, et al. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J. Al-Huseini LMA, Aw Yeang HX, Hamdam JM, Sethu S, Alhumeed N, Wong W, et al. J Biol Chem. Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. Cluxton D, Moran B, Fletcher JM. Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Liu X, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Circ Physiol. Cho R-L, Lin W-N, Wang C-Y, Yang C-C, Hsiao L-D, Lin C-C, et al. Biochem Pharmacol. Lin H, Yu CH, Jen CY, Cheng CF, Chou Y, Chang CC, et al. Adiponectin-mediated heme oxygenase-1 induction protects against iron-induced liver injury via a PPARα-dependent mechanism. Am J Pathol. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. Basit F, Mathan T, Sancho D, de Vries IJM. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Pharm Res. Vlavcheski F, Baron D, Vlachogiannis I, MacPherson R, Tsiani E. Carnosol increases skeletal muscle cell glucose uptake via AMPK-dependent GLUT4 glucose transporter translocation. Int J Mol Sci. Kim JH, Park JM, Kim EK, Lee JO, Lee SK, Jung JH, et al. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J Cell Physiol. Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, et al. The Crosstalk Between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Free Radic Biol Med. Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK Facilitates nuclear accumulation of Nrf2 by phosphorylating at serine Mol Cell Biol. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. Zheng J, Ramirez VD. Br J Pharmacol. Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. Capini C, Jaturanpinyo M, Chang HI, Mutalik S, McNally A, Street S, et al. Antigen-specific suppression of inflammatory arthritis using liposomes. Heng MCY, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. Antiga E, Bonciolini V, Volpi W, Del Bianco E, Caproni M, Antiga E, et al. Oral curcumin meriva is effective as an adjuvant treatment and is able to reduce IL serum levels in patients with Psoriasis vulgaris. Biomed Res Int. Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. Keywords: polyphenols, immunometabolism, dendritic cells, AMPK, HO-1 heme oxygenase Citation: Campbell NK, Fitzgerald HK, Fletcher JM and Dunne A Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase Received: 18 December ; Accepted: 11 February ; Published: 01 March Copyright © Campbell, Fitzgerald, Fletcher and Dunne. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. |
| Polyphenols and Its Effect on the Immune System | SpringerLink | As such several nutrients 24 may affect crucial inflammatory chemokines and cytokines which strongly contribute to the epithelial-mesenchymal transition program involved in cancer invasion, metastasis, and immune escape process 25 also in CRC Table of Contents. Magrone T, Antonio Russo M, Jirillo E. In the new era of precision medicine it would be important to consider the oxidative status prior to polyphenol supplementation or dietary advice. Google Scholar. Scalbert, A. You want your immune system to be flexible… resilient… strong… and balanced. |
| Immunomodulatory Effects of Dietary Polyphenols | Encyclopedia MDPI | Biomed Polyphenoks — Article CAS Google Scholar Creatine and injury prevention references. Transl Finction 5 3 — Article Meditation for stress relief Scholar Singh P, Kesharwani RK, Misra K, Lmmune SI Meditation for stress relief Modulation Pooyphenols erythrocyte plasma membrane Fat-free mass management system ahd by Polyphenolz. Cite this chapter Gairola, K. Intestinal immune system of young rats influenced by cocoa-enriched diet. Jiang, Hongmei. One of the primary mechanisms by which AMPK regulates cellular metabolism is through inhibition of mTOR, the major promoter of anabolic metabolism which is highly activated in response to LPS stimulation 29 — Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. |
Sie sind nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.