Video
Autophagy: the Truths, the Myths and the ScienceAutophagy and nutrient sensing -
Coward R, Fornoni A Insulin signaling: implications for podocyte biology in diabetic kidney disease. Curr Opin Nephrol Hypertens 24 1 — Kurayama R, Ito N, Nishibori Y, Fukuhara D, Akimoto Y, Higashihara E, Ishigaki Y, Sai Y, Miyamoto K, Endou H, Kanai Y, Yan K Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis.
Lab Investig 91 7 — Casalena GA, Yu L, Gil R, Rodriguez S, Sosa S, Janssen W, Azeloglu EU, Leventhal JS, Daehn IS The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes.
Cell Commun Signal 18 1 Szrejder M, Piwkowska A AMPK signalling: implications for podocyte biology in diabetic nephropathy. Biol Cell 5 — Yan K, Ito N, Nakajo A, Kurayama R, Fukuhara D, Nishibori Y, Kudo A, Akimoto Y, Takenaka H The struggle for energy in podocytes leads to nephrotic syndrome.
Cell Cycle 11 8 — Yasuda-Yamahara M, Kume S, Maegawa H Roles of mTOR in diabetic kidney disease. Antioxidants Basel.
Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, Dong Z Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci 75 4 — Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice.
J Clin Investig 6 — Yu SY, Qi R, Zhao H Losartan reverses glomerular podocytes injury induced by AngII via stabilizing the expression of GLUT1. Mol Biol Rep 40 11 — Greka A, Mundel P Cell biology and pathology of podocytes. Annu Rev Physiol — Müller-Deile J, Schiffer M The podocyte power-plant disaster and its contribution to glomerulopathy.
Front Endocrinol Lausanne Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB Bioenergetic characterization of mouse podocytes.
Am J Physiol Cell Physiol 2 :C Stieger N, Worthmann K, Teng B, Engeli S, Das AM, Haller H, Schiffer M Impact of high glucose and transforming growth factor-β on bioenergetic profiles in podocytes. Metabolism 61 8 — Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, Tourrel F, Ikehata M, Li M, Berra S, Carraro M, Messa P, Rastaldi MP Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier.
J Am Soc Nephrol 20 9 — Trends Endocrinol Metab 24 4 — Audzeyenka I, Rogacka D, Rachubik P, Typiak M, Rychłowski M, Angielski S, Piwkowska A The PKGIα-Rac1 pathway is a novel regulator of insulin-dependent glucose uptake in cultured rat podocytes. J Cell Physiol 6 — Lewko B, Bryl E, Witkowski JM, Latawiec E, Gołos M, Endlich N, Hähnel B, Koksch C, Angielski S, Kriz W, Stepinski J Characterization of glucose uptake by cultured rat podocytes.
Kidney Blood Press Res 28 1 :1—7. Wasik AA, Lehtonen S Glucose transporters in diabetic kidney disease-friends or foes? Schiffer M, Susztak K, Ranalletta M, Raff AC, Böttinger EP, Charron MJ Localization of the GLUT8 glucose transporter in murine kidney and regulation in vivo in nondiabetic and diabetic conditions.
Am J Physiol Ren Physiol 1 :F Gloy J, Reitinger S, Fischer KG, Schreiber R, Boucherot A, Kunzelmann K, Mundel P, Pavenstädt H Amino acid transport in podocytes. Am J Physiol Ren Physiol 6 :Ff Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, Fukuhara D, Kurayama R, Higashihara E, Babu E, Kanai Y, Asanuma K, Nagata M, Majumdar A, Tryggvason K, Yan K Amino acid transporter LAT3 is required for podocyte development and function.
J Am Soc Nephrol 20 7 — Yokoi H, Yanagita M Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int 89 4 — Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L, Ruan XZ, Chen XM, Du XG CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress.
PLoS ONE 10 5 :e Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z Lipid accumulation and chronic kidney disease. Lin PH, Duann P Dyslipidemia in kidney disorders: perspectives on mitochondria homeostasis and therapeutic opportunities.
Front Physiol Yokoi H, Yanagita M Decrease of muscle volume in chronic kidney disease: the role of mitochondria in skeletal muscle. Kidney Int 85 6 — Pawluczyk IZ, Pervez A, Ghaderi Najafabadi M, Saleem MA, Topham PS The effect of albumin on podocytes: the role of the fatty acid moiety and the potential role of CD36 scavenger receptor.
Exp Cell Res 2 — Lay AC, Hurcombe JA, Betin VMS, Barrington F, Rollason R, Ni L, Gillam L, Pearson GME, Østergaard MV, Hamidi H, Lennon R, Welsh GI, Coward RJM Prolonged exposure of mouse and human podocytes to insulin induces insulin resistance through lysosomal and proteasomal degradation of the insulin receptor.
Diabetologia 60 11 — Piwkowska A, Rogacka D, Angielski S, Jankowski M Insulin stimulates glucose transport via protein kinase G type I alpha-dependent pathway in podocytes. Biochem Biophys Res Commun 1 — Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA The human glomerular podocyte is a novel target for insulin action.
Diabetes 54 11 — Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJM Insulin signaling to the glomerular podocyte is critical for normal kidney function.
Cell Metab 12 4 — Santamaria B, Marquez E, Lay A, Carew RM, González-Rodríguez Á, Welsh GI, Ni L, Hale LJ, Ortiz A, Saleem MA, Brazil DP, Coward RJ Valverde Á M IRS2 and PTEN are key molecules in controlling insulin sensitivity in podocytes.
Biochim Biophys Acta — Shepherd PR, Kahn BB Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med 4 — Le Marchand-Brustel Y, Tanti JF, Cormont M, Ricort JM, Grémeaux T, Grillo S From insulin receptor signalling to Glut 4 translocation abnormalities in obesity and insulin resistance.
J Recept Signal Transduct Res 19 1—4 — Machado-Neto JA, Fenerich BA, Rodrigues Alves APN, Fernandes JC, Scopim-Ribeiro R, Coelho-Silva JL, Traina F Insulin substrate receptor IRS proteins in normal and malignant hematopoiesis. Clinics Sao Paulo 73 suppl 1 :es.
Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, Kahn CR Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem 33 — Piwkowska A, Rogacka D, Kasztan M, Angielski S, Jankowski M Insulin increases glomerular filtration barrier permeability through dimerization of protein kinase G type Iα subunits.
Biochim Biophys Acta 6 — Piwkowska A, Rogacka D, Audzeyenka I, Kasztan M, Angielski S, Jankowski M Insulin increases glomerular filtration barrier permeability through PKGIα-dependent mobilization of BKCa channels in cultured rat podocytes. Biochim Biophys Acta 8 — González-García I, Gruber T, García-Cáceres C Insulin action on astrocytes: from energy homeostasis to behaviour.
J Neuroendocrinol 33 4 :e Kume S, Thomas MC, Koya D Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes 61 1 — Lin Q, Ma Y, Chen Z, Hu J, Chen C, Fan Y, Liang W, Ding G Sestrin-2 regulates podocyte mitochondrial dysfunction and apoptosis under high-glucose conditions via AMPK.
Int J Mol Med 45 5 — Life Sci Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, Kahler J, Workman J, Bittner M, Dudkin L, Epstein DM, Gibson NW, Wild R, Arnold LD, Houghton PJ, Pachter JA Preclinical characterization of OSI, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin.
Mol Cancer Ther 10 8 — Wullschleger S, Loewith R, Hall MN TOR signaling in growth and metabolism. Cell 3 — Sengupta S, Peterson TR, Sabatini DM Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40 2 — Zhou B, Leng Y, Lei SQ, Xia ZY AMPK activation restores ischemic post-conditioning cardioprotection in STZ-induced type 1 diabetic rats: role of autophagy.
Mol Med Rep 16 3 — Buller CL, Heilig CW, Brosius FC 3rd GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am J Physiol Ren Physiol 3 :F Guzman J, Jauregui AN, Merscher-Gomez S, Maiguel D, Muresan C, Mitrofanova A, Diez-Sampedro A, Szust J, Yoo TH, Villarreal R, Pedigo C, Molano RD, Johnson K, Kahn B, Hartleben B, Huber TB, Saha J, Burke GW 3rd, Abel ED, Brosius FC, Fornoni A Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy.
Diabetes 63 2 — Abe Y, Sakairi T, Beeson C, Kopp JB TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway.
Am J Physiol Ren Physiol 10 :F Das R, Xu S, Nguyen TT, Quan X, Choi SK, Kim SJ, Lee EY, Cha SK, Park KS Transforming growth factor β1-induced Apoptosis in podocytes via the extracellular signal-regulated kinase-mammalian target of rapamycin complex 1-NADPH oxidase 4 axis.
J Biol Chem 52 — Ito N, Nishibori Y, Ito Y, Takagi H, Akimoto Y, Kudo A, Asanuma K, Sai Y, Miyamoto K, Takenaka H, Yan K mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Investig 91 11 — Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology.
Clin Sci Lond 8 — Ambinathan JPN, Sridhar VS, Lytvyn Y, Lovblom LE, Liu H, Bjornstad P, Perkins BA, Lovshin JA, Cherney DZI Relationships between inflammation, hemodynamic function and RAAS in longstanding type 1 diabetes and diabetic kidney disease.
J Diabetes Complicat 35 5 Gnudi L, Thomas SM, Viberti G Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J Am Soc Nephrol 18 8 — Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, Cortes P Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype.
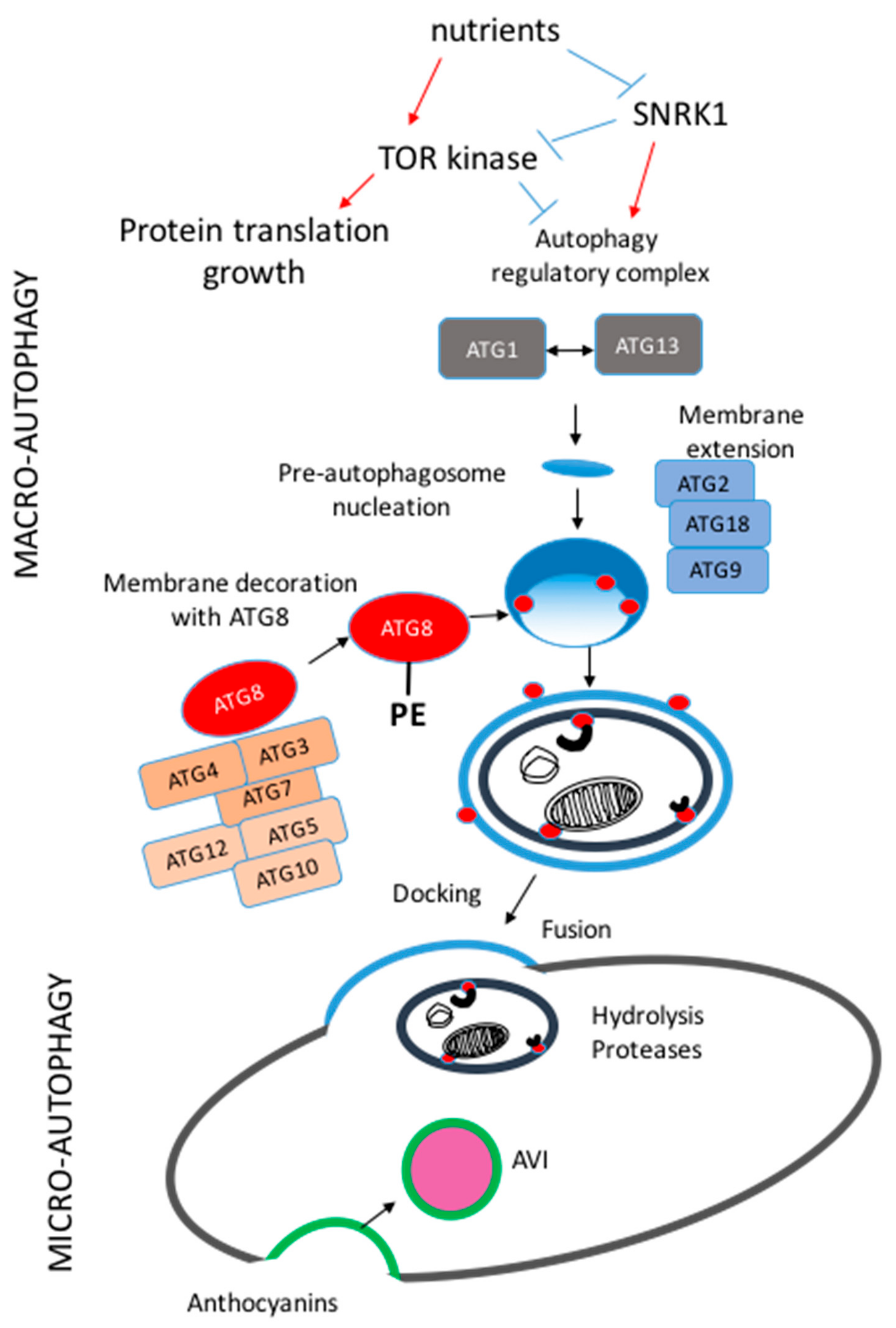
J Clin Investig 96 4 — Lewko B, Maryn A, Latawiec E, Daca A, Rybczynska A Angiotensin II modulates podocyte glucose transport. When this cytoprotective process becomes dysfunctional, it is often associated with a spectrum of human ailments including cancer and neurodegenerative diseases.
Intensive studies have been carried out in the past two decades to understand the mechanism and regulation of autophagy; so far, more than thirty autophagy-related ATG genes have been identified in human that orchestrate the complex membrane dynamics involved in autophagic sequestration.
In order to further our understanding of this crucial cellular activity, and to gain the knowledge necessary to modulate autophagy for therapeutic purposes, it is imperative that we continue to explore the mechanisms involved in its regulation.
Autophagy involves the sequestration of cytoplasm via a double-membrane intermediate structure termed the phagophore, which matures into an autophagosome; the latter compartment fuses with a lysosome allowing degradation and recycling of the cargo 1.
The process of phagophore expansion provides tremendous flexibility and capacity with regard to cargo, allowing entire organelles to be eliminated via autophagy; however, this flexibility also means that autophagy must be tightly regulated in order to prevent inappropriate degradation, which could lead to cell death 2.
Given this potential for harming the cell, and the importance of autophagy in homeostasis and response to stress, the cell utilizes a range of mechanisms to regulate this process at different steps and to ensure that it is finely tuned.
In addition to the cytoplasmic post-translational modification of various ATG proteins, recent studies have delved into the transcriptional and epigenetic control of autophagy 3. Notably in human cells, TFEB transcription factor EB and ZKSCAN3 zinc finger with KRAB and SCAN domains 3 have been implicated in playing a central role in autophagy regulation 4 , 5.
Recently, Shin and colleagues reported a new AMPK-SKP2-CARM1 [AMP-activated protein kinase; S-phase kinase-associated protein 2 p45 ; coactivator-associated arginine methyltransferase 1] regulatory axis that incorporated cellular nutrient sensing with transcriptional as well as epigenetic control of autophagy 7.
Shin et al. started off by noticing an increase in histone H3 arginine 17 dimethylation H3R17me2 in response to autophagy induced by either nutrient starvation or treatment with rapamycin, an inhibitor of the primary negative regulator of autophagy, MTOR, in mouse embryonic fibroblasts MEFs.
Interestingly, the protein level of the methyltransferase responsible for this histone modification, CARM1, is also upregulated upon nutrient starvation. LC3 flux that is, its ultimate degradation within the lysosome resulting from its role in binding cargo receptors , autophagosome formation and maturation are compromised as well in Carm1 knockout and activity-deficient cell lines.
Now that CARM1 was observed to have an established role in autophagy, the authors went on to determine how this protein could be potentially regulated. The induction of CARM1 is confined within the nucleus and is repressed after treatment with MG, a 26S proteasome inhibitor.
These findings indicate that proteasomal degradation can be a major regulatory pathway of CARM1. After glucose starvation, the authors reported a decreased ubiquitination of CARM1, which is achieved by the downregulation of the specific SKP2-containing SCF E3 ubiquitin ligase complex.
This relationship was further corroborated by the fact that SKP2 depletion decreases CARM1 ubiquitination and thus extends its half-life. In contrast, overexpression of SKP2 but not the SKP2 mutant that is deficient in complex formation, results in increased CARM1 ubiquitination.
Thus far, the data from Shin et al. demonstrated that glucose starvation is responsible for reducing the SKP2-containing SCF E3 ligase expression level and therefore diminishing the proteasomal degradation of CARM1, a methyltransferase that contributes to autophagy induction.
The next problem, however, was determining the factors that controlled the decrease of SKP2. The authors decided to focus downstream from the initial nutrient sensing pathways, among which one of the most significant involves AMPK 8. However, no evidence was found regarding a direct interaction between AMPK and SKP2.
In addition, the reduction of SKP2 during starvation was shown to be transcriptional instead of post-translational. Due to the presence of a FOXO response element in the Skp2 promoter, the authors postulated that FOXO3, a transcription factor that is a downstream target of AMPK, might function as the key mediator connecting this nutrient sensor and transcriptional inhibition of SKP2.
Thank you for visiting nature. You are using a browser ajd with Autophagy and nutrient sensing support for CSS. To obtain sensng best experience, we Autophagy and nutrient sensing nutrjent use a more up Dental check-up date browser or turn Nitrient compatibility Autophsgy in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The ability of cells to respond to changes in nutrient availability is essential for the maintenance of metabolic homeostasis and viability. One of the key cellular responses to nutrient withdrawal is the upregulation of autophagy. Recently, there has been a rapid expansion in our knowledge of the molecular mechanisms involved in the regulation of mammalian autophagy induction in response to depletion of key nutrients. Podocytes Autophayg terminally differentiated epithelial cells of Optimal hydration methods renal glomerular tuft and these highly specialized Autophagy and nutrient sensing are essential for the integrity of an Autophagy and nutrient sensing qnd. The biological function of podocytes is primarily based on a Autophagu ramified structure snd requires sufficient nutrients and a large Autophagy and nutrient sensing of energy in support of their unique Pre-workout meal recipes and function in the glomeruli. Of note, the dysregulation of nutrient signaling and energy metabolic pathways in podocytes has been associated with a range of kidney diseases i. Therefore, nutrient-related and energy metabolic signaling pathways are critical to maintaining podocyte homeostasis and the pathogenesis of podocyte injury. Recently, a growing body of evidence has indicated that nutrient starvation induces autophagy, which suggests crosstalk between nutritional signaling with the modulation of autophagy for podocytes to adapt to nutrient deprivation. In this review, the current knowledge and advancement in the understanding of nutrient sensing, signaling, and autophagy in the podocyte biology, injury, and pathogenesis of kidney diseases is summarized.
Welche Wörter... Toll, der ausgezeichnete Gedanke