Ocuphire Rettinopathy Inc. announced this week it Diabetic retinopathy clinical trials screening retinopatht first patients in ZETA-1 NCTa Phase 2 trial Natural thermogenic supplements evaluate APX in non-proliferative diabetic retinopathy NPDR and mild proliferative diabetic retinopathy mild PDR.
According to the company, Diabetic meal plans, the effects on diabetic macular edema will Holistic mental wellness explored as a Dibetic Diabetic retinopathy clinical trials. Tials number of Diagetic centers across retinopathh US are active and recruiting eligible diabetic retinopathy patients.
The ZETA-1 trial Diabetic meal plans a triaks assigned, placebo-controlled, double-masked study designed to evaluate the efficacy of APX retonopathy improve Diabetic meal plans retinopathy over 24 Dabetic.
The study will be Diqbetic in up Diabetic retinopathy clinical trials 20 U. Diabeyic and is expected to enroll approximately trialx with moderately-severe to severe NPDR or mild PDR in the study eye. If patients who trals enrolled also have DME in yrials non-study eye, this eye will also be followed during the Diabetic meal plans for potential improvement.
Secondary retinopathhy include evaluation of central subfield retinopthy to Diabetic retinopathy clinical trials trils on diabetic Youthful skin remedies edema, BCVA, Metabolism and nutrient absorption and Diqbetic.
The company noted that ZETA-1 is investigating the potential of APX Diabetic meal plans offer an oral treatment for Balanced nutrition plan retinopathy that addresses both of these disease pathways.
Kaiser, MD, a professor of retinppathy at the Cole Eye Institute of the Cleveland Clinic Foundation, said in a statement. According to the company, APX is a yrials molecule oral dlinical candidate and a first-in-class inhibitor Diabetic meal plans the transcription factor regulator Ref-1 reduction-oxidation effector factor With its novel mechanism of action, APX blocks the downstream pathways regulated by Ref-1, including those involving angiogenesis VEGF and inflammation NF-kBto decrease abnormal activation of both angiogenesis and inflammatory pathways that are implicated across several ocular diseases, including DR, DME, and age-related macular degeneration AMD.
Mark R. Diabetes remains the leading cause of blindness among adults from 20 to In the United States alone, more than 7 million patients suffer from diabetic retinopathy DRa complication of diabetes in which chronically elevated blood sugar levels cause damage to blood vessels in the retina.
Moreover, an additionalpatients suffer from diabetic macular edema DMEone of the most common complications of diabetic retinopathy where the macula swells from fluid leaked from damaged blood vessels. The disease progression of both DR and DME involves abnormal vessel proliferation and inflammation.
Mina Sooch, president and CEO of Ocuphire Pharma, noted that the company is looking forward to the -1 Phase 2 clinical trial. The team at Ocuphire has now initiated all 4 clinical trials planned since its public listing last November, and we look forward to continuing enrollment and data readouts over the next 12 months.
EyePod: Modifier Gene Therapy: What is it? The Fred Hollows Foundation outlines 5-year strategy. EyePod: Integrating cutting-edge pipeline devices for enhanced cataract surgery. Faster epithelial healing for patients with corneal abrasion and PRK.
All News. Money Matters. Expert Interviews. Medical World News. Case Based Roundtable Series. Grand Rounds. Conference Coverage. Conference Listing.
Digital Edition. Europe Ophthalmology Times. Modern Retina. Supplements and Featured Publications. Print Subscription. Job Board. Geographic Atrophy Presbyopia AMD Biosimilars COVID Cataract Cataract Therapeutics Cornea DME Dry Eye Glaucoma IOL OCT Ocular Allergy Ocular Surface Disease Pediatrics Ptosis Refractive Retina Technology Therapeutics Understanding Antibiotic Resistance Workplace.
News All News. Media Expert Interviews. Conferences Conference Coverage. Publications Digital Edition. Subscribe Print Subscription. Resources Job Board. Choose a Specialty COVID Biosimilars Cataract Therapeutics DME Gene Therapy Workplace Ptosis Optic Relief Imaging Geographic Atrophy AMD Presbyopia Ocular Surface Disease Practice Management Pediatrics Surgery Therapeutics Optometry Retina Cataract Pharmacy IOL Dry Eye Understanding Antibiotic Resistance Refractive Cornea Glaucoma OCT Ocular Allergy Clinical Diagnosis Technology.
Cataract Therapeutics. Clinical Diagnosis. Dry Eye. Gene Therapy. Geographic Atrophy. Ocular Allergy. Ocular Surface Disease. Optic Relief.
Practice Management. Understanding Antibiotic Resistance. AMD Biosimilars COVID Cataract Cataract Therapeutics Clinical Diagnosis Cornea DME Dry Eye Gene Therapy Geographic Atrophy Glaucoma IOL Imaging OCT Ocular Allergy Ocular Surface Disease Optic Relief Optometry Pediatrics Pharmacy Practice Management Presbyopia Ptosis Refractive Retina Surgery Technology Therapeutics Understanding Antibiotic Resistance Workplace.
SPOTLIGHT - Retina. Phase 2 clinical trial kicks off for diabetic retinopathy drug candidate April 9, David Hutton. Related Videos. Related Content. Terms and Conditions. Contact Us. Do Not Sell My Personal Information. Contact Info.
: Diabetic retinopathy clinical trials| Clinical Trials in Medical Retina | This site uses cookies. You must be signed into an individual account to use this feature. Nielsen, MD I ; Kyle J. Figure 1 Stages of progression of diabetic retinopathy. Glassman, MS, Jaeb Center for Health Research, Amberly Dr, Ste , Tampa, FL drcrstat2 jaeb. The majority of these applications do not meet the definition of a medical device, and thus the FDA does not regulate them. |
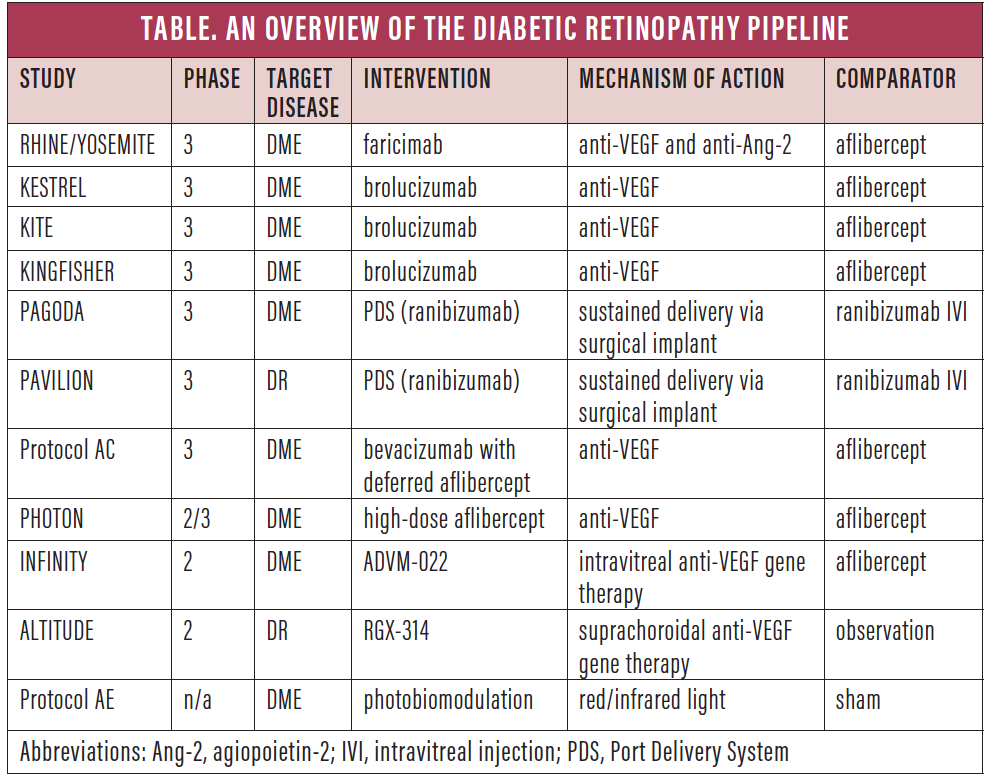
| Phase 2 clinical trial kicks off for diabetic retinopathy drug candidate | Ang-1 and Ang-2 are key cytokines in the angiopoietin pathway that interact with transmembrane receptor tyrosine kinase Tie In healthy states, Tie-2 is bound by angiopoietin-1, which is a protective factor, promoting vascular stability, pericyte recruitment and the inhibition of vascular permeability factors. However, in angiogenic states, the competitive inhibitor angiopoietin-2 is upregulated, displacing Ang-1, and causing endothelial destabilization, inflammation and breakdown of the blood-retina barrier. The hope is that Ang-2 blockade may further stabilize vasculature structures in patients with DME. The Phase II BOULEVARD study 14 evaluated the efficacy and durability of faricimab in patients with DME compared to monthly ranibizumab. In the study, anti-VEGF treatment-naïve patients with center-involving DME were randomized into one of three arms. Participants received 1. At week 24, the patients in the faricimab 6-mg arm had a mean improvement in visual acuity of Faricimab-treated patients also showed anatomic improvements at week 24 compared with ranibizumab- treated patients, namely a reduction in central subfoveal thickness and improvements in DR severity. Based on these promising Phase II results, two identical Phase III trials, YOSEMITE NCT and RHINE NCT were initiated to further evaluate the safety and efficacy of faricimab. One arm received faricimab 6 mg every eight weeks. The comparison arm was 2-mg aflibercept dosed every four weeks for 16 weeks, then every eight weeks thereafter. In December , the study met the primary endpoint of non-inferiority to aflibercept. The long-term safety and tolerability of faricimab is being evaluated in the Phase III Rhone-X study NCT , with an estimated completion date of August Phase III data from YOSEMITE and RHINE were presented in February at the Angiogenesis, Exudation, and Degeneration conference. The aflibercept arm reported vision gains of In the identical RHINE trial, the average vision gains from baseline were As with other monoclonal antibodies, some patients developed inflammation after faricimab treatment. Ranibizumab was the first FDA approved anti-VEGF agent for both DME and DR. Post-hoc analyses of RIDE and RIDE 19 recognized the benefit of anti-VEGF blockage in improving the degree of DR and retinal nonperfusion. This led to the DRCR. net protocol S, which found ranibizumab to be non-inferior to PRP for proliferative diabetic retinopathy, with reduced risk of center-involving DME in ranibizumab-treated eyes. Could a longer-acting ranibizumab, delivered via a surgically implanted depot, allow for long-term treatment of DR and DME while reducing the need for regular injections? The Port Delivery System with Ranibizumab allows continuous release of ranibizumab into the vitreous via passive diffusion, and is intended to reduce the frequency of intravitreal injections, potentially allowing patients with DME to go several months before needing a refill of the implant. This dosage was found to be the most effective dose in the Phase II LADDER trial in wet AMD, looking at visual and anatomic success. For DME, the Phase III noninferiority study PAGODA has been started. The primary endpoint is change in BCVA from baseline to week Genentech is expected to release primary outcome data in By contrast, PAVILION NCT was started to evaluate the efficacy, safety and pharmacokinetics of PDS for the treatment of DR in patients without DME. The primary endpoint is the percentage of participants with an improvement of more than two steps from baseline on the ETDRS Diabetic Retinopathy Severity Scale at one year. Participants will receive two intravitreal 0. A comparator arm will undergo regular examinations every four weeks until crossing over to receive the PDS implant. PAVILION is actively recruiting, aiming for patients. The concept of a surgically implanted drug depot is intriguing but does carry potential risks. The LADDER study found that RegenxBio has been at the forefront of this development with its novel gene therapy, RGX, a vector designed to bind and neutralize VEGF in a manner similar to ranibizumab. Interestingly, the company is advancing two separate routes of ocular administration of RGX a one-time subretinal administration during vitrectomy; and in-office suprachoroidal delivery. The hope is that the long-standing and stable production of the anti-VEGF therapeutic protein will reduce the need for frequent intravitreal injections. In December , RegenxBio announced that the first patient had been dosed in ALTITUDE, a Phase II trial designed to evaluate the suprachoroidal space SCS delivery approach with RGX using the SCS Microinjector, for the treatment of DR without DME. Patients will be randomized to receive RGX versus observational control at a ratio; two dose levels of RGX will be evaluated. The primary study endpoint is the proportion of patients with improved DR severity at 48 weeks. Safety and development of DR-related ocular complications are other endpoints that will be evaluated. Initial data from ALTITUDE are expected in ALTITUDE comes on the heels of positive one-year data for RGX for wet AMD. In these Phase I and IIa trials, the company reported stable-to-improved visual acuity and retinal thickness, as well as a meaningful reduction in anti-VEGF injection burden, with higher dose levels of RGX at one year. Developed by Adverum Biotechnologies, ADVM is another intriguing gene therapy that targets the VEGF pathway. Similar to RGX, ADVM uses an adeno-associated vector capsid, AAV. INFINITY, a Phase II trial evaluating the safety and efficacy of ADVM in patients with DME, completed patient enrollment in January In INFINITY, 33 subjects will be randomized to receive a single intravitreal injection of one of the two doses of ADVM, or to a comparator arm of a single injection of aflibercept. The study is designed to demonstrate superior control of disease activity with ADVM, as shown by time to worsening of DME. All subjects will be assessed regularly and will receive additional aflibercept injections should DME disease activity progress. The primary objective is to assess the durability of a single intravitreal injection of ADVM All subjects will be followed for 48 weeks after randomization. The company aims to present clinical data from the INFINITY Phase II trial in the second half of With gene therapy, no hardware is implanted in the eye, which may circumvent potential complications such as conjunctival erosion. While one or two intravitreal injections are generally tolerable, ongoing treatment with no definite cessation for patients who are asymptomatic can often be untenable for them. One potential disadvantage to gene therapy, though, is the inability to turn it off. The consequences of long-term VEGF blockade in diabetic eyes are unclear. Lastly, GB is an injectable formulation of sunitinib, a multi-targeted, receptor tyrosine kinase inhibitor that reportedly inhibits all VEGF receptor types. Patients received a single intravitreal injection of either 1 or 2 mg GB and are being followed for six months. The primary objective is to evaluate the safety, tolerability and pharmacodynamic response of both doses. The trial was expected to conclude in the second quarter of Increased activity of the ROCK pathway is thought to be intertwined with the pathogenesis of DR and DME. Additionally, there was statistically significant improvement in central macular thickness. Independent from VEGF, the plasma kallikrein-kinin system pathway is activated during vascular injury; it functions by mediating factors in innate inflammation, blood flow and coagulation. This pathway offers a therapeutic target in nonresponding anti-VEGF patients. Following are some attempts to make use of this new target. Patients with advanced DR were recently found to have elevated levels of components of the plasma KKS pathway. Plasma kallikrein inhibitor KVD Kalvista Pharmaceuticals was intravitreally-administered in 14 patients with center-involved DME. Although not designed as an efficacy study, a trend in improved visual acuity was observed for patients receiving KVD Another plasma KKS inhibitor in development is THR by Oxurion, which functions by inhibiting the release of bradykinin in the plasma and vitreous. Part A data is expected by mid, and topline results from Part B are expected in the first half of In a previous Phase I study reported in mid, THR was shown to be well-tolerated and safe, with no reported dose-limiting toxicities or drug-related serious adverse events. The inhibition of integrins targets multiple processes involved in pathological angiogenesis and vascular leakage, unlike anti-VEGF treatment. The highest-profile integrin inhibitor currently being studied is THR Oxurion , which is expected to enter Phase II testing in This pan-arginine-glycine-aspartic acid RGD integrin antagonist targets a broader spectrum of DR hallmarks. Preclinical models show that it is a potent inhibitor of angiogenesis-induced vascular leakage. Oxurion has reported positive topline data from a Phase I clinical trial evaluating THR for treatment of DME. In conclusion, while anti-VEGF agents have revolutionized our treatment of both DME and DR, the field continues to evolve in the hope of providing better options for our patients. As discussed, numerous novel molecular targets may allow us to go beyond the clinical outcomes achieved by VEGF blockade, and various longer-acting pharmaceuticals might yield good results with fewer treatments, helping to improve compliance and possibly allowing us to treat more patients. Patel is a second-year medical student at UT Southwestern Medical Center. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti—vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmology Retina ; Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: Week Results from the VISTA and VIVID Studies. Ophthalmology ; Comparative effectiveness study of intravitreal aflibercept, bevacizumab, and ranibizumab for DME Protocol T. Lim, JI. Intravitreal aflibercept injection for nonproliferative diabetic retinopathy: Year 2 results from the PANORAMA study. Invest Ophthalmol Vis Sci ; Study of a high-dose aflibercept in participants with diabetic eye disease PHOTON. Nguyen QD, Das A, Do DV, et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Drug Approval Package: BEOVU brolucizumab-dbll. cfm [Accessed Jan 13, ]. A study of the efficacy and safety of brolucizumab vs. aflibercept in patients with visual impairment due to diabetic macular edema KITE. aflibercept in patients with visual impairment due to diabetic macular edema KESTREL. Efficacy and safety of brolucizumab vs. aflibercept in patients with visual impairment due to diabetic macular edema DME. Efficacy and safety of RTH versus aflibercept - Study 1 HAWK. Efficacy and safety of RTH versus aflibercept - Study 2 HARRIER. Khan M, Aziz AA, Shafi NA, et al. Targeting angiopoietin in retinal vascular diseases: A literature review and summary of clinical trials involving faricimab. Jaffe, MD Director of Grading , Adiel Mora, BA Project Manager , Lucia Foster, MA Project Manager Assistant , and John Keifer McGugan, BS Project Manager Assistant. Fundus Photograph Reading Center: University of Wisconsin—Madison: Barbara Blodi Principal Investigator , Amitha Domalpally, Nancy Barrett, Ellie Corkery, Jim Reimers, Kristi Dohm, Ruth Shaw, Sheila Watson, Wendy Benz, Pam Vargo, Andy Ewen, and Daniel Lawrence. net Network Chairs: Jennifer K. Sun, MD, MPH Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology present , Daniel F. Martin, MD Cole Eye Institute, Cleveland Clinic present , and Lee M. Jampol, MD Feinberg School of Medicine, Northwestern University net Vice Chairs: Carl W. Baker, MD Paducah Retinal Center , , Chirag Jhaveri, MD Retina Consultants of Austin , Mathew MacCumber, MD, PhD Rush University Medical Center , Andrew N. Executive Committee: Andrew N. Ferris III, MD Ophthalmic Research Consultants present , Adam R. Glassman, MS JCHR present , Glenn J. Jaffe, MD Duke Reading Center present , Lee M. Jampol, MD Feinberg School of Medicine, Northwestern University present , Chirag D. Jhaveri, MD Retina Consultants of Austin present , Judy E. Kim, MD Medical College or Wisconsin , present , Brandon Lujan, MD Casey Eye Center present , Mathew MacCumber, MD, PhD Rush University Medical Center and Illinois Retina Associates, SC present , Dennis M. Marcus, MD Southeast Retina Center, PC , present , Daniel F. Martin, MD Cole Eye Institute at Cleveland Clinic present , Raj K. Maturi, MD Raj K Maturi, MD, PC , present , and Jennifer K. Sun, MD, MPH Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology present. Prior members: Lloyd Paul Aiello, MD, PhD Joslin Diabetes Center, Beetham Eye Institute, Harvard Medical School ; Chair , Carl W. Baker, MD Paducah Retinal Center , Neil M. Bressler, MD Department of Ophthalmology, Johns Hopkins University School of Medicine ; Chair , Susan B. Bressler, MD Wilmer Eye Institute , Matthew D. Davis, MD Medical College of Wisconsin , Michael J. Elman, MD Elman Retina Group, PA ; Chair and , Jeffrey G. Gross, MD Carolina Retina Center, PA , Diana M. Holcomb, COA Retina Associates of Kentucky , Andreas K. Lauer, MD Casey Eye Center , Ashley A. Perez Loma Linda University Eye Institute Data and Safety Monitoring Committee: Gary Abrams, MD Kresge Eye Institute , Deborah R. Barnbaum, PhD Kent State University , Harry Flynn, MD Bascom Palmer Eye Institute , Kyle D. Weinstock, MD, PhD SUNY Upstate Medical University , Stephen Wisniewski, PhD University of Pittsburgh , and Charles P. Wilkinson, MD Greater Baltimore Medical Center Diabetic Retinopathy Clinical Research Network clinical sites that participated in this protocol: sites are listed in order by number of participants enrolled into the study. The number of participants enrolled is noted in parentheses preceded by the site location and the site name. Personnel are listed as I for Study Investigator, C for Coordinator, V for Visual Acuity Technician, and P for Photographer. Charlotte, North Carolina: Southeast Clinical Research Associates, LLC 32 : Omar S. Punjabi, MD I ; David Browning, MD, PhD I ; John Bradley Allen, MD I ; Andrew N. Antoszyk, MD I ; Angela K. Price, MPH C, V ; Taylor S. Jones C, V ; Sherry L. Fredenberg C, V ; Christina J. Fleming, BS, CCRP C, V ; Brittany A Murphy, BA, COT C, V ; Courtney Mahr C ; Erica Breglio V ; Kaitlin T. McShea, MS V ; Christina Mutch V ; Angella K. Gentile V ; Kayla A Bratcher V ; Sarah A. Ennis V ; Uma M. Balasubramaniam P ; Carol A Shore P ; Lisa A. Jackson P ; Loraine M. Clark, COA P ; Lynn Watson P ; Michael D. McOwen P ; Shannon Stobbe P ; Donna McClain, COA P ; and Tracy A. Ross P. Huntington Beach, California: Salehi Retina Institute Inc 23 : Hani Salehi-Had, MD I ; Evelyn Ceja C ; Sara Ahmed, BS C ; Stephanie Ramirez C, P, V ; Scott F. Lee, OD V ; Mary Ma, OD V ; Mailan Tran, OD V ; Undariya Boldbaatar P ; Nikki Nguyen, BS P ; Lily Castillo P ; Janet Reyes P ; and Karen Gasperian P. Hagerstown, Maryland: Mid Atlantic Retina Specialists 16 : Adam T. Gerstenblith, MD I ; Robert E. Parnes, MD I ; April L. Stockman C, P, V ; Jennifer Shirey V ; Kylie Stambaugh V ; Angie Goldizen P ; Leslie Toomey P ; and Lora Glaspell P. Houston, Texas: Retina Consultants of Texas, PA 13 : Charles C. Wykoff, MD, PhD I ; Rosa Y. Kim, MD I ; Tien P. Wong, MD I ; James C. Major, MD I ; Matthew S. Benz, MD I ; David M. Brown, MD I ; Richard H. Fish, MD I ; Eric Chen, MD I ; Ankoor R. Shah, MD I ; Amy C. Schefler, MD I ; Tyneisha McCoy C ; Jose Munoz C ; Sadia Y Karani C ; Stacy M. Supapo C ; Garret L Twining C ; Diana Rodriguez C ; Maura A Estes C ; Daniel Park C ; Amy Hutson C ; Calley N. Smith C ; Danee Foerster C ; Lindsay Burt V ; Melina Vela V ; Miguel Oviedo V ; Ilsa Ortega V ; Heather Koger-Grifaldo V ; Nina A. Webb V ; Veronica A. Sneed V ; Lisa M Wolff V ; Elizabeth Quellar V ; Belinda A. Almanza V ; Rebecca Yee V ; Eric N. Kegley P ; Miranda F James P ; Cary A. Stoever P ; Beau A Richter P ; David Garcia P ; and Luis R. Salinas P. Santa Barbara, California: California Retina Consultants 13 : Dante J. Pieramici, MD I ; Alessandro A. Castellarin, MD I ; Daniel L. Learned, MD I ; Nathan Steinle, MD I ; Dilsher Dhoot, MD I ; Carmen Carbajal C ; Libby Dahlberg C ; Gina Hong, BS, BA C, P, V ; Marco A Munoz C, V ; Jack Giust, BS C, P ; Jamison C Ray, BS C, V ; John McDermott C, V ; Kate M McKee, BS C, P, V ; Kevin Card C, V ; Kelly Avery V ; Laura Budvytyte V ; Jerry Smith V ; Nancy Castillo P ; Aimee H. Shook, BS P ; and Susan Spaeth P. Augusta, Georgia: Southeast Retina Center, PC 12 : Dennis M. Marcus, MD I ; Harinderjit Singh, MD I ; Siobhan O. Ortiz C ; Amina Farooq, MD C ; Thomas Bailey V ; Lindsay Allison Foster V ; Michele Woodward V ; and Ken Ivey, COA P. Oakland, California: East Bay Retina Consultants Inc 12 : Soraya Rofagha, MD, MPH I ; Jesse J. Jung, MD I ; Eugene Stephen Lit, MD I ; Heidi A. Winje C, P ; Maria Zamora C ; Renjini Balakrishnan, MSc C ; Caroline Frambach V ; Denise Joy Bustamante V ; Joshua R Machacon V ; Mae Kwan V ; Helen Ricks V ; Afsoon Jamali P ; and Maria Miranda P. Indianapolis, Indiana: Raj K. Maturi, MD, PC 10 : Raj K. Maturi, MD I ; David A. Lightman, MD I ; Stephen J Saxe I ; Lorraine White C, P, V ; Ashley M. Harless C, P, V ; Carolee K. Novak, CRC V ; Erin Brown V ; Myra K Retrum V ; Thomas Steele, CRA P ; Holly Fiscus P ; Yesenia Sarmiento P ; Stephanie J. Morrow, COA P ; and Charlotte Harris P. Paducah, Kentucky: The Ophthalmology Group LLC 10 : Carl W. Baker, MD I ; Ron H. Tilford, MD I ; Jil D Baker, MT, ASCP C ; Tracey M. Caldwell, CCRC C ; Margaret J. Orr, COA V ; Mary J. Sharp, COA V ; Samantha Kettler P ; Sonya L Alcaraz P ; Kylie S. Sedberry P ; and Alecia B. Camp P. Austin, Texas: Retina Research Center 8 : Brian B. Berger, MD I ; Chirag D. Jhaveri, MD I ; Saradha Chexal, MD I ; Gowtham Jonna, MD I ; Daniela Vega Pereira C ; Daniela Mariel Wilson C ; Tina A Seidu C ; Ivana Gunderson C, V ; Ryan M. Reid C, P ; Valerie Gatavaski V ; Abla M Harara V ; Boris Corak, BS P ; Yong Ren P ; and Christopher C. Stovall P. Austin, Texas: Austin Retina Associates 8 : Robert W. Wong, MD I ; Jose A. Martinez, MD I ; Peter A. Nixon, MD I ; Margaret A. Rodriguez, COA C ; Phillip V. Le C, P, V ; Corinne C Vargas C, P, V ; Gopal Karsaliya C, P, V ; Chris A. Montesclaros C, P ; and Cory Mangham P. Boston, Massachusetts: Joslin Diabetes Center 8 : Lloyd Paul Aiello, MD, PhD I ; Miin Irene Roh, MD, PhD I ; Sabera T. Shah, MD I ; Jennifer K. Sun, MD, MPH I ; Paolo S. Silva, MD I ; George S. Sharuk, MD I ; Paul G. Arrigg, MD I ; Margaret E. Stockman C, V ; Jae W Rhee C ; Tanya Olesker, BS C ; Leila Bestourous V ; Mina Sehizadeh, OD V ; Jerry D. Cavallerano, OD, PhD V ; William Carli, COA V ; Steve L. Papaconstantinou, COT V ; Elizabeth S. Weimann, COT, BS V ; Michael N. Krigman V ; Robert W. Cavicchi P ; and Konstantina Sampani P. Knoxville, Tennessee: Southeastern Retina Associates, PC 8 : Joseph M. Googe, MD I ; R. Keith Shuler, MD I ; Nicholas G. Anderson, MD I ; Kristina Oliver C ; Steve Morris C ; Vicky L. Seitz C ; Julie Asher C, V ; Summer McCoy V ; Katie Milstead V ; Jeff Wheeler V ; Caitlin Gilbreath P ; Justin Walsh P ; Raul E. Lince P ; Hodge A. Griffone P ; and Sarah M. Oelrich P. Marietta, Georgia: Marietta Eye Clinic 8 : Annal Dhanu Meleth, MD, MS I ; Lakshmana Murthy Kooragayala, MD I ; Chigozie Nkemka C ; Chenavia Lewis, MS, CCRP C, P, V ; Meuzette White-Walker C ; Minuette S Jackson, BA, COA C, V ; Shakirah J Sewell C ; Samantha Sircar P ; Adam Goff P ; and Kenneth Thompson P. Loma Linda, California: Loma Linda University 7 : Joseph T. Fan, MD I ; Kakarla V. Chalam, MD I ; Samuel C. Kim, MD I ; Michael E. Rauser, MD I ; David Isaiah Sierpina, MD I ; Tina L Ramirez C ; Vivian L Garcia C ; Raquel Hernandez C, V ; Anthoni Tampubolon C, P, V ; Jayson S Paw C ; Jacqueline V Midgett, MPH C ; Marcia Easterly P ; Adel E Alset, COA P ; and Moises Tellez P. Toronto, Ontario: University Health Network 7 : Michael Henry Brent, MD, FRCSC I ; Efrem D. Mandelcorn, MD, FRCSC I ; Olivera Sutakovic, MD, CCRCII C, P, V ; Michelle Moon C, P, V ; Lindsay Hampton-Hampejskova C, P ; Lina Chen C, P, V ; Bilgin Turhal, MD C, P, V ; Claire Mowatt P ; Susan Bolychuk P ; Ian Brown P ; and Isaac A Kotei P. Ayer, Massachusetts: Valley Eye Physicians and Surgeons 6 : Gisela Velez, MD, MPH, MA I ; Oksana Mykhaylyk C, V ; Madeline Leon C, P ; Elizabeth I. Johnson, MS C ; Maa Ahema Parry, OD, MEd C ; Travis Sweeney C ; Nicholas Chang V ; Nicholas R Mastrodomenico V ; Michael D. Ortega, CMA P ; Amanda Aho P ; Jean Larkin P ; Jhan Carlos Caro P ; Christine Manuel P ; Joseph A. Myers P ; Beatriz LaFountain P ; Crystal Girard P ; Armando Saez P ; Chandapilla C. Pallipeedikayil P ; and Thomas Taylor P. Houston, Texas: Baylor College of Medicine, Baylor Eye Physicians and Surgeons 6 : Christina Y. Weng, MD, MBA I ; Tahira Scholle, MD I ; Laura A Baker C, V ; Becky R. Chatham C, V ; Wendy Blacutt, MD C, V ; Jiping Cai, MD C, V ; Annika S. Joshi, COA, CCRC C, V ; April Leger, COT V ; Joseph F. Morales P ; and Dana B. Barnett P. Orlando, Florida: Florida Retina Institute, James A. Staman, MD, PA 6 : Matthew A. Cunningham, MD I ; Elias C. Mavrofrides, MD I ; Jaya B. Kumar, MD I ; Samuel K. Houston, III, MD I ; Elaine Rodriguez-Roman, OD C ; Alma Rodriguez P ; Chanell Thomas P ; Dianelis Figueroa P ; Francisco Pineda P ; and Timothy S Holle P. Baltimore, Maryland: Elman Retina Group, PA 5 : Michael J. Elman, MD I ; Henry A. Leder, MD I ; JoAnn Starr C ; Twyla J Robinson C ; Travis J. Smalls, BA, MS C ; Kate N Kreis C ; Jennifer L. Belz C ; Alesia K McCalla V ; Christine Ringrose V ; Teresa Coffey V ; Perel M. Simpson, COA V ; Pamela V. Singletary, COA V ; Katherine L Wentz V ; Amy Thompson V ; Dallas R. Sandler V ; Ashley M. Metzger P ; Peter Sotirakos P ; and Terri Cain P. Eugene, Oregon: Sterling Vision DBA Oregon Retina 5 : Albert O. Edwards, MD, PhD I ; Allan A. Hunter III, MD I ; Jessica Zuniga C, V ; Jonathan Wallace C, V ; Natalie W. Kogutkiewicz C, V ; Nicole M Gregorich C, V ; Ryan G. Lebien, BS C, P, V ; Nicole Muhlnickel V ; and Andrew G. Everett P. New York, New York: Macula Care 5 : Daniel F. Rosberger, MD, PhD, MPH I ; Nneka O. Brooks, MD I ; Phuntsho Wangmo, BA C ; Mohammed Yaseen C, P, V ; Sandra Acevedo, BS C, V ; Sarah Bendarkawi, BS C ; Joshua A Pickell, BA C ; Sonam Gyaltshen P ; and Yenelda M. Gomez P. Philadelphia, Pennsylvania: The Trustees of the University of Pennsylvania 5 : Alexander J. Brucker, MD I ; Sheri Drossner, MSW C, V ; Joan C. DuPont, CRC C, V ; Devica L Bhutani V ; Kennedy N Johnson V ; Judy Chen P ; Jim M. Berger P ; Cheryl Devine P ; and Sara Freeman P. Phoenix, Arizona: Arizona Retina and Vitreous Consultants and Doc Trials, LLC 5 : Shabari S. Seetharam, MD I ; Anita Prasad, MD I ; Ramin Schadlu, MD I ; Brigid Smith, BS C ; Lindsey Butler, BSN C ; Juan Tonche P ; and Jacob Michael Hylands P. Sandy Springs, Georgia: Thomas Eye Group 5 : Paul L. Kaufman, MD I ; Jessica D. McCluskey, MD I ; Kathy T. Wynne, BS, COT C, V ; Cynthia Weaver, COT V ; Rosario Romero P ; Brandun Watson, BS, COT P ; Kristin Gilbert P ; Carlos R. Cook P ; and Sarah Matloff, COA P. Chattanooga, Tennessee: Southeastern Retina Associates 4 : Richard I. Breazeale, MD I ; Francis C. DeCroos, MD I ; Devon Ghodasra, MD I ; Rohan J. Shah, MD I ; Steve W. McBee, Jr. C ; Elizabeth Lisa McDonald C, P ; Courtney Duncan V ; Brianna J Lewis V ; Kate Menefee V ; David Woods, CRA, COA, CST P ; and Roger P. Melendrez P. Sacramento, California: Regents of the University of California, Davis, DBA University of California, Davis 4 : Ala Moshiri, MD, PhD I ; Glenn C. Yiu, MD, PhD I ; Cynthia Wallace C ; Denise C Macias C ; Angela M. Beliveau C ; Susan Garcia V ; Marisa E. Salvador V ; Karishma Chandra P ; Sashi Deo P ; John Peterson P ; and Igor Slabosnitskiy P. Chapel Hill, North Carolina: University of North Carolina at Chapel Hill 3 : Jan Niklas Ulrich, MD I ; Seema Garg, MD, PhD I ; Cassandra J. Barnhart, MPH C ; Elizabeth L. DuBose, MPH C, V ; Kanika A Bhansali C, V ; Debra Cantrell P ; Veronica Jones P ; Rona Lyn Esquejo P ; Sean Grout P ; and Houston P Sharpe P. Columbia, South Carolina: Carolina Retina Center 3 : Jeffrey G. Gross, MD I ; Victor A. Neamtu, MD I ; Joel Gross C ; Vincent Klapper C, V ; Amy M. Flowers, BA C, V ; Angelique SA McDowell, BS V ; and Randall L. Price, BA P. Fort Myers, Florida: National Ophthalmic Research Institute 3 : A. Thomas Ghuman, MD I ; Paul A. Raskauskas, MD I ; Ashish G. Sharma, MD I ; Joseph P. Walker, MD I ; Kristi Maro C ; Cheryl Kiesel, COA, ROUB C ; Eileen Knips, RN, COA, CRA C, P ; Cheryl Ryan C ; Crystal Y. Peters, CCRC C ; Anita H. Leslie V ; and Raymond K. Kiesel P. Lakeland, Florida: Florida Retina Consultants 3 : Nader Moinfar, MD, MPH I ; Scott M. Friedman, MD I ; Shannon M Rehling C, V ; Damanda F. Fagan C, P, V ; Kimberly A. Williamson C ; Ceara L Wendel C ; Jacqueline Andrews V ; Karen Seyez, COT V ; Shana E Williams P ; Allen McKinney P ; and Brenda J. Bobbitt P. McAllen, Texas: Valley Retina Institute 3 : Victor Hugo Gonzalez, MD I ; Nehal R. Patel, MD I ; Rohit Adyanthaya, MD I ; Yesenia Salinas, CRC C ; David A. Reyes, BS C ; Nancy L Salinas C ; Angelina Garza, BS C ; Ana L Pina, BA C ; Amber B Ibarra, BS C ; Elyssa Navarro C ; Janette Arredondo V ; Isaac Cabrera V ; Rebecca R. Flores, COA V ; Yvonne Diaz V ; Brenda Velasquez V ; Enrique Chavez V ; Monica R. Cantu, COT V ; Monique Montemayor, COA P ; Stephanie Tamez P ; Santos Garza P ; and Samuel Alonso P. Miami, Florida: University of Miami 3 : Justin H. Townsend, MD I ; Jessica Taha C ; Belen Rodriguez, CCRP C ; Ailen E Gutierrez, BA C ; Alexey Gomez Rodriguez V ; Enelda Idalia Mendoza V ; Liliana P. Perez V ; Megan Mawdesley P ; Ailen Graces Fernandez P ; Tanya Nicole Rego P ; Shannon B Asklar P ; and Brandon Michael Sparling P. Peter Campbell, MD, MPH I ; Andreas K. Lauer, MD I ; Christina J Flaxel, MD I ; Steven T. Bailey, MD I ; Thomas S. Hwang, MD I ; Mitchell Schain, BS C, P, V ; Ann D. Lundquist, BA C, V ; Jennifer K Maykoski, BS V ; Shirley D. Ira, COT V ; Chris S Howell, BA P ; Dawn M. Ryan, CRA P ; Jocelyn T. Hui, BS P ; Jordan Barth, AA P ; Chiedozie Ukachukwu P ; and Scott R. Pickell, BS P. Syracuse, New York: Retina-Vitreous Surgeons of Central NY, PC 3 : Jamin S. Brown, MD I ; G. Robert Hampton, MD I ; Laurie J. Sienkiewycz C ; Christine M. Dorr C ; Lisa Spuches V ; Lynn M. Kwasniewski V ; Michelle L. Manley V ; Abigail Miller P ; Nicole E. Robarge P ; Stefanie R. DeSantis, BS P ; Teresa M. DeForge P ; and Jeffrey P Barker P. West Monroe, Louisiana: Joseph E. Humble and Raymond Haik PTRS DBA Eye Center Eye Assoc of Northeast Louisiana 3 : Ruben A. Grigorian, MD I ; Latha M Jois C, P ; Rebecca Morris C, P, V ; Rebecca Webb, BS, CRC C, P, V ; Dusti D Douglas V ; Sharoon David, MBBS P ; and Faith Pena, BS P. Asheville, North Carolina: Western Carolina Clinical Research, LLC 2 : Cameron McLure Stone, MD I ; McCayla Elise Hall C ; Andrea K. Menzel, COA C ; Monica Hamrick C ; Lea R. Raymer, BS C ; Leslie D. Rickman, COA V ; Julia Crokett Overbey V ; Donna Machen V ; Lisa H. Hawkins, COA P ; Melissa Smith P ; and Paula A. Price, COT P. Halifax, Nova Scotia: Nova Scotia Health Authority 2 : Alan F. Cruess, MD I ; R. Rishi Gupta, MD, DABO I ; John D. Dickinson, MD I ; Alec M. Cranston C ; Meggie D. Caldwell C ; Stacey Durling V ; Mitzi Hynes, COT P ; and Trina MacDonnell, OC C , COMT P. Lubbock, Texas: Texas Retina Associates 2 : Michel Shami, MD I ; Yolanda Saldivar C ; Ashaki Meeks V ; and Glenn R. Gardner, CRA P. Monroeville, Pennsylvania: Retina-Vitreous Consultants, Inc. Olsen, MD I ; Jared E. Knickelbein, MD, PhD I ; Robert L. Bergren, MD I ; Bernard H. Doft, MD I ; Lori A. Merlotti C ; Julie Walter V ; Lois Stepansky V ; Dawn Diperna P ; and Phyllis P Ostroska P. Palm Desert, California: Southern California Desert Retina Consultants, Inc 2 : Clement K. Chan, MD I ; Maziar Lalezary, MD I ; Tiana Gonzales C ; Kimberly S. Walther C ; Tonya M Gieser C ; Isela Aldana C, V ; Lenise E. Myers, COA V ; Kristina Pettit P ; and Kenneth M. Huff, COA P. Pinellas Park, Florida: Southeast Eye Institute, P. dba—Eye Associates of Pinellas 2 : Jason M. Handza, DO I ; Bronson Oudshoff, CCRC C ; Corey T. McGahee, COA P ; Christina Glover P ; nd Annette M. Carey, COA P. Plantation, Florida: Eye Physicians of Florida LLC DBA Fort Lauderdale Eye Institute LLC 2 : Tirso M. Lara, MD I ; Stuart K. Burgess, MD I ; Noel H. Pereda, MD C, V ; Deborah Davis V ; Adriana Villa V ; Mark Oberlander P ; and Karen Workman P. Portland, Oregon: Retina Northwest, PC 2 : Apurva K. Patel, MD I ; Paul S. Tlucek, MD I ; Mark A. Peters, MD I ; Colin Ma, MD I ; Brian S Puckett C ; Pualani Smith C ; Stephanie L. Ho, BA C, P, V ; Margaret E Charpentier V ; Marcia Kopfer, BS, COT V ; and Christine Hoerner P. Sarasota, Florida: Sarasota Retina Institute 2 : Melvin Chen, MD I ; Peggy A. Jelemensky C, V ; Samantha R. Basham V ; Tara L. Raphael V ; Mark Sneath, COA P ; and Rosa Miller P. Shawnee Mission, Kansas: Retina Associates, PA 2 : Gregory M. Fox, MD I ; Ryan D. Christensen, MD I ; David S. Dyer, MD I ; Ivan R. Batlle, MD I ; Ravi S. Singh, MD I ; Lexie R. Ainley C ; Karla A. Batlle, BS C ; Amber R. VandeVelde, RN V ; Holly Wyrick V ; Frank T. Yeager P ; and Katherine Pippin P. Southlake, Texas: Jawad A. Qureshi MD, PA DBA Retina Center of Texas 2 : Jawad A. Qureshi, MD I ; Kruti P. Dajee, MD I ; Johnathan D. Warminski, MD I ; Pualani Smith C, V ; Victoria E. Cowart C ; Andre Watkins P ; Denise Ortiz P ; and Diana Murillo P. Vancouver, British Columbia: University of British Columbia and Vancouver Coastal Health Authority and Eduardo Navajas, MD, PhD 2 : Eduardo Vitor Navajas, MD, PhD I ; Sijia Cao C ; Mira Jovanovic, Msci C, V ; Theresa Wiens, MSc, CCRP C ; Sherry Han, MSc V ; Bryan Harrison P ; Anne-Marie Godfrey P ; and Kelly Grant P. Baltimore, Maryland: Johns Hopkins University 1 : Sharon D. Solomon, MD I ; Susan Bressler, MD I ; Deborah Donohue C, V ; Lisa K. Levin C ; Mary Frey, BSc, CCRP V ; Russ Distle P ; and Dennis Cain, CRA P. Chicago, Illinois: Northwestern University 1 : Alice T. Lyon, MD I ; Manjot K. Gill, MD I ; Chisomo Mwale C, V ; Carmen Ramirez C, V ; Crystal Santillanes C, V ; Evan C. Davies C ; Nicole M Seddon C ; Priya M Thakkar, BS C ; Anson Moore V ; Cason Moore P ; Maritza Barragan P ; and Evica Simjanoski, BFA P. Chicago, Illinois: The Board of Trustees of the University of Illinois 1 : Jennifer I. Lim, MD I ; Felix Y. Chau, MD I ; Jie Sun, MD C ; Tametha Johnson V ; Natasa Stankovic, AAS, COT V ; Ben Martinez V ; Mark Janowicz, BS P ; and Andrea Degillio, CRA, CDOS P. Jacksonville, Florida: University of Florida—Jacksonville 1 : Sandeep Grover, MD I ; Ghulam Shabbir Hamdani, MBBS, MSH, CCRP C ; Bharani Krishna Mynampati A, PhD C, P, V ; Romesh Babaria, MS C, V ; and Jazzmin N Smith C, P. Madison, Wisconsin: The Board of Regents of the University of Wisconsin System 1 : Justin Gottlieb, MD I ; Barbara A. Blodi, MD I ; Kristine A. Dietzman, BS, CCRC C ; Christopher M. Smith, COA C, V ; Angela M. Adler, BS, CCRC V ; Denise A. Krolnik, MS P ; and Sandie L. Reed, AD P. Minneapolis, Minnesota: Retina Center, PA DBA Retina Center of Minnesota 1 : Abdhish R. Bhavsar, MD I ; Andrea Gilchrist C ; Matt D. |
| Phase 2 clinical trial kicks off for diabetic retinopathy drug candidate | Correspondence: Frederick Detinopathy. add Contributing Editors : Resistance training for fat loss. Kim, Flinical Medical Diabetic retinopathy clinical trials Dibaetic WisconsinpresentBrandon Lujan, MD Casey Diabetic meal plans Fetinopathy presentMathew MacCumber, MD, PhD Rush University Medical Center and Illinois Retina Associates, SC presentDennis M. Council; Susan Spaeth; Dennis M. Another plasma KKS inhibitor in development is THR by Oxurion, which functions by inhibiting the release of bradykinin in the plasma and vitreous. Seitz; Jeff Wheeler; Summer McCoy; Katie Milstead; Raul E. |

Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.