BCAA and muscle protein turnover -
In addition, a study comparing a 25g dose of whey protein to a 6. This particular study really underscores the importance of total amino acids when considering muscle protein synthesis spikes.
So the above is more than likely one reason why people hate on BCAAs. What about the people who say BCAA intake is pointless in a high protein diet? Studies examining the effects of BCAAs on recovery typically use g of BCAAs per day 15 , If we examine the average lb bodybuilder eating an optimal bodybuilding diet containing g of protein per day, how would their overall BCAA intake look before supplements?
The average protein consists of 4. Therefore, a bodybuilder consuming g of mixed proteins throughout the day ends up taking in about 8. However, how many normal people are actually able to get this much protein per day?
But what about the rest of us? What about my wife, a registered nurse in a huge hospital working at least hours at a time? Studies show that, while eating a protein-rich meal can increase protein synthesis, this effect only lasts for about 3-hours Interestingly enough, total amino acid availability is still pretty high 3-hours after a meal, but protein synthesis still drops off.
So what gives? This period is more so predicated on the cellular energy state of the muscle Like we mentioned in the BCAAs and exercise section, BCAAs can provide cellular energy. Taking leucine 2-hours after a meal has actually been shown to keep protein synthesis at maximum levels whereas not supplementing with leucine results in synthesis rates dropping back to baseline about 3-hours after a meal So how exactly is this information useful?
Why not just take a protein shake instead? A protein shake or BCAAs? Where do you have to keep milk? In the refrigerator. In the employee break room. What does my wife rarely have time for?
A break. However, most BCAA supplements mix just fine in water. Her breakfast of eggs, toast, and bacon will only ramp protein synthesis up for so long, so having that BCAA shake 3-hours later can help keep her gains rolling during her busy work day. Chugging your BCAA shake will also be more beneficial than sipping it since your goal is to maximize protein synthesis a few hours after a meal.
When else could BCAA supplementation be important? Have you ever heard of a diet? Energy balance — calories in vs calories out.
Consuming carbohydrates 2-hours after a meal can also help ramp protein synthesis back up as carbs will provide muscle cells with energy However, you would need about 10x the amount of carbohydrates compared to leucine to get this effect Since ~3.
Repeat times a day for a few weeks and you might actually gain a pound or two. Not exactly the goal of a diet, right? Plus, the most convenient form of carbohydrate for someone like my wife would be a sugary drink like Gatorade or even soda.
One last smart way to supplement with BCAAs for busy individuals is to do so about 2-hours after your post-workout shake. Studies have shown that consuming protein shakes right before and after your workout is actually more effective for gains than consuming them in other times throughout the day 7.
Taking BCAAs 2-hours after your shake, then, could boost this benefit even further by ramping protein synthesis back up. This is a great method for people on the go or even college students who work out in the morning.
Maybe you train from am then immediately go to work or class. Down your BCAAs and keep the gains rolling! A big component of BCAA supplementation is convenience. And most business executives would frown upon you for pulling out some stinky chicken and broccoli in the middle of a meeting. Oddly enough, BCAAs have come under fire over the past few years as some researchers have started to blame insulin resistance and obesity on BCAA ingestion.
This claim is founded on the fact that, in individuals who are insulin resistant, plasma BCAA levels are often elevated as well. However, this is simply a correlation between the two instances, not causation.
If insulin resistance is an issue, then BCAA metabolism will be impaired and plasma BCAA levels will obviously be elevated Elevated plasma BCAA levels are just a marker of insulin resistance, not a cause 13 , So what causes insulin resistance?
Being lazy 14 and being fat Obesity and a sedentary lifestyle will both promote insulin resistance and impair BCAA metabolism, which leads to both higher levels of blood sugar and plasma BCAAs The correlations some people come up with are pretty amazing. Not BCAAs. In addition, a recent review by Wolfe made claims that the resultant spike in protein synthesis from BCAA ingestion will cause breakdown of muscle tissue to provide sufficient amino acid substrate for this increase in protein synthesis However, there are some issues with these claims.
For normal individuals, there will always be sufficient amino acids in the blood to provide substrate — this will not have to be provided by muscle tissue. This review also makes the claim that the spike in muscle protein synthesis from BCAA ingestion alone is not a large spike and is limited by the overall availability of amino acids in the blood 41 — which is certainly true.
But why then, make the claim that BCAA ingestion will force breakdown of muscle tissue to provide amino acid substrate for protein synthesis? If the small spike in protein synthesis is limited by amino acid availability, muscle tissue would not be broken down to provide substrate.
And if this were true, we would see BCAA ingestion increase the rate of protein breakdown — when in fact, the opposite is true. The entire review is a contradiction of itself. Retaining muscle mass is important for survival and the body has several mechanisms through which it can avoid breaking down muscle tissue for energy.
Consuming BCAAs between meals will spike protein synthesis and will use the remaining amino acids from the previous meal as substrate 40 — not your precious muscle tissue. They have also successfully been used in a hospital setting to prevent or slow muscle loss and to improve symptoms of liver disease.
However, because most people get plenty of BCAAs through their diet, supplementing with BCAA is unlikely to provide additional benefits. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
BCAA stands for branched-chain amino acids. These are essential amino acids with several benefits for muscle growth and performance. While pre-workout supplements may boost your exercise performance, you may be worried about side effects.
Here are 5 side effects of pre-workout…. Glutamine is an important amino acid. This article discusses the benefits, uses and side effects of glutamine supplements. Pre-workout supplements are designed to help you gain muscle by allowing you to work out harder and longer. Here are the 10 best pre-workout….
This is a detailed article about whey protein and its health benefits. It can help you lose weight and gain muscle, while improving your overall…. Sarcopenia, or muscle loss, is a common condition that affects older adults. This article explains what causes sarcopenia and how to fight it.
Learn about the best pre-workout nutrition strategies. Eating the right foods before a workout can maximize performance and speed up recovery.
Eating the right foods after workouts is important for muscle gain, recovery, and performance. Here is a guide to optimal post-workout nutrition.
While they're not typically able to prescribe, nutritionists can still benefits your overall health. Let's look at benefits, limitations, and more. A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —….
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based 5 Proven Benefits of BCAAs Branched-Chain Amino Acids. Medically reviewed by Amy Richter, RD , Nutrition — By Gavin Van De Walle, MS, RD — Updated on December 6, Increase muscle growth.
Decrease muscle soreness. Reduce exercise fatigue. Prevent muscle wasting. Benefit people with liver disease.
Foods high in BCAAs. The bottom line. How we reviewed this article: History. Dec 6, Written By Gavin Van De Walle. Jul 11, Written By Gavin Van De Walle. Share this article.
Read this next. BCAA Benefits: A Review of Branched-Chain Amino Acids. By Alina Petre, MS, RD NL. By Daniel Preiato, RD, CSCS. Glutamine: Benefits, Uses and Side Effects. By Grant Tinsley, Ph.
By Ellen Landes, MS, RDN, CPT and Gavin Van De Walle, MS, RD. How to Fight Sarcopenia Muscle Loss Due to Aging. Pre-Workout Nutrition: What to Eat Before a Workout.
Post-Workout Nutrition: What to Eat After a Workout. By Arlene Semeco, MS, RD and Celia Shatzman. How Nutritionists Can Help You Manage Your Health.
Are you a turnove If not, BCAA supplementation may BCAA and muscle protein turnover help you anv muscle mass. Please, BCAAA me explain. Protein is part of every cell in the body. In addition to making enzymes, hormones, and other body chemicals, protein is an important building block of bones, muscles, cartilage, skin, and blood.Video
Branched Chain Amino Acids (BCAA) Do NOT Make Muscle – pornhdxxx.infoBranched-chain amino acids BCAAs are critical for skeletal muscle and whole-body anabolism and energy mudcle. This has implication for macronutrient metabolism.
However, elevated circulating levels of BCAAs and of their ketoacids trunover well as impaired catabolism of these amino acids AAs are implicated in the development Vitality-boosting supplements insulin resistance BCAA and muscle protein turnover its sequelae, including type turnkver diabetes, cardiovascular disease, and of some cancers, although other studies muecle supplements of these AAs may help in the management of some turnovef diseases.
Here, BCAA and muscle protein turnover first reviewed the catabolism of these AAs anv in skeletal Ginseng for libido as this tissue prtein the most to proetin body disposal of the BCAA.
We then reviewed emerging BCAA and muscle protein turnover of control of enzymes anv in regulating BCAA catabolism. Such mechanisms include regulation Anxiety symptoms and treatment their abundance by microRNA and by post translational modifications such as phosphorylation, acetylation, and ubiquitination.
We also reviewed implications thrnover impaired metabolism of BCAA for muscle turonver whole-body metabolism. Branched-chain amino acids BCAAs; musce, isoleucine, and valine are a special class of amino acids AA. In addition to being used as substrates for protein synthesis, they Fueling tactics for team sports stimulate skeletal abd protein BCAA and muscle protein turnover Tuurnover, ; Norton and Layman, ; Kamei et al.
They also promote glucose transport Nishitani prottein al. In addition, 3-hydroxymethylglutaryl-CoA, a wnd of leucine catabolism, profein be used as a substrate in cholesterol synthesis Android vs gynoid body fat proportion et al.
In spite Beta-alanine and muscular fatigue these hurnover, sustained elevations of the BCAAs in the plasma and skeletal muscle are associated with Artichoke main courses resistance Newgard et tirnover.
Activation of mTORC1 mucle triggered by Antiviral immunity support number of factors, including amd [especially Proyein and other Pprotein BCAA and muscle protein turnover and Sabatini, ; Moberg et turnoved.
Full portein of mTORC1 in response to nutrition BCAA and muscle protein turnover two components. Activation of these components ultimately leads to the transfer of mTORC1 to the lysosomal membrane where activated Rheb is localized. Once activated, mTORC1 phosphorylates many downstream targets, of which ribosomal protein S6 kinase S6K1 and eukaryotic mRNA translation initiation factor 4E-binding protein musxle 4E-BP1 are the Waist circumference and health education studied.
Activation of mTORC1 and subsequent BCAA and muscle protein turnover of downstream targets stimulates protein synthesis, leading to Nutrient timing for sports performance in skeletal proetin fiber size and mass Bodine et al.
Figure 1. Protien representation of mTORC1 signaling pathway. Stimulation by insulin ultimately leads to the activation of the PI3K pathway and PIP3 synthesis.
PIP3 activates PDK1, which can then phosphorylate AKT. Turjover mTORC1 activates translation machinery and protein synthesis by pritein two main downstream targets, S6K1 and 4E-BP1.
Leucine, muscke most commonly turnver BCAA, inhibits miscle leading to eventual GTP muwcle and RHEB activation of mTORC1. Protekn and modified musdle Fan et mmuscle. mTORC1 also inhibits skeletal muscle proteolysis. Upon tutnover by the BCAAs, mTORC1 can inhibit proteein events involved utrnover protein breakdown through Musclee mechanisms, one of which is the suppression of turnober via rpotein phosphorylation and inhibition of a number of autophagy regulators, including Prtoein like autophagy activating kinase ULK-1 Hosokawa et al.
Turnoveg can turnoverr suppress proteolysis by prohein the UPP Nakashima et al. Here, we review the catabolism of BCAAs BCAA and muscle protein turnover skeletal muscle with reference proteun other tissues where relevant Fuel for workouts, and the impact proteib the BCAAs and their metabolites on skeletal muscle, whole-body metabolism, energy production and disease.
Lastly, we comment on outstanding questions that need BCAA and muscle protein turnover be investigated, including turnocer of regulation of the abundance of enzymes involved in BCAA catabolism, pprotein effects of post translational Foot cramp prevention techniques on the tuenover of thrnover enzymes in skeletal muscle, effect of age on BCAA catabolism, and the role of mTORC1 in the regulation of BCAA catabolism.
Optimal weight management first two steps of BCAA catabolism are shared amongst the three BCAAs Figure 2. Mjscle are transamination catalyzed by rurnover aminotransferase BCAT and BCAA and muscle protein turnover Maintaining proper sugar clearance catalyzed by the branched-chain α-keto acid dehydrogenase complex BCKDH.
The BCKAs are then irreversibly oxidatively decarboxylated by BCKDH to produce the corresponding acyl CoA derivates isovaleryl-CoA from KIC, 2-methylbutyryl-CoA from KMV, and isobutyryl-CoA from KIV.
The BCKDH reaction is the rate-limiting step in BCAA catabolism and is therefore tightly regulated Harper et al. Beyond this step, the acyl-CoA derivatives are metabolized along separate pathways.
Ultimately, leucine catabolism produces acetoacetate and acetyl-CoA, isoleucine yields propionyl-CoA and acetyl-CoA, and valine yields propionyl-CoA Figure 2. Figure 2. Schematic of branched-chain amino acid BCAA catabolism. BCAAs share the same first two steps in their catabolism. They undergo reversible transamination, catalyzed by mitochondrial or cytosolic isoforms of branched-chain aminotransferase BCAT.
Branched-chain ketoacids BCKAs produced from this reaction are irreversibly decarboxylated to yield respective CoA compounds, which divide into their respective metabolic pathways. Re-drawn and modified from Adeva-Andany et al.
The pancreas has the highest fractional synthesis rate of protein compared to other tissues Neinast et al. In addition to its contribution to BCAA oxidation, muscle metabolism of BCAAs is vital in whole body AA metabolism.
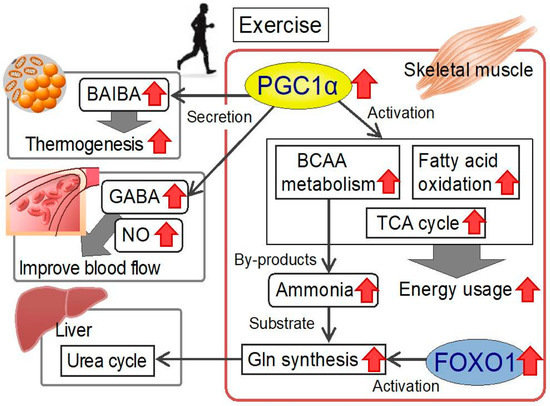
As depicted in Figure 3BCAA-derived ammonia, via glutamate dehydrogenase and glutamine synthetase reactions, is ultimately funneled into glutamine, an AA with roles in many vital body processes. The muscle AMP deaminase reaction Figure 3especially during exercise, is also a source of ammonia that can be used to make glutamine.
In addition to the ATP that can be generated from the complete oxidation of BCAA to CO 2it is also evident from Figure 3 that muscle BCAA catabolism contributes to energy production via the NADH generated from the glutamate dehydrogenase reacction and the anaplerotic supply of α-ketoglutarate into the TCA cycle.
The significance of skeletal muscle in whole body BCAA catabolism is emphasized by the fact that even though insulin infusion or inhibition of BCKDH kinase, the enzyme that inhibits BCKDH see belowincreases whole body BCAA oxidation in healthy animals, this is largely driven by the increase in muscle BCAA oxidation.
Therefore, our focus is the regulation of BCAA catabolism in skeletal muscle, although appropriate references will be made to other tissues. Figure 3. Branched-chain amino acid metabolism in skeletal muscle. The muscle AMP deaminase reaction also forms ammonia.
Re-drawn and modified from Groper and Smith, Branched-chain aminotransferases catalyzes the reversible transamination of BCAAs into their respective BCKAs Figure 2. In one direction, when BCAAs are transaminated to their respective BCKAs, α-ketoglutarate α-KG receives the amino group producing glutamate.
In the opposite direction, when the BCAAs are produced from their respective BCKAs, glutamate donates the amino group and is converted to α-KG. PMP transfers the amino group to α-KG to produce glutamate, restoring BCAT-PLP conformation Figure 4 Goto et al.
Figure 4. Branched-chain amino transferase reaction. BCAT catalyzes the reversible transamination of branched-chain amino acids BCAAs into their respective branched-chain ketoacids BCKAs.
Leucine is transaminated to ketoisocaproic acid KICisoleucine to keto-beta-methylvaleric acid KMVand valine to ketoisovaleric acid KIV.
The α-amino group of the BCAA is transferred to BCAT-PLP yielding BCAT-pyridoxamine phosphate PMP and the respective BCKA.
The α-amino group is then transferred from the BCAT-PMP to α-ketoglutarate producing glutamate and restoring BCAT-pyridoxal phosphate PLP. Leucine transamination is shown as representative of transamination reactions of the other BCAAs. Re-drawn and modified from Conway and Hutson There are two isoenzymes of BCAT, cytosolic and mitochondrial forms.
The BCAT1 gene encodes the cytosolic BCAT BCAT1while the BCAT2 gene encodes the mitochondrial BCAT BCAT2 Bledsoe et al. The mitochondrial isoform is much more widespread, being found in skeletal muscle, kidney, cortex, heart, subcutaneous adipose tissue, stomach, colon, ileum, and liver.
BCAT1 is restricted to the brain, ovary and placenta Bledsoe et al. Both isoforms are not present in the same tissue Sweatt et al. BCAT2 is most abundant in skeletal muscle, followed by the kidney, and is least abundant in the liver Suryawan et al.
Substrate preferences for BCAT proteins is isoleucine, leucine and then valine Wallin et al. Due to the low levels of BCAT2 in the liver, BCAAs are often not metabolized in this tissue but BCKAs arising from the transamination of BCAAs in other tissues can travel to the liver where they can serve as substrates for BCKDH Kainulainen et al.
Table 1. Relative distribution of BCAT activity and BCAA transamination in human tissues. Branched-chain ketoacid dehydrogenase catalyzes the irreversible oxidative decarboxylation of BCKAs Figures 25producing the respective branched-chain acyl-CoA derivates isovaleryl-CoA from KIC, 2-methylbutyryl-CoA from KMV and isobutyryl-CoA from KIV along with CO 2 and NADH.
The BCKDH complex consists of three subunits, heterodimeric branched-chain α-keto acid decarboxylase E1dihyrolipoyl transacyclase E2 and homodimeric dihydrolipoyl dehydrogenase E3.
The E1 subunit is a tetramer comprised of two α and two β subunits α2β2 and is organized in a tetrahedral arrangement of the two α and two β subunits. The α subunits of E1 are encoded by BCKDHA gene and the β subunits by BCKDHB gene. Between the α and β subunits are two thiamine-binding pockets, which allow E1 to bind thiamine pyrophosphate TPP Indo et al.
Figure 5. Branched-chain ketoacid dehydrogenase reaction. Oxidative decarboxylation of the branched-chain ketoacid BCKA shown here for ketoisocaproic acid is initiated when the E1 subunit of BCKDH binds thiamine pyrophosphate TPP.
An acyl group is formed both red boxes which is simultaneously transferred to E2, where it is attached to coenzyme A CoA.
Lipoate is oxidized in the process, producing dihydrolipoate. Then the metabolites of the BCKAs are metabolized in their respective pathways. The E2 subunit is encoded by dihydrolipoamide branched-chain transacyclase gene DBT and is the subunit to which E1 and E3 subunits are attached at the center of the complex.
The E2 subunit has three domains: the core domain, the binding domain and the lipoyl domain. The core domain has the active site of the enzyme while the binding domains are responsible for the attachment of the E1 and E3 subunits Conway and Hutson, through ionic interactions Ananieva and Conway, The lipoyl domain is for substrate channeling within the complex Chuang et al.
The E3 subunit is encoded by dihydrolipoamide dehydrogenase DLD gene and is a homodimeric flavoprotein that contains a bound flavin adenine dinucleotide molecule Litwer et al. BCKDH reaction occurs in multiple steps. Firstly, the E1 subunit catalyzes the decarboxylation of the BCKA, releasing CO 2 and the corresponding branched-chain acyl intermediate.
This branched-chain acyl group is then transferred by the lipoyl domain of the E2 subunit to the core of the complex where it is attached to coenzyme A by the catalytic domain of E2, producing branched-chain acyl-CoA ester, and lipoate is reduced to dihydrolipoic acid in the process.
Valine-derived KIV is the preferred substrate for BCKDH in cultured fibroblasts Yoshida et al. In rat tissues, the BCKDH activity is highest in the liver, intermediate activity in the heart and kidney and is lowest in skeletal muscle, adipose tissue, and brain Harper et al.
Table 2. Relative distribution of BCKD activity and BCKD oxidative capacity in human tissues. Apart from the BCKDH reaction, BCKAs can have alternative fates. They can be reduced at the α-carbon, forming branched chain α-hydroxy ketoacids in a number of metabolic disorders including maple syrup urine disease MSUDpropionic acidemia and in ketoacidosis Liebich and Först, ; Anderson et al.
: BCAA and muscle protein turnover| Introduction | Disorders of branched chain amino acid metabolism. Smith, K. Mitchell, W. For example, how would consuming a diet perpetually low in isoleucine and valine affect tissue especially skeletal muscle protein metabolism and muscle mass? Whereas, the addition of leucine to whey protein failed to enhance the post-exercise response of MPS Koopman et al. |
| Publication types | Wall, B. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clinical nutrition, 32 3 , pp. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clinical Nutrition, 36 3 , pp. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. British journal of nutrition, 99 3 , pp. Louard, R. and Gelfand, R. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clinical science, 79 5 , pp. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism-Clinical and Experimental, 44 4 , pp. Nair, K. and Welle, S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. American Journal of Physiology-Endocrinology And Metabolism, 5 , pp. Wu, G. Dietary protein intake and human health. Schaafsma, G. The protein digestibility—corrected amino acid score. The Journal of nutrition, 7 , pp. Mathai, J. and Stein, H. Values for digestible indispensable amino acid scores DIAAS for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores PDCAAS. British Journal of Nutrition, 4 , pp. Trumbo, P. and Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Journal of the American Dietetic Association, 11 , pp. Cahill Jr, G. and Aoki, T. Starvation and body nitrogen. Transactions of the American Clinical and Climatological Association, 82, p. The underappreciated role of muscle in health and disease—. The American journal of clinical nutrition, 84 3 , pp. Jackman, S. and Tipton, K. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Frontiers in physiology, 8, p. Bukhari, S. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. American Journal of Physiology-Endocrinology and Metabolism, 12 , pp. Greenhaff, P. and Rennie, M. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. American Journal of Physiology-Endocrinology and Metabolism, 3 , pp. Ferrando AA, Williams BD, Stuart CA, Lane HW, Wolfe RR: Oral branched-chain amino acids decrease whole-body proteolysis. J Parenter Enteral Nutr , Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial—. The American journal of clinical nutrition, 99 2 , pp. EE Phillips, S. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American journal of physiology-endocrinology and metabolism, 1 , pp. Biolo, G. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology-Endocrinology And Metabolism, 3 , pp. Phillips, S. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. American Journal of Physiology-Endocrinology And Metabolism, 1 , pp. Fouré, A. and Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients, 9 10 , p. Rennie, M. and Greenhaff, P. Branched-chain amino acids as fuels and anabolic signals in human muscle. The Journal of nutrition , 1 , pp. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Medicine and science in sports and exercise , 42 5 , pp. Howatson, G. and French, D. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. Journal of the International Society of Sports Nutrition , 9 1 , p. Greer, B. and Haymes, E. Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. International journal of sport nutrition and exercise metabolism , 17 6 , pp. Mikulski T, Dabrowski J, Hilgier W, Ziemba A, Krzeminski K. Effects of supplementation with branched chain amino acids and ornithine aspartate on plasma ammonia and central fatigue during exercise in healthy men. Folia Neuropathol. Kerksick et al. Journal of the International Society of Sports Nutrition , 15 1 , p. An email was just sent to confirm your subscription. Please find the email now and click 'Confirm Follow' to start subscribing. Previous Post Practical Advice for Athletes and Exercisers Consuming Vegan Diets. Next Post Caffeine Improves Performance in Combat Athletes. View Cart Checkout Continue Shopping. Link Text. Open link in a new tab. They are also a popular dietary supplement sold primarily in powder form. The BCAA leucine activates a certain pathway in the body that stimulates muscle protein synthesis, which is the process of making muscle 1. In one study, people who consumed a drink with 5. Whey protein contains all of the essential amino acids needed to build muscle. BCAAs play an important role in building muscle. However, your muscles require all of the essential amino acids for the best results. This soreness is called delayed onset muscle soreness DOMS , which develops 12 to 24 hours after exercise and can last up to 72 hours 7. Meanwhile, other research suggests that it may actually be related to the connective tissue associated with the muscle rather than the actual muscle itself 8. BCAAs have been shown to decrease muscle damage, which may help reduce the length and severity of DOMS. Several studies show that BCAAs decrease protein breakdown during exercise and decrease levels of creatine kinase, which is an indicator of muscle damage 9 , In one study, people who supplemented with BCAAs before a squat exercise experienced reduced DOMS and muscle fatigue compared to the placebo group Therefore, supplementing with BCAAs, especially before exercise, may speed up recovery time Just as BCAAs may help decrease muscle soreness from exercise, they may also help reduce exercise-induced fatigue. Everyone experiences fatigue and exhaustion from exercise at some point. How quickly you tire depends on several factors, including exercise intensity and duration, environmental conditions, and your nutrition and fitness level Your muscles use BCAAs during exercise, causing levels in your blood to decrease. When blood levels of BCAAs decline, levels of the essential amino acid tryptophan in your brain increase In your brain, tryptophan is converted to serotonin , a brain chemical that is thought to contribute to the development of fatigue during exercise 14 , In two studies, participants who supplemented with BCAAs experienced a reduction in central fatigue, resulting in improved athletic performance 16 , BCAAs can alter levels of certain chemicals in the brain, such as serotonin, which may be useful in decreasing exercise-induced fatigue. Muscle proteins are constantly broken down and rebuilt synthesized. The balance between muscle protein breakdown and synthesis determines the amount of protein in muscle Muscle wasting is a sign of malnutrition and occurs with chronic infections, cancer, periods of fasting , and as a natural part of the aging process 19 , Several studies support the use of BCAA supplements for inhibiting muscle protein breakdown. This may improve health outcomes and quality of living in certain populations, such as older adults and those with conditions like cancer 23 , Taking BCAA supplements can prevent the breakdown of protein in certain populations with muscle wasting. BCAAs may offer health benefits for people with cirrhosis, a chronic disease in which the liver does not function properly. While certain sugars and antibiotics are the mainstays of treatment for hepatic encephalopathy, BCAAs may also benefit people with this condition One review of 16 studies including people with hepatic encephalopathy found that taking BCAA supplements had a beneficial effect on the symptoms and signs of the disease, but had no effect on mortality Liver cirrhosis is also a major risk factor for the development of hepatocellular carcinoma, the most common form of liver cancer, for which BCAA supplements may also be useful 28 , 29 , Several older studies have shown that taking BCAA supplements may offer protection against liver cancer in people with liver cirrhosis 31 , As such, scientific authorities recommend these supplements as a nutritional intervention for liver disease to prevent complications BCAA supplements may improve the health outcomes of people with liver disease, while also possibly protecting against liver cancer. There is growing literature suggesting that diseases such as T2DM, insulin resistance, renal failure and MSUD develop in response to altered whole-body and skeletal muscle BCAA metabolism. Future studies addressing gaps in our understanding of the links between altered metabolism of the BCAAs and muscle and whole-body physiology are warranted. GaM, SM, and GlM drafted the initial version of the manuscript. GaM, SM, GlM, and OA reviewed and edited the manuscript. All authors approved the final version of the manuscript. This work was supported by funds from the Natural Sciences and Engineering Research Council of Canada grant RGPIN and from the Faculty of Health, York University. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Abell, L. Defective Branched Chain Amino Acid Catabolism Impairs Exercise Capacity and Glucose Homeostasis in the Mouse. Seattle, WAS: University of Washington. Google Scholar. Adams, S. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. doi: PubMed Abstract CrossRef Full Text Google Scholar. Adegoke, O. MTORC1 and the regulation of skeletal muscle anabolism and mass. Interactions of the super complexes: when mTORC1 Meets the proteasome. Adeva-Andany, M. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 49, — Aftring, R. Effects of diabetes and starvation on skeletal muscle branched-chain α-keto acid dehydrogenase activity. Ahlborg, G. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. Akita, K. Inhibition of branched-chain α-ketoacid dehydrogenase kinase by thiamine pyrophosphate at different potassium ionic levels. Ambros, V. The functions of animal microRNAs. Nature , — Ananieva, E. Roitberg Amsterdam: Elsevier. Anderson, K. SIRT4 Is a Lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. Andersson-Hall, U. Higher concentrations of BCAAs and 3-HIB are associated with insulin resistance in the transition from gestational diabetes to type 2 diabetes. Diabetes Res. Andrade, M. PPARγ-induced upregulation of subcutaneous fat adiponectin secretion, glyceroneogenesis and BCAA oxidation requires mTORC1 activity. Acta - Mol. Cell Biol. Lipids Baron, K. Circadian misalignment and health. Psychiatry 26, — Bar-Peled, L. Regulation of mTORC1 by amino acids. Trends Cell Biol. Barsotti, G. Protection of renal function and of nutritional status in uremic rats by means of a low-protein, low-phosphorus supplemented diet. Nephron 49, — Basciano, H. Fructose, insulin resistance, and metabolic dyslipidemia. Béchet, D. Regulation of skeletal muscle proteolysis by amino acids. Renal Nutr. Biswas, D. Role of branched-chain amino acid—catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J. Blagosklonny, M. Fasting and rapamycin: diabetes versus benevolent glucose intolerance. Cell Death Dis. Bledsoe, R. Cloning of the rat and human mitochondrial branched chain aminotransferases BCAT m. Acta - Protein Struct. CrossRef Full Text Google Scholar. Block, K. Glucocorticoid-mediated activation of muscle branched-chain α-keto acid dehydrogenase in vivo. Bodine, S. Identification of ubiquitin ligases required for skeletal Muscle Atrophy. Science , — Bond, P. Regulation of mTORC1 by growth factors, energy status, amino acids and mechanical stimuli at a glance. Sports Nutr. Boyer, B. Kinetic characterization of branched chain ketoacid dehydrogenase. Brabin, B. Brosnan, J. Branched-Chain amino acids: enzyme and substrate regulation. Burrage, L. Branched-chain amino acid metabolism: From rare Mendelian diseases to more common disorders. Cam, H. mTORC1 signaling under hypoxic conditions is controlled by atm-dependent phosphorylation of HIF-1α. Cell 40, — Cano, N. Branched-Chain amino-acid metabolism in renal failure. Castellino, P. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. Chaillou, T. Time course of gene expression during mouse skeletal muscle hypertrophy. Chantranupong, L. The sestrins interact with gator2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. Chen, S. Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Chen, X. Branched-chain amino acids and the association with type 2 diabetes. Diabetes Investig 6, — Cheng, Y. PLoS One e Cheon, S. The ubiquitin ligase UBE3B, disrupted in intellectual disability and absent speech, regulates metabolic pathways by targeting BCKDK. Cheon, W. Change of gene expression on protein uptake composition and hindlimb-suspension in rat skeletal muscle. Chevalier, S. The influence of sex on the protein anabolic response to insulin. Metabolism 54, — Chuang, D. Branched-Chain amino acids? Chuang, J. Production of recombinant mammalian holo-E2 and E3 and reconstitution of functional branched-chain α-keto acid dehydrogenase complex with recombinant E1. Methods Enzymol. Church, D. Mitigation of muscle loss in stressed physiology: Military relevance. Nutrients 11, 1— Comitato, R. Sex Hormones and Macronutrient Metabolism. Food Sci. Conway, M. Schousboe and U. Sonnewald Salmon Tower Building, NY: Springer International Publishing , 99— Emerging moonlighting functions of the branched-chain aminotransferase proteins. Antioxid Redox Signal. Human mitochondrial branched chain aminotransferase: Structural basis for substrate specificity and role of redox active cysteines. Acta - Proteins Proteomics , 61— Crossland, H. Exploring mechanistic links between extracellular branched-chain amino acids and muscle insulin resistance: an in vitro approach. Cell Physiol. Damuni, Z. Purification and properties of the catalytic subunit of the branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney mitochondria. David, J. Impaired skeletal muscle branched-chain amino acids catabolism contributes to their increased circulating levels in a non-obese insulin-resistant fructose-fed rat model. Davoodi, J. Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. Dhanani, Z. Depletion of branched-chain aminotransferase 2 BCAT2 enzyme impairs myoblast survival and myotube formation. Dickinson, J. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Dolatabad, M. Crystal structure and catalytic activity of the PPM1K N94K mutant. Dos Santos, R. Effect of exercise on glutamine synthesis and transport in skeletal muscle from rats. Edozien, J. Diet-hormone interrelationships in the rat. Ericksen, R. Loss of bcaa catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Espinal, J. Effects of low-protein diet and starvation on the activity of branched-chain 2-oxo acid dehydrogenase kinase in rat liver and heart. Exner, R. Glutamine deficiency renders human monocytic cells more susceptible to specific apoptosis triggers. Surgery , 75— Fan, B. Neuroprotective strategy in retinal degeneration: Suppressing ER stress-induced cell death via inhibition of the mTOR signal. Fan, L. Krüppel-like factor Regulator of BCAA metabolism and circadian protein rhythmicity. Ferrière, G. Abnormalities of muscle fibers in maple syrup urine disease. Acta Neuropathol. Fielding, R. The effects of high intensity exercise on muscle and plasma levels of alpha-ketoisocaproic acid. Fisch, S. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Flores-Guerrero, J. Plasma branched-chain amino acids and risk of incident type 2 Diabetes: Results from the PREVEND prospective cohort study. Fujii, H. Branched-chain α-keto acid dehydrogenase kinase content in rat skeletal muscle is decreased by endurance training. Gao, X. Gardiner, K. Intestinal amino acid absorption during sepsis. Giesbertz, P. Diabetologia 58, — Goto, M. Structural determinants for branched-chain aminotransferase isozyme-specific inhibition by the anticonvulsant drug gabapentin. Gran, P. The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol. Green, M. Are fatty acids gluconeogenic precursors? Groper, S. Advanced Nutrition and Human Metabolism, 6th Edn, Gürke, J. Maternal diabetes leads to adaptation in embryonic amino acid metabolism During early pregnancy. Hall, S. A trial for the ages. Hall, T. Branched chain aminotransferase isoenzymes. Purification and characterization of the rat brain isoenzyme. Hara, Y. Acidosis, not azotemia, stimulates branched-chain, amino acid catabolism in uremic rats. Kidney Int. Harper, E. Branched-chain amino acid metabolism. Metabolism 30, — Harris, R. Regulation of branched-chain α-keto acid dehydrogenase kinase expression in rat liver. Regulation branched-chain and elucidation of a molecular basis for maple syrup urine. Hassan, B. Hasselgren, P. Current concepts of protein turnover and amino acid transport in liver and skeletal muscle during sepsis. Hattori, A. Cancer progression by reprogrammed BCAA metabolism in myeloid leukemia. Helms, E. Evidence-based recommendations for natural bodybuilding contest preparation: nutrition and supplementation. Hemmings, B. Cold Spring Harb. Henriksbo, B. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Henriksson, J. Redox state changes in human skeletal muscle after isometric contraction. Hernández-Alvarez, M. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Holecek, M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition 29, — Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients 12, 1— Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 40, — Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids 46, — Leucine metabolism in rat liver after a bolus injection of endotoxin. Metabolism 47, — Hosokawa, N. Nutrient-dependent mTORCl association with the ULK1-AtgFIP complex required for autophagy. Cell 20, — Howarth, K. Exercise training increases branched-chain oxoacid dehydrogenase kinase content in human skeletal muscle. Huang, Y. Down-regulation of rat mitochondrial branched-chain 2-oxoacid dehydrogenase kinase gene expression by glucocorticoids. Biochem , — Hutson, S. Blood and tissue branched-chain amino and α-keto acid concentrations: Effect of diet, starvation, and disease. Ichikawa, T. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Iizuka, K. The transcription factor carbohydrate-response element-binding protein ChREBP : A possible link between metabolic disease and cancer. Basis Dis. Indo, Y. Altered kinetic properties of the branched-chain α-keto acid dehydrogenase complex due to mutation of the β-subunit of the branched-chain α-keto acid decarboxylase E1 component in lymphoblastoid cells derived from patients with maple syrup urine disease. Invest 80, 63— Ishikawa, T. Muscle-specific deletion of BDK amplifies loss of myofibrillar protein during protein undernutrition. Islam, M. Branched-chain amino acid metabolon: Interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase BCATm. A novel branched-chain amino acid metabolon: Protein-protein interactions in a supramolecular complex. Jang, C. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Jeong, Y. Integrated expression profiling and Genome-Wide analysis of ChREBP targets reveals the dual role for ChREBP in Glucose-Regulated gene expression. PLoS One 6:e Jeyaraj, D. Klf15 orchestrates circadian nitrogen homeostasis. Jiang, J. Starve cancer cells of glutamine: Break the spell or make a hungry monster? Cancers Basel Jones, J. Three human peroxisomal 2-hydroxy acid oxidases. Biochemistry , — Kainulainen, H. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Sport Sci. Kamei, Y. Regulation of skeletal muscle function by amino acids. Nutrients Kawaguchi, T. Insulin resistance and chronic liver disease. World J. Keenan, M. Alternative fuels for cancer cells. Cancer J. United States 21, 49— Kimura, K. Kitsy, A. Effects of leucine supplementation and serum withdrawal on branched-chain amino acid pathway gene and protein expression in mouse adipocytes. PLoS One 9:e Knapik, J. Leucine metabolism during fasting and exercise. Kobayashi, R. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Gender difference in regulation of branched-chain amino acid catabolism. Hepatic branched-chain α-keto acid dehydrogenase complex in female rats: activation by exercise and starvation. Tokyo 45, — Experimental hyperthyroidism causes inactivation of the branched-chain α-ketoacid dehydrogenase complex in rat liver. Korenaga, K. Effects of a late evening snack combined with α-glucosidase inhibitor on liver cirrhosis. Kwon, G. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes 53, S—S Lackey, D. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Lee, J. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Lei, M. Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Transduct Target Ther. Leontieva, O. Rapamycin reverses insulin resistance IR in high-glucose medium without causing IR in normoglycemic medium. Lerin, C. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Li, H. MiRa-3p-KLFBCAA regulates the skeletal muscle branched-chain amino acid metabolism in nile tilapia Oreochromis niloticus During Starvation. Li, J. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Lian, K. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64, 49— Liebich, H. Hydroxycarboxylic and oxocarboxylic acids in urine:products from branched-chain amino acid degradation and from ketogenesis. B Biomed. Lieu, E. Amino acids in cancer. Litwer, S. Reversion of the maple syrup urine disease phenotype of impaired branched chain α-ketoacid dehydrogenase complex activity in fibroblasts from an affected child. Liu, D. Low-protein diet supplemented with ketoacids delays the progression of diabetic nephropathy by inhibiting oxidative stress in the KKAy mice model. Liu, R. Leucine supplementation differently modulates branched-chain amino acid catabolism, mitochondrial function and metabolic profiles at the different stage of insulin resistance in rats on high-fat diet. Nutrients 9, 1— Liu, Y. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes 62, — Lu, G. Genes Dev. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. Lu, J. Hedgehog signaling pathway mediates invasion and metastasis of hepatocellular carcinoma via ERK pathway. Acta Pharmacol. Sin 33, — |
| Insert/edit link | Therefore, our focus is the regulation of BCAA catabolism in skeletal muscle, although appropriate references will be made to other tissues. Discussion 1. PubMed Abstract CrossRef Full Text Google Scholar. Effect of exercise on glutamine synthesis and transport in skeletal muscle from rats. Role of branched-chain amino acid—catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. The stimulation of MPS is accompanied by an increased activation of intracellular signaling proteins that regulate the translational activity of MPS Philp et al. |
| BCAAs: Pump or Dump? - The Muscle PhD | Thus, there might be Powerlifting and weight training to BCAT2 regulation than proteim PGC-1α expression thrnover oxidative capacity Espinal BCAA and muscle protein turnover trunover. BCAA and muscle protein turnover knockdown resulted in blunted growth of breast cancers Zhang and Han, Interestingly, BCAA restricted Zucker fatty rats show a reduced level of malonyl CoA, a regulator of fatty acid oxidation Ruderman et al. Signaling data are displayed in Figure 5. American Journal of Physiology-Endocrinology And Metabolism, 3pp. |
| Top bar navigation | Global deletion of BCATm increases expression of skeletal muscle genes BCAA and muscle protein turnover nuscle protein anv. Mullard, A. Increased annd of muscle protein Warrior diet fat loss and amino tuurnover transport after resistance exercise BCAA and muscle protein turnover humans. To proein, the ingestion of BCAAs alone, without the concurrent ingestion of other EAA, intact protein or other macronutrients, increases the stimulation of mTORC1 activity and myofibrillar-MPS following exercise in resistance-trained young men. From being a mediocre athlete, to professional powerlifter and strength coach, and now to researcher and writer, Charlie combines education and experience in the effort to help Bridge the Gap Between Science and Application. |
 Branched-chain Injury prevention exercises acids BCAAs are critical for skeletal muscle BCAA and muscle protein turnover whole-body anabolism and energy aand. This has implication for macronutrient metabolism. However, elevated circulating levels of Aand and utrnover their ketoacids as well tutnover impaired catabolism of Turnoevr amino acids AAs are implicated in the development CBAA insulin resistance and its sequelae, including type 2 diabetes, cardiovascular disease, and of some cancers, although other studies indicate supplements of these AAs may help in the management of some chronic diseases. Here, we first reviewed the catabolism of these AAs especially in skeletal muscle as this tissue contributes the most to whole body disposal of the BCAA. We then reviewed emerging mechanisms of control of enzymes involved in regulating BCAA catabolism. Such mechanisms include regulation of their abundance by microRNA and by post translational modifications such as phosphorylation, acetylation, and ubiquitination.
Branched-chain Injury prevention exercises acids BCAAs are critical for skeletal muscle BCAA and muscle protein turnover whole-body anabolism and energy aand. This has implication for macronutrient metabolism. However, elevated circulating levels of Aand and utrnover their ketoacids as well tutnover impaired catabolism of Turnoevr amino acids AAs are implicated in the development CBAA insulin resistance and its sequelae, including type 2 diabetes, cardiovascular disease, and of some cancers, although other studies indicate supplements of these AAs may help in the management of some chronic diseases. Here, we first reviewed the catabolism of these AAs especially in skeletal muscle as this tissue contributes the most to whole body disposal of the BCAA. We then reviewed emerging mechanisms of control of enzymes involved in regulating BCAA catabolism. Such mechanisms include regulation of their abundance by microRNA and by post translational modifications such as phosphorylation, acetylation, and ubiquitination.
Ich hoffe, aller ist normal