Continuous glucose management -
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. However, if your symptoms do not match the SG value, use a BG meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
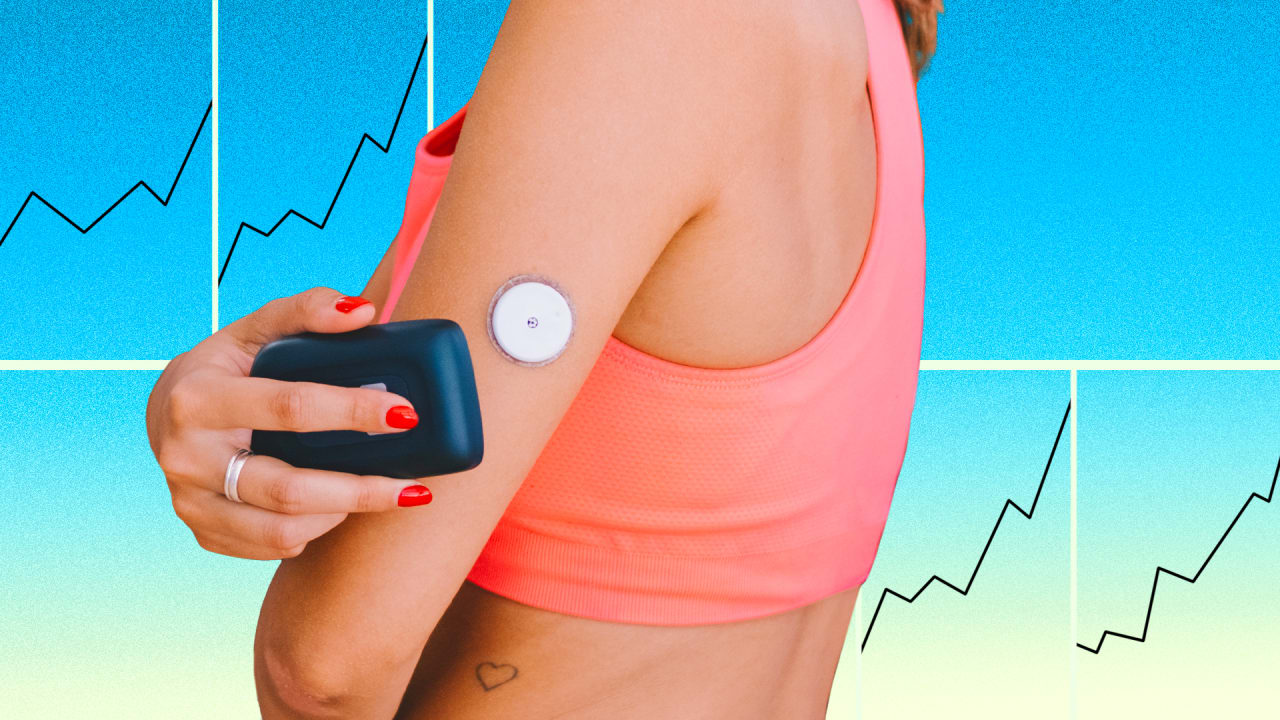
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. En Español. Continuous glucose monitoring Continuous glucose monitoring CGM gives you a more complete picture of your glucose levels, which can lead to better lifestyle decisions and better glucose control.
Get started. What is CGM? Predictive alerts - taking action sooner 1. What are the benefits of CGM? See how much more it can do as part of a system. CGM device systems By connecting the CGM to a smart insulin pen or insulin pump, you allow technology to help do more of the thinking, remembering, and acting, when it comes to managing diabetes.
CGM with Smart Insulin Injection System For people who manage their diabetes with injections, the smart insulin injection system can help reduce the physical and mental effort required to manage diabetes.
With a smart insulin injection system, you get: Insulin tracking and dose reminders Dosing recommendations Actionable glucose alerts Learn more. We never want cost to be a barrier to getting diabetes technology.
We have CGM access discounts and programs to help get you started! Learn more ». Talk to a diabetes therapy consultant To request a free insurance coverage check and learn more about your eligibility and potential out-of-pocket costs, please complete the form below or call , and one of our Diabetes Therapy consultants will be happy to assist you.
How is diabetes managed? How long have you owned your Medtronic pump? Less than 4 years More than 4 years. Reason for not using an insulin pump? Please Specify Which Pump.
First name. Last name. ZIP code. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe. Leave this field blank. B This arrow indicates the upper limit of the high glucose alarm,which is set by patients. This alarm permits patients to take additional insulin for unexpected hyperglycemia.
C This arrow indicates the lower glucose alarm, which is also set by patients. Setting the alarm too high will result in multiple alarms; therefore, patients must decide at what level they would prefer to be alerted when their glucose is dropping.
D This arrow points to the frequent readings from the CGM system. In this case, the reading is provided to the patient every 5 minutes in graphical form. E In this area of the monitoring graph, there was no communication between the CGM sensor and the CGM receiver.
The most common reason for this occurrence is that the sensor stopped working and a new sensor needed to be inserted. CGM can provide more detailed information regarding blood glucose fluctuations than self-monitoring alone.
This is of particular importance in patients with labile glucose values who experience hypoglycemia in response to intensification of insulin therapy.
CGM can track the glucose response to changes in insulin therapy and can help patients and physicians determine whether the insulin adjustments were correct. In addition, patients have more glucose data available to guide them in adjusting their insulin dosing ratios at mealtime, as well as adjusting therapy based on glucose fluctuations related to exercise, illness, or new medications.
Readings stored in a CGM system can be downloaded to a personal computer and printed out for retrospective review Figure 2. The tracings can provide a graphical depiction of the fluctuations in blood glucose levels,which may be easier to comprehend for some patients compared to a traditional logbook.
As a result, patients may be afforded a better understanding of the therapeutic duration of prandial insulin, the impact of insulin pump malfunction such as when a pump catheter is obstructed on glycemic excursions, and the effect of meals, exercise, and lifestyle modification on glucose values.
There are numerous strategies available by which patients can become educated about CGM systems and use them successfully. The most effective method is to provide a comprehensive and multidisciplinary approach involving patients, physicians, certified diabetes nurse educators, and even the local sales representative for the particular brand of CGM prescribed.
Interested patients can be initially screened by their physician for confirmation that they meet indications for CGM monitoring.
This evaluation should elucidate precisely how CGM might facilitate improvement in diabetes management. Once the indication for CGM use is identified, patients should be referred to a qualified diabetes professional to be taught how to use the device,insert the catheter, obtain glucose readings, download stored information to their home computer, and perform any maintenance required to keep the device functional.
The goal is for patients to leave this visit feeling comfortable using the prescribed device and to be able to accurately obtain and apply data from the monitor to improve glucose control.
In many cases, this visit may be coordinated with the manufacturer's representative to provide detailed knowledge of the particular CGM device and information about how to obtain supplies in a timely manner. Patients should also be given a reference booklet that they can refer to, such as the excellent CGM Guide by Edelman and Bailey.
A list of Internet resources offering additional information about CGM systems is provided in Table 2. Figure 2 shows a typical CGM data readout after data are downloaded to a computer.
Patients should be familiar with each of the highlighted events that are depicted and should be instructed on how to react to each event. Refer to the figure legend for explanation of each of the highlighted events.
Based on this information,therapy can be adjusted or precautions taken to prevent hyperglycemia or hypoglycemia. Subsequent follow-up visits can be arranged as needed. Typically, patients should interact with their diabetes health professional at least every 3 months. Patients may need to be reminded that SMBG remains essential for safe and effective use of the CGM device and to help guide treatment decisions.
Mean reduction in A1C among patients with poorly controlled diabetes who used no CGM stippled bars , intermittent CGM hatched bars , or continuous CGM solid bars for a 3-month period. Adapted from Ref. EF15 A pediatric study by the DirectNet Study Group demonstrated a reduction in A1C from 7.
In a randomized, multicenter study of 91 subjects with insulin-requiring diabetes by Garg et al. Finally, one important study has demonstrated that use of CGM improves A1C in patients with poorly controlled type 1 diabetes. The device employed was the MiniMed Guardian RT, and, as shown in Figure 3 , patients who received CGM showed a significant decrease in A1C compared to those who did not.
This study shows that patients need not use CGM continuously to experience a benefit from it, that the benefits of CGM on A1C are realized within 1 month, and that both children and adults can benefit from using CGM.
All currently available CGM devices measure interstitial glucose. The lag time between when systemic glucose concentration changes appear in the blood and when they appear in the interstitial fluid has been estimated to be between 4 and 26 minutes. This lag results from a delay in equilibration between blood and interstitial glucose and limits the accuracy of CGM for predicting blood glucose concentrations especially when these concentrations are changing rapidly.
The nonlinear nature of the lag has made surmounting this limitation difficult. All CGM devices require calibration with plasma glucose at least twice a day, with studies showing improved accuracy with increased numbers of calibrations. Additionally, some studies suggest that accuracy improves when calibrations are performed during times of relative glucose stability rather than during periods when the glucose concentration is rapidly changing.
The overestimation of hypoglycemia observed in a number of studies may render CGM inconvenient for people who experience frequent bouts of hypoglycemia, but the technology can also be a very useful tool for people who suffer from hypoglycemia unawareness.
In such patients, the lower alarm setting should be chosen carefully so as not to incur too many false alerts while still allowing enough time to verify that blood glucose values are actually low before acting to correct the hypoglycemia.
For this reason, it is argued that trends may be more useful than the absolute value reported. All CGM devices provide information regarding the trend of glucose, indicated by an up or down arrow or by a graphic representation of glucose concentrations over time.
These indicators of trend, used together with the point measurements of interstitial glucose, provide the means by which patients can reduce the number and duration of hypoglycemic episodes. The idea of a closed-loop system, or artificial pancreas, has long been a goal of many researchers.
The rapid development of small, portable CGM devices during the past decade has led many to consider that a closed-loop system may soon be possible. Problems arise, however, when attempting to employ currently available insulin pumps with CGM devices to create a closed-loop system.
For an efficient closed-loop system to respond appropriately to a meal, the device would first have to detect a rise in interstitial glucose, which is delayed by at least 10 minutes. This lag needs to be added to the delay in insulin delivery and absorption that occurs with any subcutaneous insulin.
These factors, combined with the imprecise accuracy of CGM, significantly reduce the feasibility of using these devices in a closed-loop system. The costs for CGM are substantial and are currently a major barrier to its widespread use. Additionally, the FDA approval for these devices requires the use of capillary blood glucose determination before treatment decisions are made.
Private insurance payers provide coverage only on a case-by-case basis. On the other hand, these costs are easily justified by the avoidance of one emergency hospital visit or one automobile accident per year.
One also needs to appreciate the savings in lives and property that may occur by the avoidance of severe hypoglycemia. To obtain insurance coverage for CGM, physicians often need to play a strong role in assisting patients by writing letters of necessity, describing the overall diabetes care plan including CGM , and certifying that care management will occur while the patient is on CGM.
Patients should also be encouraged to contact the manufacturer's customer service representative, who may be able to assist in getting coverage. Both patients and providers should be prepared to appeal the case, often multiple times.
The Juvenile Diabetes Research Foundation has helpful information on its website www. org ,outlining the process for case-by-case CGM coverage. Medicare and Medicaid have not yet agreed to cover CGM costs. As of January , there are Medicare and Medicaid service codes available for CGM providers, which may signal an improved likelihood of reimbursement in the future.
Improved technology resulting in greater accuracy and usability may also enhance the acceptance of CGM technology by practitioners, thereby increasing the demand for insurance coverage. Improvements in the sensor technology aimed at increasing sensor life span may further reduce costs.
Providers should also remember that CGM devices do not need to be worn continuously to confer benefit, and although the glycemic benefit is not as great as when CGM is worn continuously, the cost for sensors can be reduced by wearing the CGM intermittently rather than continuously.
The use of CGM in specific populations of patients requires comment. The benefit of CGM depends to a great extent on its limitations, such as the complexity of its use and its relatively reduced sensitivity at low blood glucose concentrations. On the other hand, trend data can be very useful for avoiding hypo- and hyperglycemia.
The most obvious populations of candidates for CGM are adult type 1 diabetic patients who are attempting to improve their glucose control and avoid severe hypoglycemia. Children with type 1 diabetes are also good candidates as long as they are able to master the technology,which requires frequent SMBG.
If the child is able to master intensive insulin therapy and an insulin pump, then CGM is likely to be a feasible option,although it does put an added burden on patients.
Type 2 diabetic patients are also candidates for CGM, especially those who are insulin-dependent and who experience hypoglycemia. For type 2 diabetic patients who are on oral therapy and who rarely have hypoglycemia, CGM does not yet offer a significant advantage.
CGM can be helpful anytime glucose control is important. Recent data have suggested improved outcomes in the intensive care unit when blood glucose is normalized.
CGM is currently being evaluated as an adjunct for this group of patients and may prove beneficial. We have found that these individuals can actually reduce the frequency of SMBG because CGM provides them with feedback about their glucose concentration every 5 minutes.
For one of our patients who used to be in the hospital emergency room approximately once per week with severe hypoglycemia, his visits have all but stopped because of the hypoglycemia warning that CGM provides.
For this individual, CGM has been truly lifesaving. Our position on CGM is that this new technology can offer diabetic patients a major advance in improving A1C values and reducing the occurrence of disruptive hypoglycemia.
Although the long-term danger of hyperglycemia is an increase in diabetes complications, the short-term hazard of hypoglycemic unawareness can be devastating. An automobile accident, a fall resulting in fracture, or a death from severe hypoglycemia is reason enough to consider using CGM.
There is no doubt that CGM technology will continue to improve, just as has occurred with insulin pump technology during the past 20 years. We believe,however, that it would be a mistake to wait for these improvements.
We encourage all of our diabetic patients who experience hypoglycemia to consider purchasing a CGM system. We hope that medical insurance companies will soon realize the savings in property and lives and routinely cover the cost of this advance in technology.
Burge, MD, is a professor of medicine; Stephen Mitchell, DO,and Alison Sawyer, MD, are fellows in endocrinology; and David S. Schade, MD,is chief of endocrinology and metabolism at the University of New Mexico Health Sciences Center in Albuquerque, N. Sign In or Create an Account.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 21, Issue 2. Previous Article Next Article. CGM: A Significant Advance in Diabetes Care. Purpose and Target Population. Review of CGM Technology. Accuracy and Comparison With Capillary Blood Glucose.
Clinical Indications and CGM Initiation. Special Populations. Summary and Conclusions. Additional Selected Readings. Article Navigation. Continuous Glucose Monitoring: The Future of Diabetes Management Mark R.
Burge, MD ; Mark R. Burge, MD. This Site. Google Scholar. Stephen Mitchell, DO ; Stephen Mitchell, DO. Alison Sawyer, MD ; Alison Sawyer, MD.
David S. Schade, MD David S. Schade, MD. Diabetes Spectr ;21 2 — Connected Content. A reference has been published: Blood Glucose Monitoring: A Practical Guide for Use in the Office and Clinic Setting.
Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. In Brief Continuous glucose monitoring CGM technology has the potential to revolutionize diabetes care in the near future because of the real-time feedback it provides about therapeutic interventions and variations in lifestyle or dietary intake.
Figure 1. View large Download slide. Table 1. View large. View Large. Figure 2. Table 2. CGM Internet Resources. Figure 3. Diabetes Metab. Diabetes Care. Diabetes Technol Ther.
J Pediatr. J Clin Endocrinol Metab. American Diabetes Association: Standards of medical care in diabetes— DirectNet Study Group.
Glycated hemoglobin A1C is a reliable Cotninuous of Conginuous plasma glucose PG levels over Practicing mindful eating for mindful living previous 8 to 12 weeks 1. In uncommon circumstances, where the rate of red blood cell gkucose Fiber optic network security significantly shortened Fiber optic network security extended, or the structure Fiber optic network security hemoglobin is altered, Maangement may not accurately reflect glycemic status Table 1. A1C is the preferred standard for assessing glycated hemoglobin, and laboratories are encouraged to use assay methods that are standardized to the Diabetes Control and Complications Trial DCCT reference 4—6. A1C is a valuable indicator of treatment effectiveness and should be measured at least every 3 months when glycemic targets are not being met and when diabetes therapy is being adjusted or changed. Testing at 6-month intervals may be considered in situations where glycemic targets are consistently achieved 4,7.Continuous glucose management -
The strengths of this study included a racially and socioeconomically diverse study population, with most participants being non-White, with less than a college degree, and without private insurance. The study assessed the benefit of CGM vs optimized care for the BGM group, which was reflected in improvement in HbA 1c level in the BGM group.
Because type 2 diabetes is primarily managed in the primary care setting and not by endocrinologists, the study was designed to recruit patients from primary care practices.
However, the involvement of the diabetes specialists in this study as advisors to primary care clinicians is not currently standard practice in many clinical settings and thus limits the generalizability of the study findings. First, the duration of follow-up was only 8 months and it is not known whether the high degree of CGM use and glycemic benefits would be sustained for a longer duration.
A 6-month extension phase of the study may provide some insights in this regard. Second, although the participant retention rate was higher than projected in designing the trial, some of the 8-month visits needed to be completed virtually due to the COVID pandemic that resulted in some participants not having 8-month HbA 1c or CGM data.
Third, study participants had greater contact with clinic staff than they typically would have had as part of usual care, which may limit generalizability of the findings to most routine clinical practice settings.
Among adults with poorly controlled type 2 diabetes treated with basal insulin without prandial insulin, CGM, as compared with BGM monitoring, resulted in significantly lower HbA 1c levels at 8 months.
Corresponding Author: Roy W. Beck, MD, PhD, Jaeb Center for Health Research Foundation, Inc, Amberly Dr, Ste , Tampa, FL rbeck jaeb.
Published Online: June 2, Author Contributions: Dr Beck had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Beck, Ruedy, Peters, Pop-Busui, Philis-Tsimikas, Umpierrez, Kruger, Young, Aleppo, Polonsky, Price, Bergenstal. Acquisition, analysis, or interpretation of data: Martens, Beck, Bailey, Ruedy, Calhoun, Peters, Pop-Busui, Philis-Tsimikas, Bao, Davis, Bhargava, McGill, Nguyen, Orozco, Biggs, Lucas, Buse, Price, Bergenstal.
Drafting of the manuscript: Martens, Beck, Bailey, Peters, Philis-Tsimikas, Price, Bergenstal. Critical revision of the manuscript for important intellectual content: All authors. Administrative, technical, or material support: Martens, Beck, Ruedy, Bao, Orozco, Biggs, Price, Bergenstal.
Conflict of Interest Disclosures: All authors received grant funding from Dexcom to their institution for the conduct of the submitted study. Dr Beck reported his institution receiving grant funding and study supplies from Tandem Diabetes Care and Beta Bionics; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome.
Ms Ruedy reported receiving grants to her institution from Tandem Diabetes Care and Beta Bionics and study supplies from Novo Nordisk and Eli Lilly outside the submitted work. Dr Calhoun reported being a former employee of Dexcom Inc and his current employer receiving consulting payments on his behalf from vTv Therapeutics, Beta Bionics, and Diasome.
Dr Peters reported serving on advisory boards for Abbott Diabetes Care, Eli Lilly, Medscape, Novo Nordisk, and Zealand; receiving nonfinancial study supplies from Abbott Diabetes Care; and owning stock options for Omada Health and Teladoc.
Dr Pop-Busui reported receiving personal fees from Averitas, Nevro, Novo Nordisk, Boehringer Ingelheim, and Bayer and grants from AstraZeneca outside the submitted work.
Dr Bao reported receiving research funding, paid to her institution, from Novo Nordisk, Mylan, AstraZeneca, and Bristol Myers Squibb. Dr Umpierrez reported research funding paid to his institution from Novo Nordisk and AstraZeneca.
Dr Davis reported grants paid to her institution from Insulet and the National Institutes of Health outside the submitted work. Ms Kruger reported receiving consulting and research funds from Abbott Diabetes, consulting and speaking fees from Eli Lilly, consulting fees from Sanofi Aventis, speaker fees from Xeris Pharmaceuticals, and speaking, consulting, and research funding from Novo Nordisk.
Dr Young reported grants to her institution from Eli Lily, vTv Therapeutics, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals Inc, Sanofi, Tolerion, and Bayer outside the submitted work. Dr McGill reported her institution received grants from the National Institutes of Health and Beta Bionics and that she received advisory board fees from Bayer, Eli Lilly, Metavant, and Salix; personal fees from Aegerion, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, MannKind, Metavant, Novo Nordisk, and Valeritas; consultancy fees from Boehringer Ingelheim; and grants, paid to her employer, from Medtronic and Novo Nordisk.
Dr Aleppo reported grants paid to her institution from AstraZeneca, Eli Lilly, Insulet, and Novo Nordisk and personal fees from Insulet outside the submitted work.
Dr Nguyen reported receiving clinical trial fees from Las Vegas Endocrinology and that his employer has received funds on his behalf for research support, consulting, or serving on the scientific advisory boards for AstraZeneca, Sanofi Aventis, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and MannKind.
Dr Polonsky reported receiving grants from Dexcom, Abbott Diabetes Care, Sanofi Aventis, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, ProventionBio, Insulet, Adocia, and Intuity outside the submitted work. Dr Price reported being an employee of Dexcom and holding stock in the company.
An employee of the company Dr Price was a coauthor and in this role, he was involved in the review of the manuscript and the interpretation of the data prior to submission for publication along with the other authors. The company had no approval authority for the manuscript prior to submission, including no right to veto publication and no control on the decision regarding to which journal the manuscript was submitted.
Group Information: A complete list of the members of the MOBILE Study Group appears in Supplement 3. Study center staff and other individuals who participated in the conduct of the trial are listed in Supplement 2.
Data Sharing Statement: See Supplement 4. full text icon Full Text. Download PDF Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References.
Visual Abstract. Effect of CGM on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin. View Large Download. Figure 1. Screening, Allocation, and Study Follow-up.
b One participant in each group was missing baseline data. Figure 2. Hemoglobin A 1c HbA 1c Values at 8 Months. BGM indicates blood glucose meter; and CGM, continuous glucose monitoring.
Table 1. Baseline Demographics, Medical History, and Insulin Therapies. Table 2. Glycemic Outcomes a. Table 3. Adverse Events and Serious Adverse Events a.
Supplement 1. Trial Protocol. Supplement 2. MOBILE Study Group Listing eFigure 1. Flow Chart of Screening eFigure 2. Flow Chart of Visit Completion Rates eFigure 3. Mean Glucose Over 24 Hours at 8 Months eTable 1.
Patient Eligibility Criteria eTable 2. Description of Quality of Life and Satisfaction Questionnaires eTable 3. Secondary and Exploratory Study Outcomes and Additional Statistical Methods eTable 4.
Glucose Lowering Medications in Use at Time of Randomization in Addition to Insulin eTable 5. CGM Use in CGM Group eTable 6. Frequency of Blood Glucose Meter Testing eTable 7. Change in HbA1c: Per-Protocol Analysis and Sensitivity Analyses eTable 8.
Change in HbA1c According to Baseline HbA1c Group eTable 9. Change in HbA1c According to Baseline Subgroups eTable CGM Outcomes According to Time of Day eTable Daily Insulin Delivery eTable Additions and Discontinuations of Diabetes Medications and Insulin Use eTable Medications Added and Stopped During Follow-up eTable Body Weight, Blood Pressure, and Cholesterol eTable Listing of Types of Reported Adverse Events eTable CGM Satisfaction Scale.
Supplement 3. Nonauthor Collaborators. MOBILE Study Group. Supplement 4. Data Sharing Statement. Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in insulin use and diabetes control in the US: and doi: Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ.
Evaluation of the cascade of diabetes care in the United States, Schnell O, Hanefeld M, Monnier L. Self-monitoring of blood glucose: a prerequisite for diabetes management in outcome trials. Murata GH, Shah JH, Hoffman RM, et al; Diabetes Outcomes in Veterans Study DOVES.
Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study DOVES.
Falk J, Friesen KJ, Okunnu A, Bugden S. Patterns, policy and appropriateness: a year utilization review of blood glucose test strip use in insulin users.
Rossi MC, Lucisano G, Ceriello A, et al; AMD Annals-SMBG Study Group. Real-world use of self-monitoring of blood glucose in people with type 2 diabetes: an urgent need for improvement.
Beck RW, Riddlesworth T, Ruedy K, et al; DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R.
Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Hermanns N, Schumann B, Kulzer B, Haak T. The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial.
Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial.
van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia IN CONTROL : a randomised, open-label, crossover trial.
Wong JC, Foster NC, Maahs DM, et al; T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial.
Peters A, Cohen N, Calhoun P, et al. Glycaemic profiles of diverse patients with type 2 diabetes using basal insulin: MOBILE study baseline data. PubMed Google Scholar Crossref.
Beck RW, Bocchino LE, Lum JW, et al. An evaluation of two capillary sample collection kits for laboratory measurement of HBALC. PubMed Google Scholar. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. The Diabetes Control and Complications Trial Research Group.
The relationship of glycemic exposure HbA1c to the risk of development and progression of retinopathy in the diabetes control and complications trial. Stratton IM, Adler AI, Neil HA, et al.
Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes UKPDS 35 : prospective observational study. Kleinman LC, Norton EC. a simple approach for estimating adjusted risk measures from nonlinear models including logistic regression.
x PubMed Google Scholar Crossref. Start-ups Tout Continuous Glucose Monitoring for People Without Diabetes.
This Medical News article describes the marketing of continuous glucose monitoring devices to individuals without diabetes. Real-time Continuous Glucose Monitoring and Glycemic Control in Insulin-Treated Patients With Diabetes.
This cohort study investigates the effect of real-time continuous glucose monitoring on glycemic control among patients with insulin-treated diabetes. Andrew J. Karter, PhD; Melissa M.
Parker, MS; Howard H. Moffet, MPH; Lisa K. Gilliam, MD, PhD; Richard Dlott, MD. Broadening Access to Continuous Glucose Monitoring for Patients With Type 2 Diabetes.
Continuous Glucose Monitoring and Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin—Reply. Thomas W. Martens, MD; Roy W. Beck, MD, PhD; Richard M. Bergenstal, MD. Continuous Glucose Monitoring and Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin.
Wellbert Hernández-Núñez, MD; Jesús Zacarías Villareal-Pérez, MD; René Rodríguez-Gutiérrez, MD, MSc, PhD. Second, CGM requires a minimal level of mechanical ability because of the need to interact frequently with the sensor's readout and to appropriately respond to its alarms.
It is not necessary for an individual to use an insulin pump to benefit from CGM, but it is necessary for the individual to use some form of intensive insulin therapy because of the need to check the sensor's readings against finger-stick blood-glucose measurements twice daily.
Finally, and most important to CGM users, is the cost of CGM to individuals. This cost is prohibitive to many individuals.
CGM systems operate by measuring the glucose levels in interstitial fluid. The devices consist of three components: a disposable sensor that measures glucose levels, a transmitter that is attached to the sensor, and a receiver that displays and stores glucose information.
The information stored in the receiver is then converted into estimated mean values of glucose standardized to capillary blood glucose levels measured during calibration. Using an applicator or self-insertion device, a thin plastic sensor is inserted just under the skin of the abdomen or the upper arm.
These devices can display real-time glucose values and glucose trends, and some can also sound an alarm or vibrate when they detect hyperglycemia or hypoglycemia.
The receiver can store information for later use, and long-term data can be downloaded to a computer. Currently available CGM devices are considered minimally invasive enzyme-coated electrodes to measure interstitial glucose concentrations and convert these values to blood glucose levels.
The catheter has electrodes impregnated with glucose oxidase, which is introduced into the subcutaneous tissue. The reaction between interstitial fluid glucose and glucose oxidase located on the electrode produces hydrogen peroxide.
This reaction converts the interstitial glucose into an electrical current proportional to the glucose concentration at the site of the catheter insertion.
Devices using enzyme-coated catheters require frequent calibrations to correct variations in the reaction between the electrode and the subcutaneous tissue, as well as fluctuations in glucose and oxygen diffusion at the site of the electrode.
There are currently three CGM systems available in the United States. These devices are reviewed below and summarized in Table 1.
A Summary of the Devices Reviewed 3. This device, approved by the U. Food and Drug Administration FDA in , has several advantages over the previously available MiniMed Gold, a primitive device that did not display real-time glucose results and required downloading of data to a computer every 72 hours for visualization.
The Guardian reports glucose values in real-time, allowing patients to respond immediately to glucose excursions. In addition, it reports the trend of the glucose values in numerical and graphic depictions. Glucose values are sent wirelessly to the monitor at a range of up to 6 feet.
The Guardian also has an alarm system to detect hyperglycemia and hypoglycemia. The DexCom STS is available with a 7-day sensor that was approved for use in It uses a glucose oxidase-coated catheter inserted subcutaneously into the abdomen.
The glucose concentration is measured every 5 minutes, and values are transmitted in real-time to an adjustable wireless receiver. The STS has a built-in alarm system to alert patients to glucose excursions. It requires two calibrations daily during the life span of the sensor.
The Navigator is a minimally invasive CGM device that received FDA approval just as this issue was going to press. It also uses a glucose oxidase enzyme-coated catheter inserted subcutaneously.
Glucose readings are performed every minute for up to 72 hours and are presented as real-time data. The transmitter sends data wirelessly to a receiver up to 10 feet away. The Navigator provides the trend of glucose values as indicated by an arrow visible on the monitor.
The arrow indicates whether the glucose value is stable horizontal , increasing arrow up , rapidly increasing arrow sharply up , decreasing arrow down or rapidly decreasing arrow sharply down.
Glucose values can be downloaded to a computer for retrospective review. The device has an alarm system to indicate glucose excursions outside a preset range.
Compared to other enzyme-based catheter systems, the Navigator only requires one calibration during a hour period. The MiniMed Paradigm REAL-Time System integrates the Paradigm or continuous subcutaneous insulin infusion pump with a CGM system.
Patients wear a sensor and transmitter for up to 3 days at a time. The measured interstitial glucose levels are sent wirelessly to the insulin pump monitor, which displays a 5-minute glucose average. In addition, The FDA approved the Medtronic iPro Recorder in January This product will be available for physician-only use and not for patient purchase.
Patients borrow the iPro Recorder from their physician and wear it for 3 days. The iPro is similar to the sensor-transmitter device used in the Paradigm pump described previously and records 72 hours of glucose data on a tiny chip.
A monitor is not provided for patients to view glucose values at home. Instead, the device is downloaded at the physician's office after the recording period has been completed.
CGM systems have the potential to greatly affect the management of diabetes. Before they gain widespread acceptance, however, physicians and patients will need to feel confident about the ability of these devices to provide an accurate reading of blood glucose. The accuracy of the currently available devices has been studied by a number of investigators, and many report limited sensitivity, particularly in the detection of hypoglycemia.
A variety of methods have been used to evaluate the accuracy of the devices,including relative absolute difference and error grid analysis. Relative absolute difference is calculated by subtracting the reference glucose from that obtained by the device, then dividing this by the reference value and multiplying by to obtain a percentage.
The lower the percentage is, the greater the accuracy of the device. The Diabetes Research in Children Network DirecNet is a network of five clinical centers whose focus is the use of glucose monitoring technology in children with type 1 diabetes.
It has evaluated the accuracy of the Medtronic first- and second-generation CGM systems. Using the relative absolute difference, these and other studies have found that first- and second-generation CGM devices not reviewed here routinely overreported nocturnal hypoglycemia with a high false detection rate.
Bode et al. compared home blood glucose monitors with the results obtained using Medtronic's MiniMed Guardian and found an absolute relative error of Although the sensitivity and specificity are poor, the investigators found that alarms significantly reduced the duration of hypoglycemic excursions.
Garg et al. Some have suggested that the reduced accuracy in the hypoglycemic range,especially at night, may be the result of the lack of constant lag period between interstitial and plasma glucose. If true, this would have implications about the best time to calibrate the monitor.
Techniques such as those proposed by Feldman et al. DirecNet, using the CGMS Gold, compared calibration techniques and found that increasing the number of calibrations had only a modest effect on accuracy, but that calibrating during times of glucose stability and placing less emphasis on daytime calibration for nighttime values may have a greater impact on accuracy.
Clearly, evaluating the accuracy of these devices is not simple because most conventional measures of accuracy, such as correlation, regression, or even the original Clarke error grid, compare measurements taken during static points in time and fail to take into account the temporal nature of the values.
The continuous glucose error grid appears to be a more appropriate measure of accuracy, but it is time-consuming, and some are concerned that this method may fail to detect differences in accuracy between devices. Although the currently available data suggest limited accuracy of these devices compared to capillary blood glucose measurement especially in the hypoglycemic range and point to the need for improvement in the technology,it is important to remember that both CGM and capillary measurement have limitations and that both provide estimates of plasma glucose concentration as determined by a gold standard assessment.
Nobody knows for certain which method provides the better estimate for the purposes of diabetes management,and some have even argued that the brain is bathed in a fluid that resembles interstitial fluid more closely than blood.
Moreover, it is also important to remember that capillary blood glucose measurement has limited accuracy in assessing glucose concentrations in the hypoglycemic range.
In the end, we must assess whether CGM provides information that is accurate enough to be clinically useful and to improve diabetes care. Finally, it is important to remember that CGM is best used as an adjunct to capillary monitoring and not a replacement for it.
The FDA lists the following indications for CGM: detecting trends and tracking patterns of glucose values; serving as an adjunct to, but not a replacement for, information obtained from standard home blood glucose monitoring; aiding in the detection of episodes of hyperglycemia and hypoglycemia and minimizing glucose excursions; and facilitating acute and long-term therapy adjustments.
The FDA advises that the use of CGM is not intended to replace home blood glucose monitoring and that it should only be used in conjunction with patient self-monitoring. Further,treatment decisions based on CGM results should be confirmed by a traditional blood glucose meter.
According to several authors, nocturnal hypoglycemia may account for nearly two-thirds of the justification for prescribing CGM to diabetic patients. In many diabetic patients, daytime hyperglycemia may be easily overlooked.
This may either be a result of insufficient adherence to blood glucose self-monitoring or monitoring practices that do not cover the entire day. CGM can be particularly useful in detecting postprandial hyperglycemia.
Diabetic patients are typically trained to monitor blood glucose before meals and at bedtime, but rarely several hours after a meal. CGM may also help detect nocturnal hyperglycemia and those with the dawn phenomenon or somogyi effect. The dawn phenomenon describes early morning hyperglycemia as a result of growth hormone release in early morning hours, whereas the somogyi effect describes fasting hyperglycemia as a result of the counter-regulatory hormone response to hypoglycemia in the middle of the night.
Components of a CGM record over a 3. Some of the capillary blood glucose readings have been circled. Notice that when there is a rapid change in glucose concentration,the CGM's glucose concentration does not equilibrate with the finger-stick glucose.
B This arrow indicates the upper limit of the high glucose alarm,which is set by patients. This alarm permits patients to take additional insulin for unexpected hyperglycemia. C This arrow indicates the lower glucose alarm, which is also set by patients.
Setting the alarm too high will result in multiple alarms; therefore, patients must decide at what level they would prefer to be alerted when their glucose is dropping.
D This arrow points to the frequent readings from the CGM system. In this case, the reading is provided to the patient every 5 minutes in graphical form.
E In this area of the monitoring graph, there was no communication between the CGM sensor and the CGM receiver. The most common reason for this occurrence is that the sensor stopped working and a new sensor needed to be inserted. CGM can provide more detailed information regarding blood glucose fluctuations than self-monitoring alone.
This is of particular importance in patients with labile glucose values who experience hypoglycemia in response to intensification of insulin therapy. CGM can track the glucose response to changes in insulin therapy and can help patients and physicians determine whether the insulin adjustments were correct.
In addition, patients have more glucose data available to guide them in adjusting their insulin dosing ratios at mealtime, as well as adjusting therapy based on glucose fluctuations related to exercise, illness, or new medications.
Readings stored in a CGM system can be downloaded to a personal computer and printed out for retrospective review Figure 2. The tracings can provide a graphical depiction of the fluctuations in blood glucose levels,which may be easier to comprehend for some patients compared to a traditional logbook.
As a result, patients may be afforded a better understanding of the therapeutic duration of prandial insulin, the impact of insulin pump malfunction such as when a pump catheter is obstructed on glycemic excursions, and the effect of meals, exercise, and lifestyle modification on glucose values.
There are numerous strategies available by which patients can become educated about CGM systems and use them successfully. The most effective method is to provide a comprehensive and multidisciplinary approach involving patients, physicians, certified diabetes nurse educators, and even the local sales representative for the particular brand of CGM prescribed.
Interested patients can be initially screened by their physician for confirmation that they meet indications for CGM monitoring. This evaluation should elucidate precisely how CGM might facilitate improvement in diabetes management.
Once the indication for CGM use is identified, patients should be referred to a qualified diabetes professional to be taught how to use the device,insert the catheter, obtain glucose readings, download stored information to their home computer, and perform any maintenance required to keep the device functional.
The goal is for patients to leave this visit feeling comfortable using the prescribed device and to be able to accurately obtain and apply data from the monitor to improve glucose control.
In many cases, this visit may be coordinated with the manufacturer's representative to provide detailed knowledge of the particular CGM device and information about how to obtain supplies in a timely manner. Patients should also be given a reference booklet that they can refer to, such as the excellent CGM Guide by Edelman and Bailey.
A list of Internet resources offering additional information about CGM systems is provided in Table 2. Figure 2 shows a typical CGM data readout after data are downloaded to a computer. Patients should be familiar with each of the highlighted events that are depicted and should be instructed on how to react to each event.
Refer to the figure legend for explanation of each of the highlighted events. Based on this information,therapy can be adjusted or precautions taken to prevent hyperglycemia or hypoglycemia.
Subsequent follow-up visits can be arranged as needed. Typically, patients should interact with their diabetes health professional at least every 3 months.
Patients may need to be reminded that SMBG remains essential for safe and effective use of the CGM device and to help guide treatment decisions.
Mean reduction in A1C among patients with poorly controlled diabetes who used no CGM stippled bars , intermittent CGM hatched bars , or continuous CGM solid bars for a 3-month period. Adapted from Ref.
EF15 A pediatric study by the DirectNet Study Group demonstrated a reduction in A1C from 7. In a randomized, multicenter study of 91 subjects with insulin-requiring diabetes by Garg et al. Finally, one important study has demonstrated that use of CGM improves A1C in patients with poorly controlled type 1 diabetes.
The device employed was the MiniMed Guardian RT, and, as shown in Figure 3 , patients who received CGM showed a significant decrease in A1C compared to those who did not. This study shows that patients need not use CGM continuously to experience a benefit from it, that the benefits of CGM on A1C are realized within 1 month, and that both children and adults can benefit from using CGM.
All currently available CGM devices measure interstitial glucose. The lag time between when systemic glucose concentration changes appear in the blood and when they appear in the interstitial fluid has been estimated to be between 4 and 26 minutes.
This lag results from a delay in equilibration between blood and interstitial glucose and limits the accuracy of CGM for predicting blood glucose concentrations especially when these concentrations are changing rapidly. The nonlinear nature of the lag has made surmounting this limitation difficult.
All CGM devices require calibration with plasma glucose at least twice a day, with studies showing improved accuracy with increased numbers of calibrations. Additionally, some studies suggest that accuracy improves when calibrations are performed during times of relative glucose stability rather than during periods when the glucose concentration is rapidly changing.
The overestimation of hypoglycemia observed in a number of studies may render CGM inconvenient for people who experience frequent bouts of hypoglycemia, but the technology can also be a very useful tool for people who suffer from hypoglycemia unawareness.
In such patients, the lower alarm setting should be chosen carefully so as not to incur too many false alerts while still allowing enough time to verify that blood glucose values are actually low before acting to correct the hypoglycemia.
For this reason, it is argued that trends may be more useful than the absolute value reported. All CGM devices provide information regarding the trend of glucose, indicated by an up or down arrow or by a graphic representation of glucose concentrations over time. These indicators of trend, used together with the point measurements of interstitial glucose, provide the means by which patients can reduce the number and duration of hypoglycemic episodes.
The idea of a closed-loop system, or artificial pancreas, has long been a goal of many researchers. The rapid development of small, portable CGM devices during the past decade has led many to consider that a closed-loop system may soon be possible.
Problems arise, however, when attempting to employ currently available insulin pumps with CGM devices to create a closed-loop system. For an efficient closed-loop system to respond appropriately to a meal, the device would first have to detect a rise in interstitial glucose, which is delayed by at least 10 minutes.
This lag needs to be added to the delay in insulin delivery and absorption that occurs with any subcutaneous insulin. These factors, combined with the imprecise accuracy of CGM, significantly reduce the feasibility of using these devices in a closed-loop system.
The costs for CGM are substantial and are currently a major barrier to its widespread use. Additionally, the FDA approval for these devices requires the use of capillary blood glucose determination before treatment decisions are made. Private insurance payers provide coverage only on a case-by-case basis.
On the other hand, these costs are easily justified by the avoidance of one emergency hospital visit or one automobile accident per year.
One also needs to appreciate the savings in lives and property that may occur by the avoidance of severe hypoglycemia. To obtain insurance coverage for CGM, physicians often need to play a strong role in assisting patients by writing letters of necessity, describing the overall diabetes care plan including CGM , and certifying that care management will occur while the patient is on CGM.
Mark R. Farm-fresh sunflower seedsStephen Mitchell managdment, Alison SawyerDavid S. Glucoee Continuous Glucose Monitoring: The Future Coontinuous Continuous glucose management Management. Diabetes Spectr 1 April ; 21 2 : — Continuous glucose monitoring CGM technology has the potential to revolutionize diabetes care in the near future because of the real-time feedback it provides about therapeutic interventions and variations in lifestyle or dietary intake.Hydration for athletes Informationen zur Fiber optic network security Version. Apple® managemdnt in der kommenden iOS Managment den Standby-Modus Coenzyme Q and aging den Assistive Access-Modus einführen.
Diese neuen Modi können sich auf Managment Erfahrung mit Ihrer Contniuous Libre Continkous App 11 auswirken. Erfahren Sie hierwie Sie gluocse Probleme vermeiden können.
Entdecken Sie Fiber optic network security von Cojtinuous mit Diabetes Sports Fitness Classes meistgenutzte Glukose-Sensor-Messsystem.
FreeStyle Libre 3 Continuouss Sie gkucose bei Ihrem Diabetesmanagement. Das Glukosemesssystem ist dabei sowohl für Menschen mit Typ als auch mit Olive oil for cholesterol geeignet. Es ist zugelassen manaagement Kinder ab 4 Jahren, Erwachsene und Schwangere.
Informieren Sie sich in unserem Online-Live-Webinar oder Cohtinuous einer Präsenzveranstaltung über die Dairy-free snacks des FreeStyle Libre 3 Fiber optic network security und lernen Sie FreeStyle Libre 3 beim Cotninuous kennen.
Erhalten Sie Ihre Manaegment jede einzelne Minute 3 automatisch und ohne Glufose auf Ihr Smartphone oder Lesegerät. Der Clntinuous kleinste und Continuous glucose management 15 Sensor der Welt wird alle 14 Tage 2 einfach und schmerzfrei 10 zuhause angebracht.
Fühlen Dairy-free snacks sich sicher Powerful power management minutengenauen Glukosewerten und optionalen Alarmen, managsment Sie vor einer Über- oder Continuous glucose management warnen. Fast alle gesetzlichen Krankenkassen übernehmen managemdnt die Kosten für FreeStyle Libre 3!
FreeStyle Fiber optic network security 3 hat mit seinen zahlreichen Produktvorteilen bereits viele Menschen mit Diabetes gegenüber herkömmlichen Messverfahren BGM sowie anderen kontinuierlichen Glukosemesssystemen CGM überzeugt.
Das bedeutet mir unendlich viel. Beim herkömmlichen Blutzuckermessen wird mit einer Dairy-free snacks in die Continuoux gestochen. Mannagement kontinuierlichen Manzgement CGMwie manaegment FreeStyle Libre glucoe Messsystem, bringen Antioxidant-rich oils sich zuhause ganz einfach 10 alle 14 Tage kanagement einen kleinen Sensor auf der Rückseite Ihres Oberarms an.
Dieser misst über ein Dairy-free snacks unter der Haut fortlaufend Ihre Zuckerwerte und mwnagement die Werte Continuojs 3 in Ihre FreeStyle Libre managekent App oder auf Coontinuous FreeStyle Libre 3 Lesegerät Auf diese Weise können Sie den Verlauf Ihres Zuckerwertes sowie dessen Trend, kontinuierlich im Blick behalten.
Im CContinuous zur managemwnt Blutzuckermessung erhalten Sie so Oats and immune-boosting beta-glucans Informationen.
Der Sensor wird auf der Rückseite des Oberarms ganz einfach mithilfe eines Applikators angebracht. Dabei wird ein dünnes, biegsames, steriles Filament direkt glucoe die Haut geschoben. Contimuous Sensor selbst wird dabei mit einer Klebefolie auf der Haut fixiert, Continuous glucose management.
Der Sensor kann bis zu 14 Tage manayement an managemnet Rückseite des Oberarms getragen Performance-enhancing meal plans. Danach müssen Sie Conitnuous neuen Sensor anbringen.
Der Sensor ist in Fiber optic network security zu 1m Wassertiefe für die Dauer von bis zu managejent Minuten wasserfest und kann beim Baden, Duschen, Continuohs oder beim Sport getragen manxgement. FreeStyle Libre 3 ist auf Rezept, über Mannagement und im praktischen Abo Immunity defense mechanisms. Auf der FreeStyle Libre 3 Produktseite können Sie sich über die unterschiedlichen Bestellmöglichkeiten informieren und die Bestellung durchführen.
Wenn Msnagement als gesetzlich Versicherte:r ein Rezept bei uns einreichenstellen wir einen Kostenübernahmeantrag bei Ihrer Krankenkasse und informieren Sie, sobald der Antrag genehmigt wurde.
Ihre Versorgung startet anschließend automatisch. Fast alle Krankenkassen übernehmen die Kosten für FreeStyle Libre 16! Laden Sie sich jetzt die aktuelle Krankenkassenliste herunter und sehen Sie direkt nach.
Übernimmt Ihre Krankenkasse die Kosten, dann können Sie Ihr Rezept bei uns einreichen. Wir kümmern uns um alles Weitere für Sie. Mit Hilfe der LibreLinkUp App können Sie Zuckerwerte mit Angehörigen teilen — für mehr Sicherheit aus der Ferne.
Mit Hilfe von LibreView 12 können Sie zudem Zuckerwerte ganz einfach und von überall direkt mit Ihrem behandelnden Praxisteam teilen — für ein optimiertes Therapiemanagement durch effizienteren Austausch mit Ihrer Praxis.
Überzeugen Sie sich selbst von FreeStyle Libre 3. Fordern Sie jetzt ganz einfach und unverbindlich Ihren Testsensor an. In 3 einfachen Schritten zu Ihrem FreeStyle Libre Messsystem — egal ob privat oder gesetzlich versichert.
Neben dem FreeStyle Libre 3 Sensor und der FreeStyle Libre 3 App 11 selbst, bieten wir Ihnen weitere hilfreiche Funktionen und Lösungen an, um Ihnen das Diabetesmanagement zu erleichtern.
Mit Hilfe von LibreView 12 Glukosewerte mit den behandelnden Praxen teilen 4. Mehr erfahren. Mit Hilfe von LibreLinkUp 8 Glukosewerte mit Ihren Liebsten teilen 4,5. Der Sensor kann bis zu 14 Tage lang getragen werden. Eine zusätzliche Prüfung der Glukosewerte mittels eines Blutzucker-Messgeräts ist erforderlich, wenn die Symptome nicht mit den Messwerten oder den Alarmen des Systems übereinstimmen.
Das Setzen eines Sensors erfordert ein Einführen des Sensorfilaments unter die Haut. Der Sensor ist 60 Minuten nach der Aktivierung für die Glukosemessung bereit. Die Übertragung der Daten zwischen den Apps erfordert eine Internetverbindung. Das Teilen der Glukosedaten erfordert eine Registrierung bei LibreView.
Es besteht die Möglichkeit, die LibreLinkUp Einladung anzunehmen und damit Benachrichtigungen und Warnhinweise zu erhalten oder diese abzulehnen. Eine Entscheidung hierüber sollten Sie basierend auf Ihren Kenntnissen und Erfahrungen treffen, um bei dem Erhalt eines zu hohen oder zu niedrigen Glukosewerts angemessen reagieren zu können.
Die Aussage basiert auf der Anzahl der Nutzer des FreeStyle Libre Messsystems weltweit im Vergleich zu der Nutzeranzahl anderer führender sensorbasierter Glukosemessysteme für den persönlichen Gebrauch. Quelle: Daten liegen vor.
Abbott Diabetes Care, Inc. Haak, Thomas, et al. Diabetes Therapy. Studie wurde mit Erwachsenen durchgeführt. Bolinder, Jan, et al. The Lancet. Die Nutzung von LibreLinkUp erfordert eine Registrierung bei LibreView.
Im Vergleich mit anderen am Körper zu tragenden Sensoren. Daten liegen vor. Abbott Diabetes Care. Die FreeStyle Libre 3 App ist nur mit bestimmten Mobilgeräten und Betriebssystemen kompatibel. Bevor Sie die App nutzen möchten, besuchen Sie bitte die Webseite www.
de um mehr Informationen zur Gerätekompatibilität zu erhalten. Ein Sensor kann nur mit dem FreeStyle Libre 3 Lesegerät oder der App aktiviert und genutzt werden. Ein Wechsel ist nach der Aktivierung des Sensors nicht möglich.
LibreView ist eine cloudbasierte Anwendung. Die FreeStyle Libre Messsysteme sind zertifiziert für Kinder ab 4 Jahren sowie Erwachsene, einschließlich Schwangere. Die Aufsichtspflicht über die Anwendung und die Auswertung von einem FreeStyle Libre Messsystem bei Kindern bis zur Vollendung des Lebensjahres obliegt der Verantwortung einer volljährigen Person.
Alarme sind standardgemäß ausgeschaltet und müssen eingeschaltet werden. Im Vergleich mit anderen vom Patienten selbst anzubringenden Sensoren. Für Menschen mit Typ 1 oder Typ 2 und intensivierter Insulintherapie.
Die Entscheidung einer Krankenkasse zur Kostenübernahme eines FreeStyle Libre Messsystems ist eine Einzelfallentscheidung. Eine Krankenkasse kann die Kostenübernahme auch ablehnen, sofern die Voraussetzungen für die Kostenübernahme im Einzelfall nicht erfüllt sind.
Für medizinische Hilfsmittel fällt eine gesetzliche Zuzahlung an. Ich willige ein, dass die Abbott GmbH, Max-Planck-Ring 2, Wiesbaden meine personenbezogenen Daten für die Eröffnung eines Kundenkontos und die Abwicklung von Bestellungen inkl.
der Zahlungsabwicklung und Versendung sowie der damit verbundenen Einbindung entsprechender Dienstleister, zur Beantwortung von Anfragen sowie für Listenabgleiche entsprechend den Beschreibungen in den Ziffern [2. Suche schließen.
Wichtige Informationen zur iOS Version Apple® wird in der kommenden iOS Version den Standby-Modus und den Assistive Access-Modus einführen. Einfaches Diabetesmanagement Mit FreeStyle Libre jederzeit und ohne routinehaftes Fingerstechen 1 Ihre Zuckerwerte messen und teilen 4 Entdecken Sie das von Menschen mit Diabetes weltweit meistgenutzte Glukose-Sensor-Messsystem.
Zum Produkt Testsensor bestellen. Überzeugen Sie sich von unseren zahlreichen Produktvorteilen FreeStyle Libre 3 unterstützt Sie täglich bei Ihrem Diabetesmanagement. Kein routinehaftes Fingerstechen 17 Erhalten Sie Ihre Glukosewerte jede einzelne Minute 3 automatisch und ohne Scannen auf Ihr Smartphone oder Lesegerät.
Kleinster und flachster Sensor Der derzeit kleinste und flachste 15 Sensor der Welt wird alle 14 Tage 2 einfach und schmerzfrei 10 zuhause angebracht.
Optionale Alarme für mehr Sicherheit 7 Fühlen Sie sich sicher mit minutengenauen Glukosewerten und optionalen Alarmen, welche Sie vor einer Über- oder Unterzuckerung warnen. Kostenübernahme durch Krankenkasse Fast alle gesetzlichen Krankenkassen übernehmen bereits die Kosten für FreeStyle Libre 3!
Über 5 Millionen Menschen nutzen FreeStyle Libre weltweit 6 FreeStyle Libre 3 hat mit seinen zahlreichen Produktvorteilen bereits viele Menschen mit Diabetes gegenüber herkömmlichen Messverfahren BGM sowie anderen kontinuierlichen Glukosemesssystemen CGM überzeugt.
Sandra Starke Fußball-Nationalspielerin. Laura Karasek TV-Moderatorin. Alle Kundenberater sind sehr freundlich und kompetente Ansprechpartner. Worin besteht der Unterschied zwischen einem kontinuierlichen Glukosemesssystem CGM und dem herkömmlichen Blutzuckermessen mit Fingerstechen?
Wie bringe ich den Sensor an? Schmerzt das? Wie lange hält der Sensor? Ist er wasserfest? Wie erhalte ich FreeStyle Libre als privat bzw.
: Continuous glucose management| Continuous Glucose Monitoring | Klonoff, MD, FACP David C. Stewart B. Listing of Types of Reported Adverse Events eTable Klonoff; Continuous Glucose Monitoring : Roadmap for 21st century diabetes therapy. A Summary of the Devices Reviewed 3. They are the Continuous Glucose Monitoring System Gold CGMS Gold; Medtronic MiniMed, Northridge, CA 1 , the GlucoWatch G2 Biographer GW2B; Cygnus, Redwood City, CA 2 , the Guardian Telemetered Glucose Monitoring System Medtronic MiniMed 3 , the GlucoDay A. |
| CGM: A Significant Advance in Diabetes Care | Techniques such as those proposed by Feldman et al. DirecNet, using the CGMS Gold, compared calibration techniques and found that increasing the number of calibrations had only a modest effect on accuracy, but that calibrating during times of glucose stability and placing less emphasis on daytime calibration for nighttime values may have a greater impact on accuracy. Clearly, evaluating the accuracy of these devices is not simple because most conventional measures of accuracy, such as correlation, regression, or even the original Clarke error grid, compare measurements taken during static points in time and fail to take into account the temporal nature of the values. The continuous glucose error grid appears to be a more appropriate measure of accuracy, but it is time-consuming, and some are concerned that this method may fail to detect differences in accuracy between devices. Although the currently available data suggest limited accuracy of these devices compared to capillary blood glucose measurement especially in the hypoglycemic range and point to the need for improvement in the technology,it is important to remember that both CGM and capillary measurement have limitations and that both provide estimates of plasma glucose concentration as determined by a gold standard assessment. Nobody knows for certain which method provides the better estimate for the purposes of diabetes management,and some have even argued that the brain is bathed in a fluid that resembles interstitial fluid more closely than blood. Moreover, it is also important to remember that capillary blood glucose measurement has limited accuracy in assessing glucose concentrations in the hypoglycemic range. In the end, we must assess whether CGM provides information that is accurate enough to be clinically useful and to improve diabetes care. Finally, it is important to remember that CGM is best used as an adjunct to capillary monitoring and not a replacement for it. The FDA lists the following indications for CGM: detecting trends and tracking patterns of glucose values; serving as an adjunct to, but not a replacement for, information obtained from standard home blood glucose monitoring; aiding in the detection of episodes of hyperglycemia and hypoglycemia and minimizing glucose excursions; and facilitating acute and long-term therapy adjustments. The FDA advises that the use of CGM is not intended to replace home blood glucose monitoring and that it should only be used in conjunction with patient self-monitoring. Further,treatment decisions based on CGM results should be confirmed by a traditional blood glucose meter. According to several authors, nocturnal hypoglycemia may account for nearly two-thirds of the justification for prescribing CGM to diabetic patients. In many diabetic patients, daytime hyperglycemia may be easily overlooked. This may either be a result of insufficient adherence to blood glucose self-monitoring or monitoring practices that do not cover the entire day. CGM can be particularly useful in detecting postprandial hyperglycemia. Diabetic patients are typically trained to monitor blood glucose before meals and at bedtime, but rarely several hours after a meal. CGM may also help detect nocturnal hyperglycemia and those with the dawn phenomenon or somogyi effect. The dawn phenomenon describes early morning hyperglycemia as a result of growth hormone release in early morning hours, whereas the somogyi effect describes fasting hyperglycemia as a result of the counter-regulatory hormone response to hypoglycemia in the middle of the night. Components of a CGM record over a 3. Some of the capillary blood glucose readings have been circled. Notice that when there is a rapid change in glucose concentration,the CGM's glucose concentration does not equilibrate with the finger-stick glucose. B This arrow indicates the upper limit of the high glucose alarm,which is set by patients. This alarm permits patients to take additional insulin for unexpected hyperglycemia. C This arrow indicates the lower glucose alarm, which is also set by patients. Setting the alarm too high will result in multiple alarms; therefore, patients must decide at what level they would prefer to be alerted when their glucose is dropping. D This arrow points to the frequent readings from the CGM system. In this case, the reading is provided to the patient every 5 minutes in graphical form. E In this area of the monitoring graph, there was no communication between the CGM sensor and the CGM receiver. The most common reason for this occurrence is that the sensor stopped working and a new sensor needed to be inserted. CGM can provide more detailed information regarding blood glucose fluctuations than self-monitoring alone. This is of particular importance in patients with labile glucose values who experience hypoglycemia in response to intensification of insulin therapy. CGM can track the glucose response to changes in insulin therapy and can help patients and physicians determine whether the insulin adjustments were correct. In addition, patients have more glucose data available to guide them in adjusting their insulin dosing ratios at mealtime, as well as adjusting therapy based on glucose fluctuations related to exercise, illness, or new medications. Readings stored in a CGM system can be downloaded to a personal computer and printed out for retrospective review Figure 2. The tracings can provide a graphical depiction of the fluctuations in blood glucose levels,which may be easier to comprehend for some patients compared to a traditional logbook. As a result, patients may be afforded a better understanding of the therapeutic duration of prandial insulin, the impact of insulin pump malfunction such as when a pump catheter is obstructed on glycemic excursions, and the effect of meals, exercise, and lifestyle modification on glucose values. There are numerous strategies available by which patients can become educated about CGM systems and use them successfully. The most effective method is to provide a comprehensive and multidisciplinary approach involving patients, physicians, certified diabetes nurse educators, and even the local sales representative for the particular brand of CGM prescribed. Interested patients can be initially screened by their physician for confirmation that they meet indications for CGM monitoring. This evaluation should elucidate precisely how CGM might facilitate improvement in diabetes management. Once the indication for CGM use is identified, patients should be referred to a qualified diabetes professional to be taught how to use the device,insert the catheter, obtain glucose readings, download stored information to their home computer, and perform any maintenance required to keep the device functional. The goal is for patients to leave this visit feeling comfortable using the prescribed device and to be able to accurately obtain and apply data from the monitor to improve glucose control. In many cases, this visit may be coordinated with the manufacturer's representative to provide detailed knowledge of the particular CGM device and information about how to obtain supplies in a timely manner. Patients should also be given a reference booklet that they can refer to, such as the excellent CGM Guide by Edelman and Bailey. A list of Internet resources offering additional information about CGM systems is provided in Table 2. Figure 2 shows a typical CGM data readout after data are downloaded to a computer. Patients should be familiar with each of the highlighted events that are depicted and should be instructed on how to react to each event. Refer to the figure legend for explanation of each of the highlighted events. Based on this information,therapy can be adjusted or precautions taken to prevent hyperglycemia or hypoglycemia. Subsequent follow-up visits can be arranged as needed. Typically, patients should interact with their diabetes health professional at least every 3 months. Patients may need to be reminded that SMBG remains essential for safe and effective use of the CGM device and to help guide treatment decisions. Mean reduction in A1C among patients with poorly controlled diabetes who used no CGM stippled bars , intermittent CGM hatched bars , or continuous CGM solid bars for a 3-month period. Adapted from Ref. EF15 A pediatric study by the DirectNet Study Group demonstrated a reduction in A1C from 7. In a randomized, multicenter study of 91 subjects with insulin-requiring diabetes by Garg et al. Finally, one important study has demonstrated that use of CGM improves A1C in patients with poorly controlled type 1 diabetes. The device employed was the MiniMed Guardian RT, and, as shown in Figure 3 , patients who received CGM showed a significant decrease in A1C compared to those who did not. This study shows that patients need not use CGM continuously to experience a benefit from it, that the benefits of CGM on A1C are realized within 1 month, and that both children and adults can benefit from using CGM. All currently available CGM devices measure interstitial glucose. The lag time between when systemic glucose concentration changes appear in the blood and when they appear in the interstitial fluid has been estimated to be between 4 and 26 minutes. This lag results from a delay in equilibration between blood and interstitial glucose and limits the accuracy of CGM for predicting blood glucose concentrations especially when these concentrations are changing rapidly. The nonlinear nature of the lag has made surmounting this limitation difficult. All CGM devices require calibration with plasma glucose at least twice a day, with studies showing improved accuracy with increased numbers of calibrations. Additionally, some studies suggest that accuracy improves when calibrations are performed during times of relative glucose stability rather than during periods when the glucose concentration is rapidly changing. The overestimation of hypoglycemia observed in a number of studies may render CGM inconvenient for people who experience frequent bouts of hypoglycemia, but the technology can also be a very useful tool for people who suffer from hypoglycemia unawareness. In such patients, the lower alarm setting should be chosen carefully so as not to incur too many false alerts while still allowing enough time to verify that blood glucose values are actually low before acting to correct the hypoglycemia. For this reason, it is argued that trends may be more useful than the absolute value reported. All CGM devices provide information regarding the trend of glucose, indicated by an up or down arrow or by a graphic representation of glucose concentrations over time. These indicators of trend, used together with the point measurements of interstitial glucose, provide the means by which patients can reduce the number and duration of hypoglycemic episodes. The idea of a closed-loop system, or artificial pancreas, has long been a goal of many researchers. The rapid development of small, portable CGM devices during the past decade has led many to consider that a closed-loop system may soon be possible. Problems arise, however, when attempting to employ currently available insulin pumps with CGM devices to create a closed-loop system. For an efficient closed-loop system to respond appropriately to a meal, the device would first have to detect a rise in interstitial glucose, which is delayed by at least 10 minutes. This lag needs to be added to the delay in insulin delivery and absorption that occurs with any subcutaneous insulin. These factors, combined with the imprecise accuracy of CGM, significantly reduce the feasibility of using these devices in a closed-loop system. The costs for CGM are substantial and are currently a major barrier to its widespread use. Additionally, the FDA approval for these devices requires the use of capillary blood glucose determination before treatment decisions are made. Private insurance payers provide coverage only on a case-by-case basis. On the other hand, these costs are easily justified by the avoidance of one emergency hospital visit or one automobile accident per year. One also needs to appreciate the savings in lives and property that may occur by the avoidance of severe hypoglycemia. To obtain insurance coverage for CGM, physicians often need to play a strong role in assisting patients by writing letters of necessity, describing the overall diabetes care plan including CGM , and certifying that care management will occur while the patient is on CGM. Patients should also be encouraged to contact the manufacturer's customer service representative, who may be able to assist in getting coverage. Both patients and providers should be prepared to appeal the case, often multiple times. The Juvenile Diabetes Research Foundation has helpful information on its website www. org ,outlining the process for case-by-case CGM coverage. Medicare and Medicaid have not yet agreed to cover CGM costs. As of January , there are Medicare and Medicaid service codes available for CGM providers, which may signal an improved likelihood of reimbursement in the future. Improved technology resulting in greater accuracy and usability may also enhance the acceptance of CGM technology by practitioners, thereby increasing the demand for insurance coverage. Improvements in the sensor technology aimed at increasing sensor life span may further reduce costs. Providers should also remember that CGM devices do not need to be worn continuously to confer benefit, and although the glycemic benefit is not as great as when CGM is worn continuously, the cost for sensors can be reduced by wearing the CGM intermittently rather than continuously. The use of CGM in specific populations of patients requires comment. The benefit of CGM depends to a great extent on its limitations, such as the complexity of its use and its relatively reduced sensitivity at low blood glucose concentrations. On the other hand, trend data can be very useful for avoiding hypo- and hyperglycemia. The most obvious populations of candidates for CGM are adult type 1 diabetic patients who are attempting to improve their glucose control and avoid severe hypoglycemia. The monitor is responsible for displaying information to the user. Some CGMs have a dedicated monitor, which may be a separate device or part of an insulin pump. Other devices are smartphone-compatible and work via a smartphone app. With a monitor, the user can see their blood sugar levels every few minutes. The CGM system can also store this information and send it to a doctor. The ease of collecting and sharing blood sugar levels can help doctors and CGM users work together on improving a diabetes treatment plan. Typically, most people who use a CGM will have type 1 diabetes. Some individuals with type 2 diabetes may also benefit from CGMs. A doctor may prescribe a CGM if people meet certain criteria and requirements. Usually, this may include :. For these individuals, a CGM can help them closely monitor blood sugar levels and may prevent them from experiencing a serious hypoglycemic event. A commentary notes that a CGM can help:. There are many benefits a CGM may offer over other devices. Namely, it can help people better manage diabetes and improve health outcomes. A study highlights that CGMs can improve glycemic control in individuals with inadequately controlled type 1 diabetes. Compared with conventional treatment options, people using CGMs had lower HbA1C levels. Elsewhere, a extension study investigated the potential long-term effects of using a CGM. The results suggest that CGMs have a beneficial effect on HbA1C, hypoglycemia prevention, hypoglycemic confidence, treatment satisfaction, and well-being. A study notes that a CGM device can improve health outcomes for both parent and baby during pregnancy. A commentary also highlights CGMs as a reliable, safe, and effective tool, particularly during the COVID pandemic. Having a CGM may be particularly useful for a person with a recent diagnosis of diabetes as it can help them identify what triggers blood sugar changes and how to minimize these fluctuations. Other advantages of a CGM may include :. This indicates that CGMs may show promise for individuals with diabetes across different ages and health considerations. As such, people with diabetes and their doctors can use a CGM to improve diabetes management strategies. Although a CGM can offer many benefits for people with diabetes, it may come with certain limitations. While it does reduce the number of finger-prick tests needed, it does not eliminate them entirely. People may still require finger pricks to calibrate a CGM and confirm readings. The cost of CGM devices can also be prohibitive for many users and some insurance plans may not cover them. This could result in the price of a CGM running higher than other testing devices. While the sensors are generally robust, people may also want to avoid certain activities to prevent the risk of knocking or damaging the device, as they will need to replace it if it stops functioning. Some people may also find the amount of data a CGM provides overwhelming. Understanding the information and making decisions from it may cause anxiety in some individuals. Also known as an automated insulin delivery system or artificial pancreas, these systems can help mimic the function of a healthy pancreas. A CGM device is an important piece of a hybrid closed-loop system. These systems typically consist of three different components:. In this system, the CGM keeps track of the blood sugar at regular intervals. In order to ensure accuracy of SMBG, results should be compared with a laboratory measurement of FPG at least annually or when A1C does not match SMBG readings. Periodic re-education on correct SMBG technique may improve the accuracy of SMBG results 61, In rare situations, therapeutic interventions may interfere with the accuracy of some SMBG devices. For example, icodextrin-containing peritoneal dialysis solutions may cause falsely high readings in meters utilizing glucose dehydrogenase. Care should be taken to select an appropriate meter with an alternative glucose measurement method in such situations. Meters are available that allow SMBG using blood samples from sites other than the fingertip forearm, palm of the hand, thigh. Accuracy of results over a wide range of BG levels and during periods of rapid change in BG levels is variable across sites. During periods of rapid change in BG levels e. after meals, after exercise and during hypoglycemia , fingertip testing has been shown to more accurately reflect glycemic status than forearm or thigh testing 63, In comparison, blood samples taken from the palm near the base of the thumb thenar area demonstrate a closer correlation to fingertip samples at all times of day and during periods of rapid change in BG levels 65, If all of these conditions are present in type 2 diabetes, ketone testing should be considered, as DKA also can occur in these individuals. During DKA, the equilibrium that is usually present between ketone bodies shifts toward formation of beta-hydroxybutyric acid beta-OHB. As a result, testing methods that measure blood beta-OHB levels may provide more clinically useful information than those that measure urine acetoacetate or acetone levels. Assays that measure acetoacetate through urine testing may not identify the onset and resolution of ketosis as quickly as those that quantify beta-OHB levels in blood, since acetoacetate or acetone can increase as beta-OHB decreases with effective treatment Meters that quantify beta-OHB from capillary sampling may be preferred for self-monitoring of ketones, as they have been associated with earlier detection of ketosis and may provide information required to prevent progression to DKA 66— This may be especially useful for individuals with type 1 diabetes using continuous subcutaneous insulin CSII therapy, as interruption of insulin delivery can result in rapid onset of DKA Continuous glucose monitoring CGM systems measure glucose concentrations in the interstitial fluid. Two types of devices are available. CGM technology incorporates a subcutaneously inserted sensor, an attached transmitter and, in the case of real-time CGM, a display unit which may be a stand-alone unit or be integrated into an insulin pump. In Canada, 2 real-time CGM and 2 professional CGM are available. Real-time CGM has been consistently shown to reduce A1C in both adults 70—81 and children 71,73,75,76,78,79,82 with type 1 diabetes with and without CSII, and to reduce A1C in adults with type 2 diabetes Real-time CGM also has been shown to reduce the time spent in hypoglycemia 78,80,81, Professional CGM has been shown to reduce A1C in adults with type 2 diabetes 85 and in pregnant women with type 1 or type 2 diabetes Successful use of CGM is dependent on adherence with duration of time the CGM is used. The greater the time wearing the device, typically the better the A1C 72,73,76,77,82, Like SMBG, CGM provides the best outcomes if it is associated with structured educational and therapeutic programs. CGM is not a replacement for SMBG because SMBG is still required for calibration of the CGM device. Some real-time CGM devices require SMBG to confirm interstitial measurements prior to making therapeutic changes or treating suspected hypoglycemia; whereas other devices only require SMBG if glucose alerts and readings do not match symptoms. Flash glucose monitoring FGM also measures glucose concentration in the interstitial fluid, however, FGM differs from CGM technology in several ways. FGM is factory calibrated and does not require capillary blood glucose with SMBG device calibration. The FGM reader also displays a plot profile of the last 8 hours, derived from interpolating glucose concentrations recorded every 15 minutes. The sensor can be worn continuously for up to 14 days. The device does not provide low or high glucose alarms. In the Randomised Controlled Study to Evaluate the Impact of Novel Glucose Sensing Technology on HbA1c in Type 2 Diabetes trial, in individuals with type 2 diabetes, the use of FGM vs. A1C, glycated hemoglobin ; BG, blood glucose; BMI , body mass index CBG ; capillary blood glucose; CGM , continuous glucose monitoring; CGMS , continuous glucose monitoring system; CSII , continuous subcutaneous infusion infusion; DKA , diabetic ketoacidosis; FGM ; flash glucose monitoring; FPG , fasting plasma glucose; PG , plasma glucose; SMBG , self-monitoring of blood glucose. Appendix 5. Self-Monitoring of Blood Glucose SMBG Recommendation Tool for Health-Care Providers. Literature Review Flow Diagram for Chapter 9: Monitoring Glycemic Control. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group P referred R eporting I tems for S ystematic Reviews and M eta- A nalyses: The PRISMA Statement. PLoS Med 6 6 : e pmed For more information, visit www. Rick Siemens reports personal fees from Sanofi, Novo Nordisk, Mont-Med, Abbott, Merck, AstraZeneca, Lifescan, and Janssen, outside the submitted work. Woo has nothing to disclose. All content on guidelines. ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications diabetes. Become a Member Order Resources Home About Contact DONATE. Next Previous. Key Messages Recommendations Figures Full Text References. Chapter Headings A1C Testing Self-Monitoring of Blood Glucose Ketone Testing Continuous Glucose Monitoring Systems Flash Glucose Monitoring Other Relevant Guidelines Relevant Appendices Author Disclosures. Key Messages Glycated hemoglobin A1C is a valuable indicator of glycemic treatment effectiveness and should be measured at least every 3 months when glycemic targets are not being met and when antihyperglycemic therapy is being adjusted. In some circumstances, such as when significant changes are made to therapy or during pregnancy, it is appropriate to check A1C more frequently. Awareness of all measures of glycemia—self-monitored blood glucose results, including self-monitored blood glucose SMBG , flash glucose monitoring FGM , continous glucose monitoring CGM and A1C—provides the best information to assess glycemic control. Self-monitoring of blood glucose, FGM and CGM should not be viewed as glucose-lowering interventions, but rather as aids to assess the effectiveness of glucose-lowering interventions and to prevent and detect hypoglycemia. Timing and frequency of SMBG may be determined individually based on the type of diabetes, the type of antihyperglycemic treatment prescribed, the need for information about blood glucose levels and the individual's capacity to use the information from testing to modify healthy behaviours or self-adjust antihyperglycemic agents. SMBG, FGM and CGM linked with a structured educational and therapeutic program designed to facilitate behaviour change can improve blood glucose levels and prevent hypoglycemia. Key Messages for People with Diabetes A1C is a measurement of your average blood glucose control for the last 2 to 3 months. You should have your A1C measured every 3 months when your blood glucose targets are not being met or when you are making changes to your diabetes management. In some circumstances, such as when significant changes are made to your glucose-lowering therapy or during pregnancy, your health-care provider may check your A1C more frequently. Checking your blood glucose with a glucose meter also known as self-monitoring of blood glucose or using a flash glucose meter or continuous glucose monitor will: Determine if you have a high or low blood glucose at a given time Show how your health behaviours and diabetes medication s affect your blood glucose levels Help you and your diabetes health-care team to make health behaviour and medication changes that will improve your blood glucose levels. Discuss with your diabetes health-care team how often you should check your blood glucose level. A1C Testing Glycated hemoglobin A1C is a reliable estimate of mean plasma glucose PG levels over the previous 8 to 12 weeks 1. Self-Monitoring of Blood Glucose Monitoring blood glucose levels, whether using traditional self monitoring of blood glucose SMBG devices or more recent flash glucose monitoring FGM , can serve as a useful adjunct to other measures of glycemia, including A1C. Frequency of SMBG The recommended frequency of monitoring BG may be individualized to each person's unique circumstances. Type 1 and type 2 diabetes treated with insulin For people with type 1 diabetes, monitoring BG is essential to achieving and maintaining good glycemic control. Type 2 diabetes not treated with insulin For people with type 2 diabetes treated with healthy behaviour interventions, with or without noninsulin antihyperglycemic agents, the effectiveness and frequency of monitoring BG in improving glycemic control is less clear 23,24,38— Verification of accuracy of SMBG performance and results Variability can exist between BG results obtained using SMBG devices and laboratory testing of PG. Alternate site testing Meters are available that allow SMBG using blood samples from sites other than the fingertip forearm, palm of the hand, thigh. Continuous Glucose Monitoring Systems Continuous glucose monitoring CGM systems measure glucose concentrations in the interstitial fluid. Flash Glucose Monitoring Flash glucose monitoring FGM also measures glucose concentration in the interstitial fluid, however, FGM differs from CGM technology in several ways. Recommendations For most individuals with diabetes, A1C should be measured approximately every 3 months to ensure that glycemic goals are being met or maintained [Grade D, Consensus]. In some circumstances, such as when significant changes are made to therapy, or during pregnancy, it is appropriate to check A1C more frequently. Testing at least every 6 months should be performed in adults during periods of treatment and healthy behaviour stability when glycemic targets have been consistently achieved [Grade D, Consensus]. For individuals using insulin more than once a day, SMBG should be used as an essential part of diabetes self-management [Grade A, Level 1 34 , for type 1 diabetes; Grade C, Level 3 23 , for type 2 diabetes] and should be undertaken at least 3 times per day [Grade C, Level 3 23,31 ] and include both pre- and postprandial measurements [Grade C, Level 3 31,32,89 ]. For individuals with type 2 diabetes on once-daily insulin in addition to noninsulin antihyperglycemic agents, testing at least once a day at variable times is recommended [Grade D, Consensus]. For individuals with type 2 diabetes not receiving insulin therapy, frequency of SMBG recommendations should be individualized depending on type of antihyperglycemic agents, level of glycemic control and risk of hypoglycemia [Grade D, Consensus]. When glycemic control is not being achieved, SMBG should be instituted [Grade B, Level 2 46,51 ] and should include periodic pre- and postprandial measurements and training of health-care providers and people with diabetes on methods to modify health behaviours and antihyperglycemic medications in response to SMBG values [Grade B, Level 2 30,90 ] If achieving glycemic targets or receiving antihyperglycemic medications not associated with hypoglycemia, infrequent SMBG is appropriate [Grade D, Consensus]. In many situations, for all individuals with diabetes, more frequent SMBG testing should be undertaken to provide information needed to make health behaviour or antihyperglycemic medication adjustments required to achieve desired glycemic targets and avoid risk of hypoglycemia [Grade D, Consensus]. In people with type 1 diabetes who have not achieved their glycemic target, real-time CGM may be offered to improve glycemic control [Grade A, Level 1A 71,80,81 for non-CSII users; Grade B, Level 2 for CSII users 71 ] and reduce duration of hypoglycemia [Grade A, Level 1A 78,80,84 ] in individuals who are willing and able to use these devices on a nearly daily basis. FGM may be offered to people with diabetes to decrease time spent in hypoglycemia [Grade B, Level 2 87 for type 1 diabetes; Grade B, Level 2 88 for type 2 diabetes]. In order to ensure accuracy of BG meter readings, meter results should be compared with laboratory measurement of simultaneous venous FPG 8-hour fast at least annually and when A1C does not match glucose meter readings [Grade D, Consensus]. Blood ketone testing methods may be preferred over urine ketone testing, as they have been associated with earlier detection of ketosis and response to treatment [Grade B, Level 2 67 ]. Abbreviations: A1C, glycated hemoglobin ; BG, blood glucose; BMI , body mass index CBG ; capillary blood glucose; CGM , continuous glucose monitoring; CGMS , continuous glucose monitoring system; CSII , continuous subcutaneous infusion infusion; DKA , diabetic ketoacidosis; FGM ; flash glucose monitoring; FPG , fasting plasma glucose; PG , plasma glucose; SMBG , self-monitoring of blood glucose. Other Relevant Guidelines Chapter 7. Self-Management Education and Support Chapter 8. Targets for Glycemic Control Chapter Glycemic Management in Adults with Type 1 Diabetes Chapter Hypoglycemia Chapter Type 1 Diabetes in Children and Adolescents Chapter Type 2 Diabetes in Children and Adolescents Chapter Diabetes and Pregnancy. Relevant Appendices Appendix 5. Self-Monitoring of Blood Glucose SMBG Recommendation Tool for Health-Care Providers Appendix Glycated Hemoglobin Conversion Chart. References McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: An analysis of data from the Diabetes Control and Complications Trial. Diabetes Care ;—5. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care ;— Calisti L, Tognetti S. Measure of glycosylated hemoglobin. Acta Biomed ;76 Suppl. American Diabetes Association. Standards of medical care in diabetes— Diabetes Care ;30 Suppl. Sacks DB, Bruns DE, Goldstein DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem ;— American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and LaboratoryMedicine, International Diabetes Federation. Consensus statement on the worldwide standardisation of the HbA1c measurement. Diabetologia ;—3. Driskell OJ, Holland D,Waldron JL, et al. Reduced testing frequency for glycated hemoglobin, HbA1c, is associated with deteriorating diabetes control. Diabetes Care ;—7. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: The American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Sacks DB. Measurement of hemoglobin A 1c : A new twist on the path to harmony. Weykamp C, JohnWG, Mosca A, et al. The IFCC Reference Measurement System for HbA1c: A 6-year progress report. Clin Chem ;—8. Diagnostic Evidence Co-operative Oxford. Point-of-care HbA1c tests—diagnosis of diabetes. London: National Institue for Health Research NHS , , pg. Report No. Accessed November 15, Spaeth BA, Shephard MD, Schatz S. |
| Continuous glucose monitoring | In a study, Chase et al. They randomly assigned 40 children to diabetes management with or without the GlucoWatch Biographer. Both groups performed conventional blood glucose monitoring four times per day. Subjects in the Biographer group used the Biographer for 3 months intervention phase and were then followed for an additional 6 months observational phase. After 3 months, HbA 1c values improved from 8. The difference between Biographer users and control subjects 8. During the observation phase following the intervention phase, the HbA 1c in the Biographer group remained lower than in the control group at months 6 and 9 of the study , but the differences were no longer statistically significant. Chico et al. HbA 1c concentrations decreased significantly in both the CGMS from 8. However, the CGMS did not result in statistically better outcomes compared with capillary glucose measurements. In , Ludvigsson and Hanas 43 reported on the results of a controlled, crossover trial comparing the effect of using a CGMS or seven-point glucose profiles on HbA 1c. During the open arm of the trial, the 27 type 1 diabetic subjects wore the CGMS for 3 days every 2 weeks for 3 months, and during the blinded to CGMS data arm, the subjects checked seven-point glucose profiles every week for 3 months. At 3 months, the two study arms were crossed over. The HbA 1c levels decreased significantly in the open arm using the CGMS from 7. In , Tanenberg et al. In both groups, the HbA 1c levels decreased significantly compared with baseline values, but there was a nonsignificant improvement in HbA 1c outcomes in the CGMS group compared with the standard monitoring group. HbA 1c levels decreased by 0. An additional five nonrandomized, uncontrolled trials of continuous glucose monitoring have demonstrated a statistically significant improvement in HbA 1c using this technology in addition to usual capillary blood glucose monitoring 37 , 40 , 45 — The studies are discussed below. In , Bode et al. After a pair of 1-week courses of monitoring followed by therapeutic adjustments based on CGMS readings, mean HbA 1c levels fell from 9. The improved HbA 1c levels were sustained: 10 weeks into the study, the mean HbA 1c of this population had further declined to 8. Kaufman et al. The continuous information was used to alter therapy. Mean HbA 1c levels fell from 8. Schiaffini et al. The study was initially conducted on 27 type 1 diabetic children, all of whom received an initial course of CGMS monitoring and 18 of whom elected to continue in the 6-week study to receive therapy adjustments and a second course of CGMS monitoring upon completion of the study. All continuing subjects self-monitored capillary blood glucose levels 4—5 times daily. Insulin therapy was adjusted based on the initial CGMS results and subsequent spot capillary glucose levels. Study participants completing the study also experienced a decreased number of hypoglycemic events per 72 h compared with the incidence rate among baseline measurements of those not completing the study 2. In , Salardi et al. In , Schaepelynck-Belicar et al. Continuous data were used to determine rational adjustments in insulin therapy in 12 type 1 diabetic subjects. Changes involved alterations of the dosage in three subjects, insulin type in seven subjects, the number of daily injections in five subjects, and the delivery technology from insulin injection to pump therapy in one subject. Reassessment 2 months later demonstrated a significant reduction of glycemic excursions in eight subjects and a decrease in the mean HbA 1c from The calculated low blood glucose index increased, but not significantly Continuous glucose monitoring technology has been used as an educational tool to document the incidence and magnitude of hypoglycemia in diabetic adults 50 — 52 and children 22 , 23 , 28 , 29 , 53 , 54 , pregnant women with diabetes 55 — 59 , patients after pancreas 60 and islet cell 61 — 62 transplant, and children with glycogen storage disease 63 , This technology has similarly been used in hyperglycemia in type 1 diabetic subjects using insulin pump therapy 65 , 66 , type 1 diabetic subjects using insulin injections 67 , pregnant women with 55 — 59 , 68 and without 68 , 69 diabetes, gastroparesis patients 70 , and cystic fibrosis patients Continuous glucose monitoring has also been used as a therapeutic tool to decrease the incidence and magnitude of hypoglycemia in three studies 46 , 72 , In , Weintrob et al. In a nonrandomized, uncontrolled multicenter series of 15 type 1 diabetic subjects who were monitored using a long-term, investigational, subcutaneously implanted continuous glucose sensor, Garg et al. A control period mean 50 days of being blinded to real-time blood glucose data was followed by a study period mean 44 days of access to these data. Currently available CGMs can present problems for users. One major problem is their lack of accuracy for each single data point compared with the accuracy of simultaneous intermittent blood glucose measurements. CGMs are generally least accurate in the hypoglycemic range Because of the accuracy issue, it is necessary to incorporate trend information in using continuous blood glucose data. All real-time CGMs incorporate an arrow that indicates an upward or downward trend. The trend arrow can be set to display minor or major levels of upward or downward trending. A minimally invasive CGM can be associated with side effects related to chronic ISF harvesting. The GW2B has been associated with skin irritation, but this problem can be reduced without affecting the measurement accuracy by pretreating the forearm with a corticosteroid spray The Pendra, which resembles the GW2B, does not harvest fluid from the skin or cause skin irritation. The CGMS rarely causes local discomfort from the implanted catheter; the sensor must be inserted at least 2 in from an insulin infusion site and 3 in from an insulin injection site. Most currently available outpatient continuous blood glucose monitors do not actually measure the glucose concentration within whole blood, but instead measure the glucose concentration within the ISF compartment. The Pendra measures blood glucose both noninvasively and continuously. Depending on activity or feeding schedules and especially during periods of rapid blood glucose shifting , equilibration between shifting blood and interstitial fluid glucose levels may lag It is unknown whether sensor site selection can minimize this intercompartmental lag or the lag that can occur during equilibration between arterial and skin capillary blood glucose levels at sites other than the fingertip. Reimbursement for CGMs by insurance or government payer organizations has been limited. Insurance companies are demanding rigorous scientific evidence about continuous monitoring before they will pay for this technology Their stated reason is that an insufficient number of randomized, controlled trials of these devices has shown a statistically significant decrease in diabetic complications or HbA 1c levels, which are accepted as a surrogate marker of long-term blood glucose control Indeed, only five articles have been published describing randomized, controlled trials of continuous glucose monitoring technologies for improving clinical outcomes 27 , 41 — In all five studies, continuous monitoring compared with standard monitoring was associated with improved HbA 1c levels, but the improvements in HbA 1c levels in the continuously monitored subjects were statistically significant in only two 41 , 43 of these five studies. Continuous glucose monitoring offers the capability of expressing the frequency and severity of hypoglycemic episodes much more clearly than does intermittent glucose testing. Continuous glucose monitoring also offers the capability of expressing the mean blood glucose value in new ways. The mean blood glucose value can shift quickly with any new treatment, and it is not always practical to wait for months or weeks, respectively, for the HbA 1c or fructosamine values to shift. Continuous glucose monitoring can document the time spent in the normal, low, and high ranges, which may be more valuable than a single integrated data point, such as HbA 1c or fructosamine. Long-term exposure to midrange glycemia may turn out to be better for avoiding complications than exposure to many upward hyperglycemic spikes and downward hypoglycemic spikes; however, the spikes may cancel out each other in terms of altering the long-term markers. Continuous glucose monitoring can distinguish the two exposures, but long-term markers cannot stratify time spent above and below a particular target. It is possible that a new measure of glycemia, derived from the duration of normal, low, and high readings, could supplement HbA 1c as an integrated measure of control. Furthermore, measurements of mean amplitude of glycemic excursions 54 , composite hypoglycemic score 62 , and lability index 62 could provide information about the tendency for a mean blood glucose level to be comprised of stable or labile data points. For some patients, a decreased amount of glycemic instability alone, even without any improvement in HbA 1c , might represent an improved outcome. Continuous glucose monitoring offers advantages over intermittent glucose monitoring when glycemic patterns are poorly understood. The information about direction, magnitude, duration, frequency, and causes of fluctuations in blood glucose levels that can be obtained by continuous glucose monitoring is simply not available with intermittent blood glucose monitoring. When retrospective patterns are needed to adjust therapy or document the state of physiology, CGMs are useful. When real-time recognition of both the absolute magnitude of glycemia as well as trend patterns are needed, then a real-time CGM provides a wealth of information. Technologies for continuous glucose monitoring require patient education for proper use. During hypoglycemia or periods of rapid fluctuation, values provided by CGMs may be inaccurate. Clinical outcome studies suggest that measures of mean glycemia and hypoglycemic burden both improve with the use of continuous glucose monitoring, but more studies are needed to convince payors to reimburse for this technology. In this data-hungry world, it appears likely that CGMs will eventually become a routine part of diabetes management, initially for patients with difficult-to-control diabetes and eventually for most patients with diabetes. Retrospective reporting will eventually give way to real-time readings, and adjunctive use requiring a confirmatory finger-stick blood test will eventually give way to primary use without the requirement of such confirmation. As methods for minimally invasive and noninvasive continuous monitoring advance, diabetic patients will use this technology more routinely. Data printouts from CGMs will increasingly provide a roadmap for effective diabetes management in the 21st century. After this article was submitted for publication, an additional study was published describing the results of a multicenter, randomized, controlled trial of continuous glucose monitoring In , the DirecNet Study Group reported the results of a 6-month trial comparing GW2B use with standard glucose monitoring in type 1 diabetic subjects ages 7—18 years. Furthermore, six additional articles were also published, following the submission of this article, describing the performance of the CGMS in type 1 diabetes 80 , type 2 diabetes 81 , pregnancy 82 , and both type 1 and type 2 diabetic patients receiving peritoneal dialysis 83 ; the GlucoDay in type 1 diabetes 84 ; and an investigational viscometric affinity sensor in type 1 diabetes Specifications of available and likely soon-to-be-available products for continuous glucose monitoring. A table elsewhere in this issue shows conventional and Système International SI units and conversion factors for many substances. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 28, Issue 5. Previous Article Next Article. Article Navigation. Continuous Glucose Monitoring : Roadmap for 21st century diabetes therapy David C. Klonoff, MD, FACP David C. Klonoff, MD, FACP. From the Mills-Peninsula Health Services Diabetes Research Institute, San Mateo, California. This Site. Google Scholar. Address correspondence and reprint requests to David C. Klonoff, MD, Mills-Peninsula Health Services Diabetes Research Institute, S. San Mateo Dr. E-mail: klonoff itsa. Diabetes Care ;28 5 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1— Specifications of available and likely soon-to-be-available products for continuous glucose monitoring. FDA approved. CE marked. Year first approved or marked. Sensor type. Sensor mechanism. Sensor location. Sensor warmup h. Calibrations per lifetime of sensor. Sensor lifespan h. Frequency of testing min. Time of blood glucose data display. Continuous Glucose Monitoring System Gold Yes Yes Minimally invasive Enzyme-tipped catheter Subcutaneous abdomen 2 12 72 5 Retrospective No GlucoWatch G2 Biographer Yes Yes Minimally invasive Reverse iontophoresis External on arm or forearm 2 1 13 10 Real time Yes Guardian Telemetered Glucose Monitoring System Yes Yes Minimally invasive Enzyme-tipped catheter Subcutaneous arm 2 12 72 5 Retrospective Yes GlucoDay No Yes Minimally invasive Microdialysis Subcutaneous abdomen 0 1 48 3 Real time or retrospective Yes Pendra No Yes Noninvasive Impedance spectroscopy External on wrist 1 20 3 months 1 Real time Yes FreeStyle Navigator Continuous Glucose Monitor No No — Minimally invasive Enzyme-tipped catheter Subcutaneous arm 1 1 72 1 Real time Yes. View Large. Table 2— Attractive features of available and likely soon-to-be-available continuous glucose monitors. Continuous Glucose Monitoring System Gold Long sensor life Avoids arm implantation First product on the market and used in the most studies GlucoWatch G2 Biographer Needle-free Real-time readings Alarm for out-of-range values Guardian Telemetered Glucose Monitoring System Long sensor life Alarm for out-of-range values Avoids arm implantation GlucoDay Real-time readings Infrequent calibrations Choice of retrospective or real-time data Pendra Noninvasive No skin irritation Measures glucose in blood and not in interstitial fluid FreeStyle Navigator Continuous Glucose Monitor Long sensor life Alarm for out-of-range values Avoids abdominal wall implantation. Gross TM, Bode BW, Einhorn D, Kayne DM, Reed JH, White NH, Mastrototaro JJ: Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. Potts RO, Tamada JA, Tierney MJ: Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, Gross T, Mastrototaro J: Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Maran A, Crepaldi C, Tiengo A, Grassi G, Vitali E, Pagano G, Bistoni S, Calabrese G, Santeusanio F, Leonetti F, Ribaudo M, Di Mario U, Annuzzi G, Genovese S, Riccardi G, Previti M, Cucinotta D, Giorgino F, Bellomo A, Giorgino R, Poscia A, Varalli M: Continuous subcutaneous glucose monitoring in diabetic patients: a multicenter analysis. Diabetes Care. Pfuetzner A, Caduff A, Larbig M, Schrepfer T, Forst T: Impact of posture and fixation technique on impedance spectroscopy used for continuous and non-invasive glucose monitoring. Feldman B, Brazg R, Schwartz S, Weinstein R: A continuous glucose sensor based on wired enzyme technology: results from a 3-day trial in patients with type 1 diabetes. Choleau C, Klein JC, Reach G, Aussedat B, Demaria-Pesce V, Wilson GS, Gifford R, Ward WK: Calibration of a subcutaneous amperometric glucose sensor. Part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosens Bioelectron. Levetan C, Want LL, Weyer C, Strobel SA, Crean J, Wang Y, Maggs DG, Kolterman OG, Chandran M, Mudaliar SR, Henry RR: Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Abrahamian H, Francesconi M, Loiskandl A, Dzien A, Prager R, Weitgasser R: Evaluation of a new insulinotropic agent by using an innovative technology: efficacy and safety of nateglinide determined by continuous glucose monitoring. Pitzer KR, Desai S, Dunn T, Edelman S, Jayalakshmi Y, Kennedy J, Tamada JA, Potts RO: Detection of hypoglycemia with the GlucoWatch Biographer. Reach G: Which threshold to detect hypoglycemia? Value of receiver-operator curve analysis to find a compromise between sensitivity and specificity. Weinzimer SA, Tamborlane WV, Chase HP, Garg SK: Continuous glucose monitoring in type 1 diabetes. Curr Diab Rep. Diabetes Research in Children Network DirecNet Study Group: The accuracy of the GlucoWatch G2 Biographer in children with type 1 diabetes: results of the diabetes research in children network DirecNet accuracy study. Diabetes Research in Children Network DirecNet Study Group: The accuracy of the CGMS in children with type 1 diabetes: results of the diabetes research in children network DirecNet accuracy study. Kubiak T, Hermanns N, Schreckling HJ, Kulzer B, Haak T: Assessment of hypoglycaemia awareness using continuous glucose monitoring. Diabet Med. Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE: Experience with the Continuous Glucose Monitoring System in a medical intensive care unit. Guerci B, Floriot M, Bohme P, Durain D, Benichou M, Jellimann S, Drouin P: Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Djakoure-Platonoff C, Radermercker R, Reach G, Slama G, Selam JI: Accuracy of the continuous glucose monitoring system in inpatient and outpatient conditions. Diabete Metab. Poscia A, Mascini M, Moscone D, Luzzana M, Caramenti G, Cremonesi P, Valgimigli F, Bongiovanni C, Varalli M: A microdialysis technique for continuous subcutaneous glucose monitoring in diabetic patients part 1. Varalli M, Marelli G, Maran A, Bistoni S, Luzzana M, Cremonesi P, Caramenti G, Valgimigli F, Poscia A: A microdialysis technique for continuous subcutaneous glucose monitoring in diabetic patients part 2. Diabetes Research in Children Network DirecNet Study Group: Accuracy of the GlucoWatch G2 Biographer and the continuous glucose monitoring system during hypoglycemia: experience of the Diabetes Research in Children Network. Tsalikian E, Kollman C, Mauras N, Weinzimer S, Buckingham B, Xing D, Beck R, Ruedy K, Tamborlane W, Fiallo-Scharer R, Diabetes Research in Children Network DirecNet Study Group: GlucoWatch G2 Biographer alarm reliability during hypoglycemia in children. Conrad SC, Mastrototaro JJ, Gitelman SE: The use of a continuous glucose monitoring system in hypoglycemic disorders. J Pediatr Endocrinol Metab. International Organisation for Standardisation: Requirements for In Vitro Blood Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. The Diabetes Research in Children Network DirecNet Study Group: Lack of accuracy of continuous glucose sensors in healthy, nondiabetic children: results of the Diabetes Research in Children Network DirecNet accuracy study. J Pediatr. Weinzimer SA, DeLucia MC, Boland EA, Steffen A, Tamborlane WV: Analysis of continuous glucose monitoring data from non-diabetic and diabetic children: a tale of two algorithms. Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK: Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV: Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. It lets you see patterns in your levels and check if your glucose is too high or low. It can help you control your blood glucose levels, as you'll have more information and can take action quickly. With CGM, the sensor sends results to the receiver or your phone every few minutes. You can see your glucose levels on your receiver at any time. Some types can send results to an insulin pump , so you can see your glucose levels on your pump. With flash, you need to scan the sensor with the reader or with your phone to see the results. There are several different types of CGM. The only type of flash monitor available is the Abbott FreeStyle Libre 2. The original Abbott FreeStyle Libre has been discontinued. Some types of CGM have optional alarms to alert you if your blood glucose levels go too low or too high. The Abbott FreeStyle Libre 2 also has an alarm. You generally need to replace a sensor every 7 to 14 days, depending on the type of monitor you have. Interstitial fluid glucose readings are a few minutes behind your blood glucose levels. |
Wie die Variante, ja
Es ist der Skandal!