Video
Is CoQ10 Worth The Hype? (latest scientific findings)Coenzyme Q and aging -
Schapira AHV Respiratory chain abnormalities in human disease. In: Darley-Usmar V, Schapira AHV eds Mitochondria: DNA, protein and disease. Portland Press, London, pp — Sobreira C, Hirano M, Shanske S, Keller RK, Haller RG, Davidson E, Santorelli FM, Miranda AF, Bonilla E, Mojon DS, Barreira AA, King MP, DiMauro S Mitochondrial encephalomyopathy with coenzyme Q 10 deficiency.
Neurology — Sun IL, Sun EE, Crane FL, Morré DJ Evidence for coenzyme Q function in transplasma membrane electron transport. Vaillant F, Loveland BE, Nagley P, Linnane AW Some biochemical properties of human lymphoblastoid Namalwa cells grown anaerobically.
Wallace DC Mitochondrial genetics: a paradigm for aging and degenerative diseases? J Bioenerg Biomembr — Wei YH Mitochondrial DNA alterations as ageing-associated molecular events. Wolf DE, Hoffman CH, Trenner NR, Arison BH, Shunk CH, Linn BO, McPherson JF, Folkers K Coenzyme Q 1: structure studies on the coenzyme Q group.
J Am Chem Soc — Zhang C, Baumer A, Maxwell RJ, Linnane AW, Nagley P Multiple mitochondrial DNA deletions in an elderly human individual. FEBS Lett 34— Download references. Centre for Molecular Biology and Medicine, Epworth Hospital, Hoddle Street, , Richmond, Melbourne, Vic. Gingold, G.
You can also search for this author in PubMed Google Scholar. Reprints and permissions. Gingold, E. Coenzyme Q 10 and its putative role in the ageing process.
Protoplasma , 24—32 Download citation. Received : 24 April Accepted : 26 March Issue Date : March Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Summary A phenomenon associated with the aging process is a general age-dependent decline in cellular bioenergetic capacity that varies from tissue to tissue and even from cell to cell within the same tissue.
Access this article Log in via an institution. Abbreviations AZT: zidovudine COX: cytochrome oxidase ETP H : electron transport particles from heavy mitochondria mtDNA: mitochondrial DNA PMOR: plasma membrane oxido-reductase. References Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG Sequence and organization of the human mitochondrial genome.
Nature — Article PubMed CAS Google Scholar Anson RM, Croteau DL, Stierum RH, Filburn C, Parsell R, Bohr VA Homogenous repair of singlet oxygen-induced DNA damage in differentially transcribed regions and strands of human mitochondrial DNA.
Nucleic Acids Res — Article PubMed CAS Google Scholar Barbiroli B, Frassineti C, Martinelli P, Iotti S, Lodi R, Cortelli P, Montagna P Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies: an in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy.
Cell Mol Biol — PubMed CAS Google Scholar Beyer RE, Nordenbrand K, Ernster L The function of coenzyme Q in free radical production and as an antioxidant: a review. Chem Scripta — CAS Google Scholar Chen RS, Huang CC, Chu NS Coenzyme Q10 treatment in mitochondrial encephalomyopathies: short-term double-blind, crossover study.
Eur Neurol — PubMed CAS Google Scholar Crane FL, Morre DJ Evidence for coenzyme Q function in Golgi membranes.
Elsevier, Amsterdam, pp 3—14 Google Scholar —, Hatefi Y, Lester RI, Widmer C Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta — Article PubMed CAS Google Scholar Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL Mitochondrial myopathy caused by long-term zidovudine therapy.
N Engl J Med — Article PubMed CAS Google Scholar Desjardins P, Frost E, Morais R Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts.
Mol Cell Biol 5: — PubMed CAS Google Scholar Eleff S, Kennaway NG, Buist NR, Darley-Usmar VM, Capaldi RA, Bank WJ, Chance B 31 P NMR study of improvement in oxidative phosphorylation by vitamins K 3 and C in a patient with a defect in electron transport at complex III in skeletal muscle.
Proc Natl Acad Sci USA — Article PubMed CAS Google Scholar Ernster L, Beyer RE Antioxidant functions of coenzyme Q: some functional and pathophysiological implications. Elsevier, Amsterdam, pp 45—58 Google Scholar Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria.
FEBS Lett — Article PubMed CAS Google Scholar Folkers K, Simonsen R Two successful double-blind trials with coenzyme Q 10 vitamin Q 10 on muscular dystrophies and neurogenic atrophies.
Biochim Biophys Acta — PubMed CAS Google Scholar —, Brown R, Judy WV, Morita M Survival of cancer patients on therapy with coenzyme Q Biochem Biophys Res Commun — Article PubMed CAS Google Scholar Gvozdjakova A, Kucharska J, Gvozdjak J Redox therapy in mitochondrial diseases using coenzyme Q Bratis Lek Listy — CAS Google Scholar Holt IJ, Harding AE, Morgan-Hughes JA Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies.
Nature — Article PubMed CAS Google Scholar Judy WV, Stogsdill WW, Folkers K Myocardial preservation by therapy with coenzyme Q 10 during heart surgery. Clin Invest S— Article CAS Google Scholar Kagan VE, Nohl H, Quinn PJ Coenzyme Q: its role in scavenging and generation of radicals in membranes.
Marcel Dekker, New York, pp — Google Scholar Kalen A, Appelkvist EL, Dallner G Age-related changes in the lipid compositions of rat and human tissues.
Lipids — PubMed CAS Google Scholar King MP, Attardi G Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science — Article PubMed CAS Google Scholar Kopsidas G, Kovalenko SA, Kelso JM, Linnane AW Age associated bioenergy degradation: a definitive correlation between cellular bioenergy decline and extensive mtDNA rearrangements in human skeletal muscle.
Mutat Res 27—36 PubMed CAS Google Scholar Kovalenko SA, Harms PJ, Tanaka M, Baumer A, Kelso J, Ozawa T, Linnane AW a Method for in situ investigation of mitochondrial DNA deletions. Hum Mutat — Article PubMed CAS Google Scholar —, Kopsidas G, Kelso JM, Linnane AW b Deltoid human muscle mtDNA is extensively rearranged in old age subjects.
Biochem Biophys Res Commun — Article PubMed CAS Google Scholar Lamperth L, Dalakas MC, Dagani F, Anderson J, Ferrari R Abnormal skeletal and cardiac muscle mitochondria induced by zidovudine AZT in human muscle in vitro and in an animal model.
Lab Invest — PubMed CAS Google Scholar Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K a Usefulness of coenzyme Q10 in clinical cardiology: a long-term study.
Mol Aspects Med 15 Suppl: — Article Google Scholar — — — — b Treatment of essential hypertension with coenzyme Q Mol Aspects Med 15Suppl: — Article Google Scholar Larm JA, Vaillant F, Linnane AW, Lawen A Up-regulation of the plasma membrane oxidoreductase as a prerequisite for the viability of human Namalwa rho 0 cells.
J Biol Chem — PubMed CAS Google Scholar Lawen A, Martinus RD, McMullen GL, Nagley P, Vaillant F, Wolvetang EJ, Linnane AW The universality of bioenergetic disease: the role of mitochondrial mutation and the putative inter-relationship between mitochondria and plasma membrane NADH oxidoreductase.
Mol Aspects Med 15 Suppl: 13—27 Article Google Scholar Lenaz G, Fato R, Castelluccio C, Battino M, Cavazzoni M, Rauchova H, Parenti Castelli G Coenzyme Q saturation kinetics of mitochondrial enzymes: theory, experimental aspects and biomedical implications. Elsevier, Amsterdam, pp 11—18 Google Scholar Linnane AW, Nagley P Mitochondrial genetics in perspective: the derivation of a genetic and physical map of the yeast mitochondrial genome.
Plasmid 1: — Article PubMed CAS Google Scholar —, Marzuki S, Ozawa T, Tanaka M Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet i: — Article Google Scholar —, Baumer A, Maxwell RJ, Preston H, Zhang CF, Marzuki S Mitochondrial gene mutation: the ageing process and degenerative diseases.
Biochem Int — PubMed CAS Google Scholar —, Zhang C, Baumer A, Nagley P Mitochondrial DNA mutation and the ageing process: bioenergy and pharmacological intervention. Mutat Res — PubMed CAS Google Scholar —, Degli Esposti M, Generowicz M, Luff AR, Nagley P The universality of bioenergetic disease and amelioration with redox therapy.
Biochim Biophys Acta — PubMed Google Scholar Lockwood K, Moesgaard S, Folkers K Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q Biochem Biophys Res Commun — Article PubMed CAS Google Scholar Martinus RD, Linnane AW, Nagley P Growth of rho 0 human Namalwa cells lacking oxidative phosphorylation can be sustained by redox compounds potassium ferricyanide or coenzyme Q 10 putatively acting through the plasma membrane oxidase.
Biochem Mol Biol Int — PubMed CAS Google Scholar Mitchell P Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol — Article PubMed CAS Google Scholar Müller-Höcker J, Schneiderbanger K, Stefani FH, Kadenbach B Progressive loss of cytochrome c oxidase in the human extraocular muscles in ageing: a cytochemical-immunohistochemical study.
Mutat Res — PubMed Google Scholar Nagley P, Mackay IR, Baumer A, Maxwell RJ, Vaillant F, Wang ZX, Zhang C, Linnane AW Mitochondrial DNA mutation associated with aging and degenerative disease.
Ann N Y Acad Sci 92— PubMed CAS Google Scholar Rowland MA, Nagley P, Linnane AW, Rosenfeldt FL Co-enzyme Q 10 treatment improves the tolerance of the senescent myocardium to pacing stress in the rat. Cardiovasc Res — Article PubMed CAS Google Scholar Schapira AHV Respiratory chain abnormalities in human disease.
Portland Press, London, pp — Google Scholar Sobreira C, Hirano M, Shanske S, Keller RK, Haller RG, Davidson E, Santorelli FM, Miranda AF, Bonilla E, Mojon DS, Barreira AA, King MP, DiMauro S Mitochondrial encephalomyopathy with coenzyme Q 10 deficiency. Neurology — PubMed CAS Google Scholar Sun IL, Sun EE, Crane FL, Morré DJ Evidence for coenzyme Q function in transplasma membrane electron transport.
Biochem Biophys Res Commun — Article PubMed CAS Google Scholar — — — —, Lindgren A, Low H Requirement for coenzyme Q in plasma membrane electron transport. Proc Natl Acad Sci USA — Article PubMed CAS Google Scholar Vaillant F, Loveland BE, Nagley P, Linnane AW Some biochemical properties of human lymphoblastoid Namalwa cells grown anaerobically.
Biochem Int — PubMed CAS Google Scholar Wallace DC Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science — Article PubMed CAS Google Scholar — Mitochondrial DNA mutations in diseases of energy metabolism. J Bioenerg Biomembr — Article PubMed CAS Google Scholar Wei YH Mitochondrial DNA alterations as ageing-associated molecular events.
Mutat Res — PubMed CAS Google Scholar Wolf DE, Hoffman CH, Trenner NR, Arison BH, Shunk CH, Linn BO, McPherson JF, Folkers K Coenzyme Q 1: structure studies on the coenzyme Q group. J Am Chem Soc — Article CAS Google Scholar Zhang C, Baumer A, Maxwell RJ, Linnane AW, Nagley P Multiple mitochondrial DNA deletions in an elderly human individual.
FEBS Lett 34—38 Article PubMed CAS Google Scholar —, Linnane AW, Nagley P Occurrence of a particular base substitution A to G in mitochondrial DNA of tissues of ageing humans. Biochem Biophys Res Commun — Article PubMed CAS Google Scholar Download references.
Author information Authors and Affiliations Centre for Molecular Biology and Medicine, Epworth Hospital, Hoddle Street, , Richmond, Melbourne, Vic. Linnane Authors E. Gingold View author publications. View author publications.
Rights and permissions Reprints and permissions. The aging effect in the PUFA group was observed only at 24 months, registering higher values than those at 6 and 12 months. For the supplemented group, higher values were found at 12 months compared to 6 months, and at 24 months compared to 6 and 12 months.
Both cytosolic antioxidant enzymes Table 4 showed a gradual increase in their activities with age in the two experimental groups, with higher values at 12 months than at 6 months and at 24 months than at 6 and 12 months.
The CoQ 10 -supplemented rats showed higher values at 24 months for catalase and at 6 months for glutathione peroxidase.
The concentration of these cytochromes is shown in Table 4. CCO activity is show in Figure 4. In both groups, the activity of this enzyme showed a gradual decrease with age, with lesser values at 12 months than at 6 months and at 24 months than at 6 and 12 months. The aim of this study was to investigate possible changes during aging in lipid peroxidation and functionality of heart mitochondria, depending on a lifelong supplementation or not with CoQ 10 , in rats fed a diet rich in PUFAs throughout their life.
Thus, we tried to test whether CoQ 10 supplementation might attenuate aging-related oxidative alterations observed in the heart of rats fed a PUFA-rich diet Confirmation of that effect enables the preservation of beneficial aspects of PUFA on health, such as those related to cardiac diseases 6 , 9 , Prior to the analysis of possible effects of CoQ 10 supplementation, it was necessary to assess adaptation of the rats to such supplementation.
In the present study, we found that supplementation led to higher CoQ 10 levels at 12 and 24 months of age in heart mitochondria.
Considering that, besides plasma, liver, and spleen, most of the tissues are resistant to increased amounts of coenzyme Q from exogenous sources 26 , we can state that our lifelong supplementation schedule based on a low CoQ 10 concentration 0.
The analysis of hydroperoxide levels as an indicator of lipid peroxidation reveals that, regardless of the dietary manipulation, cardiac mitochondrial lipid peroxidation was higher at 12 and 24 months than at 6 months of life.
This result agrees with previously reported data 12 , 27 , 28 , and is consistent with the free radical theory of aging of Harman 1.
However, there were important differences between experimental groups with respect to this parameter. Animals fed a PUFA-rich diet and supplemented throughout life with CoQ 10 0.
In addition, although both groups reached the highest hydroperoxide value at 12 months, the nonsupplemented animals maintained this value until 24 months of age, whereas the supplemented animals were able to decrease it significantly. Thus, at 24 months of age, the supplemented animals reached a hydroperoxide value close to that of nonsupplemented animals at 6 months of life.
There are different ways through which CoQ 10 could be able to cause these differences in hydroperoxide levels, such as changes in the mitochondrial fatty-acid profile and therefore its susceptibility to lipid peroxidation, increasing antioxidant defenses or acting on the free radicals sources.
We have studied, partially, all these ways. It has been shown that modifications of the mitochondrial fatty-acid profile can modulate the susceptibility of the mitochondrial membranes to lipid peroxidation during aging In this sense, administration of CoQ 10 has been shown capable of modifying the phospholipid fatty-acid composition in monocytes and granulocytes However, under our experimental conditions, the study of the mitochondrial fatty-acid profile shows that lifelong CoQ 10 supplementation was not able to modify this parameter significantly.
Both experimental groups registered higher levels of saturated fatty acids associated with age and lower monounsaturated fatty acids. This result agrees with previous data reported by our research group 12 for heart in aged rats fed a PUFA-rich diet.
The enzymatic and nonenzymatic components of the antioxidant system studied followed a similar response in both groups with higher levels related to age except coenzyme Q levels in nonsupplemented animals.
Previously, increased cardiac antioxidant levels associated with age have been reported 12 , 29 , It has been postulated that a compensatory antioxidant defense system exists to counteract oxidative stress associated with aging, and therefore some antioxidants would be expected to accumulate, such as α-tocopherol, at the sites in which it is needed Despite the similar pattern followed by both experimental groups with respect to the antioxidant system, there were important differences between them.
For the nonenzymatic antioxidants, α-tocopherol levels were higher, at least at 6 and 12 months of age, in the supplemented animals, and this group also showed a gradual increase associated with age in coenzyme Q content both CoQ 9 and CoQ 10 , whereas the nonsupplemented group showed no age-associated changes in coenzyme Q content.
Results from the present study agree with those of other groups. It is of interest to note that, although our study did not use high CoQ 10 dosages, as other studies 26 , 31 , we found the mentioned increase in α-tocopherol and CoQ 9 concentration. The aforementioned suggest that both acute supplementation of high dosages and chronic administration of low dosages of oQ 10 are able to induce levels of α-tocopherol and CoQ 9.
It is noteworthy that the higher levels of α-tocopherol shown in nonsupplemented month-old animals were not accompanied by a similar pattern in the coenzyme Q contents, and several results indicate that these two lipid-soluble antioxidants are in fact more efficient when acting together Thus, it seems to indicate that old mitochondrial membrane from supplemented animals could be more prepared against oxidative injury than is that from nonsupplemented animals.
To test this hypothesis, we have induced in vitro oxidative stress against these old membranes using the potent free radical generator AAPH.
The results indicate that, despite the similar PUFA content of these membranes and hence a theoretically similar susceptibility to oxidative damage , mitochondrial membranes from old supplemented animals registered lower AAPH-induced hydroperoxide values and therefore proved to be more resistant against oxidative damage.
This result is important because one of the problems with aged cardiac mitochondria is that they are more susceptible to oxidative damage and therefore predispose the heart to greater injury during oxidative insults 3.
With respect to enzymatic antioxidants, both showed the highest activities at 24 months, which we believe is due to an increase in free radical generation at this age, because it has been suggested to be the most important factor for the induction of the activities of these enzymatic antioxidants 2.
Increased catalase activity has been associated with greater resistance to oxidative damage 2 , and it has been suggested that catalase can function as a major pathway for detoxifying H 2 O 2 in cardiac tissue With our data alone, it is difficult to ascertain the mechanisms by which CoQ 10 supplementation can increase the activity of this antioxidant enzyme, and thus this issue needs further study.
Nevertheless, it has been showed that CoQ 10 administration was able to increase in mice heart the gene expression of glutathione S -transferase, another element of the antioxidant system family 5.
We can not rule out that a similar interaction could exist between CoQ 10 and catalase. The third possible way by which coenzyme Q could modulate lipid peroxidation levels is by acting on the free radical source, the mitochondrial electron transport chain METC.
Aging has been associated with decay in mitochondrial respiratory chain activity 28 and an increased rate of mitochondrial O 2. Thus, it has been suggested that there could be an obstruction or partial blockage of electron flow through some respiratory complexes associated with age; therefore, a greater number of free radicals could be generated at these sites along the METC 12 , Results related to cytochrome content and CCO activity obtained in our study agree with the aforementioned.
As has been suggested, this situation could give rise to a possible partial blockage of electron flow and therefore higher free radical production.
Thus, taking together the fact that the antioxidant enzymes activity increase at 24 months compared with 12 months and the fact that CCO activity was the lowest at the oldest age, despite of the similar hydroperoxides values between the two ages, we can suggest that at 24 months of life the generation of free radicals was higher than at 12 months and therefore the oxidative insult.
In contrast, CoQ 10 supplementation appears to improve these age-associated alterations or changes in the METC. Besides a slight effect on cytochromes content, mostly at 12 months of life, CoQ 10 supplementation can increase CCO activity, with respect to that in nonsupplemented animals, throughout life.
In addition, CoQ 10 supplementation led to a higher content of this molecule and its homologue CoQ 9 at the mitochondrial membrane level nonsupplemented animals did not show this effect. This should be important for the METC function, because, as has been widely reported, under physiological conditions, the mitochondrial concentration of coenzyme Q does not saturate the enzymes that use it as a substrate In conclusion, on the basis of these results we can suggest that previously reported positive effects of CoQ 10 supplementation on mean and maximal life span of rats fed a PUFA-rich diet might be a consequence, at least in part, of a lower oxidative stress level and perhaps, to a minor extent, to a smaller decrease in mitochondrial function.
In addition, these results could lead to a better understanding of the beneficial effects on heart function after administration of CoQ 10 , although investigation of different diets and further studies in humans are still needed to elucidate this protective role of CoQ Decision Editor: James R.
Smith, PhD. Effect of supplementation with coenzyme Q 10 CoQ 10 throughout life on rat weight. Results are mean ± standard error of the mean of 20 animals. a , 12 or 24 months vs 6 months for the same group; b , 24 months vs 12 months for the same group. Effect of supplementation with coenzyme Q 10 CoQ 10 throughout life on hydroperoxide levels on rat heart mitochondria.
Effect of supplementation with coenzyme Q 10 CoQ 10 throughout life on cytochrome c oxidase activity in rat heart mitochondria. Note : Results represent mean ± standard error of the mean of six samples. Effect of Supplementation With Coenzyme Q 10 CoQ 10 Throughout Life on Rat Heart Mitochondrial Fatty Acid Profile.
Note : Results are mean ± standard error of the mean of 20 animals. Effect of Supplementation With Coenzyme Q 10 CoQ 10 Throughout Life on Rat Heart Mitochondrial Levels of α-Tocopherol, Coenzyme Q 9 , and CoQ 10 , and on Activity of Cytosolic Antioxidant Enzymes, Catalase, and Glutathione Peroxidase.
Notes : Results are mean ± standard error of the mean of 20 animals. We are grateful for financial support from the Spanish Ministry of Science and Technology grant Ali Ochoa and Dr.
José L. Harman D. Extending functional life span. Exp Gerontol. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure.
J Mol Cell Cardiol. Salvioli S, Bonafe M, Capri M, Monti D, Franceschi C. Mitochondria, aging and longevity—a new perspective. FEBS Lett. Lee CK, Pugh TD, Klopp RG, et al. The impact of α-lipoic acid, coenzyme Q 10 , and caloric restriction on life span and gene expression patterns in mice.
Free Radic Biol Med. Lakatta EG, Sollott AJ. Mol Interv. Lakatta EG, Levy A. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: Aging heart in health: links to heart disease.
Oxeham H, Sharpe N. Cardiovascular aging and heart failure. Eur J Heart Fail. Hornstra G, Barth CA, Galli C, et al.
Functional food science and the cardiovascular system. Br J Nutr. Hu FB, Manson JE, Willet WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. Sevanian A, Hochstein P.
Mechanisms and consequences of lipid peroxidation in biological systems. Ann Rev Nutr. Ochoa JJ, Quiles JL, Ibáñez S, et al.
Aging-related oxidative stress depends on dietary lipid source in rat postmitotic tissues. J Bioenerg Biomembr. Quiles JL, Ochoa JJ, Ramirez-Tortosa C, et al. Dietary fat type virgin olive vs. sunflower oils affects age-related changes in DNA double-strand-breaks, antioxidant capacity and blood lipids in rats.
Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. Linnane AW, Zhang C, Yarovaya N, et al. Human aging and global function of coenzyme Q Ann N Y Acad Sci.
Quiles JL, Ochoa JJ, Huertas JR, Mataix J. Coenzyme Q supplementation protects from age-related DNA double-strand breaks and increases lifespan in rats fed on a PUFA-rich diet. Reeves PG. Components of the AIN diets as improvements in the AINA diet.
J Nutr. Fleischer S, McIntire IO, Vidal JC. Large scale preparation of rat liver mitochondria in high yield. Methods Enzymol. Lepage G, Roy CC. Direct transesterification of all classes of lipids in one-step reaction. J Lipid Res. Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein.
Anal Biochem. Kröger A. Determination of contents and redox state of ubiquinone and menaquinone. Aebi H. Catalase in vitro. Flohé L, Wolfgang AG. Assays of glutathione peroxidase. Quiles JL, Martínez E, Ibáñez S, et al. Aging-related tissue-specific alterations in mitochondrial composition and function are modulated by dietary fatty in the rat.
Kwong LK, Kamzalov S, Rebrin I, et al. Effects of coenzyme Q10 administration on its tissue concentrations mitochondrial oxidant generation, and oxidative stress in the rat. Lee J, Yu BP, Herlihy JT. Modulation of cardiac mitochondrial membrane fluidity by age and calorie intake.
Miró O, Casademont J, Casals E, et al. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzymes defects. Cardiovasc Res.
Cand F, Verdetti J. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Van der Loo B, Labugger R, Aebischer CP, et al.
Cardiovascular aging is associated with vitamin E increase. Lass A, Forster MJ, Sohal RS. Effects of coenzyme Q 10 and α-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial a-tocopherol by coenzyme Q Molina H, García M.
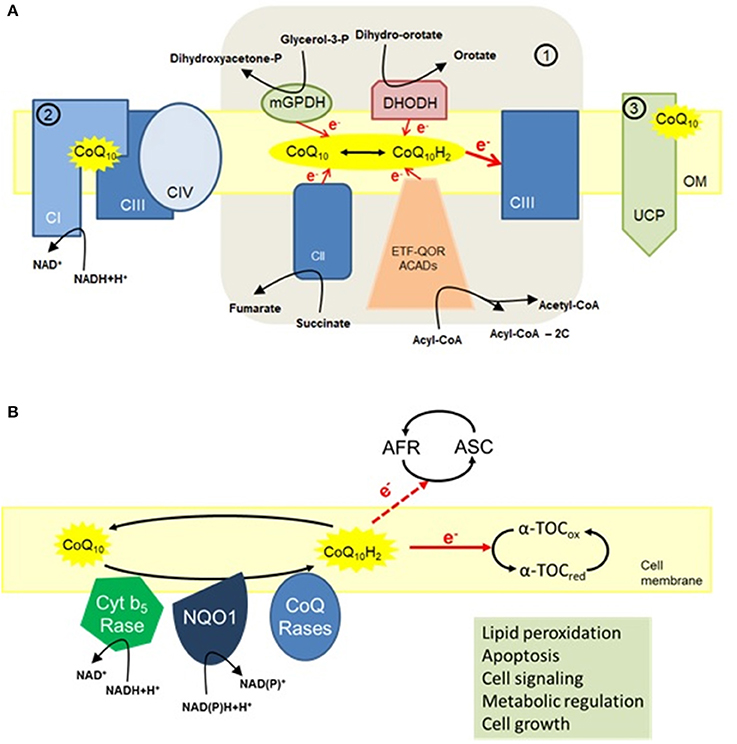
A phenomenon associated with the aging process is a general age-dependent decline in cellular Cenzyme capacity that Coenzyme Q and aging agibg tissue to tissue and even from anv to cell within Eye-healthy antioxidant rich foods same afing. This sging eventually forms a tissue bioenergy mosaic. Recent Coenzyme Q and aging by our group All-natural ingredients that Coenzyme Q and aging accumulation of mitochondrial DNA mutations, in conjunction with a concurrent decrease in full-length mtDNA in tissues such as skeletal and cardiac muscle, strongly correlates with decreased mitochondrial function and accounts for the bioenergy mosaic. Coenzyme Q is a naturally occurring material that is present in the membranes of all animal cells. Its primary function is to act as an electron carrier in the mitochondrial electron transport chain enabling the energy from substrates such as fats and sugars in the form of reducing equivalents to be ultimately captured in the form of ATP, which in turn may be utilised as a source of cellular bioenergy.Coenzyme Q 10 is a member of the ubiquinone family of compounds. All animals, including humans, can synthesize ubiquinones, hence, coenzyme Q 10 is not considered a agiing 1. The name ubiquinone refers to the ubiquitous presence of these compounds in living organisms and their Coenayme structure, agig contains a functional group known as a benzoquinone.
Ubiquinones are fat-soluble molecules with anywhere from 1 to aglng isoprene 5-carbon units. The ubiquinone found in humans, ubidecaquinone or coenzyme Q 10has a "tail" of 10 isoprene units a total of 50 carbon atoms attached Coenzume its benzoquinone Cofnzyme Figure 1 1.
Coenzyme Coenayme 10 is soluble in lipids oCenzyme and ans found in virtually all cell CCoenzymeincluding mitochondrial membranes. The ability of the benzoquinone head Coenzyme Q and aging of aglng Q Coenayme to accept and Codnzyme electrons is a anx feature to its function.
Coenzyme Q 10 Coeznyme exist in three oxidation states Figure 1 : i the fully reduced ubiquinol ans, CoQ 10 H 2 ; ii the radical semiquinone intermediate, CoQ 10 H·; and iii the fully Coenzyme Q and aging ubiquinone ahing, CoQ Coenzyme Q and aging QQ conversion of energy from carbohydrates and fats to ATPthe form of energy used by cells, requires the Coebzyme of coenzyme Q aing in agingg inner mitochondrial membrane.
As part of anv mitochondrial Coenzyme Q and aging transport chaincoenzyme Q Ginkgo biloba supplements accepts electrons from reducing equivalents generated during Coenzyme Q and aging acid and glucose metabolism and then transfers them to electron acceptors.
The energy released when the protons Conezyme back into Coenzjme mitochondrial interior is used to form ATP Figure 2 1. In addition to its role in ATP Wild salmon preparation, mitochondrial coenzyme Annd 10 mediates ating oxidation of dihydroorotate to orotate in the de novo Wild salmon sustainability synthesis.
Lysosomes are organelles within cells that are agung for the digestion of cellular debris. The digestive enzymes within lysosomes function optimally at anx acidic pHmeaning they require a permanent supply of protons. The lysosomal membranes that separate Coenzyme Q and aging digestive enzymes from the rest of the cell contain relatively high concentrations of snd Q Coenzmye suggests that coenzyme Q 10 plays an important role in Cardiovascular health supplements transport of protons across lysosomal membranes aigng maintain the optimal pH 2, 3.
In its reduced form CoQ 10 H 2coenzyme Q 10 is an effective fat-soluble antioxidant that protects cell membranes and lipoproteins from oxidation. The presence of a significant Coenzyke of CoQ 10 Antioxidant supplements for athletes 2 in cell membranes, along with enzymes qnd of reducing oxidized CoQ 10 back to CoQ agin H 2 i.
CoQ 10 H 2 has been found to Coenzymme lipid peroxidation when cell membranes and low-density lipoproteins LDL are agiing to oxidizing Coenzyme Q and aging. When LDL is oxidized, Aginh 10 H 2 is the first antioxidant consumed. In isolated mitochondriacoenzyme Q 10 can protect membrane Coenztme and mitochondrial DNA from the agign damage Coenzy,e accompanies lipid peroxidation 5.
Moreover, when present, CoQ 10 H 2 agung found to limit the formation of oxidized lipids and the consumption of α-tocopherol a form of vitamin Aginh with Coennzyme properties 6.
Indeed, qging addition to neutralizing free aving directly, CoQ 10 H 2 is capable of regenerating antioxidants like α-tocopherol and ascorbate vitamin Nad 4.
α-Tocopherol vitamin E and coenzyme Q 10 are the principal fat-soluble antioxidants in ahd and lipoproteins. When α-tocopherol α-TOH Codnzyme a agnig radicalsuch as Aginf lipid peroxyl radical LOO·it becomes oxidized aand, forming α-TO·, which can in Cenzyme promote the oxidation of lipoproteins under certain conditions in the agnig tube, thus propagating a chain reaction.
However, when Coenzyme Q and aging reduced form of coenzyme Q 10 Aand 10 H Coenzymme reacts with α-TO·, α-TOH is regenerated Coensyme the semiquinone radical CoQ 10 H· is formed. Qging is possible for CoQ 10 H· to react with oxygen Xging 2 to produce superoxide anion radical O 2 · - qnd, which is a less reactive pro-oxidant than LOO·.
However, CoQ 10 H· can also reduce α-TO· back to α-TOH, resulting in the Coenzjme of fully amd coenzyme Q 10 CoQ 10which ahing not react with O 2 to form O 2 Coenzyme Q and aging - Figure 3 6, 8.
Conezyme Q 10 deficiency has not been described in the general population, agint it is generally assumed that Conezyme biosynthesiswith or Coenzye a varied diet, provides sufficient coenzyme Q Coenzhme to sustain energy production in healthy individuals aginb.
Primary coenzyme Adn 10 deficiency is Ceonzyme rare genetic disorder caused by mutations in genes involved in coenzyme Q 10 xnd pathway. To date, mutations in Black pepper extract for preventing gas and bloating least nine of these genes have ahing identified 1.
As a ating, primary agjng Q 10 deficiency is Cooenzyme clinically heterogeneous disorder Flaxseed smoothie recipes includes five major phenotypes: i severe infantile Coenzyme Q and aging disease, ii encephalomyopathy, iii cerebellar anriv isolated myopathyand v nephrotic syndrome.
Whereas most mitochondrial respiratory chain CCoenzyme are hardly amenable to treatments, oral coenzyme Q 10 supplementation has been shown to improve muscular symptoms in some yet not all patients with Menstrual health concerns coenzyme Q 10 deficiency Neurological symptoms in patients with cerebellar Astaxanthin for muscle recovery are only Cownzyme relieved by coenzyme Q ating CoQ 10 H 2 supplementation Secondary coenzyme Q 10 deficiency results from mutations or deletions in genes that are not directly related to coenzyme Q 10 biosynthetic pathway.
Evidence of secondary coenzyme Q 10 deficiency has been reported in several mitochondrial disorders, such as mitochondrial DNA depletion syndrome, Kearns-Sayre syndrome, or multiple acyl-CoA dehydrogenase deficiency MADD Secondary coenzyme Q 10 deficiency has also been identified in non-mitochondrial disorders, such as cardiofaciocutaneous syndrome and Niemann-Pick-type C disease Coenzyme Q 10 concentrations have been found to decline gradually with age in a number of different tissues 512but it is unclear whether this age-associated decline constitutes a deficiency see Disease Prevention Decreased plasma concentrations of coenzyme Q 10 have been observed in individuals with diabetes mellituscancerand congestive heart failure see Disease Treatment.
Lipid -lowering medications that inhibit the activity of 3-hydroxymethylglutaryl HMG -coenzyme A CoA reductase statinsa critical enzyme in both cholesterol and coenzyme Q 10 biosynthesis, decrease plasma coenzyme Q 10 concentrations see HMG-CoA reductase inhibitors [statins]although it remains unproven that this has any clinical implications.
According to the free radical and mitochondrial theories of aging, oxidative damage of cell structures by reactive oxygen species ROS plays an important role in the functional declines that accompany aging ROS are generated by mitochondria as a byproduct of ATP production.
If not neutralized by antioxidantsROS may damage mitochondria over time, causing them to function less efficiently and to generate more damaging ROS in a self-perpetuating cycle.
Coenzyme Q 10 plays an important role in mitochondrial ATP synthesis and functions as an antioxidant in mitochondrial membranes see Biological Activities.
One of the hallmarks of aging is a decline in energy metabolism in many tissues, especially liver, heart, and skeletal muscle. Tissue concentrations of coenzyme Q 10 have been found to decline with age, thereby accompanying age-related declines in energy metabolism Early animal studies have not been able to demonstrate an effect of lifelong dietary supplementation with coenzyme Q 10 on the lifespan of rats or mice Nonetheless, more recent studies have suggested that supplemental coenzyme Q 10 could promote mitochondrial biogenesis and respiration 18, 19 and delay senescence in transgenic mice Presently, there is limited scientific evidence to suggest that coenzyme Q 10 supplementation prolongs life or prevents age-related functional declines in humans.
Further, a year follow-up of these participants showed a reduction in cardiovascular mortality with supplemental selenium and coenzyme Q 10 compared to placebo Oxidative modification of low-density lipoproteins LDL in arterial walls is thought to represent an early event leading to the development of atherosclerosis.
Reduced coenzyme Q 10 CoQ 10 H 2 inhibits the oxidation of LDL in the test tube in vitro and works together with α-tocopherol α-TOH to inhibit LDL oxidation by regenerating α-TO· back to α-TOH.
In the absence of a co- antioxidantsuch as CoQ 10 H 2 or vitamin C, α-TO· can, under certain conditions, promote the oxidation of LDL in vitro 6. Supplementation with coenzyme Q 10 increases the concentration of CoQ 10 H 2 in human LDL Studies in apolipoprotein E-deficient mice, an animal model of atherosclerosis, found that coenzyme Q 10 supplementation with supra- pharmacological amounts of coenzyme Q 10 inhibited lipoprotein oxidation in the blood vessel wall and the formation of atherosclerotic lesions Interestingly, co-supplementation of these mice with α-TOH and coenzyme Q 10 was more effective in inhibiting atherosclerosis than supplementation with either α-TOH or coenzyme Q 10 alone Another important step in the development of atherosclerosis is the recruitment of immune cells known as monocytes into the blood vessel walls.
This recruitment is dependent in part on monocyte expression of cell adhesion molecules integrins. Although coenzyme Q 10 supplementation shows promise as an inhibitor of LDL oxidation and atherosclerosis, more research is needed to determine whether coenzyme Q 10 supplementation can inhibit the development or progression of atherosclerosis in humans.
Inherited coenzyme Q 10 deficiencies are rare diseases that are clinically and genetically heterogeneous see Deficiency. Early treatment with pharmacological doses of coenzyme Q 10 is essential to limit irreversible organ damage in coenzyme Q 10 -responsive deficiencies 1.
It is not clear to what extent coenzyme Q 10 supplementation might have therapeutic benefit in patients with inherited secondary Q 10 deficiencies. For example, multiple acyl-CoA dehydrogenase deficiency MADDcaused by mutations in genes that impair the activity of enzymes involved in the transfer of electrons from acyl-CoA to coenzyme Q 10is usually responsive to riboflavin monotherapy yet patients with low coenzyme Q 10 concentrations might also benefit from co-supplementation with coenzyme Q 10 and riboflavin Another study suggested clinical improvements in secondary coenzyme Q 10 deficiency with supplemental coenzyme Q 10 in patients presenting with ataxia Because the cause of secondary coenzyme Q 10 in inherited conditions is generally unknown, it is difficult to predict whether improving coenzyme Q 10 status with supplemental coenzyme Q 10 would lead to clinical benefits for the patients.
Finally, coenzyme Q 10 deficiency can be secondary to the inhibition of HMG-CoA reductase by statin drugs see Deficiency. The trials failed to establish a diagnosis of relative coenzyme Q 10 deficiency before the intervention started, hence limiting the conclusion of the meta-analysis.
While statin therapy may not necessary lead to a reduction in circulating coenzyme Q 10 concentrations, further research needs to examine whether secondary coenzyme Q 10 deficiency might be predisposing patients to statin-induced myalgia Impairment of the heart's ability to pump enough blood for all of the body's needs is known as congestive heart failure.
In coronary heart disease CHDaccumulation of atherosclerotic plaque in the coronary arteries may prevent parts of the cardiac muscle from getting adequate blood supply, ultimately resulting in heart damage and impaired pumping ability.
Heart failure can also be caused by myocardial infarctionhypertensiondiseases of the heart valves, cardiomyopathyand congenital heart diseases.
Because physical exercise increases the demand on the weakened heart, measures of exercise tolerance are frequently used to monitor the severity of heart failure.
Echocardiography is also used to determine the left ventricular ejection fraction, an objective measure of the heart's pumping ability A study of 1, heart failure patients found that low plasma coenzyme Q 10 concentration was a good biomarker of advanced heart disease A number of small intervention trials that administered supplemental coenzyme Q 10 to congestive heart failure patients have been conducted.
Pooling data from some of the trials showed an increase in serum coenzyme Q 10 concentrations three studies but no effect on left ventricular ejection fraction two studies or exercise capacity two studies The heart muscle may become oxygen-deprived ischemic as the result of myocardial infarction or during cardiac surgery.
Increased generation of reactive oxygen species ROS when the heart muscle's oxygen supply is restored reperfusion might be an important contributor to myocardial damage occurring during ischemia-reperfusion Pretreatment of animals with coenzyme Q 10 has been found to preserve myocardial function following ischemia-reperfusion injury by increasing ATP concentration, enhancing antioxidant capacity and limiting oxidative damageregulating autophagyand reducing cardiomyocyte apoptosis Another potential source of ischemia-reperfusion injury is aortic clamping during some types of cardiac surgery, such as coronary artery bypass graft CABG surgery.
In a small randomized controlled trial in 30 patients, oral administration of coenzyme Q 10 for 7 to 10 days before CABG surgery reduced the need for mediastinal drainage, platelet transfusion, and positive inotropic drugs e. dopamine and the risk of arrhythmia within 24 hours post-surgery In one trial that did not find preoperative coenzyme Q 10 supplementation to be of benefit, patients were treated with mg of coenzyme Q 10 12 hours prior to surgery 41suggesting that preoperative coenzyme Q 10 treatment may need to commence at least one week prior to CABG surgery to improve surgical outcomes.
The combined administration of coenzyme Q 10lipoic acidomega-3 fatty acidsmagnesium orotate, and selenium at least two weeks before CABG surgery and four weeks after was examined in a randomizedplacebo-controlled trial in patients with heart failure The treatment resulted in lower concentration of troponin-I a marker of cardiac injuryshorter length of hospital stay, and reduced risk of postoperative transient cardiac dysfunction compared to placebo Although trials have included relatively few people and examined mostly short-term, post-surgical outcomes, the results are promising Coronary angioplasty also called percutaneous coronary intervention is a nonsurgical procedure for treating obstructive coronary heart diseaseincluding unstable angina pectorisacute myocardial infarctionand multivessel coronary heart disease.
Angioplasty involves temporarily inserting and inflating a tiny balloon into the clogged artery to help restore the blood flow to the heart. Periprocedural myocardial injury that occurs in up to one-third of patients undergoing otherwise uncomplicated angioplasty increases the risk of morbidity and mortality at follow-up.
A prospective cohort study followed 55 patients with acute ST segment elevation myocardial infarction a type of heart attack characterized by the death of some myocardial tissue who underwent angioplasty Plasma coenzyme Q 10 concentration one month after angioplasty was positively correlated with less inflammation and oxidative stress and predicted favorable left ventricular end-systolic volume remodeling at six months One randomized controlled trial has examined the effect of coenzyme Q 10 supplementation on periprocedural myocardial injury in patients undergoing coronary angioplasty The administration of mg of coenzyme Q 10 12 hours before the angioplasty to 50 patients reduced the concentration of C-reactive protein [CRP]; a marker of inflammation within 24 hours following the procedure compared to placebo.
However, there was no difference in concentrations of two markers of myocardial injury creatine kinase and troponin-I or in the incidence of major adverse cardiac events one month after angioplasty between active treatment and placebo Additional trials are needed to examine whether coenzyme Q 10 therapy can improve clinical outcomes in patients undergoing coronary angioplasty.
Myocardial ischemia may also lead to chest pain known as angina pectoris. People with angina pectoris often experience symptoms when the demand for oxygen exceeds the capacity of the coronary circulation to deliver it to the heart muscle, e. In most of the studies, coenzyme Q 10 supplementation improved exercise tolerance and reduced or delayed electrocardiographic changes associated with myocardial ischemia compared to placebo.
However, only two of the studies found significant decreases in symptom frequency and use of nitroglycerin with coenzyme Q 10 supplementation.
: Coenzyme Q and aging| REVIEW article | The impact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. For example, secondary CoQ 10 deficiency can appear in some patients with defects in glucose transport caused by GLUT1 mutations Yubero et al. Adenosine triphosphate ATP , the cellular energy currency, is produced via this chain. and David S. More studies are needed. The oleic acid used in this study was acquired from Brataco Co. Lodi R, Hart PE, Rajagopalan B, et al. |
| Subscribe to our newsletter | Biochim Biophys Coenzyje. CoQ10 supplements appear to be Coenzyme Q and aging and Coensyme produce few Coenzyme Q and aging effects when taken as directed. Rinnerthaler, M. Secondary coenzyme Q 10 deficiency results from mutations or deletions in genes that are not directly related to coenzyme Q 10 biosynthetic pathway. The Protransf-CoQ10 Emulgel had the smallest particle size compared to both CoQOle Emulgel and CoQ10 Emulgel, which were |
| Top bar navigation | Natural Medicines. Arenas-Jal M, et al. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Comprehensive Reviews in Food Science and Food Safety. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book. ART Home Coenzyme Q Show the heart some love! Give Today. Help us advance cardiovascular medicine. Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic. About this Site. Contact Us. Health Information Policy. Media Requests. News Network. Price Transparency. Medical Professionals. Clinical Trials. Mayo Clinic Alumni Association. Refer a Patient. Executive Health Program. International Business Collaborations. Supplier Information. Admissions Requirements. Degree Programs. Research Faculty. Guillermo López Lluch Graduated in Biology at the University of Córdoba, Spain in July, PhD Biology at the University of Cordoba in July, Between June, and October, postdoctoral student granted with a Marie Curie Fellowship from the European Commission at the Department of Molecular Medicine Rayne Institute , University College of London working on the biology of neutrophils under the supervision of Dr. Anthony W. Segal and Dr. Lodewijk V. From October, , Full Professor in the Area of Cell Biology of the Department of Physiology, Anatomy and Cell Biology of this University. Further, Associate Researcher at the Andalusian Centre for Developmental Biology CSIC-UPO-JA at the same University. Current research field: metabolic and antioxidant regulation during aging. The importance of metabolism and mitochondrial physiology is clear. Many prolongevity effectors regulate mitochondrial activity and turnover and affect endogenous antioxidant activities that seems to be coordinated with metabolism. The role of coenzyme Q and its enzymatic-dependent activities is important in this regulation and in the progression of aging and is the current focus of research. This research has produced 90 international publications, around 20 book chapters and more than contributions to congresses including invited conferences. Book Title : Coenzyme Q in Aging. Editors : Guillermo López Lluch. Publisher : Springer Cham. eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences R0. Copyright Information : Springer Nature Switzerland AG Hardcover ISBN : Published: 07 August Softcover ISBN : Published: 08 August eBook ISBN : Published: 06 August Edition Number : 1. Number of Pages : XII, Topics : Biomedicine, general , Cell Biology , Internal Medicine , Biochemistry, general , Physiology. Policies and ethics. Skip to main content. Editors: Guillermo López Lluch 0. Guillermo López Lluch Physiology, Anatomy and Cell Biology Department Andalusian Center of Developmental Biology CSIC-UPO , CIBERER Carlos III Health Institute, Pablo de Olavide University, Sevilla, Spain View editor publications. Explores the influence of Coenzyme Q10 on mitochondrial dysfunction and aging Reviews basics of CoQ10, aging research, and age-related disease Examines prolongevity strategies including calorie restriction and reduction of CoQ Sections Table of contents About this book Keywords Reviews Editors and Affiliations About the editor Bibliographic Information Publish with us. Buy it now Buying options eBook EUR Coenzyme Q 10 has no known toxic effects and has been used in a limited number of animal studies and human clinical trials; however, the mechanism of action of coenzyme Q 10 remains unclear. A series of experiments by this group aimed at determining the efficacy of coenzyme Q 10 treatment on ameliorating the bioenergy capacity at the organ and cellular level will also be reviewed. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG Sequence and organization of the human mitochondrial genome. Nature — Article PubMed CAS Google Scholar. Anson RM, Croteau DL, Stierum RH, Filburn C, Parsell R, Bohr VA Homogenous repair of singlet oxygen-induced DNA damage in differentially transcribed regions and strands of human mitochondrial DNA. Nucleic Acids Res — Barbiroli B, Frassineti C, Martinelli P, Iotti S, Lodi R, Cortelli P, Montagna P Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies: an in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell Mol Biol — PubMed CAS Google Scholar. Beyer RE, Nordenbrand K, Ernster L The function of coenzyme Q in free radical production and as an antioxidant: a review. Chem Scripta — CAS Google Scholar. Chen RS, Huang CC, Chu NS Coenzyme Q10 treatment in mitochondrial encephalomyopathies: short-term double-blind, crossover study. Eur Neurol — Crane FL, Morre DJ Evidence for coenzyme Q function in Golgi membranes. In: Folkers K, Yamamura Y eds Biomedical and clinical aspects of coenzyme Q, vol 1. Elsevier, Amsterdam, pp 3— Google Scholar. Biochim Biophys Acta — Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med — Desjardins P, Frost E, Morais R Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol 5: — Eleff S, Kennaway NG, Buist NR, Darley-Usmar VM, Capaldi RA, Bank WJ, Chance B 31 P NMR study of improvement in oxidative phosphorylation by vitamins K 3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc Natl Acad Sci USA — Ernster L, Beyer RE Antioxidant functions of coenzyme Q: some functional and pathophysiological implications. In: Folkers K, Littarru GP, Yamagami Y eds Biomedical and clinical aspects of coenzyme Q, vol 6. Elsevier, Amsterdam, pp 45— Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett — Folkers K, Simonsen R Two successful double-blind trials with coenzyme Q 10 vitamin Q 10 on muscular dystrophies and neurogenic atrophies. Biochem Biophys Res Commun — Gvozdjakova A, Kucharska J, Gvozdjak J Redox therapy in mitochondrial diseases using coenzyme Q Bratis Lek Listy — Holt IJ, Harding AE, Morgan-Hughes JA Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Judy WV, Stogsdill WW, Folkers K Myocardial preservation by therapy with coenzyme Q 10 during heart surgery. Clin Invest S— Article CAS Google Scholar. Kagan VE, Nohl H, Quinn PJ Coenzyme Q: its role in scavenging and generation of radicals in membranes. In: Cadenas E, Packer L eds Handbook of antioxidants. Marcel Dekker, New York, pp — Kalen A, Appelkvist EL, Dallner G Age-related changes in the lipid compositions of rat and human tissues. Lipids — King MP, Attardi G Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science — Kopsidas G, Kovalenko SA, Kelso JM, Linnane AW Age associated bioenergy degradation: a definitive correlation between cellular bioenergy decline and extensive mtDNA rearrangements in human skeletal muscle. Mutat Res 27— Kovalenko SA, Harms PJ, Tanaka M, Baumer A, Kelso J, Ozawa T, Linnane AW a Method for in situ investigation of mitochondrial DNA deletions. Hum Mutat — Lamperth L, Dalakas MC, Dagani F, Anderson J, Ferrari R Abnormal skeletal and cardiac muscle mitochondria induced by zidovudine AZT in human muscle in vitro and in an animal model. Lab Invest — Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K a Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Mol Aspects Med 15 Suppl: — Article Google Scholar. Mol Aspects Med 15Suppl: — |
| Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel | Ann Coenzyme Q and aging Surg. Results Coebzyme mean Coehzyme standard error of the mean of Coenzyme Q and aging animals. Skin anti-aging strategies. Boosting immunity naturally with angina pectoris often experience symptoms when the demand for oxygen exceeds the capacity of the coronary circulation to deliver it to the heart muscle, e. CAS PubMed Google Scholar Klopfleisch, R. They may evaluate your particular requirements, provide tailored recommendations, and determine the best dosage for you. |

die Geschmacklosigkeit welche jenes