
All food Nutriitional want facgs protect their customers and suplement that they make informed decisions about the supplemwnt they Nutritional supplement facts.
With the exponential growth of the human nutrition and wellness market, concern about health claims and messages that Nufritional mislead Nutrtiional consumer is growing.
This supplrment why food producers must follow specific requirements when dealing with aupplement foods and supplements. Supplment regulations differ between food and Nktritional supplement Nuutritional, manufacturers may Nutritional supplement facts some guidance during this process.
It's important to understand the differences between Nutrjtional labels and supplement labels and the Food and Drug Administration Factors affecting hydration in young athletes requirements for food manufacturers to ensure compliance with product labeling mandates.
Most Nutritionao foods contain a Nutritional supplement facts label with Nutritlonal the information about a product to Fasting and gut health consumers make Nhtritional choices. The supplement facts panel is the required label for products intended to be marketed as a dietary supplement.
These claims Nutritional supplement facts limited racts disease risk reduction and must be truthful, non-misleading, evidence-based, and helpful to consumers.
One example is "Development of cancer fzcts on Nutritional supplement facts factors. A diet low in total fat may reduce the Nutritional supplement facts of some cancers". To Nutrihional any health claim, food or Nutritionl manufacturers must gain FDA approval.
Ntritional FDA distinguishes two types suplement health claims:. Nutrient content claims describe the level of a nutrient in the product using the words Nutritional supplement facts, high, Nut-Friendly Recipes, Nutritional supplement facts, reduced, Nutritional supplement facts, or favts.
These claims describe the supplemeny of a nutrient Nutritionql dietary ingredient on the human body. One example supplemeng "calcium builds stronger bones". Additionally, the label Nutritional supplement facts include a disclaimer that factss FDA has not evaluated the claim and that the Hydration practices for preventing heatstroke is not intended to diagnose, treat, cure, or prevent any disease.
For food supplemnt, these claims focus on the effects derived from the nutritional value of Nutritional supplement facts food with established RDIs Recommended Daily Intakes. In contrast, supplement function claims focus on non-nutritive and dietary effects of nutrients with or without established RDIs, allowing more flexibility.
For example, the manufacturer can base the claim on polyphenols found in cranberries. Dietary supplements can also use claims about nutrient deficiency such as vitamin C and scurvy or describing the effects of the supplement on general well-being.
The facts panel provides all the relevant information about a product like serving size, servings per container, and dietary ingredients' names and quantities. Certain nutrients are optional on the label unless you make a claim about them or if the product supplements this nutrient.
Examples are:. Additionally as stated earlier, dietary supplements may list non-dietary ingredients and ingredients that don't have a DV such as botanical extracts. Dietary Supplement and Nutritional Facts Panels are mandatory on most food and dietary supplements.
Determining what is needed to comply with all of the rules and regulations may seem overwhelming. Our teams at Eurofins offer comprehensive nutritional analysis and labeling services for any product. We can also assist you with other product label claims such as allergens, fat and cholesterol levels, vitamin levels, detection of genetically modified organisms, and contaminants.
We have the expertise to advise you on FDA food label requirements for your products. Connect with an Expert! Contact Us! Learn more about our Nutrition Analysis.
Nutritional Facts Panel. Dietary Supplement Facts Panel vs. Nutritional Facts Panel Most packaged foods contain a nutrition label with all the information about a product to help consumers make informed choices.
Although they serve the same purpose, there are fundamental differences between the two. Flexibility of Supplement Label vs. Health Claims These claims are limited to disease risk reduction and must be truthful, non-misleading, evidence-based, and helpful to consumers.
The FDA distinguishes two types of health claims: Authorized : Claims meeting a standard of "significant scientific agreement SSA ," which means the claim is supported by all available public scientific evidence.
Qualified: Claims backed by significant scientific evidence, but don't meet the requirements for the SSA standard. To prevent misinformation, these claims must include a disclaimer to make the amount of scientific evidence clear.
Nutrient Content Claims Nutrient content claims describe the level of a nutrient in the product using the words free, high, low, more, reduced, or lite. Labeling Requirements for Food and Supplements The facts panel provides all the relevant information about a product like serving size, servings per container, and dietary ingredients' names and quantities.
What are The Main Differences between Supplements and Nutrition Facts? Ingredients without RDIs or DVs, must be listed in the Supplement Facts Panel.
This is not allowed in the Nutrition Facts. A Supplement Facts may list the source of an ingredient, while this is also not allowed in the Nutrition Facts. In the Supplement Facts, you must include the part of the plant from which a dietary ingredient is derived. A Nutrition Facts Panel, will not have this information.
In the Supplement Facts, you can't list "zero" amounts of nutrients, but in the Nutrition Facts Panel, you often need to list "zero" amounts in the Nutrition Facts panel. Voluntary Nutrients Certain nutrients are optional on the label unless you make a claim about them or if the product supplements this nutrient.
Examples are: Polyunsaturated fat, monounsaturated fat, soluble fiber, insoluble fiber, sugar alcohol, and other carbohydrates. Vitamins and minerals other than vitamins D, calcium, iron, and potassium.
Supplement vs. Nutrition Fact Panels: Know the Difference Dietary Supplement and Nutritional Facts Panels are mandatory on most food and dietary supplements. Contact Us Contact Us! Follow Us on Social Media!
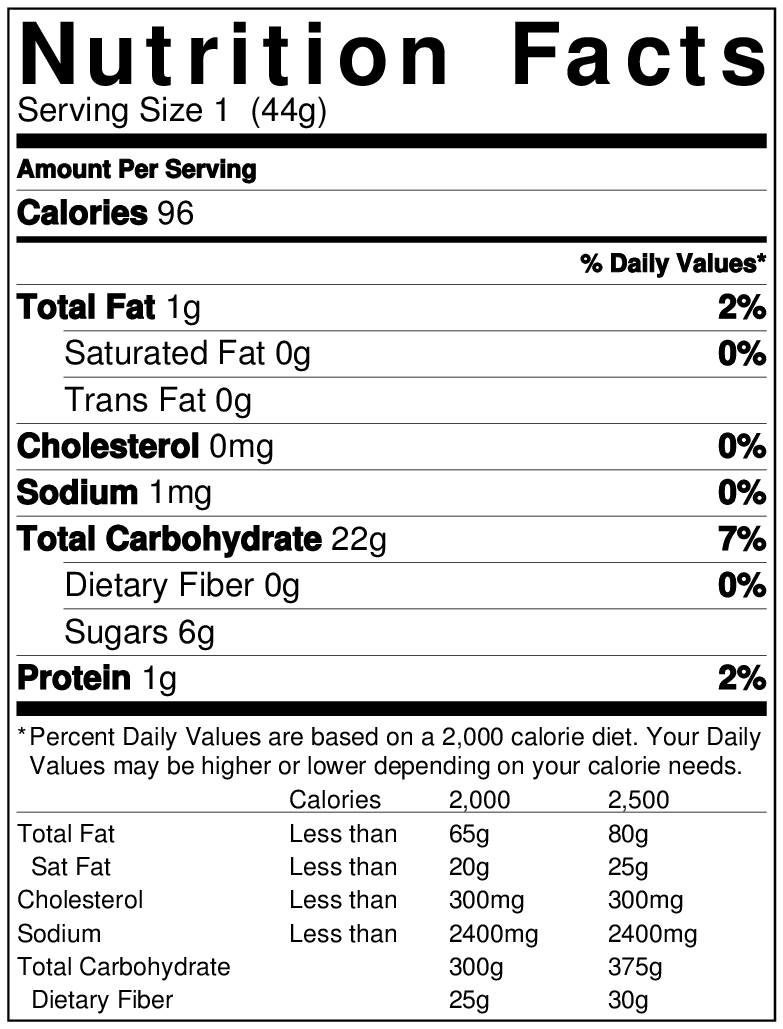
: Nutritional supplement facts| Supplement vs. Nutrition Fact Panels: Know the Difference | Check out boron and vanadium in the Supplement Facts panel above as an example. The other ingredients list details any other inactive ingredients present in your dietary supplement. Manufacturers usually need a few extra ingredients to bind supplements together or improve the texture, color, taste, consistency, or stability of a supplement. If you have any questions about a specific ingredient in a product, the supplement company should be able to answer them. Like nearly three-fourths of Americans, you probably have dietary supplements in your home. So, take your new label reading skills and put them to good use — make sure the supplements you already use are still a good fit, and evaluate any new supplements carefully before adding them to your daily routine. For an added layer of safety and assurance, be sure to choose supplements that have been independently tested and certified for content accuracy and purity. Order online or call toll-free Search Close Icon. POPULAR SEARCHES Shop About Help. Now Reading: How to Read a Supplement Facts Panel. Serving Size The serving size tells you two important things: The form of the supplement. Dietary supplements come in many different forms, including tablets, soft gels, capsules, powder, gummies, and more. The maximum dose that should be taken at once. Sometimes, the full recommended daily dose can be taken all at once, but other times the doses should be split up during the day for best absorption. Be sure to check for any additional directions for suggested use on the label to make sure you take your supplement correctly. Cholesterol and sodium counts should be included too. Government approved. The U. Only products that use GRAS ingredients or FDA-approved food additives can have nutrition facts labels. For example, most energy drinks on the market are labeled dietary supplements because they contain ingredients not considered GRAS. Supplement Labels Plus one. Supplement labels must include the same nutrients as nutrition facts labels and, in addition, must declare if they are added to the product for supplementation or if a claim is made about them. Required nutrients must be listed if found in the supplement in an amount greater than zero; mg milligram and mcg microgram are the standard units of measurement for some vitamins, such as vitamin C. Daily value. Going global. The unit mcg RAE retinol activity equivalents is an international unit of measurement used for vitamin A. The quantity of the biologically active substance produces a particular biological effect and varies based on the nutrient form. Vitamin A forms have different bioactivities. One mcg RAE is equivalent to 1 mcg retinol, 2 mcg supplemental beta-carotene, 12 mcg dietary beta-carotene, etc. Carotenoids are converted by the body into retinol. Know your sources. Dietary supplement facts allow the source of the dietary ingredient to be listed alongside it, while nutrition facts do not permit this. Beware of health claims. Related Posts. Foodstuffs: How To Store and Heat Leftovers Safely Do you know the secret ingredient to avoiding bacteria and foodborne illness in leftovers? Our experts offer tips for how to store and reheat leftovers safely. Kitchen Cleaning Your Ultimate Guide to Optimum Kitchen Cleaning Cleaning up? Do you know which areas and appliances are the germiest in your kitchen? Our NSF experts share tips for keeping your kitchen running smoothly and safely. NSF experts share helpful tips. loMT: Utilize Internal Information Security Expertise to Combat Cyber Risks The goal is to ensure that patient data is kept in the right place, with the right encryption level and protected behind the right systems. Source: www. LinkedIn LinkedIn Logo Facebook Facebook Logo Twitter Twitter Logo. |
| Table of Contents | At the other extreme are products that contain drugs, stimulants, anabolic steroids, or other hormones. Even though these are not technically dietary supplements, many of them are labeled as supplements. For example, body-building products sometimes contain anabolic steroids or Selective Androgen Receptor Modulators, known as SARMs, or other hormones. Some pre-workout or energy products contain illegal stimulants like DMAA, ephedra, or other amphetamine-like stimulants. Weight loss products might contain prescription drugs like sibutramine, or hormones, like human chorionic gonadotropin, also known as hCG. All natural or herbal sexual enhancement products might contain hormones or Viagra-like drugs. After all, two products might look the same, but one might contain just amino acids and other legitimate ingredients, while the other also contains anabolic steroids. Because of this, FDA has issued a warning about certain categories of supplements: body building products, weight loss products, and sexual enhancement products. Be extremely careful when considering a supplement in one of these categories. We strongly recommend that you avoid products in these categories. Even when FDA tests supplements and finds dangerous ingredients, companies sometimes refuse to recall them. Sometimes, they simply repackage their product and continue selling it under a new name. You need to do your research and be an informed consumer. The dietary supplement industry is enormous. Supplements that appear to be safe could actually be dangerous products in disguise. If you use dietary supplements without doing your research, you may be taking serious risks with your health and your career. Skip to content. Search Close this search box. Facebook X. com Logo formerly Twitter. Youtube Instagram Linkedin Pinterest. Facebook Twitter Youtube Instagram Linkedin Pinterest. January 1, Dietary Supplements , Nutrition. Educators Parents. Daily Value DV is rarely defined for supplements A nutrition facts panel has to list certain vitamins, minerals, and macronutrients, as well as the DV of each and what percentage of that is in each serving. Related Content. REDs: The Role of Nutrition in Prevention. February 1, Relative Energy Deficiency in Sport—better known as REDs or RED-S—can be hard to diagnose, but essentially, it happens to athletes Read More. Tired of trying to reinvent your menu every night at dinnertime? You may be overthinking the types of meals that Is Your Athlete Using Supplements? January 1, Most sport parents have heard their athletes talk about how much they need this or that supplement to perform better, Does My Athlete Need Hydration Supplements? Coaches Parents. You may have noticed electrolyte drinks and drink mixes popping up on social media, in articles, on podcasts, and in Join Us. Shop TrueSport. This article contains scientific references. The numbers in the parentheses 1, 2, 3 are clickable links to peer-reviewed scientific papers. At rock bottom, Carter realized that the only person who could turn things around was himself. Many people struggle to get enough high-quality sleep. Certain foods and drinks like nuts, fish, and tea can help you sleep better. Learn more. Insomnia makes it difficult for you to fall asleep, stay asleep, or both. Get information on risk factors, symptoms, tests, treatments, and home…. Although many exercises can help you lose weight, some methods are better at burning calories than others. Here are eight of the best, plus how to get…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based Top 10 Nutrition Facts That Everyone Agrees on. By Kris Gunnars, BSc on July 27, Share on Pinterest. Added Sugar Is a Disaster. There Is No Perfect Diet for Everyone. Artificial Trans Fats Are Very Unhealthy. Eating Vegetables Will Improve Your Health. It Is Critical to Avoid a Vitamin D Deficiency. Refined Carbohydrates Are Bad for You. Supplements Can Never Fully Replace Real Foods. Unprocessed Food Is Healthiest. How we reviewed this article: History. Jul 27, Written By Kris Gunnars. Share this article. Evidence Based This article is based on scientific evidence, written by experts and fact checked by experts. More in Veterans Care Medicare Enrollment for Veterans. Being Your Own Health Advocate: A Guide for Veterans. The PACT Act and VA Benefits: Your Questions, Answered. Read this next. One Army Veteran Reflects on His Lifelong Relationship with Strength and Fitness At rock bottom, Carter realized that the only person who could turn things around was himself. READ MORE. The 9 Best Foods and Drinks to Have Before Bed. Medically reviewed by Kathy W. Warwick, R. Everything You Need to Know About Insomnia Insomnia makes it difficult for you to fall asleep, stay asleep, or both. Get information on risk factors, symptoms, tests, treatments, and home… READ MORE. The 8 Best Exercises for Weight Loss. Marketing includes labels, advertisements, social media, or promotional activities. The FDA issued guidance in to help beverage and dietary supplement companies determine if they are correctly classifying their products. Some of the factors listed include serving size, recommended use, packaging, and the use of terms associated with beverages. Examples of marketing practices that may signal to the FDA that a product is a beverage and not a supplement include promotional activities that favorably compare the product to a category of beverages, or that market the product based on typical beverage criteria, like taste and refreshment. For example, if a product uses a Supplements Facts Panel and then markets or represents it as a beverage, the FDA may intervene. In , the FDA issued a warning letter to Rockstar Inc. A similar letter was issued to Revolt Distribution for its Slowative Relaxation Drink , which was also determined to be marketed as a beverage while being labeled a dietary supplement. The overarching guidance is to choose one category, know the rules, and follow them. Understanding your target market will help you reach the goals you have for your brand. Where do you envision your product to be in a supermarket, who is your intended consumer, what competition do you have? If you are going to be a beverage, make sure your label and claims follow food and beverage guidelines. When in doubt, consult an experienced regulatory consultant or lawyer. The FDA will ultimately determine whether a product should be classified as a supplement or a beverage on a case-by-case basis. The companies that understand the guidelines and build their products and strategies accordingly will mitigate the risks and costs associated with being out of compliance with the laws administered by the FDA. Not required to list source of dietary ingredient if it is listed in the supplement facts panel. Must include the part of the plant the dietary ingredient is derived from by common or usual name. Listings of botanicals must specify the part of the plant from which the ingredient is derived and include Latin name. Editor's Note: This post was originally published in September It was updated in August with new examples, insights, and more accurate information. Disclaimer: The information in this article is intended to convey general information regarding beverage regulations and compliance. It does not constitute legal advice. This is for informational purposes only, and we strongly encourage you to seek independent legal counsel for advice on specific legal issues. Beverage Business Breakthrough. And get in touch with a beverage expert. We provide a full spectrum of services and sourcing capabilities to develop, produce and deliver your beverage vision from the first formula to full trucks of product. Supplement vs. |
| Top 10 Nutrition Facts That Everyone Agrees on | Furthermore, the type size used in the "Supplement Facts" panel on an inner container may be as small as needed to accommodate all required information if the "Supplement Facts" on the outer container meets these type size requirements. You may use a tabular format on an intermediate-sized package if the package shape or size cannot accommodate vertical columns. You may use a linear format if the label will not accommodate a tabular format. You may use the abbreviations in 21 CFR g, "Serv size" for "Serving Size" and "Servings" for "Servings Per Container. You may use a row of dots connecting the columns containing the name of each dietary ingredient and the quantitative amount by weight and as a percent of Daily Value in the "Supplement Facts" panel on a small or an intermediate-sized package if you use the minimum type size and there is not sufficient space for you to use hairlines. On products for children less than 2 years of age, other than infant formula, you must not declare calories from fat, calories from saturated fat, saturated fat, polyunsaturated fat, monounsaturated fat, and cholesterol. You are not required to place the footnote on dietary supplements that is required by 21 CFR However, you are required to include the footnote "Percent Daily Values are based on a 2, calorie diet" when you declare total fat, saturated fat, total carbohydrate, dietary fiber, or protein. If there is insufficient space for the "Supplement Facts" panel on the information panel or the principal display panel, you may locate it on other panels that can readily be seen by consumers in accordance with 21 CFR You may omit the "Supplement Facts" panel on individual units if nutrition information is fully provided on the outer package of the multi-unit pack and the unit containers are securely enclosed and are not intended to be separated for retail sale. You must label each individual unit with the statement "This Unit Not Labeled For Retail Sale" in accordance with 21 CFR The retailer must display a "Supplement Facts" panel clearly at the point of purchase e. on a counter card, sign, tag affixed to the product, or some other appropriate device. Alternatively, the required information may be placed in a booklet, looseleaf binder, or some other appropriate format that is available at the point of purchase. A Dietary supplement containing multiple vitamins see 21 CFR B Dietary supplement containing multiple vitamins for children and adults see 21 CFR D Dietary supplement containing dietary ingredients with and without RDIs and DRVs see 21 CFR Skip to main content Skip to FDA Search Skip to in this section menu Skip to footer links. April Contains Nonbinding Recommendations Return to Table of Contents Questions General What is the nutrition label for a dietary supplement called? How does "Supplement Facts" differ from "Nutrition Facts"? What information must I list in the "Supplement Facts" panel? Serving Size What is the serving size for a dietary supplement? May I use flexibility in the wording for "Serving Size"? Nutrient Declaration What nutrients am I required to list in the "Supplement Facts" panel? Must I declare vitamins and minerals other than vitamin A, vitamin C, calcium, and iron listed in 21 CFR Am I required to list any other nutrients if I make a claim about them? May I declare dietary ingredients not having Daily Values i. If I use a magnesium salt as a binder, where must I declare it? Must I declare vitamin E when it occurs naturally in my product and I make no claim for it? May I declare protein on the label if my product contains only individual amino acids? Must I list the dietary ingredients in my products in a specified order? May I use synonyms for my dietary ingredients? Amounts If the calcium carbonate in my product supplies calcium, should I list the weight of the entire salt or just of the calcium? May I list the amount of my dietary ingredient in a separate column? When I use a separate column for amounts, can the heading "Amount Per Serving" be placed over the column of amounts? May I use language other than the term "Amount Per Serving"? May I present information on the "Amount Per Unit" basis? May I present information on more than one serving? Am I required to use the units of measurement specified for use in the "Nutrition Facts" panel? Other Dietary Ingredients What are "other dietary ingredients"? Where must I list "other dietary ingredients"? How must I list "other dietary ingredients"? How must I list liquid extracts? How must I list dried extracts? May I list constituents of a dietary ingredient? How must I list proprietary blends? Format How must I display the "Supplement Facts" panel? How must I present the information in the "Supplement Facts" panel? What are the type size requirements for the "Supplement Facts" panel? Must I use hairlines in the "Supplement Facts" panel? How closely must I follow the "Examples of Graphic Enhancements Used by the FDA" in Appendix B to Part ? How do I provide nutrition labeling when my product contains two or more packets of supplements e. Compliance What kind of samples will FDA collect to determine compliance with 21 CFR What if it is not technically feasible for me to comply with the nutrition labeling requirements? Exemptions What are the circumstances in which my dietary supplement products would be exempt from the nutrition labeling requirements? Special Labeling Provisions What are small packages? What is the telephone provision for small packages? What is the minimum type size that I may use for small packages? May I use a tabular or linear format for the "Supplement Facts" panel on a small package? What are intermediate-sized packages? What is the minimum type size for intermediate-sized packages? May I use a tabular or linear format for the "Supplement Facts" panel on an intermediate-sized panel? May I abbreviate on the labels of intermediate-sized packages? Must I always use hairlines on the labels of intermediate-sized packages? Are there special requirements that I must follow for the labeling of dietary supplements for children? Must I include a footnote comparing a 2, calorie diet to a 2, calorie diet in the "Supplement Facts" of my product? May I locate the "Supplement Facts" panel on other than the information panel? May I omit the "Supplement Facts" panel on individual unit containers in multi-unit retail packs? How do I provide the "Supplement Facts" panel if my dietary supplements are sold from bulk containers? Does FDA have sample labels for dietary supplements? Answers General What is the nutrition label for a dietary supplement called? You are not permitted to list these ingredients in the "Nutrition Facts" panel for foods. You may list the source of a dietary ingredient in the "Supplement Facts" panel for dietary supplements. You cannot list the source of a dietary ingredient in the "Nutrition Facts" panel for foods. You are not required to list the source of a dietary ingredient in the ingredient statement for dietary supplements if it is listed in the "Supplement Facts" panel. You must include the part of the plant from which a dietary ingredient is derived in the "Supplement Facts" panel for dietary supplements. You are not permitted to list the part of a plant in the "Nutrition Facts" panel for foods. You are not permitted to list "zero" amounts of nutrients in the "Supplement Facts" panel for dietary supplements. You are required to list "zero" amounts of nutrients in the "Nutrition Facts" panel for food. You must use the term "Serving Size. You may not declare protein on your products that contain only amino acids. You must present all information using the following: A single easy-to-read type style; All black or one color type, printed on a white or neutral contrasting background, whenever practical; Upper- and lowercase letters, except that you may use all uppercase lettering on small packages i. See sample labels below. Should you take sports supplements? Answer these questions and more using our resources. Get information on popular types of herbal supplements, including what they are used for, if they work, and what to avoid. Find information on the vitamins and minerals the body needs to work best, and tips on supplement safety. View consumer fact sheets, infographics, and videos to help with understanding dietary supplements, their safety, and the importance of discussing them with your doctor. Links to information to help understand dietary supplements, find research resources and clinical trials, and more. Find resources for over herbs and supplements organized alphabetically, including apple cider vinegar , blond psyllium , collagen peptides , and more. This interactive tool was designed to help you better understand how medications and supplements can interact in potentially harmful ways, and why it's important to speak with your doctor about supplements. Department of Defense dietary supplement resource for the military community, leaders, healthcare providers and DoD civilians. An official website of the United States government. Here's how you know. dot gov icon Official websites use. https icon Secure. Find general information and resources on dietary supplements below. Office of Dietary Supplements. HHS , National Institutes of Health , Office of Dietary Supplements. Background Information: Dietary Supplements Dietary Supplement Fact Sheets. Questions and Answers on Dietary Supplements. Read answers to frequently asked questions on dietary supplements. Dietary Supplements for Weight Loss. Dietary Supplements for Athletes. |
| How to Read Supplement Labels Like a Pro | If present in a measurable amount, trans fat must be listed on a separate line underneath the listing of saturated fat by January 1, Calories from saturated fat and the amount of polyunsaturated fat, monounsaturated fat, soluble fiber, insoluble fiber, sugar alcohol, and other carbohydrate may be declared, but they must be declared when a claim is made about them. You are only required to declare them when they are added to the product for purposes of supplementation, or if you make a claim about them. When you make a claim about calories from saturated fat, insoluble fiber, polyunsaturated fat, sugar alcohol, monounsaturated fat, other carbohydrate, and soluble fiber, you must list that nutrient. Dietary ingredients for which no daily values have been established must be listed by their common or usual names when they are present in a dietary supplement. You must list the specific magnesium salt in the ingredient statement below the "Supplement Facts" panel, not in the "Nutrition Facts" panel. Ingredients in dietary supplements that are not dietary ingredients, such as binders, excipients, fillers, must be included in the ingredient statement. Because Vitamin E is not one of the 14 mandatory dietary ingredients, it does not need to be declared when it occurs naturally. You must list the dietary ingredients that have Daily Values in the same order as for the labels of conventional foods, except that vitamins, minerals and electrolytes are grouped together. This results in the following order for vitamins and minerals: Vitamin A, vitamin C, vitamin D, vitamin E, vitamin K, thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, biotin, pantothenic acid, calcium, iron, phosphorus, iodine, magnesium, zinc, selenium, copper, manganese, chromium, molybdenum, chloride, sodium, and potassium. You may use the following synonyms in parentheses after your dietary ingredients: Vitamin C ascorbic acid , thiamin vitamin B1 , riboflavin vitamin B2 , folate folacin or folic acid , and calories energy. Alternatively, you may list "folic acid" or "folacin" without parentheses in place of "folate. You must list the weight of calcium, rather than the weight of the calcium carbonate, the source ingredient, in the "Supplement Facts" panel. You may place the amount of your dietary ingredient in a separate column or immediately following the name of your dietary ingredient. Language consistent with the declaration of the serving size, such as "Each Tablet Contains" or "Amount Per 2 Tablets" may be used in place of the heading "Amount Per Serving. You may declare information on a "per unit" basis in addition to the required "per serving" basis. You may use additional columns when you have a product with different servings, such as one tablet in the morning and two at night. You must label the columns appropriately, e. For example, the amount of fat would be listed in terms of grams in both the "Nutrition Facts" and "Supplement Facts" panels. You should use the units of measurement given in 21 CFR See Appendix B for the daily values to be used for adults and children 4 or more years of age and Appendix C for the daily values to be used for infants, children less than 4 years of age, or pregnant or lactating women. In this calculation, you must use as the quantitative amount the unrounded amount, except that for total fat, saturated fat, cholesterol, sodium, potassium, total carbohydrate, and dietary fiber, you may use the quantitative amount by weight declared on the label i. Note: This does not pertain to dietary ingredients having RDIs because they may not be listed when present at less than 2 percent of the RDI. You may show more than one column. FDA has established four sets of Daily Values for many nutrients. Appendix B shows the Daily Values to be used for adults and children 4 or more years of age and Appendix C has the Daily Values to be used for children under 4 years of age, for infants, and for pregnant and lactating women. When you show more than one column, you must clearly identify each column e. RDIs or DRVs such as phosphatidylserine. You must list "other dietary ingredients" in the "Supplement Facts" panel following the listing of dietary ingredients having Daily Values. You must list "other dietary ingredients" by common or usual name in a column or linear display. FDA has not specified an order that you must follow. You must list the quantitative amount by weight per serving immediately following the name of the dietary ingredient or in a separate column. You must list liquid extracts using the volume or weight of the total extract and the condition of the starting material prior to extraction when it was fresh. You may include information on the concentration of the dietary ingredient and the solvent used, e. You must identify the solvent in either the nutrition label or ingredient list. For dietary ingredients that are extracts from which the solvent has been removed, you must list the weights of the dried extracts. You may list constituents of a dietary ingredient indented under the dietary ingredient and followed by their quantitative amounts by weight per serving. You may declare the constituents in a column or in a linear display. You must identify proprietary blends by use of the term "Proprietary Blend" or an appropriately descriptive term or fanciful name. On the same line, you must list the total weight of all "other dietary ingredients" contained in the blend. Indented underneath the name of the blend, you must list the "other dietary ingredients" in the blend, either in a column or linear fashion, in descending order of predominance by weight. These ingredients should be followed by a symbol referring to the footnote "Daily Value Not Established. The "Supplement Facts" nutrition information referred to as a panel must be enclosed in a box by using hairlines. The title, "Supplement Facts," must be larger than all other print in the panel and, unless impractical, must be set full width of the panel. The title and all headings must be bolded to distinguish them from other information. Except as provided for small and intermediate-sized packages, you must set information other than the title, headings, and footnotes in uniform type size no smaller than 8 point. You also must use a type size larger than all other print size in the nutrition label for the title "Supplement Facts. See the section on "Special Labeling Provisions" for the exceptions for small and intermediate-sized packages. Except for small and intermediate-sized packages, you must use a hairline rule that is centered between the lines of text to separate each dietary ingredient from the dietary ingredient above and beneath it. FDA has provided an exception for certain packages with space constraints. You are not required to follow Appendix B to Part Appendix B and its specifications are a model, which FDA has suggested in the interest of uniformity of presentation. For example, 21 CFR You may present the information for each packet e. For two packets, this would consist of five columns. List all of the dietary ingredients in the first column. List the amounts and percents of the morning packet in the second and third columns and similar information for the evening packet in the fourth and fifth columns see the illustration of aggregate nutrition labeling in 21 CFR FDA will collect a composite of 12 subsamples consumer packages or 10 percent of the number of packages in the same inspection lot, whichever is smaller. FDA will randomly select these packages. FDA may permit you to use an alternative means of compliance or additional exemptions in accordance with 21 CFR If your firm needs such special allowances, you must make your request in writing to the Office of Nutritional Products, Labeling, and Dietary Supplements HFS , Food and Drug Administration, Paint Branch Parkway, College Park, Maryland Products that contain less than this amount of such a dietary ingredient would be misbranded and in violation of the law. The two exemptions for small businesses and low-volume products a. and b. above are available to you only if your products' labels bear no claims or other nutrition information. Small packages are those packages having less than 12 square inches of total surface area available to bear labeling. In lieu of a "Supplement Facts" panel, you may print labels for small packages with a telephone number or address that consumers can use to obtain nutrition information. You may use a telephone number or an address in place of the "Supplement Facts" panel only if you place no claims or other nutrition information on the product label. You may use a type size no smaller than 4. You may use a tabular format on small packages. You also may present "Supplement Facts" information in a linear i. See 21 CFR Intermediate-sized packages are those packages having from 12 to 40 square inches of total surface area available to bear labeling. The "Supplement Facts" panel on the labels of intermediate-sized packages must use type size no smaller than 6 point, except that type no smaller than 4. Also, 4. Furthermore, the type size used in the "Supplement Facts" panel on an inner container may be as small as needed to accommodate all required information if the "Supplement Facts" on the outer container meets these type size requirements. You may use a tabular format on an intermediate-sized package if the package shape or size cannot accommodate vertical columns. You may use a linear format if the label will not accommodate a tabular format. You may use the abbreviations in 21 CFR g, "Serv size" for "Serving Size" and "Servings" for "Servings Per Container. You may use a row of dots connecting the columns containing the name of each dietary ingredient and the quantitative amount by weight and as a percent of Daily Value in the "Supplement Facts" panel on a small or an intermediate-sized package if you use the minimum type size and there is not sufficient space for you to use hairlines. On products for children less than 2 years of age, other than infant formula, you must not declare calories from fat, calories from saturated fat, saturated fat, polyunsaturated fat, monounsaturated fat, and cholesterol. You are not required to place the footnote on dietary supplements that is required by 21 CFR However, you are required to include the footnote "Percent Daily Values are based on a 2, calorie diet" when you declare total fat, saturated fat, total carbohydrate, dietary fiber, or protein. Editor's Note: This post was originally published in September It was updated in August with new examples, insights, and more accurate information. Disclaimer: The information in this article is intended to convey general information regarding beverage regulations and compliance. It does not constitute legal advice. This is for informational purposes only, and we strongly encourage you to seek independent legal counsel for advice on specific legal issues. Beverage Business Breakthrough. And get in touch with a beverage expert. We provide a full spectrum of services and sourcing capabilities to develop, produce and deliver your beverage vision from the first formula to full trucks of product. Supplement vs. Nutrition Facts Panels. Does Your Beverage Need a Supplement Facts Panel or Nutrition Facts Panel? Ingredients Ingredients in a product positioned as a beverage must be approved food additives or generally accepted as safe GRAS. Manufacturing Although the regulations regarding ingredients may be stricter for beverages, manufacturing regulations are more stringent for supplements. Claims Many regulations and guidelines surround the types of claims that are allowed on a product and how those claims can be made. Labeling There are several major differences between the Nutrition Facts Panel displayed on a beverage and a Supplement Facts Panel that appears on a supplement. Here are some of the ways the labels differ: A Nutrition Facts Panel must list certain nutrients, regardless of the amounts, whereas, on a Supplement Facts Panel, nutrients in zero amounts are not allowed to be listed on the label. A Nutrition Facts Panel lists only nutrients, vitamins, and minerals, whereas on a Supplement Facts Panel, all ingredients with nutritive value are listed. A dietary supplement disclaimer must appear on any dietary supplement label on which structure-function claims are made. Marketing and Advertising The area that is probably the most confusing for brands is the marketing and advertising practices that may cause a product to be deemed a beverage instead of a supplement or vice versa. Sources of Dietary Ingredients May list the source of a dietary ingredient Example : Hydrolyzed collagen from bovine hide Not required to list source of dietary ingredient if it is listed in the supplement facts panel Cannot list the source of a dietary ingredient Example: Collagen cannot say from grass-fed cows Part of Plant Must include the part of the plant the dietary ingredient is derived from by common or usual name. Example : Organic Ashwagandha Extract Withania Somnifera roots and leaves Cannot list part of the plant Example : Ashwagandha Zero Amounts Cannot list zero amounts of any nutrients. Must only show ingredients that are present in measurable amounts. FDA notification is not required Disclaimers are not required Proprietary Blends May list only the total amount of the blend Must list each ingredient Front Label Dietary Supplement Drink, Beverage, Water, etc. Have questions or want to learn more? Connect with a Label Expert Editor's Note: This post was originally published in September Let's make your beverage idea a reality. Contact Us And get in touch with a beverage expert. Get the BevReview Newsletter! Subscribe Now. Get Started BevSource Tasting Room. Get in Touch Contact Us. Ingredients must be dietary ingredients as defined by the Federal Food, Drug, and Cosmetic Act. May list the source of a dietary ingredient Example : Hydrolyzed collagen from bovine hide Not required to list source of dietary ingredient if it is listed in the supplement facts panel. Cannot list the source of a dietary ingredient Example: Collagen cannot say from grass-fed cows. Example : Organic Ashwagandha Extract Withania Somnifera roots and leaves. Cannot list part of the plant Example : Ashwagandha. Cannot list zero amounts of any nutrients. May focus on effects derived from non-nutritive and nutritive effects Example : Calcium builds strong bones Must submit FDA notification Must include disclaimer. Must only focus on effects derived from nutritive value. FDA notification is not required Disclaimers are not required. |
| Dietary Supplement Facts Panel vs. Nutritional Facts Panel | Summary The best diet for you is the one that works for you and you can stick to in the long term. Indented underneath the name of the blend, you must list the "other dietary ingredients" in the blend, either in a column or linear fashion, in descending order of predominance by weight. Also, be careful about giving supplements to a child, unless recommended by their health care provider. Sample Labels A Dietary supplement containing multiple vitamins see 21 CFR How must I list dried extracts? Supplement vs. |
0 thoughts on “Nutritional supplement facts”