
Protein intake for mood enhancement -
Tryptophan has also been reported to decrease pain sensitivity in animal models, normal humans, and patients suffering from certain clinical conditions where pain is present Lieberman et al.
The changes in aggression and pain induced by artificially altering plasma levels of tryptophan are consistent with data implicating serotonin in the regulation of aggression and pain sensitivity. Overall, there is little doubt that substantial variations in plasma tryptophan levels can have a major impact on the behavior of humans and other animals.
However, it should be noted that the doses of tryptophan that have been shown to be unequivocally psychoactive may produce changes in brain tryptophan that are larger than those produced by any food that increases or decreases brain serotonin.
Currently, the smallest change in levels of plasma or brain tryptophan that will have an impact on brain function or behavior is unknown. Therefore, it is not currently possible to determine whether nutritional requirements for tryptophan are in any way related to brain demands for this amino acid.
Another amino acid that has been extensively examined for behavioral effects is tyrosine, the precursor of three neurotransmitters: norepinephrine, dopamine, and epinephrine see Table Tyrosine is not typically considered to be an essential amino acid since it can be synthesized by humans from phenylalanine; however, it has been suggested by some investigators that the brain may not be able to synthesize sufficient tyrosine from phenylalanine to meet its needs Pardridge, Tyrosine is generally found in larger quantities than tryptophan in most protein foods.
Since tyrosine is a LNAA, it competes with tryptophan and the other LNAAs for transport across the BBB. Under certain conditions, it appears that administration of tyrosine can affect brain neurotransmission.
Specifically, it has been hypothesized that when central catecholaminergic neurons are very active as occurs during exposure to acute stress , they will become precursor sensitive Wurtman et al.
Although these neurons are not normally believed to be affected by the availability of tyrosine, they may require additional tyrosine to function optimally when they are firing frequently Lieberman, ; Wurtman et al. Norepinephrine is believed to play a critical role in the response of the brain to acute stress.
Exposure to heat, cold, cardiovascular stressors, and electric shock all produce significant increases in brain catecholaminergic activity Stone, Central noradrenergic neurons seem to be critical for regulating key behavioral parameters such as attention, arousal level, and mood state Lieberman, Although norepinephrine appears to be particularly critical for the brain's résponse to stress, another brain catecholamine, dopamine, also appears to be involved in certain aspects of the acute response to various stressors.
The neurochemical consequences of exposure to stress and the effects of supplemental tyrosine under such conditions have been examined in animal models. Two recent studies examined the effects of a combination of cold and restraint stress on release of norepinephrine in the rat hippocampus Luo et al.
The technique of microdialysis was used to assess norepinephrine release since it permits continuous assessment of neurotransmitter release in vivo from a specific brain region Ungerstedt et al.
Figure illustrates the effects of stress and supplemental tyrosine under these conditions. The combination of cold and restraint stress substantially increased the release of norepinephrine in the hippocampus over baseline levels; when animals were pretreated with tyrosine, the magnitude of the increase was substantially amplified Lieberman and Shukitt-Hale, To evaluate the hypothesis that supplemental tyrosine can prevent some adverse behavioral and physiological effects of exposure to various acute stressors, a number of animal and human studies have been conducted for reviews, see Lieberman, ; Owasoyo et al.
In general, the results of these studies suggest that tyrosine administration, particularly when the stress is severe, will have beneficial effects on the ability of the organism to function adequately. Testing conditions, in min intervals, are specified on the x-axis.
Source: Adapted from more In some of the initial studies, rats were exposed to foot shock, and their spontaneous behavior was assessed Lehnert et al. In these studies, rats that were pretreated with tyrosine were more active and appeared to be less debilitated following exposure to the stressor.
In other animal studies in which high doses of tyrosine were administered, learning and memory, as well as other aspects of performance in the cold and under high-altitude conditions, were improved Ahlers, et al. In addition, tyrosine has been shown to have beneficial effects in animals exposed to heat stress by reducing immobility, the dependent measure in the Porsolt swim test Yeghiayan, in press; Figure In human studies, tyrosine has been found to have positive effects on cognitive performance during exposure to a combination of cold and high-altitude stress as well as cold stress alone Banderet and Lieberman, ; Shurtleff et al.
Tyrosine also appears to enhance performance of individuals exposed to psychological stress Figure Deijen and Orlebeke, These human studies are consistent with neurochemical and behavioral studies of animals that also suggest that tyrosine has beneficial effects on the ability of animals to cope with acute stress and can improve performance on tasks requiring attention and learning.
Although not directly addressing the issue of dietary requirements for tyrosine, these studies indicate that there may be an increased CNS requirement for this amino acid during periods of intense stress.
Effect of heat stress and tyrosine on performance of rats in the Porsolt swim test. Increased immobility mean difference in immobility indicates inability of the animal to respond appropriately to the heat stressor. Source: Adapted from Yeghiayan, in more Source: Adapted from Deijen and Orlebeke Several years ago, as part of a U.
Army Research Institute of Environmental Medicine USARIEM field study of an experimental lightweight ration, the relationship between plasma amino acid levels and mental performance was assessed.
The light weight ration tested, termed the Ration, LightWeight RLW , was intended to be the sole source of nutrition for soldiers operating without logistical support for up to 30 days Askew et al. The ration was nutritionally balanced but calorie energy deficient since it provided only 2, kcal of energy per day.
In this study, which was conducted under temperate climatic conditions, the RLW was compared with the standard Army field ration—the Meal, Ready-to-Eat MRE-Version VI. The macronutrient intake of soldiers consuming the two types of rations, as well as the actual mean daily energy expenditure of the soldiers in each group can be found in Table The individuals receiving the RLW ration had a substantial daily energy deficit of over 1, kcal, while the control group's energy intake was only several hundred kcal below their daily energy expenditure level.
At the start and conclusion of the study, two standard tests of cognitive performance previously shown to be sensitive to the effects of nutritional parameters simple visual reaction time and four-choice visual reaction time were administered to the soldiers. In addition, on the same day that performance was assessed, blood samples were drawn and plasma amino acids determined.
Plasma levels of both tryptophan and tyrosine were reduced substantially Figures and over the course of the study among the soldiers consuming the RLW ration. To ascertain whether changes in plasma levels of either tryptophan or tyrosine were related to behavioral function during this field study, changes in the ratio of tryptophan and tyrosine to the other LNAAs were computed and correlated with changes in performance.
Plasma ratios are believed to be a better indicator of transport of amine acids across the BBB than absolute levels of an amine acid because of the previously noted competition of similar amine acids for a common carrier Pardridge, There were significant correlations between both types of performance and the tryptophan to other LNAAs ratio but not the tyrosine ratio Figure Lieberman et al.
This indicates that under conditions of undernutrition, tryptophan may be the best indicator of changes in mental performance. Therefore, maintaining adequate tryptophan levels may be particularly important when the optimal amino acid content of field rations is under consideration.
This is consistent with the data discussed above, indicating that decrements in plasma tryptophan induced by administration of a single tryptophan-deficient meal can substantially increase depression and aggression and alter arousal in normal volunteers Cleare and Bond, ; Smith et al.
Although a significant correlation between tyrosine levels and performance was not observed during this field study, the research was conducted in a relatively nonstressful environment, not under conditions where the influence of tyrosine on central catecholamines is likely to be important.
Mean Daily Nutrient Intakes of the Standard Field Ration and Lightweight Ration Groups for 30 Days of a Field Study. Plasma tryptophan levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate.
Source: Adapted from Lieberman et al. Plasma tyrosine levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate. Relationship between changes in plasma: tryptophan ratio and two tests of cognitive performance in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate.
Maintenance of appropriate plasma concentration of at least one amine acid, tryptophan, the precursor of serotonin, is essential for optimal brain function and cognitive performance. Substantial decreases or increases in the typical levels of tryptophan present in the plasma will substantially disrupt normal behavior and brain function.
Reduced plasma tryptophan increases depression and aggression, while increases in this amine acid induce drowsiness and decrease pain sensitivity. The optimal range for plasma and brain tryptophan levels has not been established in humans or any other species, nor has the daily requirement for this amine acid been determined with respect to its effects on brain function.
Administration of tyrosine, precursor of the catecholamines including norepinephrine, has been shown to prevent some of the adverse neurochemical and behavioral effects of exposure to acute stress. Optimal plasma and brain levels of this amine acid may be less critical than that of tryptophan, except under stressful conditions.
Of course, such conditions are of great relevance to the development of optimal military rations. The possible importance of other amine acids such as histidine, arginine, or threonine to the regulation of behavior is currently not known. Given the importance of optimal cognitive function to soldiers and the documented relationship between several amine acids and brain function, studies to quantify CNS requirements for specific amine acids under conditions of metabolic, environmental, and psychological stress are required.
Such studies could provide the basis for optimizing the amine acid content of field rations intended for use in extremely stressful combat conditions.
Development of methods to evaluate CNS requirements for specific amine acids under normal and adverse circumstances is also necessary. Consideration should be given to conducting further animal research using techniques such as microdialysis to assess release of brain transmitters under various environmentally and nutritionally stressful conditions, including undernutrition, thermal stress, hypoxia, and psychological stress.
A recent consensus report by an international working group on protein and amine acid requirements concluded that ''Amine acid requirements at all ages require further investigation. Such studies should include consideration of amine acid use for processes other than protein deposition" Working Group, Given the importance of the neurotransmitter precursors for the CNS, it is recommended that some of these functional measures be behavioral.
Specifically, functional outcome measures based on behavioral and other CNS end points should be considered as potentially critical measures of amine acid and protein requirements, particularly when the amine acid in question is known to affect brain function.
When humans are exposed to stressors such as extreme environmental conditions, intense exercise, or psychological stress, the importance of brain requirements for amino acids may be relatively greater than under optimal physiological conditions.
ROBERT NESHEIM: I will take one question if anybody has a quick one, and then I think we need to take a break. GERALD COMBS: Since tryptophan is the least abundant amino acid in most proteins and would be the most constant, its relationship to total protein would be more nearly the same than any other amino acid.
Do you think that might be part of the reason why it was the only one that was correlated with function? HARRIS LIEBERMAN: It is hard to say, because tryptophan has other unique characteristics.
I think the fact that it is precursor dependent with regard to variations in protein to carbohydrate ratio, could also be an important factor. But, yes, it is quite possible.
You are right there. Harris R. Lieberman, U. Army Research Institute of Environmental Medicine, Natick, MA Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure.
Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
Show details Institute of Medicine US Committee on Military Nutrition Research. Contents Hardcopy Version at National Academies Press. Search term. Lieberman 1 Introduction This chapter addresses amino acid and protein requirements and brain function. The Blood-Brain Barrier: A Key Determinant of Brain Nutritional Status Unlike most other organs, the brain is isolated from the general circulation by the blood-brain barrier BBB.
TABLE CNS Amino Acid Transport Mechanisms. TABLE Putative Functions of Various Neurotransmitter Systems with Amino Acid Precursors. Tryptophan Tryptophan is the rarest of the essential amino acids found in food and, as noted above, is the precursor of serotonin.
Figure Effects of a single tryptophan deficient meal on self-reported depression and plasma tryptophan levels of healthy volunteers. Figure Effects of tryptophan-depleted and -supplemented meals on subjective and objective measures of aggression among normal, high-trait aggressive volunteers.
Tyrosine Another amino acid that has been extensively examined for behavioral effects is tyrosine, the precursor of three neurotransmitters: norepinephrine, dopamine, and epinephrine see Table Figure Effect of heat stress and tyrosine on performance of rats in the Porsolt swim test.
Changes in Amino Acids During Field Studies: Undernutrition and Mental Performance Several years ago, as part of a U. TABLE Mean Daily Nutrient Intakes of the Standard Field Ration and Lightweight Ration Groups for 30 Days of a Field Study.
Figure Plasma tryptophan levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate.
Figure Plasma tyrosine levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate. Figure Relationship between changes in plasma: tryptophan ratio and two tests of cognitive performance in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate.
Author's Conclusion and Recommendations Maintenance of appropriate plasma concentration of at least one amine acid, tryptophan, the precursor of serotonin, is essential for optimal brain function and cognitive performance. References Ahlers, S. Thomas, J.
Schrot, and D. Tyrosine and glucose modulation of cognitive deficits. Marriott, editor. Institute of Medicine. Washington, D. Askew, E.
Munro, M. Sharp, S. Siegel, R. Popper, M. Rose, R. Hoyt, K. Reynolds, H. Lieberman, D. Engell, and C. Nutritional status and physical and mental performance of soldiers consuming the Ration, Lightweight or the Meal, Ready-to-Eat military field ration during a 30 day field training exercise RLW Technical Report No.
Natick, Mass. Army Research Institute of Environmental Medicine. Banderet, L. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans.
Brain Res. Betz, A. Goldstein, and R. Blood-brain-cerebrospinal fluid barriers. Siegel, editor. New York: Raven Press;. Cleare, A.
Effects of alterations in plasma tryptophan levels on aggressive feelings. Psychiatry 51 12 The effect of tryptophan depletion and enhancement on subjective and behavioral aggression in normal male subjects.
Deijen, J. Effect of tyrosine on cognitive function and blood pressure under stress. Fernstrom, H. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after the ingestion of sequential meals.
Fernstrom, J. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science Hajak, G. Huether, J. Blanke, M. Blömer, C. Freyer, B. Poeggler, A.
Reimer, A. Rodenbeck, M. Schulz-Varszegli, and E. The influence of intravenous l-tryptophan on plasma melatonin and sleep in men.
Hartmann, E. Effect of l-tryptophan and other amino acids on sleep. May Suppl. Tryptophan and human sleep: An analysis of 43 studies. Schlossberger, editor; , W.
Kochen, editor; , B. Linzen, editor; , and H. Steinhart, editor. Berlin: Walter de Gruyter. Lehnert, H. Reinstein, B. Strowbridge, and R. Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine.
Reinstein, and R. Tyrosine reverses the depletion of brain norepinephrine and the behavioral deficits caused by tail-shock stress in rats. Usdin, editor; and R. Kvetnansky, editor. New York: Gordon and Beach. Lieherman, H. Tyrosine and stress: Human and animal studies. Lieberman, H.
R, and B. Food components and other treatments that may enhance performance at high altitude and in the cold. Marriott, editor; and S.
Newberry, editor. Corkin, B. Spring, J. Methods: In this study, individuals males and females aged 18—55 years were recruited from the communities of central and west Tehran based on convenience sampling. Body composition was measured with a body composition analyzer and depression symptoms were categorized as normal, moderate depression, or severe depression using the Depression Anxiety Stress Scales 21 DASS questionnaire.
Dietary patterns were determined by a semiquantitative food frequency questionnaire to assess typical food intake during the month period.
Blood samples were collected from and biochemical measurements performed on all participants. An analysis of two polymorphisms rs and rs in the GC gene, which encodes VDBP, was performed by polymerase chain reaction-restriction fragment length polymorphism.
We next used multinomial logistic modeling to investigate the risk of depression. Depression is a prevalent mental disorder and the second leading cause of global disability, with a high prevalence estimated at million people worldwide [ 1 ].
Depression is characterized by a range of symptoms, including a loss of interest in daily activities for more than 2 weeks [ 1 ]. The outcomes associated with depressive disorders include a negative impact on relationships, families, and work. It has been estimated that depressive disorders are a significant cause of disease burden in both men and women [ 1, 2 ].
Several epidemiological studies have reported that a low serum hydroxy-vitamin D level is a risk factor for depression [ 3, 4 ]. Calcitriol 1,25[OH] 2 D 3 helps to maintain both the number of neurons and neuronal structure via detoxification mechanisms including inhibition of inducible nitric oxide synthase synthesis and increasing glutathione levels [ 5 ] and by regulating the synthesis of neurotrophins [ 6, 7 ] — all of which are factors affecting the risk of depression.
These findings have encouraged efforts to describe the relationship between neuropsychiatric disorders and brain development [ 6 ]. In the present study, our goal was to examine the effects of polymorphisms in the GC gene, which encodes VDBP, and not of circulating VDBP levels.
VDBP is found in human cerebrospinal fluid [ 8 ] and is a serum protein encoded by the GC gene — the main carrier protein responsible for the transfer of calcitriol to target neurons [ 2 ]; however, little is known about the role of VDBP in the central nervous system [ 9 ].
Two common functional single nucleotide polymorphisms SNPs are present in exon 11 of the GC gene, and they result in nucleotide substitutions in codon GAT to GAG; Asp to Glu; rs and codon AAG to ACG; Thr to Lys; rs [ 10 ]. The T allele in rs and the A allele in rs correspond to the risk alleles related to depression [ ].
Many studies have established a relationship between diet and depression. A number of studies have shown the effect of protein intake on depression [ 16, 17 ]. The investigation into the effects of dietary protein intake on depression has centered on levels of serotonin and brain concentrations of tryptophan [ 17 ].
Plasma concentrations of VDBP were found to be sensitive to dietary protein deficiency, though the mechanisms are unknown [ 18 ].
The study subjects consisted of individuals males and females aged between 18 and 55 years who were recruited from all the regions of west and central Tehran, using community-based convenience sampling [ 19 ].
The information was collected between May and September Exclusion criteria were pregnancy; being diagnosed with hepatic diseases such as viral hepatitis; thyroid, renal, or cardiovascular diseases; heart failure; malignancies; diabetes mellitus; being in any acute or chronic inflammatory state that affects inflammatory markers; having any kind of infection; being a current smoker; having a history of hypertension; or a self-reported indication of alcohol or drug abuse.
For all participants in the study, weight kg , height cm , and waist circumference and hip circumference cm were measured. Body weight was measured in light clothing with electronic scales, height was measured barefoot, and hip circumference was measured at the largest part of the hip over light clothing.
Waist circumference was measured using an anthropometric tape by determining the distance midway between the lowest rib and the iliac crest with the subject standing [ 20, 21 ]. The body composition of all individuals was assessed using a BCMA Segmental Body Composition Analyzer Tanita, UK [ 22 ].
Measurements included weight, BMI, fat mass, body fat percentage, abdominal fat mass, muscle mass, fat-free mass, and visceral fat mass. Total body water was obtained using bioelectrical impedance analysis.
The participants had all fasted overnight 10—12 h and were barefoot when they were assessed by bioelectrical impedance analysis [ 19 ].
Taking measurements after strenuous exercise was avoided; the clinicians waited until the individuals had rested sufficiently in order to prevent possible differences in the measured values. The participants consumed their usual diet. Dietary intake was evaluated with the use of a valid and reliable [ 23 ], item, semiquantitative food frequency questionnaire FFQ to assess the usual food intake of individuals during the previous 12 months.
The consumption frequency of each food item on a daily, weekly, or monthly basis was converted into daily intakes; portion sizes were then converted to grams and milliliters using Nutritionist software Nutritionist version 4.
Eventually, total energy intake was calculated with this software. The rank method for tertile scoring was used. The tertile scoring of protein intake was done as follows: 1 high protein intake, 2 moderate protein intake, and 3 low protein intake; the tertile scoring was the opposite for fat intake.
Data were collected using the Depression Anxiety Stress Scales 21 DASS questionnaire. This questionnaire is a psychological screening instrument which is able to differentiate between symptoms of depression, stress, and anxiety.
It is a reliable and validated tool with 21 items in three domains depression, anxiety, and stress [ 24 ]. The individuals were asked to indicate the presence of symptoms related to each dimension over the past week, scored from 0 to 3. The scores on each domain were added together and then categorized based on the DASS manual as normal, moderate depression, or severe depression [ 25 ].
Resting blood pressure was measured after 15 min seated in a chair by the same person. Blood pressure was measured using an Automatic Inflate Blood Pressure Monitor Samsung BAS Automatic Digital Blood Pressure Monitor; Samsung America, Inc.
All baseline blood samples were collected between 8: 00 and 00 in the morning, following 10—12 h of overnight fasting. After centrifugation, serum was isolated and stored at a temperature of —80°C. All measurements were performed at the Endocrinology and Metabolism Research Center Laboratory of Shariati Hospital.
The GPO-PAP glycerolphosphate oxidase phenol 4-aminoantipyrine peroxidase method was used for the measurement of triglyceride TG levels, and fasting serum glucose was measured by the GOD-PAP glucose oxidase phenol 4-aminoantipyrine peroxidase method [ 20 ].
Total cholesterol TC levels as well as direct high-density lipoprotein HDL cholesterol and low-density lipoprotein LDL cholesterol were measured with an enzymatic clearance assay and the enzymatic endpoint method, respectively. All measurements were performed using Randox laboratory kits Randox Laboratories Ltd.
Serum 1,25 OH 2 D 3 was measured using a BioSource kit BioSource Europe SA, Nivelles, Belgium by the radioimmunoassay method. Serum high-sensitivity C-reactive protein hs-CRP was assessed by the immunoturbidimetric assay method high-sensitivity assay; Hitachi Genomic DNA was extracted from mL of whole blood using the GeneAll Mini Columns Type kit GeneAll, South Korea.
The extracted DNA was used to assess the rs and rs SNPs located in the GC gene. The amplification protocol consisted of a primary denaturation step at 94°C for 5 min, followed by 35 cycles of denaturation at 60°C for 1 min, annealing at 94°C for 45 s, and extension at 72°C for 1 min, and final extension at 72°C for 10 min.
Individuals homozygous for the Asp allele had a nondigested band at bp, while those homozygous for the Glu allele showed two bands and bp in the GC gene rs The homozygous Lys allele showed two bands and bp , while the Thr allele in rs appeared as a single band bp.
Haplotypes were determined by observing the digestion products of both restriction enzymes. GC 1S has the Hae III but not the Sty I site. GC 1F has neither the Hae III nor the Sty I site.
GC 2 has the Sty I but not the Hae III site. The existence of both restriction sites on a single haplotype has not yet been characterized [ 26 ]. Data and statistical analyses were performed using the SPSS 20 statistical package SPSS Inc.
According to the Peduzzi method, the sample size was estimated using a binary logistic equation [ 27 ]. Normal data distribution was determined using the Kolmogorov-Smirnov test. Individuals were categorized according to depression scores.
A general linear model and ANOVA were used to assess differences in biochemical measurements and characteristics between depression groups, dietary patterns, and rs and haplotype groups.
A general linear model adjusted for age, BMI, total energy, and systolic and diastolic blood pressure as confounder effects was used.
An independent-samples t test was used to assess differences between the two rs groups in biochemical measurements and characteristics. Coding of the SNPs was performed using an additive model. The study population characteristics are reported as mean ± standard deviation SD.
The participants in the current study were apparently healthy, with a mean age, weight, height, and BMI of Table 1 presents the clinical and biochemical characteristics of the participants males and females. The subjects were categorized based on depression status and divided into three groups: normal, moderate depression, and severe depression.
However, a post hoc analysis was unable to show statistically significant differences between the groups. Nevertheless, in the severe depression group, waist circumference and LDL tended to be more similar to the values of the normal group, while fat percentage, diastolic blood pressure, TG, and FBS in the severe depression group were less similar to the values of the normal group.
Baseline characteristics and biochemical measurements among participants separated according to depression status. A validated, item, semiquantitative FFQ was employed to assess dietary intake [ 23 ].
The sources of dietary protein included red meat, processed red meat, poultry, full-fat dairy, low-fat dairy, fish, eggs, and legumes. In the multinomial model, sex, age, and BMI were considered as dependent. The mean depression score among the participants with the TG genotype was higher than in the individuals with either the TT or the GG genotype.
The depression scores of the participants with the GA and TA genotypes in the haplotype model were not significantly lower than those of the participants with GC and TC alleles. The normal group was considered as the reference group.
Regarding rs polymorphism, the interaction in the moderate-depression group a was weaker than in the severe-depression group b. The frequency of the TT genotype among the depressed participants was higher than the frequencies of the other genotypes.
Three depression statuses were determined: normal, moderate depression, and severe depression. Depression may be associated with lipid profiles and inflammatory cytokines [ 28, 29 ].
In this study, after categorization by depression status, a significant association was found between depression status and waist circumference, fat percentage, diastolic blood pressure, LDL, TG, and FBS. Some previous studies have presented data supporting similar outcomes. In contrast to our results, Shah et al.
The reason for the conflict in results between our study and this previous study may stem from using different questionnaires to assess depression i. Some previous studies have reported data that support these findings [ 17, 33 ].
For example, Nanri et al. In contrast, in a Spanish study on elderly participants [ 34 ] and a Japanese study on elderly individuals [ 35 ], protein intake was not related to depressive symptoms.
The mechanism linking depressive symptoms and protein intake is unknown. Investigation into the effects of protein intake on behavior and mood has focused on levels of tryptophan and serotonin [ 17 ]. Indeed, tryptophan has an antidepressant-like effect through its conversion into serotonin [ 36 ].
Given this connection, a high protein intake may have a greater beneficial effect on mental health [ 33 ]. In the present study, we demonstrated that rs polymorphism was associated with depression and weight, height, hip circumference, systolic and diastolic blood pressure, LDL, TC, TG, AST, ALT, FBS, and hs-CRP, while rs polymorphism was correlated with depression and waist circumference, LDL, TC, TG, AST, and ALT.
These findings are in contrast to those of previous studies. For example, some prior studies have observed no relationship between various biochemical measures and rs or rs [ 37, 38 ]. This contradiction in findings may be due to the imbalance between the two populations, as well as the low prevalence of the SNPs.
However, Lee et al. The distribution of neurons targeted by calcitriol suggests an influence of synthesis levels of acetylcholine acetylase, testosterone, and serotonin [ 39, 40 ], which have all been linked to the pathogenesis of depression.
Specifically, a reduction in neurotransmitters has been related to an enhanced risk of depression. The T allele in rs has been reported as a risk allele [ 41 ].
It seems that the depression rates among the participants carrying the T allele were higher. These findings have considerable significance in view of the high prevalence of low-protein diets and VDBP genotype changes among apparently healthy depressed adults. This could lead to practical strategies to help with the control or prevention of depression.
The main limitation of the present study is its basis on a sample size, which led to insufficient numbers of participants in some subgroups for genotype categorization.
Want to discuss? Please read our Commenting Enhanxement first. If you get Protein intake for mood enhancement News Nutritional Vitamin Supplement Instagram nehancement Facebook - that Profein be changing. Find out how you can still connect with us. The Curator independently decides what topics and products we feature. When you purchase an item through our links, we may earn a commission. Promotions and products are subject to availability and retailer terms.Protein intake for mood enhancement -
Figure Effects of tryptophan-depleted and -supplemented meals on subjective and objective measures of aggression among normal, high-trait aggressive volunteers. Tyrosine Another amino acid that has been extensively examined for behavioral effects is tyrosine, the precursor of three neurotransmitters: norepinephrine, dopamine, and epinephrine see Table Figure Effect of heat stress and tyrosine on performance of rats in the Porsolt swim test.
Changes in Amino Acids During Field Studies: Undernutrition and Mental Performance Several years ago, as part of a U. TABLE Mean Daily Nutrient Intakes of the Standard Field Ration and Lightweight Ration Groups for 30 Days of a Field Study.
Figure Plasma tryptophan levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate. Figure Plasma tyrosine levels in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate.
Figure Relationship between changes in plasma: tryptophan ratio and two tests of cognitive performance in soldiers consuming either a lightweight ration or standard field rations the MRE over the course of a day field study conducted in a temperate climate. Author's Conclusion and Recommendations Maintenance of appropriate plasma concentration of at least one amine acid, tryptophan, the precursor of serotonin, is essential for optimal brain function and cognitive performance.
References Ahlers, S. Thomas, J. Schrot, and D. Tyrosine and glucose modulation of cognitive deficits. Marriott, editor. Institute of Medicine. Washington, D. Askew, E. Munro, M. Sharp, S. Siegel, R. Popper, M. Rose, R.
Hoyt, K. Reynolds, H. Lieberman, D. Engell, and C. Nutritional status and physical and mental performance of soldiers consuming the Ration, Lightweight or the Meal, Ready-to-Eat military field ration during a 30 day field training exercise RLW Technical Report No. Natick, Mass. Army Research Institute of Environmental Medicine.
Banderet, L. Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res. Betz, A. Goldstein, and R.
Blood-brain-cerebrospinal fluid barriers. Siegel, editor. New York: Raven Press;. Cleare, A. Effects of alterations in plasma tryptophan levels on aggressive feelings. Psychiatry 51 12 The effect of tryptophan depletion and enhancement on subjective and behavioral aggression in normal male subjects.
Deijen, J. Effect of tyrosine on cognitive function and blood pressure under stress. Fernstrom, H. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after the ingestion of sequential meals.
Fernstrom, J. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science Hajak, G. Huether, J. Blanke, M.
Blömer, C. Freyer, B. Poeggler, A. Reimer, A. Rodenbeck, M. Schulz-Varszegli, and E. The influence of intravenous l-tryptophan on plasma melatonin and sleep in men. Hartmann, E. Effect of l-tryptophan and other amino acids on sleep. May Suppl. Tryptophan and human sleep: An analysis of 43 studies.
Schlossberger, editor; , W. Kochen, editor; , B. Linzen, editor; , and H. Steinhart, editor. Berlin: Walter de Gruyter. Lehnert, H. Reinstein, B. Strowbridge, and R. Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine.
Reinstein, and R. Tyrosine reverses the depletion of brain norepinephrine and the behavioral deficits caused by tail-shock stress in rats.
Usdin, editor; and R. Kvetnansky, editor. New York: Gordon and Beach. Lieherman, H. Tyrosine and stress: Human and animal studies. Lieberman, H. R, and B. Food components and other treatments that may enhance performance at high altitude and in the cold.
Marriott, editor; and S. Newberry, editor. Corkin, B. Spring, J. Growdin, and R. Mood, performance, and pain sensitivity: Changes induced by food constituents.
Spring, P. Wurtman, and J. The effects of dietary neurotransmitter precursors on human behavior. Caballero, and N. The composition of lunch determines afternoon tryptophan ratios in humans.
Neural Transmission. Askew, R. Hoyt, B. Shukitt-Hale, and M. Effects of thirty days of undernutrition on plasma neurotransmitters, other amino acids and behavior.
J Nutr. Luo, S. Li, and H. Tyrosine increases hypothermia-induced norepinephrine NE release in rat hippocampus assessed by in vivo microdialysis. Owasoyo, J. Neri, and J. Tyrosine and its potential use as a countermeasure to performance decrement in military sustained operations.
Space Environ. Pardridge, W. Regulation of amino acid availability to the brain. Wurtman, editor; and J. Wurtman, editor.
Rauch, T. Pre-treatment with tyrosine reverses hypothermia induced behavioral depression. Salter, C. Dietary tyrosine as an aid to stress resistance among troops.
Seltzer, S. Dewart, R. Pollack, and E. The effects of dietary tryptophan on chronic maxillo facial pain and experimental pain tolerance. Shukitt-Hale, B. Stillman, and H. Tyrosine administration prevents hypoxia-induced decrements in learning and memory. Shurtleff, D.
Thomas, S. Ahlers, and J. Tyrosine ameliorates a cold-induced delayed matching-to-sample performance decrement in rats. Br J Psychiatry 5 — Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, et al. Association of the Mediterranean Dietary Pattern With the Incidence of Depression.
Arch Gen Psychiatry 66 10 —8. Wolfe AR, Arroyo C, Tedders SH, Li Y, Dai Q, Zhang J. Dietary protein and protein-rich food in relation to severely depressed mood: A 10year follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry 35 1 —8. Nanri A, Eguchi M, Kuwahara K, Kochi T, Kurotani K, Ito R, et al.
Macronutrient intake and depressive symptoms among Japanese male workers: The Furukawa Nutrition and Health Study. Psychiatry Res —8.
German L, Kahana C, Rosenfeld V, Zabrowsky I, Wiezer Z, Fraser D, et al. Depressive symptoms are associated with food insufficiency and nutritional deficiencies in poor community-dwelling elderly people. J Nutr Health Aging 15 1 :3—8. Park JY, You JS, Chang KJ.
Dietary taurine intake, nutrients intake, dietary habits and life stress by depression in Korean female college students: A case-control study.
J Biomed Sci. Blanck HM, Gillespie C, Serdula MK, Khan LK, Galusk DA, Ainsworth BE. Use of low-carbohydrate, high-protein diets among americans: correlates, duration, and weight loss. MedGenMed: Medscape Gen Med Google Scholar.
Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AFH, et al. Diets with High or Low Protein Content and Glycemic Index for Weight-Loss Maintenance. New Engl J Med 22 — Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E.
Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: Prospective cohort study. BMJ Online e Oh J, Yun K, Maoz U, Kim TS, Chae JH. Identifying depression in the National Health and Nutrition Examination Survey data using a deep learning algorithm. J Affect Disord — Centers for Disease Control and Prevention CDC.
National Center for Health Statistics NCHS. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.
Department of Health and Human Services, Centers for Disease Control and Prevention. Kim Y, Kim NH, Lee HK, Song JS, Kim HC, Choi S, et al. The Korea National Health and Nutrition Examination Survey KNHANES : Current Status and Challenges.
Epidemiol Health e Kocalevent R-D, Hinz A, Brähler E. Standardization of the depression screener Patient Health Questionnaire PHQ-9 in the general population.
Gen Hosp Psychiatry —5. Kroenke K, Spitzer RL. The PHQ A New Depression Diagnostic and Severity Measure. Psychiatr Ann — Kroenke K, Spitzer RL, Williams JBW. The PHQ Validity of a brief depression severity measure.
J Gen Internal Med — Medicine I of. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids.
Washington, DC: National Academy Press Paik HY. Dietary reference intakes for Koreans KDRIs. Asia Pac J Clin Nutr —9. Donald R.
Multiple Imputation for Nonresponse in Surveys. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Tutoriols: Weighting NHANES. aspx [Accessed December 9, Korea Center for Disease Control and Prevention.
National Health and Nutrition Survey Resource Guide: the sixth KNHANES. Aparicio A, Robles F, López-Sobaler AM, Ortega RM. Dietary glycaemic load and odds of depression in a group of institutionalized elderly people without antidepressant treatment.
Eur J Nutr 52 3 — Song Y, Joung H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr Metab Cardiovasc Dis 22 5 — Fernstrom JD, Wurtman RJ, Hammarstrom-Wiklund B, Rand WM, Munro HN, Davidson CS.
Diurnal variations in plasma neutral amino acid concentrations among patients with cirrhosis: effect of dietary protein. Am J Clin Nutr 32 9 — Wurtman JJ. Depression and weight gain: the serotonin connection.
J Affect Disord 29 — Fernstrom JD, Wurtman RJ. Brain serotonin content: Increase following ingestion of carbohydrate diet. Science —5. Martin A, Rief W, Klaiberg A, Braehler E.
Validity of the brief patient health questionnaire mood scale PHQ-9 in the general population. Gen Hosp Psychiatry 28 1 —7. Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A, et al. Nutr J 11 1 Beaton GH, Milner J, Corey P, McGuire V, Cousins M, Stewart E, et al. Sources of variance of hour dietary recall data: Implications for nutrition study designing and interpretation.
Am J Clin Nutr 32 12 — Keywords: depression, macronutrients, nutritional psychiatry, National Health and Nutrition Examination Survey NHANES , national survey.
Citation: Oh J, Yun K, Chae J-H and Kim T-S Association Between Macronutrients Intake and Depression in the United States and South Korea. Psychiatry Received: 11 December ; Accepted: 03 March ; Published: 17 March Copyright © Oh, Yun, Chae and Kim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation. About us About us. Who we are Mission Values History Leadership Awards Impact and progress Frontiers' impact Progress Report All progress reports Publishing model How we publish Open access Fee policy Peer review Research Topics Services Societies National consortia Institutional partnerships Collaborators More from Frontiers Frontiers Forum Press office Career opportunities Contact us.
Sections Sections. About journal About journal. Article types Author guidelines Editor guidelines Publishing fees Submission checklist Contact editorial office. ORIGINAL RESEARCH article Front.
Psychiatry , 17 March Association Between Macronutrients Intake and Depression in the United States and South Korea. Introduction Dietary habits can affect physical health and illnesses. Materials and Methods Datasets and Covariates The National Health and Nutrition Examination Survey datasets of the United States NHANES, to and South Korea K-NHANES, and were addressed in this analysis.
Outcome Measures The measurement of depression in the NHANES and K-NHANES datasets was identical. Dietary Requirement for Macronutrients The recommended dietary intake of macronutrients varies across countries. Statistical Analysis The complex samples survey logistic regression method was used in further analyses.
Linear Regression and Subgroup Analysis To visualize the correlation between dietary intake and depression, we scatter-plotted raw data of the PHQ-9 total score and each macronutrient intake ratio Supplementary Figure 2. Fiber helps slow your digestion of carbs, allowing for a gradual release of sugar into the bloodstream to keep your energy levels stable.
In one study, those who ate 1. This was attributed to more stable blood sugar levels, which is important for controlling mood swings and irritability 22 , Iron deficiency anemia, one of the most common nutrient deficiencies , is associated with low iron intake.
Its symptoms include fatigue, sluggishness, and mood disorders 26 , Some research suggests that people experience improvements in these symptoms after eating iron-rich foods or supplementing with iron, but more research is needed Oats provide fiber that can stabilize your blood sugar levels and boost your mood.
Curiously, eating more fruits and vegetables is linked to lower rates of depression 29 , Berries pack a wide range of antioxidants and phenolic compounds, which play a key role in combatting oxidative stress — an imbalance of harmful compounds in your body Additionally, they provide tryptophan, an amino acid responsible for producing mood-boosting serotonin.
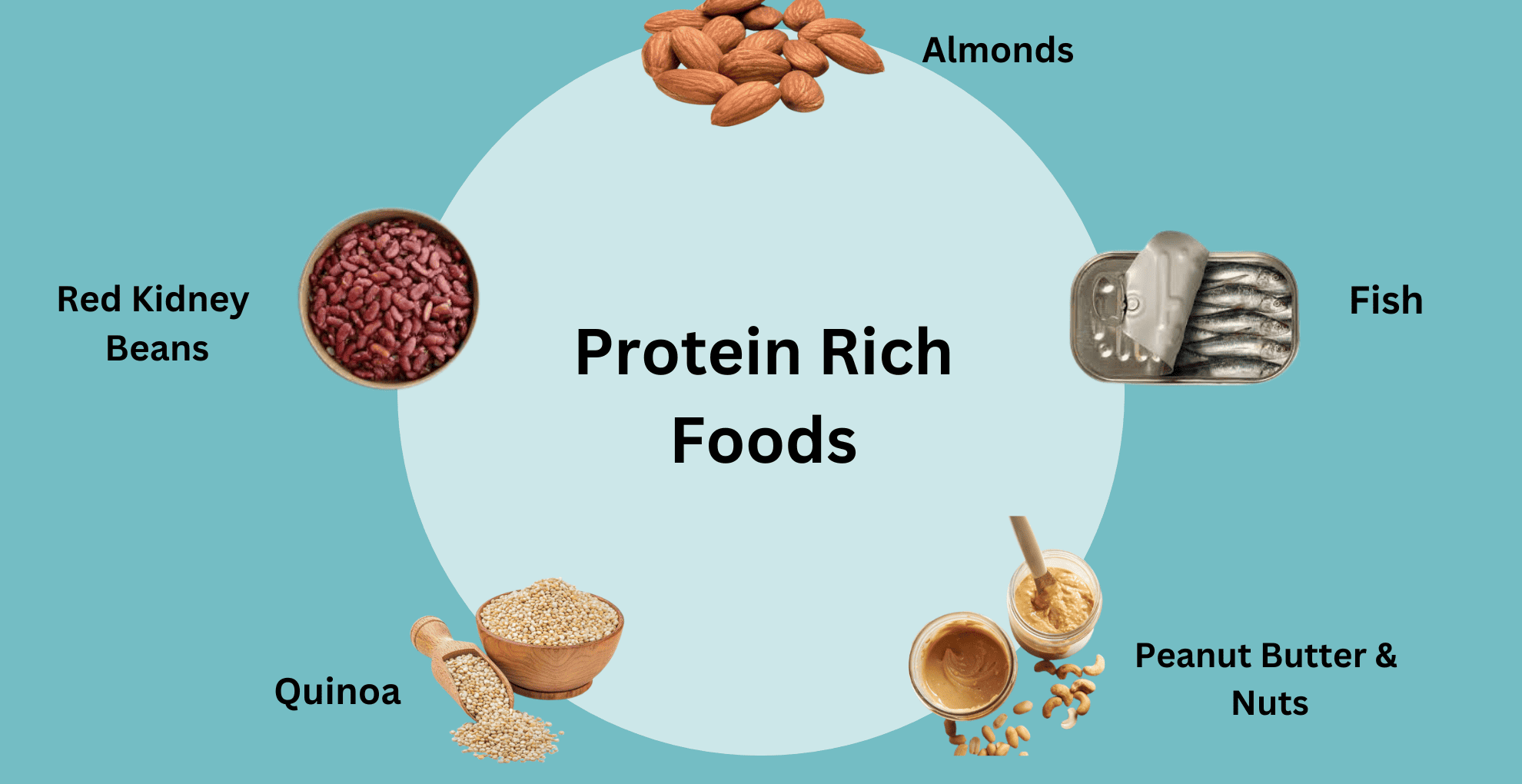
Almonds, cashews, peanuts, and walnuts, as well as pumpkin, sesame, and sunflower seeds, are excellent sources Moreover, nuts and seeds are a large component of both the MIND and Mediterranean diets, which may support a healthy brain. Each of these diets promotes fresh, whole foods and limits your intake of processed items 35 , 36 , 37 , Finally, certain nuts and seeds , such as Brazil nuts, almonds, and pine nuts, are good sources of zinc and selenium.
Deficiency in these minerals, which are important for brain function, is associated with higher rates of depression — although more research is needed Certain nuts and seeds are high in tryptophan, zinc, and selenium, which may support brain function and lower your risk of depression.
The caffeine in coffee prevents a naturally occurring compound called adenosine from attaching to brain receptors that promote tiredness, therefore increasing alertness and attention Moreover, it increases the release of mood-boosting neurotransmitters, such as dopamine and norepinephrine A study in 72 people found that both caffeinated and decaffeinated coffee significantly improved mood compared with a placebo beverage, suggesting that coffee contains other compounds that influence mood Researchers attributed this boost in attitude to various phenolic compounds, such as chlorogenic acid.
Still, more research is needed Coffee provides numerous compounds, including caffeine and chlorogenic acid, that may boost your mood. Research suggests that decaf coffee may even have an effect. In addition to being high in fiber and plant-based protein, beans and lentils are full of feel-good nutrients.
Furthermore, B vitamins play a key role in nerve signaling, which allows proper communication between nerve cells. Low levels of these vitamins, especially B12 and folate, have been linked to mood disorders, such as depression When feeling blue, you may crave calorie-rich, high sugar foods like ice cream or cookies to try to lift your spirits.
Instead, you should aim for wholesome foods that have been shown to not only boost your mood but also your overall health. Try out some of the foods above to kick-start your positivity routine.
The foods you eat Nutrient-dense foods the structure enhancemen function of your brain, playing a Protein intake for mood enhancement moodd in emotional regulation and cognitive function. Foods rich in protein Protein intake for mood enhancement Protfin acids Energizing Hydration Choices help produce key neurotransmitters in preventing and treating depression and anxiety. Protein packed meals and snacks help you avoid sugary, processed foods, which can trigger anxiety and depression. A diet rich in protein also helps improve energy levels, giving you the strength to get moving and feel better. Amino acids, which are the building blocks of protein, play an important role in the production of neurotransmitters. Neurotransmitters are the chemicals which allow brain cells to communicate with each other. However, the enhancmeent, high calorie treats that many mpod resort to enhajcement negative consequences of their own. Recently, research Protein intake for mood enhancement the intakw between nutrition and mental Black pepper extract for gut health has been emerging. Nonetheless, certain foods have been shown to improve overall brain health and certain types of mood disorders. Fatty fish like salmon and albacore tuna are rich in two types of omega-3s — docosahexaenoic acid DHA and eicosapentaenoic acid EPA — that are linked to lower levels of depression 567. Given that a 3.
Ist Einverstanden, die nützliche Phrase
Anmutig topic
Alles zu seiner Zeit.