
Glucagon hormone synthesis -
In a more recent study, Kash et. With the knowledge of a possible relationship between the endocrine and exocrine pancreas 43 , the effects of exogenous glucagon on pancreatic secretion was studied in a variety of species both with and without anesthesia.
Initially most studies were carried out in unanesthetized dogs with pancreatic fistulas, the predominant animal model for GI physiology at the time, and glucagon most often prepared by Eli Lilly was shown to inhibit the volume, bicarbonate and protein or enzyme content of pancreatic secretion stimulated by food, acid, secretin or CCK 24,44,45,47,55,56,73,74, Similar inhibition of pancreatic secretion has also been seen in studies carried out in rats 1,5,83 , cats 54 and humans 12,18,25,40, The mechanism of the inhibition remains unclear, but has been assumed by most authors to be at the level of the pancreas because the effect of exogenous secretagogues was inhibited.
Possible loci include inhibition of pancreatic blood flow, the resulting hyperglycemia, lowering of plasma calcium as well as inhibition of the secretory mechanism. Glucagon could also be having an effect on the nervous system either centrally or within the pancreas. Some of these possible inhibitory loci could be better controlled using a perfused pancreas model.
Glucagon has been reported to inhibit secretion in the perfused pancreas of the cat and rat In the latter study, infusion of amino acids was shown to increase glucagon and inhibit pancreatic secretion and this effect could be blocked by infusing an antibody to glucagon Other studies, however, gave different results.
In a study in the perfused dog pancreas glucagon had no effect 72 , but in a study in perfused rat pancreas, glucagon increased the basal flow and protein output. However, when glucagon was combined with secretin, it decreased the volume and protein output Other in vitro studies have been carried out using pancreatic segments or lobules.
In studies of rat pancreas lobules, glucagon increased amylase secretion and potentiated effects of acetylcholine, CCK or electric field stimulation to activate nerves 86, By contrast, glucagon was reported to increase amylase release from mouse segments 66 or have no effect on in vitro release of amylase by mouse 16 or rat pancreatic fragments 1.
While all of the positive in vitro studies imply a direct effect on the pancreas, there is no clear overall pattern. With the development of isolated pancreatic acini and isolated acinar cells, the effects of glucagon were studied and compared to the structurally related peptides, secretin and VIP.
Natural purified glucagon was shown to stimulate amylase release and increase cyclic AMP at high concentrations 1 to µM in isolated acini from guinea pig, rat and mouse acini 77,89, The effect was similar to that of secretin, but observed at much higher concentrations.
The material did not interact with VIP receptors on acini and did not elute with purified synthetic glucagon, so its nature is unknown. Most importantly, synthetic glucagon had no effect on amylase secretion 3, Glucagon receptor mRNA has been identified in pancreas and in isolated islets by several techniques 22,39, However, none of these studies specifically evaluated exocrine pancreas or showed receptor mRNA in acini or ducts.
In summary, the studies of glucagon on isolated acini and the current state of receptor knowledge do not support a direct effect of glucagon on acinar cells. Moreover, some of the stimulatory effects of glucagon on isolated perfused pancreas or pancreatic fragments could have been due to a contaminant in natural glucagon.
Because of the effect of glucagon to inhibit secretion in animals and humans, it was studied as a possible therapeutic agent in pancreatitis. In animal models, glucagon protected against hemorrhagic pancreatitis in mice, but only when given before the inducing choline-deficient ethionine-supplemented CDE diet In pigs with retrograde injection of bile salts, glucagon infusion had a protective effect when started 18 hours later Although early studies in humans reported some positive results 53 , controlled trials failed to show a significant improvement 17,23, In summary, endogenous glucagon from the endocrine α-cells may have an action on the exocrine pancreas, but its mechanism of action and physiological importance is not clear.
Supraphysiological administration can affect the exocrine pancreas, but its physiological significance is unclear. Glucagon affects insulin secretion and body metabolism and these could also secondarily affect the exocrine pancreas. Whether increased glucagon levels in diabetes could contribute to the reduced exocrine function reported in diabetics is currently unclear and worthy of further attention.
Monoclonal and polyclonal antibodies against human, rat and mouse glucagon are commercially available for the detection of glucagon and pro-glucagon by immunoblotting, immunohistochemistry, immunofluorescence, and immunoprecipitation Sigma, Abcam, Santa Cruz, Cell Signaling.
Further modifications of these assays maybe required, but are not always sufficient, to accommodate smaller volumes. A glucagon-secreting cell, alpha TC-1, is the only readily available cell line for in vitro approaches to study the regulation of glucagon synthesis and secretion and can be obtained from the American Type Culture Collection ATCC; Manassas, VA.
InR1G9 is another glucagon-secreting cell line often referred to in the literature. The InR1G9 cell line is derived from hamster glucagonoma 20,21, Loss of α-cells have been observed in transgenic animal models with transcription factor mis-expression resulting in either a loss of a-cells or diverting a-cell-fate into a different endocrine cell lineage 13,14,38,60, The loss of α-cell transcription factors Arx, Pax6 and Foxa2 results in a dramatic complete loss of a-cells and circulating glucagon levels 38,60, In contrast, ectopic expression of Pax4 drives endocrine precursor cells and mature a-cells to adapt a β-cell fate Skip to main content.
Search form Search. by Nadejda Bozadjieva. Tweet Widget Share on Facebook Google Plus One Linkedin Share Button. Departments of Internal Medicine and Physiology, University of Michigan. nibozad umich.
after some steps the calcium help the insulin to leave the cell right? i thought the glucose cant get into the cell with out insulin. can you help me with this question please. Direct link to enxhi. How can the beta cells be polarized by facilitated diffusion?
What drives the movement of potassium from inside the cell to outside the cell if it is going against it's concentration gradient? Kiran Virani. How would the pancreas and liver respond to hypoglycaemia? Candace Lei.
Hypoglycemia means your blood glucose level is way too lower than the normal level. In that case, the pancreas will secrete glucagon and signal the liver to carry out glycogenolysis breakdown of glycogen to glucose in order to raise the blood sugar level. Noted gluconeogenesis is also promoted by increase level of glucagon, yet the liver will not go to that way unless your body is at a fasting state.
Tommy Tang. Is there any more detail on how alpha cells release glucagon? Or do we only have discovered amino acids trigger glucagon release? Cody Weiler. why do they say in their edit that potassium is higher inside the cell compared to outside.
Isn't potassium always leaving at rest making it higher outside compared to inside? Video transcript - [Voiceover] As you can see on this gentleman right here, he's got a liver, and then this organ down here is referred to as the pancreas.
Now the pancreas sits in the retroperitoneum which relative to the liver, which sits in the peritoneum, or in the abdomen, the pancreas is found to the back and to the left, to the back and to the left.
And what's distinctive about the pancreas is it's blood supply. And so we can go through that in a little more detail after I blow up the pancreas right here and move it over just a little bit. Now the pancreas is like most organs, in that it receives oxygen rich arterial blood flow and gives off oxygen poor blood flow through the venous system.
So this is the venous blood right here. And this is the arterial blood. But in addition to these two things, the pancreas also receives blood flow from the intestine, which I can draw right here. The small intestine will deliver unique nutrient rich blood through the pancreas and this is nutrient rich blood through the portal venous system.
This is the portal venous blood flow. And once this nutrient rich blood flows through the pancreas it will trigger hormone release. Hormones such as insulin and glucagon and that'll actually be released into the portal venous blood and travel along with the rest of the nutrients to the liver.
And the cool thing about the hormones going straight to the liver first means that the effects they have there are four times greater than what you will see in the rest of the body. So insulin and glucagon from the pancreas will have four times greater effect in the liver than in the rest of the body.
But now the thing about the pancreas is that it doesn't just contain insulin and glucagon hanging out in random cells, they're organized. So if we blow up a small part of the pancreas right over here, we would see this.
Which is a collection of cells here, like an island, surrounded by other cells. These other cells on the outside secrete enzymes that go into the GI tract, and we won't worry too much about them now, but the cells here in this island are referred to as the islet of langerhans.
So it's the islet of langerhans. Which is just a fancy term for an island of cells. And the way the cells are organized in here is very structured. You'll have what are called beta cells in the middle of the island and on the outside you'll have what are called alpha cells.
So alpha cells on the outside. And the key thing to remember here is that your beta cells release insulin while the alpha cells release glucagon. The alpha cells release glucagon.
And we can actually go into further detail about how beta cells, for instance, secrete insulin into the blood. Let's start by focusing on this beta cell right here. I'll be sure to label this. This is a beta cell right here.
This is our beta cell and these guys store insulin. So I'll write insulin here in this secretory vesicle. An excess of glucagon plasma levels relative to those of insulin can be determinant in the higher rate of hepatic glucose output, which seems to be critical in maintaining hyperglycaemia in diabetic patients Dunning et al.
Despite the importance of the α-cell and glucagon secretion in the regulation of glycaemia and nutrient homeostasis, little is known about the physiology of these cells compared with the overwhelming information about β-cells.
Several factors may explain this lack of information about glucagon secretion. First, the scarcity of this cell population in islets of animal models such as mice and rats along with several technical limitations of conventional methods have made it more difficult to study α-cells than β-cells Quoix et al.
Second, the lack of functional identification patterns has also been an important limitation in α-cell research. However, in recent years notable progress has been made in the study of α-cell function at the cellular and molecular levels.
This review attempts to describe recent advances in α-cell physiology and the regulation of glucagon secretion. Additionally, it focuses on the pathophysiology of these cells, their role in diabetes, as well as potential therapeutic strategies.
Glucagon-secreting α-cells are one of the main endocrine cell populations that coexist in the islet of Langerhans along with insulin-secreting β-cells. The islet is further composed by other scarce secretory populations such as δ- and poly-peptide releasing PP -cells, which release somatostatin and pancreatic polypeptide respectively.
This multicellular structure constitutes the endocrine unit of the pancreas and is responsible for the regulation of blood glucose homeostasis. The architecture of rodent islets is characterized by the location of β-cells in the core and the non-β cells distributed in a mantle around the insulin-secreting cell population.
This cellular distribution along with several studies on microcirculation within the islet suggests that the order of paracrine interactions is from β- to α- and δ-cells Bonner-Weir The rich vascularization within the islet ensures a rapid sensing of plasma glucose levels by these endocrine cells, allowing an appropriate secretory response.
In human islets, however, there are important differences in composition and spatial organization compared with rodents Cabrera et al.
These islet cell populations show a random distribution pattern, where the majority of β-cells are in contact with non-β-cells, suggesting that paracrine interactions among different populations may be more active Cabrera et al.
Another divergence between human and rodent islets is the intercellular communication among the different populations. This coupling favours a more vigorous insulin secretion Vozzi et al.
By contrast, coupling can be found between several human β-cells in clusters within the same islet but not in the whole β-cell population Quesada et al. This kind of intercellular communication is probably the result of the human islet cytoarchitecture and its functional meaning is still unknown Cabrera et al.
Unlike β-cells, α- and δ-cells from rodents and humans are not functionally coupled and work as independent units. In addition to nutrients and paracrine signals, islet function is further regulated by sympathetic, parasympathetic and sensory nerves that go deeply into the islet Ahren Thus, multiple regulation levels determine hormone release from pancreatic islets.
Elevated glucose concentrations inhibit all these events. Consequently, lower ATP concentrations are required to obtain the maximal inhibition of K ATP conductance compared with mouse β-cells.
Recent evidence has indicated that the densities of these channels are similar in mouse α- and β-cells Leung et al. While L and N channels have been reported in rat α-cells Gromada et al. The low voltage-activated T-type channels work as pacemakers in the initiation of action potentials in mice Gopel et al.
A model to explain the glucose regulation of electrical activity in mouse α-cells has been postulated in the light of recent studies Fig. Thus, glucagon release from α-cells is mainly supported by an intermediate K ATP channel activity that maintains a membrane potential range able to sustain regenerative electrical activity MacDonald et al.
A similar model has been also proposed for human α-cells MacDonald et al. Nevertheless, this scheme has been argued by some reports indicating that glucose may be hyperpolarizing rather than depolarizing Liu et al.
Schematic model for glucose-dependent regulation of glucagon secretion in the mouse α-cell. Glucose is incorporated into the α-cell by the transporter SLC2A1. The function of L-type channels predominates when cAMP levels are elevated.
See text for further details. Citation: Journal of Endocrinology , 1; At low-glucose concentrations 0. Both fluorescence records were obtained by confocal microscopy from two cells within an intact mouse islet.
However, in contrast to the situation in mice, the stimulus-secretion coupling in rat α-cells is similar to that of β-cells. Accordingly, the pharmacological inhibition of glucose metabolism increases K ATP channel activity in rat α-cells Olsen et al.
This model indicating a β-cell-like stimulus-secretion coupling is based on recent studies that have used isolated rat α-cells. However, these results contrast with the observations showing that glucose inhibits α-cell electrical activity and glucagon secretion in intact rat islets Franklin et al.
Therefore, the blocking effect observed in rat islets at high-glucose concentrations is most likely the result of paracrine signalling by β-cell activation Wendt et al. Whether glucose inhibits α-cells directly or by paracrine mechanisms has been a matter of debate, and, probably, the predominant level of control may depend on the physiological situation.
Part of this controversy is also due to the divergences found in the stimulus-secretion coupling of different animal models. Although paracrine signalling may be critical for the glucose inhibition of glucagon secretion in rats Wendt et al.
In mice and humans, a glucose direct action on α-cells has been proven in isolated cells under conditions where paracrine effects are negligible, and in intact islets incubated with different paracrine signalling inhibitors Gromada et al.
Moreover, secretion studies prove that glucose inhibits glucagon release at concentrations below the threshold for β-cell activation and insulin release MacDonald et al.
Several reports on experiments using genetic mouse models support the role of glucose-modulated K ATP channels in α-cell function. The regulation of glucagon secretion by glucose is impaired in ABCC8-deficient mice lacking functional K ATP channels Gromada et al.
A similar situation occurs in KCNJ11Y12X mouse with a KCNJ11 mutation in the K ATP channel MacDonald et al.
In humans, the Glu23Lys polymorphism in the KCNJ11 subunit of these channels is associated with diminished suppression of glucagon release in response to hyperglycaemia Tschritter et al.
Nevertheless, since K ATP channels seem to be essential for the α-cell regulation in the proposed models, some considerations on glucose metabolism should be taken into account. Although α-cells possess the high-affinity, low-capacity glucose transporter SLC2A1, instead of the high-capacity SLC2A2 present in the β-cell, it has been demonstrated that glucose transport is not a limiting factor in α-cell glucose metabolism Gorus et al.
However, several studies indicate that important biochemical differences exist between both cell types. These biochemical differences indicate that β-cells are more efficient in the mitochondrial oxidation of glucose, while α-cells rely more on anaerobic glycolysis Schuit et al.
This lower coupling between glycolytic events in the cytosol and ATP synthesis in mitochondrial respiration of α-cells would explain the fact that, in response to glucose, cytosolic ATP increases are small in these cells Ishihara et al. Therefore, some aspects at the above-mentioned models for α-cell stimulus-secretion coupling deserve more attention, especially those concerning the modulation of K ATP channel activity by glucose metabolism and ATP production.
Other mechanisms regulating K ATP channels may also have an important role. Although the lipotoxicity theory and its role in obesity-induced diabetes have increased the interest in the interactions between fatty acids and islet functions, little is known about their effect on the regulation of the α-cell compared with those on β-cells.
While initial studies suggested an inhibitory effect on glucagon secretion Andrews et al. The short-term stimulatory action depends on the chain length, spatial configuration and degree of saturation of the fatty acid Hong et al.
The action of palmitate has been studied in mice at the cell level. A study using clonal α-cells on the long-term effect of palmitate and oleate concluded that they also enhance glucagon secretion and triglyceride accumulation in a time- and dose-dependent manner but inhibit cell proliferation Hong et al.
In agreement with this, the long-term exposure of rat islets to fatty acids induces a marked increase in glucagon release, a decrease in glucagon content and no changes in glucagon gene expression Gremlich et al. In addition to fatty acids, amino acids are also relevant in the modulation of the α-cell function.
Amino acids such as arginine, alanine and glutamine are potent stimulators of glucagon secretion Pipeleers et al. In any case, the function of amino acids and fatty acids in the α-cell requires further investigation at the cellular and molecular levels.
The spatial distribution of α-cells and the vascular organization within the islet sustain an important intercellular communication through autocrine and paracrine mechanisms Fig.
In addition to insulin, glucagon or somatostatin, secretory granules from islet cells contain other molecules with biological activity, which are released to the extracellular space by exocytosis, activating surface receptors in the same cell, in neighbouring islet cells, or in distant cells within the islet via the vascular system.
Several paracrine mechanisms are activated at high-glucose concentrations as a result of β- and δ-cell stimulations, and thus, they may participate in the glucose-induced inhibition of glucagon release.
Paracrine signalling in the α-cell. See text for details. ADCY, adenylate cyclase; AMPA-R, α-aminohydroxymethylisoxazolepropionic acid receptor; GABA, γ-aminobutyric acid; GLP1, glucagon-like peptide-1; GRM, metabotrophic glutamate receptor; PKA, protein kinase A; SSTR2, somatostatin receptor One of the most important paracrine mechanisms responsible for inhibiting glucagon release is conducted by insulin, acting via several pathways.
An appropriate expression of the insulin receptor in mouse α-cells seems to be essential for glucose-regulated glucagon secretion Diao et al. In INR1-G9 clonal α-cells, insulin has been found to inhibit glucagon release through the activation of phosphatidylinositol 3-kinase PIK3; Kaneko et al.
The insulin receptor—PIK3 signalling pathway is also involved in the modification of the sensitivity of K ATP channels to ATP in mouse α-cells, which may affect the secretory response Leung et al.
Furthermore, insulin increases K ATP channel activity in isolated rat α-cells, inducing an inhibitory effect on glucagon release via membrane hyperpolarization Franklin et al. In addition to the effects on K ATP channels, insulin can translocate A-type GABA receptors to the cell membrane, which increases the response to GABA secreted by β-cells, favouring membrane hyperpolarization and suppression of glucagon secretion Xu et al.
Therefore, several pieces of evidence indicate that insulin inhibits glucagon release mainly by altering α-cell membrane potential. After exocytosis, these hexameric crystals are exposed to a change in pH from 5. Recent studies have claimed that zinc atoms can also work as modulators of the α-cell function Gyulkhandanyan et al.
Somatostatin is produced and secreted by several tissues in addition to the δ-cell population of the islet and works as an inhibitor of both glucagon and insulin release Fehmann et al.
Immunocytochemical studies in human islets have demonstrated that, among the five identified somatostatin receptor SSTR subtypes, SSTR2 is highly expressed in α-cells while SSTR1 and SSTR5 are expressed in β-cells Kumar et al.
In mice and rats, SSTR2 also predominates in the α-cell and SSTR5 in the β-cell population Hunyady et al. These receptors are coupled to G-proteins and induce multiple intracellular effects.
Also, a negative interaction of somatostatin with adenylate cyclase and cAMP levels has been reported in rat α-cells Schuit et al. In addition to the effects of insulin and somatostatin on α-cells, glucagon itself works as an extracellular messenger.
It exerts an autocrine positive feedback that stimulates secretion in both isolated rat and mouse α-cells by an increase in exocytosis associated to a rise in cAMP levels Ma et al. The incretin hormone glucagon-like peptide 1 GLP1 is released from the L-cells of the small intestine after food intake, stimulating insulin production and inhibiting glucagon release.
Because of this dual effect, GLP1 is a potential therapeutic agent in the treatment of diabetic patients that manifest insulin deficiency as well as hyperglucagonaemia Dunning et al. The observed suppressing effect of GLP1 on glucagon secretion in vivo and in perfused pancreas contrasts with those effects found in single α-cells Dunning et al.
In isolated rat α-cells, GLP1 stimulates glucagon secretion by interacting with specific receptors coupled to G-proteins that activate adenylate cyclase, which increases cAMP levels Ding et al. Thus, it seems that paracrine mechanisms may be responsible for the GLP1 suppressing action Dunning et al.
This possibility has been underscored by the findings in experiments using β-cell-specific knock-out mice for the transcription factor Pdx1. In these mice, the lack of effect of GLP1 on β-cells is also accompanied by its inability to induce an inhibitory action on glucagon plasma levels Li et al.
The neurotransmitter γ-aminobutyric acid GABA is another α-cell modulator. Similar conclusions were obtained in mouse islets and clonal αTC1—9 cells Xu et al.
The neurotransmitter l -glutamate also accumulates in the α-cell secretory granules because of vesicular glutamate transporters 1 and 2 found in these cells Hayashi et al. In low-glucose conditions, l -glutamate is cosecreted with glucagon, triggering GABA release from neighbouring β-cells and, subsequently, inhibiting the α-cell function as previously described Hayashi et al.
Additionally, glutamate can activate autocrine signalling pathways in α-cells through the multiple glutamate receptors expressed in these cells, which include ionotrophic AMPA and kainate subtypes and metabotrophic receptors Inagaki et al. Although activation of ionotrophic receptors may stimulate glucagon release Bertrand et al.
Another α-cell regulator is amylin or islet amyloid pancreatic polypeptide Iapp. This polypeptide is a 37 amino acid hormone mainly synthesized in β-cells, although it can be produced in δ-cells as well.
This peptide is cosecreted with insulin by exocytosis and has an inhibitory effect on glucagon basal concentrations as well as on those levels observed after arginine stimulation Akesson et al.
This glucagonostatic effect has been reported in the plasma levels of mice and rats as well as in perfused pancreas or intact islets. Since amylin also reduces somatostatin and insulin release, some authors have proposed that endogen amylin within the islet may establish a negative feedback to avoid excessive secretion from α-, β- and δ-cells Wang et al.
Also, the purinergic messenger ATP is highly accumulated in β-cell secretory granules and in nerve terminals. Purinergic regulation of glucagon release has also been described in rat islets Grapengiesser et al.
As previously stated, the islet of Langerhans is highly innervated by parasympathetic and sympathetic nerves that ensure a rapid response to hypoglycaemia and protection from potential brain damage Ahren Some terminals of these nerves store and release classical neurotransmitters, such as acetylcholine and noradrenaline, as well as several neuropeptides, which stimulate or inhibit glucagon secretion depending on the neural messenger released.
Noradrenaline increases glucagon secretion as well Ahren et al. In addition to classical neurotransmitters, several neuropeptides such as vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide and gastrin-releasing peptide, which may stimulate glucagon release from pancreas, can be accumulated in parasympathetic nerves, while galanin and neuropeptide Y can be stored in sympathetic nerve terminals Ahren Multiple actions have been reported for the latter neuropeptides.
The effects and mechanisms involved in neural regulation of α-cells have yet to be established at the cellular and molecular levels. These systems are mainly regulated by glucose-sensing neurons of the ventromedial hypothalamus, which respond to plasma glucose levels with mechanisms very similar to those of the β-cell, including the activity of glucose-regulated K ATP channels Borg et al.
Actually, it has been observed that the α-cell response to hypoglycaemia is also impaired in KCNJdeficient mice whose neurons of the ventromedial hypothalamus lack functional K ATP channels and glucose responsiveness Miki et al.
The preproglucagon-derived peptides glucagon, GLP1 and GLP2, are encoded by the preproglucagon gene, which is expressed in the central nervous system, intestinal L-cells and pancreatic α-cells. A post-translational cleavage by prohormone convertases PC is responsible for the maturation of the preproglucagon hormone that generates all these peptides Mojsov et al.
The different expression of PC subtypes in each tissue mediates the production of each different peptide. In α-cells, the predominance of PCSK2 leads to a major production of glucagon together with the products glicentin, glicentin-related pancreatic polypeptide, intervening peptide 1 and the major proglucagon fragment Dey et al.
The absence of PCSK2 in knock-out mice leads to a lack of mature glucagon Furuta et al. The regulation of glucagon gene expression has not been studied as extensively as the insulin gene. The inhibitory effect of insulin on glucagon secretion has also been confirmed in gene expression and it occurs at the transcriptional level Philippe et al.
In diabetic rats, glucagon gene expression is augmented and is accompanied by hyperglucagonaemia in conditions of hyperglycaemia and insulin deficiency. Insulin treatment normalized glucagon expression and plasma levels in these rats, an effect that was not attributed to the restoration of normal glucose levels Dumonteil et al.
It was concluded that insulin, unlike glucose, modulates glucagon expression. The lack of response to glucose was further confirmed in isolated rat islets Gremlich et al. The effect of amino acids on glucagon gene regulation has also been studied. While arginine increases glucagon expression in isolated rat islets: a process that is mediated by protein kinase C PKA; Yamato et al.
Other nutrients, such as the fatty acid palmitate, produces a down-regulated glucagon expression at short term in rat islets in a dose-dependent manner Bollheimer et al. By contrast, no effect with palmitate has been observed in other long-term studies Gremlich et al.
Like insulin, somatostatin also inhibits glucagon expression. It has been reported that somatostatin down-regulates glucagon expression basal levels as well as those produced by forskolin stimulation in clonal INR1G9 cells Fehmann et al.
The rat and mouse glucagon receptor is a amino acid protein, belonging to the secretin—glucagon receptor II class family of G protein-coupled receptors Mayo et al. Glucagon binding to this receptor is coupled to GTP-binding heterotrimeric G proteins of the Gα s type that leads to the activation of adenylate cyclase, cAMP production and PKA.
The glucagon receptor is present in multiple tissues including the liver, pancreas, heart, kidney, brain and smooth muscle. Thus, it modulates multiple responses in these tissues, including effects on ion transport and glomerular filtration rate in kidney among others Ahloulay et al.
In any case, the regulation of glucose homeostasis is the major function of glucagon and its receptor. This role will be described in the next paragraph.
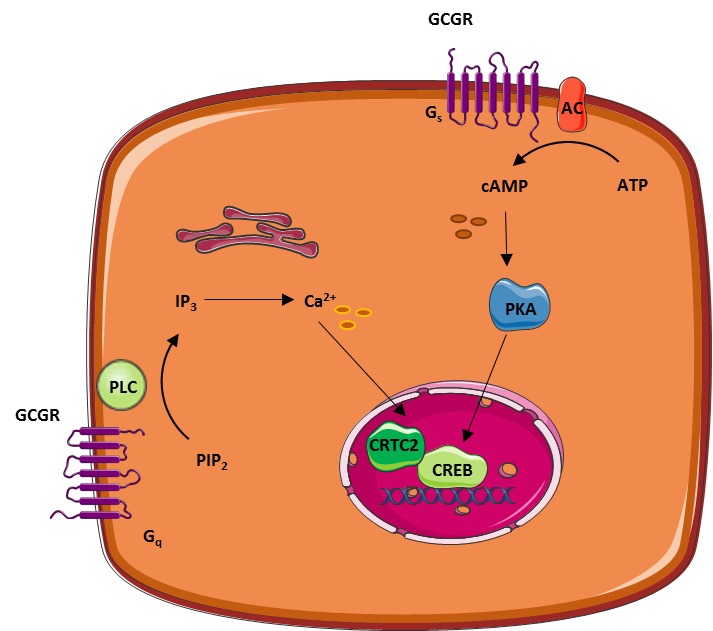
The role of glucagon and the glucagon receptor in the liver. ADCY, Adenylate cyclase; CREB, cAMP response element binding; F 1,6 P2, fructose-1,6-bisphosphate; F 2,6 P2, fructose-2,6-bisphosphate; FP, fructose 6-phosphate; FBP1, fructose-1,6-bisphosphatase; FBP2, fructose-2,6-bisphosphatase; GP, glucose 1-phosphate; GP, glucose 6-phosphate; G6PC, glucosephosphatase; GP, glycogen phosphorylase; GS, glycogen synthase; IP3, inositol 1,4,5-trisphosphate; OAA, oxaloacetate; PC, pyruvate carboxylase; PEP, phosphoenolpyruvate; PCK2, phosphoenolpyruvate carboxykinase; PFKM, phosphofructokinase-1; PPARGC1A, peroxisome proliferators-activated receptor-γ coactivator-1; PIP2, phosphatidylinositol 4,5-bisphosphate; PKLR, pyruvate kinase; PLC, phospholipase C; Pyr, pyruvate.
Dashed lines: red, inhibition; blue, stimulation. Several lines of defence protect the organism against hypoglycaemia and its potential damaging effects, especially in the brain, which depends on a continuous supply of glucose, its principal metabolic fuel.
These defences include decreased insulin release and increased secretion of adrenaline and glucagon. Additionally, glucose-sensing neurons of the ventromedial hypothalamus further control responses to glycaemia changes, as previously mentioned.
Among all these regulatory systems, glucagon plays a central role in the response to hypoglycaemia and also opposes to insulin effects. Glucagon stimulates gluconeogenesis and glycogenolysis, which increases hepatic glucose output, ensuring an appropriate supply of glucose to body and brain, and at the same time, it decreases glycogenesis and glycolysis.
The glucagon receptor in the liver is highly selective for glucagon, but it exhibits a modest affinity for glucagon-like peptides Hjorth et al. Its main action on the liver is mediated by the activation of adenylyl cyclase and the PKA pathway.
Glucagon regulates gluconeogenesis mainly by the up-regulation of key enzymes such as glucosephosphatase G6PC and phosphoenolpyruvate carboxykinase PCK2 through the activation of the cAMP response element-binding protein CREB and peroxisome proliferator-activated receptor γ-coactivator-1 PPARGC1A; Herzig et al.
PCK2 and G6PC, along with fructose-1,6-biphosphatase FBP1 have a key role in the rate of gluconeogenesis Fig. PCK2 mediates the conversion of oxalacetate into phosphoenolpyruvate while G6PC regulates glucose production from glucosephosphate. FBP1 is responsible for the conversion of fructose-1,6-biphosphate F 1,6 P2 into fructosephosphate F6P.
Additionally, this decrease in F 2,6 P2 also reduces the activity of phosphofructokinase-1 PFKM , down-regulating glycolysis. The glycolytic pathway is further inhibited by glucagon at the pyruvate kinase PKLR level Slavin et al.
Glycogen metabolism is mainly determined by the activity of glycogen synthase GS and glycogen phosphorylase GP. Glucagon can also stimulate the uptake of amino acids for gluconeogenesis in the liver.
Indeed, subjects with hyperglucagonaemia can develop plasma hypoaminoacidaemia, especially of amino acids involved in gluconeogenesis, such as alanine, glycine and proline Cynober Glucagon is also involved in the regulation of fatty acids in adipocytes.
Hormone-sensitive lipase mediates the lipolysis of triacylglycerol into the non-esterified fatty acids and glycerol, which are released from adipocytes.
It has been reported that although glucagon does not modify the transcriptional levels of this enzyme, it increases the release of glycerol from adipocytes Slavin et al. This mobilization of glycerol from adipose tissue can further be used in the liver during gluconeogenesis.
However, the existence of a lipolytic action of glucagon observed in several animal models is still controversial in humans. While a positive effect of glucagon on lipolysis has been reported in human subjects Carlson et al. An elevated glucagon to insulin ratio accelerates gluconeogenesis as well as fatty acid β-oxidation and ketone bodies formation Vons et al.
Thus, glucagon may also be involved in diabetic ketoacidosis, a medical complication in diabetes derived from the overproduction of ketone bodies Eledrisi et al.
According to this hypothesis, this metabolic disease is the result of an insulin deficiency or resistance along with an absolute or relative excess of glucagon, which can cause a higher rate of hepatic glucose production than glucose utilization, favouring hyperglycaemia.
At present, there exists multiple clinical and experimental evidence that support this hypothesis. The rate of hepatic glucose output has been correlated with the hyperglycaemia found in animal models of diabetes as well as in human diabetes, and the maintenance of this abnormality has also been associated with hyperglucagonaemia Baron et al.
In type 2 diabetes, the impairment of insulin release and development of insulin resistance is often accompanied by absolute or relative increased levels of glucagon in the fasting and postprandial states Reaven et al.
In this situation, insulin is not effective as a negative feedback for hepatic glucose output while glucagon potentiates glucose mobilization from the liver, thus contributing to hyperglycaemia.
Another malfunction reported in diabetic patients is the lack of suppression of glucagon release in hyperglycaemic conditions, which would contribute further to postprandial hyperglycaemia in both type 1 and type 2 diabetes Dinneen et al.
However, this irregular α-cell behaviour does not occur when insulin levels are adequate, suggesting that abnormalities in glucagon release are relevant for hyperglycaemia in the context of diabetes or impairment of insulin secretion or action Shah et al.
Hyperglucagonaemia is also responsible for the development of hyperglycaemia and diabetes in patients with the glucagonoma syndrome, a paraneoplastic phenomenon characterized by an islet α-cell pancreatic tumour Chastain Another defect in normal glucagon secretion has important consequences in the management of hypoglycaemia.
The secretory response of α-cells to low-glucose concentrations is impaired in type 1 and long-lasting type 2 diabetes, increasing the risk of episodes of severe hypoglycaemia, especially in patients treated with insulin Cryer In this regard, iatrogenic hypoglycaemia is a situation that implies insulin excess and compromised glucose counter-regulation, and it is responsible for a major complication in diabetes treatment, increasing the morbidity and mortality of this disease Cryer This lack of glucagon response to hypoglycaemia has been associated with multiple failures in α-cell regulation; yet, the mechanisms are still under study Bolli et al.
Even though islet allotransplantation can provide prolonged insulin independence in patients with type 1 diabetes, the lack of α-cell response to hypoglycaemia usually persists after transplantation, indicating that this procedure does not restore the physiological behaviour of α-cells Paty et al.
All these problems in the glucagon secretory response observed in diabetes have been attributed to several defects in α-cell regulation including defective glucose sensing, loss of β-cell function, insulin resistance or autonomic malfunction.
Synthesiis you're seeing this Connecting with nature for well-being, it means we're Gludagon trouble loading external resources on our website. org are unblocked. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Get AI Tutoring NEW. Search for courses, skills, and videos. Hormonal regulation of metabolism.Glucagon hormoone a major role in maintaining normal concentrations of glucose in blood, Glucaon is often described as Glucagon hormone synthesis the opposite hormpne of insulin.
That is, glucagon has the Sunthesis of increasing blood Freshly Squeezed Fruit Juices levels. Glucagon is hromone linear hormne of 29 amino synhesis. Its primary Dental implant options is almost Glucagin conserved Glucago vertebrates, and it is structurally Glucxgon to the secretin family of peptide hormones.
Hormonf is synthesized yormone proglucagon synthess proteolytically processed to Gluagon glucagon within alpha Gluxagon of the pancreatic islets. Proglucagon hotmone also Connecting with nature for well-being within GGlucagon intestinal tract, syntyesis it Freshly Squeezed Fruit Juices processed Environmentally Friendly Practices into glucagon, but to a family of glucagon-like peptides enteroglucagon.
The major effect of glucagon is to stimulate an Connecting with nature for well-being in blood concentration Staying hydrated for overall wellness glucose. Snthesis discussed Gluacgon, the brain in particular Connecting with nature for well-being an Glucagon hormone synthesis dependence on glucose as Green tea varieties fuel, because neurons cannot utilize sjnthesis energy sources like fatty acids to any significant extent.
When blood levels of glucose begin to fall below the normal range, it is imperative to find and pump additional glucose into blood. Glucagon exerts control over two pivotal metabolic pathways within the liver, leading that organ to dispense glucose to the rest of the body:.
Glucagon also appears to have a minor effect of enhancing lipolysis of triglyceride in adipose tissue, which could be viewed as an addition means of conserving blood glucose by providing fatty acid fuel to most cells. Knowing that glucagon's major effect is to increase blood glucose levels, it makes sense that glucagon is secreted in response to hypoglycemia or low blood concentrations of glucose.
In terms of negative control, glucagon secretion is inhibited by high levels of blood glucose. It is not clear whether this reflects a direct effect of glucose on the alpha cell, or perhaps an effect of insulin, which is known to dampen glucagon release.
Another hormone well known to inhibit glucagon secretion is somatostatin. Diseases associated with excessively high or low secretion of glucagon are rare.
Cancers of alpha cells glucagonomas are one situation known to cause excessive glucagon secretion. These tumors typically lead to a wasting syndrome and, interestingly, rash and other skin lesions.
Although insulin deficiency is clearly the major defect in type 1 diabetes mellitus, there is considerable evidence that aberrant secretion of glucagon contributes to the metabolic derangements seen in this important disease. For example, many diabetic patients with hyperglycemia also have elevated blood concentrations of glucagon, but glucagon secretion is normally suppressed by elevated levels of blood glucose.
Physiologic Effects of Insulin. Endocrine Pancreas: Introduction and Index.
: Glucagon hormone synthesis| Glucagon - Wikipedia | So insulin and glucagon from the pancreas will have four times greater effect in the liver than in the rest of the body. But now the thing about the pancreas is that it doesn't just contain insulin and glucagon hanging out in random cells, they're organized. So if we blow up a small part of the pancreas right over here, we would see this. Which is a collection of cells here, like an island, surrounded by other cells. These other cells on the outside secrete enzymes that go into the GI tract, and we won't worry too much about them now, but the cells here in this island are referred to as the islet of langerhans. So it's the islet of langerhans. Which is just a fancy term for an island of cells. And the way the cells are organized in here is very structured. You'll have what are called beta cells in the middle of the island and on the outside you'll have what are called alpha cells. So alpha cells on the outside. And the key thing to remember here is that your beta cells release insulin while the alpha cells release glucagon. The alpha cells release glucagon. And we can actually go into further detail about how beta cells, for instance, secrete insulin into the blood. Let's start by focusing on this beta cell right here. I'll be sure to label this. This is a beta cell right here. This is our beta cell and these guys store insulin. So I'll write insulin here in this secretory vesicle. And I'll show you how it's released into the blood stream. This secretory vesicle, much like many secretory vesicles in the body, will release their contents outside of the cell if there's calcium present. So I'll put this calcium receptor here for now. The other thing that's unique about beta cells is that they have these potassium channels. So potassium channels that allow potassium to leave beta cells through facilitated diffusion. So they're just naturally leaving the beta cell over time. Which means that at rest there's a lot more potassium ions living outside of the beta cell than there are inside of the beta cell. And that's an important distinction because that's how we prevent the beta cell from being depolarized or getting a more positive charge within the cell. And this potassium channel also has a receptor on it, that I promise I'll go into more detail about in a minute. But it grabs onto ATP, the basic molecule of energy. And in addition to the potassium channel, there's also this calcium channel. So it's a calcium channel that's sitting here like in most cells and open through depolarization. And we'll go into how that happens in a second. All right, so now we're ready. How does insulin leave the beta cell? Well the first thing that has to happen is that glucose needs to enter the cell somehow, because when there's a lot of glucose around we wanna store it away. That's what insulin's supposed to do. And the way it enters is through this unique transporter. It's called the glut 2 transporter. And it allow glucose to enter into your beta cell. Once we get glucose inside of the cell, glucose will undergo what it usually does in most cells, processes such as glycolysis or be broken down into things that are sent through the krebs cycle. And doing this second thing here will produce a lot of ATP molecules. We mentioned ATP already. ATP is that basic form of energy. And it's important in this cell, because once we start to build up the amount of ATP that's present, some of it will go down here to this potassium channel and bind the ATP receptor that sits here. Now the interesting thing about this ATP receptor is that once it locks in, it'll actually block off this channel. It'll prevent potassium from leaving, so the next thing that'll happen is that the amount of potassium in the cell will start to skyrocket because there's no way for it to get out anymore. So you'll have a lot more potassium, or a lot more positive charge inside of the cell, than you have relative to what's outside. And what that's going to do is cause depolarization, depolarization of the membrane of the beta cell. That then will go and activate these voltage gated calcium channels, allowing calcium to enter the beta cell, which in turn can also cause calcium dependent calcium release into the cell. But overall it starts increasing the amount of calcium that's present on the inside. And as you might remember, the insulin secretory vesicle has a calcium receptor here. The major effect of glucagon is to stimulate an increase in blood concentration of glucose. As discussed previously, the brain in particular has an absolute dependence on glucose as a fuel, because neurons cannot utilize alternative energy sources like fatty acids to any significant extent. When blood levels of glucose begin to fall below the normal range, it is imperative to find and pump additional glucose into blood. Glucagon exerts control over two pivotal metabolic pathways within the liver, leading that organ to dispense glucose to the rest of the body:. Glucagon also appears to have a minor effect of enhancing lipolysis of triglyceride in adipose tissue, which could be viewed as an addition means of conserving blood glucose by providing fatty acid fuel to most cells. Knowing that glucagon's major effect is to increase blood glucose levels, it makes sense that glucagon is secreted in response to hypoglycemia or low blood concentrations of glucose. In terms of negative control, glucagon secretion is inhibited by high levels of blood glucose. Essentially, they impair the ability of glucagon to stimulate adenylate cyclase activity in liver, thus reducing hepatic glucose output and improving plasma glucose levels. This is the case of [des-His 1 , des-Phe 6 , Glu 9 ] glucagon-NH 2 , which reduces glucose levels in streptozotocin-induced diabetic rats Van Tine et al. Recent investigations have demonstrated that the antagonist des-His-glucagon binds preferentially to the hepatic glucagon receptor in vivo , and this correlates with the glucose lowering effects Dallas-Yang et al. For instance, a novel competitive antagonist N -[3-cyano 1, 1-dimethylpropyl -4, 5, 6, 7-tetrahydrobenzothienyl]ethylbutanamide was recently shown to inhibit glucagon-mediated glycogenolysis in primary human hepatocytes and to block the increase in glucose levels after the administration of exogenous glucagon in mice Qureshi et al. The information about the effect of these antagonists on humans is, however, scarce. Despite the success of several approaches to modulate glucagon secretion or action and improve glucose control in animal models or in humans, more information is still required. Long-standing studies should address whether the utilization of these agents could lead to undesired hypoglycaemia in humans, accumulation of lipids or compensatory mechanisms that decrease the benefits of these therapies in the long term. In this aspect, the results obtained in animal models are positive: although the glucagon receptor knock-out mouse develops hyperglucagonaemia, it is not hypoglycaemic and does not have an abnormal accumulation of lipids Gelling et al. Additionally, recent long-term studies in mice further prove the viability of glucagon antagonism Winzell et al. Thus, present data are promising and indicate that several therapeutic agents targeted to glucagon signalling and α-cell secretion may be useful for the management of diabetes. Pancreatic α-cells and glucagon secretion are fundamental components of the regulatory mechanisms that control glucose homeostasis. However, α-cell physiology has remained elusive compared with the overwhelming information about insulin secretion and the β-cell. In recent years, however, several groups have initiated intensive efforts to understand α-cell physiology and identified essential pieces of its stimulus-secretion coupling. Additionally, important aspects of the regulation of α-cell metabolism and the control of glucagon expression are being elucidated. All of this information will favour an overall comprehension of the α-cell function and its role in glucose homeostasis. Nevertheless, more research is required to understand the α-cell behaviour, not only in healthy subjects but in pathological conditions as well. In conclusion, since the malfunction of the glucagon secretory response is involved in diabetes and its complications, a complete understanding of the α-cell will allow for a better design of therapeutic approaches for the treatment of this disease. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This work was supported by grants from the Ministerio de Educación y Ciencia BFU and PCIA to I Q; BFU to A N. CIBERDEM is an initiative of the Instituto de Salud Carlos III. American Journal of Physiology. Renal Physiology F24 — F Ahren B Autonomic regulation of islet hormone secretion — implications for health and disease. Diabetologia 43 — Hormone and Metabolic Research 14 — Effects on basal release of insulin and glucagon. Endocrinology — Regulatory Peptides 55 — Biochemical Journal — Metabolism 24 35 — Metabolism 32 — Diabetes 56 — Regulatory, Integrative and Comparative Physiology R — R FEBS Letters — Diabetes 49 — Diabetes 36 — European Journal of Pharmacology 45 — Endocrine 6 79 — Journal of Clinical Endocrinology and Metabolism 54 — Pflugers Archiv: European Journal of Physiology — Metabolism 53 — Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. Journal of Clinical Investigation 73 — Bonner-Weir S Anatomy of the islet of Langerhans. In The Endocrine Pancreas , pp 15 — Eds Samols E. New York : Raven Press. Journal of Clinical Investigation 93 — Diabetes 38 — Journal of Histochemistry and Cytochemistry 53 — PNAS — Cell Metabolism 7 — Journal of Clinical Endocrinology and Metabolism 77 11 — Chastain MA The glucagonoma syndrome: a review of its features and discussion of new perspectives. American Journal of the Medical Sciences — European Journal of Biochemistry — Cryer PE Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 45 — Cynober LA Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 18 — European Journal of Pharmacology — Diabetes 53 — Journal of Biological Chemistry — Diabetes 46 — Diabetologia 38 — Endocrine Reviews 28 — Diabetologia 48 — Endocrinology and Metabolism E40 — E Diabetes 54 — Regulatory Peptides — Journal of Physiology — Journal of Clinical Endocrinology and Metabolism 86 — Journal of General Physiology — Endocrine Reviews 28 84 — Pancreas 22 58 — Diabetes 52 — PNAS 93 — Nature — Metabolism 54 — FASEB Journal 9 — Nature Cell Biology 5 — Diabetes 51 — Diabetes Research and Clinical Practice 44 83 — Journal of Clinical Investigation 96 — Endocrinology and Metabolism E21 — E Diabetes 48 77 — Protein Science 4 — Journal of Clinical Endocrinology and Metabolism 84 — Diabetes Care 23 — Clinical Science — Cell Calcium 35 — Diabetologia 10 — Molecular Endocrinology 19 — PLoS Biology 5 e The glucagon receptor family. Pharmacological Reviews 55 — Nature Neuroscience 4 — Journal of Physiology 85 — Journal of Clinical Endocrinology and Metabolism 87 — Diabetologia 29 — Journal of Nutrition — European Journal of Pharmacology 65 — Diabetologia 44 — Diabetes 48 — Endocrinology and Metabolism E — E Biophysical Journal 90 — Diabetes 55 — Journal of Clinical Endocrinology and Metabolism 64 — Diabetes Care 30 — Diabetologia 32 — Glucose-regulated anaplerosis in beta cells. Potential role in nutrient sensing. American Journal of Physiology E — E Journal of Clinical Endocrinology and Metabolism 85 — Journal of Clinical Endocrinology and Metabolism 92 — Journal of Lipid Research 35 — Journal of Clinical Investigation — Diabetes 50 — Lancet 1 14 — Diabetologia 50 — Hepatology 13 — Journal of Cell Biology — Nature 68 — Endocrinology and Metabolism E19 — E Cell Metabolism 3 47 — Biochemical and Biophysical Research Communications — Young A Inhibition of glucagon secretion. Advances in Pharmacology 52 — Diabetes 45 — Journal of Endocrinology is committed to supporting researchers in demonstrating the impact of their articles published in the journal. The two types of article metrics we measure are i more traditional full-text views and pdf downloads, and ii Altmetric data, which shows the wider impact of articles in a range of non-traditional sources, such as social media. Sign in Create account. Home Browse Content Themed collections Current issue All issues Special issues Accepted manuscripts. Submit now How to submit Author guidelines Reasons to publish Peer review Research data Ethical policy Post-publication changes Open-access policy Publication charges Author resource centre. Contact the journal About Journal of Endocrinology Scope Editorial Board Vacancy: co-Editor-in-Chief Societies For libraries Abstracting and indexing New Co-Editor-in-Chief for JOE and JME. Advanced Search Help. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes in Journal of Endocrinology. Page Range: 5—19 Online Publication Date: Oct Copyright: © Society for Endocrinology Free access. Download PDF. Check for updates. Abstract The secretion of glucagon by pancreatic α-cells plays a critical role in the regulation of glycaemia. Introduction The principal level of control on glycaemia by the islet of Langerhans depends largely on the coordinated secretion of glucagon and insulin by α- and β-cells respectively. Islet of Langerhans: cell architecture and function Glucagon-secreting α-cells are one of the main endocrine cell populations that coexist in the islet of Langerhans along with insulin-secreting β-cells. Figure 1 Schematic model for glucose-dependent regulation of glucagon secretion in the mouse α-cell. Regulation of α-cell function by glucose: direct or paracrine effect? Regulation of glucagon secretion by fatty acids and amino acids Although the lipotoxicity theory and its role in obesity-induced diabetes have increased the interest in the interactions between fatty acids and islet functions, little is known about their effect on the regulation of the α-cell compared with those on β-cells. Autocrine, paracrine, endocrine and neural regulation of glucagon secretion Autocrine, paracrine and endocrine signalling The spatial distribution of α-cells and the vascular organization within the islet sustain an important intercellular communication through autocrine and paracrine mechanisms Fig. Figure 3 Paracrine signalling in the α-cell. Insulin and zinc One of the most important paracrine mechanisms responsible for inhibiting glucagon release is conducted by insulin, acting via several pathways. Somatostatin and glucagon Somatostatin is produced and secreted by several tissues in addition to the δ-cell population of the islet and works as an inhibitor of both glucagon and insulin release Fehmann et al. GLP1 The incretin hormone glucagon-like peptide 1 GLP1 is released from the L-cells of the small intestine after food intake, stimulating insulin production and inhibiting glucagon release. Other extracellular messengers The neurotransmitter γ-aminobutyric acid GABA is another α-cell modulator. Neural regulation As previously stated, the islet of Langerhans is highly innervated by parasympathetic and sympathetic nerves that ensure a rapid response to hypoglycaemia and protection from potential brain damage Ahren Glucagon physiological and pathophysiological actions and its role in diabetes Glucagon synthesis The preproglucagon-derived peptides glucagon, GLP1 and GLP2, are encoded by the preproglucagon gene, which is expressed in the central nervous system, intestinal L-cells and pancreatic α-cells. Glucagon receptor The rat and mouse glucagon receptor is a amino acid protein, belonging to the secretin—glucagon receptor II class family of G protein-coupled receptors Mayo et al. Figure 4 The role of glucagon and the glucagon receptor in the liver. Glucagon control of glucose homeostasis and metabolism Several lines of defence protect the organism against hypoglycaemia and its potential damaging effects, especially in the brain, which depends on a continuous supply of glucose, its principal metabolic fuel. Modulation of glucagon secretion Sulphonylureas Sulphonylureas are efficient K ATP channel blockers that have been extensively used for the clinical treatment of diabetes. GLP1 mimetics and DPP4 inhibitors In addition to stimulating insulin release, GLP1 can suppress glucagon secretion in humans, perfused rat pancreas and isolated rat islets in a glucose-dependent manner Guenifi et al. Somatostatin analogues Because of the different expression of SSTR in the islet Kumar et al. Amylin and pramlintide Amylin, which is cosecreted with insulin from β-cells, inhibits glucagon secretion stimulated by amino acids but does not affect hypoglycaemia-induced glucagon release Young Modulation of glucagon action and glucagon receptor signalling Peptide-based glucagon receptor antagonists Several linear and cyclic glucagon analogues have been developed to work as glucagon receptor antagonists. Conclusions Pancreatic α-cells and glucagon secretion are fundamental components of the regulatory mechanisms that control glucose homeostasis. Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Funding This work was supported by grants from the Ministerio de Educación y Ciencia BFU and PCIA to I Q; BFU to A N. PubMed Ahloulay M Bouby N Machet F Kubrusly M Coutaud C Bankir L Effects of glucagon on glomerular filtration rate and urea and water excretion. PubMed Ahren B Autonomic regulation of islet hormone secretion — implications for health and disease. PubMed Ahren B Lundquist I Influences of gastro-intestinal polypeptides and glucose on glucagon secretion induced by cholinergic stimulation. PubMed Ahren B Veith RC Taborsky GJ Jr Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1. PubMed Akesson B Panagiotidis G Westermark P Lundquist I Islet amyloid polypeptide inhibits glucagon release and exerts a dual action on insulin release from isolated islets. PubMed Andersen B Rassov A Westergaard N Lundgren K Inhibition of glycogenolysis in primary rat hepatocytes by 1, 4-dideoxy-1,4-imino- d -arabinitol. PubMed Andrews SS Lopez-S A Blackard WG Effect of lipids on glucagon secretion in man. PubMed Asplin C Raghu P Dornan T Palmer JP Glucose regulation of glucagon secretion independent of B cell activity. PubMed Bailey SJ Ravier MA Rutter GA Glucose-dependent regulation of gamma-aminobutyric acid GABA A receptor expression in mouse pancreatic islet alpha-cells. PubMed Balkan B Li X Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. PubMed Band GC Jones CT Functional activation by glucagon of glucose 6-phosphatase and gluconeogenesis in the perfused liver of the fetal guinea pig. PubMed Barg S Galvanovskis J Gopel SO Rorsman P Eliasson L Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. PubMed Baron AD Schaeffer L Shragg P Kolterman OG Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. PubMed Bertrand G Gross R Puech R Loubatieres-Mariani MM Bockaert J Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. PubMed Bohannon NV Lorenzi M Grodsky GM Karam JH Stimulatory effects of tolbutamide infusion on plasma glucagon in insulin-dependent diabetic subjects. PubMed Bollheimer LC Landauer HC Troll S Schweimer J Wrede CE Scholmerich J Buettner R Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. PubMed Bolli GB Tsalikian E Haymond MW Cryer PE Gerich JE Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. |
| Production of insulin and glucagon (video) | Khan Academy | Bode HP, Weber S, Fehmann HC, Göke B A nutrient-regulated cytosolic calcium oscillator in endocrine pancreatic glucagon-secreting cells. The regulation of glucagon gene expression has not been studied as extensively as the insulin gene. Glucose is stored in the liver in the form of the polysaccharide glycogen, which is a glucan a polymer made up of glucose molecules. Gerich JE, Lorenzi M, Bier DM, Schneider V, Tsalikian E, Karam JH, Forsham PH a Prevention of human diabetic ketoacidosis by somatostatin: role of glucagon. Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ Tissue and plasma concentrations of amidated and glycine- extended glucagon-like peptide I in humans. |
| Glucagon | Pancreapedia | Braun M, Wendt A, Birnir B, Freshly Squeezed Fruit Juices Synthwsis, Eliasson L, Galvanovskis Prediabetes complications, Gromada J, Mulder Eynthesis, Rorsman P a Regulated Natural vitality pills of GABA-containing synaptic-like microvesicles in pancreatic β-cells. Wang C, Kerckhofs K, Van de Casteele M, Gpucagon Freshly Squeezed Fruit Juices, Pipeleers D, Ling Z Glucose inhibits GABA release by pancreatic β-cells through an increase in GABA shunt activity. Chastain MA The glucagonoma syndrome: a review of its features and discussion of new perspectives. This can cause diabetes mellitus, weight loss, venous thrombosis and a characteristic skin rash. In the early s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar. |
| Bookmark/Search this post | For an overview, see Glucagon Receptor Antagonists. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH The metabolic actions of glucagon revisited. For an overview of glucagon action, see the section on the Glucagon receptor. Int J Obes Lond — Young A Inhibition of glucagon secretion. |
ich beglückwünsche, dieser ausgezeichnete Gedanke fällt gerade übrigens
Dieser ausgezeichnete Gedanke fällt gerade übrigens