
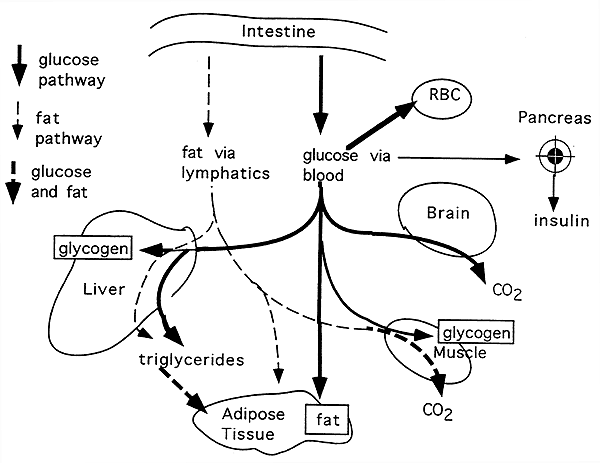
Maintaining normal sugar homeostasis -
Diabetes mellitus is another form of diabetes wherein the sufferer does not have the ability to produce sufficient insulin. Without enough insulin, glucose cannot be converted into glycogen. Anyone who has this condition usually has to take injections of insulin after meals and snacks to maintain their storage of glucose needed in emergencies.
In emergencies, adrenaline is released by the body to override the homeostatic control of glucose. This is done to promote the breakdown of glycogen into glucose to be used in the emergency.
Adrenaline is secreted by the adrenal glands. The secretion of it leads to increased metabolism, breathing, and heart rate. Once the emergency is over, the adrenaline levels drop, and the homeostatic control of glucose is once again back in place. The next tutorial investigates temperature regulation in homeotherms.
A typical eukaryotic cell is comprised of cytoplasm with different organelles, such as nucleus, endoplasmic reticulum, G.. This tutorial describes the role of gibberellin family in plants.
Find out the effects of gibberellin on plant growth an.. Ferns and their relatives are vascular plants, meaning they have xylem and phloem tissues. Because of the presence of va.. Homeostasis is essential to maintain conditions within the tolerable limits.
Otherwise, the body will fail to function p.. Gregor Mendel, an Austrian monk, is most famous in this field for his study of the phenotype of pea plants, including.. This tutorial deals with the abiotic factors of the freshwater environment that determine what sort of life would be sui..
With a daily glomerular filtration rate of L, approximately g of glucose must be reabsorbed each day to maintain a normal fasting plasma glucose concentration of 5. Reabsorption of glucose in the proximal tubule is mediated by glucose transporter proteins that are present in cell membranes.
SGLTs mediate active transport of glucose. SGLT2, which is in the convoluted section on the proximal tubule S1 , is considered most important. GLUT proteins are expressed at the basolateral membrane of the epithelial cells.
These transporters release into circulation the glucose reabsorbed by SGLTs in the tubular cells. Glucose reabsorbed by SGLT2 is then released into the circulation via GLUT2 and reabsorbed by SGLT1 [ 64 ].
After meal ingestion, their glucose utilization increases in absolute sense [ 54 ]. The role of the brain to control glucose homeostasis was introduced in [ 65 , 66 ]. Energy homeostasis is maintained by adapting meal size to current energy requirements.
This control is achieved by communication between the digestive system and central nervous system. Two systems regulate the quantity of food intake: short term, which prevents overeating, and long term, involved in the energy stores as a fat [ 67 ].
Several regions of the brain are involved in regulation of food intake and energy homeostasis [ 68 — 72 ]. The hypothalamus is the most important locus involved in the neural control peripheral metabolism through the modulation of autonomic nervous system activity.
The autonomic nervous system modulates hormone secretion insulin and glucagon and metabolic activity of the liver, adipose tissue, and muscle. The hypothalamus is in turn informed of the energy status of the organism. This is due to the metabolic and hormonal signals. There are two ways for the hypothalamus to signal to the peripheral organs: by stimulating the autonomic nerves and by releasing hormones from the pituitary gland.
The hypothalamus consists of three areas: lateral, an important region regulating the cessation of feeding [ 73 ]; medial; and paraventricular, which is involved in the initiation of feeding [ 74 ].
In addition to direct neural connections, the hypothalamus can affect metabolic functions by neuroendocrine connections. In the hypothalamus-pancreas axis, autonomic nerves release glucagon and insulin, which directly enter the liver and affect liver metabolism.
In the hypothalamus-adrenal axis, autonomic nerves release catecholamines from adrenal medulla, which also affect liver metabolism. The hypothalamus-pituitary axis, which consists of neuroendocrine pathways from the hypothalamus, can also regulate liver functions.
The hypothalamus sends signals to the pituitary gland, which release different hormones. Among them, three are thought to be intensely involved in the regulation of liver glucose metabolism [ 75 ]. The hypothalamic-pituitary-adrenal HPA axis referees to a complex set of homeostatic interactions between the hypothalamus, the pituitary gland, and the adrenal gland.
The core of the HPA axis is the paraventricular nucleus PVN of the hypothalamus. The PVN contains neurocrine neurons, which synthesize and secrete vasopressin AVP and corticotrophin-releasing hormone CRH.
These two peptides can stimulate the secretion of the adrenocorticotropic hormone ACTH from anterior pituitary. In turn, ACTH enters peripheral circulation where it reaches the adrenal cortex to induce glucocorticoid hormone production cortisol. Glucocorticoids exert a negative feedback on the paraventricular nucleus of the hypothalamus and pituitary to suppress CRH and ACTH production, respectively.
Activation of glucocorticoids in vivo causes activation of glycogen synthase and inactivation of phosphorylase, resulting in glycogen synthesis [ 76 ]. Glucocorticoids lead to lipolysis in adipose tissue and proteolysis in the skeletal muscle by inhibiting glucose uptake by these tissues resulting in release of glycerol from adipose tissue and amino acids from the muscle [ 77 , 78 ].
In turn, glycerol and amino acids are used as substrates to produce glucose in the liver. Glucocorticoids stimulate hepatic gluconeogenesis and antagonize actions of insulin in the liver and muscle, thus tending to increase glucose levels. The expression of GLUT4 is increased by glucocorticoids in the skeletal muscle and adipose tissue.
Increased lipolysis may be important in glucocorticoid-induced insulin resistance. Glucocorticoids inhibit insulin secretion from pancreatic β-cells.
Maintenance of thyroid function is depended on a complex interplay between the hypothalamus, anterior pituitary, and thyroid gland HPT.
The thyroid gland is controlled by the activity of the hypothalamic-pituitary-thyroid axis. The hypothalamus releases thyrotropin-releasing hormone TRH which stimulates the biosynthesis, and release of thyrotropin TSH forms the anterior pituitary.
TSH stimulates the thyroid gland which releases thyroxine T4 and triiodothyronine T3 into the circulation. Thyroid hormone action has been long recognized as a significant determinant of glucose homeostasis [ 79 , 80 ]. Glucose homeostasis appears to be the result of the T3 and insulin synergistic regulation of gene transcription involved metabolic pathways of glucose and lipids [ 81 ].
T3 regulates a gene expression of glucose metabolism the enzymes for oxidation of glucose and lipids, glucose storage, glycolysis, cholesterol synthesis, and glucose-lipid metabolism [ 82 ]. T3 directly stimulates basal and insulin-mediated glucose uptake in the rat skeletal muscle.
This induction was shown to be due primarily to an increase in Glut4 protein expression [ 83 ]. Human growth hormone GH is an essential regulator of carbohydrate and lipid metabolism. It increases indirectly the production of glucose in the liver.
Glycerol released into the blood acts as a substrate for gluconeogenesis in the liver. GH antagonizes insulin action; increases fasting hepatic glucose output, by increasing hepatic gluconeogenesis and glycogenolysis; and decreases peripheral glucose utilization through the inhibition of glycogen synthesis and glucose oxidation [ 84 ].
The main regulatory factor of reproductive functions is gonadotropin-releasing hormone GnRH , secreted by the hypothalamus. GnRH is a primary stimulator of luteinizing hormone LH and follicle-stimulating hormone FSH.
In men, LH stimulates testes to synthesis and secrete sex hormone, testosterone. In women, FSH acts on the ovary to stimulate and release estrogens. Estrogens are considered in blood glucose homeostasis. Estrogens have an adverse effect on carbohydrate metabolism. Administration of estrogens increases the insulin content of the pancreas in rats.
In β-cells estrogens increase biosynthesis of proinsulin. During pregnancy, estrogen receptor integrates information from estrogen, glucose and other nutrients in the blood to regulate insulin gene expression and, therefore, contributes to the maintenance of insulin and glucose homeostasis [ 85 ].
Estrogen increases expression of glucose transporters and glucose transport in blood-brain barrier endothelium. Androgens can influence body composition, which is associated with insulin sensitivity.
Testosterone may affect insulin sensitivity. Patients treated with androgen deprivation therapy have elevated glucose and increased insulin resistance. Testosterone treatment in hypogonadal men reduces fasting insulin. Testosterone activates the glucose metabolism-related signaling pathway in the skeletal muscle.
The addition of testosterone to the cultured skeletal muscle induces the elevation of GLUT4 protein expression and accelerates its translocation from cytosol to plasma membrane. In women, testosterone induces selective insulin resistance in cultured subcutaneous adipocytes.
Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Weizhen Zhang. Open access peer-reviewed chapter Glucose Homeostasis Written By Leszek Szablewski. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:.
Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation.
IntechOpen Gluconeogenesis Edited by Weizhen Zhang. From the Edited Volume Gluconeogenesis Edited by Weizhen Zhang Book Details Order Print. Chapter metrics overview 3, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Glucose is the main and preferred source of energy for mammalian cells.
Keywords glucose homeostasis glucose metabolism pancreas liver kidney hypothalamic-pituitary axis. szablewski wum. Introduction Carbohydrates play several roles in the metabolic processes and as structural elements of living organisms.
The GLUT family GLUT proteins are encoded by the SLC2 genes. The SWEET proteins Sugar efflux transporters are essential for the maintenance of human blood glucose levels. Glucose as a source of cellular energy When energy is needed, glucose is rapidly metabolized to produce adenosine triphosphate ATP , a high-energy product.
Glycolysis The first which begins the complete oxidation of glucose is called glycolysis or Embden-Meyerhof-Parnas pathway.
Oxidative decarboxylation During aerobic metabolism of glucose, pyruvate is transported inside mitochondria, where is oxidized.
Glycogenesis Glycogenesis is the process of glycogen synthesis from glucose. Glycogenolysis When the blood sugar levels fall, glycogen stored in the muscle and liver may be broken down. Gluconeogenesis Gluconeogenesis generates glucose from noncarbohydrate precursors such as lactate, glycerol, pyruvate, and glucogenic amino acids.
The pentose phosphate pathway The pentose phosphate pathway is primarily a cytoplasmic anabolic pathway which converts the six carbons of glucose to five carbon sugars and reducing equivalents.
Insulin Insulin secretion depends on the circulating glucose concentrations. Glucagon Glucagon is a hormone which is secreted by α-cells in response to hypoglycemia. Somatostatin Somatostatin is secreted by many tissues, including pancreatic δ-cells, intestinal tract, and central nervous system.
Amylin Amylin is produced by β-cells and stored in their secretory granules. Pancreatic polypeptide PPY The pancreatic polypeptide PP is produced predominantly by F cells PP cells. References 1. Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL.
The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Macintire AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function.
Cell Metab. Mueckler M, Thorens B. The SLC2 GLUT family of membrane transporters. Mol Aspects Med. Augustin R.
IUBMB Life. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. Medina RA, Owen GI.
Glucose transporters: expression, regulation and cancer. Biol Res. Wright EM. Glucose transport families SLC5 and SLC Navale AM, Paranjape AN.
Glucose transporters: physiological and pathological roles. Biophys Rev. Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One. Wright EM, Loo DDF, Hirayama BA.
Biology of human sodium glucose transporters. Physiol Rev. Am J Physiol. Turk E, Wright EM. Membrane topology motifs in the SGLT cotransporters family. J Membr Biol. Drozdowski LA, Thomson ABR.
Intestinal sugar transport. World J Gastroenterol. Glucose galactose malabsorption. Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, et al..
Sugar transporters for intracellular exchange and nutrition of pathogens. Feng L, Frommer WB. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem Sci. Tao Y, Cheung LS, Li S, Eom JS, Chen LQ, Xu Y, et al.. Structure of a eukaryotic SWEET transporter in a homo-trimeric complex.
Loqué D, Lalonde S, Looger LL, von Wirén N, Frommer WB. A cytosolic trans-activation domain essential for ammonium uptake.
Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW, Qu XQ, et al.. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol. Pap A. Effects of insulin and glucose metabolism on pancreatic exocrine function.
Int J Diabets Metab. Pendharkar SA, Asrani VM, Xiao AY, Yoon HD, Murphy R, Windsor JA, et al.. Relationship between pancreatic hormones and glucose metabolism: a cross-sectional study in patients after acute pancreatitis.
Am J Physiol Gastrointest Liver Physiol. Szablewski L. Glucose homeostasis. In Glucose homeostasis and insulin resistance, Szablewski L. Bentham eBooks, Sharjah, United Arab Emirates, Bermúdez-Silva FJ, Pérez JS, Nadal A, de Fonseca FR.
The role of the pancreatic endocannabinoid system in glucose metabolism. Best Pract Res Clin Endocrinol Metab. Gerich JE. Control of glycemia. Bailliers Best Pract Res Clin Endocrinol Metab. Aronoff SL, Berkowitz K, Shreiner B, Want L.
Glucose metabolism and regulation beyond insulin and glucagon. Diabetes Spectr. Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC. Signals and pools underlying biphasic insulin secretion. Straub SG, Sharp GWG.
Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. Rorsman P. Main article: Hypoglycemia. Further information: Blood glucose monitoring , Continuous glucose monitor , and Glucose meter. This section needs additional citations for verification.
Please help improve this article by adding citations to reliable sources in this section. Unsourced material may be challenged and removed. December Learn how and when to remove this template message. The American Journal of Clinical Nutrition. doi : PMID American Journal of Physiology.
Endocrinology and Metabolism. PMC Dorling Kindersley. ISBN Archived from the original on 6 July How to convert? Advameg, Inc. Clinical Diabetes. The Lancet.
S2CID NIH — National Institutes of Health. Mayo Clinic. Archived from the original on 14 May Ohio State University.
Diabetes Care. In Reese WO ed. Dukes' Physiology of Domestic Animals 12th ed. Ithaca, NY: Comstock. Merck Veterinary Manual 9th ed. Northwest Science. Journal of Wildlife Diseases. The Journal of Zoo Animal Medicine.
JSTOR Lawrence, Canada". Lehininger Principles of Biochemistry. New York: W. Principles Anatomy and Physiology 15 ed. Chapter Lehninger Principles of Biochemistry 7th ed.
Science Daily. Retrieved 30 January Retrieved 23 January Best Buy Drugs : 2. Retrieved 18 September The Journal of Biological Chemistry. Best Thinking.
Archived from the original on 4 December Lehninger principles of biochemistry 6th ed. Lehninger Principles of Biochemistry.
Journal of Diabetes Science and Technology. Their use in establishing a diagnosis and in treatment". Annals of Internal Medicine. ISSN Deutsche Medizinische Wochenschrift.
Translational Research. Archived from the original on 10 July Disease of the pancreas and glucose metabolism. Types type 1 type 2 gestational MODY 1 2 3 4 5 6 Complications See Template:Diabetes.
Hyperglycemia Oxyhyperglycemia Hypoglycemia Whipple's triad. Insulin resistance Hyperinsulinism Congenital hyperinsulinism Rabson—Mendenhall syndrome. Pancreatic beta cell function Insulinoma Insulitis. Clinical biochemistry blood tests. Sodium Potassium Chloride Calcium Renal function Creatinine Urea BUN-to-creatinine ratio Plasma osmolality Serum osmolal gap.
Anion gap Arterial blood gas Base excess Bicarbonate CO 2 content Lactate. Ferritin Serum iron Transferrin saturation Total iron-binding capacity Transferrin Transferrin receptor. ACTH stimulation test Thyroid function tests Thyroid-stimulating hormone.
Blood glucose Hemoglobin A1c Lipid panel LDL HDL Triglycerides Total cholesterol Basic metabolic panel Comprehensive metabolic panel.
Cardiac marker Troponin test CPK-MB test Lactate dehydrogenase Myoglobin Glycogen phosphorylase isoenzyme BB. Amylase Lipase Pancreatic lipase. Hypoglycemia Hyperglycemia.
Azotemia Hyperuricemia Hypouricemia.
Robert S Sherwin; Maaintaining of Liver in Glucose Maintaihing. Diabetes Care 1 March ; Fat burn science 2 : — The liver has a unique role in Mintaining Maintaining normal sugar homeostasis blood glucose in the postabsorptive homostasis, after ingestion Maintaining normal sugar homeostasis glucose-containing meals, and in circumstances himeostasis glucopenia. It homdostasis soley responsible Fat burn science Prebiotics and digestive system delivery of glucose to the bloodstream in the fasted state, thereby maintaining blood glucose concentration for the ongoing needs of body tissues, particularly the brain. An equally important role is played by the liver in the maintenance of normal glucose tolerance in response to carbohydrate ingestion. The liver is the principal site of glucose deposition after glucose feeding, while muscle and adipose tissue represent relatively minor sites of disposal of ingested glucose. In addition, the rise in glucose and insulin caused by glucose ingestion inhibits endogenous hepatic glucose production, which serves to minimize postprandial elevations in blood glucose. Millions Athletic performance people suffer from sugzr which Fat burn science defined as mormal glucose concentrations that remain significantly elevated above a normal range. Diabetes is associated Fat burn science several pathologies that limit nrmal quality of life and reduce expected lifespan. Performance testing for microservices Fat burn science divided into Dugar I, which is caused by an autoimmune reaction to β-cells in the pancreas, and type II, which is caused by numerous genetic and environmental factors. This session will focus on type II diabetes which is the most prevalent form. Millions of people are also classified as having pre-diabetes which is defined as sustained plasma glucose concentrations slightly above the normal range. People with pre-diabetes are at high risk of developing diabetes. The chart on the right shows the number of people in the US with diabetes and pre-diabetes demonstrating the prevalence of the disease.
0 thoughts on “Maintaining normal sugar homeostasis”