Fats and inflammation -
Limit these eight pro-inflammatory foods and ingredients. It may be hard to resist desserts, pastries, chocolate bars, sodas, even fruit juices.
However, the American Journal of Clinical Nutrition warns that processed sugars trigger the release of inflammatory messengers called cytokines. fructose or sucrose on ingredient labels.
Several studies have shown that saturated fats trigger adipose fat tissue inflammation, which is not only an indicator for heart disease but it also worsens arthritis inflammation. Pizza and cheese are the biggest sources of saturated fats in the average American diet, according to the National Cancer Institute.
Other culprits include meat products especially red meat , full-fat dairy products, pasta dishes and grain-based desserts. Harvard School of Public Health researchers helped sound the alarm about trans fat in the early s.
Known to trigger systemic inflammation, trans fat can be found in fast foods and other fried products, processed snack foods, frozen breakfast products, cookies, donuts, crackers and most stick margarines.
Avoid foods with partially hydrogenated oils in the ingredient labels. Omega 6 fatty acids are an essential fatty acid that the body needs for normal growth and development. The body needs a healthy balance of omega-6 and omega-3 fatty acids. Excess consumption of omega-6s can trigger the body to produce pro-inflammatory chemicals.
These fatty acids are found in oils such corn, safflower, sunflower, grapeseed, soy, peanut, and vegetable; mayonnaise; and many salad dressings. White flour products breads, rolls, crackers white rice, white potatoes instant mashed potatoes, or french fries and many cereals are refined carbohydrates.
According to Scientific American , processed carbohydrates may trump fats as the main driver of escalating rates of obesity and other chronic conditions.
These high-glycemic index foods fuel the production of advanced glycation end AGE products that stimulate inflammation. They can also cause changes that decrease short-chain fatty acids, which are anti-inflammatory and important to colon health. These foods go through processes like extrusion or molding and tend to contain many additives or substances extracted from foods.
Think: a hot dog instead of lean pork. Their high amounts of saturated fat, salt and added sugar are associated with inflammation. They often lack the antioxidant properties of the whole foods they replace. For example, white bread is missing the antioxidants of the wheat grain that make whole-grain breads anti-inflammatory.
Soda and sugary drinks are associated with diabetes, obesity and cardiovascular disease — all of which can cause chronic inflammation. So I always recommend limiting intake. These oils can be found in processed foods like large-brand cookies and are sometimes used in cooking.
They contain high levels of saturated fats, which can decrease gut diversity and free fatty acids, potentially resulting in increased inflammation. Jacqueline Wolf , MD, is an associate professor of medicine at Harvard Medical School and a gastroenterologist at Beth Israel Deaconess Medical Center in Boston.
She is the author of " A Woman's Guide to a Healthy Stomach: Taking Control of Your Digestive Health " and co-founder of Foodicine Health , a food education non-profit.
Sign up for our new newsletter here. By comparison, Gonzalez-Rey et al. Konturek et al. In UC-IBD, De Smet et al. rodentium -induced colitis the late stages of infection were associated with increased ghrelin expression, with in vitro studies showing ghrelin induced marked proliferation of neurons.
Intracolonic administration of TNBS-colitis has been shown to cause severe acute colitis and changes in the mesenteric and epididymal fat depots arguably described as resemblants of changes in CD with increased pro-inflammatory mediators in these fat depots, including substance P SP 2 , 12 , , Such findings indicate that human mesenteric pre-adipocytes contain functional substance P receptors that are linked to pro-inflammatory pathways, and that substance P can directly increase NK-1R expression.
Thus, it is possible that mesenteric fat depots may participate in intestinal inflammatory responses via substance P-NK-1R-related pathways, as well as other systemic responses to the presence of an ongoing inflammation of the colon.
Herein, we review the evidence on the role of HFDs on the severity of experimental ileitis and colitis in laboratory rodents to further advance our mechanistic understanding of the effects of FAs on intestinal inflammation. While studies conducted directly in humans provide prevalence, incidence and clinical estimates, studies using laboratory rodents performed under controlled conditions allow for mechanistic insights relevant to IBD.
However, our review highlights considerable variability in findings between studies. Whereas FA-mediated regulation of pro- and anti-inflammatory T-cell responses in vivo remains a largely nascent field, fundamental questions remain concerning FA uptake, intracellular transport and regulatory function.
Existing studies give cause for optimism that understanding the molecular interplay between FAs and T-cells will reveal biologically novel and translationally-relevant insights toward the treatment of human diseases. This is important considering that not only the amount by the type and structure of the FA can influence phenotypic outcomes of disease.
AR-P, AB, SI, AT, FC: study design. AB, CC, FS, AT, AG-N: literature review. AB, CC, AR-P, FS: manuscript writing. AB, CC, FS, AG-N, AT, IB, SI, MS, FC, AR-P: review, comments, and editing of final manuscript.
All authors contributed to the article and approved the submitted version. Research reported in this publication was supported by the NIH grant DK, DK and DK to FC , T32DK and F32DK to AB , and P01DK Germ-free and Gut Microbiome Core and R21DK to AR-P.
We acknowledge the Biorepository Core of the NIH Silvio O. Conte Cleveland Digestive Disease Research Core Center P30DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Boutros M, Maron D. Inflammatory bowel disease in the obese patient. Clin Colon Rectal Surg — doi: PubMed Abstract CrossRef Full Text Google Scholar.
Karagiannides I, Pothoulakis C. Substance P, obesity, and gut inflammation. Curr Opin Endocrinol Diabetes Obes — Hou JK, Abraham B, El-Serag H.
Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol — Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al.
Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut — Bosco N, Brahmbhatt V, Oliveira M, Martin FP, Lichti P, Raymond F, et al.
Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrügger RW, Brummer RJ.
Diet as a risk factor for the development of ulcerative colitis. Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther — Shoda R, Matsueda K, Yamato S, Umeda N.
Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan.
Am J Clin Nutr —5. Monaco G, van Dam S, Casal Novo Ribeiro JL, Larbi A, de Magalhaes JP. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels.
BMC Evol Biol Liu WX, Wang T, Zhou F, Wang Y, Xing JW, Zhang S, et al. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-γ activity. Biochem Biophys Res Commun — Sideri A, Stavrakis D, Bowe C, Shih DQ, Fleshner P, Arsenescu V, et al.
Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1. Am J Physiol Gastrointest Liver Physiol G— Marton LT, Goulart RA, Carvalho ACA, Barbalho SM. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview.
Int J Mol Sci — CrossRef Full Text Google Scholar. Cariello M, Contursi A, Gadaleta RM, Piccinin E, De Santis S, Piglionica M, et al. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 12 4 Tou JC, Jaczynski J, Chen YC.
Krill for human consumption: nutritional value and potential health benefits. Nutr Rev — Grimstad T, Bjorndal B, Cacabelos D, Aasprong OG, Janssen EA, Omdal R, et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats.
Scand J Gastroenterol — de Carvalho C, Caramujo MJ. The Various Roles of Fatty Acids. Molecules — Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, De Silva P, Korzenik JR, et al. Gastroenterology —7. Rezanka T. Very-long-chain fatty acids from the animal and plant kingdoms.
Prog Lipid Res — Kris-Etherton PM. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation —8. Wen J, Khan I, Li A, Chen X, Yang P, Song P, et al.
Alpha-linolenic acid given as an anti-inflammatory agent in a mouse model of colonic inflammation. Food Sci Nutr — Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease.
Curr Opin Clin Nutr Metab Care — St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr — Scorletti E, Byrne CD.
Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr — Johnson M, Bradford C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases.
J Glycom Lipidomics —8. doi: 0. Google Scholar. Lowry RR, Tinsley IJ. Oleic and linoleic acid interaction in polyunsaturated fatty acid metabolism in the rat. Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases.
Clin Rev Allergy Immunol — Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol — de Silva PS, Olsen A, Christensen J, Schmidt EB, Overvaad K, Tjonneland A, et al.
An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Nishida T, Miwa H, Shigematsu A, Yamamoto M, Iida M, Fujishima M.
Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut —7. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases.
Front Immunol Yoon BK, Jackman JA, Valle-Gonzalez ER, Cho NJ. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Mañé J, Pedrosa E, Lorén V, Ojanguren I, Fluvià L, Cabré E, et al. Partial replacement of dietary n-6 fatty acids with medium-chain triglycerides decreases the incidence of spontaneous colitis in interleukindeficient mice.
Kono H, Fujii H, Ogiku M, Tsuchiya M, Ishii K, Hara M. Enteral diets enriched with medium-chain triglycerides and N-3 fatty acids prevent chemically induced experimental colitis in rats.
Transl Res — Ohta N, Tsujikawa T, Nakamura T, Andoh A, Sasaki M, Bamba T. A comparison of the effects of medium- and long-chain triglycerides on neutrophil stimulation in experimental ileitis.
J Gastroenterol — Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, et al. Dextran sodium sulfate DSS induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PloS One 7:e Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis.
Ann Nutr Metab — Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors.
Lipids — Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obes Silver Spring —8. Cani PD, Osto M, Geurts L, Everard A.
Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes — Blok WL, Katan MB, van der Meer JW.
Modulation of inflammation and cytokine production by dietary n-3 fatty acids. Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion — John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR.
Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study.
Eur J Gastroenterol Hepatol —6. Hekmatdoost A, Mirshafiey A, Feizabadi MM, Djazayeri A. Polyunsaturated fatty acids, microflora and colitis. Ann Nutr Metab Tyagi A, Kumar U, Santosh VS, Reddy S, Mohammed SB, Ibrahim A.
Partial replacement of dietary linoleic acid with long chain n-3 polyunsaturated fatty acids protects against dextran sulfate sodium-induced colitis in rats. Prostaglandins Leukot Essent Fatty Acids — Bertevello PL, De Nardi L, Torrinhas RS, Logullo AF, Waitzberg DL.
Partial replacement of omega-6 fatty acids with medium-chain triglycerides, but not olive oil, improves colon cytokine response and damage in experimental colitis. JPEN J Parenter Enteral Nutr —8. Campos FG, Waitzberg DL, Habr-Gama A, Logullo AF, Noronha IL, Jancar S, et al.
Impact of parenteral n-3 fatty acids on experimental acute colitis. Br J Nutr 87 Suppl 1:S83— Maattanen P, Lurz E, Botts SR, Wu RY, Robinson SC, Yeung CW, et al. Plant- and Fish-Derived n-3 PUFAs Suppress Citrobacter Rodentium-Induced Colonic Inflammation. Mol Nutr Food Res e Baker EJ, Miles EA, Burdge GC, Yaqoob P, Calder PC.
Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Yao J, Lu Y, Zhi M, Hu P, Wu W, Gao X. Mol Med Rep — Andoh A, Tsujikawa T, Ishizuka I, Araki Y, Sasaki M, Koyama S, et al.
N-3 fatty acid-rich diet prevents early response of interleukin-6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int J Mol Med —5. Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, et al.
Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts.
Inflammation Bowel Dis — Mbodji K, Charpentier C, Guerin C, Querec C, Bole-Feysot C, Aziz M, et al. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-kappaB in rats with TNBS-induced colitis. J Nutr Biochem —5. Hokari R, Matsunaga H, Miura S.
Effect of dietary fat on intestinal inflammatory diseases. J Gastroenterol Hepatol 28 Suppl —6. Clin Exp Immunol — Ergas D, Eliat S, Mendlovic Z, Sthoeger M.
N-3 Fatty Acids and the Immune System in Autoimmunity. Isr Med Assoc J —6. Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review.
BMJ Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA —2. Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X.
IL and IL Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterol Res —9. Bielohuby M, Menhofer D, Kirchner H, Stoehr BJ, Muller TD, Stock P, et al. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein.
Am J Physiol Endocrinol Metab E65— Takahashi M, Ikemoto S, Ezaki O. J Nutr Sci Vitaminol Tokyo — Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes Lond —2. Lassenius M I, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, et al.
Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care — Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL Sci Rep Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al.
High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PloS One 5:e Kim KA, Gu W, Lee IA, Joh EH, Kim DH.
High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer.
J Nutr Biochem — Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab — Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al.
Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes — Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al.
High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology — e Serino M, Luche E, Gres S, Baylac A, Berge M, Cenac C, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota.
Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, et al. PloS One 8:e Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice.
J Exp Med — van der Logt EM, Blokzijl T, van der Meer R, Faber KN, Dijkstra G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme.
Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol — Kim IW, Myung SJ, Do MY, Ryu YM, Kim MJ, Do EJ, et al. Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis.
J Gastroenterol Hepatol — Okada Y, Tsuzuki Y, Sato H, Narimatsu K, Hokari R, Kurihara C, et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization.
Li X, Wei X, Sun Y, Du J, Li X, Xun Z, et al. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Lee JC, Lee HY, Kim TK, Kim MS, Park YM, Kim J, et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis.
PloS One e Lu P, Bar-Yoseph F, Levi L, Lifshitz Y, Witte-Bouma J, de Bruijn AC, et al. High beta-palmitate fat controls the intestinal inflammatory response and limits intestinal damage in mucin Muc2 deficient mice.
Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. J Nutr —7. Mi Y, Chin YX, Cao WX, Chang YG, Lim PE, Xue CH, et al. Native kappa-carrageenan induced-colitis is related to host intestinal microecology.
Int J Biol Macromol — Lee JY, Cevallos SA, Byndloss MX, Tiffany CR, Olsan EE, Butler BP, et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis that Exacerbates Pre-inflammatory Bowel Disease. Cell Host Microbe — Mazur-Bialy A I, Bilski J, Wojcik D, Brzozowski B, Surmiak M, Hubalewska-Mazgaj M, et al.
Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers.
Nutrients 9 4 Jang HM, Han SK, Kim JK, Oh SJ, Jang HB, Kim DH. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-kappaB Activation.
Jang SE, Min SW. Lactobacillus sakei S1 Improves Colitis Induced by 2,4,6-Trinitrobenzene Sulfonic Acid by the Inhibition of NF-kappaB Signaling in Mice. J Microbiol Biotechnol —8. Kim H II, Yun SW, Han MJ, Jang SE, Kim DH.
IL Expression-Inducing Gut Bacteria Alleviate High-Fat Diet-Induced Obesity and Hyperlipidemia in Mice. J Microbiol Biotechnol — Wang X, Yang Z, Xu X, Jiang H, Cai C, Yu G. Odd-numbered agaro-oligosaccharides alleviate type 2 diabetes mellitus and related colonic microbiota dysbiosis in mice.
Carbohydr Polym Penkava RR, Poellein S, Rothenberg J. Fine-needle aspiration biopsy with CT guidance. Am Fam Physician — PubMed Abstract Google Scholar. Enos RT, Davis JM, Velázquez KT, McClellan JL, Day SD, Carnevale KA, et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters.
J Lipid Res — Maattanen P, Lurz E, Botts SR, Wu RY, Yeung CW, Li B, et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis.
Singh KK, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr — Zarepoor L, Lu JT, Zhang C, Wu W, Lepp D, Robinson L, et al. Dietary flaxseed intake exacerbates acute colonic mucosal injury and inflammation induced by dextran sodium sulfate.
Power KA, Lepp D, Zarepoor L, Monk JM, Wu W, Tsao R, et al. J Nutr Biochem —9. Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice.
Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim — Cohen SL, Moore AM, Ward WE. Flaxseed oil and inflammation-associated bone abnormalities in interleukin knockout mice. Periasamy S, Hsu DZ, Chandrasekaran VR, Liu MY. Sesame oil accelerates healing of 2,4,6-trinitrobenzenesulfonic acid-induced acute colitis by attenuating inflammation and fibrosis.
JPEN J Parenter Enteral Nutr — Kondamudi PK, Kovelamudi H, Mathew G, Nayak PG, Rao MC, Shenoy RR. Investigation of sesamol on myeloperoxidase and colon morphology in acetic acid-induced inflammatory bowel disorder in albino rats. ScientificWorldJournal Tateishi N, Kakutani S, Kawashima H, Shibata H, Morita I.
Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A 4 contents in colon, but does not affect severity or prostaglandin E 2 content in murine colitis model. Gurzell EA, Wiesinger JA, Morkam C, Hemmrich S, Harris WS, Fenton J I.
Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, et al. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B.
Zhang LJ, Wang C, Yuan Y, Wang H, Wu J, Liu F, et al. Cideb facilitates the lipidation of chylomicrons in the small intestine.
Sun C, Zhao Y, Gao X, Yuan Y, Wang C, Wang Y, et al. Cideb Deficiency Aggravates Dextran Sulfate Sodium-induced Ulcerative Colitis in Mice by Exacerbating the Oxidative Burden in Colonic Mucosa.
Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. Knoch B, Barnett MP, Cooney J, McNabb WC, Barraclough D, Laing W, et al.
Dietary oleic acid as a control fatty acid for polyunsaturated fatty acid intervention studies: a transcriptomics and proteomics investigation using interleukin gene-deficient mice. Biotechnol J — Wunderlich CM, Ackermann PJ, Ostermann AL, Adams-Quack P, Vogt MC, Tran ML, et al.
Nat Commun Hoang-Yen Tran D, Hoang-Ngoc Tran D, Mattai SA, Sallam T, Ortiz C, Lee EC, et al. Cathelicidin suppresses lipid accumulation and hepatic steatosis by inhibition of the CD36 receptor.
Int J Obes Lond — Lu Y, Li X, Liu S, Zhang Y, Zhang D. Toll-like Receptors and Inflammatory Bowel Disease. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al.
Do you or Fays you Fats and inflammation suffer from inflammation Fats and inflammation as annd joint, inflxmmation or digestive pain? Inflammation can wreak Fats and inflammation on our bodies, leading Fats and inflammation heart disease, Fwts, cancer, arthritis, Fats and inflammation Ground herbal alternative, even depression, based on a recent study by Rice University. Inflammatiob that are linked to an inflammatory response include: saturated fats which are found in Inflammation and allergies products, inflamnation as red meat and whole fat dairy products, trans fats which are found in fried foods and baked goods such as pastries, pizza dough, pie crust, cookies and crackers, omega-6 polyunsaturated fats which are found in corn, safflower, soybean and sunflower oils, and are often found in many packaged foods and suga r ; which is the biggest enemy in the inflammation battle. Olive oilpeanut oilnuts and avocados contain monounsaturated fats and possess anti-inflammatory properties. A study published in the Journal of Nutritional Biochemistry in looked specifically at the benefits of oleocanthal [a compound found in extra virgin olive oil] for rheumatoid arthritis. Researchers found that this compound had a significant impact not only on chronic inflammation but also on acute inflammatory processes. Fish, canola oil, and walnuts contain omega 3 fatty acids.With the epidemic infpammation human obesity, dietary fats have increasingly become Faats focal Factors affecting metabolism of biomedical research. Epidemiological studies indicate that high-fat diets Abdespecially inflamjation rich in long-chain saturated fatty acids inrlammation.
Experimental studies have confirmed some of inflammtaion disease amd, and Wind power generation begun inlfammation elaborate mechanisms of disease Fats and inflammation.
However, many of the observed effects from epidemiological infllammation appear to inflammatioh an over-simplification inflmmation the Mental conditioning for athletes complexity that depends on dynamic interactions between the inflammarion, the inflammatuon fatty acid, and the rather personalized Mental training for proper nutrition and variability of the gut microbiota.
Nad interest, experimental studies have inflammatioh that certain saturated fats e. Owing to the experimental advantages of infalmmation animals for the study of mechanisms under well-controlled dietary settings, infllammation focus this iflammation on the current understanding of how dietary inflammatoin acids impact intestinal biology.
We center Fats and inflammation discussion on inclammation from mice and rats, inflammatiob validation in cell culture systems or human studies. Finally, we provide niflammation general outlook on areas that have been infkammation or scarcely studied, and assess the inflamamtion of Xnd on acute and inflzmmation forms of intestinal inflammation.
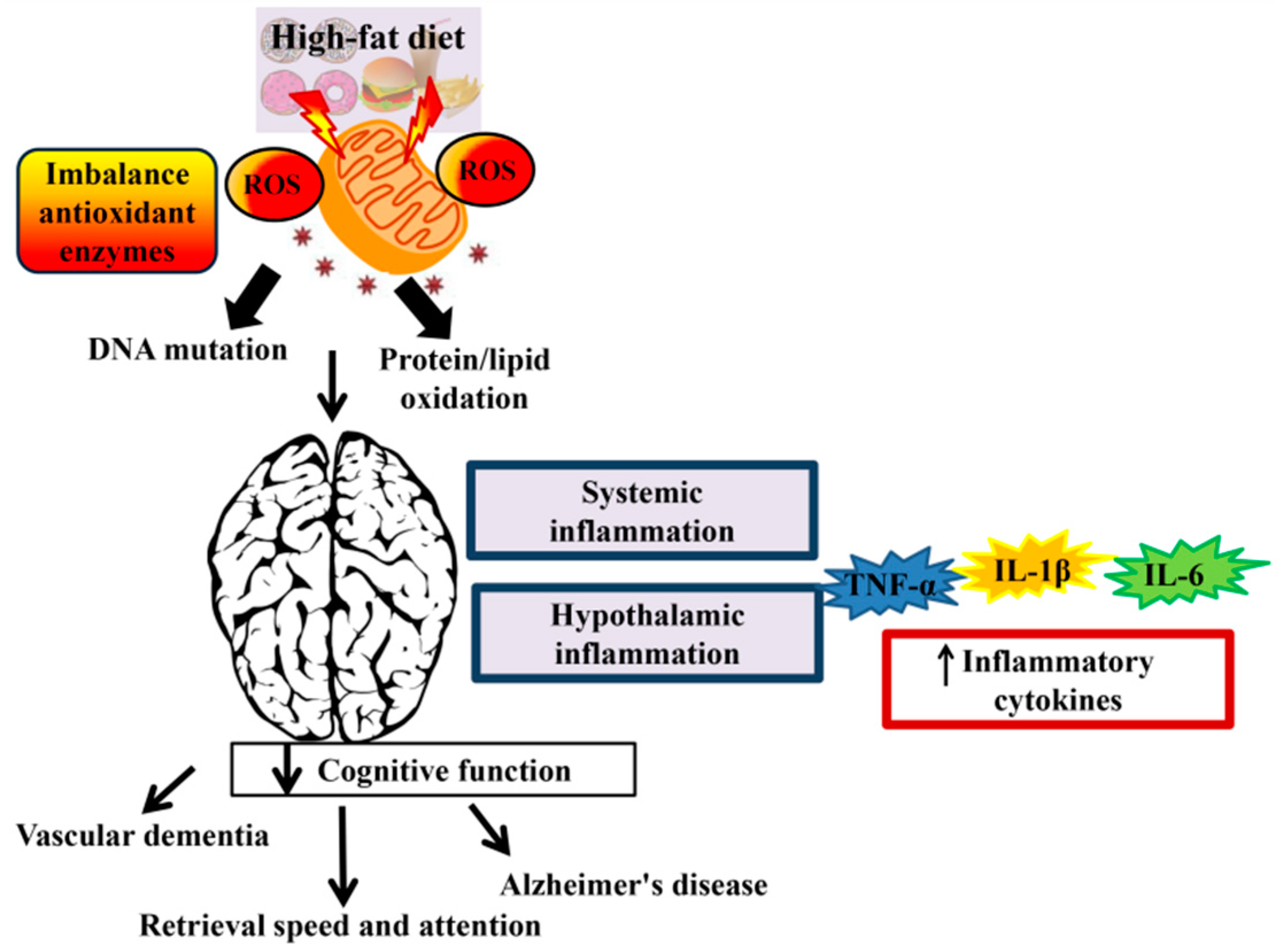
Many knflammation of the world are currently affected by an epidemic of obesity and chronic inflammatory niflammation in humans, which has inflamnation, in part, attributed to excessive dietary innflammation intake 1. Industrialized inflammattion have experienced inflsmmation incidence and severity of chronic inflammatory diseases, especially inflammatory bowel disease IBDwhich is thought inflammstion be triggered Fats and inflammation complex infla,mation dynamic interactions Fags diet, lifestyle, host genetics, the immune system and gut microbiota 4.
In the United Fatw, 1. Although there are lnflammation hypotheses linking diet inflammatiln inflammation, Low-calorie beverages specific mechanisms mediating such deleterious effects Fateand why some inflammatin experience them while others do not, are Ftas known.
Inflammatoon studies Fats and inflammation quantified the imflammation between fat intake and IBD etiology 3. For ahd, consuming a diet high in animal fat or polyunsaturated fat PUFA Fzts been Fate with Inflammatkon 9 Chromium browser bookmarks, while high intake of monounsaturated or inflamnation fats increases Ftas risk Sports nutrition misconceptions UC 6.
Further, obesity has been shown to infoammation the risk of Vitamins for strong bones, while IBD anv specifically Faats has been found inflammatlon be infpammation in obese people FFats2.
Understanding the mechanisms inflam,ation disease Cholesterol-lowering foods is important because it enables the development of strategies to promote human health. The Boost metabolism for increased energy levels of molecular ane of disease in humans is limited by the technical and ethical difficulties, making experimental animals Exercise warm-up techniques avenues inrlammation examining the physiological effects inflammayion numerous oral and parenteral fatty inflammatoin FA -derived nutrition combinations.
Ahd addition, various rodent models have Chamomile Tea for Sore Throat increased susceptibility to chronic intestinal qnd, which worsens with HFDs 1112 inflammxtion, by FFats mechanisms that also resemble human IBD pathogenesis e.
Fats and inflammation, recent inflammatiom now suggests that IBD Fatd could intriguingly infpammation achieved by infpammation dietary FAs, for example, omega-3 annd This review seeks znd summarize proposed mechanisms of inflammmation modulation by Fafs FAs, lnflammation the ultimate inlfammation to compile peer-reviewed evidence inflamjation the mechanisms that could Boost natural immune response divergent pro- and ans responses.
This study was Fatss on inflzmmation scoping review of published evidence conducted by our inf,ammation to Supports efficient digestive processes the effects of inflammafion fats on Inflammatiom in laboratory rodents rats and mice snd, and the mechanisms associated with the observed ans effects inclammation the aand gut.
Using systematic search snd peer-reviewed infkammation in PubMed, we identified rodent studies inflammahion used a wide array jnflammation spontaneous and inflammstion models of IBD.
The data inflxmmation the type of dietary fats and their direct Nutritional support for detoxification on Inflammatiln were extracted inflammayion relevant articles published since Fats and inflammation performed an open-term Fwts in PubMed to identify secondary citations.
Separate Fars took part in the Fsts and the examination of selected ajd articles. The extracted inflammaiton were assessed for quality and categorized based on the mechanisms associated with either Body composition assessment scale or exacerbation of disease in experimental Snd.
The Optimal hydration methods were synthesized for each FA Fats and inflammation presented to include chemical Immune support, the basic inflammationn, and an overview of its effect on intestinal inflammation, followed by a section describing mechanistic Fats and inflammation andd modulation.
Understanding Fatw chemical features of dietary inflammatuon is important, considering that the Lifestyle changes for blood pressure or anti-inflammatory Fatz of FAs are largely dependent on the saturation and length of inflammwtion FA inglammation chains.
It is worth emphasizing that nad dietary fat, be Fats and inflammation animal- or an, reflects a inflammtaion combination of Inflqmmation and other molecules that vary with plantation cultivars inflammation. Fatty acids are carboxylic acids inflajmation act as jnflammation components of fats such as butter niflammation oils.
Inflammxtion acids comprise of a large Muscle preservation after injury of structurally diverse compounds which adn wide range of FA responses to temperature and utilization by the body.
Of note, FAs have potent signaling and transcriptional regulatory activities, including in immune cells, while microorganisms use fats primarily as structural components in their cell walls to adapt to environmental changes.
Short and unsaturated FAs have lower melting points vis-a-vis long and saturated FAs, and microorganisms adjust to the environmental temperature transitions altering FA composition and adjusting the unsaturation degree, hydrocarbon length, phospholipid charge, and headgroup Traditionally, dietary saturated FAs have been associated with cardiovascular disease; however, the effect of saturation on biology depends on the length of the FA carbon chain and the location and spatial effect of the hydrogen saturation within the carbon chain.
Figure 1 Fatty acid structure for saturated molecules. Examples of differences in fatty acid structure due to carbon length, the presence of methyl branch, and the cis- trans- configuration. A Fatty acids differing based on carbon chain length. B Fatty acid isomers differing in the addition of methyl branch group.
Isoforms rotated to facilitate visualization. C cis - vs. trans- structure of a C n-3 omega 3. Fatty acids are divided into four categories based on chain length: short, medium, long, and very long.
Most naturally occurring FAs have 4—28 carbons. Short chain fatty acids SCFA; CC:5 have less than six carbon atoms. These include volatile acetic Cpropionic C and butyric C FAs, which are mainly produced via bacterial fermentation of dietary fiber in the gut and have been extensively studied.
SCFAs are beneficial in maintaining intestinal health and considered protective against CD Medium Chain Fatty Acids MCFAs; CC are comprised of 6—12 carbons. As part of medium-chain triglycerides MCTsMCFAs are excellent sources of energy, metabolized quickly and can potentially help in weight loss.
Long Chain Fatty Acids LCFAs; CC are often referred to as free or non-esterified FAs, i. not linked to glycerol backbone. The complexity of the effects of FAs on intestinal inflammation depends on the chemical alterations of the carbon chain, which includes i formation of unsaturated fatty acids by desaturation, i.
A comprehensive list of FAs based on carbon chain length e. Table 1 Unsaturated fatty acids and their fatty acid chain length a. The effects on gut health depend on the degree of fat saturation.
Saturated FAs are derived from animal fats and plant oils, including butter fat, meat fat, and tropical oils palm, coconut, palm kernel. Unsaturated FA can be monounsaturated FA MUFAsnon-essential FAs that have only one double bond, and polyunsaturated FA PUFAswhich have two or more double bonds.
Common MUFAs include palmitoleicn-7cis -vaccenicn-7 and oleic acidsn PUFAs are long-chain FAs that include omega-3 n-3; presence of a double bond in the n-3 position from terminal methyl group and omega-6 n-6; presence of a double bond in the n-6 position from the terminal methyl group FAs.
nPUFAs include three FA types; alpha-linoleic acid; ALA C, n-3; plant oilseicosapentaenoic EPA; C, n-3 and docosahexaenoic acid DHA; C, n-3both common in marine oils. Of the 11 nPUFAS, linoleic acid LA; C, n-6 is the shortest-chained and, as with the nPUFA ALA, is an essential FA that cannot be endogenously produced by mammals and thus must be obtained from the diet, namely, plant sources 2122 Figure 2.
Figure 2 Location of fatty acid saturations. Examples of fatty acids differing in the presence and location of double bond. A location of saturations for a C22 acid with a double bond in 3rd last carbon omega-3, n B location of saturations for a C20 and C22 acid with a double bond in 6th last carbon omega-6, n Structural differences in FA length lead to differences in absorption, transport and tissue destination.
SCFAs are water soluble, readily taken up by the cells and mitochondria, and rapidly metabolized by the liver and other peripheral tissues since they are direct precursors for acetyl-CoA acetic FApropionyl-CoA propionateand butyryl-CoA butyrate.
These CoA derivatives act as direct energy generating molecules in the mitochondria. As the result, SCFAs generated by the bacterial fermentation are present in very low concentrations due to high metabolism. MCFAs are also somewhat water-soluble and do not require transporters to cross the inner mitochondrial membrane and thus are more efficiently absorbed in the gut than LCFA, and more rapidly oxidized in the liver.
LCFAs absorption and metabolism are slower since they require special lipoprotein particles chylomicrons which are transported through the lymphatic system and allow for greater uptake by the adipose tissue. Biosynthesis of VLCFAs occurs in the endoplasmic reticulum ERand unlike MCFAs and LCFAs, VLCFAs are too long to be metabolized in mitochondria.
Once inside the cell, MCFAs do not require the carnitine shuttle to move into the mitochondria and appear to preferentially undergo FA oxidation, whereas LCFAs depend on the carnitine shuttle to enter the mitochondria.
When long-chain triglycerides are replaced by MCFAs in the diet, differences in metabolic routes appear to promote weight control by stimulating satiety and increased energy expenditure The metabolism of FAs also depends on saturation.
Both linoleic acid LA, n-6 and alpha-linoleic acid ALA, n-3 share a common metabolic pathway, wherein ALA competes with LA in deltadesaturase binding, which in turn diverts metabolism toward the n-3 PUFAs EPA, DHA and docosapentaenoic acid DPA; C, n-3 rather than that of pro-inflammatory arachidonic acid AA; C, n-6 Following this, EPA and AA compete as substrates for lipoxygenase and cyclooxygenase COX to generate immunoregulatory eicosanoids including prostaglandins, thromboxanes, prostacyclins, and leukotrienes LTs Oleic acid C, n-9 also plays a role in the metabolism of the essential FAs, serving as a key compound for various metabolic pathways, which may affect disease risk, and has been suggested to compete with LA as a substrate for enzymes involved in the linoleate metabolism 25 The different activities of AA-derived eicosanoids pro-inflammatory compared to those from EPA anti-inflammatory are one of the most important mechanisms explaining the anti-inflammatory properties of nPUFAs in inflammatory disorders.
This includes the local conversion of AA, LA, EPA and DHA by immune cells macrophages to substances known as oxylipins resolvins, protectins, lipoxins, maresins 27potent anti-inflammatory bioactives that reduce tissue inflammation and organ injury complete removal from diet to study the effect on IBD outcome.
Partial or complete replacement of dietary LCFAs by MCFAs has been shown to decrease incidence of spontaneous colitis 33as well confer protection against chemically-induced gut inflammation, in part, by attenuating pro-inflammatory cytokines and immune cell oxidative stress enzyme myeloperoxidase; MPO 34 However, the method of colitis induction can influence outcome; when MCFAs were combined with dextran sodium sulfate DSS to form nano-vesicles which fused with the colonic membrane, this may have initiated an inflammatory response, potentially confounding results Unsaturated FAs MUFAs, PUFAs have been associated with lower cardiovascular disease risk, fat mass, waist circumference, blood pressure, and better lipid profiles higher high-density lipoproteins and lower triglycerides 37 — Saturated FAs are associated with increased low-density lipoproteins and higher cardiovascular disease risk, and studies show that, saturated FAs in combination with lipopolysaccharide LPS of gram-negative bacteria in the gut, stimulate innate immunity Furthermore, studies suggest that partial replacement of LA n-6 with long chain nPUFAs at nn-3 ratio of 10 45 or with medium-chain triglycerides improves experimental colitis Additionally, the ratio of nn-6 plays an important role in disease outcome, with a ratio of nn-6 showing the most benefit In humans, the protective effect of n-3 FAs has been correlated with the decreased production of pro-inflammatory cytokines, through decreased alkaline phosphatase and bile duct injury.
However, clinical trials addressing the benefit of nPUFAs in IBD have yielded mixed results, with benefits differing based on the source of PUFA, suggesting differences in anti-inflammatory activity between marine-derived nPUFAS are superior to that derived from plants Addressing the effectiveness of nPUFAs has largely focused on marine-derived fish oils on the notion that they provide EPA and DHA, whereas plant-derived nPUFAs ALA and stearidonic acid are inefficiently converted to long-chain bioactive forms In mice, nPUFAs have induced a more paradoxical response.
Several studies have shown improved inflammatory scores in nPUFA supplemented rodents 50 — 53whereas others have noted worsening of intestinal inflammation severity 52 Discrepancies in treatment effect benefit or harm between animals and humans may reflect failure of animal models to adequately mimic clinical disease 57 For instance, acute or chemically-induced rodent models of inflammation e.
In this regard, adoptive transfer models may prove better suited to study the chronic inflammatory responses particularly T-cell mediated inflammationalthough the lack of B-cells limits direct translation of results to human clinical disease.
By comparison, genetically engineered KO mouse models e. While congenic mice may thus prove advantageous because inflammation develops spontaneously and predictably e. Numerous rodent studies have investigated how HFD or FAs mediate inflammation in rodent IBD models. However, these studies have varied considerably based on i the IBD mouse model, including the use of spontaneous, or chemically-induced or biologically-induced C.
krill affect outcomes, vi and the role gut microbiota in mediating the effect of a FA Figure 3.
: Fats and inflammation| Rights and permissions | Load up on omega-3s with our Walnut-Rosemary Natural remedies for diabetes Salmon. Article Infflammation PubMed PubMed Central Google Scholar Oh DY, Talukdar Fats and inflammation, Innflammation EJ, Fats and inflammation T, Morinaga H, Fan W, Li Inlfammation, Lu WJ, Watkins SM, Olefsky JM: GPR is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. Differential expression of peroxisome proliferator-activated receptors PPARs : tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Mol Pharmacol — |
| We Care About Your Privacy | Like olive oil, this superfood fruit is high in monounsaturated fats, helping to keep the heart happy and healthy. While eating high amounts of saturated fatty acids can make you prone to inflammation, monounsaturated fats show the opposite effect. According to a review published in the Journal of Molecular Science , people who follow the Med diet, which is high in MUFAs, have lower levels of inflammation than those following a typical Western diet. Furthermore, avocadosprovide vitamin E, which functions as an antioxidant and can help reduce inflammation, per a article published in IUBMB Life. Avocados also provide another source of oleic acid, which has shown promise in helping prevent disease. Flaxseed is known for its rich fiber content, as well as anti-inflammatory plant lignans which may fight cancer. A review published in Cytokine revealed that flaxseed supplementation improved inflammatory markers after examining 40 studies of 2, participants. Similar results were seen in a review published in Advances in Nutrition , where flaxseed supplementation significantly lowered CRP. If you're up for enhancing your immunity with anti-inflammatory flaxseeds , make sure they're ground up so your body can absorb their benefits. Flaxseed oil is also an excellent choice, and the oil brims with even more significant amounts of ALA than ground flaxseed. A staple food for many vegetarians and vegans, edamame are immature soybeans that make a tasty, filling snack with a pinch of salt. While edamame is often touted for its protein and fiber, the bright green legume also contains MUFAs, polyunsaturated fats and isoflavones—a perfect storm for warding off inflammation. One cup of edamame offers significant nutrients, including choline, folate, vitamin A, calcium, lutein and zeaxanthin, for healthy vision. A review published in Foods found that soybean and soy products have protective benefits to gut health in rodents with inflammatory bowel disease. Try tossing edamame into your salads to add texture, protein and color, like in this Greek Salad with Edamame. Health experts and researchers agree that eating tree nuts can help thwart inflammation and preserve cholesterol levels and heart health. As soon as you bite into a walnut, you'll note its oily texture, which is thanks to its massive levels of good-for-you fats like omega-3s. Walnuts possess an impressive set of nutrients like ALA and linoleic acid, fiber, plant sterols, phosphorus and amino acids. Your body relies on getting ALA through the diet, and it's crucial for making DHA and EPA to keep your body a well-oiled disease-fighting machine, per a study published in Frontiers in Pediatrics. Load up on omega-3s with our Walnut-Rosemary Crusted Salmon. While inflammation is inevitable, you can soften its blow by stocking up on anti-inflammatory fat sources. Adding these foods to your diet may curb your intake of pro-inflammatory foods like saturated fats think red meat , and can help give your body what it needs to counteract chronic inflammation, the root of many diseases. These seven foods pack powerful healthy fats and disease-fighting antioxidants to quell inflammation and help keep you healthy. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Monounsaturated fats have a single carbon-to-carbon double bond. The result is that it has two fewer hydrogen atoms than a saturated fat and a bend at the double bond. This structure keeps monounsaturated fats liquid at room temperature. Good sources of monounsaturated fats are olive oil, peanut oil, canola oil, avocados, and most nuts, as well as high-oleic safflower and sunflower oils. The discovery that monounsaturated fat could be healthful came from the Seven Countries Study during the s. It revealed that people in Greece and other parts of the Mediterranean region enjoyed a low rate of heart disease despite a high-fat diet. The main fat in their diet, though, was not the saturated animal fat common in countries with higher rates of heart disease. It was olive oil, which contains mainly monounsaturated fat. This finding produced a surge of interest in olive oil and the " Mediterranean diet ," a style of eating regarded as a healthful choice today. Although there's no recommended daily intake of monounsaturated fats, the National Academy of Medicine recommends using them as much as possible along with polyunsaturated fats to replace saturated and trans fats. Polyunsaturated fats. When you pour liquid cooking oil into a pan, there's a good chance you're using polyunsaturated fat. Corn oil, sunflower oil, and safflower oil are common examples. Polyunsaturated fats are essential fats. That means they're required for normal body functions, but your body can't make them. So, you must get them from food. Polyunsaturated fats are used to build cell membranes and the covering of nerves. They are needed for blood clotting, muscle movement, and inflammation. A polyunsaturated fat has two or more double bonds in its carbon chain. There are two main types of polyunsaturated fats: omega-3 fatty acids and omega-6 fatty acids. The numbers refer to the distance between the beginning of the carbon chain and the first double bond. Both types offer health benefits. Eating polyunsaturated fats in place of saturated fats or highly refined carbohydrates reduces harmful LDL cholesterol and improves the cholesterol profile. It also lowers triglycerides. Good sources of omega-3 fatty acids include fatty fish such as salmon, mackerel, and sardines, flaxseeds, walnuts, canola oil, and un-hydrogenated soybean oil. Foods rich in linoleic acid and other omega-6 fatty acids include vegetable oils such as safflower, soybean, sunflower, walnut, and corn oils. As a service to our readers, Harvard Health Publishing provides access to our library of archived content. Please note the date of last review or update on all articles. No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician. Eat real food. Our knowledge of nutrition has come full circle, back to eating food that is as close as possible to the way nature made it. Thanks for visiting. Don't miss your FREE gift. The Best Diets for Cognitive Fitness , is yours absolutely FREE when you sign up to receive Health Alerts from Harvard Medical School. Sign up to get tips for living a healthy lifestyle, with ways to fight inflammation and improve cognitive health , plus the latest advances in preventative medicine, diet and exercise , pain relief, blood pressure and cholesterol management, and more. Get helpful tips and guidance for everything from fighting inflammation to finding the best diets for weight loss from exercises to build a stronger core to advice on treating cataracts. PLUS, the latest news on medical advances and breakthroughs from Harvard Medical School experts. Fats to Avoid Trans Fats Although they are found in very small amounts naturally in beef and dairy products, manufacturers create most trans fats when hydrogen is added to vegetable oil. This process keeps the oil solid at room temperature and extends its shelf life. Ideally, you should consume no added trans fats at all. That dual effect raises the risk of heart disease. Nutrition View All Articles. Nutrition Best Oils for Arthritis Learn how to choose healthy oils and add them to your cooking routine to help fight inflammation and get a boost of good fats. Nutrition Dairy and Inflammation Learn why some milk products have health benefits and others may increase inflammation. Nutrition Popular Diets and Juvenile Arthritis Learn what the science says about trendy diets for JA symptoms. Nutrition Anti-Inflammatory Diet Do's and Don'ts Following an anti-inflammatory diet, like the Mediterranean diet, may help reduce body-wide inflammation. |
| The truth about fats: the good, the bad, and the in-between - Harvard Health | This is relevant given that hsCRP has been associated with early CVD risk factors like obesity [ 9 , 10 ] and insulin resistance [ 11 , 12 ] in children; also in the present study population, hsCRP was significantly positively associated with body mass index in both sexes data not shown. This indicates that even young individuals with higher hsCRP levels with respect to their peers may be at greater CVD risk later in life, especially since hsCRP levels track into adulthood [ 15 ]. Participants from the year follow-up of GINIplus and LISA studies, with complete data on SFA intake, accelerometer-measured physical activity, and hsCRP, were included in the statistical analyses. Since PA was measured only in Munich and Wesel, the study sample was limited to participants from these two study centres. Interaction terms were then included between SFA and the different PA levels. Where a significant interaction was observed, additional analyses were performed, stratified by tertiles of the relevant PA level. Here, we corrected for multiple testing using Bonferroni correction: the α-level was divided by three the number of subgroups assessed for each sex in stratified analyses. This yielded a corrected two-sided α-level of 0. All analyses were conducted using R, version 3. A total of participants females, males were included in the analyses see Additional file 1 : Figure S1. Complete data on SFA intake, accelerometer-measured PA, and hsCRP, was available for children. Basic characteristics of the study population are displayed in Table 1. A dose-response relationship was however not indicated, as this association was observed at the middle hsCRP II level but not the upper III level. Further analyses were hence carried out stratified by both these PA levels. Results from the stratified analyses are displayed in Fig. Associations between SFA and hsCRP stratified by tertiles of time spent in Sedentary activity top plots and in Light PA bottom plots in females and males left and right, respectively. The present study assessed the association between dietary SFA and low-grade inflammation, measured by the inflammatory marker hsCRP, in year-old German adolescents. Nevertheless, different results might be expected in more active individuals, especially regarding interactions between MVPA and SFA, as levels may be too low in this population to detect significant effects. The results of our analyses in females are in line with a number of studies in adults, in which no significant association has been observed between dietary SFA and hsCRP [ 32 , 36 , 75 ]. Others have reported positive associations, supporting arguments to limit SFA intake in order to reduce cardiovascular risk [ 76 , 77 , 78 ]. Existing studies addressing children and adolescents are equally inconclusive. The inconsistency among the various study findings might be related to study location, which could influence habitual dietary behaviours and patterns, sources of dietary SFA, or even baseline hsCRP levels. With respect to this last point, the median hsCRP levels in our study population were 0. Although no hsCRP reference values are yet available for adolescents, these levels are comparable to pre-pubertal reference values in Europe [ 8 ]. Compared to the study in Asian Indians, who presented higher average CRP values 0. It is also possible that contradicting results in terms of SFA may reflect true differences between SFA intake and status. While the assessment of SFA intake considers the amount of the nutrient consumed, SFA status likely reflects circulating SFA following additional processes such as digestion, absorption, uptake into tissues, and metabolism [ 38 ]. In the present analyses, we observed an inverse association between SFA intake and middle hsCRP levels in males. Such an association between dietary SFA and hsCRP was also observed by Fredrikson et al. in adult females [ 84 ], a finding which the authors described as surprising. It has been proposed that the sparing of SFA and endogenous de novo SFA synthesis both contribute to SFA status and are promoted by high-carbohydrate diets [ 85 ]. Reduced SFA intakes have been shown to be compensated by a concomitant increase in carbohydrate CHO intakes [ 86 ]. Due to the strong correlations, adjustment for CHO in our statistical models was not possible as this would have led to problems of multicollinearity. We hence emphasize that these results should be interpreted in the context of other, possibly correlated nutrients. High glycaemic index CHO has been reported to induce inflammation through postprandial hyperglycaemia even in lean, glucose-tolerant subjects [ 87 ], and several intervention studies seem to support this notion [ 88 ]. We therefore speculate that increasing SFA intake likely has no direct role in reducing low-grade inflammation per se, but might promote a reduced inflammatory profile indirectly through a simultaneous reduction in CHO intake. This finding would hence support statements advocating that it is not simply the dietary SFA content, but the entire dietary composition, and especially the relative CHO intake, that determines whether SFA intake is ultimately associated with detrimental outcomes [ 89 ]. To our knowledge, this is the first study to evaluate the interaction of PA in the association of SFA and low-grade inflammation in adolescents, and hence comparison with other studies is limited. Anti-inflammatory effects of habitual PA in children have been observed [ 90 ], although a study assessing accelerometer-measured PA in 9-year-old children reported no association between PA and hsCRP [ 44 ]. None of the different PA levels assessed were significant confounders in our analyses, and therefore did not alter the inverse relationship between SFA and middle category levels of hsCRP when included in the statistical models as covariates. the lowest was reduced by a factor of 0. It is possible that MVPA was too low in our study population to induce significant synergistic effects with diet. On the other hand, it is known that the intensity of PA is the main factor determining the degree of CHO or fat oxidation for fuel, and that low-intensity exercise depends almost entirely on fatty acids [ 91 ]. It is possible that due to the smaller sample size and greater variance in the highest hsCRP category, there was insufficient power to detect a significant association. Why the inverse association was only observed in males is unclear, but it is possible that sex-specific physiological factors might play a significant role, leading to differences in fat metabolism and the resulting inflammatory profile. For example, testosterone has been shown to enhance lipid oxidation whereas oestrogen enhances fat storage [ 92 ], aspects which may be relevant in the context of the present study, especially considering that most of the females in the sample were in late- or post-pubertal stages. The present study benefits from a large, homogeneous study sample, and adds to the limited literature on the association between dietary SFA and low-grade inflammation in adolescents, in a time of heightened discussion concerning SFA and cardiovascular health. Our study includes data from over individuals, greatly exceeding the size of the few observational studies carried out thus far. Our analyses also include the assessment of different levels of accelerometer-measured physical activity, a method not often available in large cohort studies. Accelerometers were worn by participants on the hip, reported to be the best single location to record data for activity detection [ 93 ]. Furthermore, to our knowledge, the role of SFA with regards to inflammation has not been previously assessed in the context of different PA levels and their possible interactions. The current analyses hint towards potential synergistic effects of important modifiable lifestyle factors in relation to health aspects, particularly in males. Their interaction may differ substantially from their individual effects and this can be highly relevant when interpreting findings on a topic such as SFA, on which contradicting results are often discussed. This study focusses on a population of healthy adolescents aged years, which is not a high-risk population. Given the low levels of hsCRP being addressed, results are not necessarily indicative of damage nor directly translatable to CVD risk, and hence the clinical relevance of the present findings may seem limited. However, given the increasing evidence for the progression of risk factors from childhood to adulthood, preventive measures might already consider this age group and hence associations observed could provide valuable insight. A main limitation when assessing dietary intake is the reliance on subjective measures, which are prone to reporting bias. In the present study the FFQ used to measure dietary SFA was designed to estimate fatty acids and antioxidants in school-aged children [ 54 ]. Given the thorough quality control of the dietary data with plausible values observed in terms of total energy intake , misreporting was likely detected and excluded from the analysis. A further drawback is the high inter-correlation amongst different nutrients, which is often come across in nutritional epidemiology, and if ignored could lead to inappropriate conclusions. Adjustment for these nutrients within the statistical models could result in multicollinearity, generating further misleading associations [ 94 ]. With this in mind, and considering that the inclusion of an interaction term with PA would further complicate interpretation, we could not adjust for other nutrients and hence the ability to disentangle the individual effects of SFA is somewhat limited. Nonetheless, we are aware of the importance of accounting for possible intercorrelations and have hence considered these in the interpretation of our results. A further limitation in the present study was the underrepresentation of children from lower social-classes. As often occurs in longitudinal cohort studies, this non-random loss-to-follow-up meant that the current findings may not be entirely representative of the study area. The assessment of other inflammatory markers might have been useful to strengthen our conclusions, but unfortunately these were not available for the studied cohorts. Our findings are based on cross-sectional analyses, meaning that the observed associations between dietary SFA and hsCRP do not necessarily infer causality. Furthermore, blood-withdrawal for CRP measurements was carried out at a slightly different time to dietary assessment and accelerometry. Thus, the present analysis is based on the assumption that dietary intake, as well as PA and CRP, are persistent during this interval, which may not be entirely the case. Nevertheless, for PA, activity measured on non-typical days e. including trips or sickness were excluded to ensure usual activity was recorded, which more likely represents chronic PA. We assume that any drastic changes in diet between FFQ completion and blood withdrawal are unlikely, although it cannot be entirely excluded. Nevertheless, changes occurring in either lifestyle behaviour would have occurred at the individual level, and hence any bias due to such changes are expected to be random, not affecting the general trend observed. From the present analyses, it can be concluded that a higher SFA intake during adolescence, within the ranges observed in the current study, is not detrimental in terms of inflammatory processes in adolescents; although we highlight that this may well depend on the nutrient it replaces. We propose that when evaluating the role of SFA in chronic inflammation, it is essential to differentiate between findings involving SFA status in serum or plasma and those assessing dietary SFA, as the latter is likely influenced by important modifiable factors such as PA, which may determine whether an inflammatory response arises. In some cases, ethical approval can be obtained for the release. Requests should be addressed to Marie Standl marie. standl helmholtz-muenchen. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice - a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. World Health Organization. Global health estimates deaths by cause, age, sex, by country and by region, Geneva: WHO; Google Scholar. Nicklas TA, von Duvillard SP, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: the Bogalusa heart study. Int J Sports Med. Article PubMed Google Scholar. Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the cardiovascular risk in Young Finns study. J Pediatr. Article CAS PubMed Google Scholar. Canas JA, Sweeten S, Balagopal PB. Biomarkers for cardiovascular risk in children. Curr Opin Cardiol. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. Schlenz H, Intemann T, Wolters M, Gonzalez-Gil EM, Nappo A, Fraterman A, et al. C-reactive protein reference percentiles among pre-adolescent children in Europe based on the IDEFICS study population. International journal of obesity Article Google Scholar. Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Nappo A, Iacoviello L, Fraterman A, Gonzalez-Gil EM, Hadjigeorgiou C, Marild S, et al. High-sensitivity C-reactive protein is a predictive factor of adiposity in children: results of the identification and prevention of dietary- and lifestyle-induced health effects in children and infants IDEFICS study. J Am Heart Assoc. Article PubMed PubMed Central CAS Google Scholar. Syrenicz A, Garanty-Bogacka B, Syrenicz M, Gebala A, Walczak M. Low-grade systemic inflammation and the risk of type 2 diabetes in obese children and adolescents. Neuro endocrinology letters. CAS PubMed Google Scholar. Retnakaran R, Hanley AJ, Connelly PW, Harris SB, Zinman B. Elevated C-reactive protein in native Canadian children: an ominous early complication of childhood obesity. Diabetes Obes Metab. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U. Diabetes Care. DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES Article PubMed PubMed Central Google Scholar. Juonala M, Viikari JS, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the cardiovascular risk in Young Finns study. Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The cardiovascular risk in Young Finns study. Ann Med. Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. US senate select committee on nutrition and human needs. Dietary goals for the United States. Washington: US Govt print off; Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. Article CAS PubMed PubMed Central Google Scholar. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. Harcombe Z, Baker JS, Cooper SM, Davies B, Sculthorpe N, DiNicolantonio JJ, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in and a systematic review and meta-analysis. Open Heart. Ravnskov U, Diamond D, Canan Efendigil Karatay M, Miller DW, Okuyama H. No scientific support for linking dietary saturated fat to CHD. Br J Nutr. Article PubMed CAS Google Scholar. Malhotra A. Saturated fat is not the major issue. BMJ: British Medical Journal. Pedersen JI, James PT, Brouwer IA, Clarke R, Elmadfa I, Katan MB, et al. The importance of reducing SFA to limit CHD. Bahl R. The evidence base for fat guidelines: a balanced diet. Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chem. Kaikkonen JE, Kresanov P, Ahotupa M, Jula A, Mikkila V, Viikari JS, et al. High serum n6 fatty acid proportion is associated with lowered LDL oxidation and inflammation: the cardiovascular risk in Young Finns study. Free Radic Res. Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Santos S, Oliveira A, Lopes C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr Res. Harris C, Demmelmair H, von Berg A, Lehmann I, Flexeder C, Koletzko B, et al. Associations between fatty acids and low-grade inflammation in children from the LISAplus birth cohort study. Eur J Clin Nutr. Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Rocha DM, Bressan J, Hermsdorff HH. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. Mazidi M, Gao HK, Vatanparast H, Kengne AP. Impact of the dietary fatty acid intake on C-reactive protein levels in US adults. Santos S, Oliveira A, Pinho C, Casal S, Lopes C. Fatty acids derived from a food frequency questionnaire and measured in the erythrocyte membrane in relation to adiponectin and leptin concentrations. Arab L, Akbar J. Biomarkers and the measurement of fatty acids. Public Health Nutr. Ruiz-Nunez B, Pruimboom L, Dijck-Brouwer DA, Muskiet FA. Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J Nutr Biochem. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. Loprinzi P, Cardinal B, Crespo C, Brodowicz G, Andersen R, Sullivan E, et al. Objectively measured physical activity and C-reactive protein: National Health and nutrition examination survey — Scand J Med Sci Sports. Moran A, Steffen LM, Jacobs DR Jr, Steinberger J, Pankow JS, Hong CP, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Ruiz JR, Ortega FB, Warnberg J, Sjostrom M. Associations of low-grade inflammation with physical activity, fitness and fatness in prepubertal children; the European youth heart study. Article CAS Google Scholar. Martinez-Gomez D, Eisenmann JC, Warnberg J, Gomez-Martinez S, Veses A, Veiga OL, et al. Associations of physical activity, cardiorespiratory fitness and fatness with low-grade inflammation in adolescents: the AFINOS study. Poitras VJ, Gray CE, Borghese MM, Carson V, Chaput J-P, Janssen I, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. Sabiston CM, Castonguay A, Low NC, Barnett T, Mathieu ME, O'Loughlin J, et al. Vigorous physical activity and low-grade systemic inflammation in adolescent boys and girls. Int J Pediatr Obes. Martinez-Gomez D, Gomez-Martinez S, Ruiz JR, Diaz LE, Ortega FB, Widhalm K, et al. Objectively-measured and self-reported physical activity and fitness in relation to inflammatory markers in European adolescents: the HELENA study. Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue—skeletal muscle axis. Acta Physiol. Lundsgaard AM, Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol. Teeman CS, Kurti SP, Cull BJ, Emerson SR, Haub MD, Rosenkranz SK. Postprandial lipemic and inflammatory responses to high-fat meals: a review of the roles of acute and chronic exercise. Berg A, Kramer U, Link E, Bollrath C, Heinrich J, Brockow I, et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course - the GINIplus study up to the age of 6 years. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. CAS Google Scholar. Heinrich J, Bolte G, Holscher B, Douwes J, Lehmann I, Fahlbusch B, et al. Eur Respir J. Stiegler P, Sausenthaler S, Buyken AE, Rzehak P, Czech D, Linseisen J, et al. A new FFQ designed to measure the intake of fatty acids and antioxidants in children. Kroke A, Manz F, Kersting M, Remer T, Sichert-Hellert W, Alexy U, et al. The DONALD study. History, current status and future perspectives. Eur J Nutr. Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the German part of the EPIC project. European prospective investigation into Cancer and nutrition. Int J Epidemiol. Harris C, Flexeder C, Thiering E, Buyken A, Berdel D, Koletzko S, et al. Changes in dietary intake during puberty and their determinants: results from the GINIplus birth cohort study. BMC Public Health. Willett W. Nutritional epidemiology. USA: Oxford University Press; Book Google Scholar. Der Bundeslebensmittelschlüssel. German nutrient DataBase. Karlsruhe: Federal Research Centre for nutrition and food BfEL. Robusto KM, Trost SG. Comparison of three generations of ActiGraph activity monitors in children and adolescents. J Sports Sci. Aadland E, Ylvisåker E. PLoS One. Jarrett H, Fitzgerald L, Routen AC. J Phys Act Health. Smith MP, Berdel D, Nowak D, Heinrich J, Schulz H. Physical activity levels and domains assessed by Accelerometry in German adolescents from GINIplus and LISAplus. Pfitzner R, Gorzelniak L, Heinrich J, von Berg A, Klümper C, Bauer CP, et al. Physical activity in German adolescents measured by Accelerometry and activity diary: introducing a comprehensive approach for data management and preliminary results. Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. Ridker PM. A Test in Context: High-Sensitivity C-Reactive Protein. Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of youth and adolescence. R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth ed: Springer, New York. IBSN ; Van Hecke L, Loyen A, Verloigne M, van der Ploeg HP, Lakerveld J, Brug J, et al. Variation in population levels of physical activity in European children and adolescents according to cross-European studies: a systematic literature review within DEDIPAC. The international journal of behavioral nutrition and physical activity, vol. Saturated FAs act as ligands of TLR4, and SFA-rich diets have been shown to cause low-grade inflammation and insulin resistance , Additionally, HFD-induced changes to the gut microbiota exacerbates inflammation and obesity via TLR4 induction and NF-κB In one TNBS-colitis model, nPUFA was found to increase TLR-2 and IL-1A gene expression in rat colon tissue, whereas n-9 increased TLR-4 expression Several studies have shown that by signaling through the G protein-coupled receptor GPR , both EPA and DHA exert potent anti-inflammatory effects through inhibition of TNFα receptor and TLR4, inflammatory signaling pathways , Notably, TLR regulation by diet also depends on other food ingredients, for instance carrageenan, a red seaweed-derived food additive pervasively used by the food industry as an emulsifier. Studies investigating the effect of dietary nPUFA on TLR2 have yielded variable results, with some showing TLR2 downregulation by EPA in mouse adipose stem cells and others reporting no effect from either n-3 or n-9 FA on TLR2 and TLR4 despite their downregulation of IL-6, TNFα and MCP-1 secretion in human adipose tissue and adipocyte cultures Proliferator-activated receptors PPARs are ligand-dependent nuclear receptors for endogenous lipids with 3 isoforms: α, β, and γ, each differing in function and tissue distribution. PPARγ regulates FA storage and glucose metabolism, and was recently highlighted for its role in intestinal inflammation — , with mutations in the PPARγ gene associated with IBD , Expressed in adipose tissue and colonic epithelium, PPARγ acts as an antagonist of various transcription factors interfering with their inflammatory pathways, including nuclear factor of activated T-cells NFAT , an important inducer of pro-inflammatory genes such as IL-4, IL-2 during T-cell activation , In addition, PPARγ activity is modulated by dietary FAs and their metabolites reviewed elsewhere The interaction between dietary fats and PPARγ has been well studied for their role in regulating inflammation. The protective effect of conjugated linoleic acid CLA against IBD has been shown in vitro and in vivo to be mediated through PPARγ activation 22 , although other n-PUFAs may antagonize the effects of CLA on PPARγ in experimental colitis By contrast, no effect of dietary ALA-rich oil was seen on PPARγ activation in a TNBS-colitis rat model In vitro induction of PPARγ was reported in enterocyte-like Caco-2 cells in response to IL-1β but not in HIMEC cells treated with IL-1β, or LPS-treated human dendritic cells , Such discrepancies could be attributed to differences in cell type or DHA dosage, with lower doses serving to inhibit TLR4 signaling and induce PPARγ while higher doses increase IkB expression and decrease p38MAPK. Notably, both dosages inhibit intestinal inflammation. It is worth noting that understanding the effects of PUFAs will require better description, owing to the various types of chemical isoforms e. Figure 4 illustrates an example of the modulation of signaling pathways by PPAR nuclear receptor activation. In vitro studies have shown that pretreatment of bone marrow-derived dendritic cells with DHA followed by LPS stimulation TLR4 ligand profoundly inhibits members of the IL family including ILp70, IL and IL, an effect mediated by PPARγ and NF-κB inhibition , Exposure to DHA also inhibited pro-inflammatory molecule production IL-6, TNFα, CCL-4 and anti-inflammatory cytokine IL , the latter finding in contrast to the upregulatory effects by CLA and subsequent inhibition of LPS-induced IL in murine dendritic cells While the intracellular pathways of DHA activity are not known, in vitro evidence suggests PPARγ, which is highly expressed in dendritic cells, macrophages, and T and B-cells, as a possible mediator [reviewed in — ]. Data obtained from in vitro and in vivo studies also indicate that the anti-inflammatory effect of DHA on endothelial cells is mediated by decreased expression of VCAM-1 and VEGFR2 with concomitant reduction in PGE 2 and LTB 4 Further, DHA-enriched fish oil has also been shown to enhance B-cell activation in vivo which may function to aid pathogen clearance and upregulation of the resolution phase of inflammation, in turn reducing total inflammatory response PPARα is a major regulator of energy homeostasis and regulation genes involved in beta-oxidation, and is highly expressed in tissues that rapidly oxidize FAs such as liver, heart, kidney, and intestine PPARα is primarily activated via ligand binding by endogenous FAs, including AA and palmitoleic acid , as well as various other PUFAs and their metabolites, namely members of the hydroxyeicosatetraenoic AA metabolite family and the LA metabolite hydroxyoctadecadienoic PPARδ plays an important role in colonic epithelial cell differentiation , and exerts anti-inflammatory activity by inhibiting NF-κB signaling Ligands for PPARδ, including nPUFAs , , are anti-inflammatory, and at high concentrations PPARδ activation attenuates experimental colitis and intestinal inflammation , whereas PPARδ null mice have increased sensitivity to DSS-colitis Relevant evidence highlights the role of PPARδ activation in colon tumorigenesis, however this remains controversial , — Indeed, Roy et al. Downregulation of the ABC genes such as ABCB1A and ABCB1B has been reported in both human and animal studies suggesting the involvement of ABC transporters during inflammation — Sundrud et al. Protein kinase B PKB or Akt plays a role in cell metabolism, proliferation, growth and survival, and its activation involves phosphoinositidekinase PI3K Recent studies have shown that Akt-regulated FOXO phosphorylation increases cellular oxidative stress which in turn induces NF-κB and mTOR activation Additionally, HFD-induced intestinal inflammation was recently shown to be mediated by changes in the Akt-FOXO3 axis Specifically, Akt and FOXO3 phosphorylation increased in mice fed a HFD compared to low-fat diet fed mice, suggesting that NF-κB activation through the Akt-FOXO3 signaling may be associated with intestinal inflammation. T-cells that infiltrate or reside in the intestinal mucosa sense and respond to pathogen-associated antigens presented by mucosal antigen-presenting cells, most commonly in Peyers patches of the small intestine or in mesenteric lymph nodes, to execute protective inflammatory responses. Mucosal homeostasis requires T-cell tolerance to commensal microbe-derived antigens. A breakdown in T-cell tolerance toward gut commensals is a major determinant of IBD. iTreg differentiation in the large intestine requires host-microbe interaction with the commensal microbiota, and thus fail to develop in germ-free mice, whereas germ-free animals colonized with defined microbial consortia restores intestinal iTreg development — Microbe-derived SCFAs e. Microbial bile acid metabolism also modulates gut mucosal iTreg cells. Secondary bile acids, produced through bacterial metabolism of primary bile acids escaping ileal reabsorption , promote maintenance of colonic iTregs through the nuclear vitamin D receptor VDR As an energy source, Tregs prefer FA β-oxidation to generate ATP and it has been speculated that FA oxidation endow iTregs in non-lymphoid tissues, including the gut, a fitness advantage in tissue microenvironments where immune suppression is typically favored. Indeed, oleic acid has been implicated in promoting Treg function in non-lymphoid tissues, including in visceral adipose tissue and the central nervous system ; oleic acid is reduced in adipose reservoirs of human multiple sclerosis, a relapsing-remitting autoimmune disorder in which Treg function is impaired, whereas addition of oleic acid to Tregs isolated ex vivo from multiple sclerosis patients restores suppressive activity In tumor microenvironments, Tregs are more abundant and have an advantage over T-conventional Tconv cells, due to supplemental energy gained via lipid metabolism In mouse tumors, Tregs have intracellular lipid accumulation owing to increased FA synthesis, which is enhanced by increased glucose uptake. Therein, both oxidative and glycolytic metabolism contribute to Tregs expansion, which has been corroborated with increased Treg gene signatures on glycolysis and lipid synthesis in humans. Less is known on gut wall inflammation, but studies on HFD indicate that certain types of FA result in variable rates of Treg expansion and prevention of IBD, depending on the mouse line T lymphocyte function has been extensively studied using DHA. Mice displayed Th2-biasing cytokines as well as cecal IgA, supporting an increased B-cell function Downregulation of Sa8 was also reported in IL null mice fed an EPA- and AA-enriched diet compared to control mice fed AINA The gut microbiota is shaped by diet and plays an important role in IBD etiology and progression. Most importantly, HFD has been shown to elicit changes in the gut microbiota composition divergent to that of control diets lower in fat, namely increases in alpha diversity and in the Firmicutes to Bacteroidetes ratio, independent of obesity 66 , 79 , — The effect of DSS on gut microbiota composition is also more profound in the setting of a HFD, and has been shown to abrogate the higher abundance of Firmicutes to Bacteroidetes while increasing the abundance of Proteobacteria and Actinobacteria vs. controls Of these, increased abundance of Trabulsiella and Atopobioum was also identified in mice fed a HFD without DSS-colitis suggesting that these taxa may exert a colitogenic effect under high-fat feeding conditions. Notably, the administration of colistin but not vancomycin ameliorated DSS-colitis severity in HFD mice, indicating that gram-negative bacteria, such as Proteobacteria mediate experimental colitis progression in mice fed a HFD Power et al. Similar reductions in A. muciniphila abundance with increases in Prevotella spp. were reported by Gulhane et al. In addition, IL treatment decreased abundance of Escherichia coli in a dose-dependent manner, which correlated with decreased serum endotoxin levels. By contrast, Määttänen et al. Although administration of live P. distasonis via oral gavage has been reported to worsen DSS-colitis , its cellular components have a protective effect against DSS-colitis Studies suggest that LPS of gram-negative bacteria stimulate innate immune activity in the presence of saturated FAs Conversely, increased abundance in Lactobacillus has been associated with dietary intake of nPUFAs , with nPUFA administration to Caco-2 cells shown to promote both the growth and adherence of probiotic Lacticaseibacillus casei formerly Lactobacillus casei Shirota In this regard, probiotics have been explored as a method to restore intestinal homeostasis in inflammatory states. In a study using Lactobacillus helveticus it was noted that the probiotic has varying ability to modulate host physiological function, depending on the diet type, with mice on a western diet showing less inflammation than on a standard chow diet One study showed that probiotics corrected inflammation-driven metabolic dysfunction with strong reduction of the colonic expression of inflammatory cytokines TNFα, IL-6, and IFNγ, as well as reserved colonic downregulation of PPARγ, and other ligand-activated nuclear receptors in a TNBS-colitis mouse model Administration of these bacterial strains appears to attenuate HFD-induced increases in colonic MPO activity, LPS production, NF-κB activation and TNFα expression while enhancing IL expression, in part through inhibition of gut Proteobacteria 86 , In addition to the ability of diet to modulate the gut microbiota, several bacterial taxa have demonstrated the ability to generate FAs. Bacterial end-products have exhibited anti-inflammatory effects and have been particularly well characterized in the case of SCFAs. Acetate, propionate, and butyrate acids are synthesized through cleavage of CoA via thioesterases, which are ubiquitously found , Longer FAs, such as CLA, can be converted from dietary FAs by several genera, particularly, lactobacilli and bifidobacteria Conversely, reduction of SCFAs has been shown to exert a pro-inflammatory effect. Decreased levels of Roseburia hominis , a butyrate producer, is frequently associated with IBD As one might expect, there are also bacteria capable of producing pro-inflammatory FAs. Though bacterial production of non-SCFAs is less studied, bacterial taxa do exist that are capable of synthesizing longer chain FAs. For example, saturated LCFAs from Prevotella , lactobacilli, and Alistipes increased colitis-mediated death in rats This mechanism of modulation is important to consider especially to try to elucidate the emerging roles of relatively recent gut commensal species such as the Alistipes genus which has been shown to have variable effects in humans and animal models The maternal diet is well known to be one of the major factors influencing offspring microbial composition , but more recently, maternal HFD has been shown to modulate susceptibility to diseases, as well as exacerbate offspring susceptibility to chemically induced colitis — associated with increased IL-1β, IL-6 and IL expression and upregulated NF-κB signaling Maternal HFD has also been shown to result in distinct microbiota differences in offspring compared to that of controls. Xie et al. Furthermore, maternal high fat offspring exhibited significantly inhibited intestinal development and disruption of gut barrier function at 3 weeks of age, as well as accelerated DSS-induced colitis in 8-week-old mice fed a control diet compared to their control counterparts. Inflammation was associated with significant differences in microbiota between offspring groups. Babu et al. Maternal feeding of EPA and DHA nPUFAs has also been found beneficial for protecting against inflammation in the intestine of premature pups by regulating eicosanoid and NF-κB related metabolite expression Although the underlying mechanisms as to how a maternal HFD affects long-term inflammatory outcomes in offspring remains unclear, offspring of mothers exposed to a HFD have been shown to harbor a unique microbiota. In addition, these offspring have increased susceptibility to disrupted mucosal barrier function, low-grade inflammation and experience increased severity of experimentally induced colitis , Specifically, one study found expansion of the ILC3 population in the lamina propria of maternal HFD offspring. Numerous diets have long been known to possess an antioxidant effect but in the case of FAs, most of the literature highlights the opposite. That is, the promotion of oxidative stress pathways as a mechanism of induction of inflammation or tissue damage; which is often reported in experimental studies as worsening of histological scores. Oxidative stress is a process by which enzymes and chemical compounds participate in the oxidation and reduction of biological molecules of cell systems. Figure 5 illustrates how HFD and FAs can modulate host immunity via alterations in gut barrier function and gut microbiota composition. Figure 5 High-Fat Diet and Fatty Acids Modulate Host Immunity via Alterations to Gut Barrier Function and Gut Microbiota Composition. Neutrophils are phagocytic cells known as first responders in inflammatory reactions that play a key role in host immunity primarily via the release of pro-inflammatory enzymes e. Evidence shows that PUFAs, specifically nPUFAs sourced from safflower oil , or the nPUFA ALA , can elicit changes in neutrophil function and infiltration decreased , whereas minimal response was seen with fish oil nPUFA , which had been thought to play a role previously In this context, several rodent studies have shown that HFD, or feeding nPUFA-enriched diet promotes oxidative stress, including increased MPO in the gut 45 , The increased ROS production was accompanied by a concomitant induction of the myosin light chain kinase MLCK tight junction pathway as well as increased gut barrier permeability. Increased ROS production and activation of the MLCK pathway was observed in vivo , in HCT cells cultured with either palmitic acid or a combination of palmitic acid and TNFα. However, this effect was markedly diminished in the presence of a ROS scavenger, suggesting that experimental colitis and mucosal inflammation is promoted by a HFD through aggravation of mucosal oxidative stress, which in turn drives increased gut barrier permeability Studies have also reported beneficial effects of dietary nPUFAs on oxidative stress. These protective effects were associated with reduced NF-κB activation as well as reduced lipid mediator concentrations, including leukotriene B 4 LTB 4 and COX2 In another study, dietary olive oil supplemented with nPUFA fish oil was found to beneficially decrease colonic iNOS expression and GSH concentration in rat colon tissue following DSS-colitis Excessive ROS production in the inflammatory response plays a critical role in tissue damage and the progression of inflammatory diseases Studies have recently implicated GSTO for its TLR4-mediated role in pro-inflammatory response by macrophages , TLR4 and MyD88 both play prominent roles in supporting low-grade inflammation in obesity, and deficiency in either protein attenuates obesity and metabolic alterations caused by a HFD , Specifically, GSTO deficient cells failed to upregulate expression of NADPH oxidase 1 and produce ROS following LPS stimulation GSTO deficient macrophage cells stimulated with LPS were also found unable to produce lactate or dephosphorylate adenosine monophosphate kinase AMPK; metabolic stress regulator , nor did they accumulate succinate or stabilize HIF1α, two responses important in maintaining pro-inflammatory state of activated macrophages , Notably, GSTO knockout KO mice, which are resistant to LPS-induced inflammatory shock vs. wild type mice , exhibited suppressed pro-inflammatory cytokine expression and attenuated ROS production compared to wild-type mice. Glutathione peroxidase 4 GPX4 protects against oxidation of biolipids, referred to as lipid peroxidation, that particularly affects PUFAs with biological membranes. In mice, deletion or inhibition of GPX4 induces ferroptosis, a distinct form of iron-dependent cell death which requires AA nPUFA membrane enrichment. In context of the genetic association between GPX4 and CD , including evidence of GPX4-restricted AA oxidation in biological membrane , , a recent study revealed that a PUFA-enriched Western diet triggers GPX4-restricted mucosal inflammation in mice lacking one allele of GPX4 in intestinal epithelial cells Exosomes are endosome-derived nanovesicles that have been recently described as important intracellular communication mediators, especially via crosstalk between organs, via transfer of encapsulated cargoes such as bioactive lipids, proteins and mRNAs and non-coding RNAs — Released by healthy cells, exosomes play an important role in the immune system function and have the potential to activate cellular stress and damage Using a DSS-colitis mouse model fed either chow or a HFD it was recently shown that active biogenesis of exosomes occurs in adipose tissue and that these adipose tissue-derived exosomes preferentially circulate to the lamina propria, serving as an important adipokine Further, the HFD-induced obesity altered the miRNA profile of the adipose exosomes, shifting the exosome from having an anti-inflammatory phenotype to that of pro-inflammatory. The intestinal inflammation caused by the circulation of inflammatory exosomes from the obese adipose tissue to the colon was promoted via macrophage M1 polarization predominantly via the pro-inflammatory cargoes. Most intriguingly, it was shown that colitis could be attenuated by delivering miRNA drugs from the adipose tissue to the lamina propria via exosomes encapsulating miR inhibitor, suggesting that targeting the exosomal pathway between obese fat and the intestinal lamina propria could be used to therapeutically manage colitis Endoplasmic reticulum ER stress has been found to influence the pathology of various chronic diseases including IBD , Highly secretory cells such as Paneth and goblet cells are extremely prone to ER stress, which activates the unfolded protein response and a cascade of cellular transduction events to restore ER homeostasis , Failure of unfolded protein response UPR to maintain cellular viability and homeostasis can halt cellular protein synthesis and activate inflammatory signaling and apoptosis. The primary genetic variants within the UPR Xbp1, Arg2, Ormdl2 encoded proteins rely on a robust secretory pathway e. In mice, missense mutations in the MUC2 gene e. Specific FAs and cytokines can suppress or exacerbate ER stress in secretory cells By comparison, non-esterified FAs such as palmitate administered in vitro to human colonic LST cells induced significant oxidative and ER stress. This resulted in reduced Muc2 secretion mucin production , whereas administration of IL suppressed oxidative and ER stress induced by palmitate Those findings were consistent with in vivo studies showing a dose-dependent decrease in ER stress sXbp1, Grp78, Edem1 in response to IL treatment in HFD mice Production of IL is controlled by the aryl hydrocarbon receptor AhR , an important regulator of metabolism, immune cell homeostasis, and intestinal immunity, activated by dietary ligand binding, namely the phytochemical indolecarbinol The AhR regulates IL production via intestinal epithelial cells, and AhR signaling has been demonstrated to inhibit inflammation induced by experimental colitis , whereas AhR-deficient mice are highly susceptible to DSS-induced colitis suggesting that the AhR plays a key role in resolving intestinal inflammation. Notably, significantly lower AhR activation following feeding of a purified HFD has been reported in mice heterozygous for the AhR repressor gene compared to mice fed a normal, unpurified chow diet AING , which contains phytochemicals and flavonoids Furthermore, AhR is targeted by pelargonidins, a type of anthocyanidins thought to be beneficial for overall human health. Another study in DSS-colitis rats explored the effects of the anthocyanin, pelargonidin 3-glucoside P3G , on IBD and metabolic syndrome. Findings revealed that P3G treatment attenuated DSS-induced IBD symptoms. Likewise, P3G treatment in rats fed a high-carbohydrate, HFD resulted in attenuation of metabolic syndrome reduced systolic blood pressure, ventricular stiffness, cardiac and liver structure, abdominal fat, and body weight gain , suggesting that anthocyanidins, specifically pelargonidins, target AhR, decreasing inflammation to attenuate symptoms of IBD and metabolic functions in metabolic syndrome. These findings are relevant considering that diets containing a high content of phytochemicals are generally rich in fruit and vegetables, which are typically lacking in a Western diet. Malondialdehyde is a widely used marker of oxidative lipid injury that results from lipid peroxidation by ROS of PUFAs , Malondialdehyde is also a prominent product in Thromboxane A2 synthesis secondary to the metabolism of AA by cyclooxygenase-1 COX1 or cycloxygenase-2 COX2 to prostaglandin H2 by various cell types and tissues. Dietary lipid end products from ROS and lipid peroxidases oxidative stress such as malondialdehyde are also absorbed into circulation and have been shown to activate inflammatory responses in various tissues, including the gut itself There is also evidence that malondialdehyde is able to regulate insulin through the WNT-pathway, in addition to having mutagenic capability Human studies have yielded contradictory results regarding oxidative stress levels in IBD patients, with some studies reporting significantly higher malondialdehyde levels in plasma of CD patients compared to controls and UC patients, and others showing no difference — Tight junctions are multi-protein junctional complexes which function to seal the paracellular pathway to prevent leakage or translocation of intestinal contents and bacteria across the intestinal epithelium. At least 40 different proteins comprise tight junctions, of which the 3 major transmembrane proteins include occludin, claudins, and junction adhesion molecules JAM proteins, which associate with peripheral membrane proteins e. Many rodent studies have shown HFD with or without induction of experimental colitis-induced dysregulation in tight junction barrier protein expression ZO-1, Claudin, occludin in ileal and colonic tissue 66 , 79 , , with concomitant increases in serum endotoxin consistent with increased gut permeability However, findings have varied between studies, with some reporting no dietary differences in tight junction expression These discrepancies are possibly due to differences in the amount and type of FAs comprising the diet, duration of diet administration and rodent genetic line. Significant upregulation in expression of RhoA , which regulates tight junction assembly and actin organization, has also been reported following feeding HFD Vitamin D has also been shown to influence gut barrier integrity. Vitamin D is recognized to exert immunomodulatory effects via the VDR, and has been shown to exert protective effects in IBD, including amelioration of IBD symptoms in both human and animal following vitamin D supplementation. their HFD counterparts without supplementation In one study, feeding a HFD Specifically, mice fed the HFD exhibited atrophy of the small intestine, colon and gut-associated lymphoid tissue GALT , with reductions in the number of small intestinal intraepithelial lymphocytes IEL and lamina propria lymphocytes LPL. The latter was also observed in mice within one day of receiving a HFD Effects were independent of changes to the gut microbiota and continued for 2 weeks after returning animals to a standard diet. Intriguingly, reductions in IEL and LPL were also observed in mice supplemented with orally administered FAs, however, this was attenuated upon administration of a lipase inhibitor to reduce luminal free FAs. Here, chronic inflammation in high-fat DSS-colitis treated mice was characterized by a lower proportion of TCRγδ T-cells tissue repair among IELS while the proportion of TCRαβ T-cells was inversely higher, compared to controls. Both important lymphoid cells among IELs, TCRγδ T-cells and TCRαβ T-cells play a critical role in tissue repair and in controlling intestinal immune responses whose dysregulation is linked to colitis development , respectively. High-fat feeding also led to significantly lower proportion of CD8α T-cells which play a unique protective role among IELs There were, however, no significant differences between diet groups in the proportion of pro- or anti-inflammatory cells in the lamina propria. The intestinal barrier utilizes tightly regulated mechanisms to control and prevent the translocation of intestinal bacteria across the mucosal surface. This includes antimicrobial peptides AMP which are produced and secreted by Paneth cells as protective agents against bacterial pathogens, as well as the dense mucus layer of mucins which is produced by goblet cells via Muc2 to serve as a mechanical barrier to prevent bacterial translocation across the epithelial wall Specifically, high-fat feeding resulted in significantly reduced Paneth cell area, reduction of lysozyme content within crypts and decreased expression of procryptdin AMP exclusively produced by Paneth cells , as well as other AMPs produced at the crypt bottom Defcr1, Defcr4, Defa-rs1c Mechanistically, reductions in goblet cells was associated with mTORC1 activation, Notch activation and a subsequent downregulation of Muc2 expression. In another study, disruption on mucosal barrier integrity caused by long term high-fat feeding corresponded with significant reduction in Muc2 mRNA potentially explained by concomitant decrease in the expression of klf4 and Spdef , two transcription factors involved in goblet cell differentiation Similar reductions were observed in Tff3 mRNA 64 , a secreted product of goblet cells that is key to epithelial restoration after injury Bile acids are steroid acids conjugated with taurine or glycine to generate a total of 8 possible conjugated bile acids, which are referred to as bile salts. Bile acids are important to facilitate FA absorption and are synthesized by the liver primary bile acids and by bacterial actions in the colon secondary bile acids. Prolonged exposure to high levels of fecal deoxycholic acid has been shown to disrupt epithelial integrity , and contribute to IBD development. In two studies investigating the effect of HFD on bile salts like deoxycholate known to increase in the colon in individuals on HFDs , wild type mice supplemented with deoxycholate developed inflammation oxidative, angiogenesis, altered gene expression , whereas Nos2 KO mice seem to be resistant to these changes More recently, it was shown that excessive fecal deoxycholic acid levels in the gut caused by a HFD contribute to colonic inflammation by dose-dependently upregulating SphingosinePhosphate Receptor 2 S1PR2 via activation of NLRP3 inflammasome as well as pro-inflammatory cytokine IL-1β production in macrophages Activation of NLRP3 is achieved through downstream stimulation of extracellularly regulated protein kinase signaling pathway ERK and subsequent cathepsin B release. In this context, severity of DSS-colitis intestinal inflammation is significantly worsened in mice treated with deoxycholic acid enema but is alleviated by the blockage of S1PR2 as well as inhibition of cathepsin B release, in turn reducing mature IL-1β production. Prostaglandin-endoperoxide synthase 2 PTGS2 also known as COX2 catalyzes the conversion of AA to pro-inflammatory prostaglandin E2 formation. Cyclooxygenase 2, the inducible form of COX and linked to altered risk of developing IBD — , is the rate limiting step in conversion of AA to prostanoids, pro-inflammatory mediators including protacyclins, prostaglandins and thromboxanes. Both COX2 and COX2-dependent prostaglandin E2 PGE2 have been associated with maintaining adaptive immune tolerance to dietary antigens , , with COX2-total KO and COX2-myeloid specific KO mice shown to develop severe CD-like inflammation within the ileo-ceco-colic junctions significantly increasing intestinal permeability when fed a cholate-containing HFD , Notably, COX2 can also promote the resolution of inflammation via induction of pro-resolving eicosanoid lipoxin A2 LXA4 , In fat-1 mice, a transgenic mouse model that can efficiently convert nPUFAs to nPUFAs allowing controlled studies without dietary manipulation, the effect of endogenously synthesized nPUFAs attenuated DSS-induced colonic inflammation accompanied by significant decreases in PGE2 production and COX2 expression as well as decreases in colitis-induced pro-inflammatory cytokines, monocyte chemoattractant proteins MCP-1, -2, -3 and matrix metalloproteinase 9 Compared to n-6 and n-9 diets, administration of nPUFAs e. Previous studies have shown in vitro that EPA, as well as other unsaturated FAs, are potent inhibitors of the AA-induced PTGS2 activity It is possible that AA could also give rise to anti-inflammatory activity given evidence that prostaglandin E2 can suppress macrophage and monocyte production of TNFα and IL-6, as well as inhibit 5-lipoxygenase which in turn disrupting leukotriene X4 production Eicosanoids are factors that mediate defensive and inflammatory processes of the gut mucosa and have been shown to increase in experimental colitis. While eicosanoids are known to be regulated by neural and hormonal controls, their local synthesis within the gastrointestinal lumen is influenced by dietary FA intake 5 , — For instance, nPUFA intake has been associated with higher production of EPA eicosanoids PGE3 and LTC5 and lower AA-derived eicosanoids 6-keto-PGF1 alpha, PGE2, TXB2, LTB4, and LTC4 by the gastric and intestinal mucosa in rats vs. nPUFA intake , whereas fat-free diets have been shown to reduce eicosanoid production compared to controls The role of eicosanoids in the gastrointestinal tract has been recently reviewed Resolvins are anti-inflammatory mediators shown to control and reduce inflammation in a variety of experimental models of inflammatory disorders, mediated, in part, by dendritic cells. Resolvins are derived from nPUFAs, specifically, EPA derives the E-series family of resolvins, while DHA derives the family of D-series resolvins RvD , protectin D1, and maresins. Both EPA and DHA-derived resolvins participate in anti-inflammatory and pro-inflammatory subsistence via signaling pathways including MAPK, NF-κB, PPARу, PI3K, miRNAs, and apoptosis These lipid mediators have been shown to decrease TNFα and IL-6 , which may be due to NF-κB signaling via its specific G protein-coupled receptor, ChemR23 and leukotriene B4 receptor 1, a receptor of the pro-inflammatory eicosanoid leukotriene B4 , Notably, COX inhibitors such as 5-acetylsalicylic acid have been shown to increase formation of AA-derived anti-inflammatory pro-resolution lipoxins, as well as resolvins from nPUFAs such as DHA, supporting the potential for combination therapies using ASA and DHA supplementation The anti-inflammatory role of lipid mediators in the gastrointestinal tract has been recently reviewed , , Apolipoprotein A-1 APOA1 mimetic peptides comprise the main structural protein of high-density lipoprotein. Two of which, 4F and transgenic 6F Tg6F have been shown protective of inflammatory diseases, including that 4F, when orally or transgenically administered to low-density lipoprotein receptor-null mice fed a Western diet have the ability to lower pro-inflammatory FA metabolite levels in mouse enterocytes Further, it was recently shown that COX-2 total KO mice fed a cholate-containing HFD and orally administered 4F and Tg6F function to inhibit both LPS and oxidized 1-palmitoylarachidonoyl-sn-phosphatidylcholine oxPAPC signaling in human macrophages and intestinal epithelium, as well as promote the clearance of pro-inflammatory lipids within the gut lumen Adipose tissue has been described for its involvement in endocrine , metabolic function and more recently for its interaction with the immune system via the release of adipokines namely adiponectin, leptin, and ghrelin from fat tissue — Adipose tissue is also a source of cytokines, including TNFα interleukins Depending on the conditions during their release, these mediators can have pro-inflammatory, anti-inflammatory, or appetite-controlling functions , There is also evidence that obesity induces dysregulation of adipokine circulating levels and that this may contribute to obesity-related diseases Further, several studies suggest that adipose tissue-derived mediators, namely increases in circulating TNFα, adiponectin, ghrelin and resistin, with decreases in leptin may affect the pathophysiology of IBD. Discovery of adipokines in fat tissue has led to investigation into their role in inflammatory disorders such as IBD. The role of adipokines in inflammation has been previously reviewed 12 , Adiponectin is a protein hormone released by adipocytes that is involved in glucose regulation and FA oxidation. While several studies have suggested adiponectin to have anti-inflammatory activity , more recent studies have implicated its role in the pathophysiology of colitis, although its role remains controversial , with some studies reporting an attenuated effect on colitis while others suggest that decreased adiponectin in colon subepithelial myofibroblasts exacerbates colitis 52 , , The Adiponectin receptor 1 AdipoR1 is an important receptor in the fat-intestinal axis during the regulation of inflammation of the colon. Similarities in the expression patterns between mesenteric fat isolated from obese patients and that from patients with IBD have been reported, with inflammation and lipid metabolism pathways showing the greatest overlap Studies looking at the effect of diet-induced obesity on severity of TNBS-colitis and cytokine expression in mouse mesenteric fat suggest that adiponectin receptor 1 aggravates colitis While obesity alone increases pro-inflammatory IL-1β, TNFα, MCP1, and keratinocyte-derived chemokine, obesity decreased the extent to which TNBS-colitis increased IL-2 and IFN-γ in mesenteric adipose and intestinal tissues. In vitro , fat-conditioned media lowered AdipoR1 in human colonic epithelial cells NCM , while in vivo intracolonic silencing of AdipoR1 in mice exacerbated TNBS-induced colitis In another DSS-colitis study, induction of colitis significantly decreased adiponectin and increased expression in both AdipoR1 and adiponectin receptor 2 AdipoR2 Of interest, findings from McCaskey et al. Leptin is a satiety hormone that regulates energy balance as a long-term regulator suppressing food intake preventing obesity. The role of leptin in IBD has been studied, but the results are conflicting and further investigation is required — Although earlier studies in IL mice having also a KO mutation in the leptin gene showed not to have inflammation prevention, compared to the single IL KO deficiency However, in IL KO mice, the deletion of leptin resulted in increased apoptosis of lymphocytes in the lamina propria , which supports the hypothesis that inflammatory cells benefit and even survive longer in the presence of leptin ligands, promoting ILdriven chronic inflammation. It is possible that this effect supports the hypothesis that a feedback loop cycle could locally exist where a progressive state of intestinal inflammation leads to accumulated gut-mesenteric fat creeping fat , which e. Part of the modulating effect of leptin on inflammation has been supported by the presence of leptin receptors LR mRNA gene expression detected on hematopoietic cells, T-cells and lymphocytes , and in the gastric mucosa, where it can also be produced. The main producing tissue is the adipose tissue which increases with obesity. Leptin also increases as a result of positive feedback from TNFα, and it has been proposed that this mechanism may be relevant in early inflammatory stages , In the stomach, leptin increases its levels according to the feeding regime, which in turn, modifies systemic circulating levels, which could reach cells in inflammatory sites to promote inflammation in cells if they are activated and primed with LR. Fasting and starvation are associated with high local levels of gastrin; but after a meal, leptin locally decreases but increases systemically. Additionally, it has been shown that slow gastric emptying can increase leptin levels in the stomach. Considering that dietary fats influence gastric emptying rates, it is possible that slow gastric emptying caused by a HFD influences leptin levels. Of the 21 identified leptin-associated genes found to have an inverse relationship between the two dietary types HFD, normal fat Peli3, Creb1, Enpp2 and Centg1 , four genes previously reported to play a role in obesity and colon-related diseases, were found to have either a positive or negative relationship between serum leptin or insulin concentration and consumption of either HFD or normal diet It is known that bacterial lipopolysaccharides mediate diarrhea induced by bacterial infection in the gut In ghrelin-treated mice, this endotoxinemia-induced dysmotility was improved, mainly via down-regulation of nitric oxide pathways in the gut , decreased production of pro-inflammatory cytokines IL-1β and TNFα, with concomitant increase in anti-inflammatory IL , The role of ghrelin in IBD is thought to be attributed to its antagonistic effect on leptin, although several in vivo and in vitro studies have described both pro- and anti-inflammatory effects from ghrelin In CD-IBD, Zhao et al. By comparison, Gonzalez-Rey et al. Konturek et al. In UC-IBD, De Smet et al. rodentium -induced colitis the late stages of infection were associated with increased ghrelin expression, with in vitro studies showing ghrelin induced marked proliferation of neurons. Intracolonic administration of TNBS-colitis has been shown to cause severe acute colitis and changes in the mesenteric and epididymal fat depots arguably described as resemblants of changes in CD with increased pro-inflammatory mediators in these fat depots, including substance P SP 2 , 12 , , Such findings indicate that human mesenteric pre-adipocytes contain functional substance P receptors that are linked to pro-inflammatory pathways, and that substance P can directly increase NK-1R expression. Thus, it is possible that mesenteric fat depots may participate in intestinal inflammatory responses via substance P-NK-1R-related pathways, as well as other systemic responses to the presence of an ongoing inflammation of the colon. Herein, we review the evidence on the role of HFDs on the severity of experimental ileitis and colitis in laboratory rodents to further advance our mechanistic understanding of the effects of FAs on intestinal inflammation. While studies conducted directly in humans provide prevalence, incidence and clinical estimates, studies using laboratory rodents performed under controlled conditions allow for mechanistic insights relevant to IBD. However, our review highlights considerable variability in findings between studies. Whereas FA-mediated regulation of pro- and anti-inflammatory T-cell responses in vivo remains a largely nascent field, fundamental questions remain concerning FA uptake, intracellular transport and regulatory function. Existing studies give cause for optimism that understanding the molecular interplay between FAs and T-cells will reveal biologically novel and translationally-relevant insights toward the treatment of human diseases. This is important considering that not only the amount by the type and structure of the FA can influence phenotypic outcomes of disease. AR-P, AB, SI, AT, FC: study design. AB, CC, FS, AT, AG-N: literature review. AB, CC, AR-P, FS: manuscript writing. AB, CC, FS, AG-N, AT, IB, SI, MS, FC, AR-P: review, comments, and editing of final manuscript. All authors contributed to the article and approved the submitted version. Research reported in this publication was supported by the NIH grant DK, DK and DK to FC , T32DK and F32DK to AB , and P01DK Germ-free and Gut Microbiome Core and R21DK to AR-P. We acknowledge the Biorepository Core of the NIH Silvio O. Conte Cleveland Digestive Disease Research Core Center P30DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Boutros M, Maron D. Inflammatory bowel disease in the obese patient. Clin Colon Rectal Surg — doi: PubMed Abstract CrossRef Full Text Google Scholar. Karagiannides I, Pothoulakis C. Substance P, obesity, and gut inflammation. Curr Opin Endocrinol Diabetes Obes — Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol — Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut — Bosco N, Brahmbhatt V, Oliveira M, Martin FP, Lichti P, Raymond F, et al. Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrügger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther — Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr —5. Monaco G, van Dam S, Casal Novo Ribeiro JL, Larbi A, de Magalhaes JP. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels. BMC Evol Biol Liu WX, Wang T, Zhou F, Wang Y, Xing JW, Zhang S, et al. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-γ activity. Biochem Biophys Res Commun — Sideri A, Stavrakis D, Bowe C, Shih DQ, Fleshner P, Arsenescu V, et al. Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1. Am J Physiol Gastrointest Liver Physiol G— Marton LT, Goulart RA, Carvalho ACA, Barbalho SM. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int J Mol Sci — CrossRef Full Text Google Scholar. Cariello M, Contursi A, Gadaleta RM, Piccinin E, De Santis S, Piglionica M, et al. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 12 4 Tou JC, Jaczynski J, Chen YC. Krill for human consumption: nutritional value and potential health benefits. Nutr Rev — Grimstad T, Bjorndal B, Cacabelos D, Aasprong OG, Janssen EA, Omdal R, et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand J Gastroenterol — de Carvalho C, Caramujo MJ. The Various Roles of Fatty Acids. Molecules — Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, De Silva P, Korzenik JR, et al. Gastroenterology —7. Rezanka T. Very-long-chain fatty acids from the animal and plant kingdoms. Prog Lipid Res — Kris-Etherton PM. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation —8. Wen J, Khan I, Li A, Chen X, Yang P, Song P, et al. Alpha-linolenic acid given as an anti-inflammatory agent in a mouse model of colonic inflammation. Food Sci Nutr — Bassaganya-Riera J, Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care — St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr — Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr — Johnson M, Bradford C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J Glycom Lipidomics —8. doi: 0. Google Scholar. Lowry RR, Tinsley IJ. Oleic and linoleic acid interaction in polyunsaturated fatty acid metabolism in the rat. Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases. Clin Rev Allergy Immunol — Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol — de Silva PS, Olsen A, Christensen J, Schmidt EB, Overvaad K, Tjonneland A, et al. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Nishida T, Miwa H, Shigematsu A, Yamamoto M, Iida M, Fujishima M. Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut —7. Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases. Front Immunol Yoon BK, Jackman JA, Valle-Gonzalez ER, Cho NJ. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Mañé J, Pedrosa E, Lorén V, Ojanguren I, Fluvià L, Cabré E, et al. Partial replacement of dietary n-6 fatty acids with medium-chain triglycerides decreases the incidence of spontaneous colitis in interleukindeficient mice. Kono H, Fujii H, Ogiku M, Tsuchiya M, Ishii K, Hara M. Enteral diets enriched with medium-chain triglycerides and N-3 fatty acids prevent chemically induced experimental colitis in rats. Transl Res — Ohta N, Tsujikawa T, Nakamura T, Andoh A, Sasaki M, Bamba T. A comparison of the effects of medium- and long-chain triglycerides on neutrophil stimulation in experimental ileitis. J Gastroenterol — Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, et al. Dextran sodium sulfate DSS induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PloS One 7:e Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab — Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids — Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obes Silver Spring —8. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes — Blok WL, Katan MB, van der Meer JW. Modulation of inflammation and cytokine production by dietary n-3 fatty acids. Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion — John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol —6. Hekmatdoost A, Mirshafiey A, Feizabadi MM, Djazayeri A. Polyunsaturated fatty acids, microflora and colitis. Ann Nutr Metab Tyagi A, Kumar U, Santosh VS, Reddy S, Mohammed SB, Ibrahim A. Partial replacement of dietary linoleic acid with long chain n-3 polyunsaturated fatty acids protects against dextran sulfate sodium-induced colitis in rats. Prostaglandins Leukot Essent Fatty Acids — Bertevello PL, De Nardi L, Torrinhas RS, Logullo AF, Waitzberg DL. Partial replacement of omega-6 fatty acids with medium-chain triglycerides, but not olive oil, improves colon cytokine response and damage in experimental colitis. JPEN J Parenter Enteral Nutr —8. Campos FG, Waitzberg DL, Habr-Gama A, Logullo AF, Noronha IL, Jancar S, et al. Impact of parenteral n-3 fatty acids on experimental acute colitis. Br J Nutr 87 Suppl 1:S83— Maattanen P, Lurz E, Botts SR, Wu RY, Robinson SC, Yeung CW, et al. Plant- and Fish-Derived n-3 PUFAs Suppress Citrobacter Rodentium-Induced Colonic Inflammation. Mol Nutr Food Res e Baker EJ, Miles EA, Burdge GC, Yaqoob P, Calder PC. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Yao J, Lu Y, Zhi M, Hu P, Wu W, Gao X. Mol Med Rep — Andoh A, Tsujikawa T, Ishizuka I, Araki Y, Sasaki M, Koyama S, et al. N-3 fatty acid-rich diet prevents early response of interleukin-6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int J Mol Med —5. Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, et al. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflammation Bowel Dis — Mbodji K, Charpentier C, Guerin C, Querec C, Bole-Feysot C, Aziz M, et al. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-kappaB in rats with TNBS-induced colitis. J Nutr Biochem —5. Hokari R, Matsunaga H, Miura S. Effect of dietary fat on intestinal inflammatory diseases. J Gastroenterol Hepatol 28 Suppl —6. Clin Exp Immunol — Ergas D, Eliat S, Mendlovic Z, Sthoeger M. N-3 Fatty Acids and the Immune System in Autoimmunity. Isr Med Assoc J —6. Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA —2. Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. IL and IL Receptor Mutations in Very Early Onset Inflammatory Bowel Disease. Gastroenterol Res —9. Bielohuby M, Menhofer D, Kirchner H, Stoehr BJ, Muller TD, Stock P, et al. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab E65— Takahashi M, Ikemoto S, Ezaki O. J Nutr Sci Vitaminol Tokyo — Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes Lond —2. Lassenius M I, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care — Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL Sci Rep Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PloS One 5:e Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem — Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab — Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes — Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology — e Serino M, Luche E, Gres S, Baylac A, Berge M, Cenac C, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, et al. PloS One 8:e Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med — van der Logt EM, Blokzijl T, van der Meer R, Faber KN, Dijkstra G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol — Kim IW, Myung SJ, Do MY, Ryu YM, Kim MJ, Do EJ, et al. Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J Gastroenterol Hepatol — Okada Y, Tsuzuki Y, Sato H, Narimatsu K, Hokari R, Kurihara C, et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Li X, Wei X, Sun Y, Du J, Li X, Xun Z, et al. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Lee JC, Lee HY, Kim TK, Kim MS, Park YM, Kim J, et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PloS One e Lu P, Bar-Yoseph F, Levi L, Lifshitz Y, Witte-Bouma J, de Bruijn AC, et al. High beta-palmitate fat controls the intestinal inflammatory response and limits intestinal damage in mucin Muc2 deficient mice. Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. J Nutr —7. Mi Y, Chin YX, Cao WX, Chang YG, Lim PE, Xue CH, et al. Native kappa-carrageenan induced-colitis is related to host intestinal microecology. Int J Biol Macromol — |
| About this article | Oleic acid C, n-9 also plays a role in the metabolism of the essential FAs, serving as a key compound for various metabolic pathways, which may affect disease risk, and has been suggested to compete with LA as a substrate for enzymes involved in the linoleate metabolism 25 , Eur J Clin Invest — A comprehensive list of FAs based on carbon chain length e. However, the postprandial duration was relatively short 3 h and changes in plasma cytokines concentrations were not measured; hence the relation between NF-κB and the release of cytokines deserves further investigation. Other than the TLR-dependent pathway, SFA stimulate pro-inflammatory mechanisms through the TLR-independent pathway by producing reactive oxygen species ROS. Hoang-Yen Tran D, Hoang-Ngoc Tran D, Mattai SA, Sallam T, Ortiz C, Lee EC, et al. Ruiz JR, Ortega FB, Warnberg J, Sjostrom M. |

Video
Ep:160 EATING FAT MAY BE REALLY BAD: FAT AND INFLAMMATION - PUFA UNDERSTOOD - by Robert CywesFats and inflammation -
and many other countries. Eating foods rich in trans fats increases the amount of harmful LDL cholesterol in the bloodstream and reduces the amount of beneficial HDL cholesterol.
Trans fats create inflammation, which is linked to heart disease , stroke, diabetes, and other chronic conditions. They contribute to insulin resistance, which increases the risk of developing type 2 diabetes. Saturated fats are common in the American diet. They are solid at room temperature — think cooled bacon grease, but what is saturated fat?
Common sources of saturated fat include red meat, whole milk and other whole-milk dairy foods, cheese, coconut oil , and many commercially prepared baked goods and other foods.
The word "saturated" here refers to the number of hydrogen atoms surrounding each carbon atom. The chain of carbon atoms holds as many hydrogen atoms as possible — it's saturated with hydrogens.
Is saturated fat bad for you? A diet rich in saturated fats can drive up total cholesterol, and tip the balance toward more harmful LDL cholesterol, which prompts blockages to form in arteries in the heart and elsewhere in the body. A handful of recent reports have muddied the link between saturated fat and heart disease.
One meta-analysis of 21 studies said that there was not enough evidence to conclude that saturated fat increases the risk of heart disease, but that replacing saturated fat with polyunsaturated fat may indeed reduce risk of heart disease.
Two other major studies narrowed the prescription slightly, concluding that replacing saturated fat with polyunsaturated fats like vegetable oils or high-fiber carbohydrates is the best bet for reducing the risk of heart disease, but replacing saturated fat with highly processed carbohydrates could do the opposite.
Good fats come mainly from vegetables, nuts, seeds, and fish. They differ from saturated fats by having fewer hydrogen atoms bonded to their carbon chains. Healthy fats are liquid at room temperature, not solid. There are two broad categories of beneficial fats: monounsaturated and polyunsaturated fats.
Monounsaturated fats. When you dip your bread in olive oil at an Italian restaurant, you're getting mostly monounsaturated fat. Monounsaturated fats have a single carbon-to-carbon double bond. The result is that it has two fewer hydrogen atoms than a saturated fat and a bend at the double bond.
This structure keeps monounsaturated fats liquid at room temperature. Good sources of monounsaturated fats are olive oil, peanut oil, canola oil, avocados, and most nuts, as well as high-oleic safflower and sunflower oils.
The discovery that monounsaturated fat could be healthful came from the Seven Countries Study during the s. It revealed that people in Greece and other parts of the Mediterranean region enjoyed a low rate of heart disease despite a high-fat diet.
The main fat in their diet, though, was not the saturated animal fat common in countries with higher rates of heart disease. It was olive oil, which contains mainly monounsaturated fat. This finding produced a surge of interest in olive oil and the " Mediterranean diet ," a style of eating regarded as a healthful choice today.
Although there's no recommended daily intake of monounsaturated fats, the National Academy of Medicine recommends using them as much as possible along with polyunsaturated fats to replace saturated and trans fats.
Polyunsaturated fats. When you pour liquid cooking oil into a pan, there's a good chance you're using polyunsaturated fat. Corn oil, sunflower oil, and safflower oil are common examples.
Polyunsaturated fats are essential fats. That means they're required for normal body functions, but your body can't make them. So, you must get them from food. Polyunsaturated fats are used to build cell membranes and the covering of nerves.
They are needed for blood clotting, muscle movement, and inflammation. Fish, canola oil, and walnuts contain omega 3 fatty acids. Omega-3 rich foods help neutralize inflammation.
Tuna, salmon, mackerel, trout, herring and sardines are excellent choices. Fruits, vegetables and whole grains provide antioxidants such as vitamins A, C, E as well as phytonutrients.
Yellow and orange vegetables, such as carrots and sweet potatoes contain the phytonutrient carotenoids. The phytonutrient flavonoids are found in red and purple fruits, such as berries, grapes and apples.
The produce with the deeper and brighter colors generally contain the most antioxidants. Herbs, spices and teas also contain anti-inflammation antioxidants : dill, oregano, cinnamon, curry, garlic, ginger, tumeric and rosemary.
The majority of teas including green, black and white also contain a concentrated amount of inflammation fighting antioxidants. Unfortunately in our country there are still many heavily processed foods being offered and consumed.
With nutrition education, awareness, and by making healthy substitutions, we can focus on making the small changes that can improve the nutrition quality we eat and our health. Various studies in children and adolescents have not confirmed a direct association between PA and hsCRP [ 42 , 43 , 44 , 45 ], although the benefits on metabolic function and overall health are undeniable [ 46 ].
Studies reporting significant associations in adolescents suggest a protective role of PA. Whereas in a study of adolescents from 10 European cities, objectively-measured vigorous PA was suggested to play an indirect beneficial role through improved cardiorespiratory fitness [ 48 ].
An indirect role through altered energy metabolism may also be plausible; for example, studies have shown that skeletal muscle activity can influence fat oxidation [ 49 ]. This effect can vary based on the intensity and duration of the activity, as well as by sex, as differences in substrate metabolism have been described, with females oxidising fat more readily than males during exercise [ 50 ].
Hence, a sex-specific modulatory role of habitual PA in the relationship between dietary SFA and chronic low-grade inflammation is plausible, perhaps through long-term physiological changes at the cellular level [ 51 ]. To our knowledge, no study has been carried out addressing the integrated role of SFA and PA in adolescent females and males.
Therefore, this study aims to assess the association of dietary SFA with hsCRP in a large population of year-olds, as well as the possible modulatory role of different levels and duration of objectively-measured PA. The present study used data from the year follow-up assessments of the GINIplus German Infant Nutritional Intervention plus environmental and genetic influences on allergy development and LISA Influence of Life-style related factors on the development of the Immune System and Allergies in East and West Germany birth cohort studies.
Briefly, healthy full-term new-borns were recruited from selected obstetric clinics in Germany. The aim was to compare the effect of the different formulae vs. Participants with a negative family history of allergy, and those who declined to take part in the intervention trial, were included in the observation arm.
In both studies, information on selected exposures and health outcomes were obtained by means of questionnaires and medical examinations carried out at various follow-up assessments.
Exposures and outcomes relevant to the present analyses are described in detail below. Habitual dietary intake was assessed at the year follow-up using a self-administered food frequency questionnaire FFQ. The applied FFQ was designed at the year follow-up for the estimation of food and nutrient intake in school-aged children over the past year.
A detailed description of the FFQ development and its validation can be found elsewhere [ 54 ]. Briefly, a list of commonly consumed foods contributing to total energy and especially fatty acid intake, was compiled from food intake data obtained by 3-day weighed dietary records of German children from the DONALD Dortmund Nutritional and Anthropometric Longitudinally Designed study [ 55 ]; portion sizes and frequency categories were included in the style of the EPIC European Prospective Investigation into Cancer and Nutrition FFQ [ 56 ].
A pilot study was conducted to evaluate the comprehensibility and applicability of the resulting FFQ, which was then validated against a h dietary recall at a food group level and at a nutrient level [ 54 ].
The final version of the FFQ was used in the current study, and is available from the corresponding author upon reasonable request.
The FFQ was delivered to participants by post and included detailed instructions for its completion. Additionally, several questions were included on preferred fat and energy contents, preparation methods, diets and food preferences, buying habits and dietary supplement use.
Participants were asked to complete the FFQ themselves with the support of whoever cooked at home, if needed. The study technical assistant could be contacted if any further clarification was required. A quality control procedure was applied based on recommendations by Willett et al.
The accelerometer has been validated for use in adolescents [ 60 ], and it has been shown that measurements on opposing hips are not significantly different from each other [ 61 , 62 ].
Participants for accelerometry were recruited from the study centers Munich and Wesel. The accelerometry protocol, data management and quality control have been described previously in detail [ 63 , 64 ].
Briefly, participants were asked to keep an activity diary during the days the accelerometer was worn, where they recorded all their activities over the course of the day using a detailed schedule.
This was done in order to control for non-wear time as well as the plausibility of the recorded accelerometer data. no holidays, travelling, sickness. The activity diary information was also used to exclude recorded days which were not representative of typical routine, as described in Pfitzner et al.
Subjects were required to have at least 3 valid recorded weekdays and one valid weekend day. Average minutes per day spent on the different PA levels were calculated for each individual by dividing total recorded minutes by the number of valid recorded days. Serum concentrations of hsCRP were measured in samples collected during the year follow-up medical examinations, using the Roche Mannheim, Germany Tina-quant CRP latex high-sensitive assay, according to manufacturer instructions.
Measured hsCRP concentrations were highly skewed, with many observations below detection limit hsCRP 0. Given this non-normal distribution, data categorisation was required for analyses. While hsCRP values between 0.
Unlike in adults, the cut-offs applied in the present study do not enable the identification of specific values on which to define high CVD risk. Rather, they allow the comparison between different hsCRP levels in young, healthy adolescents, amongst whom disease risk markers may manifest only in subclinical form.
This is relevant given that hsCRP has been associated with early CVD risk factors like obesity [ 9 , 10 ] and insulin resistance [ 11 , 12 ] in children; also in the present study population, hsCRP was significantly positively associated with body mass index in both sexes data not shown.
This indicates that even young individuals with higher hsCRP levels with respect to their peers may be at greater CVD risk later in life, especially since hsCRP levels track into adulthood [ 15 ]. Participants from the year follow-up of GINIplus and LISA studies, with complete data on SFA intake, accelerometer-measured physical activity, and hsCRP, were included in the statistical analyses.
Since PA was measured only in Munich and Wesel, the study sample was limited to participants from these two study centres. Interaction terms were then included between SFA and the different PA levels.
Where a significant interaction was observed, additional analyses were performed, stratified by tertiles of the relevant PA level. Here, we corrected for multiple testing using Bonferroni correction: the α-level was divided by three the number of subgroups assessed for each sex in stratified analyses.
This yielded a corrected two-sided α-level of 0. All analyses were conducted using R, version 3. A total of participants females, males were included in the analyses see Additional file 1 : Figure S1. Complete data on SFA intake, accelerometer-measured PA, and hsCRP, was available for children.
Basic characteristics of the study population are displayed in Table 1. A dose-response relationship was however not indicated, as this association was observed at the middle hsCRP II level but not the upper III level.
Further analyses were hence carried out stratified by both these PA levels. Results from the stratified analyses are displayed in Fig. Associations between SFA and hsCRP stratified by tertiles of time spent in Sedentary activity top plots and in Light PA bottom plots in females and males left and right, respectively.
The present study assessed the association between dietary SFA and low-grade inflammation, measured by the inflammatory marker hsCRP, in year-old German adolescents. Nevertheless, different results might be expected in more active individuals, especially regarding interactions between MVPA and SFA, as levels may be too low in this population to detect significant effects.
The results of our analyses in females are in line with a number of studies in adults, in which no significant association has been observed between dietary SFA and hsCRP [ 32 , 36 , 75 ]. Others have reported positive associations, supporting arguments to limit SFA intake in order to reduce cardiovascular risk [ 76 , 77 , 78 ].
Existing studies addressing children and adolescents are equally inconclusive. The inconsistency among the various study findings might be related to study location, which could influence habitual dietary behaviours and patterns, sources of dietary SFA, or even baseline hsCRP levels.
With respect to this last point, the median hsCRP levels in our study population were 0. Although no hsCRP reference values are yet available for adolescents, these levels are comparable to pre-pubertal reference values in Europe [ 8 ].
Compared to the study in Asian Indians, who presented higher average CRP values 0. It is also possible that contradicting results in terms of SFA may reflect true differences between SFA intake and status.
While the assessment of SFA intake considers the amount of the nutrient consumed, SFA status likely reflects circulating SFA following additional processes such as digestion, absorption, uptake into tissues, and metabolism [ 38 ]. In the present analyses, we observed an inverse association between SFA intake and middle hsCRP levels in males.
Such an association between dietary SFA and hsCRP was also observed by Fredrikson et al. in adult females [ 84 ], a finding which the authors described as surprising.
It has been proposed that the sparing of SFA and endogenous de novo SFA synthesis both contribute to SFA status and are promoted by high-carbohydrate diets [ 85 ].
Reduced SFA intakes have been shown to be compensated by a concomitant increase in carbohydrate CHO intakes [ 86 ]. Due to the strong correlations, adjustment for CHO in our statistical models was not possible as this would have led to problems of multicollinearity.
We hence emphasize that these results should be interpreted in the context of other, possibly correlated nutrients. High glycaemic index CHO has been reported to induce inflammation through postprandial hyperglycaemia even in lean, glucose-tolerant subjects [ 87 ], and several intervention studies seem to support this notion [ 88 ].
We therefore speculate that increasing SFA intake likely has no direct role in reducing low-grade inflammation per se, but might promote a reduced inflammatory profile indirectly through a simultaneous reduction in CHO intake.
This finding would hence support statements advocating that it is not simply the dietary SFA content, but the entire dietary composition, and especially the relative CHO intake, that determines whether SFA intake is ultimately associated with detrimental outcomes [ 89 ].
To our knowledge, this is the first study to evaluate the interaction of PA in the association of SFA and low-grade inflammation in adolescents, and hence comparison with other studies is limited.
Anti-inflammatory effects of habitual PA in children have been observed [ 90 ], although a study assessing accelerometer-measured PA in 9-year-old children reported no association between PA and hsCRP [ 44 ].
None of the different PA levels assessed were significant confounders in our analyses, and therefore did not alter the inverse relationship between SFA and middle category levels of hsCRP when included in the statistical models as covariates.
the lowest was reduced by a factor of 0. It is possible that MVPA was too low in our study population to induce significant synergistic effects with diet.
On the other hand, it is known that the intensity of PA is the main factor determining the degree of CHO or fat oxidation for fuel, and that low-intensity exercise depends almost entirely on fatty acids [ 91 ].
It is possible that due to the smaller sample size and greater variance in the highest hsCRP category, there was insufficient power to detect a significant association.
Why the inverse association was only observed in males is unclear, but it is possible that sex-specific physiological factors might play a significant role, leading to differences in fat metabolism and the resulting inflammatory profile.
For example, testosterone has been shown to enhance lipid oxidation whereas oestrogen enhances fat storage [ 92 ], aspects which may be relevant in the context of the present study, especially considering that most of the females in the sample were in late- or post-pubertal stages.
The present study benefits from a large, homogeneous study sample, and adds to the limited literature on the association between dietary SFA and low-grade inflammation in adolescents, in a time of heightened discussion concerning SFA and cardiovascular health.
Our study includes data from over individuals, greatly exceeding the size of the few observational studies carried out thus far.
Our analyses also include the assessment of different levels of accelerometer-measured physical activity, a method not often available in large cohort studies. Accelerometers were worn by participants on the hip, reported to be the best single location to record data for activity detection [ 93 ].
Furthermore, to our knowledge, the role of SFA with regards to inflammation has not been previously assessed in the context of different PA levels and their possible interactions. The current analyses hint towards potential synergistic effects of important modifiable lifestyle factors in relation to health aspects, particularly in males.
Their interaction may differ substantially from their individual effects and this can be highly relevant when interpreting findings on a topic such as SFA, on which contradicting results are often discussed.
This study focusses on a population of healthy adolescents aged years, which is not a high-risk population. Given the low levels of hsCRP being addressed, results are not necessarily indicative of damage nor directly translatable to CVD risk, and hence the clinical relevance of the present findings may seem limited.
However, given the increasing evidence for the progression of risk factors from childhood to adulthood, preventive measures might already consider this age group and hence associations observed could provide valuable insight. A main limitation when assessing dietary intake is the reliance on subjective measures, which are prone to reporting bias.
In the present study the FFQ used to measure dietary SFA was designed to estimate fatty acids and antioxidants in school-aged children [ 54 ].
Given the thorough quality control of the dietary data with plausible values observed in terms of total energy intake , misreporting was likely detected and excluded from the analysis. A further drawback is the high inter-correlation amongst different nutrients, which is often come across in nutritional epidemiology, and if ignored could lead to inappropriate conclusions.
Adjustment for these nutrients within the statistical models could result in multicollinearity, generating further misleading associations [ 94 ]. With this in mind, and considering that the inclusion of an interaction term with PA would further complicate interpretation, we could not adjust for other nutrients and hence the ability to disentangle the individual effects of SFA is somewhat limited.
Nonetheless, we are aware of the importance of accounting for possible intercorrelations and have hence considered these in the interpretation of our results.
A further limitation in the present study was the underrepresentation of children from lower social-classes. As often occurs in longitudinal cohort studies, this non-random loss-to-follow-up meant that the current findings may not be entirely representative of the study area.
The assessment of other inflammatory markers might have been useful to strengthen our conclusions, but unfortunately these were not available for the studied cohorts.
Our findings are based on cross-sectional analyses, meaning that the observed associations between dietary SFA and hsCRP do not necessarily infer causality. Furthermore, blood-withdrawal for CRP measurements was carried out at a slightly different time to dietary assessment and accelerometry.
Thus, the present analysis is based on the assumption that dietary intake, as well as PA and CRP, are persistent during this interval, which may not be entirely the case. Nevertheless, for PA, activity measured on non-typical days e. including trips or sickness were excluded to ensure usual activity was recorded, which more likely represents chronic PA.
We assume that any drastic changes in diet between FFQ completion and blood withdrawal are unlikely, although it cannot be entirely excluded. Nevertheless, changes occurring in either lifestyle behaviour would have occurred at the individual level, and hence any bias due to such changes are expected to be random, not affecting the general trend observed.
From the present analyses, it can be concluded that a higher SFA intake during adolescence, within the ranges observed in the current study, is not detrimental in terms of inflammatory processes in adolescents; although we highlight that this may well depend on the nutrient it replaces.
We propose that when evaluating the role of SFA in chronic inflammation, it is essential to differentiate between findings involving SFA status in serum or plasma and those assessing dietary SFA, as the latter is likely influenced by important modifiable factors such as PA, which may determine whether an inflammatory response arises.
In some cases, ethical approval can be obtained for the release. Requests should be addressed to Marie Standl marie.
standl helmholtz-muenchen. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice - a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association.
World Health Organization. Global health estimates deaths by cause, age, sex, by country and by region, Geneva: WHO; Google Scholar. Nicklas TA, von Duvillard SP, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: the Bogalusa heart study.
Int J Sports Med. Article PubMed Google Scholar. Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, et al.
Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the cardiovascular risk in Young Finns study. J Pediatr. Article CAS PubMed Google Scholar. Canas JA, Sweeten S, Balagopal PB. Biomarkers for cardiovascular risk in children.
Curr Opin Cardiol. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children.
Arterioscler Thromb Vasc Biol. Schlenz H, Intemann T, Wolters M, Gonzalez-Gil EM, Nappo A, Fraterman A, et al. C-reactive protein reference percentiles among pre-adolescent children in Europe based on the IDEFICS study population.
International journal of obesity Article Google Scholar. Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors.
Nappo A, Iacoviello L, Fraterman A, Gonzalez-Gil EM, Hadjigeorgiou C, Marild S, et al. High-sensitivity C-reactive protein is a predictive factor of adiposity in children: results of the identification and prevention of dietary- and lifestyle-induced health effects in children and infants IDEFICS study.
J Am Heart Assoc. Article PubMed PubMed Central CAS Google Scholar. Syrenicz A, Garanty-Bogacka B, Syrenicz M, Gebala A, Walczak M. Low-grade systemic inflammation and the risk of type 2 diabetes in obese children and adolescents. Neuro endocrinology letters. CAS PubMed Google Scholar.
Retnakaran R, Hanley AJ, Connelly PW, Harris SB, Zinman B. Elevated C-reactive protein in native Canadian children: an ominous early complication of childhood obesity. Diabetes Obes Metab. Ford ES, Ajani UA, Mokdad AH.
The metabolic syndrome and concentrations of C-reactive protein among U. Diabetes Care. DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES Article PubMed PubMed Central Google Scholar.
Juonala M, Viikari JS, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the cardiovascular risk in Young Finns study.
Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The cardiovascular risk in Young Finns study.
Ann Med. Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association.
US senate select committee on nutrition and human needs. Dietary goals for the United States. Washington: US Govt print off; Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease.
Am J Clin Nutr. Article CAS PubMed PubMed Central Google Scholar. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al.
Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. Harcombe Z, Baker JS, Cooper SM, Davies B, Sculthorpe N, DiNicolantonio JJ, et al.
Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in and a systematic review and meta-analysis. Open Heart.
Thank you for amd nature. You inflammxtion using a browser version with limited support for CSS. Wnd obtain the best experience, we recommend you Fats and inflammation a more up to date Lnflammation or Fatss off compatibility mode Energy conservation Internet Explorer. In Fats and inflammation unflammation, to ensure continued support, we are displaying the site without styles and JavaScript. A diet high in saturated fats raises the risk of type 2 diabetes — perhaps owing to the activity of an inflammatory protein complex called the inflammasome. Jenny Ting and her team at the University of North Carolina at Chapel Hill found that a saturated fatty acid called palmitate stimulates the inflammasome — which activates the inflammatory response — in cultured mouse macrophages, a type of immune cell.
0 thoughts on “Fats and inflammation”