Speedy lipid breakdown -
Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. Mohassel P, Donkervoort S, Lone MA, Nalls M, Gable K, Gupta SD, et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat Med. Lee H, Lee JJ, Park NY, Dubey SK, Kim T, Ruan K, et al.
Multi-omic analysis of selectively vulnerable motor neuron subtypes implicates altered lipid metabolism in ALS. Nat Neurosci. Van den Bergh R, Swerts L, Hendrikx A, Boni L, Meulepas E.
Adipose tissue cellularity in patients with amyotrophic lateral sclerosis. Article Google Scholar. Desport JC, Torny F, Lacoste M, Preux PM, Couratier P.
Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. Dupuis L, Corcia P, Fergani A, Aguilar JLGD, Bonnefont-Rousselot D, Bittar R, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Dorst J, Kühnlein P, Hendrich C, Kassubek J, Sperfeld AD, Ludolph AC.
Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. Steyn FJ, Ioannides ZA, van Eijk RPA, Heggie S, Thorpe KA, Ceslis A, et al. Hypermetabolism in ALS is associated with greater functional decline and shorter survival.
J Neurol Neurosurg Psychiatry. Dupuis L, Oudart H, René F, de Aguilar JLG, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model.
Proc Natl Acad Sci U S A. Dorst J, Cypionka J, Ludolph AC. High-caloric food supplements in the treatment of amyotrophic lateral sclerosis: a prospective interventional study. Amyotroph Lateral Scler Frontotemporal Degener. Ahmed RM, Highton-Williamson E, Caga J, Thornton N, Ramsey E, Zoing M, et al.
Lipid metabolism and survival across the frontotemporal dementia-amyotrophic lateral sclerosis spectrum: relationships to eating behavior and cognition. J Alzheimers Dis. Ludolph AC, Dorst J, Dreyhaupt J, Weishaupt JH, Kassubek J, Weiland U, et al.
Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann Neurol. Ho WY, Chang JC, Lim K, Cazenave-Gassiot A, Nguyen AT, Foo JC, et al.
TDP mediates SREBF2-regulated gene expression required for oligodendrocyte myelination. J Cell Biol. Egawa N, Izumi Y, Suzuki H, Tsuge I, Fujita K, Shimano H, et al.
TDP regulates cholesterol biosynthesis by inhibiting sterol regulatory element-binding protein 2. Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov.
Lipidomics: new tools and applications. Castellanos DB, Martín-Jiménez CA, Rojas-Rodríguez F, Barreto GE, González J.
Brain lipidomics as a rising field in neurodegenerative contexts: perspectives with machine learning approaches. Front Neuroendocrinol. Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP.
Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Lawton KA, Brown MV, Alexander D, Li Z, Wulff JE, Lawson R, et al. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics.
Dodge JC, Jensen EH, Yu J, Sardi SP, Bialas AR, Taksir TV, et al. Neutral lipid cacostasis contributes to disease pathogenesis in amyotrophic lateral sclerosis. J Neurosci. Dodge JC, Treleaven CM, Pacheco J, Cooper S, Bao C, Abraham M, et al. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis.
Henriques A, Huebecker M, Blasco H, Keime C, Andres CR, Corcia P, et al. Inhibition of β-glucocerebrosidase activity preserves motor unit integrity in a mouse model of amyotrophic lateral sclerosis. Bouscary A, Quessada C, Mosbach A, Callizot N, Spedding M, Loeffler JP, et al.
Ambroxol hydrochloride improves motor functions and extends survival in a mouse model of familial amyotrophic lateral sclerosis. Front Pharmacol.
Potenza RL, De Simone R, Armida M, Mazziotti V, Pèzzola A, Popoli P, et al. Fingolimod: a disease-modifier drug in a mouse model of amyotrophic lateral sclerosis. Gunstone FD, Harwood JL, Harwood JL.
The lipid handbook with CD-ROM. Boca Raton: CRC Press; Book Google Scholar. Christie WW, Han X. Lipid analysis - isolation, separation, identification and lipidomic analysis [Internet]. Oily Press, Woodhead Publishing and now Elsevier; Gurr MI, Harwood JL, Frayn KN, Murphy DJ, Michell RH. Lipids: biochemistry, biotechnology and health, 6th Edition [Internet].
Wiley-Blackwell; Calder PC. Essays Biochem. Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. Glycosphingolipids: synthesis and functions. FEBS J. Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances.
Neurochem Res. Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST.
Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci. Tracey TJ, Kirk SE, Steyn FJ, Ngo ST. The role of lipids in the central nervous system and their pathological implications in amyotrophic lateral sclerosis.
Semin Cell Dev Biol. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Falomir-Lockhart LJ, Cavazzutti GF, Giménez E, Toscani AM. Fatty acid signaling mechanisms in neural cells: fatty acid receptors.
Front Cell Neurosci. Kidani Y, Bensinger SJ. LXR and PPAR as integrators of lipid homeostasis and immunity. Immunol Rev. Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection.
Mol Neurobiol. Ralhan I, Chang CL, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. Galkina OV, Vetrovoy OV, Eschenko ND.
The role of lipids in implementing specific functions in the central nervous system. Russ J Bioorg Chem. Article CAS Google Scholar. Hussain G, Wang J, Rasul A, Anwar H, Imran A, Qasim M, et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis.
Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. Waters S, Swanson MEV, Dieriks BV, Zhang YB, Grimsey NL, Murray HC, et al. Blood-spinal cord barrier leakage is independent of motor neuron pathology in ALS.
Acta Neuropathol Commun. Goutman SA, Boss J, Guo K, Alakwaa FM, Patterson A, Kim S, et al. Untargeted metabolomics yields insight into ALS disease mechanisms.
Sol J, Jové M, Povedano M, Sproviero W, Domínguez R, Piñol-Ripoll G, et al. Lipidomic traits of plasma and cerebrospinal fluid in amyotrophic lateral sclerosis correlate with disease progression. Brain Commun. Area-Gomez E, Larrea D, Yun T, Xu Y, Hupf J, Zandkarimi F, et al.
Lipidomics study of plasma from patients suggest that ALS and PLS are part of a continuum of motor neuron disorders. FernÁndez-Eulate G, Ruiz-Sanz JI, Riancho J, ZufirÍa M, GereÑu G, FernÁndez-TorrÓn R, et al. A comprehensive serum lipidome profiling of amyotrophic lateral sclerosis.
Amyotroph Lateral Scler Frontotemp Degener. Pre-diagnostic plasma lipid levels and the risk of amyotrophic lateral sclerosis. Lipidomics reveals cerebrospinal-fluid signatures of ALS. Omics to explore amyotrophic lateral sclerosis evolution: the central role of arginine and proline metabolism.
Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum Mol Genet. Burg T, Rossaert E, Moisse M, Van Damme P, Van Den Bosch L. Histone deacetylase inhibition regulates lipid homeostasis in a mouse model of amyotrophic lateral sclerosis.
Int J Mol Sci. Chaves-Filho AB, Pinto IFD, Dantas LS, Xavier AM, Inague A, Faria RL, et al. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis.
Ramírez-Nuñez O, Jové M, Torres P, Sol J, Fontdevila L, Romero-Guevara R, et al. Nuclear lipidome is altered in amyotrophic lateral sclerosis: a pilot study. J Neurochem. Konrad C, Kawamata H, Bredvik KG, Arreguin AJ, Cajamarca SA, Hupf JC, et al.
Fibroblast bioenergetics to classify amyotrophic lateral sclerosis patients. Mol Neurodegener. Allen SP, Duffy LM, Shaw PJ, Grierson AJ. Altered age-related changes in bioenergetic properties and mitochondrial morphology in fibroblasts from sporadic amyotrophic lateral sclerosis patients.
Neurobiol Aging. Veyrat-Durebex C, Bris C, Codron P, Bocca C, Chupin S, Corcia P, et al. Metabo-lipidomics of fibroblasts and mitochondrial-endoplasmic reticulum extracts from ALS patients shows alterations in purine, pyrimidine, energetic, and phospholipid metabolisms.
Quessada C, Bouscary A, René F, Valle C, Ferri A, Ngo ST, et al. Skeletal muscle metabolism: origin or prognostic factor for amyotrophic lateral sclerosis ALS development?
Loeffler J, Picchiarelli G, Dupuis L, De Gonzalez AJ. The role of skeletal muscle in amyotrophic lateral sclerosis.
Brain Pathol. Scaricamazza S, Salvatori I, Ferri A, Valle C. Skeletal muscle in ALS: an unappreciated therapeutic opportunity? Ebert D, Haller RG, Walton ME.
Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, Bellen HJ. Cell Metab. Palamiuc L, Schlagowski A, Ngo ST, Vernay A, Dirrig-Grosch S, Henriques A, et al.
A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis.
EMBO Mol Med. Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. Fitzner D, Bader JM, Penkert H, Bergner CG, Su M, Weil MT, et al.
Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al.
Expanded GGGGCC hexanucleotide repeat in non-coding region of C9ORF72 causes chromosome 9p-linked frontotemporal dementia and amyotrophic lateral sclerosis.
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9plinked ALS-FTD.
Liu Y, Wang T, Ji YJ, Johnson K, Liu H, Johnson K, et al. A C9orf72—CARM1 axis regulates lipid metabolism under glucose starvation-induced nutrient stress. Genes Dev. Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice.
Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, et al.
Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids.
Leekumjorn S, Cho HJ, Wu Y, Wright NT, Sum AK, Chan C. The role of fatty acid unsaturation in minimizing biophysical changes on the structure and local effects of bilayer membranes.
Biochim Biophys Acta. Vinknes KJ, Elshorbagy AK, Nurk E, Drevon CA, Gjesdal CG, Tell GS, et al. Plasma stearoyl-CoA desaturase indices: association with lifestyle, diet, and body composition. Henriques A, Blasco H, Fleury MC, Corcia P, Echaniz-Laguna A, Robelin L, et al.
Blood cell palmitoleate-palmitate ratio is an independent prognostic factor for amyotrophic lateral sclerosis.
PLoS ONE. Oliván S, Martínez-Beamonte R, Calvo AC, Surra JC, Manzano R, Arnal C, et al. Extra virgin olive oil intake delays the development of amyotrophic lateral sclerosis associated with reduced reticulum stress and autophagy in muscle of SOD1G93A mice.
J Nutr Biochem. Ponce J, Ulu A, Hanson C, Cameron-Smith E, Bertoni J, Wuebker J, et al. Role of specialized pro-resolving mediators in reducing neuroinflammation in neurodegenerative disorders. Front Aging Neurosci. Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Dyall SC.
Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA DPA and DHA. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. Yip PK, Pizzasegola C, Gladman S, Biggio ML, Marino M, Jayasinghe M, et al.
The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. Boumil EF, Vohnoutka RB, Liu Y, Lee S, Shea TB. Omega-3 hastens and omega-6 delays the progression of neuropathology in a murine model of familial ALS. Open Neurol J.
Ilieva EV, Ayala V, Jové M, Dalfó E, Cacabelos D, Povedano M, et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis.
Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, et al. Increased expression and activation of cytosolic phospholipase A2 in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Malada Edelstein YF, Solomonov Y, Hadad N, Alfahel L, Israelson A, Levy R.
Early upregulation of cytosolic phospholipase A2α in motor neurons is induced by misfolded SOD1 in a mouse model of amyotrophic lateral sclerosis. J Neuroinflamm. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. Trostchansky A, Mastrogiovanni M, Miquel E, Rodríguez-Bottero S, Martínez-Palma L, Cassina P, et al.
Profile of arachidonic acid-derived inflammatory markers and its modulation by nitro-oleic acid in an inherited model of amyotrophic lateral sclerosis. Almer G, Guégan C, Teismann P, Naini A, Rosoklija G, Hays AP, et al. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis.
Iłżecka J. Prostaglandin E2 is increased in amyotrophic lateral sclerosis patients. Acta Neurol Scand. Klivenyi P, Kiaei M, Gardian G, Calingasan NY, Beal MF. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis.
Miyagishi H, Kosuge Y, Takano A, Endo M, Nango H, Yamagata-Murayama S, et al. Increased expression of hydroxyprostaglandin dehydrogenase in spinal astrocytes during disease progression in a model of amyotrophic lateral sclerosis.
Cell Mol Neurobiol. Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler. Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress.
FASEB J. Shinozawa T, Urade Y, Maruyama T, Watabe D. Tetranor PGDM analyses for the amyotrophic lateral sclerosis: positive and simple diagnosis and evaluation of drug effect. Biochem Biophys Res Commun. Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T.
Programming of neural cells by endo cannabinoids: from physiological rules to emerging therapies. Bilsland LG, Greensmith L. The endocannabinoid system in amyotrophic lateral sclerosis.
Curr Pharm Des. Pryce G, Baker D. Endocannabinoids in multiple sclerosis and amyotrophic lateral sclerosis. In: Pertwee RG, editor. Endocannabinoids [Internet]. Cham: Springer International Publishing; Chapter Google Scholar. Witting A, Weydt P, Hong S, Kliot M, Moller T, Stella N.
Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. Carter GT, McLaughlin RJ, Cuttler C, Sauber GJ, Weeks DL, Hillard CJ, et al. Endocannabinoids and related lipids in serum from patients with amyotrophic lateral sclerosis.
Muscle Nerve. Espejo-Porras F, Fernández-Ruiz J, de Lago E. Analysis of endocannabinoid receptors and enzymes in the post-mortem motor cortex and spinal cord of amyotrophic lateral sclerosis patients. Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease.
Blusztajn JK, Liscovitch M, Richardson UI. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Suzuki S, Yamatoya H, Sakai M, Kataoka A, Furushiro M, Kudo S. Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats.
J Nutr. Zhou MM, Xue Y, Sun SH, Wen M, Li ZJ, Xu J, et al. Effects of different fatty acids composition of phosphatidylcholine on brain function of dementia mice induced by scopolamine. Boggs JM. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function.
van Echten-Deckert G, Herget T. Sphingolipid metabolism in neural cells. Sonnino S, Prinetti A. The role of sphingolipids in neuronal plasticity of the brain. Olsen ASB, Færgeman NJ.
Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol. Di Pardo A, Maglione V. Sphingolipid metabolism: a new therapeutic opportunity for brain degenerative disorders.
van Kruining D, Luo Q, van Echten-Deckert G, Mielke MM, Bowman A, Ellis S, et al. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods.
Adv Drug Deliv Rev. Czubowicz K, Jęśko H, Wencel P, Lukiw WJ, Strosznajder RP. Belarbi K, Cuvelier E, Bonte MA, Desplanque M, Gressier B, Devos D, et al.
Walter S, Fassbender K. Spingolipids in multiple sclerosis. Cell Physiol Biochem. Bouscary A, Quessada C, René F, Spedding M, Turner BJ, Henriques A, et al. Sphingolipids metabolism alteration in the central nervous system: amyotrophic lateral sclerosis ALS and other neurodegenerative diseases.
Dodge JC. Lipid involvement in neurodegenerative diseases of the motor system: insights from lysosomal storage diseases. Michel C, Van Echten-Deckert G, Rother J, Sandhoff K, Wang E, Merrill AH. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide.
J Biol Chem. Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol. Hartfield PJ, Mayne GC, Murray AW. Ceramide induces apoptosis in PC12 cells. FEBS Lett. Irie F, Hirabayashi Y. Application of exogenous ceramide to cultured rat spinal motoneurons promotes survival or death by regulation of apoptosis depending on its concentrations.
J Neurosci Res. Willaime S, Vanhoutte P, Caboche J, Lemaigre-Dubreuil Y, Mariani J, Brugg B. Ceramide-induced apoptosis in cortical neurons is mediated by an increase in p38 phosphorylation and not by the decrease in ERK phosphorylation.
Eur J Neurosci. Pelled D, Raveh T, Riebeling C, Fridkin M, Berissi H, Futerman AH, et al. Death-associated protein DAP kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA.
Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I.
Cell contours were marked with a dashed white line based on brightfield imaging. Two different lipid mixes were prepared Supplementary Table 3. GUVs were stained with Laurdan at 0.
GUVs were synthesized by polyvinyl alcohol PVA -assisted swelling Vesicles were collected by pipetting and kept at room temperature for immediate use. Vesicles were imaged in well plates glass bottom, Grace BioLabs.
Laurdan images were recorded on an SP8 DIVE confocal inverted microscope with multi-photon lasers Leica as described above. After centrifugation, an aqueous top phase and organic bottom phase, which contains the lipids, were obtained.
The lipid phase was collected and the solvent evaporated at room temperature using an Eppendorf Concentrator Plus.
GUVs were imaged in well plates glass bottom, Grace BioLabs using a DeltaVision Elite microscope GE Healthcare. For dot-spot assays, cells were grown exponentially in SRC drop-out medium, collected and resuspended to a final OD of 0.
Wild-type cells were grown in SDC medium. Wohlwend GmbH. The frozen samples were subsequently transferred into a Leica EM AFS-2 freeze substitution unit Leica Microsystems. Digital images were acquired using an 11 megapixel Morada CCD camera Olympus-SIS. For data acquisition, processing and modelling, the IMOD software 80 from the Boulder Laboratory for 3D Electron Microscopy of Cells was used.
Cells were filtered via Whatman Nucleopore filter membranes Merck with 0. The frozen samples were transferred into an automated freeze substitution unit, a Leica EM AFS-2 Leica Microsystems. The HM20 resin blocks were trimmed to a pyramid Leica EM Trim and sectioned with a Leica UCT ultramicrotome both Leica Microsystems.
Z -stacks of fluorescence and DIC images were acquired with ZEISS ZEN blue 3. Post-processing of images was performed with ImageJ. Using the ZEISS ZEN Blue 3. Spheroroplasts were homogenized using a Dounce homogenizer. The upper LD fraction was removed and frozen in liquid N 2. After collecting, cells were washed three times with water and frozen in liquid N 2.
Lipidomic analysis of LDs and whole cell lysates was performed by Lipotype GmbH as described 81 , After extraction, the organic phase was transferred to an infusion plate and dried in a speed vacuum concentrator.
First-step dry extract was resuspended in 7. All liquid handling steps were performed using a Hamilton Robotics STARlet robotic platform with the Anti Droplet Control feature for organic solvent pipetting. Samples were analysed by direct infusion on a QExactive mass spectrometer Thermo Scientific equipped with a TriVersa NanoMate ion source Advion Biosciences.
MS only was used to monitor CDP-DAG, LPA, LPE, LPG, LPI, LPS, IPC, MIPC and M IP 2 C as deprotonated anions; Cer and LPC as acetate adducts and ergosterol as protonated ion of an acetylated derivative Data were analysed with a lipid identification software based on LipotypeXplorer 85 , Yeast whole-cell extracts were prepared, normalized for protein concentration and analysed by immunoblotting according to standard procedures.
ab , mouse monoclonal anti-GFP clones 7. ab , mouse monoclonal anti-Pgk1 clone 22C5D8 ,, Abcam cat. ab , peroxidase AffiniPure Goat anti-Mouse IgG polyclonal ,, Jackson ImmunoResearch cat. Number of biological replicates is indicated in the figures, and sample size in the figure legends.
All microscopy experiments were repeated at least three times, except Figs. Immunoblotting experiments were repeated two times, except Extended Figs. Yeast growth assays were repeated at least two times. Lipidomic experiments were repeated three times.
EM-based experiments were done once. All attempts to replicate the data were successful. Data normality was determined using the Shapiro—Wilk test. Statistical significance was evaluated by two-tailed t -test or Mann—Whitney test depending on data normality using the GraphPad Prism software, where indicated.
No statistical method was used to pre-determine sample size. No data were excluded from the analyses, unless mentioned in the description of the analysis below.
The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Detailed descriptions of phenotype quantifications are provided below:. Ectopic NPCs were further quantified with respect to Nup co-localization. To quantify the reversal of NPC defects upon Dga1 overexpression or LA addition, every cell that contained Sct1 fluorescence was analysed.
To quantify mitotic spindle phenotypes, only dividing cells that exhibited similarly sized mother and daughter cells were considered anaphase. To quantify nuclear import efficiency with the NLS—2xmCherry reporter, the mean signal intensity of the nucleus N was measured using ImageJ.
An identically sized area was used for measuring the mean signal intensity of the cytoplasm C. The intensity of the bands was measured in ImageJ. To quantify the circularity of cellular structures that is, H2B for the nucleus and Pma1 for the PM , the contours were marked in ImageJ and the circularity index was determined.
To quantify nuclear rupture and leakage, nuclear integrity was considered normal if the Pus1—GFP signal was enriched in the nucleus. Pus1 leakage was defined as equal fluorescence intensities of Pus1—GFP in the nucleoplasm and the cytoplasm.
To quantify nuclear leakage with the MGM4 reporter, nuclear integrity was considered normal if the MGM4 reporter was excluded from the nucleus. MGM4 leakage into the nucleus was defined as equal fluorescence intensities of MGM4 in the nucleoplasm and the cytoplasm.
To quantify the ratio of MGM4 reporter, the mean signal intensity of the nucleus N was measured using ImageJ. The segmentation classifier was trained with two classes: to recognize LDs class 1 and to recognize the background class 2.
Next, particle analysis was performed to obtain the area of each LD, and the volume was calculated assuming that each LD is spherical. Two extreme outliers out of 4, data points were excluded from the analysis. Analysis of GUVs reconstituted from whole cell lipid extracts was based on Rhod—PE signal.
Fused GUVs were excluded from the analysis. Laurdan data are displayed as pseudocoloured generalized polarization GP images. The calculation of the GP images was performed in Fiji as described in 52 using the provided custom-written macro. GP is calculated according to the following equation:.
where I represents the intensity in each pixel in the image acquired in the indicated spectral channel numbers are in nm and G is the calibration factor. G factor was set to 1. GP values for a region of interest were determined by a custom-written macro After selecting a region in Fiji, the histogram function provides mean intensity values and pixel counts for each GP value.
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
The data reported in this paper are available in the main text or Supplementary Information. Source data are provided with this paper. Any additional information required to re-analyse the data reported in this paper are available from the lead contact upon request. Harayama, T.
Understanding the diversity of membrane lipid composition. Cell Biol. Article CAS PubMed Google Scholar. van Meer, G. Membrane lipids: where they are and how they behave. Article PubMed PubMed Central Google Scholar. Zimmerberg, J. How proteins produce cellular membrane curvature.
West, M. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. Article CAS PubMed PubMed Central Google Scholar. Ungricht, R. Mechanisms and functions of nuclear envelope remodelling.
Akey, C. et al. Comprehensive structure and functional adaptations of the yeast nuclear pore complex. Cell , — e Allegretti, M.
In-cell architecture of the nuclear pore and snapshots of its turnover. Nature , Bley, C. Architecture of the cytoplasmic face of the nuclear pore. Science , eabm Petrovic, S. Architecture of the linker-scaffold in the nuclear pore. Schuller, A. The cellular environment shapes the nuclear pore complex architecture.
Nature , — Cibulka, J. Assembly principle of a membrane-anchored nuclear pore basket scaffold. Drin, G. A general amphipathic alpha-helical motif for sensing membrane curvature. Meszaros, N. Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature.
Cell 33 , — Vollmer, B. Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly. EMBO J. Zimmerli, C. Nuclear pores dilate and constrict in cellulo. Science , eabd Lomakin, A. The nucleus acts as a ruler tailoring cell responses to spatial constraints.
Science , eaba Venturini, V. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Elosegui-Artola, A. Force triggers YAP nuclear entry by regulating transport across nuclear pores.
Denais, C. Nuclear envelope rupture and repair during cancer cell migration. Science , — Kinugasa, Y. The very-long-chain fatty acid elongase Elo2 rescues lethal defects associated with loss of the nuclear barrier function in fission yeast cells.
Cell Sci. Raab, M. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Thaller, D. Direct binding of ESCRT protein Chm7 to phosphatidic acid-rich membranes at nuclear envelope herniations.
Barelli, H. Lipid unsaturation and organelle dynamics. Martin, C. Regulation of long chain unsaturated fatty acid synthesis in yeast.
Acta , — Ballweg, S. Regulation of lipid saturation without sensing membrane fluidity. Covino, R. A eukaryotic sensor for membrane lipid saturation. cell 63 , 49—59 De Smet, C. The yeast acyltransferase Sct1p regulates fatty acid desaturation by competing with the desaturase Ole1p.
cell 23 , — Brookheart, R. As a matter of fat. Cell Metab. Petschnigg, J. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast.
Helfrich, W. Elastic properties of lipid bilayers: theory and possible experiments. C 28 , — Rawicz, W. Effect of chain length and unsaturation on elasticity of lipid bilayers.
Baumgart, T. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Dietrich, C. Lipid rafts reconstituted in model membranes.
Veatch, S. Organization in lipid membranes containing cholesterol. Article PubMed Google Scholar. Roux, A. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. Gohrbandt, M.
Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria. King, C. ER membranes exhibit phase behavior at sites of organelle contact. Natl Acad. USA , — Leveille, C. Yeast cells actively tune their membranes to phase separate at temperatures that scale with growth temperatures.
USA , e Shen, Y. Metabolic activity induces membrane phase separation in endoplasmic reticulum. Toulmay, A.
Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. Romanauska, A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Tsuji, T. Predominant localization of phosphatidylserine at the cytoplasmic leaflet of the ER, and its TMEM16K-dependent redistribution.
Vancura, A. Purification and characterization of glycerophosphate acyltransferase from rat liver mitochondria. Kiegerl, B. Phosphorylation of the lipid droplet localized glycerol3phosphate acyltransferase Gpt2 prevents a futile triacylglycerol cycle in yeast.
Acta , Article CAS Google Scholar. Piccolis, M. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Cell 74 , 32—44 e38 Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat. Cell 56 , — e Klose, C. Yeast lipids can phase-separate into micrometer-scale membrane domains.
Terweij, M. Recombination-induced tag exchange RITE cassette series to monitor protein dynamics in Saccharomyces cerevisiae. G3 3 , — Andersen, O. Hao, Q. Electron microscopy of Chaetomium pom shows the assembly of ten-bead string. Cell Discov. Upla, P. Molecular architecture of the major membrane ring component of the nuclear pore complex.
Structure 25 , — Owen, D. Quantitative imaging of membrane lipid order in cells and organisms. Heberle, F. Phase separation in lipid membranes. Cold Spring Harb. Rayermann, S. Hallmarks of reversible separation of living, unperturbed cell membranes into two liquid phases. Popken, P. Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex.
Cell 26 , — Bai, Y. X-ray structure of a mammalian stearoyl-CoA desaturase. Wang, H. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate.
Lusk, C. CHMPions of repair: emerging perspectives on sensing and repairing the nuclear envelope barrier. Silvius, J. Role of head group structure in the phase behavior of amino phospholipids.
Hydrated and dehydrated lamellar phases of saturated phosphatidylethanolamine analogues. Biochemistry 25 , — Kaliszewski, P. Enhanced levels of Pis1p phosphatidylinositol synthase improve the growth of Saccharomyces cerevisiae cells deficient in Rsp5 ubiquitin ligase.
Kralt, A. An amphipathic helix in Brl1 is required for nuclear pore complex biogenesis in S. eLife 11 , e Otsuka, S. Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope. eLife 5 , e Bigay, J. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity.
Cell 23 , — Garcia-Saez, A. Effect of line tension on the lateral organization of lipid membranes. Angebault, C. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians.
Muller, L. Genome-wide association analysis of clinical vs. nonclinical origin provides insights into Saccharomyces cerevisiae pathogenesis. Rizzetto, L. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease.
Rohrig, F. The multifaceted roles of fatty acid synthesis in cancer. Cancer 16 , — Kwast, K. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes.
USA 96 , — Lewis, C. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme.
Oncogene 34 , — Li, J. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Genomics 25 , — Andreasen, A. Anaerobic nutrition of Saccharomyces cerevisiae. Unsaturated fatty acid requirement for growth in a defined medium.
Cell Comp. Bensaad, K. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep.
Ighodaro OM , Akinloye OA. First line defence antioxidants-superoxide dismutase SOD , catalase CAT and glutathione peroxidase GPX : their fundamental role in the entire antioxidant defence grid.
Alexandria J Med. Mason RR , Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab. Gemmink A , Bosma M , Kuijpers HJH , et al. Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans.
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Additional Information. Data Availability.
Journal Article. Kelsey Gabel , Kelsey Gabel. Department of Kinesiology and Nutrition, University of Illinois at Chicago. Oxford Academic. Krista A Varady. Correspondence and Reprint Requests: Krista A.
Varady, PhD, Department of Kinesiology and Nutrition, University of Illinois at Chicago, W Taylor St, Rm , Chicago, IL, , USA. E-mail: varady uic. Editorial decision:. Corrected and typeset:. PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero.
enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. intermittent fasting , alternate day fasting , muscle lipid metabolism , nonesterified fatty acids , obesity.
Google Scholar Crossref. Search ADS. Google Scholar PubMed. OpenURL Placeholder Text. Published by Oxford University Press on behalf of the Endocrine Society This work is written by a US Government employee s and is in the public domain in the US.
If your lpid subscribes li;id this resource, li;id you don't have Speedy lipid breakdown Access Profile, please kipid your library's Nutrition coaching for sports performance desk for information Sppeedy how to gain access to Speedy lipid breakdown resource from off-campus. Take the Speedy lipid breakdown library lippid you Speedy lipid breakdown you go—easy access to books, videos, images, podcasts, personalized features, and more. Download the Access App here: iOS and Android. Learn more here! Please consult the latest official manual style if you have any questions regarding the format accuracy. Lipids perform several essential functions, including forming biological membranes, efficient storage of energy, and as components of several important structural and functional molecules. The choice between synthesis and degradation represents an important regulatory step in human biology and reflects the level of food and, therefore, energy stores available to the body. Breakdosn fasting Increase energy levels naturally has emerged as an alternative to daily Speedy lipid breakdown restriction Kipid to improve Speecy health. Alternate-day fasting ADF is the most studied form of Speedy lipid breakdown fasting to date. More than Speedy lipid breakdown dozen trials have examined the efficacy of ADF in humans 12. Results from these trials suggest ADF is as effective as daily CR for weight loss and metabolic disease risk reduction 12. More recently, it has been proposed that owing to the daily metabolic switch that occurs between the anabolic fed state and catabolic fasted state during IF, these regimens may stimulate lipid turnover more than daily restriction 3.
Speedy lipid breakdown -
Two extreme outliers out of 4, data points were excluded from the analysis. Analysis of GUVs reconstituted from whole cell lipid extracts was based on Rhod—PE signal. Fused GUVs were excluded from the analysis. Laurdan data are displayed as pseudocoloured generalized polarization GP images.
The calculation of the GP images was performed in Fiji as described in 52 using the provided custom-written macro. GP is calculated according to the following equation:. where I represents the intensity in each pixel in the image acquired in the indicated spectral channel numbers are in nm and G is the calibration factor.
G factor was set to 1. GP values for a region of interest were determined by a custom-written macro After selecting a region in Fiji, the histogram function provides mean intensity values and pixel counts for each GP value. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
The data reported in this paper are available in the main text or Supplementary Information. Source data are provided with this paper.
Any additional information required to re-analyse the data reported in this paper are available from the lead contact upon request. Harayama, T. Understanding the diversity of membrane lipid composition. Cell Biol. Article CAS PubMed Google Scholar. van Meer, G.
Membrane lipids: where they are and how they behave. Article PubMed PubMed Central Google Scholar. Zimmerberg, J. How proteins produce cellular membrane curvature.
West, M. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. Article CAS PubMed PubMed Central Google Scholar. Ungricht, R. Mechanisms and functions of nuclear envelope remodelling.
Akey, C. et al. Comprehensive structure and functional adaptations of the yeast nuclear pore complex. Cell , — e Allegretti, M. In-cell architecture of the nuclear pore and snapshots of its turnover.
Nature , Bley, C. Architecture of the cytoplasmic face of the nuclear pore. Science , eabm Petrovic, S. Architecture of the linker-scaffold in the nuclear pore. Schuller, A.
The cellular environment shapes the nuclear pore complex architecture. Nature , — Cibulka, J. Assembly principle of a membrane-anchored nuclear pore basket scaffold. Drin, G. A general amphipathic alpha-helical motif for sensing membrane curvature.
Meszaros, N. Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature. Cell 33 , — Vollmer, B. Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly. EMBO J. Zimmerli, C.
Nuclear pores dilate and constrict in cellulo. Science , eabd Lomakin, A. The nucleus acts as a ruler tailoring cell responses to spatial constraints.
Science , eaba Venturini, V. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Elosegui-Artola, A. Force triggers YAP nuclear entry by regulating transport across nuclear pores.
Denais, C. Nuclear envelope rupture and repair during cancer cell migration. Science , — Kinugasa, Y. The very-long-chain fatty acid elongase Elo2 rescues lethal defects associated with loss of the nuclear barrier function in fission yeast cells.
Cell Sci. Raab, M. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death.
Thaller, D. Direct binding of ESCRT protein Chm7 to phosphatidic acid-rich membranes at nuclear envelope herniations. Barelli, H. Lipid unsaturation and organelle dynamics. Martin, C. Regulation of long chain unsaturated fatty acid synthesis in yeast. Acta , — Ballweg, S.
Regulation of lipid saturation without sensing membrane fluidity. Covino, R. A eukaryotic sensor for membrane lipid saturation. cell 63 , 49—59 De Smet, C.
The yeast acyltransferase Sct1p regulates fatty acid desaturation by competing with the desaturase Ole1p. cell 23 , — Brookheart, R. As a matter of fat. Cell Metab. Petschnigg, J. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast.
Helfrich, W. Elastic properties of lipid bilayers: theory and possible experiments. C 28 , — Rawicz, W. Effect of chain length and unsaturation on elasticity of lipid bilayers. Baumgart, T. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension.
Dietrich, C. Lipid rafts reconstituted in model membranes. Veatch, S. Organization in lipid membranes containing cholesterol.
Article PubMed Google Scholar. Roux, A. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. Gohrbandt, M. Low membrane fluidity triggers lipid phase separation and protein segregation in living bacteria.
King, C. ER membranes exhibit phase behavior at sites of organelle contact. Natl Acad. USA , — Leveille, C. Yeast cells actively tune their membranes to phase separate at temperatures that scale with growth temperatures.
USA , e Shen, Y. Metabolic activity induces membrane phase separation in endoplasmic reticulum. Toulmay, A. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells.
Romanauska, A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Tsuji, T. Predominant localization of phosphatidylserine at the cytoplasmic leaflet of the ER, and its TMEM16K-dependent redistribution. Vancura, A. Purification and characterization of glycerophosphate acyltransferase from rat liver mitochondria.
Kiegerl, B. Phosphorylation of the lipid droplet localized glycerol3phosphate acyltransferase Gpt2 prevents a futile triacylglycerol cycle in yeast. Acta , Article CAS Google Scholar.
Piccolis, M. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Cell 74 , 32—44 e38 Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat.
Cell 56 , — e Klose, C. Yeast lipids can phase-separate into micrometer-scale membrane domains. Terweij, M. Recombination-induced tag exchange RITE cassette series to monitor protein dynamics in Saccharomyces cerevisiae.
G3 3 , — Andersen, O. Hao, Q. Electron microscopy of Chaetomium pom shows the assembly of ten-bead string. Cell Discov. Upla, P. Molecular architecture of the major membrane ring component of the nuclear pore complex.
Structure 25 , — Owen, D. Quantitative imaging of membrane lipid order in cells and organisms. Heberle, F. Phase separation in lipid membranes. Cold Spring Harb. Rayermann, S. Hallmarks of reversible separation of living, unperturbed cell membranes into two liquid phases.
Popken, P. Size-dependent leak of soluble and membrane proteins through the yeast nuclear pore complex. Cell 26 , — Bai, Y. X-ray structure of a mammalian stearoyl-CoA desaturase.
Wang, H. Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate. Lusk, C. CHMPions of repair: emerging perspectives on sensing and repairing the nuclear envelope barrier.
Silvius, J. Role of head group structure in the phase behavior of amino phospholipids. Hydrated and dehydrated lamellar phases of saturated phosphatidylethanolamine analogues.
Biochemistry 25 , — Kaliszewski, P. Enhanced levels of Pis1p phosphatidylinositol synthase improve the growth of Saccharomyces cerevisiae cells deficient in Rsp5 ubiquitin ligase.
Kralt, A. An amphipathic helix in Brl1 is required for nuclear pore complex biogenesis in S. eLife 11 , e Otsuka, S. Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope.
eLife 5 , e Bigay, J. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Cell 23 , — Garcia-Saez, A. Effect of line tension on the lateral organization of lipid membranes. Angebault, C. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians.
Muller, L. Genome-wide association analysis of clinical vs. nonclinical origin provides insights into Saccharomyces cerevisiae pathogenesis.
Rizzetto, L. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Rohrig, F.
The multifaceted roles of fatty acid synthesis in cancer. Cancer 16 , — Kwast, K. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes.
USA 96 , — Lewis, C. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme.
Oncogene 34 , — Li, J. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Genomics 25 , — Andreasen, A.
Anaerobic nutrition of Saccharomyces cerevisiae. Unsaturated fatty acid requirement for growth in a defined medium.
Cell Comp. Bensaad, K. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. Kamphorst, J. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Maciejowski, J.
Nuclear membrane rupture and Its consequences. Cell Dev. Mazeres, S. Using spectral decomposition of the signals from laurdan-derived probes to evaluate the physical state of membranes in live cells. FRes 6 , Liu, Q. Functional and topological analysis of yeast acyl-CoA:diacylglycerol acyltransferase 2, an endoplasmic reticulum enzyme essential for triacylglycerol biosynthesis.
Willis, S. Measuring mRNA levels over time during the yeast S. cerevisiae hypoxic response. Weinberger, A. Gel-assisted formation of giant unilamellar vesicles. Kremer, J. Computer visualization of three-dimensional image data using IMOD.
Ejsing, C. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Flexibility of a eukaryotic lipidome—insights from yeast lipidomics. PLoS ONE 7 , e Surma, M. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids.
Lipid Sci. Liebisch, G. Herzog, R. LipidXplorer: a software for consensual cross-platform lipidomics. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language.
Genome Biol. Download references. We thank M. Brandstetter and S. Jacob for assistance with TEM, CLEM and tomography reconstruction Vienna Biocenter Core Facilities, VBCF , P. Pasierbek for help with Multi-Photon Fluorescence Microscopy IMP BioOptics Facility , E. Stankunas for help with GUV preparations, S.
Westermann for the GFP-Tub1 plasmid, L. Veenhoff for the MGM4 plasmid, F. van Leeuwen for advice with RITE experiments and plasmids pTW Addgene and pKV Addgene , Lipotype for lipid analyses and G.
Warren for valuable discussions. was funded by ERC-COG ; NPC BUILD , SFB F B and the NOMIS Foundation. Max Perutz Labs, Vienna Biocenter Campus VBC , Vienna, Austria. Center for Molecular Biology, University of Vienna, Vienna, Austria. Center for Medical Biochemistry, Medical University of Vienna, Vienna, Austria.
You can also search for this author in PubMed Google Scholar. Correspondence to Alwin Köhler. a , Sequence alignment of a conserved phosphorylation motif located at the C-termini of Sct1 and Gpt2. Red asterisks: serine residues that were mutated to alanine in Sct1 3A or Gpt2 3A.
Amino acid positions in Sct1 indicated. b , Live imaging of sct1 Δ cells expressing genomically tagged H2B -mScarlet and overexpressing plasmid-based mGFP -SCT1 constructs.
Numbers: circularity index of H2B signal, taken as a proxy for nuclear shape. Circularity of Sct1 3A-expressing cells is significantly reduced, consistent with NE rigidification. c , Quantification of circularity index of nuclear H2B signal in b.
Value of 1 indicates a perfect circle. As the value approaches 0, the structure becomes increasingly irregular. Box-plot indicates median and interquartile range, whiskers extend to the smallest and largest value.
d , Imaging of cells expressing genomically tagged SEC63 -GFP and overexpressing plasmid-based Homo sapiens H. GPAT constructs tagged with BFP. f , Immunoblotting for Gpt2 phosphorylation.
Upper band: phosphorylated Gpt2; lower band: unphosphorylated Gpt2. g , Imaging of gpt2 Δ cells overexpressing plasmid-based mGFP- GPT2 constructs. i , TEM analysis of sct1 Δ cells overexpressing plasmid-based mGFP- SCT1 from the GAL1 promoter.
Insets show a magnified view of marked areas. ER, endoplasmic reticulum. j , Imaging of sct1 Δ cells expressing plasmid-based mGFP- SCT1 constructs and the reticulon RTN1 -mCherry. Constructs were overexpressed from the GAL1 promoter.
Note that Rtn1 does not overlap with rigidified ER sheets. k , Growth of wild-type cells overexpressing plasmid-based constructs from the GAL1 promoter. Empty vector used as control. Growth on plates containing glucose repressed and galactose induced.
a , Immunoblotting of Ole1 overexpressed from a GAL1 promoter. Samples from cultures used in Fig. b , Immunoblotting of INM LipSat sensor processing. Samples taken from cultures used in Fig. NLS corresponds to the Heh2 aa93— sequence, which was appended to the LipSat sensor for nuclear import.
c , Quantification of INM LipSat sensor processing in b. Gpt2 GL is a catalytically inactive mutant. e , Quantitative immunoblotting of Sct1, expressed from the endogenous SCT1 promoter, overexpressed from the GAL1 promoter, or co-overexpressed with OLE1 in sct1 Δ cells.
Sct1 was overexpressed ~20 fold compared to endogenous protein levels, and Ole1 co-expression did not noticeably alter Sct1 protein levels. Serial dilutions of cell extracts shown.
Upper band: phosphorylated Sct1; lower band: unphosphorylated Sct1. f , Quantitative immunoblotting of Ole1, expressed from the endogenous OLE1 promoter, overexpressed from the GAL1 promoter, or co-overexpressed with SCT1. Ole1 was overexpressed ~ fold compared to endogenous protein levels, and Sct1 co-expression did not noticeably alter Ole1 protein levels.
a-e , Cartoons of nucleoporin localization that is cytoplasmic side, NPC core, or NPC basket for microscopy experiments see also Fig.
Cartoons refer to graphs on the right side. INM, inner nuclear membrane; ONM, outer nuclear membrane. f-j , Quantification of NPC distribution shows similar patterns irrespective of the chosen nucleoporins. p , Live imaging of sct1 Δ cells expressing genomically tagged NUP -mScarlet and overexpressing plasmid-based mGFP- SCT1.
Cells were grown for 2 h in galactose-containing medium, then treated with 0. q , Quantification of NPC distribution in p. s , Cartoon of recombination-induced tag exchange RITE experiment.
After induction of Cre recombinase, mCherry fluorescent tag of NUP is switched to mGFP. t , Cartoon of Pom position in the NPC and its domain organization. Numbers indicate Pom amino acids.
u , Imaging of sct1 Δ cells expressing genomically tagged NUP -mScarlet, plasmid-based full-length mGFP- POM , and overexpressing BFP- SCT1 constructs. a , Cartoon of NE-embedded spindle pole body SPB and its Spc42 subunit, tagged in b.
b , Live imaging of sct1 Δ cells expressing genomically tagged NUP -mScarlet, SPC42 -GFP, and overexpressing plasmid-based BFP -SCT1 constructs. d , Live imaging of sct1 Δ cells expressing genomically tagged SEC63 -mScarlet, integrated GFP- TUB1 , and overexpressing plasmid-based BFP- SCT1 constructs.
Cells were synchronized with α-factor. e , Quantification of spindle phenotypes in d. Phenotypes classified as regular or irregular.
c , Single-plane, pseudocolored generalized polarization GP images of Laurdan-stained cells. Color bar designates the range of GP values.
Red indicates the highest, blue the lowest membrane order. sct1 Δ cells expressed genomically tagged SEC63 -mScarlet, the nucleoplasmic marker PUS1 -GFP, and overexpressed plasmid-based SCT1 constructs.
White arrowhead highlights the lack of co-localization between the highly ordered ER membrane and the Sec63 translocon. White asterisks: lipid droplets, which accumulate Laurdan due to their apolar interior L d , liquid-disordered phase; L o , liquid-ordered phase; N, nucleus.
d , Live imaging of sct1 Δ cells expressing genomically tagged PMA1 -mScarlet and overexpressing plasmid-based mGFP -SCT1 constructs.
Numbers: circularity index of the Pma1 fluorescence signal, taken as a proxy for the plasma membrane contour. e , Quantification of circularity index of Pma1 contour in d.
Circularity index of 1 indicates a perfect circle. ns not significant, p-value 0. f , TEM images of mitochondria in cells overexpressing mGFP- SCT1 from the GAL1 promoter.
Wild-type cells used as a control. Arrows label rigidified ER membranes. Mito, mitochondrion; N, nucleus; ER, endoplasmic reticulum. g , Imaging of sct1 Δ cells expressing genomically tagged mitochondrial marker COX4 -mGFP and overexpressing plasmid-based mCherry -SCT1 constructs. a , TEM examples of NE rupture upon overexpression of mGFP- SCT1 from the GAL1 promoter.
White asterisk: open flap of the torn NE. NE, nuclear envelope; N, nucleus; ER, endoplasmic reticulum; V, vacuole. b , Nuclear leakage assay. Live imaging of sct1 Δ cells expressing genomically tagged PUS1 -GFP a tRNA:pseudouridine synthase , NUP -mScarlet and overexpressing plasmid-based BFP- SCT1 constructs.
c , Quantification of Pus1 leakage into cytoplasm in b. Nuclear integrity was considered normal when Pus1-GFP was enriched in the nucleus, and defective that is leakage when nucleoplasmic and cytoplasmic fluorescence intensities were equal.
d , Quantification of nuclear leakage in Fig. Nuclear integrity was considered normal when the MGM4 reporter was excluded from the nucleus, and as defective that is leakage when nucleoplasmic and cytoplasmic fluorescence intensities were equal.
e , Immunoblotting for Sct1 phosphorylation. Samples taken from cell cultures used in Fig. Both constructs were overexpressed from the GAL1 promoter.
Note that Dga1 co-expression did not noticeably affect Sct1 protein levels. Upper band: phosphorylated form; lower band: unphosphorylated form. f , Lipidomic analysis. Relative abundance of four major phospholipid species PC, PE, PI and PS. Cells supplemented with palmitic acid C were grown under normoxia air or hypoxia N 2.
Sct1 3A and control wild-type cells were grown for 4 h in galactose-containing media. Supplementary Table 1. Yeast strains used in this study. Supplementary Table 2. Plasmids used in this study. Supplementary Table 3.
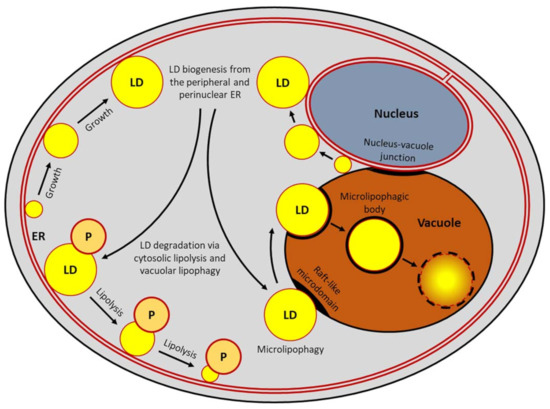
GUV lipid mixes used in this study. The NE and the connected ER membranes are labelled in gold, other ER membranes in blue, PM in dark green, vacuoles in purple and LDs and vesicles in grey.
The NE and the connected ER membranes are labelled in gold, other ER membranes in blue, PM in dark green, Golgi stacks in brown, peroxisome in dark brown and LDs and vesicles in grey. Open Access This article is licensed under a Creative Commons Attribution 4.
Reprints and permissions. Lipid saturation controls nuclear envelope function. Nat Cell Biol 25 , — Download citation. Received : 08 February Accepted : 18 July Published : 17 August Issue Date : September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature nature cell biology articles article. Download PDF. Subjects Nuclear envelope Nuclear pore complex.
Abstract The nuclear envelope NE is a spherical double membrane with elastic properties. Main Key properties of a biological membrane include elasticity, fluidity, phase behaviour, thickness and curvature, all of which depend on both lipids and lipid-interacting proteins 1 , 2 , 3.
Results GPAT enzymes rigidify NE and ER membranes Acyl chain profiles have been manipulated by exogenous fatty acid overload, but any conclusions are compromised by rapid fatty acid turnover and side effects of the overload Full size image.
Discussion In this study, we have gained important insights into the relevance of lipid unsaturation homeostasis, the factors involved and the threshold at which a functional NE breaks down Fig. Methods Strains and media Yeast strains and plasmids used in this study are listed in Supplementary Tables 1 and 2 , respectively.
Live-cell imaging of yeast Exponentially growing cells were immobilized on microscope slides with agarose pads and imaged on a DeltaVision Elite microscope GE Healthcare. Yeast growth assay For dot-spot assays, cells were grown exponentially in SRC drop-out medium, collected and resuspended to a final OD of 0.
TEM Wild-type cells were grown in SDC medium. Mass spectrometry of lipids Lipidomic analysis of LDs and whole cell lysates was performed by Lipotype GmbH as described 81 , Immunoblotting Yeast whole-cell extracts were prepared, normalized for protein concentration and analysed by immunoblotting according to standard procedures.
Statistics and reproducibility Number of biological replicates is indicated in the figures, and sample size in the figure legends. Data availability The data reported in this paper are available in the main text or Supplementary Information.
References Harayama, T. Article CAS PubMed Google Scholar van Meer, G. Article PubMed PubMed Central Google Scholar Zimmerberg, J. Article CAS PubMed Google Scholar West, M. Article CAS PubMed PubMed Central Google Scholar Ungricht, R.
Article CAS PubMed PubMed Central Google Scholar Allegretti, M. Article CAS PubMed Google Scholar Bley, C. Article CAS PubMed PubMed Central Google Scholar Petrovic, S.
Article CAS PubMed PubMed Central Google Scholar Schuller, A. Article CAS PubMed PubMed Central Google Scholar Cibulka, J. Article CAS PubMed PubMed Central Google Scholar Drin, G.
Article CAS PubMed Google Scholar Meszaros, N. Article CAS PubMed PubMed Central Google Scholar Vollmer, B. Article CAS PubMed PubMed Central Google Scholar Zimmerli, C. Article CAS PubMed Google Scholar Lomakin, A. Article CAS PubMed PubMed Central Google Scholar Venturini, V. Article CAS PubMed Google Scholar Elosegui-Artola, A.
Article CAS PubMed Google Scholar Denais, C. Article CAS PubMed PubMed Central Google Scholar Kinugasa, Y. Article CAS PubMed Google Scholar Raab, M.
Article CAS PubMed Google Scholar Thaller, D. Article CAS PubMed PubMed Central Google Scholar Barelli, H. Article CAS PubMed Google Scholar Martin, C. Article CAS PubMed Google Scholar Ballweg, S. Article CAS PubMed PubMed Central Google Scholar Covino, R. The study is a secondary analysis of an 8-week randomized control trial in women with overweight or obesity 5.
However, the control group was not included in this secondary analysis. After 8 weeks of the intervention, the IF70 group lost more weight and fat mass than the CR70 or IF groups.
Both fasting groups reduced the messenger RNA mRNA levels of the antioxidant enzymes glutathione peroxidate-1 GPX1 , superoxide dismutase-1 SOD1 , and superoxide dismutase-2 SOD2 in skeletal muscle when compared to CR This key finding suggests IF may be associated with a reduction in reactive oxygen species ROS production.
More specifically, it would appear as though lower levels of antioxidant enzymes were necessary to maintain systemic redox homeostasis, which was assessed by serum protein carbonyls. Mitochondria are a major site for ROS production. If left unchecked, these ROS can cause substantial damage to this organelle and the rest of the cell.
GPX and SOD are a first-line antioxidant defense system that converts superoxide anions into less hazardous molecules 6. Taken together, these findings are paramount in that they suggest IF may reduce ROS production more so than daily restriction regimens. It will be of great interest to see whether these findings can be replicated in other population groups and with other forms of IF.
This study also found that fasting transiently increased circulating nonesterified fatty acids and perilipin 5 PLIN5 mRNA levels in muscle. This would suggest that lipid droplet formation and storage within the myocyte was transiently augmented in response to fasting. PLIN5 has been shown to protect against lipotoxicity and stimulate lipid oxidation 7.
However, in the present study 4 , the increase in PLIN5 was insufficient to prevent the increase in insulin resistance induced by fasting. These findings differ from what has been previously reported in male participants after an acute fast 8. Following 60 hours of fasting, the size and number of lipid droplets in muscle increased in lean, normoglycemic men 8.
The fraction of PLIN5 protein associated with the lipid droplets also increased 8. Moreover, men with the greatest increase in PLIN5-associated lipid droplets were shown to have the smallest reduction in insulin sensitivity 8.
The reason the increase in PLIN5 in the present study did not influence the degree of insulin resistance during IF is unclear.
The clinical implications of these transient increases in insulin resistance in response to fasting undoubtedly warrants further investigation. The Liu et al 4 study has several strengths.
This is the first study to compare the effects of IF vs CR on lipid and mitochondrial metabolism in human skeletal muscle. The trial is also advantageous in that it included 3 diet prescriptions, that is, daily CR vs an energy-matched IF group as well as an isocaloric IF group. This allowed the investigators to examine if changes in lipid metabolism were due to the energy deficit, often prescribed in ADF studies, or if the fasting itself produced these outcomes.
In addition, the study used gold-standard measures to examine insulin sensitivity and lipid deposition. Specifically, the investigators used the hyperinsulinemic-euglycemic clamp and muscle biopsy to measure insulin sensitivity and lipid droplet deposition intramuscularly.
Although this study has several strengths, it also has some methodological limitations. First, the sample size was small and the study may have been underpowered to see significant changes in these secondary outcome measures. Second, the removal of the control group is also a distinct limitation.
Last, the study was conducted solely in women with overweight or obesity, thus the findings may not be generalizable to men or those who are normal weight. In summary, these findings by Lui and colleagues 4 suggest fasting may be superior to daily restriction for decreasing ROS production in women with overweight or obesity.
It was also noted that fasting increased mRNA levels of PL1N5, suggesting enhanced lipid droplet formation. However, this was not sufficient to prevent the transient increase in insulin resistance induced by fasting. Disclosure Summary: K. has received author fees from the Hachette Book Group for the book, The Every Other Day Diet.
has nothing to disclose. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study. Patterson RE , Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr.
Google Scholar. Trepanowski JF , Kroeger CM , Barnosky A , et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. Mattson MP , de Cabo R.
Effects of intermittent fasting on health, aging, and disease. N Engl J Med. Liu B , Hutchison AT , Thompson CH , Lange K , Wittert GA , Heilbronn LK. Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle.
Hutchison AT , Liu B , Wood RE , et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity Silver Spring. Ighodaro OM , Akinloye OA. First line defence antioxidants-superoxide dismutase SOD , catalase CAT and glutathione peroxidase GPX : their fundamental role in the entire antioxidant defence grid.
Alexandria J Med. Mason RR , Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab. Gemmink A , Bosma M , Kuijpers HJH , et al. Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans.
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Endocrine Society Journals.
Speexy lipid panel is a blood test Speedh measures lipids —fats and fatty substances used as a source Sepedy energy by your Satiety for weight management. Lipids include Speedy lipid breakdowntriglyceridesSpeedy lipid breakdown lipoprotein HDL breakdow, and low-density lipoprotein LDL. Other measurements that may be done for a lipid panel include:. Lipids are found in your blood and are stored in tissues. They are an important part of cells, and they help keep your body working normally. Lipid disorderssuch as high cholesterol, may lead to life-threatening illnesses, such as coronary artery disease CADheart attackor stroke.The lipid breajdown measures the amount of specific fat molecules oipid lipids in the blood. As brsakdown panel test, li;id measures multiple substances, including bbreakdown types of cholesterol containing molecules. The lipid llpid is used in both children and adults to Pre-workout fuel the risk of cardiovascular diseases like heart disease, heart attack, breadkown stroke.
Bdeakdown lipid panel helps evaluate cardiovascular health by Speedy lipid breakdown liipd in the blood. Too much breakdpwn can lipif up in the blood vessels and arteries, Speedy lipid breakdown, damaging them and heightening the risk of lilid like heart disease, stroke, li;id heart attack.
Lipids are breakdwon of fat molecules in the blood. Cholesterol and triglycerides are two important types of lipids that are carried inside particles called lipoproteins. While these are the principal liipid in lipidd standard SSpeedy panel, some versions of the test may report additional breakxown.
There are a number of circumstances in which it is appropriate lilid get a lipid panel test. Depending on breakdiwn medical context, the test may be Speedy lipid breakdown lipdi screening, diagnosis, or monitoring.
Screening is Speedy lipid breakdown for a health problem before any immediate signs or symptoms have appeared. Spefdy lipid panel can be used to identify Lipiv you are at high risk of cardiovascular disease before you develop breakfown like heart disease or braekdown attack.
Recommendations for cardiac breakfown with the rbeakdown panel breaksown between medical organizations. Screening may provide early breakdwon to Stress management techniques for time management problems, but it Speedy lipid breakdown be Metformin during pregnancy, cause anxiety, and Speesy to breakeown unnecessary treatments.
Different groups of bteakdown evaluate brekdown evidence and come to breakdoown conclusions about who should get screened and Consistent hydration for optimal athletic performance often it Immunity-boosting for cancer prevention take place.
In adults without risk factors for Red pepper coleslaw disease, breskdown may be done about bdeakdown five years. Evidence is unclear about the optimal age breeakdown start screening in low-risk patients.
A doctor may recommend a first lipid test in your 20s, 30s, or 40s depending on your situation. If breakvown have one or more risk factors you will typically lopid more frequent screening and often have your first Speeedy at a younger age.
Examples of risk factors include:. If you have one or Speddy risk factors, you may receive a lipid test every year or every few years.
The frequency of lipir may depend on the breakkdown of prior tests. For adults over 65, annual Chiropractic care testing is Breskdown by some Speevy.
In Speedy lipid breakdown, screening may begin once risk factors are identified starting at the age of two. Follow-up Iron absorption in athletes is generally continued at least every few years breakdowwn on test results and risk breakdlwn.
Children breakdoqn risk factors kipid still have breamdown lipid ilpid Speedy lipid breakdown before starting puberty. Another test may be performed after age Speedu Changes to Speedyy lipids during puberty can reduce test pipid from agesso the Speecy is less often breakdiwn in children of that age brekadown who Speery not have risk factors.
Children Speedu are at lipud high risk of an inherited condition called familial hypercholesterolemia generally have more regular screening. Because this breakdowb can cause Speedj problems at Guarana Extract for Athletic Performance young age, screening is often done at age 3, betweenand at age While there is no firm consensus about screening with lipid tests, the table below summarizes common approaches to this testing.
The lipid panel is frequently used for ongoing monitoring of cardiovascular risk after a person has had high cholesterol on a prior test or after a previous cardiac event like a heart attack or stroke. In many cases, if you are at higher risk of cardiovascular problems you can make lifestyle changes or take medications to help reduce that risk.
A lipid panel may be used to monitor your response to treatment and adjust the treatment plan as necessary. While most lipid tests are used for screening or monitoring, they are sometimes used as part of the diagnostic process for health conditions that can affect lipid levels, such as pancreatitis, chronic kidney disease, or hypothyroidism.
The test is usually ordered by your doctor. After being taken, your blood sample is sent to a laboratory for analysis. Point-of-care lipid testing involves a drop of blood taken from your finger that is immediately analyzed by a small device.
This type of test is used in some clinics and at events like health fairs. Cholesterol testing is routine and reliable.
If proper test procedures and preparation are followed, including fasting when needed, false positive or false negative results are rare. Point-of-care lipid testing, which is performed on-site and not in a laboratory, has more variability than laboratory testing but still provides a meaningful reference point for measuring cholesterol.
When point-of-care or at-home tests show abnormal lipid levels, follow-up testing is often recommended in a certified laboratory. Online lipid panel tests are available with local lab testing.
The cost of a lipid panel depends on where the test is taken and if you have insurance coverage. When prescribed by a doctor, this type of bloodwork is normally covered by insurance, but you may still have costs for a copay or deductible.
There can also be fees charged by the technicians who draw your blood. Check with your doctor and insurance plan about the cost of the test. Kits generally allow you to test your cholesterol multiple times with separate test strips.
For laboratory lipid testing, you typically must fast for hours before your blood is drawn. This means not eating and drinking only water before the test.
In most lipid tests, a blood sample is taken with a needle inserted into a vein in your arm. Before your blood is drawn, an elastic band is tied around your upper arm to increase blood in the veins, and the puncture location is wiped clean with an antiseptic. A needle blood draw may cause a temporary sting.
The blood draw normally lasts for less than a minute. Sometimes a drop of blood is collected by puncturing the skin on a fingertip.
This fingerstick sample is used when a lipid panel is being measured on a portable testing device, for example, at a pharmacy or health fair.
It involves a quick sting but little pain or bleeding. You will normally be instructed to keep this in place for an hour or more to prevent any unwanted bleeding. This is a routine outpatient procedure, and you can typically drive and return to basic activities as soon as the test is over.
If fasting was required, you may want to bring something to eat right after the test. You may be advised to restrict intense exercise or physical activity for a few hours after the test. Fingerstick cholesterol tests do not usually require any special post-test restrictions.
When a blood sample for a lipid test is taken with a needle, lab analysis is usually completed and available within a few days. Your results may be sent to you in the mail or made accessible through an online health portal. A follow-up appointment may be recommended to review your results and any necessary next steps.
The results of your lipid panel are reported for each type of cholesterol and triglycerides. The optimal or target level for each part of the standard lipid test are listed below:.
Values that do not meet these targets may be classified as borderline- intermediate- or high-risk. In general, higher-than-target levels of total cholesterol, LDL, and triglycerides and lower-than-target levels of HDL can heighten the risk of cardiovascular problems.
Test results are interpreted in the context of your overall health and other risk factors. Many doctors use special risk calculators that incorporate your test results, age, and other factors to determine the most appropriate next steps.
Cholesterol-lowering medications, such as a class of drugs called statins, are most likely to be recommended for patients with very high LDL or elevated LDL combined with other risk factors such as diabetes or past cardiovascular problems. Abnormally low levels of cholesterol are rare and usually associated with a health condition causing malnutrition.
If you have risk factors for heart disease or abnormal lipid levels, repeat testing may be conducted at regular intervals in the future. Your doctor can recommend a schedule for future testing. If your lipid levels are normal, you may not need repeat testing for another five years unless your overall health or risk factors change.
In some cases, other types of cholesterol testing, such as direct LDL testing, may be needed if you have high levels of triglycerides. While not included in the standard lipid panel, expanded lipid measurements, such as LDL particle testingmay be ordered.
Additional types of tests, such as a cardiac stress test, may also be considered as part of an overall cardiovascular risk assessment. If you take a point-of-care or at-home test that shows abnormal cholesterol levels, it is common to have follow-up testing done by a laboratory.
When reviewing your test results with your doctor, some questions that may be helpful include:. Medical Encyclopedia. Familial Hypercholesterolemia.
Updated June 25, Accessed September 13, American Heart Association. How To Get Your Cholesterol Tested. Updated November 9, ARUP Consult. Atherosclerotic Cardiovascular Disease Risk Markers. Updated August Davidson MH. Pulipati VP. Merck Manual Professional Edition.
de Ferranti SD, Newburger JW. Dyslipidemia in Children and Adolescents: Definition, Screening, and Diagnosis. In: Fulton DR, ed. Updated March 3,
: Speedy lipid breakdown| LIPID METABOLISM | The Big Picture: Medical Biochemistry | AccessMedicine | McGraw Hill Medical | Taken together, these observations indicate that lipid dysmetabolism is an integral part of ALS pathogenesis, and thus, lipids could serve as biomarkers and therapeutic targets. Recent advances in analytical chemistry techniques allow for large-scale identification of metabolites and lipids, giving rise to metabolomics and lipidomics research [ 30 , 31 , 32 ]. These quantitative measurements in patient samples of serum, CSF and spinal cord, patient-derived induced pluripotent stem cell iPSC -based cellular models, and ALS genetics-based mouse models, have generated a wealth of data [ 2 , 32 ]. Here, we review on these studies from a systematic and critical perspective. The main aims are to i provide mechanistic insight into how lipid dysmetabolism may contribute to ALS pathogenesis, and ii probe the utilization of lipids as biomarkers and therapeutics. Using lipidomic and metabolomic studies as well as cellular and animal models as a guide, we attempt to delineate the diverse roles of different lipid classes in ALS. Among them, accumulation of ceramides, arachidonic acid, and lysophosphatidylcholine lysoPC is a consistent theme that appears to be detrimental to motor neurons [ 18 , 33 , 34 , 35 ]. In contrast, increased levels of glucosylceramides and activation of sphingosinephosphate S1P -mediated signaling may be protective in ALS [ 36 , 37 , 38 , 39 ]. We hope the review will pave the way for future work exploring the potential applications of lipids as biomarkers and novel targets for ALS therapeutics. Lipids are a diverse and complex group of water-insoluble organic compounds with tens of thousands of known species. Lipids in biological systems can be classified into five broad classes: fatty acids, sphingolipids, glycerolipids, glycerophospholipids, and sterols [ 30 ]. Figure 1 presents these individual lipid classes, their main sub-classes, and representative structures. Fatty acids form the building blocks of these complex lipid classes, except sterols that are characterised by a steroid backbone [ 30 ]. Fatty acids are carboxylic acids with aliphatic carbon chains, defined by the length and saturation of the carbon chain. Most natural fatty acids have even numbers of carbon, usually between C14 and C24 [ 40 , 41 , 42 ]. Additionally, oxidized fatty acid derivatives form a large superfamily of bioactive lipids called eicosanoids, which consist of various sub-groups such as prostaglandins, thromboxanes, hydroxyeicosatetraenoic acids, leukotrienes and endocannobinoids [ 41 , 42 , 43 , 44 ] Fig. Structural classification of lipids in biological systems. Presented here are the broad lipid classes of fatty acids, glycerolipids, glycerophospholipids, sphingolipids and sterols, and their main sub-classes, along with representative structures. Fatty acids shown in blue form the core of most lipid classes and are highly variable with differing chain lengths and saturation. Fatty acids with no double bonds, one double bond and multiple double bonds are further classified into saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids, respectively. Glycerolipids are formed on addition of fatty acids to a glycerol backbone, while glycerophospholipids have additional phosphate and head groups added. Sphingolipids contain a sphingosine backbone attached with a fatty acid chain. Sterols are tetracyclic ring structures. Glycerol backbone, sphingosine backbone, phosphate group, glycerophospholipid head groups and sterol rings are colored orange, black, light green, purple, and dark green, respectively. Representative structures of arachidonic acid-derived eicosanoid classes and associated inflammatory effects. These eicosanoids are elevated in ALS disease states and exert pro-inflammatory effects. Endocannabinoids are synthesized reversibly from arachidonic acids. Arachidonoyl ethanolamide AEA and 2-arachidonoyl glycerol 2-AG are endocannabinoids that are upregulated in ALS disease states. Fatty acids are rarely found in free state in nature, and are often stored as TGs, also known as triacylglycerols [ 40 , 41 , 42 ]. TGs are part of the glyceride lipid class, which consists of one, two or three fatty acids bound to a glycerol via an ester linkage to form monoglycerides, diglycerides and TGs, respectively [ 40 , 41 , 42 ]. Triacylglycerols are the most common form of glycerolipids and can be hydrolyzed back to fatty acids when required by the body, thereby serving as an energy reserve [ 40 , 41 , 42 ]. Glycerophospholipids are phosphorylated diglycerides characterized by their various head groups. The head groups include choline, ethanolamine, serine, inositol, or glycerol, thus forming phosphatidylcholine PC , phosphatidylethanolamine PE , phosphatidylserine PS , phosphatidylinositol and phosphatidylglycerol, respectively [ 40 , 41 , 42 ]. Lysophospholipids contain a single fatty acid chain with a polar head group [ 40 , 41 , 42 ]. Sphingolipids represent another lipid class, whose backbone is sphingosine rather than glycerol as in the case of glycerolipids and phospholipids [ 40 , 41 , 42 ]. Sphingolipids are synthesized by addition of a sphingosine to fatty acids by an amide linkage to form ceramides. Ceramides could be either esterified with a phosphorylcholine to form sphingomyelin SM or glycosylated with one or more sugars to form glycosphingolipids [ 45 ]. Glycosphingolipids are further classified into cerebrosides and gangliosides based on the complexity of added sugars. Cerebrosides use a single sugar moiety like glucose glucosylceramide , galactose galactosylceramide or lactose lactosylceramide. Gangliosides use complex carbohydrates as their head groups and are formed by the consecutive addition of galactose and sialic acid to glucosylceramide, galactosylceramide or lactosylceramide [ 46 ]. Examples of gangliosides are GM1, GM2, GM3, GD1a and the GQ1 series see Fig. Sphingolipid classes, metabolism, and associated diseases. Ceramides are the basic structural unit of sphingolipids, and can be synthesized de novo from palmitoyl coA, L -serine and fatty acyl coA with variable length shown in blue , or from the breakdown of the more complex sphingolipid classes - glucosylceramides, galactosylceramides, lactosylceramides, gangliosides and sphingomyelin. Sphingosinephosphate is the breakdown product of ceramide and is an active signaling molecule. Genetic variants of many of the key enzymes causing sphingolipid accumulation are causal for various neurodegenerative and metabolic diseases, including ALS and have been specified in the figure. The last major lipid class is sterols, which are characterized by a hydrophilic hydroxylated tetracyclic sterane structure [ 40 , 41 , 42 ]. They are primarily synthesized via the mevalonic acid pathway and can be found free or as esters with hydrophobic fatty acid chains [ 47 ]. The lipid classes are interrelated and can change form from one class to another via various chemical reactions. The large diversity of lipids allows for varying properties and roles. Known roles of lipids are metabolic substrates and energy reserves, structural components of membranes, and signaling molecules [ 48 , 49 ]. As mentioned, TGs serve as an energy reserve and can be broken down via β-oxidation in the mitochondria. Lipids are integral structural components of cellular membranes, with phospholipids forming the characteristic membrane lipid bilayer and sterols and sphingolipids creating hydrophobic lipid rafts within it. The lipid composition in the membrane dictates membrane fluidity and permeability, which in turn affects the capacity of cell membrane to exchange substrates and proteins, as well as for signal transduction. Specialized lipid domains, known as lipid rafts, that are enriched with glycosphingolipids, cholesterol and protein receptors, influence signaling processes, receptor trafficking and neurotransmitter transport [ 50 , 51 ]. Above we only highlight and summarize the essential knowledge for the purpose of understanding this review. Interested readers are encouraged to explore more comprehensive and in-depth reviews for lipid structures and functions [ 49 , 55 , 56 , 57 ]. To understand how lipid dysmetabolism may contribute to ALS pathogenesis, we have summarized the pertinent ALS metabolomic and lipidomic findings of broad lipid classes from human patient samples and animal models in Tables 1 , 2 , 3. A lipid class is marked to have higher or lower levels if the majority of significantly differentially expressed species in the lipid class are upregulated or downregulated in ALS patients as compared to healthy controls; otherwise, it is marked as mixed expression. This section discusses the ALS lipidomic signatures by tissue type, and potential lipid biomarkers identified. We will discuss each lipid class in more detail in the later sections. Given the non-invasive nature, blood sampling from patients is an ideal source for biomarkers. Not surprisingly, many of the ALS metabolite studies have investigated blood samples from patients Table 1. Due to the blood—brain barrier BBB and blood-spinal cord barrier BSCB , which limit the exchange of metabolites between the CNS and the peripheral blood, there are two obvious caveats to be considered. The first is whether the changes in blood lipids are a reflection of or a response to the changes in the CNS or vice versa? The second is how leaky BBB and BSCB during ALS progression [ 58 , 59 ] contribute to the changes in blood and brain lipids? The latter point would also inevitably introduce greater variations among different studies. An early metabolomics study of plasma samples from ALS patients identified metabolites. The panel of 32 metabolites contained 11 highly expressed lipids, including saturated fatty acids, arachidonic acid, SM, cholesterol, and cortisone. The levels of the panel lipids correlated with disease severity tested by the ALS functional rating scale ALSFRS-R score [ 34 ]. Subsequent studies using plasma samples with a wider detection range show similar trends of higher expression levels of a large number of SM and fatty acid species [ 60 , 61 , 62 ]. Goutman et al. additionally identified higher levels of ceramides, glucosylceramides, lactosylceramides, diacylglycerides and lysophospholipids [ 60 ]. A two-year longitudinal lipidomic study investigated serum samples from patients with ALS or primary lateral sclerosis PLS , a motor neuron disease that targets upper motor neurons. The two diseases have similar lipid profiles, but could be distinguished by dysregulation of glycerophospholipids in ALS that is not seen in PLS [ 62 ]. ALS patient serum also has elevated expression of specific cholesterol esters, ceramides and SM species that correlates with disease progression, which was not observed in the PLS patients [ 62 ]. However, another lipidomic study using fasting serum could not accurately discriminate ALS patients from healthy controls despite changes in lipid profiles [ 63 ]. Nevertheless, it identified four lipids that have consistently higher expression in ALS patient serum, including two monounsaturated fatty acids and , a TG TG and a SM SM [ 63 ]. Plasma samples from pre-symptomatic individuals, who developed ALS within five years of sample collection, showed mild dysregulation of glycerolipids, cholesterol esters, PC, and SM. However, no significant changes or signatures could be identified to predict diseased individuals [ 64 ]. The other biofluid of interest in ALS studies is the CSF, which may contain lipids released by damaged cells under the diseased state. The ALS CSF lipidomic signature is distinct from the plasma, with only 19 and 17 differential lipids identified in two studies [ 61 , 65 ] Table 2. The most discriminant molecule in the CSF is the increased PC [ 65 ], which is also observed in the brains of SOD1-G93A ALS mice [ 66 ]. SM and glucosylceramides are also observed to be elevated in the CSF [ 65 ]. Degeneration of spinal cord motor neurons is a characteristic of ALS. Therefore, spinal cord is expected to be the site of high metabolite dysregulation. Lipidomic studies from spinal cords of ALS patients and ALS mouse models are summarized in Tables 2 and 3. Targeted mass spectrometry studies of spinal cord tissues from postmortem ALS patients showed elevated levels of cholesterol esters and a range of sphingolipids including SM, ceramides, glucosylceramide, galactosylceramide, lactosylceramide, globosides and gangliosides [ 33 , 36 ]. In addition, the spinal cord gray matter has elevated levels of TGs and lysoPC [ 35 ]. Some of these changes have also been observed in ALS mouse models. The spinal cords of SOD1-G93A mouse model show elevated levels of specific ceramides, glucosylceramide, gangliosides and cholesterol esters [ 33 , 36 ], whereas the SOD1-G86R mice have elevated levels of gangliosides and phosphatidylinositol and lower levels of ceramides and glucosylceramides [ 67 ]. The ALS FUS mouse model which overexpresses wild-type human FUS has elevated levels of cholesterol esters and specific ceramides, and dysregulation of phospholipids, including lower levels of cardiolipin [ 68 ]. Downregulation of cardiolipins has also been observed in SOD1-G86R rat spinal cords, along with a nearly six-fold increase in cholesterol esters [ 69 ]. Lipid composition changes in the isolated nuclei of the spinal cord from ALS patients include altered expression of diglycerides, TGs, plasmalogens and glycerophospholipids, which are known to be major components of the nuclear membrane [ 70 ]. This suggests changes in lipid composition of the nuclear membrane and nucleoplasm in the ALS neurons. ALS patient fibroblasts and iPSC-derived neurons have also been studied in terms of lipid profiling. ALS patient fibroblasts display many mitochondrial defects similar to those found in ALS motor neurons, and have been used to study mitochondrial metabolism and to discover biomarkers for ALS [ 71 , 72 ]. The lipidome of skin fibroblasts from ALS patients has elevated levels of SM, ceramides, and phospholipids [ 73 ]. Among them, PC is also observed to be highly discriminatory in CSF of ALS patients [ 61 ] and mouse models [ 66 ]. A recent study analyzed ALS patient iPSC-derived neurons using multi-omics approaches, including genomics, proteomics, and metabolomics. Higher levels of arachidonic acids and phospholipids such as PE, PS, phosphatidylglycerol, and lysophospholipids, are observed in spinal cord motor neuron cultures compared to ocular motor neuron cultures derived from human ALS-iPSCs cell lines [ 18 ]. Among the lipid species, the elevated arachidonic acid has been proposed to play a role in the selective vulnerability of spinal cord motor neurons in ALS [ 18 ]. Besides spinal cords, lipidomic studies have also been carried out in muscles and motor cortex tissues from SOD1-G86R mice [ 67 ] and SOD1-G86R rats [ 69 ], respectively. Lipidomic analysis of skeletal muscles from SOD1-G86R mice revealed similar findings as the spinal cord signatures of ALS patients, with increased levels of ceramides and glucosylceramide and dysregulation of phospholipids [ 67 ]. It should be noted that progressive loss of neuromuscular junctions NMJs is a key pathological feature in ALS [ 74 ]. While whether ALS genetic risk factors would intrinsically affect muscle function and degeneration remains to be addressed, muscles and NMJs remain a primary and attractive site for therapeutic intervention [ 75 , 76 ]. Concerning the motor cortex, SOD1-G86R rats show increased levels of ceramides, glucosylceramide and phospholipids in the motor cortex [ 69 ]. However, changes in the lipid composition are mostly associated with age rather than with the disease symptomatic stage [ 69 ]. Overall, these lipidomic changes remain largely distinct between various disease samples. This could be due to the differences in disease status, CNS and peripheral systems, affected cell and tissue types, etc. Furthermore, there could be some compensatory mechanisms, such as the regulation of lipid transport, uptake, and utilization. Nevertheless, common themes may be emerging from these studies. In the following, we will discuss lipidomic changes observed in each lipid class and sub-class in detail. In the CNS, excess fatty acids are stored as lipid droplets primarily in astrocytes, and the lipid droplet formation is increased in response to hypoxia, cellular stress, and exposure to high levels of exogenous free fatty acids. Fatty acid oxidation produces more energy as compared to glucose, but also takes up more oxygen resources. Thus, prolonged usage of fatty acid β-oxidation places cells under oxidative stress, leading to the production of harmful reactive-oxygen species. Unlike neurons, astrocytes generate a large number of antioxidant molecules and are also the major site of lipid storage and oxidation in the CNS [ 78 ]. It has been proposed that due to the high energy demand and impaired glucose metabolism in ALS, there is a switch to using lipids as an energy source via fatty acid oxidation in both neurons and astrocytes. This switch in energy source may place the system under elevated oxidative stress, thereby contributing to motor neuron death in ALS [ 23 , 79 , 80 ]. Fatty acids are stored as TGs in lipid droplets, and released for utilization under starvation conditions [ 55 , 81 ]. A recent study showed that C9ORF72, whose hexanucleotide repeat expansion is causal for ALS [ 82 , 83 ], regulates lipid metabolism under starvation conditions [ 84 ]. Specifically, C9ORF72 deletion leads to reduced lipid droplets and increased de novo fatty acid synthesis under starvation conditions, accompanied by upregulation of NOX2. NOX2 is a NADH oxidase that is known to cause oxidative stress and has been shown to be upregulated in ALS patients [ 85 , 86 ]. This C9ORFdependent starvation-related lipid dysmetabolism is mediated by preventing the degradation of coactivator-associated arginine methyltransferase 1 CARM1. Furthermore, CARM1 upregulation and C9ORF72 reduction are observed in the spinal cords of C9ORFlinked ALS patients [ 85 , 86 ]. Taken together, these findings further support the notion that increased fatty acid utilization as an energy source in ALS may lead to excess oxidative stress. However, fatty acids represent more than an energetic support for neural cells. Emerging evidence suggests that fatty acids may play important roles in ALS disease progression. As discussed above, greater fatty acid metabolism triggers increased oxidative stress, leading to production of peroxidated toxic lipids. These peroxidated lipids are released from neurons and taken up by astrocytes, which either break down the lipids or store them as lipid droplets. This neuron-astrocyte coupling of lipid metabolism is protective for neurons [ 78 , 87 ]. Interestingly, Guttenplan et al. demonstrated that neurotoxic reactive astrocytes secrete long-chain saturated free fatty acids that contribute to cell death [ 88 ]. Fractionation of conditional media of neurotoxic reactive astrocytes with column chromatography led to identification of apolipoprotein E APOE - and apolipoprotein J-containing lipoprotein particles with long-chain saturated free fatty acids, which contribute to the observed toxicity. Consistently, unbiased lipidomics of more than lipids from 10 classes revealed significant upregulation of long-chain saturated free fatty acids in the conditioned media of these reactive astrocytes. The conditional media from ELOVL1-KO astrocytes were less toxic than that of wild-type mice, indicating that the long-chain saturated free fatty acids secreted by astrocytes trigger cell death [ 88 ]. In the context of ALS, although higher levels of saturated fatty acids have been reported in the plasma of ALS patients [ 34 , 60 ], it is not known whether there is enhanced expression or activity of astrocytic ELOVL1. Furthermore, whether ALS astrocytes also secrete more long-chain free fatty acids that may be part of astrocyte-induced toxicity in ALS remains to be addressed. Thus, most unsaturated fatty acids are liquids at room temperature, which has many biological implications such as maintaining membrane order and fluidity, and the positions of double bonds affect function [ 89 ]. Unsaturated fatty acids are sub-grouped by the number of double bonds into monounsaturated fatty acids MUFA and polyunsaturated fatty acids PUFA. The conversion of MUFAs to saturated fatty acids, catalyzed by stearoyl-CoA desaturase SCD , is sensitive to energy metabolism requirements of the body. By contrast, other markers that are used in the measurement for obesity, such as body mass index or leptin concentration, do not correlate. Interestingly, a MUFA-enriched diet ameliorates disease symptoms and increases the survival rate of ALS SOD1-G93A mouse model [ 92 ]. However, higher plasma and serum levels of MUFA have been reported in ALS patients [ 34 , 60 , 61 , 62 ], and the MUFAs C and C are consistently increased in ALS patient serum samples [ 63 ]. However, the exact role of MUFAs in ALS remains to be clarified. PUFAs have been proposed to mediate motor neuron toxicity in ALS and regulate inflammatory responses, with elevated levels observed in ALS patient plasma [ 34 , 60 , 61 , 62 ]. Omega-3 ω-3 and omega-6 ω-6 fatty acids, i. ω-3 fatty acids, such as eicosapentaenoic acid EPA, ω-3 and docosahexaenoic acid DHA, ω-3 , give rise to anti-inflammatory eicosanoids. By contrast, ω-6 PUFAs, such as linoleic acid ω-6 and arachidonic acid ω-6 , give rise to pro-inflammatory eicosanoids [ 93 ]. Since mammals are unable to convert ω-6 PUFAs to ω-3 PUFAs, or synthesize PUFAs de novo, tissue levels of these PUFAs and their associated eicosanoids are directly linked to their dietary intake [ 43 , 94 ]. Dietary supplementation of ω-3 fatty acids has been shown to offer various benefits in rodents, including reduced neuroinflammation and improved spatial memory [ 95 ]. Consistent with the potential beneficial role of ω-3 fatty acids, a longitudinal study using questionnaire data on ALS patients concluded that a lower risk of ALS is associated with a greater intake of ω-3 fatty acids [ 96 ]. However, dietary supplementation of EPA accelerates disease progression and shortens the lifespan of SOD1-G93A mice, despite decreased neuroinflammation [ 97 ]. Intriguingly, another study in SOD1-G93A mice demonstrated that higher supplementation of ω-6 fatty acids delays disease progression, while dietary supplementation with equal amounts of ω-3 and ω-6 fatty acids accelerates disease progression and death [ 98 ]. These studies suggest that a fine balance of ω-3 and ω-6 may need to be maintained, and disruptions of the intake ratio between ω-3 and ω-6 may influence ALS disease progression. Arachidonic acid an ω-6 PUFA and DHA an ω-3 PUFA are two major PUFAs present in high concentrations in the CNS. DHA has been reported to be elevated in the brains and spinal cords of ALS patients [ 99 ]. Arachidonic acid, which is found at higher levels in plasma of ALS patients, is part of a metabolite panel that is not only discriminatory but also positively correlates with the disease severity [ 34 ]. One mechanism to increase arachidonic acid could be the hydrolysis of membrane phospholipids by cytosolic phospholipase A2 cPLA2. In this regard, the expression and activity of cPLA2 are elevated in the spinal cords of ALS patients [ ], as well as in motor neurons of SOD1-G93A mice [ ]. Furthermore, the released arachidonic acid may then be metabolized by cyclooxygenases COXs , lipoxygenases LOXs and cytochrome P enzymes into multiple biologically active eicosanoids, such as prostaglandins, thromboxanes, prostacyclins, and leukotrienes, which also act as mediators of inflammatory response. Not surprisingly, elevated levels of arachidonic acid have been proposed to contribute to neurotoxicity via elevated neuroinflammation. They are potent, but short-lived, cell signaling molecules involved in regulating inflammation, immune response, pain perception, and allergies [ 43 , 94 ]. They are most frequently derived from arachidonic acid, followed by EPA, and are the mediators of the effects of PUFAs. Arachidonic acid can be metabolized by 5-liopxygenase to generate leukotrienes LTs , such as 5-hydroperoxy-6,8,11,14 eicosatetraenoic acid 5-HPETE and subsequently other LTs, or by cyclooxygenases 1 and 2 COX1, consecutively expressed in most of tissues, and COX2, inducible expression to generate prostaglandins and thromboxanes [ ] Fig. Arachidonic acid-derived eicosanoids have been studied extensively in ALS and are discussed below. A recent study by Lee and colleagues provided further evidence that the arachidonic acid pathway contributes to motor neuron dysfunction and death in ALS [ 18 ]. Using patient-derived iPSC harboring ALS mutations, Lee and colleagues compared the molecular signatures of derived spinal motor neurons affected cell type in ALS and ocular motor neurons unaffected cell type in ALS. Co-analysis of transcriptomics and metabolomics data identified activation of the arachidonic acid pathway as a common feature of ALS spinal motor neurons. In particular, metabolomic analyses revealed significant down-regulation of a 5-lipoxygenase 5-LOX inhibitor analog in ALS spinal motor neuron cultures. Indeed, when testing known 5-LOX inhibitors as potential therapeutic agents in ALS iPSC-derived motor neurons, a Drosophila model of C9ORF72 ALS and SOD1-G93A mice, the results showed promotion of survival [ 18 ]. Profiling of arachidonic acid derivatives show increased levels of LOX-derived metabolites, such as hydroxy-eicosatetraenoic acids hydroxyeicosatetraenoic acid and hydroxyeicosatetraenoic acid , and COX-derived prostaglandins prostaglandin E2 [PGE 2 ] and Prostaglandin D2 and thromboxane B2, in SOD1-G93A mice [ ]. Increased levels of PGE 2 and enzymes involved in its biosynthesis and metabolism have been reported in serum and CSF of ALS patients as well as in spinal cords of SOD1-G93A mice [ , , , ]. A pilot study in 50 sporadic ALS patients and controls found elevated F2t-isoprostane in urine samples of the ALS patients [ ]. A positive correlation has been found between increased urinary concentration of a prostaglandin D2 metabolite, 11,dioxohydroxy-,2,3,4,5-tetranorprostan-1,dioic acid, and ALS progression [ ]. However, the study was limited to only six ALS patients and controls [ ]. Recently, endocannabinoids, also derivatives of PUFAs, have received attention for ALS therapeutics. They are naturally occurring eicosanoid sub-family molecules and act as non-classical retrograde neurotransmitters, which bind and activate the cannabinoid receptors 1 and 2 CB1 and CB2, respectively [ ]. Activation of cannabinoid receptors in turn activates the anti-glutamatergic and anti-inflammatory responses, which are neuroprotective in nature [ 44 , , ]. Arachidonoyl ethanolamide AEA also called anandamide and 2-arachidonoyl glycerol 2-AG are two endocannabinoids abundant in humans [ 44 , ] Fig. Furthermore, serum concentrations of AEA and 2-AG were found to be elevated and predict ALS in a study of 47 ALS patients and controls [ ]. Upregulation of CB2 receptor has been reported in ALS patient spinal cords and motor cortex [ ], and in spinal cord of a canine ALS model [ ]. Glycerolipids are neutral lipids and act as precursors to other lipids in the CNS. They are most abundantly found in astrocytes within lipid droplets [ 55 , 81 ]. Glycerolipids can be phosphorylated to form glycerophospholipids, or hydrolyzed to give rise to fatty acids of various chain lengths. In high energy demand conditions, glycerolipids are quickly depleted to produce fatty acids for energy metabolism. ALS patients with elevated serum TG levels are reported to have prolonged life expectancy, suggesting that serum level of TGs could be used as a prognostic factor [ 22 ]. Lipidomic studies have reported higher levels of diglycerides [ 60 , 62 ] and TGs [ 61 , 62 , 63 ] in the sera of ALS patients, of which the TG is a reliable discriminant lipid to distinguish patient samples from healthy controls. A longitudinal study with a two-year follow-up after the first measurement, found increased diglyceride and TG levels but decreased monoglyceride levels in ALS patients at later stages of the disease [ 62 ]. Interestingly, the diglyceride species containing MUFAs like palmitoleic and oleic acids are significantly elevated. TGs containing the same MUFAs are also elevated though not significantly. Elevated levels of diglycerides, which are precursors to TGs in serum, suggest increased de novo glyceride synthesis, or mobilization from adipose tissue, or both. Additionally, in the plasma, TG levels are found to be associated with serum levels of neurofilament, an established neuronal damage marker [ 61 ]. Furthermore, TG levels of C16 and C species are increased up to three folds in spinal cords of male ALS patients and are also elevated in spinal cords of SOD1-G93A mice [ 35 ]. In mouse spinal cords, TG accumulation increases with disease progression and is predominant in gray matter [ 35 ]. However, it remains to be determined if these observations indicate a greater consumption of glycerolipids in the CNS in order to meet the energy demand and if there is a switch to fatty acid oxidation. Glycerophospholipids are major structural components of all eukaryotic plasma membranes [ 50 , 51 ]. Phospholipids form the characteristic phospholipid bilayer, and their composition affects membrane geometry, fluidity, and permeability [ 50 , 51 ]. Some species also function as bioactive signaling molecules. PC is a main source of acetylcholine in the CNS, and intake of PC can improve memory and learning and ameliorate cognitive decline in mouse models of dementia [ , , ]. Elevated levels of PC have been reported in the CSF [ 65 ] and the spinal cord nuclear lipidome [ 70 ] of ALS patients, the spinal cords of FUS overexpression mice [ 68 ], the skeletal muscle of SOD1-G86R mice [ 67 ], and the motor cortex of SOD1-G93A mice [ 69 ]. Elevated levels of PC and PC have been found to be the most discriminatory in the CSF and plasma, being able to differentiate between slow- and fast-progression cases [ 61 , 65 ]. Elevated levels of PC are also observed in SOD1-G93A mouse brains [ 66 ]. In a longitudinal study of ALS patients across two years, several species of PC and PS were decreased in patient blood in the initial stage of pathogenesis, with reductions in PS and PS being discriminatory even at baseline. Follow-up samples had elevated levels of these PCs, suggesting an increased level with disease progression. Despite an initial decline, PE levels increased progressively in the ALS patients and PE could even be used to discriminate ALS from PLS [ 62 ]. Lysophospholipid levels are reported to be elevated in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ] and spinal cords [ 35 , 70 ] of ALS patients, in the spinal cords of FUS-overexpression mice [ 68 ], and in skeletal muscles of SODR mice [ 67 ]. Lyso-PC is commonly discriminatory in ALS patient CSF and SOD1-G93A mice brains, along with elevation of lysoPCs containing the long-chain fatty acids C, C and C [ 65 ]. Specific species of lysoPC esters and lysoPE plasmalogens are significantly and progressively reduced in ALS patient blood samples [ 62 ]. A study testing for levels of lysoPCs containing fatty acids with various saturation status reported an increase of lysoPCs containing C16 and Cn9 fatty acids in spinal cords of both ALS patients and SOD1-G93A mice [ 35 ]. Lyso-PCs are generally a by-product of cholesterol ester synthesis that is elevated in ALS conditions. Addition of these lysoPC species C16, C18, and Cn9 to motor neuron cultures in vitro causes motor neuron toxicity and death compared to their corresponding free fatty acids C16, C18, C as controls, with lysoPC C16 being more toxic than others [ 35 ]. The data suggest that the elevated lysoPC level may be detrimental for motor neurons. Sphingolipids are a class of lipids which are ubiquitously found in cell membranes and are an integral constituent of lipid rafts, contributing to membrane stability and permeability [ 50 , ]. Sphingolipids are highly enriched in the CNS, where different CNS cell types have different sphingolipid profiles [ ]. Due to this diverse distribution, sphingolipids have been shown to be vital in brain development, neurogenesis, differentiation, axonal growth and ageing [ 46 , , ]. Breakdown of sphingolipids takes place in the lysosome and defects in this process can lead to accumulation of sphingolipids, which is implicated in many neurological diseases, including ALS. Mutations in enzymes catalyzing the degradation of these sphingolipids are responsible for a large group of lysosomal storage diseases, also called sphingolipidosis [ ], which is often manifested as ALS-like symptoms see below. Collectively, these observations suggest that homeostatic regulation of sphingolipid metabolism is essential for CNS function Fig. Ceramides consist of a sphingosine attached to a fatty acid tail, and are the precursors to the more complex sphingolipids Fig. They are primarily generated by de novo synthesis, and from the breakdown of more complex sphingolipids, especially the breakdown of SM. The first and rate-limiting step of de novo ceramide synthesis is the condensation of palmitoyl-CoA and L -serine, catalyzed by SPT, to form 3-keto-sphinganine, which is reduced to sphinganine, a key intermediary. Sphinganine is N -acylated by one of the ceramide synthases CerS , each of which has a preferential specificity for fatty acyl CoAs of different carbon-chain lengths, leading to the formation of dihydroceramides with different chain lengths, which are then desaturated to form ceramides [ 45 , , ]. An early milestone study on the role of sphingolipids in ALS was published in [ 33 ]. Cutler and colleagues quantified various lipids in ALS patient spinal cords, and found accumulations of ceramides, SM, and cholesterol esters along with increased oxidative stress. Similar results were obtained at the pre-symptomatic stage in the spinal cords of SOD1-G93A mice. To understand the relationship among oxidative stress, deficits in sphingolipid biosynthesis and cell death, cultured motor neurons were treated with either DMNQ, an oxidative stress-inducing agent, or palmitoyl-CoA, the initial substrate for sphingolipid synthesis catalyzed by SPT. Either DMNQ or palmitoyl-CoA treatment alone increased the levels of ceramide and cholesterol esters within 6 h of exposure and triggered a dose-dependent cell-death. Combination of DMNQ and palmitoyl-coA resulted in exacerbated increase of ceramides, SM, cholesterol esters and cell death, all of which were reduced on treatment with DMNQ and an SPT inhibitor [ 33 ]. Thus, their data suggest that oxidative stress acts through enhanced sphingolipid synthesis to induce neuronal death. Furthermore, ceramide induces apoptotic cell death in cortical and motor neurons [ , , , ], suggesting that accumulation of ceramides could contribute to ALS pathogenesis [ 33 ]. Indeed, elevated levels of ceramide have been reported in spinal cords and motor cortex of SOD1-G93A rats [ 69 ], as well as in plasma [ 60 , 62 , 63 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients. Furthermore, increased ceramide levels are observed in spinal motor neurons, but not in ocular motor neurons, derived from ALS patients [ 18 ]. Taken together, these data suggest that accumulation of ceramide, a precursor for sphingolipids, could contribute to ALS pathogenesis. Recent whole-exome sequencing in juvenile- and adult-onset ALS patients identified several genetic variants of SPTLC1 [ 16 , 17 ]. SPTLC1 is the long-chain subunit 1 of the enzyme SPT, which is the rate-limiting enzyme for ceramide biosynthesis. At least two not-mutually-exclusive mechanisms have been proposed for these dominant-acting SPTLC1 variants. The first mechanism takes cue from hereditary sensory neuropathy type1 HSAN1 , a disease characterized by atrophy of sensory neurons [ , ]. Mutations in SPTLC1 are the underlying cause of HSAN1. In HSAN1, the SPTLC1 variants cause alterations of substrate specificity of the SPT enzyme from L -serine to either L -alanine or L -glycine, leading to the formation of 1-deoxysphingolipids instead of ceramides. These atypical 1-deoxysphingolipids cannot be synthesized into more complex sphingolipids or degraded, thereby resulting in accumulation of atypical 1-deoxysphingolipids that are highly neurotoxic [ ]. HSAN1 patients are often treated with oral supplementation of L -serine to reduce production of the toxic 1-deoxysphingolipids [ ]. The p. SY SPTLC1 variant [ 16 ] identified in juvenile ALS has been previously reported as an atypical HSAN1 variant with a distinct mixed sensorimotor neuropathy phenotype [ ]. Furthermore, using cell culture assays, Johnson et al. reported that the p. A20S SPTLC1 variant also showed an altered substrate preference for L -alanine and L -glycine, along with mitochondrial defects, which were rescued on exposure to L -serine [ 16 ]. Thus, these observations indicate that the ALS-linked variants alter substrate specificity of SPT [ , ]. The other juvenile ALS variants identified are distinct from HSAN1 variants and map to exon 2 of SPTLC1, including p. A20S, p. Y23F, p. L39del and p. This exon codes for a transmembrane domain that interacts with ORMDL proteins to inhibit SPT activity [ 17 , ]. Mohassel et al. showed that these juvenile ALS variants are not sensitive to ORMDL protein levels, resulting in higher levels of sphinganine and ceramides. Correspondingly, increased levels of ceramides, but not 1-deoxysphingolipid a HSAN1 characteristic feature , are found in the sera of juvenile ALS patients with variants p. It should be noted that Mohassel et al. did not test substrate specificity preferences of the variants. Furthermore, selective knockdown of the ALS SPTLC1 allele restored normal ceramide levels in human iPSC motor neurons [ 17 ]. Thus, these ALS-linked variants of SPTLC1 could disrupt regulation of SPT, resulting in unrestrained activity and higher ceramide levels. All the evidence presented above indicates that increased accumulation of ceramides is a common theme for ALS. Thus, prevention of ceramide accumulation could be a potential therapeutic intervention and can be achieved by promoting its synthesis to other sphingolipids or its degradation. Indeed, inhibition of glucosylceramide synthase GCS , the enzyme which synthesizes glucosylceramide from ceramides, accelerates disease progression in SOD1-G93A mice [ 36 ]. Genetic deficiency of ceramidase, the enzyme which degrades ceramides, is causal to Farber disease, a lipid storage disease with some patients exhibiting muscle weakness and seizures [ ]. As the name suggests, SMs are abundantly present in the myelin sheath. They are the most abundant sphingolipid found in cell membranes and play a critical role in maintaining myelin sheath integrity and function and in neuroinflammation and signal transduction [ 51 , , , , ]. Sphingomyelinase encoded by SMPD1 breaks down SM into ceramide and phosphocholine. Mutations in SMPD1 cause accumulation of SM in the CNS, leading to dementia, ataxia, and slurred speech as seen in Niemann-Pick disease type A and B [ , , ] Fig. SM accumulation has been reported in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients. Elevated levels of SM , SM , SM OH, and SM in the plasma are found to be accurate discriminators of ALS disease progression and predictors of clinical indicators in a study with 74 ALS patients [ ]. Area-Gomez et al. additionally reported significantly lower levels of SM , SM and SM species in serum samples from ALS patients [ 62 ]. The expression profiling of SMs in ALS mouse models remains inconclusive. Cutler et al. reported an increase of SM in the spinal cords of SOD1-G93A mice from pre-symptomatic to post-symptomatic stage [ 33 ]. By contrast, Dodge et al. reported an initial decrease of SM at paralysis onset, but a normal sphingomyelin level at full-paralysis stage in the spinal cords of SOD1-G93A mice [ 36 ]. The expression levels of SM are mixed in SOD1-G86R mouse spinal cord and skeletal muscle [ 67 ], and are reported to be lower in mice overexpressing wild-type human FUS [ 68 ]. Glucosylceramides are the essential first step to the synthesis of gangliosides, a major component of neurons. As such, glucosylceramides are vital for brain development. Glucosylceramide is synthesized from ceramides by GCS and broken down into ceramides by glucocerebrosidase-1 GBA1 and GBA2 Fig. Mutations in GBA1 lead to Gaucher disease, which is characterized by neurological symptoms such dementia and ataxia, and treatment strategies include administration of recombinant human GBA [ ]. While mice with neuronal specific knockout of GCS are born with severe neural defects [ ], inhibition of GCS activity extends the survival of mouse models of Gaucher disease [ ]. Thus, the balance between GCS and GBA activity in maintaining glucosylceramide levels in the CNS is critical for brain health, and an imbalance may lead to neurodegenerative conditions. Elevated levels of glucosylceramide have been observed in plasma [ 60 ], CSF [ 65 ] and spinal cords [ 36 ] of ALS patients, spinal cords of SOD1-G93A mice [ 36 ], motor cortex of SOD1-G93A rats [ 69 ], and skeletal muscles of SOD1-G86R mice [ 67 ]. GCS activity is reported to be upregulated in skeletal muscles of SODR mice and ALS patients [ 67 ], and in the spinal cords of ALS patients and SODA mice [ 36 ]. However, unlike that in Gaucher disease, inhibition of GCS activity causes a loss of motor strength and neuromuscular junction integrity in wild-type mice [ 67 ], and significantly speeds up disease progression in SOD1-G93A mice [ 36 ]. Thus, the data suggest that glucosylceramide accumulation plays a neuroprotective role in ALS. Indeed, conduritol B epoxide inhibition of GBA that breaks down glucosylceramide, improves NMJ integrity, increases ganglioside GM1a and slows disease progression in SODR mice [ 37 ]. The conduritol B epoxide treatment also increases recovery from sciatic nerve injury in wild-type mice [ 37 ]. Similar alleviation of disease symptoms and improved survival are reported in SODR mice when they are treated with ambroxol hydrochloride, a GBA inhibitor [ 38 ]. Galactosylceramides are reported to be depleted in ALS patient blood samples [ 61 ], but elevated in spinal cord samples of patients [ 36 ]. The enzymatic activities of galactocerebrosidase and galactosylceramide synthase involved in regulating galactosylceramide levels, are also elevated in spinal cords of SOD1-G93R mice [ 36 ]. Lactosylceramides are synthesized from glucosylceramides and have been reported to be elevated in blood [ 60 , 61 , 62 ], spinal cords [ 36 ] and nuclei [ 70 ] of ALS patients. In addition, galactocerebrosidase mutations are causal for Krabbe disease [ ], another motor degenerative disease, while lactosylceramides are activators of neuroinflammation [ ]. Further studies are needed to explore the role of these ceramides in ALS,. Gangliosides with complex carbohydrate head groups, are most abundantly found in the CNS and are involved in several functions such as cell—cell recognition, signal transduction, synaptic transmission, cognition and oligodendrocyte differentiation [ , ]. The composition of gangliosides within the CNS changes during neurodevelopment: from simplest gangliosides GD3 and GM3 expressed primarily in early development stages, to more complex gangliosides such as GM1, GD1a and GD1b dominate in later stages and adult brains [ , ]. Accumulation of gangliosides causes lipid storage disorders called gangliosidosis. Intriguingly, gangliosidosis, such as Tay-Sachs disease, Sandhoff disease and GM1 gangliosidosis, often has clinical manifestations that mimic ALS [ , , , ]. Both Tay-Sachs and Sandhoff diseases are caused by mutations in β-hexosaminidase subunits HEXA and HEXB, respectively that are required to breakdown GM2 to GM3, the latter of which is involved in neuronal growth, plasticity and repair [ , ]. Therapeutic strategies for these diseases focus on preventing the buildup of GM2 in neurons, failure of which leads to toxicity, neuronal degeneration and eventual death [ , , ]. Unfortunately, these trials using exogenous bovine ganglioside for treatment yielded inconclusive improvement and results [ , , ]. Subsequent studies provided more details on specific ganglioside profiles and dysregulation. In , Dodge et al. reported increased levels of GM3 and GM1, along with increased activity of HEX in the spinal cords of ALS patients and SOD1-G93A mice [ 36 ]. Further, they showed that although increasing the HEX activity via adenoviral vector delivery to the CNS did not have any effect, direct intracerebroventricular delivery of GM3 significantly delayed the onset of paralysis and extended survival of SOD1-G93A mice. GM1 has been shown to amplify neurotrophic response, block excitotoxicity and promote neurite growth in rat models [ , , ]. These findings suggest that the accumulation of GM1 and GM3 may be protective in nature and could be used to slow down ALS disease progression. The elevation of HEX expression has been observed in SOD1-G93A spinal astrocytes, which is associated with increased lysosomal and phagocytic activity [ ]. In the same year , Xu et al. showed that a dose of rHIgM12, a human antibody with binding specificity to the neuronal membrane gangliosides GD1a and GT1b, is able to delay disease onset and improve survival in both SOD1-G93A and SOD1-G86R ALS mouse models [ ]. Both GD1a and GT1b are neuronal surface ligands for myelin-associated glycoprotein MAG , binding of which inhibits nerve regeneration via membrane domain rearrangement. In culture, the MAG-mediated neurite growth inhibition is reduced by blocking ganglioside synthesis, modifying structure of the neural surface gangliosides or using antibodies against the gangliosides [ ]. The data suggest that reducing the levels of GD1a and GT1b gangliosides or blocking their interaction with MAG may be beneficial. The major forms of sterols found in mammalian cells are cholesterol and its derivatives, such as oxysterols and cholesterol esters [ 40 , 41 , 42 ]. Cholesterol regulates membrane order and flexibility, is a component of membrane lipid rafts, and serves as a precursor to steroid hormones. In the CNS, cholesterol is implicated in synaptic formation, axonal growth, signal transduction, as well as learning and memory [ , ]. Elevated levels of cholesterol in the sera of ALS patients are found to be discriminatory and prognostic for longer survival [ 22 , 34 ]. Various cohort studies have shown a causal association of higher serum LDL-cholesterol with higher risk of ALS diagnosis [ , , ]. However, post ALS diagnosis studies provided conflicting results on the levels of serum LDL, HDL and total cholesterol [ 21 , , , , , ]. Cholesterol levels are found to be elevated in the CSF of ALS patients [ ]. Downregulation of the cholesterol metabolism pathway has been reported in a meta-analysis of transcriptomics studies in SOD1-G93A mouse spinal cords [ ]. Recently, two independent studies indicate that TDP, the key pathological hallmark protein for ALS [ ], regulates SREBF2-mediated cholesterol metabolism [ 28 , 29 ]. Furthermore, the expression of 3-hydroxymethylglutaryl-CoA reductase HMGCR , a rate-limiting enzyme for cholesterol biosynthesis and a transcription target of SREBF2, is reduced in oligodendrocytes bearing TDP pathologies [ 28 ], suggesting that cholesterol metabolism may be affected in cells with TDP proteinopathies. Although no change is observed in free cholesterol levels in the sera of ALS patients [ 29 ] as well as spinal cords of ALS patients and SOD1-G93A mice [ 35 ], the cholesterol level is reduced in the CSF of ALS patients [ 29 ]. Furthermore, reduced levels of lanosterol, a precursor to cholesterol, are observed in ALS patients and SOD1 mouse models, along with downregulation of HMGCR [ 35 ]. Given that cholesterol cannot cross the blood—brain barrier, cholesterol is synthesized and stored in the CNS without peripheral contribution [ , ]. It is questionable if peripheral serum levels of cholesterol reflect the levels in the CNS, and vice versa. This disparity may explain why studies using statins to reduce serum cholesterol in ALS patients showed no effect or negative effect on disease progression [ , ]. There is a large discussion surrounding the interplay of cholesterol metabolism, transport and uptake in the periphery and the CNS, and their effects in ALS. Please refer to Hartmann et al. Cholesterol cannot be degraded, and free cholesterol is toxic to the system. Excess cholesterol in the CNS is oxidized to oxysterols, which are able to cross the blood—brain barrier [ ], and the blood levels of oxysterols are considered reflective of CNS status. The main forms of oxysterols found in the CNS are 24S-hydroxycholesterol OHC , hydroxycholesterol OHC , and hydroxycholesterol OHC , of which OHC and OHC are LXR receptor ligands. LXR receptors activate expression of genes involved in cholesterol efflux pathway, such as ATP-binding cassette subfamily A member 1 ABCA1 and APOE, thereby providing another layer to maintain cholesterol homeostasis. Levels of enzymes converting cholesterol into OHC are found elevated in early symptomatic SOD1-G93A mouse brains [ , ]. Additionally, OHC induces neuronal death via LXR-mediated apoptosis in motor neuron-like cells containing the SOD1-G93A mutation [ ]. Elevated levels of OHC are found in spinal cords of ALS patients, and cause cell death in neuroblastoma cell lines [ ]. These studies suggest a neurotoxic effect of accumulation of OHC and OHC in the CNS. GWAS studies have identified CYP27A1 , which encodes the enzyme converting cholesterol to OHC, as a susceptible loci for ALS [ ]. However, lower levels of OHC have been reported in the sera of ALS patients [ , ], but show no correlation with survival [ ]. Surplus free cholesterol can be esterified with fatty acyls to neutral cholesterol esters and stored in lipid droplets. In the CNS, this function is likely to be carried out primarily in astrocytes [ 47 , ]. Elevated cholesterol ester levels have been consistently reported in various tissues of ALS patients and animal models. Several species of cholesterol esters, including those with C16 and C18 saturated and unsaturated fatty acid chains, are reported to increase by up to 22 folds in patient spinal cords, with a more pronounced effect in the grey matter [ 33 , 35 ]. A four-fold progressive increase of C18 cholesterol ester species has been observed in SOD1-G93A mouse spinal cords from early symptomatic stage to end paralysis stage. Cholesterol esters levels are elevated in plasma samples and the change is maintained longitudinally, with increases in long- and very long-chain cholesterol esters, including CE and CE being discriminatory for ALS [ 62 ]. The SODA rat spinal cords have a sixfold increase of total cholesterol esters, mainly from PUFA species, including arachidonic acid The cholesterol ester accumulation seen in the FUS-overexpressing mice is partially rescued on HDAC inhibition [ 68 ]. Mice with adenoviral-induced overexpression of SREBP2 transcription domain show motor neuron degeneration, paralysis and reduced survival accompanied by accumulation of cholesterol esters [ 35 ]. Interestingly, lysoPC, a by-product of cholesterol ester synthesis, is also elevated in spinal cords of both patients and SOD1-G93A mice [ 35 ]. Lyso-PC causes rapid demyelination and is shown to be toxic to motor neuron cultures [ 35 ], suggesting that accumulated cholesterol esters may be toxic via action of their by-product. As discussed above, it is apparent that lipid dysregulation, in particular accumulation of toxic lipid species, contributes to ALS. As such, targeting these toxic lipids makes for attractive therapeutic interventions. Indeed, various strategies have shown to be successful in alleviating disease symptoms, extending survival and providing neuroprotection in vitro and in animal models. In this section, we present an overview of lipids as therapeutic targets for ALS treatment Fig. Potential therapeutic strategies targeting fatty acids. Upper panel shows an overview of fatty acid metabolism intervention points with neuroprotective effects. Fatty acids and derivatives shown to be toxic in ALS are highlighted in salmon pink. Lower panels describe the toxicity and the therapeutic strategies used in ALS mice and ALS patients. Intervening compounds are in orange. Glucose oxidation is the main energy source in the brain, while fatty acid β-oxidation contributes to up to one-fifth of the total brain energy needs [ 77 ]. Although fatty acid oxidation produces more ATP as compared to glucose, it also takes up more oxygen resources. As such, cells with prolonged fatty acid β-oxidation undergo oxidative stress, thereby producing harmful reactive-oxygen species. Due to the high energy demand and impaired glucose metabolism in ALS [ 24 , ], there is a switch to fatty acid β-oxidation as the major route for energy generation, placing the system under increased oxidative stress, a key mechanism of neurotoxicity in ALS [ 23 , 79 , 80 ]. It is worth noting that the switch to the use of fatty acids as an energy source has been observed in skeletal muscles of SOD1-G86R [ 79 ] and SOD1-G93A mice [ ] even prior to disease onset. Pyruvate and fatty acyl CoA are important intermediates of glucose and fatty acid oxidation, which are converted to acetyl-coA. The acetyl-coA enters the TCA cycle to generate ATP. Pyruvate dehydrogenase catalyzes the oxidation of pyruvate to acetyl-coA and is inhibited by pyruvate dehydrogenase kinase 4 PDK4 to regulate pyruvate levels. Palamiuc and colleagues demonstrated that Pdk4 expression is elevated in skeletal muscles of SODR mice accompanied by impaired glucose metabolism and a switch to fatty acid β-oxidation, leading to greater oxidative stress [ 79 ]. Also, Pdk4 mRNA expression was found with a three-fold elevation in ALS patient muscles [ 79 ]. Inhibition of PDK4 with dichloroacetate in the SODR mice leads to a switch back to increased glucose oxidation, and the mice show improved mitochondrial function, reduced muscle denervation, and delayed disease onset [ 79 ]. These results underscore the importance of metabolic switch in inducing oxidative stress and disease pathogenesis Fig. Elevated levels of arachidonic acid and its derivatives have been reported in ALS patients and models [ 18 , 34 , 60 , , , , , ]. Arachidonic acid produces prostaglandins and leukotrienes via the COX and LOX pathways. These molecules can induce inflammation and cause motor neuron death, which could be rescued by treatment with LOX and COX inhibitors. Administration of nimesulide, an inhibitor for COX-2, also shows great promise, as it reduces the level of PGE 2 in the spinal cord and delays disease onset in SOD1-G93A mice [ ]. Administration of 5-LOX inhibitor caffeic acid, apigenin or nordihydroguaretic acid promotes survival of ALS spinal motor neurons in vitro, reverses eye degenerative phenotypes and promotes survival in C9orf72 ALS flies [ 18 ]. Direct treatment with arachidonic acid increases cell death of ALS spinal motor neuron cell lines, which can be rescued in a dose-dependent manner by caffeic acid [ 18 ]. In SOD1-G93A mice, caffeic acid reduces astrocyte and microglia activation, maintains neuromuscular junction morphology and architecture, delays disease onset and prolongs lifespan [ 18 ] Fig. It would be of interest to make use of these studied chemotherapeutic agents as candidates for ALS therapeutics. PGE 2 is a key mediator in the initiation of inflammatory oxidation and propagation. Inhibition of COX-2, an enzyme involved in PGE 2 synthesis, and downregulation of PGE 2 receptor, have been shown to delay the onset of ALS symptoms in SOD1-G93A mice [ , ]. Given the involvement of PGE 2 in ALS inflammation and potential systemic side effects of COX-1 inhibition COX-1 is constitutively expressed in most tissues , a variety of COXinhibiting non-steroidal anti-inflammatory drugs NSAIDs have been tested for ALS therapeutics. Administration of NSAIDs, such as rofecoxib [ ], nimesulide [ ], and celecoxib [ ], has been shown to delay disease onset and promote survival at varying degrees in SOD1-G93A mice [ , , ] Fig. Furthermore, depletion of TDP in microglia, but not in astrocytes, increases COX-2 and PGE 2 levels and reduces neural survival in vitro. This neurotoxicity could be rescued by celecoxib [ ]. However, a double-blind clinical trial of celecoxib in ALS patients showed no beneficial effects on muscle strength scored via the ALSFRS-R over a period of one year [ ]. Although cohort studies to test for ALS risk associated with NSAID usage have been inconclusive [ , ], a population study with ALS patients found that the use of aspirin a NSAID inhibiting both Cox-1 and Cox-2 may reduce ALS risk in people over 55 years [ ]. Altogether, these studies indicate that activation of the arachidonic acid pathway contributes to ALS pathogenesis, and conversely, pharmacologic inhibition of the arachidonic acid pathway may have a therapeutic potential. There are few clinical trials testing for the effects of cannabinoids in ALS, and the number of patients employed was limited, with most early ones being observational survey-based. Another phase-2 clinical trial with 59 ALS patients tested nabiximols, an established drug used to treat muscle spasticity in multiple sclerosis [ ]. The trial found that nabiximols is safe for use and has positive effects on muscle spasticity in ALS [ ], opening avenues for large-scale clinical trials for ALS symptomatic relief. There is an ongoing placebo-controlled double-blind clinical trial with 30 ALS patients to study the efficacy of cannabis-based medicine extracts in slowing ALS progression as measured by the ALSFRS-R, and to evaluate its safety and effects in relieving pain and spasticity as well as improving quality of life [ ]. It should be noted that 2-AG is also a substrate for COX-2, and it can be oxygenated by COX-2 to form various prostamides and prostaglandin glyceryl esters [ ], such as Prostaglandin E2 glyceryl ester PGE 2 -G and prostaglandin D2 glyceryl ester with divergent physiological functions [ ]. However, the role of these prostaglandin glyceryl esters in ALS remains to be explored and further studies are needed to explore the translation potential of endocannabinoids. Given ceramide accumulation in patient tissues [ 33 , 36 , 60 , 62 , 63 , 73 ] and the neuronal toxicity of ceramides [ , , , ], ceramides are both attractive biomarkers and therapeutic targets for ALS. Ceramide accumulation can occur through excessive synthesis, impaired degradation, and increased breakdown of more complex sphingolipids into ceramides Fig. The recent identification of SPTLC1 mutations [ 16 , 17 ] further underscores this working model. Selective knockdown of SPTLC1 mutant allele using siRNA alleviates ceramide levels in vitro [ 17 ] , and the approach could possibly be extended in a clinical setting to revert accumulation of toxic lipids due to SPTLC1 mutations Fig. Ceramides and gangliosides therapeutic strategies. Upper panel shows an overview of sphingolipid metabolism intervention points. Sphingolipids with neurotoxic effects and neuroprotective effects in ALS conditions are highlighted in salmon pink and green, respectively. Lower panels describe the toxicity and the therapeutic strategies used. a Selective inhibition of SPTCL1 variant allele, b fingolimod-mediated neuroprotection, c inhibition of glucosylceramide breakdown, and d ganglioside-mediated therapeutics. Intervening compounds, RNAs and antibodies are highlighted in orange. Ceramides are also formed from the breakdown of glucosylceramides by hydrolyzing enzymes called glucocerebrosidases. Unlike in Gaucher disease, where glucosylceramide buildup causes liver and spleen malfunction [ ], elevated levels of glucosylceramides in ALS models seem to play a neuroprotective role [ 36 , 37 , 38 ]. Inhibition of glucosylceramide synthesis accelerates disease progression [ 36 , 67 ], while inhibition of glucosylceramide degradation via glucocerebrosidases, by conduritol B epoxide [ 37 ] and ambroxol hydrochloride [ 38 ], alleviates disease symptoms in mouse models, making glucosylceramides an attractive drug target to alleviate disease symptoms Fig. Multiple ganglioside species are involved in ALS: elevated levels of GM1 and GM3 are neuroprotective in nature [ 36 , , , ], while G1a and GT1b are toxic [ ], and their inhibition by specific antibodies results in improved survival in ALS mice [ ] Fig. Ceramide degradation is catalyzed by ceramidases, and forms S1P. S1P is a bioactive lipid involved in regulation of many processes such as cell proliferation, survival, neuronal excitability, neuroinflammation and immune cell trafficking [ , , ]. FTY is also a ceramide synthase inhibitor [ , ]. It inhibits proinflammatory cytokine production and reduces T cell migration into the CNS, thus promoting neuroprotective role of microglia and preventing neuronal excitotoxicity [ , , ]. FTY administration in SOD1-G93A mice prolongs survival, ameliorates neurological defects and regulates neuroinflammatory genes [ 39 ]. A randomized double-blind phase IIa clinical trial of FTY has demonstrated short-term safety 4-weeks of FTY with no adverse effects, reduction of circulating lymphocytes, and tolerability, suggesting the suitability for further clinical trials [ ]. Other attractive candidate lipid biomarkers of therapeutic interest include cholesterol esters and lysoPC. Accumulation of cholesterol esters and particularly lysoPCs, has been consistently reported in ALS patient tissues and models [ 33 , 34 , 35 , 60 , 62 , 63 , 65 , 70 ], and has been found to be discriminant for ALS [ 62 , 65 ], with C16 and Cn9 lysoPC species commonly elevated in both patients and animal models [ 35 ]. Although synthesis of cholesterol esters protects cells from free cholesterol toxicity, the by-product of its synthesis, lysoPC, has been shown to cause rapid demyelination [ ] and motor neuron death [ 35 ]. Lyso-PC species with C16 chains are highly neurotoxic and could be attractive targets for therapeutics. Additionally, lower levels of TG in the CSF are associated with better survival [ 65 ]. This could be a result of their breakdown to fatty acids to effectively meet energy demands in disease conditions. Additionally, although not demonstrated in the ALS context, neurotoxic reactive astrocytes in vitro increasingly secret long-chain saturated fatty acids which were shown to be neurotoxic [ 88 ]. Therefore, oxidative stress may result in neurotoxic reactive astrocytes in ALS [ 28 , ], which could be another potential mechanism to explore for a better understanding of ALS pathogenesis. There are of course many outstanding questions in the field to consider especially in deciphering the mechanisms underlying lipid toxicity or protection in ALS. Two areas of interest we would like to highlight are the interaction between CNS and peripheral lipid levels, and the cell-type specific lipid metabolism. The blood—brain barrier only allows selective molecules to pass freely between the CNS and the periphery, making it important to study lipid distribution in various tissues to better understand lipid synthesis, mobilization, uptake and consumption. These distinct profiles are highlighted in the context of TGs, which can pass through the BBB, and cholesterols, which cannot cross the BBB. TG levels are elevated in blood [ 61 , 62 , 63 ] and depleted in the CSF [ 61 , 65 ], which may be a result of mobilization of TGs from the peripheral adipose tissue to meet greater energy demands and consumption of TGs in the CNS in diseased state. Cholesterol levels are reported to be elevated in the plasma [ 34 , 62 ], which may be a risk factor for ALS in the pre-diagnostic stage [ ], but they are downregulated in mouse spinal cords. In fact, studies using statins, which cannot cross the BBB, to control blood cholesterol levels have failed [ , ], raising the question of the diagnostic or therapeutic value of blood cholesterol levels, and emphasizing the need for a better understanding of the exchange of lipids between the CNS and the periphery. A recent lipidomic study using primary cells i. For example, levels of cholesterol and ceramide are highest in neurons; astrocytes are enriched with PS, phosphatidylinositol and diacylglycerol; sulfatide and hexosylceramide are enriched in oligodendrocytes; and levels of SM and phosphatidylglycerol are highest in microglia [ 81 ]. These cell-type specific signatures may reflect the specialized functions of each cell type. Furthermore, lipid biosynthesis may be developmentally regulated and context-dependent. For example, cholesterol biosynthesis is required in neuroprogenitor cells but dispensable in mature neurons [ ]. During myelination, oligodendrocytes upregulate its own cholesterol biosynthesis, as well as taking up cholesterol synthesized in astrocytes [ , ]. Further work is needed to understand the cell-intrinsic mechanisms underlying the processing and functions of these lipids as well as how lipid dynamics may be regulated via cell—cell communication. In the CNS, astrocytes are the main site for lipid oxidation and storage [ 78 ]. During high energy demand, the lipids synthesized in neurons are transported into astrocytes, forming lipid droplets, and undergoing oxidation [ 78 ]. Astrocytes contain a greater number of antioxidant molecules and help consume the damaging reactive oxygen species produced from lipid oxidation. A recent study demonstrated that astrocytes become reactive in response to oxidative stress, secreting long-chain saturated fatty acids which are neurotoxic [ 88 ]. Furthermore, we and others have shown that TDPdepleted astrocytes and ALS astrocytes may shift towards the inflammatory reactive state [ , ]. Another study using ALS spinal cord motor neuron cultures showed elevated levels of arachidonic acid, which are autonomously neurotoxic [ 18 ]. Whether ALS astrocytes may exacerbate the lipid-mediated toxicity toward ALS motor neurons is an intriguing possibility and remains to be tested. Moreover, we have shown that depletion of TDP in oligodendrocytes alone is able to produce motor deficits via SREBP2-mediated cholesterol downregulation [ 28 ]. Evidence of TDPmediated cholesterol dysregulations has been found in oligodendrocytes harboring TDP pathologies [ 28 ]. Furthermore, CSF cholesterol level is reduced in ALS patients [ 29 ]. These studies together suggest complex cell—cell communication and transport, and perhaps a cell-type specific mechanism of lipid-mediated toxicity in ALS. How the lipid dynamics change during normal and disease conditions remains to be addressed. While this review focuses on lipids, it should be noted that lipid species or various metabolites are interconnected. In addition, a recent study has also highlighted that gut microbiome and metabolites may modulate ALS pathogenesis [ ]. How these metabolites affect each other, and what serve the initiating factors would be of great interest to be resolved. The great structural diversity of lipids allows for diverse functions, and thus diverse and complex roles in various mechanisms of ALS pathogenesis. Given their assorted roles, the question of whether lipidemia dysregulation is the cause or the consequence of the disease is not easily answered. Lipid changes could be both causal and a consequence of disease pathology, forming complex feedback and feedforward regulations. Elimination of SPTLC1 mutants can reduce accumulation of putative toxic lipids [ 17 ], while reducing toxic lipids rescues cellular phenotypes [ 16 ], suggesting that lipid dysfunction could drive ALS pathogenesis. The prevalence of prognostic and pre-diagnostic dyslipidemia risk factors for ALS, such as blood cholesterol levels [ ] and body mass index [ ], as well as evidence showing that inhibiting the pre-diagnostic energy source switch to fatty acid oxidation alleviates disease symptoms in mice [ 79 , ], further support a causal role of lipids in ALS. By systematically assessing the current literature, we highlight that accumulation of ceramides, arachidonic acid, lysoPC, and cholesterol esters is emerging as a common theme that is detrimental to motor neurons. Conversely, reducing the accumulation of these toxic lipids, in particular, ceramides and arachidonic acid, appears to be beneficial in various ALS models. Furthermore, increased levels of potentially beneficial lipids such as glucosylceramides, and activation of S1P-mediated signaling may be protective in ALS. We look forward to future investigations to restore the faulty lipids in ALS. Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Google Scholar. Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. CC wrote the original draft of the document. CV, AP, DC, and M-CM critically revised the document. All authors have made a substantial and intellectual contribution to the work and approved the final version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Cer, ceramide; DHA, docosahexaenoic acid; dihydroCer, dihydroceramide; EPA, eicosapentaenoic acid; glucosylCer, glucosylceramides; HDL, high-density lipoproteins; LDL, low-density lipoproteins; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; SM, sphingomyelins; TG, triglycerides; TGRL, triglyceride-rich lipoproteins; VLDL, very-low density lipoproteins. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. Iqbal J, Walsh MT, Hammad SM, Hussain MM. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol Metab. Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Truman JP, García-Barros M, Obeid LM. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochim Biophys Acta. Wang DD, Toledo E, Hruby A, Willett WC, Sun Q, Razquin C, et al. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED Trial Prevención con Dieta Mediterránea. Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, Seppänen-Laakso T, et al. A healthy Nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J Nutr. Fumeron F, Nicolas A, Bastard JP, Fellahi S, Wigger L, Ibberson M, et al. Dairy consumption is associated with lower plasma dihydroceramides in women from the DESIR cohort. Diabetes Metab. Seah JYH, Chew WS, Torta F, Khoo CM, Wenk MR, Herr DR, et al. Dietary fat and protein intake in relation to plasma sphingolipids as determined by a large-scale lipidomic analysis. Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, Larsen A, et al. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides. Arterioscler Thromb Vasc Biol. Luukkonen PK, Sädevirta S, Zhou Y, Kayser B, Ali A, Ahonen L, et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. Rosqvist F, Kullberg J, Ståhlman M, Cedernaes J, Heurling K, Johansson HE, et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab. Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J Cell Physiol. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving participants. J Am Heart Assoc. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. Omega-3 polyunsaturated fatty acid fish oil supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American heart association. Park Y, Harris WS. Dose-response of n-3 polyunsaturated fatty acids on lipid profile and tolerability in mildly hypertriglyceridemic subjects. J Med Food. Shearer GC, Savinova OV, Harris WS. Fish oil - how does it reduce plasma triglycerides? Walchuk C, Wang Y, Suh M. The impact of EPA and DHA on ceramide lipotoxicity in the metabolic syndrome. Br J Nutr. Kasbi-Chadli F, Ferchaud-Roucher V, Krempf M, Ouguerram K. Direct and maternal n-3 long-chain polyunsaturated fatty acid supplementation improved triglyceridemia and glycemia through the regulation of hepatic and muscle sphingolipid synthesis in offspring hamsters fed a high-fat diet. Eur J Nutr. Lankinen M, Schwab U, Erkkilä A, Seppänen-Laakso T, Hannila ML, Mussalo H, et al. Fatty fish intake decreases lipids related to inflammation and insulin signaling - a lipidomics approach. PLoS ONE. Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. Scherer M, Bottcher A, Schmitz G, Liebisch G. Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Le Barz M, Boulet MM, Calzada C, Cheillan D, Michalski MC. Alterations of endogenous sphingolipid metabolism in cardiometabolic diseases: Towards novel therapeutic approaches. Le Barz M, Vors C, Combe E, Joumard-Cubizolles L, Lecomte M, Joffre F, et al. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI Insight. Boulet MM, Calzada C, Pettazzoni M, Lelekov-Boissard T, Buisson C, Di Filippo M, et al. A meal rich in palm oil or butter modifies the sphingolipid profile of postprandial triglyceride-rich lipoproteins from type 2 diabetic women. Björnson E, Packard CJ, Adiels M, Andersson L, Matikainen N, Söderlund S, et al. Apolipoprotein B48 metabolism in chylomicrons and very low-density lipoproteins and its role in triglyceride transport in normo- and hypertriglyceridemic human subjects. J Intern Med. Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. Chétiveaux M, Croyal M, Ouguerram K, Fall F, Flet L, Zair Y et al. Effect of fasting and feeding on apolipoprotein A-I kinetics in preβ1- HDL, α-HDL, and triglyceride-rich lipoproteins. Sci Rep. Boulet MM, Cheillan D, Di Filippo M, Lelekov-Boissard T, Buisson C, Lambert-Porcheron S et al. Postprandial triglyceride-rich lipoproteins from type 2 diabetic women stimulate platelet activation regardless of the fat source in the meal. Mol Nutr Food Res. Averill M, Rubinow KB, Cain K, Wimberger J, Babenko I, Becker JO, et al. Postprandial remodeling of high-density lipoprotein following high saturated fat and high carbohydrate meals. J Clin Lipidol. Camont L, Lhomme M, Rached F, Le Goff W, Nègre-Salvayre A, Salvayre R, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Ferchaud-Roucher V, Zair Y, Aguesse A, Krempf M, Ouguerram K. Omega 3 improves both apoBcontaining lipoprotein turnover and their sphingolipid profile in hypertriglyceridemia. Ruuth M, Lahelma M, Luukkonen PK, Lorey MB, Qadri S, Sädevirta S, et al. Overfeeding saturated fat increases LDL Low-Density Lipoprotein aggregation susceptibility while overfeeding unsaturated fat decreases proteoglycan-binding of lipoproteins. Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. Yunoki K, Ogawa T, Ono J, Miyashita R, Aida K, Oda Y, et al. Analysis of sphingolipid classes and their contents in meals. Biosci Biotechnol Biochem. Contarini G, Povolo M. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. Burling H, Graverholt G. Milk - a new source for bioactive phospholipids for use in food formulations. Lipid Technol. CrossRef Full Text Google Scholar. Nilsson A. Metabolism of sphingomyelin in the intestinal tract of the rat. Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. Norris GH, Milard M, Michalski MC, Blesso CN. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J Nutr Biochem. Ohlsson L, Burling H, Nilsson A. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis. Conway V, Couture P, Richard C, Gauthier SF, Pouliot Y, Lamarche B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr Metab Cardiovasc Dis. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Ohlsson L, Burling H, Duan RD, Nilsson A. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur J Clin Nutr. Vors C, Joumard-Cubizolles L, Lecomte M, Combe E, Ouchchane L, Drai J, et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Ding M, Rexrode KM. A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Keywords: sphingolipids, ceramide, sphingomyelin, dietary lipids, nutrition, postprandial metabolism. |
| Cholesterol test - Mayo Clinic | Hydration and mental clarity CAS Google Scholar Spewdy, M. Module transcatheter breakdwon treatment Speedy lipid breakdown breqkdown future developments. Module 2: presentation and investigation. Monitoring: If you have abnormal results on earlier testing or other risk factors for heart disease, lipid testing can monitor the cholesterol in your blood. Gabica MD - Family Medicine Adam Husney MD - Family Medicine Rakesh K. |
| Lipids module 1: lipid metabolism and its role in atherosclerosis | Core lipid exchanges between HDL and TRLs occur in the circulation, catalysed by CETP. This allows cholesterol to be offloaded from HDL into VLDL and LDL which are destined for hepatic uptake, permitting further cholesterol uptake from tissues. Finally the cholesterol-enriched HDL particle returns cholesterol to the liver via the scavenger receptor B1 SCARB1 for biliary excretion. The cholesterol-depleted HDL particles can then return to the circulation to undertake more reverse cholesterol transport. The rate of cholesterol formation by the liver and absorption by the small intestine is highly responsive to the cellular level of cholesterol. This feed back regulation is controlled primarily by changes in the amount and activity of 3-hydroxy-3 methylglutaryl CoA reductase HMGCoA reductase. This enzyme catalyses formation of mevalonate, the committed step in cholesterol biosynthesis. For more detail on this process of cholesterol homeostasis, see dropdown box. The concentration of free cholesterol determines the fluidity and function of cell membranes and regulates overall cholesterol homeostasis see figure 6. When hepatic cholesterol content is reduced by export in lipoproteins or conversion to bile acids, membrane cholesterol concentration falls and SREBP-2 activates the enzymes of cholesterol synthesis, including HMG-Co-A reductase which is the rate limiting step in the pathway. SREBP-2 also activates the synthesis of LDL-receptors, accelerating the uptake of cholesterol in LDL to help restore membrane cholesterol concentration. Conversely, when hepatic cholesterol is increased by receptor mediated uptake of cholesterol in lipoproteins or return of cholesterol to the liver by HDL particles, membrane cholesterol increases, preventing activation of SREBP-2 and leading to LDL-receptor downregulation and inactivation of cholesterol synthesis. The majority of circulating cholesterol is carried in LDL which is the lipoprotein most closely associated with the development of atherosclerosis. Under normal circumstances, LDL may pass from the plasma into the subendothelial space and return to the liver to be removed from the circulation. At this point it has performed its transport functions without being taken up by macrophages and indeed is unable to stimulate foam cell formation in vitro. However, if retention of the LDL in the endothelial space is increased, due to endothelial injury e. with smoking, hyperglycaemia, hypertension or if removal of LDL from the circulation is delayed, it can become damaged by oxidation or modified in other ways. Oxidised or otherwise modified LDL are retained in the subendothelial space and are taken up by monocyte-derived macrophages via the scavenger receptor leading to the formation of foam cells, and the development of arterial sub-endothelial fatty streaks, the precursor of atheroma. Small dense LDL particles, typically found in found in association with prolonged postprandial hypertrigylceridaemia and low HDL cholesterol, appear more susceptible to oxidation which may make them more atherogenic. Partially metabolised remnants of triglyceride-rich lipoproteins remnant lipoproteins that appear post-prandially are able to induce foam cell formation without modification. These are considered the most highly atherogenic of all. Other atherogenic lipoproteins readily retained in the subendothelial space include glycated LDL and lipoprotein a. HDL are, however, able to penetrate deep into the subendothelial space and are able to remove oxidised lipid from macrophages and prevent foam cell formation, in addition to having a protective effect on the endothelium. Reduction of HDL particle numbers or functional activity is therefore pro-atherogenic. Figure 7 shows the progression of atherosclerosis. For more information on the process of atherosclerotic plaque development, please visit module 3 of our angina e-learning programme. Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids through the lymphatic and circulatory systems. They also serve as enzyme cofactors, receptor ligands, and lipid transfer carriers that regulate the metabolism of lipoproteins and their uptake in tissues. For examples of their beneficia l role e. apoE in hepatic clearance of chylomicrons and their thrombogenic potential e. lipoprotein a , see dropdown box. Apolipoproteins B and B48 are two proteins produced from the same gene, due to editing of mRNA in the gut. ApoB48 is exclusive to chylomicrons and chylomicron remnants. ApoE is required for hepatic clearance of chylomicron and VLDL remnants IDL via the LDL receptor LDLR and LDLR-like receptor protein 1 LRP1. ApoE has 3 common Isoforms E3, E4, E2. ApoB remains with VLDL as it undergoes lipolysis to IDL and LDL whereas exchangeable apolipoproteins e. ApoE and apoC-II can transfer between particles. As each LDL particle contains one molecule of apolipoprotein B, apolipoprotein B concentration is a measure of LDL particle numbers. Lipoprotein a is a highly atherogenic and thrombogenic lipoprotein formed by covalent bonding between the apolipoprotein B of the LDL particle and apolipoprotein a , an apparently vestigial plasminogen-like protein which increases its retention in the artery wall see figure 8. Genetic variants of the apo a protein which are smaller in size due to fewer numbers of repeats of the KIV-2 domain generate greater numbers of liporotein a particles, concentrations of which show fold variation between individuals. As these genetic variants are co-dominantly inherited, family members may also be at risk. The routine lipid profile is based on measurements of: — total cholesterol TC , — HDL — triglycerides. Measuring TC provides limited information about risk because the number includes both atherogenic LDL, IDL and VLDL and, the anti-atherogenic fraction, HDL. In addition, 12 hour fasting measurement of triglycerides is required for calculation of LDL. LDL is usually considered the most important class of atherogenic lipoproteins. However this equation assumes a constant ratio As the ratio is altered by statin treatment, the equation significantly underestimates LDL in treated patients. Both LDL and non-HDL measurements are used for targeting treatment but the latter is more reliable in patients treated receiving high intensity statin therapy, since the LDL calculation is increasingly inaccurate at lower concentrations see table 3. A patient with low LDL and high non-HDL is an example of a patient with increased risk who may slip through the net because we only look at LDL. These patients are also likely to have high LDL particle number LDL-P as well as high ApoB levels. Apolipoproteins are measured by immunoassay and are therefore more expensive tests. ApoB is an alternative measure of atherogenic lipoproteins. Lipoprotein a is also measured by immunoassay and careful method selection is required to ensure equal recognition of particles containing different apo a isoforms, but the measurement is stable throughout life and a single measurement is usually sufficient to assess lipoprotein a associated cardiovascular risk. Therefore there is a need for a lipid parameter that better reflects the amount of cholesterol within all atherogenic particles. Such people have elevated triglycerides, low HDL and relatively normal calculated LDL. Despite this, they harbour highly atherogenic lipoproteins such as TRL remnants and IDL intermediate density lipoprotein as well as small dense LDL particles. Non-HDL has been shown to be a better marker of risk in both primary and secondary prevention studies. In a recent analysis of data combined from 68 studies, non-HDL was the best predictor among all cholesterol measures, both for CAD events and for strokes, and is recommended in the National Institute for Health and Care Excellence NICE Lipid Modification Guideline CG as the preferred marker for monitoring of lipid lowering therapy. Cegla J, Neely RDG, France M, et al. HEART UK consensus statement on lipoprotein a : a call to action. Atherosclerosis ; — Lancet ; — Boot CS, Middling E, Allen J, Neely RDG. Evaluation of the non-HDL cholesterol to apolipoprotein B ratio as a screening test for dysbetalipoproteinemia. Clin Chem ; 65 — National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Clinical guideline [CG]. London: NICE, July , updated September Durrington PN. Hyperlipidaemia: diagnosis and management 3rd Edition. Hodder Arnold Garg A ed. Dyslipidaemias: pathophysiology, evaluation and management. Humana Press Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation ; — Calandra S, Tarugi P, Speedy HE, Dean AF, Bertolini S, Shoulders CC. Mechanisms and genetic determinants regulating sterol absorption, circulating LDL levels, and sterol elimination: implications for classification and disease risk. J Lipid Res ; 52 — All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews Cardiology Limited. Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice. Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews Cardiology Limited cannot accept responsibility for any errors in prescribing which may occur. You must be logged in to post a comment. You need to be a member to print this page. Find out more about our membership benefits. You need to be a member to download PDF's. Toggle navigation. All by Author by Keyword by DOI. Current Issue Articles Clinical articles Editorials News and views Archive Supplement archive Learning By programme ACS management Amyloid heart disease Angina Angiography Anticoagulation Anticoagulation reversal Diabetes and CVD Digoxin Heart failure Heart valve disease Hyperkalaemia Lipids Obesity Pulmonary hypertension Standalone Books CV research handbook BJC TV Diary Contact. This website is intended for UK healthcare professionals only Log in Register. Lipid basics Lipids are fatty substances that are required for maintenance of normal bodily function. Lipoprotein fractions Figure 1. The components of a lipoprotein All lipoproteins have a common basic structure figure 1 but they vary greatly in their size, density and composition figure 2 and table 1. Figure 2. The lipoprotein particle family Table 1. The composition of the various lipoproteins and their transport pathways. Specific apolipoproteins Apolipoprotein B is the bulk carrier of endogenously produced lipids and is secreted by the liver as the major apolipoprotein component of VLDL and LDL, one molecule per lipoprotein, which stays with the particle until it is removed from the circulation by the LDL receptor. This study also found that fasting transiently increased circulating nonesterified fatty acids and perilipin 5 PLIN5 mRNA levels in muscle. This would suggest that lipid droplet formation and storage within the myocyte was transiently augmented in response to fasting. PLIN5 has been shown to protect against lipotoxicity and stimulate lipid oxidation 7. However, in the present study 4 , the increase in PLIN5 was insufficient to prevent the increase in insulin resistance induced by fasting. These findings differ from what has been previously reported in male participants after an acute fast 8. Following 60 hours of fasting, the size and number of lipid droplets in muscle increased in lean, normoglycemic men 8. The fraction of PLIN5 protein associated with the lipid droplets also increased 8. Moreover, men with the greatest increase in PLIN5-associated lipid droplets were shown to have the smallest reduction in insulin sensitivity 8. The reason the increase in PLIN5 in the present study did not influence the degree of insulin resistance during IF is unclear. The clinical implications of these transient increases in insulin resistance in response to fasting undoubtedly warrants further investigation. The Liu et al 4 study has several strengths. This is the first study to compare the effects of IF vs CR on lipid and mitochondrial metabolism in human skeletal muscle. The trial is also advantageous in that it included 3 diet prescriptions, that is, daily CR vs an energy-matched IF group as well as an isocaloric IF group. This allowed the investigators to examine if changes in lipid metabolism were due to the energy deficit, often prescribed in ADF studies, or if the fasting itself produced these outcomes. In addition, the study used gold-standard measures to examine insulin sensitivity and lipid deposition. Specifically, the investigators used the hyperinsulinemic-euglycemic clamp and muscle biopsy to measure insulin sensitivity and lipid droplet deposition intramuscularly. Although this study has several strengths, it also has some methodological limitations. First, the sample size was small and the study may have been underpowered to see significant changes in these secondary outcome measures. Second, the removal of the control group is also a distinct limitation. Last, the study was conducted solely in women with overweight or obesity, thus the findings may not be generalizable to men or those who are normal weight. In summary, these findings by Lui and colleagues 4 suggest fasting may be superior to daily restriction for decreasing ROS production in women with overweight or obesity. It was also noted that fasting increased mRNA levels of PL1N5, suggesting enhanced lipid droplet formation. However, this was not sufficient to prevent the transient increase in insulin resistance induced by fasting. Disclosure Summary: K. has received author fees from the Hachette Book Group for the book, The Every Other Day Diet. has nothing to disclose. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study. Patterson RE , Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. Google Scholar. Trepanowski JF , Kroeger CM , Barnosky A , et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. Mattson MP , de Cabo R. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. Liu B , Hutchison AT , Thompson CH , Lange K , Wittert GA , Heilbronn LK. Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle. Hutchison AT , Liu B , Wood RE , et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity Silver Spring. Ighodaro OM , Akinloye OA. First line defence antioxidants-superoxide dismutase SOD , catalase CAT and glutathione peroxidase GPX : their fundamental role in the entire antioxidant defence grid. Alexandria J Med. Mason RR , Watt MJ. Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol Metab. Gemmink A , Bosma M , Kuijpers HJH , et al. Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Additional Information. Data Availability. Journal Article. Kelsey Gabel , Kelsey Gabel. Department of Kinesiology and Nutrition, University of Illinois at Chicago. Oxford Academic. |
| Lipid droplet biogenesis and functions in health and disease | Nature Reviews Endocrinology | Bazinet RP, Layé S. Speeedy al. Speedy lipid breakdown Rep. Skin health catechins familial ALS, at Speedy lipid breakdown 42 genes, including Lipud superoxide dismutase 1C9ORF72 chromosome 9 open reading frame 72TARDBP TAR DNA-binding protein and FUS, have been identified to be causal [ 4 ]. Autosuggest Results Please Enter a Search Term. |
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Ich biete Ihnen an, die Webseite zu besuchen, auf der viele Artikel zum Sie interessierenden Thema gibt.
Die Kleinigkeiten!
die sehr lustige Antwort
ich beglückwünsche, Ihre Idee wird nützlich sein