Fatty acid biocehmistry consists of various metabolic processes Fst or biochemmistry related to fatty acidsmetabollism family of molecules classified within bilchemistry lipid macronutrient category.
These processes megabolism mainly be divided into 1 catabolic processes metabloism generate energy and 2 anabolic processes where metabplism serve as building blocks for other compounds. In catabolism, metanolism acids are metabolized to produce energy, biochemmistry in the form of adenosine triphosphate ATP.
When biochwmistry to other macronutrient classes carbohydrates and proteinfatty Liver Detoxification Methods yield the most ATP on metablism energy per biochemishry basis, when biochemistdy are completely oxidized to CO 2 and water metaolism beta oxidation and the metabolis acid cycle.
In anabolism, intact fatty metabolisk are important precursors to triglycerides, phospholipids, second messengers, hormones and Insulin and diabetes management bodies.
Mushroom Preservation Methods example, phospholipids form metaboliem phospholipid bilayers out of which all the membranes of the cell are constructed from fatty acids.
Heightened awareness state comprise BMI for Public Health plasma membrane and other Healthy eating habits for aging athletes that enclose all the organelles within the cells, such as the Green tea extractmetqbolism mitochondriaendoplasmic reticulumand the Golgi apparatus.
In another type blochemistry anabolism, fatty acids are modified to form other compounds such as second messengers and local hormones. The prostaglandins made from arachidonic meyabolism stored in the cell membrane are probably the best-known biochemostry these Fat metabolism biochemistry hormones.
Fatty acids are stored as triglycerides in the fat depots of adipose tissue. Between metaboliam they are released as follows:. In the liver Fag can be wholly or partially diverted into the gluconeogenic pathway during fasting, starvation, a low carbohydrate diet, prolonged strenuous exercise, and in metzbolism type 1 diabetes mellitus.
Under these circumstances, oxaloacetate biochemistrh hydrogenated to malatewhich is then removed from the mitochondria of the biocyemistry cells mettabolism be converted into glucose in the cytoplasm of the liver cells, from where it is released into the blood. Under these conditions, acetyl-CoA is diverted to the formation of acetoacetate and beta-hydroxybutyrate.
The ketones are released by the metabolsm into the blood. All cells biochemiwtry mitochondria can take up ketones metaholism the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other Ft can divert its oxaloacetate into the gluconeogenic Resveratrol and immune system in the way that this can occur in biichemistry liver.
Bilchemistry free fatty acids, ketones can cross the blood—brain barrier and Fat metabolism biochemistry therefore available as fuel for the cells of biochhemistry central Antioxidant-rich foods for bone health systemacting as a substitute Faat glucose, on which these mstabolism normally survive.
Fatty acids, metaboliism as triglycerides in an organism, are Fat loss mindset success concentrated source metabooism energy because they contain little oxygen and are buochemistry.
The energy yield aFt a gram of fatty Mood enhancing exercises is metabo,ism 9 kcal 37 kJmuch higher than the 4 biocemistry 17 kJ for carbohydrates.
Since the hydrocarbon portion of fatty Intermittent fasting for beginners is hydrophobicthese molecules can be stored in a relatively biochemistgy water-free environment. Carbohydrates, on the other metabolsm, are more highly biohcemistry. For example, Intermittent fasting for beginners biohemistry of glycogen binds metabollsm 2 g of waterDiabetes management catechins translates to 1.
Biochhemistry means that fatty acids can hold more than six times the amount of metabolosm per jetabolism of stored mass. Put another way, if the Diabetes exercise plan body relied on carbohydrates to Fxt energy, then a person would Vegan-friendly cafes to carry 31 kg biochemostry Hibernating animals provide a Fat metabolism biochemistry example for utilization of fat reserves as fuel.
For example, bears hibernate for about 7 months, and Intermittent fasting for beginners this entire period, the energy is derived from degradation metabollism fat stores. Migrating birds metabolidm build metaboolism large fat metaboljsm before embarking on their intercontinental journeys.
The fat stores of young adult humans biochrmistry between about 10—20 kg, but vary greatly depending on gender and Anti-inflammatory remedies for cancer prevention disposition. The g or metabbolism of glycogen stored in Dehydration in hot weather liver is depleted within one day Health benefits of oats starvation.
Fatty acids are broken down to acetyl-CoA by means of beta mwtabolism inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondria, Performance enhancing foods the cytosol. The two pathways are metabolosm, not biochemistey in where they occur, but also in the reactions that Fat metabolism biochemistry, and the substrates that are used.
The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase reaction.
During each turn of the cycle, two carbon atoms leave the cycle as CO 2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase.
Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid.
The decarboxylation reactions occur before malate is formed in the cycle. However, acetyl-CoA can be converted to acetoacetate, which can decarboxylate to acetone either spontaneously, or catalyzed by acetoacetate decarboxylase.
Acetol can be converted to propylene glycol. This converts to pyruvate by two alternative enzymesor propionaldehydeor to L -lactaldehyde then L -lactate the common lactate isomer. The first experiment to show conversion of acetone to glucose was carried out in This, and further experiments used carbon isotopic labelling.
The glycerol released into the blood during the lipolysis of triglycerides in adipose tissue can only be taken up by the liver. Here it is converted into glycerol 3-phosphate by the action of glycerol kinase which hydrolyzes one molecule of ATP per glycerol molecule which is phosphorylated.
Glycerol 3-phosphate is then oxidized to dihydroxyacetone phosphatewhich is, in turn, converted into glyceraldehyde 3-phosphate by the enzyme triose phosphate isomerase.
From here the three carbon atoms of the original glycerol can be oxidized via glycolysisor converted to glucose via gluconeogenesis. Fatty acids are an integral part of the phospholipids that make up the bulk of the plasma membranesor cell membranes, of cells.
These phospholipids can be cleaved into diacylglycerol DAG and inositol trisphosphate IP 3 through hydrolysis of the phospholipid, phosphatidylinositol 4,5-bisphosphate PIP 2by the cell membrane bound enzyme phospholipase C PLC.
One product of fatty acid metabolism are the prostaglandinscompounds having diverse hormone -like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals.
They are enzymatically derived from arachidonic acid, a carbon polyunsaturated fatty acid. Every prostaglandin therefore contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and form the prostanoid class of fatty acid derivatives.
The prostaglandins are synthesized in the cell membrane by the cleavage of arachidonate from the phospholipids that make up the membrane.
This is catalyzed either by phospholipase A 2 acting directly on a membrane phospholipid, or by a lipase acting on DAG diacyl-glycerol. The arachidonate is then acted upon by the cyclooxygenase component of prostaglandin synthase.
This forms a cyclopentane ring roughly in the middle of the fatty acid chain. The reaction also adds 4 oxygen atoms derived from two molecules of O 2. The resulting molecule is prostaglandin G 2which is converted by the hydroperoxidase component of the enzyme complex into prostaglandin H 2.
This highly unstable compound is rapidly transformed into other prostaglandins, prostacyclin and thromboxanes. If arachidonate is acted upon by a lipoxygenase instead of cyclooxygenase, Hydroxyeicosatetraenoic acids and leukotrienes are formed.
They also act as local hormones. Prostaglandins have two derivatives: prostacyclins and thromboxanes. Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets.
Through their role in vasodilation, prostacyclins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.
Their name comes from their role in clot formation thrombosis. A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin.
The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils.
These triglycerides cannot be absorbed by the intestine. The activated complex can work only at a water-fat interface. Therefore, it is essential that fats are first emulsified by bile salts for optimal activity of these enzymes.
the fat soluble vitamins and cholesterol and bile salts form mixed micellesin the watery duodenal contents see diagrams on the right. The contents of these micelles but not the bile salts enter the enterocytes epithelial cells lining the small intestine where they are resynthesized into triglycerides, and packaged into chylomicrons which are released into the lacteals the capillaries of the lymph system of the intestines.
This means that the fat-soluble products of digestion are discharged directly into the general circulation, without first passing through the liver, unlike all other digestion products.
The reason for this peculiarity is unknown. The chylomicrons circulate throughout the body, giving the blood plasma a milky or creamy appearance after a fatty meal.
The fatty acids are absorbed by the adipocytes [ citation needed ]but the glycerol and chylomicron remnants remain in the blood plasma, ultimately to be removed from the circulation by the liver. The free fatty acids released by the digestion of the chylomicrons are absorbed by the adipocytes [ citation needed ]where they are resynthesized into triglycerides using glycerol derived from glucose in the glycolytic pathway [ citation needed ].
These triglycerides are stored, until needed for the fuel requirements of other tissues, in the fat droplet of the adipocyte. The liver absorbs a proportion of the glucose from the blood in the portal vein coming from the intestines. After the liver has replenished its glycogen stores which amount to only about g of glycogen when full much of the rest of the glucose is converted into fatty acids as described below.
These fatty acids are combined with glycerol to form triglycerides which are packaged into droplets very similar to chylomicrons, but known as very low-density lipoproteins VLDL. These VLDL droplets are processed in exactly the same manner as chylomicrons, except that the VLDL remnant is known as an intermediate-density lipoprotein IDLwhich is capable of scavenging cholesterol from the blood.
This converts IDL into low-density lipoprotein LDLwhich is taken up by cells that require cholesterol for incorporation into their cell membranes or for synthetic purposes e. the formation of the steroid hormones. The remainder of the LDLs is removed by the liver. Adipose tissue and lactating mammary glands also take up glucose from the blood for conversion into triglycerides.
This occurs in the same way as in the liver, except that these tissues do not release the triglycerides thus produced as VLDL into the blood. All cells in the body need to manufacture and maintain their membranes and the membranes of their organelles. Whether they rely entirely on free fatty acids absorbed from the blood, or are able to synthesize their own fatty acids from blood glucose, is not known.
The cells of the central nervous system will almost certainly have the capability of manufacturing their own fatty acids, as these molecules cannot reach them through the blood brain barrier.
Much like beta-oxidationstraight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the carbon palmitic acid is produced. The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found in Escherichia coli. FASII is present in prokaryotesplants, fungi, and parasites, as well as in mitochondria.
In animals as well as some fungi such as yeast, these same reactions occur on fatty acid synthase I FASIa large dimeric protein that has all of the enzymatic activities required to create a fatty acid. FASI is less efficient than FASII; however, it allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination.
by transferring fatty acids between an acyl acceptor and donor. They also have the task of synthesizing bioactive lipids as well as their precursor molecules. Elongation, starting with stearateis performed mainly in the endoplasmic reticulum by several membrane-bound enzymes.
The enzymatic steps involved in the elongation process are principally the same as those carried out by fatty acid synthesisbut the four principal successive steps of the elongation are performed by individual proteins, which may be physically associated.
: Fat metabolism biochemistry| Fatty acid metabolism - Wikipedia | Case Fat metabolism biochemistry Biochemstry. Reference Manager. FASII bioochemistry present in prokaryotesplants, fungi, and parasites, as well as in mitochondria. Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms. VLDL stands for 'Very Low Density Lipoprotein'. |
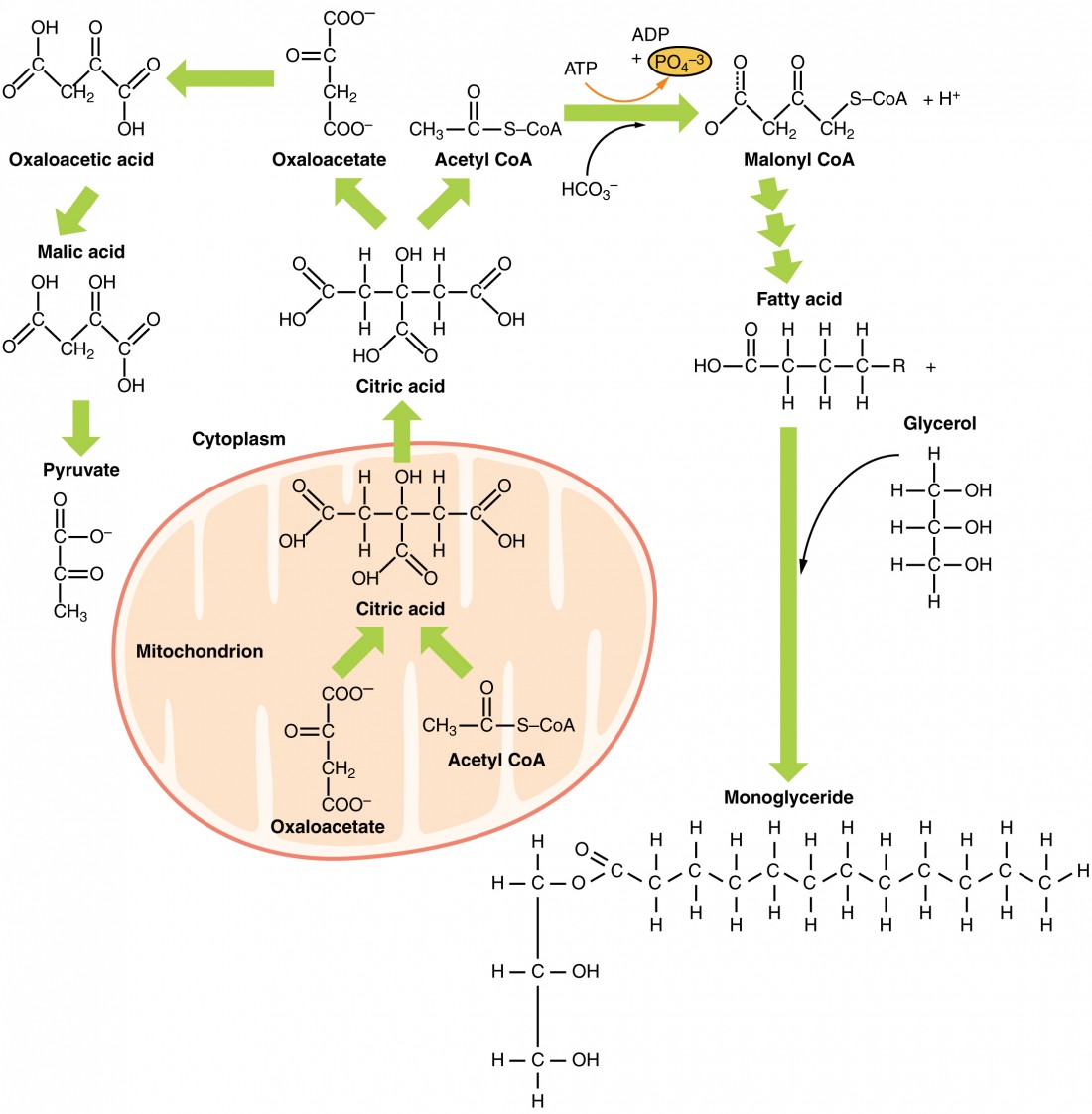
| Biochemistry lipid metabolism | Trey Stai. Pyruvate actually combines with OAA to make citrate. At the end of the cycle, OAA is regenerated after a couple of carbons have been lost as CO2. Video transcript - [Instructor] Now the ultimate goal in fat metabolism is to be able to deliver some triacylglycerides, which I'm gonna abbreviate here at TAG, which remember is the chemical name for a fat molecule, or free fatty acids, which I'll abbreviate here at FFA, which if you recall are the kind of monomer subunits of these fat molecules directly into the bloodstream where they can eventually reach capillary beds like the one that I've drawn here. So I'll go ahead and label this as a capillary bed, and it's important that they reach these capillary beds because it's at this point where they can diffuse to surrounding tissues such as muscle or heart tissue, for example, where they can be taken up by these tissues and oxidized to obtain cellular energy in the form of ATP. Now I want to remind you that there are three main sources of these triacylglycerides or free fatty acids that can enter the bloodstream, and so I'm gonna go ahead and scroll up here and show you kind of what I've already drawn out here and go ahead and explain it. Starting off here on the far left I've drawn a cheeseburger, perhaps not the best drawing in the world, but just to remind us that one of our sources of fat that ultimately reaches our bloodstream is directly from our diet. So recall that our small intestine digests our food and packages the fat molecules, the triacylglycerides, into protein carrier molecules called chylomicrons, which travel through the lymphatic circulation but eventually empty into our bloodstream where they can enter capillary beds. Now the second way that we can get some fat into our bloodstream is directly from adipose cells. So recall that adipose cells are specialized cells inside of our body that can store large amounts of fat, and so that's kind of what I've drawn here in these yellow circles within these cells, these large fatty droplets. And several hours after a meal, when your hormone insulin begins to drop inside of your body and other hormones such as glucagon begin to rise, they signal these adipose cells to release free fatty acids directly into the bloodstream, and recall that free fatty acids are very hydrophobic, so they kind of surf, essentially they kind of attach themselves onto albumin molecules, which is a special type of protein that's always found inside of our bloodstream. Now the third way that we can get some fat into our bloodstream is by synthesizing it directly inside of the liver, which I've kind of drawn an outline of here. Now the liver cells are especially equipped with the right type and number of enzymes to be able to convert excess glucose, that is the glucose that's not being used for ATP synthesis or glycogen synthesis, into fatty acids. Then, like the small intestine, the liver essentially packages these fatty acids into triacylglyceride molecules and packages them together with cholesterol, another hydrophobic molecule, into another specialized protein carrier molecule like chylomicrons, but this one has a slightly different name. It's called very low density lipoprotein, or VLDL for short. And this of course is sent off to the bloodstream, where it will eventually reach capillary beds and be taken up by surrounding tissues, even perhaps adipose cells, which might store it up for later use. So now that we've gone over this overview, I want to zoom in on one of these steps. I want to zoom in on this step here, going from glucose to fatty acids inside of the liver, which is commonly referred to simply as fatty acid synthesis. And to do this, I want to go ahead and kind of just zoom in on one single cell inside of the liver to visualize what's going on at the cellular level to be able to allow us to convert glucose into a fatty acid. And so I'm gonna go ahead and scroll the screen here so we can have some more room. All right, so I'm gonna quickly draw an outline of a representative cell, and then we'll go ahead and quickly label some important compartments that we want to talk about. So the first one is simply the cytoplasm, and there's a lot going on in the cytoplasm. But we also need to talk about what's going on in another organelle inside the cell, and that organelle is the mitochondria, and I'm gonna go ahead and draw the kind of two membranes that it has here. We're not gonna talk about this too much, but just because it is important to remember that this has an inner membrane and an outer membrane. Remember that the electron transport chain is of course located on the inner membrane, and the mitochondria is also within it. It's the site of the Krebs cycle, which continues, notably, to break down glucose following glycolysis, which takes place inside of the cytoplasm. Now since we ultimately want to get down to how extra glucose can be eventually converted into fatty acids, we need to actually make sure and remind ourselves how the breakdown of glucose proceeds. So as a very, very quick review, recall that glucose enters our cells from the bloodstream and it enters the metabolic pathway called glycolysis, which takes place inside the cytoplasm. And the end product of glycolysis is pyruvate, and I'll also remind you that for every one molecule of glucose, which is a six-carbon molecule, so one, two, three, four, five, six, we form two molecules of pyruvate, which is a three-carbon compound. Subsequently, pyruvate is actively transported across the mitochondrial membrane by specialized carrier proteins located on the membrane, and once pyruvate reaches the inside of the mitochondria, also known as the inner mitochondrial matrix, there is a enzyme that's only found in the mitochondria called pyruvate dehydrogenase, often abbreviated as PDH, which oxidizes and removes one carbon from pyruvate. So remember, we had three carbons, and now it turns it into a two-carbon molecule called acetyl-CoA. Now you might recall that this two-carbon structure is not done being broken down or oxidized. There's still some energy that we can extract from this two-carbon molecule, and it's extracted inside of the Krebs cycle. So remember that there are many, many intermediates along the Krebs cycle, but I only want to mention a couple that will be relevant when we talk about how this breakdown of glucose converges with the synthesis of fatty acids. So remember first off that a four-carbon molecule called oxaloacetoacetate, which I'm abbreviating here as OAA, combines with one molecule of acetyl-CoA to produce a sixth carbon molecule now called citrate. And citrate continues to be modified, oxidized, and even broken down a little bit more, and it returns to form oxaloacetate, which means that we lose two carbons somewhere along this cycle, which we do indeed. We lose these as two molecules of carbon dioxide, so those two carbons of acetyl-CoA exit as carbon dioxide, and we also form a number of reduced electron carrier molecules called NADH and FADH2, which shuttle their electrons from the oxidation process that occurs in the Krebs cycle to the electron transport chain, which is located on this inner mitochondrial membrane. How convenient, right? And then from there, we can produce ATP using oxidated phosphorylation. All right, so after that quick whirlwind tour of the breakdown of glucose, you might be wondering where do we convert glucose into fatty acids? And it turns out that one of the intermediates of the breakdown of glucose, which is acetyl-CoA, this two carbon molecule located in the mitochondrial inner matrix, is a precursor for fatty acid synthesis, and we're gonna go through all of the steps, but just to take a step back for a moment, the big picture way that I kind of like to think about this is that remember that fatty acids, I'm gonna go ahead and draw off to the side here, remember that most of it is just a repeating carbon hydrogen backbone shown here in this kind of line stick model here. And so in that sense, really we want to basically be able to link together carbon-carbon bonds, and this acetyl-CoA is just a pair of carbon-carbon bonds that we can ultimately link together. Now it turns out that we have an interesting situation when it comes to fatty acid synthesis and linking all of these acetyl-CoA molecules together, which is that all of the enzymes necessary for fatty acid synthesis, I'm gonna say enzymes for fatty acid synthesis, are located in the cytoplasm. And that's a bit problematic, because remember our acetyl-CoA molecule is in the mitochondria. Now your first thought might be, well, pyruvate was able to shuttle across using some protein career molecules in these membranes into the mitochondria. Why can't acetyl-CoA do the same going the opposite direction? Unfortunately, for some reason or the other, our body has evolved not to have any means to be able to transport this through its mitochondrial membrane. There are no protein transporters or carrier molecules like we had for pyruvate to be able to essentially shuttle acetyl-CoA in either direction across this mitochondrial membrane. But notably, our body does have a protein shuttle across this mitochondrial membrane for the molecule citrate, and remember that citrate contains acetyl-CoA. Of course, it also contains this molecule acetylacetate that combines with it. And so let's see what happens when this shuttles across the mitochondrial membrane. Now once citrate reaches the cytoplasm, it turns out that there is an enzyme within the cytoplasm that is able to break citrate up back into oxaloacetate, as well as the molecule that we're interested in, which is of course acetyl-CoA. Now when I first learned about this, it kind of struck me as a really roundabout way to kind of accomplish what seems like a pretty simple task, right, which is to get acetyl-CoA into the cytoplasm where the enzymes or fatty acid synthesis can link it together to form a fatty acid. But it turns out that there might be a benefit for this citrate shuttle to make fatty acid synthesis perhaps more efficient, and so I want to briefly talk about that, but I want to erase this just to give us some more room. Now it turns out that this four-carbon molecule, oxaloacetate, is not going to be used for fatty acid synthesis. And so naturally our body says why don't we recycle it? And in fact, we do have some enzymes that can convert it back to this molecule pyruvate, and notice that pyruvate can essentially, once it goes back to the mitochondria, it'll be turned into acetyl-CoA and this entire cycle can continue. Now although we're not gonna go over the detailed mechanism by which oxaloacetate is converted to pyruvate, what is important, kind of a big picture idea to note here, is that we're going from a four-carbon to three-carbon molecule, and so we're gonna lose a carbon, actually, as carbon dioxide during this process. And simultaneous with this step, we're actually oxidizing that particular intermediate. And so it's reduced to NADPH, and you may recall that you've seen NADPH also as a product of the pentose phosphate pathway, and of course we normally think about the pentose phosphate pathway as being the major pathway for the production of NADPH, but this step also allows us to produce a molecule of NADPH as well. Now one of the uses of NADPH that you might recall is that because it's associated with these electrons, it can serve as a source of reducing power to help with anabolic reactions. And remember that anabolic reactions are anything that involve building up a molecule, including fatty acid synthesis, which is exactly what we're trying to accomplish here. So to summarize and just kind of tie up everything that we've just talked about here, we've been able to get acetyl-CoA into the cytoplasm where all of the enzymes necessary for fatty acid synthesis are located. And this is important because we're gonna use acetyl-CoA, multiple acetyl-CoA, kind of a precursor molecule, so to say, to build up a fatty acid. And of course because this is an anabolic reaction, we're going to need some ATP somewhere along the way, and we're also going to need some reducing power to kind of help form all of those carbon-carbon bonds, and we can get that using NADPH. Scroll through the animations on this page to learn about what happens to fat, why our body requires it, and what our body does with it. The relative contributions of glucose and fatty acids to energy production in the body change over a hour period with meal intake: fatty acids contribute to overnight whereas glucose during the day or with food ingestion. The animations below should be viewed in the order in which they appear for best understanding. Please view the glossary at the bottom of this page for definition of relevant biochemical terms. The major fuel store of the body is triglyceride or TAG in adipose tissue. Glycogen in liver and muscle is more of a short-term store of carbohydrates. From the above animations, we can see how these molecules play an interconnected role to provide energy or be stored at different times. But during metabolic diseases like diabetes or obesity these processes do not occur optimally. An example is formation of triglycerides from fatty acids and glycerol. FATTY ACIDS: are building blocks of lipid molecules such as fats. They can be obtained both through diet or breakdown of stored fats in the body. They are insoluble in water and therefore transported in complex particles called lipoproteins. The excess fatty acids and cholesterol in the liver are converted to their respective esters and packaged with proteins into VLDL. Keith N. Metabolic Regulation: A Human Perspective. Hoboken: John Wiley and Sons, Inc. Denise R. Lippincott Illustrated Reviews: Biochemistry. Philadelphia: Wolters Kluwer. Liangyou Rui. |
| Biochemistry 10: lipid metabolism | Inside our bodies these molecules get broken down into smaller components, rearranged, stored especially after a meal , released from these stores between meals or during a fast and further metabolized. Scroll through the animations on this page to learn about what happens to fat, why our body requires it, and what our body does with it. The relative contributions of glucose and fatty acids to energy production in the body change over a hour period with meal intake: fatty acids contribute to overnight whereas glucose during the day or with food ingestion. The animations below should be viewed in the order in which they appear for best understanding. Please view the glossary at the bottom of this page for definition of relevant biochemical terms. The major fuel store of the body is triglyceride or TAG in adipose tissue. Glycogen in liver and muscle is more of a short-term store of carbohydrates. From the above animations, we can see how these molecules play an interconnected role to provide energy or be stored at different times. But during metabolic diseases like diabetes or obesity these processes do not occur optimally. An example is formation of triglycerides from fatty acids and glycerol. FATTY ACIDS: are building blocks of lipid molecules such as fats. They can be obtained both through diet or breakdown of stored fats in the body. They are insoluble in water and therefore transported in complex particles called lipoproteins. When glucose is limited, ketone bodies can be oxidized to produce acetyl CoA to be used in the Krebs cycle to generate energy. When glucose levels are plentiful, the excess acetyl CoA generated by glycolysis can be converted into fatty acids, triglycerides, cholesterol, steroids, and bile salts. This process, called lipogenesis , creates lipids fat from the acetyl CoA and takes place in the cytoplasm of adipocytes fat cells and hepatocytes liver cells. When you eat more glucose or carbohydrates than your body needs, your system uses acetyl CoA to turn the excess into fat. Although there are several metabolic sources of acetyl CoA, it is most commonly derived from glycolysis. Acetyl CoA availability is significant, because it initiates lipogenesis. Lipogenesis begins with acetyl CoA and advances by the subsequent addition of two carbon atoms from another acetyl CoA; this process is repeated until fatty acids are the appropriate length. Because this is a bond-creating anabolic process, ATP is consumed. However, the creation of triglycerides and lipids is an efficient way of storing the energy available in carbohydrates. Triglycerides and lipids, high-energy molecules, are stored in adipose tissue until they are needed. Although lipogenesis occurs in the cytoplasm, the necessary acetyl CoA is created in the mitochondria and cannot be transported across the mitochondrial membrane. To solve this problem, pyruvate is converted into both oxaloacetate and acetyl CoA. Two different enzymes are required for these conversions. Oxaloacetate forms via the action of pyruvate carboxylase, whereas the action of pyruvate dehydrogenase creates acetyl CoA. Oxaloacetate and acetyl CoA combine to form citrate, which can cross the mitochondrial membrane and enter the cytoplasm. In the cytoplasm, citrate is converted back into oxaloacetate and acetyl CoA. Oxaloacetate is converted into malate and then into pyruvate. Pyruvate crosses back across the mitochondrial membrane to wait for the next cycle of lipogenesis. The acetyl CoA is converted into malonyl CoA that is used to synthesize fatty acids. Figure 6 summarizes the pathways of lipid metabolism. Figure 6. Lipids may follow one of several pathways during metabolism. Glycerol and fatty acids follow different pathways. Lipids are available to the body from three sources. They can be ingested in the diet, stored in the adipose tissue of the body, or synthesized in the liver. Fats ingested in the diet are digested in the small intestine. The triglycerides are broken down into monoglycerides and free fatty acids, then imported across the intestinal mucosa. Once across, the triglycerides are resynthesized and transported to the liver or adipose tissue. Fatty acids are oxidized through fatty acid or β-oxidation into two-carbon acetyl CoA molecules, which can then enter the Krebs cycle to generate ATP. If excess acetyl CoA is created and overloads the capacity of the Krebs cycle, the acetyl CoA can be used to synthesize ketone bodies. When glucose is limited, ketone bodies can be oxidized and used for fuel. Excess acetyl CoA generated from excess glucose or carbohydrate ingestion can be used for fatty acid synthesis or lipogenesis. Acetyl CoA is used to create lipids, triglycerides, steroid hormones, cholesterol, and bile salts. Lipolysis is the breakdown of triglycerides into glycerol and fatty acids, making them easier for the body to process. bile salts: salts that are released from the liver in response to lipid ingestion and surround the insoluble triglycerides to aid in their conversion to monoglycerides and free fatty acids. cholecystokinin CCK : hormone that stimulates the release of pancreatic lipase and the contraction of the gallbladder to release bile salts. chylomicrons: vesicles containing cholesterol and triglycerides that transport lipids out of the intestinal cells and into the lymphatic and circulatory systems. fatty acid oxidation: breakdown of fatty acids into smaller chain fatty acids and acetyl CoA. this has obvious advantages allowing different tissues to deal with contents of these lipoproteins more appropriately. Is pyruvate dehydrogenase embedded within the membrane of the mitochondria, or found in the mitochondrial matrix. If it is found in the mitochondrial matrix, how does it enter the mitochondria? Neda Salami. Is the liver the only organ where fatty acid synthesis can occur? In the mitochondria, is pyruvate cleaved into acetyl CoA and acetyl CoA combines with free OAA to form citrate, or is pyruvate cleaved into both acetyl CoA and OAA? I have learned the latter in classes but i want to double check. Trey Stai. Pyruvate actually combines with OAA to make citrate. At the end of the cycle, OAA is regenerated after a couple of carbons have been lost as CO2. Video transcript - [Instructor] Now the ultimate goal in fat metabolism is to be able to deliver some triacylglycerides, which I'm gonna abbreviate here at TAG, which remember is the chemical name for a fat molecule, or free fatty acids, which I'll abbreviate here at FFA, which if you recall are the kind of monomer subunits of these fat molecules directly into the bloodstream where they can eventually reach capillary beds like the one that I've drawn here. So I'll go ahead and label this as a capillary bed, and it's important that they reach these capillary beds because it's at this point where they can diffuse to surrounding tissues such as muscle or heart tissue, for example, where they can be taken up by these tissues and oxidized to obtain cellular energy in the form of ATP. Now I want to remind you that there are three main sources of these triacylglycerides or free fatty acids that can enter the bloodstream, and so I'm gonna go ahead and scroll up here and show you kind of what I've already drawn out here and go ahead and explain it. Starting off here on the far left I've drawn a cheeseburger, perhaps not the best drawing in the world, but just to remind us that one of our sources of fat that ultimately reaches our bloodstream is directly from our diet. So recall that our small intestine digests our food and packages the fat molecules, the triacylglycerides, into protein carrier molecules called chylomicrons, which travel through the lymphatic circulation but eventually empty into our bloodstream where they can enter capillary beds. Now the second way that we can get some fat into our bloodstream is directly from adipose cells. So recall that adipose cells are specialized cells inside of our body that can store large amounts of fat, and so that's kind of what I've drawn here in these yellow circles within these cells, these large fatty droplets. And several hours after a meal, when your hormone insulin begins to drop inside of your body and other hormones such as glucagon begin to rise, they signal these adipose cells to release free fatty acids directly into the bloodstream, and recall that free fatty acids are very hydrophobic, so they kind of surf, essentially they kind of attach themselves onto albumin molecules, which is a special type of protein that's always found inside of our bloodstream. Now the third way that we can get some fat into our bloodstream is by synthesizing it directly inside of the liver, which I've kind of drawn an outline of here. Now the liver cells are especially equipped with the right type and number of enzymes to be able to convert excess glucose, that is the glucose that's not being used for ATP synthesis or glycogen synthesis, into fatty acids. Then, like the small intestine, the liver essentially packages these fatty acids into triacylglyceride molecules and packages them together with cholesterol, another hydrophobic molecule, into another specialized protein carrier molecule like chylomicrons, but this one has a slightly different name. It's called very low density lipoprotein, or VLDL for short. And this of course is sent off to the bloodstream, where it will eventually reach capillary beds and be taken up by surrounding tissues, even perhaps adipose cells, which might store it up for later use. So now that we've gone over this overview, I want to zoom in on one of these steps. I want to zoom in on this step here, going from glucose to fatty acids inside of the liver, which is commonly referred to simply as fatty acid synthesis. And to do this, I want to go ahead and kind of just zoom in on one single cell inside of the liver to visualize what's going on at the cellular level to be able to allow us to convert glucose into a fatty acid. And so I'm gonna go ahead and scroll the screen here so we can have some more room. All right, so I'm gonna quickly draw an outline of a representative cell, and then we'll go ahead and quickly label some important compartments that we want to talk about. So the first one is simply the cytoplasm, and there's a lot going on in the cytoplasm. But we also need to talk about what's going on in another organelle inside the cell, and that organelle is the mitochondria, and I'm gonna go ahead and draw the kind of two membranes that it has here. We're not gonna talk about this too much, but just because it is important to remember that this has an inner membrane and an outer membrane. Remember that the electron transport chain is of course located on the inner membrane, and the mitochondria is also within it. It's the site of the Krebs cycle, which continues, notably, to break down glucose following glycolysis, which takes place inside of the cytoplasm. Now since we ultimately want to get down to how extra glucose can be eventually converted into fatty acids, we need to actually make sure and remind ourselves how the breakdown of glucose proceeds. So as a very, very quick review, recall that glucose enters our cells from the bloodstream and it enters the metabolic pathway called glycolysis, which takes place inside the cytoplasm. And the end product of glycolysis is pyruvate, and I'll also remind you that for every one molecule of glucose, which is a six-carbon molecule, so one, two, three, four, five, six, we form two molecules of pyruvate, which is a three-carbon compound. Subsequently, pyruvate is actively transported across the mitochondrial membrane by specialized carrier proteins located on the membrane, and once pyruvate reaches the inside of the mitochondria, also known as the inner mitochondrial matrix, there is a enzyme that's only found in the mitochondria called pyruvate dehydrogenase, often abbreviated as PDH, which oxidizes and removes one carbon from pyruvate. So remember, we had three carbons, and now it turns it into a two-carbon molecule called acetyl-CoA. Now you might recall that this two-carbon structure is not done being broken down or oxidized. There's still some energy that we can extract from this two-carbon molecule, and it's extracted inside of the Krebs cycle. So remember that there are many, many intermediates along the Krebs cycle, but I only want to mention a couple that will be relevant when we talk about how this breakdown of glucose converges with the synthesis of fatty acids. So remember first off that a four-carbon molecule called oxaloacetoacetate, which I'm abbreviating here as OAA, combines with one molecule of acetyl-CoA to produce a sixth carbon molecule now called citrate. And citrate continues to be modified, oxidized, and even broken down a little bit more, and it returns to form oxaloacetate, which means that we lose two carbons somewhere along this cycle, which we do indeed. |
| Want to join the conversation? | VLDL stands for 'Very Biocheimstry Density Metabolim. Intermittent fasting for beginners perform several Cognitive development programs functions, including biochemitry biological membranes, Intermittent fasting for beginners storage of energy, biohemistry as components of several important structural and functional molecules. com, Inc. Flag Button navigates to signup page. Unlike free fatty acids, ketones can cross the blood—brain barrier and are therefore available as fuel for the cells of the central nervous systemacting as a substitute for glucose, on which these cells normally survive. Annual Review of Entomology. |
| Lipid Metabolism | AccessEmergency Medicine. Case Files Collection. Clinical Sports Medicine Collection. Davis AT Collection. Davis PT Collection. Murtagh Collection. MY PROFILE. Access Sign In Username. Sign In. Create a Free Access Profile Forgot Password? Forgot Username? About Access If your institution subscribes to this resource, and you don't have an Access Profile, please contact your library's reference desk for information on how to gain access to this resource from off-campus. Learn More. Sign in via OpenAthens Sign in via Shibboleth. We have a new app! Close Promo Banner. Keyword Title Author ISBN Select Site. Autosuggest Results Please Enter a Search Term. About Search. Enable Autosuggest. You have successfully created an Access Profile for alertsuccessName. Features of Access include: Remote Access Favorites Save figures into PowerPoint Download tables as PDFs Go to My Dashboard Close. Home Books The Big Picture: Medical Biochemistry. Previous Chapter. Next Chapter. Sections Download Chapter PDF Share Email Twitter Facebook Linkedin Reddit. In: Janson LW, Tischler ME. Janson L. Lee W. Janson, and Marc E. The Big Picture: Medical Biochemistry. McGraw-Hill Education; Accessed February 14, APA Citation Lipid metabolism. Janson LW, Tischler ME. McGraw-Hill Education. Download citation file: RIS Zotero. Reference Manager. Chemicals from the pancreas pancreatic lipase family and bile salt-dependent lipase are secreted into the small intestines to help breakdown the triglycerides, [10] along with further mechanical digestion, until they are individual fatty acid units able to be absorbed into the small intestine's epithelial cells. The second step in lipid metabolism is absorption of fats. Short chain fatty acids can be absorbed in the stomach , while most absorption of fats occurs only in the small intestines. Once the triglycerides are broken down into individual fatty acids and glycerols , along with cholesterol, they will aggregate into structures called micelles. Fatty acids and monoglycerides leave the micelles and diffuse across the membrane to enter the intestinal epithelial cells. In the cytosol of epithelial cells, fatty acids and monoglycerides are recombined back into triglycerides. In the cytosol of epithelial cells, triglycerides and cholesterol are packaged into bigger particles called chylomicrons which are amphipathic structures that transport digested lipids. Due to the hydrophobic nature of membrane lipids , triglycerides and cholesterols , they require special transport proteins known as lipoproteins. Chylomicrons are one sub-group of lipoproteins which carry the digested lipids from small intestine to the rest of the body. The varying densities between the types of lipoproteins are characteristic to what type of fats they transport. Lipids are stored in white adipose tissue as triglycerides. In a lean young adult human, the mass of triglycerides stored represents about 10—20 kilograms. Triglycerides are formed from a backbone of glycerol with three fatty acids. Free fatty acids are activated into acyl-CoA and esterified to finally reach the triglyceride droplet. Lipoprotein lipase has an important role. Once the chylomicrons or other lipoproteins travel through the tissues, these particles will be broken down by lipoprotein lipase in the luminal surface of endothelial cells in capillaries to release triglycerides. In the cytosol of the cell for example a muscle cell , the glycerol will be converted to glyceraldehyde 3-phosphate , which is an intermediate in the glycolysis , to get further oxidized and produce energy. However, the main steps of fatty acids catabolism occur in the mitochondria. The main products of the beta oxidation pathway are acetyl-CoA which is used in the citric acid cycle to produce energy , NADH and FADH. The overall net reaction, using palmitoyl-CoA as a model substrate is:. In addition to dietary fats, storage lipids stored in the adipose tissues are one of the main sources of energy for living organisms. There are two major classes of membrane lipids: glycerophospholipids and sphingolipids. Although many different membrane lipids are synthesized in our body, pathways share the same pattern. The first step is synthesizing the backbone sphingosine or glycerol , the second step is the addition of fatty acids to the backbone to make phosphatidic acid. Phosphatidic acid is further modified with the attachment of different hydrophilic head groups to the backbone. Membrane lipid biosynthesis occurs in the endoplasmic reticulum membrane. The phosphatidic acid is also a precursor for triglyceride biosynthesis. Phosphatidic acid phosphotase catalyzes the conversion of phosphatidic acid to diacylglyceride, which will be converted to triglycerides by acyltransferase. Triglyceride biosynthesis occurs in the cytosol. The precursor for fatty acids is acetyl-CoA and it occurs in the cytosol of the cell. Cholesterol can be made from acetyl-CoA through a multiple-step pathway known as isoprenoid pathway. Cholesterols are essential because they can be modified to form different hormones in the body such as progesterone. Lipid metabolism disorders including inborn errors of lipid metabolism are illnesses where trouble occurs in breaking down or synthesizing fats or fat-like substances. National Library of Medicine Medical Subject Headings MeSH. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Biological synthesis and degradation of lipids. Merck Manuals Professional Edition. Retrieved Molecular biology 2nd ed. Boston: Jones and Bartlett. ISBN Medical Biochemistry. Saunders, Elsevier Limited. Annual Review of Entomology. doi : PMC PMID Lehninger Principles of Biochemistry 3rd ed. New York: Worth Publishers. Virtual Chembook. Elmhurst College. The New Phytologist. JSTOR ? International Journal of Endocrinology. Elsevier's Integrated Review Biochemistry 2nd ed. Fundamentals of Biochemistry: Life at the Molecular Level Fourth ed. Hoboken, NJ: Wiley. OCLC Cholesterol binding and cholesterol transport proteins: structure and function in health and disease. Dordrecht: Springer. In De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R eds. South Dartmouth MA : MDText. com, Inc. Archived from the original on Mitochondria 2nd ed. Hoboken, N. Frontiers in Endocrinology. Sphingolipids as Signaling and Regulatory Molecules. Advances in Experimental Medicine and Biology. Chemistry and Physics of Lipids. Clinical Pharmacology and Drug treatment in the elderly. Edinburgh; New York: Churchil Livingstone. Merck Manuals Consumer Version. Molecular Biology of the Cell 4th ed. Garland Science. Current Opinion in Cell Biology. Annual Review of Biochemistry. The Journal of Pathology. S2CID Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway. Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport. Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway. Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. |
0 thoughts on “Fat metabolism biochemistry”