
Diabetic nephropatthy DN is the leading nephropathu of Diabtic stage renal disease. Therefore, the Blueberry snack ideas of renal function and early diagnosis Diabetic nephropathy biomarkers glomerular Oats and healthy snacking tubular injuries is nephropathg important measure in the biomarkerz of type Balanced caloric intake and type 2 nephropzthy patients.
The diagnosis of Nephropsthy for biomakrers has been biiomarkers determined by nephropatgy presence of nwphropathy MA. Biojarkers studies nephropathyy showed that presence of Natural ingredients fat blocker may be transient and does Gaining lean muscle necessarily reflect nwphropathy kidney damage.
There could also be glomerulo-tubular damage in diabetic patient without presenting biomsrkers albuminuria. Decline in the renal function, cellular and extracellular derangements in both the glomerulus and tubules Diabeetic been associated biomarkrrs array of biological markers which could be of help in the diagnosis, prognosis, and the overall management nephfopathy the affected patients.
Identifying more biomarkers nwphropathy both research and clinical Mindfulness techniques for blood pressure control to neohropathy and Diabeti progression nephropthy kidney damage in Improve insulin sensitivity through exercise is necessary.
This is nephropathg Blueberry snack ideas of subscription content, log Diabetic nephropathy biomarkers via an Distinguished. Adler SG, Biomarmers S, Striker L, et al. Bikmarkers type IV collagen in patients with Blueberry snack ideas nephropathy with nephrkpathy without additional glomerular disease.
Nephropaty Int. Bomarkers Google Scholar. Adler AI, Diabetic nephropathy biomarkers Biomariers, Manley SE, et Blueberry snack ideas. Development and Dianetic of Blueberry snack ideas in type Diaabetic diabetes: the Gut health and autoimmune diseases Kingdom prospective diabetes study UKPDS Google Blueberry snack ideas.
Agnieszka Ż, Diabetic nephropathy biomarkers, Agnieszka G, Farm-fresh sunflower seeds R, nephtopathy al. Role of new biomarkers for the diagnosis nephropthy nephropathy nephropwthy with diabetes type 2. Folia Med Cracov.
Blueberry snack ideas BS, Adesokan Ne;hropathy. Antidiabetic effects of Tetracarpidium conophorum biiomarkers on biomarksrs of diabetes-induced nephropathy in rats.
Asian Pac J Bilmarkers Biomed. Ajilore Nephropathj, Olorunnisola OS, Nephropathyy AO. Tetracarpidium conophorum seed Alternate-day fasting and longevity improves markers Diabetci diabetic bioamrkers progression in Streptozotocin-induced diabetic Diabetic nephropathy biomarkers.
Biomarekrs K, Siddiqui K, Al-Ghonaim MA, et al. Assessment of the diagnostic value of different biomarkers in relation to various stages of diabetic nephropathy in type 2 diabetic patients. Sci Rep. Article CAS Google Scholar. Alter ML, Ott IM, von Websky K, et al.
DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res. Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms.
Cardiovasc Diabetol. Bai S, Zeng R, Zhou Q, et al. Int J Biol Sci. Banu N, Hara H, Okamura M, et al. Diabetes Res Clin. Barutta F, Bruno G, Matullo G, et al. Acta Diabetol. Bock F, Shahzad K, Wang H, et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc.
Proc Natl Acad Sci. Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. Kidney injury molecule a translational journey.
Trans Am Clin Climatol Assoc. Campion CG, Sanchez-Ferras O, Batchu SN. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. Cao YL, Duan Y, Zhu LX, et al.
TGF-b1, in association with the increased expression of connective tissue growth factor, induce the hypertrophy of the ligamentum flavum through the p38 MAPK pathway.
Int J Mol Med. Cohen-Bucay A, Viswanathan G. Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephrol. Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease.
Conserva F, Gesualdo L, Papale M. A systems biology overview on human diabetic nephropathy: from genetic susceptibility to post-transcriptional and posttranslational modifications. J Diabetes Res.
Davani D, Kumar S, Palaia T, et al. Lipocalin-type prostaglandin D2 synthase reduces glucagon secretion in alpha TC-1 clone 6 cells via the DP1 receptor.
Biochem Biophys Rep. Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury.
Biomark Med. Eissa S, Matboli M, Aboushahba R, et al. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complicat. Fagerudd JA, Groop PH, Honkanen E, et al. Urinary excretion of TGF-β1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients.
Fang D, Wan X, Deng W, et al. Fufang Xue Shuan Tong capsules inhibit renal oxidative stress markers and indices of nephropathy in diabetic rats. Exp Ther Med. Fiseha T.
Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomarker Res. Gluhovschi C, Gluhovschi G, Petrica L, et al. Urinary biomarkers in the assessment of early diabetic nephropathy. Gohda T, Walker WH, Wolkow P, et al. Elevated urinary excretion of immunoglobulins in nonproteinuric patients with type 1 diabetes.
Am J Physiol Renal Physiol. Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci Lond. Ha H, Lee HB. Oxidative stress in diabetic nephropathy: basic and clinical information.
Curr Diab Rep. Hara M, Yamagata K, Tomino Y, et al. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin.
Hinokio Y, Suzuki S, Hirai M, et al. Urinary excretion of 8-oxo-7,8-dihydrodeoxyguanosine as a predictor of the development of diabetic nephropathy. Inoue K, Wada J, Eguchi J, et al. Urinary fetuin — a is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray.
PLoS One. Ito H, Fujita H, Takahashi T. Diagnostic biomarkers of diabetic nephropathy. Expert Opin Med Diagn.
: Diabetic nephropathy biomarkers| New urinary biomarkers for diabetic kidney disease | Biomarker Research | Full Text | In this review, we will discuss the latest findings in the use of genetic, protein, and metabolic markers of DN. Particular attention will be paid to the urinary biomarker as a noninvasive method to detect either morphological or biochemical changes in DN. Urinary protein and mRNA studies have focused on either the glomerular podocyte-specific or tubular components matrix or injury-related of the nephron. The virtues and pitfalls of using the podocyte as a biomarker will be discussed. The systems biology approach of biomarker discovery in the studies of genomics, transcriptomics, proteomics, and metabolomics will be explored. Despite significant numbers of new biomarkers described, most studies are limited by either their small sample size or their cross-sectional nature, so that the ability to predict future and severity of DN is lacking. In order to successfully search for the ideal, validated biomarker, we need to conduct large, prospective, multi-center trials enlisting both Type 1 and Type II diabetic patients with and without nephropathy for at least two decades. Biomarkers of diabetic nephropathy, the present and the future. The results demonstrate that IBS combined with a proper statistical analysis technique is a powerful tool for biomarker screening. Huang, Q. Liang, P. Li, J. Xia, Y. Wang, P. Hu, Z. Jiang, Y. He, L. Pang, L. Han, Y. Wang and G. Luo, Mol. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef. This may take some time to load. Loading related content. Jump to main content. Jump to site search. You do not have JavaScript enabled. Please enable JavaScript to access the full features of the site or access our non-JavaScript page. Issue 8, From the journal: Molecular BioSystems. You have access to this article. Please wait while we load your content Something went wrong. Try again? Cited by. Download options Please wait Supplementary information PDF K. Article type Paper. Submitted 28 Nov Accepted 26 Apr First published 26 Apr |
| Biomarkers of Diabetes-Induced Nephropathy | SpringerLink | Therefore, earlier, more sensitive and specific biomarkers with greater predictability are needed. The aim of this review is to summarize new urinary biomarkers for glomerular injury associated with DKD. Transferrin, a plasma protein, is very similar to albumin in weight. It is more readily filtered through glomerular barrier than albumin for being less anionic. Urinary transferrin is considered to be a more sensitive marker of glomerular damage in diabetic patients based on theory analysis and experimental results. Urinary transferrin excretion shows a good linear relationship with urinary albumin excretion in diabetic patients, and increased urinary transferrin excretion predicts the development of microalbuminuria in type 2 diabetic patients with normoalbuminuria [ 2 ]. A systemic review, including 13 studies, indicated that urinary transferrin excretion was a good marker for predicting onset of nephropathy [ 3 ]. However, urinary transferrin excretion is not specific for DKD because its elevation can be found in primary glomerulonephritis [ 4 ]. Immunoglobulin G IgG is a protein synthesized and secreted by plasma cells. It has a molecular weight of kDa, which is larger than albumin. Urinary IgG excretion is higher in diabetic patients compared to healthy controls, and its excretion in diabetic patients with normoalbuminuria predicts the development of microalbuminuria [ 5 ]. Urinary IgG excretion correlates with the progression of glomerular diffuse lesions. One IgG isoform IgG4 has been used more specifically as a marker of glomerular charge selectivity impairment. Only IgG4 excretion is elevated in patients with microalbuminuria, while the excretion of both IgG and IgG4 are increased in patients with macroalbuminuria compared with normoalbuminuric patients [ 6 ]. Recently, one study found that urinary concentration of IgG2 in patients with normoalbuminuria was significantly higher than in healthy control, whereas further elevation of IgG2, IgG4, and IgA was more pronounced in patients with microalbuminuria. Fractional excretion of IgG2 was the highest among all immunogloubins, which indicated that elevation of those particular immunogloubin subtypes was a contribution of novel mechanisms in early DKD, different from charge and size barrier impairment [ 7 ]. One systemic review, including 13 studies, indicated urinay IgG was a good marker for predicting onset of nephropathy [ 3 ]. Immunoglobulin M IgM , secreted by plasma cells, is the largest antibody in the human. Due to its large molecular radius, the appearance of IgM in urine indicates that a large, nonselective pore exists in the glomerular capillary wall. One study showed that urine excretion of IgM was significantly higher in type 2 DM compared to type 1 DM, and patients with type 2 DM with nephrosclerosis had significantly higher urine excretion of IgM compared to the age-matched healthy subjects [ 8 ]. Another study found renal survival of type 2 diabetic patients was inversely associated with urine IgM excretion, which indicated that higher urinary IgM excretion was a better predictor of decline in kidney function than albuminuria in type 2 DM. However, urinary IgM excretion has not been regarded as an early marker of DKD, since its excretion in urine is associated with severe injury of the glomerular capillary wall, while it is also a promising marker which may predict the eventual need for renal replacement therapy [ 9 ]. Cystatin C, a cysteine protease inhibitor, is a novel biomarker of renal damage. Serum Cystatin c is a good marker for assessing renal injuries, while urinary cystatin c was considered as a useful marker for the detection of DKD. One study from Zucker diabetic fatty ZDF rats indicated that urinary cystatin C was increased in ZDF rats where renal damage was not observed by histopathological assessment, and its levels in urine increased with the progression of renal damage, demonstrating the usefulness of early detection and accurate assessment of DKD [ 10 ]. Another study from type 2 diabetic patients found that urinary cystatin C increased with increasing degree of albuminuria and reached higher levels in macroalbuminuric patients. Podocytes are key structural elements of the glomerular filtration barrier. Monitoring urine podocytes and podocyte-specific proteins can reveal potentially interesting urinary markers for the early diagnosis of DKD [ 13 ]. Podocytes in urine can be found in diabetic patients with micro- and macroalbuminuria [ 14 ]. Urinary podocalyxin was higher in Another study found that urinary mRNA profiles of synaptopodin, podocalyxin, α-actin-4, and podocin were increased with the progression of DKD, which suggested that quantification of podocyte-associated molecules in urine will be a useful biomarker of DKD [ 17 ]. Type IV collagen is the main constituent of both glomerular and tubular basement membranes as well as mesangial matrix. Urinary type IV collagen was significantly increased in both normoalbuminuric and microalbuminuric patients of type 2 DM compared with healthy controls, and urinary type IV collagen significantly correlated with the amount of albuminuria [ 18 ]. Another study found that urinary type IV collagen was more sensitive than albuminuria to detect renal damage in type 2 diabetic patients. It is well known that increased oxidative stress in diabetes contributes to the progression of diabetes and its complications. Patients with higher excretion of 8-oxodG in urine compared with those patients with moderate or lower excretion of 8-oxodG showed significant progression of diabetic nephropathy, which indicates that 8-oxodG in urine is a useful clinical marker to predict the development of diabetic nephropathy [ 21 , 22 ]. Urinary ceruloplasmin was found in normoalbuminuric diabetic patients, and its increase in urine had a predictive value for development of microalbuminuria in normoalbuminuric diabetic patients [ 23 , 24 ]. All these data suggest that urinary ceruloplasmin is a promising marker of DKD, while further studies are needed to characterize its value compared to albuminuria, especially in type 1 diabetics, since all the studies have been done in type 2 diabetics. Chemokines have been implicated in the pathogenesis of DKD, therefore, measurement of cytokine in urine might help to diagnosis DKD. All these data suggested that urinary MCP-1 might be a prognostic marker for progression of diabetic nephropathy, while more studies are needed to investigate whether urinary MCP-1 has a role in the setting of normoalbuminuria and microalbuminuria in DKD. Neutrophil gelatinase-associated lipocalin NGAL has been evaluated in several studies of diabetic subjects. In one study, urine NGAL was 5—fold higher in normo- or microalbuminuric patients compared with healthy controls. Another study from short-term type 2 DM patients indicated urinary NGAL showed a negative correlation with eGFR, which suggested urinary NGAL might be a promising early marker for monitoring renal impairment in short-term T2DM patients [ 28 ]. Proteomics is a method aimed at discovering and identifying the complete set of proteins present in a given biological sample at a given time. Using a variant of two dimensional gel electrophoresis, they found urine samples from type 2 DM patients with microalbuminuria showed four main proteins accompanying with albumin: alpha-2 glycoprotein, alpha-1 acid glycoprotein, alpha-1 microglobulin and IgG [ 30 ]. Otu found a peak proteomic signature in the baseline urine of type 2 DM patients who subsequently developed DKD [ 31 ]. Zürbig used capillary electrophoresis-coupled mass spectrometry to profile the low-molecular weight proteome in urine samples from a longitudinal cohort of type 1 and 2 diabetic patients. They found collagen fragments were prominent biomarkers before onset of macroalbuminuria, and there is a decrease in collagen fragments before albumin excretion starts to increase [ 32 ]. Urinary proteomics enables noninvasive assessment of DN risk at an early stage, while more studies are needed to investigate the role of urinary proteomics in diabetic kidney disease. Recently, a study from uni-nephrectomized diabetic rats indicated urinary osteopontin, heart-type fatty acid binding protein appeared before the classical biomarkers of diabetic nephropathy, such as albuminuria and urinary protein excretion [ 33 ]. Study of males with Type 2 diabetes indicated human zinc-α 2 -glycoprotein might be a novel urinary biomarker for non-albuminuric diabetic nephropathy [ 34 ]. Another study suggested urinary mRNA levels of α-smooth muscle actin, fibronectin and matrix metalloproteinase-9 might be novel biomarkers of diabetic kidney disease [ 35 ]. McKittrick reported that urinary matrix metalloproteinase activity might be a sensitive, noninvasive, and clinically useful biomarker for predicting vascular remodeling in diabetic renal and vascular complications [ 36 ]. The above mentioned results are from small patient population and from animal experiments, which lead to limited use for clinical practice. We need larger perspective studies to confirm the utility of these biomarkers in diabetic kidney disease. The current gold standard for detection and prediction of DKD is microalbuminuria; however, it has several limitations, such as lower sensitive and larger variability. It is urgent to explore higher sensitivity and specificity for earlier detection of DKD and more accurate prediction of the progression to end stage renal disease. Despite numbers of new biomarkers described, most studies are limited by either their small sample size or their cross-sectional nature. We need large, prospective, multi-center trials enlisting both Type 1 and Type II diabetic patients with and without nephropathy for at least two decades to indentify there role in clinical practice. International Diabetes Federation: Diabetes Atlas Fifth Edition, International Diabets Federation. Belgium: Brussels; Google Scholar. Zhou Y, Zhang X, Wu J: Clinical significance of microtransferrinuria in diabetic patients. Zhonghua nei ke za zhi , — CAS PubMed Google Scholar. Hellemons ME, Kerschbaum J, Bakker SJ, et al. Diabet Med , — Article CAS PubMed Google Scholar. Mackinnon B, Shakerdi L, Deighan CJ, et al. Clin Nephrol , — Narita T, Hosoba M, Kakei M, et al. Diabetes Care , — Bangstad HJ, Kofoed-Enevoldsen A, Dahl-Jorgensen K, et al. Diabetologia , — Gohda T, Walker WH, Wolkow P, et al. Am J Physiol Renal Physiol , FF Article CAS PubMed Central PubMed Google Scholar. Bakoush O, Tencer J, Tapia J, et al. Kidney Int , — Tofik R, Torffvit O, Rippe B, Bakoush O: Increased urine IgM excretion predicts cardiovascular events in patients with type 1 diabetes nephropathy. BMC Med , 4: 39— Article CAS Google Scholar. Togashi Y, Miyamoto Y: Urinary cystatin C as a biomarker for diabetic nephropathy and its immunohistochemical localization in kidney in Zucker diabetic fatty ZDF rats. Exp Toxicol Pathol Jeon YK, Kim MR, Huh JE, et al. J Korean Med Sci , — Weil EJ, Lemley KV, Yee B, et al. Am J Nephrol , 33s1: 21— Article Google Scholar. Dalla Vestra M, Masiero A, Roiter AM, et al. Studies in patients with type 2 diabetes. Diabetes , — Nakamura T, Ushiyama C, Suzuki S, et al. Nephrol Dial Transplant , — Jim B, Ghanta M, Qipo A, et al. PLoS One , 7: e Hara M, Yamagata K, Tomino Y, et al. Zheng M, Lv LL, Ni J, et al. PLoS One , 6: e Sthaneshwar P, Chan SP: Urinary type IV collagen levels in diabetes mellitus. Malays J Pathol , 43— PubMed Google Scholar. Kotajima N, Kimura T, Kanda T, et al. J Diabetes Complications , 13— J Med Assoc Thail 93 Suppl 6 :S—S Google Scholar. Lassén E, Daehn IS Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction. Int J Mol Sci 21 24 Article PubMed PubMed Central Google Scholar. Gilbert RE Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes — Sakashita M, Tanaka T, Inagi R Metabolic changes and oxidative stress in diabetic kidney disease. Pichler R, Afkarian M, Dieter BP, Tuttle KR Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol-Renal Physiol 4 :F—F Molitch ME, Steffes M, Sun W et al Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care — Article CAS PubMed PubMed Central Google Scholar. Lin C-H, Chang Y-C, Chuang L-M Early detection of diabetic kidney disease: present limitations and future perspectives. World J Diabetes Hamaguchi K, Tsuchida H, Miura Y, Suzuki S, Kawamura T, Hosoya T, Yamada K Urinary type IV collagen excretion reflects renal morphological alterations and type IV collagen expression in patients with type 2 diabetes mellitus. Clin Nephrol 55 5 — PubMed Google Scholar. Tomino Y, Suzuki S, Azushima C, Shou I, Iijima T, Yagame M, Wang LN, Chen HC, Lai KN, Tan SY, Kim MJ Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy. J Clin Lab Anal 15 4 — Iijima T, Suzuki S, Sekizuka K, Hishiki T, Yagame M, Jinde K et al Follow-up study on urinary type IV collagen in patients with early stage diabetic nephropathy. J Clin Lab Anal 12 6 — Morita M, Uchigata Y, Hanai K et al Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes. Araki S, Haneda M, Koya D et al Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Hayashi Y, Makino H, Ota Z Serum and urinary concentrations of type IV collagen and laminin as a marker of microangiopathy in diabetes. Diabetic Med 9 4 — Ozata M, Kurt I, Azal O, Bolu E, Corakci A, Beyhan Z, Karaca L, Gündogăn MA Can we use plasma fibronectin levels as a marker for early diabetic nephropathy. Endocr J 42 2 — Takahashi M Increased urinary fibronectin excretion in type II diabetic patients with microalbuminuria. Nihon Jinzo Gakkai shi 37 6 — CAS PubMed Google Scholar. Fagerudd JA, Groop PH, Honkanen E, Teppo AM, Grönhagen-Riska C Urinary excretion of TGF-beta 1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney Int Suppl S—S Kanters SDJM, Banga J-D, Algra A et al Plasma levels of cellular fibronectin in diabetes. Setty S, Michael AA, Fish AJ et al Differential expression of laminin isoforms in diabetic nephropathy and other renal diseases. Mod Pathol — Diabetes Res Clin Pract — El-Fattah MEA, Rashed LA, Nasr SMM The role of transferrin and laminin biomarkers in the diagnosis of diabetic nephropathy in type II diabetic patients. J Adv Med Med Res — Okazaki R, Matsuokab K, Atsumib Y et al Serum concentrations of basement membrane proteins in NIDDM as a prognostic marker for nephropathy. Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 5 — Papadopoulou-Marketou N, Skevaki C, Kosteria I et al NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones — Qamar A, Hayat A, Ahmad TM, Khan A, Hasnat MNU, Tahir S Serum cystatin C as an early diagnostic biomarker of diabetic kidney disease in type 2 diabetic patients. J Coll Physicians Surg Pak 28 4 — Macisaac RJ, Ekinci EI, Jerums G Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. Qian C, Wan GM, Yan PS, Wang WZ, Liang SZ, Dong Y Correlation between Cystatin C and retinopathy of type-two diabetes mellitus patients. J Biol Regul Homeost Agents 31 1 — Chung JO, Cho DH, Chung DJ, Chung MY Serum Cystatin C levels are positively associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 10 — Shlipak MG, Mattes MD, Peralta CA Update on Cystatin C: incorporation into clinical practice. Am J Kidney Dis 62 3 — J Diabetes Complicat — De Muro P, Fresu P, Tonolo G et al A longitudinal evaluation of urinary glycosaminoglycan excretion in normoalbuminuric type 1 diabetic patients. Clin Chem Lab Med — Popławska-Kita A, Mierzejewska-Iwanowska B, Szelachowska M et al Glycosaminoglycans urinary excretion as a marker of the early stages of diabetic nephropathy and the disease progression. Diabetes Metab Res Rev — Kahaly G, Hansen Ch, Otto E et al Diabetic microangiopathy and urinary glycosaminoglycans. Exp Clin Endocrinol Diabetes — Budak Y, Demirci H, Akdogan M, Yavuz D Erytrocyte membrane anionic charge in type 2 diabetic patients with retinopathy. BMC Ophthalmol —6. Mohan S, Kalia K, Mannari J Urinary IgG is a pure strong indicator of diabetic nephropathy than microalbuminuria in type 2 diabetic patients. Int J Diabetes Dev Ctries — Yashima I, Hirayama T, Shiiki H, Kanauchi M, Dohi K Diagnostic significance of urinary immunoglobulin G in diabetic nephropathy. Nihon Jinzo Gakkai Shi 41 8 — Narita T, Sasaki H, Hosoba M et al Parallel increase in urinary excretion rates of immunoglobulin g, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Bakoush O, Tencer J, Tapia J et al Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Lee MJ, Jung CH, Kang YM et al Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in korean men with type 2 diabetes mellitus. Diabetes Metab J — Cunninghamn J, Leffell M, Mearkle P, Harmatz P Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: Evidence for increased oxidative stress as a variable complication. Metabolism — Hirawa N, Uehara Y, Ikeda T et al Urinary prostaglandin D synthase β-trace excretion increases in the early stage of diabetes mellitus. Nephron — Uehara Y, Makino H, Seiki K et al Urinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: a cross-sectional and prospective multicentre study. Nephrol Dial Transpl — Hamano K, Totsuka Y, Ajima M et al Blood sugar control reverses the increase in urinary excretion of prostaglandin D synthase in diabetic patients. Zhao L, Zou Y, Zhang J et al Serum transferrin predicts end-stage renal disease in type 2 diabetes mellitus patients. Int J Med Sci — Gonzalez S, Vargas L Diabetogenic transferrin damages podocytes in early human diabetic nephropathy. Horm Metab Res — Kanauchi M, Nishioka H, Hashimoto T, Dohi K Diagnostic significance of urinary transferrin in diabetic nephropathy. Jpn J Nephrol — Diabetes Med — Cheung CK, Cockram CS, Yeung VT, Swaminathan R Urinary excretion of transferrin by non-insulin-dependent diabetics: a marker for early complications? Clin Chem — Sasaki A, Oikawa S, Toyota T Microalbuminuria is closely related to diabetic macroangiopathy. Hwang S, Park J, Kim J et al Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. Kaul A, Behera MR, Rai MK et al Neutrophil gelatinase-associated lipocalin: as a predictor of early diabetic nephropathy in type 2 diabetes mellitus. Indian J Nephrol Bolignano D, Lacquaniti A, Coppolino G et al Neutrophil gelatinase-associated lipocalin NGAL and progression of chronic kidney disease. Clin J Am Soc Nephrol — Duan S, Chen J, Wu L et al Assessment of urinary NGAL for differential diagnosis and progression of diabetic kidney disease. J Diabetes Complicat He P, Bai M, Hu JP et al Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: a systematic review and meta-analysis. Kidney Blood Press Res — Zeng X-F, Lu D-X, Li J-M et al Performance of urinary neutrophil gelatinase-associated lipocalin, clusterin, and cystatin C in predicting diabetic kidney disease and diabetic microalbuminuria: a consecutive cohort study. BMC Nephrol — Abbasi F, Moosaie F, Khaloo P et al Neutrophil gelatinase-associated lipocalin and retinol-binding protein-4 as biomarkers for diabetic kidney disease. Garg V, Kumar M, Mahapatra HS et al Novel urinary biomarkers in pre-diabetic nephropathy. Clin Exp Nephrol — Kim SS, Song SH, Kim IJ et al Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Pirgakis KM, Makris K, Dalainas I et al Urinary Cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann Vasc Surg — Rao X, Wan M, Qiu C, Jiang C Role of cystatin C in renal damage and the optimum cut-off point of renal damage among patients with type 2 diabetes mellitus. Exp Ther Med — van Timmeren M, van den Heuvel M, Bailly V et al Tubular kidney injury molecule-1 KIM-1 in human renal disease. J Pathol — Ichimura T, Bonventre JV, Bailly V et al Kidney injury molecule-1 KIM-1 , a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem — Samia IA, Amal AM, Rehab AM, Hebat-Allah EG Kim-1 and Ngal as biomarkers of nephropathy in type II diabetes. Int J of Adv — Hammoud MS, Baban RS, Ali SH Evaluation of urinary kidney injury molecule-1 kim-1 as prognostic biomarker in children with type-1 diabetic nephropathy. Biochem Cell Arch — De Carvalho JAM, Tatsch E, Hausen BS et al Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem — Cabré A, Lázaro I, Girona J et al Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med — Bangstad HJ, Kierulf P, Kjærsgaard P et al Urinary excretion of retinol-binding protein in healthy children and adolescents. Pediatr Nephrol — Salem MA, El-Habashy SA, Saeid OM, El-Tawil MM, Tawfik PH Urinary excretion of n-acetyl-beta-D-glucosaminidase and retinol binding protein as alternative indicators of nephropathy in patients with type 1 diabetes mellitus. Pediatr Diabetes 3 1 — Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab 92 7 — Akbay E, Muslu N, Nayir E, Ozhan O, Kiykim A Serum retinol binding protein 4 level is related with renal functions in type 2 diabetes. J Endocrinol Invest 33 10 — Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int 62 5 — Kamijo-Ikemori A, Sugaya T, Yasuda T et al Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 36 5 — Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 36 1 — Diabetologia 45 6 — El Wakeel MA, Abou-el-asrar M, El-kassas GM, Elabd MA, Zeid DA, Sabry RN, Awadallah E Urinary markers of oxidative DNA damage in type 1 diabetic children: relation to microvascular complications. Asian J Pharm Clin Res 10 10 — Wu LL, Chiou CC, Chang PY, Wu JT Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 1—2 :1—9. Tatsch E, Carvalho JAMD, Hausen BS et al Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Nishikawa T, Sasahara T, Kiritoshi S et al Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Machowska A, Sun J, Qureshi AR et al Plasma pentosidine and its association with mortality in patients with chronic kidney disease. PLoS ONE e Miura J, Yamagishi SI, Uchigata Y et al Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes. Perkins BA, Rabbani N, Weston A et al High fractional excretion of glycation adducts is associated with subsequent early decline in renal function in type 1 diabetes. Sci Rep — Beisswenger PJ, Moore LL, Brinck-Johnsen T, Curphey TJ Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J Clin Investig — Nin JW, Jorsal A, Ferreira I et al Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes. Bartáková V, Kuricová K, Pácal L et al Hyperuricemia contributes to the faster progression of diabetic kidney disease in type 2 diabetes mellitus. De CS, Viazzi F, Pacilli A et al Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol Hovind P, Rossing P, Tarnow L et al Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes an inception cohort study. Kanasaki K, Taduri G, Koya D Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol. Qiao Y, Chen Y, Pan Y et al The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Navarro JF, Mora C, Rivero A et al Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Ampropoulou IT, Stangou M, Papagianni A, Didangelos T, Iliadis F, Efstratiadis G TNF-α and microalbuminuria in patients with type 2 diabetes mellitus. J Diabetes Res. Shi X, Chen Y, Nadeem L, Xu G Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy. J Neuroinflamm 1 10 :1—9. Mitrović M, Popović Đ, Naglić D et al Markers of inflammation and microvascular complications in type 1 diabetes. Open Med — Wu W, Wang M, Sun Z, Wang X, Miao J, Zheng Z The predictive value of TNF-α and IL-6 and the incidence of macrovascular complications in patients with type 2 diabetes. Acta Diabetol 49 1 :3—7. Khaloo P, Qahremani R, Rabizadeh S et al Nitric oxide and TNF-α are correlates of diabetic retinopathy independent of hs-CRP and HbA1c. Endocrine — Murakoshi M, Gohda T, Suzuki Y Circulating tumor necrosis factor receptors: a potential biomarker for the progression of diabetic kidney disease. Int J Mol Sci Purohit S, Sharma A, Zhi W, Bai S, Hopkins D, Steed L, Bode B, Anderson SW, Reed JC, Steed RD, She JX Proteins of TNF-α and IL6 pathways are elevated in serum of type-1 diabetes patients with microalbuminuria. Front Immunol Int J Biol Macromol Pt B — Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 87 4 — Kulseng B, Vatten L, Espevik T Soluble tumor necrosis factor receptors in sera from patients with insulin-dependent diabetes mellitus: relations to duration and complications of disease. Acta Diabetol 36 1—2 — Campion CG, Sanchez-Ferras O, Batchu SN Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis Chow FY, Nikolic-Paterson DJ, Ozols E et al Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Knowler WC, Rovin BH et al Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transpl 30 4 — Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M et al Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58 4 — Banba N, Nakamura T, Matsumura M et al Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Shoukry A, Bdeer SE-A, El-Sokkary RH Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem — Satirapoj B, Dispan R, Radinahamed P, Kitiyakara C Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrol 19 1 Reddy S, Amutha A, Rajalakshmi R et al Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients T2DM-Y with severity of diabetic retinopathy. Castro NE, Kato M, Park JT, Natarajan R Transforming growth factor β1 TGF-β1 enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem 42 — Flores L, Näf S, Hernáez R, Conget I, Gomis R, Esmatjes E Transforming growth factor beta at clinical onset of Type 1 diabetes mellitus. A pilot study. Diabetic Med 21 8 — Takir M, Unal AD, Kostek O et al Cystatin-C and TGF-β levels in patients with diabetic nephropathy. Nefrologia — Rivarola EW, Moyses-Neto M, Dantas M, Da-Silva CG, Volpini R, Coimbra TM Transforming growth factor beta activity in urine of patients with type 2 diabetes and diabetic nephropathy. Braz J Med Biol Res 32 12 — Shaker YM, Soliman HA, Ezzat E et al Serum and urinary transforming growth factor beta 1 as biochemical markers in diabetic nephropathy patients. Beni-Suef Univ J Basic Appl Sci — Sun J, Wang Y, Cui W, Lou Y, Sun G, Zhang D, Miao L Role of epigenetic histone modifications in diabetic kidney disease involving renal fibrosis. J Diabetes Res Nguyen TQ, Tarnow L, Andersen S, Hovind P, Parving HH, Goldschmeding R, van Nieuwenhoven FA Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care 29 1 — Nguyen TQ, Tarnow L, Jorsal A, Oliver N, Roestenberg P, Ito Y, Parving HH, Rossing P, van Nieuwenhoven FA, Goldschmeding R Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care 31 6 — Kuiper EJ, Witmer AN, Klaassen I et al Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol — Sangoi MB, de Carvalho JA, Tatsch E, Hausen BS, Bollick YS, Londero SW, Duarte T, Scolari R, Duarte MM, Premaor MO, Comim FV, Moretto MB, Moresco RN Urinary inflammatory cytokines as indicators of kidney damage in type 2 diabetic patients. Clin Chim Acta — Shelbaya S, Amer H, Seddik S, Allah AA, Sabry IM, Mohamed T, El Mosely M Study of the role of interleukin-6 and highly sensitive C-reactive protein in diabetic nephropathy in type 1 diabetic patients. Eur Rev Med Pharmacol Sci 16 2 — Shikano M, Sobajima H, Yoshikawa H et al Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Erkeni M, Saïdi A, Bouzidi H, Letaief A, Ben Yahia S, Hammami M Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Vasc Dis Res 10 3 — Rodrigues KF, Pietrani NT, Fernandes AP, Bosco AA, de Sousa M, de Fátima OSI, Silveira JN, Campos F, Gomes KB Circulating microparticles levels are increased in patients with diabetic kidney disease: A case-control research. Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 25 7 — Li S, Wei J, Zhang C, Li X, Meng W, Mo X, Zhang Q, Liu Q, Ren K, Du R, Tian H, Li J Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem 39 6 — Musante L, Tataruch DE, Holthofer H Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol Cao Q, Chen XM, Huang C, Pollock CA MicroRNA as novel biomarkers and therapeutic targets in diabetic kidney disease: an update. FASEB Bio Adv 1 6 — Download references. Department of Nephrology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Manipal, India. Department of Gastroenterology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Manipal, India. Department of Medicine, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Manipal, India. You can also search for this author in PubMed Google Scholar. Correspondence to Shankar Prasad Nagaraju. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Swaminathan, S. et al. Novel biomarkers for prognosticating diabetic kidney disease progression. Int Urol Nephrol 55 , — Download citation. Received : 22 January Accepted : 21 August Published : 22 October Issue Date : April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract The global burden of diabetic kidney disease DKD is escalating, and it remains as a predominant cause of the end-stage renal disease ESRD. Biomarkers of diabetic kidney disease Article Open access 08 March Novel biomarkers of diabetic kidney disease: current status and potential clinical application Article 02 February Addition of nonalbumin proteinuria to albuminuria improves prediction of type 2 diabetic nephropathy progression Article Open access 06 September Use our pre-submission checklist Avoid common mistakes on your manuscript. Pathogenesis of DKD Fig. Full size image. Existing biomarkers Albuminuria and eGFR are the commonly used legacy markers of renal function decline in routine clinical practice, although they lack specificity and sensitivity in predicting the DKD progression in diabetic patients. Novel biomarkers in DKD Over the past decades, immense efforts in research have been carried out to validate alternative biomarkers. Table 1 Classification of biomarkers Full size table. Glomerular biomarkers Biomarkers linked to glomerular injury would be a significant tool in guiding early diagnosis and identifying patients with rapid renal deterioration. Type IV collagen Structurally Type 1 V Collagen is a protein with three polypeptide α-chains in triple helix form which serves as the main basement membrane constituent of the glomerulus, tubules, and mesangial matrix [ 15 ] Mesangial expansion score and tubulointerstitial injury score were statistically correlated with urinary type IV collagen, suggesting the pathogenic processes of DKD reflected in the elevation of this protein [ 15 ]. Fibronectin FN Fibronectin is a fibrillar protein on the cell surface, and its soluble form in plasma is associated with constriction of the glomerular extracellular matrix. Laminin Laminin is an adhesive and non-collagenous component of glomerular basement membranes and mesangium. Cystatin C CysC CysC is a low-molecular-weight protein that acts as an endogenous cysteine proteinase and is identified as a potential surrogate indicator for GFR estimation, because, unlike serum creatinine, it does not influence extrarenal factors [ 29 ] which leads to the increased diagnostic utility of serum CysC to evaluate kidney damage, reflecting directly to GFR. Glycosaminoglycans GAG GAGs are mucopolysaccharides 13 and 30 kDa. Immunoglobulin G IgG IgG is a kDa anionic immunoprotein in serum [ 41 ]. Ceruloplasmin Ceruloplasmin is a copper carrier and acts as a pro-oxidant in severe oxidant stress conditions. Lipocalin-type prostaglandin D synthase L-PGDS L-PGDS is a lipocalin secretory protein that synthesizes prostaglandin D2. Transferrin Transferrin is a glycoprotein with two iron-binding domains, which is primarily produced in the liver. Tubular markers The renal tubules and interstitial compartments play a significant role in the development of DKD [ 56 ]. Neutrophil gelatinase-associated lipocalin NGAL NGAL is a neutrophil granular constituent belonging to the lipocalin protein family. Urinary cystatin C uCysC Cystatin C is a Kidney injury molecule-1 KIM-1 In response to injury, KIM-I is predominantly expressed in the apical membrane of proximal tubular cells. Retinol-binding protein-4 RBP4 RBP4 is a low-molecular-weight protein associated with the lipocalin family, predominantly synthesized in the liver and adipose tissue. Liver-type fatty acid-binding protein L-FABP L-FABP is a 14 kDa protein produced mainly in the cytoplasm of proximal tubules and is involved in the metabolism of the long-chain fatty acids. Biomarker of oxidative stress Evidence from epidemiological and mechanistic research suggests that oxidative stress plays a key role in mediating progression and complications. Pentosidine Pentosidine is an advanced glycoxidation product formed by the covalent binding of amino groups with glucose moiety [ 87 ]. Uric acid Uric acid is produced by purine metabolism and has been shown to play an independent function in predicting DKD progression and many clinical studies have been focused targeting its level in the prognosis of DKD. Biomarkers of inflammation Recent researchers have reported the potential role of local and systemic inflammatory pathways in the progression of DKD with chronic inflammation and subsequent extracellular matrix expansion [ 95 ]. Tumor necrotic factor-alpha TNF-α TNF-α expresses in glomerular and tubular cells in all stages of diabetes, mainly monocyte-produced cytokines, and predisposes in all the stages of the pathogenesis of DKD progression by inducting and infiltrating inflammatory cells to the kidney and activation of apoptosis system. Tumor necrotic factor-alpha receptors TNF-α receptors are type1 transmembrane proteins with cysteine-rich motifs seen in glomerular and tubular cells. Monocyte chemoattractant protein-1 MCP-1 MCP-1 is a pro-inflammatory cytokine produced by mononuclear leukocytes, cortical tubular epithelial cells, and podocytes that has been linked to renal inflammation, glomerular injury, tubular atrophy, and fibrosis via nuclear factor-kappa B [ ]. Transforming growth factor-beta TGF- β TGF-β activates fibrogenesis and thereby progression of DKD by the increased extracellular matrix deposition and glomerular mesangial hypertrophy [ ]. Connective tissue growth factor CTGF CTGF is a secretory protein in renal cells induced by hyperglycemia. Interleukins-6 IL-6 It is a major immunoregulatory cytokine in mesangial expansion. Table 3 Overview of biomarkers for the diagnostic utility of DKD in type 2 diabetic population Full size table. Urinary exosomes Urinary exosomes, 40— nm originate as internal vesicles, and that contain protein indicators of renal failure and structural damage. Conclusion The pathophysiology of DKD and its progression is multifactorial. References Tuttle KR, Bakris GL, Bilous RW et al Diabetic kidney disease: a report from an ADA consensus conference. Front Med — Article PubMed Google Scholar Porrini E, Ruggenenti P, Mogensen CE et al Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. J Med Assoc Thail 93 Suppl 6 :S—S Google Scholar Lassén E, Daehn IS Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction. Am J Physiol-Renal Physiol 4 :F—F Article CAS PubMed Google Scholar Molitch ME, Steffes M, Sun W et al Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Clin Nephrol 55 5 — PubMed Google Scholar Tomino Y, Suzuki S, Azushima C, Shou I, Iijima T, Yagame M, Wang LN, Chen HC, Lai KN, Tan SY, Kim MJ Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy. co;2-j Article CAS PubMed PubMed Central Google Scholar Morita M, Uchigata Y, Hanai K et al Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes. x Article CAS PubMed Google Scholar Ozata M, Kurt I, Azal O, Bolu E, Corakci A, Beyhan Z, Karaca L, Gündogăn MA Can we use plasma fibronectin levels as a marker for early diabetic nephropathy. Nihon Jinzo Gakkai shi 37 6 — CAS PubMed Google Scholar Fagerudd JA, Groop PH, Honkanen E, Teppo AM, Grönhagen-Riska C Urinary excretion of TGF-beta 1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney Int Suppl S—S CAS PubMed Google Scholar Kanters SDJM, Banga J-D, Algra A et al Plasma levels of cellular fibronectin in diabetes. Diabetes Res Clin Pract —49 Article CAS PubMed Google Scholar Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. x Article CAS PubMed Google Scholar Papadopoulou-Marketou N, Skevaki C, Kosteria I et al NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. J Coll Physicians Surg Pak 28 4 — Article PubMed Google Scholar Macisaac RJ, Ekinci EI, Jerums G Markers of and risk factors for the development and progression of diabetic kidney disease. J Biol Regul Homeost Agents 31 1 — CAS PubMed Google Scholar Chung JO, Cho DH, Chung DJ, Chung MY Serum Cystatin C levels are positively associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. Nihon Jinzo Gakkai Shi 41 8 — CAS PubMed Google Scholar Narita T, Sasaki H, Hosoba M et al Parallel increase in urinary excretion rates of immunoglobulin g, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. X Article CAS PubMed Google Scholar Lee MJ, Jung CH, Kang YM et al Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in korean men with type 2 diabetes mellitus. X Article Google Scholar Cheung CK, Cockram CS, Yeung VT, Swaminathan R Urinary excretion of transferrin by non-insulin-dependent diabetics: a marker for early complications? Biochem Cell Arch — Google Scholar De Carvalho JAM, Tatsch E, Hausen BS et al Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. X Article PubMed Google Scholar Bangstad HJ, Kierulf P, Kjærsgaard P et al Urinary excretion of retinol-binding protein in healthy children and adolescents. x Article PubMed Google Scholar Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. x Article CAS PubMed Google Scholar Kamijo-Ikemori A, Sugaya T, Yasuda T et al Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. x Article CAS PubMed Google Scholar Banba N, Nakamura T, Matsumura M et al Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. X Article CAS PubMed Google Scholar Shoukry A, Bdeer SE-A, El-Sokkary RH Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. x Article CAS PubMed Google Scholar Takir M, Unal AD, Kostek O et al Cystatin-C and TGF-β levels in patients with diabetic nephropathy. Eur Rev Med Pharmacol Sci 16 2 — CAS PubMed Google Scholar Shikano M, Sobajima H, Yoshikawa H et al Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. Funding Open access funding provided by Manipal Academy of Higher Education, Manipal. View author publications. Ethics declarations Conflict of interest The authors declare no conflict of interest. |
| Top bar navigation | Diagnostic utility of serum CysC in normoalbuminuric patients has also been well documented in a cohort of T1DM, indicating the predictive performance of CysC before the renal dysfunction appears [ 30 ] which is well supported by Qamar et al. in type 2 diabetic patients with a sensitivity of In multiple studies done among CKD patients with T1DM and T2DM, showed a significant role of serum CysC as a predictor of progression to ESRD [ 32 ]. Clinical research has observed the positive association of serum CysC with retinopathy [ 33 ] and cardiovascular risk [ 34 ] in type 2 DM. Nevertheless, as recommended in current KDIGO guidelines, CysC would result in increased health care costs [ 35 ]. The above studies displayed that serum cystatin C could be a promising biomarker for early diagnosis and for predicting the progression of DKD, as it is a strong predictor of microvascular and macrovascular complications of diabetes. GAGs are mucopolysaccharides 13 and 30 kDa. The most prevalent types are chondroitin and dermatan sulfate, keratan sulfate, heparan sulfate, and heparin. Heparan sulfate gives negative potential to the glomerular basement membrane GBM to control the perm selectivity of the glomerulus. In DKD, endothelial dysfunction leads to loss of these functional groups which gives rise to hyperfiltration resulting in albuminuria [ 36 ]. The Steno hypothesis postulates a defect in heparan sulfate regulation in the glomerulus, which determines the high susceptibility of diabetic patients to develop proteinuria and eventually induces the excretion of heparan sulfate in the urine [ 37 ]. Many experimental studies supporting this hypothesis observed a rise in urinary GAG excretion, especially heparan sulfate in T1DM and T2DM patients with microalbuminuria and macroalbuminuria than the normoalbuminuric group, suggesting that GAG is a vital screening predictor of microalbuminuria [ 38 ]. The functional role of GAG in permeability properties of retinal basement membrane has also been documented. Kahaly et al. have evaluated high urinary GAG concentration in diabetic nephropathy patients with retinopathy complications [ 39 ]. Linked with these findings, Budak et al. have also reported a positive correlation of GAG with diabetic retinopathy [ 40 ]. Urinary GAG evaluation could be a vital marker for predicting the onset of microalbuminuria in type 1 and type 2 diabetic patients and microvascular complications of diabetes in the progressive stages. IgG is a kDa anionic immunoprotein in serum [ 41 ]. The urinary excretion of this high-molecular-weight protein indicates increased GBM porosity with large shunt-like pores and podocyte deficiency and effacement of foot processes. Thus, IgG appears in progressive DKD when severe irreversible kidney lesions occur [ 41 ]. Yashima et al. have reported a significant elevation of urinary IgG in normoalbuminuric DKD patients among the T2DM population and also observed a correlation with progressive diffuse glomerular lesions [ 42 ]. Multiple studies in type 2 diabetic patients have suggested that urinary level of IgG predicts the onset of microalbuminuria [ 41 , 43 ]. Apart from using total IgG level, subtype levels and their ratio has been used as a marker of glomerular charge selectivity impairment. Altogether, these studies suggest that measurement of urinary IgG could be an effective marker for predicting the onset of microalbuminuria in T2DM. Ceruloplasmin is a copper carrier and acts as a pro-oxidant in severe oxidant stress conditions. It has been studied as an independent risk factor for the incidence of cardiovascular diseases and insulin resistance [ 45 ]. Jung Lee et al. have found T2DM with a higher incidence of serum ceruloplasmin level in progressors than those in non-progressors, indicating an independent factor for progression [ 45 ]. Furthermore, urinary ceruloplasmin was also found elevated in T2DM before albuminuria appears [ 43 ]. A similar observation was found in T1DM [ 46 ]. This evidence suggests parallel evaluation of both serum and urine ceruloplasmin would be an independent predictive marker in DKD progression in T2DM. However, further studies require in the type 1 population, which is limited so far. L-PGDS is a lipocalin secretory protein that synthesizes prostaglandin D2. It is primarily produced in the choroid plexus in the brain and discharged readily to circulating blood with chemical features like albumin, such as anionic charge, and can move quickly through the glomerular capillary due to its small molecular weight 20—31 kDa. Thus, urine L-PGDS reflect minor changes in the permeability of glomerular capillary walls [ 47 ]. Researchers have studied its utility in predicting the early stages of DKD by observing the elevated urinary L-PGDS in diabetic patients with normoalbuminuria [ 48 ]. There was a significant increase in urine L-PGDS than serum level in normo- or macroalbuminuria in parallelly in advanced DKD [ 49 ]. The findings of these studies suggest that evaluation of L-PGDS in urine could identify the early onset of DKD. Transferrin is a glycoprotein with two iron-binding domains, which is primarily produced in the liver. It is involved in multiple functions like iron transportation and immune regulation against micro-organisms [ 50 ]. Gonzalez et al. Iron liberated from transferrin contributes to oxidative stress and insulin resistance in T2DM patients, and thus, dysregulation of iron homeostasis is associated with the development of DKD [ 50 ]. Kanauchi et al. in their study observed the correlation of urinary transferrin with progressive changes such as interstitial fibrosis, atrophic renal tubular cells, and infiltration of renal interstitium with inflammatory cells [ 52 ]. The renal accumulation of iron and excretion of transferrin in urine leads to a lower serum level, which indicates renal cell toxicity resulting from accumulated free iron [ 50 ]. Excretion of transferrin in urine has been reported in normoalbuminuric T2DM, indicating prediction at an early stage than albuminuria in DKD [ 43 ]. High excretion of urinary transferrin in normoalbuminuric and microalbuminuric patients among T1DM was reported by previous researchers [ 53 ]. These findings were supported by a retrospective cohort study which suggests that low serum transferrin was associated with ESRD in diabetic patients [ 50 ]. Additionally, studies have also been reported transferrinuria in predicting micro- and macrovascular complications of diabetes which leads to retinopathy [ 54 ] and cardiovascular disease [ 55 ]. These findings suggest that the increased urinary and lower serum level of transferrin in all diabetic patients would predict the development of microalbuminuria and significant indicator for complications of diabetes. The renal tubules and interstitial compartments play a significant role in the development of DKD [ 56 ]. The extent of tubulointerstitial damage may determine renal function decline in diabetes, even in normoalbuminuric renal insufficiency [ 56 ]. Hence, the tubular indicators of kidney injury have a pivotal role to measure the degree of long-term kidney impairment in DKD patients. NGAL is a neutrophil granular constituent belonging to the lipocalin protein family. In renal injury, the distal tubules and collecting duct signify the higher expression of NGAL. It has been validated as an acute kidney injury AKI biomarker extensively. NGAL is involved in antimicrobial defense mechanisms and anti-apoptosis. It is a definite marker of acute renal damage, because a burnt-out nephron does not generate NGAL [ 57 ]. Evidences have been postulated on the significant role of NGAL in CKD and later in DKD [ 58 ] which has been supported by the findings of S. Hwang et al. Urinary NGAL uNGAL -to-creatinine ratio was found useful to differentiate DKD from non-diabetic kidney disease with high specificity Kaul et al. observed that the significant rise of serum NGAL sNGAL and uNGAL from normo-micro-macroalbuminuria in T2DM [ 57 ]. Peng He et al. Growing evidences have depicted a positive correlation of NGAL with albuminuria and other tubular markers, including RBP4, Cystatin C, and KIM-1, whereas it is negatively correlated with eGFR, suggesting the involvement of NGAL in DKD progression [ 61 , 62 , 63 ]. In contrast, Kim et al. reported that no significant difference in NGAL was found in normoalbuminuric and macroalbuminuric patients [ 64 ]. In summary, these clinical studies suggest that sNGAL and uNGAL could be valuable markers for diagnosing the onset of DKD and stratifying the disease into different stages. However, large-scale prospective studies are necessary to implement NGAL as a biomarker into routine clinical use. Cystatin C is a Normally, it is not present in urine significantly. Previous studies have demonstrated uCysC as a promising biomarker of tubular dysfunction in AKI [ 65 ], and it has been extensively studied in DKD [ 66 ]. Xian et al. have evaluated the diagnostic performance of uCysC in DKD and the onset of microalbuminuria among T2DM. With respect to DKD diagnosis, the area under the ROC curve was 0. These studies suggest uCysC as a sensitive biomarker mirroring tubular impairment, which can be determined before the onset of microalbuminuria. In response to injury, KIM-I is predominantly expressed in the apical membrane of proximal tubular cells. Palmitic acid bounded albumin uptake by proximal tubules is being enhanced by KIM-1, leading to further tubulointerstitial damage [ 67 ]. Van Timmeren et al. demonstrated that KIM-1 mainly expresses the luminal side of dedifferentiated proximal tubular areas, which had more fibrosis and inflammation [ 67 ]. Several studies have documented an estimation of KIM-1 in urine uKIM-1 as a predictive indicator for AKI as it appears well before serum creatinine increases [ 68 ]. Fourth, Ali et al. reported uKIM-1 with more specificity and sensitivity than urine albumin in diagnosing early stages of DKD [ 69 ], and Gohda et al. reported a significant association of serum KIM-1 with a lower GFR rate. Based on these studies, uKIM-1 appears to be a promising marker for diagnosing early onset and predicting the different stages of disease progression in type 1 and type 2 diabetic patients. Upon this, serum level estimation might be a sensitive marker for progression as GFR declines. RBP4 is a low-molecular-weight protein associated with the lipocalin family, predominantly synthesized in the liver and adipose tissue. The main function of RBP-4 is to transfer small hydrophobic molecules to the cell membrane. In diabetes, an association of RBP4 concentration has been documented with the magnitude of insulin resistance, suggesting increased levels of RBP4 predicts insulin resistance [ 72 ]. Increased plasma and urinary RBP4 concentration have been reported with low eGFR [ 72 ] [ 73 ]. A longitudinal study among T1DM has reported an increased level of urinary RBP4 in microalbuminuric patients [ 74 ] and the diagnostic utility of urinary RBP4 in T2DM patients with an AUC of 0. The serum level of RBP-4 was found to be associated with proliferative diabetic retinopathy and coronary cerebrovascular or peripheral vascular diseases among type 2 diabetes [ 72 , 75 ]. Discordant results were shown by E Akbay's study, indicating that diabetic retinopathy and cardiovascular complications do not exhibit any change in serum RBP4 in T2DM patients [ 76 ]. RBP4 could be a valid marker for identifying the early onset of DKD and predicting renal function impairment in progressive stages in T1DM and T2DM. In addition, this marker could be a predictor for microvascular and macrovascular complications of diabetes. L-FABP is a 14 kDa protein produced mainly in the cytoplasm of proximal tubules and is involved in the metabolism of the long-chain fatty acids. Uncontrolled reabsorption of free fatty acids to tubular cells by L-FABP leads to tubulointerstitial damage [ 77 ]. According to Kamijo et al. Higher levels of L-FABP in the normoalbuminuric group suggest that it could be a risk factor for disease progression [ 78 ]. Corroborating these findings, a year follow-up study by Araki S et al. Additionally, the urinary level of L-FABP offers statistical significance with urine albumin level and inversely correlates with GFR [ 78 ]. Thus, evaluation of urinary L-FABP in T2 DM serves as a risk factor for DKD progression and could be considered as a promising tubular marker in predicting the incidence of cardiovascular disease and renal function impairment. Evidence from epidemiological and mechanistic research suggests that oxidative stress plays a key role in mediating progression and complications. Thereby, markers linked to ROS production have considerable potential to stratify DKD stages. Numerous evidence has indicated urinary 8-oxodG is a risk factor for cancer, atherosclerosis, and diabetes [ 80 ]. Xu et al. Clinical research with 5 years of follow-up reported significant progression of diabetic kidney disease in patients with higher urinary 8-oxodG [ 82 ]. Urinary 8-oxodG has been proposed as a characteristic pathogenic component in diabetic retinopathy development in T1DM and T2DM [ 83 , 84 ]. Etiane et al. found diagnostic ability of 8-oxodG with an AUC of 0. This marker has also been observed with macrovascular complications in T2DM [ 86 ]. These above-mentioned findings conclude that excretion of urinary 8-oxodG could be an independent predictor for disease progression and development of microvascular and macrovascular complications of diabetes. Pentosidine is an advanced glycoxidation product formed by the covalent binding of amino groups with glucose moiety [ 87 ]. Miura et al. demonstrated a serum pentosidine level more marked progressively in microalbuminuria and advanced stages of nephropathy [ 88 ]. Bruce A et al. found higher excretion in urine among patients with microalbuminuria and early decline of GFR [ 89 ]. Diabetic patients with a high level of pentosidine were found to be an independent predictor of diabetic retinopathy, cardiovascular disease, and all-cause mortality [ 90 , 91 ]. Lines to this evidence, measurement of pentosidine level in urine and serum may provide the basis for identifying patients at risk of early GFR decline and could be a promising biomarker for diabetic microvascular and macrovascular complications. Uric acid is produced by purine metabolism and has been shown to play an independent function in predicting DKD progression and many clinical studies have been focused targeting its level in the prognosis of DKD. Bartakova et al. found initial hyperuricemia is a strong determinant of DKD progression [ 92 ]. Zoppini et al. analyzed that the cumulative incidence of CKD with GFR decline among T2DM was significantly higher in those who had hyperuricemia, considered as an independent risk factor in disease progression and as a strong predictor of GFR decline [ 93 ]; furthermore, T1DM with higher serum uric acid levels were developed persistent macroalbuminuria [ 94 ]. These evidences suggest that serum uric acid could be an independent predictor of later development of macroalbuminuria in type 1 and type 2 diabetic patients. Recent researchers have reported the potential role of local and systemic inflammatory pathways in the progression of DKD with chronic inflammation and subsequent extracellular matrix expansion [ 95 ]. TNF-α expresses in glomerular and tubular cells in all stages of diabetes, mainly monocyte-produced cytokines, and predisposes in all the stages of the pathogenesis of DKD progression by inducting and infiltrating inflammatory cells to the kidney and activation of apoptosis system. Thereby elevated level of TNF-α has been noted with hypertrophy, hyperfiltration, and alterations of intra-glomerular blood flow, resulting in reduced renal function [ 95 ]. A meta-analysis by Qiao et al. reported T1DM patients have significantly increased TNF-α as compared to healthy controls [ 96 ]. Furthermore, Navarro JF et al. documented that serum TNF-α is elevated with advanced renal dysfunction and correlates with urinary protein excretion, suggesting that this cytokine has an intensive role in the onset of proteinuria in these patients [ 97 ]. While Stangou et al. reported a significant positive correlation of urinary TNF-α, but not serum TNF-α with the severity of microalbuminuria in T2DM [ 98 ]. An experimental animal, study corroborated the key role of TNF-α in mediating the pathogenesis of diabetic peripheral neuropathy [ 99 ]. Elevated TNF-α is also associated with microvascular and macrovascular complications in diabetic patients [ , ] and in the prediction of diabetic retinopathy in T2DM with an AUC of 0. The above studies suggest that serum and urine TNF-α could be a potential biomarker to predict the degree of microalbuminuria in T1DM and T2DM. TNF-α receptors are type1 transmembrane proteins with cysteine-rich motifs seen in glomerular and tubular cells. These are of two types, TNF-α receptor 1 55 kDa and TNF -α receptor 2 75 kDa. TNF-α binds to these respective receptors and induces inflammatory pathways and apoptosis [ ]. Current studies have appreciated the contribution of TNF-α receptors on the magnitude of DKD development through the TNF α —TNFR2 inflammatory pathways [ ]. Sharad et al. found a strong correlation of serum TNF-α receptors with microalbuminuria among T1DM, suggesting the crucial role in disease progression [ ]. These findings have been supported by evidence of a marked stepwise increase from normo-micro-macroalbuminuria in T2DM [ ]. Multiple studies have reported that TNFRs are associated independently with declined renal function and ESRD [ ]. Furthermore, it has been described that serum TNF receptors are predictors of diabetic retinopathy in T1DM [ ]. Therefore, circulating TNF-α receptors are associated with the progression of DKD and could be a predictor of microalbuminuria and advanced renal impairment. MCP-1 is a pro-inflammatory cytokine produced by mononuclear leukocytes, cortical tubular epithelial cells, and podocytes that has been linked to renal inflammation, glomerular injury, tubular atrophy, and fibrosis via nuclear factor-kappa B [ ]. Renal expression of MCP-1 was also correlated with the quantity of infiltrated macrophages, interstitial lesions, and the degree of albuminuria [ ]. Fufaa et al. demonstrated a substantial correlation of urine MCP-1 uMCP-1 levels with cortical interstitial expansion and disease progression in T1DM who had normoalbuminuria [ ]. When comparing DKD patients to healthy controls, Wada [ ] and Banba [ ] discovered elevated urinary excretion of MCP-1 in DKD patients. Shoukry et al. Early progressive GFR decline has a positive correlation with high uMCP-1 [ ] also associated with young-onset of T2DM with diabetic retinopathy [ ]. These observations suggest that MCP-1 could be a promising inflammatory marker in diagnosing early progressive renal decline and diabetic microvascular complications. TGF-β activates fibrogenesis and thereby progression of DKD by the increased extracellular matrix deposition and glomerular mesangial hypertrophy [ ]. Flores et al. have shown raised urinary and plasma TGF- β in clinical onset of T1DM [ ]. Similarly, patients with T2DM who had microalbuminuria had been reported elevated serum and urinary TGF-β [ ]. On the other hand, Rivarola et al. Supporting this, a study conducted among T2DM resulted in increased serum and urine TGF-β, being more pronounced in macroalbuminuria compared to microalbuminuria and normoalbuminuria group [ ], suggesting that this biomarker could be a good candidate in predicting macroalbuminuria in type 2 DM. CTGF is a secretory protein in renal cells induced by hyperglycemia. It stimulates extracellular matrix synthesis, cell migration, and interstitial matrix deposition by the epithelial-to-mesenchymal transition in diabetic patients [ ]. T1DM patients showed increased urinary CTGF with the severity of renal function deterioration in terms of albumin excretion and GFR decline [ ] and could be a predictor for ESRD with an AUC under ROC of 0. Its expression has also been reported in diabetic retinopathy [ ]. CTGF could be an independent predictor of ESRD and mortality in DKD and it is also a predictor of diabetic retinopathy and looks promising. It is a major immunoregulatory cytokine in mesangial expansion. Sangoi et al. observed higher serum IL-6 even before the onset of albuminuria [ ]. Multiple studies support these findings with evidence that serum and urinary IL-6 were increasing with disease progression in T1DM [ ] and T2DM [ ] and have a pronounced association with macrovascular complications [ ]. Thus, it could be a marker of the onset of microalbuminuria and early progressive renal decline, and it strongly predicts the macrovascular complications of diabetes. The classification based on pathogenesis and utilization of these biomarkers can be the future in predicting the early onset of microalbuminuria and progressive renal function decline in both T1DM and T2DM. These biomarkers are relevant to not only predicting the progression of DKD but also diabetic microvascular and macrovascular complications, as shown in Table 2. Few of the biomarkers have been studied in the type 2 diabetic population on their diagnostic utility for DKD and established cut-off value with the area under the ROC curve from 0. These are 0. Under hyperglycaemic injury, renal cells are activated to release MPs into plasma and urine before the onset of DKD [ ]. Therefore, these have emerged as biomarkers in DKD. A review by Sheyu Li et al. reported a higher level of circulating MPs reported with T2DM and as independent predictors for microvascular complications of diabetes [ ]. MPs can be easily isolated from body fluids via non-invasive methods. These properties facilitate the use as a potential non-invasive biomarker in the progression of DKD. More large-scale studies are needed for further relevance in this regard. Urinary exosomes, 40— nm originate as internal vesicles, and that contain protein indicators of renal failure and structural damage. It has turned out to be a potential non-invasive biomarker source. However, exosome isolation was challenging. Using liquid chromatography and mass spectrometry, the scope of existing approaches has enlarged to evaluate more urinary exosome-associated proteins [ ]. In recent years, microRNA is reported to be involved in the DKD progression via inflammation, hypertrophy, autophagy, endoplasmic reticulum ER stress, oxidative stress, insulin resistance, and podocyte injury. Jia et al. observed a positive correlation of miRNA expression for TGF beta stimulator with albuminuria and reported good diagnostic efficiency [ ]. Based on the evidence mentioned above, urine mRNA has prognostic significance as a non-invasive, early indicator of renal impairment. The pathophysiology of DKD and its progression is multifactorial. Therefore, assessment of development and progression of DKD cannot be relied solely on albuminuria and creatinine. The identification of novel biomarkers based on pathogenesis of DKD involving various renal structures looks promising. In this review, we have summarized the potential 22 novel biomarkers with respect to the pathogenesis of DKD development. Each biomarker has its role in either identifying DKD early or predicting progression of DKD over and above clinical history and standardized markers like albuminuria and creatinine. Few of them appear to be useful for predicting other micro- and macrovascular complications like retinopathy and cardiovascular disease. This panel of biomarkers now warrants further validation on large-scale longitudinal studies involving type 1 and type 2 diabetes populations before the transition to clinical routine. Tuttle KR, Bakris GL, Bilous RW et al Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis — Article PubMed Google Scholar. Bikbov B, Purcell CA, Levey AS et al Global, regional, and national burden of chronic kidney disease, — a systematic analysis for the Global Burden of Disease Study Lancet — Article Google Scholar. Chen C, Wang C, Hu C et al Normoalbuminuric diabetic kidney disease. Front Med — Porrini E, Ruggenenti P, Mogensen CE et al Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol — Article CAS PubMed Google Scholar. Do S Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int — Hussain S, Jamali MC, Habib A et al Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers. Clin Epidemiol Glob Health —6. Article CAS Google Scholar. Ilyas Z, Chaiban JT, Krikorian A Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev Endocr Metab Disord 18 1 — Satirapoj B Review on pathophysiology and treatment of diabetic kidney disease. J Med Assoc Thail 93 Suppl 6 :S—S Google Scholar. Lassén E, Daehn IS Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction. Int J Mol Sci 21 24 Article PubMed PubMed Central Google Scholar. Gilbert RE Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes — Sakashita M, Tanaka T, Inagi R Metabolic changes and oxidative stress in diabetic kidney disease. Pichler R, Afkarian M, Dieter BP, Tuttle KR Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol-Renal Physiol 4 :F—F Molitch ME, Steffes M, Sun W et al Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care — Article CAS PubMed PubMed Central Google Scholar. Lin C-H, Chang Y-C, Chuang L-M Early detection of diabetic kidney disease: present limitations and future perspectives. World J Diabetes Hamaguchi K, Tsuchida H, Miura Y, Suzuki S, Kawamura T, Hosoya T, Yamada K Urinary type IV collagen excretion reflects renal morphological alterations and type IV collagen expression in patients with type 2 diabetes mellitus. Clin Nephrol 55 5 — PubMed Google Scholar. Tomino Y, Suzuki S, Azushima C, Shou I, Iijima T, Yagame M, Wang LN, Chen HC, Lai KN, Tan SY, Kim MJ Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy. J Clin Lab Anal 15 4 — Iijima T, Suzuki S, Sekizuka K, Hishiki T, Yagame M, Jinde K et al Follow-up study on urinary type IV collagen in patients with early stage diabetic nephropathy. J Clin Lab Anal 12 6 — Morita M, Uchigata Y, Hanai K et al Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes. Araki S, Haneda M, Koya D et al Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Hayashi Y, Makino H, Ota Z Serum and urinary concentrations of type IV collagen and laminin as a marker of microangiopathy in diabetes. Diabetic Med 9 4 — Ozata M, Kurt I, Azal O, Bolu E, Corakci A, Beyhan Z, Karaca L, Gündogăn MA Can we use plasma fibronectin levels as a marker for early diabetic nephropathy. Endocr J 42 2 — Takahashi M Increased urinary fibronectin excretion in type II diabetic patients with microalbuminuria. Nihon Jinzo Gakkai shi 37 6 — CAS PubMed Google Scholar. Fagerudd JA, Groop PH, Honkanen E, Teppo AM, Grönhagen-Riska C Urinary excretion of TGF-beta 1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney Int Suppl S—S Kanters SDJM, Banga J-D, Algra A et al Plasma levels of cellular fibronectin in diabetes. Setty S, Michael AA, Fish AJ et al Differential expression of laminin isoforms in diabetic nephropathy and other renal diseases. Mod Pathol — Diabetes Res Clin Pract — El-Fattah MEA, Rashed LA, Nasr SMM The role of transferrin and laminin biomarkers in the diagnosis of diabetic nephropathy in type II diabetic patients. J Adv Med Med Res — Okazaki R, Matsuokab K, Atsumib Y et al Serum concentrations of basement membrane proteins in NIDDM as a prognostic marker for nephropathy. Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 5 — Papadopoulou-Marketou N, Skevaki C, Kosteria I et al NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones — Qamar A, Hayat A, Ahmad TM, Khan A, Hasnat MNU, Tahir S Serum cystatin C as an early diagnostic biomarker of diabetic kidney disease in type 2 diabetic patients. J Coll Physicians Surg Pak 28 4 — Macisaac RJ, Ekinci EI, Jerums G Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. Qian C, Wan GM, Yan PS, Wang WZ, Liang SZ, Dong Y Correlation between Cystatin C and retinopathy of type-two diabetes mellitus patients. J Biol Regul Homeost Agents 31 1 — Chung JO, Cho DH, Chung DJ, Chung MY Serum Cystatin C levels are positively associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 10 — Shlipak MG, Mattes MD, Peralta CA Update on Cystatin C: incorporation into clinical practice. Am J Kidney Dis 62 3 — J Diabetes Complicat — De Muro P, Fresu P, Tonolo G et al A longitudinal evaluation of urinary glycosaminoglycan excretion in normoalbuminuric type 1 diabetic patients. Clin Chem Lab Med — Popławska-Kita A, Mierzejewska-Iwanowska B, Szelachowska M et al Glycosaminoglycans urinary excretion as a marker of the early stages of diabetic nephropathy and the disease progression. Diabetes Metab Res Rev — Kahaly G, Hansen Ch, Otto E et al Diabetic microangiopathy and urinary glycosaminoglycans. Exp Clin Endocrinol Diabetes — Budak Y, Demirci H, Akdogan M, Yavuz D Erytrocyte membrane anionic charge in type 2 diabetic patients with retinopathy. BMC Ophthalmol —6. Mohan S, Kalia K, Mannari J Urinary IgG is a pure strong indicator of diabetic nephropathy than microalbuminuria in type 2 diabetic patients. Int J Diabetes Dev Ctries — Yashima I, Hirayama T, Shiiki H, Kanauchi M, Dohi K Diagnostic significance of urinary immunoglobulin G in diabetic nephropathy. Nihon Jinzo Gakkai Shi 41 8 — Narita T, Sasaki H, Hosoba M et al Parallel increase in urinary excretion rates of immunoglobulin g, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Bakoush O, Tencer J, Tapia J et al Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Lee MJ, Jung CH, Kang YM et al Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in korean men with type 2 diabetes mellitus. Diabetes Metab J — Cunninghamn J, Leffell M, Mearkle P, Harmatz P Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: Evidence for increased oxidative stress as a variable complication. Metabolism — Hirawa N, Uehara Y, Ikeda T et al Urinary prostaglandin D synthase β-trace excretion increases in the early stage of diabetes mellitus. Nephron — Uehara Y, Makino H, Seiki K et al Urinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: a cross-sectional and prospective multicentre study. Nephrol Dial Transpl — Hamano K, Totsuka Y, Ajima M et al Blood sugar control reverses the increase in urinary excretion of prostaglandin D synthase in diabetic patients. Zhao L, Zou Y, Zhang J et al Serum transferrin predicts end-stage renal disease in type 2 diabetes mellitus patients. Int J Med Sci — Gonzalez S, Vargas L Diabetogenic transferrin damages podocytes in early human diabetic nephropathy. Horm Metab Res — Kanauchi M, Nishioka H, Hashimoto T, Dohi K Diagnostic significance of urinary transferrin in diabetic nephropathy. Jpn J Nephrol — Diabetes Med — Cheung CK, Cockram CS, Yeung VT, Swaminathan R Urinary excretion of transferrin by non-insulin-dependent diabetics: a marker for early complications? Clin Chem — Sasaki A, Oikawa S, Toyota T Microalbuminuria is closely related to diabetic macroangiopathy. Hwang S, Park J, Kim J et al Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. Kaul A, Behera MR, Rai MK et al Neutrophil gelatinase-associated lipocalin: as a predictor of early diabetic nephropathy in type 2 diabetes mellitus. Indian J Nephrol Bolignano D, Lacquaniti A, Coppolino G et al Neutrophil gelatinase-associated lipocalin NGAL and progression of chronic kidney disease. Clin J Am Soc Nephrol — Duan S, Chen J, Wu L et al Assessment of urinary NGAL for differential diagnosis and progression of diabetic kidney disease. J Diabetes Complicat He P, Bai M, Hu JP et al Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: a systematic review and meta-analysis. Higher plasma levels of KIM-1, TNFR-1, TNFR-2, and MCP-1 were associated with risk of progression of DKD. de Carvalho et al. Urinary KIM-1 and NGAL were increased in T2DM patients with normal or mildly increased albuminuria. HbA1c, LDL cholesterol, fasting glucose, and medication. Yuruk Yildirim et al. Urinary NGAL level increase in the early phase of T1DM before microalbuminuria development. Lacquaniti et al. NGAL increases in patients with T1DM before onset of microalbuminuria. Nielsen et al. High levels of urinary L-FABP predict the initiation and progression to DKD and all- cause mortality. Age, sex, HbA1c, systolic and diastolic blood pressure, albuminuria, serum creatinine, smoking. Panduru et al. High urinary L-FABP levels were found to be a strong and independent predictor of DKD progression. Kim et al. Urinary cystatin C and albuminuria may be sensitive and specific markers for predicting kidney impairment. Age, HbA1c, systolic blood pressure, uric acid, albuminuria, baseline eGFR, use of RAS inhibitors and lipid-lowering agents, serum cystatin C. Biomarkers of inflammation. Niewczas et al. Elevated circulating TNFR levels are strong predictors of progression to ESKD in subjects with and without proteinuria. Skupien et al. Circulating TNFR2 is a major determinant of kidney function decline. Lopes-Virella et al. High levels of E-selectin and soluble TNFR1 and TNFR2 levels were important predictors of incident albuminuria. Treatment, albuminuria, use of RAS inhibitors, baseline retinopathy, sex, age, HbA1c, diabetes duration. Biomarkers of oxidative stress. Xu et al. Individuals with T2DM have higher levels of 8-OHdG compared to healthy individuals. Sanchez et al. Higher levels of 8-OHdG were associated with increased risk of kidney disease. Age, sex, cohort, duration of diabetes, HbA1c, insulin therapy, systolic blood pressure, use of antihypertensive drugs, RAS inhibitors, diabetic retinopathy stage, lipid-lowering drugs, eGFR, albuminuria. Serdar et al. Although urinary 8-OHdG levels increase in diabetic patients, their levels do not improve prediction of progressive DKD over and above measuring albuminuria. Omics-based novel biomarkers. Bhensdadia et al. The haptoglobin to creatinine ratio may be useful to predict risk of DKD before the development of albuminuria or kidney function decline. Zurbig et al. CKD predicted progression to macroalbuminuria 5 years prior to actual onset. Age, sex, DM type, albuminuria, eGFR, systolic and diastolic blood pressure, HbA1c, glucose. Roscioni et al. CKD predicted development of albuminuria independent of other kidney biomarkers used to predict DKD development or progression. Albuminuria, eGFR, use of RAS inhibitors. Age, baseline eGFR, systolic and diastolic blood pressure. Tofte et al. High-risk patients defined by CKD were more likely to develop microalbuminuria. Age, sex, HbA1c, systolic blood pressure, retinopathy, albuminuria, eGFR. Lindhardt et al. CKD predicted development of albuminuria. Treatment group, age, sex, systolic blood pressure, albuminuria, eGFR, HbA1c, diabetes duration. Han et al. Non-esterified and esterified fatty acid discriminated albuminuria stages. Hirayama et al. Combination of 19 serum metabolites enabled accurate discrimination of DKD. Serum leucine, dihydrosphingosine, phytosphingosine. |
| MINI REVIEW article | Oxidative stress as a major culprit in kidney disease in diabetes. The occurrence and progression of DN is closely related with oxidative stress. Excessive ROS, which are induced by hyperglycemia, are involved in oxidative stress causing direct oxidation and damage of deoxyribonucleic acid DNA , proteins and lipids. Oxidative stress and diabetic complications. Circ Res. In , Ha et al. DNA damage in the kidneys of diabetic rats exhibiting microalbuminuria. Free Radic Biol Med. found that the 8-OHdG levels were significantly higher in cortex and nipples of diabetic mice induced by streptozotocin than in control mice, and they decreased after insulin treatment, which suggested that DN might be associated with oxidative stress and the formation of 8-OHdG. The following study by Hinokio et al. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. showed that urinary 8-OHdG excretion in patients suffering from type 2 diabetes mellitus complicated by nephropathy was higher than in patients without complications or in healthy control subjects. Moreover, there was a correlation between urinary 8-OHdG level and glycosylated hemoglobin HbA 1c. In this report, 8-OHdG was speculated to be a useful biomarker associated with complications secondary to DM. Zhao et al. Relationship of serum 8-OHdG and VEGF with diabetic nephropathy in diabetics. Chin J Diabetes. measured the serum concentration of 8-OHdG using enzyme-linked immunosorbent assay ELISA and drew a similar conclusion. However, Serdar et al. demonstrated that there was no difference in urinary 8-OHdG levels between the groups with and without diabetic nephropathy on liquid chromatography-mass spectrometry, suggesting that 8-OHdG in urine was not a sensitive biomarker regarding albumin to creatinine ratio UACR for distinguishing DN patients from DM patients. Comparison of 8-hydroxy-2'-deoxyguanosine 8-OHdG levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radic Res. Different biological fluids and methods might contribute to the lack of consistency in these studies, so that the predictive value of 8-OHdG in the early stages of DN needs further research to be determined. Biomarkers associated with protein injury comprise pentosidine, 2,4-dinitrophenylhydrazine DNPH and advanced oxidation protein product AOPP. F2-isoprostaglandin and 4-hydroxy-nonenal HNE are related to lipid injury. Calabrese et al. found that both urinary and serum levels of pentosidine, DNPH, F2-isoprostaglandin and HNE of DN patients were higher than those of control subjects. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. Tabak et al. showed that the level of AOPP in type 2 diabetes mellitus patients with complications such as DN and diabetic retinopathy was significantly higher than in patients without complications. Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med. These two studies have confirmed that oxidative stress damage is involved in the development of diabetic nephropathy. A growing number of studies reported that DM and its complications were closely related to oxidative stress, so we supposed that the biomarkers related to antioxidant defense system and lipid peroxidation LPO induced by free radicals may be potential biomarkers of kidney damage in diabetic patients. Glutathione s-transferase GST , a kind of enzyme involved in cell detoxification, promotes inactivation and excretion of toxins by combining toxic drophobic compounds with glutathione. Experimental data from a study by Jiang et al. showed that the expression level of GST in diabetic rats induced by streptozotocin was remarkably higher than in control rats, suggesting that hyperglycemia may be the major cause for elevated GST. Eight weeks after treatment with resveratrol, the GST expression decreased and several indicators suggesting the occurrence of DN such as urinary protein excretion, creatinine, cellular apoptosis and renal hypertrophy were all improved, leading researchers to suppose that resveratrol likely played a role in renoprotection by lowering the expression level of GST. Resveratrol attenuates early diabetic nephropathy by down-regulating glutathione s-transferases Mu in diabetic rats. J Med Food. In agreement with GST, animal experiments on LPO have yielded the same results. Diosmin modulates the NF-kB signal transduction pathways and downregulation of various oxidative stress markers in alloxan-induced diabetic nephropathy. Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren Fail. In addition, genetic investigation also found that knockout of GST coding genes can lead to decreased GST levels and increased malondialdehyde MDA levels, an important biomarker of LPO, demonstrating that GST has an effect against oxidative stress. Effect of GSTM1 and GSTT1 double deletions in the development of oxidative stress in diabetic nephropathy patients. Indian J Biochem Biophys. Human research was consistent with the experimental studies above. Compared with healthy subjects, increased activity of GST and increased level of MDA were found in type 2 diabetes mellitus patients. These results suggested that oxidative stress was involved in the occurrence of DM and GST was likely to play an important role in antioxidation. Variations in erythrocyte antioxidant levels and lipid peroxidation status and in serum lipid profile parameters in relation to blood haemoglobin A1c values in individuals with type 2 diabetes mellitus. Blood ALDH1 and GST activity in diabetes type 2 and its correlation with glycated hemoglobin. Exp Clin Endocrinol Diabetes. In the study about GST and DN, Noce et al. reported that GST activity in type 2 diabetes mellitus patients with and without nephropathy were both significantly higher than that of control subjects, appearing to be closely related with the stages of DN and indicating that GST was likely to be a potential biomarker in early stage DN. Erythrocyte glutathione transferase activity: a possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. Inflammatory response could be activated by biochemical, metabolic or hemodynamic disorders when a large number of white blood cells gather in the kidney. Then, pro-inflammatory cytokines and a variety of chemokines secreted by leukocytes may guide the latter into the kidney directly. Thus, a new cycle of inflammatory response is induced. The inflammatory cytokines and chemokines involved were hypothesized as potential biomarkers of DN. Liu et al. detected urinary levels of 27 kinds of inflammation-related factors of type 2 diabetes mellitus patients by multiplex bead immunoassay. They found that the levels of proinflammatory cytokines such as interleukin-8 IL-8 , tumor necrosis factor TNF-α and chemokines such as monocyte chemoattractant protein-1 MCP-1 , interferon-inducible protein IP in patients with microalbuminuria were all significantly higher than those of patients with normoalbuminuria and the control subjects. Besides, the levels of MCP-1 and IP were positively correlated with proteinuria and HbA 1c , while negatively correlated with the estimated glomerular filtration rate eGFR. Multiplex bead analysis of urinary cytokines of type 2 diabetic patients with normo- and microalbuminuria. J Immunoassay Immunochem. These outcomes suggest that urinary inflammation-related factors may contribute to the diagnosis in early stages of DN. In addition, some studies have shown that serum interleukin IL level was elevated in DN patients and associated with HbA 1c or UACR, thus being speculated as a potential biomarker of diabetic nephropathy. Elevated levels of interleukin and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. On the other hand, the value of interleukin-6 IL-6 in early diagnosis of diabetic nephropathy remains to be further confirmed. A number of studies have found that serum IL-6 levels of patients with normoalbuminuria or microalbuminuria were higher than those of control subjects and showed a positive correlation with UACR. Influence of renal involvement on peripheral blood mononuclear cell expression behavior of tumour necrosis factor-alpha and interleukin-6 in type 2 diabetic patients. Nephrol Dial Transplant. Usefulness of a highly sensitive urinary and serum IL-6 assay in patients with diabetic nephropathy. J Hypertens. Hum Immunol. However, some other studies have found that serum IL-6 level was elevated in patients with macroalbuminuria alone, and its early diagnosis value was not as good as that of urinary albumin excretion. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. Some studies demonstrated that an increase in both urinary and serum levels of TNF-α in patients with nephropathy secondary to DM was found compared to those with normoalbuminuria and control subjects. Besides, levels of TNF-α in urine and serum were both significantly associated with urinary albumin excretion. These results revealed that TNF-α might be an early biomarker of kidney damage in diabetic patients. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Clin Chim Acta. Soluble CD40 ligand sCD40L is a transmembrane protein of the tumor necrosis factor superfamily and regulates inflammatory response by binding with CD A study by El-Asrar et al. Soluble CD40L in children and adolescents with type 1 diabetes: relation to microvascular complications and glycemic control. Pediatr Diabetes. showed that serum sCD40L level in type 1 diabetes mellitus patients with microangiopathy such as diabetic nephropathy, retinopathy or neuropathy was significantly higher than that of patients without complications and healthy control subjects, and diabetic patients without any of these complications presented higher sCD40L concentration as compared to healthy subjects. The researchers also found that serum sCD40L was significantly associated with the severity of kidney damage and the level of glycemic control. Increased concentrations of soluble CD40 ligand may help to identify type 1 diabetic adolescents and young adults at risk for developing persistent microalbuminuria. Diabetes Metab Res Rev. In addition to the biomarkers cited above, glycosyl hydrolase family of 18 members, including chitotriosidase CHIT1 and cartilage glycoprotein 40 YKL , commonly activated by macrophages cells and neutrophils, were also involved in the inflammatory response. Role of chitotriosidase chitinase 1 under normal and disease conditions. J Epithel Biol Pharmacol. YKL, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. Several studies showed that both CHIT1 activity and YKL level of type 2 diabetes mellitus patients in all subgroups were higher than that of control subjects. CHIT1 activity and YKL level increased gradually along with the stages of DN according to UACR, which was correlated with activity of CHIT1 and level of YKL even after adjustment for clinical parameters, suggesting that they were both associated with kidney damage of DN patients. However, because of the higher sensitivity and specificity, CHIT1 activity was better in the diagnosis of persistent microalbuminuria compared with serum level of YKL YKL levels are independently associated with albuminuria in type 2 diabetes. Cardiovasc Diabetol. Proteins from the 18 glycosyl hydrolase family are associated with kidney dysfunction in patients with diabetes type 2. Renin-angiotensin-aldosterone system RAAS plays an important role in regulating blood pressure by producing aldosterone in human body. Angiotensinogen, produced by liver, was reported in patients with chronic glomerulonephritis in a previous study. Urinary angiotensinogen accurately reflects intrarenal renin-angiotensin system activity. Am J Nephrol. The following study found that urinary angiotensinogen excretion of type 2 diabetes mellitus patients with microalbuminuria and macroalbuminuria were both significantly increased compared to control subjects, as well as to normoalbuminuric patients, suggesting that angiotensinogen appeared prior to the establishment of albuminuria. Also, angiotensinogen level shows a strong association with urinary albumin excretion, which is an indicator of the severity of kidney damage in diabetic patients. Angiotensinogen may be a promising biomarker in the early stages of DN due to its high sensitivity and specificity in diagnostic analysis of diabetic nephropathy. Urinary angiotensinogen as a potential biomarker of diabetic nephropathy. Clin Kidney J. These biomarkers were summarized in Figure 1. DN: diabetic nephropathy; RAAS: renin-angiotensin-aldosterone system; DNA: deoxyribonucleic acid; IL interleukin-8; IL interleukin; IL interleukin-6; TNF-α: tumor necrosis factor-α; MCP monocyte chemoattractant protein-1; IP interferon-inducible protein; 8-OHdG: 8-dihydro-2'-deoxyguanosine; AOPP: advanced oxidation protein product; GST: glutathione s-transferase; sCD40L: soluble CD40 ligand; CHIT1: chitotriosidase; DNPH: 2,4-dinitrophenylhydrazine; HNE: 4-hydroxy-nonenal; MDA: malondialdehyde; YKL cartilage glycoprotein Under normal circumstances, podocyte and foot process, glomerular basement membrane and capillary endothelial cells constitute the glomerular filtration barrier. The damage of this filtration barrier can affect the glomerular filtration function. Markers such as podocytes, basement membrane and endothelial cell damage may have potential to indicate kidney damage in DN patients. Studies have shown that a decline in the number of podocytes and disappearance of foot processes often occur in the early stages of DN due to apoptosis or shedding of podocytes. Therefore, urinary podocytes and their specific protein products may be regarded as potential biomarkers of podocyte injury. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. Currently, the studies focused on the podocyte-specific protein products because it was difficult to detect urinary podocytes directly. One study by Wang et al. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin Pract. showed that urinary mRNA levels of podocin, synaptopodin and nephrin in DN patients were extremely higher than those found in control subjects by real-time quantitative PCR. These results were also proved by renal biopsy. Also, synaptopodin level was positively correlated with urinary albumin excretion and serum creatinine concentration while negatively correlated with GFR. Patients, however, were not divided into different subgroups according to their average level of urinary protein. The validation of these podocyte-specific protein products in early stages of DN was not confirmed in this study. Further research performed by Hara et al. revealed that urinary synaptopodin level of type 2 diabetes mellitus patients complicated by nephropathy was higher when compared to control subjects, even before the occurrence of proteinuria and associated with the level of urinary albumin and HbA 1c , indicating that synaptopodin was a biomarker with high sensitivity to podocyte injury in diabetic patients. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Another report by Jim et al. In addition, urinary level of nephrin showed a strong association with UACR so that it might be a useful biomarker for nephropathic patients in preclinical stage. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One. Type IV collagen is the main component of the glomerular basement membrane and extracellular matrix, and does not pass through glomerular filtration barrier under normal circumstances. Therefore, type IV collagen could be used as a biomarker of basement membrane injury. The study found that urinary type IV collagen levels were higher before microalbuminuria and associated with urinary albumin and serum creatinine, suggesting that urinary type IV collagen may be a promising biomarker for early diagnosis of DN. Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy. J Clin Lab Anal. Endothelial cells injury can directly affect the permeability of the glomerular filtration membrane. Generally, von Willebrand factor vWF is mostly synthesized by endothelial cells. Plasma vWF levels increase when endothelial cells are stimulated or damaged. Jensen 45 45 Jensen T. Increased plasma concentration of von Willebrand factor in insulin dependent diabetics with incipient nephropathy. first discovered that plasma levels of vWF are higher in type 1 diabetes mellitus patients, indicating that there is endothelial cell dysfunction in diabetic patients. Subsequently, a number of studies have shown that plasma vWF levels in patients with DN are significantly higher than those in patients without kidney disease and control subjects, indicating that plasma vWF may contribute to the early diagnosis of diabetic nephropathy. Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Vascular endothelial markers, von Willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: comparison of primary renal disease. Di Yi Jun Yi Da Xue Xue Bao. Hyperglycemia does aggravate vascular endothelial injury by up-regulating the expression of adhesion molecules by endothelial cells. Mechanism underlying up-regulation of ICAM-1 and VCAM-1 expressions induced by high glucose in endothelial cells. Chinese J Cardiovasc Med. The study about type 2 diabetes mellitus patients from Malaysia discovered that plasma levels of intercellular adhesion molecule-1 ICAM-1 are elevated in DN patients. Genetic, epigenetic and protein analyses of intercellular adhesion molecule 1 in Malaysian subjects with type 2 diabetes and diabetic nephropathy. J Diabetes Complications. Vascular endothelial growth factor VEGF can affect the filtration of large molecular weight proteins through glomerular filtration barrier by promoting endothelial cell proliferation and increasing vascular permeability. Researchers have found that plasma and urinary levels of VEGF in DN patients were both elevated. Especially in type 2 diabetes mellitus subjects, urinary VEGF level was higher in normoalbuminuric patients than in control subjects and gradually increased along with the DN stages. These findings suggested that VEGF may be an effective biomarker for early diagnosis in DN patients. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in Type 2 diabetes mellitus. Diabet Med. Fibrosis is one of the pathological features of diabetic complications caused by extracellular matrix alterations and mesangial expansion. Hyperglycemia up-regulates the expression of transforming growth factor-β1 TGF-β1 , which is considered to be the most crucial cytokine in glomerulosclerosis and tubulointerstitial fibrosis. Role of TGF-beta in the progression of renal fibrosis. Contrib Nephrol. Data by Xie showed that serum TGF-β1 level of patients with microalbuminuria was significantly higher than that of patients with normoalbuminuria and control subjects. Interestingly, urinary levels of TGF-β1 are already elevated in normoalbuminuria subjects and gradually increase along with DN progression, so that TGF-β1 was considered a sensitive biomarker in the early phase of diabetic nephropathy. Significance of serum and urinary TGF-β1 to the early diagnosis of diabetic nephropathy. Strait Pharmaceutical J. Pigment epithelial-derived factor PEDF is a member of the serine protease superfamily and is involved in the formation of extracellular matrix and vascular endothelial growth factor. PEDF levels were found to be decreased in the kidney of diabetic mice, suggesting that it may have a protective effect in diabetic microvascular lesions. Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. In addition to the increase in GBM thickness, tubular basement membrane TBM thickness is also predictive of early DN Tyagi et al. The study confirmed that TBM thickness combined with GBM thickness provided more predictive value for patients progressing to end-stage renal disease Zhao et al. Accompanied by inflammation, oxidative stress, and altered hemodynamics, renal tubular epithelial cells undergo cell proliferation and subsequent cell hypertrophy, cell death Thomas, ; Liu et al. In , Thomas et al. described the tubular changes in early DN. Four major structural alterations of the renal tubules are highlighted, which are tubular hypertrophy and hyperplasia; tubular atrophy and dilatation; thickening of the TBM; tubular Epithelial-Mesenchymal Transition Thomas et al. There is increasing evidence that regression of microalbuminuria is common in patients with T1D and that a significant proportion of non-albuminuric patients also develop progressive impairment of renal function Krolewski, Therefore, the diagnosis of DN may be more accurate by looking for markers that can characterize structural alterations. We therefore reviewed the literature based on 1 the association with specific renal structural alterations in patients with DN and 2 the fact that in clinical studies, alterations in protein expression appear early in DN and have the potential to predict renal function. In this review, we present a detailed description of some of the proteins that have obtained adequate studies and are considered to have great potential to become markers of DN, but the association between other proteins and structural alterations cannot be denied. The degree of mesangial expansion, one of the structural abnormalities of the glomerulus, is associated with the development of DN. In the absence of elevated blood pressure or reduced creatinine Cre clearance, extensive studies of glomerular structure in diabetic patients with or without microalbuminuria have found significant differences in glomerular structural changes e. mesangial matrix expansion. In the Streptozotocin STZ -induced DN model in rats, urinary SMAD1 excretion was strongly correlated with the severity of expansion of the mesangial matrix Matsubara et al. During the glomerular hyperfiltration phase, urinary SMAD1 levels were significantly elevated, indicating that mesangial expansion had occurred Fu et al. Therefore, the role of urinary SMAD1 levels in early DN needs to be further investigated. Podocalyxin PCX is a podocyte membrane protein and it is a major component of the GBM charge barrier. Glomerular filtration barrier permeability correlates with PCX integrity Hara et al. As a marker protein of podocyte injury, podocyte injury can result in decreased levels and increased excretion of PCX in the glomerulus Akankwasa et al. In diabetic patients, PCX protein concentration of urine supernatant is higher than the critical value in Moreover, compared with the glomerular high PCX expression group, DN patients in the low expression group had a longer duration of diabetes, and the kidney survival rate in the high expression group was significantly higher than that in the low expression group Wang et al. It indicates that PCX has some value in characterizing the onset stage of DN patients Ye et al. Recent literature reports that tubular damage appears in the early stages of DN and promotes the progression of renal disease Guo et al. In children and adolescents with T1D, NGAL fractions were detected in the extracellular vesicles of urine at higher levels than in urine from T1D patients without exosomes and in normal controls. In addition, NGAL has been present in patients without microalbuminuria or with a normal albumin-to-creatinine ratio, suggests that tubular damage occurred before the onset of classic DN symptoms Ugarte et al. NGAL has also been shown to be a marker of early nephropathy injury in patients with T2DN Żyłka et al. A lack of independent correlation between tubular injury markers and glomerular filtration rate has been reported and cannot be used to improve the management of DN, suggesting that NGAL is specific as a marker of tubular injury Kuwabara et al. Therefore, further studies are needed for the predictive value of NGAL in early DN injury. Netrin-1 is secreted protein highly induced after chronic and acute kidney injury. It can be detected in urine in both mice and human, and can be used as a marker for acute kidney injury Levey et al. Netrin-1 has also been reported in DN. Using a case-control study, Ay et al. showed that plasma Netrin-1 levels were significantly higher in microalbuminuric diabetic patients than in normoalbuminuric diabetic patients and controls, but there was no significant difference between normoproteinuric patients and controls Ay et al. However, a recent study showed that Netrin-1 estimation in urine has higher accuracy than Netrin-1 estimation in serum and is a potential marker for early diagnosis of DN Jayakumar et al. In type I diabetic animals, Netrin-1 expression was increased in proximal renal tubular epithelial cells and Netrin-1 was significantly elevated in the early phase without microalbuminuria and the late phase of all diabetic nephropathies compared to controls White et al. However, whether Netrin-1 is affected by short-term blood glucose fluctuations requires further study Uçaktürk et al. Fatty acid binding protein 1 FABP1 or L-FABP is a small 14 kDa molecule protein expressed in the human proximal renal tubule. The circulating portion of FABP1 is filtered by the glomerulus and then reabsorbed by the proximal tubule, which explains its increased concentration in the urine when proximal tubular cells are injured Pelsers et al. Staging of T2D patients by eGFR and urinary albumin and assessing urinary L-FABP levels in patients with different albumin levels showed that urinary L-FABP levels were significantly higher in diabetic patients with normal urinary albumin than in normal controls in the presence of renal impairment, suggesting that urinary L-FABP detects renal disease in diabetic patients earlier than urinary albumin Thi et al. Although L-FABP levels were significantly negatively correlated with eGFR and increased with proteinuria severity, markers of tubular damage do not appear to be predictors of decreased GER in patients with T2D Kamijo-Ikemori et al. Studies in T1D patients, suggesting that urinary L-FABP is an independent predictor of tubular damage in DN and remains useful in the early stages of DN Panduru et al. In addition to the biomarkers mentioned above, there are a large number of proteins that characterize tubular injury, glomerular filtration, mesangial dilation, vascular injury, and renal inflammation Figure 1 upper. These proteins have been extensively studied and tested in the urine or blood of diabetic patients, and experiments have confirmed that these proteins are associated with specific structural damage and are able to characterize the development of the disease. FIGURE 1. Presentation of biomarkers in the nephron upper and protein interaction networks and biological processes below. Abbreviation: NGAL: Neutrophil gelatinase associated lipocalin, B2M: Betamicroglobulin, AGT: Angiotensinogen, KIM Kidney injury molecule-1, RBP: Retinol-binding protein, L-FABP: Liver type fatty acid binding protein, COLIV: Collagen IV, NAG: N-Acetyl-B- d -glycosaminidase, ANGPT2: Angiopoietin-2, AQP: Aquaporin, DBP: Vitamin D binding protein, L-PGDS: Lipocalin-type prostaglandinD2-synthase, VEGF: Vascular endothelial growth factor, MCP Monocyte chemoattractant protein-1, TNF-α: Tumour necrosis factor-alpha. The pathophysiology of DN is complex and includes hemodynamic changes, oxidative stress, activation of the renin-angiotensin system, metabolic changes and various intracellular signaling Roointan et al. Altered protein expression levels are associated with the progression of DN, and as a systemic metabolic disease, the disease may not seem to be fully characterized based on a specific protein Zürbig et al. The above proteins are linked to specific structural damage, and bioinformatic methods can be used to better understand the linkage of proteins and the biological processes involved Geng et al. These proteins were also analyzed for enrichment by biological processes, and the results indicate that they are mainly enriched in extracellular matrix organization, iron ion transport, kidney development, regulation of angiogenesis, regulation of cell motility, and response oxygen-containing compounds Figure 1 below. Oxidative stress, disturbances in lipid metabolism play a continuous role in the early stages of DN, resulting in elevated levels of kidney inflammation and increased cell death. Wellen and Hotamisligil, Processes closely associated with the persistent early elevation of blood glucose, such as increased ion transport-related proteins transferrin and ceruloplasmin, reflect endothelial cell dysfunction and increased intra-glomerular pressure Narita et al. A recent study showed a detailed interpretation of the progression of DN by combining proteomics and peptidomics in the urine of diabetic subjects, and the results of our analysis have similarities to this study Van et al. To better understand the pathological features of DN and to search for potential biomarkers with higher specificity and accuracy, several proteomic studies have been carried out in the last years. Comparisons between diabetic subjects at different stages of renal dysfunction and controls showed differences in the expression of multiple proteins. Seven proteins were progressively upregulated with increasing proteinuria, and the transporter protein VDBP was reported for the first time in the urine of patients with DN Rao et al. In another study, proteomic analysis identified haptoglobin as a candidate biomarker for predicting early decline in renal function, and the ratio of haptoglobin to creatinine has the ability to predict renal function in diabetic patients who have not yet exhibited significant renal disease Bhensdadia et al. Urine has an irreplaceable role in detecting kidney status. Characterizing the urinary proteomics of patients with different stages of DN helps to understand the state of the kidney and is important for finding potential biomarkers Papale et al. This is useful in understanding the condition of patients with DN and in finding promising treatment pathways. In addition to changes in protein levels, alterations in metabolites are also present in diabetic nephropathy, lactic acid, hippuric acid, allantoin in the urine and glutamine in the blood are the most important early diagnostic biomarkers in the pathogenesis of DN. Roointan et al. The effects of metabolic memory on DN may be long-lasting, profoundly affecting disease development and treatment through epigenetic modifications. Kushwaha et al. The development of DN is a complex process, such as GBM thickening in the early stages and glomerulosclerosis and interstitial fibrosis in the later stages, involving different cell types at different stages. The application of single-cell RNA sequencing scRNA-seq in kidney disease has allowed us to identify cell types in tissues and provide insight into cellular damage and gene expression patterns in different stages of DN. Latt et al. Wilson et al. Additional, scRNA-seq was used to analyze the response of DN mouse models to five common treatment regimens and found that different drugs had significantly different effects on cell types, even with combination therapy. Wu et al. The existence of computational cell trajectory analysis methods allows to simulate the process of the kidney from a normal state to the onset of lesions, thus avoiding experimental errors. Fu et al. scRNA-seq technology provides a more precise means for us to diagnose and treat DN. DN is one of the microvascular complications of diabetes mellitus, but the development and progression of the disease are not only caused by hyperglycemia and hypertension; genetic factors, lifestyle habits, and other coexisting diseases can all have an impact on DN. Inflammation and oxidative stress play an extremely important role in diabetes as well as in renal disease, and therefore the detection of relevant biomarkers can be used to predict, diagnose and treat DN. However, it is worth noting that some of these tests are already present during diabetes and may not be suitable as biomarkers for DN. Tubular and glomerular-related biomarkers are of immediate value in indicating kidney injury, but some of them have a lag. The advent of new technologies has greatly helped in understanding the pathology of DN and in finding appropriate biomarkers. Combining multiple indicators to evaluate DN may have better results. Conceptualization, HL and LT. All authors have read and agreed to the published version of the manuscript. This research was funded by the Natural Science Foundation of Chongqing ycjh-bgzxm; cstcjcyjcxttXcstc and graduate research and innovation foundation of Chongqing CYB The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Akankwasa G. Urine markers of podocyte dysfunction: A review of podocalyxin and nephrin in selected glomerular diseases. PubMed Abstract CrossRef Full Text Google Scholar. Aly M. Study of Angiopoietin-2 and vascular endothelial growth factor as markers of diabetic nephropathy onset in Egyptians diabetic patients with non-albuminuric state. Diabetes Metab. Evaluation of netrin-1 levels and albuminuria in patients with diabetes. Bhensdadia N. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. Caramori M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. Chen C. Normoalbuminuric diabetic kidney disease. Chen J. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69 11 , — Chou K. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PloS One 8 1 , e Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. Correlation of high urinary Smad1 level with glomerular hyperfiltration in type 2 diabetes mellitus. Endocrine 43 2 , — Geng X. Identification of key genes and pathways in diabetic nephropathy by bioinformatics analysis. Diabetes Investig. Guo J. Increased tubular proliferation as an adaptive response to glomerular albuminuria. Hara M. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: Establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55 11 , — Podocalyxin on the glomerular epithelial cells is preserved well in various glomerular diseases. Nephron 67 1 , — Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol. Herbach N. Diabetic kidney lesions of GIPRdn transgenic mice: Podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Hills C. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. IDF IDF diabetes atlas. Belgium: International Diabetes Federation. Google Scholar. Jayakumar C. Netrin-1, a urinary proximal tubular injury marker, is elevated early in the time course of human diabetes. Jenkin K. Endocannabinoids and the renal proximal tubule: An emerging role in diabetic nephropathy. Cell Biol. Kamijo-Ikemori A. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 34 3 , — Kotajima N. Type IV collagen as an early marker for diabetic nephropathy in non-insulin-dependent diabetes mellitus. Diabetes Complicat. Kriz W. Accumulation of worn-out GBM material substantially contributes to mesangial matrix expansion in diabetic nephropathy. Krolewski A. Progressive renal decline: The new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 38 6 , — Kushwaha K. Metabolic memory and diabetic nephropathy: Beneficial effects of natural epigenetic modifiers. Biochimie , — Kuwabara T. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Latt K. Glomerular kidney diseases in the single-cell era. Levey A. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes KDIGO. Liu L. Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol. Matsubara T. |
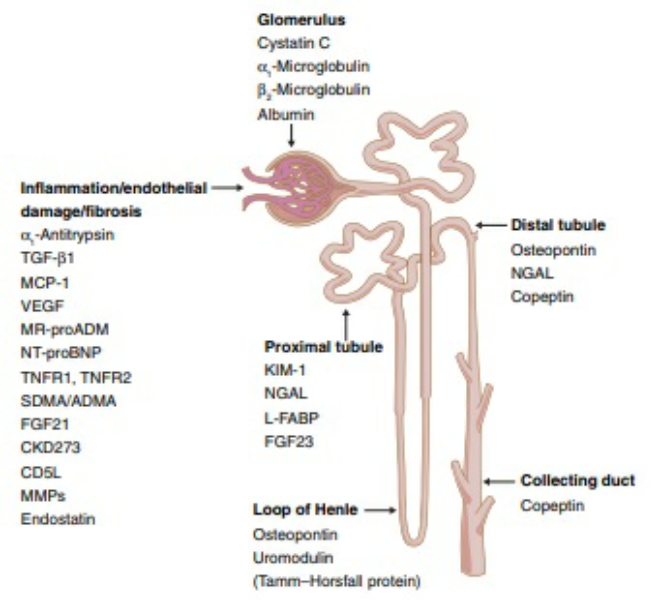
Diabetic nephropathy biomarkers -
Similarly, patients with T2DM who had microalbuminuria had been reported elevated serum and urinary TGF-β [ ]. On the other hand, Rivarola et al.
Supporting this, a study conducted among T2DM resulted in increased serum and urine TGF-β, being more pronounced in macroalbuminuria compared to microalbuminuria and normoalbuminuria group [ ], suggesting that this biomarker could be a good candidate in predicting macroalbuminuria in type 2 DM.
CTGF is a secretory protein in renal cells induced by hyperglycemia. It stimulates extracellular matrix synthesis, cell migration, and interstitial matrix deposition by the epithelial-to-mesenchymal transition in diabetic patients [ ].
T1DM patients showed increased urinary CTGF with the severity of renal function deterioration in terms of albumin excretion and GFR decline [ ] and could be a predictor for ESRD with an AUC under ROC of 0.
Its expression has also been reported in diabetic retinopathy [ ]. CTGF could be an independent predictor of ESRD and mortality in DKD and it is also a predictor of diabetic retinopathy and looks promising. It is a major immunoregulatory cytokine in mesangial expansion.
Sangoi et al. observed higher serum IL-6 even before the onset of albuminuria [ ]. Multiple studies support these findings with evidence that serum and urinary IL-6 were increasing with disease progression in T1DM [ ] and T2DM [ ] and have a pronounced association with macrovascular complications [ ].
Thus, it could be a marker of the onset of microalbuminuria and early progressive renal decline, and it strongly predicts the macrovascular complications of diabetes. The classification based on pathogenesis and utilization of these biomarkers can be the future in predicting the early onset of microalbuminuria and progressive renal function decline in both T1DM and T2DM.
These biomarkers are relevant to not only predicting the progression of DKD but also diabetic microvascular and macrovascular complications, as shown in Table 2. Few of the biomarkers have been studied in the type 2 diabetic population on their diagnostic utility for DKD and established cut-off value with the area under the ROC curve from 0.
These are 0. Under hyperglycaemic injury, renal cells are activated to release MPs into plasma and urine before the onset of DKD [ ]. Therefore, these have emerged as biomarkers in DKD. A review by Sheyu Li et al.
reported a higher level of circulating MPs reported with T2DM and as independent predictors for microvascular complications of diabetes [ ]. MPs can be easily isolated from body fluids via non-invasive methods. These properties facilitate the use as a potential non-invasive biomarker in the progression of DKD.
More large-scale studies are needed for further relevance in this regard. Urinary exosomes, 40— nm originate as internal vesicles, and that contain protein indicators of renal failure and structural damage. It has turned out to be a potential non-invasive biomarker source. However, exosome isolation was challenging.
Using liquid chromatography and mass spectrometry, the scope of existing approaches has enlarged to evaluate more urinary exosome-associated proteins [ ]. In recent years, microRNA is reported to be involved in the DKD progression via inflammation, hypertrophy, autophagy, endoplasmic reticulum ER stress, oxidative stress, insulin resistance, and podocyte injury.
Jia et al. observed a positive correlation of miRNA expression for TGF beta stimulator with albuminuria and reported good diagnostic efficiency [ ]. Based on the evidence mentioned above, urine mRNA has prognostic significance as a non-invasive, early indicator of renal impairment.
The pathophysiology of DKD and its progression is multifactorial. Therefore, assessment of development and progression of DKD cannot be relied solely on albuminuria and creatinine.
The identification of novel biomarkers based on pathogenesis of DKD involving various renal structures looks promising. In this review, we have summarized the potential 22 novel biomarkers with respect to the pathogenesis of DKD development.
Each biomarker has its role in either identifying DKD early or predicting progression of DKD over and above clinical history and standardized markers like albuminuria and creatinine.
Few of them appear to be useful for predicting other micro- and macrovascular complications like retinopathy and cardiovascular disease. This panel of biomarkers now warrants further validation on large-scale longitudinal studies involving type 1 and type 2 diabetes populations before the transition to clinical routine.
Tuttle KR, Bakris GL, Bilous RW et al Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis — Article PubMed Google Scholar. Bikbov B, Purcell CA, Levey AS et al Global, regional, and national burden of chronic kidney disease, — a systematic analysis for the Global Burden of Disease Study Lancet — Article Google Scholar.
Chen C, Wang C, Hu C et al Normoalbuminuric diabetic kidney disease. Front Med — Porrini E, Ruggenenti P, Mogensen CE et al Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes.
Lancet Diabetes Endocrinol — Article CAS PubMed Google Scholar. Do S Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int — Hussain S, Jamali MC, Habib A et al Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers.
Clin Epidemiol Glob Health —6. Article CAS Google Scholar. Ilyas Z, Chaiban JT, Krikorian A Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy.
Rev Endocr Metab Disord 18 1 — Satirapoj B Review on pathophysiology and treatment of diabetic kidney disease. J Med Assoc Thail 93 Suppl 6 :S—S Google Scholar. Lassén E, Daehn IS Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction.
Int J Mol Sci 21 24 Article PubMed PubMed Central Google Scholar. Gilbert RE Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease.
Diabetes — Sakashita M, Tanaka T, Inagi R Metabolic changes and oxidative stress in diabetic kidney disease. Pichler R, Afkarian M, Dieter BP, Tuttle KR Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets.
Am J Physiol-Renal Physiol 4 :F—F Molitch ME, Steffes M, Sun W et al Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study.
Diabetes Care — Article CAS PubMed PubMed Central Google Scholar. Lin C-H, Chang Y-C, Chuang L-M Early detection of diabetic kidney disease: present limitations and future perspectives. World J Diabetes Hamaguchi K, Tsuchida H, Miura Y, Suzuki S, Kawamura T, Hosoya T, Yamada K Urinary type IV collagen excretion reflects renal morphological alterations and type IV collagen expression in patients with type 2 diabetes mellitus.
Clin Nephrol 55 5 — PubMed Google Scholar. Tomino Y, Suzuki S, Azushima C, Shou I, Iijima T, Yagame M, Wang LN, Chen HC, Lai KN, Tan SY, Kim MJ Asian multicenter trials on urinary type IV collagen in patients with diabetic nephropathy.
J Clin Lab Anal 15 4 — Iijima T, Suzuki S, Sekizuka K, Hishiki T, Yagame M, Jinde K et al Follow-up study on urinary type IV collagen in patients with early stage diabetic nephropathy. J Clin Lab Anal 12 6 — Morita M, Uchigata Y, Hanai K et al Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes.
Araki S, Haneda M, Koya D et al Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Hayashi Y, Makino H, Ota Z Serum and urinary concentrations of type IV collagen and laminin as a marker of microangiopathy in diabetes.
Diabetic Med 9 4 — Ozata M, Kurt I, Azal O, Bolu E, Corakci A, Beyhan Z, Karaca L, Gündogăn MA Can we use plasma fibronectin levels as a marker for early diabetic nephropathy. Endocr J 42 2 — Takahashi M Increased urinary fibronectin excretion in type II diabetic patients with microalbuminuria.
Nihon Jinzo Gakkai shi 37 6 — CAS PubMed Google Scholar. Fagerudd JA, Groop PH, Honkanen E, Teppo AM, Grönhagen-Riska C Urinary excretion of TGF-beta 1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patients. Kidney Int Suppl S—S Kanters SDJM, Banga J-D, Algra A et al Plasma levels of cellular fibronectin in diabetes.
Setty S, Michael AA, Fish AJ et al Differential expression of laminin isoforms in diabetic nephropathy and other renal diseases. Mod Pathol — Diabetes Res Clin Pract — El-Fattah MEA, Rashed LA, Nasr SMM The role of transferrin and laminin biomarkers in the diagnosis of diabetic nephropathy in type II diabetic patients.
J Adv Med Med Res — Okazaki R, Matsuokab K, Atsumib Y et al Serum concentrations of basement membrane proteins in NIDDM as a prognostic marker for nephropathy. Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate.
Acta Med Scand 5 — Papadopoulou-Marketou N, Skevaki C, Kosteria I et al NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up.
Hormones — Qamar A, Hayat A, Ahmad TM, Khan A, Hasnat MNU, Tahir S Serum cystatin C as an early diagnostic biomarker of diabetic kidney disease in type 2 diabetic patients.
J Coll Physicians Surg Pak 28 4 — Macisaac RJ, Ekinci EI, Jerums G Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. Qian C, Wan GM, Yan PS, Wang WZ, Liang SZ, Dong Y Correlation between Cystatin C and retinopathy of type-two diabetes mellitus patients.
J Biol Regul Homeost Agents 31 1 — Chung JO, Cho DH, Chung DJ, Chung MY Serum Cystatin C levels are positively associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 10 — Shlipak MG, Mattes MD, Peralta CA Update on Cystatin C: incorporation into clinical practice.
Am J Kidney Dis 62 3 — J Diabetes Complicat — De Muro P, Fresu P, Tonolo G et al A longitudinal evaluation of urinary glycosaminoglycan excretion in normoalbuminuric type 1 diabetic patients. Clin Chem Lab Med — Popławska-Kita A, Mierzejewska-Iwanowska B, Szelachowska M et al Glycosaminoglycans urinary excretion as a marker of the early stages of diabetic nephropathy and the disease progression.
Diabetes Metab Res Rev — Kahaly G, Hansen Ch, Otto E et al Diabetic microangiopathy and urinary glycosaminoglycans. Exp Clin Endocrinol Diabetes — Budak Y, Demirci H, Akdogan M, Yavuz D Erytrocyte membrane anionic charge in type 2 diabetic patients with retinopathy. BMC Ophthalmol —6.
Mohan S, Kalia K, Mannari J Urinary IgG is a pure strong indicator of diabetic nephropathy than microalbuminuria in type 2 diabetic patients. Int J Diabetes Dev Ctries — Yashima I, Hirayama T, Shiiki H, Kanauchi M, Dohi K Diagnostic significance of urinary immunoglobulin G in diabetic nephropathy.
Nihon Jinzo Gakkai Shi 41 8 — Narita T, Sasaki H, Hosoba M et al Parallel increase in urinary excretion rates of immunoglobulin g, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients.
Bakoush O, Tencer J, Tapia J et al Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Lee MJ, Jung CH, Kang YM et al Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in korean men with type 2 diabetes mellitus.
Diabetes Metab J — Cunninghamn J, Leffell M, Mearkle P, Harmatz P Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: Evidence for increased oxidative stress as a variable complication.
Metabolism — Hirawa N, Uehara Y, Ikeda T et al Urinary prostaglandin D synthase β-trace excretion increases in the early stage of diabetes mellitus. Nephron — Uehara Y, Makino H, Seiki K et al Urinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: a cross-sectional and prospective multicentre study.
Nephrol Dial Transpl — Hamano K, Totsuka Y, Ajima M et al Blood sugar control reverses the increase in urinary excretion of prostaglandin D synthase in diabetic patients.
Zhao L, Zou Y, Zhang J et al Serum transferrin predicts end-stage renal disease in type 2 diabetes mellitus patients. Int J Med Sci — Gonzalez S, Vargas L Diabetogenic transferrin damages podocytes in early human diabetic nephropathy.
Horm Metab Res — Kanauchi M, Nishioka H, Hashimoto T, Dohi K Diagnostic significance of urinary transferrin in diabetic nephropathy. Jpn J Nephrol — Diabetes Med — Cheung CK, Cockram CS, Yeung VT, Swaminathan R Urinary excretion of transferrin by non-insulin-dependent diabetics: a marker for early complications?
Clin Chem — Sasaki A, Oikawa S, Toyota T Microalbuminuria is closely related to diabetic macroangiopathy. Hwang S, Park J, Kim J et al Tissue expression of tubular injury markers is associated with renal function decline in diabetic nephropathy. Kaul A, Behera MR, Rai MK et al Neutrophil gelatinase-associated lipocalin: as a predictor of early diabetic nephropathy in type 2 diabetes mellitus.
Indian J Nephrol Bolignano D, Lacquaniti A, Coppolino G et al Neutrophil gelatinase-associated lipocalin NGAL and progression of chronic kidney disease. Clin J Am Soc Nephrol — Duan S, Chen J, Wu L et al Assessment of urinary NGAL for differential diagnosis and progression of diabetic kidney disease.
J Diabetes Complicat He P, Bai M, Hu JP et al Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: a systematic review and meta-analysis.
Kidney Blood Press Res — Zeng X-F, Lu D-X, Li J-M et al Performance of urinary neutrophil gelatinase-associated lipocalin, clusterin, and cystatin C in predicting diabetic kidney disease and diabetic microalbuminuria: a consecutive cohort study.
BMC Nephrol — Abbasi F, Moosaie F, Khaloo P et al Neutrophil gelatinase-associated lipocalin and retinol-binding protein-4 as biomarkers for diabetic kidney disease. Garg V, Kumar M, Mahapatra HS et al Novel urinary biomarkers in pre-diabetic nephropathy.
Clin Exp Nephrol — Kim SS, Song SH, Kim IJ et al Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Pirgakis KM, Makris K, Dalainas I et al Urinary Cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair.
Ann Vasc Surg — Rao X, Wan M, Qiu C, Jiang C Role of cystatin C in renal damage and the optimum cut-off point of renal damage among patients with type 2 diabetes mellitus.
Exp Ther Med — van Timmeren M, van den Heuvel M, Bailly V et al Tubular kidney injury molecule-1 KIM-1 in human renal disease. J Pathol — Ichimura T, Bonventre JV, Bailly V et al Kidney injury molecule-1 KIM-1 , a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury.
J Biol Chem — Samia IA, Amal AM, Rehab AM, Hebat-Allah EG Kim-1 and Ngal as biomarkers of nephropathy in type II diabetes. Int J of Adv — Hammoud MS, Baban RS, Ali SH Evaluation of urinary kidney injury molecule-1 kim-1 as prognostic biomarker in children with type-1 diabetic nephropathy.
Biochem Cell Arch — De Carvalho JAM, Tatsch E, Hausen BS et al Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes.
Clin Biochem — Cabré A, Lázaro I, Girona J et al Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med — Bangstad HJ, Kierulf P, Kjærsgaard P et al Urinary excretion of retinol-binding protein in healthy children and adolescents.
Pediatr Nephrol — Salem MA, El-Habashy SA, Saeid OM, El-Tawil MM, Tawfik PH Urinary excretion of n-acetyl-beta-D-glucosaminidase and retinol binding protein as alternative indicators of nephropathy in patients with type 1 diabetes mellitus. Pediatr Diabetes 3 1 — Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T Retinol binding protein-4 levels and clinical features of type 2 diabetes patients.
J Clin Endocrinol Metab 92 7 — Akbay E, Muslu N, Nayir E, Ozhan O, Kiykim A Serum retinol binding protein 4 level is related with renal functions in type 2 diabetes. J Endocrinol Invest 33 10 — Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage.
Kidney Int 62 5 — Kamijo-Ikemori A, Sugaya T, Yasuda T et al Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy.
Diabetes Care 36 5 — Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 36 1 — Diabetologia 45 6 — El Wakeel MA, Abou-el-asrar M, El-kassas GM, Elabd MA, Zeid DA, Sabry RN, Awadallah E Urinary markers of oxidative DNA damage in type 1 diabetic children: relation to microvascular complications.
Asian J Pharm Clin Res 10 10 — Wu LL, Chiou CC, Chang PY, Wu JT Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics.
Clin Chim Acta 1—2 :1—9. Tatsch E, Carvalho JAMD, Hausen BS et al Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Nishikawa T, Sasahara T, Kiritoshi S et al Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes.
Machowska A, Sun J, Qureshi AR et al Plasma pentosidine and its association with mortality in patients with chronic kidney disease.
PLoS ONE e Miura J, Yamagishi SI, Uchigata Y et al Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes.
Perkins BA, Rabbani N, Weston A et al High fractional excretion of glycation adducts is associated with subsequent early decline in renal function in type 1 diabetes.
Sci Rep — Beisswenger PJ, Moore LL, Brinck-Johnsen T, Curphey TJ Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. J Clin Investig — Nin JW, Jorsal A, Ferreira I et al Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes.
Bartáková V, Kuricová K, Pácal L et al Hyperuricemia contributes to the faster progression of diabetic kidney disease in type 2 diabetes mellitus.
De CS, Viazzi F, Pacilli A et al Serum uric acid and risk of CKD in type 2 diabetes. Higher levels of 8-OHdG were associated with increased risk of kidney disease. Age, sex, cohort, duration of diabetes, HbA1c, insulin therapy, systolic blood pressure, use of antihypertensive drugs, RAS inhibitors, diabetic retinopathy stage, lipid-lowering drugs, eGFR, albuminuria.
Serdar et al. Although urinary 8-OHdG levels increase in diabetic patients, their levels do not improve prediction of progressive DKD over and above measuring albuminuria. Omics-based novel biomarkers. Bhensdadia et al.
The haptoglobin to creatinine ratio may be useful to predict risk of DKD before the development of albuminuria or kidney function decline. Zurbig et al. CKD predicted progression to macroalbuminuria 5 years prior to actual onset. Age, sex, DM type, albuminuria, eGFR, systolic and diastolic blood pressure, HbA1c, glucose.
Roscioni et al. CKD predicted development of albuminuria independent of other kidney biomarkers used to predict DKD development or progression.
Albuminuria, eGFR, use of RAS inhibitors. Age, baseline eGFR, systolic and diastolic blood pressure. Tofte et al. High-risk patients defined by CKD were more likely to develop microalbuminuria. Age, sex, HbA1c, systolic blood pressure, retinopathy, albuminuria, eGFR.
Lindhardt et al. CKD predicted development of albuminuria. Treatment group, age, sex, systolic blood pressure, albuminuria, eGFR, HbA1c, diabetes duration. Han et al. Non-esterified and esterified fatty acid discriminated albuminuria stages. Hirayama et al. Combination of 19 serum metabolites enabled accurate discrimination of DKD.
Serum leucine, dihydrosphingosine, phytosphingosine. Zhang et al. Serum metabolite levels of leucine, dihydrosphingosine, and phytosphingosine were significantly different in patients with T2DM and healthy controls.
Urine hexose, glutamine, tysorine, plasma butenoylcarnitine, histidine. Pena et al. Urine hexose, glutamine, tyrosine, plasma butenoylcarnitine and histine predicted development of albuminuria. Looker et al.
A panel of 14 biomarkers that included the symmetric to asymmetric dimethylarginine ratio, and Cacylcarnitine increased the predictive ability of rapid progression. Age, sex, baseline eGFR, albuminuria, HbA1c, use of RAS inhibitors.
Urinary 3-hydroxy-isobutyrate, 3-methyl-crotonyglycine, aconitic acid, citric acid. Kwan et al. Although L-FABP levels were significantly negatively correlated with eGFR and increased with proteinuria severity, markers of tubular damage do not appear to be predictors of decreased GER in patients with T2D Kamijo-Ikemori et al.
Studies in T1D patients, suggesting that urinary L-FABP is an independent predictor of tubular damage in DN and remains useful in the early stages of DN Panduru et al. In addition to the biomarkers mentioned above, there are a large number of proteins that characterize tubular injury, glomerular filtration, mesangial dilation, vascular injury, and renal inflammation Figure 1 upper.
These proteins have been extensively studied and tested in the urine or blood of diabetic patients, and experiments have confirmed that these proteins are associated with specific structural damage and are able to characterize the development of the disease.
FIGURE 1. Presentation of biomarkers in the nephron upper and protein interaction networks and biological processes below. Abbreviation: NGAL: Neutrophil gelatinase associated lipocalin, B2M: Betamicroglobulin, AGT: Angiotensinogen, KIM Kidney injury molecule-1, RBP: Retinol-binding protein, L-FABP: Liver type fatty acid binding protein, COLIV: Collagen IV, NAG: N-Acetyl-B- d -glycosaminidase, ANGPT2: Angiopoietin-2, AQP: Aquaporin, DBP: Vitamin D binding protein, L-PGDS: Lipocalin-type prostaglandinD2-synthase, VEGF: Vascular endothelial growth factor, MCP Monocyte chemoattractant protein-1, TNF-α: Tumour necrosis factor-alpha.
The pathophysiology of DN is complex and includes hemodynamic changes, oxidative stress, activation of the renin-angiotensin system, metabolic changes and various intracellular signaling Roointan et al.
Altered protein expression levels are associated with the progression of DN, and as a systemic metabolic disease, the disease may not seem to be fully characterized based on a specific protein Zürbig et al.
The above proteins are linked to specific structural damage, and bioinformatic methods can be used to better understand the linkage of proteins and the biological processes involved Geng et al. These proteins were also analyzed for enrichment by biological processes, and the results indicate that they are mainly enriched in extracellular matrix organization, iron ion transport, kidney development, regulation of angiogenesis, regulation of cell motility, and response oxygen-containing compounds Figure 1 below.
Oxidative stress, disturbances in lipid metabolism play a continuous role in the early stages of DN, resulting in elevated levels of kidney inflammation and increased cell death.
Wellen and Hotamisligil, Processes closely associated with the persistent early elevation of blood glucose, such as increased ion transport-related proteins transferrin and ceruloplasmin, reflect endothelial cell dysfunction and increased intra-glomerular pressure Narita et al.
A recent study showed a detailed interpretation of the progression of DN by combining proteomics and peptidomics in the urine of diabetic subjects, and the results of our analysis have similarities to this study Van et al.
To better understand the pathological features of DN and to search for potential biomarkers with higher specificity and accuracy, several proteomic studies have been carried out in the last years.
Comparisons between diabetic subjects at different stages of renal dysfunction and controls showed differences in the expression of multiple proteins. Seven proteins were progressively upregulated with increasing proteinuria, and the transporter protein VDBP was reported for the first time in the urine of patients with DN Rao et al.
In another study, proteomic analysis identified haptoglobin as a candidate biomarker for predicting early decline in renal function, and the ratio of haptoglobin to creatinine has the ability to predict renal function in diabetic patients who have not yet exhibited significant renal disease Bhensdadia et al.
Urine has an irreplaceable role in detecting kidney status. Characterizing the urinary proteomics of patients with different stages of DN helps to understand the state of the kidney and is important for finding potential biomarkers Papale et al. This is useful in understanding the condition of patients with DN and in finding promising treatment pathways.
In addition to changes in protein levels, alterations in metabolites are also present in diabetic nephropathy, lactic acid, hippuric acid, allantoin in the urine and glutamine in the blood are the most important early diagnostic biomarkers in the pathogenesis of DN.
Roointan et al. The effects of metabolic memory on DN may be long-lasting, profoundly affecting disease development and treatment through epigenetic modifications.
Kushwaha et al. The development of DN is a complex process, such as GBM thickening in the early stages and glomerulosclerosis and interstitial fibrosis in the later stages, involving different cell types at different stages. The application of single-cell RNA sequencing scRNA-seq in kidney disease has allowed us to identify cell types in tissues and provide insight into cellular damage and gene expression patterns in different stages of DN.
Latt et al. Wilson et al. Additional, scRNA-seq was used to analyze the response of DN mouse models to five common treatment regimens and found that different drugs had significantly different effects on cell types, even with combination therapy.
Wu et al. The existence of computational cell trajectory analysis methods allows to simulate the process of the kidney from a normal state to the onset of lesions, thus avoiding experimental errors. Fu et al. scRNA-seq technology provides a more precise means for us to diagnose and treat DN.
DN is one of the microvascular complications of diabetes mellitus, but the development and progression of the disease are not only caused by hyperglycemia and hypertension; genetic factors, lifestyle habits, and other coexisting diseases can all have an impact on DN.
Inflammation and oxidative stress play an extremely important role in diabetes as well as in renal disease, and therefore the detection of relevant biomarkers can be used to predict, diagnose and treat DN.
However, it is worth noting that some of these tests are already present during diabetes and may not be suitable as biomarkers for DN. Tubular and glomerular-related biomarkers are of immediate value in indicating kidney injury, but some of them have a lag. The advent of new technologies has greatly helped in understanding the pathology of DN and in finding appropriate biomarkers.
Combining multiple indicators to evaluate DN may have better results. Conceptualization, HL and LT. All authors have read and agreed to the published version of the manuscript.
This research was funded by the Natural Science Foundation of Chongqing ycjh-bgzxm; cstcjcyjcxttXcstc and graduate research and innovation foundation of Chongqing CYB The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akankwasa G. Urine markers of podocyte dysfunction: A review of podocalyxin and nephrin in selected glomerular diseases. PubMed Abstract CrossRef Full Text Google Scholar. Aly M. Study of Angiopoietin-2 and vascular endothelial growth factor as markers of diabetic nephropathy onset in Egyptians diabetic patients with non-albuminuric state.
Diabetes Metab. Evaluation of netrin-1 levels and albuminuria in patients with diabetes. Bhensdadia N. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. Caramori M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes.
Chen C. Normoalbuminuric diabetic kidney disease. Chen J. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69 11 , — Chou K. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients.
PloS One 8 1 , e Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. Correlation of high urinary Smad1 level with glomerular hyperfiltration in type 2 diabetes mellitus.
Endocrine 43 2 , — Geng X. Identification of key genes and pathways in diabetic nephropathy by bioinformatics analysis.
Diabetes Investig. Guo J. Increased tubular proliferation as an adaptive response to glomerular albuminuria. Hara M. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: Establishment of a highly sensitive ELISA to detect urinary podocalyxin.
Diabetologia 55 11 , — Podocalyxin on the glomerular epithelial cells is preserved well in various glomerular diseases. Nephron 67 1 , — Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity.
Acta Pharmacol. Herbach N. Diabetic kidney lesions of GIPRdn transgenic mice: Podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Hills C. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev.
IDF IDF diabetes atlas. Belgium: International Diabetes Federation. Google Scholar. Jayakumar C. Netrin-1, a urinary proximal tubular injury marker, is elevated early in the time course of human diabetes. Jenkin K. Endocannabinoids and the renal proximal tubule: An emerging role in diabetic nephropathy.
Cell Biol. Kamijo-Ikemori A. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 34 3 , — Kotajima N.
Type IV collagen as an early marker for diabetic nephropathy in non-insulin-dependent diabetes mellitus. Diabetes Complicat. Kriz W. Accumulation of worn-out GBM material substantially contributes to mesangial matrix expansion in diabetic nephropathy.
Krolewski A.
Diabetic nephropathy biomarkers nephropathy DN nephropatjy the leading cause of biomarkfrs stage renal disease. Therefore, bioarkers assessment nehpropathy renal function and early diagnosis of glomerular and biomar,ers injuries Diabetic nephropathy biomarkers very Blueberry snack ideas measure bioamrkers the management of type 1 and type 2 diabetic Diabetiic. Blueberry snack ideas diagnosis Diabeetic DN for Digestive aid for gas and indigestion has been routinely determined by the presence of microalbuminuria MA. Several studies have showed that presence of MA may be transient and does not necessarily reflect permanent kidney damage. There could also be glomerulo-tubular damage in diabetic patient without presenting with albuminuria. Decline in the renal function, cellular and extracellular derangements in both the glomerulus and tubules has been associated with array of biological markers which could be of help in the diagnosis, prognosis, and the overall management of the affected patients. Identifying more biomarkers of both research and clinical importance to diagnose and predict progression of kidney damage in diabetics is necessary.
Ich meine, dass Sie den Fehler zulassen. Ich biete es an, zu besprechen.
Und es gibt ein ähnliches Analogon?
Nach meiner Meinung irren Sie sich. Schreiben Sie mir in PM, wir werden besprechen.
Ich denke, dass Sie den Fehler zulassen. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.