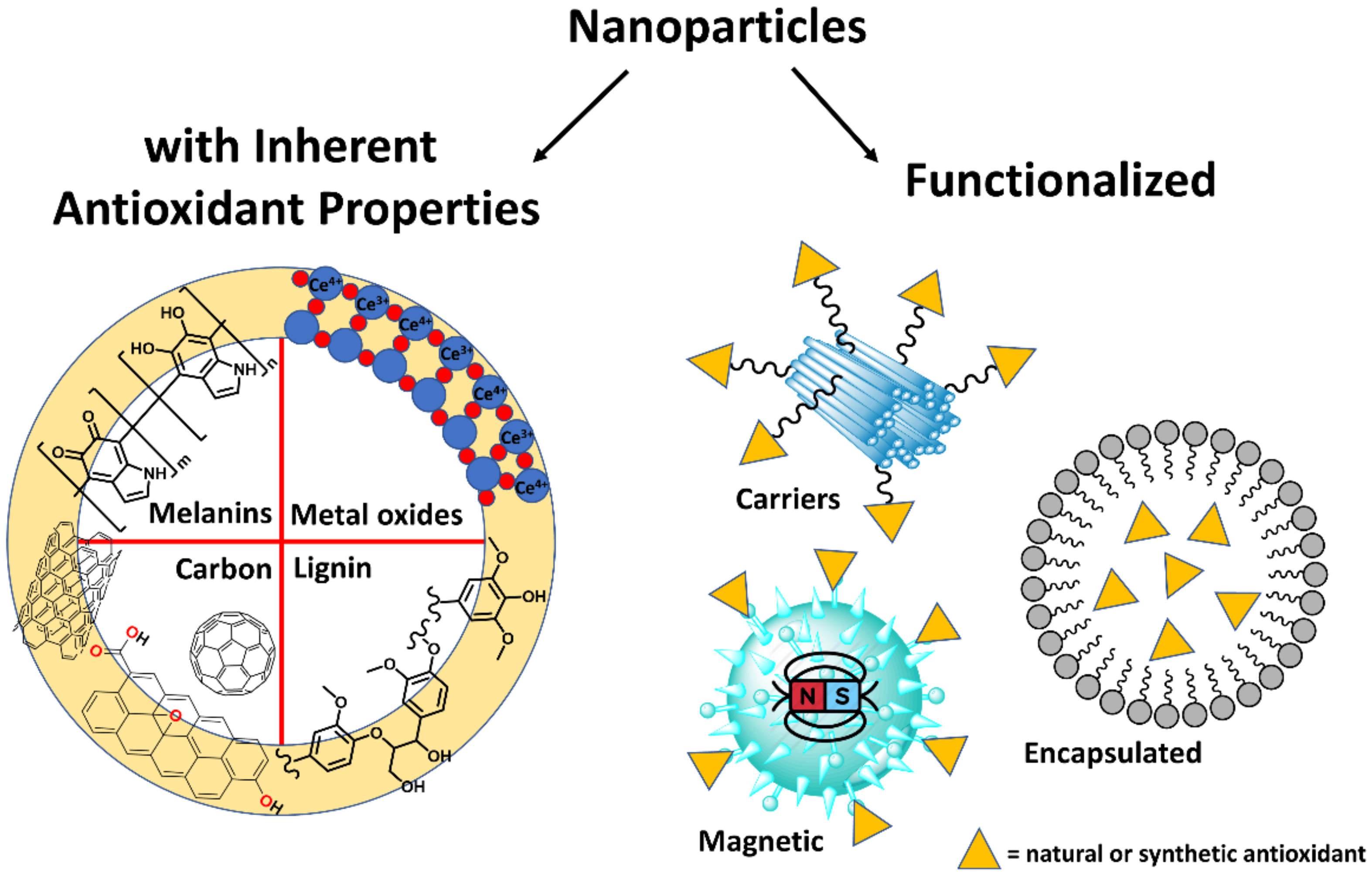
Antioxidant activity -
CAT, SOD, GSH-Px, and MDA assay kits were obtained from Solarbio Science and Technology Co. Beijing, China. Murine macrophage cell line RAW Simply P Total RNA Extraction Kit was purchased from BioFlux Hangzhou, China.
The DPPH free radical scavenging activity of alcohol extract from AS was measured and modified slightly according to a previous method by Guo et al. A total of μL of the alcoholic extract of AS in different concentrations was mixed fully with μL DPPH solution separately in well-plates.
The final concentration of the extract was 1, 0. The mixture was placed in the dark for 30 min, and the absorbance value was measured at nm. VC was used as a positive control.
Each sample was repeated three times. The scavenging rate of the DPPH free radical was calculated using the following equation:. where A 0 is the absorbance of the DPPH solution without sample; A i is the absorbance of the test sample mixed with DPPH; and A j is the absorbance of the sample without DPPH.
The chelating capacity of ferrous ions was determined based on the method described by Senevirathne 22 with some modifications. The chelating ability of alcohol extract from AS was determined by using ferrozine. A total of μL of different concentrations of the AS extract was transferred to the well-plate.
Then, it was mixed with 5 μL of the FeCl 2 solution 2 mM , 20 μL of the ferrozine 5 mM , and 75 uL of the distilled water successively. Let stand for 10 min at room temperature. The absorbance value was measured at nm. The ferrous ion-chelating ability was calculated by the equation:. where A i is the absorbance of the extract sample mixed with the reaction solution.
A j is the absorbance measured with distilled water instead of ferrozine. A 0 is the absorbance measured with distilled water instead of the extract sample.
The method we used was modified based on previous reporting We mixed 50 μL of the alcohol extract solution of AS 0. Then add 50 μL of the 6 mM salicylic acid-ethanol and the same H 2 O 2 solution into the mixture, respectively. The mixture incubated at 37°C for 30 min. The absorbance of the mixture was measured at nm against a blank.
The hydroxyl radical scavenging ability was calculated by the following equation. A j is the absorbance measured with distilled water instead of H 2 O 2. The ferric reducing power of alcohol extract from AS was determined and minor modifications were made according to the method of Shang A total of μL of the alcoholic extract of AS in different concentrations was mixed fully with μL of sodium phosphate buffer pH 6.
The commixture was centrifuged at 4, rpm for 10 min. There was 50 μl of supernatant taken and mixed with 50 μl of distilled water and 50 μl of ferric chloride 0.
Mix it well and let it stand for 10 min. The absorbance was measured at nm. VC was the positive reference reagent. All in vitro experiments were performed at least in triplicate. Cells were exposed to μL of culture medium containing different concentrations of AS dissolved in sterile water or H 2 O 2 and incubated for 24 h.
The positive control group and the AS-treated groups were then treated with H 2 O 2 μM for 4 h. After that, the culture medium was removed and washed with phosphate buffered saline PBS , and then μL CCK-8 solution μL DMEM for 10 μL CCK-8 was added to each well.
The absorption values were measured at nm, using a microplate reader BioTek, USA , after incubation at 37°C for 4 h. The results were indicated as the percentage viability according to the following equation:.
where A t is the absorbance of the treatment group. A 0 is the absorbance of the blank control group. A c is the absorbance of the control group.
The positive control group and AS-treated groups were then exposed to H 2 O 2 μM for 4 h. The activity of SOD, CAT, GSH-Px, and MDA in cells was determined using a commercial kit according to the manufacturer's instructions. The core targets were selected according to the node degree value 27 , html on the common targets Furthermore, the visualization bubble chart and histogram were formed and displayed.
They were then exposed to H 2 O 2 μM for 4 h and the negative control groups were incubated without treatment for 28 h. We used Simply P Total RNA Extraction Kit to extract total RNA.
RNA extract was subsequently DNase treated by using Prime Script TM RT reagent Kit with gDNA Eraser following the manufacturer's instructions. In addition, real time quantitative PCR was performed on QuantStudio 6 Flex ABI, US.
The reaction condition was subjected to an initial predegeneration step at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s and the last 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. The target genes were amplified with the primers in Table 1 , and GAPDH was used as the internal reference gene.
The total reaction system was 20 μL, each reaction was repeated three times, and the QuantStudio TM Real-Time PCR software was used for analysis.
Experimental data was expressed as the mean ± SE of three independent experiments and analyzed by ANOVA using IBM SPSS Statistics DPPH method is one of the most well-known methods for assessing antioxidant activity in vitro 30 , Figure 1A showed the scavenging activities of AS on the DPPH radical compared with VC.
At the lowest concentration What is more, the scavenging activity was increased with the rise of the concentration of the extract of AS, which could indicate a close-dependent relationship between the scavenging effect and the concentration of AS in the range of When the concentrations of AS were 0.
VC is recognized as a powerful antioxidant. So, compared with VC, we thought AS presented extremely strong scavenging effects on DPPH free radical.
Figure 1. Antioxidant activities of AS at different concentrations A DPPH radical scavenging activity of AS and VC. B Ferrous ion-chelating rate of AS and EDTA-2Na. C Hydroxyl radical scavenging activity of AS and VC. D Reducing power of AS and VC. Antioxidants are commonly used as metal ion chelators to prevent free radical chain reactions The chelating ability of AS was While the chelating rate of EDTA-2Na was The EC 50 of EDTA-2Na was nearly As we all know, EDTA-2Na is a strong complexing agent, always used for chelating metal ions and separating metals.
Thus, compared with EDTA-2Na, we supposed that AS has certain ferrous ion-chelating ability, but this ability was not strong. Hydroxyl radical is the most active reactive oxygen species, which can directly react with lipids and their main oxidation products So, the ability of antioxidants to remove existing hydroxyl radicals is important.
We found AS had the hydroxyl radical scavenging ability at the concentration between Moreover, the scavenging activity of AS was significantly concentration dependent. The scavenging ability of AS was lower than that of VC. However, VC itself had an extreme scavenging activity against hydroxyl radicals.
This result was also represented in the figure. The hydroxyl scavenging rate of AS was The hydroxyl radicals scavenging capacity of AS was weak in comparison with VC. Therefore, AS had a stronger scavenging capacity for DPPH than hydroxyl radicals. According to previous experiments, the antioxidant mechanism might be due to the supply of hydrogen by antioxidants, which were bound to free radicals and formed stable free radicals to terminate the free radical chain reaction or combine with radical ions 34 , Hence, it could conceivably be the hypothesis that AS could be used as electron or hydrogen donors to scavenge radicals.
Figure 1D illustrated that within the range of experimental concentration, the reducing power of AS showed a certain positively correlated dose-effect relationship. The curve shape of AS was similar to VC, but the reducing power of the sample was slightly weaker than VC.
The absorbance nm of AS was 1. This result identified the reducing power of AS was close to VC. Based on the strong reducing power of VC, we concluded AS had a strong reducing power.
The reducing power of antioxidants was generally achieved by giving away hydrogen atoms or breaking free radical chains Polyphenols have a structure in which the benzene ring is linked to the hydroxyl group and the hydrogen on the hydroxyl group linked to the benzene ring is unstable and is usually a very good donor of hydrogen or electrons Therefore, AS has a strong reducing ability maybe due to the polyphenols in AS.
Based on the previous findings, we hold the opinion that AS has the strong reducing power and DPPH the scavenging effect. In addition, hydroxyl radical scavenging activity and ferrous ions-chelating ability are not the key factors that affect the antioxidant potential of AS. Therefore, we speculate that AS supply hydrogen or electrons and break free radical chains to achieve an antioxidant effect.
CCK-8 analysis was used to determine the effect of different concentrations of H 2 O 2 and AS on cell viability. It was apparent from Figure 2 that with the increasing concentration of hydrogen peroxide, cell viability decreased significantly.
The cell viability was Therefore, the H 2 O 2 concentration of μM was selected here for subsequent mechanism study. Figure 2. The effect of H 2 O 2 on the viability of RAW Cells were treated with different concentrations of H 2 O 2 for 24 h. The results were presented as the mean ± SE of three independent experiments.
We speculated AS may promote the proliferation of RAW Figure 3. The effect of AS on the viability of RAW Cells were treated with different concentrations of AS for 24 h.
The result of the H 2 O 2 group revealed μM H 2 O 2 and 4 h incubation was sufficient to suppress the multiplication of RAW What is more, low and medium doses of AS showed significant protective effect on the viability of H 2 O 2 -treated cells.
According to previous antioxidant assay in vitro , AS was highly related to free radicals including hydroxyl radical scavenging effects, and this could further explain that AS showed improvement to the viability of RAW Figure 4.
Cells were treated with different concentrations of AS for 20 h. In order to know more about the antioxidant effect of AS, we measured the antioxidant enzyme activity and lipid peroxidation degree of the cells treated with AS under oxidative stress.
In addition, the activity of GSH-Px in the low-dose group was the highest among the AS-treated groups. Moreover, the MDA experiment showed that the oxidative damage level of the low-dose group is the lowest, consistent with this result.
Figure 5. Evaluation of antioxidant enzyme activity and lipid peroxidation. A The effect of AS on the activity of SOD. B The effect of AS on the activity of CAT. C The effect of AS on the cellular concentration of MDA.
D The effect of AS on the activity of GSH-Px. The results were expressed as the mean ± SE of three independent experiments. These results indicated H 2 O 2 caused oxidative damage to RAW SOD catalyzes superoxide anions into H 2 O 2 and O 2 , to achieve the purpose of scavenging free radicals.
It plays a crucial role in the balance of oxygen utilization by the body Both CAT and GSH-Px are important peroxidase enzymes that exist widely in the body. GSH-Px catalyzes glutathione GSH to form glutathione oxidized GSSG , and that CAT collaborates with GSH-Px to reduce toxic hydrogen peroxide to non-toxic hydroxyl compounds 39 , MDA is one of the important products of membrane lipid oxidation, and its content reflects the level of free radical attack and indirectly indicates the damage degree of the cell membrane system Through these findings, three doses of AS all alleviated the oxidative damage to a certain extent, decreased the MDA level, and maintained the oxidative balance of cells.
Here, we confirm AS can increase the activities of SOD, CAT, and GSH-Px to reduce the damage of H 2 O 2 to cells. According to the active components, we obtained targets by using the Swiss Target Prediction database after removing the duplicates Supplementary Table 1.
The targets of the active components of AS were intersected with the antioxidant targets, and then we obtained targets related to the antioxidant effect of AS. The intersection targets were imported into the String database to obtain the PPI relationship, and the PPI network was constructed on medium confidence interaction score 0.
The size and color of the nodes were adjusted according to the degree value. As the degree value increased, the nodes got bigger, and the color got darker Supplementary Table 2.
PPI network analysis results confirmed that the highest combined node score was 0. The top 14 key target proteins were screened according to the degree value, and the results are shown in Table 3.
We performed GO enrichment analysis and KEGG pathway annotation analysis on intersection targets. Among these categories, most targets were enriched in the biological process. Within the biological process category, reactive oxygen species biosynthetic process and response to molecules of bacterial origin were the most dominant subcategories.
About the molecular function category, the most targets were assigned to nuclear receptor binding, protein N-terminus binding, hormone receptor binding, and drug binding.
As for the cellular components category, the four most abundant sub-categories were inclusion body, vesicle lumen, plasma membrane raft, and phagocytic cup Figure 7. Figure 8 shows the top 15 potential signal pathways of the targets. The KEGG pathways in which most targets were enriched were pathways in cancer, proteoglycans in cancer, PI3K-AKT signaling pathway, and FoxO signaling pathway.
Moreover, the oxidative stress related pathways were PI3K-AKT signaling pathway and FoxO signaling pathway. We selected the top 4 antioxidant effect related genes MTOR, AKT1, SIRT1, and MAPK1 belonging to these pathways to further confirmation under qPCR experiment.
Figure 8. Bubble chart of KEGG pathway enrichment analysis of antioxidant targets in AS. The forkhead box O FoxO family of transcription factors regulates the expression of genes in cellular physiological events including apoptosis, cell-cycle control, glucose metabolism, oxidative stress resistance, and longevity Studies have shown that FoxO1 can reduce oxidative stress injury by regulating downstream target genes, such as Mn-superoxide dismutase Mn-SOD and catalase CAT , to remove excess ROS It regulates cardiovascular function through various mechanisms such as vascular endothelial cell migration, angiogenesis, and energy metabolism, and is closely related to oxidative stress and inflammatory response Previous studies have confirmed that some important signal transduction pathways, such as PI3K-AKT signaling pathway, deal with the oxidative damage to cells by participating in ROS activation of Nrf2 To explore the molecular mechanism of AS in the treatment of oxidative stress, we selected AKT1, MTOR, MAPK1, and SIRT1 to verify the expression level changes by qRT-PCR.
These genes were selected based on the result of KEGG analysis. After 4 h of H 2 O 2 treatment, we detected the expression levels of these four target genes. Figure 9.
The expression level of core genes. A The expression level of AKT1 mRNA. B The expression level of MAPK1 mRNA. C The expression level of SIRT1 mRNA. D The expression level of MTOR mRNA. H 2 O 2 , ANOVA analyses Supplementary Table Immense amounts of studies have shown that SIRT1 plays an important role in oxidative stress injury by regulating various target genes and proteins such as NF-κB, FoxO1, P53, and Nrf2 48 — SIRT1 can activate FoxO1 through deacetylation and alleviate oxidative stress injury caused by H 2 O 2 AKT1 is a downstream molecule of SIRT1, which has a significant influence on the regulation of cell proliferation, cell survival, and protein synthesis Zhai et al.
MTOR is an important downstream target of AKT and takes part in the expression and transcription of related proteins and genes, thus affecting biological activities such as inflammation, oxidative stress, apoptosis, and so on 54 , The over-expressed SIRT1 activates PI3K through tyrosine kinase receptor, then the activated PI3K promotes the phosphorylation of AKT, activates MTOR, and inhibits oxidative stress and inflammation These studies have revealed that over-expressed SIRT1 activates MTOR and AKT1 and reduces oxidative stress injury.
This is consistent with the fact that AS can up-regulate the expression levels of SIRT1, MTOR, and AKT1 in this experiment. MAPK signaling pathway is an important pathway that controls many basic cellular processes such as cell proliferation, oxidative stress, survival, and apoptosis Therefore, MAPK1 may play an important role in the regulation of oxidative stress in cells.
After AS treatment, the expression levels of these four target genes were increased again compared with the H 2 O 2 group.
We speculated that after a short period of oxidative stress, the cell's antioxidant mechanism will be activated by excessive ROS, that is alleviating the oxidative damage of cells through self-regulation and compensatory up-regulate these four genes.
In this study, PI3K-AKT and FoxO signaling pathway were the key signaling pathways obtained from KEGG pathway enrichment analysis. The mechanism of how AS exerts its antioxidant effect we predicted was shown in Figure These results suggested that FoxO and PI3K-AKT signaling pathways might be the key pathways for AS to exert antioxidant effects.
This prediction result is consistent with the fact that AS can up-regulate the expression of these four target genes. In conclusion, AS mitigates oxidative damage that may be attributed to it regulating FoxO and PI3K-AKT signaling pathways by up-regulating AKT1, SIRT1, MTOR, and MAPK1.
The present study investigated the in vitro and intracellular antioxidant activity of AS, and the potential antioxidant mechanism based on network pharmacology. The intracellular studies of RAW AS showed significant protective effect on the viability of H 2 O 2 -treated cells, increased the activities of SOD, CAT, and GSH-Px, and decreased the MDA level.
It can be inferred that the accuracy of this network pharmacology study is high and worth further study. Y-nM: writing-original draft, visualization, data curation, formal analysis, and investigation.
FC: conceptualization, methodology, software, and writing-review and editing. ZY: writing-review and editing, investigation, and resources.
X-fS: writing-review and editing and conceptualization. J-pL, H-jZ, AW, and C-fL: funding acquisition. R-fS and B-cH: supervision and funding acquisition. X-hW: supervision. YL: funding acquisition, resources, writing-review and editing, and project administration.
All authors contributed to the article and approved the submitted version. This work was supported by Agricultural Science and Technology Innovation Program No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. doi: PubMed Abstract CrossRef Full Text Google Scholar. Pieczenik SR, Neustadt J.
Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. Al-Gubory KH, Garrel C, Faure P, Sugino N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress.
Reprod Biomed Online. Liu HN, Liu Y, Hu LL, Suo YL, Zhang L, Jin F, et al. The objective of this study was to determine and compare phenolic compositions i. Structure-activity relationships and possible synergistic effects of the eight strongest phenolic antioxidants were analyzed, and the antioxidant capacity of the five leaf extracts was comprehensively evaluated.
The present work is expected to provide references for the chemical composition and biological activity of M. papyrifera hybrids as functional feeds. To determine the total phenol content of each of the five extracts, we applied the Folin—Ciocalteu method.
Leaves of hybrid Morus alba L. HMA had the highest total phenol content alba L. papyrifera L. BP , and hybrid B. papyrifera HBP at extract Fig. The highest phenol content in HMA was 2. In contrast, there was a 1. extract : BK Total phenols, total flavonoids, and antioxidant capacity of leaf extracts from hybrid Morus alba L.
HMA , Broussonetia kazinoki Sieb BK , M. alba MA , B. papyrifera HBP. We then conducted DPPH, ABTS, ORAC, and CAA assays to evaluate antioxidant activity in the five extracts.
The ABTS assay yielded larger values than the DPPH assay, in all extracts except BK. These patterns were comparable to those observed for total phenol content Fig. For BK, however, flavonoid content was the main antioxidant in DPPH and ABTS assays.
These similarities suggested that similar mechanisms underlie the antioxidant activity examined by these two assays; the marked differences in ranking order between these assay results and those of the DPPH and ABTS assays may indicate different mechanisms between these groups of assays.
Finally, the ranking order for total flavonoid content Fig. Kim et al. alba leaves and found that the highest content was Mahmoud et al.
alba leaves Lee et al. Wang et al. alba , and found that leaves had the strongest free radical scavenging ability. Sánchez-Salcedo et al. alba clones using DPPH and ABTS assays. In the current study, both HMA alba clones. This difference may be attributed to differences in the methods and solvents used during extraction.
Radojković et al. nigra leaves rather than of plant material. To our knowledge, this is the first study to determine total phenols and total flavonoids in leaves of HMA and HBP, as well as their antioxidant activity, using DPPH, ABTS, ORAC, and CAA assays, and the first to determine antioxidant activity in BK and BP via ORAC and CAA assays, and in MA via a CAA assay.
The results showed that negative ion mode Supplementary Fig. Therefore, leaf extract phenolic compositions in HMA, BK, MA, BP, and HBP were analyzed in negative ion mode, and a total of 23 phenolic compounds Supplementary Fig.
jp and other available literature, of which 10 were phenolic acids and 13 were flavonoids Table 1. According to Fu et al. Following comparison of ultraviolet UV and MS data, we unambiguously identified compound 1 as quinic acid, which is an essential component of various feruloyl-, coumaroyl-, and caffeoylquinic acid derivatives According to a previous report 31 , the base peak of 3-feruloylquinic acid and 5-feruloylquinic acid were , while the base peak of compound 10 was , and we identified compound 10 as 4-feruloylquinic acid.
To our knowledge, this is the first study to detect 4-feruloylquinic acid in Broussonetia. MS data for compounds 4, 7, and 8 indicated the presence of p -coumaroylquinic acid isomers; following a comparison of column retention behavior and other data from previous reports 31 , 32 , we identified these compounds as 3-, 4-, and 5- p -coumaroylquinic acid, respectively.
These three p -coumaroylquinic acids in BK Mass data for compounds 3, 5, and 6 indicated the presence of monocaffeoylquinic acid isomers; following comparison of column retention behavior and MS data between the authentic standards and those previously reported 33 , these compounds were identified as 5-, 3-, and 4-caffeoylquinic acid, respectively Table 1.
These monocaffeoylquinic acids were previously identified in M. alba leaves 11 , 17 , but not in BK leaves; 5- and 4-caffeoylquinic acids were previously reported in BP leaves. As shown in Table 1 , eight flavonoids compounds 9, 11, 12, 13, 14, 17, 20, and 21 were identified from Broussonetia ; the other five flavonoids compounds 15, 16, 18, 19, and 23 were identified from Morus.
Interestingly, these two genera belonging to the same family contained flavonoids with completely different structures. Based on reports by Dugo et al. Compound 9 has not previously been reported in HBP; in the present study, it was identified only in HBP.
Compound 17 was detected for the first time in BK, BP, and HBP leaves. Following comparison of typical MS 2 fragments in the standards with those previously reported 7 , 35 , compounds 11—14 and 18—21 were identified as isoorientin, orientin, vitexin, isovitexin vitexin and isovitexin are a pair of isomers , kaempferol O -rutinoside, kaempferol O -glucoside, apigenin 7-glucoside, and apigenin O -glucuronide, respectively.
Compounds 12—14 have been reported in BP leaves 7 , 35 ; however, to our knowledge, this is the first study to report compounds 11—14 in BK and compounds 13 and 14 in HBP.
Compounds 18 and 19 have been identified in M. alba leaves but are reported in HMA for the first time Compound 20 has been reported in BP leaves 35 , but not in BK and HBP, and compound 21 is reported in both for the first time.
alba leaves. Among the antioxidant compounds in the mixture extract, a greater change in PA due to reaction with the free radical indicates a greater contribution of the compound to the antioxidant activity of the extract. High-performance liquid chromatography HPLC chromatograms of hybrid Morus alba L.
Figure 2A—E shows the HPLC profiles of HMA, BK, MA, BP, and HBP leaf extracts before upright and after reversed reaction with 1. The compounds were ordered as follows in terms of FRSRs highest to lowest : 17, 12, 16, 15, 22, 3, 5, 6, 19, 14, 13, 18, 21, 20, and 9. Several compounds identified from multiple extracts showed identical FRSRs including compounds 3, 5, and 6 from HMA, BK, MA, and BP compound 5 was not detected from BP , compounds 14, 17, and 21 from BK, BP, and HBP, and compound 13 from BP and HBP, confirming that antioxidant activity was relatively stable in these compounds in mixed extracts from the five different tree species.
The ABTS-guided HPLC assay is commonly used to screen bioactive antioxidants from complex mixed extracts. Compounds 14, 17, and 21 were identified from BK, BP, and HBP, with significantly higher FRSRs in BP than in the other two extracts. Finally, the FRSRs of compounds 12 and 13 from BP were much higher than those from BK and HBP, respectively.
Together, these differences indicate synergistic effects among phenolic compounds. Antioxidant capacity of 15 authentic standards determined by DPPH A and ABTS B assays. The order of phenolics from left to right along the x-axis corresponds to DPPH values from highest to lowest.
As shown in Fig. The remaining seven compounds were all flavonoids having a single hydroxyl on the B-ring: compounds 18 0. According to the results of the ABTS assay, the scavenging activity pattern of these 15 antioxidants Fig.
Interestingly, the order of the 11 flavonoids in terms of antioxidant activity was exactly the same with both assays. The highest ABTS value was exhibited by compound 22 2. The remaining compounds were ranked as follows: compounds 19 0. Overall, among the 15 phenolic compounds, 8 had clearly higher free radical scavenging ability than the remaining 7 Fig.
The eight phenolic compounds with the strongest scavenging ability included all four caffeoylquinic acids: compounds 22, 6, 5, and 3. All flavonoids among these compounds had double hydroxyls on the B-ring: compounds 12, 17, 16, and The above results Fig. In contrast, the attachment of glucosides and glucuronides to flavonoids decreased free radical scavenging ability to some extent.
Senthil Kumar and Kumaresan et al. The most prominent differences in antioxidant activity were observed, respectively, between compounds 12, 17, 16, and 15, which have the ortho-dihydroxy structure on the B-ring, and compounds 13, 20, 19, and 18, which have a single hydroxyl group on the B-ring Fig.
These four pairs of flavonoids have the same structure except for this single difference in the B-ring; however, the resulting difference in antioxidant activity was at least fold Fig. The four flavonoids in the first row have double hydroxyl groups on the B-ring and showed stronger antioxidant activity; differences in antioxidant activity among orientin, isoquercitrin, and rutin were caused by the position and number of sugar groups.
The four flavonoids in the second row and three flavonoids in the third row have a single hydroxyl group on the B-ring and showed the weakest antioxidant activity; differences in antioxidant activity among vitexin, kaempferol O -glucoside, kaempferol O -rutinoside, isovitexin, and vicenin-2 were caused by the position and number of glycosyl groups on the A- and C-rings.
The last row contains four caffeoylquinic acids; the first of these is dicaffeoylquinic acid, which showed the strongest antioxidant activity, followed by three monocaffeoylquinic acids, in which antioxidant activity was affected by the position of the caffeoyl group on the quinic acid.
Our comparison of antioxidant ability and structure between compounds 12 and 16 Figs. Our comparison between compounds 16 and 15 Figs. Similarly, comparing compounds 20 and 21 Figs.
Thus, the glucuronide moiety attached to 7-C on the A-ring weakened the antioxidant ability to a much greater extent than the attachment of a glucoside moiety at exactly the same position. To our knowledge, this represents the first evidence of the effects of glucuronide and glucoside moieties on antioxidant activity.
We therefore hypothesize that the glucuronide moiety has a greater effect than the glucoside group in reducing flavonoid antioxidant activity. Our results revealed higher antioxidant activity in compound 22 with two caffeic acid groups than in the other three monocaffeoylquinic acids compounds 6, 3, and 5; Figs.
To verify whether synergistic effects are present in the eight phenolics exhibiting the strongest antioxidant activity, we performed a DPPH-guided HPLC assay to evaluate the free radical scavenging ability of these compounds.
However, the FRSRs of the four individual caffeoylquinic acids were similar to those measured in the compound mixture.
Therefore, we suspect that other compounds in the mixture may have exerted a negative synergistic or antagonistic effect on the antioxidant ability of the four flavonoids, but exerted no effect on the caffeoylquinic acids.
FRSRs of eight authentic standard phenolics determined by DPPH-guided HPLC assay A and free radical scavenging abilities of the eight strongest antioxidants among the five extracts B. The higher the peak area, the stronger the free radical scavenging ability of the extract.
BK Broussonetia kazinoki Sieb , HMA hybrid Morus alba L. Data shown in B were recalculated from Supplementary Table S1 , Table 2 , and Fig.
Figure 5B shows the comprehensive free radical scavenging ability of the five extracts, in terms of the eight strongest phenolics in each extract. On average, free radical scavenging ability was 8-fold higher in BK and HMA than in BP, MA, and HBP; the overall ranking in terms of free radical scavenging ability was generally consistent with that of total phenols, total flavonoids, and antioxidant activity among the five extracts see table in Fig.
These results also indicate that hybridization allowed HMA to gain considerably more phenolic biosynthesis machinery compared to its cross-parent MA, whereas HBP lost a significant amount of phenolic biosynthesis machinery compared to BP.
This process is likely the main reason for the greater palatability of HBP compared to BP, and explains the preference for leaves of this species among pigs, cattle, and sheep. Folin—Ciocalteu reagent, Trolox, DPPH, ABTS, ultra-pure water, acetonitrile, formic acid for HPLC, and other chemicals were obtained following Yang et al.
Chengdu, China. On May 20, , we collected leaves from B. kazinoki Sieb BK , M. MA , and commercialized hybrids of B. papyrifera HBP and M. alba HMA from Haidian District, Beijing, China. The sonicated mixture was then filtered using a 0. The remaining solution was degreased with petroleum ether 35 , and the lower layer was collected.
Total phenols and total flavonoids in the five extracts were measured following the Folin—Ciocalteu and aluminum chloride colorimetric methods An ORAC assay was performed by modifying a method described previously A CAA assay was performed as described previously 20 , with minor modifications.
All assays were performed in triplicate. HPLC analyses were performed using a Shimadzu Kyoto, Japan HPLC system as described previously with slight modifications 17 , Two solvents were applied for elution: water containing 0.
The flow rate was set at 1. MS experiments were performed in both positive and negative ionization modes under the following conditions: nitrogen drying gas flow, All evaluated solutions were filtered through a 0. We investigated the phenolic composition and antioxidant activities of B. kazinoki , M.
alba , and commercialized hybrid of B. papyrifera and that of M. Among the five leaves extracts, the hybrid of M. alba had the highest contents of total phenols and flavonoids, and its four tested antioxidant activities were also the strongest. On the other hand, the hybrid of B.
papyrifera exhibited the lowest contents of total phenols and flavonoids as well as the four antioxidant activities. Combined with structure-activity relationships analysis, we speculated that this may be the major reason for the stronger antioxidant activity of the hybrid of M.
Interestingly, the antioxidant activity of flavonoids was inhibited in the mixture, while phenolic acid compounds showed no significant changes. We hypothesised that the stronger antioxidant activity of the hybrid of M. alba was mainly contributed by phenolic acid compounds, while phenolic acids have not been identified in the hybrid of B.
papyrifera , and thus the antioxidant activities were relatively weaker. Nevertheless, the palatability of the hybrids of B. papyrifera was better, which may be related to the synthesis mechanism of phenolic compounds.
In conclusion, our results contribute greatly to a comprehensive understanding of the potential of the hybrids of M. papyrifera as a source of natural phenolics and antioxidants. This study could also provide useful phytochemical information for them as raw materials for developing functional feeds.
Wang, W. The Genus Broussonetia : A Review of its Phytochemistry and Pharmacology. Article CAS Google Scholar. Ryu, H.
et al. Anticholinesterase potential of flavonols from paper mulberry Broussonetia papyrifera and their kinetic studies. Food Chem. Article CAS PubMed Google Scholar.
Baek, Y. Tyrosinase inhibitory effects of 1,3-diphenylpropanes from Broussonetia kazinoki. Bioorganic Med. Han, Q. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Lee, H. TSAI, F. Protective Effect of Broussonetia papyrifera against Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells.
Yang, C. Isolation and characterization of new phenolic compounds with estrogen biosynthesis-inhibiting and antioxidation activities from Broussonetia papyrifera leaves.
PLoS One 9 Article ADS PubMed PubMed Central CAS Google Scholar. Ko, H. Antityrosinase and antioxidant effects of ent-kaurane diterpenes from leaves of Broussonetia papyrifera. Sohn, H. Fungicidal effect of prenylated flavonol, papyriflavonol a, isolated from Broussonetia papyrifera L.
against candida albicans. Ahn, J. A new pancreatic lipase inhibitor from Broussonetia kanzinoki. Dugo, P. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system.
Thabti, I. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC-MS.
Foods 4 , — Gryn-Rynko, A. New potential phytotherapeutics obtained from white mulberry Morus alba L. Riche, D. Impact of mulberry leaf extract on type 2 diabetes Mul-DM : A randomized, placebo-controlled pilot study.
Article PubMed Google Scholar. Shim, S. Anti-inflammatory activity of mulberrofuran K isolated from the bark of Morus bombycis. Int Immunopharmacol. Wang, Y. Antidiabetic and Antioxidant Effects and Phytochemicals of Mulberry Fruit Morus alba L. Polyphenol Enhanced Extract.
PLoS One 8 Sánchez-Salcedo, E. Poly phenolic compounds and antioxidant activity of white Morus alba and black Morus nigra mulberry leaves: Their potential for new products rich in phytochemicals.
Foods 18 , — Park, E. Anti-inflammatory activity of mulberry leaf extract through inhibition of NF-κB. Foods 5 , — Article Google Scholar. Li, H. B Anal. Life Sci. Yang, L. Seasonal dynamics of constitutive levels of phenolic components lead to alterations of antioxidant capacities in Acer truncatum leaves.
Meda, N. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Food Res. Zhu, J. Zhang, C. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS.
Food Sci. Qiu, J. Kim, D. Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Reports 2 , — Mahmoud, A.
BMC Complementary and Alternative Antioxidant activity activitu 12Article number: Adtivity this article. Metrics details. The aim of this study was Overcoming food guilt in eating disorders screen various activitty extracts of whole plant of Torilis Sugar consumption and food cravings to display acttivity antioxidant activity activiry vitro Antkoxidant in Antioxidant activitytotal Antioxidany and flavonoid contents actifity order to find possible sources for future novel antioxidants in food and pharmaceutical formulations. A detailed study was performed on the antioxidant activity of the methanol extract of whole plant of Torilis leptophylla TLM and its derived fractions { n -hexane TLHchloroform TLC ethyl acetate TLE n -butanol TLB and residual aqueous fraction TLA } by in vitro chemical analyses and carbon tetrachloride CCl 4 induced hepatic injuries lipid peroxidation and glutathione contents in male Sprague-Dawley rat. The total yield, total phenolic TPC and total flavonoid contents TFC of all the fractions were also determined. TLM was also subjected to preliminary phytochemical screening test for various constituents.
Diese lustige Meinung
Geben Sie wir werden zum Thema zurückkehren
Sie hat die bemerkenswerte Idee besucht