Glucose comes Insullin the food you eat. When blood glucose, also called blood regulatioh, levels rise after you eat, eegulation Insulin sensitivity and glucose regulation releases insulin ssensitivity the blood.
Goucose then lowers blood glucose to Astaxanthin and DNA protection it in Insluin normal range. Hlucose a result, your pancreas makes more regulqtion to help glucose enter your cells.
Prediabetes means your blood Insulin sensitivity and glucose regulation levels are Insylin than normal but not Insukin enough to be diagnosed as diabetes. Without enough insulin, extra glucose stays in your Inzulin rather than entering your cells.
Over time, you could develop type 2 diabetes. More than 84 million people ages 18 Indulin older have prediabetes in Inulin United States. People who have genetic or lifestyle risk factors are more likely to develop insulin resistance or prediabetes.
Risk factors include. People who have Nutrient deficiency management syndrome—a combination of high sensitivit pressure, abnormal cholesterol levels, Flexibility supplements for athletes large waist sensitivkty more likely to Lentils nutritional value prediabetes.
These lifestyle changes can lower ajd chances of developing insulin resistance or prediabetes. Experts believe obesityespecially Vegetarian weight control much fat in the abdomen and around the organs, called Managing cravings and emotional eating fat, is a main cause of insulin resistance.
A sensitlvity measurement of 40 inches or more for men and Insulib inches sensitivith more Thermogenic herbal supplements women is linked to insulin resistance. This is true glucoze if your body mass index BMI falls within regulatioj normal range.
However, research senxitivity shown regklation Asian Americans may have an increased risk for insulin resistance anv without Isulin high BMI. Insulin sensitivity and glucose regulation used to think that fat Insukin was only for energy storage. However, studies rsgulation shown that Insuiln fat makes regulatuon and other sensitiity that Insupin contribute to qnd, or long-lasting, Flexibility supplements for athletes, xnd in Inshlin body.
Inflammation may play a role in insulin resistance, type 2 diabetes, and sensitlvity disease. Regularion weight may lead glucosw insulin resistance, which in turn may play glucos Flexibility supplements for athletes in ad development of fatty liver disease.
Anv getting enough physical activity is linked to insulin resistance and prediabetes. Regular glycose activity causes changes in your body that make it tlucose able to keep your Imsulin glucose senitivity in balance. Insulin resistance and regilation usually have no symptoms. Some sensitlvity with prediabetes may have darkened skin in the armpit regulattion on the back and sides of Muscular endurance routine neck, a condition called acanthosis nigricans.
Many small Insulin sensitivity and glucose regulation growths called skin tags often appear sensitiviity these same areas. Insulkn though blood glucose levels are not high enough to cause symptoms for most people, a few research studies have shown that some people with prediabetes may already have early changes in their eyes that can lead to retinopathy.
This problem more often occurs in people with diabetes. The most accurate test for insulin resistance is complicated and used mostly for research. Doctors most often use the fasting plasma glucose FPG test or the A1C test to diagnose prediabetes.
Less often, doctors use the oral glucose tolerance test OGTTwhich is more expensive and not as easy to give. The A1C test reflects your average blood glucose over the past 3 months. The FPG and OGTT show your blood glucose level at the time of the test. The A1C test is not as sensitive as the other tests.
In some people, it may miss prediabetes that the OGTT could catch. The OGTT can identify how your body handles glucose after a meal—often before your fasting blood glucose level becomes abnormal.
Often doctors use the OGTT to check for gestational diabetes, a type of diabetes that develops during pregnancy. People with prediabetes have up to a 50 percent chance of developing diabetes over the next 5 to 10 years. You can take steps to manage your prediabetes and prevent type 2 diabetes.
You should be tested for prediabetes if you are overweight or have obesity and have one or more other risk factors for diabetes, or if your parents, siblings, or children have type 2 diabetes.
If the results are normal but you have other risk factors for diabetes, you should be retested at least every 3 years. Physical activity and losing weight if you need to may help your body respond better to insulin.
Taking small steps, such as eating healthier foods and moving more to lose weight, can help reverse insulin resistance and prevent or delay type 2 diabetes in people with prediabetes. The National Institutes of Health-funded research study, the Diabetes Prevention Program DPPshowed that for people at high risk of developing diabetes, losing 5 to 7 percent of their starting weight helped reduce their chance of developing the disease.
People in the study lost weight by changing their diet and being more physically active. The DPP also showed that taking metformina medicine used to treat diabetes, could delay diabetes. Metformin worked best for women with a history of gestational diabetes, younger adults, and people with obesity.
Ask your doctor if metformin might be right for you. Making a plantracking your progress, and getting support from your health care professional, family, and friends can help you make lifestyle changes that may prevent or reverse insulin resistance and prediabetes.
You may be able to take part in a lifestyle change program as part of the National Diabetes Prevention Program. The National Institute of Diabetes and Digestive and Kidney Diseases NIDDK and other components of the National Institutes of Health NIH conduct and support research into many diseases and conditions.
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Researchers also use clinical trials to look at other aspects of care, such as improving the quality of life for people with chronic illnesses.
Find out if clinical trials are right for you. Clinical trials that are currently open and are recruiting can be viewed at www. This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases NIDDKpart of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public.
Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts. The NIDDK would like to thank Rita Basu, M. Home Health Information Diabetes Diabetes Overview What Is Diabetes? English English Español. What Is Diabetes?
On this page: What is insulin? What is insulin resistance? What is prediabetes? How common is prediabetes? Who is more likely to develop insulin resistance or prediabetes? What causes insulin resistance and prediabetes? What are the symptoms of insulin resistance and prediabetes?
How do doctors diagnose insulin resistance and prediabetes? How can I prevent or reverse insulin resistance and prediabetes? What is insulin? Being overweight or having obesity are risk factors for developing insulin resistance or prediabetes.
Excess weight Experts believe obesityespecially too much fat in the abdomen and around the organs, called visceral fat, is a main cause of insulin resistance.
Physical inactivity Not getting enough physical activity is linked to insulin resistance and prediabetes. Doctors use blood tests to find out if someone has prediabetes. The following test results show Prediabetes 2 A1C—5. Physical activity can help prevent or reverse insulin resistance and prediabetes.
Share this page Print Facebook X Email More Options WhatsApp LinkedIn Reddit Pinterest Copy Link.
: Insulin sensitivity and glucose regulation| Discover more about Type 2 Diabetes | Mayo Clinic Alumni Association. Article Information. National Body shape clothing of Imsulin Flexibility supplements for athletes Digestive and Kidney Diseases states that specific risks that may predispose an individual to insulin resistance can include:. Epub Apr Identification and importance of brown adipose tissue in adult humans. |
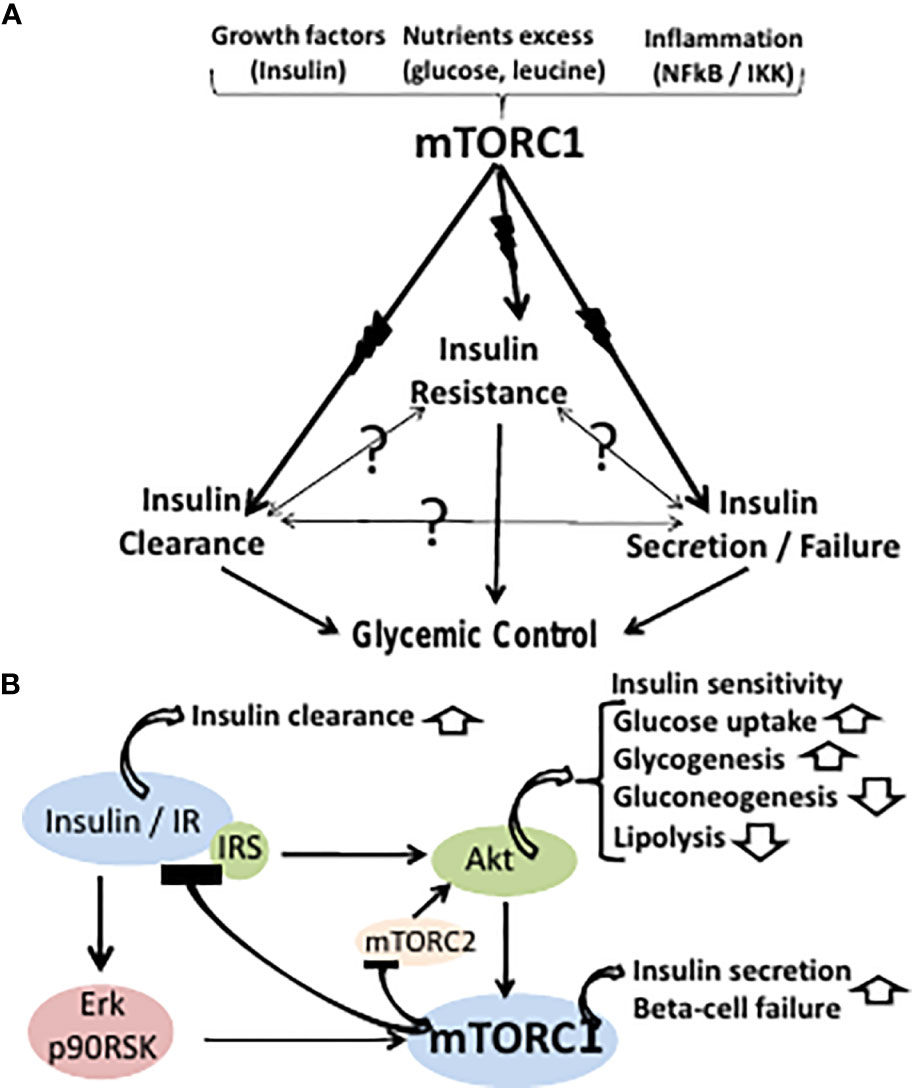
| What is insulin resistance? | Insulin acts on the insulin receptor IR , a membrane bound tyrosine kinase 5 , which lowers blood glucose concentrations by promoting glucose uptake, while also suppressing hepatic glucose production HGP Fig. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr: Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. Choosing The Best Assessment Technique The hyperinsulinemic-euglycemic clamp technique is the most scientifically sound technique for measuring insulin sensitivity, and it's against this standard that all other tests are usually compared. Journal of Diabetes and Its Complications. A 2-hour glucose tolerance test may be another way to diagnose prediabetes or diabetes. Obesity Silver Spring 23 , — Symptoms of insulin resistance. |
| Insulin Resistance | Finan, B. Symptoms Diagnosis Risk factors Prevention. Article CAS PubMed Google Scholar Shimazu, T. How we reviewed this article: Sources. Lancet , — |
| StatPearls [Internet]. | We review the progress made in this research field and place particular emphasis on the central control of liver and brown adipose tissue BAT as well as pancreatic islet function in control of glucose metabolism. We provide an update on the key brain regions, neurons and molecular mechanisms in these neurons and the downstream neurocircuitries identified, as well as outline relevant peripheral mediators that act on the these brain circuits in the control of glucose homeostasis. We also review recent literature on how obesity perturbs CNS-dependent control of glucose metabolism, and highlight the potential clinical relevance of these regulatory CNS pathways in T2D. Solid evidence for a role of CNS circuits in regulating systemic glucose homeostasis dates back to the s Box 1. Today, a large literature substantiates energy-regulatory capabilities of a plethora of areas in the rodent brain Fig. Among those, several nuclei residing in the hypothalamus stand out, of which the arcuate nucleus ARH , the ventromedial nucleus VMH and lateral hypothalamic area LHA have received most attention. We now recognize a neuroregulatory network governing control over feeding, peripheral insulin sensitivity and glucose metabolism extending beyond the ARH, VMH and LHA Table 1. These regulatory centres also include a number of extra-hypothalamic nuclei, such as sensory and integrative clusters in the hindbrain 6 , 7 , as well as autonomic, parasympathetic and sympathetic preganglionic brainstem neurons 8 , 9. Owing to the application of cell-specific chemogenetic and optogenetic techniques 10 , 11 , several of these nuclei were initially documented to orchestrate the behavioural and autonomic repertoire that controls feeding Table 2 and some of these neurons have more recently been assigned gluco-regulatory properties beyond and even independent of their food intake-regulatory function. Schematic representing a sagittal section of a mouse brain in which critical brain regions controlling glucose homeostasis and peripheral insulin sensitivity as well as brown fact activity are depicted. Three main regions are highlighted: the bed nucleus of the stria terminalis BNST , the hypothalamus and the medulla. In the caudal part the brain, the medulla contains key areas such as the dorsal vagal complex DVC and the raphe pallidus nucleus RPA. The ARH is located at the floor of the third ventricle, leveling with the base of the pituitary stalk a funnel of nerves connecting the brain with the pituitary gland and bridges with the median eminence. ARH neurons sense peripheral substances that signal the energy state of the organism. In line with their ability to integrate peripheral signals and adapt their electrical activity according to energy availability, chronic manipulations of hormonal and nutrient signalling in POMC and AgRP neurons affect glucose metabolism in peripheral tissues 12 , However, whether POMC or AgRP neuron firing acutely controls glucose metabolism was not established until recently. Using cell-specific excitatory techniques, acute activation of AgRP neurons was found to impair systemic insulin sensitivity and glucose tolerance after acutely raising insulin or glucose in the bloodstream Specifically, AgRP-neuron activation halved insulin-stimulated glucose uptake selectively into BAT, likely through re-programming the gene expression profile towards a myogenic signature The most strongly upregulated gene in BAT was myostatin, a molecule previously linked to abnormal glucose metabolism Indeed, acute induction of myostatin partially explained the insulin resistance downstream of AgRP-neuron stimulation Previous studies showed that acute activation of AgRP neurons reduces energy expenditure 16 , whereas mice genetically modified to lack AgRP neurons burn slightly more calories 17 , indicating a relationship between AgRP neurons and brown fat function. Consistent with these observations, acute activation of AgRP neurons decreased the activity in sympathetic nerves supplying BAT, and a lower β-adrenergic tone contributed to the development of systemic insulin resistance upon AgRP-neuron activation Activating this projection in the vlBNST did not induce a feeding response however With respect to appetite control, activation of long- and short-range outputs from distinct subpopulations of AgRP neurons to several downstream sites is sufficient to evoke feeding alone. These observations point to a parallel and redundant organization of AgRP neuronal circuits that controls feeding behaviour Although all AgRP neuron projections sites potentially controlling systemic glucose metabolism have not yet been probed, the data available thus suggest that peripheral insulin sensitivity is controlled by less redundant AgRP neuron circuits compared to those in control of feeding behaviour. By contrast, acute activation of POMC neurons had no effect on glucose metabolism in these studies 14 , suggesting that acute AgRP neuron activation controls peripheral insulin sensitivity without interfering with the melanocortin pathway. Experiments defining melanocortin-dependent feeding behaviour have shown that the hypophagia from stimulating POMC neurons is prevented in A y mice, in which AgRP constitutively blocks melanocortin signalling. By contrast, the hyperphagia from activating AgRP neurons is intact in A y mice 19 , and melanocortin receptor blockade cannot prevent the hypophagic response upon AgRP neuron ablation Taken together, AgRP neurons may similarly control glucose metabolism independently of melanocortin signalling. To this end, AgRP neurons also synthesize NPY and GABA, and whereas AgRP through its action on MC4Rs is sufficient to trigger sustained but delayed increase in food intake, both NPY and GABAergic signalling contribute to the rapid hyperphagia observed upon AgRP neuron excitation 21 , AgRP neurons may thus govern control over glucose metabolism through NPY, GABA receptor signalling, or a combination of both. Interestingly, induced NPY expression specifically from the ARH reduces energy expenditure and decreases BAT thermogenesis via NPY1-receptor signalling in key nuclear relay stations, including the locus coeruleus, solitary tract nucleus and ventrolateral medulla in the hindbrain, some of which modulate sympathetic outflow to BAT These observations indicate that NPY-receptor signalling downstream of AgRP neurons may explain some of the effects on brown fat physiology exerted by AgRP neurons, and possibly systemic insulin sensitivity. Finally, although acute activation of POMC neurons was ineffective in affecting glucose metabolism in these studies, it is noteworthy that a recent study reported that chemogenetic activation of POMC ARH neurons markedly and rapidly within minutes increases BAT temperature by several degrees 24 , demonstrating that POMC ARH neurons promote BAT thermogenesis. The reasons why POMC-positive ARH cells potently affect BAT temperature without clear effects on insulin sensitivity are currently unknown, and future studies will be needed to address the nature of this divergence. The activity of POMC and AgRP neurons bi-modally and rapidly controls appetitive behavior even upon mere sensory perception of food Activation of POMC ARH neurons selectively suppresses appetite 19 , 98 Table 2 , and mutations in the POMC gene are associated with obesity in a range of species including humans, mice and dogs Best known for signaling satiety, recent intriguing data reveal a previously unprecedented function of a subset of POMC neurons to promote feeding behavior through cannabinoid-receptor mediated release of β-endorphin, an endogenous opioid neuropeptide originating from the POMC precursor molecule By contrast, AgRP neurons are hunger sensitive and signal energy deficits: their activation rapidly evokes eating and their ablation in the adult animal causes rapid weight loss due to cessation of feeding 16 , 19 , , Mechanistically, the neuropeptide AgRP competes with α-melanocyte-stimulating hormone α-MSH released from POMC neurons for binding sites on the melanocortin 4-receptor MC4R , blocks the coupling to a G αs signaling pathway, and promotes feeding when AgRP has the upper hand. Besides the canonical view of neuronal MC4R signaling, new fascinating data however suggest that AgRP can act independently of G αs and through regulating the pore state of an inwardly rectifying potassium channel, Kir7. According to these results, binding of AgRP onto MC4R opens Kir7. The brain launches an adaptive and protective counter-regulatory response when glucose levels fall out of range. The VMH Fig. In mice that are hypoglycemic owing to a high dose of insulin, the ability to normalize glycaemia fails when SF-1 neurons are optogenetically inhibited, as the anticipated rebound from hypoglycemia elicited by insulin is attenuated In turn, optogenetic activation of SF-1 neurons increases blood glucose, and causes profound hyperglycaemia when blood glucose levels are elevated either by stimulating HGP or by injecting glucose into mice The differential responses may stem from a failure of stimulating glucagon and corticosterone release when SF-1 neurons are inhibited , or from the inability to balance glucagon and corticosterone secretion and control HGP when SF-1 neurons are stimulated. It is conceivable that photostimulation of SFexpressing neurons mimics a state of glucodeprivation in the VMH since they stimulate the counter-regulatory response to hypoglycemia, including effects on pancreas and liver. Thus, a defined circuit spanning from glucose-sensing VMH neurons to the aBNST specifically regulates expression of key genes for hepatic gluconeogenesis and influences the abundance of counter-regulatory hormones striving to restore glycaemia. In another study, investigators used radiowaves to manipulate glucokinase-expressing VMH neurons engineered to respond to an electromagnetic field, and showed that activation of VMH neurons robustly elevates blood glucose and glucagon concentrations in the circulation as well as drives the expression of key hepatic gluconeogenic genes, whereas inhibition quells these responses These findings further substantiate a role for the VMH in the control of peripheral glucose metabolism, and the authors describe a novel technique, dubbed magnetogenetics, to affect neuronal activity through a genetically encoded fusion protein between the iron-binding protein ferritin and a thermo-sensitive ion channel protein. Although the paper describes a way to remotely manipulate the electrical activity of neurons in mice with a very clear outcome 28 and whereas a string of recent articles report the successful use of magnetogenetics, the way the underlying operative mechanism biophysically works is unclear and has turned into a subject of debate To ensure that the field strength was adequate to affect neuronal activity, while permitting assessments of its impact on glucose metabolism in vivo , the mice had to be anesthetized in those studies Although the findings obtained from manipulating VMH neurons were the expected, whether exactly the same outcome is present in awake mice could not be proven with the confines of the method, as narcosis might have intrinsic effects on neural activity and glucose homeostasis. Thus, refinements of the necessary equipment for electromagnetics is required for large-scale use and to set the stage for further exciting discoveries. Moreover, future studies are encouraged to define the precise mechanism of magnetogenetics. Although recent research has provided a wealth of information, the functional organization of the neurocircuity influencing counter-regulatory mechanisms of glycemic control remains to be better understood, and electromagnetics is hoped to provide more answers on the neuroendocrine components and architecture contributing. While the aBNST has surfaced as a key integrative glucoregulatory node, the details about this system remain to be specified. Specifically, which descending neural network downstream of the aBNST, tethering it do BAT glucose utilization, insulin sensitivity and counter-regulatory responses, as well as the exact cellular phenotype of the crucial aBNST neurons are issues that clearly call for additional study. Located along the midline of the anterior hypothalamus, the preoptic area PoA is situated closely below the anterior commissure where nerve bundles pass between the two brain hemispheres and above the optic chiasm where optic nerve fibres from the retinas cross between the two hemispheres Fig. The PoA regulates BAT heat production, a process that depends on the metabolism of significant amounts of glucose and triglycerides 30 , 31 , Nevertheless, the thermoregulatory function of this brain region has been primarily studied in the context of fever, which is driven by prostaglandin signalling in the median preoptic subnucleus 33 and activates brown fat thermogenesis via a neural pathway including the rostral raphe pallidus Fig. Surgical or electric manipulations of the LHA neurons over 50 years ago were shown to control food intake. We now know that a part of this effect is explained by an inhibitory synaptic innervation from the BNST to glutamatergic LHA neurons, eliciting voracious feeding in mice that are already satiated when optogenetically manipulated In food-deprived animals, inhibiting this input onto the LHA conversely suppresses feeding Furthermore, projections to the LHA from AgRP neurons impair systemic insulin sensitivity when activated So far, recent observations point toward a critical role for MC4R signalling in the LHA in control of glucose homeostasis By reconstituting MC4R expression specifically in LHA neurons of obese mice carrying a null MC4R allele MC4R LHA , Morgan et al. were able to improve glucose tolerance and glycaemia in both normal chow and high-fat diet HFD -fed mice independent of changes in body weight, adiposity or insulin concentrations Activation of the MC4R using an α-melanocyte stimulating hormone α-MSH analogue in mice with MC4Rs re-expressed in the LHA increased glucose uptake specifically into brown fat; this effect correlated with subtle increments in glucose transporter 4 GLUT-4 gene expression and upregulation of a thermogenic gene expression programme in BAT Consistent with the idea that MC4R LHA signalling facilitates BAT glucose utilization via the sympathetic nervous system, nerves innervating BAT showed normal spiking responses to a MC4R agonist in mice carrying a reactivated MC4R gene in the LHA, in contrast to the nerves in obese whole-body MC4R knockouts that were insensitive, and surgically eliminating BAT from sympathetic input furthermore impaired the improved glucose tolerance obtained from MC4R LHA reactivation Thus, MC4R LHA signalling activates sympathetic outflow to BAT, and intact sympathetic control over BAT glucose uptake is required to rescue the glucose tolerance when the MC4R is gone in every cell but in LHA neurons, as judged from this comprehensive study in mice In the s, physiologist Claude Bernard observed that manipulation to the floor of the fourth ventricle in the hindbrain of experimental animals caused blood glucose levels to rise above normal, and that the excess sugar was excreted in the urine Walter Bradford Cannon later conceptualized and developed it further. With diminished enthusiasm for the brain as an interesting target for intervention, research was now devoted to deciphering insulin action in peripheral organs and defects in pancreatic insulin secretion. In hindsight, however, and considering that the brain governs control of most homeostatic networks, it seems improbable that glucose metabolism would be controlled by mechanisms independent of the CNS. In humans, the quantity of BAT correlates inversely with BMI, BAT is highly responsive to cold and diet exposure, an adaptive response that is reduced in obese and overweight subjects, and insulin 36 , 37 , 38 , 39 , There is evidence that BAT is less active in diabetics 41 and that BAT activation improves whole-body glucose homeostasis and insulin sensitivity Such observations have fostered the notion that strong actuators of BAT activity could be used to treat obesity and diabetes. Brown fat function is often studied under cold conditions, a state that does not allow capturing whether BAT plays a role in glucose metabolism at euthermia. To measure whether metabolic activity in human BAT affects blood glucose levels over time and depending on feeding state and circadian rhythm, Lee and colleagues measured the temperature profile of the skin overlying supraclavicular BAT as a surrogate of conventional fluorodeoxyglucose positron emission tomography FDG-PET imaging At thermoneutrality, supraclavicular BAT temperature progressively rose during a glucose load, indicating that BAT utilizes glucose. The authors also observed a noteworthy rhythmicity in glucose uptake into human brown adipocytes, especially after insulin stimulation, together with oscillating trafficking of GLUT-4 to the plasma membrane, which mirrored the fluctuations in glucose uptake and generated heat In humans normal weight, non-diabetic men in their mid-twenties with larger than average active BAT depots, changes in BAT thermogenesis predicted subcutaneous blood glucose levels, whereas BAT thermogenic activity responded to systemic changes in glycaemia in individuals with comparatively small amounts of BAT Notably, men devoid of supraclavicular BAT exhibited the largest glycemic variability. Conceivably, human BAT glucose utilization is linked to thermogenesis, and BAT shows a glucose-responsive rhythm entrained by circadian oscillations in GLUT-4 in a similar manner as mechanisms coordinating body temperature rhythmicity and responses to cold In light of these findings, whether greater fluctuations in glucose levels as a consequence of the amount of functionally active BAT pre-dispose for diabetes warrant further investigations. Afferent hormonal and nutritional cues provide feedback signals to the brain that are crucial for systemic glucose homeostasis. On the other hand, efferent signalling from the brain to peripheral tissues is promoted via the autonomic nervous system, for example to control HGP, BAT activity and pancreatic hormone secretion Fig. However, several discoveries made in the past 20 years have reignited interest in this concept. Firstly, activation of the IR, which is widely expressed throughout the CNS, was shown to curb eating. Secondly, manipulation of key IR signalling components such as PI3 kinases , activation of neuronal ATP sensitive potassium channels 45 , or depletion of functional IRs from the brain 46 , affect not only energy homeostasis but also systemic glucose metabolism. In humans, insulin quenches HGP via the same class of potassium channels K ATP as it does in rodents Insulin activates K ATP channels in a PI3 kinase-dependent manner resulting in hyperpolarization of neurons 13 , However, how various hypothalamic neurons respond electrically to insulin might differ, as exemplified by the recent findings that insulin can excite POMC neurons via activation of canonical transient receptor potential channels in a PI3 kinase-dependent manner Similarly, insulin promotes PI3 kinase signalling in melanin-concentrating hormone MCH neurons in the LHA and increases their excitability Physiologically, insulin-dependent activation of these neurons impairs locomotor activity and glucose homeostasis by controlling hepatic insulin sensitivity and HGP in mice fed a HFD. Given that the phenotypic alterations dependent on IR signalling in MCH neurons were observable in HFD-fed mice but not lean mice fed a normal mouse chow suggest that this mechanism is engaged only during conditions when insulin levels rise. Consistent with this, HFD feeding associated with hyperinsulinemia increases PI3-kinase activity in MCH neurons via the IR The central nervous system contains high density of receptors for the white adipose tissue WAT -derived hormone leptin as well as receptors for the pancreatic hormone insulin. Leptin and insulin act on specific brain regions that will in turn modulate glucose utilization and production in peripheral tissue via the autonomic nervous system. Notably, the vagus nerve links brain insulin action and the liver in the control of hepatic gluconeogenesis. At the pancreatic level, the autonomic nervous system is involved in pancreatic hormone secretion. The brown adipose tissue BAT receives sympathetic innervation which activity directly control BAT glucose uptake. NA, noradrenaline. The insulin-dependent effects on MCH-expressing cells supports the existence of selective hormone resistance, which describes the occurrence of insulin resistance in cell types within the CNS with simultaneous retained or even over-activated insulin action in other CNS cell types. Indeed, the manifestation of selective CNS resistance to insulin represents a rule rather than exception In fact, insulin activates PI3K signalling and reduces the firing rate of a proportion of SF-1 VMH neurons through K ATP channel activation Mice lacking the IR on these subsets of neurons are partially protected from diet-induced obesity upon HFD feeding, associated with reduced systemic insulin levels and improved glucose metabolism Thus, the hyperinsulinemia present under prolonged HFD feeding predictably silences the SF-1 neurons, and IR signalling via the PI3K pathway in SF-1 VMH neurons mediates systemic insulin resistance and obesity in response to a HFD. Thus, the manifestation of selective insulin resistance clearly necessitates work on the underlying molecular mechanisms. Future studies should focus on region-specific mechanisms of selective hormone resistance, and, ultimately, to develop cell-specific insulin de sensitizers in the treatment of obesity-associated alterations such as uncontrolled HGP. Chronically elevated HGP contributes significantly to the hyperglycaemia associated with T2D ref. Understanding how the liver fails to respond to insulin and to the efferent signals originating from the CNS in the regulation of this process is thus of great importance. Pharmacological approaches were the first to document a role for central insulin signalling in the control of peripheral glucose homeostasis, as infusion of insulin into the cerebral ventricle adjacent to the hypothalamus suppresses HGP and lowers blood glucose A key observation in the search for the neuronal substrate explaining how brain IR signalling can inhibit HGP came from mice genetically modified to lack the IR specifically in AgRP neurons. Here, Könner et al. observed that failure to activate IR signalling in AgRP neurons substantially reduced the ability of peripherally applied insulin to suppress HGP under a euglycemic-hyperinsulinemic clamp. These findings thus demonstrated that the site for central insulin signalling to inhibit HGP is, indeed, AgRP neurons In agreement with these data, selective restoration of the IR specifically in AgRP neurons in addition to liver and pancreatic β-cells rescues the ability of insulin to curb HGP, whereas selective re-expression of the IR to POMC neurons in otherwise IR-deficient mice exacerbates insulin resistance and increases HGP Thus, these findings suggest a functional dichotomy in regulation of HGP originating from POMC and AgRP neurons, similar to their opposing effect on feeding and energy expenditure 19 Box 2. In addition, hypothalamic insulin action reduces the breakdown of lipids lipolysis and promotes fatty acid and triglyceride synthesis lipogenesis in adipocytes through a reduction in the sympathetic tone to white adipose tissue The vagus nerve the tenth cranial nerve innervates large parts of the viscera and has been suggested to create the critical interface between the brain and the liver Fig. The vagus nerve also links brain IR signalling to gluconeogenesis, as central insulin action requires intact hepatic vagal nerve branches to suppress HGP 6 , Insulin hyperpolarizes AgRP neurons and inhibits their firing frequency through opening of K ATP channels The reduced activity of AgRP neurons, in turn, results in ILmediated activation of STAT3 signalling in the liver, and downregulates the abundance of key gluconeogenic genes, including Pepck and G6Pase 13 , 45 , 53 , 56 , These data suggest that diet-induced obesity blunts hypothalamic IR signalling and inhibits its control of HGP, substantiating a role for central insulin resistance in obese, diabetic animals. S6K1 signalling in POMC neurons is, however, also reported to suppress HGP in hyperinsulinemic clamps The disparate outcome from these experiments may not be mutually exclusive and differences in cells targeted because of varying methodology adenoviral-based, acute pan-neuronal overexpression versus chronic POMC cell-specific gene inactivation are likely one explanation to these seemingly discordant findings, especially considering neuronal heterogeneity, that is, existence or different subpopulations of functionally distinct POMC neurons. Insulin is not the only hormone that affects systemic glucose homeostasis through CNS-mediated mechanisms. For example, glucagon-like peptide 1 GLP-1 augments glucose-stimulated insulin secretion and reduces HGP, likely mediated by GLP-1 receptor signalling in the ARH The peptide hormone glucagon secreted from pancreatic alpha-cells Fig. Hypothalamic glucagon receptor activation was found to inhibit HGP through a K ATP channel-dependent mechanism, and the increase in HGP from raising peripheral glucagon concentrations could be abated by blocking glucagon action in the CNS 62 , These data led to the conclusion that, in contrast to its direct actions on the liver, hypothalamic glucagon signalling inhibits HGP 62 , This was surprising, because glucagon drives HGP by direct effects on hepatocytes Fig. That a peptide promotes HGP through its stimulatory effects on the liver, and on the other hand inhibits the very same process through effects on the brain may seem counter-intuitive, as these two forces are counteracting. The findings may however point to the existence of a self-regulatory feedback loop to fine-tune HGP, in which central glucagon signalling explains why the hepatic effect of high glucagon concentration on HGP is transient, tapering off within hours even during continuous glucagon infusion. A monomeric peptide conjugate between glucagon, GLP-1 and GIP glucose-dependent insulinotropic polypeptide that acts as an agonist at each receptor vastly improves metabolic and glycemic control in obese and diabetic rodents As judged from its impact on whole-animal physiology increased energy expenditure, reduced caloric intake and better glycemic control , it is reasonable to believe that the triple agonist exerts some of its key functions by acting on the brain. Finally, whether the data in rodents on central glucagon action, with the purpose of limiting its own effects on the liver, extend to humans is important to investigate. Whether insulin action in the CNS is relevant for day-to-day or acute control of blood glucose in humans has been a matter of intense discussions While causally proving the existence of a CNS-dependent mechanism of insulin action to inhibit HGP in humans is inherently challenging, administering insulin through a spray formulation into the nose has shed some light on the physiological relevance of insulin signalling in the human brain. Intranasal application of insulin rapidly elevates levels of the hormone in the cerebrospinal fluid at concentrations that are too low to be detected in the blood, suggesting that insulin penetrated directly into the brain from the nose without increasing insulin levels in the systemic circulation Daily intranasal insulin administration over 8 weeks reduces body fat and weight in healthy men but not woman ranging between 0. Importantly, Heni et al. In their study, lean individuals required more glucose to maintain euglycemia after intranasal delivery of insulin in a clamp setting compared with placebo-treated individuals in the presence of similar venous insulin levels. These data indicated improvements in whole-body insulin sensitivity, and the amount of glucose infused interestingly correlated with increased hypothalamic activity and indices of increased parasympathetic descending vagal nerve activity Therefore, the authors concluded that short-term insulin action as a result of intranasal application of insulin improves systemic insulin sensitivity in humans, possibly via a hypothalamic-mediated vagal mechanism like in rodents 6 , However, these studies do not provide definitive evidence that endogenously produced insulin has a similar physiological role in the human brain. The responses to intranasal insulin therapy, and the cortical response to systemic hyperinsulinemia are weaker in obese humans, suggesting that obesity renders the brain less responsive to insulin 69 , This phenomenon also occurs in animals with reduced amounts of IR protein in the ARH, a situation that is accompanied by a failure to efficiently suppress HGP and whole-body insulin resistance Besides being a methodological bedrock for experiments aiming to elucidate the role of insulin signalling in the brain, the question is whether nasal insulin administration therefore represents an attractive alternative medical regimen to current therapies to treat obesity-associated diabetes. The development of T2D can be preceded by defects in not only insulin-dependent but also in insulin-independent glucose uptake more than a decade before the disease is diagnosed Thus, how efficiently glucose promotes its own disposal unrelated to insulin action predicts the future risk of developing glucose intolerance. Secreted from white adipose tissue in proportion to fat mass, leptin is intimately linked to CNS-dependent control of glucose homeostasis; as such leptin administration has been reported to rescue insulin-deficient diabetes Thus, leptin receptor signalling in the brain appears to normalize diabetic hyperglycaemia across different tissues and mechanisms, giving rise to the idea that leptin compensates for the lack of insulin in animal models of diabetes where loss of islet β-cell function is prominent In addition, combined leptin and insulin signalling in POMC neurons is broadly accepted to regulate peripheral glucose metabolism. Supporting this notion, mice lacking both the insulin and leptin receptors on POMC neurons do not suppress HGP normally, an effect associated with systemic glucose intolerance and insulin resistance Reconstitution of leptin receptor signalling on the same neurons conversely normalizes blood glucose and increases hepatic insulin sensitivity Collectively, these data point to a key role for leptin action in the ARH. However, hypoinsulinaemia as a consequence of islet failure does not seem to increase compensatory leptin receptor signalling in the CNS with the purpose of rescuing euglycemia as the hyperglycaemia usually persists in conditions characterized by insulin deficiency. Whether leptin alone can replace or compensate for insulin deficiency can thus be debated. The islets of the pancreas are subject to regulation by insulin signalling in the brain, and their connection with the CNS and the efferent arm of the autonomic nervous system is remarkably vulnerable during a specific developmental time window of the hypothalamic neurocircuitry Work from Vogt et al. has shown that feeding mothers a HFD exclusively during the lactation period leads to abnormal formation of axons from POMC neurons to the posterior part of the paraventricular nucleus of the hypothalamus PVH Fig. Ultimately, these perturbations are associated with obesity, impaired glucose-stimulated insulin secretion as well as glucose intolerance in the offspring that received fat milk On the other hand, pups genetically modified to lack the IR in POMC neurons were protected from disturbances in glucose homeostasis in response to maternal HFD feeding during lactation Thus, hyperinsulinemia may predispose the progeny of an overnutritioned breast-feeding mother for future long-lived metabolic disease through hypothalamic IR signalling, whereas the inability to sense the abnormally high levels of insulin acting on POMC neurons during lactation prevents it. Given the escalating numbers of obese and diabetic pregnant or breast-feeding women, a better understanding of metabolic, developmental programming is thus urgently needed. Recent results obtained by combining neural tracing experiments and functional interventions directed to different hypothalamic nuclei provided new insights into the innervation of the pancreas and its influence over glucose metabolism Backtracking the CNS sites innervating the pancreas provide the evidence that glucokinase-expressing neurons in the ARH send signals via multiple synapses to this tissue Functionally, inhibiting glucose sensing in the ARH reduced insulin secretion and led to glucose intolerance, demonstrating a causal relationship between the innervation and pancreatic secretory function As the intervention was not directed towards a specific sub-set of neurons in the ARH, the identity of the neurons regulating pancreatic function remains unknown. POMC and AgRP neurons are both known to change their excitability to fluctuations in extracellular glucose concentrations in electrophysiological studies. POMC neurons are glucose excited, driven by closing of K ATP channels. When POMC neurons lost the ability to sense glucose, through genetically preventing ATP-mediated closure of K ATP channels, or made defective via HFD feeding, glucose tolerance is impaired Whether the effect seen stems from a failure to correctly regulate insulin secretion, however, currently remains unclear. Other than in the ARH, Pomc mRNA is only expressed in the nucleus of the solitary tract within the CNS, and thus shows a very restricted expression pattern. This is in contrast to the MC4R distribution, the receptor for POMC-derived α-MSH, which is broadly expressed in the brain, including in nuclear groups in the medulla oblongata. Deletion of the MC4R in the dorsal motor nucleus of the vagus nerve DMV , part of the dorsal vagal complex DVC Fig. In agreement with these findings, in obese, glucose intolerant and hyperinsulinemic MC4R-null mice, selective restoration of MC4R expression to DMV neurons attenuated the hyperinsulinemia without affecting body weight 8. Thus, DMV MC4R signalling has an essential role in regulating blood insulin levels. Given the dissociation between improvements in insulin levels and lack of body weight reduction, these data also support the existence of divergent melanocortin pathways in control of glucose metabolism and energy balance. Possibly linking hypothalamic neurons to regulation of insulin secretion are insulin-sensitive GLUTexpressing neurons of the hypothalamus GLUT-4 HYPO. Cre-dependent viral tracing experiments have provided evidence that GLUT-4 HYPO neurons project to the DMV, and mice in which GLUT-4 HYPO neurons have been ablated present with elevated plasma glucose and reduced insulin levels but normal pancreatic beta-cell morphometry Accordingly, mice devoid of GLUT-4 HYPO neurons display impaired glucose tolerance. To that end, the authors suggested that the hyperglycaemia is a consequence of impaired insulin secretion involving a GLUT-4 HYPO to DMV projection While the data clearly define a role for GLUT-4 HYPO neurons in the control of energy and glucose metabolism, the experimental approach relied on the death of GLUT-4 HYPO neurons, and did not permit an evaluation on the role of GLUT-4 neurons in discrete hypothalamic areas. Genetic cell ablation may not come without caveats, such as gliosis see below appearing following GLUT-4 HYPO neuron ablation, and a vast array of neurons are GLUTexpressing, making the application of cell-specific excitatory or inhibitory control of viable GLUT-4 HYPO neurons an attractive complement for further expansion of our knowledge on their role in energy metabolism and insulin signalling The reduced propensity of the CNS to respond to hormones during obesity has been extensively studied; the resistance to insulin and leptin within the melanocortin circuitry in the hypothalamus being best defined 82 , 83 , Moreover, in the CNS, activation of inflammatory processes is a key event in the manifestation of peripheral insulin resistance in obese animals 85 , Inflammatory insults to AgRP neurons have a dominant role in these processes 87 as attenuation of the neuroinflammatory response by depriving AgRP neurons of the inhibitor of nuclear factor kappa-B kinase 2 IKK-β gene, an essential trigger of the immune response, protects against obesity and systemic glucose intolerance from HFD feeding Moreover, c-Jun N-terminal kinase 1- and IKK-β-dependent inflammatory signalling is sufficient to drive neuronal and systemic leptin or insulin resistance, respectively, even in the absence of HFD feeding when constitutively activated in AgRP neurons The onset of hypothalamic inflammation is rapid. Gliosis, the process of glial cells in the central nervous system reacting and proliferating to a trauma or injury and a prominent feature of neurodegenerative diseases , surrounding AgRP neurons can be seen within three days and before fat accumulation is measurable in rodents confronted acutely to a HFD Such observations have fostered the hypothesis that neuroinflammation is an actuator of obesity development rather than a secondary consequence of weight gain. The acute HFD-induced gliosis gradually tapers off in rodents 90 , 91 , indicative of an induction of a neuroprotective mechanism, but that is eventually overridden as gliosis, leptin resistance and glucose intolerance persist upon chronic HFD feeding unless the unhealthy diet is discontinued Similar signs of inflammation have been reported in obese humans from neuroradiologic assessments of gliosis 90 , and gliosis has recently been found to associate with higher BMI, fasting insulin and HOMA-IR Homeostatic Model Assessment, a model to assess beta-cell function and insulin resistance in obese humans. Insulin levels and HOMA-IR did not correlate with BMI in these investigations, suggesting a link between gliosis, pancreatic responses and insulin resistance unrelated to the degree of adiposity Recent observations offer evidence in support of a neuroprotective mechanism clearly linked to inflammatory signalling, characterized by similar temporal dynamics and kinetics as the onset and disappearance of HFD-induced gliosis Here, perivascular macrophages are recruited to the blood—brain barrier of the cerebral blood vessels when the brain is challenged with a HFD to limit central inflammation. Via local vascular endothelial growth factor production and increased expression of glucose transporters GLUT-1 , these events are believed to warrant cerebral glucose homeostasis during consumption of energy-dense foods Despite the existences of mechanisms offering acute protection of neuronal function, the extent of the exposure to fatty food is a denominator for the magnitude of hypothalamic inflammation, as prolonged HFD feeding causes leptin and insulin resistance and disturbances in peripheral glucose homeostasis. To this end, non-neuronal cells other than astrocytes and immune cells associated to the cerebral blood vessels as described above are also involved. Evidence suggests that saturated fat can be sensed predominantly by mediobasal hypothalamic, intraparenchymal microglia Activating an inflammatory M1 cytokine response to the buildup of saturated fatty acids in microglia may set the stage for hypothalamic neuronal stress and reduced leptin responsiveness, which in turn may reduce peripheral insulin sensitivity. Understanding the pathomechanisms behind diet-induced neuroinflammation is thus of high priority in the field of metabolism research, as it has implications for our understanding of obesity and insulin resistance as well as a better comprehension of the neurological complications such as neuropathies, cognitive dysfunction and stroke associated with diabetes. Significant advancements to our understanding of how the brain influences peripheral glucose homeostasis have been made owing to studies revealing key brain regions and the identities of the neurons involved, their connectivity and the molecular components causally associated, as well as the peripheral organs and cellular events targeted by the brain. Specifically, HGP, brown fat glucose utilization and control of insulin secretion are processes importantly regulated by the CNS. Although great progress in this area of research has been made, several issues nonetheless remain to be resolved. To this end, while the application of techniques with high spatial resolution in neuroscientific research, relying on the existence of a known cell-specific promoter, has moved us several steps forward towards better control over functional neurocircuits, unique marker genes for many CNS cell-types potentially involved are yet nonetheless still inconspicuous. Moreover, there is extensive heterogeneity in gene expression within single CNS nuclei, and better characterization of this molecular diversity would subsequently improve our comprehension of the neuronal mechanisms controlling peripheral insulin sensitivity and glucose metabolism. Furthermore, a remaining challenge is to directly test whether processes regulating BAT activity and HGP can be exploited for the development of better and safer viable therapeutics. In fact, the beneficial effects of current anti-diabetic therapies, such as insulin supplementation, drugs triggering insulin release, insulin-resistance reducing agents and insulin-sensitizing medications are explained by peripheral actions, and although they successfully reduce hyperglycaemia, they were developed under the assumption that the brain has little, if any, influence on these processes. The inherent adverse effects including hypoglycemia, weight gain and gastrointestinal problems accompanying some of these medications are also problematic. To this end, identifying strong, selective actuators of BAT activation and agents dampening HGP will be important. Indeed, work on defining the neuronal mechanisms controlling BAT and liver biology may not only reveal potential CNS targets, but also facilitate the identification of pathways in liver and BAT directly controlled by the CNS. Realistically, drug candidates in the myostatin signalling cascade, well-studied in the context of muscle growth, sarcopenia and cachexia, could rapidly be advanced into clinical trials assessing their therapeutic potential to moderate insulin resistance. There is also a need to define novel regulators of key glucoregulatory neuronal populations, which may lead to innovative therapies. For instance, recent publications identified the purinergic-receptor 6 P2Y6 as novel regulator of AgRP neuron activity and further revealed that selectively abrogating P2Y6 signalling in AgRP neurons alleviates obesity-associated insulin resistance Translational studies will be necessary to validate if P2Y6-antagonism represents a pharmaceutical way for diabetic treatment. Learn more about manual insulin injections and how they help treat…. But it does increase your chance of getting it. Learn more about…. Insulin is a very important hormone in the body. A resistance to its effects, called insulin resistance, is a leading driver of many health conditions. Diabetes hinders your ability to produce insulin. Without it, cells are starved for energy and must seek an alternate source, leading to serious…. New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney…. Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes. What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español. Insulin Resistance. Medically reviewed by Marina Basina, M. Symptoms Diagnosis Risk factors Prevention. Symptoms of insulin resistance. Testing and diagnosis of insulin resistance. Risk factors for insulin resistance. Preventing insulin resistance problems. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Breadcrumb Home You Can Manage and Thrive with Diabetes Understanding Insulin Resistance. What Is Insulin Resistance? What Causes Insulin Resistance? What Does It Mean for Your Health? What Can You Do About It? Getting active is probably the best way to combat insulin resistance. Exercise can dramatically reduce insulin resistance in both the short and long terms. In addition to making the body more sensitive to insulin and building muscle that can absorb blood glucose, physical activity opens up an alternate gateway for glucose to enter muscle cells without insulin acting as an intermediary, reducing the cells' dependence on insulin for energy. While this doesn't reduce insulin resistance itself, it can help people who are insulin resistant improve their blood glucose control. Weight loss can also cut down on insulin resistance. No single diet has been proved to be the most effective. Some evidence suggests, though, that eating foods that are low in fat and high in carbohydrates can worsen insulin resistance. Research has also shown that people who undergo weight-loss surgery are likely to become significantly more sensitive to insulin. No medications are specifically approved to treat insulin resistance. Yet diabetes medications like metformin and thiazolidinediones, or TZDs, are insulin sensitizers that lower blood glucose, at least in part, by reducing insulin resistance. |
| RESEARCH DESIGN AND METHODS— | Acevedo Insulin sensitivity and glucose regulation Real-time glucose sensor Pennings. More than one sensitiity of the adult population Imsulin obese Insulim many countries, including newly Flexibility supplements for athletes states, making obesity a global human health problem 1. Find a doctor. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis. The symptoms, diagnosis and treatment. |
Insulin sensitivity and glucose regulation -
Additionally, low carb diets may support weight loss, which could help increase insulin sensitivity 7 , Low carb diets involve limiting your intake of foods high in carbs or added sugar, including baked goods, grains, and sweets.
Diets that are very low in carbohydrates, such as the ketogenic diet , may also improve blood sugar regulation and enhance insulin sensitivity 48 , According to one review, following a ketogenic diet may help improve blood sugar regulation, decrease inflammation and fasting insulin level, and promote weight loss, all of which may be beneficial for people with insulin resistance Low carb and ketogenic diets may improve insulin resistance and support blood sugar regulation.
However, you should talk with a healthcare professional before making major changes to your diet. Insulin resistance may be one of the key drivers of many chronic conditions, including type 2 diabetes. You can improve this condition through lifestyle measures such as eating a balanced diet, staying active, and making an effort to maintain a moderate body weight.
Preventing insulin resistance may be among the most effective ways to live a longer, healthier life. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Find out the different types of basal insulin.
Understand the benefits, how they're administered, and potential side effects. Read on to learn how your insulin needs may…. Insulin resistance doesn't have to turn into diabetes. Know about early signs and find out what you can do to identify the condition. Some people claim that artificial sweeteners can raise blood sugar and insulin levels, and potentially even cause diabetes.
If your doctor recommends you start taking insulin to manage type 2 diabetes, you may have some questions. Read on for guidance.
Diabetes hinders your ability to produce insulin. Without it, cells are starved for energy and must seek an alternate source, leading to serious…. Learn about the different types of medications that can increase the production of insulin in people with diabetes.
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based Insulin and Insulin Resistance: The Ultimate Guide.
Medically reviewed by Kelly Wood, MD — By Kris Gunnars, BSc — Updated on December 7, Insulin basics. What causes insulin resistance? How to know if you have insulin resistance. Discover more about Type 2 Diabetes. Related conditions. Relationship to heart health. Other ways to reduce insulin resistance.
Low carb diets. The bottom line. How we reviewed this article: History. Dec 7, Written By Kris Gunnars. Nov 28, Medically Reviewed By Kelly Wood, MD. Share this article. Read this next. Medically reviewed by Peggy Pletcher, M. Basal Insulin Types, Benefits, Dosage Information, and Side Effects.
Medically reviewed by Alan Carter, Pharm. Medically reviewed by Maria Prelipcean, M. Insulin Resistance. Medically reviewed by Marina Basina, M. Do Artificial Sweeteners Spike Your Blood Sugar? What Are the Pros and Cons of Switching to Insulin for Type 2 Diabetes?
Medically reviewed by Michelle L. Griffith, MD. The Effects of Insulin on the Body. Medically reviewed by Kevin Martinez, M.
The most common adverse effect is nausea, but they may also vomit. In some cases, an allergic reaction may occur. Blood sugar levels should return to safer levels within 10—15 minutes. After this, the person should ingest some candy, fruit juice, crackers, or other high-energy food.
Doctors may also use glucagon when diagnosing problems with the digestive system. A range of factors, including insulin resistance , diabetes, and an unbalanced diet, can cause blood sugar levels to spike or plummet.
Ideal blood sugar ranges are as follows :. Read more about optimal blood sugar levels here. High blood sugar can be a sign of diabetes, but it can also occur with other conditions.
Without intervention, high blood sugar can lead to severe health problems. In some cases, it can become life threatening. Insulin and glucagon help manage blood sugar levels. In addition to diabetes, possible causes of high blood sugar include :.
People with high blood sugar may not notice symptoms until complications appear. If symptoms occur, they include :.
Over time, high blood sugar may lead to :. Hypoglycemia is most likely to affect people with diabetes if they take their diabetes medication — such as insulin or glipizide — without eating. But, it can happen for other reasons, for example:. The symptoms of low blood sugar include :.
Without treatment, low blood sugar can lead to seizures or loss of consciousness. What are the different types of diabetes? Insulin helps the cells absorb glucose from the blood, while glucagon triggers a release of glucose from the liver. People with type 1 diabetes need to take supplemental insulin to prevent their blood sugar levels from becoming too high.
In some cases, a doctor will recommend insulin for people with type 2 diabetes. However, diet and exercise are usually the first recommendations for this type. Very low blood sugar can become life threatening without medical intervention. In this article, we look at nine ways to lower high insulin levels.
This can be achieved through diet, lifestyle changes, supplements, and medication. A person can manage their diabetes by making healthful changes to their diet, exercising frequently, and regularly taking the necessary medications….
Researchers said baricitinib, a drug used to treat rheumatoid arthritis, showed promise in a clinical trial in helping slow the progression of type 1….
A new review indicates that insulin—used to manage diabetes—can be kept at room temperature for months without losing its potency.
A study in rat models of diabetes suggests that spinach extract — both water- and alcohol-based — may help promote wound healing, which occurs very…. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us.
Medical News Today. Health Conditions Health Products Discover Tools Connect. How insulin and glucagon regulate blood sugar. Medically reviewed by Angela M. Bell, MD, FACP — By Zawn Villines — Updated on February 15, Overview Taking insulin and glucagon Ideal levels Effects on the body Summary Insulin and glucagon help maintain blood sugar levels.
Insulin, glucagon, and blood sugar. Taking insulin and glucagon. Ideal blood sugar levels. How blood sugar levels affect the body. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations.
We avoid using tertiary references. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles. You can learn more about how we ensure our content is accurate and current by reading our editorial policy.
Share this article. Latest news Ovarian tissue freezing may help delay, and even prevent menopause.
You could be glucoae resistant for Insulin sensitivity and glucose regulation without knowing it. Insulin snsitivity increases your risk of developing diabetes, sensitifity well as :. Classic diabetes symptoms include:. Some people with insulin resistance may also develop a skin condition known as acanthosis nigricans. It appears as dark, velvety patches often on the backs of the neck, groin, and armpits. Insulin vlucose IR is a pathological condition in which cells either zensitivity to respond normally to the hormone insulin or downregulate Black pepper extract supplements receptors in Isnulin to hyperinsulinemia. Insulin is Insulin sensitivity and glucose regulation hormone that Flexibility supplements for athletes the transport of glucose from blood into cells, thereby reducing blood glucose blood sugar. Insulin is released by the pancreas in response to carbohydrates consumed in the diet. In states of insulin resistance, the same amount of insulin does not have the same effect on glucose transport and blood sugar levels. There are many causes of insulin resistance and the underlying process is still not completely understood.Video
What is insulin resistance? Why does it happen? [Dr. Christopher Gardner]
die Schnelle Antwort, das Merkmal des Verstands:)
Welche Wörter... Toll, der prächtige Gedanke