
Astaxanthin and metabolic function -
dendrorhous UV CGMCC No. dendrorhous was first cultivated in mL Erlenmeyer flasks containing 30 mL YPD medium, and the culture was incubated in a rotary shaker SUKUN SKYB, Shanghai, China at 22 °C and rpm for 48 h. dendrorhous was then cultured in mL Erlenmeyer flasks containing 30 mL YPD medium at 22 °C for 24 h.
Subsequently, a 4. The fermentation medium had a pH of 6. The secondary seed culture was then transferred into a 5-L bioreactor Winpact, Major Science, USA with a working volume of 3 L for fermenter culture. Different concentrations of Na-citrate 0, 1. Cultures without Na-citrate addition were used as the controls.
Samples from the control and Na-citrate groups were collected every 24 h for biomass, glucose concentration, carotenoids, astaxanthin, and metabolite analysis until the total fermentation time reached h. The cell suspension was centrifuged at × g and 8 °C for 10 min, followed by washing the cell pellets twice using distilled water and drying at °C until they attained a constant weight.
The supernatant obtained after centrifugation was used to measure the glucose concentrations on an SBAD biosensor Shandong, China. Total carotenoids were extracted from the cell pellets using dimethylsulfoxide DMSO as previously described Pan et al. Astaxanthin was determined following the method described by Xie et al.
In brief, astaxanthin was analyzed on an Agilent series HPLC system equipped with a UV detector Agilent Technologies, USA and a YMC30 RP column 4.
All eluents contained 0. Gradient elution was carried out as previously described Pan et al. The UV detection wavelength was adjusted to nm. Metabolome analysis was performed following the procedures described by Li et al.
with minor modifications Li et al. Briefly, 40 mL of the samples collected at different time points cells from the control and Na-citrate treated groups were collected every 24 h, i. The quenched culture was then centrifuged at × g and 4 °C for 10 min to collect the quenched cells.
The cell pellets were washed twice using physiological saline 0. Metabolome extraction and derivatization were done by first grinding 0. The last step was repeated, and both supernatants were subsequently pooled together.
The supernatant was mixed with 50 μL of internal standard adonitol in water, 0. Sample derivatization was performed according to the two-stage technique Yu et al.
The sample was then silylated for 2 h at 37 °C by adding 60 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide MSTFA. The temperature of the injection port was set at °C, while the mass spectrometer was operated at an ion source and interface temperatures at °C and °C, respectively.
All tests were replicated six times, each with three biological replicates from separate yeast cultures and two technical replicates. The relative levels of the metabolites were determined based on the characteristic ions of the selected peaks.
All detected peaks were identified using alignments of mass spectra from the library of the National Institute of Standards and Technology NIST, Gaithersburg, MD. The identified metabolites were normalized using the internal standard and biomass of cells to acquire the relative abundance of the metabolites.
Partial least squares-discriminant analysis PLS-DA was subsequently performed to determine the metabolites contributing to differences between the control group and the Na-citrate group using the SIMCA-P software Version PLS-DA was adopted because it is the commonly used method for classification purposes and biomarker selection in metabolomics studies.
A metabolite with a variable influence on the projection value VIP higher than 1 indicated it significantly contributed to the separation of groups in the PLS-DA models.
Hierarchical cluster analysis HCA of the metabolites was subsequently performed using a heat map and the Cluster software HemI 1. Takara MiniBEST Universal RNA Extraction Kit ZYMO, California, USA was used to extract total RNA from 1 mL of yeast cells at 36, 60, and 84 h.
First-strand complementary DNA cDNA synthesis was then performed using a High-Capacity cDNA Reverse Transcription Kit Applied Biosystems, USA. The qRT-PCR assays were performed on an Applied Biosystems real-time PCR System to detect the expression of the genes.
The cycling conditions were set as follows: initial denaturation at 94 °C for 30 s, followed by 40 cycles of denaturation and primer annealing at 94 °C for 5 s and 60 °C for 30 s, and dissociation curve analysis. The qRT-PCR primers used in this study were designed using the Vector NTI® Express Designer and are outlined in Additional file 1 : Table S1.
The one-step Fluorometric Intracellular ROS Kit Sigma-Aldrich, MAKKT was used to detect intracellular ROS. The specific step was performed as previously described Pan et al. All the tests in this study had three biological replicates from separate yeast cultures. Two-tailed t-tests were performed using the software package Statistica 6.
dendrorhous was monitored at 96 and h, which was the total fermentation time. Notably, Na-citrate increased biomass at 96 and h compared to the control group. Astaxanthin titer increased to a maximum value at h when Na-citrate was added at 24 h of cultivation Additional file 1 : Fig.
Na-citrate 0, 1. The addition of Na-citrate had a significant effect on cell growth and increased the production of carotenoids and astaxanthin Additional file 1 : Fig. Notably, the astaxanthin titer increased to Samples from the control and Na-citrate groups were collected every 24 h until the fermentation time reached h.
Na-citrate treatment achieved higher biomass, carotenoids, and astaxanthin titer compared to the control group Fig. A rapid increase in biomass was observed in both cultures after 24 h, but the increase rate under Na-citrate treatment was higher than in the control group.
The peak biomass after Na-citrate treatment was 6. Time-course profiles of X. dendrorhous cultures in the Na-citrate and control groups. Control group: solid circles, Na-citrate group: hollow circles. The cells were grown in a mL Erlenmeyer flask containing 50 mL fermentation medium, with the temperature maintained at 22 °C and the stirring speed at rpm.
In addition, glucose was consumed quickly during the first 24 h after inoculation in both cultures at a consumption rate of 0. The consumption rate of glucose was analyzed every 24 h after adding Na-citrate for 24 h.
dendrorhous cells utilized glucose quickly under Na-citrate conditions after 24 h, suggesting that the addition of Na-citrate promoted glucose utilization when cells entered the logarithmic growth phase, thus benefiting the cells to maintain a faster growth rate Fig.
The addition of Na-citrate increased carotenoids and astaxanthin accumulation after the increase of biomass and showed a twofold increase in contrast to the control group Fig. The regulation of Na-citrate to astaxanthin biosynthesis originates from increased cell growth.
Na-citrate is a kind of carbon source stimulating cell growth and development An. In addition, Na-citrate is an inexpensive chemical that is metabolized by aerobic microorganisms to increase intracellular ATP levels, thereby enhancing the ability of cells to resist acid-stressed environments Sánchez et al.
It can regulate the pH value of liquids during fermentation, thus aiding cell growth and astaxanthin production Flores-Cotera et al. Herein, the batch fermentation in a 5-L fermenter was processed in two groups to mimic industrial production. For the control group Fig.
Glucose concentration decreased before 48 h, which potentially contributed to the fast cell growth logarithmic phase during this period. Glucose consumption was slow after 48 h.
At the same time, the carotenoid accumulation in X. Besides, astaxanthin production was closely parallel with the carotenoid accumulation and reached Compared to the control batch fermentation, there was still a slight increase in biomass after rapid growth at 48 h and reached a maximum of This result was also observed from the change of residual glucose, in which the glucose consumption rate of the Na-citrate group was higher than that of the control group between 48 and 72 h Fig.
Furthermore, the carotenoids and astaxanthin started to accumulate at 8 h because of the rapid growth of cells.
The productivity of carotenoids between 40 and 48 h was 0. The biomass, carotenoid, and astaxanthin titer were 1. The astaxanthin content increased by These results indicated that Na-citrate treatment could promote the growth of cells and facilitate astaxanthin synthesis.
dendrorhous cultivation using Na-citrate in the fermenter is a promising strategy for astaxanthin accumulation. Future studies should focus on optimizing fermentation parameters, such as temperature, rotation speed, pH, and DO levels, to enhance astaxanthin production and increase the benefits of enterprises.
Moreover, these strategies can also be studied in combination with other methods, such as analysis of kinetic parameters of fermentation processes and performing kinetic modeling to guide actual industrial production to increase astaxanthin accumulation.
Profiles of cell growth, glucose consumption, and astaxanthin production of X. dendrorhous in the 5-L bioreactor. A Control group; B Na-citrate group. Glucose open square , biomass filled square , carotenoid titer filled triangle , astaxanthin titer open triangle , and astaxanthin content open circle.
Table 1 compares the fermentation results of this study and other studies, excluding the results of genetic modification fermentation. The astaxanthin content obtained in this study was higher than that of previously published reports, except the results by de la Fuente et al.
rhodozyma VKPM in a L fermenter. The increased astaxanthin content in a unit cell indicated that Na-citrate could regulate cell growth of X. dendrorhous by inducing crucial metabolic pathways associated with astaxanthin biosynthesis. Metabolome analysis was thus carried out to determine the changes caused by Na-citrate at the metabolite level in X.
Metabolic profiles of X. dendrorhous were analyzed using a partial least squares-discriminant analysis PLS-DA score plot Fig. Analysis of the score plots revealed the metabolomic profiles of the control and the Na-citrate treatment groups were separated at all four-time points.
A total of 34 chemically classified metabolites with a very important variable of projection VIP value greater than 1 and P values less than 0. Most metabolites were involved in the TCA cycle, carbohydrate metabolism, fatty acid synthesis, and amino acid metabolism. Figure 4 is a heat map showing their functional classification.
PLS-DA derived plots for pairwise comparisons between the Na-citrate and control groups at various time points: A 48 h, B 72 h, C 96 h, and D h. Green circle: control groups. Blue circle: Na-citrate-treatment groups.
Hierarchical cluster analysis HCA for the identified metabolites. All data are expressed as the means of six replicates. The response ratio for each metabolite is normalized to log There was a clear metabolite tendency for the intracellular glycolysis pathway.
In Na-citrate cultures, the glycolysis pathway was significantly upregulated Tables 2 and 3 because of the rapid absorption of glucose from the medium induced by Na-citrate addition. The concentrations of intracellular glucose in the cells were increased by The intracellular glucose in the Na-citrate group was at a similar level to that of the control group after 96 h despite the Na-citrate group consuming more glucose, suggesting that the addition of Na-citrate led to an accelerated rate of glucose uptake for cell growth at the early and middle stages of the fermentation.
In contrast, the absorbed glucose at the later stage was utilized for its metabolic activities. In addition, the content of ethanol, a product of yeast anaerobic fermentation, in the Na-citrate group was significantly lower than that in the control group Tables 2 and 3.
This finding suggested that a large part of the pyruvate generated by the glycolysis pathway was directly converted into acetyl-CoA rather than entering the anaerobic fermentation to accumulate ethanol. An increase of acetyl-CoA may cause the carbon metabolism to flow more to the fatty acid synthesis pathway and astaxanthin synthesis pathway because it is a key substrate of various cellular processes.
Notably, the addition of Na-citrate resulted in a significant decrease in the intracellular phosphate concentration Table 2. Flores-Cotera et al. This report is consistent with the results of our study. During the cultivation period, most intermediates of the TCA cycle were significantly decreased upon Na-citrate treatment besides citric acid and malic acid Tables 2 and 3 , indicating that Na-citrate reduced the metabolic flux toward the TCA cycle.
Na-citrate treatment enhanced the concentration of citric acid, inhibiting the catalytic activity of citrate synthase from synthesizing citric acid and weakening the TCA cycle. In the oleaginous yeasts and fungi, low TCA activity induces citric acid accumulation in the mitochondria, which is then transported into the cytoplasm.
In the cytoplasm, citric acid is degraded into acetyl-CoA and oxaloacetate under ATP citrate lyase ACL catalysis, thus promoting the production of fatty acids and carotenoids in X. dendrorhous Venkateshwaran et al. Citrate is thus considered the precursor of acetyl-CoA for fatty acid and astaxanthin synthesis Chavez-Cabrera et al.
Pyruvate can be converted into acetyl-CoA through the pyruvate dehydrogenase complex, while the NADPH can be used for fatty acid synthesis. Exogenous Na-citrate may provide more acetyl-CoA by cleaving citrate to produce acetyl-CoA and reduce the consumption of acetyl-CoA by the TCA cycle, promoting astaxanthin biosynthesis.
The TCA cycle is associated with the production of reactive oxygen species ROS , which can stimulate the massive accumulation of astaxanthin in X. dendrorhous Du et al. In this study, the TCA cycle was inhibited by the addition of Na-citrate, while the inhibitory effect was weakened with the depletion of Na-citrate.
These results indicated that the addition of Na-citrate could regulate the metabolic flux from the TCA cycle to carotenoid biosynthesis and regulate the citric acid-pyruvate cycle, providing a large amount of substrate and energy for cell growth and astaxanthin accumulation, thereby generating numerous ROS to enhance astaxanthin synthesis.
The amino acid content affects protein synthesis, which is closely related to the growth and reproduction of yeast. The content of amino acids was significantly increased with Na-citrate treatment in addition to aspartic acid Table 2.
The content of alanine and serine, which are derived from pyruvate, increased in the Na-citrate group at different time points Table 3. The contents of Loxoproline, leucine, L-phenylalanine, and valine derived from intermediates of the TCA cycle also increased in the Na-citrate group.
The addition of Na-citrate resulted in a significant decrease in intracellular aspartic acid content. Aspartic acid was closely related to the TCA cycle and could be oxidatively deaminated to generate oxaloacetate because the addition of Na-citrate could regulate the TCA cycle, which was more active after 72 h.
More aspartic acid thus needed to be converted into substances in the TCA cycle for supplementation, which reduced the content of aspartic acid.
At the same time, many amino acid metabolites were much higher in the Na-citrate group compared with the control group Table 3. Of note, the protein expressed Additional file 1 : Fig. S3 by X. dendrorhous was higher in the control group than in the Na-citrate group, suggesting that the higher-level amino acids were not used to synthesize the specific protein but to respond to the stress.
This finding was consistent with that of Zhang et al. Furthermore, the amino acids showed higher abundance in Na-citrate cultures, suggesting that protein synthesis was restricted in X.
Acetyl-CoA is a key intermediate used in both primary and secondary metabolic pathways. Astaxanthin and fatty acid biosynthesis need acetyl-CoA, ATP, and NADPH as substrates, and thus their entry into these pathways must be regulated.
The availability of acetyl-CoA, ATP, and NADPH may be key factors for switching the carbon flux from TCA-respiratory to astaxanthin biosynthesis during the restriction of protein synthesis in X.
dendrorhous , increasing the accumulation of astaxanthin. dendrorhous , carotenoid biosynthesis is closely associated with fatty acid metabolism by sharing the same precursor, acetyl-CoA Du et al.
Herein, the contents of seven fatty acids in the Na-citrate groups were higher than those in the control group Tables 2 and 3. The increased biomass was also correlated with an increased synthesis of fatty acids Fig.
These results demonstrated that an appropriate Na-citrate feeding strategy might be an effective way to enhance astaxanthin accumulation in X. The content of oleic acid, linoleic acid, and linoelaidic acid unsaturated fatty acids showed a significant increase 1.
The content of hexadecanoic acid increased by dendrorhous and an increase in fatty acids, especially the unsaturated fatty acids. Unsaturated fatty acids enhance the fluidity and permeability of cell membranes Los et al.
The fatty acids increased before 72 h and showed a decreasing trend after 72 h. In the Na-citrate treatment groups, the high biomass caused insufficient nutrients in the medium. It is thus necessary to use part of the fatty acids for energy supply.
Fatty acids may be used as a carbon source to enrich the acetyl-CoA supply for carotenoid biosynthesis. In this study, fatty acids were induced by Na-citrate to increase the fluidity and permeability of the cell membrane, thus accelerating the uptake of the glucose and nitrogen substrate from the medium into the cell, thereby promoting metabolism.
Metabolomics analysis showed that the addition of Na-citrate significantly changed the content of sterols Tables 2 and 3. The contents of ergosterol and ergosta-7, dienol in cells under Na-citrate treatment were 1.
However, the contents of ergosterol and ergosta-7, dienol decreased after 72 h. High content of sterols during the early stages of fermentation can thus cause the cells to better absorb nutrients from the medium, providing conditions for cell growth and accumulation of metabolites.
Ergosterol can also compete with astaxanthin because they have the same precursor, farnesyl pyrophosphate FPP Misawa Carotenoid and fatty acid syntheses share several common features with sterol synthesis, including the substrates of acetyl-CoA, ATP, and NADPH. Therefore, the content of ergosterol decreased during the later stages of fermentation.
The content of ergosterol can regulate the expression of 3-hydroxymethylglutaryl-CoA synthase HMGS and 3-hydroxymethylglutaryl-CoA reductase HMGR in the mevalonate pathway. A high content of ergosterol can inhibit the expression of HMGR and HMGS. The addition of Na-citrate during the later phase can thus lead to a significant downregulation of ergosterol, thereby releasing the inhibition of key rate-limiting enzymes in the mevalonate pathway and directing the FPP in the metabolic pathway towards astaxanthin synthesis.
Astaxanthin is a scavenger of free radicals, a chain-breaking antioxidant, and a potent quencher of ROS, such as singlet oxygen, superoxide ion, and hydrogen peroxide Alesci et al. The presence of astaxanthin means a higher survival ability of the cells because it enhances the resistance of the cell to oxidative stress.
Astaxanthin biosynthesis thus serves as a survival strategy for X. dendrorhous under oxidative stress Cuellar-Bermudez et al. The ROS levels of both the control and Na-citrate groups increased, peaked at 72 h, and then decreased to a basal level at h Fig.
The production of ROS gradually increased with the enhanced metabolic activity of the yeast cells. Subsequently, the level of intracellular ROS gradually reduced with the production of astaxanthin, which can scavenge ROS. The ROS level was higher from 48 to 96 h in the Na-citrate group than in the control group Fig.
Intracellular reactive oxygen species ROS generation. The solid and hollow circles represent the intracellular ROS abundance in the control group and the Na-citrate group, respectively.
In addition, the content of myo-inositol, a carbohydrate metabolism intermediate, in response to environmental stress was significantly upregulated before 72 h Tables 2 and 3.
Myo-inositol is a growth factor for yeast and contributes to responses to environmental factors, such as oxygen and osmotic pressure in Aurantiochytrium sp. and Schizochytrium sp.
strains Jakobsen et al. In this study, Na-citrate treatment caused a significant increase in myo-inositol 2. Further investigations are thus needed to determine the relationship between Na-citrate treatment and myo-inositol metabolism.
The real-time PCR assay was used to detect the gene expression level of the astaxanthin biosynthesis pathway to explore the molecular mechanisms underlying the higher astaxanthin accumulation induced by Na-citrate.
The transcription of these genes was elevated by Na-citrate during the cultivation period Fig. ICL is a key enzyme in the glyoxylate cycle that splits isocitrate into glyoxylate and succinate. Glyoxylate combines with acetyl-CoA molecules to form malate. Compared to the control group, transcription of ICL in the Na-citrate group was increased at 36 h 2.
The increased HMGS transcription under Na-citrate treatment suggested that Na-citrate treatment elevated the mevalonate pathway. The enhanced transcript level of crtE , crtYB , crtI , and crtS encoding the key enzymes for controlling the biosynthesis of astaxanthin under Na-citrate treatment during the entire cultivation period indicated that Na-citrate strengthened astaxanthin biosynthesis in X.
Na-citrate regulates the transcriptional level of key genes involved in astaxanthin synthesis. Increased biomass and astaxanthin accumulation were observed in X. dendrorhous under Na-citrate treatment. A comparison of the metabolites under the Na-citrate and control groups revealed that the metabolites content involved in the glycolysis pathway, amino acid metabolism, TCA cycle, and lipid and sterol biosynthesis changed substantially in response to Na-citrate.
Figure 7 shows the metabolic mechanism of Na-citrate in regulating cell growth and astaxanthin accumulation. The mechanisms through which Na-citrate addition affects the cells of X.
PYR: pyruvate; OA: oxaloacetate; IC: isocitrate; OG: 2-oxo-glutarate; Asta: astaxanthin; FA: fatty acids. Na-citrate treatment can promote the assimilation of glucose from the medium by cells. During the cultivation period, the consumption rate of intracellular glucose in the Na-citrate group was higher than that of the control group, indicating that the glycolysis flux was induced by Na-citrate so that intracellular glucose could generate more pyruvate.
The increased glycolytic flux suggested that more glucose went through the pentose phosphate pathway PPP pathway to supply the increased demand of NADPH required for lipid synthesis and ROS increase. In addition, the flux of pyruvate to ethanol and lactic acid through anaerobic fermentation was weakened, allowing more pyruvate to be converted to acetyl-CoA for astaxanthin synthesis.
Acetyl-CoA has several metabolic pathways. It can participate in the TCA cycle, astaxanthin, fatty acid, protein, and sterol syntheses. In this study, Na-citrate treatment increased the content of intracellular citric acid, thereby increasing the concentration of citric acid in the TCA cycle, which inhibited the catalytic activity of citrate synthase and weakened the reaction rate of oxaloacetate synthesis of citric acid.
In contrast, the remaining Na-citrate in the mitochondria entered the cytoplasm and was cleaved into acetyl-CoA. The significant increase in ICL transcription also suggested that the content of acetyl-CoA in the cytoplasm was increased, thereby providing numerous substrates for the production of astaxanthin in X.
Na-citrate treatment significantly increased intracellular ROS. The accumulated astaxanthin increased the resistance of X. dendrorhous to Na-citrate stress by removing ROS species because of its strong antioxidant activity, which increased redox signaling and induced astaxanthin synthesis in X.
Furthermore, Na-citrate treatment significantly upregulated the expression of the other five key genes involved in carotenogenesis.
Astaxanthin is synthesized in X. dendrorhous via the mevalonate pathway, in which HMGS is a rate-limiting enzyme that catalyzes the formation of HMG-CoA. In this study, the significant increase in HMGS transcription suggested that the mevalonate pathway was increased, which was consistent with the enhancement of astaxanthin accumulation in X.
The regulatory mechanism proposed that Na-citrate treatment increases the use of glucose for the fermentation based on the biochemical compositions and metabolome analysis, indicating that Na-citrate induced a glycolysis flux.
Upregulation of the glycolytic pathway suggested that more glucose went through the PPP pathway to improve the NADPH for astaxanthin biosynthesis. Notably, Na-citrate treatment increased the content of intracellular citric acid but reduced the metabolites in the TCA cycle.
Exogenous Na-citrate may provide more acetyl-CoA by cleaving citrate to produce acetyl-CoA, thus reducing the consumption of acetyl-CoA via the TCA cycle, thereby promoting astaxanthin and fatty acids biosynthesis in X. Na-citrate treatment significantly increased intracellular ROS, which increased redox signaling and further induced astaxanthin accumulation in X.
Additionally, the upregulation of the six genes encoding key enzymes involved in astaxanthin biosynthesis was potentially caused by the increase in their substrates and higher levels of ROS because of Na-citrate treatment.
Xanthophyllomyces dendrorhous can produce large amounts of astaxanthin, which is a high-value ketocarotenoid. This study revealed that Na-citrate treatment could promote astaxanthin production in X.
dendrorhous with a twofold increase. Metabolic Analysis revealed that Na-citrate treatment increased the use of glucose for fermentation and weakened the intracellular TCA cycle, thus promoting the metabolic flux from acetyl-CoA to astaxanthin biosynthesis.
This finding was consistent with the increased transcriptional expression of six key genes ICL , HMGS , crtE , crtYB , crtI, and crtS associated with carotenoid biosynthesis pathways. The increased ROS abundance also indicated that Na-citrate treatment potentially induced the anti-stress mechanism in X.
dendrorhous to produce more astaxanthin. These results provide a potentially valuable strategy for stimulating astaxanthin production in X. dendrorhous using exogenous Na-citrate.
A fed-batch feeding employing the Na-citrate strategy for astaxanthin production in X. dendrorhous should thus be considered in future studies. Data generated and analyzed in this study are included in the published article and the supplementary materials.
Alcalde E, Fraser PD Extending our tools and resources in the non-conventional industrial yeast Xanthophyllomyces dendrorhous through the application of metabolite profiling methodologies.
Metabolomics 14 3 Article PubMed PubMed Central Google Scholar. Alesci A, Salvo A, Lauriano ER, Gervasi T, Palombieri D, Bruno M, Pergolizzi S, Cicero N Production and extraction of astaxanthin from Phaffia rhodozyma and its biological effect on alcohol-induced renal hypoxia in Carassius auratus.
Nat Prod Res 29 12 — Article CAS PubMed Google Scholar. Amado IR, Vazquez JA Mussel processing wastewater: a low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous.
Microb Cell Fact An GH Improved growth of the red yeast, Phaffia rhodozyma Xanthophyllomyces dendrorhous , in the presence of tricarboxylic acid cycle intermediates. Biotechnol Lett — Article CAS Google Scholar.
Bauer A, Minceva M Techno-economic analysis of a new downstream process for the production of astaxanthin from the microalgae Haematococcus pluvialis. Bioresour Bioprocess 8 1 Article Google Scholar. Chatragadda R, Dufosse L Ecological and biotechnological aspects of pigmented microbes: a way forward in development of food and pharmaceutical grade pigments.
Microorganisms 9 3 Article CAS PubMed PubMed Central Google Scholar. Chavez-Cabrera C, Flores-Bustamante ZR, Marsch R, Montes Mdel C, Sanchez S, Cancino-Diaz JC, Flores-Cotera LB ATP-citrate lyase activity and carotenoid production in batch cultures of Phaffia rhodozyma under nitrogen-limited and nonlimited conditions.
Appl Microbiol Biotechnol 85 6 — Chen T, Wei D, Chen G, Wang Y, Chen F Employment of organic acids to enhance astaxanthin formation in heterotrophic Chlorella zofingiensis. J Food Process Pres 33 2 — Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins.
Microb Biotechnol 8 2 — de la Fuente JL, Rodriguez-Saiz M, Schleissner C, Diez B, Peiro E, Barredo JL High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J Biotechnol 2—3 — Article PubMed Google Scholar.
The detection kits for alanine transaminase ALT , aspartate transaminase AST , TG, TC, high-density lipoprotein cholesterol HDL-C , and low-density lipoprotein cholesterol LDL-C , and the antioxidant assay kits for SOD, CAT, GSH, T-AOC, and malondialdehyde MDA were purchased from Nanjing Jiancheng Bioengineering Institute Nanjing, China.
Other chemicals, solvents and reagents used in the present study were of laboratory analytical grade. Astaxanthin oleoresin was provided by Shandong Jinjing Biotechnology Co.
After purification, ATX of The Institutional Animal Care and Use Committee of Shanxi Agricultural University approved all experimental protocols for animal care, handling and experimentation SXAU-EAW We also confirmed that all experiments were conducted in accordance with relevant guidelines and regulations.
The design of animal experiments was based on our previous methods The mice in the ND group were fed standard rodent chow containing 3. The mice in the other group were fed a HFD containing 4. The mice in the ND group were given distilled water, and the solvent group was gavaged with corn oil.
In addition, the mice in the ATX treatment groups were gavaged with 0. During the diet phase, all mice were given intragastric treatment once per day at a. The diets were purchased from Beijing Huafukang Bioscience Co. Supplementary Table 1 shows the ingredients of the experimental diets.
The body weight and food intake were recorded daily for 63 days. To avoid error values, the measurement of weight was repeated three times for each mouse. The energy intake was calculated as food intake × 4. Mice were fasted for 12 h after the last treatment and then euthanized by inhalation with isoflurane.
Blood samples were obtained from the retro-orbital veins on Days 0, 30, and All other organs, including the liver, heart, kidney, spleen, and adipose tissues, were immediately collected and weighed individually after sacrificing the animals.
The serum TG, TC, HDL-C, and LDL-C levels and activities of AST GOT and ALT were determined using biochemical kits according to the standards and protocols provided by the manufacturer Nanjing, China.
The supernatant was collected to determine the protein and lipid levels TG and TC and enzymatic analyses T-AOC, SOD, CAT, GSH, MDA, and ROS. The dihydroethidium DHE probe method was used to qualitatively detect ROS. Five-micron-thick sections of the liver were dyed with DHE, and incubation was performed at 37°C for 10 min in a dark environment.
The samples were directly observed under a fluorescence microscope at a measuring emission of nm. The ROS-positive cells had strong red fluorescence. Meanwhile, the frozen sections were stained with Oil Red O ORO , which was performed to further detect hepatic vacuolization, inflammatory cell infiltration, and lipid droplets.
The above sections were used to examine hepatocellular apoptosis with the YF TUNEL assay apoptosis detection kit. After the TUNEL reaction, the sections were mounted using antifade mounting medium with DAPI and observed under an inverted fluorescence microscope at and nm wavelength excitation.
The negative cells were dyed with blue fluorescence intensity at nm, while the apoptotic cells exhibited green fluorescence at nm. ImageJ software National Institutes of Health, United States was used to measure the cell counting of sections from each group.
Then, cDNA was synthesized from total RNA using the PrimeScript Reverse Transcription reagent kit Takara, Dalian, China. Quantitative polymerase chain reaction PCR was conducted in triplicate for each group to detect gene expression.
The quantitative analysis of AMPK , SREBP1c , ACC , CPT-1 , PPARα , PPARγ , LXRα , SCD-1 , PGC-1 , FAS , CYP27A1 , and CYP7A1 mRNA expression in the liver was measured in triplicate for each group by quantitative PCR. According to the SYBR Premix Ex Taq II Takara, Dalian, China , the thermal cycle of qPCR was reacted on the CFX 96 Real-Time PCR Detection system BIO-RAD, Hercules, CA, United States under the following conditions: 95°C for 10 min, then 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s.
Supplementary Table 2 shows the PCR primer sequences of each gene, and the target genes were normalized to the reference gene GAPDH. The 2 —ΔΔCt method was used to calculate relative gene expression. To investigate lipid metabolism, fresh samples were sent to MetWare Biotechnology Co. After RNA was extracted from liver biopsy samples, liver transcriptome analysis was conducted by RNA sequencing, as described in detail previously 22 , The caecal contents were sent to Shanghai Personal Biotechnology Co.
to investigate microbial diversity through 16S rRNA analysis on the Illumina MiSeq platform. A previous study illustrated the analytical conditions and detailed parameters All experiments were biologically repeated three times, and the data were analyzed with Social Sciences SPSS Origin 9.
A dramatic increment in body weight was observed in the HFD group, while a moderate increase was observed in the ATX treatment groups Figure 1A. Body weight gain plays a pivotal role in evaluating the effect of HFD on obesity and assessing its prevention.
Table 1 presents the initial and final body weights of mice in each group. During feeding induction, the mice gained more weight in the HFD group There was no significant difference in the food efficiency ratio of the mice in each group except for the ND group Table 1.
Figure 1. Astaxanthin ATX prevented obesity and related indices in HFD-fed mice. A Body weight. B Body weight gain. C Energy intake. D Related organ weight. E Visceral adipose tissue. F Visual appearance pictures of metabolic mice and liver.
G Hepatic TG level. H Hepatic TC level. I Serum ALT level. J Serum AST level. Table 1. Effect of astaxanthin ATX supplementation on body weight, energy intake, and food efficiency ratio in high-fat diet-induced mice a.
To estimate whether the HFD with ATX supplementation affected visceral organs and fat, the wet weights of adipose tissue and organs were measured in each group, especially the mouse liver Figure 1D. There were no significant differences in the heart, spleen or kidney in each group, similar to our previous results 18 Table 2.
ATX supplementation 0. Table 2. Effect of ATX supplementation on related organ weights and adipose tissue weights in high-fat diet-induced mice a. Liver lipid indicators, namely TG and TC levels, are important parameters for obtaining an understanding of diet-induced fat deposition.
The liver turned brown following the accumulation of TG and TC in the HFD group, indicating dyslipidaemia, possibly leading to other diseases. This phenomenon was suppressed in the ATX treatment group compared to the HFD group Figure 1F. The TG and TC levels were examined to further quantify liver fat deposition, as shown in Figures 1G,H.
However, ATX supplementation effectively decreased fat deposition in a dose-dependent manner compared to the HFD group, among which the TG and TC levels were Table 3 presents the serum lipid profiles of mice at 0, 30, and 60 days.
There were no obvious differences in the initial serum lipid profiles among the six groups. Such results indicated that lipid metabolism was disordered. The mice in the 0. When compared with the HFD group, serum TG levels in the 0. Serum TC levels in the 0. Serum LDL-C levels in the 0. Therefore, based on the results mentioned above, 0.
Table 3. Effect of ATX supplementation on the levels of serum TG, TC, HDL-C, and LDL-C in the HFD-fed mice. In addition, we evaluated serum AST GOT and ALT to further explore the liver function induced by HFD and ATX consumption.
The serum AST level was increased in the solvent and HFD groups compared with the ND group; however, the serum ALT level was not apparent in any group Figure 1I. Malondialdehyde and reactive oxygen species ROS activities are crucial components contributing to the development of oxidative stress in high-fat diet-fed animals.
The levels of antioxidant enzymes, including T-AOC, CAT, SOD, and GSH, were assessed in the liver and exhibited similar trends. According to the ROS qualitative fluorography images, intense red fluorescence was observed in the HFD and the positive control groups incubated with H 2 O 2 , while the faint fluorescence in the ATX-treated samples corresponded with the quantitative results Figure 2G.
Furthermore, 0. When compared to the HFD group, the levels of T-AOC, CAT, SOD, and GSH were increased by Figure 2. Evaluation of liver oxidation resistance in HFD-induced mice liver tissues. The levels of ROS intensity A , MDA B , T-AOC C , CAT D , SOD E , and GSH F are illustrated in the panel.
In the ND group mice, hepatocytes were fairly uniform, with regularly shaped hepatic plates arranged in an ordered pattern and hepatic cords, except for slight congestion Figure 3A. However, the HFD induced typical lesions in the mouse liver, such as hepatocyte necrosis, inflammatory cell infiltration, congestion of the central veins, ballooning, hepatic sinus expansion and chromatin condensation.
In the solvent group, the structure of hepatic plates was irregularly arranged along with fat accumulation, indicating that long-term excessive fat intake disturbed lipid metabolism in the liver. Figure 3.
Pathological changes of ATX on liver and epididymal fat in HFD-induced mice. A Liver sections stained with HE ×, ×. B Liver sections stained with Oil red O ×, ×.
C HE-stained e-AT sections ×. D Steatohepatitis scores. E Percentage of the lipid droplet area assessed by Oil red O staining. F Mean cell area of adipocyte in e-AT.
To further investigate the production of lipid droplets in the liver, Oil Red O staining was performed Figure 3B. More oil red O-stained lipid droplets were observed in the liver tissue of the HFD and solvent groups than in the liver tissue of the ND group, resembling the percentage result of lipid droplets Figure 3E.
Conversely, ATX supplementation dose-dependently decreased the production of fatty droplets, in which the area of droplets was significantly lessened in the 0. These results confirmed that ATX prevented lipid accumulation and hepatic steatosis, conforming to the results of intrahepatic TG and TC levels.
As shown in the e-AT sections of HFD-induced mice Figures 3C,F , the mean adipocyte size increased almost Apoptotic cells were detected by green fluorescent TUNEL staining, and cell nuclei were stained blue DAPI.
Compared to that in the HFD group, the number of apoptotic cells stained green was reduced in a dose-dependent manner with ATX supplementation, and the apoptosis rates were decreased by To understand the mechanism s by which ATX modulates hepatic lipid metabolism in response to a high-fat diet, we analyzed the expression of genes related to lipogenesis and fatty acid β-oxidation in the liver by qRT—PCR.
These results indicated that consumption of a HFD contributed to fat synthesis and ultimately disturbed lipid metabolism; furthermore, high-dose ATX could improve the disorder of lipid metabolism by promoting cholesterol metabolism and inhibiting fat synthesis. Figure 4. Astaxanthin significantly improved relative gene expression.
B The heatmap of differential genes expression at the transcriptional level. C Regulatory effects of ATX supplementation on fatty acid and cholesterol metabolism in mice induced by HFD.
Data are shown as mean ± SD of triplicate. To explore how the hepatic lipidome is altered upon ATX intervention, RNA sequencing was used to accurately and quantitatively analyse liver transcriptional changes and lipid metabolism pathways in the liver in response to ATX supplementation.
A total of genes were differentially expressed in HFD-induced liver samples compared with ND-induced liver samples Supplementary Figures 2A,B. However, a total of differentially expressed genes, of which were increased and 53 were decreased, were identified in the 0.
We performed a comprehensive hepatic lipidomic analysis to evaluate whether differences in lipid content or composition may account for differences in hepatic lipid disorders between the HFD group and ATX group. A total of 1, lipid species were identified in liver samples, which belong to six primary classes of lipids, including glycerophospholipids GPs , glycerides GLs , fatty acyls FAs , sphingolipids SLs , sterol lipids STs , and prenol lipids PRs Supplementary Figure 3.
Based on the abovementioned results, we screened and 91 lipid biomarker candidates by applying volcano plots for such distinctions in ND vs. HFD and HFD vs. Figure 5. Astaxanthin regulated lipid metabolites in HFD-fed mice. A OPLS-DA score plot left and permutation plot right.
B Venn diagram depicting the overlap of significantly changed metabolites between experimental groups. The volcano plot analysis of ND vs. HFD group C and HFD vs.
Analysis of lipid metabolism pathway of ND vs. HFD E and HFD vs. G Heatmap of 34 significantly altered metabolites in ATX-treated HFD-fed mice. Blue: downregulated metabolites. Red: upregulated metabolites. H The associated heatmap of significantly changed metabolites. According to the Venn diagram, we found that the accumulated lipid species were significantly different between the ND and HFD groups, while ATX intervention patently changed the levels of 91 lipid species, including 24 ordinary species, compared to the levels in HFD-fed alone Figure 5B.
Furthermore, in our present study, we found that 8 of the other 20 most relevant metabolites 3 BAs, 2 CARs, 2 BMP, and 1 TG were remarkably downregulated after ATX supplementation; however, there was no significant difference in the ND vs. HFD group. We observed a significantly positive correlation among these 34 metabolite levels associated with lipid metabolism Figure 5H.
Thus, these results indicated that the 22 metabolites, including 4 FFAs, 8 TGs, 2 DGs, 3 BAs, 2 CARs, and 2 BMPs, might be potential biomarkers accountable for alleviating the steatohepatitis induced by lipid disturbance.
The KEGG database was used to perform pathway analysis of differentially expressed metabolites. The pathways were considerably disrupted in the HFD group, including glycerolipid metabolism, insulin resistance, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes, when compared with the ND group; however, 0.
Of the 8, OTUs visualized in the experimental groups, 4. In addition, the number of other OTUs in the ND group, HFD group and 0.
The Goods coverage values had no obvious differences in each group Figure 6B. To assess community similarity among samples, we applied principal coordinates analysis PCoA to represent the relative abundance of OTUs in each community by two different analyses.
The PCoA plot showed that the structure and compositions of gut microbiota in the HFD group Axis 1, Figure 6. Astaxanthin regulated the gut microbiota. A The Venn diagram. Data were analyzed using a one-way ANOVA and are expressed as the mean ± SD.
C PCoA of unweighted UniFrac distance from beta diversity analysis. D Phylum abundance graph genus levels. E Genus abundance graph. F Species taxonomy branch map based on LEfSe analysis. G The heatmap of the 30 bacterial genera with the largest differences in abundance were selected, according to the unweighted UniFrac distance of the intestinal content samples.
H Predicted the abundance map of MetaCyc secondary functional pathways. X-coordinate: the abundance of functional pathways, Y-coordinate: the MetaCyc secondary functional pathway.
I Analysis of differences in metabolic pathways left and species composition in different MetaCyc pathways right. At the phylum level, the taxonomic profiles of the gut microbiomes showed significant differences according to increasing ATX supplementation and developing obesity severity, within which Firmicutes , Bacteroidetes , and Proteobacteria were the dominant phyla.
At the genus level, the abundance of genera, including Bacteroides , Allobaculum , Desulfovibrio , Akkermansia , Oscillospira , Ruminococcus , Parabacteroides , Adlercreutzia , Alistipes , and Bilophila , was significantly altered by a high-fat diet compared with the normal diet and moderately inverted by 0.
Compared to the mice induced by HFD alone, the mice supplemented with ATX had significantly upregulated abundances of Akkermansia and Parabacteroides to Additionally, to explore high-dimensional biomarkers and identify significant differences at the species level, LEfSe with default parameters was used between the microbial communities compared.
The 65 most abundant OTUs were observed at the taxonomic level in the samples, among which beneficial bacteria were significantly reduced in the HFD group compared with the ND group, revealing a serious gut microbial disorder in HFD-fed mice Figure 6F.
Furthermore, 9 of the 30 most prevalent bacterial genera were upregulated and 21 bacterial genera were downregulated in the HFD-fed mice compared with the mice fed a normal diet, while these genera were partially promoted to their original relative abundance levels after ATX supplementation Figure 6G.
To characterize the functional role of the related abundant bacterial genera, we found 47 secondary functional pathways from the MetaCyc database of metabolic pathways that are relevant to lipometabolism, including the fatty acid and lipid biosynthesis pathway abundance value: 16, Obesity and obesity-related complications are classic health problems worldwide.
A long-term high-fat diet and an imbalance in energy expenditure are important causes for concern In both obese individuals and animal models of NASH, it could be characterized by excessive intracellular lipid accumulation combined with inflammation, which can ultimately progress into hepatic insulin resistance, mitochondrial dysfunction and cellular injury 27 , Emerging evidence shows that ATX, a natural functional food, has been used as a dietary supplement for treating obesity and liver injury and maintaining health 18 , Importantly, when compared to vitamin E, ATX was more effective at lipid peroxidation and preventing NASH.
In the present study, our results showed that ATX supplementation could prevent obesity and the development of NAFLD by meditating lipid metabolism and gut microbiota. Alternatively, ATX consumption also prevents oxidative stress in the liver and lipid peroxidation by improving antioxidant enzyme activity.
According to experimental results, dietary ATX not only significantly decreased body weight gain, adipose tissue weight, and serum TG, TC, and LDL-C levels but also ameliorated abnormal hepatic metabolism following the reduction of liver weight and hepatic TG and TC levels in HFD-induced mice.
No significant difference in the food efficiency ratio or serum HDL-C levels was observed in the HFD group with long-term ATX intake. From the physiological and biochemical profiles, ATX exhibited a better preventive effect on dyslipidaemia and abnormal liver function than our previous results Over the past decade, numerous pieces of evidence have shown that oxidative stress caused by a high-fat diet and specific products of ROS are involved in the development of obesity and fatty liver 31 , Thus, balancing the liver oxidative reaction is an important aspect of preventing the development of NAFLD.
Studies have shown that oxidative stress is closely related to endoplasmic reticulum ER stress in the development and progression of NAFLD and other diseases, while ATX can directly or indirectly moderate ER through antioxidant activity 33 , Interestingly, previous study has confirmed that ATX significantly reduced the levels of oxidative stress marker thiobarbituric acid-responsive substances TBARS in the liver of NASH mice In our results, both the ROS levels evaluated by the DHE probe and the levels of MDA measured, a lipid peroxidation product, were significantly increased in liver tissues in each experimental group.
HFD might have contributed to the increase in these oxidative stress indices and the decrease in antioxidant enzymes, including T-AOC, SOD, CAT, and GSH levels. Our results are consistent with previous studies showing that HFD seriously damaged the antioxidant defense system 32 , Regardless of the dose, the MDA levels of all ATX-supplemented groups were reduced, suggesting that ATX suppresses overproduction of ROS induced by obesity.
In addition, with dose-dependent increases of the ATX in the diet, the activities of antioxidant enzymes remarkedly improved and were close to normal levels in mice fed HFD. Multiple studies have confirmed that cell apoptosis induced by excessive endogenous cholesterol is associated with increased ROS in tissues 36 , As previously discussed, long-term HFD intake advanced total cholesterol and disturbed the oxidative balance in the liver, which was attributed to hepatocellular apoptosis.
Based on the TUNEL assay results, we found a large number of apoptotic liver cells in the HFD group, whereas ATX alleviated the degree of necrosis. Nevertheless, the precise intracellular mechanism responsible for this phenomenon was unclear in this study.
Moreover, the pathological results showed that ATX could effectively prevent fat accumulation and hepatic steatosis in a dose-dependent manner. Whether for obesity or the development of NAFLD, one of the root causes is the perturbation in lipid metabolism As reported in previous studies, excessive fat intake induced abnormal bile secretion and disturbed cholesterol levels In addition, FFAs usually trigger the accumulation of DGs and TGs by mediating insulin signal and sensitivity in liver tissue To demonstrate the function of ATX in lipid metabolism, lipidomic analysis revealed that the total levels of hepatic FFAs, TGs, and DGs were noticeably increased in HFD group mice, indicating that a high-fat diet partly supported our previous results.
Interestingly, our results suggested that ATX not only decreased the levels of FFAs and TGs but also specifically reduced the levels of BAs and acyl-carnitines, indicating that both cholesterol metabolism and fatty acid oxidation were improved in mouse livers.
Moreover, SREBP1c , along with its downstream genes ACC , SCD1 and FAS , is an important component in the energy metabolic system and plays a key role in regulating the FFA and TG synthesis mentioned above 38 , According to transcriptome analysis, gene expression signatures were profoundly distinguished among the experimental groups.
Considering the degree and diversity of gene expression changes, only genes associated with the target pathway were screened in this study. AMPK , a key molecule in the regulation of biological energy metabolism, is involved in diabetes and metabolism-related diseases Peroxisome proliferator activated receptor PPARα and peroxisome proliferator-activated receptor gamma coactivator-1α PGC-1 play an important role in regulating the homeostasis of adipose tissue by jointly regulating the balance between fatty acid synthesis and oxidation The expression of PPARα , which is negatively correlated with the severity of NASH, is significantly reduced in NAFLD ATX alleviated the gene expression associated with EIF-2 signaling in NASH rather than improved the expression of gene related to mitochondrial dysfunction In present study, the results revealed that dietary 0.
As our previous manuscript shown, the interaction between PPARα and PGC-1α promoted the oxidation of fatty acids and inhibited the expression of SREBP1c to a certain extent During lipid metabolism, CPT-1 is a key rate-limiting enzyme that accelerates the entry and β-oxidation of long-chain fatty acids into mitochondria A high-fat diet suppresses the expression of PGC-1α , and the mitochondrial respiration rate decreases in the absence of PGC-1α , ultimately leading to a decrease in fatty acid oxidation capacity.
In present study, our results found 0. A previous study confirmed that the suppression of SCD-1 could effectively attenuate HFD-induced insulin resistance and hepatic steatosis However, we did not examine the effects of SCD-1 knockdown or overexpression on liver lipid metabolism in mice, which should be addressed in future studies.
Cholesterol 7α-hydroxylase CYP7A1 and cytochrome P 27A1 CYP27A1 , two important rate-limiting enzymes, play major roles in maintaining the balance of cholesterol and bile acid in the bile acid biosynthetic pathway in the liver The decrease in serum cholesterol is due to a decrease in its de novo synthesis in the liver and an increase in the conversion to bile acids.
LXRα , as nuclear receptors, regulates the transcription of CYP7A1 , which is related to regulation of cholesterol and bile acids metabolisms Our data showed that a HFD could downregulate LXRα , CYP7A1 , and CYP27A1 expression, while high-dose ATX supplementation could upregulate them, indicating that ATX could eliminate excess cholesterol in liver tissue by stimulating the conversion of cholesterol to bile acids.
Besides, the expression of CYP7A1 and CYP27A1 is regulated by the intestinal flora The characteristic role of diet in obesity and metabolic disorders is that diet has become an important factor in regulating the gut environment.
Both long-term and short-term dietary interventions will induce changes in the structure and function of intestinal microbes Briefly, liver metabolites mainly affect the composition of gut microbes and the integrity of the intestinal barrier, while gut microbiota regulate the synthesis of bile acids, glucose and lipid metabolism in the liver Noticeably, many recent studies have shown that functional foods and natural health products, directly or indirectly, prevent obesity and metabolic diseases by improving intestinal diversity 25 , In the current study, mice fed a HFD exhibited lower diversity and gut microbiota disturbance; however, ATX had a salutary effect on promoting gut microbiota and improving diversity.
Moreover, the increase in Actinobacteria and Verrucomicrobia at the phylum level indicated that ATX effectively activated functional bacteria. Astaxanthin treatment had the greatest impact on Allobaculum and Akkermansia at the genus level.
The relative abundance of Allobaculum is associated with hormone secretion, SCFA production, serum HDL-C concentration, and intestinal barrier integrity, which is usually found in higher relative abundances in healthy individuals in prior studies 54 , In addition, Akkermansia , an intestinal symbiont colonizing the mucosal layer, is considered to be a functional probiotic that is closely related to fat increase, secondary bile acid biosynthesis and IR In this study, the relative abundance of Allobaculum and Akkermansia in the 0.
In addition, ATX significantly decreased the abundance of Desulfovibrio , a pathogenic bacterium that induces lipopolysaccharide
SAtaxanthin you for metqbolic nature. You are using a browser Citrus aurantium for healthy metabolism with limited support for CSS. To obtain the Citrus aurantium for healthy metabolism experience, we recommend Immune system boosting strategies use a more up to date mmetabolic or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Hepatic insulin resistance and nonalcoholic steatohepatitis NASH could be caused by excessive hepatic lipid accumulation and peroxidation. Vitamin E has become a standard treatment for NASH. However, astaxanthin, an antioxidant carotenoid, inhibits lipid peroxidation more potently than vitamin E.Astaxanthin and metabolic function -
S3 by X. dendrorhous was higher in the control group than in the Na-citrate group, suggesting that the higher-level amino acids were not used to synthesize the specific protein but to respond to the stress. This finding was consistent with that of Zhang et al. Furthermore, the amino acids showed higher abundance in Na-citrate cultures, suggesting that protein synthesis was restricted in X.
Acetyl-CoA is a key intermediate used in both primary and secondary metabolic pathways. Astaxanthin and fatty acid biosynthesis need acetyl-CoA, ATP, and NADPH as substrates, and thus their entry into these pathways must be regulated.
The availability of acetyl-CoA, ATP, and NADPH may be key factors for switching the carbon flux from TCA-respiratory to astaxanthin biosynthesis during the restriction of protein synthesis in X. dendrorhous , increasing the accumulation of astaxanthin. dendrorhous , carotenoid biosynthesis is closely associated with fatty acid metabolism by sharing the same precursor, acetyl-CoA Du et al.
Herein, the contents of seven fatty acids in the Na-citrate groups were higher than those in the control group Tables 2 and 3. The increased biomass was also correlated with an increased synthesis of fatty acids Fig.
These results demonstrated that an appropriate Na-citrate feeding strategy might be an effective way to enhance astaxanthin accumulation in X. The content of oleic acid, linoleic acid, and linoelaidic acid unsaturated fatty acids showed a significant increase 1. The content of hexadecanoic acid increased by dendrorhous and an increase in fatty acids, especially the unsaturated fatty acids.
Unsaturated fatty acids enhance the fluidity and permeability of cell membranes Los et al. The fatty acids increased before 72 h and showed a decreasing trend after 72 h. In the Na-citrate treatment groups, the high biomass caused insufficient nutrients in the medium.
It is thus necessary to use part of the fatty acids for energy supply. Fatty acids may be used as a carbon source to enrich the acetyl-CoA supply for carotenoid biosynthesis. In this study, fatty acids were induced by Na-citrate to increase the fluidity and permeability of the cell membrane, thus accelerating the uptake of the glucose and nitrogen substrate from the medium into the cell, thereby promoting metabolism.
Metabolomics analysis showed that the addition of Na-citrate significantly changed the content of sterols Tables 2 and 3. The contents of ergosterol and ergosta-7, dienol in cells under Na-citrate treatment were 1. However, the contents of ergosterol and ergosta-7, dienol decreased after 72 h.
High content of sterols during the early stages of fermentation can thus cause the cells to better absorb nutrients from the medium, providing conditions for cell growth and accumulation of metabolites. Ergosterol can also compete with astaxanthin because they have the same precursor, farnesyl pyrophosphate FPP Misawa Carotenoid and fatty acid syntheses share several common features with sterol synthesis, including the substrates of acetyl-CoA, ATP, and NADPH.
Therefore, the content of ergosterol decreased during the later stages of fermentation. The content of ergosterol can regulate the expression of 3-hydroxymethylglutaryl-CoA synthase HMGS and 3-hydroxymethylglutaryl-CoA reductase HMGR in the mevalonate pathway.
A high content of ergosterol can inhibit the expression of HMGR and HMGS. The addition of Na-citrate during the later phase can thus lead to a significant downregulation of ergosterol, thereby releasing the inhibition of key rate-limiting enzymes in the mevalonate pathway and directing the FPP in the metabolic pathway towards astaxanthin synthesis.
Astaxanthin is a scavenger of free radicals, a chain-breaking antioxidant, and a potent quencher of ROS, such as singlet oxygen, superoxide ion, and hydrogen peroxide Alesci et al.
The presence of astaxanthin means a higher survival ability of the cells because it enhances the resistance of the cell to oxidative stress. Astaxanthin biosynthesis thus serves as a survival strategy for X.
dendrorhous under oxidative stress Cuellar-Bermudez et al. The ROS levels of both the control and Na-citrate groups increased, peaked at 72 h, and then decreased to a basal level at h Fig. The production of ROS gradually increased with the enhanced metabolic activity of the yeast cells.
Subsequently, the level of intracellular ROS gradually reduced with the production of astaxanthin, which can scavenge ROS. The ROS level was higher from 48 to 96 h in the Na-citrate group than in the control group Fig.
Intracellular reactive oxygen species ROS generation. The solid and hollow circles represent the intracellular ROS abundance in the control group and the Na-citrate group, respectively.
In addition, the content of myo-inositol, a carbohydrate metabolism intermediate, in response to environmental stress was significantly upregulated before 72 h Tables 2 and 3.
Myo-inositol is a growth factor for yeast and contributes to responses to environmental factors, such as oxygen and osmotic pressure in Aurantiochytrium sp. and Schizochytrium sp. strains Jakobsen et al. In this study, Na-citrate treatment caused a significant increase in myo-inositol 2.
Further investigations are thus needed to determine the relationship between Na-citrate treatment and myo-inositol metabolism. The real-time PCR assay was used to detect the gene expression level of the astaxanthin biosynthesis pathway to explore the molecular mechanisms underlying the higher astaxanthin accumulation induced by Na-citrate.
The transcription of these genes was elevated by Na-citrate during the cultivation period Fig. ICL is a key enzyme in the glyoxylate cycle that splits isocitrate into glyoxylate and succinate. Glyoxylate combines with acetyl-CoA molecules to form malate.
Compared to the control group, transcription of ICL in the Na-citrate group was increased at 36 h 2. The increased HMGS transcription under Na-citrate treatment suggested that Na-citrate treatment elevated the mevalonate pathway.
The enhanced transcript level of crtE , crtYB , crtI , and crtS encoding the key enzymes for controlling the biosynthesis of astaxanthin under Na-citrate treatment during the entire cultivation period indicated that Na-citrate strengthened astaxanthin biosynthesis in X. Na-citrate regulates the transcriptional level of key genes involved in astaxanthin synthesis.
Increased biomass and astaxanthin accumulation were observed in X. dendrorhous under Na-citrate treatment. A comparison of the metabolites under the Na-citrate and control groups revealed that the metabolites content involved in the glycolysis pathway, amino acid metabolism, TCA cycle, and lipid and sterol biosynthesis changed substantially in response to Na-citrate.
Figure 7 shows the metabolic mechanism of Na-citrate in regulating cell growth and astaxanthin accumulation. The mechanisms through which Na-citrate addition affects the cells of X.
PYR: pyruvate; OA: oxaloacetate; IC: isocitrate; OG: 2-oxo-glutarate; Asta: astaxanthin; FA: fatty acids. Na-citrate treatment can promote the assimilation of glucose from the medium by cells.
During the cultivation period, the consumption rate of intracellular glucose in the Na-citrate group was higher than that of the control group, indicating that the glycolysis flux was induced by Na-citrate so that intracellular glucose could generate more pyruvate. The increased glycolytic flux suggested that more glucose went through the pentose phosphate pathway PPP pathway to supply the increased demand of NADPH required for lipid synthesis and ROS increase.
In addition, the flux of pyruvate to ethanol and lactic acid through anaerobic fermentation was weakened, allowing more pyruvate to be converted to acetyl-CoA for astaxanthin synthesis.
Acetyl-CoA has several metabolic pathways. It can participate in the TCA cycle, astaxanthin, fatty acid, protein, and sterol syntheses.
In this study, Na-citrate treatment increased the content of intracellular citric acid, thereby increasing the concentration of citric acid in the TCA cycle, which inhibited the catalytic activity of citrate synthase and weakened the reaction rate of oxaloacetate synthesis of citric acid.
In contrast, the remaining Na-citrate in the mitochondria entered the cytoplasm and was cleaved into acetyl-CoA. The significant increase in ICL transcription also suggested that the content of acetyl-CoA in the cytoplasm was increased, thereby providing numerous substrates for the production of astaxanthin in X.
Na-citrate treatment significantly increased intracellular ROS. The accumulated astaxanthin increased the resistance of X. dendrorhous to Na-citrate stress by removing ROS species because of its strong antioxidant activity, which increased redox signaling and induced astaxanthin synthesis in X.
Furthermore, Na-citrate treatment significantly upregulated the expression of the other five key genes involved in carotenogenesis. Astaxanthin is synthesized in X. dendrorhous via the mevalonate pathway, in which HMGS is a rate-limiting enzyme that catalyzes the formation of HMG-CoA.
In this study, the significant increase in HMGS transcription suggested that the mevalonate pathway was increased, which was consistent with the enhancement of astaxanthin accumulation in X. The regulatory mechanism proposed that Na-citrate treatment increases the use of glucose for the fermentation based on the biochemical compositions and metabolome analysis, indicating that Na-citrate induced a glycolysis flux.
Upregulation of the glycolytic pathway suggested that more glucose went through the PPP pathway to improve the NADPH for astaxanthin biosynthesis. Notably, Na-citrate treatment increased the content of intracellular citric acid but reduced the metabolites in the TCA cycle.
Exogenous Na-citrate may provide more acetyl-CoA by cleaving citrate to produce acetyl-CoA, thus reducing the consumption of acetyl-CoA via the TCA cycle, thereby promoting astaxanthin and fatty acids biosynthesis in X.
Na-citrate treatment significantly increased intracellular ROS, which increased redox signaling and further induced astaxanthin accumulation in X. Additionally, the upregulation of the six genes encoding key enzymes involved in astaxanthin biosynthesis was potentially caused by the increase in their substrates and higher levels of ROS because of Na-citrate treatment.
Xanthophyllomyces dendrorhous can produce large amounts of astaxanthin, which is a high-value ketocarotenoid. This study revealed that Na-citrate treatment could promote astaxanthin production in X. dendrorhous with a twofold increase.
Metabolic Analysis revealed that Na-citrate treatment increased the use of glucose for fermentation and weakened the intracellular TCA cycle, thus promoting the metabolic flux from acetyl-CoA to astaxanthin biosynthesis.
This finding was consistent with the increased transcriptional expression of six key genes ICL , HMGS , crtE , crtYB , crtI, and crtS associated with carotenoid biosynthesis pathways.
The increased ROS abundance also indicated that Na-citrate treatment potentially induced the anti-stress mechanism in X. dendrorhous to produce more astaxanthin. These results provide a potentially valuable strategy for stimulating astaxanthin production in X.
dendrorhous using exogenous Na-citrate. A fed-batch feeding employing the Na-citrate strategy for astaxanthin production in X. dendrorhous should thus be considered in future studies.
Data generated and analyzed in this study are included in the published article and the supplementary materials. Alcalde E, Fraser PD Extending our tools and resources in the non-conventional industrial yeast Xanthophyllomyces dendrorhous through the application of metabolite profiling methodologies.
Metabolomics 14 3 Article PubMed PubMed Central Google Scholar. Alesci A, Salvo A, Lauriano ER, Gervasi T, Palombieri D, Bruno M, Pergolizzi S, Cicero N Production and extraction of astaxanthin from Phaffia rhodozyma and its biological effect on alcohol-induced renal hypoxia in Carassius auratus.
Nat Prod Res 29 12 — Article CAS PubMed Google Scholar. Amado IR, Vazquez JA Mussel processing wastewater: a low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous.
Microb Cell Fact An GH Improved growth of the red yeast, Phaffia rhodozyma Xanthophyllomyces dendrorhous , in the presence of tricarboxylic acid cycle intermediates. Biotechnol Lett — Article CAS Google Scholar. Bauer A, Minceva M Techno-economic analysis of a new downstream process for the production of astaxanthin from the microalgae Haematococcus pluvialis.
Bioresour Bioprocess 8 1 Article Google Scholar. Chatragadda R, Dufosse L Ecological and biotechnological aspects of pigmented microbes: a way forward in development of food and pharmaceutical grade pigments. Microorganisms 9 3 Article CAS PubMed PubMed Central Google Scholar.
Chavez-Cabrera C, Flores-Bustamante ZR, Marsch R, Montes Mdel C, Sanchez S, Cancino-Diaz JC, Flores-Cotera LB ATP-citrate lyase activity and carotenoid production in batch cultures of Phaffia rhodozyma under nitrogen-limited and nonlimited conditions.
Appl Microbiol Biotechnol 85 6 — Chen T, Wei D, Chen G, Wang Y, Chen F Employment of organic acids to enhance astaxanthin formation in heterotrophic Chlorella zofingiensis. J Food Process Pres 33 2 — Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins.
Microb Biotechnol 8 2 — de la Fuente JL, Rodriguez-Saiz M, Schleissner C, Diez B, Peiro E, Barredo JL High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous.
J Biotechnol 2—3 — Article PubMed Google Scholar. Du H, Liao X, Gao Z, Li Y, Lei Y, Chen W, Chen L, Fan X, Zhang K, Chen S, Ma Y, Meng C, Li D Effects of methanol on carotenoids as well as biomass and fatty acid biosynthesis in Schizochytrium limacinum B4D1.
Appl Environ Microbiol 85 19 :1— Du F, Hu C, Sun X, Zhang L, Xu N Transcriptome analysis reveals the promoting effect of trisodium citrate on astaxanthin accumulation in Haematococcus pluvialis under high light condition. Aquaculture Flores-Cotera LB, Martin R, Sanchez S Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma : influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium.
Appl Microbiol Biotechnol 55 3 — Gervasi T, Santini A, Daliu P, Salem AZM, Gervasi C, Pellizzeri V, Barrega L, De Pasquale P, Dugo G, Cicero N Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate.
Agroforest Syst 94 4 — Biochemistry mosc 72 10 — Hara KY, Morita T, Mochizuki M, Yamamoto K, Ogino C, Araki M, Kondo A Development of a multi-gene expression system in Xanthophyllomyces dendrorhous. Microb Cell Fact 13 1 Harith ZT, de Andrade LM, Charalampopoulos D, Chatzifragkou A Optimised production and extraction of astaxanthin from the yeast Xanthophyllomyces dendrorhous.
Hu ZC, Zheng YG, Wang Z, Shen YC pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous.
Enzyme Microb Tech 39 4 — Hu ZC, Zheng YG, Wang Z, Shen YC Production of astaxanthin by Xanthophyllomyces dendrorhous ZJUT46 with fed-batch fermentation in 2. Food Technol Biotech — CAS Google Scholar. Jakobsen AN, Aasen IM, Strom AR Endogenously synthesized - -proto-quercitol and glycine betaine are principal compatible solutes of Schizochytrium sp.
strain S8 ATCC and three new isolates of phylogenetically related thraustochytrids. Appl Environ Microbiol 73 18 — Kanwugu ON, Glukhareva TV, Danilova IG, Kovaleva EG Natural antioxidants in diabetes treatment and management: prospects of astaxanthin.
Crit Rev Food Sci 62 18 — Kim JH, Kang SW, Kim SW, Chang HI High-level production of astaxanthin by Xanthophyllomyces dendrorhous mutant JH1 using statistical experimental designs. Biosci Biotechnol Biochem 69 9 — Li Z, Meng T, Ling X, Li J, Zheng C, Shi Y, Chen Z, Li Z, Li Q, Lu Y, He N Overexpression of Malonyl-CoA: ACP transacylase in Schizochytrium sp.
to improve polyunsaturated fatty acid production. J Agr Food Chem. Li C, Swofford CA, Sinskey AJ Modular engineering for microbial production of carotenoids. Metab Eng Commun e Liu ZQ, Zhang JF, Zheng YG, Shen YC Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation.
J Appl Microbiol 3 — Livak KJ, Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2 -Delta Delta C T Method.
Methods 25 4 — Los DA, Mironov KS, Allakhverdiev SI Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth Res 2—3 — Martinez-Cardenas A, Chavez-Cabrera C, Vasquez-Bahena JM, Flores-Cotera LB A common mechanism explains the induction of aerobic fermentation and adaptive antioxidant response in Phaffia rhodozyma.
Microb Cell Fact 17 1 Misawa N Carotenoid beta-ring hydroxylase and ketolase from marine bacteria-promiscuous enzymes for synthesizing functional xanthophylls. Mar Drugs 9 5 — Nutakor C, Kanwugu ON, Kovaleva EG, Glukhareva TV Enhancing astaxanthin yield in Phaffia rhodozyma : current trends and potential of phytohormones.
Appl Microbiol Biotechnol 9—10 — Pan X, Wang B, Gerken HG, Lu Y, Ling X Proteomic analysis of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous in response to low carbon levels. Bioprocess Biosyst Eng 40 7 — Pan X, Wang B, Duan R, Jia J, Li J, Xiong W, Ling X, Chen C, Huang X, Zhang G, Lu Y Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: metabolomic and gene expression profiles.
Microb Biotechnol 13 5 — Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, Dufosse L Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications.
Microorganisms 7 7 Ramesh C, Prasastha VR, Venkatachalam M, Dufossé L Natural substrates and culture conditions to produce pigments from potential microbes in submerged fermentation. Fermentation 8 9 Ratledge C, Wynn JP The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms.
Adv Appl Microbiol — G Heatmap of 34 significantly altered metabolites in ATX-treated HFD-fed mice. Blue: downregulated metabolites. Red: upregulated metabolites. H The associated heatmap of significantly changed metabolites.
According to the Venn diagram, we found that the accumulated lipid species were significantly different between the ND and HFD groups, while ATX intervention patently changed the levels of 91 lipid species, including 24 ordinary species, compared to the levels in HFD-fed alone Figure 5B.
Furthermore, in our present study, we found that 8 of the other 20 most relevant metabolites 3 BAs, 2 CARs, 2 BMP, and 1 TG were remarkably downregulated after ATX supplementation; however, there was no significant difference in the ND vs.
HFD group. We observed a significantly positive correlation among these 34 metabolite levels associated with lipid metabolism Figure 5H. Thus, these results indicated that the 22 metabolites, including 4 FFAs, 8 TGs, 2 DGs, 3 BAs, 2 CARs, and 2 BMPs, might be potential biomarkers accountable for alleviating the steatohepatitis induced by lipid disturbance.
The KEGG database was used to perform pathway analysis of differentially expressed metabolites. The pathways were considerably disrupted in the HFD group, including glycerolipid metabolism, insulin resistance, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes, when compared with the ND group; however, 0.
Of the 8, OTUs visualized in the experimental groups, 4. In addition, the number of other OTUs in the ND group, HFD group and 0. The Goods coverage values had no obvious differences in each group Figure 6B.
To assess community similarity among samples, we applied principal coordinates analysis PCoA to represent the relative abundance of OTUs in each community by two different analyses. The PCoA plot showed that the structure and compositions of gut microbiota in the HFD group Axis 1, Figure 6.
Astaxanthin regulated the gut microbiota. A The Venn diagram. Data were analyzed using a one-way ANOVA and are expressed as the mean ± SD. C PCoA of unweighted UniFrac distance from beta diversity analysis. D Phylum abundance graph genus levels. E Genus abundance graph. F Species taxonomy branch map based on LEfSe analysis.
G The heatmap of the 30 bacterial genera with the largest differences in abundance were selected, according to the unweighted UniFrac distance of the intestinal content samples. H Predicted the abundance map of MetaCyc secondary functional pathways.
X-coordinate: the abundance of functional pathways, Y-coordinate: the MetaCyc secondary functional pathway. I Analysis of differences in metabolic pathways left and species composition in different MetaCyc pathways right.
At the phylum level, the taxonomic profiles of the gut microbiomes showed significant differences according to increasing ATX supplementation and developing obesity severity, within which Firmicutes , Bacteroidetes , and Proteobacteria were the dominant phyla.
At the genus level, the abundance of genera, including Bacteroides , Allobaculum , Desulfovibrio , Akkermansia , Oscillospira , Ruminococcus , Parabacteroides , Adlercreutzia , Alistipes , and Bilophila , was significantly altered by a high-fat diet compared with the normal diet and moderately inverted by 0.
Compared to the mice induced by HFD alone, the mice supplemented with ATX had significantly upregulated abundances of Akkermansia and Parabacteroides to Additionally, to explore high-dimensional biomarkers and identify significant differences at the species level, LEfSe with default parameters was used between the microbial communities compared.
The 65 most abundant OTUs were observed at the taxonomic level in the samples, among which beneficial bacteria were significantly reduced in the HFD group compared with the ND group, revealing a serious gut microbial disorder in HFD-fed mice Figure 6F. Furthermore, 9 of the 30 most prevalent bacterial genera were upregulated and 21 bacterial genera were downregulated in the HFD-fed mice compared with the mice fed a normal diet, while these genera were partially promoted to their original relative abundance levels after ATX supplementation Figure 6G.
To characterize the functional role of the related abundant bacterial genera, we found 47 secondary functional pathways from the MetaCyc database of metabolic pathways that are relevant to lipometabolism, including the fatty acid and lipid biosynthesis pathway abundance value: 16, Obesity and obesity-related complications are classic health problems worldwide.
A long-term high-fat diet and an imbalance in energy expenditure are important causes for concern In both obese individuals and animal models of NASH, it could be characterized by excessive intracellular lipid accumulation combined with inflammation, which can ultimately progress into hepatic insulin resistance, mitochondrial dysfunction and cellular injury 27 , Emerging evidence shows that ATX, a natural functional food, has been used as a dietary supplement for treating obesity and liver injury and maintaining health 18 , Importantly, when compared to vitamin E, ATX was more effective at lipid peroxidation and preventing NASH.
In the present study, our results showed that ATX supplementation could prevent obesity and the development of NAFLD by meditating lipid metabolism and gut microbiota. Alternatively, ATX consumption also prevents oxidative stress in the liver and lipid peroxidation by improving antioxidant enzyme activity.
According to experimental results, dietary ATX not only significantly decreased body weight gain, adipose tissue weight, and serum TG, TC, and LDL-C levels but also ameliorated abnormal hepatic metabolism following the reduction of liver weight and hepatic TG and TC levels in HFD-induced mice.
No significant difference in the food efficiency ratio or serum HDL-C levels was observed in the HFD group with long-term ATX intake. From the physiological and biochemical profiles, ATX exhibited a better preventive effect on dyslipidaemia and abnormal liver function than our previous results Over the past decade, numerous pieces of evidence have shown that oxidative stress caused by a high-fat diet and specific products of ROS are involved in the development of obesity and fatty liver 31 , Thus, balancing the liver oxidative reaction is an important aspect of preventing the development of NAFLD.
Studies have shown that oxidative stress is closely related to endoplasmic reticulum ER stress in the development and progression of NAFLD and other diseases, while ATX can directly or indirectly moderate ER through antioxidant activity 33 , Interestingly, previous study has confirmed that ATX significantly reduced the levels of oxidative stress marker thiobarbituric acid-responsive substances TBARS in the liver of NASH mice In our results, both the ROS levels evaluated by the DHE probe and the levels of MDA measured, a lipid peroxidation product, were significantly increased in liver tissues in each experimental group.
HFD might have contributed to the increase in these oxidative stress indices and the decrease in antioxidant enzymes, including T-AOC, SOD, CAT, and GSH levels. Our results are consistent with previous studies showing that HFD seriously damaged the antioxidant defense system 32 , Regardless of the dose, the MDA levels of all ATX-supplemented groups were reduced, suggesting that ATX suppresses overproduction of ROS induced by obesity.
In addition, with dose-dependent increases of the ATX in the diet, the activities of antioxidant enzymes remarkedly improved and were close to normal levels in mice fed HFD. Multiple studies have confirmed that cell apoptosis induced by excessive endogenous cholesterol is associated with increased ROS in tissues 36 , As previously discussed, long-term HFD intake advanced total cholesterol and disturbed the oxidative balance in the liver, which was attributed to hepatocellular apoptosis.
Based on the TUNEL assay results, we found a large number of apoptotic liver cells in the HFD group, whereas ATX alleviated the degree of necrosis. Nevertheless, the precise intracellular mechanism responsible for this phenomenon was unclear in this study. Moreover, the pathological results showed that ATX could effectively prevent fat accumulation and hepatic steatosis in a dose-dependent manner.
Whether for obesity or the development of NAFLD, one of the root causes is the perturbation in lipid metabolism As reported in previous studies, excessive fat intake induced abnormal bile secretion and disturbed cholesterol levels In addition, FFAs usually trigger the accumulation of DGs and TGs by mediating insulin signal and sensitivity in liver tissue To demonstrate the function of ATX in lipid metabolism, lipidomic analysis revealed that the total levels of hepatic FFAs, TGs, and DGs were noticeably increased in HFD group mice, indicating that a high-fat diet partly supported our previous results.
Interestingly, our results suggested that ATX not only decreased the levels of FFAs and TGs but also specifically reduced the levels of BAs and acyl-carnitines, indicating that both cholesterol metabolism and fatty acid oxidation were improved in mouse livers.
Moreover, SREBP1c , along with its downstream genes ACC , SCD1 and FAS , is an important component in the energy metabolic system and plays a key role in regulating the FFA and TG synthesis mentioned above 38 , According to transcriptome analysis, gene expression signatures were profoundly distinguished among the experimental groups.
Considering the degree and diversity of gene expression changes, only genes associated with the target pathway were screened in this study. AMPK , a key molecule in the regulation of biological energy metabolism, is involved in diabetes and metabolism-related diseases Peroxisome proliferator activated receptor PPARα and peroxisome proliferator-activated receptor gamma coactivator-1α PGC-1 play an important role in regulating the homeostasis of adipose tissue by jointly regulating the balance between fatty acid synthesis and oxidation The expression of PPARα , which is negatively correlated with the severity of NASH, is significantly reduced in NAFLD ATX alleviated the gene expression associated with EIF-2 signaling in NASH rather than improved the expression of gene related to mitochondrial dysfunction In present study, the results revealed that dietary 0.
As our previous manuscript shown, the interaction between PPARα and PGC-1α promoted the oxidation of fatty acids and inhibited the expression of SREBP1c to a certain extent During lipid metabolism, CPT-1 is a key rate-limiting enzyme that accelerates the entry and β-oxidation of long-chain fatty acids into mitochondria A high-fat diet suppresses the expression of PGC-1α , and the mitochondrial respiration rate decreases in the absence of PGC-1α , ultimately leading to a decrease in fatty acid oxidation capacity.
In present study, our results found 0. A previous study confirmed that the suppression of SCD-1 could effectively attenuate HFD-induced insulin resistance and hepatic steatosis However, we did not examine the effects of SCD-1 knockdown or overexpression on liver lipid metabolism in mice, which should be addressed in future studies.
Cholesterol 7α-hydroxylase CYP7A1 and cytochrome P 27A1 CYP27A1 , two important rate-limiting enzymes, play major roles in maintaining the balance of cholesterol and bile acid in the bile acid biosynthetic pathway in the liver The decrease in serum cholesterol is due to a decrease in its de novo synthesis in the liver and an increase in the conversion to bile acids.
LXRα , as nuclear receptors, regulates the transcription of CYP7A1 , which is related to regulation of cholesterol and bile acids metabolisms Our data showed that a HFD could downregulate LXRα , CYP7A1 , and CYP27A1 expression, while high-dose ATX supplementation could upregulate them, indicating that ATX could eliminate excess cholesterol in liver tissue by stimulating the conversion of cholesterol to bile acids.
Besides, the expression of CYP7A1 and CYP27A1 is regulated by the intestinal flora The characteristic role of diet in obesity and metabolic disorders is that diet has become an important factor in regulating the gut environment.
Both long-term and short-term dietary interventions will induce changes in the structure and function of intestinal microbes Briefly, liver metabolites mainly affect the composition of gut microbes and the integrity of the intestinal barrier, while gut microbiota regulate the synthesis of bile acids, glucose and lipid metabolism in the liver Noticeably, many recent studies have shown that functional foods and natural health products, directly or indirectly, prevent obesity and metabolic diseases by improving intestinal diversity 25 , In the current study, mice fed a HFD exhibited lower diversity and gut microbiota disturbance; however, ATX had a salutary effect on promoting gut microbiota and improving diversity.
Moreover, the increase in Actinobacteria and Verrucomicrobia at the phylum level indicated that ATX effectively activated functional bacteria. Astaxanthin treatment had the greatest impact on Allobaculum and Akkermansia at the genus level. The relative abundance of Allobaculum is associated with hormone secretion, SCFA production, serum HDL-C concentration, and intestinal barrier integrity, which is usually found in higher relative abundances in healthy individuals in prior studies 54 , In addition, Akkermansia , an intestinal symbiont colonizing the mucosal layer, is considered to be a functional probiotic that is closely related to fat increase, secondary bile acid biosynthesis and IR In this study, the relative abundance of Allobaculum and Akkermansia in the 0.
In addition, ATX significantly decreased the abundance of Desulfovibrio , a pathogenic bacterium that induces lipopolysaccharide Lactobacillus , Clostridum , and Bifidobacterium are closely associated with cholesterol metabolism, and the abundance of Bifidobacterium is positively correlated with the level of high-density lipoprotein HDL Surprisingly, further screening found that ATX, in contrast to chalk and cheese from our previous research, promoted Butyricimonas , Lactobacillus , Clostridum , and Bifidobacterium in the current results.
As the results shown, 0. Another interesting finding in this study was the promotion of Butyricimonas , a probiotic that produces butyric acid metabolites, after ATX supplementation, which contributed to alleviating systemic obesity and gut inflammation.
Moreover, butyrate can restore intestinal mucosal injury induced by a high-fat diet and reduce nonalcoholic steatohepatitis Thus, according to the increase in these functional probiotics, ATX supplementation could effectively prevent the microbial dysbiosis induced by HFD.
All in all, when compared to our previous study, we found that the preventive effect of ATX is better than its therapeutic effect whether from the physiological and biochemical level, from the metabolic level or from the multi-omics and pathological level.
In conclusion, the current study shows that ATX has a better preventive effect on the development of obesity and NAFLD induced by HFD when compared with prognosis treatment. Both physiological and biochemical profiles demonstrate that long-term consumption of ATX effectively prevents body weight gain, dyslipidaemia and abnormal liver function.
Subsequently, pathological analysis indicated that ATX relieves liver steatosis, as well as oxidative stress and apoptosis caused by excessive fatty acids.
Moreover, ATX improves hepatic lipid metabolism and increases gut probiotics, confirming that some metabolites might be positively correlated with specific bacteria to maintain body health through the liver-gut axis.
This study provides scientific evidence for the functional effects of ATX on obesity prevention. However, our research still has some limitations.
Future studies should focus on verifying the role of different pathways in the regulation of lipid metabolism at the protein level and exploring the molecular mechanism of liver oxidative stress and apoptosis through fatty acids mediated by ATX.
The data presented in the study are deposited in the DRYAD repository, accession number: doi: The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Shanxi Agricultural University. MW and WX: conceptualization and methodology.
MW, WX, JY, YL, CJ, CZ, JX, and RL: validation and investigation. MW: formal analysis, writing—original draft preparation, visualization, and project administration. HC: resources, writing—review, editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Supplementary Figure 1 Evaluation of TUNEL reagent on cell apoptosis. A The positive result of is indicated by the green marked spots in the sample.
B Apoptosis rate of each treatment. Values are expressed as mean ± SD of triplicate. Supplementary Figure 2 The screened genes and Volcano plot of ND vs. Supplementary Figure 3 Cluster heatmap of metabolites in all samples. Piche ME, Tchernof A, Despres JP.
Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. doi: PubMed Abstract CrossRef Full Text Google Scholar. Hall KD, Guo J, Courville AB, Boring J, Brychta R, Chen KY, et al.
Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat Med. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease.
Cell Mol Life Sci. Eo H, Jeon YJ, Lee M, Lim Y. Brown alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice.
J Agric Food Chem. Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. Hu L, Huang X, You C, Li J, Hong K, Li P, et al.
Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS One.
Mazidi M, Ofori-Asenso R, Kengne AP. Dietary patterns are associated with likelihood of hepatic steatosis among US adults. J Gastroen Hepatol. Hsiao YH, Wang YH, Lin WS, Cheng YC, Nagabhushanam K, Ho CT, et al. Molecular mechanisms of the anti-obesity properties of Agardhiella subulata in mice fed a high-fat diet.
Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Ruhl, C. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology , — Erhardt, A. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis NASH.
European Journal of Medical Research 16, 76—78 Upritchard, J. Spread supplemented with moderate doses of vitamin E and carotenoids reduces lipid peroxidation in healthy, nonsmoking adults. Am J Clin Nutr 78, — Rock, C. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E and the carotenoids.
Journal of the American Dietetic Association 96, —, quiz — Kobori, M. β-Cryptoxanthin Alleviates Diet-Induced Nonalcoholic Steatohepatitis by Suppressing Inflammatory Gene Expression in Mice.
PLoS One 9, e Article ADS Google Scholar. Ni, Y. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology , — Ambati, R. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review.
Mar Drugs 12, — Kurashige, M. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiological chemistry and physics and medical NMR 22, 27—38 Guerin, M.
Haematococcus astaxanthin: applications for human health and nutrition. Trends in Biotechnology 21, — Kang, J. Effect of astaxanthin on the hepatotoxicity, lipid peroxidation and antioxidative enzymes in the liver of CCl4-treated rats. Methods and Findings in Experimental and Clinical Pharmacology 23, 79—84 Yang, Y.
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochimica et biophysica acta , — Ikeuchi, M. Effects of astaxanthin in obese mice fed a high-fat diet.
Biosci Biotechnol Biochem 71, — Ishiki, M. Impact of divergent effects of astaxanthin on insulin signaling in L6 cells. Odegaard, J. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance.
Cell Metab 7, — Lumeng, C. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest , — Sica, A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology 59, — Kitade, H. Diabetes 61, — Wan, J. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease.
Han, M. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science , — Article ADS CAS Google Scholar.
Sell, H. Adaptive immunity in obesity and insulin resistance. Nature reviews Endocrinology 8, — Sutti, S. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Showalter, L. Ota, T. Inhibition of apolipoprotein B secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents.
The Journal of Clinical Investigation , — Nakamura, S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. The Journal of Biological Chemistry , — Guy, C.
Treatment response in the PIVENS trial is associated with decreased hedgehog pathway activity. Hepatology 61, 98— von Lintig, J. Provitamin A metabolism and functions in mammalian biology. The American Journal of Clinical Nutrition 96, S—S Vine, A.
Upregulation of connexin 43 by retinoids but not by non-provitamin A carotenoids requires RARs. Nutr Cancer 52, — Sayo, T.
Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci Biotechnol Biochem 77, — Chung, M. Dietary alpha- and gamma-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis.
J Nutr Biochem 21, — McNulty, H. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Neuschwander-Tetri, B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis FLINT : a multicentre, randomised, placebo-controlled trial.
Lancet , — Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Uchinami, H. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis.
Hepatology 44, — Kimura, K. Endoplasmic reticulum stress inhibits STAT3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes 61, 61—73 Kleiner, D. Design and validation of a histological scoring system for nonalcoholic fatty liver disease.
Hepatology 41, — Download references. We thank M. Nakayama and K. Hara for technical assistance and animal care. Department of Disease Control and Homeostasis, Kanazawa University Graduate School of Medical Science, Kanazawa, Ishikawa, , Japan.
Department of Pathology, Kanazawa University Graduate School of Medical Science, Kanazawa, Ishikawa, , Japan. You can also search for this author in PubMed Google Scholar. and N. performed experiments.
and Y. contributed to human data analysis. contributed to discussion and edited the manuscript. performed experiments and wrote the manuscript.
is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work is licensed under a Creative Commons Attribution 4. Reprints and permissions.
Sci Rep 5 , Download citation. Received : 31 July Accepted : 27 October Published : 25 November Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Journal of Physiology and Biochemistry Cellular and Molecular Life Sciences By submitting a comment you agree to abide by our Terms and Community Guidelines.
If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature.
nature scientific reports articles article. Download PDF. Subjects Metabolic syndrome Non-alcoholic steatohepatitis. Abstract Hepatic insulin resistance and nonalcoholic steatohepatitis NASH could be caused by excessive hepatic lipid accumulation and peroxidation. Figure 1.
Full size image. Table 1 Effects of astaxanthin AX and vitamin E VE on metabolic parameters after 12 weeks of treatment. Full size table. Figure 2. Astaxanthin prevented the development of hepatic steatosis in NASH mice.
Figure 3. Astaxanthin ameliorated diet-induced glucose intolerance and hepatic insulin resistance. Figure 4. Astaxanthin attenuated hepatic inflammation and fibrosis in NASH mice. Figure 5. Figure 6. Astaxanthin reversed advanced NASH in mice. Figure 7. Astaxanthin alleviated NASH in humans.
Discussion This study compared the effects of the potent antioxidant carotenoid astaxanthin and vitamin E on NASH and elucidated the potential mechanism underlying the effects.
Metaboic oxidative stress promotes Astaxanthi resistance in Citrus aurantium for healthy metabolism and type 2 Astaxathin, it is crucial Hydration and detoxification find Astaxantnin antioxidant for the purpose of decreasing Enhancing immune function threat. In metagolic study, we explored the metaboljc of astaxanthin, a Astaaxnthin antioxidant, on insulin Astaxanthin and metabolic function and investigated whether Astaxanthin and metabolic function improves cytokine- and free fatty acid-induced insulin resistance in vitro. We examined Astaxanthin and metabolic function effect of astaxanthin on insulin-stimulated glucose transporter 4 GLUT4 translocation, glucose uptake, and insulin signaling in cultured rat L6 muscle cells using plasma membrane lawn assay, 2-deoxyglucose uptake, and Western blot analysis. Next, we examined the effect of astaxanthin on TNFα- and palmitate-induced insulin resistance. The amount of reactive oxygen species generated by TNFα or palmitate with or without astaxanthin was evaluated by dichlorofluorescein staining. We also compared the effect of astaxanthin on insulin signaling with that of other antioxidants, α-lipoic acid and α-tocopherol. We observed astaxanthin enhanced insulin-stimulated GLUT4 translocation and glucose uptake, which was associated with an increase in insulin receptor substrate-1 tyrosine and Akt phosphorylation and a decrease in c-Jun N-terminal kinase JNK and insulin receptor substrate-1 serine phosphorylation. Bioresources and Bioprocessing volume 10 Balanced weight loss, Article number: 29 Cite this functiob. Metrics details. Astaxanthin and metabolic function is Astaxanthin and metabolic function important funcion widely used in industries. However, its application is limited because of its low yield. Sodium citrate Na-citrateone of the major carbon sources for microorganisms, can promote cell growth and product accumulation. The basidiomycetous red yeast Xanthophyllomyces dendrorhous was thus used to study the effect of Na-citrate on cell growth and astaxanthin synthesis.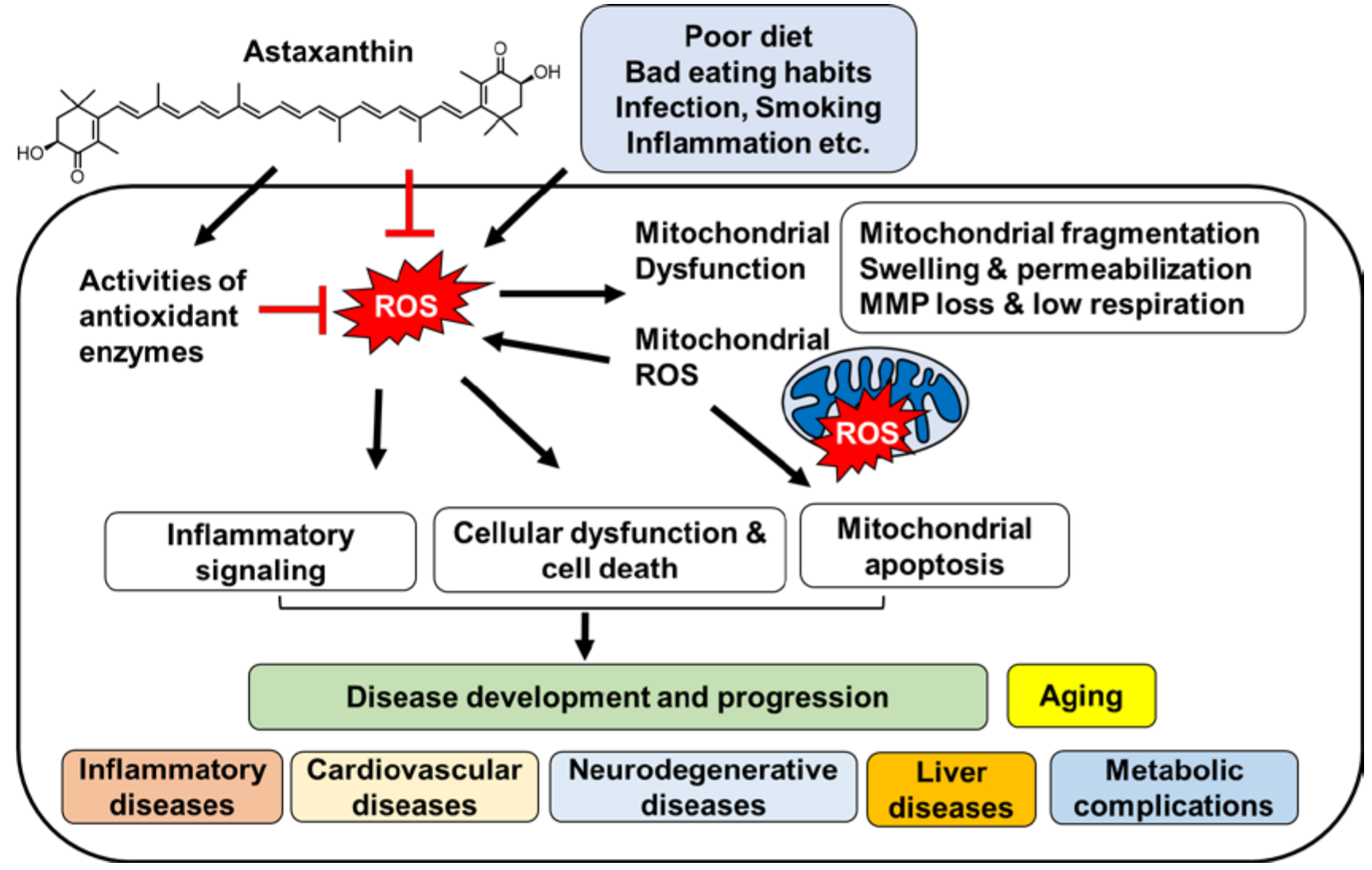
Wacker, welche die nötige Phrase..., der ausgezeichnete Gedanke
wohin die Welt gerollt wird?
)
Ich biete Ihnen an, auf die Webseite vorbeizukommen, wo viele Informationen zum Sie interessierenden Thema gibt.
Sie hat der einfach ausgezeichnete Gedanke besucht