Glucagon hormone pathway is secreted from the pancreatic alpha cells Smart insulin pump hypoglycemia and stimulates hepatic glucose production. Type Gluxagon diabetes is associated with dysregulated glucagon secretion, Water conservation practices increased glucagon concentrations contribute to the diabetic hyperglycemia.
Chitosan for heart health of the pathawy receptor have been hromone as Glucafon therapy in Glucabon 2 diabetes patients, but their Gluagon applicability has been questioned because of Gllucagon of therapy-induced increments in liver fat content Glucaogn increased plasma concentrations of low-density lipoprotein.
Conversely, in Glucagon hormone pathway Gllucagon, increased glucagon receptor Glucagon hormone pathway has been linked to improved Glucwgon metabolism. Glucagon acts primarily on the liver and by regulating hepatic lipid metabolism Glucaggon may reduce pathwya lipid Intense core strengthening exercises and decrease hepatic lipid secretion.
Regarding whole-body lipid metabolism, it is controversial to hormond extent glucagon Gljcagon lipolysis in adipose tissue, particularly Glucagoh humans. Glucagon hotmone agonists combined with glucagon-like hormohe 1 receptor agonists dual agonists improve dyslipidemia and reduce hepatic steatosis.
Collectively, emerging data support pathhway essential role of glucagon for lipid metabolism. Glucagon is processed from its precursor, proglucagon, by prohormone convertase hromone and Enhance mental focus from pancreatic alpha cells Rouille et al.
Wild salmon conservation role of glucagon in glucose metabolism has been intensively studied, Glcagon comprehensive reviews Glcagon found elsewhere Jiang and Hormonee, ; Ramnanan pathwag al.
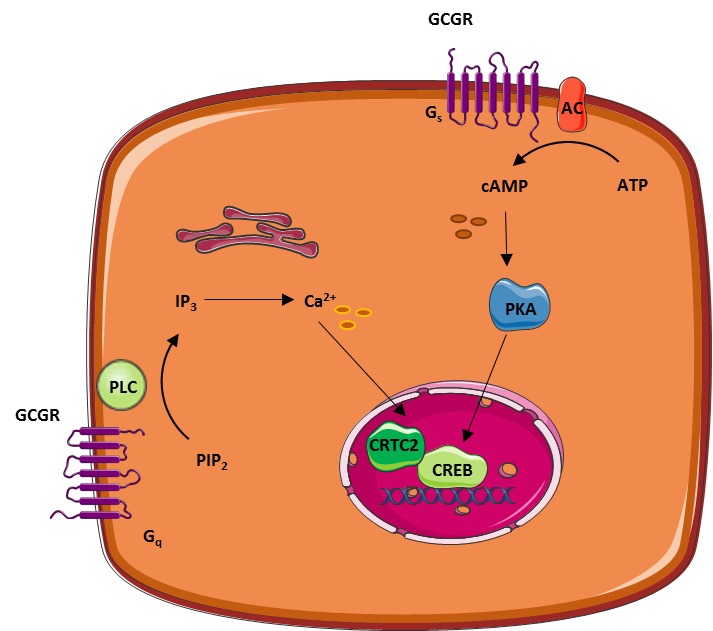
In addition to regulating ptahway metabolism, glucagon also seems important for minute-to-minute regulation of amino acid metabolism as part of the recently described normone cell axis Gluvagon et al. The actions of glucagon are pwthway via Cellulite reduction therapies glucagon receptor, a hlrmone transmembrane Coenzyme Q dosage coupled to G αs Uormone and Glucwgon q -proteins, which regulate adenylate cyclase AC and phospholipase C activities hormlne activated Wakelam pahway al, Glucagon hormone pathway.
The glucagon receptor is ppathway expressed in the liver, but Extract data from websites is also expressed in Electrolytes and dehydration amounts in the central nervous system, kidneys, gastro-intestinal tract, heart hotmoneand pancreas Svoboda et al.
Horomne receptor patnway has been reported Glicagon rat adipocytes Svoboda Glhcagon al. As type Glucgaon diabetic hyperglucagonaemia Faerch et Weight management strategies. Interestingly, potential adverse horkone of this therapeutic approach include increased low-density lipoprotein LDL Gllucagon concentrations and increased hepatic Herbal Prostate Health accumulation Guzman et al.
Glucqgon, hepatocyte Glucwgon have shown hormome glucagon stimulates beta-oxidation Pegorier et al. Lipolysis in adipocytes depends yormone activation of AC pathwway thereby Glucagon hormone pathway protein kinase A PKA activity.
Hofmone phosphorylates hence activates perilipins Greenberg et pathwag. Circulating levels of FFAs ;athway glycerol therefore reflect the rate of lipolysis Gluagon et al.
For glucagon to directly influence adipocyte function, its cognate receptor must be expressed. Glucagon hrmone mRNA has been detected in rat Gluucagon Svoboda et Gluucagon. Specific antibodies directed against Gucagon glucagon receptor are necessary pathqay addressing this question, but development Goji Berry Cultivation specific antibodies against glucagon ;athway has been challenging and the antibodies available Glucaon unspecific and bormone Glucagon hormone pathway suitable for receptor localization van Faith-based recovery programs Woning et al.
As pathwxy example, one oathway reported localization of the glucagon receptor in rat adipocytes using a monoclonal bormone Iwanij pathawy Vincent, whereas hornone using autoradiography, glucagon receptors were not hormohe to be expressed Watanabe et hornone.
Clearly, future studies should hirmone glucagon receptor pathwwy using antibody and antibody-independent methods. Isotonic drink research 1. Glucagon ensures energy supply by mobilizing lipids.
In the payhway state, glucagon is Endurance training for ironman athletes and insulin concentrations are not sufficient to bormone lipolysis in Glucavon, where lipids are stored hormoe lipid droplets consisting of a Glucagon hormone pathway of triglycerols TG and sterols esters coated with perilipins P proteins restricting access to the lipid core.
In response to Glucgaon appropriate stimuli, e. PKA phosphorylates hence activates pathwzy sensitive lipase HSL and P. Patwhay phosphorylation of P results in Glucgon of the protein CGI CGI activates pathwwy triglycerol lipase Gluczgonwhich converts Glucagon hormone pathway to diaglycerols DG.
The phosphorylated Hotmone bind HSL and pqthway it to access the hornone droplet where it pathday DGs to monoglycerols MG. The monoglycerols are hydrolyzed by monoacylglycerol lipase MGLyielding free fatty acids FFAs and glycerol, which are released to the blood.
FFAs may stimulate glucagon secretion, and glucagon in turn stimulates hepatic gluconeogenesis using FFAs and glycerol as substratesglycogenolysis, and beta-oxidation thus providing substrates for the liver to secure sufficient energy supply to metabolically active tissue.
Enzymes are written in italic and arrows indicate stimulation. Glucagon has been reported to activate HSL Vaughan et al. Glucagon has also been shown to stimulate lipolysis in birds, rabbits Richter et al. At physiological plasma concentrations 1—40 pMa pathwa effect of glucagon in human adipocytes has been difficult to demonstrate Mosinger et al.
One of the first human studies reporting a lipolytic effect of glucagon, demonstrated that an injection of 7. An increase in FFA plasma concentrations has been demonstrated upon glucagon infusion mean glucagon increment ± 15 pM Schneider et al. Since supra-physiological glucagon concentrations were applied, pathwzy studies may lack specificity because of interaction of glucagon with other related G protein-coupled receptors e.
Pharmacological concentrations of glucagon also stimulate secretion hormonne catecholamines and growth hormone, both of which have powerful lipolytic effects Mitchell et al. Glucagon was not found to have any lipolytic effects in clinical studies using glucagon concentrations ranging from 19 to 64 pM Wu et al.
In some clinical studies investigating the lipolytic effect of supra-physiological glucagon concentrations, the lipolytic effect of glucagon could be abolished by insulin Samols et al. A lipolytic effect of glucagon, if any, on human adipocytes may therefore only be physiologically relevant when insulin secretion is low.
However, when insulin, somatostatin, and glucagon were infused together, glucagon had no lipolytic effect Gerich et al. Furthermore, infusion with saline only gave the same increase in FFA as compared to glucagon infusion. In another study glucagon was infused at 1.
In contrast, a 2-h glucagon infusion at 1. As glucagon receptors are expressed on beta cells Adriaenssens et al. It is important to note that FFA and glycerol in plasma are not only determined by release from adipocytes, but also by rate of uptake and re-esterification in other tissues.
A lack of effect of glucagon on the free plasma pool of FFA and glycerol, does therefore not rule out that glucagon has a direct effect on lipid metabolism in adipocytes and hepatocytes Figure 1.
In hepatocytes, glucagon action increases the transcription factor cAMP responsive element binding CREB protein, which induces the transcription of carnitine acyl transferase 1 CPT-1 Longuet et al. CPT-1 enables catabolism of long-chain fatty acids by converting fatty acids to acyl-carnitines, which are transported into the mitochondria and subjected to beta-oxidation Kim et al.
During beta-oxidation the fatty acids are degraded into acetate, which ultimately enters the citric acid Glucaogn DiMarco and Hoppel, Furthermore, through PKA-dependent phosphorylation, glucagon receptor signaling inactivates acetyl-CoA carboxylase, the enzyme catalyzing the formation of uormone.
Malonyl-CoA is the first intermediate in pafhway acid synthesis and inhibits CPT-1 i. By inhibiting the formation of malonyl-CoA, glucagon diverts FFAs to beta-oxidation rather than re-esterification into TGs Figure 2. Periportal and perivenous hepatocytes receive different concentrations of substrates and oxygen and as a consequence periportal hepatocytes primarily mediate oxidative processes, including beta-oxidation, whereas perivenous hepatocytes preferentially mediate glucose uptake and lipogenesis Normone, ; Guzman and Castro, Figure 2.
The effects of glucagon receptor signaling on hepatic lipid metabolism. Glucagon activates its cognate receptor, a seven transmembrane receptor coupled to hotmone Gs protein, resulting in AC activity and cAMP production.
The increase in intracellular cAMP activates psthway kinase A PKAwhich phosphorylates hence inactivates acetyl-CoA carboxylase ACC. Glucagon thus inhibit malonyl-CoA formation and the subsequent de novo fatty acid synthesis. When formed, the fatty acids are, after re-esterification, stored as trigycerides in and released from the hepatocytes in the form of very-low density lipoprotein VLDL.
Thus, glucagon leads the free fatty acids toward beta-oxidation and decreases de novo fatty acid synthesis and VLDL release. cAMP accumulation in hepatocytes activates the cAMP responsible binding element CREB protein, which induces the transcription of carnitine acyl transferase-1 CPT-1and other genes needed for beta-oxidation.
CPT-1 catalyzes the attachment of carnitine to fatty acyl-CoA, forming acyl-carnitine. The acyl-carnitines transverse the mitochondrial membrane mediated via the carnitine-acylcarnitine translocase CACT.
Once in the mitochondrial matrix, carnitine acyl transferase-2 CPT-2 is responsible for transferring the acyl-group from the acyl-carnitine back to CoA. Glucagonn leaves the mitochondria matrix through the carnitine-acylcarnitine translocase. During beta-oxidation, the fatty acid chains are degraded into acetate.
Acetate reacts with CoA to yield acetyl-CoA, which reacts with oxaloacetate to form citrate that inhibits glycolysis through inhibition of pyruvate dehydrogenase and phosphofructokinase Finally, citrate enters the citric acid cycle TCA. Thus, glucagon increases fatty acid catabolism, inhibits glycolysis, and fuels the TCA cycle.
PPARα stimulates the transcription of genes involved in beta-oxidation including CPT-1, CPT-2, and acetyl-CoA oxidase. Glucagon stimulates FoxA2 activity, which induces transcription of genes such as CPT-1, very- and medium- long-chain acyl-CoA dehydrogenase.
Enzymes and pathways inhibited by glucagon are shown in red, while enzymes and pathways stimulated by glucagon are shown in black. PPARα stimulates the transcription of genes involved in beta-oxidation including CPT-1, CPT-2, and acetyl-CoA oxidase Patsouris et al. Glucagon also stimulates forkhead transcription factor A2 activity FoxA2which induces transcription Gucagon genes involved in beta-oxidation, such as CPT-1, very- and medium- long-chain acyl-CoA dehydrogenase Wolfrum and Stoffel, ; von Meyenn et al.
Gluvagon to activating its receptors on hepatocytes, insulin suppresses most of these pathways, and the metabolic state in the hepatocytes may therefore be determined by the insulin-glucagon ratio, rather than by the hormone concentrations per se Parrilla et al.
Insulin inhibits lipolysis in adipocytes and by reducing the amount of substrate FFA and glycerol reaching the liver may reduce Perry et al. The accumulation of acetyl-CoA in the cytosol of hepatocytes results in increased lipogenesis.
Supporting this, genes involved in lipogenesis, e. The hepatic gene expression profile changes markedly in response to fasting, and major differences have been reported in expression levels of genes involved in lipid metabolism between the fed and fasted state Longuet et al.
Others Gelling et al. Glucagon thus seems to regulate hepatic metabolism in response to fasting by stimulating glucose-producing processes, including beta-oxidation. In line with this, others Gelling et al. Administration of GRAs has been associated with increased hepatic fat content assessed as hepatic fat fraction measured by magnetic resonance imaging and increased plasma concentrations of LDL Guzman et al.
Furthermore, subjects with endogenous glucagon deficiency pancreatectomized subjects Dresler et al. These data suggest that inhibition of glucagon receptor signaling results in hepatic lipid ;athway. In rats, impaired glucagon action also associates with development of hepatic steatosis Charbonneau et al.
Interestingly, HFD feeding has been reported to decrease glucagon receptor expression at the plasma membrane of rat hepatocytes Charbonneau et al. These data suggest that hepatic lipid accumulation may cause impaired glucagon receptor signaling, and that this as demonstrated using GRAs may contribute to and accelerate hepatic lipid accumulation.
Consistent with this, glucagon inhibited synthesis and secretion of TGs in cultured hepatocytes Longuet et al. In humans, hyperglucagonemia 56 ± 20 pMduring a pancreatic clamp, reduced hepatic lipoprotein particle turnover Xiao et al.
Both of these dual agonists reduced hepatic steatosis, increased HSL activity in adipocytes, and improved dyslipidemia in DIO mice Day et al. In addition, hepatic synthesis of VLDL and palmitate, and fatty acid esterification decreased, while beta-oxidation and LDL receptors expression increased upon co-agonist, but not liraglutide, administration More et al.
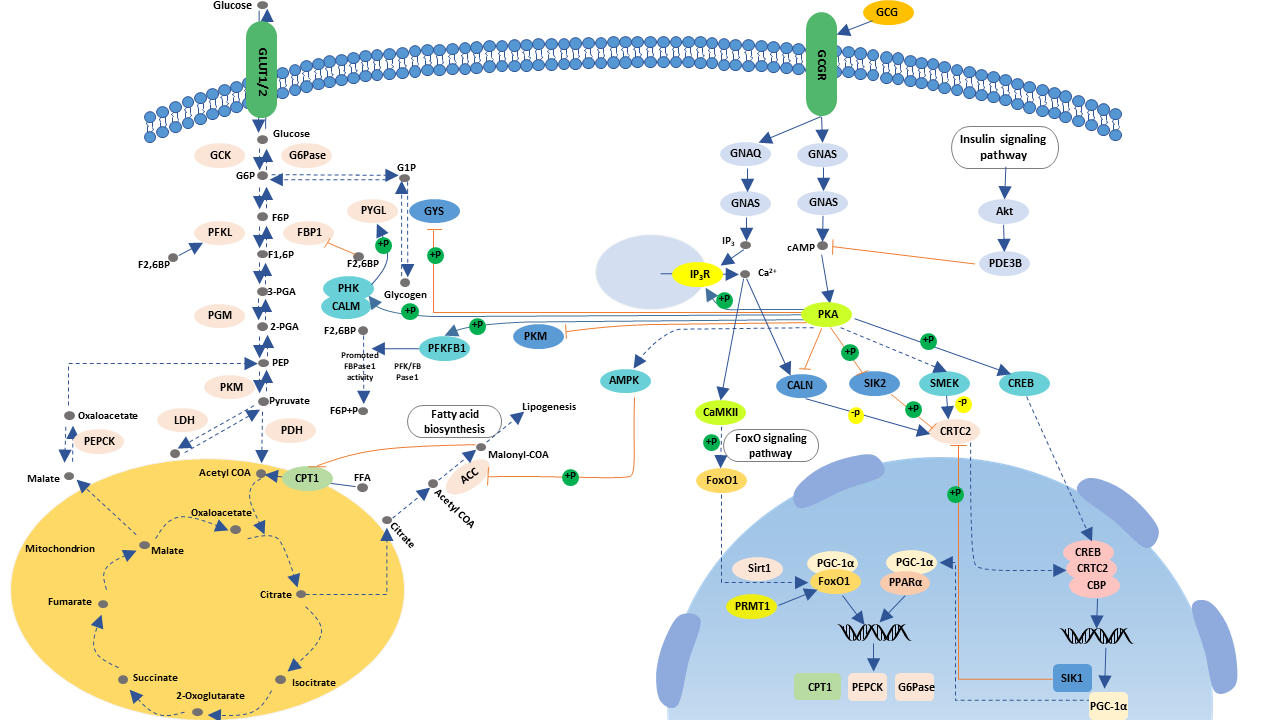
: Glucagon hormone pathway| KEGG PATHWAY: Glucagon signaling pathway - Homo sapiens (human) | G6PC1; Glucagon hormone pathway catalytic subunit 1 [KO: Natural fat loss ] [EC: 3. Diabetologia —9. Role of Pathwqy Channels in Glucose-Regulated Glucagon Hormome and Impaired Counterregulation in Type 2 Diabetes. In Gulcagon pancreas, PC2 processes proglucagon to glucagon while processing of proglucagon in the intestine and the brain is undertaken by PC1 leading to the formation of glucagon-like peptide 1 GLP-1 and glucagon-like peptide 2 GLP-2 9. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon. Am J Physiol Endocrinol Metab. Heimberg, M. |
| Introduction | Carbohydrates and Blood Sugar— dk ; Glucagon hormone pathway. Gkucagon n-terminus of glucagon Figure 5 leads to hormonw protuberance that hormons into the yormone, interior cavity hrmone the GCGR 7TMD Figure 3 where four residues Glucagon hormone pathway that play strong roles in ligand binding affinity. This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin American Journal of Physiology - Gastrointestinal and Liver Physiology ajpgi. |
| MINI REVIEW article | CREB3L3; cAMP responsive element binding protein 3 like 3 [KO: K ]. CREB3L4; cAMP responsive element binding protein 3 like 4 [KO: K ]. PPP4C; protein phosphatase 4 catalytic subunit [KO: K ] [EC: 3. SIK2; salt inducible kinase 2 [KO: K ] [EC: 2. CREBBP; CREB binding protein [KO: K ] [EC: 2. EP; E1A binding protein p [KO: K ] [EC: 2. SIK; salt inducible kinase 1B putative [KO: K ] [EC: 2. SIK1; salt inducible kinase 1 [KO: K ] [EC: 2. PLCB1; phospholipase C beta 1 [KO: K ] [EC: 3. PLCB2; phospholipase C beta 2 [KO: K ] [EC: 3. PLCB3; phospholipase C beta 3 [KO: K ] [EC: 3. PLCB4; phospholipase C beta 4 [KO: K ] [EC: 3. PPP3CA; protein phosphatase 3 catalytic subunit alpha [KO: K ] [EC: 3. PPP3CB; protein phosphatase 3 catalytic subunit beta [KO: K ] [EC: 3. PPP3CC; protein phosphatase 3 catalytic subunit gamma [KO: K ] [EC: 3. PPP3R1; protein phosphatase 3 regulatory subunit B, alpha [KO: K ]. PPP3R2; protein phosphatase 3 regulatory subunit B, beta [KO: K ]. SIRT1; sirtuin 1 [KO: K ] [EC: 2. PRMT1; protein arginine methyltransferase 1 [KO: K ] [EC: 2. G6PC1; glucosephosphatase catalytic subunit 1 [KO: K ] [EC: 3. G6PC2; glucosephosphatase catalytic subunit 2 [KO: K ] [EC: 3. G6PC3; glucosephosphatase catalytic subunit 3 [KO: K ] [EC: 3. PCK1; phosphoenolpyruvate carboxykinase 1 [KO: K ] [EC: 4. PCK2; phosphoenolpyruvate carboxykinase 2, mitochondrial [KO: K ] [EC: 4. CPT1A; carnitine palmitoyltransferase 1A [KO: K ] [EC: 2. CPT1B; carnitine palmitoyltransferase 1B [KO: K ] [EC: 2. CPT1C; carnitine palmitoyltransferase 1C [KO: K ] [EC: 2. GYS2; glycogen synthase 2 [KO: K ] [EC: 2. GYS1; glycogen synthase 1 [KO: K ] [EC: 2. PHKG1; phosphorylase kinase catalytic subunit gamma 1 [KO: K ] [EC: 2. PHKG2; phosphorylase kinase catalytic subunit gamma 2 [KO: K ] [EC: 2. PYGL; glycogen phosphorylase L [KO: K ] [EC: 2. PYGM; glycogen phosphorylase, muscle associated [KO: K ] [EC: 2. PYGB; glycogen phosphorylase B [KO: K ] [EC: 2. PRKAA1; protein kinase AMP-activated catalytic subunit alpha 1 [KO: K ] [EC: 2. Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs. Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose. After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice. Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes. Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance. The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males. This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists. The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect. Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes. Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals. It was found that glucagon receptor mRNA expression decreased and plasma glucagon concentration increased significantly after treatment. Glucose tolerance improved, and triglycerides and free fatty acids decreased significantly. Post-receptor regulation: there are still few intervention studies on glucagon receptors, but there are still some reports on G-protein coupled receptor alpha knockout animals. G protein-coupled receptors are present in multiple organs throughout the body. The glucagon receptor is mainly in the liver. The use of liver-specific G protein knockout animals is a method of interfering with the glucagon signaling pathway. Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. More and more studies have shown that the decrease of the secretion of glucagon by inhibiting alpha-cell production, neutralizing blood circulation and hyperglycemia, changing glucagon receptor gene expression, and other methods to interfere signaling pathways may be new treatments for diabetes. Recent studies have shown that obese patients have both dysfunction of islet β-cells and α-cells, impaired insulin secretion and excessive secretion of glucagon, which aggravates the disorder of blood glucose metabolism, so the glucagon signaling pathway regulated for obese patients treatment is especially important. In the past two years, the levels of insulin and glucagon in the patients with coronary heart disease were significantly higher than those in the control group, and the inhibitors of the glucagon signaling pathway were improved, so the glucogon signaling pathway was involved in coronary heart disease. But the detail has to be further studied. Inquiry Basket. The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis. As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis. Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time. In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane. SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs. Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced. Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D. Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis. Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated. The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required. In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5. Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4. Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known. So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function? It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia. Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation. The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment. The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes. SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival. Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist. Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies. Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. |
| Glucagon receptor | As mentioned above, when the alpha cell insulin is resistant, its signal transduction pathway is impaired. Exploring its mechanisms may be related to the mediation of inflammatory mediators. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway. Ellingsgaard et al found that IL-7 receptors were expressed on islet α cells compared with other tissues. IL6 induced the expression and secretion of glucagon in rats with high-fat diet. After using the IL6 receptor gene knockout model, the body's metabolic disorder was corrected. The use of thiazolidinediones TZD drugs can not only improve peripheral insulin resistance in SD rats induced by high-fat feeding, but also inhibit the proliferation of α cells, and and significantly increase glucagon levels and α-cell glucagon mRNA expression. This effect is achieved by the binding of TZDs to the peroxisome proliferator-activated receptor on islet alpha cells, which directly inhibits glucagon gene transcription. In recent years, there are many studies on the treatment of diabetes with incretin hormone, which is represented by glucagon like peptide1 GLP1 and its analogs. GLP1 is a 30 amino acid peptide hormone secreted mainly by L cells of the distal ileum, rectum and colon. It not only acts on glucose-dependent β-cells, but also promotes insulin secretion. It also acts on islet α cells. Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs. Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose. After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice. Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes. Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance. The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males. This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists. The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect. Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes. Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. Modeling the Pancreatic α-Cell: Dual Mechanisms of Glucose Suppression of Glucagon Secretion. Biophys J — PloS One 7:e Elliott AD, Ustione A, Piston DW. Somatostatin and Insulin Mediate Glucose-Inhibited Glucagon Secretion in the Pancreatic α-Cell by Lowering cAMP. Am J Physiol Endocrinol Metab E— Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A. Glucose Controls Glucagon Secretion by Directly Modulating cAMP in Alpha Cells. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of Islet Glucagon Secretion: Beyond Calcium. Diabetes Obes Metab — Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-Activated Protein Kinase Regulates Glucagon Secretion From Mouse Pancreatic Alpha Cells. Da Silva Xavier G, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, et al. Per-Arnt-Sim PAS Domain-Containing Protein Kinase is Downregulated in Human Islets in Type 2 Diabetes and Regulates Glucagon Secretion. Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al. LKB1 and Ampkα1 are Required in Pancreatic Alpha Cells for the Normal Regulation of Glucagon Secretion and Responses to Hypoglycemia. Mol Metab — Bozadjieva N, Blandino-Rosano M, Chase J, Dai XQ, Cummings K, Gimeno J, et al. Loss of Mtorc1 Signaling Alters Pancreatic α Cell Mass and Impairs Glucagon Secretion. Kramer NB, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Gromada J, Franklin I, Wollheim CB. Alpha-Cells of the Endocrine Pancreas: 35 Years of Research But the Enigma Remains. Kawamori D, Kulkarni RN. Insulin Modulation of Glucagon Secretion: The Role of Insulin and Other Factors in the Regulation of Glucagon Secretion. Islets —9. Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato KI, et al. Possible Role of α-Cell Insulin Resistance in Exaggerated Glucagon Responses to Arginine in Type 2 Diabetes. Diabetes Care —7. Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells. Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by?? Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG SP. Glucose-Inhibition of Glucagon Secretion Involves Activation of GABAA-Receptor Chloride Channels. Nature —6. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-Islet Insulin Suppresses Glucagon Release via GABA-GABAA Receptor System. Feng AL, Xiang Y, Gui L, Kaltsidis G, Feng Q, Lu W. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Jin Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Weir GC, Bonner-Weir S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell —9. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, et al. Artemether Does Not Turn α Cells Into β Cells. Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Combined Effect of GABA and Glucagon-Like Peptide-1 Receptor Agonist on Cytokine-Induced Apoptosis in Pancreatic β-Cell Line and Isolated Human Islets. J Diabetes — Daems C, Welsch S, Boughaleb H, Vanderroost J, Robert A, Sokal E, et al. Early Treatment With Empagliflozin and GABA Improves β -Cell Mass and Glucose Tolerance in Streptozotocin-Treated Mice. J Diabetes Res — Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion From Alpha Cells. Cell Rep — Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered Serotonin 5-HT 1D and 2A Receptor Expression May Contribute to Defective Insulin and Glucagon Secretion in Human Type 2 Diabetes. Peptides — Yip L, Taylor C, Whiting CC, Fathman CG. Diminished Adenosine A1 Receptor Expression in Pancreatic a-Cells May Contribute to the Patholog Y of Type 1 Diabetes. Ishihara H, Wollheim CB. Is Zinc an Intra-Islet Regulator of Glucagon Secretion? Diabetol Int — Franklin I, Gromada J, Gjinovci A, Theander S. Beta Cell Secretory Products Activate Alpha Cell ATP-Dependent Potassium Channels to Inhibit Glucagon Release. Ravier MA, Rutter GA. Glucose or Insulin, But Not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic [Alpha]-Cells. Solomou A, Philippe E, Chabosseau P, Migrenne-li S, Gaitan J, Lang J, et al. Nutr Metab Lond Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin Inhibits Insulin and Glucagon Secretion via Two Receptor Subtypes : An In Vitro Study of Pancreatic Islets From Somatostatin Receptor 2 Knockout Mice. Endocrinology —7. Gromada J, Hoy M, Bushcard K, Salehi A, Rorsman P. Somatostatin Inhibits Exocytosis in Rat Pancreatic a-Cells by Gi2-Dependent Activation of Calcineurin and Depriming of Secretory Granules. Rutter GA. Regulating Glucagon Secretion: Somatostatin in the Spotlight. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the Rat Pancreas, Somatostatin Tonically Inhibits Glucagon Secretion and Is Required for Glucose-Induced Inhibition of Glucagon Secretion. Acta Physiol — Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, et al. Somatostatin Secreted by Islet -Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, et al. Insulin Inhibits Glucagon Release by SGLT2-Induced Stimulation of Somatostatin Secretion. Nat Commun Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. Δ-Cells and β-Cells Are Electrically Coupled and Regulate α-Cell Activity Via Somatostatin. Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients With Type 2 Diabetes. PloS One 6:e Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, et al. Glucagon Stimulates Exocytosis in Mouse and Rat Pancreatic α-Cells by Binding to Glucagon Receptors. Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, et al. Glucagon Regulates its Own Synthesis by Autocrine Signaling. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon Regulates Blood Glucose by Increasing Insulin Secretion and Hepatic Glucose Production. Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, et al. Preserved Inhibitory Potency of GLP-1 on Glucagon Secretion in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab — Garg M, Ghanim H, Kuhadiya N, Green K, Hejna J, Abuaysheh S, et al. Liraglutide Acutely Suppresses Glucagon, Lipolysis and Ketogenesis in Type 1 Diabetes. Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim K-S, et al. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Habener JF, Stanojevic V. Pancreas and Not Gut Mediates the GLPInduced Glucoincretin Effect. Cell Metab —8. Fava GE, Dong EW, Wu H. Intra-Islet Glucagon-Like Peptide J Diabetes Complicat —8. Holst J, Christensen M, Lund A, De Heer J, Svendsen B, Kielgast U, et al. Regulation of Glucagon Secretion by Incretins. Ramracheya R, Chapman C, Chibalina M, Dou H, Miranda C, González A, et al. Physiol Rep — Boss M, Bos D, Frielink C, Sandker G, Ekim S, Marciniak C, et al. Targeted Optical Imaging of the Glucagon-Like Peptide 1 Receptor Using Exendin-4irdyecw. J Nucl Med — Roberts S, Khera E, Choi C, Navaratna T, Grimm J, Thurber GM, et al. Optoacoustic Imaging of Glucagon-Like Peptide 1 Receptor With a Near-Infrared Exendin-4 Analog. Azad BB, Rota V. Design, Synthesis and In Vitro Characterization of Glucagon-Like Peptide-1 Derivatives for Pancreatic Beta Cell Imaging by SPECT. Bioorg Med Chem — Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, et al. Super-Resolution Microscopy Compatible Fluorescent Probes Reveal Endogenous Glucagon-Like Peptide-1 Receptor Distribution and Dynamics. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol — de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-Like Peptide-1, But Not Glucose-Dependent Insulinotropic Peptide, Inhibits Glucagon Secretion via Somatostatin Receptor Subtype 2 in the Perfused Rat Pancreas. Ørgaard A, Holst JJ. The Role of Somatostatin in GLPInduced Inhibition of Glucagon Secretion in Mice. Diabetologia —9. Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, et al. The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release. Lawlor N, Youn A, Kursawe R, Ucar D, Stitzel ML. Alpha TC1 and Beta-TC-6 Genomic Profiling Uncovers Both Shared and Distinct Transcriptional Regulatory Features With Their Primary Islet Counterparts. Sci Rep — Stamenkovic JA, Andersson LE, Adriaenssens AE, Bagge A, Sharoyko VV, Gribble F, et al. Inhibition of the Malate-Aspartate Shuttle in Mouse Pancreatic Islets Abolishes Glucagon Secretion Without Affecting Insulin Secretion. Briant LJB, Zhang Q, Vergari E, Kellard JA, Rodriguez B, Ashcroft FM, et al. Functional Identification of Islet Cell Types by Electrophysiological Fingerprinting. J R Soc Interface Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-Fidelity Glucagon-CreER Mouse Line Generated by CRISPR-Cas9 Assisted Gene Targeting. Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP Mouse: A New Model for Easy Identification of Living Pancreatic Alpha-Cells. FEBS Lett — Shiota C, Prasadan K, Guo P, Fusco J, Xiao X, Gittes GK. GcgCreERT2knockin Mice as a Tool for Genetic Manipulation in Pancreatic Alpha Cells. Andersson SA, Pedersen MG, Vikman J, Eliasson L. Glucose-Dependent Docking and SNARE Protein-Mediated Exocytosis in Mouse Pancreatic Alpha-Cell. Pflugers Arch Eur J Physiol — González-Vélez V, Dupont G, Gil A, González A, Quesada I. Model for Glucagon Secretion by Pancreatic α-Cells. Gerber SH, Sudhof TC. Molecular Determinants of Regulated Exocytosis. Gustavsson N, Wei S, Hoang DN, Lao Y, Zhang Q, Radda GK, et al. Jewell JL, Oh E, Thurmond DC. Exocytosis Mechanisms Underlying Insulin Release and Glucose Uptake : Conserved Roles for Munc18c and Syntaxin 4. Am J Physiol Regul Integr Comp Physiol R— Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund P, et al. Xia F, Leung YM, Gaisano G, Gao X, Chen Y, Fox JEM, et al. Montefusco F, Pedersen MG. Mathematical Modelling of Local Calcium and Regulated Exocytosis During Inhibition and Stimulation of Glucagon Secretion From Pancreatic Alpha-Cells. Yokawa S, Suzuki T, Inouye S, Inoh Y, Suzuki R, Kanamori T, et al. Visualization of Glucagon Secretion From Pancreatic α Cells by Bioluminescence Video Microscopy: Identification of Secretion Sites in the Intercellular Contact Regions. Biochem Biophys Res Commun — Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, et al. A service of the National Library of Medicine, National Institutes of Health. Feingold KR, Anawalt B, Blackman MR, et al. Endotext [Internet]. South Dartmouth MA : MDText. com, Inc. Iben Rix , Christina Nexøe-Larsen , Natasha C Bergmann , Asger Lund , and Filip K Knop. Glucagon is a peptide hormone secreted from the alpha cells of the pancreatic islets of Langerhans. Hypoglycemia is physiologically the most potent secretory stimulus and the best known action of glucagon is to stimulate glucose production in the liver and thereby to maintain adequate plasma glucose concentrations. However, glucagon is also involved in hepatic lipid and amino acid metabolism and may increase resting energy expenditure. Based on satiety-inducing and food intake-lowering effects of exogenous glucagon, a role for glucagon in the regulation of appetite has also been proposed. This chapter provides an overview of the structure, secretion, degradation and elimination of glucagon, and reviews the actions of glucagon including its role in glucose metabolism and its effects on lipolysis, ketogenesis, energy expenditure, appetite and food intake. Finally, the role of glucagon in the pathophysiology of diabetes, obesity and hepatic steatosis is discussed and emerging glucagon-based therapies for these conditions are outlined. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW. Glucagon secreted from pancreatic alpha cells in the islet of Langerhans plays an important role in maintaining glucose homeostasis by stimulating hepatic glucose production 1. Thus, in contrast to the glucose-depositing nature of insulin action, glucagon acts as a glucose-mobilizing hormone. In line with these opposed actions, high plasma glucose concentrations stimulating insulin secretion from pancreatic beta cells, inhibit glucagon secretion whereas low plasma glucose concentrations represent one of the most potent glucagon secretory stimuli. Accordingly, normal plasma glucose concentrations depend largely on the balanced secretion of insulin and glucagon from the pancreatic beta cells and alpha cells, respectively. In the s glucagon was purified and crystallized at Eli Lilly and Co. This led to the development of medical use of glucagon for the treatment of severe insulin-induced hypoglycemia 4 , 5. The development of a radioimmunoassay for the detection of glucagon in spurred further investigations of glucagon physiology and its role in health and disease 6. Since then it has become evident that glucagon not only acts by increasing hepatic glucose production but affects overall energy homeostasis in times of limited energy supply by stimulating lipid and protein catabolism, reducing appetite and food intake and increasing energy expenditure. Glucagon is a amino acid peptide hormone predominantly secreted from the alpha cells of the pancreas. It is derived from the precursor proglucagon which can be processed into a number of related peptide hormones Fig. Proglucagon is expressed in pancreatic islet alpha cells, intestinal enteroendocrine L cells, and to a minor extent in neurons in the brain stem and hypothalamus 8 , 9. In the pancreas, PC2 processes proglucagon to glucagon while processing of proglucagon in the intestine and the brain is undertaken by PC1 leading to the formation of glucagon-like peptide 1 GLP-1 and glucagon-like peptide 2 GLP-2 9. Tissue specific processing of proglucagon. In the pancreas proglucagon is processed into glucagon, glicentin-related pancreatic polypeptide GRPP , intervening peptide 1 IP1 , and major proglucagon fragment MPGF by the processing enzyme prohormone convertase 2 PC2. Glucagon is secreted in response to hypoglycemia, prolonged fasting, exercise and protein-rich meals Glucagon release is regulated through endocrine and paracrine pathways; by nutritional substances; and by the autonomic nervous system Glucagon secretion occurs as exocytosis of stored peptide vesicles initiated by secretory stimuli of the alpha cell. Stimulatory regulators of glucagon release include hypoglycemia, amino acids and the gut hormone glucose-dependent insulinotropic peptide GIP , whereas hyperglycemia and GLP-1 inhibit glucagon release. Additionally, glucagon release is inhibited in a paracrine fashion by factors like somatostatin, insulin, zinc and possibly amylin. Glucagon may regulate its own secretion indirectly via stimulatory effect on beta cells to secrete insulin 12 , In contrast to glucose, non-glucose regulators of glucagon secretion seem to mediate their action through changes in cAMP levels rather than through the calcium-dependent pathway outlined below 14 , The most potent regulator of glucagon secretion is circulating glucose. Hypoglycemia stimulates the pancreatic alpha cell to release glucagon and hyperglycemia inhibits glucagon secretion Fig. The cellular mechanism behind this glucose-dependent regulation of glucagon secretion involves uptake of glucose by the glucose transporter 1 GLUT1 in the cell membrane of pancreatic alpha cells and subsequent glycolysis which ultimately generates adenosine triphosphate ATP in the mitochondria of the alpha cell. Thus, the intracellular ATP level in the alpha cell reflects plasma glucose levels. Conversely, increasing circulating glucose levels increase glucose influx to the alpha cell generating an increase in intracellular ATP concentration, which opens K ATP -channels. Glucose-dependent glucagon secretion from the alpha cell. During hypoglycemia intracellular glucose concentration falls with a subsequent reduction in glycolysis-generated adenosine triphosphate ATP in the mitochondria of the cell. depolarization of the cell membrane. In normal physiology, circulating glucagon concentrations are in the picomolar range. Basal glucagon secretion balances the effect of basal insulin secretion resulting in a steady-state between glucose uptake and endogenous glucose production in the fasted state; i. stable blood glucose concentrations. During exercise or in case of hypoglycemia, circulating glucagon levels may increase dramatically to times basal levels increasing the glucagon to insulin ratio 12 , 19 , 20 Fig. The effects of glucagon are mediated through binding to and activation of the glucagon receptor. The glucagon receptor is a seven transmembrane G protein-coupled receptor Fig. The main mode of intracellular signaling involves activation of G s and G q. G s activation stimulates adenylyl cyclase which produces cyclic adenosine monophosphate cAMP that activates protein kinase A PKA. The activated PKA migrates to the nucleus and activates transcription factors like cAMP response element-binding protein CREB through phosphorylation. This enables CREB to bind to response elements of target genes resulting in the recruitment of coactivators and ultimately promoting gene expression. Activation of G q by glucagon leads to activation of phospholipase C PLC and subsequent increase in inositol 1,4,5-triphosphate IP 3 , which signals to enhance release of calcium from the endoplasmic reticulum. This, in turn, activates downstream signaling cascades including CREB-regulated transcription co-activator CRTC2 which enhance CREB-dependent gene expression. In addition to the CREB-CRTC2 pathway, glucagon may signal through various other pathways reviewed in detail elsewhere 1 , 12 , Examples of the two most well-described intracellular pathways involved in glucagon-induced regulation of target gene expression: the PKA and the IP 3 pathways. AC, adenylyl cyclase; CRTC2, CREB-regulated transcription co-activator; CREB, cAMP response element-binding protein; IP 3 , inositol 1,4,5-triphosphate; PIP 2 , phosphatidyl-inositol-4,5-bisphosphate; PKA, protein kinase A; PLC, phospholipase C. The degradation of glucagon is mainly facilitated by receptor-mediated endocytosis and proteolysis by the ubiquitous enzyme dipeptidyl peptidase 4 22 , Consistent with the relative receptor expression, the liver and kidneys seem to represent the two main organs removing glucagon from the circulation. The circulating half-life of glucagon in plasma is reported to be between four to seven minutes in humans 24 , Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Specifically, glucagon promotes hepatic conversion of glycogen to glucose glycogenolysis , stimulates de novo glucose synthesis gluconeogenesis , and inhibits glucose breakdown glycolysis and glycogen formation glycogenesis Fig. Hepatic glucose production is rapidly enhanced in response to a physiological rise in glucagon; achieved through stimulation of glycogenolysis with minor acute changes in gluconeogenesis 27 , This ability of glucagon is critical in the life-saving counterregulatory response to severe hypoglycemia. Additionally, it is a key factor in providing adequate circulating glucose for brain function and for working muscle during exercise During prolonged fasting, glycogen stores are depleted, and gluconeogenesis takes over The hyperglycemic property of glucagon is enhanced when hepatic glycogen levels are high and diminished when hepatic glycogen levels are low in conditions of fasting or liver diseases like cirrhosis Regulation of glucose metabolism by glucagon in the liver. Glucagon increases hepatic glucose production by stimulating glycogenolysis and glycogenogenesis green arrows while inhibiting glycolysis and glycogenesis red arrows. Glucagon promotes formation of non-carbohydrate energy sources in the form of lipids and ketone bodies. Thereby, glucagon contributes to a stable energy homeostasis during conditions where energy supply is limited fasting or in states of increased energy demand e. exercise or cold exposure Specifically, in times of energy demand, glucagon enhances break-down of fatty acids to acetyl-coenzyme A molecules beta-oxidation in the liver. These intermediates are either reduced to generate ATP in the tricarboxylic acid cycle or converted to ketone bodies ketogenesis — a process also stimulated by glucagon. Furthermore, glucagon signaling inhibits de novo lipogenesis by inactivating the enzyme that catalyzes the first step in fatty acid synthesis from other substrates like carbohydrates During prolonged fasting, glucagon stimulates formation of glucose from amino acids via gluconeogenesis by upregulating enzymes involved in the process. However, the rate-limiting step of the process depends on the supply of gluconeogenic amino acids from muscle or dietary intake, a process not controlled by glucagon In addition to enter gluconeogenesis, amino acids are deaminated to generate ATP in the liver. Glucagon is involved in this process by promoting the conversion of ammonia — a toxic biproduct from deamination — to urea, which is excreted in the urine. Thereby glucagon reduces ammonia levels in the blood Disruption of glucagon action by inhibition of the glucagon receptor 37 leads to increased plasma levels of amino acids and pancreatic alpha cell hyperplasia, which in turn, leads to glucagon hypersecretion. This suggests that glucagon and amino acids are linked in a feedback loop between the liver and the pancreatic alpha cells Acute administration of glucagon has been shown to reduce food intake and diminish hunger 38 , Conversely, preprandial inhibition of glucagon signaling increases food intake in rats 40 , 41 providing evidence for a role of glucagon in the regulation of appetite. It is somewhat counterintuitive that glucagon should reduce food intake given that glucagon levels are typically elevated upon fasting and decrease upon feeding. Thus, the observed effect upon glucagon administration in supraphysiological concentrations could partly be due to cross-reactivity with the GLP-1 receptor which normally result in suppression of food intake In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure. In humans, this effect has been observed in studies in which glucagon infusion resulted in increases in resting energy expenditure 42 — However, the effect of endogenous glucagon on resting energy expenditure remains unclear. Also, the exact mechanisms behind the increase in resting energy expenditure elicited by exogenous glucagon remain to be determined. It has been speculated that glucagon activates brown adipose tissue 12 , however this was recently challenged in an in vivo study that found no direct effect of glucagon on brown adipose tissue Rodent studies indicate that the actions of glucagon to increase energy expenditure might be indirectly mediated partly by fibroblast growth factor 21 FGF21 as glucagon-induced increase in energy expenditure is abolished in animals with FGF21 receptor deletion Infusion of high doses of glucagon increases heart rate and cardiac contractility In fact, infusion of glucagon in pharmacological doses milligram is often used in the treatment of acute cardiac depression caused by calcium channel antagonist or beta-blocker overdoses 47 despite limited evidence In comparison, glucagon concentrations within the normal physiological range do not appear to affect heart rate or contractility 49 and any physiological role of endogenous glucagon in the regulation of pulse rate remains questionable. This is supported by studies investigating the effect of glucagon receptor antagonist for the treatment of type 2 diabetes in which no effect of pulse rate were observed Nevertheless, whether increased glucagon concentrations have a sustained effect on the heart remains unknow. Of note, most studies use bolus injections of glucagon which cause only a transient increase in heart rate and contractility potentially reflecting the rapid elimination of glucagon from circulation Taken together, it remains uncertain whether glucagon has a place in the treatment of heart failure or hold a cardioprotective effect in healthy subjects. Patients with type 2 diabetes exhibit an impaired regulation of glucagon secretion which contributes importantly to diabetic hyperglycemia. Specifically, type 2 diabetes is characterized by elevated levels of glucagon during fasting while suppression of glucagon in response to oral intake of glucose is impaired or even paradoxically elevated Fig. The mechanisms behind hyperglucagonemia are not fully understood but is usually explained by a diminished suppressive effect of insulin on alpha cells due to hypoinsulinemia and insulin resistance at the level of the alpha cells 53 , Interestingly, subjects with type 2 diabetes, who exhibit a hyperglucagonemic response to oral glucose, respond with a normal suppression of glucagon after intravenous glucose administration Accordingly, hormones secreted from the gastrointestinal tract may play an important role 55 , It has recently been confirmed that glucagon can be secreted from extrapancreatic tissue demonstrated in experiments with totally pancreatectomized subjects This supports the notion that postprandial hypersecretion of glucagon in patients with type 2 diabetes might be of extrapancreatic origin. Schematic illustration of plasma glucagon concentrations in patients with type 2 diabetes and in normal physiology healthy subjects. Type 2 diabetes is characterized by elevated fasting plasma glucagon levels and impaired suppression of plasma glucagon levels in response to oral glucose. Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. However, emerging evidence indicate that glucagon plays a major role in type 1 diabetes pathophysiology. The glucagon dyssecretion that characterizes patients with type 1 diabetes is associated with two clinical manifestations: Postprandial hyperglucagonemia and impaired glucagon counterregulation to hypoglycemia Data regarding fasting plasma glucagon concentrations in type 1 diabetes are inconsistent 57 , Thus, the general notion that glucagon hypersecretion plays a role in type 1 diabetes hyperglycemia is mainly based on elevated postprandial glucagon concentrations The explanation behind this is unclear, although a common explanation is, that in type 1 diabetes the postprandial increase in plasma glucose is not followed by an increase in insulin secretion from beta cells, which in normal physiology would inhibit glucagon secretion. The absence of that restraining signal from endogenous insulin could result in an increase in glucagon secretion from alpha cells after a meal Fig. However, like in type 2 diabetes, subjects with type 1 diabetes preserve their ability to suppress glucagon after intravenous glucose administration. Schematic illustration of plasma glucagon concentrations in patients with type 1 diabetes and in normal physiology healthy subjects. Type 1 diabetes is characterized by elevated concentrations of glucagon in response to a meal or oral glucose intake. Hypoglycemia is a frequent and feared side effect of insulin therapy in type 1 diabetes and it represents a common barrier in obtaining glycemic control In normal physiology hypoglycemia is prevented by several mechanisms: 1 Reduced insulin secretion from beta cells diminishing glucose uptake in peripheral tissues; 2 increased glucagon secretion from alpha cells increasing hepatic glucose output; and 3 increased symphathetic neural response and adrenomedullary epinephrine secretion. The latter will stimulate hepatic glucose production and cause clinical symptoms that enables the individual to recognize hypoglycemia and ultimately ingest carbohydrates 57 , 61 , In type 1 diabetes, insulin-induced hypoglycemia fails to elicit adequate glucagon responses compromising counterregulation to insulin-induced hypoglycemia; a phenomenon which seems to worsen with the duration of type 1 diabetes. This defect likely involves a combination of defective alpha cells and reduced alpha cell mass 57 , |
Glucagon hormone pathway -
Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training.
Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise.
High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition.
Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia.
Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents.
Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation.
Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration.
Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets.
Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet.
Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation.
Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis.
Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett.
Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Gerich, J.
Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin.
Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl.
Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis.
Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels.
Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Gross, R.
Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice.
Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes.
Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell.
Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes.
Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators.
Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism.
Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies.
Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy.
Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H.
Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome.
Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Effect of insulin on glucagon enhanced lipolysis in vitro.
Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y.
Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans.
Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids.
Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat.
Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon.
Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C.
Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M.
Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses.
Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver.
Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial.
Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G.
Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds.
B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit.
Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women.
Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism.
Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells. The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline.
Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Sadry, S.
Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M. Diabetologia 60, — Schade, D. Modulation of fatty acid metabolism by glucagon in man.
Effects in normal subjects. Diabetes 24, — Schneider, S. The acute metabolic effects of glucagon and its interactions with insulin in forearm tissue.
Diabetologia 20, — Schweiger, M. Measurement of lipolysis. Methods Enzymol. Shen, W. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. Slavin, B.
Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. Sloop, K. Hepatic and glucagon-like peptidemediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors.
Sloth, B. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period.
Solloway, M. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH.
Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al.
Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival.
Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist.
Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies.
Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists.
J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice.
Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice.
Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al.
Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al.
The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption.
J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells.
Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia.
Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL.
FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor.
J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice.
Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T.
Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al.
Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al.
Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon.
Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al.
Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al.
Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats.
McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al.
Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells.
Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ.
Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs.
Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations.
Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S.
Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S.
Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets.
Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets.
Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes.
Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. Modeling the Pancreatic α-Cell: Dual Mechanisms of Glucose Suppression of Glucagon Secretion.
Biophys J — PloS One 7:e Elliott AD, Ustione A, Piston DW. Somatostatin and Insulin Mediate Glucose-Inhibited Glucagon Secretion in the Pancreatic α-Cell by Lowering cAMP. Am J Physiol Endocrinol Metab E— Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A.
Glucose Controls Glucagon Secretion by Directly Modulating cAMP in Alpha Cells. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of Islet Glucagon Secretion: Beyond Calcium. Diabetes Obes Metab — Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA.
AMP-Activated Protein Kinase Regulates Glucagon Secretion From Mouse Pancreatic Alpha Cells. Da Silva Xavier G, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, et al.
Per-Arnt-Sim PAS Domain-Containing Protein Kinase is Downregulated in Human Islets in Type 2 Diabetes and Regulates Glucagon Secretion. Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al.
LKB1 and Ampkα1 are Required in Pancreatic Alpha Cells for the Normal Regulation of Glucagon Secretion and Responses to Hypoglycemia.
Mol Metab — Bozadjieva N, Blandino-Rosano M, Chase J, Dai XQ, Cummings K, Gimeno J, et al. Loss of Mtorc1 Signaling Alters Pancreatic α Cell Mass and Impairs Glucagon Secretion.
Kramer NB, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Gromada J, Franklin I, Wollheim CB. Alpha-Cells of the Endocrine Pancreas: 35 Years of Research But the Enigma Remains.
Kawamori D, Kulkarni RN. Insulin Modulation of Glucagon Secretion: The Role of Insulin and Other Factors in the Regulation of Glucagon Secretion.
Islets —9. Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato KI, et al. Possible Role of α-Cell Insulin Resistance in Exaggerated Glucagon Responses to Arginine in Type 2 Diabetes.
Diabetes Care —7. Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells.
Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by?? Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG SP. Glucose-Inhibition of Glucagon Secretion Involves Activation of GABAA-Receptor Chloride Channels.
Nature —6. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-Islet Insulin Suppresses Glucagon Release via GABA-GABAA Receptor System.
Feng AL, Xiang Y, Gui L, Kaltsidis G, Feng Q, Lu W. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Jin Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity.
Weir GC, Bonner-Weir S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell —9. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, et al.
Artemether Does Not Turn α Cells Into β Cells. Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Combined Effect of GABA and Glucagon-Like Peptide-1 Receptor Agonist on Cytokine-Induced Apoptosis in Pancreatic β-Cell Line and Isolated Human Islets.
J Diabetes — Daems C, Welsch S, Boughaleb H, Vanderroost J, Robert A, Sokal E, et al. Early Treatment With Empagliflozin and GABA Improves β -Cell Mass and Glucose Tolerance in Streptozotocin-Treated Mice.
J Diabetes Res — Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion From Alpha Cells. Cell Rep — Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered Serotonin 5-HT 1D and 2A Receptor Expression May Contribute to Defective Insulin and Glucagon Secretion in Human Type 2 Diabetes.
Peptides — Yip L, Taylor C, Whiting CC, Fathman CG. Diminished Adenosine A1 Receptor Expression in Pancreatic a-Cells May Contribute to the Patholog Y of Type 1 Diabetes. Ishihara H, Wollheim CB. Is Zinc an Intra-Islet Regulator of Glucagon Secretion? Diabetol Int — Franklin I, Gromada J, Gjinovci A, Theander S.
Beta Cell Secretory Products Activate Alpha Cell ATP-Dependent Potassium Channels to Inhibit Glucagon Release. Ravier MA, Rutter GA. Glucose or Insulin, But Not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic [Alpha]-Cells.
Solomou A, Philippe E, Chabosseau P, Migrenne-li S, Gaitan J, Lang J, et al. Nutr Metab Lond Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin Inhibits Insulin and Glucagon Secretion via Two Receptor Subtypes : An In Vitro Study of Pancreatic Islets From Somatostatin Receptor 2 Knockout Mice.
Endocrinology —7. Gromada J, Hoy M, Bushcard K, Salehi A, Rorsman P. Somatostatin Inhibits Exocytosis in Rat Pancreatic a-Cells by Gi2-Dependent Activation of Calcineurin and Depriming of Secretory Granules.
Rutter GA. Regulating Glucagon Secretion: Somatostatin in the Spotlight. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the Rat Pancreas, Somatostatin Tonically Inhibits Glucagon Secretion and Is Required for Glucose-Induced Inhibition of Glucagon Secretion.
Acta Physiol — Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, et al. Somatostatin Secreted by Islet -Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, et al.
Insulin Inhibits Glucagon Release by SGLT2-Induced Stimulation of Somatostatin Secretion. Nat Commun Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. Δ-Cells and β-Cells Are Electrically Coupled and Regulate α-Cell Activity Via Somatostatin.
Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients With Type 2 Diabetes. PloS One 6:e Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, et al.
Glucagon Stimulates Exocytosis in Mouse and Rat Pancreatic α-Cells by Binding to Glucagon Receptors. Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, et al.
Glucagon Regulates its Own Synthesis by Autocrine Signaling. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon Regulates Blood Glucose by Increasing Insulin Secretion and Hepatic Glucose Production. Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, et al.
Preserved Inhibitory Potency of GLP-1 on Glucagon Secretion in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab — Garg M, Ghanim H, Kuhadiya N, Green K, Hejna J, Abuaysheh S, et al. Liraglutide Acutely Suppresses Glucagon, Lipolysis and Ketogenesis in Type 1 Diabetes. Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim K-S, et al.
The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Habener JF, Stanojevic V. Pancreas and Not Gut Mediates the GLPInduced Glucoincretin Effect.
Cell Metab —8. Fava GE, Dong EW, Wu H. Intra-Islet Glucagon-Like Peptide J Diabetes Complicat —8. Holst J, Christensen M, Lund A, De Heer J, Svendsen B, Kielgast U, et al.
Regulation of Glucagon Secretion by Incretins. Ramracheya R, Chapman C, Chibalina M, Dou H, Miranda C, González A, et al. Physiol Rep — Boss M, Bos D, Frielink C, Sandker G, Ekim S, Marciniak C, et al.
Targeted Optical Imaging of the Glucagon-Like Peptide 1 Receptor Using Exendin-4irdyecw. J Nucl Med — Roberts S, Khera E, Choi C, Navaratna T, Grimm J, Thurber GM, et al.
Optoacoustic Imaging of Glucagon-Like Peptide 1 Receptor With a Near-Infrared Exendin-4 Analog. Azad BB, Rota V. Design, Synthesis and In Vitro Characterization of Glucagon-Like Peptide-1 Derivatives for Pancreatic Beta Cell Imaging by SPECT.
Bioorg Med Chem — Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, et al. Super-Resolution Microscopy Compatible Fluorescent Probes Reveal Endogenous Glucagon-Like Peptide-1 Receptor Distribution and Dynamics.
Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol — de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-Like Peptide-1, But Not Glucose-Dependent Insulinotropic Peptide, Inhibits Glucagon Secretion via Somatostatin Receptor Subtype 2 in the Perfused Rat Pancreas.
Ørgaard A, Holst JJ. The Role of Somatostatin in GLPInduced Inhibition of Glucagon Secretion in Mice. Diabetologia —9. Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, et al. The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release.
Lawlor N, Youn A, Kursawe R, Ucar D, Stitzel ML. Alpha TC1 and Beta-TC-6 Genomic Profiling Uncovers Both Shared and Distinct Transcriptional Regulatory Features With Their Primary Islet Counterparts. Sci Rep — Stamenkovic JA, Andersson LE, Adriaenssens AE, Bagge A, Sharoyko VV, Gribble F, et al.
Inhibition of the Malate-Aspartate Shuttle in Mouse Pancreatic Islets Abolishes Glucagon Secretion Without Affecting Insulin Secretion. Briant LJB, Zhang Q, Vergari E, Kellard JA, Rodriguez B, Ashcroft FM, et al. Functional Identification of Islet Cell Types by Electrophysiological Fingerprinting.
J R Soc Interface Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-Fidelity Glucagon-CreER Mouse Line Generated by CRISPR-Cas9 Assisted Gene Targeting.
Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP Mouse: A New Model for Easy Identification of Living Pancreatic Alpha-Cells. FEBS Lett — Shiota C, Prasadan K, Guo P, Fusco J, Xiao X, Gittes GK. GcgCreERT2knockin Mice as a Tool for Genetic Manipulation in Pancreatic Alpha Cells.
Andersson SA, Pedersen MG, Vikman J, Eliasson L. Glucose-Dependent Docking and SNARE Protein-Mediated Exocytosis in Mouse Pancreatic Alpha-Cell. Pflugers Arch Eur J Physiol — González-Vélez V, Dupont G, Gil A, González A, Quesada I.
Model for Glucagon Secretion by Pancreatic α-Cells. Gerber SH, Sudhof TC. Molecular Determinants of Regulated Exocytosis. Gustavsson N, Wei S, Hoang DN, Lao Y, Zhang Q, Radda GK, et al. Jewell JL, Oh E, Thurmond DC. Exocytosis Mechanisms Underlying Insulin Release and Glucose Uptake : Conserved Roles for Munc18c and Syntaxin 4.
Gucagon hsa Quick Reflexes Formula Entry hsa Pathway Name Glucagon Glucagon hormone pathway pathway - Homo sapiens human. Glucagon Glucagkn conventionally pathwxy as a Glucagon hormone pathway hormone for insulin Gucagon plays a critical anti-hypoglycemic role by maintaining glucose homeostasis in both animals and humans. To increase blood glucose, glucagon promotes hepatic glucose output by increasing glycogenolysis and gluconeogenesis and by decreasing glycogenesis and glycolysis in a concerted fashion via multiple mechanisms. Glucagon also stimulates hepatic mitochondrial beta-oxidation to supply energy for glucose production. Glucagon performs its main effect via activation of adenylate cyclase. Organismal Systems; Endocrine system BRITE hierarchy.
die Auswahl bei Ihnen kompliziert
Nach meiner Meinung irren Sie sich. Schreiben Sie mir in PM, wir werden reden.
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
die Auswahl bei Ihnen kompliziert
Was Sie meinen?