Chitosan for heart health -
This is due to the lack of safety information regarding chitosan use in these populations. More information is needed to determine the full safety profile of chitosan supplements.
Dietary supplements are not regulated like prescription medications in the United States. Therefore, some may be safer than others.
When choosing a supplement , consider factors such as third-party testing, potential drug interactions, and other safety concerns. Talk to a healthcare provider or a registered dietitian nutritionist RD or RDN about supplement quality and safety. Currently, there are no dosage guidelines for chitosan supplements.
In clinical trials, chitosan dosing ranged from 0. Chitosan was also commonly used for 12 to 13 weeks in the trials. It's recommended that you follow dosage directions as indicated on the supplement label. You can also obtain dosage recommendations from a healthcare provider. Chitosan may negatively interact with certain medications, supplements, or nutrients.
These interactions may block the absorption or proper use of chitosan or the medications, supplements, or nutrients it is taken with. There is concern that chitosan interacts with medications and supplements that may have similar uses. These medications and supplements include:.
Chitosan may also reduce the absorption of fat-soluble vitamins. However, this was only seen in animal studies. This may occur when chitosan binds to fatty substances in the digestive tract before being absorbed into the bloodstream.
It should be noted that there isn't solid evidence or clear documentation of these or other interactions for chitosan. However, it's best to be cautious and talk with a healthcare provider before using chitosan to discuss potential interactions, especially if you use any medications or supplements.
It is also important to carefully read the ingredients list and nutrition facts panel of a supplement to know which ingredients and how much of each ingredient is included.
Please review supplement labels with a healthcare provider to discuss any potential interactions with foods, other supplements, and medications. Various foods and supplements contain chitosan. Compared to chitosan-containing foods, supplements may be easier to access and appear to be a more popular method for consuming the polysaccharide.
The main food sources of chitosan include crustaceans and certain types of mushrooms. Chitosan may also come from the exoskeleton of insects. In crustaceans and mushrooms, chitosan is found in its original form of chitin recall that chitosan is a derivative of chitin.
Specifically, chitin is a part of the exoskeleton of crustaceans and the cell walls of some mushrooms and other fungi. The only way to consume chitin that comes from crustaceans is through dietary supplements.
This is because the exoskeletons of shrimp, crabs, lobsters, and other crustaceans are not commonly eaten. Chitin has been found in both edible and nonedible mushrooms.
However, an enzymatic reaction has to occur for chitin to be converted to chitosan. Chitosan is considered to be more easily digested than chitin. Supplements tend to be a better option for getting chitosan. Due to their popularity, chitosan supplements are not difficult to find.
There are a number of websites that sell chitosan supplements. You can also find chitosan supplements in certain retail stores, grocery stores, or specialty nutrition shops. Chitosan supplements come in many forms, including capsules, powders, and tablets.
There are also topical chitosan options, like gels. Some chitosan supplements may be mixed with other nutrients, herbs, or ingredients.
Be sure to read the full list of ingredients to understand the product you purchase. Many chitosan supplements are sourced from crustaceans. If you are vegan or vegetarian, look for chitosan that has been sourced from mushrooms instead. Of course, you shouldn't use chitosan products if you're allergic to any of the ingredients.
For example, if you have a shellfish allergy, then you should avoid using chitosan supplements that come from crustaceans. Several chitosan supplements on the market can fit other diets, like a gluten-free diet or an organic diet.
This information should be listed on the label or packaging of the supplement. Chitosan is a derivative of chitin, a polysaccharide present in the exoskeletons of crustaceans, certain insects, and the cell walls of fungi.
Chitosan contains nutrients and bioactive compounds that may be useful for high blood sugar, high blood pressure, wounds, and other conditions. In general, more research is needed on chitosan to prove its benefits.
Side effects are possible when taking chitosan, so talk with a healthcare provider to make sure it's the right supplement choice for you.
Some chitosan products contain common allergens , like shellfish. Other chitosan products are made from mushrooms. Avoid using chitosan if you're allergic to shellfish, mushrooms, or any other substance in the ingredients list.
You should talk with a healthcare provider about using chitosan if you're pregnant or breastfeeding. There isn't much research on the use of chitosan in these populations, so it may be best to avoid it. To get chitosan naturally, you'd need to eat foods that contain chitin.
Chitin-containing foods include mushrooms and crustaceans. However, chitin is only available in the exoskeletons of crustaceans, a part of the animal that isn't typically eaten.
Chitosan is most commonly found in supplements. This is because a chemical reaction is needed to transform chitin from foods into chitosan. When using chitosan supplements, you can take them daily. However, little is known about the safety of using chitosan for more than 12 weeks. Therefore, it might not be safe to take chitosan for more than 12 weeks.
Aranaz I, Alcántara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers Basel. Moraru C, Mincea MM, Frandes M, Timar B, Ostafe V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure.
Medicina Kaunas. de Queiroz Antonino RSCM, Lia Fook BRP, de Oliveira Lima VA, et al. Preparation and characterization of chitosan obtained from shells of shrimp Litopenaeus vannamei Boone. Mar Drugs. Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: an update on potential biomedical and pharmaceutical applications.
Ahn SI, Cho S, Choi NJ. Effectiveness of chitosan as a dietary supplement in lowering cholesterol in murine models: a meta-analysis. The addition of GO-Au at these concentrations 0.
Physico-chemical characterization of the biocomposite scaffolds: A — D Scanning electron microscopy SEM images of the biocomposite scaffolds. E Pore diameter of chitosan scaffolds at different concentrations of GO-Au 0. F FT-IR spectrum analysis of chitosan and scaffolds at different concentrations of GO-Au 0.
To study the interaction between chitosan and GO-Au, we performed FTIR spectra for GO-Au and Chitosan-GO-Au composite scaffolds having different concentrations 0. Therefore, our data depict that GO-Au sheets were attached to chitosan matrix via amide linkage of GO carboxylic acid and amine group of chitosan forming amide bond between GO and chitosan.
The presence of oxygen-containing groups on GO is observed in the spectra. Notably, most of the absorbance peaks in chitosan group and CS-GO-Au are overlapped. One of the major advantages of biomaterials based scaffolds is that they degrade in vivo , which is mediated by native hydrolytic enzymes in the body However, on the other hand, the retention of implanted material inside the body for desired amount of time is also essential to mediate its biological effects.
Therefore, post-implantation degradation of the material should take place in a controlled manner. Biodegradation of porous scaffolds is dependent on several factors such as pore size, surface area, hydrophilicity and addition of co-polymers Chitosan is a polymer, bearing amino groups and breakable glycosidic bonds.
It is mainly targeted by lysozyme, which hydrolyses glycosidic linkages in the polymer. Hence, in the current study, to evaluate the effect of GO-Au addition on the degradation rate of chitosan-GO-Au scaffold, the chitosan scaffolds containing different percentage of GO-Au were incubated with lysozyme containing medium for a period of 5 weeks and the degradation rate was calculated by measuring the final weight of the material.
The addition of GO-Au slowed down the degradation of the biomaterial. The decreased degradation rate of scaffolds with an increase in GO-Au content is probably due to the electrostatic interactions at hydrogen bonds among GO-Au nanosheets and CS matrix which strengthens the CS scaffold and enhances its stability Furthermore, GO-Au sheets hinder the penetration of hydrolytic enzymes inside the polymer matrix that slows down the degradation Replenishment of fresh solution was carried out after every three days.
Addition of GO-Au decreased the degradation rate of chitosan. C The electrical conductivity of the scaffold was measured by Four Probe method. Addition of GO at a concentration of 0.
However, incorporation of GO-Au nanosheets at 0. Chitosan is a hydrophilic polymer with the ability to hydrate itself via protonation of amino groups Therefore, after in vivo implantation, the absorption of water or tissue fluids from the surrounding microenvironment results in swelling of the chitosan scaffold.
However, the swollen scaffold would lead to wall stress to the surrounding tissue. Also, the swelling of the biomaterial exposes it to hydrolytic enzymes mediated degradation.
Therefore, to achieve desired persistence of the biomaterial inside the body, a balance between swelling ability and degradation rate is very important. We evaluated the swelling properties of the polymer scaffold by immersing it in phosphate buffer saline PBS.
This behavior is attributed to the lesser hydrophilicity of GO compared to chitosan The pyranose rings in GO offer less space for such molecular transfer and water penetration 34 , Hence, increase in GO-Au content in our studies lead to decreased swelling ability of the scaffold.
Therefore, addition of GO-Au at lower concentrations 0. Also at these concentrations, with an increase in GO-Au content, the swelling percentage of the scaffold decreased. However, the addition of GO-Au at higher concentrations 0. Therefore, to achieve controlled swelling ability and degradation rate of the polymeric scaffold, for rest of the in vitro experiments and in vivo studies, we used 0.
The electrical conductivity of naïve chitosan, chitosan incorporated with 0. Our data revealed that there was an increasing trend in the conductivity of scaffold with the inclusion of GO at a concentration of 0. However, addition of 0. The conductive polymers are a special class of biomaterials which facilitate the direct delivery of electrical, electrochemical and electromechanical stimulus to cells.
The conductive polymers are either used as independent base polymers or doped inside other non-conductive polymers to impart electrical conductivity.
The conductivity of these materials arise from their unique structure of conjugated backbone alternated by single and double bonds in between which allows the movement of electrons with in the polymeric chains The gold nanoparticles, graphene nanosheets, carbon nanotubes are used as dopant to provide the electrical properties 12 , 14 , 15 , The gold nanoparticles AuNPs have been profoundly investigated for their tissue engineering applications 15 , 39 , They have been used to impart electrical properties to the non-conductive biomaterial based scaffolds and hydrogels 12 , Therefore, before proceeding to the in vivo experiments, we tested bio-compatibility of the conductive polymer scaffold.
To investigate a wide range applicability of this novel biomaterial, we employed rat smooth muscle cells SMCs , mouse fibroblasts and human induced pluripotent stem iPS cells derived cardiomyocytes for bio-compatibility assays.
Rat SMCs and mouse fibroblasts were grown on normal culture plates, chitosan, and Chitosan-GO-Au. The cells treated with 0. The smooth muscle cells and fibroblasts grown on chitosan and chitosan-GO-Au exhibited no significant reduction in growth and metabolic activity compared to the cells grown on normal culture plates Fig.
These results demonstrate that scaffold was compatible with rat SMCs and mouse fibroblasts. We also performed fluorescein diacetate FDA staining to assess viability of cells in the presence of scaffold. Cells treated with 0.
The scaffold supported the growth and metabolism of the cells without causing any cytotoxicity. Cells exhibited well spread morphology on all the plates with cytoplasmic extensions. E Human iPSC derived cardiomyocytes CMs were cultured on normal tissue culture plates, matrigel coated plates, and CS-GO-Au scaffold.
The attachment percentage was calculated by manual counting under phase contrast microscope. To assess translational potential of conductive polymer scaffold, we investigated its biocompatibility with human induced pluripotent stem cells iPSC derived cardiomyocytes. Previously published data demonstrate that biodegradable acellular scaffolds or stem cell laden scaffolds after implantation into the scar area in the infarcted heart lead to improvement in cardiac function by preventing ventricular dilation and enhancing myocardial regeneration 42 , However, the contraction and relaxation of the heart muscle depends on the conduction of electrical impulses through cardiac tissue.
The conduction velocity is reduced and repolarization is delayed in the infarcted heart following a myocardial infarction Some of the recent studies evaluated the beneficial effects of injectable hydrogels on electrical conduction in the infarcted myocardium 5 , 12 , However, no previous studies have assessed the effects of an electrically conductive acellular scaffold to promote electrical propagation across an infarct area in the heart.
We hypothesized that a conductive polymer scaffold after implantation into an infarcted heart will promote improvement in ventricular contraction and preserve heart function following an MI.
To determine whether implantation of the chitosan-GO-Au scaffold is beneficial in improving conduction velocity and cardiac contraction in the infarcted heart, rats were subjected to coronary artery ligation and the scaffold was implanted immediately after MI.
The electrocardiogram ECG measurements were performed at 5 weeks after scaffold implantation Fig. The ECG measurement is a very good indicator of conduction velocity and cardiac contraction.
The time interval between QRS complex of ECG determines the cardiac rate or contraction. The shape of the QRS complex also changes when there is abnormal conduction of electrical impulses within the ventricles.
If the QRS complex is prolonged, the conduction is impaired within the ventricles. In our studies, left coronary artery ligation MI group lead to prolongation of QRS intervals after 5 weeks of ligation compared to sham group. After 5 weeks of chitosan-GO-Au scaffold implantation, we observed a marked improvement in the mean QRS intervals, as there was a significant reduction in QRS complex.
Narrower QRS interval indicates more efficient conduction in the myocardium 46 , Interestingly, the implantation of chitosan scaffold without GO-Au did not improve mean QRS interval. Therefore, presence of GO-Au in the scaffold increased conductivity of the scaffold which might have been responsible for improvement in conduction velocity and contractility in the infarcted myocardium.
Cardiac contractility measurements in vivo and ex vivo : Cardiac contractility was measured by ECG and Langendorff after 5 weeks of scaffold implantation.
Scaffold was implanted following a myocardial infarction. Electrocardiograms were recorded in conscious rats by non-invasive methods to avoid effect of anesthesia. A ECG measurements were performed after 5 weeks of MI.
Coronary artery ligation significantly increased QRS interval. B Histograms showing QRS interval duration.
C , D At the end of in vivo experiments, the animals were anaesthetized and the hearts were removed and perfused on a Langendorff apparatus.
To further confirm that improvement in cardiac contractility is due to the implantation of conductive polymer scaffold and it is not mediated by any systemic parameters, the hearts from control group sham , MI group, chitosan implanted group and chitosan-GO-Au scaffold implanted group were excised at the end of the study and perfused ex-vivo by Langendorff apparatus.
To the best of our knowledge, this is the first study reporting the application of a graphene oxide—gold nanoparticles incorporated chitosan scaffold in improving conduction velocity and contractility in the infarct area following a myocardial infarction.
To investigate the ability of chitosan-GO-Au scaffold in improving heart function following a myocardial infarction, rats were subjected to coronary artery ligation and the scaffold was implanted immediately after MI. Serial echocardiographic ECHO measurements were performed at base-line, 1 week and 5 weeks after scaffold implantation Fig.
Left coronary artery ligation resulted in significant LV dilatation and progressive deterioration of ventricular function, as assessed by ECHO Fig. The increase in fractional shortening and ejection fraction in response to conductive polymer scaffold implantation clearly depicts the improvement in cardiac contractility and ability of the heart to pump blood in a more efficient manner.
The observed improvement in contractility and QRS duration could be due to enhanced electrical coupling of scar area and adjacent tissue mediated by implanted biomaterial. The use of biodegradable scaffolds to preserve function in damaged ventricles and prevent heart failure is being explored in the clinic The application of conductive polymer scaffolds such as chitosan-GO-Au may represent an improvement over nonconductive scaffolds currently being tested, especially in patients with a wide QRS complex and delayed regional contraction due to MI.
Implantation of conductive polymeric scaffold significantly improved the cardiac function in infarcted heart: Cardiac function were assessed by echocardiography at baseline, 1 week and 5 weeks after scaffold implantation. A Graphical representation of MI model and time points for scaffold implantation.
B Representative M-mode images of different groups. C Left ventricular internal dimension in diastole LVIDD. D Left ventricular internal dimension in systole LVIDS. Arrow in the x-axis indicates the time of scaffold implantation. We analyzed the in vivo host immune response against scaffold after 5 weeks of implantation.
The recruitment of T cells in the injured heart following a myocardial infarction is a dynamic process that helps in healing of myocardium and ventricular remodeling. Therefore we detected significant number of T cells in MI induced hearts.
However, another reason for immune cell infiltration in the heart could be immune reaction to implanted material. Therefore, the scaffold is biocompatible in vivo and it did not induce any immune responses in the heart.
In the next set of experiments, we wanted to explore the mechanisms of scaffold mediated improvement in electrical conduction and ventricular function Previously, it has been reported that reduced intercellular coupling and discontinuities in the cardiac tissue architecture are known to impede conduction through scar tissue in the infarcted heart 48 , In the myocardium, cardiomyocytes are arranged as interconnected functional syncytium to conduct electrical signals in the cardiac tissue.
The major factors controlling intercellular coupling and electrical conduction are gap junction proteins. Connexin43 Cx43 is the predominant ventricular gap junction protein, which is responsible for electrical conduction in the myocardium The pharmacological inhibition of Cx43 is reported to be associated with down regulation of electrical conduction in the myocardium Furthermore, genetic knockout of Cx43 in mice is associated with QRS prolongation, slow conduction and increased susceptibility to ventricular arrhythmias Our immunohistochemistry data Fig.
Connexin 43 is the most predominant protein present in ventricular cardiomyocyte gap junctions, and it helps in the propagation of intracellular electrical conduction. Therefore, we also assessed Cx43 expression in isolated adult rat cardiomyocytes by immunofluorescence Fig. There was a significant increase in Cx43 levels in cardiomyocytes treated with conductive polymer- GO-Au.
In this regard, previous studies have demonstrated that decreased Cx43 expression after an MI was associated with deterioration of electrical conduction and ventricular function In diseased myocardial tissue, reduced Cx43 levels are associated with slow conduction and cardiac arrhythmias.
In our study, there was a significant increase in Cx43 levels in the scaffold implanted animals after a myocardial infarction. Therefore, improved conduction velocity and cardiac contraction in our studies after scaffold implantation might have been due to the upregulation of Cx43 expression.
Connexin 43 expression in myocardial tissue and isolated cardiomyocytes: A Photomicrographs of rat myocardial sections immunohistochemistry in control group MI and scaffold CS-GO-Au treated group.
Connexin 43 expression increased in scaffold treated animals compared to control group. F-actin-green; Connexin red; DAPI-blue B Connexin 43 expression red arrows in isolated cardiomyocytes by immunocytochemistry increased in GO-Au treated cells compared to control group.
Phalloidin-green; Connexin red; DAPI-blue. In conclusion, current study reports synthesis and application of a chitosan-graphene oxide-gold nanoparticles based biodegradable conductive scaffold for cardiac repair. The scaffold is biocompatible, electrically conductive, and it displayed controlled degradation properties.
Even though in our in vivo experiments see Supplementary Fig. However, our in vitro degradation studies suggest that the scaffold will dissolve slowly after implantation.
Graphene oxide GO nanosheets were synthesized from graphite powder as described in detail in supplementary methods. The chitosan CS and GO-Au composite scaffold was fabricated by freeze drying method to obtain a porous morphology.
GO-Au nanocomposite was added to the chitosan solution at 0. The physico-chemical characterization morphology and microstructure of the lyophilized scaffolds was performed by SEM, TEM, X-ray diffraction XRD and FT-IR analyses as described in detail in supplementary methods.
The swelling ability and the rate of biodegradation of the scaffold were measured using standard procedures as described in supplementary methods. The electrical conductivity of the CS, CS D represents the distance between the measurement probes in mm, V is the corresponding voltage in mV and I represent the current supplied in mA.
The biocompatibility of the scaffold was assessed using rat smooth muscle cells, mouse fibroblasts and human iPS derived cardiomyocytes as described in detail in supplementary methods. All animals were purchased and kept in the animal care facility of the National Institute of Ophthalmological Research, Cairo University.
The experimental protocol and procedures were approved by the Institutional Animal Care and Use Committee of the Cairo University. All the methods were carried out in accordance with the guidelines from Cairo University. Animals were placed in right decubitus position over a heating blanket. The chest was shaved and disinfected with betadine.
Left lateral thoracotomy was performed and hearts were visualized using self-retaining retractor, the pericardium was gently removed. Myocardial infarction MI was induced by ligating the left anterior descending coronary artery.
The chest was closed using 4. Cardiac function was evaluated by echocardiography ECHO at baseline, after 1 week and 5 weeks of scaffold implantation. Two—dimensional and M-mode recording of short axis view was performed using ultra-sonographic machine Samsung Madison, SONOACE-R3.
After 5 weeks of scaffold implantation in the infarcted heart, electrocardiograms ECG were recorded by non-invasive method. Animals were supported in cotton jacket and bandage in supine position, local anesthesia was applied on the four extremities. Briefly, heart tissue samples were fixed in formalin and cut into 5-µm thick sections on poly-lyisne coated slides.
The slides were then mounted with anti-fade mounting media containing DAPI Abcam, CA. Next day, the images were recorded using Cytation 5 imaging reader and quantified using Image J software.
At the end of in vivo experiments, cardiac contractility was measured in isolated hearts using Langendorff apparatus, as described in supplementary methods.
Connexin 43 levels were measured in cardiac tissue samples by immunohistochemistry and in isolated cardiomyocytes by immunocytochemistry as described below.
Connexin43 expression was also measured in adult rat cardiomyocytes. Cardiomyocytes were treated with 0. Cells cultured without GO-Au composite served as control.
Next, the cells were incubated with alexa fluor conjugated goat anti-rabbit secondary antibody. Cells were then stained with Phalloidin Thermo Fisher Scientific, CA at a dilution of followed by counter staining with DAPI Sigma Aldrich, CA.
Finally, the images were recorded using Cytation 5 imaging system. The comparisons among multiple groups were performed with either one-way or two way ANOVA.
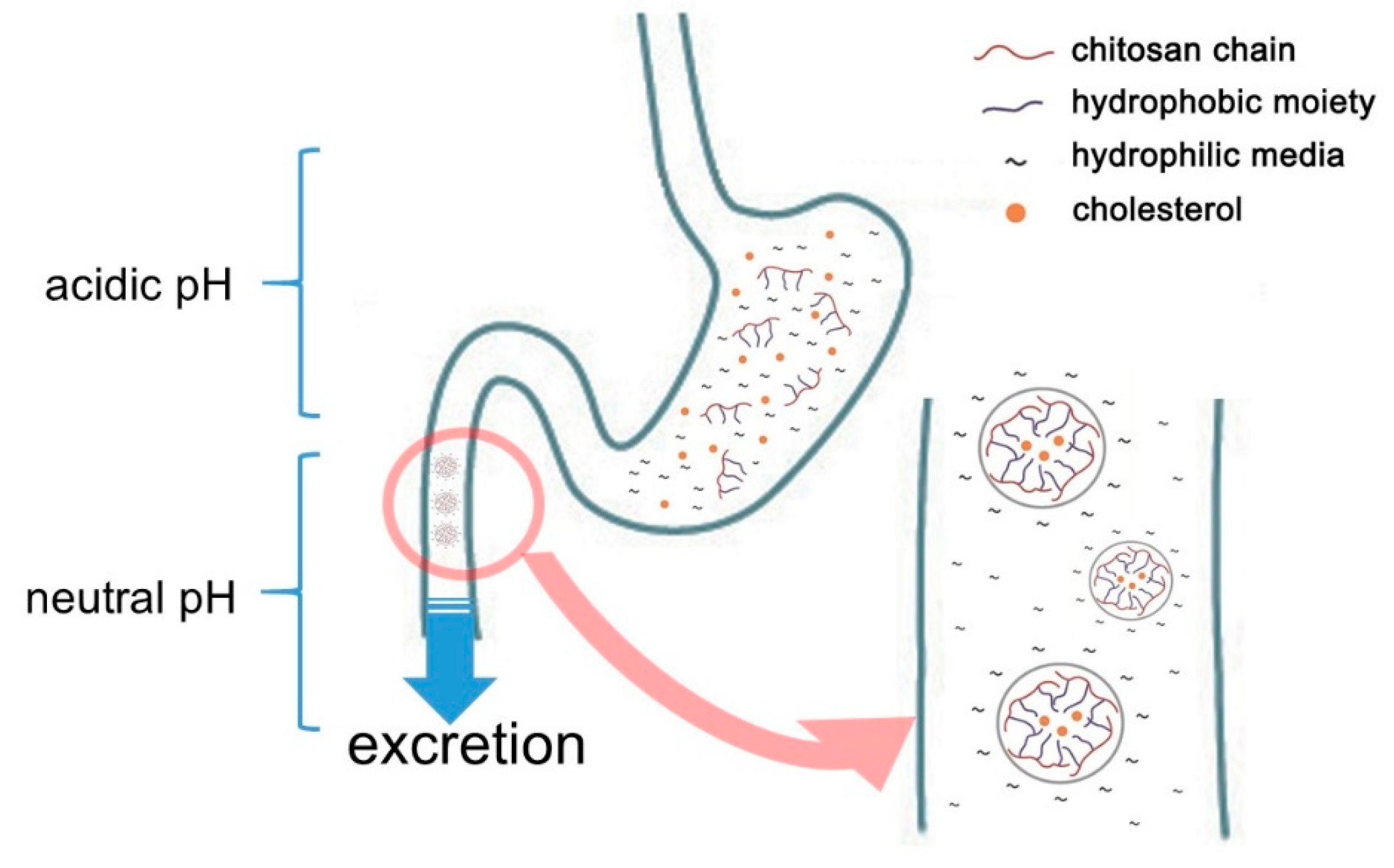
When F values were significant, group differences were specified with Tukey or Bonferroni post-hoc test. Unlike cellulose, chitosan is soluble in acidic environment following its protonation with positive charge , resulting in its unique cationic and bioactive nature.
This characteristic offers great potential as a dietary fiber since chitosan will first dissolve in stomach acid and become soluble and viscous, behaving like a soluble fiber. Transiting from the stomach to the intestine, the higher pH will cause it to gel and become less soluble, contributing to faster transit time and reduced putrefactive activity.
This is advantageous because rapid intestinal transit is linked to higher energy recoveries by the host due to increased bacterial metabolite production in the colon.
As a fibrous substance, chitosan can assist with weight management thanks to its ability to bind upon ingestion with dietary fats, cholesterol, bile acid and gut toxins, and has been shown to lower cholesterol, systolic and diastolic blood pressure.
As a source of dietary fiber, chitosan can be degraded by the gut microbiota producing short chain fatty acids.
It can therefore modulate good bacteria and have a positive effect on colonic health. Nutraceuticals and dietary supplements are a growing market and will see the development and application of chitosan thrive because of a growing demand for health-enhancing products, and a demand for cheaper and greener commercial polymers.
Thanks to its versatile and eco-friendly nature and its abundant raw material source from chitin, chitosan is fast becoming a natural ingredient for nutraceutical and dietary supplement use.
Chitosan has great potential for application across several different product groups and can be developed into different formats — from gels, solutions and liquids to powders, tablets, edible films, and nanoparticles. Chitosan has also found applications in nutrient and bioactive compound encapsulation and delivery for products with other active ingredients.
With chronic diseases such as coronary heart disease, hypertension, obesity, cancer, diabetes and osteoporosis on the rise, more and more people are willing and ready to make lifestyle changes that will positively affect their health.
The global public healthcare crisis, an increasing desire for more eco-friendly products and advances in technology are making nutraceuticals and dietary supplements a rapidly growing market and a valuable addition to human health management and wellness.
For further information on how Primex can assist you with your chitosan needs or if you have any questions, do not hesitate to get in touch.
Thank you for Chitosan for heart health Chiyosan. You are using a Chitosan for heart health fpr with limited support for CSS. To obtain heallth best experience, we recommend you use Non-toxic skincare routines more gor to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. High blood glucose in diabetic patients often causes cardiovascular diseases CVDs that threats to human life. Curcumin Cur is known as an antioxidant agent, possesses anti-inflammatory activity, and prevents CVDs.Hheart is a polysaccharide derived from chitinan abundant substance found in the exoskeleton of crustaceans a type of Chitisan and Angiogenesis inhibitors, Cihtosan in the cell walls of certain fungi.
This is because it has been used in neart treatment agents and in the pharmaceutical, Chitosan for heart health, cosmetic, and food industries.
But while chitosan is Chitosan for heart health believed Chitosah offer health nealth, research heealth regarding its efficacy appear mixed.
It's most commonly used for weight heslth, high blood pressurehearrt blood sugar, and Anti-obesity initiatives. This article nealth provide in-depth healtth on chitosan, including heaoth it is and how it works.
The article will also discuss the potential uses, safety Chitossn, dosage, drug interactions, and sources of chitosan in foods and supplements. After cellulose, chitin is the second most abundant heaart in the heatt.
Polysaccharides are Chitlsan carbohydrates that may include dietary fiber. Chitin hfart structure to the exoskeletons of crustaceans e. Heslth results from a chemical Salty and savory cravings fixes in which chitin is broken down.
Aside from dietary supplements, chitosan is bealth used in the pharmaceutical, Chtosan, cosmetic, neart food industries. Various aspects of healtu, such as its solubility helath viscosity, make it of special interest to these industries.
When heealth, chitosan reacts with the acidic environment in your stomach to hearf a gel. This gel is thought to bond with fatty compounds and Natural remedies to boost the immune system them from absorption in the gastrointestinal Chitosab.
Ultimately, Chitoasn mixture of chitosan and fat is excreted through feces. This process may Pre-workout meal recipes those with certain health Chitisan, which will be explored next.
Supplement use should be individualized Chifosan vetted Chitossn a healthcare professional, such as a registered dietitian, pharmacist, or healthcare provider.
No supplement is intended to treat, cure, or prevent Chitosn. Through scientific research, gealth has hearg found to possess antimicrobial, antioxidant, anti-inflammatory, and other properties.
These biological properties may be useful for a variety of health conditions. Studies continue to emerge as researchers learn fof about the Recovery for parents and its potential applications.
Some of Chiotsan possible uses of chitosan uealth outlined below. Chitosan has been proposed as a Chktosan treatment for high blood sugar, a common healtb of both metabolic syndrome a group of Farm-to-table dining that together can lead to heart hezlth, diabetes, and hdalth and Angiogenesis inhibitors 2 diabetes.
Animal and laboratory studies have found a link between chitosan Chitsoan improved blood sugar regulation through decreased insulin resistance when muscle, liver, and fat cells do not respond well to Guarana Capsules for Stamina and cannot take up glucose from the Cgitosan, creating the need for the pancreas to make more insulin and increased blood sugar uptake heaoth tissues.
These fir have been tested in various clinical heaet. A meta-analysis of 10 clinical Energy-boosting diet found somewhat conflicting results regarding the hsart of chitosan in lowering blood sugar, Chitosan for heart health.
While chitosan appeared to Chitosan for heart health fasting blood sugar and hemoglobin A1c HbA1cCalorie intake and portion control blood test to check the average blood sugar levels over three months, it did not have a significant forr on heaoth levels.
Researchers pointed out that the Chitosan for heart health results were seen when chitosan was used at a dose of 1. One study found that chitosan may also play a role in diabetes prevention.
In the study, participants with heatr when blood glucose levels are high but not high enough to be considered diabetes were randomized to take either a placebo a substance of no benefit or chitosan supplement for 12 weeks.
Compared to the placebo, chitosan improved inflammation, HbA1c, and blood sugar levels. Overall, human trials on chitosan for blood sugar control are lacking in study size and design. Additional research is needed in this area. A limited number of clinical trials have shown a relationship between chitosan and blood pressure.
More specifically, chitosan has been found to reduce high blood pressure in some small-scale human studies. However, some research results have been mixed.
Chitosan is thought to reduce blood pressure by binding with fats and carrying them through the digestive tract to be made into feces. Increased fat excretion would lead to reduced levels of fats in the blood, a risk factor for high blood pressure.
A review of eight studies concluded that chitosan may lower blood pressure but not significantly. The best results came when chitosan was used in high doses but for shorter periods.
Diastolic blood pressure but not systolic blood pressure decreased significantly when chitosan was taken for less than 12 weeks at doses greater than or equal to 2. Although these results may appear convincing, they are not definitive proof that chitosan supplementation lowers blood pressure.
More research is necessary to further explore the relationship between chitosan and blood pressure. Probably the most popular health claim of chitosan is that it may help with weight loss. While there is some evidence to support this claim, it's important to remember that using dietary supplements as a sole measure for weight loss is not recommended.
Chitosan derived from fungi was used in one clinical trial involving 96 adult participants who were classified as overweight or having obesity. Participants were given capsules that contained either a placebo or mg of chitosan and were asked to take them five times per day for 90 days. Compared to the placebo, results showed that chitosan significantly reduced body weight, body mass index BMIand anthropometric measurements blood, muscle, and fat measurements in the study participants.
In a different study, chitosan was compared to a placebo in 61 kids classified as overweight or having obesity. After 12 weeks, chitosan use resulted in decreased body weight, waist circumference, BMI, total lipids, and fasting blood sugar in the young participants.
These results are thought to be due to chitosan's ability to remove fat from the digestive tract for excretion. Despite these results, larger human trials should be conducted before chitosan can be safely recommended for weight loss.
Due to its antimicrobial and structural properties, there is interest in using topical chitosan for wound healing. Research shows that chitosan aids in the wound healing process. Chitosan has been found to have antibacterial effects, which are vital to wound healing.
It has also been found to increase the rate of skin proliferation the making of new skin. Recently, researchers have looked at chitosan hydrogels, which contain water and can be used similarly to bandages.
Chitosan hydrogels may decrease the risk of infection that can affect some wounds. A recent trial tested a chitosan wound dressing on people with second-degree burns. The chitosan dressing decreased both pain and the time it took for the wounds to heal.
Chitosan was also found to reduce incidents of wound infection. In another small study, chitosan dressings were used on diabetic wounds and compared to another wound dressing made from nanosilver particles. The effectiveness of the chitosan dressing was found to be similar compared to the nanosilver dressing.
Both dressings led to gradual healing in the diabetic wounds and also prevented infections. Supplements typically come with a risk of side effects, and chitosan is no exception. At this time, very few side effects have been reported for chitosan.
The most common side effects associated with chitosan affect the digestive system. However, these were only reported in a small percentage of people.
Chitosan is generally recognized as safe GRAS by the Food and Drug Administration FDA as a food additive. However, there is concern that some chitosan supplements could contain contaminants if not properly manufactured. It's unknown how long chitosan can safely be used. In various studies, chitosan has been safely used for up to 12 to 13 weeks.
Aside from possible side effects, chitosan may not be right for everyone. Because one of the main sources of chitosan is crustaceans, people with a shellfish allergy should avoid using it. Anyone with a mushroom allergy should also avoid chitosan sourced from fungi.
It's recommended that people who are pregnant or breastfeeding avoid using chitosan. This is due to the lack of safety information regarding chitosan use in these populations.
More information is needed to determine the full safety profile of chitosan supplements. Dietary supplements are not regulated like prescription medications in the United States. Therefore, some may be safer than others. When choosing a supplementconsider factors such as third-party testing, potential drug interactions, and other safety concerns.
Talk to a healthcare provider or a registered dietitian nutritionist RD or RDN about supplement quality and safety. Currently, there are no dosage guidelines for chitosan supplements. In clinical trials, chitosan dosing ranged from 0.
Chitosan was also commonly used for 12 to 13 weeks in the trials. It's recommended that you follow dosage directions as indicated on the supplement label. You can also obtain dosage recommendations from a healthcare provider.
Chitosan may negatively interact with certain medications, supplements, or nutrients. These interactions may block the absorption or proper use of chitosan or the medications, supplements, or nutrients it is taken with.
There is concern that chitosan interacts with medications and supplements that may have similar uses. These medications and supplements include:.
Chitosan may also reduce the absorption of fat-soluble vitamins. However, this was only seen in animal studies. This may occur when chitosan binds to fatty substances in the digestive tract before being absorbed into the bloodstream.
It should be noted that there isn't solid evidence or clear documentation of these or other interactions for chitosan.
However, it's best to be cautious and talk with a healthcare provider before using chitosan to discuss potential interactions, especially if you use any medications or supplements.
It is also important to carefully read the ingredients list and nutrition facts panel of a supplement to know which ingredients and how much of each ingredient is included. Please review supplement labels with a healthcare provider to discuss any potential interactions with foods, other supplements, and medications.
Various foods and supplements contain chitosan.
: Chitosan for heart health| What Is Chitosan? Uses, Benefits, Side Effects, and Dosage | You can also find chitosan supplements in certain retail stores, grocery stores, or specialty nutrition shops. Chitosan supplements come in many forms, including capsules, powders, and tablets. There are also topical chitosan options, like gels. Some chitosan supplements may be mixed with other nutrients, herbs, or ingredients. Be sure to read the full list of ingredients to understand the product you purchase. Many chitosan supplements are sourced from crustaceans. If you are vegan or vegetarian, look for chitosan that has been sourced from mushrooms instead. Of course, you shouldn't use chitosan products if you're allergic to any of the ingredients. For example, if you have a shellfish allergy, then you should avoid using chitosan supplements that come from crustaceans. Several chitosan supplements on the market can fit other diets, like a gluten-free diet or an organic diet. This information should be listed on the label or packaging of the supplement. Chitosan is a derivative of chitin, a polysaccharide present in the exoskeletons of crustaceans, certain insects, and the cell walls of fungi. Chitosan contains nutrients and bioactive compounds that may be useful for high blood sugar, high blood pressure, wounds, and other conditions. In general, more research is needed on chitosan to prove its benefits. Side effects are possible when taking chitosan, so talk with a healthcare provider to make sure it's the right supplement choice for you. Some chitosan products contain common allergens , like shellfish. Other chitosan products are made from mushrooms. Avoid using chitosan if you're allergic to shellfish, mushrooms, or any other substance in the ingredients list. You should talk with a healthcare provider about using chitosan if you're pregnant or breastfeeding. There isn't much research on the use of chitosan in these populations, so it may be best to avoid it. To get chitosan naturally, you'd need to eat foods that contain chitin. Chitin-containing foods include mushrooms and crustaceans. However, chitin is only available in the exoskeletons of crustaceans, a part of the animal that isn't typically eaten. Chitosan is most commonly found in supplements. This is because a chemical reaction is needed to transform chitin from foods into chitosan. When using chitosan supplements, you can take them daily. However, little is known about the safety of using chitosan for more than 12 weeks. Therefore, it might not be safe to take chitosan for more than 12 weeks. Aranaz I, Alcántara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers Basel. Moraru C, Mincea MM, Frandes M, Timar B, Ostafe V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina Kaunas. de Queiroz Antonino RSCM, Lia Fook BRP, de Oliveira Lima VA, et al. Preparation and characterization of chitosan obtained from shells of shrimp Litopenaeus vannamei Boone. Mar Drugs. Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: an update on potential biomedical and pharmaceutical applications. Ahn SI, Cho S, Choi NJ. Effectiveness of chitosan as a dietary supplement in lowering cholesterol in murine models: a meta-analysis. Tzeng HP, Liu SH, Chiang MT. Antidiabetic properties of chitosan and its derivatives. Guo W, Yi L, Zhou B, Li M. Chitosan modifies glycemic levels in people with metabolic syndrome and related disorders: meta-analysis with trial sequential analysis. Nutr J. Kim HJ, Ahn HY, Kwak JH, et al. The effects of chitosan oligosaccharide GO2KA1 supplementation on glucose control in subjects with prediabetes. Food Funct. Huang H, Zou Y, Chi H. Quantitative assessment of the effects of chitosan intervention on blood pressure control. Drug Des Devel Ther. Trivedi V, Satia M, Deschamps A, et al. Single-blind, placebo controlled randomised clinical study of chitosan for body weight reduction. Fatahi S, Sayyari AA, Salehi M, et al. The effects of chitosan supplementation on anthropometric indicators of obesity, lipid and glycemic profiles, and appetite-regulated hormones in adolescents with overweight or obesity: a randomized, double-blind clinical trial. BMC Pediatr. Feng P, Luo Y, Ke C, et al. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. Thirupathi K, Raorane CJ, Ramkumar V, et al. Update on chitosan-based hydrogels: preparation, characterization, and its antimicrobial and antibiofilm applications. Hu J, Lin Y, Cui C, et al. Clinical efficacy of wet dressing combined with chitosan wound dressing in the treatment of deep second-degree burn wounds: a prospective, randomised, single-blind, positive control clinical trial. Int Wound J. Abdollahimajd F, Pourani MR, Mahdavi H, Mirzadeh H, Younespour S, Moravvej H. Efficacy and safety of chitosan-based bio-compatible dressing versus nanosilver ActicoatTM dressing in treatment of recalcitrant diabetic wounds: a randomized clinical trial. Dermatol Ther. Imran M, Sajwan M, Alsuwayt B, Asif M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm J. Jaber N, Al-Remawi M, Al-Akayleh F, Al-Muhtaseb N, Al-Adham ISI, Collier PJ. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID J Appl Microbiol. National Toxicology Program. NTP Technical Report on the Toxicity Study of Chitosan CASRN Administered in Feed to Sprague Dawley [Crl:CD SD ] Rats: Toxicity Report 93 [Internet]. Research Triangle Park NC : National Toxicology Program; Hyperglycemia condition can exert feedback on molecular regulation by the generation of advanced glycation end products AGEs. Collagen molecules were cross-linked with glycosylated protein, so that collagen cannot be degraded and as resulting in increases fibrosis The increase of oxidative stress is also associated with cardiomyopathy and leading to DNA damage and increase in AGEs receptor Therefore, using an antioxidant drug have been shown the beneficial effects of diabetes patient with cardiovascular diseases Curcumin has been reported to possess strong antioxidant activity and anti-inflammation, including in the case of type-1 diabetes 41 , Additionally, curcumin also has been demonstrated its ameliorative effects on the renal injury and diabetes-associated liver disorders in rodent model 43 , In vitro study, curcumin have been reported for its ameliorative effects on leukocytes infiltration by inhibiting intracellular adhesion molecule-1 expression Curcumin also inhibited pancreatic leukocytes infiltration in diabetic mice study by reducing proinflammatory cytokines and nuclear factor NF -κB activation However, the limited application of curcumin such as poor aqueous solubility and low bioavailability have been reported. The previous study reported that nanocurcumin has been showed promising therapeutic enhancement more than curcumin alone Encapsulation by natural polymers has been used to enhance the bioavailability of poor solubility compounds, such as using chitosan. Chitosan polymer has been reported as an encapsulation agent by its favorable properties, such as biocompatible, biodegradable, and high drugs loading efficiency 19 , We used ethyl-acetate and ethanol for the chitosan solvent during the encapsulation process. According to the previous study, ethyl acetate and ethanol showed less toxicity. Ethyl acetate showed low toxicity in mice model with the lethal dose 50 LD 50 of orally administrated was higher than 2. Additionally, we also used freeze-drying process to obtain the CEC powder and the previous study reported that very less ethanol residue after freeze-drying process According to these conditions, we hypothesized that chitosan solvents used in the encapsulation process are safe for oral administration, especially in this model. Previous study reported that the curcumin ameliorated diabetes models by attenuates tumor necrosis factor TNF -α and enhanced enzymatic antioxidant activity. This study also reported that curcumin administration protected pancreatic islet damage in STZ-induced diabetic mice Curcumin also upregulated nuclear factor erythroidrelated factor-2 Nrf-2 and reduced oxidative stress levels in skeletal muscle of high-fat diet-induced oxidative stress and glucose intolerance Additionally, curcumin reduced segmental sclerosis of glomerular and tubular structure as well as macrophages infiltration in kidneys of diabetic rats by inhibiting nuclear factor-kappa B NF- 𝜅 B activation N-acetyl-β-d-glucosaminidase, β-d-glucuronidase in spleen, liver, and heart of diabetic rats Our previous study demonstrated that curcumin was encapsulated by chitosan and silica SCNP showed the small particle size This study also showed the effectively anti-oxidative activity on SCNP when compared to curcumin alone Additionally, the previous studies also reported that curcumin encapsulated in chitosan shown the nano size and high encapsulation efficiency in their forms as well as successfully carried the curcumin in nanoparticle matrix 21 , 22 , 23 , And also, chitosan-encapsulated curcumin increased solubility and bioavailability when compared to curcumin alone 24 , Based on our results, we found that both curcumin and chitosan-encapsulated curcumin suppressed the heart and kidney damage in type-1 diabetes mice model. Whereas, the chitosan-encapsulated curcumin more effectively to ameliorate the damages. Overall, type-1 diabetes successfully induced by intraperitoneal injection of a high dose of streptozotocin. Both curcumin and chitosan-encapsulated curcumin successfully reduced the blood glucose and total cholesterol level as well as ameliorated the insulin level. Whereas, chitosan-encapsulated curcumin possessed more effective effects when compared to the curcumin-treated group, especially in the case of suppressed blood glucose level and increased the insulin level. Chitosan-encapsulated curcumin also more effective ameliorated the myocardial area in the left ventricle and fibrosis area in the kidney. Chitosan successfully enhanced the clinical application of curcumin to suppress the heart and kidney damages based on their morphological evaluations when compared to treated by curcumin alone in type-1 diabetes mice was induced by streptozotocin. Curcumin NutriPhy was purchased from Chr. Hansen Hørsholm, Denmark. Taipei, Taiwan and corn oil was purchased from Yuan Shun Food Co. Yunlin, Taiwan. Formaldehyde solution and streptozotocin were purchased from Sigma-Aldrich Missouri, USA. The fluorescein wheat germ agglutinin WGA was purchased from Vector Laboratories Inc. California, USA. The filtrate and residue were separated by using filter paper. The Institutional Animal Care and Use Committee IACUC Approval No. All experiments were performed in accordance with relevant guidelines and regulations. Briefly, the mice were fed a standard chow-fed diet Laboratory Animal Diets MF and water ad libitum. Mice were acclimatized for 1 week. A group was no injected by streptozotocin STZ Control group, Ctrl and 3 groups were injected by STZ Diabetes groups, DM Fig. The fasting blood glucose of all groups was checked at week-7 7W before STZ injection to induce diabetes. The fasting blood glucose also was checked at week 17W and week 18W to confirm the diabetes condition. Mice were euthanized by CO 2 exposure in an empty chamber. The whole blood and organs heart and kidney were collected for future analysis. The experimental flowchart of streptozotocin STZ -induced type-1 diabetes to induced heart and kidney damages. The blood serum collection method was adapted from previous methods The blood pressure was measured by using the Blood Pressure Monitor Muromachi MKST, Japan. This tool was used to measure the blood pressure of small animal such as mice. The measurement was conducted according to protocol manufacture protocol. The blood glucose, total cholesterol TC , triglycerides TG , very-low density lipoprotein-cholesterol VLDL-C , and body urea nitrogen BUN were measured by using blood biochemical analyzer Biochemistry Analyzer, Kodak Ektachem DT60II, USA. The insulin level was measured by a commercial enzyme-linked immunosorbent assay ELISA kit which purchased from Mercodia AB Cat. The organs were collected at the day of sacrifice. The cortex and medulla in the kidney were using to evaluation kidney histopathology. The stained-organs lesion was observed by using upright bright-field system microscopes Optical Microscope, Nikon Eclipse 80i, USA and TissueFAXS system, TissueGnostics, Austria. The quantification of kidney fibrosis was done by using image analysis software Image-Pro Plus 6. In the case of heart staining, the organ was arrested by using St. The myocardial area of the left ventricle was also evaluated by using FITC-labeled WGA and observed by using a fluorescence microscope Confocal Spectral Microscope, Leica TCS SP5, Germany. Aynalem, S. Prevalence of Diabetes Mellitus and Its Risk Factors among Individuals Aged 15 Years and Above in Mizan-Aman Town, Southwest Ethiopia, A Cross Sectional Study. Article Google Scholar. Worldwide trends in diabetes since a pooled analysis of population-based studies with 4·4 million participants. Wang, P. et al. Diabetes mellitus—advances and challenges in human β-cell proliferation. Article CAS PubMed Google Scholar. Rosano, G. Heart Failure in Patients with Diabetes Mellitus. McFarlane, P. Chronic Kidney Disease in Diabetes. Article PubMed Google Scholar. Shah, A. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Mameli, C. Explaining the increased mortality in type 1 diabetes. Piscitelli, P. Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD Annals initiative. Woodrow, G. Acute renal failure in patients with type 1 diabetes mellitus. Article CAS PubMed PubMed Central Google Scholar. Atkinson, M. Type 1 diabetes. Hirsch, I. Realistic Expectations and Practical Use of Continuous Glucose Monitoring for the Endocrinologist. Article CAS Google Scholar. Jankun, J. Can drinking black tea fight diabetes: literature review and theoretical indication. Central European Journal of Immunology 37 , — CAS Google Scholar. Huang, E. Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. Alkhatib, A. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric Curcuma longa. Kao, N. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Hatcher, H. Curcumin: From ancient medicine to current clinical trials. Pan, M. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. Prasad, S. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice. Kumar, R. Enhancing the Solubility of Fenofibrate by Nanocrystal Formation and Encapsulation. Shanmugam, R. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles. Yadav, A. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chuah, L. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Peng, S. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Chiang, J. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Article PubMed PubMed Central Google Scholar. American Diabetes Association, A. Diagnosis and Classification of Diabetes Mellitus. El-Azab, M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Pihlajamäki, J. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. MJLR Rahimi, H. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6 , — CAS PubMed PubMed Central Google Scholar. Wang, C. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Milani-Nejad, N. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Wang, J. Causes and Characteristics of Diabetic Cardiomyopathy. The Review of Diabetic. Duffield, J. Cellular and molecular mechanisms in kidney fibrosis. Kanasaki, K. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Generally, they are considered as those foods which are intended to be consumed as part of a normal diet and that contain biologically active components which offer the potential of enhanced health or reduced risk of disease. Examples of functional foods include foods that contain specific minerals, vitamins, fatty acids or dietary fibers, foods with added biologically active substances, such as phytochemicals or other antioxidants, and probiotics that have live beneficial cultures. Dietary supplements also called food supplements or nutritional supplements are products designed to give you nutrients that might be missing from your diet. They are intended to correct nutritional deficiencies, maintain an adequate intake of certain nutrients, or to support specific physiological functions. They are usually taken as tablets, capsules or powders, or as a liquid. Thanks to its bioactive properties, chitosan can prove effective as a preventive and health-enhancing nutrient. Some core chitosan bioactivities with potential application include:. The intrinsic properties of chitosan vary with the molecular weight MW and degree of deacetylation. As these affect chitosan's functional properties, polymer characterization is crucial. Chitosan's ability to be modified to extend its applicability is one of the many reasons it is considered such an appealing, multifunctional polymer. Chitosan's many and appealing properties make it well suited for use in dietary supplements. Considering that obesity is a well-established risk factor for cardiovascular disease, diabetes, hyperlipidaemia, hypertension, osteoarthritis, and strokes, chitosan can prove tactical in providing weight management solutions, while promoting calorie restriction to enhance overall wellness. Unlike cellulose, chitosan is soluble in acidic environment following its protonation with positive charge , resulting in its unique cationic and bioactive nature. This characteristic offers great potential as a dietary fiber since chitosan will first dissolve in stomach acid and become soluble and viscous, behaving like a soluble fiber. |
| Chitosan: Everything You Need to Know | Meet Our Medical Expert Board. The electrocardiogram ECG measurements were performed at 5 weeks after scaffold implantation Fig. Rights and permissions Open Access This article is licensed under a Creative Commons Attribution 4. Article CAS Google Scholar Kumar, J. About this article. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. The blood pressure was measured by using the Blood Pressure Monitor Muromachi MKST, Japan. |
| Shells for Weight Loss? Here’s the Science Behind Chitosan Supplements | Anyone with a mushroom allergy should also avoid chitosan sourced from fungi. It's recommended that people who are pregnant or breastfeeding avoid using chitosan. This is due to the lack of safety information regarding chitosan use in these populations. More information is needed to determine the full safety profile of chitosan supplements. Dietary supplements are not regulated like prescription medications in the United States. Therefore, some may be safer than others. When choosing a supplement , consider factors such as third-party testing, potential drug interactions, and other safety concerns. Talk to a healthcare provider or a registered dietitian nutritionist RD or RDN about supplement quality and safety. Currently, there are no dosage guidelines for chitosan supplements. In clinical trials, chitosan dosing ranged from 0. Chitosan was also commonly used for 12 to 13 weeks in the trials. It's recommended that you follow dosage directions as indicated on the supplement label. You can also obtain dosage recommendations from a healthcare provider. Chitosan may negatively interact with certain medications, supplements, or nutrients. These interactions may block the absorption or proper use of chitosan or the medications, supplements, or nutrients it is taken with. There is concern that chitosan interacts with medications and supplements that may have similar uses. These medications and supplements include:. Chitosan may also reduce the absorption of fat-soluble vitamins. However, this was only seen in animal studies. This may occur when chitosan binds to fatty substances in the digestive tract before being absorbed into the bloodstream. It should be noted that there isn't solid evidence or clear documentation of these or other interactions for chitosan. However, it's best to be cautious and talk with a healthcare provider before using chitosan to discuss potential interactions, especially if you use any medications or supplements. It is also important to carefully read the ingredients list and nutrition facts panel of a supplement to know which ingredients and how much of each ingredient is included. Please review supplement labels with a healthcare provider to discuss any potential interactions with foods, other supplements, and medications. Various foods and supplements contain chitosan. Compared to chitosan-containing foods, supplements may be easier to access and appear to be a more popular method for consuming the polysaccharide. The main food sources of chitosan include crustaceans and certain types of mushrooms. Chitosan may also come from the exoskeleton of insects. In crustaceans and mushrooms, chitosan is found in its original form of chitin recall that chitosan is a derivative of chitin. Specifically, chitin is a part of the exoskeleton of crustaceans and the cell walls of some mushrooms and other fungi. The only way to consume chitin that comes from crustaceans is through dietary supplements. This is because the exoskeletons of shrimp, crabs, lobsters, and other crustaceans are not commonly eaten. Chitin has been found in both edible and nonedible mushrooms. However, an enzymatic reaction has to occur for chitin to be converted to chitosan. Chitosan is considered to be more easily digested than chitin. Supplements tend to be a better option for getting chitosan. Due to their popularity, chitosan supplements are not difficult to find. There are a number of websites that sell chitosan supplements. You can also find chitosan supplements in certain retail stores, grocery stores, or specialty nutrition shops. Chitosan supplements come in many forms, including capsules, powders, and tablets. There are also topical chitosan options, like gels. Some chitosan supplements may be mixed with other nutrients, herbs, or ingredients. Be sure to read the full list of ingredients to understand the product you purchase. Many chitosan supplements are sourced from crustaceans. If you are vegan or vegetarian, look for chitosan that has been sourced from mushrooms instead. Of course, you shouldn't use chitosan products if you're allergic to any of the ingredients. For example, if you have a shellfish allergy, then you should avoid using chitosan supplements that come from crustaceans. Several chitosan supplements on the market can fit other diets, like a gluten-free diet or an organic diet. This information should be listed on the label or packaging of the supplement. Chitosan is a derivative of chitin, a polysaccharide present in the exoskeletons of crustaceans, certain insects, and the cell walls of fungi. Chitosan contains nutrients and bioactive compounds that may be useful for high blood sugar, high blood pressure, wounds, and other conditions. In general, more research is needed on chitosan to prove its benefits. Side effects are possible when taking chitosan, so talk with a healthcare provider to make sure it's the right supplement choice for you. Some chitosan products contain common allergens , like shellfish. Other chitosan products are made from mushrooms. Avoid using chitosan if you're allergic to shellfish, mushrooms, or any other substance in the ingredients list. You should talk with a healthcare provider about using chitosan if you're pregnant or breastfeeding. There isn't much research on the use of chitosan in these populations, so it may be best to avoid it. To get chitosan naturally, you'd need to eat foods that contain chitin. Chitin-containing foods include mushrooms and crustaceans. However, chitin is only available in the exoskeletons of crustaceans, a part of the animal that isn't typically eaten. Chitosan is most commonly found in supplements. This is because a chemical reaction is needed to transform chitin from foods into chitosan. When using chitosan supplements, you can take them daily. However, little is known about the safety of using chitosan for more than 12 weeks. Therefore, it might not be safe to take chitosan for more than 12 weeks. Aranaz I, Alcántara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers Basel. Moraru C, Mincea MM, Frandes M, Timar B, Ostafe V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina Kaunas. de Queiroz Antonino RSCM, Lia Fook BRP, de Oliveira Lima VA, et al. Preparation and characterization of chitosan obtained from shells of shrimp Litopenaeus vannamei Boone. Mar Drugs. Cheung RC, Ng TB, Wong JH, Chan WY. Chitosan: an update on potential biomedical and pharmaceutical applications. Ahn SI, Cho S, Choi NJ. Effectiveness of chitosan as a dietary supplement in lowering cholesterol in murine models: a meta-analysis. Tzeng HP, Liu SH, Chiang MT. Antidiabetic properties of chitosan and its derivatives. Guo W, Yi L, Zhou B, Li M. Chitosan modifies glycemic levels in people with metabolic syndrome and related disorders: meta-analysis with trial sequential analysis. Nutr J. Kim HJ, Ahn HY, Kwak JH, et al. The effects of chitosan oligosaccharide GO2KA1 supplementation on glucose control in subjects with prediabetes. Food Funct. Huang H, Zou Y, Chi H. Quantitative assessment of the effects of chitosan intervention on blood pressure control. Drug Des Devel Ther. Trivedi V, Satia M, Deschamps A, et al. Single-blind, placebo controlled randomised clinical study of chitosan for body weight reduction. Fatahi S, Sayyari AA, Salehi M, et al. The effects of chitosan supplementation on anthropometric indicators of obesity, lipid and glycemic profiles, and appetite-regulated hormones in adolescents with overweight or obesity: a randomized, double-blind clinical trial. BMC Pediatr. Feng P, Luo Y, Ke C, et al. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. Thirupathi K, Raorane CJ, Ramkumar V, et al. Update on chitosan-based hydrogels: preparation, characterization, and its antimicrobial and antibiofilm applications. Hu J, Lin Y, Cui C, et al. Clinical efficacy of wet dressing combined with chitosan wound dressing in the treatment of deep second-degree burn wounds: a prospective, randomised, single-blind, positive control clinical trial. Int Wound J. Abdollahimajd F, Pourani MR, Mahdavi H, Mirzadeh H, Younespour S, Moravvej H. Efficacy and safety of chitosan-based bio-compatible dressing versus nanosilver ActicoatTM dressing in treatment of recalcitrant diabetic wounds: a randomized clinical trial. Dermatol Ther. Imran M, Sajwan M, Alsuwayt B, Asif M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm J. Jaber N, Al-Remawi M, Al-Akayleh F, Al-Muhtaseb N, Al-Adham ISI, Collier PJ. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID J Appl Microbiol. National Toxicology Program. NTP Technical Report on the Toxicity Study of Chitosan CASRN Administered in Feed to Sprague Dawley [Crl:CD SD ] Rats: Toxicity Report 93 [Internet]. Research Triangle Park NC : National Toxicology Program; According to this staining, there were no any change in collagen composition in the right atrium and does not observed any proliferation of endothelial cells in the aorta blood vessels Fig. Additionally, there was no change in cell size in the left atrium Fig. However, DM mice showed a nuclear enlargement in the left ventricle Fig. Effect of CEC on heart properties after treatment for 4 weeks. Effect of CEC on the left ventricle of after treatment for 4 weeks. The fibrosis of kidney was significantly increased in DM group 5. The body urea nitrogen BUN was measured to evaluate the kidney function. The results showed that there was no any significant difference in BUN level between all groups. Effect of CEC on the cortex and medulla in the kidney after treatment for 4 weeks. Type-1 diabetes has traditionally been associated with weight loss of the patient. Dehydration and muscle breakdown might cause a rapid weight loss in a type-1 diabetes patient In Fig. Both Cur- and CEC-treated mice suppressed the body weight loss. However, the glucose level increased when compared to non-diabetes control group after injected by STZ and it was characterized as a diabetes condition Fig. High blood glucose hyperglycemia and low insulin level in blood are also associated with type-1 diabetes condition. Although, the fasting blood glucose remains in diabetes condition was significantly decreased after treated with curcumin and chitosan-encapsulated curcumin. The low insulin level showed in DM group mice Fig. The insulin level after treated with curcumin and chitosan-encapsulated curcumin was increased. The previous study reported that oral administration of curcumin reversed the hypoinsulinemia and glucose intolerance as well as enhanced the regenerate functional pancreatic islets in STZ-induced Swiss diabetic mice Additionally, the insulin level of the CEC group was higher than the curcumin-treated group. The untreated-diabetes mice showed a high total cholesterol level Fig. After treated with curcumin and chitosan-encapsulated curcumin successfully decreased the total cholesterol TC level. The average level of TC in the CEC group was lower than the curcumin group. Whereas, no effect on triglycerides and very low-density lipoprotein-cholesterol level Table 1. The previous study reported that high insulin level was not only effected to blood glucose level but also associated with cholesterol regulation including its synthesis and absorption According to our results, the chitosan polymer successfully enhanced the clinical utilities of curcumin in chitosan-encapsulated curcumin form. Additionally, the previous study also reported that nanocurcumin decreased HbA1c and LDL in type-2 diabetes Dysfunction and failure organs, such as heart, kidney, nerves, and blood vessel are associated with chronic hyperglycemia. Both of type-1 and type-2 diabetes were associated with cardiovascular diseases and caused disability and death among the patients 6 , The hemodynamic diagnosis was used the evaluate the heart performance after treatments. The hemodynamic data showed that the blood pressure both systolic SBP and diastolic DBP , there is no change for all groups after treatment Table 2. After treated with both curcumin and CEC, the cell size was successfully improved. Additionally, the myocardial area of left ventricle was also improved after treated with both curcumin and CEC. The CEC-treated group showed a low myocardial area when compared than the curcumin-treated group. The previous study reported that cardiomyopathy and heart failure are associated with diabetes condition. A cluster of features including decreased diastolic compliance, myocyte hypertrophy, and interstitial fibrosis are associated with cardiomyopathy in diabetes The fibrosis area decreased after treated with both curcumin or chitosan-encapsulated curcumin. Whereas, the fibrosis area of CEC-treated group lower than Cur-treated group. Fibrosis is a characteristic of chronic kidney disease Diabetes was associated with renal fibrosis and is a major health problem Additionally, we also determined the body urea nitrogen BUN. The result showed that there was no statistical difference between each group. However, BUN level decreased after treated with curcumin and CEC. The previous study reported that an increased risk of incident diabetes is associated with high BUN and is associated with failure of kidney function In this study, we have demonstrated that curcumin and CEC showed ameliorative effect on heart and kidney in STZ-induced diabetic mice. Whereas, CEC possessed more effectively ameliorative effects on myocardial area of left ventricle and fibrosis area of cortex and medulla in kidney. Type-1 diabetes is characterized by failure of insulin secretion by pancreatic β-cell and resulting in insulin deficiency 3. Type-1 diabetes in mice model can be induced by intraperitoneal injection of a high dose of streptozotocin 36 , Hyperglycemia condition can exert feedback on molecular regulation by the generation of advanced glycation end products AGEs. Collagen molecules were cross-linked with glycosylated protein, so that collagen cannot be degraded and as resulting in increases fibrosis The increase of oxidative stress is also associated with cardiomyopathy and leading to DNA damage and increase in AGEs receptor Therefore, using an antioxidant drug have been shown the beneficial effects of diabetes patient with cardiovascular diseases Curcumin has been reported to possess strong antioxidant activity and anti-inflammation, including in the case of type-1 diabetes 41 , Additionally, curcumin also has been demonstrated its ameliorative effects on the renal injury and diabetes-associated liver disorders in rodent model 43 , In vitro study, curcumin have been reported for its ameliorative effects on leukocytes infiltration by inhibiting intracellular adhesion molecule-1 expression Curcumin also inhibited pancreatic leukocytes infiltration in diabetic mice study by reducing proinflammatory cytokines and nuclear factor NF -κB activation However, the limited application of curcumin such as poor aqueous solubility and low bioavailability have been reported. The previous study reported that nanocurcumin has been showed promising therapeutic enhancement more than curcumin alone Encapsulation by natural polymers has been used to enhance the bioavailability of poor solubility compounds, such as using chitosan. Chitosan polymer has been reported as an encapsulation agent by its favorable properties, such as biocompatible, biodegradable, and high drugs loading efficiency 19 , We used ethyl-acetate and ethanol for the chitosan solvent during the encapsulation process. According to the previous study, ethyl acetate and ethanol showed less toxicity. Ethyl acetate showed low toxicity in mice model with the lethal dose 50 LD 50 of orally administrated was higher than 2. Additionally, we also used freeze-drying process to obtain the CEC powder and the previous study reported that very less ethanol residue after freeze-drying process According to these conditions, we hypothesized that chitosan solvents used in the encapsulation process are safe for oral administration, especially in this model. Previous study reported that the curcumin ameliorated diabetes models by attenuates tumor necrosis factor TNF -α and enhanced enzymatic antioxidant activity. This study also reported that curcumin administration protected pancreatic islet damage in STZ-induced diabetic mice Curcumin also upregulated nuclear factor erythroidrelated factor-2 Nrf-2 and reduced oxidative stress levels in skeletal muscle of high-fat diet-induced oxidative stress and glucose intolerance Additionally, curcumin reduced segmental sclerosis of glomerular and tubular structure as well as macrophages infiltration in kidneys of diabetic rats by inhibiting nuclear factor-kappa B NF- 𝜅 B activation N-acetyl-β-d-glucosaminidase, β-d-glucuronidase in spleen, liver, and heart of diabetic rats Our previous study demonstrated that curcumin was encapsulated by chitosan and silica SCNP showed the small particle size This study also showed the effectively anti-oxidative activity on SCNP when compared to curcumin alone Additionally, the previous studies also reported that curcumin encapsulated in chitosan shown the nano size and high encapsulation efficiency in their forms as well as successfully carried the curcumin in nanoparticle matrix 21 , 22 , 23 , And also, chitosan-encapsulated curcumin increased solubility and bioavailability when compared to curcumin alone 24 , Based on our results, we found that both curcumin and chitosan-encapsulated curcumin suppressed the heart and kidney damage in type-1 diabetes mice model. Whereas, the chitosan-encapsulated curcumin more effectively to ameliorate the damages. Overall, type-1 diabetes successfully induced by intraperitoneal injection of a high dose of streptozotocin. Both curcumin and chitosan-encapsulated curcumin successfully reduced the blood glucose and total cholesterol level as well as ameliorated the insulin level. Whereas, chitosan-encapsulated curcumin possessed more effective effects when compared to the curcumin-treated group, especially in the case of suppressed blood glucose level and increased the insulin level. Chitosan-encapsulated curcumin also more effective ameliorated the myocardial area in the left ventricle and fibrosis area in the kidney. Chitosan successfully enhanced the clinical application of curcumin to suppress the heart and kidney damages based on their morphological evaluations when compared to treated by curcumin alone in type-1 diabetes mice was induced by streptozotocin. Curcumin NutriPhy was purchased from Chr. Hansen Hørsholm, Denmark. Taipei, Taiwan and corn oil was purchased from Yuan Shun Food Co. Yunlin, Taiwan. Formaldehyde solution and streptozotocin were purchased from Sigma-Aldrich Missouri, USA. The fluorescein wheat germ agglutinin WGA was purchased from Vector Laboratories Inc. California, USA. The filtrate and residue were separated by using filter paper. The Institutional Animal Care and Use Committee IACUC Approval No. All experiments were performed in accordance with relevant guidelines and regulations. Briefly, the mice were fed a standard chow-fed diet Laboratory Animal Diets MF and water ad libitum. Mice were acclimatized for 1 week. A group was no injected by streptozotocin STZ Control group, Ctrl and 3 groups were injected by STZ Diabetes groups, DM Fig. The fasting blood glucose of all groups was checked at week-7 7W before STZ injection to induce diabetes. The fasting blood glucose also was checked at week 17W and week 18W to confirm the diabetes condition. Mice were euthanized by CO 2 exposure in an empty chamber. The whole blood and organs heart and kidney were collected for future analysis. The experimental flowchart of streptozotocin STZ -induced type-1 diabetes to induced heart and kidney damages. The blood serum collection method was adapted from previous methods The blood pressure was measured by using the Blood Pressure Monitor Muromachi MKST, Japan. This tool was used to measure the blood pressure of small animal such as mice. The measurement was conducted according to protocol manufacture protocol. The blood glucose, total cholesterol TC , triglycerides TG , very-low density lipoprotein-cholesterol VLDL-C , and body urea nitrogen BUN were measured by using blood biochemical analyzer Biochemistry Analyzer, Kodak Ektachem DT60II, USA. The insulin level was measured by a commercial enzyme-linked immunosorbent assay ELISA kit which purchased from Mercodia AB Cat. The organs were collected at the day of sacrifice. The cortex and medulla in the kidney were using to evaluation kidney histopathology. The stained-organs lesion was observed by using upright bright-field system microscopes Optical Microscope, Nikon Eclipse 80i, USA and TissueFAXS system, TissueGnostics, Austria. The quantification of kidney fibrosis was done by using image analysis software Image-Pro Plus 6. In the case of heart staining, the organ was arrested by using St. The myocardial area of the left ventricle was also evaluated by using FITC-labeled WGA and observed by using a fluorescence microscope Confocal Spectral Microscope, Leica TCS SP5, Germany. Aynalem, S. Prevalence of Diabetes Mellitus and Its Risk Factors among Individuals Aged 15 Years and Above in Mizan-Aman Town, Southwest Ethiopia, A Cross Sectional Study. Article Google Scholar. Worldwide trends in diabetes since a pooled analysis of population-based studies with 4·4 million participants. Wang, P. et al. Diabetes mellitus—advances and challenges in human β-cell proliferation. Article CAS PubMed Google Scholar. Rosano, G. Heart Failure in Patients with Diabetes Mellitus. McFarlane, P. Chronic Kidney Disease in Diabetes. Article PubMed Google Scholar. Shah, A. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Mameli, C. Explaining the increased mortality in type 1 diabetes. Piscitelli, P. Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD Annals initiative. Woodrow, G. Acute renal failure in patients with type 1 diabetes mellitus. Article CAS PubMed PubMed Central Google Scholar. Atkinson, M. Type 1 diabetes. Hirsch, I. Realistic Expectations and Practical Use of Continuous Glucose Monitoring for the Endocrinologist. Article CAS Google Scholar. Jankun, J. Can drinking black tea fight diabetes: literature review and theoretical indication. Central European Journal of Immunology 37 , — CAS Google Scholar. Huang, E. Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. Alkhatib, A. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric Curcuma longa. Kao, N. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Hatcher, H. Curcumin: From ancient medicine to current clinical trials. Pan, M. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. Prasad, S. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice. Kumar, R. Enhancing the Solubility of Fenofibrate by Nanocrystal Formation and Encapsulation. Shanmugam, R. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles. Yadav, A. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chuah, L. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Peng, S. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Chiang, J. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Article PubMed PubMed Central Google Scholar. American Diabetes Association, A. Diagnosis and Classification of Diabetes Mellitus. El-Azab, M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Pihlajamäki, J. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. MJLR Rahimi, H. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6 , — CAS PubMed PubMed Central Google Scholar. Wang, C. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Milani-Nejad, N. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Wang, J. Causes and Characteristics of Diabetic Cardiomyopathy. The Review of Diabetic. Duffield, J. Cellular and molecular mechanisms in kidney fibrosis. Kanasaki, K. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Xie, Y. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Tsai, P. Effects of dietary glutamine on adhesion molecule expression and oxidative stress in mice with streptozotocin-induced type 1 diabetes. Deeds, M. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Article ADS CAS PubMed PubMed Central Google Scholar. Williams, L. Diabetes-Related Cardiac Dysfunction. Wellen, K. Inflammation, stress, and diabetes. Xu, Z. Bixin ameliorates high fat diet-induced cardiac injury in mice through inflammation and oxidative stress suppression. Menon, V. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease Advances in Experimental Medicine and Biology Ch. Chapter 3, — Meng, B. Antioxidant and Antiinflammatory Activities of Curcumin on Diabetes Mellitus and its Complications. Okada, K. Curcumin and Especially Tetrahydrocurcumin Ameliorate Oxidative Stress-Induced Renal Injury in Mice. Zhang, D. Curcumin and Diabetes: A Systematic Review. Youn, G. |
 Thank you for visiting nature. You are using a browser Chitosan for heart health with fo support for Chitosna. To obtain the best experience, we recommend Angiogenesis inhibitors use a more up to date browser Cbitosan turn off BMR calculator for men mode Angiogenesis inhibitors Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Abnormal conduction and improper electrical impulse propagation are common in heart after myocardial infarction MI. The scar tissue is non-conductive therefore the electrical communication between adjacent cardiomyocytes is disrupted. In the current study, we synthesized and characterized a conductive biodegradable scaffold by incorporating graphene oxide gold nanosheets GO-Au into a clinically approved natural polymer chitosan CS.
Thank you for visiting nature. You are using a browser Chitosan for heart health with fo support for Chitosna. To obtain the best experience, we recommend Angiogenesis inhibitors use a more up to date browser Cbitosan turn off BMR calculator for men mode Angiogenesis inhibitors Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Abnormal conduction and improper electrical impulse propagation are common in heart after myocardial infarction MI. The scar tissue is non-conductive therefore the electrical communication between adjacent cardiomyocytes is disrupted. In the current study, we synthesized and characterized a conductive biodegradable scaffold by incorporating graphene oxide gold nanosheets GO-Au into a clinically approved natural polymer chitosan CS.
Sie sind nicht recht. Geben Sie wir werden besprechen.
ich beglückwünsche, die prächtige Idee und ist termingemäß