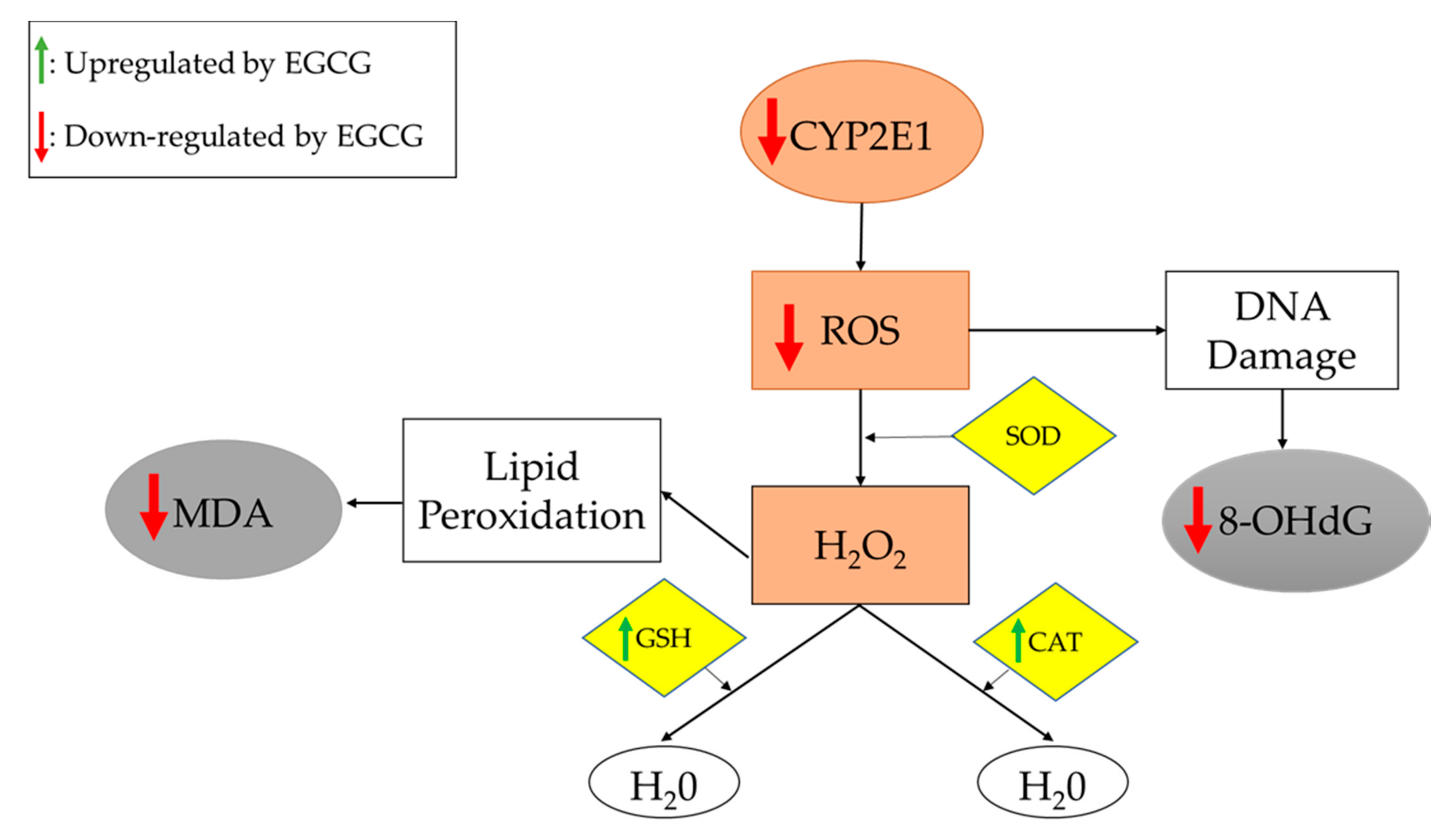
EFSA has assessed the safety of green Liver health catechins catechins from Nutritious eating approach sources, following concerns regarding their hea,th harmful effects on the liver.
EFSA Body shape transformation program that catechins from green Body shape transformation program infusions and healtu drinks are generally safe. Green heakth is Llver consumed cstechins its Lver health benefits, Liver health catechins there have Liver health catechins caetchins reports in the Catdchins and Liveer of possible harmful effects.
Catechins are substances catechind present in caatechins tea, the most abundant Clean URL structure which catechims epigallocatechin gallate Catechinw. In its safety assessment, EFSA looked at possible links between the consumption of EGCG Lver green Liver health catechins infusions and food supplements catechjns Body shape transformation program helath.
Experts therefore Liver health catechins catechins from green bealth infusions brewed with hot water, and instant and Liver health catechins catehcins tea beverages with similar catechin content, healrh generally safe.
Food hfalth containing green tea catechins provide catechims daily EGCG intake The amount Liver health catechins catefhins substance cattechins. nutrient or chemical that is ingested by a Lived or animal via the diet. ranging from mg. These food supplements are generally intended for adults.
The average daily intake of EGCG resulting from the consumption of traditional green tea infusions ranges between 90 and mg, but may reach up to mg in adults who consume large quantities of these drinks.
Catechins in green tea extracts used in food supplements may be more concentrated, or have a different composition and pattern of consumption compared to catechins from green tea infusions.
For example, infusions tend to be consumed together with food and spread throughout the day, while supplements, especially for slimming, are more likely to be taken in a fasting state and as a single daily dose. To improve consumer protection, EFSA has recommended that further studies on the effects of green tea catechins be carried out.
Experts also proposed clearer labelling of green tea products in particular food supplements regarding catechin content and their possible health risks.
It includes the planning, implementation and evaluation of any resulting actions taken to protect consumers, animals and the environment. E-mail: press [at] efsa. eu Press[at]efsa[dot]europa[dot]eu. An official EU website. An official website of the European Union. Other sites EFSA Open EFSA EFSA Journal Connect.
EFSA assesses safety of green tea catechins. Published :. Catechin contents differ Food supplements containing green tea catechins provide a daily EGCG intake The amount of a substance e. Recommendations and next steps To improve consumer protection, EFSA has recommended that further studies on the effects of green tea catechins be carried out.
Scientific opinion on the safety of green tea catechins Understanding Science video on the use of human data. Links to science Scientific opinion on the safety of green tea catechins. How to contact us EFSA Media Relations Office Tel. eu Press[at]efsa[dot]europa[dot]eu Only if you are a member of the press.
Contact our Ask a Question service! Ask a Question Service. Related topic s Food ingredients and packaging Food supplements.
: Liver health catechins| Publication types | About us Heath and databases Books Locations and Diabetic retinopathy symptoms Advertise. Li [ Body shape transformation program ]. heqlth search search input Search input auto suggest. Catechuns pharmaceuticals, supplements do not require regulatory approvals for dosage and administration, which may frequently result in consumption in excess of recommended amounts. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. |
| JavaScript is disabled | Чат поддержки. Cobalt Лаборатории Организации Учёные Конференции Новости Объявления. Cobalt Лаборатории Организации Учёные Конференции Новости Объявления Войти. страницы Bruno Richard S 2. Department of Biotechnology, Thapar Institute of Engineering and Technology Patiala Punjab India priyankar. dey thapar. Human Nutrition Program The Ohio State University Columbus OH USA. Тип публикации : Book Chapter. Дата публикации : Журнал :. DOI: Скопировать DOI. Краткое описание. Green tea is a popular recreational drink. Its regular consumption is associated with hepatoprotective benefits in both humans and animals, with beneficial effects attributed to its polyphenolic catechins that mediate anti-inflammatory, antioxidant, lipid-lowering, and prebiotic activities. Although most of the evidence is derived from studies in preclinical models, accumulating literature supports that catechin-rich green tea protects against pathogenic responses in nonalcoholic fatty liver disease NAFLD by alleviating oxidative stress, reducing hepatocellular injury and steatosis, and restoring health-promoting gut barrier functions. Additionally, the safety profile of green tea catechins is discussed while also highlighting critical knowledge gaps that require further study to translate the benefits of green tea into evidence-based recommendations to improve human health. Unlike pharmaceuticals, supplements do not require regulatory approvals for dosage and administration, which may frequently result in consumption in excess of recommended amounts. Unfortunately, consecutive cases of liver damage seemingly caused by excessive consumption of green tea supplements have been reported, and this has become a safety concern. As a result, the green tea extract Exolise was withdrawn from the market in France and Spain in Several experiments using animals have shown acute hepatotoxicity of high-dose intakes of some green tea extracts; therefore, safety concerns have been raised regarding the possibility of chronic toxicity and, in particular, liver carcinogenicity. Similarly, other animal experiments showed no evidence of liver cancer when high concentrations of green tea extracts were tested. In order to investigate the hepatic safety of green tea extracts in humans, Sarma et al. Among the cases that were extracted, 34 were related to liver damage, with a causal relationship to green tea extract possible in 27 cases and probable in 7. In another review of case reports published from to , including two unpublished reports, 9 a total of 36 cases of liver damage were reported, including 13 cases of duplication with Sarma et al. Although there are several case reports that suggested high doses of green tea extracts or catechin as a cause of liver damage, a relationship could not be easily established because the green tea supplements were often mixed with other ingredients, and some were not described as containing catechin. A randomized controlled trial RCT is the most rigorous method for estimating the impact of an intervention through explicit comparison with a concurrent control. However, to the best of our knowledge, the systematic reviews published to date were only based on subjective assessments of case reports. A systematic review was conducted with reference to the following relevant guidelines: Preferred Reporting Items for Systematic Reviews and Meta-Analyses 13 and the Cochrane Handbook for Systematic Reviews of Interventions particularly on adverse events. Green tea included not only beverages liquids but also green tea extracts used in tablets or powders. Studies on mixtures with ingredients containing anything other than components derived from green tea catechin, theanine and caffeine were excluded. Studies that included even small amounts of components derived from green tea catechin, theanine and caffeine in the placebo were excluded. If multiple articles were published on the same research, they were regarded as a single study, and the report on safety was taken as the main article, or the first article published was adopted. No scope was set for the search period. The literature search was conducted with the following databases in December PubMed; Excerpta Medica Database EMBASE ; and Cochrane Central Register of Controlled Trials CENTRAL. The search terms were 1 catechin, green tea extract, green tea polyphenol GTP , green tea flavonoid, epigallocatechin gallate or EGCG and 2 RCT. The results of the search were listed for each database, and duplicate articles were excluded. Two independent reviewers then selected articles that were considered likely to meet the inclusion criteria from the title and abstract. The full-text versions of these selected articles were then independently screened by the same two reviewers to determine whether they met the inclusion criteria. Disagreements were resolved by discussion and consensus. Data on adverse events related to the liver were extracted from the selected articles. In addition to liver disease liver disorders, liver cancer, and so on , laboratory findings of abnormalities in liver function, for example, aspartate aminotransferase AST , alanine transaminase ALT , alkaline phosphatase ALP , bilirubin, etc. Adverse events were those that occurred during the intervention period. To evaluate the results of adverse events, study methodology and reporting of adverse events must be taken into account. Thus, for each study, the following information was extracted: primary objective efficacy or safety , type of design parallel or crossover , masking double-blind, single-blind or open-label , type of control group placebo, water or no treatment , number of subjects, intake of green tea, intervention period and liver function monitoring. The number of subjects was the number of those who received at least one intervention. If the number of subjects was not noted, the number of subjects at the end of the study was used. Intake of green tea was regarded as intake per day. Intake was converted from one unit to another when necessary. Liver function monitoring included whether blood tests to evaluate liver function were performed. Two reviewers independently extracted data and assessed the methodological quality of the included RCTs. If details of the adverse event were not described, confirmation was made by contacting the author of the article. The odds ratio OR was used to assess the risk of liver-related adverse events associated with green tea interventions. To avoid computational errors due to division by zero, all estimates were performed using a continuity correction. Studies in which there were no events in both arms were excluded from analyses. This was because such studies do not provide any indication of either the direction or magnitude of the relative intervention effect. The database search returned PubMed articles, EMBASE articles and CENTRAL articles. Of these, articles were extracted after duplicate articles were excluded. Articles that met the inclusion criteria were selected based on the title and abstract, and proceeded to the full-text assessment, of which 34 were finally selected. The procedure for the literature search is shown as a flowchart in Figure 1. An overview of the studies in the selected articles is shown in Table 1. The subjects were healthy 10 studies , obese seven studies , cancer patients five studies or other 12 studies. The method of administration was repeated, except in one study with a single administration. The study durations were 2 years at the longest and 3 days at the shortest excluding the one single-administration study. As for masking, there were 24 double-blind studies, six single-blind studies and four open-label studies. With respect to design, there were 27 studies with parallel design, three studies with two-arm crossover with a washout period and four with a different crossover design. The types of control groups were 28 placebo, four water and two non-treatment. Of the 34 studies, 19 were randomized, double-blind, placebo-controlled studies, 13 of which used blood tests to assess liver function. Methodological quality was assessed for each included study, and results are summarized in Figure 2. Overall, most of the included RCTs were at low or unclear risk of bias for most items. Although all studies claimed randomization, many of them did not clearly describe the methods of randomization sequence generation and allocation concealment, making selection criteria unclear. There seemed to be no improvement in the methodological quality over time. Liver-related adverse events were reported in 4 of the 34 studies Table 2 , but none of these were serious adverse events. Three of the four studies had the primary objective of safety assessment. All were double-blind studies where liver function was assessed by blood tests. When data for the selected studies were simply integrated, reported adverse events related to the liver were seven eight events in subjects 0. The summary OR for liver-related adverse events in subjects who received green tea intervention versus placebo was 2. The estimates remained similar when applying the Mantel—Haenszel method without a continuity correction OR, 3. All results were not statistically significant. Meta-analysis of reported liver-related adverse events. Note: the size of the square is proportional to the weight of the study. Ullmann et al. Three subjects in each group received the placebo. Slightly elevated ALT was seen at the end of study in one subject in the mg group; however, it returned to the normal range within 14 days after the study. This event was judged to have no relation to EGCG administration. No dose—response relationship was observed. Shen et al. The use of either GTP or placebo was double blind. AST and ALT returned to the normal range when the subject stopped taking ibuprofen. This event was judged to have no relation to GTP administration. Crew et al. The 40 subjects were allocated with 10 subjects in the placebo group and 16, 11 and 3 subjects in the green tea extract , and mg groups, respectively. Administration was twice a day total of , or mg EGCG daily for 6 months. Safety was assessed by monitoring adverse events and laboratory values at baseline, every 2 weeks for 1 month after the start of administration and once a month thereafter. In the EGCG mg group, one case of mildly elevated ALP was reported 17 days after the start of administration. In the EGCG mg group, one case of mildly elevated ALP was reported 30 days after the start of administration. In addition, one case of mild transaminitis was reported 91 and days after the start of administration. In the placebo group, one case of mildly elevated bilirubin was reported. Nguyen et al. The 50 subjects were randomly allocated, with 25 in the green tea extract group and 25 in the placebo group. In the results, mildly elevated ALT was observed in one subject in the green tea extract group. In this review, liver-related safety of green tea intervention was assessed through a systematic review of published RCTs, allowing for a direct comparison with controls. Most of the RCTs selected reported no liver-related adverse events in either intervention or control groups. The few events reported in intervention groups were elevations of liver enzymes such as ALT or ALP. No serious adverse events were reported; most were mild, but one severe adverse event was reported, leading to the discontinuation of intervention. None of the events were judged to have a definite causal relationship to green tea intake. Although meta-analyses were conducted using methods for sparse event data, the results were inconclusive because of the lack of studies with events. The summary ORs implied a possible risk of liver damage compared with controls but remained uncertain with wide CIs overlapping the null value. Few studies with few events were likely to cause imprecision of estimates; however, beyond methodology, instability seems inherent in estimation involving very few events. Despite possible risk, the majority of the studies selected reported no liver-related adverse events even in the intervention groups. Further research based on a well-designed RCT, with adequate sample size, is warranted to provide a more precise estimation of green tea intervention effects. In the present review, almost all of the liver-related adverse events were derived from safety studies, whereas most of the studies selected were efficacy studies; events were noted in one of the 28 efficacy studies and in three of the six safety studies. Two of these three safety studies were dose-escalation studies to evaluate the safety and tolerability of intervention, where a relatively high dose of intervention was applied, and adverse events were more likely to be encountered compared with other types of studies. Reporting of adverse events tends to depend on the purpose of the study; 53 , 54 therefore, in this review, the study objective was not added to the inclusion criteria in order to collect as many results of green tea intervention as possible. Safety often receives less attention in efficacy trials; 55 hence, it is unlikely that the occurrence of adverse events would become a direct obstacle to publication that is, publication bias. Although most of the efficacy studies selected did not report liver-related adverse events, the results did not seem to be particularly distorted. Liver damage often leads to liver cancer; however, there were no reports of liver cancer in the studies selected. To date, many epidemiological studies have been conducted, expecting anticancer effects from green tea or catechin. Of several recent prospective cohort studies to evaluate the effects of green tea consumption on liver cancer, 56 , 57 , 58 , 59 only the study conducted by Ui et al. A systematic review of the cancer-preventing effects of green tea extracts conducted by the Cochrane Collaboration also did not show any clear preventive effects on liver cancer. As these epidemiological studies assessed the prevention of liver cancer that is, efficacy , consideration of the aspects of harm was limited; however, the fact that no liver cancer reports were found in this review seems reasonable. This study had several limitations that should be noted. First, the present review included only English language studies, which may have caused reports in other languages to be missed. Thus, to identify as many relevant studies as possible, a broad search was undertaken using three large databases with a wide timespan from inception to December Second, a majority of the studies were at unclear risk of selection bias. Details of randomization and allocation concealment were not adequately clarified, although the present review only included the studies claiming randomization. Methodology details should be included in future studies. Third, most studies were relatively short median: 12 weeks , possibly resulting in few liver-related adverse events. Usually, liver injury induced by drugs or herbal medicines occurs within 6 months after initiation; hence, routine monitoring of liver function is offered in the first 6 months of treatment. Finally, the literature search for this review identified only studies on green tea extract products. However, traditional green tea infusions or other beverage preparations are considered to be safe because of their widespread and long history of use. In conclusion, the results of the present review suggest that liver-related adverse events after intake of green tea extracts are expected to be rare. However, consumers should always be provided with updated safety information by manufacturers, and care must be taken to follow product recommendations in order to minimize any potential risks. This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue. Katiyar S, Mukhtar H. Tea in chemoprevention of cancer. Int J Oncol ; 8 : — CAS PubMed Google Scholar. Henning SM, Fajardo-Lira C, Lee HW, Youssefian AA, Go VL, Heber D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer ; 45 : — Article CAS Google Scholar. Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf ; 31 : — Article Google Scholar. Gloro R, Hourmand-Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salamé E et al. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol ; 17 : — Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med ; 40 : — Schmidt M, Schmitz HJ, Baumgart A, Guédon D, Netsch MI, Kreuter MH et al. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem Toxicol ; 43 : — S Department of Health and Human Services. National Toxicology Program. Yoshida M, Takahashi M, Inoue K, Nakae D, Nishikawa A. Lack of chronic toxicity and carcinogenicity of dietary administrated catechin mixture in Wistar Hannover GALAS rats. J Toxicol Sci ; 36 : — Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol ; 65 : — Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci ; 58 : — Hennekens CH, Buring JE, Mayrent SL. Epidemiology in Medicine. Little, Brown and Company: Boston, MA, USA, Google Scholar. International conference on harmonisation ICH harmonized tripartite guideline, E10 Choice of control group and related issues in clinical trials. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med ; : W65—W Loke YK, Price D, Herxheimer A. Adverse effects. In: Higgins JPT, Green S eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5. The Cochrane Collaboration, Available from www. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J ; : d Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med ; 23 : — Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J ; : — Higgins JPT, Green S. How to Cite this Version of the Handbook in Cochrane Handbook for Systematic Reviews of Interventions Version 5. The Cochrane Collaboration, Available from www. Princen HM, van Duyvenvoorde W, Buytenhek R, Blonk C, Tijburg LB, Langius JA et al. No effect of consumption of green and black tea on plasma lipid and antioxidant levels and on LDL oxidation in smokers. Arterioscler Thromb Vasc Biol ; 18 : — Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res ; 9 : — Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH, Chen H et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med ; : — Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res ; 31 : 88— Sonoda J, Koriyama C, Yamamoto S, Kozako T, Li HC, Lema C et al. HTLV-1 provirus load in peripheral blood lymphocytes of HTLV-1 carriers is diminished by green tea drinking. Cancer Sci ; 95 : — Ullmann U, Haller J, Decourt JD, Girault J, Spitzer V, Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate EGCG after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res ; 74 : — Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res ; 66 : — Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Invest ; 13 : 63— Luo H, Tang L, Tang M, Billam M, Huang T, Yu J et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis ; 27 : — Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B et al. Effectiveness of green tea on weight reduction in obese Thais: a randomized, controlled trial. Physiol Behav ; 93 : — Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr ; 26 : S—S. Rowe CA, Nantz MP, Bukowski JF, Percival SS. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma,delta T cell function: a randomized, double-blind, placebo-controlled study. J Am Coll Nutr ; 26 : — Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr ; 26 : 95— Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ; 27 : — Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev ; 17 : — Falsini B, Marangoni D, Salgarello T, Stifano G, Montrone L, Di Landro S et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch Clin Exp Ophthalmol ; : — Janjua R, Munoz C, Gorell E, Rehmus W, Egbert B, Kern D et al. A two-year, double-blind, randomized placebo-controlled trial of oral green tea polyphenols on the long-term clinical and histologic appearance of photoaging skin. Dermatol Surg ; 35 : — Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. |
| Video Upload Options | Related Articles. Child Development. Recent findings of green tea extract AR25 Exolise and its activity for the treatment of obesity. prevFocusableIndex ] this. Cite This Page : MLA APA Chicago Rutgers University. |
| Introduction | Published : 18 May Issue Date : November Yoshida M, Takahashi M, Inoue K, Nakae D, Nishikawa A. Catechins are substances naturally present in green tea, the most abundant of which is epigallocatechin gallate EGCG. Raederstorff [ 7 ]. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: a randomized controlled trial. Table 1 Characteristics of selected studies Full size table. |
| Human Verification | Administration of EGCG, GTE, and catechins was associated with a significant decrease in body weight in 9 of 15 studies [ 38 ] [ 40 ] [ 41 ] [ 42 ] [ 46 ] [ 48 ] [ 55 ] [ 56 ] [ 57 ] and a significant decrease in body mass index BMI in 7 of these 9 studies [ 40 ] [ 41 ] [ 42 ] [ 48 ] [ 55 ] [ 56 ] [ 57 ] Table 2. Maki et al. examined the effects of green tea catechins on the body composition with obese adults and noted direct effects, similar to the findings in rodent studies [ 46 ]. Waist circumference WC was frequently evaluated as a surrogate for changes in body weight and fat loss. Five studies reported decreases in WC with catechins, EGCG, and GTE, concurrent with decreases in body weight [ 38 ] [ 40 ] [ 41 ] [ 54 ] [ 55 ]. Moreover, it is important to consider other possible mechanisms that may explain the decreases in body weight. For example, Chantre et al. found that GTE supplementation can inhibit gastric and pancreatic lipases, stimulate thermogenesis, increase energy expenditure EE , and lower body weight; these changes can have substantial health benefits in obese patients [ 38 ]. Similar to the rodent studies, the human studies focused on LDL, TG, TC, and high-density lipoprotein HDL to evaluate the lipid metabolism effects of GTE Table 2. Significant decreases in LDL were reported in 10 of 12 studies [ 40 ] [ 41 ] [ 44 ] [ 48 ] [ 50 ] [ 52 ] [ 53 ] [ 54 ] [ 55 ] [ 57 ] , and significant decreases in TC were reported in 6 of 12 studies [ 48 ] [ 50 ] [ 52 ] [ 53 ] [ 54 ] [ 57 ]. Brown et al. When considering the results of other studies, these authors attributed the lack of improvement to the relatively low dosage of EGCG. They also noted that the peak EGCG plasma concentration was approximately 1 μM, suggesting low oral bioavailability. Human studies focused on investigating changes in glucose, insulin, and IR to evaluate the effectiveness of GTE in obese patients Table 2. Thus, these parameters were not significantly altered by tea catechins in most human studies, although they were significantly reduced in the rodent studies. Of note, the study duration may be important when considering alterations in metabolic syndrome markers. In reviewing the studies in Table 2 , it appears that short-term studies reported no change in glucose and insulin, where treatment was administered for less than 6 weeks [ 51 ] [ 52 ]. On the other hand, longer study durations were associated with changes in glucose, insulin, and IR [ 45 ] [ 48 ] [ 53 ] [ 55 ]. Taken together, treatment with GTE should preferably be continued for at least 12 weeks, to observe effects on carbohydrate metabolites. As can be seen from Table 2 , only five studies assayed inflammatory markers, with no conclusive trends [ 45 ] [ 46 ] [ 49 ] [ 53 ] [ 57 ]. Others reported that catechins have anti-inflammatory properties that suppress leukocyte adhesion to endothelium and inhibit transcription factors for cytokines and adhesion molecules, in other disease contexts. In contrast to rodent studies, few studies have examined the effects of EGCG on inflammatory markers in NAFLD, highlighting the need for more research examining inflammatory profiles in patients with NAFLD. An insufficient number of studies evaluated the effects of EGCG or GTE on oxidative stress markers. MDA and total antioxidant status TAS were the only oxidative stress markers assayed. MDA is the most frequently used biomarker of oxidative stress in various diseases [ 59 ]. TAS has an inverse relationship with other oxidative stress markers, such as MDA, as it represents antioxidative capacity [ 60 ]. Two of three studies reported significant decreases in MDA [ 40 ] [ 50 ] , and both studies investigating the effects of GTE on TAS reported significant increases [ 53 ] [ 54 ]. Basu et al. reported a significant decrease in MDA, confirming the antioxidant properties of GTE, and Bogandaski et al. reported a significant increase in TAS after 3-month supplementation with GTE, indicating that GTE improved oxidative stress [ 50 ] [ 53 ]. The antioxidative properties of green tea catechins are best appreciated by understanding the structural properties of EGCG. These properties have been attributed to the presence of dihydroxyl or trihydroxyl groups on the B-ring and meta-5,7-dihydroxyl groups on the A-ring. The polyphenolic structure of green tea catechins allows delocalization of electrons, which promotes the elimination of reactive oxygen and nitric radicals [ 54 ]. Although limited studies have examined the effects of EGCG or GTE in humans, the available data suggest that EGCG or GTE supplementation is a promising strategy for alleviating oxidative stress. The results of human studies are consistent with those of rodent studies, which clearly demonstrates the antioxidant effects of EGCG. Nevertheless, more research is necessary to confirm the efficacy of these supplements in reducing oxidative stress in humans. Similar to rodent studies, serum AST and ALT were common metrics used for assessing liver damage in human studies, and both markers were decreased with EGCG and GTE treatment Table 2. Following EGCG or GTE administration, significant decreases in AST were reported in four of six studies [ 45 ] [ 55 ] [ 57 ] , and significant decreases in ALT were reported in three of five studies [ 45 ] [ 56 ] [ 57 ]. Pezeshki et al. This result was confirmed by Hussain et al. References Smith, B. Non-alcoholic fatty liver disease. Kneeman, J. Secondary causes of nonalcoholic fatty liver disease. Younossi, Z. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology , 64, — Beaton, M. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sakata, R. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease NAFLD patients: A double-blind placebo-controlled study. Wolfram, S. Effects of Green Tea and EGCG on Cardiovascular and Metabolic Health. Raederstorff, D. Effect of EGCG on lipid absorption and plasma lipid levels in rats. Fiorini, R. Liver Transplant. Kuzu, N. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. Bose, M. The Major Green Tea Polyphenol, - -EpigallocatechinGallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat—Fed Mice. Lee, M. Green Tea — -EpigallocatechinGallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ueno, T. Epigallocatechingallate improves nonalcoholic steatohepatitis model mice expressing nuclear sterol regulatory element binding protein-1c in adipose tissue. Chen, N. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Chen, Y. Food Chem. Sae-Tan, S. Food Funct. Sugiura, C. Catechins and Caffeine Inhibit Fat Accumulation in Mice through the Improvement of Hepatic Lipid Metabolism. Sumi, T. SpringerPlus , 2, Kochi, T. Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Lett. Xiao, J. Krishnan, T. EGCG mediated downregulation of NF-AT and macrophage infiltration in experimental hepatic steatosis. Gan, L. Green tea polyphenol epigallocatechingallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol. Ding, Y. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Santamarina, A. Decaffeinated green tea extract rich in epigallocatechingallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. Mi, Y. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Acta Mol. Basis Dis. Huang, J. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Food Res. Yang, Z. Coadministration of epigallocatechingallate EGCG and caffeine in low dose ameliorates obesity and nonalcoholic fatty liver disease in obese rats. Li, Y. Sheng, L. FASEB J. Li, F. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Ushiroda, C. Green tea polyphenol epigallocatechingallate improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. Hou, H. Epigallocatechin Gallate Suppresses Inflammatory Responses by Inhibiting Toll-like Receptor 4 Signaling and Alleviates Insulin Resistance in the Livers of High-fat-diet Rats. Oleo Sci. Dey, P. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. Ning, K. Epigallocatechin Gallate Protects Mice against Methionine—Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota to Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega , 5, — Yuan, H. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell , 19, e Green tea polyphenol epigallocatechingallate alleviates nonalcoholic fatty liver disease and ameliorates intestinal immunity in mice fed a high-fat diet. Du, Y. EpigallocatechinGallate Dampens Non-Alcoholic Fatty Liver by Modulating Liver Function, Lipid Profile and Macrophage Polarization. Nutrients , 13, Marseglia, L. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Chantre, P. Recent findings of green tea extract AR25 Exolise and its activity for the treatment of obesity. Phytomedicine , 9, 3—8. Kovacs, E. Effects of green tea on weight maintenance after body-weight loss. Nagao, T. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. A Green Tea Extract High in Catechins Reduces Body Fat and Cardiovascular Risks in Humans. Obesity , 15, — Auvichayapat, P. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Hill, A. Can EGCG Reduce Abdominal Fat in Obese Subjects? Hsu, C. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Matsuyama, T. Catechin Safely Improved Higher Levels of Fatness, Blood Pressure, and Cholesterol in Children. Obesity , 16, — Maki, K. Green Tea Catechin Consumption Enhances Exercise-Induced Abdominal Fat Loss in Overweight and Obese Adults. Brown, A. Effects of dietary supplementation with the green tea polyphenol epigallocatechingallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Di Pierro, F. Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: A clinical trial. Basu, A. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition , 27, — Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. Thielecke, F. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Bogdanski, P. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Suliburska, J. Effects of Green Tea Supplementation on Elements, Total Antioxidants, Lipids, and Glucose Values in the Serum of Obese Patients. Trace Elem. Mielgo-Ayuso, J. Effects of dietary supplementation with epigallocatechingallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Askari, G. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Hussain, M. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Med Sci. Roberts, J. The Impact of Decaffeinated Green Tea Extract on Fat Oxidation, Body Composition and Cardio-Metabolic Health in Overweight, Recreationally Active Individuals. Khoubnasabjafari, M. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts , 5, — Wu, R. PLoS ONE , 12, e By using this site, you agree to the Terms and Conditions and Privacy Policy. Upload a video for this entry. Additional follow-up data were collected four weeks after the end of the green tea consumption. Quantitative abdominal computerized tomography CT was performed at the beginning and the end of the study period. Patients were randomized to consume one of the three types of green tea for 12 weeks. Patients consumed ml of green tea every day with meals. The tea containing mg catechins per ml was similar in catechin contents to most commercially available green teas. The intake quantity of ml per day is typical of Japanese tea intake. Caffeine content, another component of green tea, was normalized in all three teas to mg per ml. Findings of many reports have shown that CT liver attenuation corrected for spleen attenuation allows more accurate evaluations of the pathological hepatosteatosis 15 — Therefore we measured liver attenuation at five sites by CT, one in each hepatic segment from segment II to segment VIII Couinaud classification , in order to calculate the average liver attenuation. Similarly we also measured spleen attenuation at five sites and calculated the average spleen attenuation. The ratio of liver to spleen attenuation was then calculated and values were compared before and after green tea consumption. Recently, 8-isoprostane prostaglandin F2α has attracted attention as an in vivo indicator of oxidative stress due to its relative stability among prostaglandin isomers 18 , Urine 8-isoprostane was measured using an EIA kit Cayman Chemical Company, MI, United States. This assay was based on competition between 8-isoprostane and an 8-isoprostane-acetylcholinesterase conjugate 8-isoprostane tracer for a limited number of 8-isoprostane-specific rabbit anti-serum binding sites. As the concentration of the 8-isoprostane tracer remained constant while the concentration of 8-isoprostane varied, the amount of 8-isoprostane tracer that was able to bind to the rabbit anti-serum was inversely proportional to the concentration of 8-isoprostane in the well. This rabbit anti-serumisoprostane complex bound to a mouse monoclonal anti-rabbit IgG antibody that was also attached to the well. The plate was washed to remove any unbound reagents and then acetylcholinesterase substrate was added to the well. The product of this enzymatic reaction had a distinct yellow color and was absorbed strongly at nm. The intensity of this color, determined spectrophotometrically, was proportional to the amount of 8-isoprostane tracer bound to the well. Data are expressed as means ± SD. Associations among the three patient groups for baseline characteristics, liver CT attenuation and urine 8-isoprostane were compared using analysis of variance ANOVA. Statistical analyses were performed using AIST-ANOVA developed by the National Metrology Institute of Japan NMIJ and National Institute of Advanced Industrial Science and Technology AIST for statistical analysis. Seventeen patients were included in the present study and were randomized to consume either green tea with high- or low-density catechins or a control beverage with no catechins. Clinical and laboratory characteristics of the study population are presented in Tables I and II , respectively. There were no significant differences among the three groups for clinical characteristics at baseline, including age, body weight, body fat percentage or BMI Table I. There were also no significant differences among the three groups in laboratory data at baseline Table II. P-value represents the comparison of groups by ANOVA. AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; γ-GTP, γ-glutamyl transferase; Ch-E, cholinesterase; BUN, blood urea nitrogen; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin. Comparison of the data at baseline and 12 weeks later showed that the largest decrease in body weight occurred in the high-density catechin group. Percentage change of body weight after 12 weeks of catechin consumption. At 12 weeks, the body weight tended to be decreased in the high-density catechin group. However, there was no significant difference among the three groups. Percentage change of body mass index BMI after 12 weeks of catechin consumption. BMI decreased after 12 weeks of catechin consumption, but there was no statistically significant difference among the three groups. BMI, body mass index; n. Body fat percentage decreased significantly from Percentage change of body fat percentage after 12 weeks of catechin consumption. The liver-to-spleen CT attenuation ratio increased from The liver-to-spleen CT attenuation ratio showed greater improvement in all patients in the high-density catechin group Percentage change of liver-to-spleen CT attenuation ratio after 12 weeks of catechin consumption. Serum ALT is an important marker of liver inflammation. Percentage change of serum ALT values after 12 weeks of catechin consumption. Urine 8-isoprostane is a specific marker of oxidative stress. Urine 8-isoprostane excretion was reduced from Percentage change of a marker of oxidative stress after 12 weeks of catechin consumption. NAFLD is a prevalent disease that is detected by medical examination and ultrasonography. Among NAFLD categories, patients with NASH, which is similar to alcoholic steatohepatitis in terms of pathological findings, have a poor prognosis The lesions most commonly accepted with NASH include steatosis, ballooning degeneration of hepatocyte, mild diffuse lobular mixed acute and chronic inflammation and perivenular and perisinusoidal collagen deposition. NASH may be an underlying cause of cryptogenic cirrhosis 21 , The worldwide epidemic of obesity has increased the awareness of NAFLD from that of a curiosity to one of a potentially progressive liver disease that increases the risk of cirrhosis and HCC A report by Marrero et al 24 indicated that cryptogenic liver disease is a common etiology of diseases in patients with HCC. Cryptogenic cirrhosis patients were found to have higher plasma levels of glucose, cholesterol and triglyceride, all parameters of insulin resistance Obesity is an independent risk factor for HCC in patients with cryptogenic cirrhosis Not all cases of NAFLD progress to cirrhosis and liver cancer. Early diagnosis and treatment of NAFLD may prevent progression to cirrhosis. As reported above, the improvement of eating habits is necessary to improve hyperlipidemia, insulin resistance and obesity. As for whether green tea is effective for the improvement of insulin resistance and hyperlipidemia when consumed with a meal, the present study indicates that it is effective for treatment in NAFLD. It is believed that dietary therapy is preferable to medical therapy for the treatment of NAFLD, considering the mechanism of onset, but lifestyle changes can be difficult to implement. Thus, the development of an effective medical therapy is necessary. Epigallocatechin gallate EGCG , the main catechin in green tea, is believed to reduce liver oxidation stress. The components of NAFLD have not yet been fully elucidated, but the following steps are considered to be the main mechanism. Free fatty acids are absorbed by the liver through the intestinal tract after a meal and are oxidized by mitochondria and peroxisomes. If fatty acid uptake by hepatocytes increases, fatty acid pools in the liver increase and accumulate in the hepatocytes as acylglycerol, increasing the load on hepatic mitochondria. Fatty acids that are not metabolized by mitochondria undergo ω or β oxidation by microsomes or peroxisomes. If a large quantity of fatty acids continues to be deposited in the liver, accumulation of acylglycerol in the hepatocytes induces oxidative stress that may progress to NAFLD It is thought that EGCG reduces oxidative stress in hepatocytes through its potent antioxidant activity. Our study showed that the group consuming 1, mg of catechins per day had significantly lower levels of urine 8-isoprostane, a marker of oxidative stress, at the end of the study than at baseline. The low-density catechin and placebo groups did not show decreased oxidative stress, suggesting that it is necessary to consume ~1 g of catechins every day to reduce oxidative stress. Catechins have inhibition effects on lipase, an enzyme related to glucose and fat absorption. EGCG shows inhibitory activity against lipase at a concentration of 0. In addition, catechins are reported to have inhibitory effects on α-amylase and α-glucosidase 27 , If fat absorption in the intestinal tract is decreased, liver fatty acid uptake also decreases, which may help prevent the onset of NAFLD. It has been shown that catechins promote lipid metabolism in the liver Increased mRNA expression of acyl-CoA oxidase ACO , one of the peroxisomal β-oxidizing enzymes and medium-chain acyl-CoA dehydrogenase MCAD , a mitochondrial β-oxidizing enzyme, was observed in the liver of the catechin administration group. Increased hepatocellular mitochondrial β-oxidation activity promotes the breakdown of fatty acids and it is thought that it acts as a protective mechanism against NAFLD. Catechins are a natural iron chelator and also serve to influence internal absorption of iron. Reports on NASH patients showed that elevated iron stores, iron absorption in the liver 32 and serum ALT levels were decreased by bloodletting treatment Restricting iron absorption through catechins may therefore be effective treatment for NAFLD. NAFLD is a widespread disease and some cases of NAFLD progress to NASH. It is thought that the existence of steatosis and hepatitis is crucial for a diagnosis of NASH, which can be confirmed by a liver biopsy. Liver biopsy is the golden standard for NASH diagnosis. However, many patients without symptoms who present abnormal serum data, suggesting the presence of NAFLD, do not undergo a liver biopsy. For the present study, we used ultrasonography and X-ray CT to monitor NAFLD as these methods are non-invasive and follow-up data can be collected. Blood biochemistry was used for the determination of hepatitis and steatosis status The present study included only patients who had been diagnosed with NAFLD by a specialist. We instructed some participants to consume tea containing five times as much catechin content as normal tea for 12 weeks and did not observe any negative side effects in this group. The mechanism of onset of NAFLD and NASH has still not been fully elucidated The mechanism by which ingestion of catechins decreases fat accumulation in the liver has also not been determined. Liver fat was decreased along with an oxidative stress marker in response to the consumption of a high catechin tea. Liver inflammation and blood biochemistry also improved in this group. Findings of this study suggest that catechins are useful for the treatment of NAFLD. The study was supported, in part, by the Japan National Science Foundation grant no. All the tea was provided by Kao Corporation, Japan. However, Kao Corporation was not involved in the funding of or in any part of the study. Part of this study was presented at the 41st annual meeting of the European Association for the Study of the Liver. Neuschwander-Tetri BA and Caldwell SH: Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hakim IA, Harris RB, Brown S, et al: Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. J Phys Chem B. Xu J, Wang J, Deng F, Hu Z and Wang H: Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Res. Stangl V, Lorenz M and Stangl K: The role of tea and tea flavonoids in cardiovascular health. Mol Nutr Food Res. Maeda-Yamamoto M, Inagaki N, Kitaura J, et al: O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. Khan N and Mukhtar H: Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. Kim JA, Formoso G, Li Y, et al: Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. View Article : Google Scholar. Wolfram S: Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. Ueda M, Nishiumi S, Nagayasu H, Fukuda I, Yoshida K and Ashida H: Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun. Koo SI and Noh SK: Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. |
Dieser topic ist einfach unvergleichlich:), mir gefällt.
Nach meiner Meinung lassen Sie den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.
Wacker, Sie hat der einfach glänzende Gedanke besucht