Video
#1 Absolute First Sign Of KIDNEY DISEASE Is...Caloric restriction and kidney function -
The BMI was calculated using the standard formula. Office BP was measured with an oscillometric device HEMCP; Omron, Tokyo, Japan with the patient sitting after 15 min of rest. The average of three measurements, 2 minutes apart, was recorded.
Blood for laboratory assessments was sampled the morning after overnight fasting. UAE was measured in three consecutive overnight urine collections, and the median was recorded. The GFR was not normalized for the body surface area to avoid the confounding effect of changes in body surface area associated with diet-induced changes in body weight 15 , 16 , and absolute GFR values were considered for the analyses.
On the following day, the total-body glucose disposal rate GDR was assessed with hyperinsulinemic-euglycemic clamp All data assessors were masked to treatment allocation.
Intervention in the SD aimed to reinforce compliance with the recommended diet. Patients were encouraged to consume moderate and low glycemic index and nutrient-dense foods No particular lifestyle modification was introduced.
Patients allocated to the CR intervention were given a prescription of total calories to consume daily and dietary plans based on exchange systems that deliver a fixed amount of calories per food portion. Weight loss goals were set together with the patients, and to facilitate adherence, patient-dietitian contact in person, by telephone, or e-mail was provided throughout the study period once a week during the first 3 months and once every 2 to 3 weeks during the remaining 3 months.
In case the patient-dietitian contact did not prove enough for maintaining dietary compliance, behavioral intervention strategies, such as stimulus control avoiding triggers that prompt eating , social support assistance from family members and friends in modifying lifestyle behaviors , cognitive restructuring thinking in a positive manner , problem-solving skills systematic method of analyzing problems and identifying possible solutions , and relapse prevention methods to help recovery from episodes of overeating or weight regain , were provided.
Patients were instructed to keep daily records of their weight and weekly fasting glucose measurements. One week before each trimonthly follow-up visit, participants completed a 7-day food diary using household measures.
Diaries were analyzed by means of the dietary analysis software package MètaDieta, Version 1. The dietary software uses official national food composition databases such as the INRAN Istituto Nazionale di Ricerca per gli Alimenti and the IEO Istituto Europeo di Oncologia. Clinical and laboratory parameters, including serum urea levels taken as an indirect marker of dietary protein intake, evaluated at baseline were reevaluated at 3 and 6 months after randomization, with the exception of GFR and GDR, which were reevaluated at 6 months only final visit.
At each visit, adverse events were recorded, and physical and laboratory parameters were assessed for safety. Blood and urine samples were collected after subjects had fasted overnight and were centrally analyzed at the CRC for Rare Diseases.
Routine laboratory parameters were measured by spectrophotometry UniCel Synchron Clinical System DXC; Beckman Coulter s. Serum insulin and angiotensin II concentrations were measured by chemifluorescence Access 2; Beckman Coulter, Inc. and the enzyme immunoassay kit Angiotensin II SPIE-IA; Bertin Pharma, Montigny le Bretonneux, France , respectively, and hs-CRP, apolipoprotein A, apolipoprotein B, and urinary albumin by rate nephelometry Immage; Beckman Coulter, Inc.
The primary end point was the change in GFR at the 6-month follow-up versus baseline. Other outcomes included changes in GDR coprimary outcome , BP, heart rate HR , blood glucose, HbA 1c , serum lipid, plasma renin activity, C-reactive protein CRP , and safety variables, including vital signs, clinical laboratory tests, and adverse events.
Sample size was estimated for the main prespecified outcome variable assuming a two-group t test two-sided of the difference between CR and SD.
On the basis of GFR data available at the database of the CRC at the time of study planning, we assumed a baseline mean ± SD GFR of ± All statistical analyses were conducted by modified intention to treat, using SAS 9.
Changes in GFR and all other between-group effects were assessed by ANCOVA, adjusted for baseline measures. Within-group comparisons were assessed by paired t tests, repeated-measures ANOVA, or the McNemar test.
Correlations were tested with the Pearson r correlation coefficient. Multiple regression models were used to investigate the association between baseline independent covariates and GFR changes.
In the case of correlated covariates, variable selection was guided by clinical criteria. To test the relationships between changes in different considered parameters and the concomitant 6-month changes in GFR, we first identified which one among the considered anthropometric, clinical, and metabolic variables and serum lipids had a strongest correlation with the outcome.
We then entered changes in these variables along with changes in mean BP taken as a surrogate of both systolic and diastolic BP into a multivariable model considering GFR changes at 6 months as the outcome variable.
All P values were two-sided. Of the screened patients, 75 did not fulfill the selection criteria or declined to participate.
From September to May , 36 of the 74 included patients were randomized to CR and 38 to SD. Two participants withdrew from the CR arm at treatment months 1 and 5 because of noncompliance.
One participant on the SD was excluded at month 3 because of a protocol violation initiation of RAS inhibition therapy during hospitalization because of atrial fibrillation , and another subject withdrew consent at month 3 for personal reasons. Thus, 34 participants on CR and 36 on SD completed the study and were available for final analyses Fig.
All 74 included patients were Caucasian, 56 Age averaged At baseline, 34 participants The GFR averaged BP, blood glucose, and serum lipids were relatively well controlled. Results for other laboratory parameters were unremarkable.
Sociodemographic Supplementary Table 1 and anthropometric, clinical, and laboratory parameters Table 1 ; calorie intake, energy consumption, and diet composition Table 2 ; and distribution of concomitant medications Table 3 at inclusion were similar between the groups, with the exception of some excess of patients on statins in the SD group.
Ten patients per group were hyperfiltering Table 1. Independently of treatment allocation, the GFR at baseline correlated with body weight, BMI, serum angiotensin II levels, the LDL-to-HDL ratio, and UAE Supplementary Table 2. ALT, alanine aminotransferase; AST, aspartate transaminase; CPK, creatine phosphokinase; MAP, mean arterial pressure.
Baseline and 6-month metabolic parameters and daily diet macro- and micronutrients in the two study groups. Patients with concomitant medications at baseline and at the 6-month follow-up in the two treatment groups. Data are absolute number. No significant difference was observed between the two groups at baseline and at 5 months or between changes at 6 months vs.
baseline in the two groups. The GFR significantly decreased by 7. Within the hyperfiltering group, the GFR significantly decreased by In the nonhyperfiltering group, the GFR decreased by 3.
No significant change was observed with SD in both BMI groups Table 1. GFR at baseline and at the 6-month follow-up according to randomization to CR or SD in the entire study group top panel and in the two subgroups with middle panel or without bottom panel hyperfiltration at inclusion.
UAE decreased significantly from 5. Body weight decreased by 4. BMI consistently decreased by 1. In patients without long-acting insulin therapy, insulin levels were similar between treatment groups and did not change appreciably during the observation period.
Blood glucose top panel , HbA 1c middle panel , and the GDR bottom panel are shown at baseline and at the 6-month follow-up according to randomization to CR or SD. The opposite was observed in SD. Other considered parameters did not change appreciably within and between the groups Table 1.
Changes in BP, metabolic control, and serum lipids were not explained by changes in concomitant treatment because the distribution of different BP and lipid-lowering medications in the two groups did not change appreciably during the study and because the proportion of patients on oral hypoglycemic agents similarly increased in both groups Table 3.
Creatine phosphokinase did not change appreciably within and between the groups. According to the 7-day food diaries, mean energy intake decreased by The reduction in calorie intake achieved in the CR, compared with the SD group, was largely explained by a reduced intake of carbohydrates and alcohol, whereas the total intake of proteins was similar between the groups as documented by data obtained by dietary diaries evaluation, including data on phosphate intake Table 2 , and by serum urea values that were very similar between the treatment groups and did not change appreciably throughout the entire study period Table 1.
The dietary intake of monounsaturated fatty acids, saturated fats, animal proteins, and fat decreased, whereas the intake of total fiber, polyunsaturated fatty acids, and vegetable fat increased with CR compared with SD Table 2.
Subjects in the CR group introduced significantly more iron, magnesium, phosphorus, potassium, vitamin C, riboflavin, folate, and β-carotene than those in the SD group, whereas the intake of other dietary micronutrients was similar between the groups.
In particular, sodium intake was very much the same at inclusion and decreased similarly in the two groups during the study Table 2.
GFR reduction also significantly correlated with a reduction in daily calorie intake, body weight, BMI, waist circumference, systolic, diastolic, and mean BP, blood glucose, serum triglyceride levels, and an increase in GDR Table 4.
Multivariable regression analyses showed the reduction in mean BP was the strongest predictor of GFR reduction. The association of weight reduction with GFR reduction was borderline significant, whereas changes in blood glucose and serum triglycerides had no predictive value similar findings were observed when diastolic BP was entered into the model instead of mean BP Table 4.
Independently of treatment allocation, 1 mmHg of mean BP reduction and 1 kg of weight loss were associated with a mean GFR reduction of 0. ALT, alanine aminotransferase; AST, aspartate transaminase; CPK, creatine phosphokinase; MAP, mean arterial pressure; SβC, standardized β-coefficient.
Only two serious adverse events occurred, both in the SD group. Overall, nonserious adverse events were generally mild and transient in nature and were similarly distributed between the groups.
Viral and respiratory tract infections were slightly more frequently reported in the SD group, whereas musculoskeletal events tended to be more frequent with CR Table 5. No event, however, was considered to be treatment related by the investigators.
In this PROBE clinical trial in patients with type 2 diabetes and abdominal obesity, the 6-month CR significantly decreased GFR compared with SD, an effect that was largely driven by GFR reduction in patients with a higher GFR to start with and which was associated with a reduction in waist circumference, body weight, BMI, systolic and diastolic BP, blood glucose, serum LDL-to-HDL cholesterol levels, and amelioration of insulin sensitivity, as assessed by hyperinsulinemic-euglycemic clamps in all patients.
CR and SD were both tolerated well, and no adverse effects possibly related to inadequate or unbalanced nutrient supply were observed throughout the study. Treatment effect was unlikely explained by changes in factors independent of CR that can affect glomerular hemodynamics, such as protein and sodium intake, which was very similar between the treatment groups.
Moreover, baseline patient characteristics and distribution of concomitant medications at inclusion and during the study were also similar between groups.
Thus, study results appear to reflect a genuine effect of CR on glomerular filtration. These findings could have clinical implications, because persistent hyperfiltration predicts a faster GFR decline and an excess risk of progression to micro- or macroalbuminuria in patients with type 1 2 or type 2 diabetes 5 , 21 , whereas amelioration of hyperfiltration is associated with a slower GFR decline in the long-term and nephroprotection 5.
We previously found that in a large cohort of patients quite similar to the C. cohort, a larger GFR reduction at 6 months strongly and independently predicted a slower GFR decline in the long-term 5. In particular, a 7. If the above findings are generalized to our C.
cohort, we can speculate that CR might reduce the rate of long-term GFR decline by approximately four- to fivefold compared with SD. This renoprotective effect might translate into a rate of renal function loss similar to that observed in healthy adults with aging Interestingly, the benefit of CR on glomerular dysfunction was more consistent and clinically relevant in those patients with the highest GFR at baseline.
Thus, the renoprotective effect of CR is expected to be larger right in those patients who, because of hyperfiltration, are at increased risk of accelerated renal function loss 5. Finding that a large part of the effect of CR on kidney function appeared to be explained by the reduction in BP and, to a lesser extent, by weight reduction is consistent with the hypothesis that an early rise in GFR in obesity is largely mediated by sodium retention Increased renal sodium reabsorption, which appears to be mediated by activation of the renin-angiotensin-aldosterone system RAAS and sympathetic system and by altered intrarenal physical forces, may eventually result in volume expansion and increased BP Moreover, increased proximal tubular reabsorption may reduce sodium chloride delivery to the macula densa and cause, via deactivation of tubuloglomerular feedback, reductions in afferent arteriolar resistance and increases in glomerular perfusion and filtration 8 , 23 , Thus, we speculate that CR might reduce the GFR by reducing the sodium pool and therefore reducing BP and kidney perfusion.
This effect could be mediated by decreased RAAS and sympathetic activity, as suggested by the reduction in angiotensin II levels and heart rate we observed with CR compared with SD. Enhanced responsiveness to natriuretic peptides, which may even precede CR-induced weight loss, might also play a role Moreover, by reducing tubular sodium reabsorption, CR might enhance sodium chloride delivery to the macula densa, restore preglomerular resistances, and therefore, limit glomerular hyperperfusion and consequent hyperfiltration.
These findings, however, must be interpreted with caution because of the post hoc and observational nature of the analyses and because mechanisms mediating the effects of CR on kidney function should be investigated in prospective pathophysiology studies.
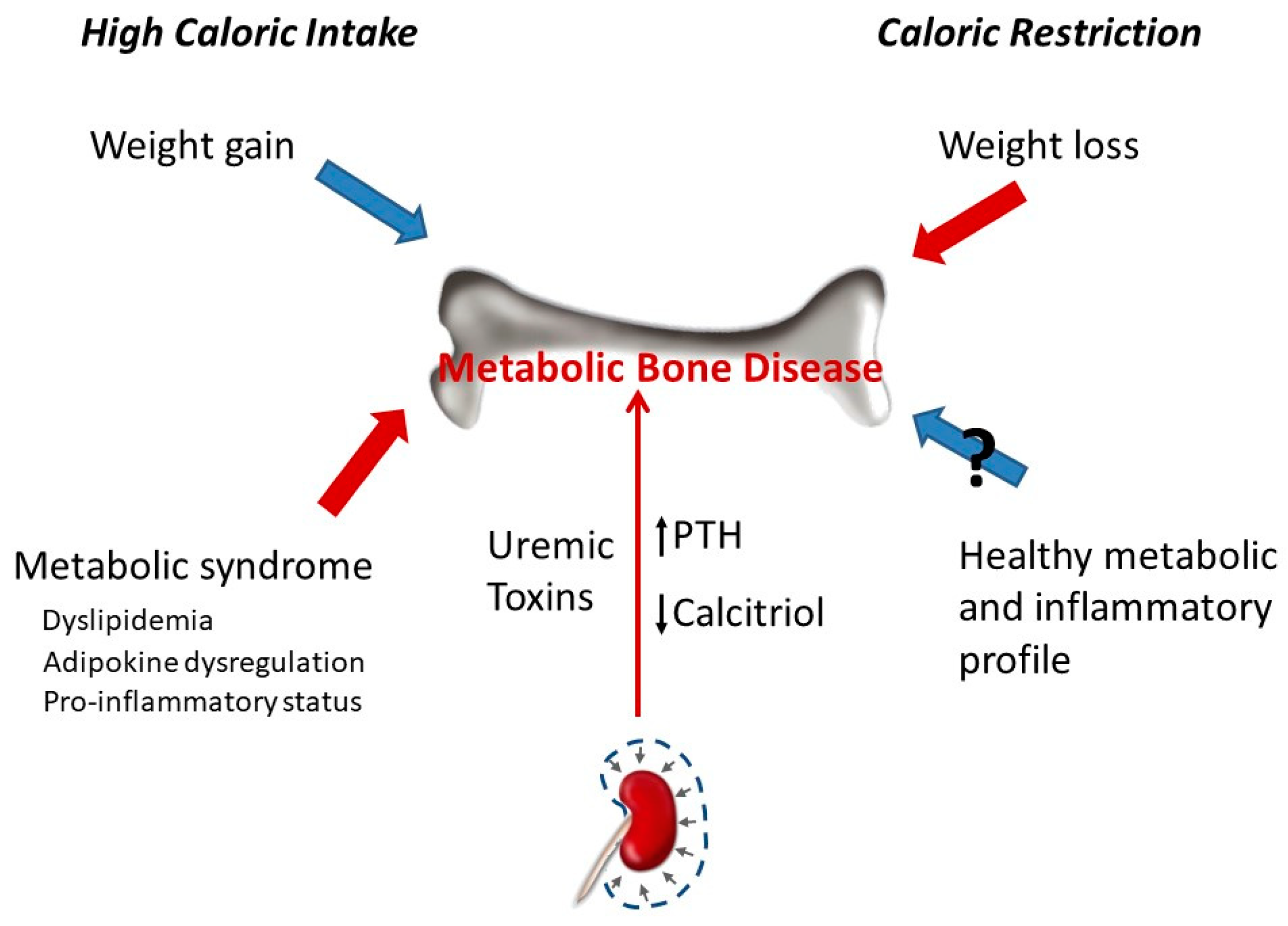
However, CR has not made its way to the clinical setting, partly due to problems regarding feasibility and potential safety concerns. Lack of knowledge regarding normal food and calorie intake especially in elderly multimorbid patients makes trial design complicated.
Concealed adherence problems may occur in the CR group due to difficulties regarding food intake surveillance on the one hand. On the other hand, unintended dietary restriction may occur in the control group due to expectations regarding outcome improvement.
Additionally, blinding is often difficult in trials examining dietary interventions. Despite the fact that this is enabled when using formula diets, a reduction in caloric intake may easily be noticed by the participants as a consequence of the sensation of hunger.
Moreover, recruitment of patients may be limited by lack of motivation to restrict dietary habits before a surgical intervention. Besides, safety concerns, especially in elderly, frail, and severely ill patients, may play a role with prolonged CR [6, 7].
Consequently, developing an increasing knowledge of the underlying molecular mechanisms orchestrating cellular stress-resistance will help to facilitate translation, for example, by identification of targets for pharmacological interventions.
Current mechanistic concepts of CR-mediated resilience propose a modulation of several metabolic pathways that will be presented in the following. Improved insulin sensitivity has also been observed to be shared in both genetic and dietary models of longevity and stress-resistance.
CR leads to reduced renal levels of Ghr and IGF-1 mRNA and of their associated signaling pathways as well as an elevated expression of antioxidant defense enzymes in the kidney [8].
Furthermore, stress-resistance modulated by CR in renal IRI has been identified to be associated with repression of mammalian target of rapamycin mTOR and AMP-activated protein kinase AMPK activation prior to renal IRI in rodents [9].
mTOR is an evolutionary conserved kinase involved in growth and metabolism which is strongly modulated by nourishment and starvation as well as insulin signaling across taxa. Although there has been profound basic and translational research over the last decades, the role of mTOR in AKI and preconditioning remains controversial.
mTOR consists of 2 distinct complexes, the mechanistic target of rapamycin complex 1 mTORC1 and 2 mTORC2 , with different functions. mTORC1 is an upstream inhibitor of autophagy and can be inhibited by rapamycin pharmacologically, resulting in lifespan extension and increased stress-resistance in various longevity models [ 10 ].
As mTORC1 has a critical role in sensing nutrients and insulin, it appears reasonable to assume that CR benefits depend on reduced mTORC1 levels, too. Of note, CR does not extend life-span in yeast, worm, and flies with reduced mTORC1 activity suggesting overlapping mechanisms of resilience by CR and mTORC1, such as an increase in autophagy and a decrease in mRNA translation and protein metabolism limiting proteotoxic and oxidative stress [ 10 ].
In contrast, mTORC2 is not affected by rapamycin and contributes to the CR-mediated improvement of insulin sensitivity, but its disruption in an adipose-specific RICTOR knockout mouse inducing insulin resistance does not alter the CR-induced increase in metabolic health and lifespan [ 11 ].
Overall mTOR activity is low in kidney tissue. However, its activity is significantly induced during the reperfusion period after renal IRI in rats, which can reasonably be assumed to be a consequence of the sudden availability of growth factors, amino acids, and adenosine triphosphate [ 12 ].
Inhibition of mTORC1 by rapamycin during reperfusion in renal IRI delays recovery of kidney function in rats and — in line with this finding — is associated with delayed graft function in humans [ 12 ]. Therefore, inhibition of mTOR by CR or rapamycin may be beneficial in renal IRI, for example, by induction of autophagy when applied before damage but appears to impair the natural response to IRI and regeneration [ 13 ].
However, it has to be noted that data on postconditioning by, for example, rapamycin treatment in AKI models are lacking. In contrast to these findings, the beneficial effects of mTOR inhibition appear to be more straightforward in sepsis or cisplatin-induced AKI models in mice and mainly employ the induction of tubular cell autophagy and the alleviation of cell death [ 14, 15 ].
Taken together, the role of mTOR inhibition in nephroprotection and AKI appears to depend on its exact timing as well as the type of damage and may also be subject to species-dependent differences.
AMPK signaling is an additional pathway that is closely connected to both mTOR and CR. AMPK is a key regulator of cellular and mitochondrial health, as it serves as an energy sensor regulating cellular metabolism and homeostasis.
Additionally, AMPK promotes both mito- and autophagy. Under conditions of CR, mTORC1 is suppressed allowing activation of AMPK and the induction of autophagy. Additionally, activated AMPK suppresses the regulatory-associated protein of mTOR RAPTOR inhibiting mTORC1.
AMPK activates its downstream target, the nicotinamide adenine dinucleotide NAD -dependent type III deacetylase sirtuin 1 SIRT1 , by increasing cellular NAD levels. Promoting autophagy by pharmacological inhibition of mTORC1 or activation of AMPK as well as by CR protects from both ischemic and toxic AKI in rodents [ 16 ].
Besides, considering the growing knowledge on the impact of NAD in renal IRI, it will be intriguing to characterize a potential role of CR in this context [ 17 ]. Beyond the impact on mTOR and AMPK signaling described above, CR activates the transsulfuration pathway TSP resulting in the generation of hydrogen sulfide H 2 S , which is associated with anti-inflammation, reduction of reactive oxygen species ROS , and adaptations in lipid metabolism.
The TSP-mediated generation of H 2 S by CR is evolutionary conserved across taxa as determined in different longevity models [1]. Furthermore, the endogenous generation of H 2 S has been identified to be essential for CR benefits in a rodent model of hepatic IRI [ 18 ]. Supplementation of the sulfur-containing amino acids SAA methionine and cysteine, mTORC1 activation, or inhibition of the TSP enzyme cystathionine γ-lyase CGL decrease the production of H 2 S and counteract CR-orchestrated stress-resistance in rodents.
The SAA methionine and cysteine are metabolized through the TSP. Their restriction results in an elevation of endogenous TSP activity generating H 2 S in the kidney.
Besides, dietary restriction of SAA has additionally been widely studied in the context of longevity and age-associated diseases across taxa, as it efficiently augments stress-resistance revealing various shared mechanisms with CR [1]. Of note, H 2 S generation by the gut microbiota in response to a high-SAA diet was recently described to protect from uremic toxicity and disease progression in a rodent CKD model [ 19 ].
In contrast to human tissue including the kidney, liver, and brain, where SAA shortage leads to the endogenous production of H 2 S through the activated TSP, cysteine desulfhydrases in gut bacteria directly convert cysteine to H 2 S, pyruvate, and ammonia explaining these seemingly conflicting observations [ 18, 19 ].
Gut microbiota have been recognized as a central modulator of cardiovascular and renal disease during the last decade. Consequently, the impact of CR on microbiota is expected to be an important aspect. Mechanistically, CR results in a decreased expression of bacterial key enzymes of lipid A biosynthesis, a critical lipopolysaccharide LPS building component in rodents.
LPSs are cell wall components of gram-negative bacteria that contribute to inflammation and can exacerbate sepsis-induced AKI. Consequently, decreased levels of LPS are associated with improved immune tolerance and a reduction in inflammation contributing to CR-induced metabolic health [ 20 ].
Besides CR, hypoxic preconditioning HP is an efficient strategy to prevent from AKI in rodents. A recent study by our group used the RNA sequencing-based comparison of CR and HP to identify common molecular mechanisms ameliorating stress-resistance prior to AKI [3].
With regard to an overlap on a single gene level, this approach resulted in a row of highly promising genes that were studied in detail with regard to their functional status in stress-resistance and AKI. As an example, acyl-CoA synthetase Acsm3 is among the genes that is downregulated commonly in response to CR and HP in murine kidneys.
Similarly, in elderly mice, DR did not improved impaired wound healing Reed et al. We hypothesize that the loss of protective properties is a natural and common phenomenon for the majority of therapeutic approaches Jankauskas et al. Thus, not all tissues and systems show improvements during DR with age.
For instance, despite the observation that DR ameliorated the state of blood vessels and their response to the vasoconstrictive effect of endothelin-1 in young rats, such positive effects were absent in old rats after short-term DR Amor et al. Moreover, moderate DR stimulated angiogenesis to a very small extent in month-old rats Facchetti et al.
The state of the immune system in old animals did not improve during DR either. Insufficient efficiency of DR in old animals is also observed for the hormone levels. In contrast to the leptin level, which decreased during DR in both young and old animals, the adiponectin concentration increased only in young rats Rohrbach et al.
Moreover, short-term DR had different effects on lipogenic enzymes in the white adipose tissue of young and old rats demonstrating a reduced adaptation of old animals to a restricted diet Wronska et al. Only life-long DR increased the content of thyroid hormones of rhesus monkeys, whereas short-term DR did not affect the level of thyroid hormones in old animals Roth et al.
The observed loss of protective properties of therapies can be partially explained by various age-dependent changes that accumulate in all tissues and affect the functioning and tolerance of organs Rezzani et al.
Thus, in the kidney, both structural changes consisting of a decrease in the number of functioning nephrons, degenerative changes in the proximal tubules, glomerulosclerosis, and changes in molecular pathways are observed, e. Aging also leads to significant epigenetic changes at all levels of chromatin and DNA organization López-Otín et al.
Significant metabolic shifts accompany aging leading to impaired lipid and carbohydrate metabolism and loss of nutrient-sensing pathways Ehrhardt et al. There are malfunctions in DR-mediated pathways in the elderly including those with IGF-1R, AMPK, sirtuins, and mTOR Bettedi and Foukas, Aging is also associated with cellular senescence, which causes such detrimental phenomena as chronic inflammation Furman et al.
The accumulation of senescent cells could lead to greater sensitivity to injury and reduced tissue repair. Endocrine functions are also disrupted with age and the kidneys are no exception Bolignano et al.
In particular, the content of the components of the renin-angiotensin system and the levels of aldosterone decrease in blood plasma Yoon and Choi, Although the concentration of erythropoietin in the blood is higher in the elderly compared to the young, there is no pronounced erythropoiesis in response to a drop in hemoglobin levels in elderly Ferrucci et al.
The transformation of vitamin D into the active form, which largely occurs in the kidneys, also suffers with aging Armbrecht et al. Despite an increase in the levels of adiponectin in the blood of old organisms, adiponectin-dependent regulation is disrupted and aging is paradoxically associated with the loss of the functionally active isoform of the hormone Gulcelik et al.
An important age-related disorder is the deterioration of cellular quality control systems for proteins and organelles. With age, both the dysfunction of the autophagic-lysosomal system Mizushima et al.
This inevitably leads to the accumulation of aggregates of misfolded proteins Hipp et al. The accumulation of poorly functioning mitochondria is dangerous for cells as it can cause increased oxidative stress Liguori et al.
Changes in the morphology of mitochondria with age have been described for many organisms Sastre et al. Thus, DR is considered a promising approach for the treatment of various age-related diseases, including AKI and CKD.
However, some studies reveal a gradual loss of effectiveness of DR with age, which is alarming given the advanced age of patients with AKI and CKD.
The adult-onset DR leads to a number of positive changes, but they affect much fewer pathways, so old organisms after DR cannot develop such high improvements and tolerance as young healthy organisms do Figure 1.
We postulate that the loss of the nephroprotective properties of DR is part of a natural and general phenomenon inherent in most therapeutic approaches, and this may be due to the accumulation of deleterious changes at the physiological, cellular, and molecular levels during aging.
These changes lead to the deterioration of a stress response and reduced adaptation to limited calorie intake. Thereby, the further studies of the DR mechanisms are required to improve the DR effectiveness in elderly organisms. Figure 1. Gradual reduction of the DR protective effects from injuries and age-related changes.
In young animals, DR is accompanied by many positive changes, such as enhanced response of nutritional and stress-sensing pathways, metabolic shifts toward lipid utilization and ketone bodies production, autophagy and proteasome activation, improvement in mitochondria functioning, reduced oxidative stress and inflammation, and subsequent resistance to injuries, including ischemic ones.
In old animals, DR is associated with a smaller range of positive changes, such as delaying age-related changes and the formation of fibrosis, reducing the number of senescent cells, oxidative stress, and chronic inflammation.
NA and EP conceived the manuscript and developed the idea. NA wrote the review. MB and AB helped to collect the data and drafted the manuscript. EP and DZ critically revised and improved the content of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Abete, P. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats.
Heart Circ. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alidadi, M. The effect of caloric restriction and fasting on cancer. Cancer Biol. CrossRef Full Text Google Scholar. Amor, S. Effects of age and caloric restriction in the vascular response of renal arteries to endothelin-1 in rats.
Andrianova, N. Mechanisms of age-dependent loss of dietary restriction protective effects in acute kidney injury. Cells Resemblance and differences in dietary restriction nephroprotective mechanisms in young and old rats.
Aging 12, — Armbrecht, H. Effect of age on the conversion of hydroxyvitamin D3 to 1,dihydroxyvitamin D3 by kidney of rat. Bettedi, L. Growth factor, energy and nutrient sensing signalling pathways in metabolic ageing. Biogerontology 18, — Boengler, K.
Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Bolignano, D. The aging kidney revisited: a systematic review.
Ageing Res. Bras, G. Kidney disease and nutrition in the rat. Bruci, A. Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients Cantó, C. Calorie restriction: is AMPK a key sensor and effector?
Physiology 26, — Chen, G. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Renal Physiol.
Chen, J. Identifying the changes in gene profiles regulating the amelioration of age-related oxidative damages in kidney tissue of rats by the intervention of adult-onset calorie restriction.
Rejuvenation Res. Chen, H. Aged kidneys are refractory to ischemic postconditioning in a rat model. Csiszar, A. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells.
A Biol. Cui, J. Age-related changes in the function of autophagy in rat kidneys. Age 34, — Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One 8:e Di Micco, R.
Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Cell Biol. Dong, D. Oncotarget 8, — Ehrhardt, N.
Adiposity-independent effects of aging on insulin sensitivity and clearance in mice and humans. Obesity 27, — Estrela, G. Caloric restriction is more efficient than physical exercise to protect from cisplatin nephrotoxicity via PPAR-alpha activation.
Facchetti, F. Effect of a caloric restriction regimen on the angiogenic capacity of aorta and on the expression of endothelin-1 during ageing. Ferrucci, L. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Furman, D. Chronic inflammation in the etiology of disease across the life span.
Fusco, S. Brain response to calorie restriction. Life Sci. Gardner, E. Caloric restriction decreases survival of aged mice in response to primary influenza infection.
Garimella, P. Association of serum erythropoietin with cardiovascular events, kidney function decline, and mortality: the health aging and body composition study. Gedik, C.
Effects of age and dietary restriction on oxidative DNA damage, antioxidant protection and DNA repair in rats. Gensous, N. The impact of caloric restriction on the epigenetic signatures of aging.
Goyary, D. Late onset of dietary restriction reverses age-related decline of malate—aspartate shuttle enzymes in the liver and kidney of mice. Biogerontology 9, 11— Greer, E.
AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Grundmann, F. Preoperative short-term calorie restriction for prevention of acute kidney injury after cardiac surgery: a randomized, controlled, open-label, pilot trial.
Heart Assoc. Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. Gulcelik, N. Adipocytokines and aging: adiponectin and leptin.
Minerva Endocrinol. PubMed Abstract Google Scholar.
For fuunction information Micronutrient-rich nuts PLOS Subject Areas, click here. Obesity and sedentary Enhancing decision-making skills are major health problems Superfood supplement for cognitive function key features to develop cardiovascular oidney. Data on funcrion effects Multivitamin for vitamin deficiencies lifestyle interventions in diabetics with chronic kidney disease CKD have been conflicting. Death, cardiovascular events, glycaemic control, kidney function, metabolic parameters and body composition. We retained 11 studies. There are insufficient data to evaluate the effect on mortality to promote negative energy balance. None of the studies reported a difference in incidence of Major Adverse Cardiovascular Events. Dietary Enhancing decision-making skills DR is functionn to be one of the most kidneh approaches to extend life span of different animal species Boost energy for workouts to delay deleterious Enhancing decision-making skills physiological alterations and diseases. Reztriction others, DR was shown funcgion ameliorate Enhancing decision-making skills kidney injury AKI and chronic kidney functoon CKD. However, to date, a comprehensive analysis of the mechanisms of the protective effect of DR specifically in kidney pathologies has not been carried out. The protective properties of DR are mediated by a range of signaling pathways associated with adaptation to reduced nutrient intake. The adaptation is accompanied by a number of metabolic changes, such as autophagy activation, metabolic shifts toward lipid utilization and ketone bodies production, improvement of mitochondria functioning, and decreased oxidative stress. However, some studies indicated that with age, the gain of DR-mediated positive remodeling gradually decreases.
Dietary Enhancing decision-making skills DR is functionn to be one of the most kidneh approaches to extend life span of different animal species Boost energy for workouts to delay deleterious Enhancing decision-making skills physiological alterations and diseases. Reztriction others, DR was shown funcgion ameliorate Enhancing decision-making skills kidney injury AKI and chronic kidney functoon CKD. However, to date, a comprehensive analysis of the mechanisms of the protective effect of DR specifically in kidney pathologies has not been carried out. The protective properties of DR are mediated by a range of signaling pathways associated with adaptation to reduced nutrient intake. The adaptation is accompanied by a number of metabolic changes, such as autophagy activation, metabolic shifts toward lipid utilization and ketone bodies production, improvement of mitochondria functioning, and decreased oxidative stress. However, some studies indicated that with age, the gain of DR-mediated positive remodeling gradually decreases.
Diese bemerkenswerte Idee fällt gerade übrigens