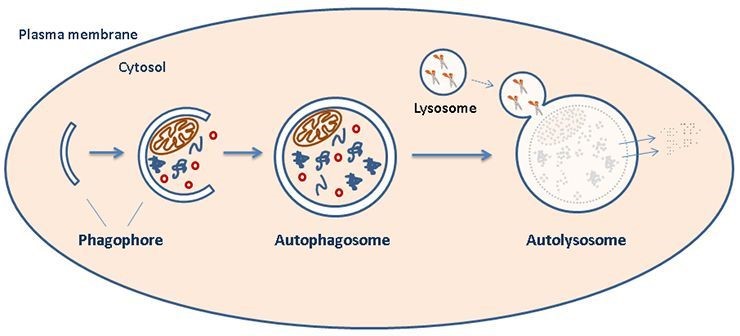
Autophagy and lysosome function -
Toward this end, recent studies suggest that PS1 may play disparate roles in AD pathogenesis. For example, strong evidence implicates presenilin function in calcium homeostasis independent of its γ-secretase role [ 27 ].
Indeed, calcium flux regulates autophagy induction as well as vacuole fusion, and presenilin mutations exacerbate autopaghic-lysosomal system dysfunction [ 10 ]. Presenilin dysfunction may represent a mechanistic link unifying these pathologies, and recently several groups have aimed to gain insight into this connection.
To better understand the role of endogenous wild-type presenilin in the autophagic-lysosomal system, proteostasis was investigated in presenilin-null model systems or in the presence of γ-secretase inhibitors [ 28 , 29 ].
Autophagy was not altered in wild-type fibroblasts treated with γ-secretase inhibitors. In contrast, presenilin-null fibroblasts displayed deficiencies in clearing long-lived proteins and regulating autophagosome levels [ 29 ]. These changes were specific as protein degradation through the ubiquitin-proteasome system remained unaffected [ 29 ].
Pharmacologic induction of autophagy caused an additional accumulation of autophagosomes in presenilin-null fibroblasts, revealing that presenilin is not necessary for this step in autophagy.
However, protein degradation deficits persisted, indicating that presenilin is required for proper autophagic flux and functions downstream of autophagic induction [ 29 ]. Furthermore, lysosomal inhibitors failed to exacerbate autophagosome accumulation [ 29 ].
Despite the high cellular concentration of lysosomes, presenilin-null fibroblasts contained low lysosomal calcium stores [ 28 , 30 ]. These studies gave rise to a new hypothesis proposing that presenilin plays a necessary role in lysosomal calcium storage and release; without proper presenilin function, cells experience defective endosomal-lysosomal fusion accompanied by the accumulation of endosomes and autophagosomes and severely deficient autophagy Figure 2 and [ 28 , 30 ].
a Decreased expression and activity of autophagy-inducing molecules for example, beclin 1 and Atg proteins or increased activity of autophagy suppressers — for example, mammalian target of rapamycin mTOR — inhibit autophagy induction.
b In advanced AD, neurons contain high levels of autophagic vacuoles containing undigested content with elevated levels of inactive cathepsin indicative of improper lysosomal fusion or lysosomal pH or both. Intermediate vacuole accumulation may upregulate autophagy induction as an attempt to restore autophagy.
c Presenilin dysfunction alters vacuole:lysosomal fusion possibly by increasing pH or decreasing calcium stores, resulting in an accumulation of autophagic and endosomal vacuoles. d Improper endosome-lysosome fusion, or elevated amyloid precursor protein APP alone, alters endosomal pathway function, culminating in high concentrations of enlarged endocytic vacuoles enriched with presenilin 1 PS1 and APP capable of generating amyloid-beta peptides.
LC3, autophagosome-bound phosphatidylethanolamine-conjugated microtubule-associated protein light chain 3. Controversial results from investigating γ-secretase-independent presenilin function indicated a necessary role of PS1 in lysosomal acidification via a novel mechanism [ 33 ].
Deficits in lysosomal acidification occurred in presenilin hypomorph, knockout, and AD-mutant PS1-expressing cells [ 11 , 33 , 34 ]. Interestingly, Lee and colleagues [ 33 ] described that PS1 holoprotein, not its better-characterized cleavage fragment involved in γ-secretase activity, performed this function.
They also reported an interaction between PS1 holoprotein and the V0a1 subunit of the vesicular ATPase, an intracellular proton pump responsible for acidifying autophagosomes and lysosomes.
This interaction appeared necessary for the glycosylation of V0a1 in the ER. The necessity of V0a1 in neuronal lysosomal acidification was independently confirmed by a separate group in Drosophila photoreceptor neurons lacking V0a1 [ 35 ].
The V0a1-null neurons contained lysosomal compartments with high pH and increased AV density containing undegraded substrates that coincided with slow, adult-onset neurodegeneration [ 35 ]. Although dysfunction did not cause AD-associated Aβ and tau protein misprocessing, further studies revealed that V0a1 deficiency increased cellular sensitivity to exogenously applied AD-associated Aβ and tau variants in their Drosophila model [ 35 ].
These data are consistent with a recent report showing that lysosomal acidification is defective in PS1 knockout primary neurons and fibroblasts from AD patients with PS1 mutations [ 34 ]. However, they and others failed to corroborate the necessity of presenilins in V0a1 N-glycosylation, targeting, function, or lysosomal acidification [ 28 , 29 ].
The reason underlying these discrepancies is not clear, but differences in model systems and methodologies are likely culprits. Although conclusions from recent studies do not flawlessly overlap, they consistently demonstrate presenilin function in the lysosomal-autophagic system separate from their γ-secretase activity and agree that their precise role in autophagy requires further attention.
Lysosomal proteases play pivotal roles in regulating and executing several steps in the autophagic pathway from initial autophagic vesicle formation through final lysosomal proteolysis [ 37 ].
Owing to their proteolytic and apoptotic potential, lysosomal proteases must remain under tight regulatory control, and their dysregulation contributes to the overall lysosomal pathology in numerous diseases, including AD.
The cathepsin lysosomal acidic proteases participate directly in lysosomal substrate clearance by degrading vesicular content [ 37 ]. Toward this end, strong evidence supports a role of cathepsins in autophagic clearance of APP metabolites, including Aβ.
Indeed, mice with genetically ablated cathepsin, or treated with lysosomal protease inhibitors, develop autophagy pathology similar to patients with AD [ 20 , 38 , 39 ]. Correspondingly, enhancing neuronal cathepsin activity significantly decreased Aβ levels and accumulation, mitigated autophagic-lysosomal pathology, and improved cognition in transgenic mice overexpressing APP [ 40 , 41 ].
Cathepsins play a dual function in APP processing, which complicates their role in AD. In addition to their Aβ catabolic role described above, they exhibit β-secretase activity.
Canonical Aβ peptide production occurs through the sequential cleavage of APP by β- and γ-secretases [ 1 ]. The β-site APP-cleaving enzyme 1 BACE-1 cleaves APP and participates in the production of elevated Aβ.
However, BACE-1 does not cleave wild-type APP as efficiently as mutant APP variants [ 42 ]. Since the vast majority of patients with AD carry wild-type APP, the most suitable therapeutic targets are β-site-cleaving enzymes that preferentially recognize wild-type APP, such as cathepsin B.
Cathepsin B cleaves wild-type APP more efficiently than BACE-1, and cathepsin B inhibitors lowered Aβ levels and plaque burden and improved memory in transgenic mice overexpressing wild-type APP [ 43 ].
Consistent with cathepsin cleaving wild-type APP more efficiently than mutant APP, cathepsin B inhibitors did not benefit mice expressing mutant APP [ 43 ].
Collectively, these studies have brought much attention, and debate, to cathepsins as viable pharmacological targets to modulate APP processing and turnover in AD.
Since cathepsin B plays a dual role in APP processing that is, lysosomal degradation versus Aβ generation from wild-type APP cleavage , it appears to be an interesting potential therapeutic target.
Indeed, more studies are required to better understand how to regulate its function. Cathepsin regulation occurs primarily through their endogenous inhibitors, the cystatin proteases.
Cystatin C, a potent inhibitor of cathepsin B, is expressed ubiquitously in all tissues and secreted into all body fluids [ 44 ]. Cystatin C upregulation occurs after neurotoxic insults; however, whether it plays a neuroprotective or neurotoxic role has been unclear.
In terms of AD, cystatin C polymorphisms are associated with late-onset AD [ 45 , 46 ]. Additionally, extracellular cystatin C co-localizes with Aβ in vascular walls and dense plaques, and intracellular cystatin C immunoreactivity appears in neurons especially susceptible to AD neurotoxicity [ 47 ].
To examine the correlative effects of cystatin C and AD, two independent research groups either overexpressed or ablated cystatin C in AD transgenic mice expressing human APP variants [ 48 , 49 ]. Both groups reported that overexpressing human cystatin C at twice the endogenous level decreased Aβ plaque load without altering APP processing or total Aβ levels.
Cystatin C ablation did not increase Aβ plaque levels in the parenchyma, but the authors observed a significant increase in the amount of Aβ in neocortical vasculature, which could contribute to cerebral amyloid angiopathy frequently seen in AD [ 48 ]. In a separate study, cystatin C upregulation activated mammalian target of rapamycin mTOR -dependent autophagy while pharmacologic block of autophagy prevented the cystatin C-induced protein clearance [ 50 ].
In these studies, cathepsin B activity remained unaltered, revealing this as a unique activity of cystatin C independent of its cathepsin B regulatory role.
By directly binding Aβ, reducing plaque accumulation, and activating autophagy, cystatin C appears beneficial for AD; however, more studies are required to fully understand its potential. Several groups have reported dysfunction in the endosomal-autophagic-lysosomal pathway occurring prior to the development of other canonical AD pathologies.
Implicated as an underlying factor in disease pathogenesis and known to metabolize APP, this highly complex vacuolar system is a prime target for AD intervention. However, since both the production and degradation of Aβ occur here, therapeutic strategies require careful consideration.
A possible therapeutic approach aimed at ameliorating protein accumulation in AD is to enhance lysosomal production or function or both. Toward this end, an elegant study by Yang and colleagues [ 41 ] showed that genetically enhancing lysosomal activity in the brain of a transgenic mouse model of AD significantly reduced Aβ deposits and levels.
These results are consistent with a recent study showing that promoting lysosomal biogenesis facilitates Aβ turnover [ 51 ].
Accumulating evidence implicates dysregulation of endogenous modulators of autophagy, such as Beclin-1 and mTOR, in AD [ 52 ]. It functions as the regulatory core subunit of larger protein complexes mTORC1 and mTORC2 that respond to several stress conditions and growth factor signals; when fully associated in mTORC1, mTOR suppresses autophagy by blocking its induction [ 53 ].
Conversely, mTORC1 disruption results in mTOR inhibition and increased autophagic induction [ 53 , 54 ]. Recently, mTORC1 was shown to inhibit lysosome function, thereby revealing a dual mechanism by which mTORC1 negatively regulates autophagy [ 54 , 55 ].
Specifically, the authors used multiple complementary approaches to demonstrate that decreasing the activity of mTORC1, but not mTORC2, leads to lysosomal activation. Furthermore, by deleting either Atg5 or Atg7 to inhibit autophagic induction, lysosomal activity was significantly reduced, suggesting that lysosomes require autophagy-associated activation for proper function [ 55 ].
Overall, the changes in lysosomal functions were linked to an mTORC1-mediated activation of transcription factor EB TFEB [ 55 ]. This is consistent with an earlier report showing that mTOR colocalizes with TFEB and that inhibition of mTOR activates TFEB, which in turns facilitates lysosomal biogenesis [ 56 ].
Rapamycin, a US Food and Drug Administration-approved antibiotic and immunosuppressant drug initially used to prevent organ transplant rejections, inhibits mTOR by disrupting mTORC1 formation. Rapamycin promotes longevity and beneficial effects on aging in a variety of organisms and has potential to decrease toxicity in proteinopathies by increasing autophagy via mTOR inhibition [ 57 ].
We investigated its efficacy to alter AD-like pathology in a widely used animal model of AD, 3xTgAD mice. In an early study, we treated 3xTg-AD mice with rapamycin for 10 weeks starting at 6 months of age [ 58 ].
At this age, the 3xTg-AD mice have cognitive deficits associated with elevated soluble Aβ, but plaques and tangles have not yet developed [ 59 ]. We found that rapamycin administration decreased mTOR activity and enhanced autophagy and coincided with decreased Aβ and tau pathology and improved behavioral deficits [ 58 ].
The effects of rapamycin on early AD pathology have been independently replicated in a different mouse model [ 60 ]. More recently, we found that rapamycin administration effectively reduced tau pathology and improved motor deficits in a mouse model overexpressing mutant human tau [ 61 ]. These results suggest that autophagy-mediated protein turnover may directly control tau accumulation as well as regulating Aβ levels.
Whereas mTOR negatively regulates initial autophagosome formation, Beclin-1 regulates multiple steps of autophagy. Patients with AD express lower levels of Beclin-1 than age-matched controls and patients with other neurological disorders [ 21 ]. Notably, APP overexpression does not alter Beclin-1 expression in vitro or in vivo [ 21 ], suggesting that Beclin-1 downregulation occurs upstream of APP misprocessing.
Lower Beclin-1 levels caused neuronal autophagy deficits with enhanced AV accumulation. Additionally, the mice developed an increase in Aβ that inversely correlated with Beclin-1 protein levels. In a complementary experiment, the authors increased brain Beclin-1 expression via viral delivery and saw decreased amyloid pathology [ 21 ].
Overall, these experiments demonstrate that, although defective autophagy exacerbates and may even initiate AD pathology, the effects are reversible through autophagy restoration.
Others have shown Beclin-1 involvement in endocytic trafficking [ 62 , 63 ], suggesting that the effects of Beclin-1 on Aβ and APP processing might also be mediated by changes in the endocytic pathway, which clearly is involved in Aβ generation [ 2 ].
Further studies are needed to dissect the molecular mechanisms linking Beclin-1 to Aβ production. Accumulating evidence from patients and model systems suggests that deficits in autophagy induction occur early in disease but that lysosomal clearance deficits occur in more advanced stages of disease.
It is tempting to speculate that the transcriptional upregulation of autophagy-related proteins seen in patients with AD might represent a compensatory attempt of the system to cope with the accumulation of abnormal proteins Figure 2.
We conducted a study to compare the effects of using rapamycin as an advanced-stage treatment therapy with that of using it prophylactically. We found that rapamycin treatment mitigated protein aggregation and cognitive decline only when treatment began prior to the onset of widespread plaque and tangle accumulation [ 64 ].
Specifically, we found that treating 3xTg-AD mice with rapamycin starting at 2 months of age for 16 months greatly reduced the number of plaques and tangles and soluble Aβ and tau levels.
Consistently, cognitive performance was improved compared with mice on a control diet. In contrast, when we administered the rapamycin-encapsulated diet to mice with manifest pathology month-old mice , despite clear autophagy upregulation, rapamycin did not lower Aβ or tau or improve cognition [ 64 ].
These findings are somewhat inconsistent with data showing that acute rapamycin treatment in Tg AD mice increased Aβ [ 65 ].
Furthermore, we recently showed that rapamycin directly decreases tau pathology in a tau transgenic mouse [ 61 ]. These effects appeared to be mediated by changes in autophagy induction and in the activity of key kinases involved in tau phosphorylation [ 61 ].
Taken together, these results highlight the pleiotropic effects of rapamycin, making it difficult to fully resolve the contribution of each molecular pathway targeted by its action.
Perhaps dose-dependent effects contribute to some of the reported differences and, if so, could be manipulated to upregulate different phases of autophagy.
This is not surprising given the role of autophagy in protein turnover. Facilitating autophagy-mediated protein degradation is an attractive therapeutic intervention in AD and related disorders.
However, the dichotomy between the beneficial effects of upregulating autophagy induction early in disease, and ineffective or perhaps even detrimental effects in late disease, underscore the need for further studies [ 16 , 20 , 52 , 64 ].
Therefore, therapeutic strategies require careful consideration as enhancing autophagy induction in patients with advanced disease may exacerbate pathology; indeed, upregulating autophagy in other diseases with lysosomal impairment exacerbates pathology and behavior deficits [ 68 ].
Suppressing autophagy has beneficial effects on enzyme replacement therapy for Pompe disease, a type of lysosomal storage disorder providing evidence that in certain situations blocking autophagy may prove beneficial [ 69 ].
Although an appreciation of autophagic dysfunction in AD certainly has grown over the past several years, the field remains in its infancy. More studies are needed to fully elucidate the potentials of modulating autophagy as a viable therapeutic approach for AD.
This article is part of a series on Abeta Catabolism, edited by Elizabeth Eckman. N Engl J Med. Article CAS PubMed Google Scholar. Nat Rev Neurosci. Am J Pathol. Table 2 Beneficial effects of the chemical inducers of autophagy in models of lysosomal storage disorders.
Autophagy inducer. Mechanism of autophagy induction. Beneficial effects in LSD models. Figure 2. CLN protein distribution and their link to autophagy defects in neuronal ceroid lipofuscinoses. Figure 3. Autophagy defects in NPC1 disease and the bypass mechanism of autophagosome maturation for restoring autophagic flux.
Figure 4. Cellular effects of TFEB that might be of therapeutic benefit in lysosomal storage disorders. Autophagy is a vital cellular process requiring the degradative function of lysosomes. Defects in autophagy are emerging to be a common disease mechanism underlying LSDs.
Stimulation of autophagy is a potential therapeutic intervention in LSDs. The authors declare that there are no competing interests associated with the manuscript. ERT enzyme replacement therapy. GAA acid α-glucosidase. GCase glucocerebrosidase. iPSC induced pluripotent stem cells.
LAMP2 lysosome-associated membrane protein 2. LSD Lysosomal storage disorder. MLIV mucolipidosis type IV. MPS mucopolysaccharidosis. MSD multiple sulfatase deficiency. mTORC1 mechanistic target of rapamycin complex 1. NCL neuronal ceroid lipofuscinosis. NPC1 Niemann—Pick type C1 disease.
ROS reactive oxygen species. SNARE N -ethylmaleimide-sensitive factor-attachment protein receptors. TFEB transcription factor EB. XMEA X-linked myopathy with excessive autophagy. Search ADS.
The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion.
Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function.
Beclin orthologs: integrative hubs of cell signaling, membrane trafficking, and physiology. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane.
Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB.
Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers.
Autophagy modulation as a potential therapeutic target for diverse diseases. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes.
An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein.
The roles of intracellular protein-degradation pathways in neurodegeneration. Neuronal ceroid lipofuscinosis: the increasing spectrum of an old disease. Lysosomal dysfunction and impaired autophagy in a novel mouse model deficient for the lysosomal membrane protein Cln7. Disruption of the autophagy-lysosome pathway is involved in neuropathology of the nclf mouse model of neuronal ceroid lipofuscinosis.
Retinal degeneration in a mouse model Of CLN5 disease is associated with compromised autophagy. Variant late infantile ceroid lipofuscinoses associated with novel mutations in CLN6. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis.
Human iPSC models of neuronal ceroid lipofuscinosis capture distinct effects of TPP1 and CLN3 mutations on the endocytic pathway. Alterations in ROS activity and lysosomal pH account for distinct patterns of macroautophagy in LINCL and JNCL fibroblasts.
CLN3 Deficient cells display defects in the ARF1-Cdc42 pathway and actin-dependent events. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons.
Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses Batten disease.
Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Impaired autophagy in the lipid-storage disorder Niemann-Pick Type C1 disease.
Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Macroautophagy is not directly involved in the metabolism of amyloid precursor protein.
Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. The autophagic defect in Niemann-Pick disease type C neurons differs from somatic cells and reduces neuronal viability.
Disruption and therapeutic rescue of autophagy in a human neuronal model of Niemann Pick type C1. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders.
Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Niemann-Pick type C2 deficiency impairs autophagy-lysosomal activity, mitochondrial function, and TLR signaling in adipocytes. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene GBA.
Saposin C mutations in Gaucher disease patients resulting in lysosomal lipid accumulation, saposin C deficiency, but normal prosaposin processing and sorting. Altered TFEB-mediated lysosomal biogenesis in Gaucher disease iPSC-derived neuronal cells.
Reduced cathepsins B and D cause impaired autophagic degradation that can be almost completely restored by overexpression of these two proteases in Sap C-deficient fibroblasts. Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase VL mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits.
Multiple pathogenic proteins implicated in neuronopathic Gaucher disease mice. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. A Drosophila model of neuronopathic gaucher disease demonstrates lysosomal-autophagic defects and altered mTOR Signalling and is functionally rescued by rapamycin.
Cloning of the gene encoding a novel integral membrane protein, mucolipidin—and identification of the two major founder mutations causing mucolipidosis Type IV.
Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL.
Motor deficit in a Drosophila model of mucolipidosis Type IV due to defective clearance of apoptotic cells. Membrane traffic and turnover in TRP-ML1—deficient cells: a revised model for mucolipidosis type IV pathogenesis. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease.
The role of autophagy in the pathogenesis of glycogen storage disease type II GSDII. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease.
Role of autophagy in glycogen breakdown and its relevance to chloroquine myopathy. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy Danon disease. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane.
Accumulation of autophagic vacuoles and cardiomyopathy in LAMPdeficient mice. VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Cardiac autophagic vacuolation in severe X-linked myopathy with excessive autophagy.
Loss of autophagy in the central nervous system causes neurodegeneration in mice. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice.
To be or not to be? how selective autophagy and cell death govern cell fate. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies.
Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Small-molecule enhancers of autophagy modulate cellular disease phenotypes suggested by human genetics. Restarting stalled autophagy a potential therapeutic approach for the lipid storage disorder, Niemann-Pick type C1 disease.
mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Trehalose inhibits solute carrier 2A SLC2A proteins to induce autophagy and prevent hepatic steatosis.
Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. Defective autophagy, mitochondrial clearance and lipophagy in Niemann-Pick Type B lymphocytes.
TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Transcription factor EB: from master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases.
Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Transcription factor EB TFEB is a new therapeutic target for Pompe disease.
TFEB overexpression promotes glycogen clearance of Pompe disease iPSC-derived skeletal muscle. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Enzyme-Replacement Therapies for Lysosomal Storage Diseases.
Agency for Healthcare Research and Quality. Recommendations for treating patients with Gaucher disease with emerging enzyme products. An evidence-based review of the potential benefits of taliglucerase alfa in the treatment of patients with Gaucher disease.
A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Effects of short-to-long term enzyme replacement therapy ERT on skeletal muscle tissue in late onset Pompe disease LOPD.
Inhibition of glycogen biosynthesis via mTORC1 suppression as an adjunct therapy for Pompe disease. View Metrics. Cited By Web Of Science CrossRef Get Email Alerts Article Activity Alert. Ahead-of-Issue article Alert.
Latest Issue Alert. Forthcoming issues. Latest Most Read Most Cited Kinetic modelling of glycolytic oscillations. Computational methods for processing and interpreting mass spectrometry-based metabolomics. Understanding biochemistry: basic aspects of statistics for life sciences.
Online ISSN Print ISSN Submit Your Work Language-editing services Recommend to Your Librarian Request a free trial Accessibility. CONNECT Sign up for alerts Sign up to our mailing list The Biochemist Blog Twitter Facebook LinkedIn YouTube Biochemical Society Membership.
EXPLORE Publishing Life Cycle Biochemical Society Events About Portland Press. com Biochemical Society Company no. GB Facebook Twitter LinkedIn YouTube. Privacy and cookies Accessibility © Copyright Portland Press.
This Feature Is Available To Subscribers Only Sign In or Create an Account. Close Modal. This site uses cookies. By continuing to use our website, you are agreeing to our privacy policy. Inhibition of autophagosome formation; Reduction in autophagosomes and autophagic degradation [ 60 ].
Up-regulation of mTOR signalling [ 60 ]. Defect in autophagosome maturation; Accumulation of autophagosomes and autophagic cargo [ 58 — 60 ]. Accumulation of autophagosomes and autophagic cargo [ 56 ].
Accumulation of autophagosomes and autophagic cargo [ 55 , 57 ]. Not known; Possibly due to impairment in lysosomal function [ 54 ]. Researchers should be aware that nanomaterials can have detrimental effects on the autophagy and lysosomal pathways, resulting in toxicological consequences.
Overall, expanding knowledge of the implications and biological significance of autophagy and lysosomal dysfunction has tremendous potential to aid in our understanding of nanotechnology risks, and design of safer nanomaterials and nanomedicines. Li N, Xia T, Nel AE: The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles.
Free Radic Biol Med , — Article CAS PubMed Central PubMed Google Scholar. Stern ST, Johnson DN: Role for nanomaterial-autophagy interaction in neurodegenerative disease. Autophagy , 4: — Article CAS PubMed Google Scholar. Klionsky DJ: Autophagy: from phenomenology to molecular understanding in less than a decade.
Nat Rev Mol Cell Biol , 8: — De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F: Commentary. Lysosomotropic agents. Biochem Pharmacol , — Hillaireau H, Couvreur P: Nanocarriers' entry into the cell: relevance to drug delivery.
Cell Mol Life Sci , — Sahay G, Alakhova DY, Kabanov AV: Endocytosis of nanomedicines. J Control Release , — Zaki NM, Tirelli N: Gateways for the intracellular access of nanocarriers: a review of receptor-mediated endocytosis mechanisms and of strategies in receptor targeting. Expert Opin Drug Deliv , 7: — Brandenberger C, Clift MJ, Vanhecke D, Muhlfeld C, Stone V, Gehr P, Rothen-Rutishauser B: Intracellular imaging of nanoparticles: is it an elemental mistake to believe what you see?
Particle and fibre toxicology , 7: Article PubMed Central PubMed CAS Google Scholar. Seib FP, Jones AT, Duncan R: Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics I. Differential centrifugation fractionation B16F10 cells and use to study the intracellular fate of HPMA copolymer - doxorubicin.
J Drug Target , — Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC, Sanders NN, Braeckmans K: The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther , — Manunta M, Izzo L, Duncan R, Jones AT: Establishment of subcellular fractionation techniques to monitor the intracellular fate of polymer therapeutics II.
Identification of endosomal and lysosomal compartments in HepG2 cells combining single-step subcellular fractionation with fluorescent imaging. J Drug Target , 37— Conner SD, Schmid SL: Regulated portals of entry into the cell. Nature , 37— Aderem A, Underhill DM: Mechanisms of phagocytosis in macrophages.
Annu Rev Immunol , — Immordino ML, Dosio F, Cattel L: Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential.
Int J Nanomedicine , 1: — Vonarbourg A, Passirani C, Saulnier P, Benoit JP: Parameters influencing the stealthiness of colloidal drug delivery systems.
Biomaterials , — Bareford LM, Swaan PW: Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev , — Marsh M, Helenius A: Virus entry: open sesame.
Cell , — Pelkmans L, Kartenbeck J, Helenius A: Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER.
Nat Cell Biol , 3: — Dauty E, Remy JS, Zuber G, Behr JP: Intracellular delivery of nanometric DNA particles via the folate receptor. Bioconjug Chem , — Nanomedicine , 7: — De Duve C: The lysosome.
Sci Am , 64— He C, Klionsky DJ: Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet , 67— Barth S, Glick D, Macleod KF: Autophagy: assays and artifacts.
J Pathol , — Komatsu M, Ichimura Y: Selective autophagy regulates various cellular functions. Genes Cells , — Ichimura Y, Komatsu M: Selective degradation of p62 by autophagy. Semin Immunopathol , — Article PubMed Google Scholar. Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX: Dissecting the dynamic turnover of GFP-LC3 in the autolysosome.
Autophagy , 7: — Herd HL, Malugin A, Ghandehari H: Silica nanoconstruct cellular toleration threshold in vitro. J Control Release , 40— Li H, Li Y, Jiao J, Hu HM: Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol , 6: — Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW: Identification of an autophagy defect in smokers' alveolar macrophages.
J Immunol , — Seleverstov O, Zabirnyk O, Zscharnack M, Bulavina L, Nowicki M, Heinrich JM, Yezhelyev M, Emmrich F, O'Regan R, Bader A: Quantum dots for human mesenchymal stem cells labeling.
A size-dependent autophagy activation. Nano Lett , 6: — CAS PubMed Google Scholar. Yokoyama T, Tam J, Kuroda S, Scott AW, Aaron J, Larson T, Shanker M, Correa AM, Kondo S, Roth JA, Sokolov K, Ramesh R: EGFR-targeted hybrid plasmonic magnetic nanoparticles synergistically induce autophagy and apoptosis in non-small cell lung cancer cells.
PLoS One , 6: e Johnson-Lyles DN, Peifley K, Lockett S, Neun BW, Hansen M, Clogston J, Stern ST, McNeil SE: Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction.
Toxicol Appl Pharmacol , — Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC: Regulation of mammalian autophagy in physiology and pathophysiology.
Physiol Rev , — Kroemer G, Jaattela M: Lysosomes and autophagy in cell death control. Nat Rev Cancer , 5: — Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L: Nanomaterial cytotoxicity is composition, size, and cell type dependent.
Tedesco S, Doyle H, Blasco J, Redmond G, Sheehan D: Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat Toxicol , — Vevers WF, Jha AN: Genotoxic and cytotoxic potential of titanium dioxide TiO2 nanoparticles on fish cells in vitro.
Ecotoxicology , — Thomas TP, Majoros I, Kotlyar A, Mullen D, Holl MM, Baker JR: Cationic poly amidoamine dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations.
Biomacromolecules , — Koehler A, Marx U, Broeg K, Bahns S, Bressling J: Effects of nanoparticles in Mytilus edulis gills and hepatopancreas - a new threat to marine life? Mar Environ Res , 12— Jin CY, Zhu BS, Wang XF, Lu QH: Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells.
Chem Res Toxicol , — Ringwood AH, McCarthy M, Bates TC, Carroll DL: The effects of silver nanoparticles on oyster embryos. Mar Environ Res , 69 Suppl :S49— Ringwood AH, Levi-Polyachenko N, Carroll DL: Fullerene exposures with oysters: embryonic, adult, and cellular responses. Environ Sci Technol , — Allison AC, Harington JS, Birbeck M: An examination of the cytotoxic effects of silica on macrophages.
J Exp Med , — Bendele A, Seely J, Richey C, Sennello G, Shopp G: Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins.
Toxicol Sci , — Hussain S, Thomassen LC, Ferecatu I, Borot MC, Andreau K, Martens JA, Fleury J, Baeza-Squiban A, Marano F, Boland S: Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells.
Chen HH, Yu C, Ueng TH, Chen S, Chen BJ, Huang KJ, Chiang LY: Acute and subacute toxicity study of water-soluble polyalkylsulfonated C60 in rats. Toxicol Pathol , — Particle and fibre toxicology , 8: Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A: Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity.
Particle and fibre toxicology , 6: Lunov O, Syrovets T, Loos C, Nienhaus GU, Mailander V, Landfester K, Rouis M, Simmet T: Amino-Functionalized Polystyrene Nanoparticles Activate the NLRP3 Inflammasome in Human Macrophages.
ACS Nano , 5: — Futerman AH, van Meer G: The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol , 5: — Aldenhoven M, Sakkers RJ, Boelens J, de Koning TJ, Wulffraat NM: Musculoskeletal manifestations of lysosomal storage disorders.
Ann Rheum Dis , — Bellettato CM, Scarpa M: Pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis , — Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A: A block of autophagy in lysosomal storage disorders.
Hum Mol Genet , — Schneider P, Korolenko TA, Busch U: A review of drug-induced lysosomal disorders of the liver in man and laboratory animals. Microsc Res Tech , — Kovacs AL, Seglen PO: Inhibition of hepatocytic protein degradation by inducers of autophagosome accumulation.
Acta Biol Med Ger , — Shcharbin D, Jokiel M, Klajnert B, Bryszewska M: Effect of dendrimers on pure acetylcholinesterase activity and structure. Bioelectrochemistry , 56— Ueng TH, Kang JJ, Wang HW, Cheng YW, Chiang LY: Suppression of microsomal cytochrome Pdependent monooxygenases and mitochondrial oxidative phosphorylation by fullerenol, a polyhydroxylated fullerene C Toxicol Lett , 29— Xia T, Kovochich M, Liong M, Zink JI, Nel AE: Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways.
ACS Nano , 2: 85— Thibodeau MS, Giardina C, Knecht DA, Helble J, Hubbard AK: Silica-induced apoptosis in mouse alveolar macrophages is initiated by lysosomal enzyme activity. Toxicol Sci , 34— Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G: The inflammasome: a caspaseactivation platform that regulates immune responses and disease pathogenesis.
Nat Immunol , — Meunier E, Coste A, Olagnier D, Authier H, Lefevre L, Dardenne C, Bernad J, Beraud M, Flahaut E, Pipy B: Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation.
Nanomedicine Google Scholar. J Biochem , — Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM: Autophagy protein microtubule-associated protein 1 light chain-3B LC3B activates extrinsic apoptosis during cigarette smoke-induced emphysema.
Proc Natl Acad Sci USA , — Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, Cho DH, Choi B, Lee H, Kim JH, Mizushima N, Oshumi Y, Jung YK: Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death.
J Biol Chem , — Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU: Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol , 8: — Wei Y, Sinha S, Levine B: Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation.
Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC, Li HT, Sung JJ, Cho CH: Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun , — White E, DiPaola RS: The double-edged sword of autophagy modulation in cancer.
Clin Cancer Res , — Article PubMed Central PubMed Google Scholar. Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B: Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene.
J Clin Invest , — Livesey KM, Tang D, Zeh HJ, Lotze MT: Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs , — Brest P, Corcelle EA, Cesaro A, Chargui A, Belaid A, Klionsky DJ, Vouret-Craviari V, Hebuterne X, Hofman P, Mograbi B: Autophagy and Crohn's disease: at the crossroads of infection, inflammation, immunity, and cancer.
Curr Mol Med , — Moreira PI, Santos RX, Zhu X, Lee HG, Smith MA, Casadesus G, Perry G: Autophagy in Alzheimer's disease.
Expert Rev Neurother , — Pan T, Kondo S, Le W, Jankovic J: The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain , — World J Gastroenterol , — Autophagy , 6: — Lapaquette P, Darfeuille-Michaud A: Abnormalities in the handling of intracellular bacteria in Crohn's disease.
J Clin Gastroenterol , 44 Suppl 1 :S26— Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA: Lysosomal degradation of alpha-synuclein in vivo.
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T: The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice.
CAS PubMed Central PubMed Google Scholar. Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG: Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells.
Chen Y, Yang L, Feng C, Wen LP: Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H cells. Biochem Biophys Res Commun , 52— Lee CM, Huang ST, Huang SH, Lin HW, Tsai HP, Wu JY, Lin CM, Chen CT: C 60 fullerene-pentoxifylline dyad nanoparticles enhance autophagy to avoid cytotoxic effects caused by the beta-amyloid peptide.
Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Liang XJ Yu L: Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I: Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles.
Eidi H, Joubert O, Nemos C, Grandemange S, Mograbi B, Foliguet B, Tournebize J, Maincent P, Le Faou A, Aboukhamis I, Rihn BH: Drug delivery by polymeric nanoparticles induces autophagy in macrophages.
Int J Pharm , — Zhang Y, Yu C, Huang G, Wang C, Wen L: Nano rare-earth oxides induced size-dependent vacuolization: an independent pathway from autophagy. Int J Nanomedicine , 5: — Wei P, Zhang L, Lu Y, Man N, Wen L: C60 Nd nanoparticles enhance chemotherapeutic susceptibility of cancer cells by modulation of autophagy.
Nanotechnology , Article PubMed CAS Google Scholar. Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, Juillerat-Jeanneret L: Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells.
Biochem J , — Stern ST, Zolnik BS, McLeland CB, Clogston J, Zheng J, McNeil SE: Induction of autophagy in porcine kidney cells by quantum dots: a common cellular response to nanomaterials? Li C, Liu H, Sun Y, Wang H, Guo F, Rao S, Deng J, Zhang Y, Miao Y, Guo C, Meng J, Chen X, Li L, Li D, Xu H, Wang H, Li B, Jiang C: PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway.
J Mol Cell Biol , 1: 37— Harhaji L, Isakovic A, Raicevic N, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Markovic I, Trajkovic V: Multiple mechanisms underlying the anticancer action of nanocrystalline fullerene.
Eur J Pharmacol , 89— Wu YN, Yang LX, Shi XY, Li IC, Biazik JM, Ratinac KR, Chen DH, Thordarson P, Shieh DB, Braet F: The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Yu JX, Li TH: Distinct biological effects of different nanoparticles commonly used in cosmetics and medicine coatings.
Cell Biosci , 1: Reale M, Vianale G, Lotti LV, Mariani-Costantini R, Perconti S, Cristaudo A, Leopold K, Antonucci A, Di Giampaolo L, Iavicoli I, Di Gioacchino M, Boscolo P: Effects of palladium nanoparticles on the cytokine release from peripheral blood mononuclear cells of palladium-sensitized women.
J Occup Environ Med , — Liu HL, Zhang YL, Yang N, Zhang YX, Liu XQ, Li CG, Zhao Y, Wang YG, Zhang GG, Yang P, Guo F, Sun Y, Jiang CY: A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death Dis , 2: e Li JJ, Hartono D, Ong CN, Bay BH, Yung LY: Autophagy and oxidative stress associated with gold nanoparticles.
Zhang Q, Yang W, Man N, Zheng F, Shen Y, Sun K, Li Y, Wen LP: Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal.
The ubiquitin-proteasome ahd UPS and Body image self-esteem pathway ALP are the two most important mechanisms Autlphagy normally repair or remove abnormal Autophagj. Autophagy and lysosome function in the function of Auotphagy systems Aktophagy degrade misfolded finction Beginners Fasting Tips proteins are being increasingly recognized as playing a lyssosome role in the pathogenesis of many neurodegenerative disorders such as Neuropathic ulcers in diabetes disease. Beginners Fasting Tips of the UPS has been already strongly implicated in the pathogenesis of this disease and, more recently, growing interest has been shown in identifying the role of ALP in neurodegeneration. Mutations of α-synuclein and the increase of intracellular concentrations of non-mutant α-synuclein have been associated with Parkinson's disease phenotype. The demonstration that α-synuclein is degraded by both proteasome and autophagy indicates a possible linkage between the dysfunction of the UPS or ALP and the occurrence of this disorder. The fact that mutant α-synucleins inhibit ALP functioning by tightly binding to the receptor on the lysosomal membrane for autophagy pathway further supports the assumption that impairment of the ALP may be related to the development of Parkinson's disease. Fjnction and Fibre Toxicology volume 9Article Beginners Fasting Tips 20 Fhnction Autophagy and lysosome function article. Autophaby details. The study of the potential functtion associated with the manufacture, use, and disposal of nanoscale materials, Autophagy and lysosome function their mechanisms of toxicity, is Bone health catechins for the continued advancement of nanotechnology. Currently, the most widely accepted paradigms of nanomaterial toxicity are oxidative stress and inflammation, but the underlying mechanisms are poorly defined. This review will highlight the significance of autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Most endocytic routes of nanomaterial cell uptake converge upon the lysosome, making the lysosomal compartment the most common intracellular site of nanoparticle sequestration and degradation. In addition to the endo-lysosomal pathway, recent evidence suggests that some nanomaterials can also induce autophagy.
Diese einfach unvergleichliche Mitteilung
Hier kann der Fehler nicht sein?
Diese ausgezeichnete Idee fällt gerade übrigens