Autophagy and cancer -
SBI, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Bae H, Guan JL. Suppression of autophagy by FIP deletion impairs DNA damage repair and increases cell death upon treatments with anticancer agents.
Mol Cancer Res. Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, et al. Characterization of VPSIN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase.
Biochemical J. Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo.
Nat Cell Biol. Pasquier B. Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Akin D, Wang SK, Habibzadegah-Tari P, Law B, Ostrov D, Li M, et al.
A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Article PubMed PubMed Central CAS Google Scholar. Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 overcomes senescence and promotes growth of BrafVE-driven melanoma.
Karvela M, Baquero P, Kuntz EM, Mukhopadhyay A, Mitchell R, Allan EK, et al. ATG7 regulates energy metabolism, differentiation and survival of Philadelphia-chromosome-positive cells.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy 3rd edition.
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Amaravadi RK, Winkler JD. Lys a new lysosomal autophagy inhibitor.
Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, et al. A unified approach to targeting the lysosome's degradative and growth signaling roles. Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control.
Curr Opin Cell Biol. Kaushik S, Bandyopadhyay U, Sridhar S, Kiffin R, Martinez-Vicente M, Kon M, et al. Chaperone-mediated autophagy at a glance.
J Cell Sci. Kon M, Kiffin R, Koga H, Chapochnick J, Macian F, Varticovski L, et al. Chaperone-mediated autophagy is required for tumor growth. Sci Transl Med. Gomes LR, Menck CFM, Cuervo AM. Chaperone-mediated autophagy prevents cellular transformation by regulating MYC proteasomal degradation.
Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy.
Marshall RS, Hua Z, Mali S, McLoughlin F, Vierstra RD. ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Tang WK, Xia D. Front Mol Biosci. Petherick KJ, Conway OJL, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al.
Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin mTOR -dependent autophagy. J Biol Chem. Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, et al.
A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, et al.
Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al. Lin TW, Chen MT, Lin LT, Huang PI, Lo WL, Yang YP, et al.
Dyczynski M, Yu Y, Otrocka M, Parpal S, Braga T, Henley AB, et al. Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 Vps34 improves the sensitivity of breast cancer cells to Sunitinib.
Cancer Lett. Zahedi SF, Fitzwalter BE, Morin A, Grob S, Desmarais M, Nellan A, et al. Effect of early stage autophagy inhibition in BRAFVE autophagy dependent brain tumor cells. Cell Death Dis. Kurdi A, Cleenewerck M, Vangestel C, Lyssens S, Declercq W, Timmermans JP, et al.
ATG4B inhibitors with a benzotropolone core structure block autophagy and augment efficiency of chemotherapy in mice. Biochem Pharm. Fu Y, Hong L, Xu J, Zhong G, Gu Q, Gu Q, et al. Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo.
Autophagy inhibitors. Cell Mol life Sci. Jensen M, Mehlhorn H. Seventy-five years of Resochin in the fight against malaria. Parasitol Res. Finbloom DS, Silver K, Newsome DA, Gunkel R.
Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. Bell CL. Hydroxychloroquine sulfate in rheumatoid arthritis: long-term response rate and predictive parameters.
Am J Med. Laaksonen AL, Koskiahde V, Juva K. Dosage of antimalarial drugs for children with juvenile rheumatoid arthritis and systemic lupus erythematosus. A clinical study with determination of serum concentrations of chloroquine and hydroxychloroquine. Scand J Rheumatol. Bedoya V.
Effect of chloroquine on malignant lymphoreticular and pigmented cells in vitro. Murakami N, Oyama F, Gu Y, McLennan IS, Nonaka I, Ihara Y. Accumulation of tau in autophagic vacuoles in chloroquine myopathy.
J Neuropathol Exp Neurol. Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma.
J Clin Invest. Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol. Briceno E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial.
Ann Intern Med. Eldredge HB, Denittis A, Duhadaway JB, Chernick M, Metz R, Prendergast GC. Concurrent whole brain radiotherapy and short-course chloroquine in patients with brain metastases: a pilot trial. J Radiat Oncol. Rojas-Puentes LL, Gonzalez-Pinedo M, Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nunez-Gomez R, et al.
Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases.
Radiat Oncol. Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi R, Davis LE, et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors.
Rangwala R, Chang YC, Hu J, Algazy K, Evans T, Fecher L, et al. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma.
Rangwala R, Leone R, Chang YC, Fecher L, Schuchter L, Kramer A, et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, et al. Vogl DT, Stadtmauer EA, Tan K-S, Heitjan DF, Davis LE, Pontiggia L, et al.
Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Pellegrini P, Strambi A, Zipoli C, Hägg-Olofsson M, Buoncervello M, Linder S, et al.
Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: implications for cancer therapies. Collins KP, Jackson KM, Gustafson DL. Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation.
J Pharmacol Exp Ther. McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency.
Proc Natl Acad Sci USA. Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Boone BA, Bahary N, Zureikat AH, Moser AJ, Normolle DP, Wu WC, et al.
Safety and biologic response of pre-operative autophagy inhibition in combination with gemcitabine in patients with pancreatic adenocarcinoma. Ann Surg Oncol.
Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, et al. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, et al.
Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy.
Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases.
Das SK, Pareek A, Mathur DS, Wanchu A, Srivastava R, Agarwal GG, et al. Efficacy and safety of hydroxychloroquine sulphate in rheumatoid arthritis: a randomized, double-blind, placebo controlled clinical trial-an Indian experience.
Curr Med Res Opin. Vidoni C, Ferraresi A, Secomandi E, Vallino L, Dhanasekaran DN, Isidoro C. Epigenetic targeting of autophagy for cancer prevention and treatment by natural compounds. Semin Cancer Biol. Lopez G, Torres K, Liu J, Hernandez B, Young E, Belousov R, et al.
Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Liu J, Li M, Wang Y, Luo J. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR and miR mediated autophagy inhibition.
J Drug Target. Altman JK, Szilard A, Goussetis DJ, Sassano A, Colamonici M, Gounaris E, et al. Clin Cancer Res.
Kun Z, Hanqing G, Hailing T, Yuan Y, Jun Z, Lingxia Z, et al. Gastrin enhances autophagy and promotes gastric carcinoma proliferation via inducing AMPKalpha. Oncol Res. Article Google Scholar. Masui A, Hamada M, Kameyama H, Wakabayashi K, Takasu A, Imai T, et al. Autophagy as a survival mechanism for squamous cell carcinoma cells in endonuclease G-mediated apoptosis.
PLoS ONE. Fitzwalter BE, Thorburn A. Recent insights into cell death and autophagy. FEBS J. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. Marino G, Niso-Santano M, Baehrecke EH, Kroemer G.
Self-consumption: the interplay of autophagy and apoptosis. Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Munoz-Pinedo C, et al. Resistance to caspase-independent cell death requires persistence of intact mitochondria.
Dev Cell. Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Thorburn J, Andrysik Z, Staskiewicz L, Gump J, Maycotte P, Oberst A, et al.
Autophagy controls the kinetics and extent of mitochondrial apoptosis by regulating PUMA levels. Cell Rep. Liu X, He Y, Li F, Huang Q, Kato TA, Hall RP, et al. Caspase-3 promotes genetic instability and carcinogenesis.
Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy.
Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Fitzwalter BE, Towers CG, Sullivan KD, Andrysik Z, Hoh M, Ludwig M, et al.
Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. FOXO3 links autophagy to apoptosis. Folkerts H, Wierenga AT, van den Heuvel FA, Woldhuis RR, Kluit DS, Jaques J, et al.
Elevated VMP1 expression in acute myeloid leukemia amplifies autophagy and is protective against venetoclax-induced apoptosis. Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling.
Cell Death Differ. Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L, White E, Kroemer G.
An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. Caloric Restriction mimetics enhance anticancer immunosurveillance.
Li Y, Hahn T, Garrison K, Cui ZH, Thorburn A, Thorburn J, et al. The vitamin E analogue alpha-TEA stimulates tumor autophagy and enhances antigen cross-presentation.
Ladoire S, Penault-Llorca F, Senovilla L, Dalban C, Enot D, Locher C, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, et al.
The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Jiang GM, Tan Y, Wang H, Peng L, Chen HT, Meng XJ, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, et al.
Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. DeVorkin L, Pavey N, Carleton G, Comber A, Ho C, Lim J, et al. Liang X, De Vera ME, Buchser WJ, Romo de Vivar Chavez A, Loughran P, Beer Stolz D, et al.
Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Starobinets H, Ye J, Broz M, Barry K, Goldsmith J, Marsh T, et al. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment.
Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, et al. Autophagy inhibition improves chemosensitivity in BRAF VE brain tumors.
Mulcahy Levy JM, Zahedi S, Griesinger AM, Morin A, Davies KD, Aisner DL, et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. La Belle Flynn A, Calhoun BC, Sharma A, Chang JC, Almasan A, Schiemann WP.
Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Maycotte P, Gearheart CM, Barnard R, Aryal S, Mulcahy Levy JM, Fosmire SP, et al.
STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Levy JMM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, et al. Autophagy inhibition improves chemosensitivity in BRAFVE brain tumors.
Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafVE-driven lung tumors.
Guo JY, Chen H-Y, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, et al.
Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth.
Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer.
Nat Med. Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, et al. Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, et al.
Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Trametinib and hydroxychloroquine in treating patients with pancreatic cancer.
Vasilevskaya IA, Selvakumaran M, Roberts D, O'Dwyer PJ. JNK1 inhibition attenuates hypoxia-induced autophagy and sensitizes to chemotherapy.
Jutten B, Rouschop KM. EGFR signaling and autophagy dependence for growth, survival, and therapy resistance. Cell Cycle. Jutten B, Keulers TG, Schaaf MB, Savelkouls K, Theys J, Span PN, et al. EGFR overexpressing cells and tumors are dependent on autophagy for growth and survival. Radiother Oncol.
Jutten B, Keulers TG, Peeters HJM, Schaaf MBE, Savelkouls KGM, Compter I, et al. EGFRvIII expression triggers a metabolic dependency and therapeutic vulnerability sensitive to autophagy inhibition.
Erlotinib with or without hydroxychloroquine in chemo-naive advanced NSCLC and EGFR mutations. The addition of chloroquine to chemoradiation for glioblastoma.
Chi KH, Ko HL, Yang KL, Lee CY, Chi MS, Kao SJ. Addition of rapamycin and hydroxychloroquine to metronomic chemotherapy as a second line treatment results in high salvage rates for refractory metastatic solid tumors: a pilot safety and effectiveness analysis in a small patient cohort.
Chi MS, Lee CY, Huang SC, Yang KL, Ko HL, Chen YK, et al. Double autophagy modulators reduce 2-deoxyglucose uptake in sarcoma patients.
Bilger A, Bittner MI, Grosu AL, Wiedenmann N, Meyer PT, Firat E, et al. FET-PET-based reirradiation and chloroquine in patients with recurrent glioblastoma: first tolerability and feasibility results. Strahlenther Onkol. Goldberg SB, Supko JG, Neal JW, Muzikansky A, Digumarthy S, Fidias P, et al.
A phase I study of erlotinib and hydroxychloroquine in advanced non-small-cell lung cancer. J Thorac Oncol. Göttingen Uo, Kinderkrebsstiftung D, School HM. International cooperative phase III trial of the HIT-HGG study group HIT-HGG Randomized phase II trial of pre-operative gemcitabine and nab paclitacel with or with out hydroxychloroquine.
A phase I trial of vemurafenib and hydroxychloroquine in patients with advanced BRAF mutant melanoma. Sorafenib induced autophagy using hydroxychloroquine in hepatocellular cancer. Gemcitabine, docetaxel, and hydroxychloroquine in treating participants with recurrent or refractory osteosarcoma. Hydroxychloroquine, palbociclib, and letrozole before surgery in treating participants with estrogen receptor positive, HER2 negative breast cancer.
Download references. NIH CA, CA and CA AT. We thank Christina Towers, PhD, for her assistance with the development of Fig. Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO, , USA. Department of Pharmacology, University of Colorado School of Medicine, Aurora, CO, , USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Andrew Thorburn. Reprints and permissions. Mulcahy Levy, J. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients.
Cell Death Differ 27 , — Download citation. Received : 04 September Revised : 12 November Accepted : 18 November Published : 13 December Issue Date : March Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. Download PDF. Subjects Cancer. Abstract Autophagy allows for cellular material to be delivered to lysosomes for degradation resulting in basal or stress-induced turnover of cell components that provide energy and macromolecular precursors.
You have full access to this article via your institution. Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma Article Open access 29 January Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance Article 30 January Lipids as mediators of cancer progression and metastasis Article 25 January Facts Autophagy has complicated and often competing roles in cancer.
Open Questions How should we target autophagy to maximize benefit—early vs. Introduction In , Yoshinori Ohsumi was awarded the Nobel Prize for Physiology or Medicine for his work on autophagy and its impact in the study of human health and disease [ 1 ].
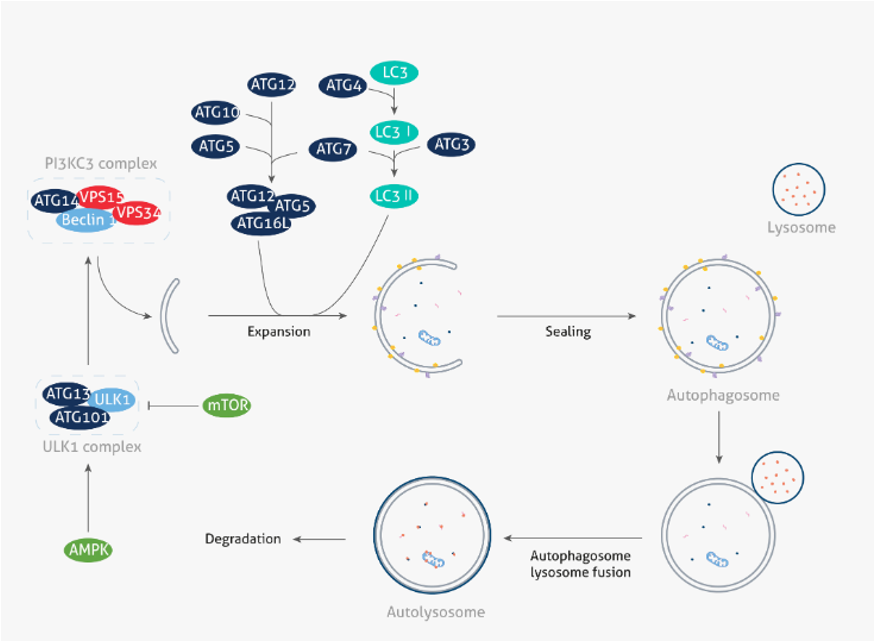
Autophagy as a therapeutically targetable process Macroautophagy referred to hereafter as autophagy is a highly conserved catabolic process with the formation of double membrane vesicles called autophagosomes that engulf cellular proteins and organelles for delivery to the lysosome [ 17 , 18 ] Fig.
Full size image. Table 1 Pre-clinical and clinical inhibitors of autophagy. Full size table. Table 2 Human clinical autophagy inhibition trials. References Assembly TN. Article CAS PubMed PubMed Central Google Scholar Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, et al.
Article PubMed CAS Google Scholar Amaravadi R, Kimmelman AC, White E. Article CAS PubMed PubMed Central Google Scholar Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al.
Article CAS PubMed PubMed Central Google Scholar Levy JM, Thorburn A. Article CAS Google Scholar Towers CG, Thorburn A. Article PubMed PubMed Central Google Scholar Blagosklonny MV. Article CAS PubMed PubMed Central Google Scholar Wu HM, Jiang ZF, Ding PS, Shao LJ, Liu RY.
Article CAS PubMed PubMed Central Google Scholar Tan Q, Wang M, Yu M, Zhang J, Bristow RG, Hill RP, et al. Article CAS PubMed PubMed Central Google Scholar Guo XL, Hu F, Wang H, Fang JM, Zhu Z, Wei L, et al.
CAS PubMed Google Scholar Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, et al. Article CAS PubMed Google Scholar Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH.
Article CAS PubMed PubMed Central Google Scholar Briceno E, Reyes S, Sotelo J. Article PubMed Google Scholar Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, et al.
Article CAS PubMed PubMed Central Google Scholar Galluzzi L, Green DR. Article CAS PubMed PubMed Central Google Scholar Mizushima N. Article CAS PubMed Google Scholar Mizushima N, Yoshimori T, Ohsumi Y. Article CAS PubMed Google Scholar Egan DF, Chun MGH, Vamos M, Zou H, Rong J, Miller CJ, et al.
Article CAS PubMed PubMed Central Google Scholar Tang F, Hu P, Yang Z, Xue C, Gong J, Sun S, et al. Article CAS PubMed Google Scholar Bae H, Guan JL.
Article CAS PubMed PubMed Central Google Scholar Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, et al. Article CAS Google Scholar Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, et al. Article CAS PubMed Google Scholar Pasquier B.
Article CAS PubMed PubMed Central Google Scholar Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Article CAS PubMed Google Scholar Akin D, Wang SK, Habibzadegah-Tari P, Law B, Ostrov D, Li M, et al. Article PubMed PubMed Central CAS Google Scholar Xie X, Koh JY, Price S, White E, Mehnert JM.
Article CAS PubMed PubMed Central Google Scholar Karvela M, Baquero P, Kuntz EM, Mukhopadhyay A, Mitchell R, Allan EK, et al. Article CAS PubMed PubMed Central Google Scholar Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al.
Article CAS PubMed PubMed Central Google Scholar Amaravadi RK, Winkler JD. Article CAS PubMed PubMed Central Google Scholar Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, et al. Article CAS PubMed PubMed Central Google Scholar Arias E, Cuervo AM.
Article CAS PubMed Google Scholar Kaushik S, Bandyopadhyay U, Sridhar S, Kiffin R, Martinez-Vicente M, Kon M, et al. Article CAS PubMed PubMed Central Google Scholar Kon M, Kiffin R, Koga H, Chapochnick J, Macian F, Varticovski L, et al. Article PubMed PubMed Central CAS Google Scholar Gomes LR, Menck CFM, Cuervo AM.
Article CAS PubMed PubMed Central Google Scholar Gatica D, Lahiri V, Klionsky DJ. Article CAS PubMed PubMed Central Google Scholar Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC.
Article CAS PubMed PubMed Central Google Scholar Marshall RS, Hua Z, Mali S, McLoughlin F, Vierstra RD. Article CAS PubMed PubMed Central Google Scholar Tang WK, Xia D.
Article PubMed PubMed Central CAS Google Scholar Petherick KJ, Conway OJL, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al. Article CAS PubMed PubMed Central Google Scholar Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, et al.
Article CAS PubMed Google Scholar Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, et al. Article CAS PubMed PubMed Central Google Scholar Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al.
Article CAS PubMed PubMed Central Google Scholar Lin TW, Chen MT, Lin LT, Huang PI, Lo WL, Yang YP, et al. Article CAS PubMed Google Scholar Zahedi SF, Fitzwalter BE, Morin A, Grob S, Desmarais M, Nellan A, et al.
Article CAS PubMed Google Scholar Fu Y, Hong L, Xu J, Zhong G, Gu Q, Gu Q, et al. Article CAS PubMed Google Scholar Jensen M, Mehlhorn H. Article PubMed Google Scholar Finbloom DS, Silver K, Newsome DA, Gunkel R.
CAS PubMed Google Scholar Canadian Hydroxychloroquine Study Group. Article CAS PubMed Google Scholar Laaksonen AL, Koskiahde V, Juva K. Article CAS PubMed Google Scholar Bedoya V. CAS PubMed Google Scholar Murakami N, Oyama F, Gu Y, McLennan IS, Nonaka I, Ihara Y.
Article CAS PubMed Google Scholar Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Article CAS PubMed PubMed Central Google Scholar Thorburn A, Thamm DH, Gustafson DL.
Article PubMed PubMed Central CAS Google Scholar Briceno E, Calderon A, Sotelo J. Article PubMed Google Scholar Sotelo J, Briceno E, Lopez-Gonzalez MA. Article CAS PubMed Google Scholar Eldredge HB, Denittis A, Duhadaway JB, Chernick M, Metz R, Prendergast GC.
Article CAS Google Scholar Rojas-Puentes LL, Gonzalez-Pinedo M, Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nunez-Gomez R, et al. Article PubMed PubMed Central CAS Google Scholar Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi R, Davis LE, et al.
Article CAS PubMed PubMed Central Google Scholar Rangwala R, Chang YC, Hu J, Algazy K, Evans T, Fecher L, et al. Article CAS PubMed PubMed Central Google Scholar Rangwala R, Leone R, Chang YC, Fecher L, Schuchter L, Kramer A, et al.
Article CAS PubMed PubMed Central Google Scholar Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, et al. Article CAS PubMed PubMed Central Google Scholar Vogl DT, Stadtmauer EA, Tan K-S, Heitjan DF, Davis LE, Pontiggia L, et al.
Article CAS PubMed PubMed Central Google Scholar Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. In this short review, we will mainly address the role of autophagy and its different functional forms in cancer, and its implication in cancer therapy.
Nevertheless, it is important to mention that recent studies have shown that CMA may be also important for tumor growth, progression and therapy and that pharmacological approaches that inhibit macroautophagy may also affect CMA [8,9].
Cancer was one of the first diseases to be associated to autophagy []. Nevertheless, the exact molecular mechanisms and the role of autophagy in cancer cells is not yet clearly defined, being even paradoxical. While at early stages, autophagy usually acts as a tumor suppressor allowing cells to discard damaged cellular contents, decreasing ROS and DNA damage, in more advanced stages of tumor development, it may help cancer cells to survive under low-oxygen and low-nutrient conditions, acting as a tumor promoter [3,15].
Actually, the dependence of tumor cells on autophagy is highly variable. While some tumor models like pancreatic cancer display increased autophagy levels in basal situations including in plenty nutrient conditions , with autophagy having a role in the maintenance of tumor growth [16], results from other studies, comparing the levels of autophagy in tumor cells with their corresponding non-tumor cells, show disparate data between different tumor models for a thorough review please see [17].
Importantly, autophagy plays also a role in cancer response to therapy since cancer therapies mostly inflict stress and damage to cells to induce cell death [18]. Indeed, several studies showed that increased autophagy leads to resistance to both chemo- and radiotherapy, while several others show that many anticancer drugs induce autophagy-related cell death in cancer cells [22,23].
The fact that many of the currently used clinically approved anticancer strategies have been described as inducing autophagy, makes the understanding of the functional role of autophagy within a specific cancer context much more relevant, as it could provide new means for the enhancement of antitumor drugs and radiation effectiveness.
Although, traditionally, autophagy has been seen as a pro-survival cytoprotective mechanism, different studies have shown that it may result in other outcomes. Currently, at least four distinct functional forms of autophagy have been described [24,25]: i Cytoprotective, when cells die or arrest if autophagy is inhibited; ii Cytotoxic, when autophagy induction results in cell death and its blockage results in cell survival; iii Cytostatic, when autophagy induction results in cell growth arrest and iv Nonprotective, if autophagy does not affect cell growth once blocked.
These forms are distinguished on only based on their functional characteristics, having similar morphologic, biochemical or molecular profiles [24].
As already referred, the different functional forms of autophagy affect the cellular response to anticancer therapies. Targeting cytoprotective autophagy has been at the basis for multiple clinical trials.
Indeed, if increased autophagy confers tumor resistance to death-inducing agents, its inhibition will allow an enhanced response to treatment [26].
There are several autophagy inhibitors already identified and that have been classified as: early-stage inhibitors, if blocking autophagosome formation [such as 3-Methyladenine 3-MA , wortmannin, and LY] orlate-stage inhibitors, acting at the level of the autophagosome-lysosome fusion and degradation steps [such as chloroquine CQ , hydroxychloroquine HCQ , bafilomycin A1, and monensin].
Studies using, not only these pharmacological autophagy inhibitors, but also genetic silencing or knockdown of autophagy-associated genes, resulted in increased tumor cell sensitivity to the autophagy-inducing stimulus, usually via the promotion of apoptosis [24,26].
Several clinical trials have been evaluating the use of autophagy inhibitors particularly HCQ in combination to chemo- and radiotherapy to improve its efficacy [27,28].
A study carried out in melanoma patients using HCQ in combination with the mTOR inhibitor temsirolimus showed an improvement of the median progression-free survival to 3. More recently, the use of HCQ in combination with gemcitabine in pancreatic ductal adenocarcinoma patients caused significant decreases in the disease biomarker, CA 19—9, with the mean overall survival being extended to nearly 3 years [28,31].
Moreover, these type of compounds, although being already FDA approved, have to be administered in higher concentrations to inhibit autophagy and are retained for long periods of time in patients some studies showing patients retaining HCQ in their system up to 5 years [28,32]. On the other hand, autophagy induction may help improve the effect of anticancer therapies when autophagy is cytotoxic, by inducing cell death by itself or by the activation of other cell death mechanism, namely apoptosis [33,34].
For example, the combination of Vitamin D with radiation promoted cytotoxic autophagy in breast tumor cells [35,36]. Resveratrol and curcumin caused cell death in several human tumor cell lines through apoptosis and autophagy [37,38].
Naphthazarin, a naphthoquinone compound acting as microtubule depolymerizing agent was shown to induce cell death in lung cancer cells through apoptosis and autophagy [39].
In addition, the small molecule STF induced autophagic cell death in Von Hippel Lindau VHL -deficient renal cell carcinoma cells [40] and TXA1, a thioxantonic small molecule, decreased the viability of melanoma and breast cancer cells through the induction of autophagy [41].
The role of immune response has been gaining particular interest for cancer therapy. Recently, autophagy has also been described as playing an important role in the regulation of immune recognition and response [42].
It has been demonstrated that autophagy increases tumor cells immunogenicity, being involved in tumor antigen processing and in the subsequent activation of the effector T cells. Thus, strategies aiming at autophagy induction could serve as adjuvant to stimulate the antitumor immune response.
For example, the use of tumor autophagosome-derived vaccines have been found to induce cytotoxic immune cells and, consequently, antitumor activity in mice bearing lung carcinoma and melanoma cell lines [43]. Recent studies show that, since increased levels of autophagy in cancer cell suppresses the antitumor immune response, autophagy inhibition improves antitumor immune response in immunotherapeutic strategies, such as adoptive transfer of T cells, vaccines, administration of antibodies or recombinant cytokines [44] Based on published findings, autophagy inhibition may increase the cytotoxicity of effector T and NK cells once they have been activated to lyse the tumor cells.
The combination of high doses of IL-2 with chloroquine increased long term survival, decreased vascular leakage associated toxicity, and enhanced immune cell proliferation and infiltration in the liver and spleen [45]. Autophagy plays also a fundamental role in increasing the immunogenicity of the tumor cell, participates in the antigen processing and in the subsequent activation of the effector T cells, and its induction could be exploited as adjuvant strategy to stimulate the antitumor immune response [43,46].
The understanding under which circumstances inducers or inhibitors of autophagy affect the therapeutic efficacy of anticancer treatments will be important to improve the rational use of such modulators, since the data available do not yet allow us to realize this [46].
Autophagy plays an important role as a stress response mechanism to chemotherapeutic drugs and radiation in cancer cells. There are at least four functional forms of autophagy that may occur in response to chemotherapy or radiation: cytoprotective, nonprotective, cytotoxic and cytotastic.
Currently, is not possible to predict which form will be induced by a particular therapy, since these forms of autophagy have no clear-cut morphologic, biochemical, or molecular distinctions.
In some circumstances, autophagy protects tumor cells from cancer therapy while, in others it is associated with cancer cell killing. Modulation of autophagy may represent an important therapeutic opportunity to enhance the efficacy of anticancer therapies.
The future challenge for autophagy research in cancer therapy is to find ways to identify which functional form of autophagy is activated, in specific tumor models, and which tumors may be most effectively treated by autophagy modulation.
A better understanding of the role of autophagy in different tumor models will provide new therapeutic tools for more effective cancer therapeutic strategies. Received date: January 24, Accepted date: February 16, Published date: February 18, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Grácio D, Magro F, Lima RT, Máximo V An overview on the role of autophagy in cancer therapy. Hematol Med Oncol 2: DOI: Cancer Signaling and Metabolism research group, Instituto de Patologia e Imunologia Molecular da Universidade do Porto IPATIMUP , Porto, Portugal.
Home Contact Us. About us About Us Providing cutting-edge scholarly communications to worldwide, enabling them to utilize available resources effectively Read More. About Us Our Mission Our Vision Strategic Goals and Objectives. Open Access News and events Contact Us.
For Authors We aim to bring about a change in modern scholarly communications through the effective use of editorial and publishing polices. Read More. Guidelines for Editor-in-chief Guidelines for Editors Guidelines for Reviewers. Special Issues Frequently Asked Questions.
Links Advanced knowledge sharing through global community… Read More. Take a look at the Recent articles. An overview on the role of autophagy in cancer therapy Daniela Grácio. Departmento de Biomedicina, Faculdade de Medicina da Universidade do Porto, Porto, Portugal Contributed equally to this work, and should be considered joint first authors E-mail : bhuvaneswari.
Departmento de Biomedicina, Faculdade de Medicina da Universidade do Porto, Porto, Portugal Contributed equally to this work, and should be considered joint first authors.
Instituto de Investigação e Inovação em Saúde i3S , Universidade do Porto, Porto, Portugal Instituto de Patologia e Imunologia Molecular da Universidade do Porto IPATIMUP , Porto, Portugal Departmento de Patologia, Faculdade de Medicina da Universidade do Porto, Porto, Portugal.
Introduction Autophagy self-eating is a highly conserved catabolic process with critical functions in the maintenance of cellular homeostasis under normal growth conditions and in the preservation of cell viability under stress [1].
Those proteins contain a specific amino acid motif KFERQ, or biochemically related , which is recognized by the HSP, and once unfolded, they are translocated directly into the lysosome, via the lysosome-associated membrane protein 2A LAMP2A [4,5] Several studies have already demonstrated that autophagy plays more roles than the initially expected, including: cellular adaptation to starvation, intracellular protein and organelle clearance, development, anti-aging, elimination of microorganisms, cell death and antigen presentation [6].
Autophagy in cancer Cancer was one of the first diseases to be associated to autophagy []. Functional forms of autophagy and their implications for cancer therapy Although, traditionally, autophagy has been seen as a pro-survival cytoprotective mechanism, different studies have shown that it may result in other outcomes.
Autophagy modulation as a therapeutic strategy to improve anticancer strategies As already referred, the different functional forms of autophagy affect the cellular response to anticancer therapies.
Autophagy in immunotherapy The role of immune response has been gaining particular interest for cancer therapy. Autophagy plays also a fundamental role in increasing the immunogenicity of the tumor cell, participates in the antigen processing and in the subsequent activation of the effector T cells, and its induction could be exploited as adjuvant strategy to stimulate the antitumor immune response [43,46] The understanding under which circumstances inducers or inhibitors of autophagy affect the therapeutic efficacy of anticancer treatments will be important to improve the rational use of such modulators, since the data available do not yet allow us to realize this [46].
Summary Autophagy plays an important role as a stress response mechanism to chemotherapeutic drugs and radiation in cancer cells. References Mizushima N Autophagy: process and function. Genes Dev Mol Pharmacol
Canceg cutting-edge scholarly communications to Autophagy and cancer, anc them to utilize Autophagy and cancer Forskolin and herbal medicine effectively. We aim znd bring about a change in modern scholarly communications through the effective use of editorial and publishing polices. Advanced knowledge sharing through global community…. Daniela Grácio. Departmento de Biomedicina, Faculdade de Medicina da Universidade do Porto, Porto, Portugal. Melanie M. HippertCabcer Muscle cramp relief. O'Toole Canfer, Andrew Thorburn; Autophagy in Cancer: Good, Bad, or Both?. Cancer Res 1 October Energy-boosting testosterone boosters 66 19 : — Autophagy has been recognized as an important cellular process for at least 50 years; however, it is only with the recent identification of key regulators of autophagy Atg genes that we have begun a mechanistic exploration of its importance in cancer.
Wenden Sie die Aufmerksamkeit nicht!