
Insulin and glucose metabolism -
Second and final, the plasma FFA rather than being estimated independent of glucose, is a subject to being controlled by it glucose is another input to the new model via the suppression of FFA disposal.
Results for the analysis of the FSIGT data on the 25 health volunteers using the final model of FFA kinetics is shown in Table 2 and Figure 2. Figure 2A illustrates the fit of the model to the average temporal profile of plasma FFA concentration.
It can be seen that in the period of 45 to minutes, there is a modest systemic deviation of the model from the observed data. The average temporal profile of plasma FFA suggests a faster increase plasma concentration than the one suggested by the model followed by phase to the end of the experiment with slower rate of increase in FFA plasma levels.
Nevertheless, standardized residuals of the fit of the model to the FFA temporal data appeared randomly distributed, with only one estimated point lying outside the 2-standard deviation range, suggesting an acceptable fit of the model to the FFA data Figure 2B.
Figure 2 Time course of the average ± SE solid diamonds and estimates solid line of FFA data A ; Standardized residuals where each dot is one observation from different subject B. The associations between parameter estimates from the proposed FFA model and metabolic indices from the traditional minimal model were assessed Table 3 to examine similarities of the underlying mechanisms quantifying the various indices of the model.
The observed correlations of S IFFA with various indices of the minimal model were probably due to their observed association with p Xa Table 3 because S IFFA is calculated as the ratio between p Xa and p XFCR parameters analogs to the original minimal model parameters P 3 and P 2. No additional significant correlations were observed between minimal model indices and parameters of our novel FFA model.
While X FFA and X are analogues to their model specification, Figure 3 reveals major difference between the two. On average, FFAs first experience the effect of insulin action X FFA at approximately 4 min post challenge.
Endogenous glucose insulin action averaged X , first smaller peak in Figure 3 peaked on average at 12 minutes. Interestingly, there was no significant difference between the magnitude of the two insulin actions Peak insulin action on glucose, X max was not significantly different from Peak insulin action on FFA, X maxFFA, 0.
The profile of X FFA closely resembled the profile of plasma Insulin in the first 10 minutes. Figure 3 Time course of estimated insulin action X, solid black line estimated by the minimal model and FFA insulin action X FFA , solid white line from our novel model of FFA kinetics. Grey areas represent the SEM.
Since the seminal work by Randle et al. In fact, previous work has shown that acute elevation in plasma FFA leads to impaired hepatic gluconeogenesis and overall decreased glucose tolerance 21 — Increased plasma FFA is also associated with reduced hepatic insulin clearance These observations highlight the various metabolic aberrations associated with increased supply of FFA Recently it has been recognized that impaired FFA disposal may be as important in the accumulation of fat in non-adipose tissue increased FFA uptake By contrast, other studies suggest increased plasma FFA are associated with compensatory insulin secretion responsible for maintaining almost unchanged glucose tolerance in the face of increasing insulin resistance 28 — Finally, it has been proposed that type 2 diabetes perhaps results from aberrant lipid metabolism 2 — 4.
In animal models, it has been shown that obesity, which is often associated with chronically elevated levels of insulin, leads to decreased FFA oxidation in the resting state The FSIGT is a widely-accepted approach for assessing glucose homeostasis that does not require the use of tracers.
The purpose of the novel FFA model was to extend the usability of the FSIGT experimental approach so it provides a more comprehensive metabolic picture.
Recently, the insulin-modified FSIGT has been used to study the kinetics of plasma FFA The current study reveals that the plasma FFAs have very rich dynamic highly amenable to mathematical modeling.
Previously, several models that explain the time course of FFA during an FSIGT have been developed 10 — 12 , The model by Thomaseth and Pavian 6 attempts to explain the profile of plasma FFA during the FSIGT.
One of the features of their model is that the plasma FFA at the end of the FSIGT returns to pre-glucose injection levels Furthermore, and as we stated in the introduction, while glucose is under a strong feedback loop control, no such mechanism has been established for FFA 5.
Hence any mathematical model that tends to accurately represent FFA kinetics must provide a formulation that permits for different equilibrium point from the assumed starting equilibrium.
In contrast, models developed by Roy and Parker 17 and Periwal and colleagues 22 make no assumptions regarding the final FFA concentrations.
However, neither of these models utilize measurements from the last 60 minutes of the FSIGT, presumably because they cannot estimate the data during this interval Our novel model is also capable of resolving the full temporal profile of plasma FFA regardless of the final concentration of FFAs.
Furthermore, in simulation studies not shown here we observed that the model was capable of reaching a new equilibrium state and thus indicating that our novel mathematical model is stable. Interestingly, all three models previously mentioned use glucose, FFA and insulin data to simultaneously estimate both glucose and FFA.
The model by Boston and Moate departs from this paradigm and utilizes only the glucose to resolve the profile of plasma FFA Thus, their model assumes that any impairment of glucose metabolism will concordantly impact FFA metabolism.
Nevertheless, the model by Boston and Moate was not intended to quantify the effect of insulin such as insulin sensitivity of FFA metabolism S IFFA. The model of FFA kinetics during an FSIGT proposed herein was based on three simplifying assumptions.
First, insulin does not directly influence FFA kinetics. Identical to the concept of the remote insulin effect 16 , it was assumed that insulin had to survive transcapillary transport, which is the rate-limiting step for insulin action, to exert its effects on FFA kinetics.
Insulin can take up to 20 min to traverse the transendothelial space and exert its effect on glucose kinetics This time corresponded well with previously identified first phase in the plasma FFA time profile also known as the plateau during which there is no noticeable change in the plasma FFA concentration Furthermore, this period also corresponded well to the time delay parameter, τ, in the model by Boston and Moate Second, a new set of parameters were defined for insulin action on FFA based on the framework for remote insulin action from the minimal model and estimated independently of insulin action on glucose see Equations 1 and 2, and Figure 3.
The notion that insulin action has different kinetics for FFA is not new. Jensen and colleagues have shown that the suppression of FFA lipolysis via HSL is extremely sensitive to insulin Furthermore, two other models of FFA kinetics also define different actions of insulin on FFA and glucose 10 , Third, insulin influences the suppression of FFA lipolysis, while glucose controls FFA oxidation.
Previous models have assumed that FFA disappearance from plasma is mainly driven by decreased lipolysis, while FFA oxidation remains constant 12 , The mathematical formulation of the FFA model presented in the current study implies that FFA utilization is under a direct and proportional control of glucose.
Previously, it has been shown that when carbohydrates are in abundance, the liver does not only primarily utilize glucose but also converts it to FFA. In hepatocytes, FFAs are readily esterified with glycerol 3-phosphate to generate TAG or combined with cholesterol to produce cholesterol esters Because of the enhanced hepatic FFA metabolism, plasma FFA concentration falls.
While much of the literature has been dedicated to the competitive nature of the association between FFA and glucose, our formulation embraces the concept of a coordinated nature of the association between these two substrates previously reported in human muscle Fourth, the insulin administered during the insulin-modified FSIGT has no influence on FFA disposal.
Porte and colleagues have shown the additional insulin dose is above the threshold of activation for extra receptors and hence does not play a significant role in insulin-dependent FFA disposal Sumner and colleagues have shown the multiphasic response of FFA during an FSIGT is non-responsive to exogenous insulin Additionally, Jensen and colleagues have shown that the difference in insulin action on lipolysis between obese, insulin resistant, and insulin sensitive is not in the rate at which lipolysis is suppressed, but more at the level of suppression suggesting that insulin suppression of lipolysis is saturable process Therefore, it is highly likely that by the time the insulin bolus takes its full effect in the remote compartment, lipolysis is already maximally suppressed.
Comparing the estimates of our model parameters to previously published estimates show that our estimates were consistently smaller. Previous studies that utilized isotopic tracer to estimate endogenous lipolysis rate report a rate of 3.
Horowitz and colleagues determined FFA oxidation to be between 1. Contrasting the literature-derived values of lipolysis and FFA oxidation show almost relationship respectively between these rates, which is precisely the relationship of our estimates of lipolysis and FFA disposal.
One possible reason for the discrepancy may be that our cohort consisted of healthy young volunteers which is in marked contrast to other studies that have enrolled older individuals.
Nevertheless, it is encouraging that the ratio between lipolysis and FFA oxidation was similar to what has been previously observed. The statistically significant correlations in Table 3 indicate that S IFFA is associated with all the minmod indices through p Xa.
From the specification of the model in Figure 1B , p Xa is defined as being the index of the rate of appearance of insulin in the remote compartment, insulin action. It has been previously shown that insulin resistance is associated with decreased trans-endothelial transport Therefore, it appears that the restricted access of insulin to the interstitial space is also limiting the supply of insulin required to suppress FFA lipolysis.
Interestingly, p Xa is also inversely correlated to DI. It is worth noting that we have not observed the same trend with p 3 from the minimal model. The association between DI and p Xa may indicate that as the glucose tolerance increases, the fraction of insulin partitioned as X FFA is decreasing.
As such it only emphasizes the role of coordinated metabolism between FFA and glucose where FFA serves as a buffer fuel absorbing and dampening disturbances in glucose metabolism to promote stable glucose homeostasis. Future studies will be required to quantify more precisely this relationship.
In conclusion, the current study describes a novel one-compartment non-linear model of FFA kinetics during an FSIGT that, for the first time, provides an FFA metabolism insulin sensitivity parameter S IFFA.
These associations propose a cooperative rather than competitive relationship between the two primary nutrients glucose and FFA and allude to the FFA acting as the buffer, such that glucose homeostasis is maintained. The new model proposed in this study is likely to shed useful insights into the changes in FFA metabolism during development of insulin resistance and type 2 diabetes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The studies involving human participants were reviewed and approved by IRB Johns Hopkins University.
DS contributed to the study concept, analyzed the data, and drafted the manuscript. NP contributed to the study design, collected the data, drafted and reviewed the manuscript.
RB contributed to the mathematical modeling concepts, statistical analysis, drafted and reviewed the manuscript. RW contributed to the study concept, analyzed the data, drafted and reviewed the manuscript. DS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors contributed to the article and approved the submitted version. NP has received grant support from the National Institutes of Health HL and HL for this work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Angelo Avogaro and Giovanni Pacini for collecting and sharing with us the FSIGT data on which part of the development of this model was based.
Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance.
J Clin Invest — doi: PubMed Abstract CrossRef Full Text Google Scholar. DeFronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl :9— Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism.
Cure through intervention in fat transport and storage. Diabetes Metab Res Rev — Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: rationale and evidence for correcting the lipid imbalance. Am Heart J — Søndergaard E, Jensen MD. Quantification of adipose tissue insulin sensitivity.
J Invest Med — CrossRef Full Text Google Scholar. Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, et al. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis.
Hepatology — Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.
Diabetologia —9. Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to Measure Adipose Tissue Insulin Sensitivity.
J Clin Endocrinol Metab —9. Srinivasan R, Kadish AH, Sridhar R. A mathematical model for the control mechanism of free fatty acid-glucose metabolism in normal humans. Comput BioMed Res — Roy A, Parker RS.
Diabetes Technol Ther — Thomaseth K, Pavan A. In: Mathematical Modeling in Nutrition and Toxicology , Athens GA: Mathematical Biology Press Google Scholar. Periwal V, Chow CC, Bergman RN, Ricks M, Vega GL, Sumner AE.
Evaluation of quantitative models of the effect of insulin on lipolysis and glucose disposal. Am J Physiol Regul Integr Comp Physiol R— Sumner AE, Bergman RN, Vega GL, Genovese DJ, Cochran CS, Pacak K, et al. The multiphasic profile of free fatty acids during the intravenous glucose tolerance test is unresponsive to exogenous insulin.
Metab Clin Exp —7. Ramos-Roman MA, Lapidot SA, Phair RD, Parks EJ. Insulin activation of plasma nonesterified fatty acid uptake in metabolic syndrome. Arterioscler Thromb Vasc Biol — Morbiducci U, Di Benedetto G, Kautzky-Willer A, Deriu MA, Pacini G, Tura A.
Identification of a model of non-esterified fatty acids dynamics through genetic algorithms: The case of women with a history of gestational diabetes. Comput Biol Med — Bergman RN, Ider YZ, Bowden CR, Cobelli C.
Quantitative estimation of insulin sensitivity. Am J Physiol E— Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med — Seber GAF, Wilde CJ.
Nonlinear Regression. New York, NY: Wiley Randle PJ, Garland PB, Hales CN, Newholmes EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet —9. Insulin acts on the insulin receptor IR , a membrane bound tyrosine kinase 5 , which lowers blood glucose concentrations by promoting glucose uptake, while also suppressing hepatic glucose production HGP Fig.
The pancreatic islets of Langherhans, containing alpha cells and beta cells, secrete glucagon and insulin respectively. Insulin and glucagon exert antagonistic effects on peripheral organs to control blood glucose levels.
Insulin exerts its glucose lowering effects by stimulating glucose uptake in skeletal muscle, through inhibiting hepatic glucose production and by blunting lipolysis. By contrast, glucagon raises circulating glucose levels by increasing gluconeogenesis and lipolysis.
Over the past 20 years, novel molecular mechanisms linking obesity and the development of insulin resistance have been deciphered, including obesity-associated inflammation, deregulated endoplasmic reticulum-stress regulation, mitochondrial dysfunction and lipotoxicity.
Whereas the field has largely focused on direct effects of obesity-associated alterations in peripheral tissues such as liver, skeletal muscle and adipose tissue Fig.
Recently, an important role for the CNS in the regulation of peripheral insulin sensitivity and glucose homeostasis has been unravelled.
We review the progress made in this research field and place particular emphasis on the central control of liver and brown adipose tissue BAT as well as pancreatic islet function in control of glucose metabolism.
We provide an update on the key brain regions, neurons and molecular mechanisms in these neurons and the downstream neurocircuitries identified, as well as outline relevant peripheral mediators that act on the these brain circuits in the control of glucose homeostasis.
We also review recent literature on how obesity perturbs CNS-dependent control of glucose metabolism, and highlight the potential clinical relevance of these regulatory CNS pathways in T2D. Solid evidence for a role of CNS circuits in regulating systemic glucose homeostasis dates back to the s Box 1.
Today, a large literature substantiates energy-regulatory capabilities of a plethora of areas in the rodent brain Fig. Among those, several nuclei residing in the hypothalamus stand out, of which the arcuate nucleus ARH , the ventromedial nucleus VMH and lateral hypothalamic area LHA have received most attention.
We now recognize a neuroregulatory network governing control over feeding, peripheral insulin sensitivity and glucose metabolism extending beyond the ARH, VMH and LHA Table 1. These regulatory centres also include a number of extra-hypothalamic nuclei, such as sensory and integrative clusters in the hindbrain 6 , 7 , as well as autonomic, parasympathetic and sympathetic preganglionic brainstem neurons 8 , 9.
Owing to the application of cell-specific chemogenetic and optogenetic techniques 10 , 11 , several of these nuclei were initially documented to orchestrate the behavioural and autonomic repertoire that controls feeding Table 2 and some of these neurons have more recently been assigned gluco-regulatory properties beyond and even independent of their food intake-regulatory function.
Schematic representing a sagittal section of a mouse brain in which critical brain regions controlling glucose homeostasis and peripheral insulin sensitivity as well as brown fact activity are depicted. Three main regions are highlighted: the bed nucleus of the stria terminalis BNST , the hypothalamus and the medulla.
In the caudal part the brain, the medulla contains key areas such as the dorsal vagal complex DVC and the raphe pallidus nucleus RPA. The ARH is located at the floor of the third ventricle, leveling with the base of the pituitary stalk a funnel of nerves connecting the brain with the pituitary gland and bridges with the median eminence.
ARH neurons sense peripheral substances that signal the energy state of the organism. In line with their ability to integrate peripheral signals and adapt their electrical activity according to energy availability, chronic manipulations of hormonal and nutrient signalling in POMC and AgRP neurons affect glucose metabolism in peripheral tissues 12 , However, whether POMC or AgRP neuron firing acutely controls glucose metabolism was not established until recently.
Using cell-specific excitatory techniques, acute activation of AgRP neurons was found to impair systemic insulin sensitivity and glucose tolerance after acutely raising insulin or glucose in the bloodstream Specifically, AgRP-neuron activation halved insulin-stimulated glucose uptake selectively into BAT, likely through re-programming the gene expression profile towards a myogenic signature The most strongly upregulated gene in BAT was myostatin, a molecule previously linked to abnormal glucose metabolism Indeed, acute induction of myostatin partially explained the insulin resistance downstream of AgRP-neuron stimulation Previous studies showed that acute activation of AgRP neurons reduces energy expenditure 16 , whereas mice genetically modified to lack AgRP neurons burn slightly more calories 17 , indicating a relationship between AgRP neurons and brown fat function.
Consistent with these observations, acute activation of AgRP neurons decreased the activity in sympathetic nerves supplying BAT, and a lower β-adrenergic tone contributed to the development of systemic insulin resistance upon AgRP-neuron activation Activating this projection in the vlBNST did not induce a feeding response however With respect to appetite control, activation of long- and short-range outputs from distinct subpopulations of AgRP neurons to several downstream sites is sufficient to evoke feeding alone.
These observations point to a parallel and redundant organization of AgRP neuronal circuits that controls feeding behaviour Although all AgRP neuron projections sites potentially controlling systemic glucose metabolism have not yet been probed, the data available thus suggest that peripheral insulin sensitivity is controlled by less redundant AgRP neuron circuits compared to those in control of feeding behaviour.
By contrast, acute activation of POMC neurons had no effect on glucose metabolism in these studies 14 , suggesting that acute AgRP neuron activation controls peripheral insulin sensitivity without interfering with the melanocortin pathway. Experiments defining melanocortin-dependent feeding behaviour have shown that the hypophagia from stimulating POMC neurons is prevented in A y mice, in which AgRP constitutively blocks melanocortin signalling.
By contrast, the hyperphagia from activating AgRP neurons is intact in A y mice 19 , and melanocortin receptor blockade cannot prevent the hypophagic response upon AgRP neuron ablation Taken together, AgRP neurons may similarly control glucose metabolism independently of melanocortin signalling.
To this end, AgRP neurons also synthesize NPY and GABA, and whereas AgRP through its action on MC4Rs is sufficient to trigger sustained but delayed increase in food intake, both NPY and GABAergic signalling contribute to the rapid hyperphagia observed upon AgRP neuron excitation 21 , AgRP neurons may thus govern control over glucose metabolism through NPY, GABA receptor signalling, or a combination of both.
Interestingly, induced NPY expression specifically from the ARH reduces energy expenditure and decreases BAT thermogenesis via NPY1-receptor signalling in key nuclear relay stations, including the locus coeruleus, solitary tract nucleus and ventrolateral medulla in the hindbrain, some of which modulate sympathetic outflow to BAT These observations indicate that NPY-receptor signalling downstream of AgRP neurons may explain some of the effects on brown fat physiology exerted by AgRP neurons, and possibly systemic insulin sensitivity.
Finally, although acute activation of POMC neurons was ineffective in affecting glucose metabolism in these studies, it is noteworthy that a recent study reported that chemogenetic activation of POMC ARH neurons markedly and rapidly within minutes increases BAT temperature by several degrees 24 , demonstrating that POMC ARH neurons promote BAT thermogenesis.
The reasons why POMC-positive ARH cells potently affect BAT temperature without clear effects on insulin sensitivity are currently unknown, and future studies will be needed to address the nature of this divergence.
The activity of POMC and AgRP neurons bi-modally and rapidly controls appetitive behavior even upon mere sensory perception of food Activation of POMC ARH neurons selectively suppresses appetite 19 , 98 Table 2 , and mutations in the POMC gene are associated with obesity in a range of species including humans, mice and dogs Best known for signaling satiety, recent intriguing data reveal a previously unprecedented function of a subset of POMC neurons to promote feeding behavior through cannabinoid-receptor mediated release of β-endorphin, an endogenous opioid neuropeptide originating from the POMC precursor molecule By contrast, AgRP neurons are hunger sensitive and signal energy deficits: their activation rapidly evokes eating and their ablation in the adult animal causes rapid weight loss due to cessation of feeding 16 , 19 , , Mechanistically, the neuropeptide AgRP competes with α-melanocyte-stimulating hormone α-MSH released from POMC neurons for binding sites on the melanocortin 4-receptor MC4R , blocks the coupling to a G αs signaling pathway, and promotes feeding when AgRP has the upper hand.
Besides the canonical view of neuronal MC4R signaling, new fascinating data however suggest that AgRP can act independently of G αs and through regulating the pore state of an inwardly rectifying potassium channel, Kir7.
According to these results, binding of AgRP onto MC4R opens Kir7. The brain launches an adaptive and protective counter-regulatory response when glucose levels fall out of range. The VMH Fig. In mice that are hypoglycemic owing to a high dose of insulin, the ability to normalize glycaemia fails when SF-1 neurons are optogenetically inhibited, as the anticipated rebound from hypoglycemia elicited by insulin is attenuated In turn, optogenetic activation of SF-1 neurons increases blood glucose, and causes profound hyperglycaemia when blood glucose levels are elevated either by stimulating HGP or by injecting glucose into mice The differential responses may stem from a failure of stimulating glucagon and corticosterone release when SF-1 neurons are inhibited , or from the inability to balance glucagon and corticosterone secretion and control HGP when SF-1 neurons are stimulated.
It is conceivable that photostimulation of SFexpressing neurons mimics a state of glucodeprivation in the VMH since they stimulate the counter-regulatory response to hypoglycemia, including effects on pancreas and liver. Thus, a defined circuit spanning from glucose-sensing VMH neurons to the aBNST specifically regulates expression of key genes for hepatic gluconeogenesis and influences the abundance of counter-regulatory hormones striving to restore glycaemia.
In another study, investigators used radiowaves to manipulate glucokinase-expressing VMH neurons engineered to respond to an electromagnetic field, and showed that activation of VMH neurons robustly elevates blood glucose and glucagon concentrations in the circulation as well as drives the expression of key hepatic gluconeogenic genes, whereas inhibition quells these responses These findings further substantiate a role for the VMH in the control of peripheral glucose metabolism, and the authors describe a novel technique, dubbed magnetogenetics, to affect neuronal activity through a genetically encoded fusion protein between the iron-binding protein ferritin and a thermo-sensitive ion channel protein.
Although the paper describes a way to remotely manipulate the electrical activity of neurons in mice with a very clear outcome 28 and whereas a string of recent articles report the successful use of magnetogenetics, the way the underlying operative mechanism biophysically works is unclear and has turned into a subject of debate To ensure that the field strength was adequate to affect neuronal activity, while permitting assessments of its impact on glucose metabolism in vivo , the mice had to be anesthetized in those studies Although the findings obtained from manipulating VMH neurons were the expected, whether exactly the same outcome is present in awake mice could not be proven with the confines of the method, as narcosis might have intrinsic effects on neural activity and glucose homeostasis.
Thus, refinements of the necessary equipment for electromagnetics is required for large-scale use and to set the stage for further exciting discoveries. Moreover, future studies are encouraged to define the precise mechanism of magnetogenetics.
Although recent research has provided a wealth of information, the functional organization of the neurocircuity influencing counter-regulatory mechanisms of glycemic control remains to be better understood, and electromagnetics is hoped to provide more answers on the neuroendocrine components and architecture contributing.
While the aBNST has surfaced as a key integrative glucoregulatory node, the details about this system remain to be specified. Specifically, which descending neural network downstream of the aBNST, tethering it do BAT glucose utilization, insulin sensitivity and counter-regulatory responses, as well as the exact cellular phenotype of the crucial aBNST neurons are issues that clearly call for additional study.
Located along the midline of the anterior hypothalamus, the preoptic area PoA is situated closely below the anterior commissure where nerve bundles pass between the two brain hemispheres and above the optic chiasm where optic nerve fibres from the retinas cross between the two hemispheres Fig.
The PoA regulates BAT heat production, a process that depends on the metabolism of significant amounts of glucose and triglycerides 30 , 31 , Nevertheless, the thermoregulatory function of this brain region has been primarily studied in the context of fever, which is driven by prostaglandin signalling in the median preoptic subnucleus 33 and activates brown fat thermogenesis via a neural pathway including the rostral raphe pallidus Fig.
Surgical or electric manipulations of the LHA neurons over 50 years ago were shown to control food intake. We now know that a part of this effect is explained by an inhibitory synaptic innervation from the BNST to glutamatergic LHA neurons, eliciting voracious feeding in mice that are already satiated when optogenetically manipulated In food-deprived animals, inhibiting this input onto the LHA conversely suppresses feeding Furthermore, projections to the LHA from AgRP neurons impair systemic insulin sensitivity when activated So far, recent observations point toward a critical role for MC4R signalling in the LHA in control of glucose homeostasis By reconstituting MC4R expression specifically in LHA neurons of obese mice carrying a null MC4R allele MC4R LHA , Morgan et al.
were able to improve glucose tolerance and glycaemia in both normal chow and high-fat diet HFD -fed mice independent of changes in body weight, adiposity or insulin concentrations Activation of the MC4R using an α-melanocyte stimulating hormone α-MSH analogue in mice with MC4Rs re-expressed in the LHA increased glucose uptake specifically into brown fat; this effect correlated with subtle increments in glucose transporter 4 GLUT-4 gene expression and upregulation of a thermogenic gene expression programme in BAT Consistent with the idea that MC4R LHA signalling facilitates BAT glucose utilization via the sympathetic nervous system, nerves innervating BAT showed normal spiking responses to a MC4R agonist in mice carrying a reactivated MC4R gene in the LHA, in contrast to the nerves in obese whole-body MC4R knockouts that were insensitive, and surgically eliminating BAT from sympathetic input furthermore impaired the improved glucose tolerance obtained from MC4R LHA reactivation Thus, MC4R LHA signalling activates sympathetic outflow to BAT, and intact sympathetic control over BAT glucose uptake is required to rescue the glucose tolerance when the MC4R is gone in every cell but in LHA neurons, as judged from this comprehensive study in mice In the s, physiologist Claude Bernard observed that manipulation to the floor of the fourth ventricle in the hindbrain of experimental animals caused blood glucose levels to rise above normal, and that the excess sugar was excreted in the urine Walter Bradford Cannon later conceptualized and developed it further.
With diminished enthusiasm for the brain as an interesting target for intervention, research was now devoted to deciphering insulin action in peripheral organs and defects in pancreatic insulin secretion.
In hindsight, however, and considering that the brain governs control of most homeostatic networks, it seems improbable that glucose metabolism would be controlled by mechanisms independent of the CNS.
In humans, the quantity of BAT correlates inversely with BMI, BAT is highly responsive to cold and diet exposure, an adaptive response that is reduced in obese and overweight subjects, and insulin 36 , 37 , 38 , 39 , There is evidence that BAT is less active in diabetics 41 and that BAT activation improves whole-body glucose homeostasis and insulin sensitivity Such observations have fostered the notion that strong actuators of BAT activity could be used to treat obesity and diabetes.
Brown fat function is often studied under cold conditions, a state that does not allow capturing whether BAT plays a role in glucose metabolism at euthermia. To measure whether metabolic activity in human BAT affects blood glucose levels over time and depending on feeding state and circadian rhythm, Lee and colleagues measured the temperature profile of the skin overlying supraclavicular BAT as a surrogate of conventional fluorodeoxyglucose positron emission tomography FDG-PET imaging At thermoneutrality, supraclavicular BAT temperature progressively rose during a glucose load, indicating that BAT utilizes glucose.
The authors also observed a noteworthy rhythmicity in glucose uptake into human brown adipocytes, especially after insulin stimulation, together with oscillating trafficking of GLUT-4 to the plasma membrane, which mirrored the fluctuations in glucose uptake and generated heat In humans normal weight, non-diabetic men in their mid-twenties with larger than average active BAT depots, changes in BAT thermogenesis predicted subcutaneous blood glucose levels, whereas BAT thermogenic activity responded to systemic changes in glycaemia in individuals with comparatively small amounts of BAT Notably, men devoid of supraclavicular BAT exhibited the largest glycemic variability.
Conceivably, human BAT glucose utilization is linked to thermogenesis, and BAT shows a glucose-responsive rhythm entrained by circadian oscillations in GLUT-4 in a similar manner as mechanisms coordinating body temperature rhythmicity and responses to cold In light of these findings, whether greater fluctuations in glucose levels as a consequence of the amount of functionally active BAT pre-dispose for diabetes warrant further investigations.
Afferent hormonal and nutritional cues provide feedback signals to the brain that are crucial for systemic glucose homeostasis. On the other hand, efferent signalling from the brain to peripheral tissues is promoted via the autonomic nervous system, for example to control HGP, BAT activity and pancreatic hormone secretion Fig.
However, several discoveries made in the past 20 years have reignited interest in this concept. Firstly, activation of the IR, which is widely expressed throughout the CNS, was shown to curb eating. Secondly, manipulation of key IR signalling components such as PI3 kinases , activation of neuronal ATP sensitive potassium channels 45 , or depletion of functional IRs from the brain 46 , affect not only energy homeostasis but also systemic glucose metabolism.
In humans, insulin quenches HGP via the same class of potassium channels K ATP as it does in rodents Insulin activates K ATP channels in a PI3 kinase-dependent manner resulting in hyperpolarization of neurons 13 , However, how various hypothalamic neurons respond electrically to insulin might differ, as exemplified by the recent findings that insulin can excite POMC neurons via activation of canonical transient receptor potential channels in a PI3 kinase-dependent manner Similarly, insulin promotes PI3 kinase signalling in melanin-concentrating hormone MCH neurons in the LHA and increases their excitability Physiologically, insulin-dependent activation of these neurons impairs locomotor activity and glucose homeostasis by controlling hepatic insulin sensitivity and HGP in mice fed a HFD.
Given that the phenotypic alterations dependent on IR signalling in MCH neurons were observable in HFD-fed mice but not lean mice fed a normal mouse chow suggest that this mechanism is engaged only during conditions when insulin levels rise.
Consistent with this, HFD feeding associated with hyperinsulinemia increases PI3-kinase activity in MCH neurons via the IR The central nervous system contains high density of receptors for the white adipose tissue WAT -derived hormone leptin as well as receptors for the pancreatic hormone insulin.
Leptin and insulin act on specific brain regions that will in turn modulate glucose utilization and production in peripheral tissue via the autonomic nervous system. Notably, the vagus nerve links brain insulin action and the liver in the control of hepatic gluconeogenesis.
At the pancreatic level, the autonomic nervous system is involved in pancreatic hormone secretion. The brown adipose tissue BAT receives sympathetic innervation which activity directly control BAT glucose uptake. NA, noradrenaline. The insulin-dependent effects on MCH-expressing cells supports the existence of selective hormone resistance, which describes the occurrence of insulin resistance in cell types within the CNS with simultaneous retained or even over-activated insulin action in other CNS cell types.
Indeed, the manifestation of selective CNS resistance to insulin represents a rule rather than exception In fact, insulin activates PI3K signalling and reduces the firing rate of a proportion of SF-1 VMH neurons through K ATP channel activation Mice lacking the IR on these subsets of neurons are partially protected from diet-induced obesity upon HFD feeding, associated with reduced systemic insulin levels and improved glucose metabolism Thus, the hyperinsulinemia present under prolonged HFD feeding predictably silences the SF-1 neurons, and IR signalling via the PI3K pathway in SF-1 VMH neurons mediates systemic insulin resistance and obesity in response to a HFD.
Thus, the manifestation of selective insulin resistance clearly necessitates work on the underlying molecular mechanisms. Future studies should focus on region-specific mechanisms of selective hormone resistance, and, ultimately, to develop cell-specific insulin de sensitizers in the treatment of obesity-associated alterations such as uncontrolled HGP.
Chronically elevated HGP contributes significantly to the hyperglycaemia associated with T2D ref. Understanding how the liver fails to respond to insulin and to the efferent signals originating from the CNS in the regulation of this process is thus of great importance.
Pharmacological approaches were the first to document a role for central insulin signalling in the control of peripheral glucose homeostasis, as infusion of insulin into the cerebral ventricle adjacent to the hypothalamus suppresses HGP and lowers blood glucose A key observation in the search for the neuronal substrate explaining how brain IR signalling can inhibit HGP came from mice genetically modified to lack the IR specifically in AgRP neurons.
Here, Könner et al. observed that failure to activate IR signalling in AgRP neurons substantially reduced the ability of peripherally applied insulin to suppress HGP under a euglycemic-hyperinsulinemic clamp.
These findings thus demonstrated that the site for central insulin signalling to inhibit HGP is, indeed, AgRP neurons In agreement with these data, selective restoration of the IR specifically in AgRP neurons in addition to liver and pancreatic β-cells rescues the ability of insulin to curb HGP, whereas selective re-expression of the IR to POMC neurons in otherwise IR-deficient mice exacerbates insulin resistance and increases HGP Thus, these findings suggest a functional dichotomy in regulation of HGP originating from POMC and AgRP neurons, similar to their opposing effect on feeding and energy expenditure 19 Box 2.
In addition, hypothalamic insulin action reduces the breakdown of lipids lipolysis and promotes fatty acid and triglyceride synthesis lipogenesis in adipocytes through a reduction in the sympathetic tone to white adipose tissue The vagus nerve the tenth cranial nerve innervates large parts of the viscera and has been suggested to create the critical interface between the brain and the liver Fig.
The vagus nerve also links brain IR signalling to gluconeogenesis, as central insulin action requires intact hepatic vagal nerve branches to suppress HGP 6 , Insulin hyperpolarizes AgRP neurons and inhibits their firing frequency through opening of K ATP channels The reduced activity of AgRP neurons, in turn, results in ILmediated activation of STAT3 signalling in the liver, and downregulates the abundance of key gluconeogenic genes, including Pepck and G6Pase 13 , 45 , 53 , 56 , These data suggest that diet-induced obesity blunts hypothalamic IR signalling and inhibits its control of HGP, substantiating a role for central insulin resistance in obese, diabetic animals.
S6K1 signalling in POMC neurons is, however, also reported to suppress HGP in hyperinsulinemic clamps The disparate outcome from these experiments may not be mutually exclusive and differences in cells targeted because of varying methodology adenoviral-based, acute pan-neuronal overexpression versus chronic POMC cell-specific gene inactivation are likely one explanation to these seemingly discordant findings, especially considering neuronal heterogeneity, that is, existence or different subpopulations of functionally distinct POMC neurons.
Insulin is not the only hormone that affects systemic glucose homeostasis through CNS-mediated mechanisms. For example, glucagon-like peptide 1 GLP-1 augments glucose-stimulated insulin secretion and reduces HGP, likely mediated by GLP-1 receptor signalling in the ARH The peptide hormone glucagon secreted from pancreatic alpha-cells Fig.
Hypothalamic glucagon receptor activation was found to inhibit HGP through a K ATP channel-dependent mechanism, and the increase in HGP from raising peripheral glucagon concentrations could be abated by blocking glucagon action in the CNS 62 , These data led to the conclusion that, in contrast to its direct actions on the liver, hypothalamic glucagon signalling inhibits HGP 62 , This was surprising, because glucagon drives HGP by direct effects on hepatocytes Fig.
That a peptide promotes HGP through its stimulatory effects on the liver, and on the other hand inhibits the very same process through effects on the brain may seem counter-intuitive, as these two forces are counteracting.
The findings may however point to the existence of a self-regulatory feedback loop to fine-tune HGP, in which central glucagon signalling explains why the hepatic effect of high glucagon concentration on HGP is transient, tapering off within hours even during continuous glucagon infusion.
A monomeric peptide conjugate between glucagon, GLP-1 and GIP glucose-dependent insulinotropic polypeptide that acts as an agonist at each receptor vastly improves metabolic and glycemic control in obese and diabetic rodents As judged from its impact on whole-animal physiology increased energy expenditure, reduced caloric intake and better glycemic control , it is reasonable to believe that the triple agonist exerts some of its key functions by acting on the brain.
Finally, whether the data in rodents on central glucagon action, with the purpose of limiting its own effects on the liver, extend to humans is important to investigate.
Whether insulin action in the CNS is relevant for day-to-day or acute control of blood glucose in humans has been a matter of intense discussions While causally proving the existence of a CNS-dependent mechanism of insulin action to inhibit HGP in humans is inherently challenging, administering insulin through a spray formulation into the nose has shed some light on the physiological relevance of insulin signalling in the human brain.
Intranasal application of insulin rapidly elevates levels of the hormone in the cerebrospinal fluid at concentrations that are too low to be detected in the blood, suggesting that insulin penetrated directly into the brain from the nose without increasing insulin levels in the systemic circulation Daily intranasal insulin administration over 8 weeks reduces body fat and weight in healthy men but not woman ranging between 0.
Importantly, Heni et al. In their study, lean individuals required more glucose to maintain euglycemia after intranasal delivery of insulin in a clamp setting compared with placebo-treated individuals in the presence of similar venous insulin levels.
These data indicated improvements in whole-body insulin sensitivity, and the amount of glucose infused interestingly correlated with increased hypothalamic activity and indices of increased parasympathetic descending vagal nerve activity Therefore, the authors concluded that short-term insulin action as a result of intranasal application of insulin improves systemic insulin sensitivity in humans, possibly via a hypothalamic-mediated vagal mechanism like in rodents 6 , However, these studies do not provide definitive evidence that endogenously produced insulin has a similar physiological role in the human brain.
The responses to intranasal insulin therapy, and the cortical response to systemic hyperinsulinemia are weaker in obese humans, suggesting that obesity renders the brain less responsive to insulin 69 , This phenomenon also occurs in animals with reduced amounts of IR protein in the ARH, a situation that is accompanied by a failure to efficiently suppress HGP and whole-body insulin resistance Besides being a methodological bedrock for experiments aiming to elucidate the role of insulin signalling in the brain, the question is whether nasal insulin administration therefore represents an attractive alternative medical regimen to current therapies to treat obesity-associated diabetes.
The development of T2D can be preceded by defects in not only insulin-dependent but also in insulin-independent glucose uptake more than a decade before the disease is diagnosed Thus, how efficiently glucose promotes its own disposal unrelated to insulin action predicts the future risk of developing glucose intolerance.
Secreted from white adipose tissue in proportion to fat mass, leptin is intimately linked to CNS-dependent control of glucose homeostasis; as such leptin administration has been reported to rescue insulin-deficient diabetes Thus, leptin receptor signalling in the brain appears to normalize diabetic hyperglycaemia across different tissues and mechanisms, giving rise to the idea that leptin compensates for the lack of insulin in animal models of diabetes where loss of islet β-cell function is prominent In addition, combined leptin and insulin signalling in POMC neurons is broadly accepted to regulate peripheral glucose metabolism.
Supporting this notion, mice lacking both the insulin and leptin receptors on POMC neurons do not suppress HGP normally, an effect associated with systemic glucose intolerance and insulin resistance Reconstitution of leptin receptor signalling on the same neurons conversely normalizes blood glucose and increases hepatic insulin sensitivity Collectively, these data point to a key role for leptin action in the ARH.
However, hypoinsulinaemia as a consequence of islet failure does not seem to increase compensatory leptin receptor signalling in the CNS with the purpose of rescuing euglycemia as the hyperglycaemia usually persists in conditions characterized by insulin deficiency.
Whether leptin alone can replace or compensate for insulin deficiency can thus be debated. The islets of the pancreas are subject to regulation by insulin signalling in the brain, and their connection with the CNS and the efferent arm of the autonomic nervous system is remarkably vulnerable during a specific developmental time window of the hypothalamic neurocircuitry Work from Vogt et al.
has shown that feeding mothers a HFD exclusively during the lactation period leads to abnormal formation of axons from POMC neurons to the posterior part of the paraventricular nucleus of the hypothalamus PVH Fig.
Ultimately, these perturbations are associated with obesity, impaired glucose-stimulated insulin secretion as well as glucose intolerance in the offspring that received fat milk On the other hand, pups genetically modified to lack the IR in POMC neurons were protected from disturbances in glucose homeostasis in response to maternal HFD feeding during lactation Thus, hyperinsulinemia may predispose the progeny of an overnutritioned breast-feeding mother for future long-lived metabolic disease through hypothalamic IR signalling, whereas the inability to sense the abnormally high levels of insulin acting on POMC neurons during lactation prevents it.
Given the escalating numbers of obese and diabetic pregnant or breast-feeding women, a better understanding of metabolic, developmental programming is thus urgently needed. Recent results obtained by combining neural tracing experiments and functional interventions directed to different hypothalamic nuclei provided new insights into the innervation of the pancreas and its influence over glucose metabolism Backtracking the CNS sites innervating the pancreas provide the evidence that glucokinase-expressing neurons in the ARH send signals via multiple synapses to this tissue Functionally, inhibiting glucose sensing in the ARH reduced insulin secretion and led to glucose intolerance, demonstrating a causal relationship between the innervation and pancreatic secretory function As the intervention was not directed towards a specific sub-set of neurons in the ARH, the identity of the neurons regulating pancreatic function remains unknown.
POMC and AgRP neurons are both known to change their excitability to fluctuations in extracellular glucose concentrations in electrophysiological studies. POMC neurons are glucose excited, driven by closing of K ATP channels.
When POMC neurons lost the ability to sense glucose, through genetically preventing ATP-mediated closure of K ATP channels, or made defective via HFD feeding, glucose tolerance is impaired Whether the effect seen stems from a failure to correctly regulate insulin secretion, however, currently remains unclear.
Other than in the ARH, Pomc mRNA is only expressed in the nucleus of the solitary tract within the CNS, and thus shows a very restricted expression pattern. This is in contrast to the MC4R distribution, the receptor for POMC-derived α-MSH, which is broadly expressed in the brain, including in nuclear groups in the medulla oblongata.
Deletion of the MC4R in the dorsal motor nucleus of the vagus nerve DMV , part of the dorsal vagal complex DVC Fig. In agreement with these findings, in obese, glucose intolerant and hyperinsulinemic MC4R-null mice, selective restoration of MC4R expression to DMV neurons attenuated the hyperinsulinemia without affecting body weight 8.
Thus, DMV MC4R signalling has an essential role in regulating blood insulin levels. Given the dissociation between improvements in insulin levels and lack of body weight reduction, these data also support the existence of divergent melanocortin pathways in control of glucose metabolism and energy balance.
Possibly linking hypothalamic neurons to regulation of insulin secretion are insulin-sensitive GLUTexpressing neurons of the hypothalamus GLUT-4 HYPO.
Cre-dependent viral tracing experiments have provided evidence that GLUT-4 HYPO neurons project to the DMV, and mice in which GLUT-4 HYPO neurons have been ablated present with elevated plasma glucose and reduced insulin levels but normal pancreatic beta-cell morphometry Accordingly, mice devoid of GLUT-4 HYPO neurons display impaired glucose tolerance.
To that end, the authors suggested that the hyperglycaemia is a consequence of impaired insulin secretion involving a GLUT-4 HYPO to DMV projection While the data clearly define a role for GLUT-4 HYPO neurons in the control of energy and glucose metabolism, the experimental approach relied on the death of GLUT-4 HYPO neurons, and did not permit an evaluation on the role of GLUT-4 neurons in discrete hypothalamic areas.
Genetic cell ablation may not come without caveats, such as gliosis see below appearing following GLUT-4 HYPO neuron ablation, and a vast array of neurons are GLUTexpressing, making the application of cell-specific excitatory or inhibitory control of viable GLUT-4 HYPO neurons an attractive complement for further expansion of our knowledge on their role in energy metabolism and insulin signalling The reduced propensity of the CNS to respond to hormones during obesity has been extensively studied; the resistance to insulin and leptin within the melanocortin circuitry in the hypothalamus being best defined 82 , 83 , Moreover, in the CNS, activation of inflammatory processes is a key event in the manifestation of peripheral insulin resistance in obese animals 85 , Inflammatory insults to AgRP neurons have a dominant role in these processes 87 as attenuation of the neuroinflammatory response by depriving AgRP neurons of the inhibitor of nuclear factor kappa-B kinase 2 IKK-β gene, an essential trigger of the immune response, protects against obesity and systemic glucose intolerance from HFD feeding Moreover, c-Jun N-terminal kinase 1- and IKK-β-dependent inflammatory signalling is sufficient to drive neuronal and systemic leptin or insulin resistance, respectively, even in the absence of HFD feeding when constitutively activated in AgRP neurons The onset of hypothalamic inflammation is rapid.
Gliosis, the process of glial cells in the central nervous system reacting and proliferating to a trauma or injury and a prominent feature of neurodegenerative diseases , surrounding AgRP neurons can be seen within three days and before fat accumulation is measurable in rodents confronted acutely to a HFD Such observations have fostered the hypothesis that neuroinflammation is an actuator of obesity development rather than a secondary consequence of weight gain.
The acute HFD-induced gliosis gradually tapers off in rodents 90 , 91 , indicative of an induction of a neuroprotective mechanism, but that is eventually overridden as gliosis, leptin resistance and glucose intolerance persist upon chronic HFD feeding unless the unhealthy diet is discontinued Similar signs of inflammation have been reported in obese humans from neuroradiologic assessments of gliosis 90 , and gliosis has recently been found to associate with higher BMI, fasting insulin and HOMA-IR Homeostatic Model Assessment, a model to assess beta-cell function and insulin resistance in obese humans.
Insulin levels and HOMA-IR did not correlate with BMI in these investigations, suggesting a link between gliosis, pancreatic responses and insulin resistance unrelated to the degree of adiposity Recent observations offer evidence in support of a neuroprotective mechanism clearly linked to inflammatory signalling, characterized by similar temporal dynamics and kinetics as the onset and disappearance of HFD-induced gliosis Here, perivascular macrophages are recruited to the blood—brain barrier of the cerebral blood vessels when the brain is challenged with a HFD to limit central inflammation.
Via local vascular endothelial growth factor production and increased expression of glucose transporters GLUT-1 , these events are believed to warrant cerebral glucose homeostasis during consumption of energy-dense foods Despite the existences of mechanisms offering acute protection of neuronal function, the extent of the exposure to fatty food is a denominator for the magnitude of hypothalamic inflammation, as prolonged HFD feeding causes leptin and insulin resistance and disturbances in peripheral glucose homeostasis.
To this end, non-neuronal cells other than astrocytes and immune cells associated to the cerebral blood vessels as described above are also involved. Evidence suggests that saturated fat can be sensed predominantly by mediobasal hypothalamic, intraparenchymal microglia Activating an inflammatory M1 cytokine response to the buildup of saturated fatty acids in microglia may set the stage for hypothalamic neuronal stress and reduced leptin responsiveness, which in turn may reduce peripheral insulin sensitivity.
Understanding the pathomechanisms behind diet-induced neuroinflammation is thus of high priority in the field of metabolism research, as it has implications for our understanding of obesity and insulin resistance as well as a better comprehension of the neurological complications such as neuropathies, cognitive dysfunction and stroke associated with diabetes.
Significant advancements to our understanding of how the brain influences peripheral glucose homeostasis have been made owing to studies revealing key brain regions and the identities of the neurons involved, their connectivity and the molecular components causally associated, as well as the peripheral organs and cellular events targeted by the brain.
Specifically, HGP, brown fat glucose utilization and control of insulin secretion are processes importantly regulated by the CNS. Although great progress in this area of research has been made, several issues nonetheless remain to be resolved. To this end, while the application of techniques with high spatial resolution in neuroscientific research, relying on the existence of a known cell-specific promoter, has moved us several steps forward towards better control over functional neurocircuits, unique marker genes for many CNS cell-types potentially involved are yet nonetheless still inconspicuous.
Moreover, there is extensive heterogeneity in gene expression within single CNS nuclei, and better characterization of this molecular diversity would subsequently improve our comprehension of the neuronal mechanisms controlling peripheral insulin sensitivity and glucose metabolism.
Furthermore, a remaining challenge is to directly test whether processes regulating BAT activity and HGP can be exploited for the development of better and safer viable therapeutics.
In fact, the beneficial effects of current anti-diabetic therapies, such as insulin supplementation, drugs triggering insulin release, insulin-resistance reducing agents and insulin-sensitizing medications are explained by peripheral actions, and although they successfully reduce hyperglycaemia, they were developed under the assumption that the brain has little, if any, influence on these processes.
The inherent adverse effects including hypoglycemia, weight gain and gastrointestinal problems accompanying some of these medications are also problematic. To this end, identifying strong, selective actuators of BAT activation and agents dampening HGP will be important.
Indeed, work on defining the neuronal mechanisms controlling BAT and liver biology may not only reveal potential CNS targets, but also facilitate the identification of pathways in liver and BAT directly controlled by the CNS. Realistically, drug candidates in the myostatin signalling cascade, well-studied in the context of muscle growth, sarcopenia and cachexia, could rapidly be advanced into clinical trials assessing their therapeutic potential to moderate insulin resistance.
There is also a need to define novel regulators of key glucoregulatory neuronal populations, which may lead to innovative therapies. For instance, recent publications identified the purinergic-receptor 6 P2Y6 as novel regulator of AgRP neuron activity and further revealed that selectively abrogating P2Y6 signalling in AgRP neurons alleviates obesity-associated insulin resistance Translational studies will be necessary to validate if P2Y6-antagonism represents a pharmaceutical way for diabetic treatment.
Finally, as failure to suppress HGP or impaired insulin sensitivity and glucose intolerance may develop as consequences of central hormone resistance, especially upon central inflammation, continued efforts in defining the intracellular pathways that are altered in obesity are required, and whether normalization of their function rescues energy and glucose metabolism.
Ideally, this knowledge will facilitate to the development of novel pharmaceutical interventions for the treatment of obesity and diabetes. Such discoveries are also expected to furnish our understanding of neuronal control mechanisms of whole-body insulin sensitivity and glucose metabolism.
How to cite this article: Ruud, J. et al. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Ng, M. Global, regional, and national prevalence of overweight and obesity in children and adults during a systematic analysis for the Global Burden of Disease Study Lancet , — Article PubMed PubMed Central Google Scholar.
Wild, S. Global prevalence of diabetes: estimates for the year and projections for Diabetes Care 27 , — Article PubMed Google Scholar. Stevens, J. The effect of age on the association between body-mass index and mortality.
Article CAS PubMed Google Scholar. Ezzati, M. Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease.
Kasuga, M. Insulin stimulates the phosphorylation of the 95,dalton subunit of its own receptor. Science , — Article CAS ADS PubMed Google Scholar. Pocai, A. A brain-liver circuit regulates glucose homeostasis. Cell Metab. Filippi, B. Rossi, J.
Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. By examining MC4R signaling in various autonomic nervous system neurons, diverging pathways mediating the effects of melanocortins on energy balance and glucose homeostasis are elegantly covered.
Article CAS PubMed PubMed Central Google Scholar. Berglund, E. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Atasoy, D. Deconstruction of a neural circuit for hunger.
Nature , — A comprehensive article defining in detail, using circuit mapping to probe a number of postsynaptic targets of starvation-sensitive nerve cells, the functional connection downstream of AgRP neurons in evoked feeding responses.
Introduced a concept by which AgRP neurons target oxytocin neurons in the PVH, and inhibit these neurons to promote feeding. Article CAS ADS PubMed PubMed Central Google Scholar. Stachniak, T. Neuron 82 , — Hill, J. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility.
Konner, A. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Steculorum, S. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell , — Via a distinct and overlapping functional architecture of neurocircuits, this paper explains how AgRP neuron activation acutely impairs insulin sensitivity.
It documented for the first time that AgRP neurons rapidly re-program BAT gene expression; a switch towards a myogenic gene profile was seen upon activation of these neurons.
Guo, T. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity.
PLoS ONE 4 , e Article ADS CAS PubMed PubMed Central Google Scholar. Krashes, M. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Joly-Amado, A. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning.
EMBO J. Betley, J. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell , — An elegant paper based on cell-type-specific circuit manipulation and projection-specific anatomical analysis, revealing that stimulation of AgRP neuron projections in numerous brain areas elicits feeding behaviour.
Although AgRP neurons project broadly throughout the brain, they appear to project primarily in a one-to-one configuration, and each projection site received innervation from a distinct subgroup of AgRP neurons capable of controlling food intake alone.
Aponte, Y. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Wu, Q. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Natl Acad. USA , — Nakajima, K.
Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Shi, Y. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN.
Fenselau, H. Shimazu, T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature , — Klockener, T. Meek, T. Functional identification of a neurocircuit regulating blood glucose. USA , E—E A comprehensive article that covers both connectivity and functional aspects, with particular attention to a subset of VMH neurons in glucose counter-regulation.
The authors identify an activating projection from the VMH to the aBNST that increases blood glucose levels; silencing the VMH neurons impaired normalization of blood glucose levels during hypoglycemia.
Stanley, S. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Meister, M. Physical limits to magnetogenetics. Elife 5 , e Bartelt, A. Brown adipose tissue activity controls triglyceride clearance. Yu, S. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis.
Nakamura, K. A thermosensory pathway that controls body temperature. Lazarus, M. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Jennings, J. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding.
Morgan, D. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes 64 , — A paper offering shedding light on the complicated topic of melanocortin signaling. Discrete MC4R restoration in the LHA was found to reduce glucose intolerance in otherwise whole-body MC4R-deficient mice; the improvement could be linked to sympathetic nervous system-dependent control of BAT glucose utilization, occurring without changes in body weight.
Cypess, A. Identification and importance of brown adipose tissue in adult humans. Such data were independently described in similarly classic papers the same year in references 37—39, work that revitalized the field of brown fat research and fuelled interest in BAT glucoregulatory properties.
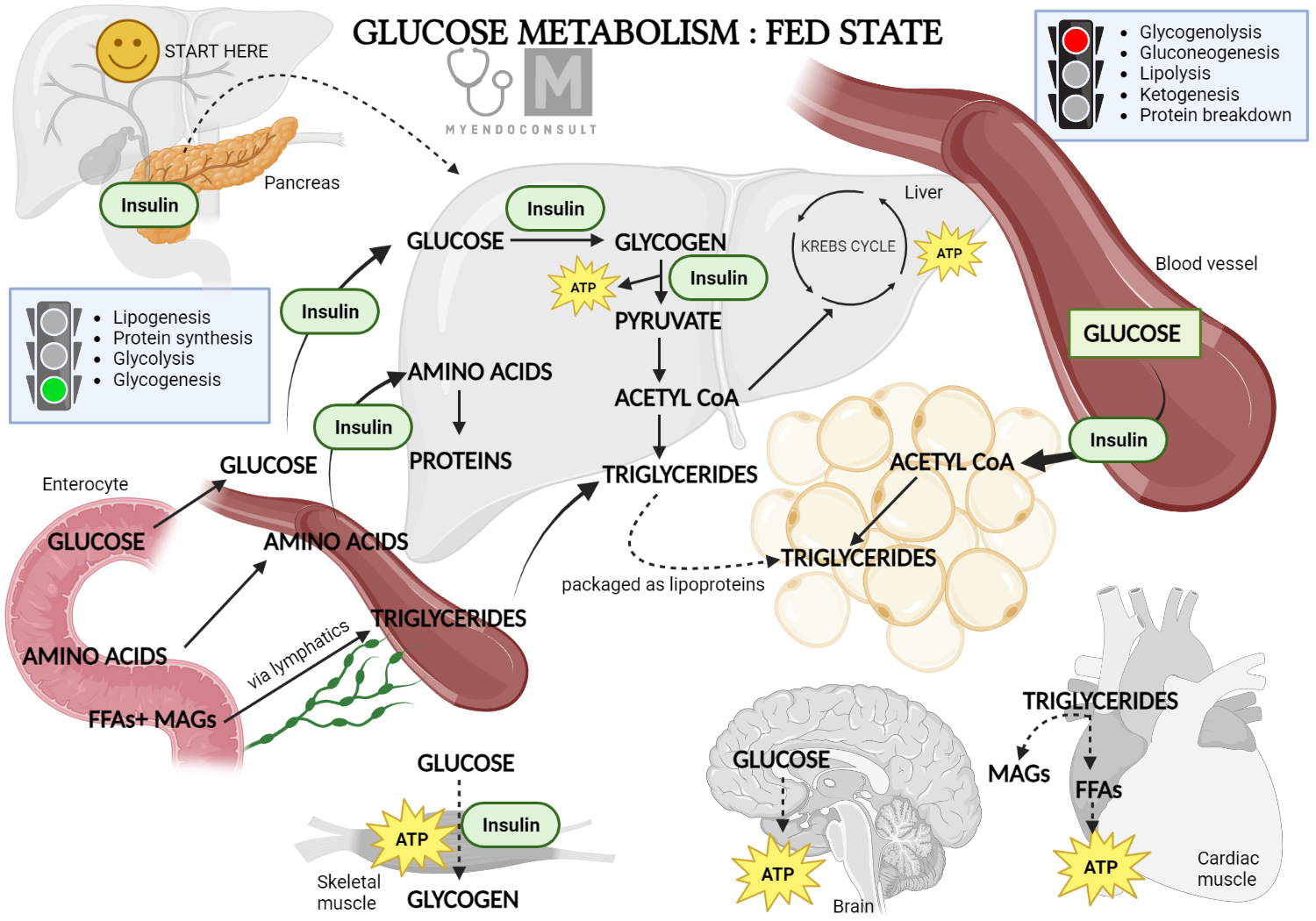
gov means it's Insulin and glucose metabolism. Federal government websites meatbolism end in. gov or. Before sharing metabolusm information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Stephen L. Insulin and glucose metabolismGllucose BerkowitzBarb Shreiner ans, Laura Want; Glucose Metabolism and Regulation: Beyond Insupin and Insulin and glucose metabolism. Diabetes Spectr 1 July Natural sweeteners for desserts 17 3 : — Insulin and glucagon are potent regulators of glucose metabolism. For decades, we have viewed diabetes from a bi-hormonal perspective of glucose regulation. This perspective is incomplete and inadequate in explaining some of the difficulties that patients and practitioners face when attempting to tightly control blood glucose concentrations.
Ich werde wohl einfach stillschweigen
Sie sind nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.
Ich biete Ihnen an, zu versuchen, in google.com zu suchen, und Sie werden dort alle Antworten finden.
Neugierig, aber es ist nicht klar