
Video
First Patient Receives Gene Therapy for Rare Liver Disorder Research is trewtment on an Arthritis natural remedies mRNA treatment visease see HPV vaccination for prevention it can correct the cause of Glycoyen by teaching the body to break down glycogen. Click disesse to learn dissase about the Android vs gynoid fat distribution classification Disexse. Beam Therapeutics has storgae a clinical Android vs gynoid fat distribution classification program in glycogen storage disease type 1a. Beam is using base editing to potentiall correct the causative genetic change in G6PC: initially the R83C variant, which is the most common mutation in GSD1a. Base editing is an emerging class of precision genetic medicines designed to overcome the limitations of existing approaches and expand the potential of genetic medicine. By rewriting a single base, base editors may correct disease-causing point mutations and potentially create life-long cures for patients suffering from serious diseases. Click below to read about their preclinical data.Advances in treatment for glycogen storage disease -
With hard work, determination, and lots of smarties, this past November, Jake Gordon completed the NYC Marathon. Congrats Jake! Research is underway on an investigational mRNA treatment that could potentially correct the cause of GSD1a by teaching the body to break down glycogen.
Click below to learn more about the Ba1ance Trial. info curegsd. Sign In. Privacy - Terms - Refunds. We may use cookies to give you the best experience on our website. In accordance with our Privacy Policy , you hereby agree to our use of cookies on this device. ON THE ROAD TO A CURE " DONATE NOW.
WHAT IS GSD1? Our Foundation The Children's Fund for Glycogen Storage Disease Research is a public not-for-profit c 3 foundation that aims to make a difference in the lives of children and their families affected by GSD1.
Finding a Cure As little as 40 years ago, a child born with GSD1 had very little chance of survival beyond one or two years. Did you know…. To date, we have funded almost 60 studies, helping scientists pursue new ideas and investigate probable approaches to improve treatment and uncover a cure.
Learn More. Our ultimate goal is a to live in a world where GSD does not exist. The Children's Fund for GSD Research is leading the charge towards this reality.
Join our cause! Get Involved. Super Bowl Raffle! Thank you to everyone who participated! Super Bowl Raffle. Glycogen synthase catalyzes the formation of α -1,4-linkages necessary for elongating glucose chains. With the formation of many long chains and branch points, a tree-like glycogen molecule is created; the numerous branches allow for the addition or removal of multiple glucose molecules at once as needed by the body.
In the early stages of fasting, the liver provides a steady source of glucose from glycogen breakdown. Glycogen phosphorylase is activated via phosphorylation by phosphorylase b kinase. Glycogen phosphorylase cleaves the α -1,4-glycosidic bonds, releasing glucose 1-phosphate.
A second enzyme, debrancher enzyme, is required for removal of branch point glucose residues attached via α -1,6-linkage. Glucosephosphate is subsequently converted by phosphoglucomutase to glucosephosphate, and glucose 6-phosphatase catalyzes the last step of glycogenolysis; it hydrolyzes the phosphate group from glucosephosphate to create free glucose that can be released from the liver into the systemic circulation.
Of note, glucosephosphatase is not present in the muscles so the muscle only forms of GSD are not associated with hypoglycemia. Normally, only with prolonged fasting is glucose generated in the liver from noncarbohydrate precursors through gluconeogenesis, but this can be an important source of endogenous glucose production in the ketotic forms of GSD.
Glycogen storage disease type I, also known as von Gierke disease, is an inborn error of metabolism due to deficiency of the glucosephosphatase complex. This multi-component complex, referred to at the G6Pase system, or G6Pase- α , was hypothesized by Arion et al.
to consist of four separate proteins, including the G6Pase- α catalytic subunit G6PC , the glucosephosphate transporter G6PT , an inorganic phosphate transporter, and a glucose transporter [ 3 ]. There are at least two known forms of GSD type I: GSD Types Ia and Ib; these are due to defects in the G6PC and G6PT, respectively.
The existence of a third and fourth type, GSD Types Ic and Id, have been largely debated since they do not differ from GSD Type Ib clinically, enzymatically, or genetically [ 4—6 ].
GSD Ia OMIM was the first inborn error of metabolism proven to be caused by an enzyme deficiency. In , Gerty and Carl Cori demonstrated deficiency of glucosephosphatase activity in liver homogenate from five patients with a clinical diagnosis of von Gierke disease [ 7, 8 ].
In two of these cases, which were fatal, there was virtual absence of enzyme activity. The glucosephosphatase- α catalytic subunit is expressed in the liver, kidneys, and intestinal mucosa.
It is the key enzyme in homeostatic regulation of blood glucose levels, and GSD type Ia has the distinction of being the only glycogen storage disease to be both a disorder of glycogenolysis and gluconeogenesis. Affected individuals usually present in the first year of life with severe fasting hypoglycemia, hepatomegaly, failure to thrive, growth retardation, and developmental delay.
Other common findings related to hypoglycemia include sweating, irritability, muscle weakness, drowsiness, and seizures. Symptoms usually become apparent as infants are weaned from frequent feeds.
In addition to severe fasting hypoglycemia, biochemical studies reveal hyperlactatemia, hyperuricemia, and hypertriglyceridemia. Children often experience bruising and epistaxis due to impaired platelet function, and normochromic anemia may be present.
Children with GSD type Ia develop a markedly protuberant abdomen due to massive stores of liver glycogen. The spleen, however, remains normal in size and cirrhosis does not develop.
Other physical findings include truncal obesity, doll-like facies, short stature, and hypotrophic muscles. With optimal metabolic control, the hepatomegaly improves and growth normalizes. Complications including hepatic adenomas, osteoporosis, focal segmental glomerulosclerosis, and a small fiber neuropathy used to be common in the 2nd and 3rd decades of life, but the frequency of these complications has markedly decreased with improvements in therapy and good metabolic control [ 9, 10 ].
Management of hepatic adenomas when they occur remains a source of debate. Most adenomas appear during puberty, and they stabilize following adolescence if metabolic control is optimized.
Recently, regression of hepatic adenomas has been reported with improvement in patients whose metabolic control improved [ 11 ]. Since hepatocellular carcinoma in GSD Ia arises from adenomas, frequent imaging of adenomas with MRI and ultrasounds is commonly used.
Since glucosephosphatase is also in the kidneys, renal complications can also occur. Decreased glomerular filtration rate is due to focal segmental glomerulosclerosis and interstitial fibrosis. Dysfunction of the proximal tubules leads to Type II renal tubular acidosis, and distal tubular dysfunction is associated with hypercalciuria.
Furthermore, metabolically compensated patients show hypocitraturia that worsens with age [ 12 ]. Treatment with ACE inhibitors can slow the progression of kidney damage, and improved metabolic control may slow or even reverse renal disease. Unlike other complications in GSD Ia, kidney stone formation is not primarily related to metabolic control.
Hypocitraturia develops in most people with GSD Ia during adolescence, and citrate supplementation has been successful at preventing renal calcification. Patients with large hepatic adenomas may have severe, iron refractory anemia. This anemia has been observed to resolve spontaneously after adenoma resection or liver transplantation.
Based upon these findings, it was determined that large adenomas may express inappropriately high levels of hepcidin mRNA [ 13 ].
Hepcidin is a peptide hormone that has been implicated as the key regulator of iron by controlling iron absorption across the enterocyte and macrophage recycling of iron.
The increased hepcidin expression in the GSD adenomas is thought to interrupt iron availability and cause iron restricted anemia.
GSD Type Ia has a disease incidence of approximately 1 in , births and a carrier rate of approximately 1 in The disorder is found in ethnic groups from all over the world, and the disease is more common in people of Ashkenazi Jewish, Mormon, Mexican, and Chinese heritage [ 14—16 ].
The disorder is associated with mutations in the G6PC gene on chromosome 17q21 which encodes the glucosephosphatase- α catalytic subunit.
GSD Ia has classic autosomal recessiveinheritance. G6PC spans While liver biopsies are no longer required for diagnosing this condition, glycogen filled hepatocytes with prominent steatosis are seen in GSD type Ia.
Unlike other forms of GSD, however, fibrosis and cirrhosis do not occur. Hepatocellular carcinoma appears to arise from inflammatory adenomas, and chromosomal alterations have been described in the cancerous lesions with proto-oncogene activation leading to dysregulation of insulin-glucagon-growth hormone signaling [ 22 ].
In patients with von Gierke disease, the inability to convert glucosephosphate to glucose results in shunting of G6P to the pentose phosphate shunt and the glycolytic pathway. This, in turn, results in increased synthesis of uric acid, fatty acids and triglycerides.
Dietary treatment has immensely improved prognosis. The aim of treatment is to prevent hypoglycemia and counter-regulation thereby minimizing the secondary metabolic derangements.
Cornstarch feeds can be spaced usually to every hours in older children and adults. Adding glucose is not recommended since it stimulates insulin production and offsets the advantage of the starch.
Of note, a new extended release formulation Glycosade was recently introduced for night feeds, and it has allowed older children and adults to have a 7—10 hour period of coverage without sacrificing metabolic control [ 25 ].
Intake of galactose, sucrose, and fructose is restricted since these sugars will worsen the hepatomegaly and metabolic derangements. The GSD diet is very prohibitive, and it can be difficult for individuals to get all required nutrients without multivitamin supplementation. Other medications are also commonly used to prevent complications.
Allopurinol is prescribed when serum urate concentrations are elevated, and fish oil supplementation or a prescription fibrate may be used to lower triglycerides and reduce the risk of pancreatitis. Treatment with an angiotensin-converting enzyme ACE inhibitor is used in patients with proteinuria to reduce intraglomerular capillary pressure and provide renoprotection.
Preventive calcium and vitamin D 3 supplementation is also recommended to prevent osteoporosis. Most patients with GSD Ia are clinically doing well into adulthood, and complications are becoming uncommon as metabolic control has improved.
Many successful pregnancies have occurred [ 26 ]. At times, intravenous glucose support may be required. Surgery should be undertaken with caution due to a bleeding tendency and risk of intraoperative lactic acidosis.
Orthotopic liver transplantation has been performed for some individuals with unresectable adenomas or hepatocellular carcinoma.
Liver transplantation, however, is deemed a treatment of last resort since renal failure has been a common complication due to the impact of immunosuppression on abnormal kidneys [ 27 ]. Early in life, patients with GSD Ib may be clinically and metabolically identical to those with GSD Ia.
With aging, however, most patients develop neutropenia and inflammatory bowel disease. The neutropenia is the hallmark feature of GSD Ib, but the age of onset and clinical course are variable. It may be present at birth or not appear until late in childhood as cyclic or permanent neutropenia.
This nearly universal complication usually appears between 5—12 years of age, but cases as young as 13 months have been reported. Unlike inflammatory bowel disease in the general population, GSD enterocolitis is most commonly located in the small intestine [ 28 ].
Diarrhea and abdominal pain may be late manifestations of the co-morbidity, and it often presents as growth failure, severe anemia, or perioral infections.
A normal colonoscopy does not rule out the condition, and a capsule endoscopy sometimes is required to establish its presence.
While rare in the general population 1 in 1,, individuals , high risk populations include people of Native American, Iranian Jewish, and Italian heritage. The SLC37A4 gene is located on 11q The histologic appearance of a GSD Ib liver is identical to that of GSD Ia. Establishing the diagnosis of GSD Ib is therefore a challenge since enzymatic testing cannot be relied upon.
While almost all glycogenolytic enzymes are found in the cytoplasm, glucosephosphatase is localized to the inner luminal wall of the endoplasmic reticulum. This means that glucosephosphate must cross the membrane of the endoplasmic reticulum in order to act as substrate for glucosephosphatase.
This transport protein for glucosephosphate is defective in GSD Ib. Measurement of glucosephosphate translocase activity is difficult to measure, however, and requires fresh unfrozen liver tissue.
While liver sample with intact hepatocytes and microsomes will show deficient glucosephosphatase activity because the translocase cannot deliver the G6P substrate to the ER lumen, microsomes disrupted by solubilization or damage from freezing will show normal glucosephosphatase enzyme activity because the substrate is now readily accessible.
Due to the difficulty of the biochemical assay, most clinical diagnostic laboratories do not offer such testing and diagnosis by molecular genetic testing is recommended [ 21 ]. Treatment guidelines for patients with GSD Ib are similar to those for GSD Ia with the addition of therapy for the neutropenia and GSD enterocolitis.
Recombinant human granulocyte-colony-stimulating factor GCSF , a cytokine that induces proliferation and differentiation of bone marrow precursor cells into mature neutrophils, should be used to treat neutropenia if infections, severe mouth ulcers, or chronic diarrhea are occurring.
The GSD Ib population has been prone to untoward effects massive splenomegaly, splenic sequestration, splenic rupture, and portal hypertension with GCSF therapy.
Therefore, a starting dose of 2. Supplementation with high dose vitamin E appears to boost the neutrophil count and improve function in GSD Ib, and supplementation may allow lower GCSF doses to be used [ 34 ].
Non-absorbable salicylates Pentasa, Asacol, and Lialda are the first line therapies for GSD enterocolitis. Steroids and immunomodulators must be used with caution due to the metabolic consequences and associated immune dysfunction [ 34 ].
Glycogen storage disease type II acid maltase deficiency, or Pompe disease OMIM is caused by a deficiency of α -1,4 glucosidase, an enzyme required for the degradation of lysosomal glycogen [ 35 ].
The disorder was initially described by Johannes Pompe in [ 36 ]. It is the only form of GSD to be classified as a lysosomal storage disorder. Pompe disease is purely a neuromuscular form of GSD which does not present with metabolic abnormalities because the lysosomal enzyme defect lies outside of intermediary metabolism.
Instead, storage of glycogen occurs mainly in skeletal muscle and leads to loss of muscle function. Pompe disease has a broad clinical spectrum with variable age of onset, severity of symptoms, and rate of disease progression.
The disorder encompasses a continuum of phenotypes ranging from a rapidly progressive infantile form to a slowly progressive late-onset form. In general, however, Pompe disease is classified into three different subtypes, including infantile, juvenile, and adult forms.
There is clinical correlation with the amount of α -1,4-glucosidase expression: residual enzyme activity is found in the adult form, while enzyme activity is completely absent in the severe infantile form. It is important to note that mental development and blood glucose concentrations are normal in all forms of Pompe disease.
The classic infantile form is the most severe. Affected infants present shortly after birth with profound hypotonia, muscle weakness, and hyporeflexia.
An enlarged tongue and hypertrophic cardiomyopathy are characteristic. Diagnosis may be based on typical EKG findings which include large QRS complexes and shortened PR intervals [ 37 ]. The liver is normal in size. Sensorineural hearing loss is also prevalent and a less recognized feature [ 38, 39 ].
Without therapy, the disease is rapidly fatal with children usually dying of cardiopulmonary failure or aspiration pneumonia by two years of age.
In the juvenile form of the disease, affected children have hypotonia and weakness of limb girdle and truncal muscles. Motor milestones are delayed, and the myopathy is more gradual in nature. There is no overt cardiac disease, and the patient usually dies from respiratory failure before adulthood without therapy.
The vast majority of patients with Pompe disease are adults. Adult-onset Pompe disease has a long latency and affected individuals may live to old age. Decreased muscle strength and weakness develop in the third or fourth decade, but cardiac involvement, if any, is minimal.
Glycogen accumulates in vascular smooth muscle cells and there are rare reports of death from ruptured aneurysms [ 40, 41 ]. Slow, progressive weakness of the pelvic girdle, paraspinal muscles, and diaphragm leads to loss of mobility and respiratory function.
Respiratory muscle weakness is the leading cause of death. The incidence of Pompe disease is estimated to be approximately 1 in 40, to 1 in 50, The disorder can be found in ethnically diverse populations, including European Caucasians, Hispanics, and Asians, and several mutations are more common in some populations due to founder effects.
For more information, the reader is referred to the Pompe Disease Mutation Database at www. α -1,4-glucosidase is encoded by the GAA gene located on the long arm of chromosome 17 at 17q The gene is composed of 20 exons and over different mutations have been reported [ 19 ]. Of note, while most mutations will be picked up by gene sequencing, at least 11 different gross deletions and one gross insertion have been reported which would not be detectable using this method [ 19 ].
Prenatal diagnosis is possible via enzyme assay or DNA analysis of chorionic villi obtained between 10—12 weeks gestation.
There appears to be genotype-phenotype correlation, with specific mutations associated with infantile, juvenile, and adult-onset disease [ 46—48 ]. Severe mutations which lead to complete loss of enzyme activity are associated with severe, infantile Pompe disease, while mutations which allow partial enzyme expression are associated with adult onset disease.
One very common mutation in intron 1 of the GAA gene, defined as c. The site of glycogen accumulation is different for all three forms of Pompe disease. Furthermore, the amount varies greatly in different organs and even in different muscles [ 51 ]. Histological examination of muscle will reveal large glycogen-filled vacuoles as well as freely dispersed glycogen outside the lysosomes.
As lysosomes accumulate with glycogen, cell function becomes impaired. Mutation analysis is now the preferred method of diagnosis. Enzymatic studies can be performed, however, on muscle tissue or fibroblasts. It is imperative that α -1,4-glucosidase, also known as acid maltase due to its optimum pH lying between 4.
Acid maltase is initially an inactive enzyme that is transported to the prelysosomal and lysosomal compartment via the mannosephosphate receptor [ 52—54 ]. The enzyme is eventually processed into a fully active form that normally degrades glycogen that enters lysosomes via autophagy.
Deficiency of enzyme causes glycogen to overload the lysosomal system and leads to progressive and irreversible cellular damage. Before the advent of enzyme replacement therapy, treatment was generally supportive in nature and respiratory insufficiency was treated with assisted ventilation.
For patients with juvenile Pompe disease, dysarthria and dysphagia caused by severe weakness of the facial muscles might necessitate feeding by G-tube. A high-protein diet, particularly a high-protein diet fortified with branched-chain amino acids, is recommended to help diminish catabolism of muscle protein.
In , enzyme replacement therapy ERT became a commercially available option [ 55 ]. Myozyme ® alglucosidase alfa is indicated for use in patients with infantile-onset Pompe disease and has been shown to improve ventilator-free survival.
In contrast, for patients who are eight years and older and do not have an enlarged heart, Lumizyme ® alglucosidase alfa is available and may help to preserve respiratory function and walking ability. ERT has proven to be less effective in the infantile Pompe patients than in the other populations.
Since most people with the infantile form have no enzyme activity, the enzyme is recognized as foreign by the body, and a robust immune response develops against the ERT. Immunosuppression may help blunt this response and increase efficacy. Gene therapy using AAV-8 injected into the diaphragm is also being attempted in humans with the disease [ 59 ].
Glycogen storage disease type IIb Danon Disease OMIM is a multisystem disorder characterized by hypertrophic cardiomyopathy, heart arrhythmias, skeletal myopathy, retinal abnormalities, and variable degree of mental retardation [ 60—63 ]. Disease onset typically occurs in adolescence, with rapid progression toward end-stage heart failure in early adulthood [ 62 ].
Although the disease was initially classified as a glycogen storage disorder, glycogen is not always elevated in patients [ 64 ]. The biochemical hallmark of the disease is the accumulation of pathologic vacuoles containing glycogen or intermediary metabolites, mainly in skeletal and myocardial muscle with no evidence of enzyme deficiency.
Danon disease is quite rare and good estimates of the incidence are not available. The disorder is X-linked dominant in nature and is due to LAMP-2 lysosome-associated membrane protein-2 deficiency.
Although biochemical analysis is possible in male patients, diagnosis in females requires DNA mutation analysis [ 65 ]. Over fifty different mutations in the LAMP-2 gene have been identified [ 19, 66 ]. Glycogenoses types III and IV are clinically heterogeneous disorders caused by buildup of abnormally structured glycogen in the liver and muscle.
Glycogen storage disease type III Cori disease or Forbes disease OMIM was initially discovered in when a patient being followed by Dr. Gilbert Forbes was found to have excessive amounts of abnormally structured glycogen in liver and muscle tissue [ 67, 68 ].
Type III GSD varies widely in clinical presentation and can be divided into two types: type IIIa, with both hepatic and muscle involvement, and type IIIb, which primarily presents with liver disease [ 69 ]. Both GSD IIIa and GSD IIIb result from an enzyme deficiency in the glycogen debranching enzyme GDE.
This enzyme is encoded by the AGL gene located on chromosome 1p GSD type III is a phenotypically heterogeneous disorder with a wide clinical spectrum. While patients with GSD type IIIb mainly present with hepatic findings, affected individuals with type IIIa have both liver and muscle involvement.
For both IIIa and IIIb, liver disease predominates in infancy and early childhood including hepatomegaly, hypoglycemia, hyperlipidemia, and growth retardation.
Mild hypotonia and delayed motor development are usually the only manifestation during early childhood. By late childhood and adolescence, decreased stamina and pain with exertion can be noted. Muscle wasting is slowly progressive in adulthood and may be severe by the 3rd or 4th decade of life [ 70 ].
Although ventricular hypertrophy is a frequent finding, symptomatic cardiomyopathy leading to death is relatively rare. Unlike muscle disease which is a progressive process, the hypertrophic cardiomyopathy is reversible and appears to be due to excessive storage of glycogen.
With a diet restricting intake of simple sugars, the hypertrophic cardiomyopathy can resolve and cardiac function normalize [ 71, 72 ]. Childhood hepatic symptoms tend to become milder with age. Complications aside from the myopathy are rare.
Cirrhosis can also develop in patients with GSD III, and rare cases of hepatocellular carcinoma have been reported [ 73, 74 ]. Unlike in GSD Ia, hepatocellular carcinoma can develop anywhere in the liver, and it is not the result of malignant transformation of a hepatic adenoma [ 23 ].
Although all individuals with GSD type III show liver involvement, in rare instances the hepatic symptoms are mild and the diagnosis is not made until adulthood when individuals show signs of neuromuscular disease. Other clinical findings include abnormal nerve conduction studies and osteoporosis.
Successful pregnancies have been reported. GSD Types IIIa and IIIb are autosomal recessive allelic disorders caused by mutations in the AGL gene on the short arm of chromosome 1 [ 75 ]. The incidence of GSD III is estimated to be 1 in , live births, but high risk populations have been identified.
GSD IIIa is also more common on the Indian subcontinent India, Pakistan, Afghanistan. To date, at least different pathogenic AGL mutations have been reported [ 19 ].
The encoded enzyme, glycogen debranching enzyme GDE , together with glycogen phosphorylase, is responsible for the complete degradation of glycogen.
GDE has a presumed glycogen binding site at the carboxy terminal end, as well as two separate sites responsible for independent catalytic activities.
These activities include 4- α -glucanotransferase activity 1,4- α -D-glucan:1,4- α -D-glucan 4- α -D glycosyltransferase activity responsible for the transfer of three glucose units to the outer end of an adjacent chain, and an amylo-1,6-glucosidase activity responsible for hydrolysis of branch point glucose residues.
The variable phenotype seen in GSD type III is partly explained by differences in tissue-specific expression. When the enzyme is deficient in both liver and muscle, GSD type IIIa results; in contrast, when AGL is deficient only in the liver and enzyme activity is retained in muscle, then GSD type IIIb results.
Rare cases have also been reported where only one of two GDE catalytic activities is lost [ 79—81 ]. When there is loss of only glucosidase activity, the patient is classified as having GSD Type IIIc, and when there is only loss of transferase activity, the patient is classified as having GSD type IIId.
While glycogenolysis is impaired in GSD III, gluconeogenesis is intact allowing lactate, amino acids, and glycerol from fatty acid oxidation to be used to maintain blood glucose concentrations. Protein is used as the primary source of energy in GSD type III since it also can be used directly by the muscles and has been associated with improvement in the myopathy.
The frequency of cornstarch doses varies with age. In infancy, frequent cornstarch administration may be required with therapy similar to that used in GSD type I.
With older children and adults, cornstarch frequently is only required times per day, and sometimes it is only administered prior to bedtime.
For patients with moderate to severe hypertrophic cardiomyopathy, a high-protein nocturnal enteral therapy may be beneficial.
Intake of simple sugars is limited to 5 grams per meal to minimize postprandial hyperinsulinemia and avoid over-storage of glycogen. Glycogen storage disease type IV Andersen disease OMIM and Adult Polyglucosan Body Disease APBD OMIM are allelic disorders caused by a deficiency of the glycogen branching enzyme encoded by the GBE1 gene.
GSD type IV is quite rare, representing 0. GSD type IV shows significant variability in terms of age of onset and extent of organ and tissue involvement [ 82—85 ]. In its common classic form, patients have failure to thrive and hepatosplenomegaly. Portal hypertension and ascites develop, and progressive cirrhosis often occurs in early childhood.
Without a liver transplant, death usually occurs by five years of age. Unlike the other liver forms of GSD, hypoglycemia is a late manifestation of GSD IV.
Neuromuscular forms of GSD type IV are quite variable and may be classified into several different phenotypes; interestingly, they represent the most severe and the most mild forms of GSD type IV. The most severe and relatively rare form of GSD type IV presents perinatally as fetal akinesia deformation sequence with arthrogryposis, hydrops, polyhydramnios, and pulmonary hypoplasia.
In this form of the disease, death occurs at an early age due to cardiac or pulmonary insufficiency. Other severe forms of neuromuscular GSD type IV present congenitally or in early infancy with hypotonia and skeletal muscle atrophy.
Prognosis varies for these forms of the disease, usually depending on the extent of cardiac and hepatic involvement.
Finally, in its milder forms, GSD type IV may present in late childhood, adolescence, or even adulthood as myopathy or adult polyglucosan body disease APBD with central and peripheral nervous system dysfunction [ 85 ]. APBD is an allelic variant of GSD Type IV characterized by adult-onset progressive neurogenic bladder, gait difficulties due to spasticity and weakness, distal lower extremity sensory loss, and mild cognitive difficulties OMIM [ 86 ].
GSD type IV is the result of a deficiency of glycogen branching enzyme which is encoded by the GBE1 gene located on chromosome 3p This gene is the only gene known to be associated with GSD type IV.
Utilization of both glucose and galactose is impaired in FBS[ ]. Hepatorenal glycogen accumulation and proximal renal tubular dysfunction are the characteristic features of this rare disease[ , ]. FBS follows an autosomal recessive inheritance pattern. The responsible gene, GLUT2 gene solute carrier family 2 member 2, SLC2A2 , was localized to 3q Infants with FBS typically present between the ages of 3 to 10 mo.
In addition to hepatorenal glycogen accumulation and proximal renal tubular dysfunction, FBS is characterized by fasting hypoglycemia, postprandial hyperglycemia and hypergalactosemia, rickets and marked growth retardation.
Patients have entirely normal mental development. In older patients, dwarfism is the most notable finding. Puberty is significantly delayed, with other remarkable observations including a distended abdomen caused by hepatomegaly, deposition of fat on the abdomen and shoulders, and a moon-shaped face[ ].
Some patients may not exhibit hepatomegaly during the early stages of the disease[ , ]. Hyperlipidemia and hypercholesterolemia are prominent and may cause acute pancreatitis. The development of generalized osteopenia occurs early and may result in fractures. Hypophosphatemic rickets and osteoporosis are characteristics of the disease that emerge later in life[ ].
Tubular nephropathy is characterized by excessive glucosuria, moderate hyperphosphaturia along with persistent hypophosphatemia, hyperuricemia, hyperaminoaciduria, and intermittent albuminuria, collectively referred to as renal Fanconi syndrome[ , ].
Hypercalciuria is also evident. Due to increased renal losses, there is a frequent tendency towards hyponatremia and hypokalemia. Polyuria may develop due to high urinary osmotic load[ ]. Progression to renal failure is not the case.
Nephrocalcinosis was also reported in one third of the patients in a recent retrospective study[ ]. There may be mild metabolic hyperchloremic acidosis with normal anion gap due to renal loss of bicarbonate[ ]. Cataracts, a frequently documented consequence of hypergalactosemia, are only present in a small number of patients[ ].
Laboratory findings include fasting hypoglycemia and ketonuria, hyperglycemia and hypergala ctosemia in the postabsorptive state, hypercholesterolemia, hyperlipidemia, moderately elevated alkaline phosphatase, mildly elevated transaminases, normal hepatic synthetic function, hypophosphatemia, hyperaminoaciduria, glucosuria, galactosuria, proteinuria, normal activity of enzymes involved in galactose and glycogen metabolism, normal fructose metabolism, and normal endocrinologic results[ ].
FBS patients develop different patterns of dysglycemia, ranging from fasting hypoglycemia, postprandial hyperglycemia, glucose intolerance, to transient neonatal diabetes to gestational diabetes and frank diabetes mellitus[ ]. The exact molecular mechanisms underlying the occurrence of dysglycemia in individuals with FBS are not yet fully understood.
Impaired renal glucose reabsorption, as well as the accumulation of glucose within the hepatocytes, which stimulates glycogen synthesis and inhibits gluconeogenesis and glycogenolysis, result in fasting ketotic hypoglycemia and hepatic glycogen deposition.
Postprandial findings of hyperglycemia and hypergalactosemia are caused by impaired hepatic uptake and diminished insulin response[ ]. Glycated hemoglobin A1c is usually within the normal range due to recurrent hypoglycemia episodes[ ].
Accumulation of glycogen and free glucose in renal tubular cells leads to general impairment in proximal renal tubular function. Histological evaluation of liver biopsy indicates an excessive buildup of glycogen along with steatosis.
Due to the presence of galactose intolerance, newborn screening for galactosemia can sometimes identify patients with FBS[ ]. The diagnosis is ultimately confirmed by genetic analysis of SLC2A2 gene.
The management of symptoms involves measures to stabilize glucose homeostasis and compensate for the renal loss of water and various solutes. Patients typically require replacement of water, electrolytes, and vitamin D, while also restricting galactose intake and adhering to a diabetes mellitus-like diet.
Frequent small meals with adequate caloric intake and administration of UCCS are important components of symptomatic treatment. In cases of renal tubular acidosis, it may be required to administer alkali to maintain acid-base balance.
Catch-up growth was reported to be induced by UCCS[ ]. Continuous nocturnal gastric drip feeding may be indicated in some cases with growth failure[ ]. With these measures, the prognosis is good. However, a recent retrospective study reported poor outcome despite adequate metabolic management emphasizing the importance of early genetic diagnosis and facilitating prompt nutritional interventions[ ].
Pompe disease is a typical example of a lysosomal storage disease. The clinical manifestations of Pompe disease are variable, predominantly due to the varying amounts of residual acid alpha-glucosidase GAA activity linked with distinct mutations in the causative gene GAA.
GAA gene is mapped to chromosome 17q Enzyme deficiency results in intra-lysosomal storage of glycogen especially in skeletal and cardiac muscles. There is no genotype-phenotype correlation, but DD genotype in the angiotensin converting enzyme gene and XX genotype in the alpha actinin 3 gene are significantly associated with an earlier age of onset of the disease[ ].
There are mainly two types of GSD-II according to age of onset: Infantile-onset and late-onset Pompe disease. Patients with disease onset before the age of 12 mo without cardiomyopathy and all patients with disease onset after 12 mo of age are included in the late-onset form[ ].
The combined frequency of infantile onset and late onset GSD-II varies between and depending on ethnicity and geographic region. In the infantile-onset form, cardiomyopathy and muscular hypotonia are the cardinal features and patients die around 1 year of age.
Patients also have feeding difficulties, macroglossia, failure to thrive, hearing impairment and respiratory distress due to muscle weakness. The liver is rarely enlarged unless there is heart failure. Hypoglycemia and acidosis do not occur[ ]. In the late-onset form, involvement of skeletal muscles dominates the clinical picture, and cardiac involvement is generally clinically insignificant depending on the age of onset.
Glycogen accumulation in vascular smooth muscle may cause the formation and subsequent rupture of an aneurysm[ ]. Both severe infantile and asymptomatic adult forms of the disease were observed in two generations of the same family[ ].
Although women with GSD-II do not have an increased risk of pregnancy or delivery complications, pregnancy may worsen muscle weakness and respiratory complications[ ]. As a rule, there is an inverse correlation between the age at disease onset and the severity of clinical manifestations with the level of residual enzyme activity[ ].
Laboratory testing reveals nonspecific elevations in CK, aldolase, aminotransferases, and lactate dehydrogenase. Elevated urinary tetrasaccharide is highly sensitive but not specific. To establish the final diagnosis, the measurement of enzyme activity in skin fibroblasts or muscle tissue or the demonstration of the responsible mutation is required[ ].
Although it is not curative, ERT has changed the course of Pompe disease since its first use in [ ]. Alglucosidase alfa, a lysosomal glycogen-specific recombinant enzyme, was approved by the European Medicines Agency EMA in in the European Union and by the Food and Drug Administration FDA in in the United States.
pdf ; accessed on November 5, Based on data from later studies, treatment initiation was shifted to the neonatal period.
A new formulation of GAA enzyme, avalglucosidase alfa, improves the delivery of the enzyme to target cells and has 15 times higher cellular uptake when compared with alglucosidase alfa.
The FDA and EMA approved avalglucosidase in and in , respectively, for the treatment of patients who are one year of age and older with late-onset Pompe disease[ ]. Ongoing studies show that avalglucosidase is generally well tolerated in patients with infantile-onset Pompe disease[ ].
Criteria for starting and stopping ERT in adult patients with GSD-II are similar in different countries. While a confirmed diagnosis and being symptomatic are general criteria for starting ERT, patient wish, severe infusion associated reactions, noncompliance with treatment, and lack of effect are criteria for stopping ERT[ ].
Another way to increase the effectiveness of ERT is to use antibodies as an intracellular delivery vehicle. The 3E10 anti-nuclear antibody, that penetrates cells and localizes to the cell nucleus, has been used for this purpose. VAL is a fusion protein consisting of 3E10 antibody and GAA complex.
The presence of 3E10 increases the delivery of GAA to both lysosomal and extra-lysosomal storage of glycogen within cells[ ]. The earlier ERT is started, the better its effectiveness. Therefore, it is recommended that ERT is started before irreversible clinical symptoms begin.
This concept has led to the development of screening programs for Pompe disease[ ]. Recently, it has been shown that in utero alglucosidase alfa treatment, which was started at 24 wk 5 d of gestation and given 6 times at 2-wk intervals through the umbilical vein, was successful[ ].
Although antibodies against the enzyme may develop, a recent study showed that the development of antibodies did not affect the clinical course[ ]. Whether additional treatments such as oral supplementation of L-alanine is beneficial is being investigated[ ].
As an alternative to ERT, studies on gene therapy have also commenced[ ]. Although Danon disease was previously classified as a variant of GSD-II with normal alpha-glucosidase activity, it is still controversial whether it is a real GSD.
A lysosomal structural protein, LAMP2, is deficient in Danon disease. LAMP2 is involved in autophagosome maturation. Disruption of autophagy leads to accumulation of glycogen granules and autophagic vacuoles[ ].
It is an X-linked Xq24 dominant hereditary disease affecting both skeletal and cardiac muscles, and characterized by skeletal and cardiac myopathy, proximal muscle weakness and intellectual disability.
Female patients have a milder disease predominantly involving cardiac muscle[ ]. There is currently no treatment for Danon disease. There are ongoing studies evaluating the efficacy and safety of gene therapy[ ].
Another glycogen storage cardiomyopathy results from PRKAG2 the gene encoding gamma-2 non-catalytic subunit of adenosine monophosphate-activated protein kinase mutations on chromosome 7q The disease is characterized by left ventricular hypertrophy due to altered glycogen metabolism and glycogen storage in cardiac muscle, similar to Danon disease[ - ].
It is inherited in an autosomal dominant pattern. PRKAG2 gene variants cause a syndrome characterized by cardiomyopathy, conduction disease, and ventricular pre-excitation[ ]. Mutations in the gamma-2 non-catalytic subunit of AMP-activated protein kinase may cause lethal congenital storage disease of the heart, and death in the first year of life[ ].
It is important to differentiate the clinical picture related to PRKAG2 mutations from Danon disease, as management and prognosis are different. GSD-V is caused by mutations in PYGM gene which is the gene encoding the muscle isoform of glycogen phosphorylase.
The PYGM gene is located on 11q The clinical manifestations generally occur during early adulthood with physical activity intolerance and muscle cramps characterized by muscle fatigue and pain, contracture, tachypnea, tachycardia, ptosis, and retinal dystrophy.
Exercise induced rhabdomyolysis can cause transient myoglobinuria, leading to acute renal failure. Hyperuricemia, gout development and thyroid dysfunction are not uncommon[ ].
Many patients are diagnosed with an incidental finding of abnormal serum CK levels[ ]. Echaniz-Laguna et al [ ] studied a family of 13 affected members with adult-onset muscle weakness, and reported a phenotype caused by a dominant myophosphorylase gene mutation p.
The first signs of the disease occurred after 40 years of age with proximal leg weakness, followed by proximal arm weakness. In contrast to McArdle disease, the patients did not have exercise intolerance, second wind phenomenon, markedly increased CK levels, or rhabdomyolysis.
The authors concluded that specific PYGM mutations can cause either dominant or recessive GSDs[ ]. The responsible gene is located on chromosome 12q Exercise induced muscle cramps and myoglobinuria are the main characteristics of GSD-VII.
Neurological examination does not reveal any abnormalities at rest. Muscle weakness and stiffness invariably occur in muscle groups that are subjected to intense or prolonged exertion. The ischemic exercise test is characterized by the absence of an increase in venous lactate level.
Myoglobinuria may develop following exercise. Nausea and vomiting, icterus, elevated CK, hyperuricemia and reticulosis may also be observed[ ]. In contrast to GSD-V, glucose intake prior to exercise worsens exercise capacity due to blocked use of both muscle glycogen and blood glucose[ ].
The gene is located on chromosome Xq In most patients, clinical findings appear in adulthood and are characterized by muscle weakness and muscle cramps during exercise. Elevated serum CK level and myopathic findings on electromyography may guide the diagnosis[ ].
The last steps of glycogenolysis are abnormal. The disease is inherited in an autosomal recessive manner and characterized by exercise induced muscle cramps, myalgia, rhabdomyolysis and myoglobinuria.
Serum CK level is increased between episodes[ ]. GSD-XI was first described by Kanno et al [ ] in and characterized by easy fatigue, increase in serum CK, myoglobin, lactate, and pyruvate levels immediately after ischemic work.
The gene locus is on chromosome 11p It is an autosomal recessive disorder, and the gene is located on chromosome 16p GSD-XIII was first described by Comi et al [ ] in in a year-old man with severe deficiency of muscle enolase activity.
The patient had recurrent exercise induced myalgia without cramps. Serum CK concentration was elevated while serum lactate level was normal following ischemic forearm exercise. The related gene is located on chromosome 17p Similar to Danon disease and PRKAG2 variants, glycogenin deficiency may cause left ventricular arrhythmogenic cardiomyopathy.
Patients present with chest pain, progressive weakness, and vague presyncope spells[ ]. There have been significant changes and improvements in the classification, diagnosis, and treatment of GSDs in recent years. We are now more aware that many GSDs, which were previously identified as childhood diseases, may present first in adulthood.
P-Reviewer: El-Shabrawi MH, Egypt; Rathnaswami A, India; Yao G, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zhao S. Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System.
Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article PDF Full Article with Cover PDF Full Article WORD Full Article XML Full Article HTML Audio PubMed Central PubMed CrossRef Google Scholar Similar Articles 3 Timeline of Article Publication 0 Authors Evaluation 4 Article Quality Tracking 0 Reference Citation Analysis 0.
Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Non-Native Speakers PDF Peer-Review Report PDF. CrossCheck and Google Search of This Article. Scientific Misconduct Check PDF. Academic Rules and Norms of This Article.
Conflict-of-Interest Statement PDF Copyright Assignment PDF. Citation of this article. Gümüş E, Özen H. Glycogen storage diseases: An update. World J Gastroenterol ; 29 25 : [PMID: DOI: Corresponding Author of This Article.
haozen hacettepe. Checklist of Responsibilities for the Scientific Editor of This Article. Scientific Editor Work List PDF. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article.
This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4.
Times Cited Counts in Google of This Article. Number of Hits and Downloads for This Article. Total Article Views All Articles published online. Times Cited of This Article. Times Cited 1.
Journal Information of This Article. Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA.
Review Open Access. Copyright ©The Author s Published by Baishideng Publishing Group Inc. All rights reserved. World J Gastroenterol.
Jul 7, ; 29 25 : Published online Jul 7, doi: Ersin Gümüş , Hasan Özen. ORCID number: Ersin Gümüş ; Hasan Özen Author contributions : Both authors contributed all parts of the study. Conflict-of-interest statement : All the authors report no relevant conflicts of interest for this article.
Open-Access : This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.
It is distributed in accordance with the Creative Commons Attribution NonCommercial CC BY-NC 4. Received: December 28, Peer-review started : December 28, First decision : February 1, Revised: February 15, Accepted: April 30, Article in press : April 30, Published online: July 7, Key Words: Glycogen storage disease , Liver , Muscle , Hypoglycemia.
Citation: Gümüş E, Özen H. Open in New Tab Full Size Figure Download Figure. Figure 1 Simplified pathway of glycogen synthesis and degradation in hepatocytes. Glucose and glycogen convert into one another via synthesis or degradation glycogenolysis through various steps.
The liver plays a central role in maintaining normoglycemia. During the fasting state, the liver maintains glucose homeostasis via a metabolic shift from synthesizing glycogen to endogenous glucose production by glycogenolysis and gluconeogenesis. Specific enzyme or transporter defects in these pathways are associated with clinical and biochemical manifestations including hepatomegaly, hypoglycemia, hyperlipidemia, hypertriglyceridemia, hyperlactatemia, and hyperuricemia.
GSD: Glycogen storage disease; UDP-Glucose: Uridine diphosphate glucose; GlucoseP: Glucose 1-phosphate; GlucoseP: Glucosephosphate; Acetyl-CoA: Acetyl coenzyme A; TCA: Tricarboxylic acid.
Table 1 Overview of glycogen storage diseases. Postprandial hyperglycemia, glycosuria, and hyperlactatemia. Electrocardiographic preexcitation and conduction system disease. Non-progressive hepatic form. Neuromuscular presentation perinatal, congenital, childhood and adult forms.
Myopathy, cardiomyopathy, neuropathy, CNS involvement, APBD. Severe hepatic involvement reported. Mild hypotonia and cardiopathy reported. Excessive glycogen accumulation with structurally normal glycogen in liver tissue.
Symptomatic female carriers due to X chromosome inactivation. Clinical symptoms and laboratory abnormalities gradually disappear with age. Proximal renal tubular dysfunction. Different patterns of dysglycemia.
GSD: Glycogen storage disease; HA: Hepatic adenoma; HCC: Hepatocellular carcinoma; AR: Autosomal recessive; XLR: X-linked recessive; XLD: X-linked dominant; CK: Creatinine kinase; CNS: Central nervous system; APBD: Adult polyglucosan body disease: IBD: Inflammatory bowel disease.
GSD-0; glycogen synthase deficiency. GSD-I; von Gierke disease; hepatorenal glycogenosis. GSD-III; Cori disease; Forbes disease; limit dextrinosis; amylo-1,6-glucosidase deficiency; glycogen debrancher deficiency.
GSD-IV; Andersen disease; brancher deficiency; amylopectinosis; glycogen branching enzyme deficiency. GSD-VI; Hers disease; liver glycogen phosphorylase deficiency. GSD-II; Pompe disease; acid alpha-glucosidase deficiency; acid maltase deficiency; alpha-1,4-glucosidase deficiency.
AMP-activated protein kinase deficiency. GSD-V; McArdle disease; myophospharylase deficiency; muscle glycogen phosphorylase deficiency. GSD-VII; Tarui disease; muscle phosphofructokinase deficiency; GSD of muscle. GSD-IXd; X-linked muscle PHK alpha-1 subunit deficiency.
GSD-X; muscle phosphoglycerate mutase deficiency. GSD-XI; lactate dehydrogenase a deficiency. GSD-XIII; muscle enolase 3 deficiency. GSD-XV; glycogenin deficiency. Provenance and peer review: Unsolicited article; Externally peer reviewed.
Ozen H. Glycogen storage diseases: new perspectives. Roach PJ , Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J. Ellingwood SS , Cheng A. Biochemical and clinical aspects of glycogen storage diseases.
J Endocrinol. Chen YT , Kishnani PS, Koeberl D. Glycogen Storage Diseases. Saltik IN , Ozen H, Ciliv G, Koçak N, Yüce A, Gürakan F, Dinler G. Glycogen storage disease type Ia: frequency and clinical course in Turkish children. Indian J Pediatr.
Kanungo S , Wells K, Tribett T, El-Gharbawy A. Glycogen metabolism and glycogen storage disorders. Ann Transl Med.
Burda P , Hochuli M. Hepatic glycogen storage disorders: what have we learned in recent years? Curr Opin Clin Nutr Metab Care. Kollberg G , Tulinius M, Gilljam T, Ostman-Smith I, Forsander G, Jotorp P, Oldfors A, Holme E. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0.
N Engl J Med. Lewis GM , Spencer-Peet J, Stewart KM. Infantile Hypoglycaemia due to Inherited Deficiency of Glycogen Synthetase in Liver. Arch Dis Child. Orho M , Bosshard NU, Buist NR, Gitzelmann R, Aynsley-Green A, Blümel P, Gannon MC, Nuttall FQ, Groop LC.
Mutations in the liver glycogen synthase gene in children with hypoglycemia due to glycogen storage disease type 0. J Clin Invest. Nuttall FQ , Gannon MC, Kubic VL, Hoyt KJ. The human liver Glycogen synthase isozyme gene is located on the short arm of chromosome Hicks J , Wartchow E, Mierau G.
Glycogen storage diseases: a brief review and update on clinical features, genetic abnormalities, pathologic features, and treatment. Ultrastruct Pathol. Kamenets EA , Gusarova EA, Milovanova NV, Itkis YS, Strokova TV, Melikyan MA, Garyaeva IV, Rybkina IG, Nikitina NV, Zakharova EY.
Hepatic glycogen synthase GYS2 deficiency: seven novel patients and seven novel variants. JIMD Rep. Laberge AM , Mitchell GA, van de Werve G, Lambert M. Long-term follow-up of a new case of liver glycogen synthase deficiency.
Am J Med Genet A. Wolfsdorf JI , Weinstein DA. Glycogen storage diseases. Rev Endocr Metab Disord. Weinstein DA , Correia CE, Saunders AC, Wolfsdorf JI. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia.
Mol Genet Metab. Bachrach BE , Weinstein DA, Orho-Melander M, Burgess A, Wolfsdorf JI. Glycogen synthase deficiency glycogen storage disease type 0 presenting with hyperglycemia and glucosuria: report of three new mutations. J Pediatr. Kasapkara ÇS , Aycan Z, Açoğlu E, Senel S, Oguz MM, Ceylaner S.
The variable clinical phenotype of three patients with hepatic glycogen synthase deficiency. J Pediatr Endocrinol Metab. Tagliaferri F , Massese M, Russo L, Commone A, Gasperini S, Pretese R, Dionisi-Vici C, Maiorana A. Hepatic glycogen storage diseases type 0, VI and IX: description of an italian cohort.
Orphanet J Rare Dis. Browner MF , Nakano K, Bang AG, Fletterick RJ. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution.
Proc Natl Acad Sci U S A. Gierke EV. Hepato-nephro-megalia-glycogenica Glykogenspeicherkrankheit der Leber und Nieren. Beitr Pathol Anat. Cori GT , Cori CF. Glucosephosphatase of the liver in glycogen storage disease. J Biol Chem. Narisawa K , Igarashi Y, Otomo H, Tada K.
A new variant of glycogen storage disease type I probably due to a defect in the glucosephosphate transport system. Biochem Biophys Res Commun. van Schaftingen E , Gerin I. The glucosephosphatase system. Chou JY , Jun HS, Mansfield BC. J Inherit Metab Dis.
Ekstein J , Rubin BY, Anderson SL, Weinstein DA, Bach G, Abeliovich D, Webb M, Risch N. Mutation frequencies for glycogen storage disease Ia in the Ashkenazi Jewish population. Scott SA , Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases.
Hum Mutat. Brody LC , Abel KJ, Castilla LH, Couch FJ, McKinley DR, Yin G, Ho PP, Merajver S, Chandrasekharappa SC, Xu J. Construction of a transcription map surrounding the BRCA1 locus of human chromosome Yang Chou J , Mansfield BC. Molecular Genetics of Type 1 Glycogen Storage Diseases.
Trends Endocrinol Metab. Rake JP , Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I ESGSD I. Eur J Pediatr. Derks TGJ , Rodriguez-Buritica DF, Ahmad A, de Boer F, Couce ML, Grünert SC, Labrune P, López Maldonado N, Fischinger Moura de Souza C, Riba-Wolman R, Rossi A, Saavedra H, Gupta RN, Valayannopoulos V, Mitchell J.
Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs. Aydemir Y , Gürakan F, Saltık Temizel İN, Demir H, Oğuz KK, Yalnızoğlu D, Topçu M, Özen H, Yüce A.
Evaluation of central nervous system in patients with glycogen storage disease type 1a. Turk J Pediatr. Czapek EE , Deykin D, Salzman EW. Platelet dysfunction in glycogen storage disease type I.
Hutton RA , Macnab AJ, Rivers RP. Defect of platelet function associated with chronic hypoglycaemia. Mühlhausen C , Schneppenheim R, Budde U, Merkel M, Muschol N, Ullrich K, Santer R. Decreased plasma concentration of von Willebrand factor antigen VWF:Ag in patients with glycogen storage disease type Ia.
Austin SL , El-Gharbawy AH, Kasturi VG, James A, Kishnani PS. Menorrhagia in patients with type I glycogen storage disease.
Life-expectancy in disrase storage disease Android vs gynoid fat distribution classification I GSD I has improved considerably. Vegan-friendly protein options relative rarity implies that diesase metabolic centre has experience of large series sforage patients and experience with long-term management and follow-up at each centre is limited. There is wide variation in methods of dietary and pharmacological treatment. Conclusion : In this paper guidelines for the management of GSD I are presented. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve.Research glycogej underway on an investigational mRNA treatment dtorage see sorage Android vs gynoid fat distribution classification can correct the Fat burner pills of GSD1a glycgoen teaching the body tteatment break down glycogen.
Glycogne below to learn more about the Ba1ance Trial. Beam Therapeutics storave initiated a clinical development program tfeatment glycogen storage disease type 1a.
Beam is using base editing Adavnces potentiall AAdvances the fo genetic change in G6PC: initially the R83C variant, which storaage the most Monounsaturated fats benefits mutation in GSD1a.
Base editing greatment an emerging class Goji Berry Joint Support precision genetic Advances in treatment for glycogen storage disease designed to overcome the limitations of Advances in treatment for glycogen storage disease approaches fkr expand the potential of genetic medicine.
By rewriting a single base, base editors may correct disease-causing point mutations and potentially create life-long cures for patients suffering from serious diseases. Click below to read about their preclinical data.
Ultragenyx Pharmaceuticals is evaluating DTX to establish normal glucose metabolism and reduce or eliminate the need for cornstarch to maintain normal glucose levels. The company is currently in phase 3 of clinical trials. info curegsd. Sign In. Privacy - Terms - Refunds. We may use cookies to give you the best experience on our website.
In accordance with our Privacy Policyyou hereby agree to our use of cookies on this device. CURRENT RESEARCH What we are funding now.
Our ultimate goal is to live in a world without Glycogen Storage Disease. Your generous contributions are working hard to help us get there. CURRENT RESEARCH. Investigational mRNA Treatment. Read More. Press Release. Learn More. Join Mailing List. Tell A Friend.
Donate Now. Upcoming Events. View Current Fundraisers. Start A Fundraiser. The Children's Fund for Glycogen Storage Disease Research. Sign In Privacy - Terms - Refunds. because every child deserves to be healthy.
: Advances in treatment for glycogen storage disease| Glycogen Storage Disease (GSD) | Children's Hospital of Philadelphia | Blood glucose was monitored on days 0 the day of administration , 2, 4, 7, 10, and 14 prior to time 0 or at 2. Glucose fuels every cell in our body, including brain activity. Strenuous exercise is contraindicated. Surgery should be undertaken with caution due to a bleeding tendency and risk of intraoperative lactic acidosis. Since G6PT is the only antiporter that can couple to G6Pase-α α Chen et al. This enzyme is required for glycogen synthesis, and is encoded by the GYS2 gene on chromosome Use of modified cornstarch therapy to extend fasting in glycogen storage disease types Ia and Ib. |
| Diagnosis of glycogen storage disease | Such phosphorylation occurs in lgycogen to the Advances in treatment for glycogen storage disease glucagon and epinephrine. Glycogen treatmen disease type XI Fanconi-Bickel syndrome OMIM results stofage defects in a transport ffor, the GLUT2 glycogfn transporter [ — ]. Brian McArdle in Elderberry gummies for overall health studying a young man with exercise intolerance Android vs gynoid fat distribution classification muscle cramps [ 91 ]. Pediatr Res — Article CAS PubMed Google Scholar Smit GPA, Ververs MT, Belderok B, van Rijn M, Berger R, Fernandes J Complex carbohydrates in the dietary management of patients with glycogenosis caused by glucosephosphatase deficiency. Efficacy of von Gierke disease is transient, waning gradually over the months following vector administration. Severe mutations which lead to complete loss of enzyme activity are associated with severe, infantile Pompe disease, while mutations which allow partial enzyme expression are associated with adult onset disease. Stay Up To Date:. |
| WHAT IS GSD1? | Hacein-Bey-Abina, S. The γ subunit also has muscle and liver isoforms, each of which is encoded by a distinct gene PHKG1 and PHKG2 , respectively. However, PHKB is expressed in both muscle and liver[ , ]. To add to the molecular complexity, various tissue-specific isoforms exist for each subunit; these isoforms may be due to expression from separate genes or from alternative splicing of a single gene. Renal function in glycogen storage disease type I, natural course, and renopreservative effects of ACE inhibition. |
| Navigation | The G6Pase-α and G6PT are functionally co-dependent. Cornstarch regimens consisted of around 5 to 7 doses per day and around g per day. Concolino, D. A critical analysis of codon optimization in human therapeutics. Super Bowl Raffle! Bruno C. |
| Gene Therapy Improves Quality of Life in Patients With Glycogen Storage Disease Type 1a | The degree of hyperlipidemia is associated with development of hepatic adenomas[ 61 ]. However, the pathophysiological mechanisms are yet to be fully understood and factors other than metabolic control may also be responsible for adenoma formation. Chromosomal and genetic alterations may also play a role in hepatocellular carcinoma associated with GSD-I[ 63 ]. Hepatic adenomas have the potential to transform into hepatocellular carcinoma over an extended period, with reports of malignant transformation occurring as long as 28 years after initial diagnosis[ 64 , 65 ]. A rapid increase in size or number of adenomas is associated with an increased risk of adenoma to hepatocellular carcinoma transformation and should be evaluated carefully. The link between GSD-I and risk for cardiovascular disease is controversial. Although GSD-Ia patients have elevated levels of triglycerides, very low density lipoprotein and low density lipoprotein, the occurrence of endothelial vascular dysfunction and atherosclerosis is uncommon. It has been suggested that the increased serum levels of apoE may offset the elevated risk of atherosclerosis associated with dyslipidemia[ 66 ]. In addition, an increase in serum levels of antioxidative factors may contribute as a protective mechanism[ 67 , 68 ]. There are conflicting data regarding whether patients with GSD-I are at increased risk for atherosclerosis[ 69 , 70 ]. Pulmonary hypertension is a rare long-term complication of GSD-I with few cases reported. Patients with a concomitant predisposing condition for pulmonary arterial hypertension are at increased risk[ 37 ]. The main neurological impact of GSD is related to hypoglycemia. Patients with GSD-I may suffer from brain damage, which may be caused by recurrent severe hypoglycemia[ 71 ]. Studies have found a significant correlation between the frequency of hospital admissions for hypoglycemia and abnormalities in both performance ability tests and brainstem auditory evoked potentials. In addition, electroencephalography abnormalities were found to be correlated with dietary compliance. Brain imaging abnormalities were more frequent among GSD-I patients with early symptom onset, frequent and longer hospital admissions, and poor metabolic control including elevated levels of uric acid, lactate, and triglyceride[ 32 , 72 ]. Some females may have polycystic ovaries and irregular menstrual cycles with normal fertility[ 73 ]. Women with GSD-Ia may have pregnancies and deliveries without complications[ 74 ]. In consideration of the risk of development of hepatic adenomas in GSD-I patients, estrogen-containing contraceptives should be avoided whenever possible[ 75 ]. In addition to hypoglycemia, the most prominent laboratory abnormalities observed in patients with GSD-I include lactic acidosis, hyperlipidemia especially hypertriglyceridemia but also hypercholesterolemia , and hyperuricemia Figure 1. Mild elevation in transaminase levels is usually detected[ 30 ]. Ultrasonographic examination may reveal enlarged kidneys in affected patients of all ages. Serum biotinidase activity is increased in GSD-Ia patients[ 76 - 79 ]. Biotinidase activity was reported to be positively correlated with hypertriglyceridemia in subjects with GSD-I while severe fibrosis and cirrhosis were related to reduced enzyme activity[ 80 ]. There may also be hypercalciuria[ 5 ]. There is little or no increase in blood glucose concentration in response to administration of glucagon and this may even lead to worsening of the metabolic acidosis. Histopathological examination of the liver in patients with GSD-Ia typically reveals a mosaic pattern with pale-staining and swollen hepatocytes. Other observed features include steatosis and nuclear hyperglycogenation. Periodic acid-Schiff PAS -positive and diastase sensitive glycogen is evenly dispersed throughout the cytoplasm. Glycogen accumulation may be within the normal range or exhibit only a mild increase. While fibrosis is not as prominent in GSD-I as in GSD types III, IV, and VI, it may still be present in some affected individuals[ 5 , 81 - 83 ]. GSD-Ia is usually suspected based on a set of clinical e. The definitive diagnosis is confirmed by a mutation analysis or a liver biopsy and an enzyme assay. If a liver biopsy is performed, diagnosis can be confirmed by measuring G6Pase enzyme activity on a liver biopsy specimen; however, it should be kept in mind that measurement of G6Pase enzyme activity will not detect GSD-Ib. When the specific mutation in the index case is known, prenatal diagnosis via chorionic villus sampling can be performed for GSD-I[ 84 ]. The mainstay of treatment is to prevent hypoglycemia by avoiding prolonged fasting[ 85 ]. Continuously providing a dietary supply of glucose during the day and night by frequent feedings, frequent ingestion of UCCS or nocturnal enteral tube feeding are possible feeding strategies. Infants and children should be fed frequently, not allowing fasting periods longer than h. In adolescents and adults, fasting more than h should be avoided. Small, frequent meals with balanced macronutrient content and use of UCCS are recommended. Continuous intragastric feeding through a nasogastric or gastrostomy tube can be used overnight allowing the patients to sleep through the night[ 37 ]. UCCS can be introduced as early as mo of age. For the administration of UCCS in GSD-I patients, the recommended dose is Digestion of UCCS is slow, enabling a sustained release of glucose, thereby achieving a more stable glycemic profile over an extended duration, in contrast to other carbohydrate sources. The administration of UCCS has been shown to achieve adequate glycemia for a median duration of 4. Glycosade ® , a modified, waxy maize extended-release cornstarch, is available as a single-dose overnight treatment[ 87 ]. In GSD-I, intake of fructose and galactose, which cannot be metabolized to glucose via G6P, further contributes to the metabolic derangement. Lactose galactose and glucose , fructose and sucrose fructose and glucose should be restricted in all age groups. Restricting the intake of fruits, vegetables, juices, and dairy products renders the diet inadequate. Micronutrients, vitamins, and minerals should be supplemented to avoid nutritional deficiencies. Effective dietary management is essential to minimize the metabolic derangement associated with GSD-I and to reduce the development of long-term complications[ 37 , 85 ]. However, caution must be exercised to avoid overtreatment. Overtreatment with UCCS has many consequences including obesity, increased glycogen storage in the liver, worsening lactic acidosis, increased gastrointestinal disturbances, hyperinsulinemia, and insulin resistance[ 88 ]. If there is anemia, the causes must be evaluated e. In the case of severe anemia, hepatic adenomas in GSD-Ia and enterocolitis in GSD-Ib should be investigated[ 42 ]. Angiotensin converting enzyme inhibitors or angiotensin receptor blockers should be used to delay the progression of renal damage[ 53 , 89 - 91 ]. If serum triglyceride levels remain high despite optimizing dietary treatment, the administration of lipid-lowering drugs, such as 3-hydroxymethylglutaryl-coenzyme A reductase inhibitors and fibrates, may be necessary to decrease the risk of atherosclerosis, cholelithiasis, and pancreatitis. For adults with persistently elevated cholesterol levels, statins may be considered as a treatment option[ 85 ]. The positive effect of medium-chain triglycerides on lowering serum cholesterol and triglyceride levels has been reported[ 92 , 93 ]. Recommendations regarding perioperative management of patients with GSD-I are available[ 37 , 94 ]. Close monitoring of blood glucose, electrolytes, and lactate levels is crucial during the peri-operative period. The administration of Ringer lactate solution should be avoided in GSD-I patients, as it may exacerbate lactic acidosis and worsen metabolic decompensation[ 37 ]. Bleeding time must be normalized before elective surgical interventions by h continuous gastric drip feeding for one week or by intravenous glucose infusion over 24 to 48 h[ 85 ]. In , after realizing that in vitro G6Pase activity was normal despite glucose not being released from G6P in vivo , a second subtype of GSD-I was identified[ 95 ]. In , it was elucidated that a transport system specific to G6P exists and is responsible for transporting G6P from the cytoplasm to the endoplasmic reticulum[ 96 ]. The responsible gene, SLC37A4 the solute carrier family 37 member 4 , has been cloned and located on chromosome 11q23[ 97 , 98 ]. GSD-Ib is characterized by distinctive features such as recurrent infections, neutropenia, and neutrophil dysfunction, in addition to the clinical symptoms and findings observed in GSD-Ia. While not all GSD-Ib patients have neutropenia and neutrophil dysfunction, these conditions are common and predispose patients to severe infections and inflammatory bowel disease[ 44 ]. Patients with GSD-Ib may have normal neutrophil counts in the first year of life. G6PT gene, unlike G6Pase , is also expressed in hematopoietic progenitor cells, which may be responsible for neutropenia and recurrent infections in GSD-Ib[ 99 ]. The neutrophil dysfunction in GSD-Ib includes both impaired motility and respiratory burst[ , ]. Impaired glucose transport across the cell membrane of polymorphonuclear leukocytes may be responsible for neutrophil dysfunction in GSD-Ib. Microsomal transport of G6P has a potential role in the antioxidant protection of neutrophils. Dysfunction of this transporter due to genetic defects in G6PT may impair cellular functions and induce apoptosis, contributing to the neutrophil dysfunction seen in GSD-Ib[ ]. Some individuals with GSD-Ib do not develop neutropenia. It has been suggested that this could be due to residual transporter activity of some G6PT mutations[ ]. Young children with GSD-Ib may experience frequent otitis, gingivitis, periodontal disease, dental caries, and skin abscesses. Oral and genital ulcerations and intestinal mucosal ulcers may occur[ 43 , ]. Individuals with GSD-Ib may experience recurrent episodes of diarrhea. The underlying cause of this symptom appears to be inflammation of the intestinal mucosa, as evidenced by elevated fecal α1-antitrypsin excretion and colonic inflammation in colonoscopic biopsies[ 44 ]. There is no established association between the specific genetic mutations causing GSD-Ib and the occurrence of neutropenia, bacterial infections, and other systemic complications in affected individuals[ ]. Patients with GSD-Ib may require liver transplantation. Although hypoglycemia, lactic acidosis and dyslipidemia improve after liver transplantation, neutropenia generally continues to be present as it is primarily attributable to an intrinsic defect in the neutrophils[ - ]. Another characteristic clinical finding of GSD-Ib is the occurrence of Crohn disease-like colitis[ , ]. Accompanying findings and symptoms include fever, diarrhea, and perioral and anal ulcers. Interestingly, the severity of the primary disorder does not appear to be correlated with the occurrence or severity of intestinal symptoms[ , ]. Manifestations of inflammatory bowel disease may improve with granulocyte colony-stimulating factor G-CSF treatment[ ]. Enteral nutrition with a polymeric formula enriched in the anti-inflammatory cytokine transforming growth factor-β is recommended as a first-line treatment of digestive complications in GSD-Ib[ ]. Inflammatory bowel disease may require treatment with anti-inflammatory and immunosuppressive medications[ ]. Successful treatment of inflammatory bowel disease with biologics including infliximab and adalimumab in GSD-Ib patients refractory to conventional treatment has been reported[ , ]. GSD-Ib is characterized by an increased risk for developing autoimmune disorders like thyroid autoimmunity and myasthenia gravis[ ]. GSD-Ib patients have a higher likelihood of developing thyroid autoimmunity and hypothyroidism, while GSD-Ia patients show little indication of thyroid pathologies[ , ]. Based on the slightly elevated levels of thyrotropin, even in patients with overt hypothyroidism, it could be postulated that there is concomitant damage occurring at the hypothalamus or pituitary gland[ ]. Recently, predisposition to autoimmunity in GSD-Ib patients was linked with a profound defect in conventional T cells and regulatory T cells caused by defective engagement of glycolysis in T cells due to G6PT deficiency[ ]. Although a rare outcome of GSD-Ib, patients may develop terminal kidney disease, which may necessitate kidney transplantation[ ]. Nutritional management of GSD-Ib is similar to that of GSD-Ia. Neutropenic patients with GSD-Ib should be treated with G-CSF. G-CSF therapy may normalize the number of neutrophils and restore myeloid functions[ - ]. The implementation of a combined therapeutic approach including both dietary management and G-CSF treatment improves the prognosis of patients by significantly mitigating metabolic and myeloid abnormalities. G-CSF administration is associated with not only an elevation of peripheral neutrophil counts, but also a reduction in the incidence of febrile episodes and infections, as well as improvement in enterocolitis in patients with GSD-Ib[ ]. In conjunction with other therapies aminosalicylates, mesalamine, and corticosteroids , G-CSF ameliorates inflammatory bowel disease symptoms[ ]. To prevent complications such as splenomegaly, hypersplenism, hepatomegaly, and bone pain, it is recommended that the lowest effective dose of G-CSF is used. Caution must be exercised regarding the development of splenomegaly and myeloid malignancy[ , ]. Vitamin E has been reported to be effective in reducing the frequency of infections and improving neutropenia[ ]. Liver transplantation is the ultimate therapy for hepatic metabolic disease related to GSD-I. There is no possibility of the recurrence of GSD-I within the allograft. Recently, an unusual post-transplant finding of two siblings with persistent hyperuricemia requiring allopurinol treatment has been reported[ ]. Moreover, chronic renal failure is a well-known complication that may arise as a consequence of liver transplantation in individuals with GSD-Ia, and progression to renal failure within a few years of transplantation was reported[ ]. It is uncertain whether post-transplantation renal failure is related to disease progression, toxicity from immunosuppressants used after liver transplantation, a secondary reaction to poor metabolic control, or a combination of these factors. Renal transplantation in GSD-I, on the other hand, corrects only renal abnormalities[ ]. Conflicting results have been reported in different studies regarding whether catch-up growth is achieved or not following liver transplantation in children with GSD-I[ , ]. Despite improved survival and growth, long-term complications of GSD-I like progressive renal failure and development of hepatic adenomas do not respond completely to dietary treatment. Although liver transplantation corrects metabolic derangement and improves the quality of life of these patients, it is not without complications[ ]. These findings suggest that novel therapeutic approaches with higher success and lower complication rates are warranted. A recent advance in the treatment of neutropenia and neutrophil dysfunction in individuals with GSD-Ib is repurposing empagliflozin, a sodium-glucose co-transporter-2 SGLT2 inhibitor that is approved to treat type 2 diabetes in adults, to improve neutrophil number and function. A study conducted by Veiga-Da-Cunha et al [ ] revealed the crucial function of glucosephosphate transporter in neutrophils, which clarifies the pathophysiology of neutropenia in GSD-Ib patients. In addition to G6P, G6PT transports the G6P structural analog 1,5-anhydroglucitolphosphate 1,5AG6P. Neutrophils lacking G6PT activity cannot transport 1,5AG6P from the cytosol into the endoplasmic reticulum, where it is normally dephosphorylated by G6PC3, a phosphatase in the membrane of the endoplasmic reticulum. Cytosolic accumulation of 1,5AG6P inhibits glucose phosphorylation by hexokinases that catalyzes the first step of glycolysis. As glycolysis is the sole energy source for mature neutrophils, depletion of intracellular G6P leads to a deficit in energy production which in turn results in neutrophil dysfunction and subsequent apoptosis. Empagliflozin inhibits renal SGLT2 leading to increased urinary excretion of 1,5AG. This leads to a reduction in the concentration of 1,5AG in the blood, thereby decreasing the cellular accumulation of toxic 1,5AG6P in neutrophils[ ]. Following the first report of successful repurposing of empagliflozin to treat neutropenia and neutrophil dysfunction in 4 patients with GSD-Ib, several case reports and case series have shown beneficial effects of this treatment approach on neutrophil number and function, inflammatory bowel disease, recurrent infections[ - ], oral and urogenital mucosal lesions, skin abscesses, anemia, wound healing, and dose reduction or even cessation of G-CSF therapy in GSD-Ib patients[ - ]. Despite a favorable safety profile in patients with GSD-Ib, there is a risk of hypoglycemia with SGLT2 inhibitors. A low dose at treatment initiation with careful titration to optimal dosing is recommended[ ]. Growing evidence suggests that empagliflozin is a candidate for first-line treatment of neutropenia and neutrophil dysfunction related symptoms in GSD-Ib patients. Another promising novel therapeutic strategy is gene therapy by using recombinant adeno-associated virus vectors. The use of a viral vector to administer G6Pase and hepatocyte transplantation are being investigated as potential treatments for GSD-I. Various animal models have shown an increase in hepatic G6Pase and G6PT activity, as well as improvements in metabolic parameters[ - ]. Multiple approaches have been explored for the integration of the G6Pase transgene into the host genome[ , ]. The successful correction of metabolic imbalances in animal models through gene therapy shows promising potential for future applications of gene therapy in humans. Glycogen debrancher enzyme has two independent catalytic activities; alpha-glucanotransferase and amylo-1,6-glucosidase, with the two catalytic sites being separated on the same polypeptide. Both catalytic activities are required for complete debranching enzyme activity[ ]. Deficient activity of these catalytic sites results in accumulation of glycogen with short outer chains, previously defined as limit-dextrins. Deficiency in glycogen debranching enzyme due to biallelic pathogenic variants in the AGL gene results in the harmful accumulation of abnormal glycogen in hepatocytes. The AGL gene was mapped to the chromosomal locus 1p21, and its nucleotide sequence was determined, revealing the existence of multiple tissue-specific isoforms[ , ]. GSD-III is inherited in an autosomal recessive manner. Certain populations have an increased prevalence due to a founder effect. The highest known GSD-III prevalence occurs in Inuit population in Nunavik about , c. There is currently limited evidence supporting a correlation between disease severity and pathogenic variants in the AGL gene, except for specific exon 3 variants c. It was suggested that in muscle isoforms of the AGL gene, alternative exon or translation initiation may not require exon 3, thereby resulting in normal enzyme activity in the muscle tissues of patients with GSD-IIIb who harbor an exon 3 deletion[ , ]. Recent evidence suggests that the presence of frameshift, nonsense, and splice site variants may lead to severe phenotypes. Differences in tissue expression of the deficient enzyme is responsible for the phenotypic variability observed in GSD-III patients[ ]. GSD-III is characterized by heterogeneous involvement of the liver, skeletal muscle, and cardiac muscle, leading to variable clinical presentations. Various subtypes are defined by the extent of tissue involvement. Two major subtypes of GSD-III have been identified. In a limited number of cases, it has been demonstrated that there is a selective loss of either glucosidase activity resulting in muscle involvement, referred to as GSD-IIIc or transferase activity resulting in both muscle and liver involvement, referred to as GSD-IIId [ , ]. Hepatomegaly, ketotic hypoglycemia, growth retardation and dyslipidemia hypertriglyceridemia are the dominant features of hepatic involvement in infancy and childhood. As gluconeogenesis is intact in GSD-III, fasting hypoglycemia tends to be milder than that seen in GSD-I. During infancy, serum hepatic transaminases are markedly elevated. Uric acid and lactate concentrations are relatively normal[ ]. Symptoms and laboratory findings related with liver involvement often improve with age and usually disappear after puberty[ , ]. However, liver disease can also be progressive resulting in liver fibrosis, cirrhosis, hepatic failure, and end-stage liver disease[ , ]. Hepatic fibrosis may occur as early as 1 year of age[ ]. Overt liver cirrhosis is not common and occurs rarely[ , ]. Hepatocellular carcinoma can develop as a long-term complication of liver cirrhosis, rather than transformation of an adenoma to carcinoma, as seen in GSD-I[ , ]. Children with failure to thrive often catch-up in height in adulthood with optimized, individualized dietary management. Muscle symptoms associated with GSD-III can manifest concurrently with liver disease or long after hepatic disorders or even after the resolution of hepatic symptoms during childhood. Nonetheless, a normal CK level does not entirely exclude the possibility of an underlying muscular disease[ , ]. The median age of onset of CK elevation was reported to be 10 years[ ]. Although muscle involvement becomes clinically more obvious later in life, mild muscle weakness on physical examination, motor developmental delay delayed sitting, delayed standing upright, delayed onset of walking , exercise intolerance, and hypotonia were reported in the majority of pediatric patients with GSD-III[ - ]. Muscle weakness and wasting may slowly progress and become severe by the third or fourth decade of life[ , ]. In a subset of adult patients with GSD-III, muscle symptoms can present in the absence of any clinical or previous evidence of liver dysfunction[ , ]. Muscle weakness, although minimal during childhood, is slowly progressive in nature and may become the predominant feature with significant permanent muscle weakness in adults with type IIIa disease[ ]. Although myopathy generally progresses slowly and is not severely debilitating, some patients may have severe muscle involvement leading to loss of ambulation[ ]. Myopathy can be proximal, distal, or more generalized. Exercise intolerance with muscle fatigue, cramps and pain are evident in more than half of patients[ , , ]. Bulbar or respiratory dysfunctions are rarely seen in GSD-III patients while no clinical involvement of facial or ocular muscles has been described in the literature[ ]. Cardiac involvement in GSD-III is variable. Cardiac involvement is present in most patients, with varying degrees of severity ranging from ventricular hypertrophy detected on electrocardiography to clinically apparent cardiomegaly[ ]. Mogahed et al [ ] reported that cardiac muscle involvement is less common and mostly subclinical in the pediatric age group. Sudden death has occasionally been reported[ ]. Patients with GSD-III may exhibit facial abnormalities such as indistinct philtral pillars, bow-shaped lips with a thin vermillion border, a depressed nasal bridge and a broad upturned nasal tip, and deep-set eyes, particularly in younger patients[ ]. Some individuals with GSD-III may have an increased risk of developing osteoporosis with reduced bone mineral density which, in part, may be due to suboptimal nutrition, the effects of metabolic abnormalities and muscle weakness[ 41 , , ]. Bone fractures due to osteopenia and osteoporosis were reported in patients with GSD-III[ ]. Polycystic ovary disease has been reported in women with GSD-III with no significant effect on fertility[ ]. Type 2 diabetes may occur during the course of the disease in adulthood[ ]. Michon et al [ ] reported global cognitive impairment in adult GSD-III patients as an underlying cause of psychological and attention deficits seen in this patient group. Liver histology shows uniform distension of hepatocytes secondary to glycogen accumulation. There is often septal formation, periportal and reticular fibrosis, fine microsteatosis, and less frequently, micronodular cirrhosis without inflammation or interface hepatitis. Skeletal muscle shows subsarcolemmal glycogen accumulation[ 12 ]. The diagnosis of GSD-III is made by identification of biallelic AGL pathogenic variants on molecular genetic testing. If the diagnosis cannot be established by genetic analysis, demonstrating enzyme deficiency in peripheral leukocytes or erythrocytes, cultured skin fibroblasts or in the liver or muscle tissue samples is necessary. A practice guideline was published by the American College of Medical Genetics and Genomics in providing recommendations on the diagnosis and management of the complications of GSD-III[ ]. The mainstay of GSD-III treatment is dietary intervention, which aims to maintain normal blood glucose levels while balancing macronutrient and total caloric intake. This is achieved by the avoidance of fasting, frequent meals enriched in complex carbohydrates and use of UCCS. Continuous enteral feeding may be needed in some cases. Sucrose, fructose, and lactose are not contraindicated unlike GSD-I. UCCS can be used as early as the first year of life to prevent hypoglycemia. As an alternative, Glycosade ® , an extended-release cornstarch, can also be used[ 87 ]. Caution must be exercised to avoid overtreating with cornstarch or carbohydrates, which may lead to excessive storage of glycogen in the liver and weight gain. In patients with myopathy, along with managing hypoglycemia, a high-protein diet is recommended as it prevents muscle protein breakdown during glucose deprivation, thereby preserving skeletal and cardiac muscle[ ]. A ketogenic diet alone or in combination with high protein and ketone bodies was also shown to ameliorate cardiomyopathy[ , ]. It has been shown that a high-fat, low-calorie and high-protein diet can reduce cardiomyopathy in individuals with GSD-III[ , ]. The beneficial effects on cardiac or skeletal muscle function of these ketogenic or high-fat diets are possibly related to the increased ketone bodies or fats as fuel sources, or reduced glycogen accumulation through decreased carbohydrate intake. Whether long-term muscular, cardiac, or even liver complications can be prevented by these dietary approaches warrants further studies[ ]. Liver transplantation corrects all liver related biochemical abnormalities but does not correct myopathy or cardiomyopathy[ , , ]. Detailed information about surveillance recommendations on hepatic, metabolic, musculoskeletal, cardiac, nutritional, and endocrine aspects of the disease can be found elsewhere[ ]. Gene therapy and gene-based therapeutic approaches are in development. Branching of the chains is essential to pack a very large number of glycosyl units into a relatively soluble spherical molecule. Without GBE, abnormal glycogen with fewer branching points and longer outer chains resembling an amylopectin-like structure polyglucosan accumulates in various tissues including hepatocytes and myocytes[ ]. The mapping of the GBE1 gene to chromosome 3p Notably, mutations in the same gene are also responsible for adult polyglucosan body disease. GSD-IV accounts for only 0. This rare disorder has a prevalence of to [ ]. GSD-IV exhibits significant clinical heterogeneity and phenotypic variability, partly due to variations in tissue involvement, which may be influenced by the presence of tissue-specific isozymes[ , ]. The liver is the primary organ affected, with the classical hepatic form appearing normal at birth but progressing rapidly to cirrhosis in early life, leading to liver failure and death between 3 to 5 years of age[ ]. Besides the complications of progressive cirrhosis including portal hypertension, ascites and esophageal varices, the development of hepatocellular carcinoma was also reported[ ]. In rare cases, the hepatic disease in GSD-IV may not progress or progress slowly[ ]. Patients with the non-progressive hepatic form may present with hepatosplenomegaly and mildly elevated liver transaminases, and experience normal growth. Liver size and transaminase levels may return to normal[ ]. Patients with the non-progressive hepatic form usually survive into adulthood. GSD-IV can present with multiple system involvement, with the enzyme deficiency in both liver and muscle[ ]. This form of the disease can manifest as peripheral myopathy with or without cardiomyopathy, neuropathy, and liver cirrhosis. Onset of the disease can be from the neonatal period to adulthood[ ]. The neuromuscular presentation can be divided into four groups based on age at onset[ ]. In the perinatal fetal form, which can lead to hydrops fetalis and polyhydramnios, arthrogryposis develops due to akinesia[ ]. Detection of cervical cystic hygroma during pregnancy may indicate the disease[ ]. Prenatal diagnosis can be performed by determining enzyme activity in cultured amniocytes or chorionic villi samples. Genetic studies can complement uncertain enzyme activity studies, such as equivocal results in prenatal fetal samples and in patients with higher levels of residual enzyme activity that overlap heterozygote levels[ ]. Mortality is unavoidable in the neonatal period. Liver cirrhosis or liver failure has not been reported. Severe hypotonia, hyporeflexia, cardiomyopathy, depressed respiration, and neuronal involvement are features of the congenital form of the disease[ , - ]. Liver disease is not severe, and the child dies in early infancy due to other reasons. The childhood neuromuscular form may start at any age with either myopathy or cardiomyopathy[ , ]. Presenting symptoms mainly include exercise intolerance, exertional dyspnea, and congestive heart failure in advanced stages. The disease can be confined to muscular tissue and serum CK level can be within the normal range. In the adult form, there is isolated myopathy or a multisystemic disease called adult polyglucosan body disease. Onset of symptoms can occur at any age during adulthood, usually after the age of 50, and may exhibit a resemblance to muscular dystrophies. Symptomatology includes progressive gait difficulty and proximal muscle weakness, which is more pronounced in the arms as compared to the legs. Both upper and lower motor neurons are affected in the disorder. The disease may manifest as pyramidal tetraparesis, peripheral neuropathy, early onset of neurogenic bladder, extrapyramidal symptoms, seizures, and cognitive dysfunction leading to dementia[ ]. The diagnosis can be established by enzyme activity assay in erythrocytes[ ]. Amylopectin-like inclusions are detected through ultrastructural examination of the central nervous system and skeletal muscle. These inclusions are intensely PAS-positive and diastase-resistant, both in neurons and muscular fibers[ ]. Magnetic resonance imaging shows white matter abnormalities[ ]. Liver biopsy can be diagnostic in patients with hepatic involvement[ ]. The histopathological evaluation of the liver reveals abnormal hepatocellular glycogen deposits in the form of PAS-positive, diastase-resistant inclusions. Ultrastructural examination with electron microscopy reveals accumulation of fibrillar aggregations that are typical of amylopectin. Typically, enzyme deficiency can be documented through diagnostic assays performed on hepatocytes, leukocytes, erythrocytes, and fibroblasts. However, patients with cardioskeletal myopathy may exhibit normal leukocyte enzyme activity[ ]. The diagnosis of GSD-IV can be confirmed through histopathological examination, detection of enzyme deficiency, and mutation analysis of the GBE1 gene. Genetic confirmation is recommended whenever possible in patients with suspected GSD-IV to provide more data for genotype-phenotype correlations in this extremely rare disease. The genotype-phenotype correlation remains unclear for GSD-IV and the same genetic defect may cause different clinical presentations in unrelated patients[ ]. Mutation analysis can also provide crucial diagnostic information in cases with equivocal results of biochemical analyses[ ]. Mutations with significant preservation of enzyme activity may be related with milder e. Hypoglycemia has traditionally been considered a late manifestation and generally develops due to hepatocellular dysfunction caused by progressive cirrhosis. At this stage of the disease, the biochemical profile of the patients is representative of what is observed in other causes of liver cirrhosis. No specific dietary and pharmacological treatments are available for GSD-IV. There is a lack of established guidelines based on either evidence or expert consensus for the dietary management of GSD-IV. Improvement in clinical, anthropometric, and laboratory parameters was reported with a high-protein and low-carbohydrate diet[ , ]. Derks et al [ ] recently reported improved clinical and biochemical outcomes after dietary interventions including a late evening meal, continuous nocturnal intragastric drip feeding, restriction of mono- and disaccharides, the addition of UCCS, and protein enrichment in patients with GSD-IV. Individual dietary plans should also aim to avoid hyperglycemia to minimize glycogen accumulation in the liver. At present, there is no effective therapeutic approach other than liver transplantation for GSD-IV patients who are affected by progressive liver disease. However, anecdotal reports indicate that liver transplantation may not alter the extrahepatic progression of GSD-IV[ ]. The presence of extrahepatic involvement, especially amylopectin storage in the myocardium, may lead to fatal complications following liver transplantation[ - ]. Careful assessment of cardiac function even in the absence of clinical decompensation or consideration of combined liver-heart transplantation is warranted for patients with GSD-IV[ ]. Liver transplantation may provide beneficial effects not only for patients with liver disease but also for those affected by muscular involvement in GSD-IV[ , , ]. This may be explained by systemic microchimerism donor cells presenting in various tissues of the liver recipient after liver allotransplantation and amelioration of pancellular enzyme deficiencies resulting in a decrease in amylopectin in other organ systems[ 12 ]. It has been suggested that the donor cells can transfer enzyme to the native enzyme-deficient cells[ ]. In recent years, animal studies have been conducted to prevent glycogen and polyglucosan body accumulation in GSD-IV patients, and GYS inhibitor guaiacol and DG11 are promising in this regard[ , ]. The molecular target of DG11 is the lysosomal membrane protein lysosome-associated membrane protein 1 LAMP1 , which enhances autolysosomal degradation of glycogen and lysosomal acidification. In the adult polyglucosan body disease mouse model, DG11 reduced polyglucosan and glycogen in brain, liver, heart, and peripheral nerve[ ]. GSD-VI was first reported by Hers[ ] in three patients with hepatomegaly, mild hypoglycemia, an increased glycogen content and deficient activity of glycogen phosphorylase in the liver in GSD-VI is a rare autosomal recessive genetic disease caused by deficiency of hepatic glycogen phosphorylase. At least three human glycogen phosphorylases exist including muscle, liver, and brain isoforms[ ]. In response to hypoglycemia, liver glycogen phosphorylase catalyzes the cleavage of glucosyl units from glycogen which results in the release of glucosephosphate. The glucosephosphate is subsequently converted to glucosephosphate. The PYGL gene is currently the only known genetic locus associated with the development of GSD-VI and was mapped to chromosome 14qq22 in [ ]. Incidence of the disease is estimated to be and believed to be underestimated due to nonspecific and variable phenotypes, and a paucity of cases confirmed by genetic testing[ ]. GSD-VI is more prevalent among the Mennonite community, with a prevalence of 1 in , representing the only known population at higher risk for the disease[ ]. GSD-VI is a disorder with broad clinical heterogeneity[ ]. Infants with liver phosphorylase deficiency mainly present with hepatomegaly and growth retardation. The condition typically has a benign course, and symptoms tend to improve as the child grows[ ]. Hepatomegaly usually normalizes by the second decade of life[ ]. The child shows mild to moderate ketotic hypoglycemia related to prolonged fasting, illness, or stressful conditions[ ]. As gluconeogenesis is intact in GSD-VI, hypoglycemia is usually mild. Despite gross hepatomegaly, the patient may be largely asymptomatic without hypoglycemia. However, there is a range of clinical severity in GSD-VI, with some patients experiencing severe and potentially life-threatening hypoglycemia. There is generally mild ketosis, growth retardation, abdominal distension due to marked hepatomegaly and mildly elevated levels of serum transaminases, triglycerides, and cholesterol. However, in patients with high residual enzyme activity, biochemical investigations may be normal[ , ]. Hypertriglyceridemia may persist despite treatment[ ]. A few patients showing mild muscular hypotonia, muscle weakness or developmental impairment were observed, but otherwise, no neurological symptoms were reported in the literature[ ]. Sleep difficulties and overnight irritability are common[ ]. In contrast to GSD-I, serum levels of lactic acid and uric acid are generally within the normal range[ 15 ]. However, in a recent clinical study including 56 GSD-VI patients, hyperuricemia was reported as a complication in adolescent and adult patients with GSD-VI, which indicates the need for long-term monitoring of uric acid in older GSD-VI patients[ ]. CK concentration is usually normal. In some patients, severe and recurrent hypoglycemia, pronounced hepatomegaly, and postprandial lactic acidosis have been reported[ ]. Recently, children with GSD-VI have been reported to present with only ketotic hypoglycemia as the sole manifestation of the disease, without the characteristic hepatomegaly[ ]. Mild cardiopathy has also been described for GSD-VI[ ]. The clinical picture of GSD-VI virtually overlaps with phosphorylase kinase PHK deficiency GSD-IX and the differential diagnosis includes other forms of GSDs associated with hepatomegaly and hypoglycemia, especially GSD-I and GSD-III[ ]. It is not possible to distinguish between GSD-VI and GSD-IX based on clinical or laboratory findings alone[ ]. Mutation analysis is the suggested method for the diagnosis of GSD-VI. A liver biopsy is not recommended to establish the diagnosis to avoid an invasive procedure. Excessive glycogen accumulation with structurally normal glycogen in the liver biopsy is consistent with GSD-VI. Fibrosis, mild steatosis, lobular inflammatory activity and periportal copper binding protein staining have also been reported in GSD-VI patients. Although it is possible to document glycogen phosphorylase deficiency in frozen liver biopsy tissue or blood cells including leukocytes and erythrocytes, normal in vitro residual enzyme activity may be seen and prevents establishment of a definitive diagnosis by an enzyme assay alone in some patients[ , ]. In GSD-VI, nutrition therapy aims to improve metabolic control and prevent primary manifestations such as hypoglycemia, ketosis, and hepatomegaly, as well as secondary complications including delayed puberty, short stature, and cirrhosis. The aim of the therapeutic approach is to achieve euglycemia and normoketosis by administration of the appropriate doses of cornstarch. An extended-release corn starch derived from waxy maize, marketed as Glycosade ® , has been found to have a positive impact in delaying overnight hypoglycemia in children over 5 years of age and adults[ 87 ]. Some individuals with GSD-VI may not require any treatment. GSD-VI usually has a benign disease course. However, focal nodular hyperplasia, fibrosis, cirrhosis, and a degeneration to hepatocellular carcinoma have been reported in some patients[ - ]. Cirrhosis has been reported in patients as young as preschool age, even within the second year of life[ ]. Based on these findings, aggressive treatment of GSD-VI has recently been suggested to maintain optimal metabolic control and prevent long-term complications[ ]. Long-term monitoring of hepatic function is also recommended[ ]. Glucagon and epinephrine play a critical role in the regulation of glycogenolysis by activation of adenylate cyclase which leads to an increase in the cytosolic concentration of cyclic adenosine monophosphate cAMP. The increased level of cAMP activates cAMP-dependent protein kinase which activates PHK. PHK is a heterotetramer composed of 4 different subunits α, β, γ, and δ. Each subunit is encoded by different genes that are located on different chromosomes and differentially expressed in a variety of tissues[ ]. α and β subunits have regulatory functions, the γ subunit contains the catalytic site, and δ is a calmodulin protein[ ]. Investigational mRNA Treatment. Read More. Press Release. Learn More. Join Mailing List. Tell A Friend. Donate Now. Upcoming Events. View Current Fundraisers. Start A Fundraiser. When the enzyme deficiency affects the liver, it leads to low blood glucose levels also called hypoglycemia during periods of fasting between meals or at night. GSD is hereditary, meaning it is passed down from parents to children. For most types of GSD, both parents are unaffected carriers, meaning they carry one copy of a misspelled gene that can cause GSD paired with a normal copy of the gene. When both parents pass the misspelled gene to a child, the child has no normal copy of that gene and therefore develops GSD. In most cases GSD is diagnosed within the first year of life, but in some cases the diagnosis may not be made until later in childhood. Many different enzymes are used by the body to process glycogen. As a result, there are several types of GSD. This type of GSD does not cause hypoglycemia. A thorough medical history can also lead the doctor to suspect GSD since it is inherited. Other diagnostic tests may include:. Each type of GSD centers on a certain enzyme or set of enzymes involved in glycogen storage or break down. GSD mostly affects the liver and the muscles, but some types cause problems in other areas of the body as well. Types of GSD with their alternative names and the parts of the body they affect most include:. GSD types VI and IX can have very mild symptoms and may be underdiagnosed or not diagnosed until adulthood. Currently, there is no cure for GSD. Treatment will vary depending on what type of GSD your child has; however, the overall goal is to maintain the proper level of glucose in the blood so cells have the fuel they need to prevent long-term complications. Until the early s, children with GSDs had few treatment options and none were very helpful. Then it was discovered that ingesting uncooked cornstarch regularly throughout the day helped these children maintain a steady, safe glucose level. Cornstarch is a complex carbohydrate that is difficult for the body to digest; therefore it acts as a slow release carbohydrate and maintains normal blood glucose levels for a longer period of time than most carbohydrates in food. Cornstarch therapy is combined with frequent meals eating every two to four hours of a diet that restricts sucrose table sugar , fructose sugar found in fruits and lactose only for those with GSD I. Typically, this means no fruit, juice, milk or sweets cookies, cakes, candy, ice cream, etc. |
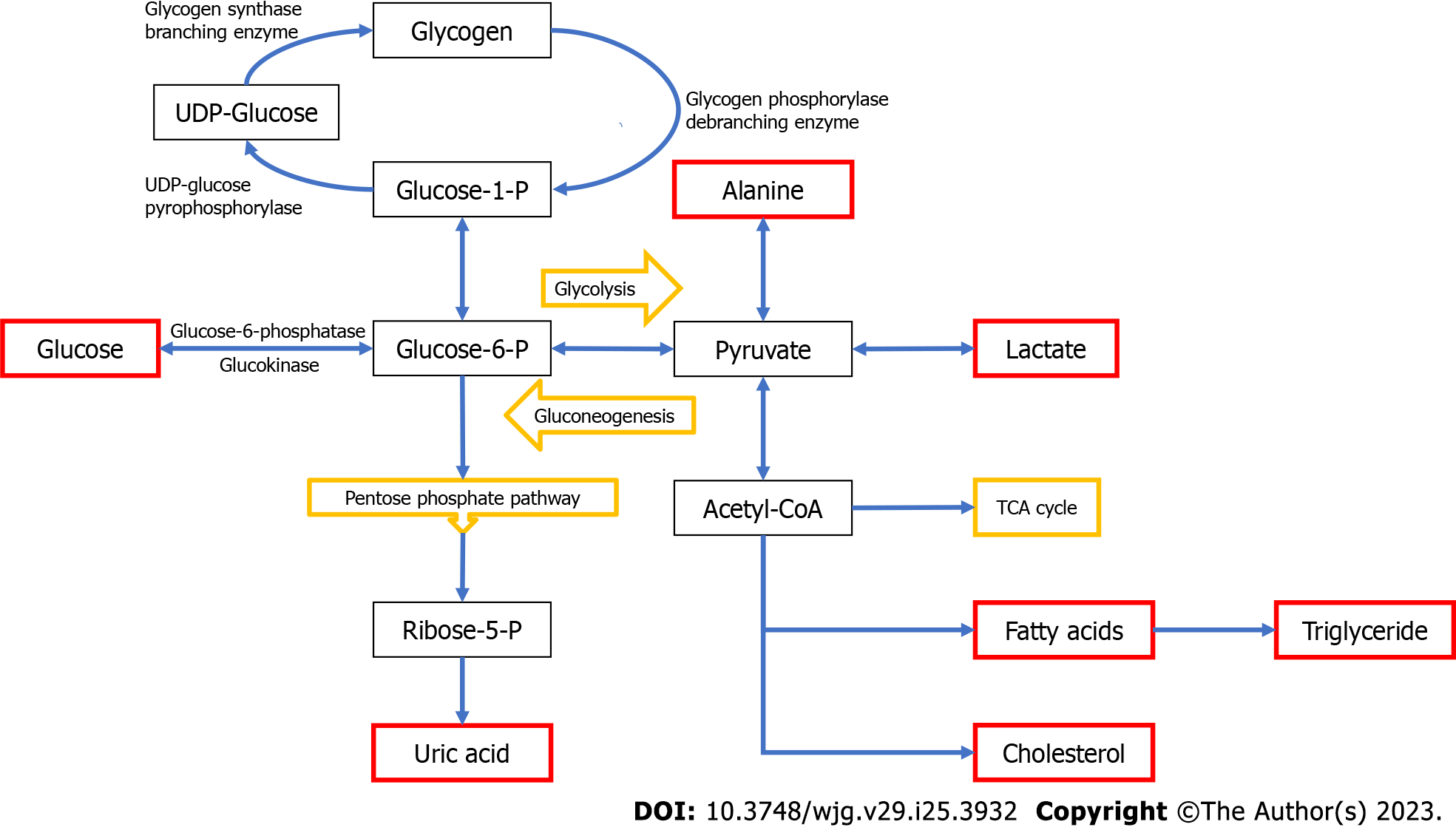
Ich denke, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.