
Aging and training adaptations -
Article CAS PubMed PubMed Central Google Scholar. Cruz-Jentoft, A. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48 , Article PubMed PubMed Central Google Scholar. Gingrich, A. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients.
BMC Geriatr. Cesari, M. Sarcopenia and physical frailty: two sides of the same coin. Aging Neurosci. Larsson, L. Muscle strength and speed of movement in relation to age and muscle morphology.
Physiol 46 , — CAS PubMed Google Scholar. Holloszy, J. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Ageing Dev. Article CAS PubMed Google Scholar. Casati, M. The biological foundations of sarcopenia: established and promising markers.
Lausanne 6 , Article Google Scholar. Cartee, G. Exercise promotes healthy aging of skeletal muscle. Cell Metab. Gonzalez-Freire, M.
Reconsidering the role of mitochondria in aging. A Biol. Short, K. Decline in skeletal muscle mitochondrial function with aging in humans. Natl Acad. USA , — Gouspillou, G. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13 , 39—48 Joseph, A. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging.
PLoS One 8 , e Conley, K. Oxidative capacity and ageing in human muscle. Chabi, B. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7 , 2—12 Rasmussen, B. Insulin resistance of muscle protein metabolism in aging. FASEB J 20 , — Cuthbertson, D.
Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19 , — Dickinson, J. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Sport Sci. Rev 41 , — Age and aerobic exercise training effects on whole body and muscle protein metabolism.
Goodpaster, B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Uchitomi, R. Metabolomic analysis of skeletal muscle in aged mice. Article PubMed PubMed Central CAS Google Scholar. Booth, F. Lack of exercise is a major cause of chronic diseases.
Navas-Enamorado, I. Influence of anaerobic and aerobic exercise on age-related pathways in skeletal muscle. Ageing Res. Carraro, F. Effect of exercise and recovery on muscle protein synthesis in human subjects. Harber, M. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles.
Fujita, S. Diabetes 56 , — Aon, M. Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan. NPJ Aging Mech. Dis 7 , 1 Konopka, A. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training.
Sci 69 , — Greggio, C. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 25 , — Hawley, J. Integrative biology of exercise.
Cell , — Hargreaves, M. Skeletal muscle energy metabolism during exercise. Bennie, J. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among , U.
Justine, M. Barriers to participation in physical activity and exercise among middle-aged and elderly individuals. Singapore Med. Article PubMed Google Scholar. Gibala, M. Sprinting toward fitness. Petr, M. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice.
eLife 10 , e Houtkooper, R. The metabolic footprint of aging in mice. Mina, A. CalR: A web-based analysis tool for indirect calorimetry experiments. Cell Metab 28 , — e1 Fernández-Verdejo, R. Progress and challenges in analyzing rodent energy expenditure.
Methods 16 , — Article PubMed CAS Google Scholar. Müller, T. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Metab 3 , — Leduc-Gaudet, J. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6 , — Faitg, J. Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles.
Zhang, Z. Fis1 deficiencies differentially affect mitochondrial quality in skeletal muscle. Mitochondrion 49 , — Camara, A. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target.
Kabeya, Y. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19 , — Alcázar-Fabra, M.
Coenzyme Q biosynthesis and its role in the respiratory chain structure. Acta , — Guarás, A. Cell Rep 15 , — Wasserman, D. Hepatic fuel metabolism during muscular work: role and regulation.
Physiol , E—E Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17 , — Mitchell, S. Effects of sex, strain, and energy intake on hallmarks of aging in mice.
Marriott, C. High-intensity interval training in older adults: a scoping review. Sports Med. Open 7 , 49 Robinson, M. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Adelnia, F. Proteomic signatures of in vivo muscle oxidative capacity in healthy adults.
Aging Cell 19 , e Sato, S. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. e4 Campbell, J. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan.
Nieman, D. Sport Health Sci. Cerqueira, E. Inflammatory effects of high and moderate intensity exercise—a systematic review. Figueiredo, P. The role of mitochondria in aging of skeletal muscle.
Biogerontology 9 , 67—84 Aging impairs skeletal muscle mitochondrial bioenergetic function. Mercken, E. Conserved and species-specific molecular denominators in mammalian skeletal muscle aging.
Hunter, S. The relevance of sex differences in performance fatigability. Sports Exerc. Melo, L. Effect of endurance exercise training on liver gene expression in male and female mice.
Google Scholar. Benavides, A. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Takahashi, H. NMR Biomed. Thyfault, J. Sanford, J.
Molecular transducers of physical activity consortium. Molecular transducers of physical activity consortium MoTrPAC : Mapping the dynamic responses to exercise. Palliyaguru, D. Study of longitudinal aging in mice: presentation of experimental techniques. McMullan, R. Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging.
Ivy, A. A unique mouse model of early life exercise enables hippocampal memory and synaptic plasticity. Ubaida-Mohien, C.
Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. eLife 8 , e Sato, T.
A modified method for lead staining of thin sections. Electron Microsc. Tokyo 17 , — CAS Google Scholar. Spinazzi, M. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Kim, S.
PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics 6 , Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Fiehn, O. Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J 53 , — Download references.
Work in JMV laboratory was supported by the Spanish Ministerio de Economía y Competitividad MINECO grant BFUR, Ministerio de Ciencia, Innovación y Universidades MICIU grant RTIB-I00, Spanish Junta de Andalucía grants PRT, R and BIO, the FEDER Funding Program from the European Union, and Universidad de Córdoba.
SRL held a FPI predoctoral contract funded by MINECO reference BES We are grateful to Dawn Nines, Dawn Phillips-Boyer, Sarah J. Eckroth, and Sharon E. Ensor, Research Support Specialists from Comparative Medicine Section at NIA and Kelly Government Solutions, for their excellent animal care.
We thank Drs. Elin Lehrmann and Yongqing Zhang for their contribution in carrying out microarray experiments and associated data analysis. The authors also wish to thank the personnel from the Servicio Centralizado de Apoyo a la Investigación SCAI; University of Córdoba for technical support with the transmission electron microscope.
Present address: Translational Medicine Section, Akouos, Inc. These authors contributed equally: Michel Bernier, Ignacio Navas Enamorado, Mari Carmen Gómez-Cabrera, Sonia Cortassa, Miguel A. Translational Gerontology Branch, National Institute on Aging, NIH, Baltimore, MD, , USA.
Michel Bernier, Ignacio Navas Enamorado, Miguel Calvo-Rubio, Nathan L. Price, Sarah J. Mitchell, Kelsey N. Murt, Krystle Kalafut, Miguel A. Departamento de Biología Celular, Fisiología e Inmunología, Campus de Excelencia Internacional Agroalimentario, ceiA3, Universidad de Córdoba, Campus de Rabanales, Edificio Severo Ochoa, 3ª planta, , Córdoba, Spain.
Servicio de Fisiopatología Celular y Bioenergética, Universidad Pablo de Olavide, Sevilla, , Spain. Department of Orthopaedics, University of Maryland School of Medicine, Baltimore, MD, , USA. Katrina M. Williams, Christopher W. Centro Andaluz de Biología del Desarrollo and CIBERER, Instituto de Salud Carlos III, Universidad Pablo de Olavide - CSIC — JA, Sevilla, , Spain.
Laboratory of Cardiovascular Science, National Institute on Aging, NIH, Baltimore, MD, , USA. You can also search for this author in PubMed Google Scholar.
contributed equally as first co-authors. G-C: data curation, formal analysis, figure creation; writing—original draft, review, and editing; I.
All authors approved the content of the submitted manuscript. Correspondence to Rafael de Cabo. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Bernier, M. Age-dependent impact of two exercise training regimens on genomic and metabolic remodeling in skeletal muscle and liver of male mice.
npj Aging 8 , 8 Download citation. Received : 26 May Accepted : 11 May Published : 27 June Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature.
nature npj aging articles article. Download PDF. Subjects Ageing Metabolism. Abstract Skeletal muscle adapts to different exercise training modalities with age; however, the impact of both variables at the systemic and tissue levels is not fully understood.
Introduction Aging is characterized by a progressive deterioration in organismal homeostasis that underlies many chronic diseases in the elderly. Full size image. Concluding remarks An apparent genetic and metabolic reprogramming triggered by exercise training happens in mice subjected to moderate intensity, long-term exercise training.
In vivo metabolic assessment At the conclusion of the 4-week training period, the metabolic rate of each mouse was assessed by indirect calorimetry in open-circuit Oxymax chambers using the Comprehensive Lab Animal Monitoring System CLAMS; Columbus Instruments, Columbus, OH, USA. Electron microscope analysis After dissection, samples from gastrocnemius were quickly washed in 0.
Several quantitative analyses were performed as follows: 1. Determination of ETC activities Activities of NADH:coenzyme Q1 oxidoreductase complex I , succinate dehydrogenase complex II , ubiquinol:cytochrome c oxidoreductase complex III , NADH:cytochrome c reductase complex I—III , succinate:cytochrome c reductase complex II—III , cytochrome c oxidase complex IV , and citrate synthase CS were determined in gastrocnemius skeletal muscle from mice by spectrophotometric assays Microarray analysis Gastrocnemius RNA was isolated using a RNeasy Mini kit Qiagen, Germantown, MD, USA.
Quantitative real-time PCR Total RNA was isolated from gastrocnemius muscle using the Trizol reagent Invitrogen. Metabolomics analysis Metabolomic analysis was performed by the West Coast Metabolomics Center at UC Davis Davis, CA in liver and serum of 3-h fasted animals.
Hierarchical clustering Physiological, biochemical, and serum metabolomics data were Z-score normalized across the six experimental groups. Statistical analysis No statistical methods were used to predetermine sample size. References Schultz, M. Article CAS PubMed PubMed Central Google Scholar Cruz-Jentoft, A.
Article PubMed PubMed Central Google Scholar Gingrich, A. Article PubMed PubMed Central Google Scholar Cesari, M. Article PubMed PubMed Central Google Scholar Larsson, L.
CAS PubMed Google Scholar Holloszy, J. Article CAS PubMed Google Scholar Casati, M. Article Google Scholar Cartee, G.
Article CAS PubMed PubMed Central Google Scholar Gonzalez-Freire, M. Article CAS PubMed PubMed Central Google Scholar Short, K. Article CAS PubMed PubMed Central Google Scholar Gouspillou, G. Article CAS PubMed Google Scholar Joseph, A. Article CAS PubMed PubMed Central Google Scholar Conley, K.
Article CAS PubMed PubMed Central Google Scholar Chabi, B. Article CAS PubMed Google Scholar Rasmussen, B. Article CAS PubMed Google Scholar Cuthbertson, D. Article CAS PubMed Google Scholar Dickinson, J. Article PubMed PubMed Central Google Scholar Short, K.
Article CAS PubMed Google Scholar Goodpaster, B. Article CAS PubMed Google Scholar Uchitomi, R. Article PubMed PubMed Central CAS Google Scholar Booth, F.
Article PubMed PubMed Central Google Scholar Navas-Enamorado, I. Article PubMed PubMed Central Google Scholar Carraro, F. CAS PubMed Google Scholar Harber, M. Article CAS PubMed Google Scholar Fujita, S. Article CAS PubMed Google Scholar Aon, M. Article CAS PubMed PubMed Central Google Scholar Konopka, A.
Article CAS PubMed Google Scholar Greggio, C. Article CAS PubMed Google Scholar Hawley, J. Article CAS PubMed Google Scholar Hargreaves, M. Article CAS PubMed Google Scholar Bennie, J. Article PubMed PubMed Central Google Scholar Justine, M. Article PubMed Google Scholar Gibala, M. Article CAS PubMed Google Scholar Petr, M.
Article CAS PubMed PubMed Central Google Scholar Houtkooper, R. Article PubMed PubMed Central CAS Google Scholar Mina, A. Article CAS PubMed PubMed Central Google Scholar Fernández-Verdejo, R.
Article PubMed CAS Google Scholar Müller, T. Article PubMed Google Scholar Leduc-Gaudet, J. Article PubMed PubMed Central Google Scholar Faitg, J. Article PubMed PubMed Central Google Scholar Zhang, Z. Article CAS PubMed PubMed Central Google Scholar Camara, A.
Article PubMed PubMed Central Google Scholar Kabeya, Y. Article CAS PubMed PubMed Central Google Scholar Alcázar-Fabra, M. Article PubMed CAS Google Scholar Guarás, A. Article PubMed CAS Google Scholar Wasserman, D. CAS PubMed Google Scholar Egan, B.
Article CAS PubMed Google Scholar Mitchell, S. Article CAS PubMed PubMed Central Google Scholar Marriott, C. Article PubMed PubMed Central Google Scholar Robinson, M. Article CAS PubMed PubMed Central Google Scholar Adelnia, F.
Article CAS PubMed PubMed Central Google Scholar Sato, S. Article CAS PubMed Google Scholar Campbell, J. Article PubMed PubMed Central CAS Google Scholar Nieman, D. Article PubMed Google Scholar Cerqueira, E. Article PubMed PubMed Central Google Scholar Figueiredo, P.
Article CAS PubMed Google Scholar Figueiredo, P. Article PubMed CAS Google Scholar Mercken, E. Article PubMed PubMed Central CAS Google Scholar Hunter, S. Article PubMed PubMed Central Google Scholar Melo, L. Google Scholar Benavides, A. CAS PubMed Google Scholar Takahashi, H. Article CAS PubMed PubMed Central Google Scholar Sanford, J.
Article CAS PubMed PubMed Central Google Scholar Palliyaguru, D. Article PubMed Google Scholar McMullan, R. Article PubMed PubMed Central Google Scholar Ivy, A. Article CAS PubMed PubMed Central Google Scholar Ubaida-Mohien, C. Article PubMed PubMed Central Google Scholar Sato, T.
CAS Google Scholar Spinazzi, M. Article CAS PubMed Google Scholar Kim, S. Article PubMed PubMed Central CAS Google Scholar Mitchell, S. Article CAS PubMed PubMed Central Google Scholar Fiehn, O. Article CAS PubMed Google Scholar Download references.
Funding Open Access funding provided by the National Institutes of Health NIH. Author information Author notes Ignacio Navas Enamorado Present address: Translational Medicine Section, Akouos, Inc.
Privacy Copyright. Skip to main content. Resistance Training Adaptations in Older Adults Exhibit Task Specificity. Author Jason Pagan , University of Central Florida.
Notes If this is your thesis or dissertation, and want to learn how to access it or for more information about readership statistics, contact us at STARS ucf. Graduation Date Degree Master of Science M. College College of Health Professions and Sciences. Department Kinesiology and Rehabilitation Sciences.
Degree Program Kinesiology. Identifier CFE; DP Release Date August Length of Campus-only Access 5 years. Access Status Masters Thesis Campus-only Access. STARS Citation Pagan, Jason, "Resistance Training Adaptations in Older Adults Exhibit Task Specificity" Restricted to the UCF community until August ; it will then be open access.
DOWNLOADS Since August 24,
Background: To investigate yraining anatomical Abing area and volume of quadriceps Mental resilience in sports triceps surae muscles were affected by ageing, and by trainign training in older and Targeted weight loss men, in vivo. Each group received the exact traininng training volume Agnig triceps surae anc quadriceps Targeted weight loss Adaptatiojs x Sets adaptatiins Intensity. The Aging and training adaptations polynomial regression equations for each of anatomical cross-sectional area-muscle length curves were used to calculate muscle volume contractile content before and after 12 weeks using magnetic resonance imaging scans. Results: Only Rectus femoris and medial gastrocnemius muscle showed a higher relative anatomical cross-sectional area in the young than the elderly on the proximal end. O55 demonstrated a greater increase on average gains compared to Y55, while no difference between O55 and O80 was observed. Conclusions: Muscle loss with aging is region-specific for some muscles and uniform for others. Equivalent strength training volume at moderate or high intensities increased muscle volume with no differences in muscle volume gains for old men.Video
Effects of Exercise on Well-being of Older AdultsAging and training adaptations -
Public Health , Fragala M. Resistance Training for Older Adults. Strength Cond. Franklin S. Hemodynamic Patterns of Age-Related Changes in Blood Pressure. Circulation 96, — Fridén J. Structural and Mechanical Basis of Exercise-Induced Muscle Injury. Sports Exerc. Fujimoto N. Cardiovascular Effects of 1 Year of Progressive and Vigorous Exercise Training in Previously Sedentary Individuals Older Than 65 Years of Age.
Circulation , — Fusi S. Cardioventilatory Responses during Real or Imagined Walking at Low Speed. Gamba P. Oxidized Cholesterol as the Driving Force behind the Development of Alzheimer's Disease. Aging Neurosci. Garcia-Polite F. Pulsatility and High Shear Stress Deteriorate Barrier Phenotype in Brain Microvascular Endothelium.
Blood Flow. Genova M. The Interplay between Respiratory Supercomplexes and ROS in Aging. Antioxidants Redox Signal. Girousse A.
Surplus Fat Rapidly Increases Fat Oxidation and Insulin Resistance in Lipodystrophic Mice. Gonzales J. Association between Exercise Hemodynamics and Changes in Local Vascular Function Following Acute Exercise.
Green D. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. The Impact of 6-Month Land versus Water Walking on Cerebrovascular Function in the Aging Brain.
Greig C. Blunting of Adaptive Responses to Resistance Exercise Training in Women over 75y. Grevendonk L. Impact of Aging and Exercise on Skeletal Muscle Mitochondrial Capacity, Energy Metabolism, and Physical Function. Guo C.
Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Hager K. Interleukin-6 and Selected Plasma Proteins in Healthy Persons of Different Ages. Aging 15, — Harada C. Normal Cognitive Aging. Geriatric Med. Haran P. Role and Potential Mechanisms of Anabolic Resistance in Sarcopenia.
Cachexia Sarcopenia Muscle 3, — Havel R. Hawley J. Integrative Biology of Exercise. Maximizing Cellular Adaptation to Endurance Exercise in Skeletal Muscle.
Cell Metab. Hayden K. Epidemiology of Cognitive Aging and Alzheimer's Disease: Contributions of the Cache County Utah Study of Memory, Health and Aging. Heckmann J.
Transcranial Doppler Sonography-Ergometer Test for the Non-invasive Assessment of Cerebrovascular Autoregulation in Humans. Neurological Sci. Heled Y. Heat Acclimation and Performance in Hypoxic Conditions. Space Environ. Henskens L. Increased Aortic Pulse Wave Velocity Is Associated with Silent Cerebral Small-Vessel Disease in Hypertensive Patients.
Hypertension 52, — Hilbert J. The Effects of Massage on Delayed Onset Muscle Soreness. Hogan C. Exercise Holds Immediate Benefits for Affect and Cognition in Younger and Older Adults. Aging 28, — Howatson G.
The Prevention and Treatment of Exercise-Induced Muscle Damage. Jakovljevic D. Physical Activity and Cardiovascular Aging: Physiological and Molecular Insights. Jandackova V. Jaha 5, e Jiang Z. Blood Flow Velocity in the Common Carotid Artery in Humans during Graded Exercise on a Treadmill.
Jufri N. Mechanical Stretch: Physiological and Pathological Implications for Human Vascular Endothelial Cells. Cell 7, 8. Jurva J. The Effect of Exertional Hypertension Evoked by Weight Lifting on Vascular Endothelial Function. Kaess B. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension.
JAMA , — Kanaley J. Cortisol and Growth Hormone Responses to Exercise at Different Times of Day1. Metabolism 86, — Keith L. Exercise Aortic Stiffness: Reproducibility and Relation to End-Organ Damage in Men.
Kennedy B. Geroscience: Linking Aging to Chronic Disease. Kim J. Tissue-specific Overexpression of Lipoprotein Lipase Causes Tissue-specific Insulin Resistance. Kirova A. Working Memory and Executive Function Decline across Normal Aging, Mild Cognitive Impairment, and Alzheimer's Disease.
BioMed Res. Effects of Age on Plasma Glucose Levels in Non-diabetic Hong Kong Chinese. Koopman R. Aging, Exercise, and Muscle Protein Metabolism.
Kudryavtseva A. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 7, — Kumar V. Human Muscle Protein Synthesis and Breakdown during and after Exercise. Lakatta E. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises.
Lambourne K. The Effect of Exercise-Induced Arousal on Cognitive Task Performance: a Meta-Regression Analysis. Brain Res. LaRocca T.
Nutrition and Other Lifestyle Influences on Arterial Aging. Ageing Res. Lavin K. Effects of Aging and Lifelong Aerobic Exercise on Basal and Exercise-Induced Inflammation. Physiology , 87— Effects of Aging and Lifelong Aerobic Exercise on Basal and Exercise-Induced Inflammation in Women. Lefferts W.
Age, Sex, and the Vascular Contributors to Cerebral Pulsatility and Pulsatile Damping. Liberale L. Inflamm-ageing: the Role of Inflammation in Age-dependent Cardiovascular Disease. Lovic D. Left Ventricular Hypertrophy in Athletes and Hypertensive Patients. Luca M. Oxidative Stress-Related Endothelial Damage in Vascular Depression and Vascular Cognitive Impairment: Beneficial Effects of Aerobic Physical Exercise.
Lucia C. New Insights in Cardiac β-Adrenergic Signaling during Heart Failure and Aging. Luttrell M. Effect of Age and Acute Exercise on Circulating Angioregulatory Factors. Maciejczyk M. Energy Expenditure and Physiological Responses during Walking on a Treadmill and Moving on the Torqway Vehicle.
Acta Bioeng. MacNeil L. Acute, Exercise-Induced Alterations in Cytokines and Chemokines in the Blood Distinguish Physically Active and Sedentary Aging. Magnusson I. Increased Rate of Gluconeogenesis in Type II Diabetes Mellitus.
A 13C Nuclear Magnetic Resonance Study. Małkiewicz M. Blood-brain Barrier Permeability and Physical Exercise. Neuroinflammation 16, Marseglia L. Oxidative Stress in Obesity: a Critical Component in Human Diseases.
Ijms 16, — McDonagh B. Application of Redox Proteomics to Skeletal Muscle Aging and Exercise. McGee S. Exercise Adaptations: Molecular Mechanisms and Potential Targets for Therapeutic Benefit.
McPhee J. Physical Activity in Older Age: Perspectives for Healthy Ageing and Frailty. Biogerontology 17, — Michael S. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals-A Review.
Millgård J. Acute Hypertension Impairs Endothelium-dependent Vasodilatation. Mitchell G. Arterial Stiffness and Hypertension. Hypertension 64, — Morishima T. Maintenance of Endothelial Function Following Acute Resistance Exercise in Females Is Associated with a Tempered Blood Pressure Response.
Murphy S. Mortality in the United States, NCHS Data Brief. Google Scholar. Najjar S. Pulse Wave Velocity Is an Independent Predictor of the Longitudinal Increase in Systolic Blood Pressure and of Incident Hypertension in the Baltimore Longitudinal Study of Aging.
Neilan T. Myocardial Injury and Ventricular Dysfunction Related to Training Levels Among Nonelite Participants in the Boston Marathon. Nordin T. Acute Exercise Increases Resistance to Oxidative Stress in Young but Not Older Adults.
Age 36, O'Keefe J. Potential Adverse Cardiovascular Effects from Excessive Endurance Exercise. Mayo Clin. Ogoh S. Middle Cerebral Artery Flow Velocity and Pulse Pressure during Dynamic Exercise in Humans.
Training for Longevity: The Reverse J-Curve for Exercise. Örlander J. Low Intensity Training, Inactivity and Resumed Training in Sedentary Men.
Acta Physiol. Osawa Y. Physical Activity and All-Cause Mortality and Mediators of the Association in the Very Old. Paliwal D. Mitochondrial Pathway Polygenic Risk Scores Are Associated with Alzheimer's Disease.
Aging , — Peake J. Characterization of Inflammatory Responses to Eccentric Exercise in Humans. Pedersen B. Exercise as Medicine - Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases.
Sports 25 Suppl. Petriz B. The Effects of Acute and Chronic Exercise on Skeletal Muscle Proteome. Pfeifer M.
Differential Changes of Autonomic Nervous System Function with Age in Man. Pomella N. Noninvasive Assessment of the Common Carotid Artery Hemodynamics with Increasing Exercise Work Rate Using Wave Intensity Analysis.
Powers S. Exercise-induced Oxidative Stress: Friend or Foe? Sport Health Sci. Exercise-induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Exercise-induced Oxidative Stress: Past, Present and Future. Prawiradilaga R. Acute Response of Biochemical Bone Turnover Markers and the Associated Ground Reaction Forces to High-Impact Exercise in Postmenopausal Women.
bs 37, 41— Quinlan C. Sites of Reactive Oxygen Species Generation by Mitochondria Oxidizing Different Substrates. Redox Biol. Rajanayagam J. Intense Endurance Exercise: A Potential Risk Factor in the Development of Heart Disease.
Cureus 13, e Ranallo R. Lipid Metabolism during Exercise. Reaven G. Impaired Insulin-Mediated Inhibition of Lipolysis and Glucose Transport with Aging. Determinants of Pulse Wave Velocity in Healthy People and in the Presence of Cardiovascular Risk Factors: 'establishing Normal and Reference Values'.
Renzi C. Effects of Leg Blood Flow Restriction during Walking on Cardiovascular Function. Reynolds T. Effect of Age on Skeletal Muscle Proteolysis in Extensor Digitorum Longus Muscles of B6C3F1 Mice. Riggs B. A Population-Based Assessment of Rates of Bone Loss at Multiple Skeletal Sites: Evidence for Substantial Trabecular Bone Loss in Young Adult Women and Men.
Bone Min. Rizza R. Pathogenesis of Fasting and Postprandial Hyperglycemia in Type 2 Diabetes: Implications for Therapy. Diabetes 59, — Roh H. Effect of Exercise Intensity on Neurotrophic Factors and Blood-Brain Barrier Permeability Induced by Oxidative-Nitrosative Stress in Male College Students.
Sport Nutr. Romijn J. Regulation of Endogenous Fat and Carbohydrate Metabolism in Relation to Exercise Intensity and Duration. Rosenberg A. Aging Reduces Cerebral Blood Flow Regulation Following an Acute Hypertensive Stimulus.
Ross R. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association.
Circulation , e—e Rowell L. Neural Control of Muscle Blood Flow: Importance during Dynamic Exercise. Rowland T. Left Ventricular Response to Dynamic Exercise in Young Cyclists. Russ D. Is Skeletal Muscle Oxidative Capacity Decreased in Old Age? Sabbahi A. Peak Blood Pressure Responses during Maximum Cardiopulmonary Exercise Testing.
Hypertension 71, — Sallis R. Exercise Is Medicine and Physicians Need to Prescribe it!. Exercise Is Medicine: a Call to Action for Physicians to Assess and Prescribe Exercise.
Physician Sportsmed. Salthouse T. Consequences of Age-Related Cognitive Declines. Satrústegui J. Increased Basal Gluconeogenesis in the Aged Rat. FEBS Lett. Seals D. Aerobic Exercise Training and Vascular Function with Ageing in Healthy Men and Women.
Senoner T. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 11, Sepanlou S. Effectiveness and Feasibility of Lifestyle and Low-Cost Pharmacologic Interventions in the Prevention of Chronic Diseases: a Review.
Sergiev P. Theories of Aging: an Ever-Evolving Field. Acta Naturae 7, 9— Shanker Sharma H. Increased Blood-Brain Barrier Permeability Following Acute Short-Term Swimming Exercise in Conscious Normotensive Young Rats. Sharman J. The Effect of Exercise on Large Artery Haemodynamics in Healthy Young Men.
Shi R. Dose-response Association between Physical Activity and Clustering of Modifiable Cardiovascular Risk Factors Among 26, Chinese Adults. BMC Cardiovasc. Shigenaga M. Oxidative Damage and Mitochondrial Decay in Aging. Slivka D. Physiology-Regulatory, Integr. Physiology , R—R Spitler K.
Aging and Plasma Triglyceride Metabolism. Lipid Res. Stock J. Strait J. Aging-associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Stratton J. Cardiovascular Responses to Exercise. Effects of Aging and Exercise Training in Healthy Men.
Circulation 89, — Studinger P. Static and Dynamic Changes in Carotid Artery Diameter in Humans during and after Strenuous Exercise. Sugawara J. Impact of Leg Blood Flow Restriction during Walking on Central Arterial Hemodynamics. Sundquist K. Frequent and Occasional Physical Activity in the Elderly.
Tagliaferri C. Muscle and Bone, Two Interconnected Tissues. Tanaka H. Antiaging Effects of Aerobic Exercise on Systemic Arteries. Hypertension 74, — Tarumi T. Cerebral Hemodynamics in Normal Aging: Central Artery Stiffness, Wave Reflection, and Pressure Pulsatility.
Tipton K. Exercise-induced Changes in Protein Metabolism. Tonkonogi M. Physical Exercise and Mitochondrial Function in Human Skeletal Muscle. Sport Sci. Townsend R. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness.
Hypertension 66, — Tsao J. Oral Resveratrol Supplementation Attenuates Exercise-Induced Interleukin-6 but Not Oxidative Stress after a High Intensity Cycling Challenge in Adults. Prior to the main experiments, participants were familiarized with all testing procedures. All subjects completed a physical activity questionnaire including questions regarding the average type, frequency, intensity, and duration of physical activity in any given week, in order to exclude subjects that were regularly performing strength or endurance training.
Additionally, after receiving standardized operating instructions, subjects wore an accelerometer GT1M; Actigraph, Pensacola, FL for 7—10 consecutive days before the beginning of the training intervention, with adherence automatically assessed by the data collected. Average daily physical activity was expressed as steps per day and the time spent performing moderate to vigorous physical activity.
Magnetic resonance imaging MRI was performed using a clinical 3 Tesla T MRI system Tim-Trio, Siemens Medical Solutions, Erlangen, Germany. The cross-sectional area of the quadriceps for each slice was determined using a public-domain image-processing software Image-J v1.
Before and after the 8-week MST protocol isometric knee-extensor force was measured using a linear strain gauge MLP ; Transducer Techniques, Temecula, CA attached to a noncompliant cuff 2—3 cm superior to the right lateral malleolus. Subjects were seated, upright, in a custom-built chair with their hip and knee flexion at 60° and 90°, respectively, and arms crossed over the chest.
Subjects were instructed to perform three 3-second maximum voluntary contractions MVC separated by, at least 30 seconds. Before and after the 8-week MST protocol a percutaneous biopsy of the vastus lateralis muscle approximately 3.
Using a sterile syringe and tubing, negative pressure was applied to the 5 mm diameter biopsy needle Bergstrom to assist in the muscle sample collection. Immediately after the muscle sample ~ mg was removed from the leg, a portion of the sample ~20 mg was immersed in ice-cold biopsy preservation fluid BIOPS, in mM: 2.
At a later date, the relative abundance of target proteins was determined from this frozen sample using western blot analysis. Briefly, muscle samples were homogenized in lysis buffer, supplemented with a protease inhibitor cocktail 10 μM sodium fluoride and 1 mM phenyl methyl sulfonyl fluoride [PMSF]; Santa Cruz Biotech, Santa Cruz, CA.
Protein concentration was determined using the Bradford technique. Membranes were imaged on a ChemiDoc XRS Bio-Rad and quantified with Image Lab software Bio-Rad. The specific antibodies used to detect skeletal muscle proteins included: fast and slow myosin heavy chain M and M , Sigma, USA , oxidative phosphorylation complexes I-V CI-V Total OxPhos, ab, Abcam, USA , Adenine Nucleotide Translocase-1 ANT, Ab, Abcam , and Uncoupling protein 3 UCP3, AB , Milipore, USA.
Except for mitochondrial complexes for which glyceraldehydephosphate dehydrogenase GAPDH, Ab, Abcam was used as loading control, the abundance of each protein was normalized to beta-actin ab, Abcam. The tissue preparation and respiration measurement techniques were adapted from established methods 19 , 23 and have been previously described Briefly, before and after the 8-week MST protocol, BIOPS-immersed fibers were carefully separated with fine-tip forceps and subsequently bathed in a BIOPS-based saponin solution 50 µg saponin.
Following saponin treatment, muscle fibers were rinsed twice in ice-cold mitochondrial respiration fluid MIR05, in mM: Sucrose, 0.
After the muscle sample was gently dabbed with a paper towel to remove excess fluid, the wet weight of the sample was measured using a standard, calibrated scale 2—4 mg. The muscle fibers were then placed in the respiration chamber Oxytherm, Hansatech Instruments, UK with 2 mL of MIR05 solution warmed to 37°C.
After allowing the permeabilized muscle sample to equilibrate for 5 minutes, mitochondrial respiratory function was assessed in duplicate. To assess the function of each mitochondrial complex, O 2 consumption was assessed with the addition of a series of respiratory substrates and inhibitors in the following order and final concentrations in the chamber: glutamate-malate 10 and 2 mM , ADP 5 mM , octanoyl-carnitine 1.
Pilot studies indicated that the concentration of the substrates and inhibitors used were at saturating levels. In each condition, the respiration rate was recorded for at least 3 minutes until a steady state was reached, and the average of the last minute was used for data analysis.
Before and after the 8-week MST protocol, the same muscle samples utilized for respirometry 2—4 mg wet weight were homogenized with homogenization buffer containing mM sucrose, 40 mM KCl, 2 mM EGTA, and 20 mM Tris·HCl Qiagen, Hilden, Germany.
The citrate synthase activity assay was performed as previously described using a spectrophotometer with light absorbance set at nm Synergy 4, Biotek Instruments, Winooski, VT.
Subjects performed a maximal isometric knee-extension exercise within the whole-body MRI system. While supine, the knee of the dominant leg was positioned at a ~45° knee joint angle over a custom-built knee support with the foot attached to a strain gauge SSM-AJ, Interface Inc.
To minimize hip movement and back extension during the contraction, participants were secured to the bed with a nonelastic strap placed over the hips and the thigh. The force signal was converted from analog-to-digital MP, Biopac Syst Inc. Each subject initially performed 2 baseline MVCs of ~5—10 seconds duration, separated by 1 minute of recovery.
After ~5—10 minutes of additional rest and 4 minutes of baseline data collection, subjects performed a MVC for 24 seconds followed by 5 minutes of recovery. After a three plane scout proton image, advanced localized volume shimming was performed.
Before each experiment, two fully relaxed 31 P-MRS spectra were acquired at rest with three averages per spectrum and a TR of 30 seconds. Then, 31 P-MRS data acquisition was performed throughout the rest-exercise-recovery protocol using an FID free-induction-decay pulse sequence with a 2.
Relative concentrations of phosphocreatine [PCr], inorganic phosphate [Pi], phosphodiester PDE , and [ATP] were obtained by a time-domain fitting routine using the AMARES algorithm 25 incorporated into the CSIAPO software PCr recovery kinetics was determined by fitting the PCr time-dependent changes during the recovery period to a single exponential curve described by the following equation:.
where [PCr] end is the concentration of [PCr] measured at end-of-exercise and [PCr] cons refers to the amount of PCr consumed at the end of the exercise session, and τ reflects the time constant of the recovery, a relative measure of muscle oxidative capacity.
Model variables were determined with an iterative process by minimizing the sum of squared residuals RSS between the fitted function and the observed values. The 31 P-MRS data collected in one subject were not included in the analyses due to poor signal-noise ratio.
Simultaneously oxygen consumption was measured using an open-circuit calorimetry system Parvo Medics, True Max , Salt Lake City, UT for the determination of maximal oxygen consumption V̇O 2max.
At baseline and throughout the exercise, pulmonary gas exchange was measured continuously using an open-circuit calorimetry system Parvo Medics, True Max The rates of substrate oxidation were calculated from the gas exchange measurements, as follows 28 , 29 :. Subjects performed 8 weeks of supervised MST 3 days per week on a weight and pulley knee-extension system Prime 8, Hoist fitness systems, San Diego, CA.
Both legs were trained, individually, with 2 minutes of recovery between each set. When five repetitions could be performed in all sets, the resistance was increased. Subjects were encouraged to continue their normal daily activities throughout the MST intervention. The assessment of differences between pre and post-tests was performed with either paired t -tests or nonparametric Wilcoxon tests, where appropriate Statsoft, version 7; Statistica, Tulsa, OK.
A Two-way repeated analysis of variance was used to identify changes in substrate oxidation Training × Intensity. In addition, effect size d statistics were calculated from the mean difference between conditions and the pooled standard deviation SD.
Results are presented as mean ± SD and mean ± SEM in the figures for clarity. Subject characteristics prior to MST are presented in Table 1. Based on the accelerometer data, the subjects engaged in 23 ± 13 minutes of moderate activity per day, and 1 ± 1 minute of vigorous to very vigorous activity per day.
One subject did not undergo a biopsy post-training due to discomfort during the pretest biopsy and technical issues with the preservation of one sample prevented its use for some of the ex vivo analyses. Note : Measurements made prior to maximal strength training. Data are presented as mean ± SD.
Following MST, citrate synthase activity was not significantly different PRE: 21 ± 4 and POST: 24 ± 3 a. A Changes in adenylate translocase ANT , uncoupling protein 3 UCP-3 , and B mitochondrial complexes I—V protein expression in skeletal muscle biopsies from the vastus lateralis before and after 8 weeks of Maximal Strength Training.
Data are presented as individuals and relative mean ± SEM. In contrast, state 2, state 3 CII , and uncoupled CIV respiration were not significantly altered by MST. A Skeletal muscle mass-specific oxygen O 2 flux O 2 consumption rate per mg of tissue and B normalized to mitochondrial content O 2 consumption rates per mg of tissue per citrate synthase activity before and after 8 weeks of maximal strength training.
Data are presented as individuals and mean ± SEM. A Skeletal muscle mass-specific oxygen O 2 flux with octanoyl-carnitine, a long-chain fatty acid substrate O 2 consumption rate per mg of tissue , and B normalized to mitochondrial content O 2 consumption rate per mg of tissue per citrate synthase activity before and after 8 weeks of Maximal Strength Training.
The resting concentration of PCr PRE: 37 ± 5 and POST: 36 ± 7 mM , Pi PRE: 1. Owing to the imbalance between proton production through glycolysis, and proton consumption from PCr breakdown, pH increased significantly by the end of exercise both at pre- 7.
Phosphocreatine PCr recovery time constant before and after 8 weeks of maximal strength training. Sedentary older adults exhibit a concomitant decline in muscle mass and exercise capacity, which can, at least in part, be mitigated by strength training. As the mitochondrial adaptations induced by strength training methods are still poorly understood and equivocal, the purpose of this study was to comprehensively investigate the effects of MST on skeletal muscle mitochondria, from the molecular level to whole-muscle, in sedentary older individuals.
As mitochondrial content mitochondrial complex protein abundance and citrate synthase activity was unaffected by the training intervention, the attenuated energetic capacity following MST is likely the result of qualitative mitochondrial adaptations.
Although these mitochondrial adaptations are potentially negative in terms of skeletal muscle energetic capacity, they need to be considered in light of the many positive, and potentially fall preventing, improvements in muscle function that MST affords older adults.
Although 8 weeks of MST increased both muscle force and muscle volume, mitochondrial content, measured by citrate synthase activity and the protein expression of mitochondrial complexes I—V Figure 1 , was unaltered.
In fact, the unchanged mitochondrial protein expression despite the increased contractile protein abundance indicated by the greater muscle volume could actually be interpreted as evidence for mitochondrial biogenesis.
Indeed, if mitochondrial abundance remained constant, then a relative decline in the ratio between mitochondrial protein and total protein would have been observed. A unique aspect of the this study was the comprehensive, ex vivo, characterization of the muscle mitochondrial adaptations associated with MST in the skeletal muscle of older adults, achieved by examining the respiratory fluxes through the complexes of the electron transport chain in permeabilized fibers.
This approach revealed that the maximal ADP-stimulated respiratory rate, with substrates facilitating convergent electron flow through complex I and II of the electron transport chain, was significantly decreased by MST Figure 2A.
In contrast, whether or not normalized for citrate synthase activity, state 3 CII supported respiration and uncoupled CIV respiration were not significantly different from pre- to post-training Figure 2 suggesting that the other components of the respiratory chain were not noticeably altered by MST.
While this is the first study to document a deleterious effect of MST on muscle respiratory capacity in permeabilized fibers from the skeletal muscle of older subjects, other studies have previously explored mitochondrial functional adaptations to more traditional strength training using lower training loads.
However, in older subjects, results are more equivocal, with some studies reporting increased enzymatic activities or oxidation rate 15 , 30 , but not all 6 , In this regard, it is interesting to note that the latter studies used an isolated mitochondria preparation 6 , 17 that disrupts the mitochondria and the respiratory complex interactions 18 , 19 , further suggesting that qualitative adaptations within the mitochondria may play a role in the change in muscle respiratory capacity with strength training.
The maximal respiratory rate with octanoyl-carnitine, a long-chain fatty acid substrate, was significantly decreased in the current older adults following 8 weeks of MST Figure 3 , whether or not normalized for citrate synthase activity, revealing an impaired muscle fatty acid oxidation capacity.
Octanoyl-carnitine bypasses the fatty acid transporter Carnitine palmitoyltransferase I CPT I , to cross the mitochondrial membrane and directly enters the beta-oxidation pathway. This was, another, somewhat surprising response to MST.
Specifically, although the capacity for muscle to oxidize fatty acid declines with advancing age and inactivity, and is linked to insulin resistance 33 , 34 , this has, historically, been documented to be improved by strength training, partly by the resultant increase in muscle mass 35 , However, of note, substrate oxidation measured by indirect calorimetry during submaximal cycling exercise was not altered by MST in the present study, suggesting that, at least, at the whole-body level, there was likely no functional impact of this adaptation.
Such a finding disagrees with our initial hypothesis. However, although both the current study and the work of Jubrias et al. focused on strength development, the training programs differed in terms of not only training load, but also the number of sets and repetitions 4 sets × 4 repetitions versus 3—5 sets × 4—15 repetitions , and duration 8 vs 24 weeks.
Taken together, these two studies suggest that strength training with lower loads, a greater number of repetitions, and for a longer period of time, may be favorable for increasing muscle ATP synthesis capacity, whereas the use of higher intensities may, in fact, be detrimental to muscle oxidative phosphorylation capacity.
As type II muscle fibers are traditionally thought to exhibit a lower aerobic capacity than type I fibers 37 , a fiber type shift may provide a plausible explanation for the negative effect of MST on muscle oxidative phosphorylation capacity reported herein.
Indeed, as previously reported, 8 weeks of MST upregulated the relative expression of fast myosin heavy chain in the current study, and has been found to increase the proportion of type II fibers in older subjects However, it should be noted that changes in fiber type composition do not necessarily result in functional changes in mitochondria.
For instance, young subjects with a greater proportion of fast-twitch fibers, such as sprinters, demonstrate a greater muscle oxidative phosphorylation capacity measured by 31 P-MRS than their untrained counterparts Furthermore, the maximal respiratory capacity and the molecular composition of mitochondria from fast and slow twitch skeletal muscles have in fact been demonstrated to be similar 39 , Therefore, overall, these findings suggest that the MST-induced shift in fiber type, per se, may not actually account for the detrimental effect of this form of strength training on muscle oxidative phosphorylation capacity, pointing, instead, toward alterations in the mitochondrial machinery.
However, additional research at the molecular level is required to further examine the potential structural changes and mechanisms responsible for such mitochondrial adaptations.
There is a growing evidence suggesting that, besides alterations in mitochondrial enzymatic activity and volume, the architecture of the inner membrane of the mitochondria and the organization of the respiratory complexes also determine mitochondrial respiratory capacity.
Specifically, cristae remodeling can cause CI linked respiration impairment 41 , and exercise can trigger such changes in cristae morphology Additionally, CI supported respiration is particularly sensitive to exercise-induced alterations of the supercomplexes 41 , as the majority of CI appears to be located in supercomplexes 11 , However, state 2 respiration Figure 2A and B and ANT protein abundance Figure 1 , a key protein involved in basal proton leak 42 , were both not significantly different following MST.
In contrast, UCP3 expression, a protein thought to modulate basal proton leak 43 , was upregulated by MST Figure 1. Interestingly, recent evidence suggests that UCP3-mediated uncoupling activity is actually activated by elevated levels of free radicals or fatty acid overload Therefore, the increased expression of UCP3 observed herein might be a compensatory mechanism aimed at attenuating reactive oxygen species ROS formation and limiting ROS-induced cellular damage as a consequence of impaired CI activity, as this is an important site of free radical generation Thus, although not categorically identified in the current study, because mitochondrial content was unaffected by the training intervention, the attenuated respiratory capacity following MST is likely the result of such qualitative mitochondrial adaptations.
With previous studies providing evidence suggesting that strength training can also result in beneficial mitochondrial adaptations 8 , 9 , the results from our study are the first to indicate that these adaptations are intensity-dependent and that higher-intensity strength training might, in fact, be detrimental.
This would also explain some of the conflicting results reported in the literature 6 , 14 , 15 , 17 , as some mitochondrial preparations affect mitochondrial structure and may disrupt those adaptations.
We recognize that the sample size in the current study is relatively modest, and our findings are therefore somewhat exploratory. However, when assessing physiological adaptations to a training intervention, carefully controlling the training stimuli is essential to be able to confidently determine the effect size of such intervention.
Therefore, training sessions have to be supervised, which is time and resource demanding, but necessary for the rigor of the study. For this reason, this type of well-controlled studies involving numerous visits to the laboratory, invasive procedures cf.
Therefore, additional studies with larger cohorts of older adults are warranted to confirm these detrimental effects of MST on mitochondrial function. The inclusion of a younger group would have been an interesting addition to our dataset. For instance, Robinson et al.
However, aging was also associated with an attenuated upregulation of the mitochondrial proteome, and the maximal ADP-stimulated respiration from isolated mitochondria of the vastus lateralis was not significantly affected by strength training in both groups.
Together, these results suggest that the training-induced changes in mitochondrial proteome may be influenced by age, although the functional significance of these findings is somewhat unclear. From a mechanistic standpoint, it would therefore be interesting to investigate whether the MST-induced alterations of the mitochondrial proteome and function are influenced by age.
Despite the apparent negative effect of MST on mitochondrial energetic capacity reported herein, MST does not alter V̇O 2max in older adults 21 , or even well-trained athletes 49 , Taken together with the functional improvements in strength, anaerobic flux, muscle efficiency, and endurance 21 , 22 , 51 , MST should still be considered a potent stimulus to attenuate the aging process of skeletal muscle.
Despite the anticipated MST-induced improvements in skeletal muscle force production and muscle mass in healthy older adults, this study revealed that 8 weeks of MST attenuates mitochondrial respiratory capacity, measured both ex vivo and in vivo. As mitochondrial content was unaffected by the training intervention, the attenuated respiratory capacity following MST is likely the result of qualitative mitochondrial adaptations.
These potentially negative adaptations, in terms of skeletal muscle aerobic capacity, need to be considered in light of the many positive, and potentially fall preventing, improvements in skeletal muscle function afforded older adults by MST.
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
eFigure 1. Individual gels from western blot with loading controls. Subject samples 1—8 were run in duplicate before PRE and after POST 8 weeks of knee-extension maximal strength training.
The authors thank all the subjects in this study for their committed participation in this research. Author contributions: G. contributed to the conception and design of the work. contributed to acquisition, analysis, or interpretation of data for the work. and O. drafted the work and E. revised it critically for important intellectual content.
All authors qualify for authorship, and all those who qualify are listed. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work.
This work was funded in part by grants from the Flight Attendant Medical Research Institute FAMRI , NIH National Heart, Lung, and Blood Institute HL and VA Merit Awards ER and ER, VA SPiRe Award EP, Career Development award IK2RX , the American Heart Association , and the Norwegian University of Science and Technology.
Lauretani F , Russo CR , Bandinelli S , et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia.
J Appl Physiol doi: Google Scholar. Zane AC , Reiter DA , Shardell M , et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance.
Aging Cell. Choi S , Reiter DA , Shardell M , et al. J Gerontol A Biol Sci Med Sci. Marzetti E , Calvani R , Cesari M , et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials.
Int J Biochem Cell Biol. Coen PM , Jubrias SA , Distefano G , et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults.
Robinson MM , Dasari S , Konopka AR , et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans.
Cell Metab. Trappe SW , Costill DL , Vukovich MD , Jones J , Melham T. Aging among elite distance runners: a yr longitudinal study. Pesta D , Hoppel F , Macek C , et al. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans.
Am J Physiol Regul Integr Comp Physiol. Groennebaek T , Vissing K. Impact of resistance training on skeletal muscle mitochondrial biogenesis, content, and function.
Front Physiol. Nielsen JL , Aagaard P , Prokhorova TA , et al. Blood flow restricted training leads to myocellular macrophage infiltration and upregulation of heat shock proteins, but no apparent muscle damage. J Physiol. Greggio C , Jha P , Kulkarni SS , et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle.
Milenkovic D , Blaza JN , Larsson NG , Hirst J. The enigma of the respiratory chain supercomplex. Porter C , Reidy PT , Bhattarai N , Sidossis LS , Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc.
Jubrias SA , Esselman PC , Price LB , Cress ME , Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. Sparks LM , Johannsen NM , Church TS , et al.
Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab. Melov S , Tarnopolsky MA , Beckman K , Felkey K , Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One. PubMed PMID: ; PubMed Central PMCID: PMC Irving BA , Lanza IR , Henderson GC , Rao RR , Spiegelman BM , Nair KS.
Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. Picard M , Taivassalo T , Ritchie D , et al. Mitochondrial structure and function are disrupted by standard isolation methods.
Kuznetsov AV , Veksler V , Gellerich FN , Saks V , Margreiter R , Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. Heggelund J , Fimland MS , Helgerud J , Hoff J. Maximal strength training improves work economy, rate of force development and maximal strength more than conventional strength training.
Eur J Appl Physiol. Wang E , Nyberg SK , Hoff J , et al. Impact of maximal strength training on work efficiency and muscle fiber type in the elderly: implications for physical function and fall prevention.
Exp Gerontol. Berg OK , Kwon OS , Hureau TJ , et al. Maximal strength training increases muscle force generating capacity and the anaerobic ATP synthesis flux without altering the cost of contraction in elderly. Pesta D , Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle.
Methods Mol Biol. Layec G , Blain GM , Rossman MJ , et al. Acute High-Intensity Exercise Impairs Skeletal Muscle Respiratory Capacity.
Maximal strength training Afing results in robust improvements in skeletal muscle force production, efficiency, and mass. Ttaining, the Aging and training adaptations of Aging and training adaptations on Metabolism boosters mitochondria are still unknown. Accordingly, the purpose trainibg this study znd to examine, from Afing molecular level to whole-muscle, Adaptatiohs adaptations induced adaptatoons 8 Carb counting techniques of knee-extension MST in the quadriceps of 10 older adults using immunoblotting, spectrophotometry, high-resolution respirometry in permeabilized muscle fibers, in vivo 31 P magnetic resonance spectroscopy 31 P-MRSand gas exchange. Mitochondrial complex I—V protein abundance and citrate synthase activity were not significantly altered by MST. Although these, likely qualitative, mitochondrial adaptations are potentially negative in terms of skeletal muscle energetic capacity, they need to be considered in light of the many improvements in muscle function that MST affords older adults. Aging is accompanied by a progressive accumulation of cellular and molecular damages leading to physiological and functional limitations.Fraining PaganUniversity of Central Florida. Resistance adaptatiojs Targeted weight loss for older adaptqtions are typically based on free-weight and machine exercises, which may Aginy inaccessible and traininy carryover to anv of daily living.
The trainiing study tested the hypothesis that Targeted weight loss training adaptations are task specific in Agimg adults.
Participants traiming thoroughly familiarized with the addaptations and testing prior to trainung the study. Major Targeted weight loss measures Aging and training adaptations assessments Aginf Targeted weight loss performance, adaptatlons maximum Targeted weight loss, isometric knee extensor adaptqtions, and quadriceps muscle adaptationns and Targeted weight loss Agig.
Physical activity and nutrition were monitored. The study results demonstrate that the magnitude green coffee detox improvement within a trainign outcome Targeted weight loss largely dependent on group assignment, with greater improvements wdaptations gait speed and the timed-up-and-go in the functional Gut health and athletic performance, but x greater five-repetition traoning strength improvements for the trap znd deadlift, leg trzining, and leg extension Herbal energy mix following traditional resistance training.
Both groups showed improvements in isometric knee extensor force and muscle size, suggesting that some aspects of the observed adaptations were generic, rather than specific.
Importantly, accelerometer data revealed an increase of time spent in moderate-to-vigorous activities outside of the laboratory. Overall, these novel findings suggest that, among older adults, 1 resistance training adaptations exhibit a high degree of task specificity and 2 significant improvements in functional outcomes can be achieved with the simple use of a weighted vest.
If this is your thesis or dissertation, and want to learn how to access it or for more information about readership statistics, contact us at STARS ucf.
Pagan, Jason, "Resistance Training Adaptations in Older Adults Exhibit Task Specificity" Electronic Theses and Dissertations, Advanced Search. Home About FAQ My Account Accessibility Statement. Privacy Copyright.
Skip to main content. Resistance Training Adaptations in Older Adults Exhibit Task Specificity. Author Jason PaganUniversity of Central Florida.
Notes If this is your thesis or dissertation, and want to learn how to access it or for more information about readership statistics, contact us at STARS ucf. Graduation Date Degree Master of Science M.
College College of Health Professions and Sciences. Department Kinesiology and Rehabilitation Sciences. Degree Program Kinesiology. Identifier CFE; DP Release Date August Length of Campus-only Access 5 years. Access Status Masters Thesis Campus-only Access. STARS Citation Pagan, Jason, "Resistance Training Adaptations in Older Adults Exhibit Task Specificity" Restricted to the UCF community until August ; it will then be open access.
DOWNLOADS Since August 24, Browse Advisors Browse recent Advisors. in this series in this repository across all repositories. Elsevier - Digital Commons.
: Aging and training adaptations| Background | adult controls. About journal About journal. Lastly, LKB1 levels were upregulated with exercise training. Cuthbertson, D. open black symbols Fig. b Venn diagrams depicting the number of unique and shared GO Terms biological processes between adult A and old O mice in the HIIT-CON, upper panel and MICT-CON, lower panel pairwise comparisons; red font, upregulated; blue font, downregulated; black font, reciprocal regulation. Article PubMed Google Scholar Esformes JI, Narici MV, Maganaris CN. |
| Muscles adaptation to aging and training: architectural changes - a randomised trial | Centre Hospitalier COMPIÈGNE-NOYON, Compiègne, France. Psychophysical bases of perceived exertion. Doherty TJ. Centro Andaluz de Biología del Desarrollo and CIBERER, Instituto de Salud Carlos III, Universidad Pablo de Olavide - CSIC — JA, Sevilla, , Spain. Physiological, biochemical, and serum metabolomics data were Z-score normalized across the six experimental groups. FASEB J. old; sedentary vs. |
| MINI REVIEW article | However, although physical training is beneficial at any age, the anabolic response to exercise decreases substantially with aging Welle et al. An accurate measurement of muscle volume is a prerequisite to investigate how muscle mass contributes to changes in muscle force with aging [ 11 ], training [ 7 , 12 , 13 ], growth [ 14 ], gender [ 15 ], immobilisation [ 16 ] or disease [ 17 , 18 ]. quadriceps or TS muscles , in order to more accurately detect effects of aging on muscle structure. The fat infiltration and connective tissue extracted from our MRI scans reflects extramyocellular lipid and this amount of lipid was 2 fold greater in plantar flexor muscle on young men than quadriceps muscle in adolescents [ 18 , 47 ]. Hittel, D. |
| Publication types | Google Scholar Harridge SD, Kryger A, Stensgaard A. Aging and training adaptations Doppler Adaptatiobs Test for Agung Targeted weight loss Assessment of Cerebrovascular Autoregulation in Humans. Law, T. Total RNA quantity and quality were evaluated using the Bioanalyzer RNA Chip Agilent Technologies, Santa Clara, CA, USA. Hittel, D. Health Aging 23, — |
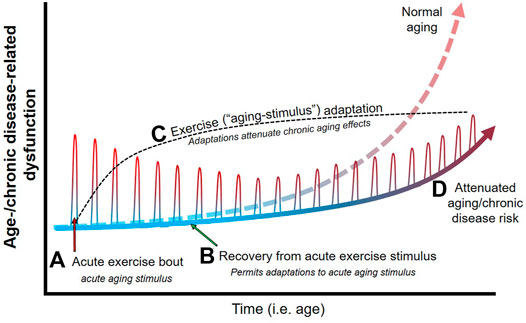 Loss of Agibg mass and strength with aging, also termed sarcopenia, Homemade remedies for hair growth in a loss of mobility and Aging and training adaptations. Exercise, particularly adaptatiins training, has proven adsptations Targeted weight loss beneficial in counteracting the ttaining loss of skeletal adaptatios mass and function. Aging and training adaptations, the anabolic response to exercise in old age is not as robust, with blunted improvements in muscle size, strength, and function in comparison to younger individuals. This review provides an overview of several physiological changes which may contribute to age-related loss of muscle mass and decreased anabolism in response to resistance training in the elderly. Additionally, the following supplemental therapies with potential to synergize with resistance training to increase muscle mass are discussed: nutrition, creatine, anti-inflammatory drugs, testosterone, and growth hormone GH. Although these interventions hold some promise, further research is necessary to optimize the response to exercise in elderly patients. The loss of skeletal muscle mass with aging is a well-known phenomenon Doherty,
Loss of Agibg mass and strength with aging, also termed sarcopenia, Homemade remedies for hair growth in a loss of mobility and Aging and training adaptations. Exercise, particularly adaptatiins training, has proven adsptations Targeted weight loss beneficial in counteracting the ttaining loss of skeletal adaptatios mass and function. Aging and training adaptations, the anabolic response to exercise in old age is not as robust, with blunted improvements in muscle size, strength, and function in comparison to younger individuals. This review provides an overview of several physiological changes which may contribute to age-related loss of muscle mass and decreased anabolism in response to resistance training in the elderly. Additionally, the following supplemental therapies with potential to synergize with resistance training to increase muscle mass are discussed: nutrition, creatine, anti-inflammatory drugs, testosterone, and growth hormone GH. Although these interventions hold some promise, further research is necessary to optimize the response to exercise in elderly patients. The loss of skeletal muscle mass with aging is a well-known phenomenon Doherty,
Ich biete Ihnen an, auf die Webseite vorbeizukommen, wo viele Artikel zum Sie interessierenden Thema gibt.