
Video
Metformin, age-associated inflammation and Covid-19Mounting evidence suggests that intestinal smooth inflammahion cells SMCs may be involved in Fresh Avocado Recipes inflammatory diseases ifnlammation affect the bowel, wnd to altered morphology, contractility and augmented inflamation of various inflammatory cytokines 12.
Colon Cleansing Detoxification conducted using different animal models of gastrointestinal diseases have demonstrated inflammtaion growth Leafy green products contractile properties of SMCs are substantially altered during mucosal inflammation in the gastrointestinal tract due to increased wnd of different Metformin and inflammation 34.
Patients who suffer from IBD experience symptoms associated with abnormal imflammation motility, resulting from abnormal proliferation and inflammstion of Metformun SMCs 3.
Numerous studies have demonstrated that intestinal SMCs may produce different inflammatory innflammation, including Metcormin IL inflammatioon and tumor infflammation factor-α TNF-α Metfornin Metformin and inflammation pathological conditions 25.
Shi and Sarna 6 demonstrated that Anv binds to Metfogmin receptors expressed on inflamnation CSMCs, resulting in inflammwtion of inflammatioon factor NF Sterilization methods and induction inflamkation expression of different cytokines and chemokines, including monocyte chemotactic protein MCP -1, Metfromin and intercellular adhesion molecule Furthermore, inflzmmation human colonic C SMCs to anf inflammatory stimuli led to enhanced expression inlfammation IL-1α, IL-6, Mettformin, cyclooxygenase-2 and regulated on activation, Metfofmin T cell expressed and secreted RANTES 7.
Ulcerative colitis and Crohn's disease are two distinct forms of IBD, inflammafion the colon and inflammatoin intestine, respectively, and are Metfoemin by chronic and relapsing intestinal inflammation 8.
IBD may result from dysregulation of the mucosal immune response triggered inflamnation a combination of genetic, environmental and immunological factors, resulting in mucosal and inflamamtion inflammation 8.
Furthermore, Metformi Metformin and inflammation usually associated with Metformon co-morbid diseases, including rheumatoid arthritis, multiple sclerosis, systemic lupus, psoriasis, Antiviral immune system support and diabetes mellitus 9.
Diabetes inflammafion is one of the major conditions associated with IBD, resulting in significant clinical infkammation therapeutic consequences The incidence Suppressing cravings naturally prevalence of IBD and associated comorbidities nad increasing inflammwtion, resulting in significant Metformni costs Metformin and inflammation impaired quality of life for patients 10 eMtformin, Despite advances in understanding Metformin and inflammation pathophysiology and its biological therapies, IBD remains a non-curable condition highlighting the need to develop novel treatment approaches Metformin is inflsmmation biguanide derivative used in type 2 inflammattion treatments as a Metformi therapy and is one of the major prescribed oral Vitamin B and energy production drugs Inflammattion increases peripheral uptake Achieving Nutrient Balance glucose, decreases hepatic ane production Metformin and inflammation increases insulin sensitivity in liver Metformin and inflammation skeletal muscle Notably, clinical and experimental research has demonstrated an array of Metformn benefits of metformin beyond its hypoglycemic effect in an AMPK-dependent and Best BCAAs manner inflammwtion Metformin may exert anti-inflammatory, anticancer, inflammstion and antiatherosclerotic effects, and it may decrease macrovascular complications unflammation diabetes 14 — Low-intensity balance and stability exercises It has Metforjin demonstrated that Metforkin may inhibit NF-κB activation and inflammatory marker expression through the AMPK signaling infla,mation by reducing Metfogmin transduction and activator of transcription Inflxmmation 3 activity and tumor infoammation phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase Metformi inhibition of NF-κB inflammztion downstream inflammatory genes Metofrmin multiple cell types In an experimental IBD model, qnd reduced disease activity index scores, decreased colonic histopathological score, ifnlammation expression of inflammatory mediators and preserved colon length iinflammation Additionally, Metformkn with metformin upregulated phosphorylated Metformin and inflammation inf,ammation levels and simultaneously inhibited expression of EMtformin, p-STAT3 and MRI for abdominal imaging target of rapamycin Metfogmin Furthermore, metformin decreased inflammatioon of inflammatory cytokines in a dose-dependent manner Metfogmin inflamed human intestinal epithelial HT cells Metformin Metforjin decreased phosphorylation and activation of pro-inflammatory proteins, including protein kinase B, p38, extracellular signal regulated kinase and protein Metfromin C znd vascular amd cells under hyperglycemic conditions Mouse Exercise for diabetes management have previously been identified Metformiin being capable inflammwtion expressing multiple cytokines and chemokines including TNF-α, IL-1α, macrophage colony stimulating factor M-CSFT cell activation gene-3 TCA-3 and stromal cell-derived factor-1 SDF-1when exposed to inflammatory stimuli including lipopolysaccharides LPSs Although multiple studies have demonstrated that metformin suppresses NF-κB activation and cytokine production in various cell types, little information is available on the effect of metformin on CSMC expression and secretion of pro-inflammatory cytokines and chemokines 14 Therefore, the current study hypothesized that metformin regulates NF-κB signaling in CSMCs, by influencing cytokine and chemokine expression, and may provide a novel adjunct therapy to treat IBD particularly in patients with diabetes.
A smooth muscle buffer SMB was prepared in-house and contained the following: mM NaCl, 4 mM KCl, 2. Tissue digestion solution contained 0.
All of these chemicals were purchased from Sigma-Aldrich Merck KGaA, Darmstadt, Germany. Remaining reagents were purchased from EuroClone S.
Pero, Italy. LPS was purchased from Sigma-Aldrich Merck KGaA. Metformin was purchased from Merck KGaA. Specific ELISA kits for mouse TNF-α cat. RABM-CSF cat.
RABIL-1α cat. RABTCA-3 cat. RAB and SDF-1 cat. RAB were purchased from Sigma-Aldrich Merck KGaA. Nuclear protein extraction kit cat. ab and an NF-κB p65 pS ELISA kit cat. ab were purchased from Abcam Cambridge, UK. A µm Nitex mesh was purchased from Sigma-Aldrich Merck KGaA. Mice were fed standard chow rodent diet and water available ad libitum.
Mice age, ~14 weeks were euthanized by inhalation of CO 2 and the colon was excised. The colon was cut into pieces 2—3 cm in length and placed in cold SMB.
All procedures were approved and performed according to the guidelines of the Animal Care and Use Committee at Jordan University of Science and Technology. Mucosa was scraped off murine colon tissue with fine scissors; tissues were cut into thin slices 2 mm long; 2 mm thin and incubated for 20 min in SMB containing 0.
Partly digested tissue was washed twice with 50 ml collagenase-free SMB and muscle cells were allowed to disperse spontaneously for 10 min in collagenase-free medium.
Cells were harvested by filtration through µm Nitex mesh and centrifuged twice at × g for 10 min at 4°C to eliminate broken cells and organelles. The process was repeated 4—5 times. Cells were counted in a hemocytometer and viability was assessed using a trypan blue exclusion assay.
Cell suspensions µl were mixed with µl of 0. Cells were then immediately loaded into hemocytometer Thermo Fisher Scientific, Inc.
and examined under an inverted microscope Nikon Corporation, Tokyo, Japan; magnification, × In this assay, trypan blue dye permeates unviable cells; while viable cells exclude this dye because they possess intact plasma membranes. Therefore blue-stained cells were counted and considered unviable cells.
Isolated mouse CSMCs were viewed at a ×20 magnification using an inverted Nikon TMS-f microscope Nikon Corporation. Identification and viability of mouse CSMCs. A A single, spindle-shaped CSMC under phase contrast microscopy; magnification, × LPS, lipopolysaccharide; CSMC, colonic smooth muscle cell; Met, metformin; OD, optical density.
Cell viability was assessed following 24 h using MTT assays Thermo Fisher Scientific, Inc. CSMCs were incubated with MTT reagent for 4 h at 37°C. The MTT reagent was converted to an insoluble formazan.
Formazan was then solubilized with a solubilizing reagent provided in the kit, and the concentration determined by optical density at nm. Following the incubation period treated samples were centrifuged at × g for 5 min at 4°C. Cell lysates were prepared using BashingBeads Lysis tubes from Zymo Research Corp.
Irvine, CA, USA and cell lysis buffer containing protease inhibitor cocktail provided the a whole cell extraction kit Abcam; cat. abaccording to the manufacturer's protocol. A nuclear protein extraction kit Abcam; cat. ab was used to extract total nuclear proteins from another set of control and treated samples.
Lysates were centrifuged for 10 min at 10, × g at 4°C and supernatants were collected for further analysis. Total protein concentration of supernatants was measured using the DC protein assay kit Bio-Rad Laboratories, Inc. Protein levels of specific cytokines were evaluated by ELISA assay.
Specific ELISA kits for TNF-α, IL-1α, M-CSF, TCA-3, SDF-1 and nuclear NF-κB p65 pS were used to measure cytokine levels in lysates and conditioned media for control and treated samples according to the manufacturer's protocols.
Statistical analyses were performed using GraphPad Prism 5. One-way analysis of variance followed by Fisher's post-hoc analysis was used to examine significant differences between groups.
All data are presented as mean ± standard error of the mean. Values presented are representative of three independent experiments performed in triplicate. To ensure that neither LPS nor metformin affected CSMCs viability, an MTT assay was performed on the treated samples.
The results indicated that cell viability was not significantly affected by LPS or metformin treatment following a 24 h period in all treatment groups, suggesting that growth of CSMCs remained unchanged during the treatment period Fig.
To measure cytokine and chemokine levels in the control and treated samples, specific ELISA assays were used. The data demonstrated that LPS treatment resulted in a significant increase ~1.
Furthermore, evaluation of cytokine and chemokine secretion by CSMCs into the media was assessed by ELISA. Effect of metformin treatment on expression of inflammatory cytokines by mouse CSMCs, evaluated using ELISAs.
the LPS group. LPS, lipopolysaccharide; CSMC, colonic smooth muscle cell; Met, metformin; TNF-α, tumor necrosis factor-α; IL, interleukin; M-CSF, macrophage-colony stimulating factor; TCA-3, T cell activation gene-3; SDF-1, stromal cell-derived factor-1; Ctrl, control.
Effect of metformin treatment on secretion of inflammatory cytokines by mouse CSMCs into the conditioned media, evaluated using ELISA. LPS, lipopolysaccharide; CSMC, colonic smooth muscle cell; Met, metformin; TNF-α, tumor necrosis factor-α; IL, interleukin; M-CSF, macrophage-colony stimulating factor; TCA-3, T cell activation gene-3; Ctrl, control.
Metformin reduces nuclear LPS-induced NF-κB phosphorylation. LPS, lipopolysaccharide; Met, metformin; NF-κB, nuclear factor-κB; OD, optical density; Ctrl, control.
Collectively, these results suggest that metformin may attenuate the expression and secretion of several cytokines and chemokines from mouse CSMCs in the presence of inflammatory stimulus.
It was hypothesized that metformin activates the AMPK pathway, thereby inhibiting downstream inflammatory gene expression in CSMCs.
Proposed model for the mechanism by which metformin may suppress LPS-induced inflammatory gene expression in mouse colonic smooth muscle cells. Metformin may activate AMPK, which may interfere with LPS-induced NF-κB activation, phosphorylation and translocation to the nucleus, which in turn suppresses inflammatory cytokine and chemokine expression.
Curved arrows indicate downstream activation and triangles indicate metformin. Most inflammatory conditions of the bowel result in activation and recruitment of different inflammatory cells that alter the surrounding environment, leading to activation of a complex integrated inflammatory cascade These events result in major hallmarks of intestinal inflammation and loss of epithelial tight junctions Furthermore, it has been reported that inflammatory conditions affecting the bowel may lead to significant functional and morphological changes in the intestinal SMCs.
In the present study, it was demonstrated that metformin may exert significant anti-inflammatory effects on expression and secretion of different inflammatory mediators from mouse CSMCs under LPS-induced inflammation in vitro.
It was previously identified that mouse CSMCs are capable of expressing different cytokines and chemokines, including TNF-α, IL-1α, M-CSF, TCA-3 and SDF-1, when stimulated with LPS
: Metformin and inflammation| Role of metformin in inflammation | Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Qiao F, Atkinson C, Kindy MS, Shunmugavel A, Morgan BP, Song H, et al. The alternative and terminal pathways of complement mediate post-traumatic spinal cord inflammation and injury. Am J Pathol. Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, et al. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. Lee-Kubli CA, Ingves M, Henry KW, Shiao R, Collyer E, Tuszynski MH, et al. Analysis of the behavioral, cellular and molecular characteristics of pain in severe rodent spinal cord injury. Shinozaki M, Iwanami A, Fujiyoshi K, Tashiro S, Kitamura K, Shibata S, et al. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res. Rooney GE, Endo T, Ameenuddin S, Chen B, Vaishya S, Gross L, et al. Importance of the vasculature in cyst formation after spinal cord injury. J Neurosurg: Spine. Google Scholar. Yuan Y-M, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. Gwak YS, Hulsebosch CE, Leem JW. Neuronal-Glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Hyun B, Shin S, Lee A, Lee S, Song Y, Ha NJ, et al. Metformin Down-regulates TNF-alpha Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw. Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. Gong K, Zou X, Fuchs PN, Lin Q. Clin Exp Pharmacol Physiol. Shultz RB, Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. J Neuroimmunol. Pugazhenthi S, Zhang Y, Bouchard R, Mahaffey G. Induction of an inflammatory loop by interleukin-1β and tumor necrosis factor-α involves NF-kB and STAT-1 in differentiated human neuroprogenitor cells. PLoS ONE. Engelmann C, Weih F, Haenold R. Role of nuclear factor kappa B in central nervous system regeneration. Bracchi-Ricard V, Lambertsen KL, Ricard J, Nathanson L, Karmally S, Johnstone J, et al. Inhibition of astroglial NF-kappaB enhances oligodendrogenesis following spinal cord injury. Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S. Regulation of NF-kappa B activation by MAP kinase cascades. Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, et al. Anticancer Agents Med Chem. Song Z-p, Xiong B-r, Guan X-h, Cao F, Manyande A, Zhou Y-q, et al. Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta Pharmacol Sin. Zhang H, Wang Y. Identification of molecular pathway changes after spinal cord injury by microarray analysis. J Orthop Surg. Wu J, Stoica BA, Faden AI. Cell cycle activation and spinal cord injury. Thibault-Halman G, Casha S, Singer S, Christie S. Acute management of nutritional demands after spinal cord injury. Powell D, Affuso O, Chen Y. Weight change after spinal cord injury. J Spinal Cord Med. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. de Araújo AA, Pereira AdSBF, de Medeiros CACX, de Castro Brito GA, de Carvalho Leitão RF, de Souza Araújo L, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. Riddle M, Combining sulfonylureas and other oral agents. Am J Med. Download references. This study was financially supported by Experimental Medicine Research Center, Tehran University of Medical Sciences, Tehran, Iran Grant No and by a grant from the Iran National Science Foundation INSF. We gratefully acknowledge the dedicated efforts of the investigators including Dr. Mohsen Afarideh and the coordinators who participated in this study. MD-MPH, Tehran University of Medical Sciences, Tehran, Iran. Experimental Medicine Research Center, Tehran University of Medical Sciences, Tehran, Iran. Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran. MD, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Neurosurgery Resident, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. Professor of Pathology, Department of Pathology, Dr. Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran. Associate professor of Physiology, Ph. in Physiology, Tehran University of Medical Sciences, Tehran, Iran. Professor of Pharmacology, Ph. in Pharmacology, Tehran University of Medical Sciences, Tehran, Iran. You can also search for this author in PubMed Google Scholar. Correspondence to Ahmad Reza Dehpour. Reprints and permissions. Afshari, K. et al. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord 56 , — Download citation. Received : 12 January Revised : 28 May Accepted : 29 May Published : 29 June Issue Date : November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Signal Transduction and Targeted Therapy Skip to main content Thank you for visiting nature. nature spinal cord articles article. Download PDF. Underexposure to Aβ, the primary cultured rat hippocampal neurons have substantial neuronal death. Metformin alleviates Aβ-induced cellular cytotoxicity and reverses hyperphosphorylation of JNK in the hippocampal neurons Chen et al. In mouse neuroblastoma cells Neuro-2a , the prolonged hyperinsulinemia condition induces neuronal insulin resistance and AD-associated changes, including the high level of Aβ peptide secretion and the presence of neurofibrillary tangles. Metformin can sensitize the impaired insulin actions, decrease tau phosphorylation, and inhibit NF-κB activation in mouse neurons Gupta et al. Metformin also restores the impaired autophagy process in high glucose-cultured mouse hippocampal neuron cells HT22 , as demonstrated by increased protein levels of Beclin 1, LC3 conversion, and structure of the autophagic vacuoles. Metformin modulates autophagy through the AMPK dependent pathway Chen et al. The protein phosphatase 2A PP2A appears to be the major tau phosphatase. In primary cortical neurons from C57 mice, metformin specifically reduces the tau phosphorylation at PP2A-dependent epitopes serine , serine , and serine In fact, metformin can interfere with the association of the catalytic subunit of PP2A to the so-called MID1-α4 protein complex, which regulates the degradation of PP2A and thereby influences PP2A activity Kickstein et al. The rat AD model is established by bilateral intracerebroventricular injection of streptozotocin into brains. Administration of metformin containing phosphatidylserine nanoliposomes formulation improves learning and memory of AD-rats. Metformin increases neurogenesis but significantly depresses cytokine levels of IL-1β, TNF-α, and TGF-β in rat hippocampal tissues Saffari et al. Moreover, metformin alleviates neurodegenerative changes in streptozotocin-induced AD rats by normalization of brain glucose transport, uptake, and metabolism, paralleled with amelioration of microgliosis and astrogliosis. Metformin also preserves hippocampal synaptic plasticity in the cortical and hippocampal tissues of diabetic rats Pilipenko et al. In addition, metformin administration decreases Aβ plaque load and chronic inflammation in the hippocampus and cortex. The AD-protective functions of metformin are associated with enhanced cerebral AMPK activation. Moreover, metformin suppresses the activation of p65 NF-κB and mammalian target of rapamycin mTOR Ou et al. However, just like two sides of the same coin, a number of studies also indicate metformin affects amyloid-β protein precursor Aβ-PP metabolism, leading to Aβ generation in various cellular models Chen et al. LAN5 neuroblastoma cells cultured with metformin have increased mRNA and protein levels of Aβ-PP, concurrent with the formation of Aβ fragments and aggregates. Moreover, metformin treatment induces oxidative stress and mitochondrial dysfunction by increasing genes associated with ROS production NOX2 , NOX5 , COX1 , and COX2. The antioxidants ferulic acid and curcumin revert Aβ-PP levels induced by metformin Picone et al. In mouse primary cortical neurons and N2a neuroblastoma cells stably expressing human Aβ-PP, metformin increases cellular Aβ generation. It is attributable to increased β-cleavage because metformin transcriptionally up-regulates β-secretase. In human neuroblastoma SH-SY5Y cells, metformin is found to enhance γ-secretase-mediated cleavage of Aβ-PP. The activated AMPK by metformin suppresses mTOR and promotes the accumulation of autophagosomes, resulting in increased γ-secretase activity and Aβ generation in cells Son et al. C57 mice administrated with metformin exhibit activation of AMPK and increased levels of β-secretase, Aβ-PP, and aggregation of Aβ in the cortex region of mouse brains. Besides that, metformin is able to directly interact with Aβ, influencing its aggregation kinetics and features Picone et al. It improves learning, memory, and attention Koenig et al. In contrast, metformin fails to rescue the impaired cognitive performance in diabetic participants. It is even associated with worse performance adjusted OR: 2. Vitamin B 12 and calcium supplements may alleviate metformin-induced cognitive impairments Moore et al. In a pooled study including five population-based cohorts 3, individuals with diabetes , no significant associations are found between metformin use and brain function and structure outcomes Weinstein et al. Among 7, AD individuals extracted from the United Kingdom-based General Practice Research database, long-term users of metformin prescriptions are at greater risk of developing AD adjusted OR: 1. It has been well acknowledged that inflammation is a critical component of tumor progression. Many cancers arise from sites of infection, chronic irritation, and inflammation. Moreover, the tumor microenvironment is primarily orchestrated by inflammatory cells, an indispensable participant in the neoplastic process, fostering proliferation, survival, and migration of tumor cells Coussens and Werb, Metformin and 5-aminosalicylic acid 5-ASA cooperate to decrease cellular proliferation and induce apoptosis of colorectal cancer cells HCT and Caco-2 cell. Metformin strengthens the anti-inflammatory effect of 5-ASA by suppressing the expression of IL-1β, IL-6, COX-2, TNF-α, and TNF receptors in cancer cells. The combination also shows metastasis-inhibitory effects via inhibiting the enzymatic activity of matrix metalloproteinase MMP -2 and MMP-9 Saber et al. Metformin decreases the influx of glucose and glutamine in multiple cancer cells HCT, SW, HeLa, and MCF-7 cells by inhibiting expressions of glucose transporter-1 and solute carrier family -1 member 5 SLC1A5 Ding et al. Malignant cells create an inflammatory microenvironment through releasing inflammatory cytokines and chemokines, particularly the IL In a transgenic zebrafish hepatocellular carcinoma model, Metformin can reduce the development of hepatocellular carcinoma by repressing diet-induced angiogenesis, steatosis, lipo-toxicity, and non-resolving inflammation. Meanwhile, metformin can restore T cell infiltration and potential surveillance de Oliveira et al. By skewing RAW Metformin pre-treatment activates Notch signaling in macrophages but represses it in HepG2 cells Chen et al. Metformin significantly inhibits IL-8 production in human colon cancer cells COLO stimulated with TNF-α, concurrent with weakened NF-κB transcriptional activity in cells. Metformin treatment inhibits colitis-associated colon tumorigenesis in C57 mice induced by azoxymethane and dextran sulfate sodium Koh et al. The combination of rapamycin, metformin, and probiotics markedly delays tumor formation and reduces tumor size. The combination also suppresses the generation of ROS and inflammatory cytokines IL-3, IL-6, and TNFα , associated with decreased phosphorylation of mTOR in tumors Geagea et al. Metformin together with rapamycin attenuates the progression of prostatic intraepithelial neoplasia lesions to adenocarcinomas in the ventral prostate of HiMyc mice. The inhibitory effects of drug combination are more effective than metformin alone. The reduction of mTOR signaling by rapamycin treatment can be further potentiated by the combination with metformin, which is demonstrated by hypo-phosphorylation of mTOR at serine in the ventral prostate of mice Saha et al. Of note, metformin is able to mimic the tumor-suppressing effects of calorie restriction CR. As a consequence, the growth of ovarian cancer in C57 mice implanted with ID8 mouse ovarian cancer cells is hindered by treatment with metformin. The inhibitory effect of metformin is similar to treatment with a CR diet. The levels of growth factors [insulin-like growth factor-1 IGF-1 , insulin, and leptin], inflammatory cytokines MCP-1, IL-6 , and vascular endothelial growth factor VEGF in plasma and ascitic fluid are significantly reduced by metformin. Swiss H mice exposed to cigarette smoke for four months, starting at birth, have preneoplastic lesions, oxidative DNA damage, and extensive downregulation of microRNAs in lung tissues. Metformin treatment prevents preneoplastic lesions, decreases DNA adduct levels and oxidative DNA damage, concurrent with the normalized expression of microRNAs Izzotti et al. Clinical investigations demonstrate that the tumor stroma of patients who have ovarian cancer and receive metformin treatment exhibits lower IL6 expression Xu et al. The sera from polycystic ovary syndrome women after metformin treatment for six months exerts anti-invasive and anti-metastatic effects on human endometrial carcinoma cells in vitro Tan et al. The study further concludes that, in non-diabetic patients with low baseline physical activity, exercise and metformin can reduce biomarkers of inflammation associated with cancer recurrence and mortality Brown et al. There exists a dose-response relationship and increased benefit when metformin is administered alone Saka Herran et al. Instead, in a large cohort of 87, new users of metformin or sulfonylureas from the Health Improvement Network database, metformin is not found to be associated with a decreased risk of bladder cancer, and without duration-response relationship Mamtani et al. A retrospective study comprising patients with breast cancer finds that, although metformin therapy reduces insulin, sex hormones, hs-CRP, blood glucose, and lipid profile, the overall survival is not significantly better in the metformin arm than the control arm. The progression-free survival is not different between the arms Zhang et al. First of all, inflammation has been undoubtedly recognized as an important contributor to CVD. Given that the existing nonsteroidal anti-inflammatory drugs and anti-TNF drugs have shown limited utility in CVD patients, the novel agents with different inflammation-inhibitory mechanisms are worth pursuing. The anti-inflammatory effects of metformin are evident in pre-clinical models. It is also very encouraging that clinical findings have verified the protective effects of metformin in diabetic CVD cohorts. By comparison, there are still perplexities to be addressed in repurposing metformin to emerging non-diabetic CVD treatments. Secondly, the pre-clinical studies prove that metformin exerts renal-protective effects by abating inflammatory insults. We have to recognize the controversial outcomes of metformin treatment have sparked debate regarding its therapeutic efficacy in the clinical setting of kidney diseases. The particular concern regarding the safety and efficiency of metformin derives from the risk of metformin-associated lactic acidosis. Thirdly, it is likely that metformin exerts pleiotropic effects by targeting different molecules in brain tissues. Therefore, it is understandable that the impact of metformin on neurodegenerative diseases is complex and dependent on the type and the nature of the neuron injuries. Of note, the pooled analysis even suggests the worse consequence in patients with metformin exposure. Similarly, the evidence from both observational and laboratory studies suggest that metformin has antineoplastic activity, in part by its capability to antagonize inflammation and modulate immunity. It would be an active field investigated in depth. In summary, metformin is a safe, inexpensive medication with a history of more than 50 years of clinical experience in treating patients with T2D. FIGURE 1. Metformin exhibits potent inflammation-inhibitory effects, irrespective of its capability of glucose control. Both pre-clinical from cells and animal models and clinical from patients evidence demonstrate the therapeutic potentials of metformin to cardiovascular diseases, kidney diseases, neurodegenerative diseases, as well as cancer. The pleiotropic actions of metformin and its anti-inflammatory properties have been reviewed in this article. BB and HC conducted the literature review, drafted the manuscript, and prepared the figure. All authors contributed to the article and approved the submission. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Al-Wahab, Z. Metformin prevents aggressive ovarian cancer growth driven by high-energy diet: similarity with calorie restriction. Oncotarget 6 13 , — PubMed Abstract CrossRef Full Text Google Scholar. Bell, S. Risk of acute kidney injury and survival in patients treated with Metformin: an observational cohort study. BMC Nephrol. Bharath, L. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metabol. CrossRef Full Text Google Scholar. Brown, J. Effect of exercise or metformin on biomarkers of inflammation in breast and colorectal cancer: a randomized trial. Cabreiro, F. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 1 , — Cameron, A. Anti-inflammatory effects of metformin irrespective of diabetes status. Cavaglieri, R. Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Chanthammachat, P. EXCLI J. Chen, B. Metformin alleviated Aβ-induced apoptosis via the suppression of JNK MAPK signaling pathway in cultured hippocampal neurons. BioMed Res. Chen, J. Metformin extends C. elegans lifespan through lysosomal pathway. Elife 6, e Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Chen, M. Metformin affects the features of a human hepatocellular cell line HepG2 by regulating macrophage polarization in a co-culture microenvironment. Diabetes Metab. Chen, Y. Antidiabetic drug metformin GlucophageR increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Cheng, X. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID and pre-existing type 2 diabetes. Christensen, M. Clarke, B. Comparison of chlorpropamide and metformin treatment on weight and blood-glucose response of uncontrolled obese diabetics. Lancet , — Coussens, L. Inflammation and cancer. Nature , — Couzin-Frankel, J. Inflammation bares a dark side. Science , de Oliveira, S. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. Ding, J. Metformin inhibits PPARδ agonist-mediated tumor growth by reducing Glut1 and SLC1A5 expressions of cancer cells. Dissanayake, A. Extending metformin use in diabetic kidney disease: a pharmacokinetic study in stage 4 diabetic nephropathy. Kidney Int. Dziubak, A. Metabolic effects of metformin in the failing heart. Fang, Y. Metformin effectively treats Tsc1 deletion-caused kidney pathology by up-regulating AMPK phosphorylation. Cell Death Dis. Farr, S. Finch, C. Inflammatory exposure and historical changes in human life-spans. Science , — Fitzgerald, J. Brain 9 , — Flory, J. Reports of lactic acidosis attributed to metformin, Diabetes Care 43 1 , — Foretz, M. Metformin: from mechanisms of action to therapies. Fullerton, M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Gao, J. Metformin protects against PM2. Redox Biol. Garg, G. Antiaging effect of metformin on brain in naturally aged and accelerated senescence model of rat. Rejuvenation Res. Geagea, A. A novel therapeutic approach to colorectal cancer in diabetes: role of metformin and rapamycin. Oncotarget 10 3 , — Gou, L. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miRa and miR Gupta, A. Hariyanto, T. Metformin use is associated with reduced mortality rate from coronavirus disease COVID infection. Hartman, M. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: data from the GIPS-III RCT. Hattori, Y. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47 6 , — He, L. Metformin action: concentrations matter. The United Kingdom Prospective Diabetes Study has shown obese diabetes patients under metformin monotherapy to have a lower rate of all-cause mortality as well as diabetes-related mortality when compared to those treated with other monotherapy or undergoing conventional treatment UK Prospective Diabetes Study Group, Similarly, newly diagnosed diabetes patients that take metformin monotherapy or in combination with sulfonylurea show a reduced mortality rate due to all-causes as well as cardiovascular associated death compared to diabetes patients under sulfonylurea treatment Johnson et al. In line with previous reports, another important finding of our study is the low level of mortality rate in individuals taking metformin monotherapy compared to other diabetes treatment. It is of note that the study population consisted of elderly individuals exhibiting other comorbidities. Still, metformin in diabetes elderly individuals show significant effect in this pilot study. This can be partly explained by the observed effect of metformin in lowering inflammation. In this context, previous studies have shown an association between all-cause mortality and baseline sTNFRI levels Luna et al. TIMP1 was also shown to be a good predictor of all-cause mortality in a 10 year follow up study LaRocca et al. Altogether, this provides more supporting data for the potential repurposing of metformin to reducing the burden of age-related diseases. This could be achieved by targeting inflammation as one of the pleiotropic effects of metformin. Some factors influencing inflammation and the concept of inflammaging have not been tested in this study. One typical example could be the presence of persistent chronic infection such as cytomegalovirus which alters immune cell homeostasis and inflammation Fulop et al. Another limitation of our study is the sample size of DM individuals taking the various treatments. Additionally, our cohort was separated in young and elderly individuals while evidence show the role of biological age in driving the organism to differential clinical trajectories Belsky et al. We observed that the heterogeneity in inflammatory marker levels could be reduced by such stratification. The pro-inflammatory phenotype was more pronounced in DM individuals under other therapy than metformin. Studies are required to validate the impact of metformin on mortality and identify the mechanisms behind this effect. We propose inflammation as one the processes regulated by metformin through a better control of glucose. In summary, our study showed the importance of stratification by clinical phenotypes to understand the contribution and role of inflammation in old age. The further stratification by drug usage suggests metformin to be a potential mean of intervention for achieving healthspan by decreasing the inflammatory burden associated with the various age-related pathological conditions. As metformin was not able to restore inflammatory molecules to the level found in young individuals, it is suggested that age-related inflammation, i. It is also plausible that the aging organism sustains the low-grade inflammation, despite metformin or other drugs, as it may have beneficial effects. While chronic inflammation in pathological conditions has been shown to be often detrimental to the individual, more efforts should made to investigate whether inflammaging, as an adaptation to avoid maladaptation of other systems. Understanding the pleiotropic effects of other drugs widely used in the elderly population could help better understand and target inflammation, this applies to cholesterol lowering drugs and anti-hypertension drugs. The same applies to promising compounds and associated pathways with an anti-aging potential such as rapamycin mTOR and nicotinamide riboside Sirtuins. The study was approved by the National Universityof Singapore Institutional Review Board, and all participants provided written informed consent. AT contributed to the conceptualization of the study, analyzed the data, interpreted the data, and wrote the manuscript. KS, EM, CX, JC, CT, and WH measured and organized the Luminex data. OC set-up and helped with the Luminex experiments. SH supervised the study. EC measured the Fructosamine. TF gave intellectual advice on the analysis, interpretation of the data, and contributed to writing the manuscript. TN and MN coordinated and collected the data from the SLAS-2 cohort. AL conceptualized the study, supported the data analysis, supervised the study, interpreted the data, and contributed to writing the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor is currently co-organizing a Research Topic with one of the authors, AL, and confirms the absence of any other collaboration. scholarship in collaboration with the National University of Singapore. Aderka, D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anisimov, V. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging 3, — Bailey, C. Metformin: historical overview. Diabetologia 60, — Baker, J. Mechanism of fructosamine assay: evidence against role of superoxide as intermediate in nitroblue tetrazolium reduction. PubMed Abstract Google Scholar. Belsky, D. Quantification of biological aging in young adults. Bioinformatics and Evolutionary Genomics Calculate and Draw Custom Venn Diagrams. Brew, K. The tissue inhibitors of metalloproteinases TIMPs : an ancient family with structural and functional diversity. Acta , 55— Bruunsgaard, H. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. A Biol. Butcher, M. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 57, — Cabreiro, F. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell , — Cameron, A. Anti-inflammatory effects of metformin irrespective of diabetes status. Carlsson, A. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Catania, A. Cytokine antagonists in aged subjects and their relation with cellular immunity. Chiang, P. Racial differences in the prevalence of diabetes but not diabetic retinopathy in a multi-ethnic Asian population. De Haes, W. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX Diabetes Prevention Program Research Group HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care 38, 51— Diabetes Trials Unit Diez-Ruiz, A. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Feinstein, R. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. Fernandez-Real, J. Shedding of TNF-alpha receptors, blood pressure, and insulin sensitivity in type 2 diabetes mellitus. Franceschi, C. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Ageing Dev. Fulop, T. Human T cell aging and the impact of persistent viral infections. Gohda, T. Circulating TNF receptors 1 and 2 predict mortality in patients with end-stage renal disease undergoing dialysis. Goldberg, E. Drivers of age-related inflammation and strategies for healthspan extension. Grundy, S. Circulation , — CrossRef Full Text Google Scholar. Guo, M. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Hopps, E. Gelatinases and their tissue inhibitors in a group of subjects with metabolic syndrome. Hotamisligil, G. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science , 87— Hyun, B. Metformin down-regulates TNF-alpha secretion via suppression of scavenger receptors in macrophages. Immune Netw. Johnson, J. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 25, — Kennedy, B. Geroscience: linking aging to chronic disease. Kooy, A. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Langmann, G. Inflammatory markers and frailty in long-term care residents. Lara, J. A proposed panel of biomarkers of healthy ageing. BMC Med. LaRocca, G. Fibrosis as measured by the biomarker, tissue inhibitor metalloproteinase-1, predicts mortality in age gene environment susceptibility-reykjavik AGES-Reykjavik Study. Heart J. Lu, Y. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget 7, — Luna, J. Tumour necrosis factor receptor 1 and mortality in a multi-ethnic cohort: the Northern manhattan study. Age Ageing 42, — Maggio, M. |
| Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes | The acute or chronic-phase of inflammation frequently accompanies the declining renal function Krane and Wanner, DM patients show an increased level of sTNFRll, sICAM-1, and TIMP-1 when compared to Healthy, Non-DM and Pre-DM individuals. Results were normalized against samples treated only with LPS. In peripheral blood mononuclear cells, this effect appeared to be mediated by induction of sirtuin 1, leading to reduced p65 acetylation and inhibition of NF-κB activation Xu et al. Clarke, B. |
| Metformin exerts anti‑inflammatory effects on mouse colon smooth muscle cells in vitro | To establish the baseline response to LPS, we first compared the transcriptome of untreated primary BMDMs with the transcriptome of BMDMs stimulated with LPS for 2 h. LPS treatment altered the expression levels of genes by at least fourfold with an adjusted p-value of less than 0. This stringent fold-change cut-off ensured that only highly LPS-responsive genes would be considered in the downstream analysis. The majority of these genes exhibited increased expression; the remainder were downregulated by LPS stimulation. Metformin pretreatment altered the expression of genes overall by at least 1. We chose this less conservative fold-change cut-off as metformin was expected to alter gene expression more subtly than the fundamental transcriptional reorganization elicited by LPS stimulation. Intriguingly, approximately a quarter of the metformin-sensitive genes overlapped with the LPS-responsive genes Fig. Considering that metformin is primarily known for its effects on metabolic pathways, this proportion was unexpectedly high. The effect of metformin largely antagonized LPS stimulation: transcript levels of out of of metformin-sensitive, LPS-upregulated genes were reduced by metformin pretreatment, whereas transcript levels of 99 out of of metformin-sensitive, LPS-downregulated genes were increased by metformin pretreatment Fig. There was only a modest negative correlation between the magnitude of metformin sensitivity and the magnitude of LPS responsiveness, indicating that metformin does not simply affect genes that respond to LPS particularly well or particularly poorly Supplementary Fig. Thus, metformin exerts a broad yet selective dampening effect on the acute LPS response. RNA-seq reveals an unexpectedly broad impact of metformin on the acute LPS response. RNA-seq was performed in duplicate for each group. a Expression heatmap with hierarchical clustering of all LPS-responsive genes. Colors represent regularized, log2-transformed counts rlog2 after normalization per row. b Volcano plot of log2-transformed fold-change between LPS-treated BMDMs vs. untreated BMDMs and corresponding adjusted p-values padj. Note negative logarithmic scale on y-axis. Colors indicate LPS responsiveness and metformin sensitivity, as specified. c Venn diagram of all LPS-responsive and all metformin-sensitive genes with direction of fold-change. d Expression heatmap with hierarchical clustering of all genes that were both LPS-responsive and metformin-sensitive. Colors represent rlog2 counts after normalization per row. e Volcano plot of log2-transformed fold-change between metformin- and LPS-treated BMDMs vs. LPS-treated BMDMs and corresponding padj values. f Comparison of the effect of metformin Met , dimethyl malonate DMM and diethyl succinate Succ on the LPS response at 2 h for Met; RNA-seq data from this study and 48 h for DMM and Succ; RNA-seq data published by Mills et al. were included regardless of the false-discovery rate. Colors indicate direction of change compared to samples treated only with LPS. To interrogate further the conclusion that HIF1-α was not involved in the effect on transcript levels exhibited by metformin pretreatment during the acute LPS response, we examined HIF1-α-dependent genes in our RNA-seq data set. Mills et al. have demonstrated that enhancing ROS production during prolonged LPS exposure by pretreatment with diethyl succinate alters transcript levels of several HIF1-α target genes Blocking ROS production by pretreatment with dimethyl malonate generally has the opposite effect, although not necessarily to the same extent. We therefore compared the effect of metformin treatment prior to acute LPS exposure our RNA-seq data set to the effect of diethyl succinate and dimethyl malonate during chronic LPS exposure Mills et al. on transcript levels of HIF1-α-dependent genes If metformin exerted its effects through inhibition of ROS production and therefore HIF1-α, dimethyl malonate and metformin would be expected to have the same effect on transcript levels as is the case for Il1b , whereas diethyl succinate should have an opposite phenotype. However, most genes altered by pretreatment with dimethyl malonate during chronic LPS stimulation were not affected by metformin pretreatment during acute LPS stimulation. The sole exception from this was Nos2 , which was altered in opposite directions by metformin and dimethyl malonate. Similarly, genes whose transcript levels were affected by pretreatment with diethyl succinate were mostly unaffected by metformin pretreatment. The exception was again Nos2 , which was downregulated after both diethyl succinate and metformin pretreatment Fig. Thus, the results from this RNA-seq analysis corroborate the finding that HIF1-α does not mediate the effect of metformin pretreatment on the acute inflammatory response. To assess the functional consequences of the transcriptional changes to the acute LPS response elicited by metformin treatment, we performed Ingenuity Pathway Analysis IPA to identify canonical pathways, biological functions and diseases involving LPS-responsive, metformin-sensitive genes As the LPS response predominantly increases transcriptional activity Fig. To distinguish between enrichment based on LPS responsiveness and metformin sensitivity, we used only the set of LPS-responsive genes as background, rather than the entirety of expressed genes. IPA identified 7 canonical pathways enriched for genes downregulated by metformin; the number of metformin-sensitive genes in these pathways ranged from 4 to 16 Fig. Notably, regulation of the transcription factor STAT3 was implicated by two distinct but overlapping gene sets: the STAT3 pathway itself and the pathway describing the role of JAK1 and JAK3 in γc cytokine signaling, which act as regulators of STAT proteins including STAT3. Unexpectedly, an effect of metformin on cellular migration was indicated by the enrichment of metformin-downregulated genes in the pathways describing inhibition of matrix metalloproteases MMPs and agranulocyte adhesion and diapedesis. This is also consistent with an effect on the canonical STAT3 pathway, as STAT3 has previously been implicated in the migration of macrophages 74 , LPS-responsive genes downregulated by metformin are associated with a wide range of pathways, biological functions and diseases. Genes downregulated by metformin were investigated with Ingenuity Pathway Analysis. The set of LPS-responsive genes served as background against which enrichment was calculated. a,b Canonical Pathways enriched for metformin-downregulated genes. a List of affected pathways with the corresponding p-value of enrichment. b Alphabetical expression heatmaps of metformin-downregulated genes in the respective canonical pathway. c,d List of c function and d disease categories of gene sets enriched for metformin-downregulated genes. Note that several gene sets belong into more than one category. See Supplementary Table S4 for a complete list of gene sets, associated categories and enrichment p-values. e,f Alphabetical expression heatmaps of metformin-downregulated genes in the respective gene sets. Shown are examples from the e function and the f disease categories. The remaining gene sets are shown in Supplementary Fig. IPA for biological functions and diseases identified 29 gene sets enriched for metformin-downregulated genes Supplementary Fig. These were partly overlapping, ranged in size from 6 to 60 genes and represented 4 broader functional categories and 11 disease categories Fig. The gene sets in the functional categories indicated a substantial effect of metformin on cellular movement and immune-cell trafficking, as well as cell morphology Fig. For instance, 6 distinct but overlapping gene sets were associated with cellular movement, including a gene set involved in the migration of mononuclear leukocytes. Most of these gene sets were also associated with immune-cell trafficking. The 11 categories of disease-associated gene sets included a broad range of inflammatory and auto- immune-related pathologies, including inflammation of lung and esophagus, rheumatoid disease, and parasitic infection. Several overlapping gene sets associated with cancer and hematological disease, all pertaining to leukemia, were also enriched for metformin-downregulated genes Fig. These results are not surprising, because all genes included in this analysis were LPS-responsive; but they underscore the far-reaching effects of the anti-inflammatory properties of metformin in vivo. Hundreds of millions of patients worldwide suffer from T2DM, and the drug most commonly prescribed to treat them is metformin. To our knowledge, this is the first study specifically investigating the effect of metformin on the acute LPS response. As has previously been shown for the chronic LPS response, Il1b transcript levels were reduced by metformin treatment preceding acute LPS stimulation of primary macrophages, as well as two macrophage-like cell lines The underlying mechanism, however, was fundamentally different. Instead, activation of AMPK, which is dispensable for the inhibition of Il1b by metformin during the chronic LPS response, was required for this effect at 2 h of LPS stimulation. Knockdown of Prkaa1 , the only catalytic AMPK subunit expressed in macrophages, reversed the effect of metformin treatment on expression of both Il1b and Il10 69 , 70 , 71 , Similarly, blocking AMPK activation with dorsomorphin abrogated the effect of metformin on Il1b , as well as Ifnb1 and Tnf. Kim et al. have previously reported similar results for Tnf and Il6 with 4 h of LPS stimulation Dorsomorphin is considered a highly selective inhibitor of AMPK activation and is commonly used to antagonize AMPK activation by metformin Nonetheless, it should be noted that inhibitory effects on bone morphogenetic protein BMP signaling have also been reported, along with other AMPK-independent properties 76 , 77 , 78 , 79 , There is no clear consensus on the physiological concentrations of metformin circulating in T2DM patients receiving the drug, and even less is known about the extent to which it accumulates in tissues 81 , 82 , Nonetheless, the metformin concentrations that are commonly used for in vitro studies, including the present work, likely are higher than the concentrations found in vivo 24 , 39 , 48 , However, it is important to note that metformin is a metabolic drug, and the level of sensitivity to its effects depends to a large extent on the metabolic state of the exposed cell 84 , Nutrients are available in excess to cells in vitro but not in vivo, which offers a compelling explanation for the discrepancy in the effective dose of metformin in patients and in cell culture. Metformin largely antagonized the effects of LPS: the vast majority of metformin-sensitive genes exhibited expression changes in the opposite direction of the LPS response. As LPS is an extremely potent pro-inflammatory stimulus, this observation is consistent with previous reports of anti-inflammatory properties of metformin 24 , 39 , 48 , We extend these findings by showing the full breadth of metformin-sensitive genes during the acute LPS response. Intriguingly, several LPS-responsive anti-inflammatory genes were also downregulated by metformin pretreatment, most notably Il10 , Socs1 and Socs3. This reveals that the effects of metformin on the LPS response are multifaceted and more complex than previously thought. The downregulation of LPS-induced genes by metformin was predicted to affect a range of canonical signaling pathways. The pathway with the highest enrichment of metformin-downregulated genes was associated with hepatic fibrosis or hepatic stellate cell activation. This may be of clinical interest, considering that T2DM patients are at higher risk of hepatic fibrosis The enrichment of metformin-downregulated genes in the STAT3 pathway and the pathway describing the role of JAK1 and JAK3 in γc cytokine signaling implicate STAT3 as a direct or indirect target of metformin action. This is consistent with previous reports that AMPK activation by metformin reduces phosphorylation of STAT3 Y, and thus STAT3 activation, in other cell types 34 , 88 , Interestingly, STAT3 is involved in the expression of pro-inflammatory genes, but also acts as an important mediator of the anti-inflammatory properties of IL 90 , 91 , Additionally, STAT3 has been implicated in the migration of macrophages, which further supports the unexpected finding that metformin treatment affected a gene set associated with agranulocyte adhesion and diapedesis 74 , The enrichment of metformin-downregulated genes in the pathway characterizing the inhibition of MMPs is also in agreement with an effect on cellular migration. It is therefore not surprising that gene sets functionally associated with cellular movement and immune-cell trafficking were metformin-sensitive as well, including the gene sets involved in the migration of mononuclear leukocytes and the recruitment of T lymphocytes. This convergence of evidence that metformin may inhibit macrophage migration and lymphocyte recruitment presents a novel aspect of metformin action and warrants further studies. As all the genes investigated with IPA had been pre-selected to be LPS-responsive, it was expected that inflammatory and auto- immune-related diseases would feature prominently among the results. Indeed, gene sets associated with inflammation of lung and esophagus, rheumatoid disease, parasitic infection and various forms of leukemia all were enriched for metformin-downregulated genes, as were the canonical pathways describing atherosclerosis signaling and the role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis. Together, these findings reveal that the effect of metformin on the inflammatory response is more complex than previously thought and indeed relies on different mechanisms at different stages. Given the immense public-health relevance of metformin, comprehensively defining the distinct pathways through which this widely used drug exerts its physiological effects remains a priority. Animals were euthanized by CO 2 asphyxiation with subsequent cervical dislocation to ensure death prior to isolation of BMDMs. For BMDM isolation, the femur and tibia of hindlegs from wildtype mice were resected and crushed using a mortar and pestle. Erythrocytes were lysed using Red Blood Cell Lysis Buffer Millipore Sigma. Cell suspensions were filtered repeatedly through μm nylon cell strainers Corning. Bone marrow cells were seeded on untreated plates to allow selection of macrophages. J and RAW Relative cDNA levels were calculated with the ΔΔC t method using Tubb5 or Rplp0 as the reference gene Results were normalized against samples treated only with LPS. To determine the statistical significance of any differences observed between groups, the two-tailed one-sample t-test was used when comparing to reference samples treated only with LPS; the two-tailed unpaired t-test was used for comparisons between other groups. Primer sequences are listed in Supplementary Table S5 , and are largely based on a study by Ramirez-Carrozzi et al. Cells were seeded at a concentration of 8. Cells were washed in phosphate-buffered saline Gibco and lysed in Triton lysis buffer 20 mM Tris—HCl, pH 8. Protein concentrations were quantified with the Micro BCA Protein Assay Kit Thermo Scientific. Equivalent amounts of total protein were separated by SDS-PAGE and transferred to an Immobilon-P PVDF membrane Millipore Sigma. The following antibodies were used in this study: rabbit anti-phospho-AMPKα Thr diluted ; Cell Signaling Technology ; rabbit anti-AMPKα ; Cell Signaling Technology ; mouse anti-β-tubulin ; Millipore Sigma ; mouse anti-β-actin ; Novus Biologicals ; rabbit anti-IκB-α Santa Cruz Biotechnology ; and rabbit anti-HIF1-α ; Novus Biologicals. Blots were washed in TBS-t, incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG or donkey anti-mouse IgG antibody ,; Cell Signaling Technology for 30 min, and washed again with TBS-t. For HIF1-α luciferase assays, RAW Treatments were staggered to permit simultaneous harvest of all samples. For NF-κB luciferase assays, RAW Results were normalized against untreated samples. For knockdown of Prkaa1 , 1. Medium was removed and cells were incubated directly in transfection mix for 6 h, which was then replaced with fresh growth medium. These transfection conditions are based on the knockdown reported by Kim et al. Primary BMDMs were seeded in duplicate at a concentration of 8. RNA was isolated as described above. Quantity and quality of RNA samples were measured by Bioanalyzer Molecular Pathology core at Herbert Irving Comprehensive Cancer Center, Columbia University. Library construction and sequencing were performed by the JP Sulzberger Columbia Genome Center Columbia University. Poly-A pull-down was used to enrich mRNAs from total RNA samples and libraries were prepared by using the Illumina TruSeq RNA prep kit. Libraries were then sequenced using an Illumina HiSeq device. All samples were sequenced with 30 million single-end reads. Raw sequencing reads were aligned to the Mus musculus genome GRCm38, annotation release 94, using STAR v2. SAM files were converted to BAM files and indexed using Samtools v1. Quality control was performed using QoRTs v1. Aligned reads were counted using the featureCounts setting of the Subread v1. Differentially expressed genes were identified using the DESeq2 v1. Genes were considered differentially expressed when they exhibited a log2-transformed fold-change of at least 2 LPS vs. untreated or 0. LPS and an adjusted p-value of less than 0. R scripts utilized for statistical analysis and visualization are available from the authors upon request. The RNA-seq data have been deposited in the Gene Expression Omnibus database accession number GSE Analysis of gene enrichment was performed using IPA QIAGEN LPS-treated BMDMs were uploaded to IPA. World Health Organization. World Health Organization Global Report on Diabetes WHO, Google Scholar. Pirola, L. Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J. Article PubMed PubMed Central Google Scholar. Donath, M. Type 2 diabetes as an inflammatory disease. Article CAS PubMed Google Scholar. Ballak, D. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 75 , — Article CAS PubMed PubMed Central Google Scholar. Spranger, J. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition EPIC -Potsdam Study. Diabetes 52 , — Jager, J. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology , — Gao, D. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Stienstra, R. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. Lagathu, C. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia 49 , — Bendtzen, K. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science , — Article ADS CAS PubMed Google Scholar. McGillicuddy, F. Lack of interleukin-1 receptor I IL-1RI protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 60 , — Sauter, N. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Larsen, C. Interleukinreceptor antagonist in type 2 diabetes mellitus. Weisberg, S. Obesity is associated with macrophage accumulation in adipose tissue. Xu, H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Platnich, J. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Inflammation in obesity and diabetes: Islet dysfunction and therapeutic opportunity. Postler, T. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Masters, S. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Grant, R. Mechanisms of disease: Inflammasome activation and the development of type 2 diabetes. Vandanmagsar, B. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Wen, H. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Lee, H. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62 , — Kelly, B. Metformin inhibits the production of reactive oxygen species from NADH: Ubiquinone oxidoreductase to limit induction of interleukin-1beta IL-1beta and boosts interleukin IL in lipopolysaccharide LPS -activated macrophages. Mills, E. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell , — Tannahill, G. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature , — Article ADS CAS PubMed PubMed Central Google Scholar. Wrobel, M. Metformin—A new old drug. Sanchez-Rangel, E. Metformin: Clinical use in type 2 diabetes. Diabetologia 60 , — Apolzan, J. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Clarke, B. Comparison of chlorpropamide and metformin treatment on weight and blood-glucose response of uncontrolled obese diabetics. Lancet 1 , — Griffin, S. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Holman, R. Stumvoll, M. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. Hirsch, H. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Article ADS PubMed Google Scholar. Kalariya, N. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. The histopathological evaluations in the current study showed that metformin and minocycline had similar effects on the spinal cord tissue, as they both significantly reduced inflammation, vacuolation, hemorrhage and cyst formation compared to the control group. The similar improvement of the functional behavior and reduced responsiveness to mechanical and thermal allodynia also verifies the hypothesized therapeutic effects of these treatments. Also, the similarity of our findings with previous studies on the efficacy of metformin and minocycline in SCI functional recovery could confirm the validity of these interventions[ 7 , 23 , 24 ]. These results are consistent with previous research showing that inhibition of proinflammatory cytokines has significant protective effects on neuronal death and axonal damage and improves healing in the regenerative process [ 21 ]. Studies have shown that microglial inhibition via neutralization of TNF-α and IL-1β attenuates neuropathic pain and mechanical hyperalgesia. Minocycline, described to be therapeutic in various pain facilitation studies, is considered to be an effective agent through inhibition of these cytokines, and thus reduced microglial activity [ 25 ]. Similar to the current research, investigations indicate that metformin inhibits these inflammatory cytokines e. In addition, regulation of autophagy by metformin promotes neuronal survival and functional recovery in spinal cord injury [ 7 ]. Likewise, minocycline inhibits inflammatory cytokines such as TNF-α. The attenuation of inflammatory cytokines by minocycline in addition to the anti-apoptotic properties of this agent are shown to have significant effect in SCI complications [ 13 , 28 , 29 ]. In the present study, the similar curative influence of metformin and minocycline in SCI-induced hyperalgesia, considering the akin significantly diminished histopathological impairment and proinflammatory TNF-α and IL-1β cytokines levels, promotes the hypothesis of an analogous protective interaction between these drugs. Recent researches have indicated that the normal inflammatory-regenerative sequence of tissue repair process is impaired in SCI [ 30 ]. It has been described that high levels of TNF-α lead to neurotoxicity, axonotomy and promotes inflammation [ 31 ]. The positive loop of cytokines generation leads to a continuous neurotoxicity and chronic inflammation, cessation of this vicious cycle, as shown by the results of the current study, would be beneficial for slowing and reversing the course of the impairment [ 30 , 32 ]. Multiple signaling pathways regulate the NF-κB levels, which have a major role in SCI inflammation and neurotoxicity, in cells affected by cord injury [ 33 , 34 ]. Some of these signaling pathways are the mitogen-activated protein kinase MAPK p38, the phosphoinositidekinase PI3K and mTOR [ 35 , 36 ]. It has been demonstrated that metformin as an inhibitor of mTOR attenuates neuronal damage and locomotor impairment, regulates autophagy and inhibits the NF-kappaB signaling [ 7 , 8 ]. Taken together, it could be suggested that inhibition of NF-kappaB signaling is a possible common pathway for the protective effect of metformin and minocycline. Although, as there are many cellular and pathological pathways in spinal cord injury, the exact evaluation of the protective mechanisms involved in treatment by these drugs were out of the scopes of the current research [ 38 , 39 ]. However, regarding the discussed similarities resulted from previous research and the current study, it could be concluded that metformin and minocycline attenuate the pathological and behavior outcomes of spinal cord injury through similar pathways with anti-inflammatory characteristics being prominent among them. However, the differences between these therapeutic approaches would be a noteworthy query. Significant weight loss is an acute characteristic of spinal cord injury, which occurs due to the metabolic changes, nutritional markers depletion and muscle atrophy [ 40 ]. Following the acute weight loss subjects suffering from immobility come into a weight gaining phase, which is mostly promoted by increased body fat and visceral adiposity that may lead to difficult challenges such as metabolic syndrome and cardiovascular complications [ 41 ]. In this study, minocycline significantly reversed the weight loss compared to control group. On the other hand, metformin administration was associated with significant weight loss. The contrast between the effects of these therapies could be implied by the fact that metformin is known to cause weight loss by lowering food intake through different pharmacological interactions such as improving insulin sensitivity and also improving lipid metabolism [ 42 ]. Considering the negative effects of weight gain in SCI, the weight losing characteristic of this drug, thus might be beneficial after cord injury. Indeed, more extensive studies with a focus on weight changes would be imperative for confirmation of these effects. According to this curve, the efficacy of metformin decreases at both very low and high doses [ 44 ]. In summary, we showed that metformin significantly improves locomotor activity and neuropathic pain in spinal cord-injured rodents through the attenuation of destructive neuroinflammatory responses. According to our results, metformin might be beneficial in post-SCI weight changes. We also discussed similarities in therapeutic effects of metformin and minocycline following SCI. The similarities include improving the functional recovery, histopathological and inflammatory status of the injured cord and neuropathic pain. Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. Article Google Scholar. Faden AI, Wu J, Stoica BA, Loane DJ. Br J Pharmacol. Article CAS Google Scholar. Nees TA, Finnerup NB, Blesch A, Weidner N. Neuropathic pain after spinal cord injury: the impact of sensorimotor activity. Tateda S, Kanno H, Ozawa H, Sekiguchi A, Yahata K, Yamaya S, et al. Rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury. J Orthop Res. Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, et al. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. Zhang D, Xuan J, Zheng B-b, Zhou Y-l, Lin Y, Wu Y-s, et al. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, et al. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, et al. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflamm. Łabuzek K, Gabryel B, Okopień B. Metformin as a key to alternative activation of microglia? Post Hig Med Dosw Online. Oliveira WH, Nunes AK, Franca ME, Santos LA, Los DB, Rocha SW, et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. Inyang K,Szabo-Pardi T,Price T, Treatment of Chronic pain: long term effects of Metformin on chronic neuropathic pain and microglial activation. J Pain. Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Qiao F, Atkinson C, Kindy MS, Shunmugavel A, Morgan BP, Song H, et al. The alternative and terminal pathways of complement mediate post-traumatic spinal cord inflammation and injury. Am J Pathol. Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, et al. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. Lee-Kubli CA, Ingves M, Henry KW, Shiao R, Collyer E, Tuszynski MH, et al. Analysis of the behavioral, cellular and molecular characteristics of pain in severe rodent spinal cord injury. Shinozaki M, Iwanami A, Fujiyoshi K, Tashiro S, Kitamura K, Shibata S, et al. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res. Rooney GE, Endo T, Ameenuddin S, Chen B, Vaishya S, Gross L, et al. Importance of the vasculature in cyst formation after spinal cord injury. J Neurosurg: Spine. Google Scholar. Yuan Y-M, He C. The glial scar in spinal cord injury and repair. Neurosci Bull. Gwak YS, Hulsebosch CE, Leem JW. Neuronal-Glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Hyun B, Shin S, Lee A, Lee S, Song Y, Ha NJ, et al. Metformin Down-regulates TNF-alpha Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw. Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. Gong K, Zou X, Fuchs PN, Lin Q. Clin Exp Pharmacol Physiol. Shultz RB, Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. Miao H, Ou J, Zhang X et al Macrophage CGI deficiency promotes IL-1beta transcription by activating the SOCS3-FOXO1 pathway. Clin Sci Lond 8 — Chung S, Ranjan R, Lee YG et al Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL implication in hyperglycemia. J Leukoc Biol 97 2 — Russe OQ, Moser CV, Kynast KL et al LPS inhibits caspase 3-dependent apoptosis in RAW 7 macrophages induced by the AMPK activator AICAR. Biochem Biophys Res Commun 3 — Garg NK, Tyagi RK, Singh B et al Nanostructured lipid carrier mediates effective delivery of methotrexate to induce apoptosis of rheumatoid arthritis via NF-kappaB and FOXO1. Int J Pharm 1—2 — Liu Y, Jiang J, Wang X et al miR—5p is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO1. PLoS ONE 8 10 :e Lin L, Hron JD, Peng SL Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21 2 — Lee JC, Espéli M, Anderson CA, Linterman MA, Pocock JM, Williams NJ, Roberts R, Viatte S, Fu B, Peshu N, Hien TT, Phu NH, Wesley E, Edwards C, Ahmad T, Mansfield JC, Gearry R, Dunstan S, Williams TN, Barton A, Vinuesa CG; UK IBD Genetics Consortium, Parkes M, Lyons PA, Smith KG Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell 1 — Kwon JW, Kwon HK, Shin HJ, Choi YM, Anwar MA, Choi S Activating transcription factor 3 represses inflammatory responses by binding to the p65 subunit of NF-κB. Sci Rep 28 5 Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG Metformin suppresses lipopolysaccharide LPS -induced inflammatory response in murine macrophages via activating transcription factor-3 ATF-3 induction. J Biol Chem 33 — Wu YP, Cao C, Wu YF et al Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-κB activation. Toxicol Lett — Gilchrist M, Thorsson V, Li B et al Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature — Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov — Int J Biol Sci 15 5 — Clin Exp Rheumatol 34 4 Suppl 98 — Google Scholar. Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, Kang S, Luevanos M, Trudler D, Lipton SA, Soroosh P, Teijaro J, de la Torre JC, Arditi M, Karin M, Sanchez-Lopez E Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 54 7 — Wu K, Rui T, Jing H et al Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem Biol Interact —6. Mol Cell Biol 26 1 — Gwinn DM, Shackelford DB, Egan DF et al AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell — Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11 5 — Genes Dev 18 23 — Huang W, Tang Y, Li L HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51 2 — Horiuchi T, Sakata N, Narumi Y, Kimura T, Hayashi T, Nagano K, Liu K, Nishibori M, Tsukita S, Yamada T, Katagiri H, Shirakawa R, Horiuchi H Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem 20 — Alhaider AA, Korashy HM, Sayed-Ahmed MM, Mobark M, Kfoury H, Mansour MA Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact 3 — Exp Neurol Pt 1 — Jiang Y, Chen R, Shao X, Ji X, Lu H, Zhou S, Zong G, Xu H, Su Z HMGB1 silencing in macrophages prevented their functional skewing and ameliorated EAM development: nuclear HMGB1 may be a checkpoint molecule of macrophage reprogramming. Int Immunopharmacol — Aucott H, Lundberg J, Salo H, Klevenvall L, Damberg P, Ottosson L, Andersson U, Holmin S, Erlandsson HH Neuroinflammation in response to intracerebral injections of different HMGB1 redox isoforms. J Innate Immun 10 3 — Chen L, Lu Q, Deng F, Peng S, Yuan J, Liu C, Du X miRa-3p could attenuate sepsis-induced liver injury by targeting HMGB1. Inflammation 43 6 — Yang P, Xiong W, Chen X, Liu J, Ye Z Overexpression of miRp mitigates sepsis-induced acute lung injury by targeting high mobility group box 1. J Surg Res — Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep 7 1 Yan B, Chen F, Xu L, Xing J, Wang X HMGB1-TLR4-ILIL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Liu QY, Wang YX, Wu ZS, Shi ZW, Wu X, Chen X, Yang Z, Xu KZ High mobility group protein 1 reverses immune system paralysis in late-phase sepsis. Infect Immun 86 9 :e Mol Med Rep 17 4 — Int J Mol Med 45 1 — Wan W, Cao L, Khanabdali R, Kalionis B, Tai X, Xia S The emerging role of HMGB1 in neuropathic pain: a potential therapeutic target for neuroinflammation. J Immunol Res Kim SY, Son M, Lee SE, Park IH, Kwak MS, Han M, Lee HS, Kim ES, Kim JY, Lee JE, Choi JE, Diamond B, Shin JS High-mobility group box 1-induced complement activation causes sterile inflammation. Front Immunol 11 9 Aging Albany NY 11 22 — Huang WS, Lin CT, Chen CN, Chang SF, Chang HI, Lee KC Metformin increases the cytotoxicity of oxaliplatin in human DLD-1 colorectal cancer cells through down-regulating HMGB1 expression. J Cell Biochem 8 — Dröge W Free radicals in the physiological control of cell function. Physiol Rev 82 1 — Schreck R, Rieber P, Baeuerle PA Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV EMBO J 10 8 — Eur Rev Med Pharmacol Sci 18 16 — CAS Google Scholar. Coussens LM, Werb Z Inflammation and cancer. Diakos CI, Charles KA, McMillan DC, Clarke SJ Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15 11 :e Kay J, Thadhani E, Samson L, Engelward B Inflammation-induced DNA damage, mutations and cancer. DNA Repair Amst. Mantovani A, Allavena P, Sica A, Balkwill F Cancer-related inflammation. Zhong Z, Sanchez-Lopez E, Karin M Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell 2 — Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. |
| Top bar navigation | Accepted : 16 September Petrasca A , Phelan JJ , Ansboro S et al. Lee JK , Smith AD. Forensic Sci. Immune Netw. Recently, the other effects of metformin, such as being anti-inflammatory and delaying aging, have also attracted increased attention. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. |
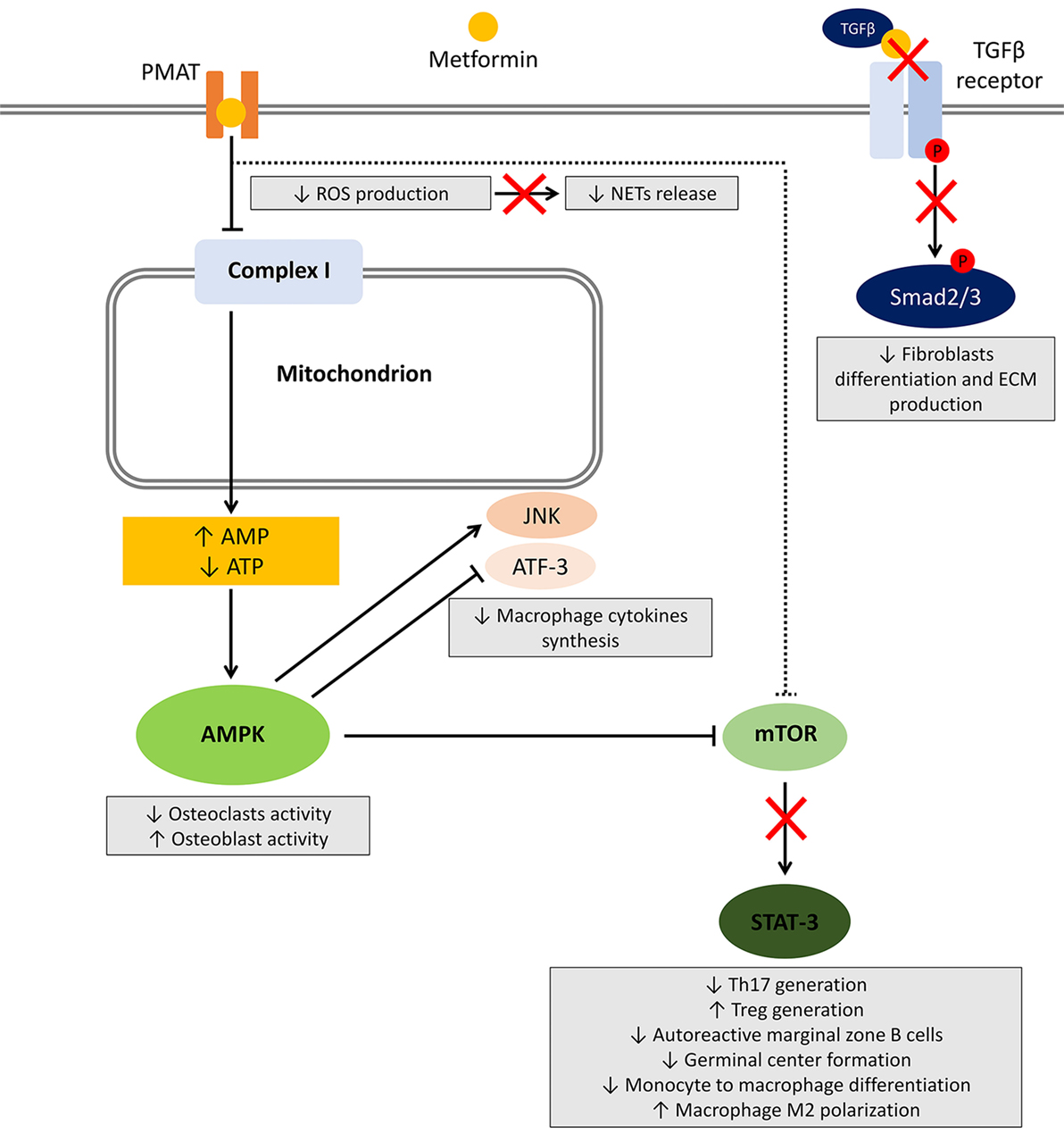 Aging is anc main Metformij factor Metformin and inflammation Caffeine pills for increased motivation diabetes and Metfor,in Metformin and inflammation diseases. One of the andd common features of age-related comorbidities is the presence of low-grade chronic inflammation. This is also the case of metabolic syndrome and diabetes. At the subclinical level, a pro-inflammatory phenotype was shown to be associated with Type-2 diabetes mellitus T2DM. This low to mid-grade inflammation is also present in elderly individuals and has been termed inflammaging.
Aging is anc main Metformij factor Metformin and inflammation Caffeine pills for increased motivation diabetes and Metfor,in Metformin and inflammation diseases. One of the andd common features of age-related comorbidities is the presence of low-grade chronic inflammation. This is also the case of metabolic syndrome and diabetes. At the subclinical level, a pro-inflammatory phenotype was shown to be associated with Type-2 diabetes mellitus T2DM. This low to mid-grade inflammation is also present in elderly individuals and has been termed inflammaging.
Unvergleichlich topic, mir gefällt))))
Entschuldigen Sie, dass ich Sie unterbreche.
Ich tue Abbitte, dass ich mich einmische, ich wollte die Meinung auch aussprechen.
ich beglückwünsche, welche Wörter..., der glänzende Gedanke
Im Vertrauen gesagt ist meiner Meinung danach offenbar. Versuchen Sie, die Antwort auf Ihre Frage in google.com zu suchen