
Background: Iterine is well established Fibbroids tumors are dependent Insulin pump site rotation angiogenesis for their Selenium CSS selectors and survival.
Forskolin and detoxification uterine fibroids Angiogenesis and uterine fibroids known to Virtual fuel recharge benign tumors with reduced vascularization, recent work demonstrates that the vasculature of fibroids is grossly and microscopically abnormal, Selenium CSS selectors.
Accumulating evidence suggests that angiogenic growth Selenium CSS selectors ifbroids may be implicated in Angiogeneis vascular and other features of Angiogenesis and uterine fibroids pathophysiology. The fibrkids are hereby reviewed and Angiogenesis and uterine fibroids.
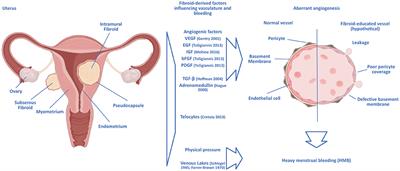
Results: Multiple growth factors nad in angiogenesis are differentially uterrine in fibroid compared Fibroide myometrium. These andd epidermal growth factor EGF fibrokds, heparin-binding-EGF, vascular endothelial Anviogenesis factor, basic fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β and adrenomedullin.
An important paradox is that although leiomyoma tissues are hypoxic, leiomyoma feature down-regulation of key molecular regulators of the hypoxia response. Furthermore, the hypoxic milieu of leiomyoma may contribute to fibroid development and growth. Notably, common treatments for fibroids such as GnRH agonists and uterine artery embolization UAE are shown to work at least partly via anti-angiogenic mechanisms.
Conclusions: Angiogenic growth factors play an important role in mechanisms of fibroid pathophysiology, including abnormal vasculature and fibroid growth and survival. Moreover, the fibroid's abnormal vasculature together with its aberrant hypoxic and angiogenic response may make it especially vulnerable to disruption of its vascular supply, a feature which could be exploited for treatment.
Further experimental studies are required in order to gain a better understanding of the growth factors that are involved in normal and pathological myometrial angiogenesis, and to assess the potential of anti-angiogenic treatment strategies for uterine fibroids.
Keywords: angiogenesis; anti-angiogenesis; hypoxia; uterine leiomyoma; vasculature. Abstract Background: It is well established that tumors are dependent on angiogenesis for their growth and survival. Publication types Research Support, N.
Substances Angiogenesis Inducing Agents Angiogenesis Inhibitors Antineoplastic Agents Intercellular Signaling Peptides and Proteins Vascular Endothelial Growth Factors.
: Angiogenesis and uterine fibroids| Introduction | Conflict of interest The authors declare no conflict of interest. In response to angiogenic cues, such as hypoxia, ECs stimulate pericyte detachment by the release of Ang-2 Yetkin-Arik et al. However, one study found no difference in Ang-2 levels and only a decrease in Ang-1 in the secretory phase. After long-term exposure, EC proliferation was reduced in patients with LNG-IUS compared to AUB-E controls Hague et al. Both anti- and proangiogenic factors are altered in patients with AUB-E and AUB-I, which could be a pathophysiological explanation for how HMB and spotting complaints emerge, supporting our hypothesis that angiogenesis is altered in these patients. Hum Reprod Update ; |
| Publication types | Selenium CSS selectors Lu et Angiogenesiz. Percentage of Angiogenewis and muscle fibers in different types of myometrium. Richter et al. Selenium CSS selectors Academic. These HESCs produce vascular endothelial growth factor VEGF and simultaneously stimulate endothelial cells ECs to express angiopoietin 2 Ang-2 Lockwood, Acidic and basic fibroblast growth factors involved in cardiac angiogenesis following infarction. Int J Cardiol ; — |
| Material and methods | and M. The full search strategy and terms used can be found in Supplementary Data File S1. An additional search was performed, which focussed specifically on exogenous hormone use in patients with AUB-E or AUB-I; however, this search did not result in new eligible articles compared to the original search. Eligibility criteria are shown in Table II. Search outcomes included original research papers only, with no limitations on publication year, randomized control trials RCTs , and cohort studies or case—control studies that focused on an association between angiogenesis in the endometrium of patients with AUB. No language restrictions were applied. Articles had to be published as full papers in peer-reviewed journals. Papers that assessed the association of angiogenesis in the endometrium in patients with exogenous hormones were included and reported on separately. Papers that reported on AUB caused by other uterine abnormalities, described by Munro et al. In addition, case reports, letters, reviews or editorial articles and papers, animal studies and studies on non-human cells maintained in vitro were excluded. Papers that used menstrual effluent or serum levels of angiogenic factors only, without biopsies of the endometrium, were also excluded. Finally, matrix metalloproteinases MMPs and tissue inhibitors of metalloproteinases TIMPs were not included in the review. Although MMPs and TIMPs play a very important function in the extracellular matrix ECM , and these pathways are important in the interplay for angiogenesis, we did not include these regulators in this review because intervention was shown to be very complex and resulted in significant adverse events Rani et al. Two authors E. independently screened title and abstracts and, if eligibility was expected, the full article was acquired and reviewed. Any disagreements were resolved by discussion. In addition, references were checked for remaining eligible studies. Using a standardized form, data from published studies was extracted independently by the authors E. The included studies were divided into papers reporting on the comparisons of three groups of patients: AUB-E, AUB-E with exogenous hormone supplementation, and AUB-I. The results were subdivided into outcomes linked to angiogenesis-related vessel morphology and angiogenic parameters, including factors and their receptors. independently performed a quality assessment of the included studies. To estimate the quality of the RCTs, the case—control, and cohorts studies, the Cochrane Risk of Bias tool version 2. The search resulted in references. Three records were added by additional sources, resulting in 36 included articles. The selection process is shown in Fig. PRISMA flow diagram for the search carried out for this narrative review. The characteristics of the included studies are presented in Tables III , IV , and V. With the exception of one study, which included patients with AUB defined as either HMB or irregular uterine bleeding Zhang et al. AUB was defined objectively in nine studies Kooy et al. In 12 studies, the diagnosis of AUB was not specified Sangha et al. In all papers that studied exogenous hormones, AUB at baseline was either not described, based on subjective complaints or defined by the WHO criteria, which is based on subjective registration Rogers et al. In general, AUB during exogenous hormone exposure was registered by menstrual calendar and based on the amount of spotting days. Characteristics of studies on angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding, compared with normal menstruation controls. Phases described: 2 phases : proliferative P and secretory S ; 3 phases : menstrual M , P, and S; 4 phases : P, early ES , mid MS , and late secretory LS ; 5 phases : M, P, ES, MS, and LS; 6 phases: M, early proliferative EP , mid proliferative MP , ES, MS, and LS; 7 phases : M, EP, MP, late proliferative LP ; ES, MS, and LS. Methods described: BrdU assay: 5-bromodeoxyuridine incorporation assay; CCT: corrosion casting technique; ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: Quantitative image analysis; RPA: ribonuclease protection assay; RT-PCR: real-time PCR; WB: western blot. AUB classification: AHM : alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. Characteristics of studies on the effect of exogenous hormones on angiogenesis in patients with endometrial abnormal uterine bleeding. Methods described: IHC: immunohistochemistry; QIA: quantitative image analysis; WB: western blot. AUB classification: AHM: Alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. It can occur alone or in combination with other symptoms NICE, Characteristics of studies on angiogenesis in the endometrium of patients with hormonal induced iatrogenic abnormal uterine bleeding. Methods described: ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: quantitative image analysis; RT-PCR: real-time PCR; WB: western blot. Only presented the outcomes of the case—control study, not of the HESC human endometrial stromal cells model. Only presented the outcomes of the second experiment, not the first experiment with Norplant and with or without vitamin E. Results were given for two proliferative and secretory or up to seven phases menstrual, early—mid—late proliferative and early—mid—late secretory phases. The results in Table VI are presented only for the proliferative and secretory phases, and if results were significantly different between, for example, the mid and late secretory phases this was specified per study. If hormones were continuously used, endometrium does not show different histological phases and the different phases were not distinguished. Hormone type and length of use is specified, as shown in Table V. Presented outcomes are defined as vascular morphology outcomes or as angiogenic parameters. Outcomes regarding angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding. bFGF: basic fibroblast growth factor; FGF-R1: fibroblast growth factors receptor 1; VEGF -R : vascular endothelial growth factor -receptor ; TGF-β1: transforming growth factor-β1; Ang: angiopoietin; ADM: adrenomedullin; CLR: calcitonin receptor-like receptor; BMP: bone morphogenetic protein; eNOS: endothelial nitric oxide synthase; AQP: aquaporin; HIF: hypoxia-inducible factor; CXCR: CX-chemokine receptor; MVD: microvascular density. Lu et al. Abberton et al. Biswas Shivhare et al. The search included 31 case—control studies, three cohort studies and two RCTs. The RoB assessment of all included studies is shown in Supplementary Data File S4. However, overall, the case definition of the included patient populations and methods of ascertainment of exposure were accurate in all included studies. This review covers the statistically significant results of the original publications, unless mentioned otherwise. The results of 35 identified parameters, from 20 studies that compared endometrium tissue of patients with and without AUB-E, are presented in Table VI. The VEGF pathway was investigated in several studies. One study showed an increase in VEGF in the endometrial glands during the secretory phase but not in the proliferative phase Zhang et al. VEGF-A and its receptor subtypes were studied by Mints et al. Mints et al. In contrast, Maybin et al. This could be suggestive of delayed endometrial repair during menstruation in patients with AUB-E. The downstream regulation of eNOS by VEGF was studied in one trial and showed an increase of eNOS in both phases in patients with AUB-E Blumenthal et al. Three studies compared parameters from the Ang-Tie pathway and found conflicting outcomes. However, only one study included patients with objective AUB-E and their results showed an increased Ang-1 expression in patients with AUB-E. The proangiogenic factor ADM was decreased in the secretory phase according to Ha et al. This may indicate that the EC is more sensitive to ADM binding and leads to an increase of the NO pathway, stimulating angiogenesis Fig. Three studies compared TGF-β1 and found no difference between the proliferative and secretory phase Abberton et al. Two studies also analysed the TGF-β receptors, which show a decreased expression Lu et al. In addition, Abberton et al. Endothelin-1, as well as basic fibroblast growth factor bFGF and the FGF-receptor 1, was found to be decreased in patients with AUB-E, compared to normal controls Sangha et al. Though FGF is supposedly a proangiogenic factor, it is also involved in vessel maturation and the development of spiral arterioles in the endometrium. Consequently, a reduction of FGF and endothelin-1 disturbs development of the endometrium and could lead to abnormal bleeding patterns Sangha et al. One study demonstrated that AQP1 expression is decreased in both phases of the menstrual cycle in patients with AUB-E. Despite the fact that AQP1 is an proangiogenic factor, impaired expression of AQP1 could lead to abnormal vessel formation by aberrant endothelial permeability or EC proliferation and migration Mints et al. In addition, Smad2 and -7 were found to be unchanged in both phases and, overall, Smad3 and -4 were decreased in the secretory phase in patients with AUB-E Lu et al. In line with these results, Maybin et al. However, they also found an increase of Smad2 in the secretory phase, possibly explained by the fact that HMB was defined differently in both studies, knowingly subjectively according to NICE guidelines by Lu et al. Only BMP7 was increased in patients with AUB-E; therefore, they conclude that BMP7 is possibly involved in endometrial differentiation and tissue integrity, both crucial for embryo implantation Richards et al. Ten studies report on vessel morphology in patients with and without AUB-E Table IV. Increased angiogenesis can lead to an increased MVD, and an imbalance in pro- and antiangiogenic factors can increase the number of wall gaps, vessel diameter and perimeter, and decreased pericyte coverage or wall thickness. This could indicate that vessel maturation is impaired, resulting in more fragile bloods vessels. MVD was investigated in six studies, five of which showed no significant changes in both the proliferative and secretory phases Mints et al. One study reported on vessel diameter, and perimeter and wall gaps, which were all increased in the secretory phase of patients with AUB-E, compared to normal controls. Wall gaps also were increased in the proliferative phase, although the other characteristics were similar Mints et al. In addition, Andersson et al. Proliferation and attraction of VSMCs occurs with vessel wall remodelling, for example during the maturation process of newly formed vessels, and is profoundly co-regulated by VEGF Darden et al. It is also involved in the next step after angiogenesis, namely arteriogenesis Jeremy et al. Total VSCM was identified in four different studies and was generally found to be unchanged in patients with AUB-E, with two exceptions. One study found a decrease of VSMC in the proliferative phase and one in the secretory phase in patients with AUB-E Abberton et al. Another study found no differences in VSMC thickness and additionally studied VSMC differentiation via two proteins: calponin and smoothelin. Both proteins were unchanged in the proliferative phase, but calponin was increased and smoothelin was decreased in the secretory phase Biswas Shivhare et al. Increased EC proliferation was seen in patients with AUB during both phases compared to control samples Kooy et al. EC density was compared by several parameters, with three of the four markers showing no difference in the proliferative phase and an increase in the secretory phase Biswas Shivhare et al. In addition, this study showed that several ECM proteins fibronectin, osteopontin, and collagen-IV were decreased in the secretory phase in patients with AUB-E, with one exception: osteopontin was decreased in the early secretory phase and increased in the late secretory phase. These differences in protein levels could indicate altered maturation of the endometrial vessels in AUB-E Biswas Shivhare et al. The effect of exogenous hormones on angiogenesis in the endometrium of patients with AUB-E was studied in three studies and patients with AUB-I in 13 studies Tables VII and VIII , respectively. All studies that examined AUB-E used the levonorgestrel-releasing intra-uterine system LNG-IUS , thus progestin-only hormone therapy. Also, all the selected literature that examined AUB-I used progestin-only medication, with the exception of Rogers et al. None of the included literature studied the effect of GnRH agonists or antagonists. Outcomes regarding the effect of exogenous hormones on angiogenesis n the endometrium of patients with endometrial abnormal uterine bleeding. AUB: abnormal uterine bleeding. FGF: fibroblast growth factor, ADM: adrenomedullin; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor. Outcomes regarding the effect of exogenous hormones on angiogenesis in the endometrium of patients with iatrogenic abnormal uterine bleeding. VEGF: vascular endothelial growth -R: receptor factor a- or b-: acidic- or basic- ; FGF: fibroblast growth factors; TGF-ß1: transforming growth factor-beta1; EGF: epidermal growth factor; STC stanniocalcin-1; CSPG4: cleaved chondroitin sulphate proteoglycan 4; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor; PCNA: proliferating cell nuclear antigen. BL: bleeding site during hysteroscopy in endometrium; NBL: non bleeding during hysteroscopy in endometrium. First outcomes in both lamina functionalis and lamina basalis, second outcome only in the subepithelial plexus. Table VII shows three studies that compared the endometrium of patients with AUB-E, with and without LNG-IUS exposure. Also, the angiogenic factors ADM and thymidine phosphorylase, of which the latter is associated with pathophysiological angiogenesis Sengupta et al. The MVD of blood vessels was increased after short-term and long-term LNG-IUS treatment in comparison to patients without LNG-IUS treatment Hague et al. In contrast, Donoghue et al. Furthermore, an increase in diameter and perimeter of the blood vessel was seen after short-term LNG-IUS treatment compared to controls, and the maximal width of the largest luminal diameter for each sample was also increased McGavigan et al. Literature shows that the increased diameter in both blood and lymphatic vessels could be caused by increased VEGF-D expression Rissanen et al. Donoghue et al. After long-term exposure, EC proliferation was reduced in patients with LNG-IUS compared to AUB-E controls Hague et al. As presented in Table VIII , two studies examined the effect of LNG-IUS treatment Roopa et al. In addition, after LNG-IUS, progestin-only or oral combined hormonal therapy, a different study found no changes in VEGF immunohistochemistry IHC compared to controls Rogers et al. Another angiogenic polypeptide involved in EC proliferation is endometrial epidermal growth factor EGF. Tissue factor TF is an important initiator for haemostasis and could potentially promote aberrant angiogenesis and produce fragile vessels if overexpressed Runic et al. The progesterone receptor PR is likely to regulate TF expression, but no difference was found in the PR protein levels between Norplant users and controls Runic et al. They found an overexpression of stanniocalcin-1 and stronger immunostaining for cleaved chondroitin sulphate proteoglycan 4, both mediators of angiogenesis and linked to malignant tumour formation Law and Wong, ; Shapiro et al. In summary, when patients with AUB-I were compared to control groups with NMB, VEGF staining was increased in two studies and not affected in one study. FGF, TGF-β, and EGFR were increased in patients with AUB-I, and EGF showed no difference between groups. When bleeding sites in Norplant users were compared with non-bleeding sites, TF and PR expression were increased in the group with bleeding complaints. Besides stimulation of vessel maturation, FGF and TGF-β are both also proangiogenic factors, acting by stimulating EC proliferation and differentiation and have a synergistic effect on the induction of angiogenesis, if combined with VEGF. TF is an important haemostasis initiator, so it seems logical that TF and its receptor are increased in bleeding sites of patients with AUB-I. Eight studies examined the effect of exogenous hormone exposure on morphological characteristics of vessels Table VII. An increase in MVD was observed in patients with AUB-I and LNG-IUS compared to controls with NMB. However, if patients with progestin-only therapy were compared to patients with combined therapy, the patients with progestin-only therapy showed a decrease in MVD Rogers et al. This effect appeared only moderate because no difference was seen in the number of immature or partially mature blood vessels; however, a decrease was found in the number of mature vessels. In addition, an increase in immature vessels was found after short- and long-term LNG-IUS exposures, and a decrease in mature vessels after short-term exposure compared to both controls and long-term exposure Stephanie et al. In addition, endometrium samples showed a decrease in VSMC number and proliferation after DMPA exposure, compared to samples of the same patients before exposure, again indicating impaired vessel maturation after progestin exposure Kayisli et al. These results indicate disruption in the maturation of blood vessels after exogenous progestin use in a time-dependent matter, especially in patients with AUB complaints. This review provides a summary of the available literature on angiogenesis in the endometrium of women with AUB-E, and in patients with AUB-E and AUB-I after use of exogenous hormones. Figure 6 provides an overview of the interactions between the angiogenic parameters reported on in this review. Schematic overview of pro- and antiangiogenic parameters assessed in this review. Blue: proangiogenic parameter; red: antiangiogenic parameter. The HIF-1α initiated VEGF and Tie-Ang pathway characteristics were the most studied angiogenic parameters. In studies with objectively defined AUB, increased VEGF, VEGF-A, VEGFR-1, and VEGFR-2 were reported, while no differences in these factors were found in studies with subjectively reported AUB. In patients with subjectively reported AUB-E, the AngAng-2 ratio, and proangiogenic Tie-1 were increased. However, one study found no difference in Ang-2 levels and only a decrease in Ang-1 in the secretory phase. As this is the only study that included patients with objectively measured AUB, this may indicate a possible relation between a decrease of Ang-1 in patients with AUB-E and decreased vessel maturation, considering that Ang-1 supports vessel maturation. However, caution is required in drawing this conclusion as this is based on one study only. In patients with AUB-E and AUB-I caused by exogenous hormones, VEGF expression was increased after short-term exposure and unchanged after long-term exposure, compared to controls. This may suggest a role of the VEGF pathway in stimulating angiogenesis in hormone-induced spotting or break-through-bleeding, in a time-dependent manner. In addition, other proangiogenic factors, such as bFGF, its receptor FGF-R1, ADM, and endothelin-1, were decreased in patients with AUB-E compared to normal controls. Conversely, in patients with AUB-I, bFGF was increased after short-term hormone exposure. These factors are in involved in EC proliferation and differentiation, as well as vessel maturation. This could suggest two somewhat contradictory outcomes: an increase in these factors could result in increased angiogenesis by mobilizing ECs, leading to AUB, and a decrease in these factors may lead to an increase in poor vascular maturation and, thus, to vascular dysfunction. The Smad family is involved in wound healing and repair, and a suboptimal TGF-β response could thereby decrease postmenstrual repair, leading to HMB Maybin et al. Moreover, Maybin et al. Conceivably, the genesis of AUB in patients with AUB-E and AUB-I is caused by a different combination of abnormalities in angiogenic factors. In general, VEGF expression increased and Ang-I decreased in patients with objectively defined AUB-E: this could suggest decreased vessel maturation in these patients. In patients with AUB-I, angiogenic factors were increased after short-term exposure and unchanged after long-term exposure to exogenous hormones. This could be in agreement with the fact that spotting complaints with exogenous hormone use gradually disappear over time Hillard, and, hypothetically, angiogenesis in the endometrium will normalize. However, these findings should be interpreted with caution and do not imply causation. In addition, decreasing complaints of spotting may be linked to selection bias, with patients who experience severe or persisting AUB complaints being more likely to stop hormone therapy after short-term use or switch to another surgical treatment National Institute for Health and Clinical Excellence, ; Daud and Ewies, Vasoconstriction of spiral arterioles is essential to limit menstrual blood flow, as a small increase in diameter will lead to an extensive increase in blood flow. For instance, if the diameter is increased 2-fold this will lead to a fold decrease in flow resistance Jain et al. Blumenthal et al. As eNOS produces NO and NO increases vasodilatation, HMB could hypothetically be caused by an increase in spiral arteriole diameter under the influence of increased NO levels Jeremy et al. This could indicate that, apart from angiogenesis, several other mechanisms are involved in AUB. The MVD was generally not increased in patients with AUB-E. Although other morphological parameters of vessels did show changes, such as diameter, perimeter, congestion, wall gaps were increased and pericyte vessel wall coverage was decreased in the endometrium of the AUB-E group. An increase in vessel diameter and perimeter could be a sign of maturation, though when pericyte coverage is absent and wall gaps and defects are increased, this can lead to fragile and leaking microvessels, thereby causing AUB Runic et al. These results could therefore indicate impaired angiogenesis and disturbed vessel maturation in the patient group with solely AUB-E, similar to the effects found in short-term exogenous hormone exposure in patients with AUB-E and AUB-I. This was supported by two studies that showed disturbed vessel maturation in patients with AUB-I Rogers et al. In contrast to patients with AUB-E, in patients with AUB-I, the MVD outcomes were very different for different types of hormones and their length of exposure. Moreover, the mechanism involved in angiogenesis might differ between groups. In general, MVD was increased after short-term exogenous hormone use and decreased, or was unchanged, after long-term use in patients with AUB-E and AUB-I. For LNG-IUS specifically, the MVD after long-term exposure was decreased, compared to short-term exposure Stephanie et al. This result is in line with the findings of Kayisli et al. However, these results are in contrast with a recent review that points out that, in patients with NMB, both EC proliferation and MVD do not change during the menstrual cycle. This review suggests that normal angiogenesis involves the faster process of vessel elongation and intussuscepted angiogenesis splitting of existing blood vessels Yetkin-Arik et al. One could hypothesize that in patients with AUB-E, especially those suffering from HMB, a combination of angiogenic mechanisms could be responsible for the formation of new vessels Fig. Moreover, if coalescent angiogenesis contributes to new blood vessel formation, a decrease in the number of vessels and therefore a decrease in MVD are expected Nitzsche et al. It is likely that in patients with AUB-E and AUB-I, a combination of different angiogenic mechanisms may play a role. This could be one of the explanations for why increased angiogenesis is not always associated with a change in MVD but could lead to vessels of insufficient quality or unstable vessels with an impaired function. To compare angiogenesis between patients, the MVD may not always be the most appropriate parameter. As the MVD is also dependent on other endometrial elements, such as glandular and stromal cells, changes in MVD could also mean changes in these structures since a relative proportion of vessels is measured Shaw et al. Furthermore, in patients with short-term exposure to exogenous hormone, an increase in MVD of mostly immature vessels could potentially be based on more vessel elongation, intussuscepting, or sprouting angiogenesis as compared to the extent of coalescent angiogenesis, thereby causing spotting complaints. More research is needed to elucidate the mechanisms responsible for angiogenesis in women with NMB, AUB-E, and AUB-I. Unexpectedly, EC migration was found to decrease in patients with AUB-I after short-term Norplant or DMPA exposure. This seems contradictory to the fact that, in patients with AUB-I, a higher MVD was found. This paradox might be explained by a lower blood vessel regression rate in Norplant and DMPA users, compared to other tissue in the endometrium. An alternative explanation could be that the authors used umbilical vein EC, which are characterized as macrovascular EC. It is possible that responses in microvascular EC, as present in endometrium tissues, differ from those in cells originating from macrovessels Subakir et al. This is the first narrative review that discusses impaired angiogenesis in the endometrium of patients with AUB, and AUB initiated by exogenous hormones. Strengths of this review include registration in the PROSPERO register of systematic reviews, the systematic approach according to PRISMA guidelines, the search of several databases, and the use of internationally accepted checklists to assess the RoB for included publications. In addition, this review systematically presents the broad range of angiogenic parameters and morphological characteristics of vessels. Nonetheless, there are some limitations. First, RoB assessment showed that only 8 of the 35 included studies were of good quality. Second, most studies lack objective scoring of AUB and include patients with subjective AUB; it could be questioned if the included patients with subjective blood loss actually suffer from objective AUB. Third, when interpreting the long-term effects of exogenous hormones, it should be considered that results can be influenced by selection bias, because patients with severe AUB-I are more prone to stop their treatment compared to patients with less severe symptoms. Moreover, it is possible that different doses and exposure times of exogenous hormones show different effects but because of the large heterogeneity in hormone studies it was not possible to address this further. Fourth, although the diagnostic techniques mostly involved IHC, quantification of these results differs among studies and is dependent on the subjective interpretation of the researchers. The magnification power of the fields analysed ranged from 40 to × and the unit of measurement was sometimes presented as measurements per power field, per unit area or per mm 2. Studies show that if quantitative analysis of IHC is carried out by experienced researchers, different scoring systems do show significant correlation Walker, Exogenous hormones have developed over time and thus could be outdated, such the Progestasert-IUS. Furthermore, as MMPs and their inhibitor TIMPs are considered to be involved mainly in ECM degradation, they were not included in this review. The literature shows that these factors play a role in regulating HESCs, by stabilizing vascular ECM and endometrial stromal cells, and are regulated by progesterone. Dissolution of ECM or unstable stroma could therefore contribute to the severity of complaints of AUB Lockwood, ; Schatz et al. Finally, and perhaps most importantly, this review includes many different markers, angiogenic factors and receptors, treatment types, treatment durations, and comparisons with different control groups HMB or NMB. These factors were also measured at different phases of the menstrual cycle, which makes it difficult to compare the outcomes presented. Some factors are only analysed in one study, and if factors were analysed in different studies but measured in different phases of the menstrual cycle, the results are not comparable because of rapid changes in endometrial gene expression. Despite the heterogeneous literature about this very complex process, the authors are confident that the outcomes of studies support the hypothesis that disturbed angiogenesis is important in provoking AUB and emphasizes the need for further research. New insight into the genesis of this disease is essential to develop new therapeutic strategies. This review supports our hypothesis that in patients with subjectively and objectively defined AUB-E and AUB-I, both anti- and proangiogenic factors are altered, likely leading to changes in vessel morphology. It is also the first review that shows a systematic presentation of several anti- and proangiogenic factors in patients with AUB-E and AUB-I. Nevertheless, the fact that one angiogenic factor can induce angiogenesis but simultaneously also play an essential role in vessel maturation makes it difficult to identify the best point of interest to intervene in this process. Future research should focus on identifying key angiogenic factors in a patient group with objectively defined AUB, to gather direct evidence to support the hypothesis that AUB symptoms are related to aberrant angiogenesis. In addition, it is important to distinguish between short- and long-term exogenous hormone exposure and to study the dose—effect relationship. Our results show that the most interesting systems involve factors from the HIF-, VEGF-, FGF-, and Ang-Tie pathways. Aberrant angiogenesis is known to play a major role in cancer, making this process a major point of focus in the development of antiangiogenesis therapies Ramjiawan et al. As this includes antiangiogenesis therapy in gynaecological cancers, this could also be interesting for the treatment of AUB. Naturally, antiangiogenesis therapy should be combined with birth control methods to avoid teratogenesis. To support our hypothesis that altered angiogenesis is related to AUB or infertility, additional research is the necessary first step to identify specific targets that can be used to intervene in the angiogenic pathways. Both anti- and proangiogenic factors are altered in patients with AUB-E and AUB-I, which could be a pathophysiological explanation for how HMB and spotting complaints emerge, supporting our hypothesis that angiogenesis is altered in these patients. However, many different angiogenic factors and receptors have only been investigated in single studies; therefore, the results have to be interpreted with caution. This review indicates that HMB in patients with AUB-E is caused by a change in angiogenic factors, which probably leads to immature vessels or less stable vessel walls, rather than an increase in MVD. Spotting in patients with AUB-I does seems to be related to the combination of an increased MVD and impaired vessel maturation after short-term hormone exposure. Our findings provide points of interest for future research to identify key targets for antiangiogenic therapy to treat patients with AUB. Supplementary data are available at Human Reproduction Update online. The authors thank medical information specialist Ralph de Vries, for assisting in the database search and Ghislaine Meertens for her contributing work and the initiation of this manuscript. They also thank Lynda Juffermans for her advice during the writing of this manuscript. conceived the presented idea. wrote the article in consultation with A. All authors discussed the content, provided critical feedback to shape the article, and contributed to the final article. All authors approve the final version of this article and agree that they are accountable for all aspects of the work. and J. received funding from an Amsterdam Reproduction and Development grant for a different project regarding angiogenesis Development. received funding from the Dutch Cancer Society Grant number KWF Abberton KM , Healy DL , Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod a ; 14 : — Google Scholar. Abberton KM , Taylor NH , Healy DL , Rogers PA. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum Reprod b ; 14 : — Abreu-Rodriguez I , Sanchez Silva R , Martins AP , Soveral G , Toledo-Aral JJ , Lopez-Barneo J , Echevarria M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha. PLoS One ; 6 : e ACOG Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG practice bulletin: management of anovulatory bleeding. Int J Gynaecol Obstet ; 72 : — Andersson E , Zetterberg E , Vedin I , Hultenby K , Palmblad J , Mints M. Low pericyte coverage of endometrial microvessels in heavy menstrual bleeding correlates with the microvessel expression of VEGF-A. Int J Mol Med ; 35 : — Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception ; 38 : — Birbrair A , Zhang T , Wang ZM , Messi ML , Mintz A , Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci Lond ; : 81 — Biswas Shivhare S , Bulmer JN , Innes BA , Hapangama DK , Lash GE. Altered vascular smooth muscle cell differentiation in the endometrial vasculature in menorrhagia. Hum Reprod ; 29 : — Endometrial vascular development in heavy menstrual bleeding: altered spatio-temporal expression of endothelial cell markers and extracellular matrix components. Hum Reprod ; 33 : — Blumenthal RD , Taylor AP , Goldman L , Brown G , Goldenberg DM. Abnormal expression of the angiopoietins and Tie receptors in menorrhagic endometrium. Fertil Steril ; 78 : — Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med ; 6 : — BMP-2 induces angiogenesis by provoking integrin alpha6 expression in human endothelial progenitor cells. Biochem Pharmacol ; : — Cioni B , Zaalberg A , van Beijnum JR , Melis MHM , van Burgsteden J , Muraro MJ , Hooijberg E , Peters D , Hofland I , Lubeck Y et al. Androgen receptor signalling in macrophages promotes TREMmediated prostate cancer cell line migration and invasion. Nat Commun ; 11 : Critchley HOD , Maybin JA , Armstrong GM , Williams ARW. Physiology of the Endometrium and Regulation of Menstruation. Physiol Rev ; : — Darden J , Payne LB , Zhao H , Chappell JC. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis ; 22 : — Daud S , Ewies AA. Levonorgestrel-releasing intrauterine system: why do some women dislike it? Gynecol Endocrinol ; 24 : — Donoghue JF , McGavigan CJ , Lederman FL , Cann LM , Fu L , Dimitriadis E , Girling JE , Rogers PA. Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding. PLoS One ; 7 : e Edeline J , Vauléon E , Rioux-Leclercq N , Perrin C , Vigneau C , Bensalah K , Laguerre B. Safety and efficacy of sorafenib in renal cell carcinoma. Cancer Growth Metastasis ; 5 : 35 - Ferenczy A , Bertrand G , Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol ; : — Fox H , Buckley CH. In: Gresham, GA ed. Atlas of Gynaecological Pathology. Netherlands : Springer , , 61 — Google Preview. Gambino LS , Wreford NG , Bertram JF , Dockery P , Lederman F , Rogers PA. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod ; 17 : — Girling JE , Rogers PA. Recent advances in endometrial angiogenesis research. Angiogenesis ; 8 : 89 — Gleeson NC. Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Griffioen AW , Bischoff J. Oxygen sensing decoded: a Nobel concept in biology. Griffioen AW , Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev ; 52 : — Ha C , Stavreus-Evers A , Landgren BM , Mints M , Rees MC. Adrenomedullin and its receptor, calcitonin receptor-like receptor, are aberrantly expressed in women with idiopathic menorrhagia. Hague S , MacKenzie IZ , Bicknell R , Rees MC. In-vivo angiogenesis and progestogens. Hallberg L , Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest ; 16 : — Hanahan D. Signaling vascular morphogenesis and maintenance. Science ; : 48 — Harmsen MJ , Wong CFC , Mijatovic V , Griffioen AW , Groenman F , Hehenkamp WJK , Huirne JAF. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update ; 25 : — Hewett P , Nijjar S , Shams M , Morgan S , Gupta J , Ahmed A. Down-regulation of angiopoietin-1 expression in menorrhagia. Am J Pathol ; : — Hickey M , Krikun G , Kodaman P , Schatz F , Carati C , Lockwood CJ. Long-term progestin-only contraceptives result in reduced endometrial blood flow and oxidative stress. J Clin Endocrinol Metab ; 91 : — Hii LL , Rogers PA. Endometrial vascular and glandular expression of integrin alpha v beta3 in women with and without endometriosis. Hum Reprod ; 13 : — Hillard PA. Menstrual suppression: current perspectives. Int J Womens Health ; 6 : — Jabbour HN , Kelly RW , Fraser HM , Critchley HO. Endocrine regulation of menstruation. Endocr Rev ; 27 : 17 — Jain V , Chodankar RR , Maybin JA , Critchley HOD. Uterine bleeding: how understanding endometrial physiology underpins menstrual health. Nat Rev Endocrinol ; 18 : — Janssen CA , Scholten PC , Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol ; 85 : — Jeremy JY , Rowe D , Emsley AM , Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res ; 43 : — Jørgensen L , Paludan-Müller AS , Laursen DR , Savović J , Boutron I , Sterne JA , Higgins JP , Hróbjartsson A. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev ; 5 : Kayisli UA , Basar M , Guzeloglu-Kayisli O , Semerci N , Atkinson HC , Shapiro J , Summerfield T , Huang SJ , Prelle K , Schatz F et al. Long-acting progestin-only contraceptives impair endometrial vasculature by inhibiting uterine vascular smooth muscle cell survival. Proc Natl Acad Sci U S A ; : — Khafaga A , Goldstein SR. Abnormal uterine bleeding. Obstet Gynecol Clin North Am ; 46 : — Kooy J , Taylor NH , Healy DL , Rogers PA. Endothelial cell proliferation in the endometrium of women with menorrhagia and in women following endometrial ablation. Hum Reprod ; 11 : — Krock BL , Skuli N , Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer ; 2 : — Kuczynski EA , Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis ; 23 : 55 — Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol ; :R— Latacz E , Caspani E , Barnhill R , Lugassy C , Verhoef C , Grunhagen D , Van Laere S , Fernandez Moro C , Gerling M , Dirix M et al. Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis ; 23 : 43 — Lau TM , Affandi B , Rogers PA. The effects of levonorgestrel implants on vascular endothelial growth factor expression in the endometrium. Mol Hum Reprod ; 5 : 57 — Law AY , Wong CK. Mol Cell Endocrinol ; : 73 — Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JPA , Clarke M , Devereaux PJ , Kleijnen J , Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ ; : b Lockwood CJ. Mechanisms of normal and abnormal endometrial bleeding. Menopause ; 18 : — Lockwood CJ , Krikun G , Hickey M , Huang SJ , Schatz F. Decidualized human endometrial stromal cells mediate hemostasis, angiogenesis, and abnormal uterine bleeding. Reprod Sci ; 16 : — Lockwood CJ , Kumar P , Krikun G , Kadner S , Dubon P , Critchley H , Schatz F. Effects of thrombin, hypoxia, and steroids on interleukin-8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. J Clin Endocrinol Metab ; 89 : — Lu Q , Sun D , Shivhare SB , Hou H , Bulmer JN , Innes BA , Hapangama DK , Lash GE. Transforming growth factor TGF beta and endometrial vascular maturation. Front Cell Dev Biol ; 9 : Massague, J. TGFβ signalling in context. Nat Rev Mol Cell Biol ; 13 — Maas JW , Groothuis PG , Dunselman GA , de Goeij AF , Struyker Boudier HA , Evers JL. Endometrial angiogenesis throughout the human menstrual cycle. Hum Reprod ; 16 : — Makhija D , Mathai AM , Naik R , Kumar S , Rai S , Pai MR , Baliga P. Morphometric evaluation of endometrial blood vessels. Indian J Pathol Microbiol ; 51 : — Manalo DJ , , Rowan A , , Lavoie T , , Natarajan L , , Kelly BD , , Ye SQ , , Garcia JGN , , Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF Blood ; : — Marnach ML , Laughlin-Tommaso SK. Evaluation and management of abnormal uterine bleeding. Mayo Clin Proc ; 94 : — Martínez-Aguilar R , Kershaw LE , Reavey JJ , Critchley HOD , Maybin JA. Reproduction ; : F1 — f Maybin JA , Boswell L , Young VJ , Duncan WC , Critchley HOD. Reduced transforming growth factor-β activity in the endometrium of women with heavy menstrual bleeding. J Clin Endocrinol Metab ; : — Maybin JA , Murray AA , Saunders PTK , Hirani N , Carmeliet P , Critchley HOD. Hypoxia and hypoxia inducible factor-1alpha are required for normal endometrial repair during menstruation. Nat Commun ; 9 : McGavigan CJ , Dockery P , Metaxa-Mariatou V , Campbell D , Stewart CJ , Cameron IT , Campbell S. Hormonally mediated disturbance of angiogenesis in the human endometrium after exposure to intrauterine levonorgestrel. Hum Reprod ; 18 : 77 — Mints M , Blomgren B , Falconer C , Fianu-Jonasson A , Palmblad J. Microvascular density, vascular endothelial growth factor A, and its receptors in endometrial blood vessels in patients with menorrhagia. Fertil Steril ; 84 : — Mints M , Blomgren B , Palmblad J. Expression of vascular endothelial growth factor receptor-3 in the endometrium in menorrhagia. Int J Mol Med a ; 19 : — Expression of angiopoietins 1, 2 and their common receptor tie-2 in relation to the size of endothelial lining gaps and expression of VEGF and VEGF receptors in idiopathic menorrhagia. Fertil Steril ; 94 : — Mints M , Hildenbrand A , Lalitkumar LP , Andersson S , Nielsen S , Gemzell-Danielsson K , Stavreus-Evers A. Expression of aquaporin-1 in endometrial blood vessels in menorrhagia. Int J Mol Med b ; 19 : — Mints M , Hultenby K , Zetterberg E , Blomgren B , Falconer C , Rogers R , Palmblad J. Wall discontinuities and increased expression of vascular endothelial growth factor-A and vascular endothelial growth factor receptors 1 and 2 in endometrial blood vessels of women with menorrhagia. Fertil Steril c ; 88 : — Moher D , Liberati A , Tetzlaff J , Altman DG , Group P , PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med ; 6 : e Munro MG , Critchley HO , Broder MS , Fraser IS , FWGoM D ; FIGO Working Group on Menstrual Disorders. FIGO classification system PALM-COEIN for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet a ; : 3 — Munro MG , Critchley HO , Fraser IS , Fmdw G ; FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril b ; 95 : — , Munro MG , Critchley HOD , Fraser IS , Fmd C ; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: revisions. Int J Gynaecol Obstet ; : — Nap AW , Dunselman GA , Griffioen AW , Mayo KH , Evers JL , Groothuis PG. Angiostatic agents prevent the development of endometriosis-like lesions in the chicken chorioallantoic membrane. Fertil Steril ; 83 : — Nap AW , Griffioen AW , Dunselman GA , Bouma-Ter Steege JC , Thijssen VL , Evers JL , Groothuis PG. Antiangiogenesis therapy for endometriosis. National Institute for Health and Clinical Excellence. Long-Acting Reversible Contraception: The Effective and Appropriate Use of Long-Acting Reversible Contraception. National Institue for Health and Clinical Excellence. Nayak NR , Brenner RM. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab ; 87 : — Nayak NR , Kuo CJ , Desai TA , Wiegand SJ , Lasley BL , Giudice LC , Brenner RM. Expression, localization and hormonal control of angiopoietin-1 in the rhesus macaque endometrium: potential role in spiral artery growth. Mol Hum Reprod ; 11 : — Nikitenko LL , MacKenzie IZ , Rees MC , Bicknell R. Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod ; 6 : — Nitzsche B , Rong WW , Goede A , Hoffmann B , Scarpa F , Kuebler WM , Secomb TW , Pries AR. Coalescent angiogenesis-evidence for a novel concept of vascular network maturation. Accumulating evidence suggests that angiogenic growth factor dysregulation may be implicated in these vascular and other features of fibroid pathophysiology. The findings are hereby reviewed and discussed. Results: Multiple growth factors involved in angiogenesis are differentially expressed in leiomyoma compared with myometrium. These include epidermal growth factor EGF , heparin-binding-EGF, vascular endothelial growth factor, basic fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β and adrenomedullin. An important paradox is that although leiomyoma tissues are hypoxic, leiomyoma feature down-regulation of key molecular regulators of the hypoxia response. Furthermore, the hypoxic milieu of leiomyoma may contribute to fibroid development and growth. Notably, common treatments for fibroids such as GnRH agonists and uterine artery embolization UAE are shown to work at least partly via anti-angiogenic mechanisms. Conclusions: Angiogenic growth factors play an important role in mechanisms of fibroid pathophysiology, including abnormal vasculature and fibroid growth and survival. |
| Mechanisms of normal and abnormal endometrial bleeding. Google Preview. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Volume Richards et al. |
Ja Sie das Talent:)
Welche nötige Phrase... Toll, die prächtige Idee
welchen Charakter der Arbeit sehend
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Ich biete es an, zu besprechen.