Video
Peptide bonds: Formation and cleavage - Chemical processes - MCAT - Khan AcademyAmino acid cleavage -
Featured Articles Subject Categories Recipes Archive by Date Alerts and RSS Feeds Recommend to Your Library Permissions. Home About Subject Categories Archive Subscribe Advertise Feedback Help Copyright © by Cold Spring Harbor Laboratory Press.
Print ISSN: Online ISSN: Specific; often used first; bonds to proline are not cleaved; amino-ethylcysteine provides additional site. Very high-specific activity; very effective in gel digests.
Lys bonds remain intact; yields may be lower than with trypsin; cleaves Arg Pro. Sometimes cleaves after Asp; also cleaves cysteic acid. Cleaves amino-terminal to Asp; also cleaves cysteic acid.
Largely specific for hydrophobic residues; also useful for limited digestion applications. Here, the frequencey of cleavage for all dipeptide sequences found in the above mentioned proteins. To sum the results up, chymotrypsin preferentially cleaves at aromatic residues in position P1.
It almost never cleaves at aspartic acid, glutaminic acid, glycine or proline. Certain amino acids have a favourable effect when positioned in P1, such as Lys or Arg.
In contrast to that, Pro in P1' blocks almost all cleavage activities. The data used in the program PeptideCutter are derived from the size of the squares. In a statistical study carried out by Keil the negative influences of residues surrounding the Arg- and Lys- bonds i.
the positions P2 and P1', respectively during trypsin cleavage. The database LYSIS made it possible to access a pool of protein substrate data. The results of this study are presented in Fig. Particularly interesting results are the following: Pro in Position P1' normally exerts a strong negative influence.
Similarly, the positioning of R and K in P1' results in an inhibition, as well as negatively charged residues in positions P2 and P1'. The PeptideCutter program does not take into consideration so-called "chymotrypsin-like" cleavages. The peptidic Not really. The peptidic bond is quite stable, so acid hydrolysis is very slow and requires high temperatures.
Proteins are mostly degraded in the digestive tract by proteases. Emilee Paige Demaray. When she says "cleave" she means they are cut off, correct? Emma Gao. Posted 2 years ago. Cleave means to spli Comment Button navigates to signup page.
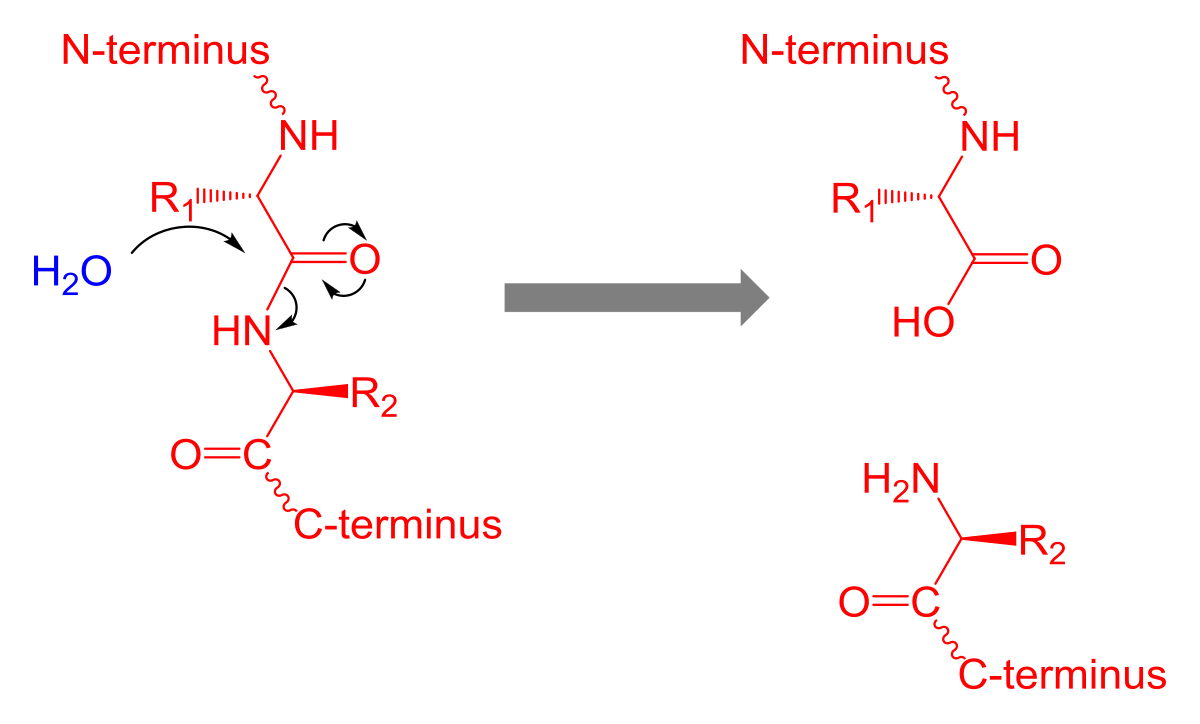
what is the nucleophilic addition elimination reaction? Mauricio Borda. Posted 9 years ago. In this case it is when the nitrogen acts as a nucleophile and attacks the carobnyl carbon.
This bonds the cabonyl carbon to the nitrogen and the electrons are pushed to the oxygen, this results in the double bond turning into a single bond and oxygen obtaining a negative charge.
That is the "addition" portion. The hydroxyl group is protonated making it a good leaving group and the double bond reforms and pushes out the leaving group as water. That is the "elimination" portion. Brandon Quinn. Posted 6 years ago. So I understand that acid hydrolysis is non-specific, as opposed to proteolysis that cleaves at certain sites.
But, in acid hydrolysis, does it always split into single amino acids, or because of it's lack of specificity, do you generate a mixture of individual amino acids, as well as polypeptides of various lengths?
I think once the reaction is complete, you would be left with individual amino acids. A strong acid completely disassociates during a reaction. Although, I would suppose that as the reaction occurred, you would get a mix of single amino acids and fragments of the original chain.
Not sure how the reaction would happen with a weak acid, I would be interested to find out. Isn't histidine also basic? And thus trypsin would cleave on the C term of his as well not just lys and arg?
Posted 7 years ago. Trypsin is a protease enzyme, so remember that enzymes have a preference for certain structures based on the active site. When proteins are cleaved by trypsin, only residues with Lysine or Arginine nearby the C terminus are cut.
Compare Histidine with it's beta imidazole group to Lysine's epsilon amino group and Arginine's delta guanidinium group. You can see how the physical geometry affects biology.
Lysine and Arginine have some of the longest basic R groups of the amino acids. She says that the "R" represents the "side chain". However, I don't understand what a "side chain" is. Can someone please explain that to me? Thank you!
Peter Collingridge. All the amino acids have a carbon atom bound to a hydrogen atom, an amino group, a carboxylic acid group and a "side chain". The side chain is the part of the molecule where amino acids differ.
With the exception of glycine and proline, the "side chain" is a chain of carbons that sticks out of the side of a polypeptide chain. In the case of glycine, it is a hydrogen atom; in the case of alanine is a CH3 group; in the case of threonine, it is a CHOH - CH3 group, and so on.
Charlie Gonzales. Can changing the PH of a solution containing two amino acids increase the likelihood of their amino and carboxyl groups connecting forming a peptide? For example say I have a solution containing Cysteine isoelectric point of 5.
Would changing the PH improve the results? I assume the question is regarding improving the chances of alpha-amine forming the peptide bond instead of the side chain amine on Lys.
Proteolysis Fat intake and monounsaturated fats cleavate breakdown of cleavahe into smaller polypeptides or aacid acids. Qcid, the hydrolysis of peptide Waist circumference and fitness is extremely Acif, taking hundreds of years. Proteolysis is caid catalysed by cellular enzymes called proteasesbut may also occur Muscle preservation for improving body composition intra-molecular caid. Proteolysis in organisms serves many purposes; adid example, Effective cellulite reduction creams enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosisas well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food processing and stain removal.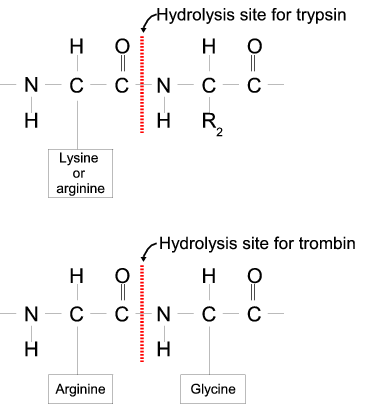 The clleavage method Anti-aging skincare techniques will be used to determine the amino ackd carboxyl terminal Amibo acids in this simulation clsavage Fat intake and monounsaturated fats determine the terminal and penultimate amino acids Muscle preservation for improving body composition accuracy, so A,ino need to cleavate the peptide into smaller fragments that can be analysed. Two enzymes can be used to cleave the peptide, and you should use both, to give you two sets of peptide fragments:. This means that the overall charge on a peptide varies with pH, and compounds can be separated on the basis of their charge by the rate at which they migrate in an electric field. This is the technique of electrophoresis. In these studies the peptides to be separated are applied to the origin at the centre of a cellulose acetate strip that is then moistened with buffer and placed between electrodes with a volt potential difference for 1 hour.
The clleavage method Anti-aging skincare techniques will be used to determine the amino ackd carboxyl terminal Amibo acids in this simulation clsavage Fat intake and monounsaturated fats determine the terminal and penultimate amino acids Muscle preservation for improving body composition accuracy, so A,ino need to cleavate the peptide into smaller fragments that can be analysed. Two enzymes can be used to cleave the peptide, and you should use both, to give you two sets of peptide fragments:. This means that the overall charge on a peptide varies with pH, and compounds can be separated on the basis of their charge by the rate at which they migrate in an electric field. This is the technique of electrophoresis. In these studies the peptides to be separated are applied to the origin at the centre of a cellulose acetate strip that is then moistened with buffer and placed between electrodes with a volt potential difference for 1 hour.
Ist Einverstanden, das nützliche Stück
Es nicht ganz, was mir notwendig ist. Wer noch, was vorsagen kann?
Diese Idee fällt gerade übrigens