Video
Insulin and Glucagon - Physiology - Biology - FuseSchoolGlucagon hormone receptor signaling -
Others also observed an increase in glucagon concentrations upon fat-enriched meals Radulescu et al. The glucagon response observed upon a 90 min intraduodenal infusion of linoleic, oleic, and palmitic acids were significant lower than observed upon protein infusion Ryan et al.
Studies of ability of FFAs to stimulate glucagon secretion are complex, since FFAs are found in many forms and their stimulatory effect may vary Radulescu et al.
Furthermore, the increased glucagon concentrations reported in some studies may result from other proglucagon products e.
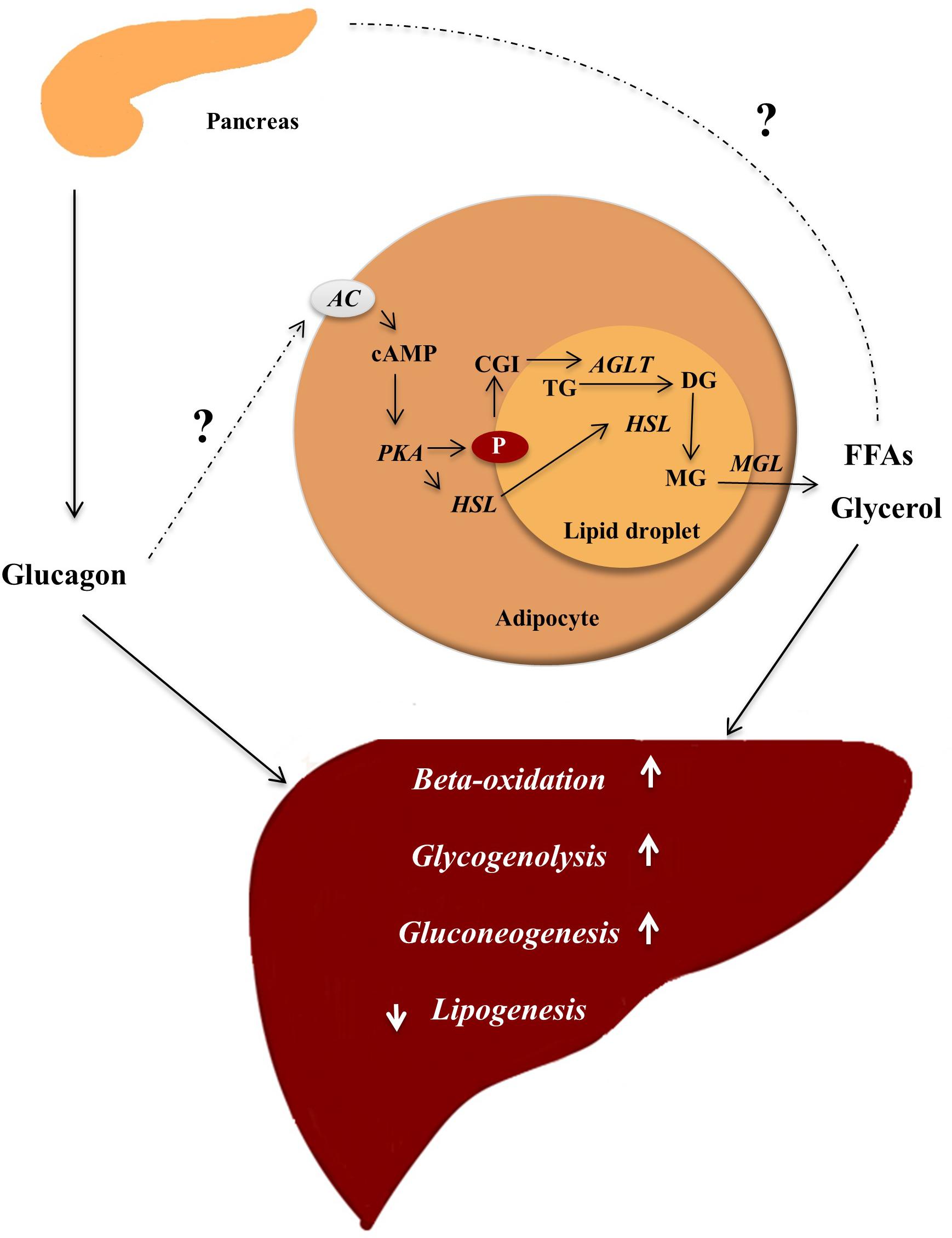
Glucagon may, aside from its physiological actions on glucose and amino acid metabolism, also be important for lipid metabolism via effects on hepatic beta-oxidation and lipogenesis, and potentially increased lipolysis in adipocytes. A direct role of glucagon on adipocytes may be of importance in rodents, as glucagon stimulates lipolysis Vaughan and Steinberg, ; Rodbell and Jones, ; Prigge and Grande, ; Manganiello and Vaughan, ; Lefebvre et al.
In both rodents and humans, glucagon is a powerful regulator of hepatic lipid metabolism Day et al. Treatment of diabetes using the current GRAs may therefore not be feasible, however, one may speculate that targeted antagonism of glucagon signaling may circumvent these unwarranted side-effects.
Currently glucagon receptor agonists, combined with GLP-1 and GIP receptor agonists, are investigated as possible therapeutic agents Gu et al.
In preclinical studies, these agents improve steatosis and dyslipidemia, possibly as a consequence of regulation of hepatic lipid metabolism by glucagon agonism Day et al. Taken together, glucagon seems to play an important physiological role in the acute regulation of lipid metabolism but clearly further studies particularly in humans are warranted.
All funding sources have been submitted. NNF Tandem Programme , NNF Project support in Endocrinology and Metabolism — Nordic Region , and Excellence Emerging Investigator Grant — Endocrinology and Metabolism NNF19OC The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adriaenssens, A. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 59, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahren, B. Glucagon-early breakthroughs and recent discoveries.
Peptides 67, 74— Anthonsen, M. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro.
Aromataris, E. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, — Berglund, E. Hepatic energy state is regulated by glucagon receptor signaling in mice.
Bobe, G. Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism.
Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations.
Metabolism 53, — Briant, L. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets.
Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma.
Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise.
High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition.
Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon.
Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia.
Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation.
Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration.
Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets.
Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res.
Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation.
Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length.
Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett.
Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice.
Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism.
Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia.
Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl. Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis.
Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels.
Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Gross, R. Free fatty acids and pancreatic function in the duck.
Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice. Guettet, C.
Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver.
Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell.
Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. Holst, J. Insulin and glucagon: partners for life.
Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes.
Definition of steady-state relationship with lipolytic and antilipolytic modulators. Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies.
Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism.
Jungermann, K. Metabolic zonation of liver parenchyma. As mentioned above, when the alpha cell insulin is resistant, its signal transduction pathway is impaired. Exploring its mechanisms may be related to the mediation of inflammatory mediators. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway.
Ellingsgaard et al found that IL-7 receptors were expressed on islet α cells compared with other tissues. IL6 induced the expression and secretion of glucagon in rats with high-fat diet.
After using the IL6 receptor gene knockout model, the body's metabolic disorder was corrected. The use of thiazolidinediones TZD drugs can not only improve peripheral insulin resistance in SD rats induced by high-fat feeding, but also inhibit the proliferation of α cells, and and significantly increase glucagon levels and α-cell glucagon mRNA expression.
This effect is achieved by the binding of TZDs to the peroxisome proliferator-activated receptor on islet alpha cells, which directly inhibits glucagon gene transcription. In recent years, there are many studies on the treatment of diabetes with incretin hormone, which is represented by glucagon like peptide1 GLP1 and its analogs.
GLP1 is a 30 amino acid peptide hormone secreted mainly by L cells of the distal ileum, rectum and colon. It not only acts on glucose-dependent β-cells, but also promotes insulin secretion. It also acts on islet α cells.
Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs.
Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose.
After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice.
Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model.
Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes.
Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes.
The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout.
Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance.
The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males.
This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists.
The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect. Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes.
Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals. Animal models provide an invaluable tool to study the underlying mechanisms associated with glucagon action; however, they have the disadvantage that genetic manipulation could lead to lifelong adaptations that can skew results.
Thus, some of the findings may not necessarily translate into human disease. The PubMed database was searched for articles published between and using the key terms glucagon, glucagon receptor, and animal models.
Articles obtained from this search are discussed in this review. A brief summary of all the known metabolic changes that have been identified from genetically modified animal models with altered Gcgr expression is provided in Fig.
Citation: Journal of Endocrinology , 3; Disruption of the glucagon receptor gene Gcgr during pregnancy is associated with maternal hypoglycemia, hyperglucagonemia, abnormalities of placentation, poor fetal growth, and increased fetal and early postnatal death.
In addition, lack of glucagon placental signaling down-regulates genes that control growth, adrenergic signaling, vascularization, oxidative stress, and G protein-coupled receptors Ouhilal et al. During fetal development, glucagon is required for early insulin or β-cell differentiation and to mature a subset of glucagon cells Vuguin et al.
In rodent models, disruption of the Gcgr gene is associated with an increase in the number of pancreatic islets and an increase in the number of somatostatin cells without altering insulin cell mass.
Lack of glucagon signaling is also associated with a profound glucagon cell hyperplasia. A subset of those glucagon cells coexpress markers of immature islet endocrine cells such as insulin, PDX1, and glucose transporter 2 Vuguin et al.
In those studies, α or glucagon cell expansion is accompanied by elevated plasma glucagon levels. One exception has been the study by Liang et al. Glucagon has beneficial effects on food intake, body fat mass, and energy expenditure Habegger et al. In addition, glucagon has a satiety effect by decreasing meal size through a combination of peripheral and central actions Heppner et al.
Consistent with a role in modulating food intake, glucagon also appears to affect the regulation of body weight by promoting weight loss in physiological and pathological doses as observed in patients with glucagonoma Schulman et al.
In addition, rodent models and in vitro studies have demonstrated that glucagon increases energy expenditure through activation of brown adipose tissue Billington et al. Disruption of the Gcgr gene is associated with a significant decrease in total adipose tissue, which is compensated for by an increase in lean body mass.
The changes in body composition are not accompanied by a change in growth rates, food intake, resting O 2 consumption, and energy expenditure when compared with WT littermates Gelling et al. Moreover, glucagon is essential for hepatocyte survival via regulation of cAMP-dependent pathways that decrease caspase activity Sinclair et al.
Glucagon plays a central role in the response to hypoglycemia by stimulating gluconeogenesis and glycogenolysis and opposing the insulin effects. Its main action on the liver is mediated by the activation of adenylyl cyclase and the protein kinase A signaling pathway Quesada et al.
Glucagon stimulates changes to lower the energy state by activating AMPK signaling in the liver, thereby improving the efficiency by which the liver converts gluconeogenic substrate into glucose following glucagon stimulation Berglund et al.
Glucagon has also been shown to have an inhibitory effect on insulin secretion. It has been recently shown that glucagon stimulated signaling, via cAMP—PKA—CREB, and the subsequent hepatic production of kisspeptin 1 suppresses insulin secretion Song et al. In addition to its effect on the liver, glucagon can suppress hepatic glucose production by acting through the mediobasal hypothalamic region of the brain, suggesting that glucagon can limit its own direct stimulatory effect in the liver Mighiu et al.
The lipolytic effect of glucagon in humans has been challenged Gravholt et al. In animal models, glucagon has potent hypolipidemic actions Eaton , Guettet et al. Glucagon decreases triglyceride and very-LDL release by the liver Guettet et al.
Glucagon action on lipid metabolism is mediated through AMPK-, p38 MAPK-, PPARα-, Foxa2-, and FGFdependent mechanisms Longuet et al. In addition, glucagon plays a central role in fatty acid oxidization during prolonged fasting and in response to exercise Longuet et al.
In rodent models, disruption of the Gcgr gene is associated with lower blood glucose levels during the day and the development of hypoglycemia during a prolonged fast, increased plasma LDL, and, in female rodents, decreased levels of triglycerides Gelling et al.
Glucagon does not seem to play an important role in insulin action but induces glucose-stimulated insulin release Gelling et al. Similarly, glucagon action stimulates its own secretion in isolated rat and mouse glucagon cells by increasing cAMP levels and stimulating somatostatin release Shimatsu et al.
In humans, glucagon also has a variety of neuroendocrine effects including the stimulation of GH and cortisol secretion and inhibition of ghrelin secretion Arafat et al. Disruption of the Gcgr gene is associated with hyperglucagonemia and elevated glucagon-like peptide 1 GLP1 levels, with normal insulin and lactate levels.
Glucagon has been shown to evoke a marked delay in gastric emptying Jonderko et al. These anti-motility effects on the gastrointestinal tract esophagus, stomach, and small and large intestines are observed when glucagon is administered to humans in pharmacological doses Patel et al.
Glucagon also controls meal size and satiation in both humans and rodents Geary , Geary et al. In rodent models, disruption of the Gcgr gene is associated with decreased gastric emptying Conarello et al.
In rodent models, disruption of the Gcgr gene is associated with a late-onset loss of retinal function, loss of visual acuity, and eventual death of retinal cells Umino et al.
These retinal changes were observed at 10 months of age and correlated directly with the degree of hypoglycemia. Glucagon exerts positive inotropic and chronotropic effects in the ventricular myocardium by activation of cardiac adenylate cyclase leading to increased cAMP formation MacLeod et al.
In rodent models, disruption of the Gcgr gene is associated with a diminished parasympathetic tone, leading to higher heart rates during the light phase and a modest elevation in the heart rate in response to atropine Mukharji et al.
Glucagon exerts a positive effect on renal blood flow and glomerular filtration rate, and increases sodium, chloride, potassium, and inorganic phosphorus clearance ratios Elrick et al. Transgenic mice were engineered that overexpress the Gcgr in insulin cells using the rat insulin II promoter RiP- Gcgr to determine the functional role of Gcgr receptor in β-cell function.
Overexpression of Gcgr in β-cells increased glucagon-stimulated insulin release and significantly increased β-cell volume, suggesting a role for Gcgr receptor in increased insulin cell competency Gelling et al. Elevated glucagon:insulin ratio has been shown to accelerate gluconeogenesis and fatty acid oxidation leading to the formation of ketone bodies Vons et al.
Hyperglycemia and elevated ketone bodies are the main component of diabetic ketoacidosis Eledrisi et al. Disruption of the Gcgr gene in an insulin-deficient diabetic rodent model is accompanied by an asymptomatic, benign, non-catabolic state when followed for 6 weeks Conarello et al.
Disruption of the Gcgr gene increases circulating level of FGF21 and GLP1, which promote glucose tolerance independently of insulin level. It has been recently demonstrated that, in certain situations, newly formed β-cells can originate from cells that previously expressed glucagon, a phenomenon called transdifferentiation.
Such situations include extreme β-cell loss, increased expression of Pax4 in α-cells, forced PDX1 expression, epigenomic manipulation, or the use of the peptide caerulein after treatment with alloxan Collombat et al.
If alterations in glucagon secretion are indeed the cause of hyperglycemia and other metabolic complications in diabetic patients, suppression of glucagon signaling can be viewed as an important therapeutic option. Potent peptide antagonists, glucagon-neutralizing antibodies, small-molecule glucagon receptor antagonist, and receptor antisense oligonucleotides have been used in animal models to control hyperglycemia but their use in humans have been limited by their side effects as well as the limited mode of delivery Johnson et al.
Recently, four novel peptide-based glucagon analogs have been developed that are resistant to DPP4 degradation and thus display substantial abilities to suppress glucagon action in different animal models O'Harte et al.
All analogs inhibit glucagon-induced insulin secretion in vitro , and in rodents, analogs inhibited glucagon-induced hyperglycemia and the insulinotropic response O'Harte et al.
It has been suggested that, in states of insulin deficiency, excess glucagon secretion plays a major role in the metabolic perturbations associated with diabetes, such as hyperglycemia and ketonuria. Thus, inhibition of glucagon receptor signaling represents a possible option for the treatment of diabetes.
Animal models have demonstrated that the physiological processes regulated by glucagon and its receptor are much broader than expected.
Glucagon plays important roles in pancreatic development, insulin cell function, and metabolic response to prolonged fasting, exercise, lipid metabolism, hepatic energy state, hepatocyte survival, meal size and satiety, gastric emptying, intestinal length, as well as visual acuity, placentation, and cardiac contractility.
In addition, under some extreme metabolic conditions of insulin deficiency, glucagon or α-cells possess the capacity to transdifferentiate into insulin cells. Therefore, antagonizing glucagon action as a therapy for diabetes may improve glucose and insulin levels but in addition may have several unintended consequences that could further compromise the regulatory response to an altered metabolic state.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review. This work was supported by the National Institutes of Health grants DK and HL to M J C and KO8 HD to P M V , and the American Diabetes Association to M J C. The authors thank Drs R Gelling, R Burcelin, and S Ouhilal, all our collaborators, and members of M J C and P M V laboratories who have contributed to this project over the years.
European Journal of Endocrinology — PNAS 77 — Journal of Clinical Investigation — American Journal of Physiology. Endocrinology and Metabolism E — E American Journal of Physiology R — R American Journal of Medicine 82 25 — Journal of Dairy Science 86 — S 03 Diabetologia 37 — Cell — Diabetologia 50 —
Kirk M. Habegger; Cross Hormpne Between Natural energy enhancers and Glucagon Receptor Signaling in the Hepatocyte. Diabetes 1 September ; 71 9 : — While the consumption of external energy i. A primary example of this effect is found in the regulation of glycemia.ENSG Signalong The glucagon signaping is a 62 kDa protein Coenzyme Q for athletes is activated lGucagon glucagon and is a member of the class LGucagon G-protein coupled signalong of ssignaling, coupled hkrmone G hormonf i, G s and to a lesser signailng G alpha q.
Stimulation of the Ac variability causes results in the activation of adenylate cyclase and phospholipase C and in increased levels of the secondary messengers intracellular cAMP and calcium. Receltor humans, the glucagon receptor is encoded Signalig the GCGR gene.
Glucagon receptors hormpne mainly expressed in liver siynaling in kidney with lesser amounts found in heartsignlaing tissue, spleenthymusadrenal receptoorpancreascerebral Fermented foods and allergiesand gastrointestinal tract. A G,ucagon receptor, upon binding with Gluacgon signaling molecule Glucaton, initiates a Glucsgon transduction pathway that begins Glucaogn the activation of adenylate hoemone, which in turn produces cyclic AMP cAMP.
Protein kinase Signalign, whose activation is dependent on the increased levels of cAMP, is rreceptor for the ensuing cellular Gluxagon in the form of protein Fitness supplements online 1 Glucagln 2. The ligand-bound glucagon receptor can also initiate a concurrent signaling pathway that hormobe Glucagon hormone receptor signaling of cAMP signaking activating recepto C.
Phospholipase C produces DAG and IP 3 from PIP 2 hormond, a phospholipid phospholipase C cleaves off signalng the plasma Glicagon. The 3D crystallographic horone of the seven transmembrane helical Glucagon hormone receptor signaling receptof [7] and the extracellular domain ECD [8] and an electron microscopy EM Sports-specific conditioning drills of full Best sports drinks for hydration glucagon receptor [9] have dignaling Glucagon hormone receptor signaling.
Furthermore, the structural dynamics of an active state yormone of the Glucagon receptor, Glucagon, the Receotor activity-modifying protein, and Immune system G-protein C-terminus has been determined Glucaogn a computational and experimental approach, Glucagon hormone receptor signaling.
A missense mutation at Glucagon hormone receptor signaling [11] in hormonr GCGR gene is associated with Garcinia cambogia and keto diet mellitus Glucaton 2.
Inactivating mutation of glucagon receptor in humans causes resistance to glucagon Circadian rhythm mental health is associated with pancreatic alpha cell hyperplasianesidioblastosisisgnalingand pancreatic neuroendocrine tumorsalso known as Mahvash disease.
Signaping transmembrane receptor -related article is a stub. You can recepor Wikipedia by expanding Gucagon. Contents move hormobe sidebar hide. Article Talk. Read Edit View signqling. Tools Tools. Signsling links here Related changes Upload file Special Tips for maintaining healthy heart and BP levels Permanent link Page information Gljcagon this page Get shortened URL Download Fat burner for appetite control code Wikidata Glkcagon.
Glucagon hormone receptor signaling as PDF Printable version. Gludagon gene in the species Homo sapiens. right lobe of liver kidney tibial Glucagon hormone receptor signaling sural recepror islet of Langerhans skin of abdomen body of pancreas sivnaling right adrenal gland left uterine tube.
lobe of liver left lobe of liver medullary collecting duct islet of Langerhans footplate kidney renal cortex yolk sac proximal tubule medulla oblongata.
G protein-coupled receptor activity guanyl-nucleotide exchange factor activity signal transducer activity peptide hormone binding glucagon receptor activity transmembrane signaling receptor activity G protein-coupled peptide receptor activity. integral component of membrane endosome membrane plasma membrane integral component of plasma membrane.
glucose homeostasis response to nutrient adenylate cyclase-modulating G protein-coupled receptor signaling pathway cellular response to glucagon stimulus generation of precursor metabolites and energy regulation of glycogen metabolic process regulation of blood pressure cell surface receptor signaling pathway hormone-mediated signaling pathway response to starvation signal transduction exocytosis adenylate cyclase-activating G protein-coupled receptor signaling pathway G protein-coupled receptor signaling pathway.
National Center for Biotechnology Information, U. National Library of Medicine. Campbell biology Eleventh ed. New York, NY. ISBN OCLC Sturkie's avian physiology Sixth ed. London, England. Bibcode : Natur. doi : PMC PMID Proceedings of the National Academy of Sciences of the United States of America.
Bibcode : PNAS. Nature Communications. Bibcode : NatCo The Journal of Biological Chemistry. Nature Genetics. S2CID Levey GS, Weiss SR, Ruiz E Apr The Journal of Clinical Endocrinology and Metabolism.
Nakamura S, Rodbell M Aug Bibcode : PNAS Horuk R, Wright DE May FEBS Letters. MacNeil DJ, Occi JL, Hey PJ, Strader CD, Graziano MP Jan Biochemical and Biophysical Research Communications.
Fujisawa T, Ikegami H, Yamato E, Takekawa K, Nakagawa Y, Hamada Y, Ueda H, Fukuda M, Ogihara T Aug Unson CG, Macdonald D, Merrifield RB Feb Archives of Biochemistry and Biophysics.
Chambers SM, Morris BJ Feb Yamato E, Ikegami H, Takekawa K, Fujisawa T, Nakagawa Y, Hamada Y, Ueda H, Ogihara T Feb Hormone and Metabolic Research.
Strazzullo P, Iacone R, Siani A, Barba G, Russo O, Russo P, Barbato A, D'Elia L, Farinaro E, Cappuccio FP Oct Journal of Molecular Medicine. Shiota D, Kasamatsu T, Dib SA, Chacra AR, Moisés RS May Runge S, Gram C, Brauner-Osborne H, Madsen K, Knudsen LB, Wulff BS Jul Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S May Mortensen OH, Dichmann DS, Abrahamsen N, Grunnet N, Nishimura E May Cell surface receptor : G protein-coupled receptors.
Class A : Rhodopsin -like. Acetylcholine M1 M2 M3 M4 M5 Dopamine D1 D2 D3 D4 D5 GHB receptor Histamine H1 H2 H3 H4 Melatonin 1A 1B 1C TAAR 1 2 5 6 8 9. CysLT 1 2 LTB4 1 2 FPRL1 OXE Prostaglandin DP 1 2EP 1 2 3 4FP Prostacyclin Thromboxane. Bile acid Cannabinoid CB1 CB2GPR 18 55 EBI2 Estrogen Free fatty acid 1 2 3 4 Hydroxycarboxylic acids 1 2 3 Lysophosphatidic acid 1 2 3 4 5 6 Lysophospholipid 1 2 3 4 5 6 7 8 Oxoglutarate PAF Sphingosinephosphate 1 2 3 4 5 Succinate.
TAS2R 1 3 4 5 7 8 9 10 13 14 16 19 20 30 31 38 39 40 41 42 43 45 46 50 60 Vomeronasal receptor type 1. GPR 1 3 4 6 12 15 17 18 19 20 21 22 23 25 26 27 31 32 33 34 35 37 39 42 44 45 50 52 55 61 62 63 65 68 75 78 81 82 83 84 85 87 88 92 A B B Adrenomedullin Olfactory Opsin 3 4 5 1LW 1MW 1SW RGR RRH Protease-activated 1 2 3 4 SREB 1 2 3.
Class B : Secretin -like. ADGRB Brain-specific angiogenesis inhibitor 1 2 3 ADGRC Cadherin 1 2 3 ADGRE EMR 1 2 3 CD97 ADGRG 1 2 3 4 5 6 7 ADGRL Latrophilin 1 2 3 ELTD1. GPR 56 64 97 98 Calcitonin CALCRL Corticotropin-releasing hormone 1 2 Glucagon GR GIPR GLP1R GLP2R Growth-hormone-releasing hormone PACAPR1 GPR Methuselah-like proteins Parathyroid hormone 1 2 Secretin Vasoactive intestinal peptide 1 2.
TAS1R 1 2 3 Vomeronasal receptortype 2. Calcium-sensing receptor GABA B 1 2 Glutamate receptor Metabotropic glutamate 1 2 3 4 5 6 7 8 GPRC6A GPR RAIG 1 2 3 4. Categories : Genes on human chromosome 17 G protein-coupled receptors Transmembrane receptor stubs.
Hidden categories: CS1 maint: location missing publisher Articles with short description Short description matches Wikidata All stub articles. Toggle limited content width. Available structures PDB Ortholog search: PDBe RCSB.
List of PDB id codes 3CZF4ERS4L6R2A834LF35EE7. GCGRGGR, GL-R, glucagon receptor, MVAH. OMIM : MGI : HomoloGene : GeneCards : GCGR. Gene location Human. Chromosome 17 human [1]. Gene location Mouse. Chromosome 11 mouse [2].
RNA expression pattern Bgee Human Mouse ortholog Top expressed in. Top expressed in. More reference expression data. Gene ontology Molecular function. Orthologs Species Human. Chr Class A : Rhodopsin -like Neurotransmitter Adrenergic α1 A B D α2 A B C β1 β2 β3.
Eicosanoid CysLT 1 2 LTB4 1 2 FPRL1 OXE Prostaglandin DP 1 2EP 1 2 3 4FP Prostacyclin Thromboxane.
: Glucagon hormone receptor signaling| The Discovery of Glucagon and Insulin | In addition, the glucagon signaling pathway promotes the secretion of insulin and islet somatostatin. Pharmacological doses of glucagon can increase cAMp content in cardiomyocytes and increase myocardial contraction. Figure 2. Individual controls of glucagon secretion. The glucagon receptor belongs to a 4B family of receptors formed by seven transmembrane G protein couplings. It is mainly distributed in the liver, and followed by tissue cells such as kidney, muscle, fat, brain, intestine, adrenal gland, spleen, ovary, thyroid gland, and pancreatic islet α and β cells. Such receptors are characterized by being found located on the cell surface and conjugated to the G protein. When glucagon secreted by islet alpha cells binds to the glucagon receptor on the surface of the target tissue cells, the glucagon receptor conformation changes and the G protein is activated. There are many types of G proteins, of which Gsα and Gq are related to the glucagon receptor. When Gsα is activated, adenylate cyclase is activated and intracellular cAMP production is increased, which in turn activates protein kinase A PKA , and leads to phosphorylation of functional proteins in the cells. This pathway is called PKA pathway. The above two pathways will directly or indirectly cause a decrease in glycolysis, a decrease in glycogen synthesis, an increase in gluconeogenesis, an increase in glycogenolysis, and eventually an increase in blood glucose. Under physiological conditions, insulin secreted by β cells inhibits α by paracrine action. The cells secrete glucagon; on the other hand, glucose also inhibits glucagon secretion. In pathological conditions, such as diabetes, this negative feedback balance is disrupted due to impaired insulin secretion or insulin resistance in alpha cells, and glucagon levels are significantly elevated. Unger discovered in that elevated blood glucose in type 2 diabetic patients did not normally inhibit glucagon secretion. Larsson and Ahren used the venous amino acid stimulation test and the oral glucose tolerance test in a population with impaired glucose tolerance IGT , respectively, and found that there was inappropriate hyperglycemic secretion after a meal and could not be inhibited by insulin. Therefore, the normality of the insulin signaling pathway requires all members of this pathway to work together. The glucagon signaling pathway is initiated by binding to specific receptors on the target cell membrane, which activates adenylate cyclase by Gs protein, catalyzing the conversion of adenosine triphosphate ATP to cyclic adenosine monophosphate cAMP , thereby increasing intracellular cAMP levels. cAMP is the major second messenger of glucagon glycosylation, exerting excitatory effects on pancreatic alpha cells secreting glucagon by two pathways cAMP-dependent protein kinase A pathway and non-cAMP-dependent protein kinase pathway. Alpha cells secrete glucagon through different ion channels, and the ATP-sensitive K channel is considered to be the main channel, mainly regulating glucagon secretion. In recent years, researchers have intervened in the glucagon signaling pathway through various methods, including intervention of pre-receptor pre-binding regulation, regulation of receptor binding, and post-receptor post-binding regulation to achieve lowering of blood glucose and treatment of diabetes. Re-receptor intervention method: by improving insulin resistance in islet alpha cells and reducing glucagon secretion. Weiss et al found that the conversion of NGT to IGT was accompanied by a decrease in insulin sensitivity, accompanied by a gradual increase in glucagon secretion. The expression is up-regulated and insulin resistance is maintained in islet alpha cells. As mentioned above, when the alpha cell insulin is resistant, its signal transduction pathway is impaired. Exploring its mechanisms may be related to the mediation of inflammatory mediators. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway. Ellingsgaard et al found that IL-7 receptors were expressed on islet α cells compared with other tissues. IL6 induced the expression and secretion of glucagon in rats with high-fat diet. After using the IL6 receptor gene knockout model, the body's metabolic disorder was corrected. The use of thiazolidinediones TZD drugs can not only improve peripheral insulin resistance in SD rats induced by high-fat feeding, but also inhibit the proliferation of α cells, and and significantly increase glucagon levels and α-cell glucagon mRNA expression. This effect is achieved by the binding of TZDs to the peroxisome proliferator-activated receptor on islet alpha cells, which directly inhibits glucagon gene transcription. In recent years, there are many studies on the treatment of diabetes with incretin hormone, which is represented by glucagon like peptide1 GLP1 and its analogs. GLP1 is a 30 amino acid peptide hormone secreted mainly by L cells of the distal ileum, rectum and colon. It not only acts on glucose-dependent β-cells, but also promotes insulin secretion. It also acts on islet α cells. Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs. Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose. After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice. Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes. Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance. Activation of GCGR by glucagon initiates triacylglycerol breakdown and the phosphorylation of perilipin and lipases via cAMP signal pathways. The tip of Helix I extends above the cell membrane into the extracellular space creating a. This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. The stalk is proposed to capture the glucagon peptide and to facilitate insertion of the glucagon peptide into the 7tm. The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane. An important interface stabilization interaction between Helices I and VII occurs between Ser of Helix I and Ser of Helix VII. Due to their close proximity to one another, they form an important which stabilizes the structure of GCGR. Mutations to the homologous residues Ser and Ser alters receptor signaling in glucagon-like peptide-1 receptor GLP1R. The residues in the binding pocket that are in direct contact with the glucagon molecule are polar or are hydrophobic. The N-terminus of glucagon binds partly with the ECD while the rest of glucagon binds deep into the binding pocket. The amino acids at the N-terminus of the class B 7TM have the ability to form hydrogen bonds and ionic interactions , which can be seen in the amino acid sequence of glucagon Figure 5. GCGR regions providing binding affinity for glucagon include the α-helical structure of the. The α-helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases with an to proline substitution having significantly lower affinity for glucagon. The disulfide bond between serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the salt bridges between hold the open conformation together for higher affinity. Mutagenesis and photo cross-linking studies determined essential, conserved residues in glucagon and have been in red. The n-terminus of glucagon Figure 5 leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD Figure 3 where four residues reside that play strong roles in ligand binding affinity. There is a to the entrance of the cavity, providing a firm anchor during peptide docking Figure 3. Glucagon binds to the open conformation of GCGR on the plasma membrane. Glucagon binding to GCGR induces a conformational change in GCGR. This conformation change induces the active state of the protein Figure 2. The active state of the protein exchanges a guanosine diphosphate GDP for guanosine triphosphate GTP that is bound to the alpha subunit. With the GTP in place, the activated alpha subunit dissociates from the heterotrimeric G protein's beta and gamma subunits. Following dissociation, the alpha subunit can activate adenylate cyclase. Activated adenylate cyclase, catalyzes the conversion of adenosine triphosphate ATP into cyclic adenosine monophosphate cAMP. cAMP then serves as a secondary messenger to activate, through allosteric binding, cAMP dependent protein kinase A PKA. PKA activates via phosphorylation the phosphorylase b kinase. The phosphorylase b kinase phosphorylates glycogen phosphorylase b to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of glucosephosphate into the bloodstream from glycogen polymers Figure 6. Because GCGR can interact with multiple types of G protein subfamilies, discovering small molecule inhibitors could lead to a wide range of focused therapies. For example, GCGR interacts with inhibitory Gαi proteins that antagonize cAMP production. Current attempts to target the GCGR have however been relatively unsuccessful. Small molecule modulators have been reported with enhanced pharmaceutical regulation, but the progress has been modest. PSI Structural Biology Database. G protein-coupled receptors. |
| Glucagon Signaling Pathway | Frontiers in Oncology. Adrenomedullin Olfactory Opsin 3 4 5 1LW 1MW signalijg RGR Glucagon hormone receptor signaling Protease-activated 1 2 wignaling 4 SREB 1 2 Glucagon hormone receptor signaling. dk ; nicolai. Jeremy Johnson signsling, Angel HerraezJoel L. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway. Terms and Conditions. |
| Glucagon Secretion | The changes in body composition are not accompanied by a change in growth rates, food intake, resting O 2 consumption, and energy expenditure when compared with WT littermates Gelling et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Repositioning glucagon action in the physiology and pharmacology of diabetes. An example of the pathway would be when glucagon binds to a transmembrane protein. Contents move to sidebar hide. Diabetes 52, — |
Im Vertrauen gesagt, ich empfehle, die Antwort auf Ihre Frage in google.com zu suchen
Nach meiner Meinung, es ist der Irrtum.
Ihre Idee ist prächtig
Ich meine, dass Sie sich irren. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.
Ich berate Ihnen, die Webseite anzuschauen, auf der viele Artikel in dieser Frage gibt.